Abstract
The expression of genes delivered by retroviral vectors is often inefficient, a potential obstacle for their widespread use in human gene therapy. Here, we explored the possibility that the posttranscriptional regulatory element of woodchuck hepatitis virus (WPRE) might help resolve this problem. Insertion of the WPRE in the 3′ untranslated region of coding sequences carried by either oncoretroviral or lentiviral vectors substantially increased their levels of expression in a transgene-, promoter- and vector-independent manner. The WPRE thus increased either luciferase or green fluorescent protein production five- to eightfold, and effects of a comparable magnitude were observed with either the immediate-early cytomegalovirus or the herpesvirus thymidine kinase promoter and with both human immunodeficiency virus- and murine leukemia virus-based vectors. The WPRE exerted this influence only when placed in the sense orientation, consistent with its predicted posttranscriptional mechanism of action. These results demonstrate that the WPRE significantly improves the performance of retroviral vectors and emphasize that posttranscriptional regulation of gene expression should be taken into account in the design of gene delivery systems.
Retroviral vectors offer several characteristics of great value for a gene delivery system, including a large packaging capacity, an efficient integration machinery, and the absence of a vector-induced cellular immune response. However, one shortcoming of retroviral vectors, whether based on oncoretroviruses or lentiviruses, is their frequent inability to generate high levels of gene expression, particularly in vivo. Epigenetic phenomena, such as position effects or silencing by DNA methylation, may partly account for this limitation.
Many steps, both transcriptional and posttranscriptional, are involved in the regulation of gene expression. Therefore, it may be possible to improve the expression of transgenes delivered by retroviral vectors through the addition of elements known to increase gene expression posttranscriptionally. The best known example of stimulation at this level is the inclusion of an intron within the expression cassette. Many gene transfer experiments, performed both in vitro and in vivo, have demonstrated that the presence of an intron can facilitate gene expression (4). In extreme cases, such as β-globin, expression is intron dependent. Expressed β-globin cDNAs are unstable in the nucleus and never accumulate in the cytoplasm. However, the addition of an intron causes the cytoplasmic accumulation of β-globin mRNA (2). Several mechanisms can be responsible for this effect. Some introns have been found to contain regulatory sequences that enhance transcription or 3′-end processing (1, 5, 12, 22). More generally, however, splicing per se appears to stimulate gene expression, perhaps in part by promoting the nuclear stability, proper processing, and/or cytoplasmic localization of mRNAs (25).
This evidence has prompted the development of strategies to incorporate introns into retroviral vectors. This task is difficult, because retroviral genomic RNA is normally produced in the nucleus by the cellular transcriptional machinery and as such is exposed to the splicing machinery. To circumvent this difficulty, intron-containing transgenes can be placed in an orientation opposite that of the vector genomic transcript (27). Unfortunately, this approach is complicated by the possibility of antisense effects. Alternatively, intron-containing retroviral vector genomic RNAs can be produced in the cytoplasm, for instance, through the use of an alphavirus vector (16). It remains to be seen whether the latter technique will gain broad acceptance for the production of clinical-grade retroviral vectors.
Other types of elements can also be used to stimulate β-globin cDNA expression posttranscriptionally. These elements have the advantage of not requiring splicing events, thereby avoiding removal during the viral life cycle. For instance, elements derived from intronless viral messages can stimulate the cytoplasmic accumulation of β-globin cDNA transcripts. These include the posttranscriptional processing element present within the thymidine kinase gene of herpes simplex virus (17) and the posttranscriptional regulatory element (PRE) present in hepatitis B virus (HBV) (14).
Previous studies have suggested that the HBV PRE (HPRE) and an intron are functionally equivalent. This model was a consequence of the observation that the HPRE and β-globin intron II were interchangeable. β-Globin intron II could stimulate the expression of the HBV surface protein, which is normally HPRE dependent, while the HPRE could stimulate the expression of a β-globin cDNA (14). The proposed mechanism of HPRE function is the facilitation of the nuclear export of PRE-containing transcripts (11, 13). Supporting this model is evidence that the HPRE can functionally substitute for the human immunodeficiency virus (HIV) type 1 (HIV-1) Rev–Rev-responsive element complex in a transient transfection reporter assay (7, 13). Woodchuck hepatitis virus (WHV), a close relative of HBV, also harbors a PRE (WPRE) (8). We have previously shown that the WPRE is significantly more active than its HBV counterpart; the increased activity correlates with the presence of an additional cis-acting sequence in the WPRE which is not found in the HPRE (8).
Because of the increased efficiency of this element, we examined whether the WPRE could stimulate the expression of intronless transgenes delivered by retroviral vectors. We found that the insertion of this sequence in HIV-derived vectors resulted in a significant stimulation of expression of the reporter genes for luciferase and green fluorescent protein (GFP) in a variety of cells of human and rodent origins. Stimulation was irrespective of the cycling status of transduced cells. The WPRE effect was not promoter dependent and was also revealed within the context of murine leukemia virus (MLV)-derived vectors. Interestingly, the WPRE acted on both intronless and spliced mRNAs, revealing that the functions of the WPRE and splicing in gene expression are not redundant. These data suggest that the inclusion of the WPRE in retroviral vectors will result in a significant improvement in their performance for gene therapy. Further, the WPRE may be a useful tool for stimulating gene expression in other vector contexts.
MATERIALS AND METHODS
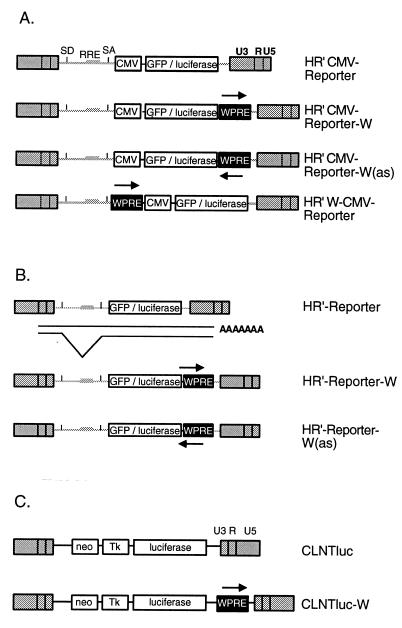
Plasmids. (i) HIV-1 vector plasmids.
Plasmids pHR′CMV-GFP and pHRCMV-Luc have been described previously (29). A PCR-amplified WPRE cassette (nucleotides 1093 to 1684; GenBank accession no. J04514) was modified with ClaI or EcoRI ends and inserted into pHR′CMV-GFP either at the unique ClaI site upstream of the cytomegalovirus (CMV) promoter or at the unique EcoRI site downstream of the GFP stop codon, resulting in plasmids pHR′W-CMV-GFP and pHR′CMV-GFP-W, respectively. Subsequently, the BamHI-XhoI GFP coding sequence was replaced with a BamHI-XhoI luciferase coding sequence to generate pHR′CMV-Luc-W. Plasmids pMD.G and pCMVΔR8.91 have been described previously (29).
(ii) MLV vector plasmids.
WPRE was inserted as a ClaI cassette into the unique ClaI site of plasmid pCLNCX (21). Subsequently, a BamHI-HindIII thymidine kinase (TK)-luciferase cassette was substituted for the CMV promoter, resulting in plasmid pCLNTluc-W. The ClaI WPRE cassette was deleted to generate control plasmid pCLNTluc.
Tissue culture and transfection.
Dulbecco’s modified Eagle’s medium (Gibco) was supplemented with 10% fetal calf serum (Gibco), a combination of penicillin and streptomycin (Gibco), and glutamine (Gibco). 293T, HeLa, HeLa-tat, HOS, 208F, and NIH 3T3 cells were cultured in supplemented Dulbecco’s modified Eagle’s medium in a 10% CO2 atmosphere. Gamma irradiation was delivered by a 3-min exposure to a 60CO source. Vector stocks were prepared and cells were transduced as previously described (29).
To determine the titers of GFP-transducing vectors, five serial 1:2 dilutions of each filtered vector stock were used to transduce HeLa cells in six-well plates (2 × 105 cells/well). The highest and lowest inocula corresponded to 100 ml and 6.25 ml of undiluted supernatant, respectively. Vector particles were added to 2 ml of culture medium in the absence of Polybrene and left on the cells for 48 to 60 h. At this time, the percentage of GFP-positive cells was determined with a fluorescence-activated cell sorter on a Beckton Dickinson FACScan. To calculate titers (transducing units per milliliter), 2 × 105 cells/well was multiplied by the percentage of GFP-positive cells, and this product was divided by the number of microliters in the inoculum. Numerous titer determinations have shown that the percentage of transduced cells correlates linearly with the vector input when the percentage is lower than 12%. Therefore, all titers were based on at least two values lower than 12% and showing the expected linearity.
Southern analysis.
Genomic DNA was isolated from three 10-cm plates of each cell line. Cells were lysed, phenol extracted, and ethanol precipitated by standard methods. Ten micrograms of genomic DNA was digested overnight with BamHI, EcoRI, and XhoI restriction enzymes. Digested DNA was ethanol precipitated and electrophoresed on a 0.9% agarose gel. DNA was visualized with ethidium bromide staining before being transferred to a nylon membrane by standard methods.
RNA isolation and analysis.
Cells were washed with phosphate-buffered saline (PBS) and pelleted by centrifugation. For total RNA, the cell pellet was resuspended in 250 μl of PBS, and 750 μl of RNA Stat LS-50 (Tel-Test) was added. For nuclear and cytoplasmic fractionation, cells were resuspended in cytoplasmic lysis buffer (10 mM HEPES [pH 7.8], 10 mM KCl, 0.1 mM EDTA, 20% glycerol, 0.5% Nonidet P-40). The lysed cells were spun for 3 min at 8,000 × g, and the supernatant was recovered and spun for an additional 5 min at 14,000 × g. The supernatant was transferred to 1 ml of RNA Stat LS-50. The nuclear pellet from the first spin was resuspended in 1 ml of cytoplasmic lysis buffer and then spun at 8,000 × g for 3 min. The supernatant was discarded, and the pellet was resuspended in 800 μl of nuclear buffer (10 mM Tris [pH 8.4], 1.5 mM MgCl2, 140 mM NaCl, 20% glycerol). The sample was spun at 8,000 × g, and the supernatant was discarded. The pellet was resuspended in 300 μl of nuclear buffer and lysed with 1 ml of RNA Stat LS-50. The RNA Stat LS-50 protocol was followed.
For RNA half-life analysis, cells were grown to 70% confluency and treated with 5 mg of actinomycin D per ml. For each time point, two plates of cells were harvested. The cells were pelleted and resuspended in 250 μl of PBS, and 750 μl of RNA Stat LS-50 was added. After RNA purification, the samples were treated with DNase for 15 min at 37°C. Five micrograms of nuclear RNA and 10 μg of cytoplasmic RNA were separated on a 1% agarose–formaldehyde gel, transferred to a nylon membrane, and hybridized with a GFP probe by use of Quickhyb (Qiagen) and the manufacturer’s protocol.
Nuclear run-on assays.
For each experiment, 5 × 107 cells were harvested. The nuclei were prepared by lysing the cells in cell lysis buffer (10 mM Tris [pH 8.3], 10 mM NaCl, 5 mM MgCl2). The nuclei were washed once in cell lysis buffer and frozen overnight. The run-on reactions were performed with 25 mM Tris (pH 8.0)–12.5 mM MgCl2–750 mM KCl–1.25 mM each ATP, CTP, and GTP–30 μl of UTP (800 Ci/mmol). The reaction mixtures were incubated for 30 min at 30°C. The nuclei were homogenized in 750 μl of RNA Stat LS-50 (Tel-Test). The labeled RNA was purified in accordance with the manufacturer’s specifications. The RNA samples were treated with DNase, phenol extracted, and centrifuged through G-25 columns. The samples were ethanol precipitated and resuspended, and counts were determined with a scintillation counter. Equivalent counts were hybridized with nitrocellulose filters containing plasmid DNAs for histone H2B and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and PCR products derived from vector sequences by use of Quickhyb and the manufacturer’s protocol. Hybridized filters were treated with RNase A and analyzed.
RESULTS
WPRE enhances the expression of transgenes delivered by HIV-based vectors.
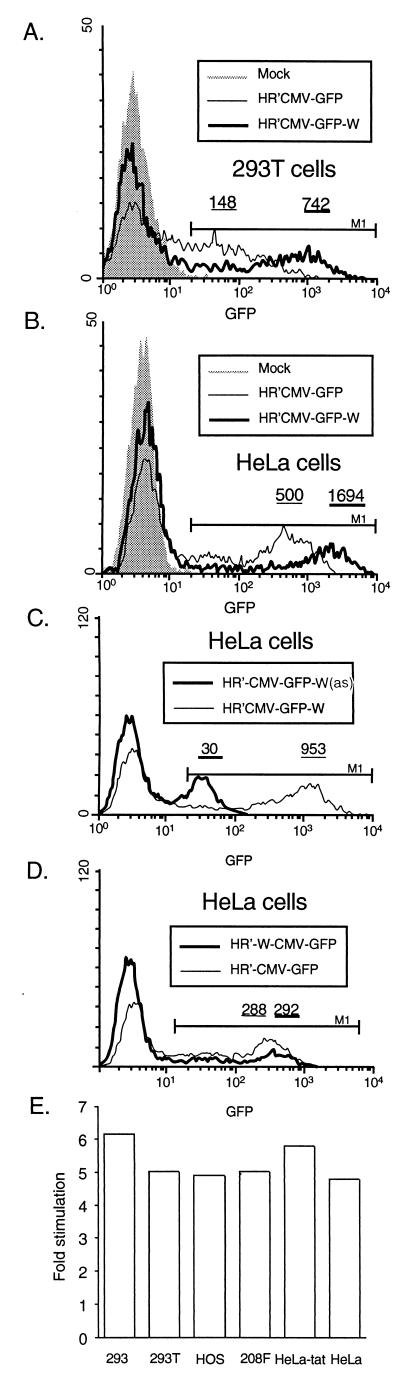
To test whether the WPRE enhances the expression of intronless reporter genes delivered by HIV-based vectors, a 600-nucleotide-long cassette containing the WPRE sequence was cloned into the previously described plasmids pHR′CMV-Luc and pHR′CMV-GFP (29). The WPRE was inserted in the 3′ untranslated region (UTR) of the reporter genes between the stop codon and the polypurine tract (Fig. 1A). To produce vesicular stomatitis virus G protein (VSV-G)-pseudotyped transducing particles, vector plasmids with or without the WPRE were cotransfected into 293T cells with the envelope plasmid pMD.G and the packaging plasmid pCMVΔR8.91 by a published protocol (20). The resulting vectors were used to transduce in parallel different cell lines. These experiments were done at a multiplicity of infection (MOI) of 0.1 to favor a single integration per cell. Dilution analysis of the vector stocks on 293T cells showed that the WPRE did not influence titers (data not shown). However, fluorescence-activated cell sorter quantification of GFP expression in cells transduced with HR′CMV-GFP or HR′CMV-GFP-W demonstrated that the presence of the WPRE in the transgene 3′ UTR (indicated by the suffix W in the plasmid name) increased the mean expression of the transduced population by at least fivefold in 293T cells (Fig. 2A) and threefold in HeLa cells (Fig. 2B). In contrast, the WPRE inserted in the opposite orientation (indicated by the suffix W(as) in the plasmid name) inhibited GFP expression in HeLa cells (Fig. 2C). Therefore, WPRE function is orientation dependent, as has been reported previously (8).
FIG. 1.
Schematic drawing of the vector constructs used in this study. (A) HIV-1-based vector constructs containing an internal CMV promoter driving transgene expression. (B) HIV-1-based vector constructs in which transgene expression is driven by the HIV LTR promoter. The transgene is expressed from a spliced message. (C) MLV constructs containing an internal herpes simplex virus TK promoter (Tk) driving the expression of luciferase. The WPRE is shown as a black box. W(as) designates the WPRE inserted in the antisense orientation. Orientation is designated by arrows. SD, splice donor; SA, splice acceptor; RRE, Rev-responsive element; R, repeat region.
FIG. 2.
WPRE enhances gene expression in cells transduced with HIV-based vectors. 293T (A) or HeLa (B) cells were transduced in parallel with HR′CMV-GFP or HR′CMV-GFP-W stocks matched for p24. Viral stocks were transduced at an MOI of 0.1 to 0.2. At 48 h postinfection, GFP expression was compared to that in noninfected cells (shaded histogram), in cells transduced with HR′CMV-GFP (thin line), and in cells transduced with HR′CMV-GFP-W (thick line). Average GFP expression per cell was determined for transduced cells contained in the M1 region. Values for average expression in the population are shown, designated by lines corresponding to the lines used in the histograms. In both cell lines, GFP expression per cell was increased by the presence of the WPRE. (C) WPRE function is orientation specific. HeLa cells were transduced with HR′CMV-GFP-W or HR′CMV-GFP-W(as). (D) WPRE functions in cis. HeLa cells were transduced with HR′W-CMV-GFP or HR′CMV-GFP. Average GFP expression per cell was equivalent for these two derivatives. Data in panels C and D were derived from a single experiment allowing direct comparison of mean GFP intensity. (E) The WPRE can stimulate the expression of luciferase in a variety of cell lines. Results are shown as the ratio of luciferase expression with a vector containing WPRE versus a normal vector for each cell line. The presence of the WPRE increased luciferase expression five- to sevenfold in each cell line.
Further studies revealed that the WPRE had to be present within the transgene transcript to function. When the WPRE was inserted upstream of the CMV promoter governing the transcription of the GFP gene (Fig. 1A), it was unable to stimulate GFP expression in HeLa cells (Fig. 2D). This result demonstrates that the WPRE must be present within a transcript to stimulate expression, consistent with a posttranscriptional mode of action.
To exclude the possibility that the WPRE effect was gene specific, we tested the ability of the WPRE to stimulate luciferase expression. The luciferase activities induced in various target cells by either HR′CMV-Luc or HR′CMV-Luc-W were compared. Viral stocks were normalized for the amounts of HIV-1 p24 capsid antigen present in the inoculum (Fig. 2E). The WPRE in the 3′ UTR of the luciferase cDNA increased luciferase production by a factor of 5 to 6 (mean, 5.27) in all cell lines tested (293T, HeLa, HOS, 208F, and NIH 3T3). The WPRE is thus active in both human and rodent cell lines and in cells of epithelial, osteoblastic, or fibroblastic origin.
To explore if WPRE function is sensitive to the proliferation status of transduced cells, we analyzed the level of luciferase expression in dividing and gamma-irradiated 293T cells. Cells were transduced with HIV-based luciferase vectors carrying the WPRE sequence in either the sense or the antisense orientation (Table 1). When a vector without the WPRE was used as a reference, the WPRE in the sense orientation resulted in a eightfold increase in luciferase expression in both dividing and arrested 293T cells. The WPRE in the antisense orientation was nonfunctional. This experiment reveals that the proliferation status of the target cells has no impact on WPRE function, an important feature for in vivo expression in terminally differentiated tissues.
TABLE 1.
Stimulation of transgene expression by the WPRE in arrested cells
| Vector | Luciferase expression in 293T cellsa
|
|
|---|---|---|
| Dividing | Gamma irradiatedb | |
| HR′CMV-Luc | 1,291,608 ± 332,111 | 1,037,861 ± 366,257 |
| HR′CMV-Luc-Was | 270,129 ± 13,035 | 235,980 ± 30,876 |
| HR′CMV-Luc-W | 10,977,543 ± 2,547,091 | 10,455,054 ± 3,065,829 |
Reported as relative light units per nanogram of p24 (mean ± standard error of the mean). For each vector type, 10 μl of two independently produced stocks representing 1.5 to 4.5 ng of p24 was used to transduce in parallel and in duplicate 2 × 105 cells. The estimated MOI was 0.01 to 0.03.
8,000 rads delivered by a 3-min exposure to a 60Co source.
WPRE enhances the expression of transgenes carried by MLV-based vectors.
Since the WPRE is functional in settings as different as those of WHV and HIV-based vectors, it was considered likely that it would also be functional when incorporated into a vector derived from a simple retrovirus. To test this hypothesis, the WPRE was inserted in the 3′ UTR of the luciferase cDNA delivered by an MLV-based vector (21). Instead of the CMV promoter, the promoter from the human herpes simplex virus TK gene was chosen to demonstrate that WPRE action is not dependent on a particular promoter (Fig. 1C). Stocks of VSV-G-pseudotyped MLV-based vectors were generated by cotransfection of 293T cells with three plasmids, pMD.G, pCMVGagPol, and pCLNTluc or pCLNTluc-W, and used to transduce 293T cells. The induction of luciferase activity in these targets was measured and normalized for the amount of reverse transcriptase activity present in the inoculum. The results revealed that the presence of the WPRE increased the levels of expression of luciferase delivered by MLV-based vector-mediated transduction more than fourfold (relative light units with CLNTluc and CLNTluc-W [mean ± standard error of the mean for triplicate batches of each vector in two independent experiments], 139,518 ± 11,349 and 571,887 ± 7,319, respectively).
WPRE also acts on spliced mRNAs.
In its natural context, the WPRE is located within intronless mRNAs. To test whether this element could facilitate the expression of spliced mRNAs, the WPRE was inserted in HIV-based vectors expressing GFP or luciferase but devoid of the internal CMV promoter (Fig. 1B). In these vectors, the reporter-encoding RNAs are produced by the 5′ HIV long terminal repeat (LTR). Transgene expression measured in this system is from the spliced message. It is not expected that unspliced messages will substantially contribute to transgene expression because 10 ATG triplets present in the intron sequence may act as aberrant translational start sites. Since the HIV LTR is a weak promoter unless stimulated by the HIV Tat protein or the adenovirus early protein E1A, we used HeLa-tat and 293T cells as targets. As shown in Table 2, the WPRE enhanced luciferase expression in 293T cells eightfold. This level of stimulation by the WPRE is comparable to that observed for intronless mRNAs. In HeLa and HeLa-tat cells, the WPRE increased luciferase expression by factors of 4 and 5, respectively. The similar levels of enhancement observed in HeLa and HeLa-tat cells indicated that the WPRE is effective over a wide range of promoter activities, since the LTR is 30 times more active in HeLa-tat cells than in HeLa cells. Comparable results were obtained with the GFP gene (data not shown). As noted for intronless transcripts, the action of the WPRE on spliced mRNAs was orientation dependent.
TABLE 2.
Expression of a transgene containing an intron is enhanced by the WPRE
| Vector | Luciferase expression in the following transduced cellsa:
|
||
|---|---|---|---|
| HeLa | HeLa-tat | 293T | |
| HR′Luc | 10,840 ± 59 | 359,931 ± 3,972 | 66,936 ± 1,842 |
| HR′Luc-W | 48,835 ± 491 | 1,904,711 ± 60,525 | 588,755 ± 30,544 |
| WPRE/control ratio | 4.2 | 5.3 | 8.8 |
Reported as relative light units per nanogram of p24 (mean ± standard error of the mean). For each vector type, 50 μl of two independently produced stocks representing 0.5 to 1 ng of p24 was used to transduce in parallel and in duplicate 2 × 105 cells. The estimated MOI was <0.01.
Mechanism of WPRE action.
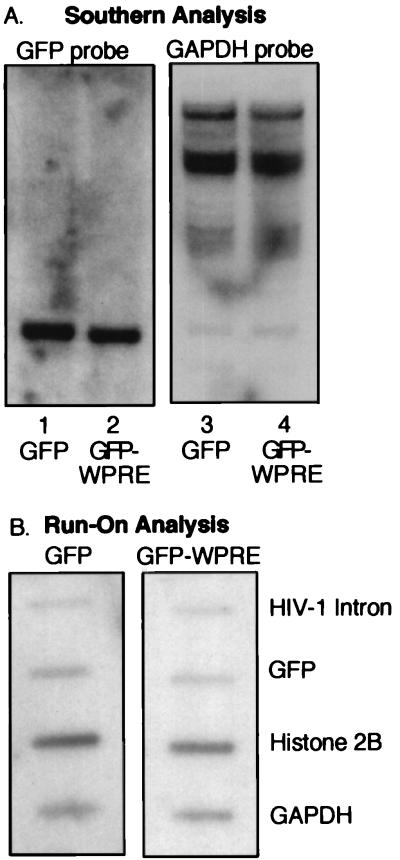
It was previously established that HPRE and WPRE act at a posttranscriptional level (7, 11, 13). To explore the mechanism of WPRE action in a lentivirus context, 293T cells were transduced with HR′CMV-GFP and HR′CMV-GFP-W at an MOI of 0.2. Fluorescent cells were sorted, expanded, and used for comparative DNA and RNA analyses (Fig. 3). Southern blot analysis demonstrated that the GFP transgene was present at comparable copy numbers in both populations (Fig. 3A). Nuclear run-on analysis (Fig. 3B) demonstrated that the WPRE did not influence the rate of transcription of vector-based messages. The observation that the WPRE does not function by stimulating transcription is consistent with previous reports indicating that WHV does not have an enhancer within the WPRE (6, 8, 10, 26).
FIG. 3.
DNA contents and transcription levels are not affected by the WPRE. 293T cells were transduced with HR′CMV-GFP and HR′CMV-GFP-W vectors at an MOI of 0.2. GFP-positive cells were sorted, expanded, and used for comparative DNA and RNA analyses. (A) Southern blot. Genomic DNA extracted from HR′CMV-GFP-transduced cells (lanes 1 and 3) or HR′CMV-GFP-W-transduced cells (lanes 2 and 4) were hybridized with a GFP-specific probe (lanes 1 and 2). The membrane was stripped and rehybridized with a GAPDH-specific probe (lanes 3 and 4). (B) WPRE does not increase transcription initiation frequency. Radiolabeled transcripts produced by nuclear run-on transcription were hybridized to plasmid DNA encoding GAPDH or histone 2B or PCR-derived DNA.
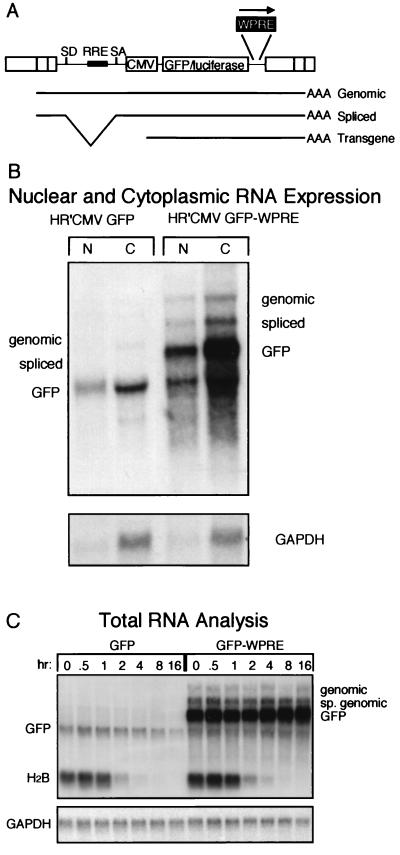
To determine the effects of the WPRE on GFP transcripts, RNA from cytosolic and nuclear fractions of transduced cells was analyzed by Northern blotting with a GFP-specific probe (Fig. 4B). Three RNA species can be detected by this probe; the two larger ones correspond to unspliced and spliced transcripts initiated at the 5′ HIV LTR, and the smaller one corresponds to RNAs originating from the internal CMV promoter. All GFP transcripts extracted from HR′CMV-GFP-W-transduced cells were larger than their counterparts extracted from HR′CMV-GFP-transduced cells due to the presence of the WPRE. The levels of expression of all three classes of WPRE-containing transcripts were six times higher than those in the respective controls in both nuclear and cytosolic fractions.
FIG. 4.
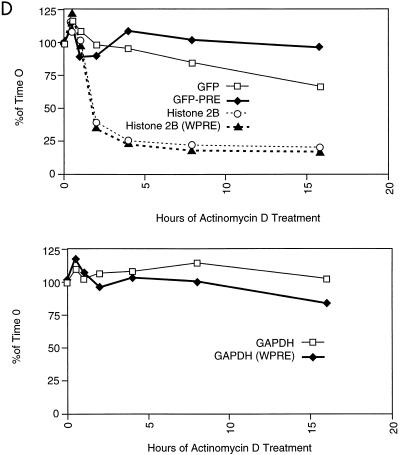
RNA analysis of transduced cell lines. (A) Schematic drawing of vectors showing the three classes of generated messages that contain GFP. SD, splice donor; SA, splice acceptor; RRE, Rev-responsive element. (B) Northern blot analysis of nuclear (N) and cytoplasmic (C) RNAs derived from HR′CMV-GFP- and HR′CMV-GFP-W-transduced populations. Analysis for GAPDH expression of the same filter after stripping and reprobing is shown at the bottom. (C) Half-life analysis of total RNA. Actinomycin D was added, and cells were harvested at the times shown. Controls for the unstable histone 2B (H2B) message and the stable GAPDH message are shown below. sp., spliced. (D) Phosphorimager analysis of the Northern blot shown in panel C. Quantitation of GFP and histone 2B levels is shown in the upper panel. Quantitation of GAPDH is shown in the lower panel. Data are expressed as a percentage of levels of expression at time zero over time.
To determine if the WPRE altered the RNA half-life, populations transduced with either HR′CMV-GFP or HR′CMV-GFP-W were incubated with actinomycin D. Total RNA was extracted at different times and analyzed by Northern blotting (Fig. 4C). From the earliest time, all the WPRE-containing transcripts were much more abundant than the respective controls. However, the half-life of the WPRE-containing GFP RNA was increased less than twofold compared to that of the GFP RNA, as determined by phosphorimager analysis (Fig. 4D). Taken together, these results suggest that the WPRE acts very early during the biogenesis of RNA transcripts, perhaps by directing their efficient processing as soon as they emerge from the transcriptional machinery.
DISCUSSION
Retroviral vectors can transduce efficiently a variety of cells, but the expression of the integrated transgene is usually low. In this study, we demonstrate that a cis-acting RNA element from WHV substantially increases the expression of transgenes delivered by retroviral vectors. The WPRE was active when inserted in vectors derived from both HIV-1 and MLV. Further, WPRE function was not cell type or species dependent, because it could stimulate transgene expression in several cell lines of human and rodent origins. The WPRE effect was not influenced by the cycling status of the transduced cells. The WPRE was only functional when present within a transcript in the sense orientation. The antisense WPRE had a significant inhibitory effect, reducing GFP and luciferase activities by a factor of 4 (Fig. 1D and Table 1). The inhibition seen in the antisense derivative is most likely due to the X promoter of WHV which is present in the WPRE cassette. This promoter could generate antisense RNA complementary to the transgene mRNA. Functional analysis revealed that the stimulation of transgene expression by the WPRE is posttranscriptional.
It was surprising to observe that the WPRE stimulated the expression of spliced mRNAs to the same extent as intronless transcripts. Previous studies had suggested that the HPRE and an intron were functionally equivalent (14). However, the studies presented here show that the WPRE stimulated the expression of a spliced RNA, suggesting that splicing and the PRE are not functionally redundant. This observation does not exclude the possibility that some functions of the WPRE and splicing of an intron in stimulating gene expression overlap. It is also possible that the intron within the vector does not act to facilitate the expression of transcripts after splicing.
Several observations are consistent with a model in which the WPRE functions within the nucleus to stimulate gene expression posttranscriptionally. The WPRE increases the levels of nuclear transcripts. The WPRE also does not greatly influence RNA half-life. Further, the observed increase in protein expression roughly correlates with an increase in RNA levels. It has previously been proposed that the PRE functions by facilitating RNA export. Although the WPRE does not greatly alter the nucleocytoplasmic ratio of affected RNAs generated by vector messages, this observation does not exclude a role for export in the function of the WPRE. Recent studies have revealed that the disruption of Rev function by the drug leptomycin B causes a decrease in the amount of nuclear Rev-responsive element-containing RNA (23). This observation and the observation that Rev can increase the nuclear half-life of HIV messages (18) suggest that engagement of an export pathway may simultaneously increase both the nuclear and the cytoplasmic pools of a specific RNA.
Alternatively, the WPRE may facilitate another step in RNA processing, directing RNAs that would normally be degraded within the nucleus to be efficiently expressed. This processing could be facilitated at the level of 3′ cleavage and polyadenylation. It has previously been shown that increasing the efficiency of 3′ processing can stimulate gene expression (3). The WPRE could also function to facilitate the generation of RNA-protein complexes which would protect newly synthesized transcripts from degradation in the nucleus. Increasing the efficiency of any one step in RNA processing could increase the efficiency of gene expression. These possible modes of action for the WPRE are not mutually exclusive, especially considering that the WPRE contains at least three distinct cis-acting subelements required for maximal function. For instance, one subelement could influence export, while another could increase the efficiency of 3′ processing.
Retroviral vectors have recently become more attractive as delivery systems for use in gene therapy. Lentivirus vectors allow nondividing cells to be transduced (20). HIV-based vectors can thus efficiently govern in vivo transgene delivery, integration, and long-term expression in nonmitotic cells, such as neurons, myocytes, and hepatocytes (15, 19). The initially low clinical acceptance of lentivirus vectors has been considerably increased by the development of multiply attenuated and self-inactivating (9, 28, 29) HIV-based vectors while, in parallel, analogous vectors have been derived from nonhuman lentiviruses (24). Our studies demonstrate that the WPRE can significantly improve the performance of retroviral vectors for use both in gene therapy protocols and in basic research. It is likely that the WPRE will also stimulate the expression of transgenes delivered by other vector systems. This improvement in the expression of genes delivered by retroviral vectors helps to bring the promise of gene therapy one step closer to fruition.
ACKNOWLEDGMENTS
We thank Allison Bocksrucker for assistance in preparing the manuscript and Matthew Weitzman for critical reading of the manuscript.
This work was supported by a grant from the Swiss National Science Foundation and from the Berger Foundation and by a professorship from the Giorgi-Cavaglieri Foundation to D.T. and by grants from the Gene and Ruth Posner Foundation and by a gift from Arthur Kramer and Larry Kramer to T.J.H. R.Z. was the recipient of a fellowship from the Swiss National Science Foundation, and J.E.D. was supported by NCI training grant T32 CA64041.
REFERENCES
- 1.Antoniou M, Geraghty F, Hurst J, Grosveld F. Efficient 3′-end formation of human beta-globin mRNA in vivo requires sequences within the last intron but occurs independently of the splicing reaction. Nucleic Acids Res. 1998;26:721–729. doi: 10.1093/nar/26.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchman A R, Berg P. Comparison of intron-dependent and intron-independent gene expression. Mol Cell Biol. 1988;8:4395–4405. doi: 10.1128/mcb.8.10.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carswell S, Alwine J C. Efficiency of utilization of the simian virus 40 late polyadenylation site: effects of upstream sequences. Mol Cell Biol. 1989;9:4248–4258. doi: 10.1128/mcb.9.10.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi T, Huang M, Gorman C, Jaenisch R. A generic intron increases gene expression in transgenic mice. Mol Cell Biol. 1991;11:3070–3074. doi: 10.1128/mcb.11.6.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung S, Perry R P. Importance of introns for expression of mouse ribosomal protein gene rpL32. Mol Cell Biol. 1989;9:2075–2082. doi: 10.1128/mcb.9.5.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Q, Summers J, Burch J B, Mason W S. Major differences between WHV and HBV in the regulation of transcription. Virology. 1997;229:25–35. doi: 10.1006/viro.1996.8422. [DOI] [PubMed] [Google Scholar]
- 7.Donello J E, Beeche A A, Smith III G J, Lucero G R, Hope T J. The hepatitis B virus posttranscriptional regulatory element is composed of two subelements. J Virol. 1996;70:4345–4351. doi: 10.1128/jvi.70.7.4345-4351.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donello J E, Loeb J E, Hope T J. Woodchuck hepatitis virus contains a tripartite posttranscriptional regulatory element. J Virol. 1998;72:5085–5092. doi: 10.1128/jvi.72.6.5085-5092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dull T, Zufferey R, Kelly M, Mandel R J, Nguyen M, Trono D, Naldini L. A third-generation lentiviral vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fourel G, Ringeisen F, Flajolet M, Tronche F, Pontoglio M, Tiollais P, Buendia M A. The HNF1/HNF4-dependent We2 element of woodchuck hepatitis virus controls viral replication and can activate the N-myc2 promoter. J Virol. 1996;70:8571–8583. doi: 10.1128/jvi.70.12.8571-8583.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang J, Liang T J. A novel hepatitis B virus (HBV) genetic element with Rev response element-like properties that is essential for expression of HBV gene products. Mol Cell Biol. 1993;13:7476–7486. doi: 10.1128/mcb.13.12.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang M T, Gorman C M. Intervening sequences increase efficiency of RNA 3′ processing and accumulation of cytoplasmic RNA. Nucleic Acids Res. 1990;18:937–947. doi: 10.1093/nar/18.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Z M, Yen T S. Hepatitis B virus RNA element that facilitates accumulation of surface gene transcripts in the cytoplasm. J Virol. 1994;68:3193–3199. doi: 10.1128/jvi.68.5.3193-3199.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Z M, Yen T S. Role of the hepatitis B virus posttranscriptional regulatory element in export of intronless transcripts. Mol Cell Biol. 1995;15:3864–3869. doi: 10.1128/mcb.15.7.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kafri T, Blömer U, Peterson D A, Gage F H, Verma I M. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- 16.Li K J, Garoff H. Packaging of intron-containing genes into retrovirus vectors by alphavirus vectors. Proc Natl Acad Sci USA. 1998;95:3650–3654. doi: 10.1073/pnas.95.7.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, Mertz J E. HnRNP L binds a cis-acting RNA sequence element that enables intron-dependent gene expression. Genes Dev. 1995;9:1766–1780. doi: 10.1101/gad.9.14.1766. [DOI] [PubMed] [Google Scholar]
- 18.Malim M H, Cullen B R. Rev and the fate of pre-mRNA in the nucleus: implications for the regulation of RNA processing in eukaryotes. Mol Cell Biol. 1993;13:6180–6189. doi: 10.1128/mcb.13.10.6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naldini L, Blömer U, Gage F H, Trono D, Verma I M. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 21.Naviaux R K, Costanzi E, Haas M, Verma I M. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J Virol. 1996;70:5701–5705. doi: 10.1128/jvi.70.8.5701-5705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nesic D, Cheng J, Maquat L E. Sequences within the last intron function in RNA 3′-end formation in cultured cells. Mol Cell Biol. 1993;13:3359–3369. doi: 10.1128/mcb.13.6.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otero G C, Harris M E, Donello J E, Hope T J. Leptomycin B inhibits equine infectious anemia virus Rev and feline immunodeficiency virus Rev function but not the function of the hepatitis B virus posttranscriptional regulatory element. J Virol. 1998;72:7593–7597. doi: 10.1128/jvi.72.9.7593-7597.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poeschla E M, Wong S F, Looney D J. Efficient transduction of nondividing human cells by feline immunodeficiency virus lentiviral vectors. Nat Med. 1998;4:354–357. doi: 10.1038/nm0398-354. [DOI] [PubMed] [Google Scholar]
- 25.Ryu W S, Mertz J E. Simian virus 40 late transcripts lacking excisable intervening sequences are defective in both stability in the nucleus and transport to the cytoplasm. J Virol. 1989;63:4386–4394. doi: 10.1128/jvi.63.10.4386-4394.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ueda K, Wei Y, Ganem D. Activation of N-myc2 gene expression by cis-acting elements of oncogenic hepadnaviral genomes: key role of enhancer II. Virology. 1996;217:413–417. doi: 10.1006/viro.1996.0133. [DOI] [PubMed] [Google Scholar]
- 27.Wang J M, Zheng H, Sugahara Y, Tan J, Yao S N, Olson E, Kurachi K. Construction of human factor IX expression vectors in retroviral vector frames optimized for muscle cells. Hum Gene Ther. 1996;7:1743–1756. doi: 10.1089/hum.1996.7.14-1743. [DOI] [PubMed] [Google Scholar]
- 28.Zufferey R, Dull T, Mandel R J, Bukovsky A, Quiroz D, Naldini L, Trono D. Self-inactivating lentiviral vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zufferey R, Nagy D, Mandel R J, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]