Abstract
The increase in antibiotic resistance calls for accelerated molecular engineering strategies to diversify natural products for drug discovery. The incorporation of non-canonical amino acids (ncAAs) is an elegant strategy for this purpose, offering a diverse pool of building blocks to introduce desired properties into antimicrobial lanthipeptides. We here report an expression system using Lactococcus lactis as a host for non-canonical amino acid incorporation with high efficiency and yield. We show that incorporating the more hydrophobic analog ethionine (instead of methionine) into nisin improves its bioactivity against several Gram-positive strains we tested. New-to-nature variants were further created by click chemistry. By azidohomoalanine (Aha) incorporation and subsequent click chemistry, we obtained lipidated variants at different positions in nisin or in truncated nisin variants. Some of them show improved bioactivity and specificity against several pathogenic bacterial strains. These results highlight the ability of this methodology for lanthipeptide multi-site lipidation, to create new-to-nature antimicrobial products with diverse features, and extend the toolbox for (lanthi)peptide drug improvement and discovery.
Keywords: antibiotics, click chemistry, methionine analogs, multi-site lipidation, nisin
Lanthipeptides are a class of ribosomally synthesized and post-translationally modified peptides (RiPPs) that are characterized by the presence of one or more (methyl)lanthionine rings. They are also known to have various unusual amino acids, such as dehydroalanine (Dha) and dehydrobutyrine (Dhb) residues (1). Lanthipeptides that have antibacterial activity are called lantibiotics (2). Nisin is the first discovered and the best-studied lanthipeptide to date. Because of its antimicrobial activity and safety, it has been used as a food preservative for many years. Nisin also has great potential for therapeutic applications. It is for instance effective against many Gram-positive antibiotic-resistant organisms, such as vancomycin-resistant Enterococcus and methicillin-resistant Staphylococcus aureus. Resistance is rare to find because nisin has two inhibition mechanisms: it binds to lipid II, thereby hampering cell wall biosynthesis, and it forms pores in membranes, resulting in leakage of cellular constituents (3). Compared with polyketides (PKs) and nonribosomal peptides (NRPs), the gene-encoded synthesis and relatively low complexity of the biosynthesis of lantibiotics make them good candidates for further engineering, with the aim to expand the diversity of the antimicrobial activity arsenal (4). One approach to engineering the properties of nisin is to introduce non-canonical amino acids (ncAAs) during ribosomal peptide synthesis. It has been shown that the ncAAs Dha and Dhb in lantibiotics play an important role in their structural stability and biological activity (5). Other ncAAs with unique moieties offer a further highly diverse pool of building blocks that can aid the design of novel lantibiotics with enhanced or special properties (e.g. stability, specificity, and bioavailability). Among the ncAAs, methionine (Met) analogs are of particular interest. For instance, the Met analog azidohomoalanine (Aha) possesses an azido moiety that can serve as a chemical handle for click chemistry with an alkyne-containing compound.
Lipopeptides represent a large class of microbial natural products with important biomedical applications, allowing their use as antibacterial and antifungal drugs. Some of them are known as powerful biosurfactants (6, 7). A well-known lipopeptide antibiotic is daptomycin. It was approved in 2003 for the non-topical treatment of skin infections caused by Gram-positive pathogens, including methicillin-resistant S. aureus (MRSA), and in 2006 for the treatment of bacteremia.
The daptomycin structure includes a cyclic peptide backbone, similar to the internal ring structures of nisin but with an N-terminal fatty acyl moiety attachment. In vivo, these types of compounds are usually synthesized by non-ribosomal peptide synthetases, giant enzyme complexes that assemble their products in a non–gene-encoded manner. This process also generates heterogeneously acylated peptides, requiring elaborate procedures to get defined compounds (8). The presence of a lipid chain can provide the lipopeptides with amphiphilic properties that confer versatile functionalities (9). The antibacterial activity of daptomycin against Gram-positive bacteria is largely dependent on the N-terminal fatty acid moiety (10, 11). The attachment of lipids to a lanthipeptide has been demonstrated to be a strong and effective strategy to improve their therapeutic potential. However, lipids can only be attached to the C-terminal residue of a peptide using chemical synthesis (12) or attached to hydroxyl-containing residues, such as Ser, Thr, or Tyr by the F-family of peptide-prenyltransferases (13).
Here, we describe a methodology for the lipidation of lanthipeptides at various positions by using Aha incorporation into peptides and subsequent in vitro click chemistry. Various expression systems have been developed for the incorporation of ncAAs, and although the incorporations were modestly successful, the yields were not satisfying, being about 0.1 to 1 mg per liter bacterial culture, which makes it very hard to perform the click reaction with sufficient yield (14, 15). In the current study, a new expression system was developed. We tested the new system with different Met analogs (azidohomoalanine, norleucine, and ethionine) incorporation. Tricine-SDS-PAGE gel and mass results showed all the analogs are very efficiently incorporated (>99.5%) at different positions (1, 17, 21, and 35) of nisin or truncated nisin variants. Notably, the yield of nisin variants improved more than 7 times compared to an earlier published expression system (14). Agar well diffusion assays showed that incorporating the more hydrophobic analog ethionine into nisin can improve its bioactivity against a Lactococcus lactis strain. Minimal inhibitory concentration (MIC) tests indicated that Aha incorporation does not highly affect the bioactivity against the tested strains. With the Aha analog and subsequent click chemistry, we obtained lipidated nisin variants at different positions of nisin (e.g. at position 1, 17, 21, or 35) or in truncated nisin variants. Some of the lipopeptides show improved bioactivity and specificity against pathogenic strains. These results highlight the suitability of this methodology for lanthipeptide lipidation, to create new products with diverse features such as higher antimicrobial activity, extending the toolbox for peptide drug improvement.
Results and discussion
An improved expression system with enhanced efficiency and yield to incorporate Met analogs into nisin
Nisin is a ribosomally synthesized and posttranslational modified peptide, and the production of nisin requires nisABTC gene expression, which encodes the nisin modification machinery NisBTC and prenisin (modified core nisin with the leader part still attached) (Figs. 1 and 2A) (16). In the model nisin expression system (Fig. S1), both the nisA and nisBTC genes were controlled by the PnisA promoter in two separate plasmids (17). As Met is essential for the expression of NisBTC and falsely incorporating analogs into enzymes may cause a non-functional nisin modification machinery, a cross-expression system that allows the production of prenisin and NisBTC at different phases was used before and tested for Met analogs incorporation (Fig. S1) (14, 15). The expression of the modification machinery started in a rich medium, after which the medium is replaced by a new synthetic medium lacking Met but containing a Met analog to express prenisin. Thus, the host starts the peptide synthesis in the presence of 19 standard amino acids and the Met analog. After the prenisin is modified by dehydratase NisB and cyclase NisC to form (methyl)lanthionine rings, the prenisin is transported out of the cell by NisT, and then the leader is cleaved off by the extracellular protease NisP to liberate the active peptide (Fig. 1). Although the expression of modification enzymes was induced beforehand, no effect on NisBTC production was observed. Using this system, four Met analogs were successfully incorporated at different positions in nisin (14).
Figure 1.
Schematic overview of the force-feeding method for non-canonical amino acid incorporation and the nisin biosynthetic pathway. The expression medium is supplemented with 19 canonical amino acids and a Met analog. Amino acids (AA) or analogs are activated by aminoacyl-tRNA synthetases (aaRSs) and then transferred off the activated AA or analog to the tRNA to form the aminoacyl-tRNA (aa-tRNA). Elongation factor Tu (EF-Tu) guides the aa-tRNA to the ribosomal aminoacyl-site (A-site). Subsequently, the nascent polypeptide chain (NPC) is transferred from the P-site (peptidyl-site) to aa-tRNA which results in one amino acid extension of the NPC. This cycle of elongation is repeated until the ribosome reaches the stop codon of the mRNA and then triggers the release of the peptide. Precursor nisin (prenisin) is a ribosomally synthesized peptide with a leader part and a core peptide part that is targeted by the nisin modification machinery NisBTC. NisB dehydrates serines and threonines forming dehydroresidues. These dehydroresidues can be coupled to cysteines thus forming (methyl)lanthionine rings catalyzed by NisC. Subsequently, the transporter NisT exports the modified prenisin outside the cells, where the protease NisP removes the leader part, releasing mature nisin. "XXX" indicates the codon or anticodon on the mRNA/tRNA.
Figure 2.
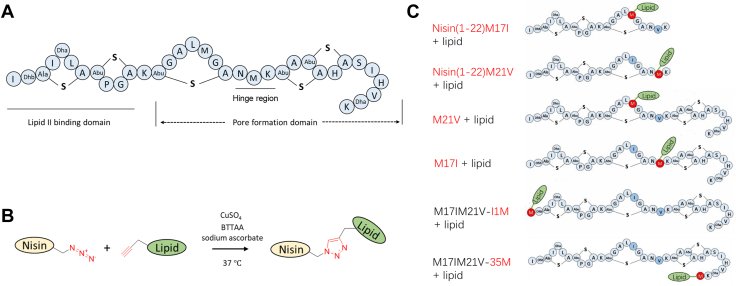
Structures of nisin and its derivatives. A, Structure of nisin A. Dha, dehydroalanine; Dhb, dehydrobutyrine; Ala-S-A, lanthionine; Abu-S-A, methyllanthionine; The functional domains, including lipid II binding site, pore formation domain, and hinge region are indicated. B, Reaction of Aha-labeled nisin with 1-undecyne (lipid C11), a copper (Cu+)-catalyzed azide-alkyne click chemistry. C, structure of various nisin constructs used in this study and each labeled with a lipid tail produced using click chemistry. In blue, Met replaced by Ile or Val; In red, the position of Aha incorporation and subsequent modification by click chemistry.
Although this approach provided a powerful tool for Met analogs incorporation, the yield and incorporation efficiency were far from satisfying, and it limited further studies and application of the low amounts of peptides produced. Efforts have been made, following the first low production phase, including optimizing analog concentrations, growth temperatures used, and variable induction times. All these tested conditions did not meet our desired production level. Here, we describe a new expression system with a promoter exchange to express the nisA and nisBTC genes. We show that in this way the yield and incorporation efficiency can be greatly improved. In the pCZ-nisin system (referring to the old system), L. lactis NZ9000 was initially transformed with a plasmid encoding the expression of nisBTC under the control of the PnisA promoter and the other plasmid encoding the expression of prenisin derivatives controlled by the PczcD promoter. PczcD is a tight zinc-inducible promoter used in L. lactis that has been cloned from the genome of Streptococcus pneumoniae (18). The PnisA promoter is a tight and efficient promoter that has been used for many years with extreme success. From the previous study, it was clear that inside the cells it is not necessary to keep a high concentration of modification enzymes, which shows the high efficiency of these enzymes. In this study, we tried to use the tight but not-so-strong zinc-inducible promoter to induce the nisBTC genes in advance, and then the nisin gene was induced by the efficient promoter PnisA (Fig. 3A). We discovered that exchanging the promoters led to 7.5 times higher peptide production with respect to that of wild-type nisin, and that the analog (Aha) incorporation efficiency was increased from 88% to above 99.5%, which makes it much easier for subsequent purifications and applications (Fig. 3). This system is the most efficient one so far for ncAA incorporation in the host strain L. lactis. Compared to the Aha incorporation efficiency with the typical strain Escherichia coli with a similar incorporation method, Lehner reported incorporated Aha into the well-studied PDZ3 domain of the postsynaptic density protein 95 (PSD-95) with about 94 % incorporation efficiency (19), while in our system using L. lactis, the efficiency is above 99.5%. With respect to the reason why L. lactis shows higher incorporation efficiency than Escherichia coli it has been suggested that the substrate specificity of L. lactis aminoacyl-tRNA synthetase (aaRS) is more relaxed than that of E. coli aaRS (20). It should be noted that as the nisin-controlled gene expression (NICE) system in L. lactis is one of the most commonly used systems for inducible expression of not only nisin and other lanthipeptides but also of proteins (21, 22), it gives the possibility that this efficient incorporation method can be extended to other peptides and proteins. Recently, the NICE system was used to mimic a non-ribosomal peptide (NRP), that is, brevicidine, resulting in an engineered mimicked peptide displaying a similar antimicrobial activity as the wild-type peptide (23). The NPRs are among the most promising sources of antibiotics, including more than 20 marketed antibacterial drugs, such as vancomycin and daptomycin (24). ncAAs play important roles in their functioning. The combination of the NICE system with a mimicking strategy and non-canonical amino acid incorporation provides an alternative way for drug modification and discovery. The system’s other advantages are that L. lactis is typified by rapid growth, ease of genetic manipulation, and is generally regarded as safe, making it a versatile system for RiPPs peptide expression and analog incorporation.
Figure 3.
Two systems for the incorporation of Met analogs into nisin. A, pCZ-nisin system, consisting of the plasmids pIL3eBTC and pCZ-nisA; pNZ-nisin system, consisting of the plasmids pTLReBTC and pNZ-nisA; A major difference between the two systems are the promoters to induce the peptide modification machinery NisBTC and the peptide nisin. SczA, encoding the repressor of PczcD; PczcD, a zinc inducible promoter; nisA, encoding NisA; PnisA, a nisin inducible promoter; nisB, nisT, and nisC, encoding nisin modification machinery NisBTC; repA and repC, encoding plasmid replication proteins; CmR, chloramphenicol resistance gene; EmR, erythromycin resistance gene. B, Tricine-SDS-PAGE analysis of prenisin labeled with Aha using pCZ-nisin and pNZ-nisin expression systems. Each lane contains isolated peptide corresponding to 0.5 ml culture. C, the yield of fully post-translationally modified nisin labeled with Aha using pCZ-nisin and pNZ-nisin expression systems. The peptide amount was quantified by HPLC according to Schmitt et al (43). D, MALDI-TOF analysis of nisin labeled with Aha using the two systems. The Aha incorporation efficiencies are indicated. −8H2O, 8 times dehydration (fully dehydrated nisin). Met1, the Met residue at position 1 in the leader part, usually, it has been cut off. Met in red, the prenisin labeled with Met.
Production of nisin variants with various types of ncAAs incorporated
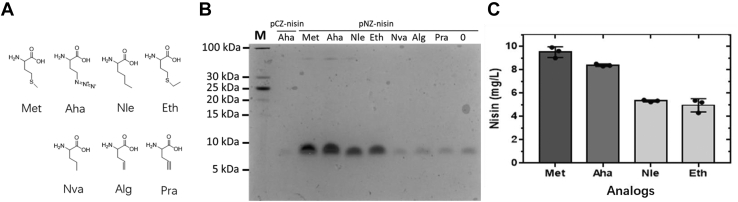
There are two Met residues in wild-type nisin, located at positions 17 and 21, respectively. The expression levels of wild-type nisin in the presence of six Met analogs, including Aha, Nle, Eth, Nva, Pra, and Alg (Fig. 4A), are shown in Figure 4B. The protein quantities in the second lane indicate yields when the pCZ-system was used, in the presence of Aha as control. Lanes 3 to 6 show that the Met, Aha, Nle, and Eth can be incorporated and their production yields are at varying levels. However, when Nva, Alg, or Pra was present in the medium, no production was observed (Fig. 4B). The highest production yield was observed when Met was supplemented, yielding 9.5 mg/L pure peptide. The production yield decreased a little bit (12%) compared with Met incorporation in the presence of Aha. A lower production yield was observed in the presence of Nle and Eth, which were 5.3 mg/L and 5 mg/L, respectively (Fig. 4C). The analog Nle was successfully incorporated before (14, 25). The structural property of Nle is similar to that of Met, displaying the same length of the side chain. The only difference is at position 4, where a Met residue has a sulfur atom, while it is a carbon atom at Nle. This illustrates that in this case, a one-atom change from sulfur to carbon for the analog does not affect the methionyl-tRNA synthetase to activate the substrate. Interestingly, also the Eth residue can be incorporated into peptides. One more methyl group attached to the side chain of Met constitutes the Eth residue. Here, we also demonstrate that multiple (i.e. 2) ncAA incorporation simultaneously is possible because the analogs could be incorporated at two positions within wild-type nisin at the same time.
Figure 4.
Production ofWTprenisin labeled with Met or Met analogs.A, structures of Met and its analogs. Met, methionine; Aha, azidohomoalanine; Nle, norleucine; Eth, ethionine; Nva, norvaline; Alg, allyglycine; Pra, propargylglycine. B, Tricine-SDS-PAGE analysis of prenisin expressed in the presence of Met or analogs using pCZ-nisin and pNZ-nisin expression systems. 0 means no Met or Met analog included in the medium. Each lane contains isolated peptide corresponding to 0.5 ml culture. C, the yield of fully modified nisin labeled with Met or analogs using pNZ-nisin expression system. The peptide amount was quantified by HPLC according to Schmitt et al (43).
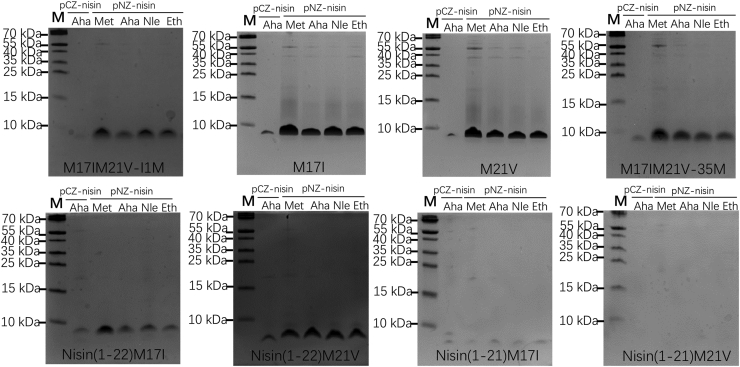
To test the effects of single Met replacements with various analogs in bioactive nisin, four single Met mutants, that is, M17I, M21V, M17IM21V-I1M, and M17IM21V-35M were constructed (Fig. 2C). The residues Ile or Val were chosen as substituents to maintain good antimicrobial activity, as both residues share the hydrophobicity of Met and their side chains are quite similar in size (26, 27). Proteolytic degradation of full-length nisin limits its potential therapeutic applications. However, previous studies have shown that C-terminally truncated nisin mutants lacking rings D and E retain significant antimicrobial activity (28). To investigate this further, we created four additional truncated nisin mutants, namely, nisin(1–21)M17I, nisin(1–21)M21V, nisin(1–22)M17I, and nisin(1–22)M21V, as depicted in Figure 2C. Met analog incorporation in M17I and M21V gave much higher peptide yields than M17IM21V-35M and M17IM21V-I1M. Two truncated nisin variants, i.e. nisin(1–21)M17I and nisin(1–21)M21V, showed no visible production when analyzed with a tricine gel. Surprisingly, one additional amino acid, i.e. Lys, attached to the tail of nisin(1–21) made the production yield of nisin(1–22) variants comparable with that of full-length nisin M17IM21V-I1M and M17IM21V-35M mutants (Fig. 5). The reason for the varied productions may be due to the levels of (in)tolerance of the modification machinery NisBC and/or the transporter NisT to charge the substrate nisin variants. To assess the efficiency of post-translational modifications and incorporation of ncAAs, all samples were further analyzed by mass spectrometry.
Figure 5.
Tricine-SDS-PAGE analysis of the expression of nisin variants using either pCZ-nisin or pNZ-nisin system in the presence of Met/Met analogs. Each lane contains prenisin peptide isolated from 0.5 ml supernatant.
The Met analog-containing nisin variants were correctly modified and the analogs were efficiently incorporated
The first Met residue (Met1) in the leader part of prenisin is usually removed by the methionine aminopeptidase (29). In the pCZ-nisin system, a large part of the peptides produced by this system contained the first Met. For the pNZ-nisin system, the peptides without Met1 showed to be prominent in all variants (Table S4). In most cases, the incorporation of ncAAs into nisin or its (truncated) variants did not affect the dehydration efficiency using this new system, as peptides with fully (8 times or 5 times, respectively) dehydrated residues were prominent. The most affected dehydration of variants was in the M17IM21V-35M peptide. By introducing Nle into M17IM21V-35M, using the new system, 7 times dehydrated peptides can also be detected. The results also indicated that Nle was hard to incorporate. It may be that the integration speed of Nle during translation is relatively slow which leads to an insufficient modification. The Aha incorporation at the different positions of nisin using the new system always yielded fully dehydrated peptides, while 7 times dehydration of M17IM21V-35M using the pCZ-nisin system can be detected, suggesting the new system significantly improved the dehydration efficiency when Aha incorporation occurs. Notably, Met can be oxidized, and masses corresponding to oxidized products were also observed (Table S4).
The incorporation efficiency indicates the ratio between the amounts of peptides containing the analogs and the total amount of peptides. For the pCZ-nisin system, the incorporation efficiency was tested by liquid chromatography–mass spectrometry (LC-MS). Here we used an easier way to test the incorporation efficiency by using MALDI-TOF (21, 30). Figure 3D shows that the incorporation efficiency of wild-type nisin labeled with Aha was 88%, the same as was found with the previous LC-MS analysis. It demonstrates that the MALDI-TOF analysis is a reliable and simple way for checking the analog incorporation. In the peptides M17IM21V-I1M, M17I, M21V, M17IM21V-35M, the Aha incorporation efficiency was 91%, 97%, and 96%, respectively, while with the new system, it was always >99.5%, indicating that our new system has a much better incorporation efficiency than the old pCZ-nisin system. The MS data also shows that the incorporation efficiency of Nle and Eth was only 51 to 88% using the old pCZ-nisin system. Remarkably, using the new system, the incorporation efficiency of Nle and Eth was also >99.5%, as the mass of peptides containing Met were always undetectable. Moreover, in the cases of truncated nisin variants, the incorporation efficiency was also >99.5% for Aha, Nle, and Eth incorporation (Table 1). The reason for the high incorporation efficiency may be due to the rate of nisin expression. When using the efficient promoter PnisA instead of the previously used zinc-inducible promotor, the majority of peptides will complete their expression within less than 2 hours (31). If the expression time takes longer, inside the cells more proteins and peptides will be degraded due to the pressure of amino acid starvation and the many active peptidases present. The rate of activation by aminoacyl-tRNA synthetases during translation with normal amino acids is faster than with ncAAs, which finally results in the incorporation efficiency being decreased.
Table 1.
Incorporation efficiency of Met analogs in nisin and its variants
| Peptide | Analog | Incorporation efficiency |
|
|---|---|---|---|
| pCZ-nisin | pNZ-nisin | ||
| Wild type | Aha | 88% | >99.5% |
| Nle | 77%a | >99.5% | |
| Eth | 56%a | >99.5% | |
| M17IM21V-I1M | Aha | 91% | >99.5% |
| Nle | 88%a | >99.5% | |
| Eth | 71%a | >99.5% | |
| M17I | Aha | 97% | >99.5% |
| Nle | 88%a | >99.5% | |
| Eth | 71%a | >99.5% | |
| M21V | Aha | 96% | >99.5% |
| Nle | 88%a | >99.5% | |
| Eth | 73%a | >99.5% | |
| M17IM21V-35M | Aha | >99.5% | >99.5% |
| Nle | 51%a | >99.5% | |
| Eth | 71%a | >99.5% | |
| nisin(1–21)M17I | Aha | n.d | >99.5% |
| Nle | n.d | >99.5% | |
| Eth | n.d | >99.5% | |
| nisin(1–21)M21V | Aha | n.d | >99.5% |
| Nle | n.d | >99.5% | |
| Eth | n.d | >99.5% | |
| nisin(1–22)M17I | Aha | n.d | >99.5% |
| Nle | n.d | >99.5% | |
| Eth | n.d | >99.5% | |
| nisin(1–22)M21V | Aha | n.d | >99.5% |
| Nle | n.d | >99.5% | |
| Eth | n.d | >99.5% | |
>99.5% means the mass of the peptides containing Met was undetectable. n.d, not detected.
Results from reference (14).
Antimicrobial activity of nisin and its Met or Met-analog–containing derivatives
All nisin variants were purified and their antimicrobial activities were tested against L. lactis (Fig. 6). Only the mutant M21V shows an enhanced antimicrobial activity compared to wild-type nisin. The M21 is located at the hinge region of nisin, in which hydrophobic amino acids play an important role. It has a profound influence on antimicrobial activity and host specificity (32, 33). In the naturally found nisin variants, various hydrophobic amino acids are within the hinge region, i.e. nisin Q-Leu21; nisin U/U2-Pro20/Leu21; nisin J-Phe20/Ala21 (34). In general, the introduction of hydrophobic amino acids in the hinge region, i.e. Leu (N20L, M21L, K22L), Ile (N20I, M21I), or Met (K22M), resulted in the retention of relatively high levels of bioactivity or improved activity, and M21V was particularly notable for that property (32). The mutation M17I resulted in the retention of relatively high levels of bioactivity, while the mutants M17IM21V-I1M and M17IM21V-35M displayed reduced activity to 64% and 76%, respectively. The first two rings of nisin can bind to lipid II by forming a pyrophosphate cage (35). A previous study showed that the I1W mutant had a twofold reduced activity (15), which was similar to that of the M17IM21V-I1M mutant. This indicates that the first Ile is not crucial for the binding. However, the substitution of the first residue of the nisin core peptide influences yield and NisP-cleavage efficiency (36). The C-terminal amino acid lysine is responsible for the initial interaction of nisin with the target membrane (37). We guess that the insertion of an additional Met at the end of full-length nisin (M17IM21V-35M variant) hampers this interaction and the translocation, thus negatively influencing the activity. The truncated variants showed a dramatic decrease in activity. The activity of nisin(1–21) was completely lost with the peptide concentrations used. One more positively charged amino acid Lys added to the C-terminus of nisin(1–21) significantly enhances its antimicrobial activity and production scale, which is consistent with a previous study (38).
Figure 6.
Antimicrobial activity of nisin and nisin variants labeled with Met/Met analogs against strain Lactococcus lactis MG1363MG1363. A, a representative image of agar well diffusion assay to test nisin variants against the target strain. B, antimicrobial activity of nisin variants against indicator strain using agar well diffusion assay. Activity expressed as the area of the zone of inhibition. In red: the activity that is improved in comparison to the nisin variants labeled with Met; In grey: lost activity under tested concentrations. Data are representative of three independent experiments.
The Aha incorporation into nisin caused the activity to either increase, decrease, or retain activity. The activity of wild-type and M21V labeled with Aha were improved by 30% and 5%, respectively. Incorporation at position M17I did not affect the activity. Incorporation of Aha into M17IM21V-I1M or truncated nisin variants decreased the activity. The incorporation of Nle had a minor influence on the activity (less than 10% change), except for truncated nisin variants. The activity increased by 65% with the nisin(1–22)M17I, while it decreased by 35% with nisin(1–22)M21V. Peptides labeled with Eth had a clear influence on the bioactivity. The activity increased by Eth incorporation in the mutants M17IM21V-I1M, M21V, and nisin(1–22)M21V to 64%, 11%, and 50%, respectively. With the Eth incorporation in M17IM21V-35M, it caused a 27% activity loss. It is also interesting to note that for all analogs incorporated in full-length nisin, M21V with Met-analog at position 17 has increased activity compared to wild-type nisin, indicating the important role of residue 17 located in nisin ring C (residues 13-19).
Lipidated nisin variants
To obtain the pure nisin variants with Aha incorporated, we first investigated the influence of the analog concentration and induction time on peptide production (Fig. S2). The optimal conditions for Aha incorporation were at a final concentration of Aha of 30 mg/L, and 3 h induction time, for which the highest yield reached 8.3 mg/L pure peptide. With the Aha incorporation (Fig. S3), the MIC results showed that the replacement of Met by Aha generally had a minor effect on antimicrobial activity (Table S1), which makes them good candidates for the next modifications. The Met analog Aha possesses the unique azide functional group that can react with alkyne substrates, commonly referred to as “click chemistry” (Fig. 2B) (39, 40). Here, 1-undecyne (C11) was chosen for further reactions and was successfully coupled at four different positions (1, 17, 21, and 35) of full-length nisin and at two positions of truncated nisin variants (Fig. 2C). The yield, which varied from 42% to 100% depending on the click position, is presented in Fig. S4 in the supplementary materials. The resulting compounds were purified and characterized by MALDI-TOF (Fig. S5). Figure 7 shows that all full-length nisin mutants and truncated nisin variants did not show antimicrobial activity against the tested strains, when used at low concentration conditions (0.01 mg/ml of full nisin variants or 0.04 mg/ml of truncated variants). However, the activity of four full-length nisin variants significantly improved against L. lactis after coupling with C11, while this was not the case for two of the truncated nisin variants. For the mutants, M17I and M17IM21V-35M, the lipidated nisin variants show selective activity against Enterococcus feacalis but not for other tested strains. Coupling the C11 at position 17 (mutation M21V) made the antimicrobial spectrum broader and also more specific, as it showed bioactivity against Listeria monocytogenes, Enterococcus faecium, and Enterococcus faecalis but did not show activity against S. aureus. There is some evidence for the important role of lipids in the activity of lanthipeptides. Zhao et.al reported that adding lipid tails to low-active lanthipeptides yielded semisynthetic macrocyclic lipo-lanthipeptides, which showed significant bactericidal activity against a number of Gram-positive and Gram-negative pathogenic bacteria (41). Different hydrophobic tails have been attached to nisin variants at the C-terminus. Deng et.al used the lipid II binding fragments of nisin (nisin 1–12 and nisin 1–20) as the backbone to conjugate different synthetic polyproline moieties, which yielded active and stable antimicrobials (42). Attaching lipids to the nisin fragment (nisin 1–12) resulted in lipopeptides that displayed antibacterial activity almost on par with that of the wild-type nisin (12). We now show that lipids can be attached at different internal positions of peptides by combining the incorporation of the analog Aha and then performing click chemistry, whereas previously, lipids and other interesting moieties could only be attached to the C-terminus of these lanthipeptides in chemical strategies (12), or these lipid attachments are catalyzed by the F superfamily of peptide prenyltransferase enzymes that use 5-carbon (prenylation) or 10-carbon (geranylation) donors attached on a Ser, Thr, or Tyr residue (13). We further demonstrate that the best position for further lipid modification is located within ring C of nisin. It is noteworthy that the method described in this study can be applied to conjugate other compatible moieties (e.g. glycans, active peptide moieties, and fluorescent moieties) that can give the lanthipeptides desired properties. Future studies will concentrate on coupling more diverse lipids to nisin variants to create novel derivatives with different characteristics (e.g. stability, activity, and/or specificity).
Figure 7.
Antimicrobial activity screening of nisin variants,with or without lipid C11,attached against various microorganisms. A representative image from three independent experiments is presented. Red arrows: activity that are improved in comparison to nisin variants without lipid attached. C11, 1-undecyne; MRSA, methicillin resistant; VRE, vancomycin resistant.
Conclusion
Here, we demonstrate a new expression system with enhanced efficiency and yield to incorporate Met analogs into lanthipeptides in L. lactis. Three Met analogs were successfully installed at various positions of the lantibiotic nisin, while peptides containing Met residues were undetectable, which means the incorporation efficiency was always above 99.5%, compared with the previous expression system, where incorporation varied from 51 to 99.5%. The improved system leads to 7.5 times more modified peptide production, making it easier for further purification, characterization, and click-chemistry application. The structural diversity of analogs incorporation resulted in antimicrobial activity against specific bacteria being reduced, retained, or even improved. In addition, this study underlines that the bio-orthogonal reactive groups of ncAAs can serve as a platform for post-biosynthetic modifications via click chemistry. We anticipate that more of these semisynthetic lipopeptides can be made for further optimization and development as novel antimicrobials.
Experimental procedures
Materials
Reagents used for molecular biology experiments were obtained from Thermo Fisher Scientific. Unless otherwise noted, all chemicals were acquired from Sigma-Aldrich. The Met analogs L-azidohomoalanine (Aha), L-norvaline (Nva), L-norleucine (Nle), and L-allyglycine (Alg) were obtained from Iris Biotech GmbH. L-ethionine (Eth) was purchased from Alfa Aesar.
Bacterial strains, plasmids, and growth conditions
The bacterial strains and plasmids used in this study are listed in Table S2 in the supplemental material. All L. lactis strains were grown in M17 broth (BD Difco) supplemented with 0.5% (w/v) glucose (GM17) at 30 °C. When appropriate, 5 μg/ml chloramphenicol (Cm) and/or erythromycin (Em) were added to the media. L. lactis NZ9000 was used as the host for cloning, plasmid maintenance, and peptide expression. Chemical defined medium lacking tryptone (CDM-P) was used for protein expression and Met analogs incorporation (43).
Molecular biology techniques
The primers used in this study for PCR and sequencing are listed in Table S3. All primers were purchased at Biolegio B.V. Plasmids encoding the mutations were constructed by amplifying the template plasmid using a phosphorylated downstream sense (or upstream antisense) primer and an upstream antisense (or downstream sense) primer (44). Phusion High-Fidelity DNA polymerase (Thermo Fisher Scientific) was used to amplify the DNA. PCR products were first checked by agarose gel (1%) electrophoresis, and the correct molecular weight band was cut and purified by NucleoSpin gel and PCR cleanup kit (Bioke, Leiden, the Netherlands). Subsequently, self-ligation of the DNA fragment was carried out with T4 DNA ligase. The ligation product was first desalted and transformed into L. lactis NZ9000 as previously described (45) using a Bio-Rad gene pulser (Bio-Rad). For the construction of plasmid pTLReBTC, the purified PCR products were mixed and fused using the Gibson assembly master mix (Bioke) according to the manufacturer’s instructions. In this procedure, ligase was not essential. The plasmid was isolated and verified by sequencing using the pNZ-sequencing primer (Table S3).
Expression of ncAAs-incorporated prenisin
To check if the ncAAs were successfully incorporated, small-scale (20 ml) expression and purification were performed. For the pCZ-nisin expression system, the precursor peptide precipitation procedure was performed as previously described (14). The expression protocol for the pNZ-nisin system was as follows: L. lactis NZ9000 cells harboring the nisBTC plasmid were electroporated with the plasmid harboring the nisA gene (100 ng), plated on GM17 agar plates supplemented with chloramphenicol (5 μg/ml) and erythromycin (5 μg/ml), and grown at 30 °C overnight. A single colony was picked and added to 4 ml of GM17CmEm medium for growth. 0.5 ml overnight culture was diluted in 20 ml of the same medium. When the OD600 reached about 0.4, 0.5 mM ZnSO4 was added to induce the expression of nisin modification machinery nisBTC. After 3 h, the cells were washed three times with phosphate-buffered saline (pH 7.2) and resuspended in 20 ml of CDM-P lacking Met. After 1-h starvation time, Met (38 mg/L) or Met analogs (50 mg/L) and 8 ng/ml nisin were added to induce peptide expression. After overnight growth, the supernatant was collected by centrifugation at 8000g for 15 min. Prenisin was precipitated with 10% trichloroacetic acid (TCA) on ice for at least 2 h, and then centrifuged at 10,000g and 4 °C for 45 min. The pellets were washed with 10 ml ice-cold acetone to remove TCA. Samples were dried in the fume hood and stored at −20 °C or resuspended in 0.4 ml 0.05% aqueous acetic acid solution for further analysis.
Tricine-SDS-PAGE analysis
The precipitated peptides were analyzed by the Tricine-SDS-PAGE system as described by Schagger (46). 10 μl of the sample was mixed with 8 μl loading dye and loaded on the 16% gel. Coomassie brilliant blue G-250 was used for protein staining.
Mass spectrometry
1 μl of the prenisin was spotted on the target, dried, and washed several times with Milli-Q water. Subsequently, an equal volume of matrix solution (5 mg/ml α-cyano-4-hydroxycinnamic acid dissolved in 50% acetonitrile containing 0.1% trifluoroacetic acid) was spotted on top of the sample. An Applied Biosystems 4800 Plus matrix-assisted laser desorption/ionization time-of-flight analyzer (MALDI-TOF) operating in linear mode using external calibration was used to obtain mass spectra. The analog incorporation efficiency was calculated by measuring the peak areas of the analog-containing peptides and the Met-containing peptides.
Agar well diffusion assay
An overnight culture of L. lactis MG1363 was added at 0.1% (v/v) to melted GM17 agar at 45 °C and then 30 ml of this solution was poured onto a plate. Once the agar was solid, wells of 8 mm were punched in the agar and filled with 30 μl 1 mg/ml of the lantibiotic solution. When necessary, lantibiotics were activated with 5 μl of NisP added directly to the well. Nisin amounts were determined by HPLC as previously described (43). The agar plate was incubated at 30 °C overnight, after which the zones of inhibition were measured. Data are representative of three independent experiments. Zone diameters were measured in millimeters and recorded as area of the zone (πr2) minus the area of the well (πr2) in millimeters.
Optimization of the expression of prenisin labeled with Aha
To achieve maximal production, the Met analog Aha concentration and induction time was investigated in detail. The effect of the Aha concentration was determined by adding 5 to 50 mg/L Aha into the medium after 3 h nisBTC induction. The impact of the induction time on the prenisin production was examined by 1 to 4 h nisBTC induction following the addition of 30 mg/L Aha to the medium.
Purification of nisin variants labeled with Aha
To obtain larger amounts of nisin variants, experiments were performed at a 2 L scale. The supernatant pH was adjusted to 7.0 and incubated with purified NisP (47) at 37 °C for 3 to 6 h to cut off the leader, and then the supernatant was applied to a C18 open column (Spherical C18, 20 g, particle size: 40–75 μm, Sigma-Aldrich). The column was washed with 40 ml of different concentrations (25%, 30%, 35%, 40%, and 60%) of buffer B (buffer A, distilled water with 0.1% TFA; buffer B, acetonitrile with 0.1% TFA). The active fractions were lyophilized and further purified using an Agilent 1200 series HPLC equipped with a C12 column (Jupiter 4 μm Proteo 90A, 250 × 4.6 mm, Phenomenex). The peak with activity and correct molecular weight was collected, lyophilized, and stored at 4 °C until further use.
Minimal inhibitory concentration assay
Minimal inhibitory concentration (MIC) values were determined by broth microdilution, according to the standard guidelines using cation-adjusted Mueller-Hinton broth (48). The inoculum was adjusted to approximately 5 × 105 CFU/ml. The MIC was defined as the lowest concentration of antimicrobial compound with no visible growth after overnight incubation at 37 °C (L. lactis MG1363 at 30 °C).
Lipidation of Aha-labeled nisin with a C11 tail
Stock solutions of copper (100 mM), sodium ascorbate (1 M), and BTTAA (2-(4-((bis((1-tert-butyl-1H-1,2,3-triazol-4-yl)methyl)amino)methyl)-1H-1,2,3-triazol-1-yl)acetic acid, 50 mM) were prepared. Nisin variants labeled with Aha (100 μg) were dissolved in 100 mM phosphate buffer (pH 7.0, final reaction volume: 200 μl), and 2 μl 1-undecyne (C11) was added to the solution. Then, stock solutions CuSO4 (4 μl): BTTAA (40 μl) premix was added followed by the addition of 20 μl sodium ascorbate. The reaction was performed at 37 °C for 1 h. After completion, the reaction mixture was quenched with 3 ml buffer (H2O: acetonitrile, 5:95 +0.1% TFA), filtered by a 0.22 μm pore size membrane filter, and purified via HPLC as described before. Product-containing fractions were lyophilized. 50 μl 0.01 mg/ml of full nisin variants (M17IM21V-I1M, M17I, M21V, M17IM21V-35M) or 0.04 mg/ml of truncated variants (nisin(1–22)M17I, and nisin(1–22)M21V) were used for the agar well diffusion test. A representative image from three independent experiments was presented.
Data availability
All data supporting the findings of this study are available within the paper and its supporting information files. Additional raw data are available from the corresponding author upon reasonable request.
Supporting information
This article contains supporting information (49, 50).
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
Author contributions
O. P. K. and J. B. conceptualization; C. W. and L. G. investigation; L. G. formal analysis; L. G. writing – original draft; L. G. Visualization; O. P. K. and J. B. writing – review and editing. O. P. K., J. B., C. W., and L. G. writing – review and editing.
Funding and additional information
L. G. and C. W. were financially supported by the China Scholarship Council (CSC).
Reviewed by members of the JBC Editorial Board. Edited by Sarah E. O'Connor
Supporting information
References
- 1.Montalbán-López M., Scott T.A., Ramesh S., Rahman I.R., van Heel A.J., Viel J.H., et al. New developments in RiPP discovery, enzymology and engineering. Nat. Prod. Rep. 2021;38:130–239. doi: 10.1039/d0np00027b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hudson G.A., Mitchell D.A. RiPP antibiotics: biosynthesis and engineering potential. Curr. Opin. Microbiol. 2018;45:61–69. doi: 10.1016/j.mib.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Shin J.M., Gwak J.W., Kamarajan P., Fenno J.C., Rickard A.H., Kapila Y.L. Biomedical applications of nisin. J. Appl. Microbiol. 2016;120:1449–1465. doi: 10.1111/jam.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z., Li Y., Zhang L., Ding Z., Shi G. Microbial production of small peptide: pathway engineering and synthetic biology. Microb. Biotechnol. 2021;14:2257–2278. doi: 10.1111/1751-7915.13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levengood M.R., Knerr P.J., Oman T.J., Van Der Donk W.A. In vitro mutasynthesis of lantibiotic analogues containing nonproteinogenic amino acids. J. Am. Chem. Soc. 2009;131:12024–12025. doi: 10.1021/ja903239s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamley I.W. Lipopeptides: from self-assembly to bioactivity. Chem. Commun. 2015;51:8574–8583. doi: 10.1039/c5cc01535a. [DOI] [PubMed] [Google Scholar]
- 7.Hutchinson J.A., Burholt S., Hamley I.W. Peptide hormones and lipopeptides: from self-assembly to therapeutic applications. J. Pept. Sci. 2017;23:82–94. doi: 10.1002/psc.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hubrich F., Bösch N.M., Chepkirui C., Morinaka B.I., Rust M., Gugger M., et al. Ribosomally derived lipopeptides containing distinct fatty acyl moieties. Proc. Natl. Acad. Sci. U. S. A. 2022;119 doi: 10.1073/pnas.2113120119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W., Separovic F., O'Brien-Simpson N.M., Wade J.D. Chemically modified and conjugated antimicrobial peptides against superbugs. Chem. Soc. Rev. 2021;50:4932–4973. doi: 10.1039/d0cs01026j. [DOI] [PubMed] [Google Scholar]
- 10.Wittmann M., Linne U., Pohlmann V., Marahiel M.A. Role of DptE and DptF in the lipidation reaction of daptomycin. FEBS. J. 2008;275:5343–5354. doi: 10.1111/j.1742-4658.2008.06664.x. [DOI] [PubMed] [Google Scholar]
- 11.Vilhena C., Bettencourt A. Daptomycin: a review of properties, clinical use, drug delivery and resistance. Mini. Rev. Med. Chem. 2012;12:202–209. doi: 10.2174/1389557511209030202. [DOI] [PubMed] [Google Scholar]
- 12.Koopmans T., Wood T.M., ’t Hart P., Kleijn L.H., Hendrickx A.P., Willems R.J., et al. Semisynthetic lipopeptides derived from nisin display antibacterial activity and lipid II binding on par with that of the parent compound. J. Am. Chem. Soc. 2015;137:9382–9389. doi: 10.1021/jacs.5b04501. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Y., Cong Y., Schmidt E.W., Nair S.K. Catalysts for the enzymatic lipidation of peptides. Acc. Chem. Res. 2022;55:1313–1323. doi: 10.1021/acs.accounts.2c00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng J., Viel J.H., Chen J., Kuipers O.P. Synthesis and characterization of heterodimers and fluorescent nisin species by incorporation of methionine analogues and subsequent click chemistry. ACS. Synth. Biol. 2020;9:2525–2536. doi: 10.1021/acssynbio.0c00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou L., Shao J., Li Q., van Heel A.J., de Vries M.P., Broos J., et al. Incorporation of tryptophan analogues into the lantibiotic nisin. Amino Acids. 2016;48:1309–1318. doi: 10.1007/s00726-016-2186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lubelski J., Rink R., Khusainov R., Moll G.N., Kuipers O.P. Biosynthesis, immunity, regulation, mode of action and engineering of the model lantibiotic nisin. Cell Mol. Life Sci. 2008;65:455–476. doi: 10.1007/s00018-007-7171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuipers O.P., Beerthuyzen M.M., de Ruyter P.G., Luesink E.J., de Vos W.M. Autoregulation of nisin biosynthesis in Lactococcus lactis by signal transduction. J. Biol. Chem. 1995;270:27299–27304. doi: 10.1074/jbc.270.45.27299. [DOI] [PubMed] [Google Scholar]
- 18.Mu D., Montalbán-López M., Masuda Y., Kuipers O.P. Zirex: a novel zinc-regulated expression system for Lactococcus lactis. Appl. Environ. Microb. 2013;79:4503–4508. doi: 10.1128/AEM.00866-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehner F., Kudlinzki D., Richter C., Müller-Werkmeister H.M., Eberl K.B., Bredenbeck J., et al. Impact of azidohomoalanine incorporation on protein structure and ligand binding. ChemBioChem. 2017;18:2340–2350. doi: 10.1002/cbic.201700437. [DOI] [PubMed] [Google Scholar]
- 20.Broos J. Fluorescence Spectroscopy and Microscopy. Humana Press; Totowa, NJ: 2014. Biosynthetic incorporation of tryptophan analogs in proteins; pp. 359–370. [DOI] [PubMed] [Google Scholar]
- 21.Petrović D.M., Leenhouts K., van Roosmalen M.L., Broos J. An expression system for the efficient incorporation of an expanded set of tryptophan analogues. Amino Acids. 2013;44:1329–1336. doi: 10.1007/s00726-013-1467-3. [DOI] [PubMed] [Google Scholar]
- 22.Mierau I., Kleerebezem M. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl. Microbiol. Biot. 2005;68:705–717. doi: 10.1007/s00253-005-0107-6. [DOI] [PubMed] [Google Scholar]
- 23.Zhao X., Li Z., Kuipers O.P. Mimicry of a non-ribosomally produced antimicrobial, brevicidine, by ribosomal synthesis and post-translational modification. Cell Chem. Biol. 2020;27:1262–1271. doi: 10.1016/j.chembiol.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Süssmuth R.D., Mainz A. Nonribosomal peptide synthesis-principles and prospects. Angew. Chem. Int. Edit. 2017;56:3770–3821. doi: 10.1002/anie.201609079. [DOI] [PubMed] [Google Scholar]
- 25.van Hest J.C., Kiick K.L., Tirrell D.A. Efficient incorporation of unsaturated methionine analogues into proteins in vivo. J. Am. Chem. Soc. 2000;122:1282–1288. [Google Scholar]
- 26.Field D., Cotter P.D., Ross R.P., Hill C. Bioengineering of the model lantibiotic nisin. Bioengineered. 2015;6:187–192. doi: 10.1080/21655979.2015.1049781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng Y., Du Y., Qiu Z., Liu Z., Qiao J., Li Y., et al. Nisin variants generated by protein engineering and their properties. Bioengineering. 2022;9:251. doi: 10.3390/bioengineering9060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rink R., Wierenga J., Kuipers A., Kluskens L.D., Driessen A.J., Kuipers O.P., et al. Dissection and modulation of the four distinct activities of nisin by mutagenesis of rings A and B and by C-terminal truncation. Appl. Environ. Microb. 2007;73:5809–5816. doi: 10.1128/AEM.01104-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuipers O.P., Rollema H.S., de Vos W.M., Siezen R.J. Biosynthesis and secretion of a precursor of nisin Z by Lactococcus lactis, directed by the leader peptide of the homologous lantibiotic subtilin from Bacillus subtilis. FEBS. Lett. 1993;330:23–27. doi: 10.1016/0014-5793(93)80911-d. [DOI] [PubMed] [Google Scholar]
- 30.Ishida Y., Park J.H., Mao L., Yamaguchi Y., Inouye M. Replacement of all arginine residues with canavanine in MazF-bs mRNA interferase changes its specificity. J. Biol. Chem. 2013;288:7564–7571. doi: 10.1074/jbc.M112.434969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J., van Heel A.J., Kuipers O.P. Subcellular localization and assembly process of the nisin biosynthesis machinery in Lactococcus lactis. mBio. 2020;11 doi: 10.1128/mBio.02825-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Field D., Connor P.M., Cotter P.D., Hill C., Ross R.P. The generation of nisin variants with enhanced activity against specific gram-positive pathogens. Mol. Microbiol. 2008;69:218–230. doi: 10.1111/j.1365-2958.2008.06279.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhou L., van Heel A.J., Kuipers O.P. The length of a lantibiotic hinge region has profound influence on antimicrobial activity and host specificity. Front. Microbiol. 2015;6:11. doi: 10.3389/fmicb.2015.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Sullivan J.N., O’Connor P.M., Rea M.C., O’Sullivan O., Walsh C.J., Healy B., et al. Nisin J, a novel natural nisin variant, is produced by Staphylococcus capitis sourced from the human skin microbiota. J. Bacteriol. 2020;202 doi: 10.1128/JB.00639-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu S.T.D., Breukink E., Tischenko E., Lutters M.A., De Kruijff B., Kaptein R., et al. The nisin–lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics. Nat. Struct. Mol. Biol. 2004;11:963–967. doi: 10.1038/nsmb830. [DOI] [PubMed] [Google Scholar]
- 36.Lagedroste M., Reiners J., Smits S.H., Schmitt L. Systematic characterization of position one variants within the lantibiotic nisin. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-018-37532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breukink E., van Kraaij C., Demel R.A., Siezen R.J., Kuipers O.P., de Kruijff B. The C-terminal region of nisin is responsible for the initial interaction of nisin with the target membrane. Biochemistry. 1997;36:6968–6976. doi: 10.1021/bi970008u. [DOI] [PubMed] [Google Scholar]
- 38.Plat A., Kuipers A., de Lange J.G., Moll G.N., Rink R. Activity and export of engineered nisin-(1-22) analogs. Polymers. 2011;3:1282–1296. [Google Scholar]
- 39.Moses J.E., Moorhouse A.D. The growing applications of click chemistry. Chem. Soc. Rev. 2007;36:1249–1262. doi: 10.1039/b613014n. [DOI] [PubMed] [Google Scholar]
- 40.Hou J., Liu X., Shen J., Zhao G., Wang P.G. The impact of click chemistry in medicinal chemistry. Expert Opin. Drug Dis. 2012;7:489–501. doi: 10.1517/17460441.2012.682725. [DOI] [PubMed] [Google Scholar]
- 41.Zhao X., Xu Y., Viel J.H., Kuipers O.P. Semisynthetic macrocyclic lipo-lanthipeptides display antimicrobial activity against bacterial pathogens. ACS. Synth. Biol. 2021;10:1980–1991. doi: 10.1021/acssynbio.1c00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng J., Viel J.H., Kubyshkin V., Budisa N., Kuipers O.P. Conjugation of synthetic polyproline moietes to lipid II binding fragments of nisin yields active and stable antimicrobials. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.575334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmitt S., Montalban-Lopez M., Peterhoff D., Deng J., Wagner R., Held M., et al. Analysis of modular bioengineered antimicrobial lanthipeptides at nanoliter scale. Nat. Chem. Biol. 2019;15:437–443. doi: 10.1038/s41589-019-0250-5. [DOI] [PubMed] [Google Scholar]
- 44.Zhao X., Yin Z., Breukink E., Moll G.N., Kuipers O.P. An engineered double lipid II binding motifs-containing lantibiotic displays potent and selective antimicrobial activity against Enterococcus faecium. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.02050-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holo H., Nes I.F. In: Nickoloff J.A., editor. Vol. 47. Humana Press; 1995. (Electroporation Protocols for Microorganisms. Methods in Molecular BiologyTM). [Google Scholar]
- 46.Schagger H. Tricine-SDS-PAGE. Nat. Protoc. 2006;1:16–22. doi: 10.1038/nprot.2006.4. [DOI] [PubMed] [Google Scholar]
- 47.Montalbán-López M., Deng J., Van Heel A.J., Kuipers O.P. Specificity and application of the lantibiotic protease NisP. Front. Microbiol. 2018;9:160. doi: 10.3389/fmicb.2018.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiegand I., Hilpert K., Hancock R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008;3:163. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 49.Kuipers O.P., de Ruyter P.G., Kleerebezem M., de Vos W.M. Controlled overproduction of proteins by lactic acid bacteria. Trends. Biotechnol. 1997;15:135–140. doi: 10.1016/S0167-7799(97)01029-9. [DOI] [PubMed] [Google Scholar]
- 50.van Heel A.J., Mu D., Montalbán-López M., Hendriks D., Kuipers O.P. Designing and producing modified, new-to-nature peptides with antimicrobial activity by use of a combination of various lantibiotic modification enzymes. ACS. Synth. Biol. 2013;2:397–404. doi: 10.1021/sb3001084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the paper and its supporting information files. Additional raw data are available from the corresponding author upon reasonable request.