Abstract
Purpose:
Small fiber neuropathy (SFN) is known to be associated with Sjögren’s Disease (SjD) and in-vivo corneal confocal microscopy can identify features compatible SFN. Here we performed a descriptive study to identify features of a SFN of the corneal sub-basal nerve plexus using in-vivo confocal microscopy.
Methods:
We recruited 10 participants from the Sjögren’s International Collaborative Clinical Alliance (SICCA), one new participant (in an effort to expand the SICCA cohort), and 22 healthy controls. All participants underwent slit-lamp examination and in vivo confocal microscopy of the central cornea’s sub-basal nerve plexus centered about the central whorl to create a 30-image montage. Each image was analyzed with automated software (ACCmetrics, Manchester, United Kingdom) to produce seven nerve metrics. We performed t-tests and age-adjusted regressions to make comparisons of nerve metrics between SjD participants and healthy controls.
Results:
Most nerve metrics were significantly lower in SjD participants compared to healthy controls. Mean corneal nerve fiber density was found to be 3.5 mm/mm2 in SjD participants compared to 10.6 mm/mm2 in healthy controls (95% CI: −8.4 to −0.93, p=0.02). Within the 11 SjD participants, 22 eyes were analyzed on confocal microscopy, and 16 of those eyes (from 9 individuals) did not have an identifiable central whorl. Within the 22 healthy controls, 22 eyes (right eye only) were analyzed on confocal microscopy, and 21 of those eyes had an identifiable central whorl.
Conclusion:
SjD exhibits lower corneal nerve metrics compared to healthy controls. These findings suggest that features compatible with SFN can distinguish SjD from healthy controls and may serve as a potential novel biomarker in identifying SjD.
Keywords: Sjögren’s disease, in vivo corneal confocal microscopy, sub-basal nerve plexus, dry eye
Introduction
Sjögren’s Disease (SjD, formerly known as Sjögren’s Syndrome) is a systemic, autoimmune condition that primarily manifests as dry eyes and dry mouth.1 Additionally, it is known to have various neurological manifestations that can include both peripheral and central nerve involvement.2,3 Examples of peripheral nervous system (PNS) manifestations of SjD include small-fiber sensory neuropathy, sensorimotor polyneuropathies, radiculoneuropathy, autonomic neuropathy, muscular disease (ranging from myalgias to myopathy), and motor neuron disease.3 Examples of central nervous system (CNS) manifestations of SjD include psychiatric changes, encephalopathy, seizures, optic neuropathy, and spinal cord involvement.3 Given such protean manifestations, it can be challenging to diagnose and longitudinally follow progression of neuropathies in SjD. Often times, the neurological symptoms may declare themselves before the patient is diagnosed with SjD.2 In these cases, their neurological manifestations may be deemed as idiopathic.4 Reports of neurological involvement in SjD has varied through the years, and it is likely due to the differing definitions and methods of detection of peripheral and central nervous system manifestations.
Current diagnostics of neuropathies may include nerve conduction studies, electromyography, and nerve biopsies to identify morphological changes. These laboratory tests along with an extensive neurological exam can help providers diagnose and longitudinally follow neuropathies that may be present in SjD patients. Additionally, brain imaging can help diagnose and track central nerve involvement. The gold standard for diagnosis of a small fiber neuropathy has traditionally been skin biopsy for assessment of epidermal nerve fiber density. However, skin biopsy is an invasive procedure and not routinely used in SjD patients. The presence of small fiber neuropathy may be an additional biomarker that could be useful in identifying SjD patients. One study found that SjD patients exhibiting a small fiber neuropathy were less likely than SjD patients without a small fiber neuropathy to have a positive anti-SSA.5 Since anti-SSA is an important test used in the classification of SjD, the absence of this antibody in a patient suspected of having SjD could be a major limitation to them being appropriately classified.6,7 Moreover, there may be unique subtypes of SjD that exhibit less B-cell activation, and treatment strategies may need to be tailored according to relevant subtypes.
Some researchers have shown that in-vivo confocal microscopy may serve as a surrogate marker of a small fiber neuropathy in diabetes and multiple sclerosis.8–11 In this study, we wished to identify quantitative and qualitative features of a small fiber neuropathy of the corneal sub-basal nerve plexus using in-vivo confocal microscopy in participants classified as SjD or non-SjD controls using the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) classification criteria.
Methods
Study Population
Eleven participants with SjD were recruited, 10 who were past participants from the Sjögren’s International Collaborative Clinical Alliance (SICCA). SICCA was comprised of an international and multidisciplinary team of ophthalmologists, oral medicine specialists, and rheumatologists and enrolled participants between 2003 and 2012 with the goal of creating standardized and universally accepted classification criteria for SjD. The SjD classification criteria developed by SICCA and two other large cohorts was ultimately validated and accepted by both ACR and EULAR.7,12 In this study, one participant was recruited who was not part of the original SICCA cohort. We recruited this participant as part of a current long-term plan to expand the SICCA cohort for future studies. Furthermore, we include the additional participant in the present study in attempts to make the current data set as robust as we are able to manage at the time. A convenience sample of healthy controls was selected from the UCSF Optometry Clinic; controls were eligible if they had no previous corneal disease in either eye, no signs or symptoms of dry eye disease on the day of enrollment, and no systemic diseases that could be associated with a small fiber neuropathy (e.g., diabetes).
Confocal Microscopy
All participants underwent in-vivo confocal microscopy (IVCM) of the cornea’s sub-basal nerve plexus centered about the central whorl. Confocal microscopy was performed by a fellowship-trained ophthalmologist (JAG). Topical proparacaine hydrochloride 0.5% was instilled into the study eye for anesthesia immediately before examination. A hypromellose gel (GenTeal Gel; Novartis Ophthalmics, East Hanover, NJ) was applied directly to the IVCM lens acting as a coupling agent bridging the space between the lens and a single-use sterile applanating cap. A Heidelberg Retinal Tomograph III Rostock Cornea Module (Heidelberg Engineering GmbH, Heidelberg Germany) was used to create a 30-image montage of the sub-basal nerve plexus for each participant.13 To create the 30-image montage, participants were asked to focus their non-examined eye on a 6 × 5 dot grid system. A central reference dot in the grid system was used as a fixation point. Participants were then instructed to look along the first line on the grid, starting with the first dot on the left and moving to each subsequent dot to the right on this top line. A single confocal image was captured as the participant fixated on each dot. At the end of each row, the participant was asked to fixate on the central reference dot to recapture the typical site of the central whorl which is found by following the nerves radially to the central location where the central whorl should be. Though some participants lacked a central whorl (which is further discussed in the Results section), the confocal operator was able to identify where the central whorl should have been by virtue of the fact that the sub-basal plexus nerves run radially toward the inferocentral aspect of the cornea. This process was repeated until all rows were captured. The image set was then reconstructed to form the 30-image montage of the central sub-basal nerve plexus, which equates to 4.8mm2 of the total area of the cornea.13 Figure 1 shows the reconstructed 30-image montage of a healthy control’s sub-basal nerve plexus.
Figure 1:

30-image montage from a healthy control participant of the cornea’s sub-basal nerve plexus within the right eye using in vivo confocal microscopy.
Image Analyses
Each image was analyzed with automated software (ACCmetrics, Manchester, United Kingdom) to produce seven nerve metrics: 1) corneal nerve fiber density (CNFD)—number of fibers/mm2, 2) corneal nerve branch density (CNBD)—number of branch points on the main fibers/mm2, 3) corneal nerve fiber length (CNFL)—total length of nerves mm/mm2, 4) nerve fiber total branch density (CTBD)—total number of branch points/mm2, 5) nerve fiber area (CNFA)—total CNFA mm2/mm2, 6) nerve fiber width (CNFW)—average CNFW (in millimeters)/mm2, and 7) nerve fiber fractal dimension. Fractal dimension is measured using a box counting method, in which differently sized boxes are used to scan the image, and only boxes containing any part of the detected nerve fibers are counted. The fractal dimension is measured as the slope of the line when the value of log (n) is plotted against log (r), where n is the number of boxes that cover the nerve fiber, and r is the inverse of the box size.
Each image was then assessed by three trained masked graders (JDK, AT, and SL) for three features: presence of nerve tortuosity, presence of neuromas, and presence of dendritic cells. All graders used a training set of images as reference (Supplemental Digital Content, SDC 1). The images included examples of neuromas, dendritic cells and nerve tortuosity. Each grader would choose a score (0 or 1) for each image with the point given for the presence of the feature being assessed. Each image was separately assessed three times for the presence of each feature. The majority consensus grade between the three graders was calculated for each image. Therefore, if the three graders had different grades, then the majority grade given out of the three graders was used. Then the proportion of images per eye with the finding was calculated as a score. The presence of a central whorl was also assessed in each 30-image confocal montage.
Statistical Analysis
Statistical analysis was performed using Stata 17.0 software (StataCorp LP, College Station, Texas, USA). T-tests and age-adjusted regressions were performed on each nerve metric assessed by ACCMetrics. Intraclass correlation coefficients (ICC) were calculated for each qualitative feature (neuromas, dendritic cells, and tortuosity).
Results
Ten participants from the original UCSF sub-cohort of the SICCA study were enrolled in this study. One additional participant, who had not participated in the SICCA baseline study, was added to the current study as part of long-term plans to expand the SICCA cohort for future studies. Hereafter, we refer to these 11 participants as “SICCA” participants. The eleven SICCA participants were classified as having SjD according to ACR/EULAR classification criteria. A separate sample of 22 healthy controls were recruited from routine UCSF optometry clinic visits. The healthy control group was a sample of convenience that was skewed younger. The average age for those classified as SjD was 67 +/− 10 years, while the average age of the healthy controls was 55 +/− 13 years (Table 1). In the healthy control group, 16 were female while 6 were male. All SjD participants were female.
Table 1:
Corneal nerve metrics of the right eye for SjD participants compared to controls.
| Sjögren’s Disease (n=11) | Controls (n=22) | Age-Adjusted Difference (95% CI) | P-value | |
|---|---|---|---|---|
| Age | 67.2 +/− 10.2 | 55.1 +/− 13.0 | N/A | N/A |
| Nerve Fiber Density | 3.5 +/− 4.5 | 10.6 +/− 4.7 | −4.7 (−8.4 to −0.93) | 0.02 |
| Nerve Branch Density | 2.7 +/− 4.9 | 15.2 +/− 9.9 | −8.7 (−15.7 to −1.7) | 0.02 |
| Nerve Fiber Length | 4.3 +/− 2.6 | 9.9 +/− 2.4 | −3.6 (−5.6 to −1.6) | 0.001 |
| Total Branch Density | 9.1 +/− 6.7 | 34.2 +/− 15.1 | −17.6 (−28.3 to −6.9) | 0.002 |
| Nerve Fiber Area | 0 | 0.006 +/− 0.001 | −0.002 (−0.003 to −0.001) | 0.001 |
| Nerve Fiber Width | 0.03 +/− 0.002 | 0.02 +/− 0.002 | 0.002 (0.0004 to 0.003) | 0.02 |
| Nerve Fiber Fractal Dimension | 1.3 +/− 0.08 | 1.4 +/− 0.03 | −0.07 (−0.13 to −0.01) | 0.02 |
The SjD participants had mean ocular staining score (OSS) of 4.6 +/− 4.3 points in the right eye and 4.9 +/− 3.9 points in the left eye. Because the healthy controls were recruited from the optometry clinic, OSS is not routinely performed in such patients. However, clinical examination in optometry did not reveal features of aqueous deficient or sufficient dry eye signs, so their OSS is expected to have been lower that the SjD participants.
SjD participants had significantly lower nerve metrics than the healthy control group (Table 1, Figure 2).
Figure 2:


(A) Bar graphs of average nerve metrics by Sjögren’s Disease classification for CNFD, CNBD, CNFL, CTBD and CNFracDim. (B) Bar graphs of average nerve metrics by Sjogren’s Disease classification for CNFA and CNFW.
While constructing the pseudo-montages for the 11 SjD participants, the presence of a central whorl was examined. Within the 11 SICCA participants, 22 eyes were analyzed on confocal microscopy, and 16 of those eyes (from 9 individuals) did not have an identifiable central whorl. Within the 22 healthy controls, 22 eyes (right eye only) were analyzed on confocal microscopy, and 21 of those eyes had an identifiable central cortex.
For the qualitative analysis of neuromas, dendritic cells and nerve tortuosity, a total of 774 confocal images from 22 eyes (11 eyes from SICCA participants and 11 eyes from the control group) were reviewed. Mean tortuosity score was 40 (SD 27) in the SICCA participant group and 2 (SD 2) in the control group (p<0.001, Wilcoxon rank sum test). Mean neuroma score was 18 (SD 15) in the SICCA participant group and 2 (SD 2) in the control group (p=0.04) (Figure 3). Mean dendritic cell score was 44 (SD 29) in the SICCA participant group and 32 (SD 27) in the control group (p=0.30) (Figure 4). There was moderate agreement between the three graders with respect to the presence of neuromas (ICC 0.55, 0.44–0.66) (Figure 5). There was poor agreement between graders with respect to the presence of dendritic cells (ICC 0.46, 0.40–0.52) and nerve tortuosity (ICC 0.38, 0.30–0.46).
Figure 3:

Examples of neuromas found in SjD participant montages.
Figure 4:
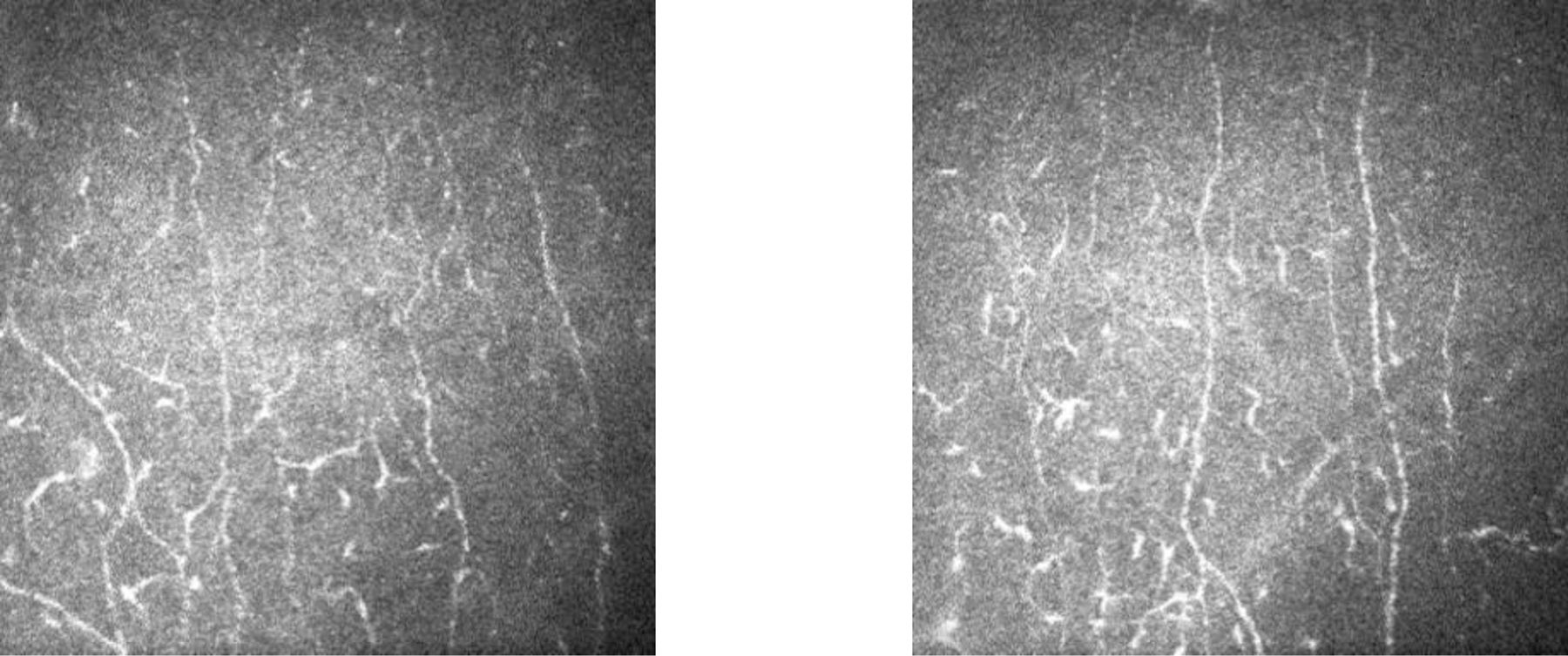
Examples of dendritic cells found in SjD participant montages.
Figure 5:

Examples of increased nerve tortuosity found in SjD participant montages.
Discussion
In this study, we investigated features of short fiber neuropathy in SjD using in-vivo confocal microscopy. Participants classified as SjD had markedly lower nerve metrics when compared to healthy controls, a frequently absent central whorl and the presence of neuromas. The presence of neuromas showed the best reliability between graders and may be a differentiating clinical feature between SICCA participants and healthy controls. Luzu et. al found a statistically significant increase of neuromas in their Sjogren’s Disease group compared to controls in their retrospective case-control study.14 They also found reduced density of nerves in the sub-basal nerve plexus and increased tortuosity. These signs taken together can point to an underlying neuropathy.
Prior studies have shown reduced intraepidermal nerve fiber density of skin biopsy in patients with SjD featuring a small fiber neuropathy based on clinical and neurophysiologic assessments.5 Interestingly, such SjD patients with a small fiber neuropathy tend to be older and have an immunologic profile that is reflective of a lower B-cell activation pattern in that they are more apt to have negative anti-SSA antibody and lower gamma globulin levels. Novel biomarkers for SjD could be helpful in identifying patients who may not meet current ACR/EULAR classification criteria. While skin biopsy with quantification of the intraepidermal nerve fiber density is intriguing, it is an invasive procedure. Less invasive procedures that are capable of identifying features of a small fiber neuropathy are therefore not only attractive but needed. Confocal microscopy has the potential to be such a biomarker in SjD. Indeed, other groups have demonstrated the value of confocal microscopy in identifying neuropathies present in diabetic patients.15–17 Malik and colleagues were able to observe reduced corneal nerve fiber density in diabetic patients within their investigation that used confocal microscopy to interrogate the sub-basal nerve plexus.15 Luzu et. al. used in vivo confocal microscopy to detect corneal nerve changes in their Sjögren’s Disease participants demonstrating its potential as a quick and non-invasive tool.14 Given that our confocal analysis showing reduced nerve fiber density in the central cornea is identified in a relatively easy and non-invasive manner, the need for epidermal biopsies may be obviated.
Our findings also show the potential use of in-vivo confocal microscopy in identifying features compatible with small fiber neuropathy. This could be used to help in identifying specific patients that should be referred for additional testing to confirm the presence of a neuropathy. Moreover, in-vivo confocal microscopy could act as a novel biomarker for SjD with potential to monitor disease progression. This is important because current methods used to monitor neuropathic manifestation of SjD include mainly qualitative questionnaires. Confocal microscopy allows for the quantification of nerves affected by SjD, and these values can easily be followed over time.
Several limitations to this study should be acknowledged. Our study involved a small sample of participants and while statistical significance was achieved when looking at the differences in nerve metrics between SjD and healthy controls, we aim to demonstrate these differences in a larger sample of participants in the future. We did not correlate our confocal microscopic findings with skin biopsies for assessment of epidermal nerve fiber density. However, others have demonstrated that confocal microscopic findings in SjD correlate with reduced epidermal nerve fiber density.5 Additionally, we did not check corneal sensation by esthesiometry. Future studies should correlate corneal sensation with nerve metrics and morphologic features of neuropathy identified on confocal microscopy. Finally, the control group was not age-matched and was skewed younger than our SjD cohort. Though statistical comparisons were adjusted for age in our study, we recognize the lack of an age-matched control group as a limitation. Nevertheless, the reduced nerve metrics we found in our SjD participants are viewed to be more than what is expected from advanced age alone.8
Future studies should consider incorporating the nerve metrics and morphologic features in the assessment of patients with Sjögren’s disease dry eye. This may allow for the stratification of a subset of SjD patients based upon confocal findings compatible with a small fiber neuropathy. Such information could be useful in identifying subset of SjD patients that should be monitored for the development of specific symptoms (such as pain) and who could benefit from specific therapies.
Conclusion
Within this pilot study, our findings showed that SjD exhibits lower corneal nerve metrics and increased number of neuromas compared to healthy controls. These findings are compatible with a small fiber neuropathy and may help distinguish SjD from healthy controls. Additionally, such features could potentially serve as a novel biomarker for some patients with SjD. While reduced nerve metrics were found in SjD participants compared to healthy controls, perhaps a lack of a central whorl in and of itself could act as a potential biomarker for the disease.
Supplementary Material
SDC 1: Training for Masked Graders. A set of images used to train the masked image graders on standard presentations of neuromas, dendritic cells and nerve tortuosity.
Source of Funding:
Analysis reported in this manuscript was supported by the National Eye Institute of the National Institutes of Health under Award Number K08EY026986 (J.A.G.). This work was supported in part by an unrestricted grant from Research to Prevent Blindness. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest
None of the authors have any conflicts of interest to disclose.
References
- 1.Baer AN, Hammitt KM. Sjögren’s Disease, Not Syndrome. Arthritis & Rheumatology 2021-07-01 2021;73(7):1347–1348. doi: 10.1002/art.41676 [DOI] [PubMed] [Google Scholar]
- 2.Mori K, Iijima M, Koike H, et al. The wide spectrum of clinical manifestations in Sjögren’s syndrome-associated neuropathy. Brain 2005-11-01 2005;128(11):2518–2534. doi: 10.1093/brain/awh605 [DOI] [PubMed] [Google Scholar]
- 3.Mccoy SS, Baer AN. Neurological Complications of Sjögren’s Syndrome: Diagnosis and Management. Current Treatment Options in Rheumatology 2017-12-01 2017;3(4):275–288. doi: 10.1007/s40674-017-0076-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delalande S, de Seze J, Fauchais AL, et al. Neurologic manifestations in primary Sjogren syndrome: a study of 82 patients. Medicine (Baltimore) Sep 2004;83(5):280–291. doi: 10.1097/01.md.0000141099.53742.16 [DOI] [PubMed] [Google Scholar]
- 5.Sene D, Cacoub P, Authier FJ, et al. Sjogren Syndrome-Associated Small Fiber Neuropathy: Characterization From a Prospective Series of 40 Cases. Medicine (Baltimore) Sep 2013;92(5):e10–e18. doi: 10.1097/MD.0000000000000005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjogren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol Jan 2017;69(1):35–45. doi: 10.1002/art.39859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiboski CH, Baer AN, Shiboski SC, et al. Natural History and Predictors of Progression to Sjogren’s Syndrome Among Participants of the Sjogren’s International Collaborative Clinical Alliance Registry. Arthritis Care Res (Hoboken) Feb 2018;70(2):284–294. doi: 10.1002/acr.23264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tavakoli M, Ferdousi M, Petropoulos IN, et al. Normative Values for Corneal Nerve Morphology Assessed Using Corneal Confocal Microscopy: A Multinational Normative Data Set. Diabetes Care 2015-05-01 2015;38(5):838–843. doi: 10.2337/dc14-2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Graham J, Dabbah MA, et al. Small Nerve Fiber Quantification in the Diagnosis of Diabetic Sensorimotor Polyneuropathy: Comparing Corneal Confocal Microscopy With Intraepidermal Nerve Fiber Density. Diabetes Care 2015-06-01 2015;38(6):1138–1144. doi: 10.2337/dc14-2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petropoulos IN, Fitzgerald KC, Oakley J, et al. Corneal confocal microscopy demonstrates axonal loss in different courses of multiple sclerosis. Scientific Reports 2021-12-01 2021;11(1)doi: 10.1038/s41598-021-01226-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia May 2003;46(5):683–8. doi: 10.1007/s00125-003-1086-8 [DOI] [PubMed] [Google Scholar]
- 12.Shiboski SC, Shiboski CH, Criswell L, et al. American College of Rheumatology classification criteria for Sjogren’s syndrome: a data-driven, expert consensus approach in the Sjogren’s International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken) Apr 2012;64(4):475–87. doi: 10.1002/acr.21591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takhar JS, Joye AS, Lopez SE, et al. Validation of a Novel Confocal Microscopy Imaging Protocol With Assessment of Reproducibility and Comparison of Nerve Metrics in Dry Eye Disease Compared With Controls. Cornea May 1 2021;40(5):603–612. doi: 10.1097/ICO.0000000000002549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luzu J, Antoine L, Annabelle RG, et al. In vivo confocal microscopic study of corneal innervation in Sjogren’s Syndrome with or without small fiber neuropathy. Ocul Surf Jul 21 2022;doi: 10.1016/j.jtos.2022.07.003 [DOI] [PubMed] [Google Scholar]
- 15.Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia 2003-05-01 2003;46(5):683–688. doi: 10.1007/s00125-003-1086-8 [DOI] [PubMed] [Google Scholar]
- 16.Edwards K, Pritchard N, Vagenas D, Russell A, Malik RA, Efron N. Utility of corneal confocal microscopy for assessing mild diabetic neuropathy: baseline findings of the LANDMark study. Clin Exp Optom May 2012;95(3):348–54. doi: 10.1111/j.1444-0938.2012.00740.x [DOI] [PubMed] [Google Scholar]
- 17.Tavakoli M, Quattrini C, Abbott C, et al. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care Aug 2010;33(8):1792–7. doi: 10.2337/dc10-0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDC 1: Training for Masked Graders. A set of images used to train the masked image graders on standard presentations of neuromas, dendritic cells and nerve tortuosity.


