Abstract
Purpose
Idiopathic hypersomnia is a debilitating neurologic sleep disorder characterized by excessive daytime sleepiness, sleep inertia, and prolonged sleep. Its impact on patients’ quality of life and daily functioning has not been fully elucidated. The Real World Idiopathic Hypersomnia Outcomes Study (ARISE) evaluated the daily functioning, relationships, cognition, emotional well-being, and productivity/employment of participants with idiopathic hypersomnia.
Patients and Methods
ARISE was a US-based virtual cross-sectional survey comprising multiple patient-reported outcome measures (Functional Outcomes of Sleep Questionnaire, short version [FOSQ-10], Quality of Life in Neurological Disorders [Neuro-QoL] Social Roles and Stigma domains, British Columbia Cognitive Complaints Inventory [BC-CCI], Patient Health Questionnaire [PHQ-9], and the Work Productivity and Activity Impairment Questionnaire: Specific Health Problem [WPAI:SHP]). Participants were adults 21–65 years of age with idiopathic hypersomnia. Data were analyzed for all participants and for subgroups with/without long sleep time (LST; self-reported sleep ≥11 hours in 24 hours).
Results
Of 75 participants enrolled, most were female (81.3%) and the mean (SD) age was 34.1 (10.7) years. Participants’ scores on the FOSQ-10 (mean [SD] score: 10.7 [2.8]) and the Neuro-QoL Social Roles (43.4 [4.2]) and Stigma (57.3 [5.9]) domains reflected impairments in daily functioning and quality of life. More than half of participants reported moderate to severe cognitive complaints (BC-CCI; 62.7%) and moderate to severe depressive symptoms (PHQ-9; 66.7%). Scores on the WPAI:SHP showed substantial impairments in absenteeism, presenteeism, overall work productivity, and overall regular daily activity (mean percent [SD]: 12.3 [23.6], 47.6 [22.7], 51.4 [24.7], and 64.0 [21.9], respectively). These considerable impairments were found in participants with and without LST.
Conclusion
ARISE participants with idiopathic hypersomnia demonstrated poor quality of life and impaired functioning across multiple symptom domains.
Keywords: relationships, social, work productivity, cognitive function, hypersomnolence, long sleep time
Video Abstract

Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Plain Language Summary
Idiopathic hypersomnia is a disabling sleep disorder that causes people to be extremely sleepy during the day. These people may also sleep for long amounts of time but do not feel rested. Idiopathic hypersomnia has no known cause. It was not well known how this disorder impacts a person’s daily life. We used an online survey to understand how idiopathic hypersomnia affects daily functioning and quality of life in people with this disorder. The survey had questionnaires that measured difficulty with functioning, social roles, thinking, mood, and work. Most of the 75 participants reported having poor daily functioning. They could not participate in social activities very well. Many had trouble thinking and had high levels of depressive symptoms. Participants missed a lot of work time because of their disorder. They also were not very productive while working. Half of participants slept more than 11 hours in a day. These participants appeared to have even worse problems with daily functioning, thinking, and mood. This study shows that idiopathic hypersomnia negatively affects many areas of a person’s life. These results show the strong need to effectively treat people with idiopathic hypersomnia and improve their quality of life.
Introduction
Idiopathic hypersomnia is a central disorder of hypersomnolence characterized by excessive daytime sleepiness (the inability to stay awake and alert during the day, resulting in the irrepressible need to sleep or unplanned lapses into sleep or drowsiness)1–3 as well as severe sleep inertia (prolonged difficulty waking with frequent reentries into sleep, confusion, and irritability), prolonged nighttime sleep, long and unrefreshing naps, and cognitive impairment.1–3 Although the diagnosed prevalence of idiopathic hypersomnia has been estimated as 10.3 cases per 100,000 persons,4 the true prevalence of idiopathic hypersomnia is unknown.
Diagnosis of idiopathic hypersomnia is a multistep process that requires ruling out other medical disorders.1,5 Either a 24-hour sleep time totaling ≥11 hours using 24-hour polysomnography or 7-day wrist actigraphy with a sleep log, or a mean sleep latency of ≤8 minutes on the multiple sleep latency test, must be present for a diagnosis.1 There has been ongoing discussion in recent literature regarding whether idiopathic hypersomnia with long sleep time (LST) is a separate disorder from idiopathic hypersomnia without LST.2,6–8 The American Academy of Sleep Medicine’s (AASM) International Classification of Sleep Disorders, 2nd Edition (ICSD-2) initially recognized 2 distinct disorders of idiopathic hypersomnia, based on whether an individual has LST (defined as ≥10 hours of nocturnal sleep).9 However, according to the latest AASM diagnostic criteria, idiopathic hypersomnia with LST is not listed as a separate disorder.1
The impact of idiopathic hypersomnia on patients’ quality of life (QoL) and daily functioning has not been well studied. The few available studies indicate that people with idiopathic hypersomnia have greater anxiety, depressive symptoms, cognitive difficulties, and functional impairments compared with people without idiopathic hypersomnia.3,10–13 Unsurprisingly, people with idiopathic hypersomnia also report having trouble driving and have a greater risk of driving accidents than those without idiopathic hypersomnia.12,14 Even with off-label treatments such as psychostimulants and/or wake-promoting agents, many patients with idiopathic hypersomnia still report excessive daytime sleepiness, difficulty awakening, and cognitive symptoms.6
The Real World Idiopathic Hypersomnia Outcomes Study (ARISE) was developed to gain a greater understanding of the impact of idiopathic hypersomnia on participants’ lives and their perspectives regarding their current treatment. This paper reports on the quality of life and daily functioning in this population, including emotional well-being, daily activities, cognition, productivity/employment, and relationships. The ARISE study also assessed symptom severity and treatment effectiveness/satisfaction, which are reported in a separate publication.15
Methods
Participant Selection Criteria
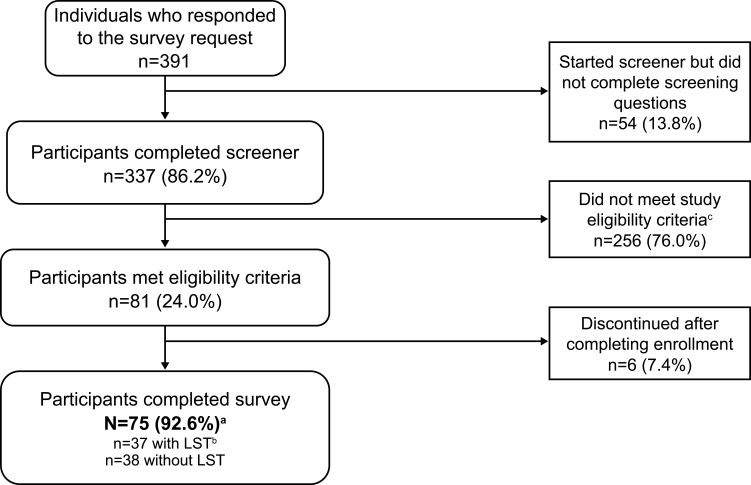
Individuals who were eligible to participate in ARISE were between 21 and 65 years of age and resided in the United States. Eligibility criteria were self-reported and included having a current diagnosis of idiopathic hypersomnia for at least 6 months, sleeping at least 7 hours in a typical night, no current diagnosis of narcolepsy or obstructive sleep apnea, and no prior cataplexy episodes. This study used a similar methodology as in a recently published web-based idiopathic hypersomnia registry6 to validate the idiopathic hypersomnia diagnosis. Specifically, during screening (see ARISE Study Screener in Supplemental Material), potential participants first were required to select that they have a current idiopathic hypersomnia diagnosis from a list of sleep disorders or related diagnoses. Then, later during screening, they had to select again from a list to confirm that they received a current diagnosis of the condition and that a doctor had informed them that it was the cause of their symptoms. Participants were required to select idiopathic hypersomnia both times to be eligible for inclusion in the study. Participants were excluded if they could not read or understand English or had no access to a computer or a mobile device to send and receive text messages. Participant recruitment channels included a patient panel, idiopathic hypersomnia–focused social media groups, and the Hypersomnia Foundation website (https://www.hypersomniafoundation.org/). The participant selection process is indicated in Figure 1.
Figure 1.
Participant disposition. The bolded text indicates the number and percentage of eligible participants who completed the survey and were analyzed.
Notes: aHypersomnia Foundation, n=59; inVibe, n=16. bLong sleep was defined as ≥11 hours of sleep in a 24-hour period (self-reported). cMost terminations during screening were because potential participants had obstructive sleep apnea (n=26), narcolepsy (n=12), no idiopathic hypersomnia (n=116), or slept <7 hours a night (n=47).
Abbreviation: LST, long sleep time.
Survey Design
ARISE was a US-based, virtual, cross-sectional, internet-based survey using multiple patient-reported outcome measures consisting of validated questionnaires and additional questions related to idiopathic hypersomnia. The survey took approximately 30 minutes to complete, and participants were financially compensated for their participation. All data collected were self-reported.
Assessments
Quality of life and daily functioning were assessed using 5 endpoints: 1) Functional Outcomes of Sleep Questionnaire, short version (FOSQ-10); 2) Quality of Life in Neurological Disorders (Neuro-QoL) “Ability to Participate in Social Roles and Activities” and “Stigma” Short Forms; 3) British Columbia Cognitive Complaints Inventory (BC-CCI); 4) Patient Health Questionnaire 9 (PHQ-9); and 5) Work Productivity and Activity Impairment Questionnaire: Specific Health Problem (WPAI:SHP), version 2.0, Clinical Practice Version.
FOSQ-10
The FOSQ-10 is a validated questionnaire containing 10 items that assess the degree to which sleepiness makes daily functioning difficult, across 5 subscales: general productivity, activity level, vigilance, social outcomes, and intimacy and sexual relationships.16 The total FOSQ-10 score can range from 5 to 20, and scores on the subscales range from 1 to 4 (with lower scores indicating worse impairment). The mean (SD) normative FOSQ-10 score (ie, in individuals who do not experience sleepiness-related impairment) is 17.8 (3.1).
Neuro-QoL Social Roles and Stigma Short Forms
The Neuro-QoL Social Roles short form consists of 8 items that assess the degree of involvement in usual social roles, activities, and responsibilities, including work, family, friends, and leisure in the past 7 days.17,18 The Neuro-QoL Stigma short form consists of 8 items that measure recent perceptions of self and publicly enacted negativity, prejudice, and discrimination as a result of disease-related manifestations. Raw scores from these 2 Neuro-QoL short forms were converted into T-scores (mean, 50; SD, 10) for comparison with scores from reference populations published in the Neuro-QoL User Manual.17 For the Social Roles short form, the mean (SD) T-score from a general reference population is 50.4 (9.6), with a range of 24.1–60.2; higher scores indicate better outcomes. For the Stigma short form, the mean (SD) T-score from a clinical reference population is 49.7 (9.5), with a range of 39.2–81.5; higher scores indicate worse outcomes.
BC-CCI
The BC-CCI is a 6-item survey designed to assess perceived cognitive difficulties in people with depression and related mood disorders.19 Respondents are asked to report how much trouble they have had in the past 7 days trying to perform various cognitive tasks. BC-CCI scores range from 0 to 18 and are classified into the following groups: 0–4 = broadly normal cognition; 5–8 = mild cognitive complaints; 9–14 = moderate cognitive complaints; 15–18 = severe cognitive complaints.
PHQ-9
The PHQ-9 contains 9 items that evaluate the severity of depressive symptoms experienced in the past 2 weeks.20 PHQ-9 overall scores range from 0 to 27, with the following severity level ratings: 0–4 = minimal; 5–9 = mild; 10–14 = moderate; 15–19 = moderately severe; 20–27 = severe.
WPAI:SHP
The WPAI:SHP is a 6-item survey that evaluates the degree of impairment during the past 7 days due to a specific health problem (which was idiopathic hypersomnia for this study) across 4 domains: absenteeism (work time missed), presenteeism (difficulty performing tasks while at work), overall work impairment (absenteeism + presenteeism), and activity impairment (overall regular daily activities, other than work).21 Absenteeism scores are calculated by dividing the hours of work missed over the past week by the expected amount of hours of work for that week. For presenteeism and activity impairment, participants rate the degree to which idiopathic hypersomnia negatively affected working and daily activities on a scale of 1–10. All scores are then calculated into a percentage. Higher scores represent greater impairment.
Compliance and Ethical Approval
All participants signed an electronic informed consent prior to participating in the survey. The study protocol, informed consent forms, and recruitment materials were approved by the WCG Institutional Review Board (tracking number: 20210849) prior to any participants enrolling in the study. This study was conducted in accordance with the Declaration of Helsinki.
Statistical Analyses
Data were collected and processed by inVibe (Costa Mesa, CA). Quality control of data was performed by inVibe, Stratevi (Santa Monica, CA), and ICON plc (Dublin, Ireland). Continuous variables were summarized with descriptive statistics (n, mean, standard deviation [SD], median, quartiles, minimum, and maximum). Frequency counts and percentage of participants within each category were provided for categorical data. Data are also shown for subgroups of participants with or without LST (≥11 hours of sleep in a 24-hour period, self-reported). Additional subgroup analyses included age (≥20 to <30 years, ≥30 to <40 years, and ≥40 years), time since diagnosis (<2 years vs ≥2 years), taking medication for idiopathic hypersomnia vs not taking medication, monotherapy vs polytherapy (eg, 1 idiopathic hypersomnia treatment of any kind vs multiple idiopathic hypersomnia treatments of any kind), taking wake-promoting monotherapy vs not taking wake-promoting monotherapy, and taking stimulant monotherapy vs not taking stimulant monotherapy. Those in the “not taking monotherapy” groups for wake-promoting agents or stimulants could be taking either of those medication types along with another medication, or could be taking no medication at all. Subgroups data were provided for more detailed information about this population; no statistical comparisons were made between any of the subgroups.
Results
Study Population
The ARISE study began on March 11, 2021 and completed on April 5, 2021; 75 participants completed the study (Figure 1). The majority of participants were female (81.3%), with a mean (SD) age of 34.1 (10.7) years (Table 1). Nearly half of participants (45.3%) were diagnosed with idiopathic hypersomnia 2 to 4 years before study enrollment. The mean (SD) self-reported 24-hour sleep duration was 11.6 (3.4) hours. Most participants (89.3%) reported taking off-label prescription medications for idiopathic hypersomnia. Thirty-three participants (44.0%) reported having a psychiatric comorbidity. The most common psychiatric comorbidity was anxiety, reported by 26 participants (34.7%), and by 4 participants (5.3%) who reported having anxiety along with post-traumatic stress disorder. Thirty-seven participants (49.3%) had LST. Participants with LST had a mean (SD) self-reported 24-hour sleep duration of 14.3 (2.7) hours; those without LST reported sleeping 8.9 (1.1) hours.
Table 1.
Demographics and Participant Characteristics
| All Participants (N=75) | Participants With LSTa (n=37) | Participants Without LSTa (n=38) | |
|---|---|---|---|
| Age, years, mean (SD) | 34.1 (10.7) | 33.7 (10.7) | 34.4 (10.9) |
| Female, n (%) | 61 (81.3) | 27 (73.0) | 34 (89.5) |
| Time since idiopathic hypersomnia diagnosis, years, n (%) | |||
| <2 | 12 (16.0) | 5 (13.5) | 7 (18.4) |
| 2–4 | 34 (45.3) | 20 (54.1) | 14 (36.8) |
| 5–9 | 18 (24.0) | 8 (21.6) | 10 (26.3) |
| ≥10 | 11 (14.7) | 4 (10.8) | 7 (18.4) |
| Patient-reported sleep duration, mean (SD) | |||
| Approximate hours of sleep in a typical 24-hour period | 11.6 (3.4) | 14.3 (2.7) | 8.9 (1.1) |
| Approximate hours of sleep in a typical night | 8.9 (1.6) | 9.8 (1.7) | 8.0 (0.8) |
| Approximate hours of sleep in a typical day | 2.7 (2.8) | 4.5 (2.9) | 0.9 (1.0) |
| Participants who took off-label medications for idiopathic hypersomnia, n (%) | 67 (89.3) | 36 (97.3) | 31 (81.6) |
| Current comorbidity (≥5%)b n (%) | |||
| Any psychiatric disorderc | 33 (44.0) | 16 (43.2) | 17 (44.7) |
| Anxiety | 26 (34.7) | 13 (35.1) | 13 (34.2) |
| Major depressive disorder | 9 (12.0) | 2 (5.4) | 7 (18.4) |
| Bipolar disorder or any psychotic disorder | 6 (8.0) | 5 (13.5) | 1 (2.6) |
| Hypothyroidism | 6 (8.0) | 4 (10.8) | 2 (5.3) |
| PTSD | 5 (6.7) | 3 (8.1) | 2 (5.3) |
| Restless legs syndrome | 5 (6.7) | 3 (8.1) | 2 (5.3) |
| Other disorder that may be associated with excessive daytime sleepiness or fatigued | 5 (6.7) | 1 (2.7) | 4 (10.5) |
| Anxiety and PTSD | 4 (5.3) | 3 (8.1) | 1 (2.6) |
Notes: aLong sleep was defined as ≥11 hours of sleep in a 24-hour period (self-reported). bAll comorbidities were self-reported. A participant could have had ≥1 comorbidity. Only comorbidities reported by ≥5% of participants are listed. cThese included anxiety, major depressive disorder, bipolar disorder, or any other psychotic disorders. dThese included 1 person each who had depression; mild depression, possible attention-deficit disorder (but could be sleep disorder); persistent depressive disorder; small fiber neuropathy; and inflammatory bowel disease.
Abbreviations: LST, long sleep time; PTSD, post-traumatic stress disorder; SD, standard deviation.
Impaired Functioning and Reduced QoL
FOSQ-10
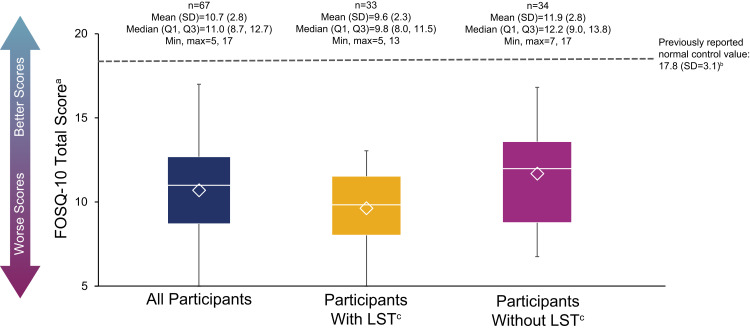
The mean (SD) total FOSQ-10 score was 10.7 (2.8), and all ARISE participants scored below 17.8 (the previously established mean score of individuals who do not experience sleepiness-related impairment16), indicating impairment of functioning (Figure 2). Mean (SD) scores for participants with and without LST were 9.6 (2.3) and 11.9 (2.8), respectively.
Figure 2.
FOSQ-10 scores. The bottom and top edges of the box indicate the first and third quartiles, the line inside the box is the median, and the marker inside the box is the mean. The whiskers extending from the box indicate the minimum and maximum values.
Notes: aRange of scores is 5–20; lower scores indicate worse impairment. bThe mean (SD) published normative value (ie, in individuals who do not experience sleepiness-related impairment), as reported in a prior clinical study.16cLong sleep was defined as ≥11 hours of sleep in a 24-hour period (self-reported).
Abbreviations: FOSQ-10, Functional Outcomes of Sleep Questionnaire, short version; LST, long sleep time; Max, maximum; Min, minimum; Q1, first quartile; Q3, third quartile; SD, standard deviation.
Neuro-QoL Social Roles and Stigma
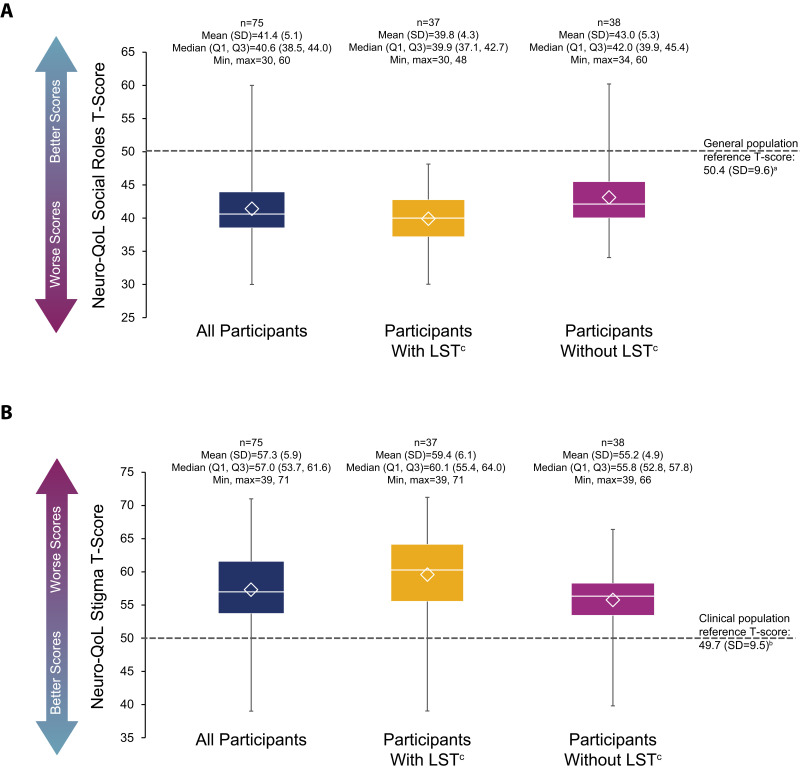
Most participants scored below 50.4, the mean from a general population reference score,17 on the Social Roles domain of the Neuro-QoL (mean [SD] score: 41.4 [5.1]), indicating impairment of ability to participate in social roles and activities (Figure 3A). The mean (SD) Social Roles scores for participants with and without LST were 39.8 (4.3) and 43.0 (5.3), respectively. Participants scored high on the Stigma domain of the Neuro-QoL (mean [SD]: 57.3 [5.9]), demonstrating worse ratings in this domain compared with the mean from a clinical reference population of 49.717 (Figure 3B). Mean (SD) Stigma scores for participants with and without LST were 59.4 (6.1) and 55.2 (4.9), respectively.
Figure 3.
Neuro-QoL short form scores in Social Rolesa (A) and Stigmab (B) domains. The bottom and top edges of the box indicate the first and third quartiles, the line inside the box is the median, and the marker inside the box is the mean. The whiskers extending from the box indicate the minimum and maximum values.
Notes: aAbility to participate in social roles and activities. Raw scores were converted into T-scores (mean, 50; SD, 10) for comparison with the mean score from a general reference population, as published in the Neuro-QoL User Manual. Range of possible T-scores is 24.1–60.2; higher scores indicate better outcomes.17bPerceptions of self and publicly enacted negativity, prejudice, and discrimination as a result of disease-related manifestations. Raw scores were converted into T-scores (mean, 50; SD, 10) for comparison with the mean score from a clinical reference population, as published in the Neuro-QoL User Manual. Range of possible T-scores is 39.2–81.5; higher scores indicate worse outcomes.17cLong sleep was defined as ≥11 hours of sleep in a 24-hour period (self-reported).
Abbreviations: LST, long sleep time; Max, maximum; Min, minimum; Neuro-QoL, Quality of Life in Neurological Disorders; Q1, first quartile; Q3, third quartile; SD, standard deviation.
BC-CCI
On the BC-CCI, 62.7% of participants reported overall moderate to severe cognitive complaints (Table 2). Severe cognitive complaints were reported by 35.1% and 18.4% of participants with and without LST, respectively.
Table 2.
BC-CCI and PHQ-9 Scores
| All Participants (N=75) | Participants With LSTa (n=37) | Participants Without LSTa (n=38) | |
|---|---|---|---|
| BC-CCI total score | |||
| Mean (SD) | 10.8 (4.8) | 11.6 (4.9) | 10.1 (4.6) |
| Median | 12.0 | 13.0 | 10.5 |
| Min, Max | 2, 18 | 2, 18 | 2, 18 |
| BC-CCI score categories, n (%) | |||
| Broadly normal cognition (0–4) | 11 (14.7) | 5 (13.5) | 6 (15.8) |
| Mild cognitive complaints (5–8) | 17 (22.7) | 7 (18.9) | 10 (26.3) |
| Moderate cognitive complaints (9–14) | 27 (36.0) | 12 (32.4) | 15 (39.5) |
| Severe cognitive complaints (15–18) | 20 (26.7) | 13 (35.1) | 7 (18.4) |
| PHQ-9 total score | |||
| Mean (SD) | 12.3 (5.4) | 14.1 (5.2) | 10.6 (5.1) |
| Median | 12.0 | 14.0 | 10.0 |
| Min, Max | 2, 26 | 5, 26 | 2, 25 |
| PHQ-9 score categories (severity of depressive symptoms), n (%) | |||
| Minimal (<5) | 2 (2.7) | 0 (0.0) | 2 (5.3) |
| Mild (5–9) | 23 (30.7) | 8 (21.6) | 15 (39.5) |
| Moderate (10–14) | 24 (32.0) | 11 (29.7) | 13 (34.2) |
| Moderately severe (15–19) | 19 (25.3) | 13 (35.1) | 6 (15.8) |
| Severe (20–27) | 7 (9.3) | 5 (13.5) | 2 (5.3) |
Notes: aLong sleep was defined as ≥11 hours of sleep in a 24-hour period (self-reported).
Abbreviations: BC-CCI, British Columbia Cognitive Complaints Inventory; LST, long sleep time; Max, maximum; Min, minimum; PHQ-9, Patient Health Questionnaire-9; SD, standard deviation.
PHQ-9
Overall, 66.7% of participants reported PHQ-9 total scores consistent with moderate to severe levels of depressive symptoms (Table 2); this proportion was 78.4% for participants with LST and 55.3% for those without LST. Overall, 9.3% of participants reported PHQ-9 total scores indicative of severe levels of depressive symptoms. Only 2 participants reported PHQ-9 total scores that correspond to minimally severe levels of depressive symptoms, neither of whom had LST.
WPAI:SHP
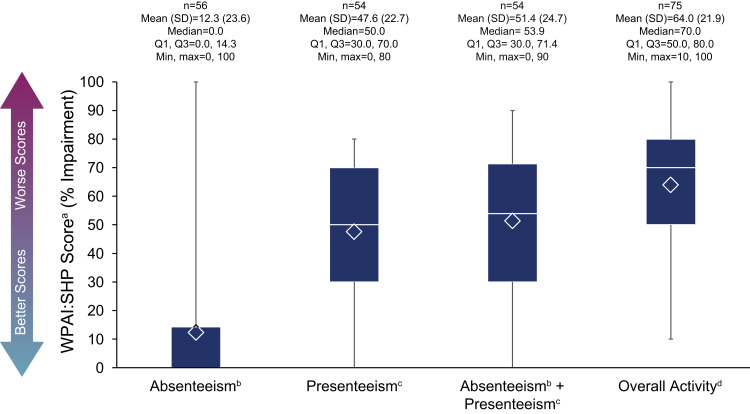
Of the 56 (74.7%) employed ARISE participants surveyed about the past week, there was a mean (SD) percent absenteeism rate of 12.3 (23.6; Figure 4). The mean (SD) percent impairment from presenteeism was 47.6 (22.7), and overall work productivity impairment (absenteeism and presenteeism) was 51.4 (24.7). In all ARISE participants (both employed and unemployed), the percent impairment in overall activity (other than work) was rated the highest out of all WPAI:SHP components (mean [SD]: 64.0 [21.9]). For participants with and without LST, respectively, mean (SD) WPAI:SHP scores were as follows: absenteeism (14.2 [26.5] and 11.1 [21.8]), presenteeism (57.1 [21.9] and 41.5 [21.4]), absenteeism + presenteeism (60.1 [24.1] and 45.8 [23.8]), and overall activity (72.2 [17.3] and 56.1 [23.2]) (Supplemental Figure 1A and B).
Figure 4.
WPAI:SHP scoresa in all participants. The bottom and top edges of the box indicate the first and third quartiles, the line inside the box is the median, and the marker inside the box is the mean. The whiskers extending from the box indicate the minimum and maximum values.
Notes: aItems relating to absenteeism and work productivity were completed only by participants who were employed; the item relating to activity impairment was completed by all participants. bAbsenteeism was defined as percent work time missed due to idiopathic hypersomnia. cPresenteeism was defined as percent impairment while working. dRefers to overall daily activity, other than working.
Abbreviations: Max, maximum; Min, minimum; Q1, first quartile; Q3, third quartile; SD, standard deviation; WPAI:SHP, Work Productivity and Activity Impairment Questionnaire: Specific Health Problem.
Subgroup Analyses
Additional subgroup analyses for age and for whether participants were taking wake-promoting agents or stimulants as monotherapy (vs not taking them as monotherapy) are reported in Supplemental Table 1.
Discussion
The ARISE study was conducted to assess daily functioning and QoL in patients with idiopathic hypersomnia, an area that has not been well studied. The characteristics of this study population were broadly consistent with other larger studies of people with idiopathic hypersomnia (eg, over half were female, and typically ≈30–40 years of age).6,22 Overall, ARISE participants showed impairment in daily functioning and social aspects compared with means from normative populations (ie, individuals who do not experience sleepiness-related impairment, or a general or clinical reference population) using the same scales,16,17 as well as substantial impairment in work productivity and general activity. A large proportion of participants reported moderate to severe cognitive complaints and depressive symptoms. These findings add much-needed clarity to the experience of people with idiopathic hypersomnia regarding the multiple aspects of this disorder on daily life, and demonstrate a symptom burden that extends far beyond just excessive daytime sleepiness.
The association of idiopathic hypersomnia with decreased daily functioning and QoL was notable: all ARISE participants had total FOSQ-10 scores below a previously reported normative value of 17.816 and also reported an impaired ability to participate in social roles and activities, as assessed with the Neuro-QoL Social Roles domain. The mean FOSQ-10 score of ARISE participants was similar to that of 154 participants in a clinical study at baseline (10.7 and 11.9, respectively), which is perhaps surprising given that 89.3% of ARISE participants were taking off-label medications for their idiopathic hypersomnia, compared with 55.8% of those from the clinical trial, and is suggestive of the inadequacy of available treatments, all of which were used off-label for idiopathic hypersomnia at the time of this study.23 Multiple studies have shown that people with idiopathic hypersomnia are impaired on most, or all, health-related QoL domains of the SF-36 (role physical, role emotional, general health perception, vitality, social functioning, mental health, physical function, and bodily pain) compared with national norms,12,24,25 even when the majority are treated with wake-promoting agents.25 Individuals with idiopathic hypersomnia also report significantly worse health-related QoL than healthy controls on the EuroQoL 5-Dimension 5-Level index and the EuroQoL Visual Analog Scale.10 One study has even reported that people with idiopathic hypersomnia scored significantly lower than people with narcolepsy (type 1, type 2, or either type) on most domains of the Veteran’s RAND 36-item Health Survey, including the social functioning domain.26
More than half of ARISE participants reported moderate to severe cognitive complaints on the BC-CCI. These data are in line with several previous studies, which indicate that over half of patients with idiopathic hypersomnia experience cognitive dysfunction, such as difficulties with memory and attention.13,27 In a study in which people were interviewed and completed a questionnaire, significantly greater proportions of participants with idiopathic hypersomnia reported memory problems, attention deficit, forgetfulness, mind going blank, and not remembering the beginning of an activity, compared with healthy controls.13 In another study, using a 30-minute sustained attention task (Perception and Attention Functions test battery–Vigilance; WAFV), people with idiopathic hypersomnia showed longer response times and more response omissions compared with healthy controls, especially during the second half of the test.28 This suggests a reduced ability to maintain attention over a period of time in the absence of a stimulating environment. Brain fog (defined as “being unable to think clearly or concentrate at any time throughout the day”) is a major cognitive complaint of people with idiopathic hypersomnia, but it appears to be an under-recognized symptom for diagnosis. In the real-world Hypersomnia Foundation registry study, brain fog was the most common symptom after excessive daytime sleepiness, reported by ≈80% of all participants.6 In addition, brain fog was reported by 54% of participants currently receiving treatment.6 Despite the prominence of this symptom in patients, brain fog is not included as a feature of idiopathic hypersomnia in the ICSD-3, which may lead to a lack of recognition in undiagnosed people with idiopathic hypersomnia.
The results of the ARISE study underscore the psychological and psychiatric burden of idiopathic hypersomnia in the study population. The degree to which ARISE participants reported feeling stigmatized because of their idiopathic hypersomnia was high, relative to the mean score from a clinical reference population on the Neuro-QoL Stigma domain (57.3 vs 49.7, respectively).17 This novel finding illustrates a deeper negative social impact (eg, embarrassment, blame, social isolation, and feeling avoided/ignored by others as a result of having idiopathic hypersomnia) that has not been previously described. Although 12.0% of ARISE participants reported having major depressive disorder, 34.7% of participants reported moderately severe to severe levels of depressive symptoms on the PHQ-9. Similarly, a retrospective observational study of patients with idiopathic hypersomnia from the Cleveland Clinic reported that 58% scored their depressive symptoms as moderate to severe on the PHQ-9.29 Other studies have reported significantly greater severity of depressive symptoms (assessed with the PHQ-9) in people with idiopathic hypersomnia as compared with healthy controls,10 as well as significantly greater anxiety and depression (evaluated with the Hospital Anxiety and Depression scale).11 It is important to note, however, that several items on the PHQ-9 assess “feeling tired” and “sleeping too much”, which could be rated worse based on idiopathic hypersomnia symptoms, and not necessarily depressive symptoms. Thus, PHQ-9 findings in people with idiopathic hypersomnia may be skewed by this overlap. Nevertheless, depression and other psychiatric comorbidities (such as anxiety) are known to be common in people with idiopathic hypersomnia,10,11 and 44% of ARISE participants reported a psychiatric comorbidity. A US insurance claims database analysis of nearly 5000 patients with newly diagnosed idiopathic hypersomnia showed that 31% had a depressive disorder, compared with only 7% of the entire claims database population.30 In a cross-sectional study, patients with idiopathic hypersomnia reported scores on the Beck Depression Inventory II that were similar to those of patients with psychiatric disorders (eg, major depressive disorder, bipolar disorder).27
Participants in ARISE reported high levels of absenteeism, presenteeism, and overall activity impairment on the WPAI:SHP due to idiopathic hypersomnia. This is not entirely surprising, given that excessive daytime sleepiness, cognitive difficulties, and brain fog symptoms experienced by people with this disorder could all interfere heavily with work responsibilities. The mean impairment scores on presenteeism (47.6%) and overall activity (64.0%) were lower than or similar to those observed in participants with idiopathic hypersomnia at baseline in a clinical trial (55.3% and 65.2%, respectively).23 However, ARISE participants reported a mean absenteeism rate (12.3%) that was higher than that reported by participants in the idiopathic hypersomnia clinical trial (7.1%),23 and even greater than that reported by people with narcolepsy in another clinical trial (6.1%).31 Although ARISE participants’ absenteeism scores were highly variable, this rate is greatly concerning given that the absenteeism rate due to illness or injury in the overall US population is 1.3%.32 While presenteeism impairment may indicate an inability to be productive while at work, high absenteeism suggests that even the task of showing up to work was often too difficult for people with idiopathic hypersomnia participating in the ARISE trial. The lack of consistency in the ARISE absenteeism rate with that of the clinical trial participants may be partially due to the fact that ARISE was an internet-based survey and had fewer participants than the clinical trial.
Notably, these impairments in daily functioning, cognition, mood, and work productivity were observed in participants regardless of LST phenotype. Although ARISE was not designed to compare differences between participants with and without LST, several studies have reported greater symptom prevalence in participants with LST, such as brain fog, difficulty waking up, fatigue, and sleep inertia,6,7 which could be expected to lead to greater impairments in daily functioning and quality of life. Future studies, including clinical trials, should aim to formally compare differences in these areas between people with idiopathic hypersomnia with LST and those without LST.
This study highlights the association of idiopathic hypersomnia with impairments in daily functioning, social aspects, cognition, affect, and work productivity. An important point is that these impairments were observed despite most participants (89.3%) taking off-label medications to treat idiopathic hypersomnia, primarily stimulants, wake-promoting agents, and antidepressants.15 This suggests that off-label treatment options are not sufficient to manage symptoms in most people with idiopathic hypersomnia. Baseline scores for daily functioning and work productivity were similarly low in participants of a Phase 3 trial (of whom 55.8% were taking off-label alerting agents), which further supports the inadequacy of off-label treatment options.23 Following the ARISE study, however, in August 2021, the United States Food and Drug Administration approved calcium, magnesium, potassium and sodium oxybates (Xywav®; low-sodium oxybate)33–36 for the treatment of idiopathic hypersomnia in adults.22 This approval was based on data from a randomized, double-blind, placebo-controlled clinical study that demonstrated efficacy over excessive daytime sleepiness and other idiopathic hypersomnia symptoms, as well as improved daily functioning and work productivity (on the FOSQ-10 and WPAI:SHP scales, respectively).22 The current findings from ARISE help to more clearly define the symptom burden of idiopathic hypersomnia, which is important to evaluating the impact of therapeutic options.
A strength of the ARISE study is that the proportion of females (81.3%) is reflective of other survey studies that have larger idiopathic hypersomnia populations (eg, 85.8%;6 89.1%),37 which supports the validity and generalizability of the present findings. Of note, the proportions of females in this study, and the 2 cited above, are higher than that from a phase 3 clinical trial (68.0%),22 perhaps due to gender bias in the willingness to participate in online surveys.38 Another strength is the inclusion of summary data for participants who did or did not have LST (defined as ≥11 hours of sleep in a 24-hour period). Given the heterogeneity of idiopathic hypersomnia, defining a distinct clinical phenotype can be valuable in developing treatment plans for patients. Although this study was not designed to evaluate differences between these 2 groups, the inclusion of these subgroups may be informative for future studies. Further strengths include the assessment of the Social Roles and Stigma subscales of the Neuro-QoL, as well as the WPAI:SHP, in a real-world population. To the authors’ knowledge, these assessments have not been previously reported in a real-world population with idiopathic hypersomnia.
A limitation of ARISE is that comprehensive demographic data, such as race and ethnicity, were not collected as a part of the survey. Because the study was virtual, people who did not have access to the internet or an appropriate electronic device were excluded. It is also possible that ARISE participants were skewed toward being less satisfied with their symptom management because those who had better symptom control may have been less motivated to take part in the study, which could potentially bias the results.
Conclusion
Findings from this real-world study demonstrate the associations of idiopathic hypersomnia with substantial impairments in quality of life, daily functioning, cognition, mood, relationships, and work productivity. These results indicate that measures of functional impairments are important to include in effectiveness trials for idiopathic hypersomnia treatments, and provide a baseline against which treatment effectiveness may be evaluated.
Acknowledgments
The authors thank the study participants. This study was supported by Jazz Pharmaceuticals. Under the direction of the authors, Emily C. Bruggeman, PhD of Peloton Advantage, LLC, an OPEN Health company, provided medical writing and editorial support for this manuscript, which was funded by Jazz Pharmaceuticals.
Some of the findings from this study have been presented in posters at Psych Congress (2021) and the 36th Annual Meeting of the Associated Professional Sleep Societies (2022), and in an oral presentation at the 74th Annual Meeting of the American Academy of Neurology (2022). An abstract was also published in Neurology. 2022;98(18 Supplement).
Abbreviations
AASM, American Academy of Sleep Medicine; ARISE, Real World Idiopathic Hypersomnia Outcomes Study; BC-CCI, British Columbia Cognitive Complaints Inventory; FOSQ-10, Functional Outcomes of Sleep Questionnaire, short version; ICSD-2, International Classification of Sleep Disorders, 2nd Edition; ICSD-3, International Classification of Sleep Disorders, 3rd edition; LST, long sleep time; Neuro-QoL, Quality of Life in Neurological Disorders; PHQ-9, Patient Health Questionnaire-9; QoL, quality of life; SF-36, Short Form-36; WAFV, Perception and Attention Functions test battery—Vigilance; WPAI:SHP, Work Productivity and Activity Impairment Questionnaire: Specific Health Problem.
Data Sharing Statement
All relevant data are provided within the article and its Supplemental Material. Jazz has established a process to review requests from qualified external researchers for data from Jazz-sponsored clinical trials in a responsible manner that includes protecting patient privacy, assurance of data security and integrity, and furthering scientific and medical innovation. Additional details on Jazz Pharmaceuticals data sharing criteria and process for requesting access can be found at: https://www.jazzpharma.com/science/clinical-trial-data-sharing/.
Author Contributions
All authors made a significant contribution to the work reported, whether in conception, study design, execution, acquisition of data, analysis and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
J Stevens: Conceptualization of the study (equal); Methodology of the study (equal); Project administration of the study (lead); Supervision of the study (lead); Writing of the article – review and editing (equal).
LD Schneider: Conceptualization of the study (equal); Methodology of the study (equal); Writing of the article – review and editing (equal).
AM Husain: Conceptualization of the study (equal); Methodology of the study (equal); Writing of the article – review and editing (equal).
D Ito: Conceptualization of the study (equal); Methodology of the study (equal); Writing of the article – review and editing (equal).
DS Fuller: Data curation of the study (lead); Formal analysis of the study results (lead); Methodology of the study (equal); Visualization of the study results (equal); Writing – review and editing (equal).
PC Zee: Writing of the article – review and editing (equal).
W Macfadden: Conceptualization of the study (supporting); Methodology of the study (equal); Supervision of the study (supporting); Writing of the article – review and editing (equal).
Disclosures
J Stevens is a full-time employee of Jazz Pharmaceuticals who, in the course of this employment, has received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals, plc. LD Schneider is an employee of Alphabet, Inc. and is a compensated member of advisory boards and speakers bureaus for Jazz Pharmaceuticals, Eisai, and Harmony Biosciences. AM Husain has received consultancy fees and/or research funding from Jazz Pharmaceuticals, UCB, BlackThorn, Merck, Pipeline, Sage, Eisai, Marinus, and Neurelis, as well as royalties from Springer, Demos Medical, and Wolters Kluwer, and holds an editorship role with Wolters Kluwer. D Ito is an employee of Stratevi, a consulting firm that received research funding from Jazz Pharmaceuticals to conduct this study. DS Fuller is a full-time employee of Jazz Pharmaceuticals who, in the course of this employment, has received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals, plc. PC Zee serves on scientific advisory boards for Jazz, Eisai, Idorsia, and Harmony Biosciences. She is also a consultant for CVS Caremark, Septerna and reports institutional grant to Northwestern University from Sleep Number. She owns stock from Teva. W Macfadden is a full-time employee of Jazz Pharmaceuticals who, in the course of this employment, has received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals, plc. The authors report no other conflicts of interest in this work.
References
- 1.American Academy of Sleep Medicine. Idiopathic hypersomnia. In: International Classification of Sleep Disorders. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2.Billiard M, Sonka K. Idiopathic hypersomnia. Sleep Med Rev. 2016;29:23–33. doi: 10.1016/j.smrv.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 3.Trotti LM. Idiopathic hypersomnia. Sleep Med Clin. 2017;12(3):331–344. doi: 10.1016/j.jsmc.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acquavella J, Mehra R, Bron M, Suomi JMH, Hess GP. Prevalence of narcolepsy, other sleep disorders, and diagnostic tests from 2013–2016: insured patients actively seeking care. J Clin Sleep Med. 2020;16(8):1255–1263. doi: 10.5664/jcsm.8482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dauvilliers Y, Bogan RK, Arnulf I, Scammell TE, St Louis EK, Thorpy MJ. Clinical considerations for the diagnosis of idiopathic hypersomnia. Sleep Med Rev. 2022;66:101709. doi: 10.1016/j.smrv.2022.101709 [DOI] [PubMed] [Google Scholar]
- 6.Trotti LM, Ong JC, Plante DT, Friederich murray C, King R, Bliwise DL. Disease symptomatology and response to treatment in people with idiopathic hypersomnia: initial data from the Hypersomnia Foundation registry. Sleep Med. 2020;75:343–349. doi: 10.1016/j.sleep.2020.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nevsimalova S, Susta M, Prihodova I, Maurovich Horvat E, Milata M, Sonka K. Idiopathic hypersomnia: a homogeneous or heterogeneous disease? Sleep Med. 2021;80:86–91. doi: 10.1016/j.sleep.2021.01.031 [DOI] [PubMed] [Google Scholar]
- 8.Lammers GJ, Bassetti CLA, Dolenc-Groselj L, et al. Diagnosis of central disorders of hypersomnolence: a reappraisal by European experts. Sleep Med Rev. 2020;52:101306. doi: 10.1016/j.smrv.2020.101306 [DOI] [PubMed] [Google Scholar]
- 9.American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic & Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 10.Wasling HB, Bornstein A, Wasling P. Quality of life and procrastination in post-H1N1 narcolepsy, sporadic narcolepsy and idiopathic hypersomnia, a Swedish cross-sectional study. Sleep Med. 2020;76:104–112. doi: 10.1016/j.sleep.2020.10.014 [DOI] [PubMed] [Google Scholar]
- 11.Vernet C, Arnulf I. Idiopathic hypersomnia with and without long sleep time: a controlled series of 75 patients. Sleep. 2009;32(6):753–759. doi: 10.1093/sleep/32.6.753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozaki A, Inoue Y, Nakajima T, et al. Health-related quality of life among drug-naive patients with narcolepsy with cataplexy, narcolepsy without cataplexy, and idiopathic hypersomnia without long sleep time. J Clin Sleep Med. 2008;4(6):572–578. doi: 10.5664/jcsm.27352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vernet C, Leu-Semenescu S, Buzare MA, Arnulf I. Subjective symptoms in idiopathic hypersomnia: beyond excessive sleepiness. J Sleep Res. 2010;19(4):525–534. doi: 10.1111/j.1365-2869.2010.00824.x [DOI] [PubMed] [Google Scholar]
- 14.Pizza F, Jaussent I, Lopez R, et al. Car crashes and central disorders of hypersomnolence: a French study. PLoS One. 2015;10(6):e0129386. doi: 10.1371/journal.pone.0129386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider LD, Stevens J, Husain AM, et al. Symptom severity and treatment satisfaction in patients with idiopathic hypersomnia: the real world idiopathic hypersomnia outcomes study (ARISE). Nat Sci Sleep. 2023;15:89–101. doi: 10.2147/NSS.S386021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chasens ER, Ratcliffe SJ, Weaver TE. Development of the FOSQ-10: a short version of the functional outcomes of sleep questionnaire. Sleep. 2009;32(7):915–919. doi: 10.1093/sleep/32.7.915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Institute of Neurological Disorders and Stroke. User Manual for the Quality of Life in Neurological Disorders (Neuro-Qol) Measures, Version 2.0. Bethesda, MD: National Institute of Neurological Disorders and Stroke; 2015. [Google Scholar]
- 18.Cella D, Lai JS, Nowinski CJ, et al. Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology. 2012;78(23):1860–1867. doi: 10.1212/WNL.0b013e318258f744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iverson GL, Lam RW. Rapid screening for perceived cognitive impairment in major depressive disorder. Ann Clin Psychiatry. 2013;25(2):135–140. [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365. doi: 10.2165/00019053-199304050-00006 [DOI] [PubMed] [Google Scholar]
- 22.Dauvilliers Y, Arnulf I, Foldvary-Schaefer N, et al. Safety and efficacy of lower-sodium oxybate in adults with idiopathic hypersomnia: a phase 3, placebo-controlled, double-blind, randomised withdrawal study. Lancet Neurol. 2022;21(1):53–65. doi: 10.1016/S1474-4422(21)00368-9 [DOI] [PubMed] [Google Scholar]
- 23.Thorpy MJ, Arnulf I, Foldvary-Schaefer N, et al. Efficacy and safety of lower-sodium oxybate in an open-label titration period of a phase 3 clinical study in adults with idiopathic hypersomnia. Nat Sci Sleep. 2022;14:1901–1917. doi: 10.2147/NSS.S369122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vignatelli L, D’Alessandro R, Mosconi P, et al. Health-related quality of life in Italian patients with narcolepsy: the SF-36 health survey. Sleep Med. 2004;5(5):467–475. doi: 10.1016/j.sleep.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 25.Dauvilliers Y, Paquereau J, Bastuji H, Drouot X, Weil JS, Viot-Blanc V. Psychological health in central hypersomnias: the French Harmony study. J Neurol Neurosurg Psychiatry. 2009;80(6):636–641. doi: 10.1136/jnnp.2008.161588 [DOI] [PubMed] [Google Scholar]
- 26.Kowalczyk S, DeBassio WA. Health-related quality of life in narcolepsy and idiopathic hypersomnia. Sleep. 2017;40(suppl 1):A241–A242. doi: 10.1093/sleepj/zsx050.651 [DOI] [Google Scholar]
- 27.Bušková J, Novák T, Miletínová E, et al. Self-reported symptoms and objective measures in idiopathic hypersomnia and hypersomnia associated with psychiatric disorders: a prospective cross-sectional study. J Clin Sleep Med. 2022;18(3):713–720. doi: 10.5664/jcsm.9702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramm M, Boentert M, Lojewsky N, Jafarpour A, Young P, Heidbreder A. Disease-specific attention impairment in disorders of chronic excessive daytime sleepiness. Sleep Med. 2019;53:133–140. doi: 10.1016/j.sleep.2018.09.021 [DOI] [PubMed] [Google Scholar]
- 29.Pascoe M, Bena J, Foldvary-Schaefer N. Effects of pharmacotherapy treatment on patient-reported outcomes in a narcolepsy and idiopathic hypersomnia cohort. J Clin Sleep Med. 2019;15(12):1799–1806. doi: 10.5664/jcsm.8088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saad R, Prince P, Taylor B, Ben-Joseph RH. Characteristics of adults newly diagnosed with idiopathic hypersomnia in the United States. Sleep Epidemiol. Accepted manuscript. Published online July 18, 2023. doi: 10.1016/j.sleepe.2023.100059 [DOI] [Google Scholar]
- 31.Emsellem HA, Thorpy MJ, Lammers GJ, et al. Measures of functional outcomes, work productivity, and quality of life from a randomized, phase 3 study of solriamfetol in participants with narcolepsy. Sleep Med. 2020;67:128–136. doi: 10.1016/j.sleep.2019.11.1250 [DOI] [PubMed] [Google Scholar]
- 32.US Bureau of Labor Statistics. Household data annual averages. 47. Absences from work of employed full-time wage and salary workers by occupation and industry; 2022. Available from: https://www.bls.gov/cps/aa2021/cpsaat47.pdf. Accessed July 20, 2023.
- 33.Jazz Pharmaceuticals, Inc. Xywav® (Calcium, Magnesium, Potassium, and Sodium Oxybates) Oral Solution, CIII. Palo Alto, CA: Jazz Pharmaceuticals, Inc.; 2022. [Google Scholar]
- 34.Szarfman A, Kuchenberg T, Soreth J, Lajmanovich S. Declaring the sodium content of drug products. N Engl J Med. 1995;333(19):1291. doi: 10.1056/NEJM199511093331917 [DOI] [PubMed] [Google Scholar]
- 35.US Food and Drug Administration. Clinical Review for Binosto, NDA 202344. US Food and Drug Administration; 2012. [Google Scholar]
- 36.US Food and Drug Administration. Quantitative labeling of sodium, potassium, and phosphorus for human over-the-counter and prescription drug products. Guidance for Industry; 2022. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/quantitative-labeling-sodium-potassium-and-phosphorus-human-over-counter-and-prescription-drug. Accessed July 20, 2023.
- 37.Neikrug AB, Crawford MR, Ong JC. Behavioral sleep medicine services for hypersomnia disorders: a survey study. Behav Sleep Med. 2017;15(2):158–171. doi: 10.1080/15402002.2015.1120201 [DOI] [PubMed] [Google Scholar]
- 38.Smith WG. Does gender influence online survey participation? A record-linkage analysis of university faculty online survey response behavior; 2008. Available from: https://files.eric.ed.gov/fulltext/ED501717.pdf. Accessed July 20, 2023.