Graphical abstract

Keywords: Arsenic, Fenugreek, Brain-derived neurotrophic factor, Learning and memory
Highlights
-
•
Fenugreek seeds powder used as a common ingredient in Bangladesh and Indian dishes and often taken as a supplement.
-
•
It maintains a healthy nutritional profile, rich in fiber, minerals and various bioactive compounds including flavonoids, saponins, tannins, etc.
-
•
It neutralizes anxiety-like behavior, learning and memory impairments in mice.
-
•
It ameliorates As-induced neurotoxicity through upregulation of BDNF and antioxidant enzymes activity in mice brain.
Abstract
The current study was designed to evaluate the protective effect of fenugreek seed powder against As-induced neurobehavioral and biochemical perturbations using a mouse model. Mice exposed to arsenic at 10 mg/kg body weight showed development of anxiety-like behavior and memory impairment compared to control mice in elevated plus maze and Morris water maze tests, respectively. A significantly decreased acetyl and butyrylcholinesterase, superoxide dismutase and glutathione reductase activities and brain-derived neurotrophic factor levels were found in the brain of arsenic-exposed mice compared to control mice. Interestingly, supplementation of fenugreek seed powder to arsenic-treated mice significantly restored the activity of cholinesterase and antioxidant enzymes (e.g. superoxide dismutase, glutathione reductase) as well as brain-derived neurotrophic factor levels in the brain tissue of arsenic-exposed mice. Consequently, reduced anxiety-like behavior, improved learning and memory were observed in fenugreek supplemented arsenic treated mice compared to only arsenic-exposed mice group. Thus, this study suggests that fenugreek seed powder reduces arsenic-induced neurotoxicity in mice.
Introduction
Arsenic (As) is one of the most toxic metals that exists naturally at high levels in many countries’ ground water, including Bangladesh (Argos et al., 2010). In Bangladesh, chronic As-exposure has not only caused human suffering, but also created socio-economic problems. The main source of drinking water in Bangladesh is tube well water. It is likely that millions of people are exposed to As mainly through drinking contaminated tube well water (WHO, Regional Office for South-East Asia, , 2002, Biswas et al., 2019). Daily consumption of high levels of As-contaminated drinking water is associated with various malignant and non-malignant diseases including peripheral neuropathy, skin diseases, cardiovascular disorders, diabetes etc. (Tyler and Allan, 2014, Mitra et al., 2020, Medda et al., 2020, Xu et al., 2020, Rangel-Moreno et al., 2022). Preclinical and clinical studies have demonstrated that As is a key player in the development of neurotoxicity (Vahidnia et al., 2007). In general, arsenic bind to glutathione followed by methylation, which is involved in the development of oxidative stress in animals and humans (Thomas et al., 2004, Kumagai and Sumi, 2007). Arsenic can accumulate in various parts of the brain and induce the generation of ROS (reactive oxygen species) and thus, modulate the oxidative stress-mediated pathways, resulting in reduce locomotor activity (Von Ehrenstein et al., 2007). A selective accumulation of As in the hippocampus and cerebral cortex of As-exposed juvenile mice brains was found to be associated with learning locomotor behavior and brain monoamine levels (Markowski et al., 2011). Consumption of contaminated drinking water induces anxiety-like behavior, impaired learning and spatial memory in experimental mice (Aktar et al., 2017, Biswas et al., 2020). In addition, associations between prenatal As exposure and children’s neurocognitive development have also been reported (von Ehrenstein et al., 2007, Hamadani et al., 2011, Chen et al., 2023).
Plants have been used since ancient times for diverse purposes in every part of the world. In particular, they are used as food for human nutrition, and medication for various illness. Herbs and spices generally play an important role in improving the overall health and well-being of people. The herb relieves nutritional deficiencies and restores proper functioning of the body. Among them, fenugreek is a popular herb, which is widely distributed in South Asia including Bangladesh. It has many applications commonly used in cooking, to make medicine or to hide the taste of other medicines in different countries. A significant number of health benefits of fenugreek have been observed in both humans and laboratory animals such as anti-diabetic, hypocholesterolemia, anti-lipidemia, antioxidant, hepatoprotective, anti-inflammatory and neuroprotective effects (Kodumuri et al., 2019, Almatroodi et al., 2021, Salam et al., 2023). Fenugreek is also considered a rich source of dietary fiber and other important nutrients required for proper growth and development. Studies have also confirmed the nutraceutical and pharmacological properties of fenugreek, supporting the potential application of fenugreek in the development of numerous functional food products and pharmaceutical products (Wani and Kumar, 2018). Bioactive phytochemicals such as alkaloids, carbohydrates, steroid, saponins, amino acids and minerals are rich in Fenugreek. Therefore, it has the potential to be used as nutritional and dietary supplement along with pharmacological applications (Khorshidian et al., 2016). Its seeds have widely been using as a flavoring agent in various classical cuisines and have a free radical scavenging activity on lipid peroxidation (Hilles and Mahmood, 2017, Akbari et al., 2019). Furthermore, saponins extracted from plant sources (Trichosanthis kirilowii Maxim) showed a neuroprotective effect by increasing the activity of antioxidant enzymes and inhibiting the expression of p-p38 and p53 in experimental animals (Chen et al., 2015). Together, phytochemical analysis reveals that fenugreek seed contain bioactive compound such as saponins, steroids, alkaloids, thus, we hypothesized that its powder may have protective functionality against As-induced toxicity. Therefore, this study was undertaken to evaluate the protective effect of fenugreek seed powder against As-induced neurobehavioral and biochemical changes using a mouse model.
Materials and methods
Animal maintenance
Swiss Albino mice of 4 weeks of ages (average body weight 20–25 g) were distributed into four groups: a) Control, b) Arsenic treated (As), c) Fenugreek, and d) As + Fenugreek. Each group was consisted of ten (10) mice and were kept in the animal house at the Department of Biochemistry and Molecular Biology, Rajshahi University in controlled conditions (25 ± 2 °C) with natural light and dark cycle. Sodium arsenite was provided to As and As + Fenugreek groups animals with drinking water at a concentration of 10 mg per kg body weigh per day for 60 consecutive days before the behavioral test, while the control and Fenugreek groups received normal drinking water and food pellets (ad libitum) during the experiment. The doses of As was selected according to the previously studies (Aktar et al., 2017, Biswas et al., 2020). Mice from Fenugreek, and As + Fenugreek groups received fenugreek seed powder containing standard pelleted diet (5 % w/w) all through the experimental period. All behavioral tests were performed between 9.00 a.m. and 3.00p.m. during light cycle. The doses of fenugreek seed powder and crushing process were as chosen according to the literature (Arafa et al., 2014). The animal experimental design and timeline is shown in Fig. 1. Institutional ethical approval of the protocol of the animal experiment was collected from the Institute of Biological Sciences, Rajshahi University, Rajshahi-6205, Bangladesh (No: 126/320/IAMEBBC/IBSc).
Fig. 1.
Graphic diagram and schedule of animal experiment Arsenic (As) and As + Fenugreek groups received As-water, while Control and Fenugreek mice groups received normal water. The Fenugreek, and As + Fenugreek groups received fenugreek seed powder containing food pellets. Morris water maze test including 2 days training and probe trail was conducted from day 61 to day 70. Thereafter, all the experimental mice were sacrificed, and serum and brain tissues homogenate were prepared for further analysis.
Qualitative phytochemical screening of fenugreek seeds
The fenugreek seeds powder has been tested for identifying the presence of potent phytochemicals. The presence of saponins, alkaloids, tanins, phenolic and flavonoid compounds in Fenugreek seed powder tested according to the previously published protocols (Islam et al., 2023).
Assessing anxiety of experimental mice in the elevated plus maze
The elevated plus maze (EPM) is used to evaluate anxiety in laboratory animals. The EPM is a plus sign shaped experimental tool, consists of two arms (50 × 10 cm) and two closed arms (5 × 10 × 40 cm) raised 50 cm height above the floor (Aktar et al., 2017). Mice were placed in the center of the platform independently in front of closed arms to travel the maze (5 min), and the time spent in the individual arms was recorded as previously described (Aktar et al., 2017, Biswas et al., 2019). Four paws of a mouse inside the enclosure were considered an entry (Schneider et al., 2011). To prevent any biased results for olfactory cues, 70% ethanol was used for cleaning after each test. The time spent in open arms and closed arms were used to study the anxiety-like behaviors of mice (Anjum et al., 2019).
Morris water maze for evaluation of spatial memory and learning in the mice
This test is broadly used laboratory tool in behavioral neuroscience for the evaluation of spatial memory and learning in rodents. The maze is a circular water pool (diameter 150 cm, height 60 cm and water depth 30 cm) with black interior and water kept in room temperature (25 ± 2 °C). A platform of black color (28 cm height) was positioned in the center of the North-East (NE) quadrant approximately 1 cm lower the water surface. A colored cue was placed on the wall of the maze and mice were placed in the pool from different starting points for each trial. The location of the platform was recognized to the mice by two days training. A maximum time of 60 s (cut-off time) was given to locate the water submerged platform and permitted to remain on it for 20 s. A mouse that was unable to identify the water-hidden platform within the given time was placed on the platform by an experimenter for 20 s. Three trials were conducted at 30-min intervals per day as described (Barnhart et al., 2015). After each maze test, mice were manually dried with a towel, placed in a warmed cage (5 min) and then return to the original cage. The mean latency time of the animal for each day was calculated from the three trials of escapes latency time to reach the platform. The animals did not meet the learning criteria within 7 days were excluded from the study. A significant decrease in latency times as compared with the 1st session was reflected as effective learning. At the end of the seven days training, a probe test was carried out without platform, and the residence time in the desired quadrant was recorded within 60 s. More time spent in the target quadrant designated better performance during the probe trial.
Biochemical analysis from serum and brain tissue homogenates
Diethyl ether was used to anesthetize the experimental mice, and blood was taken from the thoracic artery a few days after the behavioral experiments. Blood was kept for 30 min at room temperature for coagulation and serum was separated after centrifugation at 4000 rpm for 15 min at 4 °C and kept at −80 °C until use (Biswas et al., 2019). Butyrylcholinesterase (BChE) assay kit (RANDOX, UK) was used to analyze the activity of BChE in serum using an analyzer (Humalyzer-3000, Germany) according to the manufacturer's protocols.
An electronic balance was used to weigh the cleared whole brain and then homogenized in 0.5% Triton-X100 (Sigma Aldrich) containing phosphate buffer (100 mmol/L, pH 7). After the supernatant was collected followed by centrifugation at 5000 rpm (at 4 °C) and subjected to various biochemical tests as mentioned in Reza et al. (2018). Acetylcholinesterase (AChE) and BChE activity, and total protein in brain tissue homogenates were analyzed according to the methods described in previously published article (Islam et al., 2022; Islam et. al., 2023). The activities of superoxide dismutase (SOD) and glutathione reductase (GR) in brain tissue homogenates were analyzed following the methods described in Islam et al. (2023). Brain derived neurotrophic factor (BDNF) protein was estimated using BDNF ELISA kit (Elabscience, USA) following the protocol of the manufacturer. Every sample was analyzed twice, average values were used for analysis. Data from two mice that did not meet learning criteria in the behavioral tests were not included in all experimental analyses.
Statistical analysis
All data are expressed as mean ± SEM (standard error of mean) as indicated. Welch's test and ordinary one-way ANOVA tests were used to assess the statistical significance among the different groups. P value < 0.05 was thought to be statistically significant. Data was evaluated and graph was made with GraphPad Prism 7.05.
Results
Phytochemicals in fenugreek seed powder
The findings of fenugreek seed powder's phytochemical screening tests showed that fenugreek contains significant amounts of several phytochemicals, including phenols, tannins, flavonoids, alkaloids, saponins, and coumarins. These phytochemicals are responsible for the therapeutic efficacy of fenugreek against As-induced neurotoxicity.
Attenuating effects of fenugreek against As-induced anxiety-like behavior in mice
Percent of time spent of experimental mice in open and closed arms of EPM test was shown in Fig. 2. Time percentage spent in open arms of a) Control b) As-treated c) Fenugreek and d) As + Fenugreek groups were 58.46 ± 1.95, 40.38 ± 1.95, 57.21 ± 1.41, and 55.46 ± 1.33, respectively. As-treatment significantly decreased time spent in open arms and increased time spent in closed arms when compared to control mice (p < 0.0001). On the other hand, fenugreek seed powder supplementation to As-exposed mice significantly increased the time spent in open arms and decreased the time spent in closed arms compared to only As-exposed mice (p < 0.001).
Fig. 2.
Effect of fenugreek on percentage of time staying in the open arms and closed arms of the EPM The staying time in open and closed arms of Control, Arsenic (As), Fenugreek, and As + Fenugreek treated mice is indicated in box plots. Data were expressed as Mean ± SEM, where n = 8 for each group of mice. The maximum, minimum, and median values are indicated by the upper and lower limits of the boxes, as well as the middle line across the boxes. Welch's t-test was used to compare groups (****p < 0.0001) and ordinary one-way ANOVA (p < 0.0001).
Protective effect of fenugreek on As-induced spatial memory and learning impairment
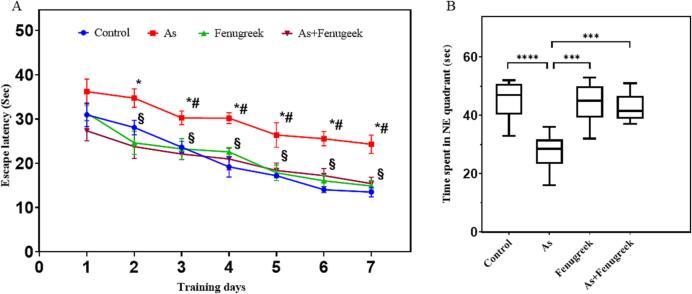
Morris water maze (MWM) test was performed on 61st day after the start of the experiment to assess the protective effect of fenugreek on As-induced learning and spatial memory impairment. Fig. 3A presented the results of MWM task. The figure shows the escape latency to find the platform. In the beginning of the experiment, all the mice tended to swim around the boundary looking for a way to escape, however, after being placed on a platform at the end of each experiment, the mice gradually learned that there was a platform, and escape latency gradually decreased for 7 days training course. The results of this study showed that the mean escape latency (mean ± SEM) of the control group mice to find the hidden platform was 30.96 ± 2.67 s on day-1 and gradually decreased over the 7-days training course and average escape latency was 13.50 ± 1.12 s on day-7. However, the mean latency on day 7 of learning was greater in the As-treated groups (Fig. 3A). A significant difference between As-treated and control groups was noted from day-3 to day-7 (p < 0.01). However, fenugreek supplementation reduced the time latency in the As + Fenugreek mice group indicating the attenuation of As-induced learning and memory impairment (p < 0.01).
Fig. 3.
Protective effects of fenugreek on As-induced learning and spatial of mice in MWM A. Time latency of the experimental mice. Latency time of Control, Arsenic (As), Fenugreek, and As + Fenugreek were expressed as Mean ± SEM, where n = 8 for each group of mice. *Significantly different from control group at p < 0.05 in Welch’s t-test. #Significantly different from control group at p < 0.05 in ordinary one-way ANOVA. §Significantly different from Arsenic (As) group at p < 0.05 in both Welch’s t-test and ordinary one-way ANOVA. B. Morris water maze probe trial for experimental mice. Time spent in the NE quadrant of Control, Arsenic (As), Fenugreek, and As + Fenugreek mice were stated as Mean ± SEM values, where n = 8 for each group of mice. The boxes and bars are as in Fig. 1. Group assessments were performed by Welch’s t-test (***p = 0.0001, ****p < 0.0001) and ordinary one-way ANOVA (p < 0.0001).
Seven days later, the platform was removed from the maze and a probe trail was performed to understand the spatial memory and learning of mice about the platform location. In the 60 sec trail, the time spent in the target quadrant of the Control, As treated, Fenugreek and As + Fenugreek groups were 45.38 ± 3.54, 27.38 ± 2.22, 44.25 ± 2.40 and 42.63 ± 1.68 sec, respectively (Fig. 3B). The results showed that the control as well as the mice of the Fenugreek group spent more time in the desired quadrant compared with the As-treated group (p < 0.001). However, the supplantation of fenugreek to the As-exposed mice increased the time spent in the target quadrant compared with the As treated mice (p < 0.001), showing a protective effect of fenugreek seed powder against As-induced learning and memory disorders.
Ameliorating effect of fenugreek on BChE activity of As-induced mice
Reduced BChE activity implies the association between neurotoxicity and dementia in experimental mice (Fig. 4A). A significant reduction of BChE activity noted in serum of As-exposure mice compared to control and fenugreek treated groups mice (p < 0.0001). However, fenugreek seed powder treatment restored the BChE activity in As-exposed mice significantly (p < 0.0001). On the other hand, BChE activity in brain tissue were 39.1 ± 2.60, 22.42 ± 1.78, 41.05 ± 1.77 and 31.63 ± 1.17 mU/mg in Control, As-treated, Fenugreek and As + Fenugreek group mice brain, respectively (Fig. 4B). Similarly, BChE activity in brain tissue of As-treated mice group significantly reduced when compared to control group (p < 0.0001). Interestingly, treatment with fenugreek also significantly restored the activity of BChE in the brain tissue of the As-exposure group (p < 0.01).
Fig. 4.
Protective effect of fenugreek on BChE activity in serum (A) and brain tissue (B) of experimental mice The results from Control, Arsenic (As), Fenugreek, and As + Fenugreek treated mice were expressed as Mean ± SEM, where n = 8 for each group of mice. Significantly different among means were calculated by Welch’s t-test (**p < 0.01, ***p < 0.001 ****p < 0.0001) and ordinary one-way ANOVA (p < 0.0001).
Effects of fenugreek on brain enzymes activity in As-induced mice
To evaluate whether fenugreek seed powder could reduce the disorder of neurotransmitter system, we measured AChE activity in brain tissues of mice (Table 1). It was noted that activity of AChE reduced significantly in brain tissue of As-exposed mice in comparison to that of control animals (p < 0.0001). Interestingly, AChE activity was found to be restored in the brain tissue of Fg supplemented As-exposed mice group compared to As-exposure mice (p < 0.01).
Table 1.
AChE, GSH and SOD activities in the brain tissues of the experimental mice.
| Enzyme activity | Experimental groups |
p-value |
|||
|---|---|---|---|---|---|
| Control | As | Fenugreek | As + Fenugreek | ||
| AChE(mU/mg) | 143.53 ± 3.10 | 89.58 ± 5.35a | 145.39 ± 5.58b | 127.22 ± 5.80c | ap < 0.0001, bp < 0.0001, cp = 0.0032 |
| GSH (umol/mg) | 3.73 ± 0.14 | 2.77 ± 0.05a | 4.12 ± 0.19b | 3.53 ± 0.13c | ap < 0.0001, bp < 0.0001, cp = 0.0002 |
| SOD(U/mg) | 6.93 ± 0.34 | 4.53 ± 0.17a | 7.84 ± 0.86b | 5.94 ± 0.12c | ap = 0.0002, bp = 0.008, cp < 0.0001 |
Data are expressed as Mean ± SEM, n = 8 for each group of mice. aSignificantly different from control, and b,cSignificantly different from Arsenic (As) group. Group comparisons were performed by Welch’s t-test and ordinary one-way ANOVA (p < 0.0001).
The antioxidant system in the animal body plays a decisive role in preventing any damage caused by free radicals, but exposure to heavy metals alters the antioxidant defense system. Two crucial enzymes such as SOD and GR, whose antioxidant activities were determined in brain tissue of mice (Table 1). The activity of SOD in Control, As-treated, Fenugreek and As + Fenugreek group mice were 6.93 ± 0.34, 4.53 ± 0.17, 7.84 ± 0.86 and 5.94 ± 0.12 U/mg, respectively. In As-exposure mice the SOD activity was significantly reduced in brain in comparison to control animals (p < 0.0001), however, supplementation of fenugreek seed powder significantly restored the activity of SOD in As + Fenugreek mice in comparison to that of As-treated mice (p < 0.0001). The GR level in brain tissues of Control, As-treated, Fenugreek and As + Fenugreek groups mice were found as 3.73 ± 0.14, 2.77 ± 0.05, 4.12 ± 0.19, and 3.53 ± 0.13 umol/mg, respectively. A significantly reduced GR activity was found in As-treated mice compared to control mice (p < 0.0001), while activity of GR was significantly recovered in As + Fenugreek group mice in comparison to that of As-treated mice (p < 0.001).
Protective effect of fenugreek on BDNF level in the brain tissue of As-induced mice
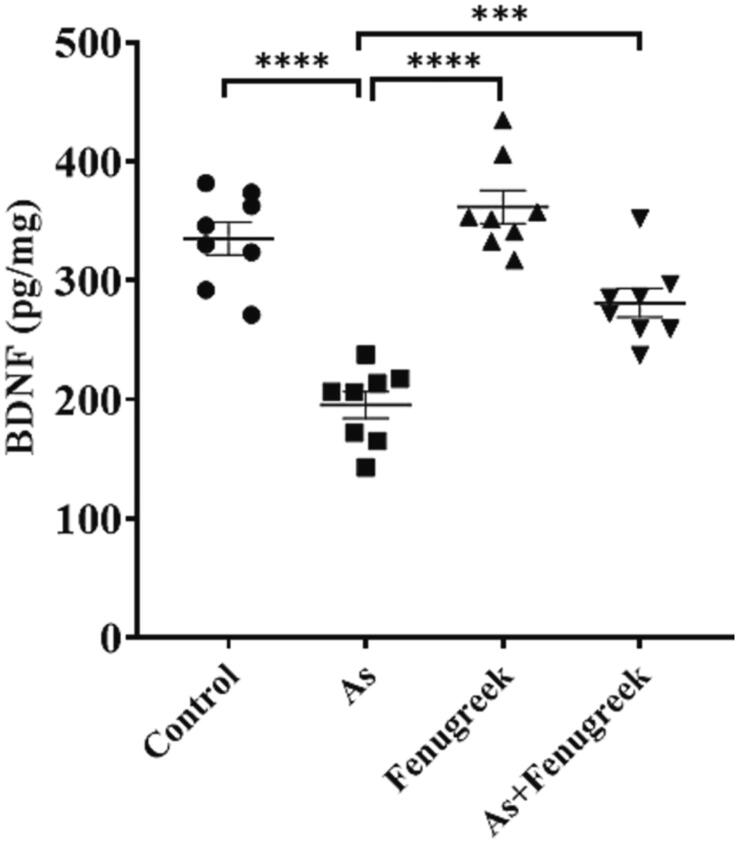
In this study, we found that BDNF levels were 333.16 ± 13.81, 195.29 ± 11.20, 361.79 ± 13.82 and 281.08 ± 12.13 pg/mg in brain tissue of Control, As-treated, Fenugreek and As + Fenugreek group mice, respectively (Fig. 5). BDNF level was significantly reduced in As-exposed mice compared to control mice (p < 0.0001). However, co-administration of fenugreek with As significantly increased the BDNF level in brain of As + Fenugreek group mice compared to As-exposed animals (p < 0.001).
Fig. 5.
Protective effect of fenugreek on BDNF level of brain tissue in experimental mice Control, Arsenic (As), Fenugreek, and As + Fenugreek mice were stated as Mean ± SEM, where n = 8 for each group of mice. A method significantly different among the means of different groups were performed by Welch’s t-test (***p = 0.0001 ****p < 0.0001) and ordinary one-way ANOVA (p < 0.0001).
Discussion
Plant materials such as herbal products are a good choice for the prevention of many diseases, including metal-induced toxicity, as they have no or very few side effects. Recently, researchers have been paying great attention, focusing on the use of antioxidants from natural sources such as plant materials, which can protect the human body from free radical-mediated oxidative damage, especially brain tissues (Hassan et al., 2017, Islam et al., 2022). Like other phytochemical plants/herbs, fenugreek is a popular herb with numerous health benefits, demonstrated in both humans and laboratory animals. In brain tissue, reduced activity of antioxidant enzymes and elevated level of ROS in As-exposed experimental animals reflect brain damage. It is reported that As-exposure induces lipid peroxidation as well as inhibits the body's antioxidant mechanisms, which indirectly lead to elevated ROS and oxidative stress (Jomova et al., 2011). Disruption of the antioxidant defense system is a major cause of memory impairment and development of anxiety-like behavior in in As-induced rodents (Salim, 2017, Fedoce et al., 2018). It was reported that As-exposure persuades anxiety-like behavior, learning and memory impairment in experimental animals (Aktar et al., 2017, Zhang et al., 2022). Thus, As is a crucial player for the development of anxiety-like behavior, learning and memory impairment in mice. Earlier, we showed that As-exposed mice have anxiety-like behavior in the EPM test and take longer to find the hidden platform during the MWM test, indicating arsenic-induced memory impairment (Biswas et al., 2019, Islam et al., 2022). However, in the present study, we observed that administration of fenugreek in As-exposed mice dramatically restored learning and memory impairments and reduced the development of anxiety in As-treated mice. It has been reported that bioactive compounds found in vegetables and fruits have a great capacity to attenuate scopolamine-induced learning and memory-impairment in experimental animals (Carrillo et al., 2019).
Exposure of As, even at a lower dose could modulate the cognitive and motor functions, and the alterations are linked with adverse consequences of cholinergic system (Tyler and Allan, 2014). Cholinesterase enzymes are involved in hydrolyzing the neurotransmitter acetylcholine into choline and acetic acid, allowing cholinergic neurons to return its resting stage after activation. Perinatal As-exposure has been reported to be associated with inhibition of normal development of the cholinergic neuron in the brain (Chandravanshi et al., 2019). Decreased BChE activity in serum is also associated with inflammation and oxidative stress that imply the progression of neurotoxicity and eventually memory loss as well as β-amyloid (Aβ) plaque formation in experimental animals with Alzheimer's disease (Peres et al., 2016). Studies shown that cognitive function is closely linked to the central cholinergic system, and decreased cholinesterase activity indicates the development of inflammation, oxidative stress, and neurotoxicity (Santarpia et al., 2013, Bono et al., 2015, Lockridge, 2015, Darvesh, 2016). Another study suggested that chronic As-exposure impairs spatial memory by modulating the NR2A and β-subunits of the glutamate receptor N-methyl D-aspartate (NMDAR) (Ramos-Chávez et al., 2015). In addition, exposure to As affects cholinergic function, and cholinergic dysfunction is correlated with reduced cholinesterase activity in the brain of laboratory animals and also learning and memory impairments (Flora et al., 2009, Chandravanshi et al., 2019, Anjum et al., 2019). Our present data demonstrated a significant reduction of BChE enzyme activity not only in blood serum, and but also in the brain of As-exposed mice. Furthermore, AChE activity reduced in the brain of As-exposed animals. Intriguingly, supply of fenugreek to As-treated animals showed a potential neuroprotective effect by attenuating the reduction of cholinesterase activity both in serum and brain tissues. Moreover, Islam et al. (2022) stated that mulberry leaves juice is rich in phenolic acids, flavonoids, flavonols etc., and supplementation of mulberry leave juice prevent the reduction of cholinesterase activity in As-exposure mice.
The generation of oxidative stress is a natural cellular phenomenon, caused by an imbalance between the production and accumulation of ROS in cells and tissues, and antioxidant enzymes regulate ROS levels to protect cells (Pizzino et al., 2017). As-exposure induces the formation of intracellular ROS, which mediate many changes in cellular behavior by modifying signaling pathways and epigenetic alterations or inducing direct oxidative damage to molecules (Hu et al., 2020). Oxidative stress is the most ubiquitous mechanism that explains As-induced toxicity, and several reports have shown that increased levels of ROS are associated with anxiety-like disorders, hippocampus-dependent and hippocampus-independent memory formation, synaptic plasticity-related signaling molecules, receptors, and channels including the N-methyl-D-aspartate (NMDA) receptor (Flora et al., 2009, Betzen et al., 2009, Massaad and Klann, 2011). In experimental animals, ROS play crucial roles in As-induced oxidative stress, which in turn induces numerous pathophysiological conditions. Also, decreased activity of antioxidant enzymes may influence oxidative damage to the brain and other stored organs of As-exposure mice (Betzen et al., 2009, Sharma et al., 2010). Similar to the other enzymes of antioxidant system, GR and SOD could play important roles in nullifying ROS, thus, aggregate As-methylation for cellular uptake (Vahter, 2002, Medina et al., 2023). Antioxidants prevent free radical attacks and may improve cognitive function in Alzheimer's disease. In the present study, a significantly reduced SOD and GR activity was detected in the brain tissue of As-exposed mice. However, a marked recovery of both enzyme activities was found in brain tissue of As-exposed mice those received fenugreek supplemented normal diet. Moreover, the results of the neurotransmitter systems as well as antioxidant enzymes activity related to behavioral tests indicated that fenugreek seed powder neutralized As-induced ROS in the brain tissue of mice. From the above findings, we hypothesized that fenugreek has the ability to neutralize As-induced ROS generation in mice brain. Therefore, fenugreek can be used to alleviate As-induced pathophysiology and restore the activity of antioxidant enzymes in the brain. Antioxidant enzymes are endogenous proteins that have specific free radical scavenging properties (Bora and Sharma, 2011). SOD acts as a front-line defense against superoxide radicals and neutralize oxidative stress-induced ROS (Fukai and Ushio-Fukai, 2011). Mathews et al. (2012) reported that tissue accumulation of As leads to decreased antioxidants and antioxidant enzymes in serum and tissue. Another study reported that GR is almost exclusively present in fairly high concentrations in humans and is directly or indirectly involved in the removal of ROS (Espinosa-Diez et al., 2015). Thus, fenugreek may provide a protective function against As-induced neurotoxicity by enhancing antioxidant properties. We assumed that active compounds present in fenugreek seed powder can plausibly bind and enhance the activity of antioxidant enzymes. In this study, we found that fenugreek seed powder is rich in flavonoids. A previous study reported that flavonoids modulate oxidative stress by reducing lipid peroxidase and increasing glutathione levels in liver cells (Boadi et al., 2021).
BDNF is an important factor in learning and memory, and also regulates gene expression involved in memory formation (Rahn et al., 2013, Leal et al., 2014). In the present study, significantly decreased BDNF level was detected in the brain tissue of As-exposure mice in comparison to that of control animal, and co-administration of fenugreek with As significantly regained the level of BDNF in brain tissue compared to As-exposed mice. Considering the present results, we hypothesized that the reduction of BDNF levels in the brain of the experimental mice group supports our behavioral data, where As-exposure mice showed weaker learning and spatial memory compared to control mice in the MWM test. Previously we and other groups revealed that As-exposure disrupts learning and memory functions and the reduction of BDNF level is associated with cognitive decline (Sun et al., 2015, Srivastava et al., 2018, Biswas et al., 2019, Karim et al., 2019, Mehta et al., 2021). Another study also stated that decreased BDNF levels found in depressed patients and decreased level of BDNF indicates lessened synaptic plasticity (Karege et al., 2005). It is also hypothesized that several important antioxidants and bioactive compounds present in fenugreek seed powder may prevent As-induced neurotoxicity in experimental mice. Fenugreek seeds contain various alkaloids such as trigonelline, gentianine, carpaine, flavonoids and higher concentration of saponin (Kumar et al, Srinivasa and Naidu, 2021). Flavonoids upregulate the CREB-BDNF pathway through interactions with receptors for ion channels, NMDA, and TrkB, and ultimately improve cognitive and memory functions (Sharma et al., 2019). Saponins, an active compound in fenugreek seeds activate NMDR receptors and NMDR receptors play an important role in learning and spatial memory (Wang et al., 2019, Zhou et al., 2020). Previous reports have shown that fenugreek seed powder, mixed with standard rodent diet, restores pyridoxine-induced nerve fiber function, and also prevents aluminum-induced memory and learning impairment in experimental animals (Moghadam et al., 2013, Prema et al., 2016, Almatroodi et al., 2021). Recently we have shown that Clerodendrum viscosum leaves are rich in saponin along with many other flavonoids and inhibit the reduction the neurochemicals and cholinergic enzymes activity through upregulation of BDNF levels in the brain tissue of metal-exposed mice (Islam et al., 2023). Furthermore, another study reported that saponin improved hindlimb motor function through upregulation of BDNF in rodents (Wang et al., 2015). In this study, we also found the presence of saponin in the fenugreek seeds powder. However, further research is necessary to elucidate the precise molecular mechanisms of the active compounds present in fenugreek seed powder to prevent As-induced neurobehavioral and biochemical alterations.
Conclusion
In conclusion, the present study showed that fenugreek seed powder supplementation reduced anxiety-like behavior, memory impairment and biochemical alterations in experimental mice. In addition, the presence of antioxidant and anti-inflammatory substances in fenugreek seeds suppresses As-mediated toxicity by increasing antioxidant enzymes activity, neurochemical system and upregulation of BDNF in brain. Thus, our data will provide a bridge between clinicians and basic scientists from which help to develop effective and specific strategies to reduce As toxicity.
Ethical approval
Institutional ethical approval of the protocol of the animal experiment was collected from the Institute of Biological Sciences, Rajshahi University, Bangladesh (No: 126/320/IAMEBBC/IBSc).
CRediT authorship contribution statement
Jahidul Islam: Investigation, Formal analysis, Writing – original draft. Zohurul Islam: Investigation, Formal analysis, Writing – original draft. Nazmul Haque: . Moriom Khatun: . Farhadul Islam: Writing – review & editing. Shakhawoat Hossain: . Md Ashraful Hoque: . Farjana Nikkon: Funding acquisition. Khaled Hossain: Writing – review & editing. Zahangir Alam Saud: Conceptualization, Funding acquisition, Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The current research was carried out using the funding (Grant No-901/5/52/RABI/BINGAN-24/20-21) from Rajshahi University, Bangladesh.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crtox.2023.100114.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Akbari S., Abdurahman N.H., Yunus R.M., Alara O.R., Abayomi O.O. Extraction, characterization and antioxidant activity of fenugreek (Trigonella-Foenum Graecum) seed oil. Mater. Sci. Technol. 2019;2(2):349–355. doi: 10.1016/j.mset.2018.12.001. [DOI] [Google Scholar]
- Aktar S., Jahan M., Alam S., Mohanto N.C., Arefin A., Rahman A., Haque A., Himeno S., Hossain K., Saud Z.A. Individual and Combined Effects of Arsenic and Lead on Behavioral and Biochemical Changes in Mice. Biol. Trace Elem. Res. 2017;177(2):288–296. doi: 10.1007/s12011-016-0883-0. [DOI] [PubMed] [Google Scholar]
- Almatroodi S.A., Almatroudi A., Alsahli M.A., Rahmani A.H. Fenugreek (Trigonella Foenum-Graecum) and its Active Compounds: A Review of its Effects on Human Health through Modulating Biological Activities. Pharmacogn. Mag. 2021;13(3):813–821. [Google Scholar]
- Anjum A., Biswas S., Rahman M., Rahman A., Siddique A.E., Karim Y., Aktar S., Nikkon F., Haque A., Himeno S., Hossain K., Saud Z.A. Butyrylcholinesterase—a potential plasma biomarker in manganese-induced neurobehavioral changes. Environ. Sci. Pollut. Res. 2019;26(7):6378–6387. doi: 10.1007/s11356-018-04066-1. [DOI] [PubMed] [Google Scholar]
- Arafa M.H., Mohammad N.S., Atteia H.H. Fenugreek Seed Powder Mitigates Cadmium-induced Testicular Damage and Hepatotoxicity in Male Rats. Exp. Toxicol. Pathol. 2014;66(7):293–300. doi: 10.1016/j.etp.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Argos M., Kalra T., Rathouz P.J., Chen Y., Pierce B., Parvez F., Islam T., Ahmed A., Rakibuz-Zaman M., Hasan R., Sarwar G. Arsenic Exposure from Drinking Water, and All-Cause and Chronic-Disease Mortalities in Bangladesh (HEALS): A Prospective Cohort Study. Lancet. 2010;376(9737):252–258. doi: 10.1016/s0140-6736(10)60481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart C.D., Yang D., Lein P.J., Chapouthier G. Using the Morris Water Maze to Assess Spatial Learning and Memory in Weanling Mice. PLoS One. 2015;10(4):e0124521. doi: 10.1371/journal.pone.0124521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzen C., White R., Zehendner C.M., Pietrowski E., Bender B., Luhmann H.J., Kuhlmann C.R. Oxidative Stress Upregulates the NMDA Receptor on Cerebrovascular Endothelium. Free Radic. Biol. Med. 2009;47(8):1212–1220. doi: 10.1016/j.freeradbiomed.2009.07.034. [DOI] [PubMed] [Google Scholar]
- Biswas S., Anjum A., Banna H.U., Rahman M., Siddique A.E., Karim Y., Nikkon F., Haque A., Hossain K., Saud Z.A. Manganese Attenuates the Effects of Arsenic on Neurobehavioral and Biochemical Changes in Mice Co-Exposed to Arsenic and Manganese. Environ. Sci. Pollut. Res. 2019;26(28):29257–29266. doi: 10.1007/s11356-019-06112-y. [DOI] [PubMed] [Google Scholar]
- Biswas S., Banna H.U., Jahan M., Anjum A., Siddique A.E., Roy A., Nikkon F., Salam K.A., Haque A., Himeno S., Hossain K. In vivo Evaluation of Arsenic-Associated Behavioral and Biochemical Alterations in F0 and F1 Mice. Chemosphere. 2020;245 doi: 10.1016/j.chemosphere.2019.125619. [DOI] [PubMed] [Google Scholar]
- Boadi W.Y., Stevenson C., Johnson D., Mohamed M.A. Flavonoids Reduce Lipid Peroxides and Increase Glutathione Levels in Pooled Human Liver Microsomes (HLMs) Adv. Biol. Chem. 2021;11(6):283–295. doi: 10.4236/abc.2021.116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bono G.F., Simão-Silva D.P., Batistela M.S., Josviak N.D., Dias P.F.R., Nascimento G.A., Souza R.L.R., Piovezan M.R., Souza R.K.M., Furtado-Alle L. Butyrylcholinesterase: K Variant, Plasma Activity, Molecular Forms and Rivastigmine Treatment in Alzheimer's Disease in a Southern Brazilian Population. Neurochem. Int. 2015;81:57–62. doi: 10.1016/j.neuint.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Bora K.S., Sharma A. Evaluation of Antioxidant and Free-Radical Scavenging Potential of Artemisia Absinthium. Pharm. Biol. 2011;49(12):1216–1223. doi: 10.3109/13880209.2011.578142. [DOI] [PubMed] [Google Scholar]
- Carrillo J.Á., Zafrilla M.P., Marhuenda J. Cognitive Function and Consumption of Fruit and Vegetable Polyphenols in a Young Population: Is There a Relationship? Foods. 2019;8(10):507. doi: 10.3390/foods8100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandravanshi L.P., Gupta R., Shukla R.K. Arsenic-Induced Neurotoxicity by Dysfunctioning Cholinergic and Dopaminergic System in Brain of Developing Rats. Biol. Trace Elem. Res. 2019;189(1):118–133. doi: 10.1007/s12011-018-1452-5. [DOI] [PubMed] [Google Scholar]
- Chen Y., Sun H., Huang L., Li J., Zhou W., Chang J. Neuroprotective Effect of Radix Trichosanthis Saponins on Subarachnoid Hemorrhage. Evid. Based Complementary Altern. Med. 2015;2015:1–10. doi: 10.1155/2015/313657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Zhang H., Wang X., Wu Y., Zhang Y., Chen S., Zhang W., Sun X., Zheng T., Xia W., Xu S., Li Y. Prenatal arsenic exposure, arsenic metabolism and neurocognitive development of 2-year-old children in low-arsenic areas. Environ Int. 2023;174 doi: 10.1016/j.envint.2023.107918. [DOI] [PubMed] [Google Scholar]
- Darvesh S. Butyrylcholinesterase as a Diagnostic and Therapeutic Target for Alzheimer's Disease. Curr. Alzheimer Res. 2016;13(10):1173–1177. doi: 10.2174/1567205013666160404120542. [DOI] [PubMed] [Google Scholar]
- Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sánchez-Pérez P., Cadenas S., Lamas S. Antioxidant Responses and Cellular Adjustments to Oxidative Stress. Redox Biol. 2015;6:183–197. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoce A.D.G., Ferreira F., Bota R.G., Bonet-Costa V., Sun P.Y., Davies K.J. The Role of Oxidative Stress in Anxiety Disorder: Cause or Consequence? Free Radic. Res. 2018;52(7):737–750. doi: 10.1080/10715762.2018.1475733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora S.J.S., Mittal M., Mishra D. Co-Exposure to Arsenic and Fluoride on Oxidative Stress, Glutathione Linked Enzymes, Biogenic Amines and DNA Damage in Mouse Brain. J. Neurol. Sci. 2009;285(1–2):198–205. doi: 10.1016/j.jns.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Fukai T., Ushio-Fukai M. Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Diseases. Antioxid. Redox Signal. 2011;15(6):1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamadani J.D., Tofail F., Nermell B., Gardner R., Shiraji S., Bottai M., Arifeen S.E., Huda S.N., Vahter M. Critical Windows of Exposure for Arsenic-Associated Impairment of Cognitive Function in Pre-School Girls and Boys: a Population-Based Cohort Study. Int. J. Epidemiol. 2011;40(6):1593–1604. doi: 10.1093/ije/dyr176. [DOI] [PubMed] [Google Scholar]
- Hassan W., Noreen H., Rehman S., Gul S., Amjad Kamal M., Paul Kamdem J., Zaman B., da Rocha B.T., J. Oxidative Stress and Antioxidant Potential of One Hundred Medicinal Plants. Curr. Top. Med. Chem. 2017;17(12):1336–1370. doi: 10.2174/1568026617666170102125648. [DOI] [PubMed] [Google Scholar]
- Hilles A.R., Mahmood S. A review on phytochemistry and pharmacological effects of Trigonella foenum-graecum. Adv. Herb. Med. 2017;3(3):61–67. [Google Scholar]
- Hu Y., Li J., Lou B., Wu R., Wang G., Lu C., Wang H., Pi J., Xu Y. The Role of Reactive Oxygen Species in Arsenic Toxicity. Biomolecules. 2020;10(2) doi: 10.3390/biom10020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam Z., Islam J., Tony S.R., Anjum A., Ferdous R., Roy A.K., Hossain S., Salam K.A., Nikkon F., Hossain K., Saud Z.A. Mulberry Leaves Juice Attenuates Arsenic-induced Neurobehavioral and Hepatic Disorders in Mice. Food Sci. Nutr. 2022;10:4360–4370. doi: 10.1002/fsn3.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam J., Shila T.T., Islam Z., Kabir E., Haque N., Khatun M., Khan S., Jubayar A.M., Islam F., Nikkon F., Hossain K., Saud Z.A. Clerodendrum viscosum leaves attenuate lead-induced neurotoxicity through upregulation of BDNF-Akt-Nrf2 pathway in mice. J. Ethnopharmacol. 2023;304 doi: 10.1016/j.jep.2022.116024. [DOI] [PubMed] [Google Scholar]
- Jomova K., Jenisova Z., Feszterova M., Baros S., Liska J., Hudecova D., Rhodes C.J., Valko M. Arsenic: toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011;31(2):95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- Karege F., Bondolfi G., Gervasoni N., Schwald M., Aubry J.M., Bertschy G. Low Brain-Derived Neurotrophic Factor (BDNF) Levels in Serum of Depressed Patients Probably Results from Lowered Platelet BDNF Release Unrelated to Platelet Reactivity. Biol. Psychiatry. 2005;57(9):1068–1072. doi: 10.1016/j.biopsych.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Karim Y., Siddique A.E., Hossen F., Rahman M., Mondal V., Banna H.U., Hasibuzzaman M.M., Hosen Z., Islam M.S., Sarker M.K., Nikkon F., Saud Z.A., Xin L., Himeno S., Hossain K. Dose-dependent relationships between chronic arsenic exposure and cognitive impairment and serum brain-derived neurotrophic factor. Environ. Int. 2019;131:105029. doi: 10.1016/j.envint.2019.105029. [DOI] [PubMed] [Google Scholar]
- Khorshidian N., Yousefi Asli M., Arab M., Adeli Mirzaie A., Mortazavian A.M. Fenugreek: Potential Applications as a Functional Food and Nutraceutical. Nutr. Food Sci. 2016;3(1):5–16. [Google Scholar]
- Kodumuri P.K., Thomas C., Jetti R., Pandey A.K. Fenugreek seed extract ameliorates cognitive deficits in streptozotocin-induced diabetic rats. J. Basic Clin. Physiol. Pharmacol. 2019;30(4) doi: 10.1515/jbcpp-2018-0140. [DOI] [PubMed] [Google Scholar]
- Kumagai Y., Sumi D. Arsenic: Signal Transduction, Transcription Factor, and Biotransformation Involved in Cellular Response and Toxicity. Annu. Rev. Pharmacol. 2007;47:243–262. doi: 10.1146/annurev.pharmtox.47.120505.105144. [DOI] [PubMed] [Google Scholar]
- Kumar, P., R. K. Kale, P. McLean, and N. Z. Baquer. 2012. “Antidiabetic and Neuroprotective Effects of Trigonella Foenum-Graecum Seed Powder in Diabetic Rat Brain.” Prague Medical Report 113 (1): 33-43. 10.14712/23362936.2015.35. [DOI] [PubMed]
- Leal G., Comprido D., Duarte C.B. BDNF-Induced Local Protein Synthesis and Synaptic Plasticity. Neuropharmacology. 2014;76:639–656. doi: 10.1016/j.neuropharm.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Lockridge O. Review of Human Butyrylcholinesterase Structure, Function, Genetic Variants, History of Use in the Clinic, and Potential Therapeutic Uses. Pharmacol. Ther. 2015;148:34–46. doi: 10.1016/j.pharmthera.2014.11.011. [DOI] [PubMed] [Google Scholar]
- Markowski V.P., Currie D., Reeve E.A., Thompson D., Wise J.P., Sr Tissue-Specific and Dose-Related Accumulation of Arsenic in Mouse Offspring Following Maternal Consumption of Arsenic-Contaminated Water. Basic Clin. Pharmacol. Toxicol. 2011;108(5):326–332. doi: 10.1111/j.1742-7843.2010.00660.x. [DOI] [PubMed] [Google Scholar]
- Massaad C.A., Klann E. Reactive Oxygen Species in the Regulation of Synaptic Plasticity and Memory. Antioxid. Redox Signal. 2011;14(10):2013–2054. doi: 10.1089/ars.2010.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews V.V., Binu P., Sauganth Paul M.V., Abhilash M., Manju A., Nair R.H. Hepatoprotective efficacy of curcumin against arsenic trioxide toxicity. Asian Pacific Journal of Tropical Biomedicine. 2012;2(2):S706–S711. [Google Scholar]
- Medda N., Patra R., Ghosh T.K., Maiti S. Neurotoxic mechanism of arsenic: synergistic effect of mitochondrial instability, oxidative stress, and hormonal-neurotransmitter impairment. Biol. Trace Elem. Res. 2020;198:8–15. doi: 10.1007/s12011-020-02044-8. [DOI] [PubMed] [Google Scholar]
- Medina, S., Zhang, H., Santos-Medina, L. V., Yee, Z. A., Martin, K. J., Wan, G., Bolt, A. M., Zhou, X., Stýblo, M., Liu, K. J., 2023. Arsenite Methyltransferase Is an Important Mediator of Hematotoxicity Induced by Arsenic in Drinking Water. Water, 15(3): 448. 10.3390/w15030448. [DOI] [PMC free article] [PubMed]
- Mehta K., Pandey K.K., Kaur B., Dhar P., Kaler S. Resveratrol Attenuates Arsenic-induced Cognitive Deficits via Modulation of Estrogen-NMDAR-BDNF Signalling Pathway in Female Mouse Hippocampus. Psychopharmacology. 2021;238(9):2485–2502. doi: 10.1007/s00213-021-05871-2. [DOI] [PubMed] [Google Scholar]
- Mitra A., Chatterjee S., Gupta D.K. Environmental arsenic exposure and human health risk. Arsenic water resources contamination: challenges and solutions. 2020;103–129 doi: 10.1007/978-3-030-21258-2_5. [DOI] [Google Scholar]
- Moghadam F.H., Vakili-Zarch B., Shafiee M., Mirjalili A. Fenugreek seed extract treats peripheral neuropathy in pyridoxine induced neuropathic mice. Excli J. 2013;25(12):282–290. [PMC free article] [PubMed] [Google Scholar]
- Peres T.V., Schettinger M.R.C., Chen P., Carvalho F., Avila D.S., Bowman A.B., Aschner M. Manganese-Induced Neurotoxicity: A Review of Its Behavioral Consequences and Neuroprotective Strategies. BMC Pharmacol. Toxicol. 2016;17(1):57. doi: 10.1186/s40360-016-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., Squadrito F., Altavilla D., Bitto A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017;2017:8416763. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prema A., Thenmozhi A.J., Manivasagam T., Essa M.M., Akbar M.D., Akbar M., Padmanabhan J. Fenugreek Seed Powder Nullified Aluminium Chloride Induced Memory Loss, Biochemical Changes, Aβ Burden and Apoptosis via Regulating Akt/GSK3β Signaling Pathway. PLoS ONE. 2016;11(11):e0165955. doi: 10.1371/journal.pone.0165955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn E.J., Guzman-Karlsson M.C., Sweatt J.D. Cellular, Molecular, and Epigenetic Mechanisms in Non-Associative Conditioning: Implications for Pain and Memory. Neurobiol. Learn. Mem. 2013;105:133–150. doi: 10.1016/j.nlm.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Chávez L.A., Rendón-López C.R., Zepeda A., Silva-Adaya D., Del Razo L.M., Gonsebatt M.E. Neurological Effects of Inorganic Arsenic Exposure: Altered Cysteine/Glutamate Transport, NMDA Expression and Spatial Memory Impairment. Front. Cell. Neurosci. 2015;9 doi: 10.3389/fncel.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Moreno K., Gamboa-Loira B., López-Carrillo L., Cebrián M.E. Prevalence of type 2 diabetes mellitus in relation to arsenic exposure and metabolism in Mexican women. Environ. Res. 2022;210 doi: 10.1016/j.envres.2022.112948. [DOI] [PubMed] [Google Scholar]
- Reza A.S.M., Hossain M.S., Akhter S., Rahman M.R., Nasrin M., Uddin M.J., Sadik G., Khurshid Alam A.H.M. In vitro Antioxidant and Cholinesterase Inhibitory Activities of Elatostema papillosum Leaves and Correlation with their Phytochemical Profiles: A Study Relevant to the Treatment of Alzheimer's Disease. BMC Complement. Altern. Med. 2018;18(1):123. doi: 10.1186/s12906-018-2182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salam S.G.A., Rashed M.M., Ibrahim N.A., Rahim E.A.A., Aly T.A., Al-Farga A. Phytochemical screening and in-vitro biological properties of unprocessed and household processed fenugreek (Trigonella foenum-graecum Linn.) seeds and leaves. Sci. Rep. 2023;13(1):7032. doi: 10.1038/s41598-023-31888-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017;360(1):201–205. doi: 10.1124/jpet.116.237503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia L., Grandone I., Contaldo F., Pasanisi F. Butyrylcholinesterase as a Prognostic Marker: a Review of the Literature. J. Cachexia Sarcopenia Muscle. 2013;4(1):31–39. doi: 10.1007/s13539-012-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P., Ho Y.J., Spanagel R., Pawlak C.R. A Novel Elevated Plus-Maze Procedure to Avoid the One-Trial Tolerance Problem. Front. Behav. Neurosci. 2011;5:43. doi: 10.3389/fnbeh.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R., Buras E., Terashima T., Serrano F., Massaad C.A., Hu L., Bitner B., Inoue T., Chan L., Pautler R.G., Wang Y.i. Hyperglycemia Induces Oxidative Stress and Impairs Axonal Transport Rates in Mice. PLoS One. 2010;5(10):e13463. doi: 10.1371/journal.pone.0013463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P., Kumar A., Singh D. Dietary Flavonoids Interaction with CREB-BDNF Pathway: An Unconventional Approach for Comprehensive Management of Epilepsy. Curr. Neuropharmacol. 2019;17(12):1158–1175. doi: 10.2174/1570159X17666190809165549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasa U.M., Naidu M.M. Fenugreek (Trigonella foenum-graecum L.) seed: promising source of nutraceutical. Stud. Nat. Prod. Chem. 2021;71:141–184. doi: 10.1016/B978-0-323-91095-8.00014-3. [DOI] [Google Scholar]
- Srivastava P., Dhuriya Y.K., Gupta R., Shukla R.K., Yadav R.S., Dwivedi H.N., Pant A.B., Khanna V.K. Protective Effect of Curcumin by Modulating BDNF/DARPP32/CREB in Arsenic-Induced Alterations in Dopaminergic Signaling in Rat Corpus Striatum. Mol. Neurobiol. 2018;55(1):445–561. doi: 10.1007/s12035-016-0288-2. [DOI] [PubMed] [Google Scholar]
- Sun B.-F., Wang Q.-Q., Yu Z.-J., Yu Y., Xiao C.-L., Kang C.-S., Ge G., Linghu Y., Zhu J.-D., Li Y.-M., Li Q.-M., Luo S.-P., Yang D., Li L., Zhang W.-Y., Tian G., Sutherland R. Exercise Prevents Memory Impairment Induced by Arsenic Exposure in Mice: Implication of Hippocampal BDNF and CREB. PLoS One. 2015;10(9):e0137810. doi: 10.1371/journal.pone.0137810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D.J., Waters S.B., Styblo M. Elucidating the Pathway for Arsenic Methylation. Toxicol. Appl. Pharmacol. 2004;198(3):319–326. doi: 10.1016/j.taap.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Tyler C.R., Allan A.M. The Effects of Arsenic Exposure on Neurological and Cognitive Dysfunction in Human and Rodent Studies: A Review. Curr. Environ. Health Rep. 2014;1:132–147. doi: 10.1007/s40572-014-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahidnia A., van der Voet G.B., de Wolff F.A. Arsenic Neurotoxicity–A Review. Hum. Exp. Toxicol. 2007;26(10):823–832. doi: 10.1177/0960327107084539. [DOI] [PubMed] [Google Scholar]
- Vahter M. Mechanisms of Arsenic Biotransformation. Toxicology. 2002;181–182:211–217. doi: 10.1016/s0300-483x(02)00285-8. [DOI] [PubMed] [Google Scholar]
- von Ehrenstein O.S., Poddar S., Yuan Y., Mazumder D.G., Eskenazi B., Basu A., Hira-Smith M., Ghosh N., Lahiri S., Haque R., Ghosh A., Kalman D., Das S., Smith A.H. Children's Intellectual Function in Relation to Arsenic Exposure. Epidemiology. 2007;18(1):44–51. doi: 10.1097/01.ede.0000248900.65613.a9. [DOI] [PubMed] [Google Scholar]
- Wang N., He F., Li W., Fang X., Li H. Purification of the Total Steroidal Saponins from Fenugreek Seeds (Trigonella Foenum-Graecum L.) Using Aqueous Two-Phase System and Determination of Diosgenin Content Using Micellar Electrokinetic Chromatography Method. Nat. Prod. Res. 2019;33(3):453–456. doi: 10.1080/14786419.2018.1455047. [DOI] [PubMed] [Google Scholar]
- Wang B., Li Y., Li X.P., Li Y. Panax Notoginseng Saponins Improve Recovery after Spinal Cord Transection by Upregulating Neurotrophic Factors. Neural Regen. Res. 2015;10(8):1317–1320. doi: 10.4103/1673-5374.162766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani S.A., Kumar P. Fenugreek: A Review on its Nutraceutical Properties and Utilization in Various Food Products. J. Saudi Soc. Agric. 2018;17(2):97–106. doi: 10.1016/j.jssas.2016.01.007. [DOI] [Google Scholar]
- WHO, Regional Office for South-East Asia, Country Health System Profile: Bangladesh. 2002. http://www.who.int/gho/countries/bgd/country_profiles/en/ Available at:
- Xu L., Mondal D., Polya D.A. Positive association of cardiovascular disease (CVD) with chronic exposure to drinking water arsenic (As) at concentrations below the WHO provisional guideline value: a systematic review and meta-analysis. International. Int. J. Environ. Res. Public Health. 2020;17(7):2536. doi: 10.3390/ijerph17072536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Mei D., Li Y., You M., Wang D., Yao D., Xu Y., Zhai L., Wang Y. Arsenic exposure via drinking water during pregnancy and lactation induces autism-like behaviors in male offspring mice. Chemosphere. 2022;290 doi: 10.1016/j.chemosphere.2021.133338. [DOI] [PubMed] [Google Scholar]
- Zhou Y.J., Chen J.M., Sapkota K., Long J.Y., Liao Y.J., Jiang J.J., Liang B.Y., Wei J.B., Zhou Y. Pananx Notoginseng Saponins Attenuate CCL2-Induced Cognitive Deficits in Rats Via Anti-Inflammation and Anti-Apoptosis Effects that Involve Suppressing Over-Activation of NMDA Receptors. Biomed. Pharmacother. 2020;127 doi: 10.1016/j.biopha.2020.110139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.