Abstract
In normal tissue homeostasis, bidirectional communication between different cell types can shape numerous biological outcomes. Many studies have documented instances of reciprocal communication between fibroblasts and cancer cells that functionally change cancer cell behavior. However, less is known about how these heterotypic interactions shape epithelial cell function in the absence of oncogenic transformation. Furthermore, fibroblasts are prone to undergo senescence, which is typified by an irreversible cell cycle arrest. Senescent fibroblasts are also known to secrete various cytokines into the extracellular space; a phenomenon that is termed the senescence-associated secretory phenotype (SASP). While the role of fibroblast-derived SASP factors on cancer cells has been well studied, the impact of these factors on normal epithelial cells remains poorly understood. We discovered that treatment of normal mammary epithelial cells with conditioned media from senescent fibroblasts (SASP CM) results in a caspase-dependent cell death. This capacity of SASP CM to cause cell death is maintained across multiple senescence-inducing stimuli. However, the activation of oncogenic signaling in mammary epithelial cells mitigates the ability of SASP CM to induce cell death. Despite the reliance of this cell death on caspase activation, we discovered that SASP CM does not cause cell death by the extrinsic or intrinsic apoptotic pathway. Instead, these cells die by an NLRP3, caspase-1, and gasdermin D–dependent induction of pyroptosis. Taken together, our findings reveal that senescent fibroblasts can cause pyroptosis in neighboring mammary epithelial cells, which has implications for therapeutic strategies that perturb the behavior of senescent cells.
Keywords: cell death, senescence, fibroblast, epithelial cell, caspase 1, pyroptosis, SASP, gasdermin D
Heterotypic interactions between distinct cell types underlie a variety of diverse biological functions including shaping tissue architecture during normal development and maintaining tissue homeostasis (1, 2, 3). In addition, aberrant heterotypic signaling has been linked to a number of pathological conditions including neurodegenerative, cardiovascular, and autoimmune diseases (2, 4, 5, 6). There has also been substantial research into heterotypic signaling in a variety of cancerous conditions (7, 8, 9). Heterotypic signaling during tumorigenesis often takes place between cells found in the tumor microenvironment (TME) and the cancer cells themselves (10, 11, 12, 13). The TME is characterized by an array of cell types (e.g., fibroblasts, endothelial cells, and immune cells) that are now appreciated to contribute significantly to tumor progression (14, 15). While numerous studies have documented instances of reciprocal communication between fibroblasts and cancer cells that functionally change cancer cell behavior, much less is known about how these types of heterotypic interactions shape epithelial cell function in the absence of oncogenic transformation.
Relatedly, fibroblasts present in close proximity to epithelial cells have the propensity to undergo senescence, a process that is linked to aging and characterized by long-term loss of proliferative capacity (16, 17, 18). Senescent fibroblasts are well known to secrete inflammatory cytokines, which can have ramifications for cells in the local microenvironment. This phenomenon is characterized as the senescence-associated secretory phenotype (SASP) and has been observed in response to multiple, distinct senescence inducers (19, 20, 21). Numerous lines of investigation have now demonstrated that the milieu of cytokines secreted by senescent fibroblasts can create a permissive environment for cancer cells (22, 23, 24, 25, 26). These data have motivated significant interest in the use of senolytic drugs, which specifically kill senescent cells, as a novel therapeutic strategy in multiple cancer types (27, 28, 29). However, the use of senolytic drugs to treat cancers is complicated by the fact that our understanding of how senescent fibroblasts can impact normal (noncancerous) cells remains quite limited. For example, recent evidence suggests that senescent fibroblasts in the lung can sense injury-induced tissue inflammation, alter the stem cell niche, and facilitate the regeneration of epithelial barrier integrity (30). Therefore, additional studies that better clarify the relationship between senescent fibroblasts and noncancerous epithelial cells are critically important to better understand the efficacy of this therapeutic strategy.
Here, we demonstrate that the induction of senescence (by distinct stimuli) in fibroblasts can result in the secretion of SASP factors that negatively impact the viability of normal mammary epithelial cells. In addition, the expression of activated oncogenes in normal mammary epithelial cells protects these cells from cell death caused by SASP factors. These findings suggest that cell death caused by SASP factors could functionally restrict extraneous growth of mammary epithelial cells prior to oncogenic transformation. When investigating the mechanism underlying cell death by SASP factors, we found that despite the necessity of caspase activation, this cell death was neither a consequence of extrinsic nor intrinsic pathway–mediated apoptosis. Instead, we found that SASP factors cause a pyroptotic cell death that is dependent on the Nod-like receptor protein 3 (NLRP3) inflammasome, caspase-1, and gasdermin D (GSDMD). Taken together, our findings reveal that senescent fibroblasts can cause pyroptosis in neighboring mammary epithelial cells, which has implications for therapeutic strategies that eliminate senescent cells.
Results
Conditioned media from senescent fibroblasts promotes caspase-dependent cell death in noncancerous mammary epithelial cells
In order to study the effects of the SASP in fibroblasts on the viability of noncancerous mammary epithelial cells, we first induced senescence in BJ fibroblasts, which have been utilized extensively for studies examining fibroblast senescence (31, 32, 33, 34, 35). To do so, we treated BJ cells with the DNA-damaging agent bleomycin, a well-known inducer of senescence and SASP in this cell line (25, 36). To confirm senescence induction in bleomycin-treated cells, we measured senescence-associated-β-galactosidase activity (Fig. 1A) and determined lamin B1 protein abundance (Fig. 1B), which is known to decrease during senescence induction (37). Additionally, we found elevated levels of p21, a cyclin-dependent kinase inhibitor that is evidenced to be induced during senescence (38), in bleomycin-treated cells (Fig. 1C). Furthermore, we assessed the mRNA levels of IL-6 and IL-8 in bleomycin-treated cells and confirmed that the expression of these factors, known to be associated with the SASP (39), are significantly elevated upon bleomycin treatment (Fig. 1D).
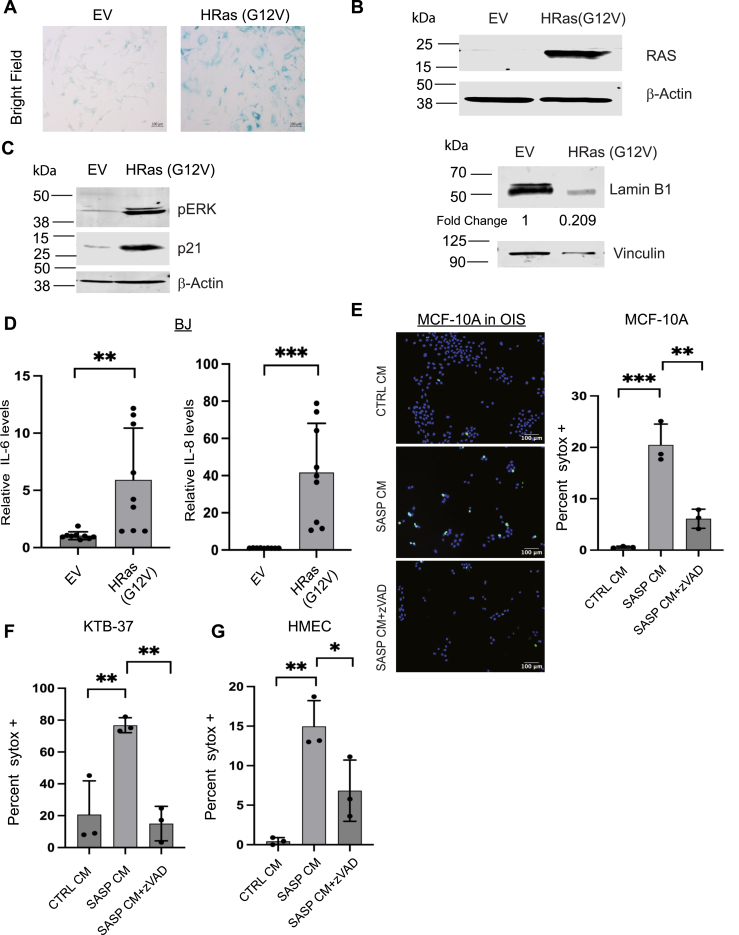
Figure 1.
SASP CM from BIS fibroblasts causes cell death in normal mammary epithelial cells.A, BJ fibroblasts were stained for senescence-associated β-galactosidase (SA-β-Gal) activity to confirm the induction of senescence in fibroblasts treated with DMSO or bleomycin. Images were taken at 10× in brightfield. Scale bar is 100 μm. B, cell lysates from BJ fibroblasts treated with DMSO or bleomycin were immunoblotted for lamin B1 protein levels as a marker for senescence induction. Densitometry is represented as fold change relative to EV control. C, cell lysates from BJ fibroblasts treated with DMSO or bleomycin were immunoblotted for the cell cycle inhibitor p21 as a marker of senescence. D, qRT-PCR was performed to measure the relative mRNA levels of IL-6 and IL-8 in BJ fibroblasts treated with either DMSO or bleomycin to validate SASP induction. n = 3 E, (left) representative immunofluorescence images of MCF-10A cells treated with CTRL or SASP conditioned media (CM) from bleomycin-induced senescent (BIS) fibroblasts and stained with Hoechst and SYTOX Green. Images were taken at 10×, and scale bar is 100 μm. z-VAD-fmk treatment was included to assess the impact of caspase activation on the abundance of SYTOX Green positive cells. (Right) F, KTB-37 cells were treated with CTRL, SASP CM, or SASP CM+z-VAD-fmk collected from BIS BJ fibroblasts and stained for Hoescht and SYTOX Green. G, HMEC cells were treated with CTRL, SASP CM, or SASP CM+z-VAD-fmk collected from BIS BJ fibroblasts. H and I, BJ fibroblasts (H) or HuVEC cells (I) were treated with CTRL or SASP CM from BIS BJ fibroblasts and stained for Hoescht and SYTOX Green. All SYTOX quantification is represented as the percentage of SYTOX Green positive cells out of the total number of cells (Hoescht +). Unpaired two-tail t test was performed for qRT-PCR data where ∗∗∗∗p < 0.0001, ∗∗∗p < 0.001. Data are presented as mean ± STDEV. SYTOX data were analyzed by one-way ANOVA followed by Tukey comparison test. Graphs are representative data collected from a minimum of three biological replicates. SASP, senescence-associated secretory phenotype.
Following confirmation of senescence induction by bleomycin, we collected conditioned media (CM) from control (nonsenescent) or senescent fibroblasts (hereafter referred to as CTRL CM or SASP CM, respectively) and assessed cell death in MCF-10A cells treated with each type of CM by SYTOX Green staining. Interestingly, treatment with SASP CM resulted in a significant induction of cell death in MCF-10A cells (Fig. 1E). Moreover, SASP CM-induced cell death in MCF-10A cells was blocked by treatment with z-VAD-fmk (a pan-caspase inhibitor), suggesting that the mechanism of cell death is dependent on caspase activation (Fig. 1E). To assess whether the induction of cell death by SASP CM was specific to MCF-10A cells, we expanded our studies to additional mammary epithelial cell lines. Indeed, SASP CM treatment resulted in robust cell death in both KTB-37 (Fig. 1F) and HMEC cells (Fig. 1G), which was averted by treatment with z-VAD-fmk. In contrast, when we extended our studies to other cell types, we found that SASP CM does not induce cell death in untreated BJ fibroblasts (Fig. 1H) or human umbilical vein endothelial cells (HuVECs) (Fig. 1I).
To complement these studies, we next evaluated whether SASP CM could promote cell death when senescence was induced in fibroblasts through an alternative mechanism. We engineered BJ fibroblasts to undergo oncogene-induced senescence (OIS) through overexpression of the oncogene HRas (G12V) and confirmed senescence induction through measurement of senescence-associated β-galactosidase activity (Fig. 2A) and immunoblotting for lamin B1 (Fig. 2B). Additionally, we confirmed Ras activation by immunoblotting for p-ERK and assessed the levels of p21 as a marker of senescence induction (Fig. 2C). Furthermore, we measured the expression of SASP genes through qRT-PCR to validate the generation of a SASP (Fig. 2D). Indeed, we found that SASP CM collected from OIS fibroblasts also stimulated the induction of cell death in MCF-10A (Fig. 2E), KTB-37 (Fig. 2F), and HMEC (Fig. 2G) cells, and in each case, the SASP CM–mediated cell death was abrogated by treatment with z-VAD-fmk. Taken together, these data suggest that senescence induction (by distinct mechanisms) can cause fibroblasts to secrete factors that provoke cell death in normal mammary epithelial cells.
Figure 2.
SASP CM from OIS fibroblasts causes cell death in normal mammary epithelial cells.A, BJ fibroblasts were stained for SA-β-Gal activity to confirm the induction of senescence in BJ fibroblasts engineered to undergo oncogene-induced senescence (OIS) through overexpression of HRas (G12V). Empty vector (EV) control is also included. Representative images were taken at 10× in brightfield and scale bar is 100 μm. B, cell lysates from BJ fibroblasts with EV or HRas (G12V) were immunoblotted for Ras or lamin B1 protein levels as a marker for senescence induction. Densitometry is represented as fold change relative to EV control. C, cell lysates from BJ OIS fibroblasts were immunoblotted for the cell cycle inhibitor p21 and phosphorylated ERK. D, qRT-PCR was performed to measure the relative mRNA levels of IL-6 and IL-8 in BJ fibroblasts engineered to overexpress EV or HRas (G12V). n = 3. E, representative immunofluorescence images of MCF-10A cells treated with CTRL or SASP conditioned media (CM) from OIS fibroblasts stained with Hoechst and SYTOX green. Images were taken at 10× and the scale bar is 100 μm. Quantification of images represented as the percentage of SYTOX Green positive cells out of the total number of cells was depicted. F, KTB-37 cells treated with CTRL or SASP conditioned media (CM) from OIS were stained with Hoechst and SYTOX Green. Quantification of images represented as the percentage of SYTOX Green positive cells out of the total number of cells was depicted. G, HMEC cells treated with CTRL or SASP CM from OIS stained with Hoechst and SYTOX Green. Quantification of images represented as the percentage of SYTOX Green positive cells out of the total number of cells was depicted. Unpaired two-tail t test was performed for qRT-PCR data where ns is no statistical significance, ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. Data are presented as mean ± STDEV. SYTOX data were analyzed by one-way ANOVA followed by Tukey comparison test. Graphs are representative data collected from a minimum of three biological replicates. SA-β-Gal, senescence-associated β-galactosidase; SASP, senescence-associated secretory phenotype.
Oncogenic signaling desensitizes breast cancer cells to SASP CM-induced cell death
Given our results demonstrating that SASP CM can cause cell death in noncancerous mammary epithelial cells, we questioned whether breast cancer cells would respond similarly to exposure to SASP CM. This is a particularly important factor to consider given that senescent fibroblasts are well-known constituents of the breast TME (40, 41, 42). To assess the capacity of senescent fibroblasts to secrete factors that alter cell death in breast cancer cells, we treated MDA-MB-231 (Fig. 3A) or MDA-MB-436 (Fig. 3B) cells with SASP CM and measured cell death by SYTOX Green staining. Using SASP CM from both bleomycin-induced senescence (BIS) and OIS fibroblasts, we found that SASP CM treatment did not induce cell death in either cell line (Fig. 3, A and B). We next sought to extend these studies in an isogenic background using MCF-10A cells, which we previously demonstrated were sensitive to cell death caused by SASP CM (Figs. 1E and 2E). To do so, we stably expressed HRas [G12V or an empty vector (EV)] control in MCF-10A cells and confirmed successful expression by immunoblotting (Fig. 3C). We also confirmed that, unlike with BJ cells, HRas activation in MCF-10A cells does not induce appreciable senescence as measured by senescence-associated β-galactosidase activity (Fig. 3D). Additionally, we stably expressed the oncogene ErbB2 in MCF-10A cells and validated successful expression by immunoblotting (Fig. 3F). We then exposed MCF-10A cells expressing these oncogenic mutations (or the EV control) to SASP CM (collected after induction of BIS or OIS) and measured cell death. Indeed, expression of either HRas (G12V) (Fig. 3E) or ErbB2 (Fig. 3G) was sufficient to block the ability of SASP CM to induce cell death. Thus, while noncancerous epithelial cells are sensitive to cell death caused by SASP CM, the activation of oncogenes in normal mammary epithelial cells prevents the induction of SASP CM–mediated cell death.
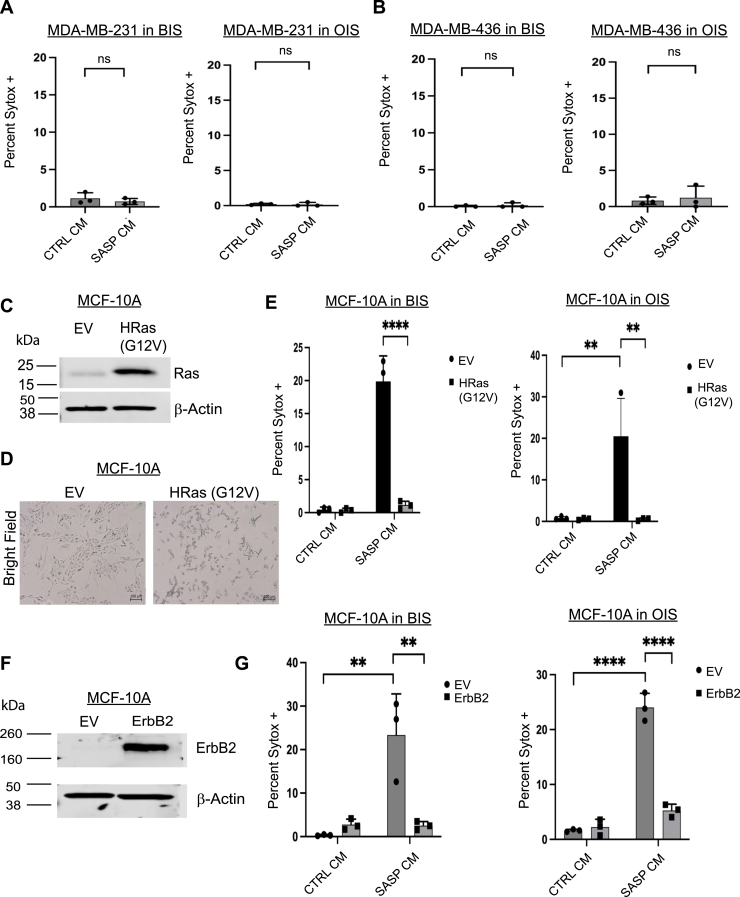
Figure 3.
Breast cancer cells are resistant to SASP CM induced cell death.A and B, MDA-MB-231 (A) and MDA-MB-436 (B) breast cancer cell lines were treated with CTRL or SASP CM collected from BIS or OIS fibroblasts and imaged using immunofluorescence microscopy. Cells were stained with Hoechst and SYTOX Green. Quantification of the percentage of SYTOX Green positive cells out of the total number of cells was depicted. C, MCF-10A cells were engineered to overexpress either empty vector (EV) or the oncogene HRas (G12V). D, MCF-10A EV and HRas (G12V) cell lysates were immunoblotted for lamin B1, and cells were stained for senescence-associated β-galactosidase (SA β-Gal) staining. All images taken at 10×. E, MCF-10A EV and HRas (G12V) overexpressing cells were treated with CTRL or SASP CM collected from BIS or OIS fibroblasts and imaged using immunofluorescence microscopy. Cells were stained with Hoechst and SYTOX Green. Quantification of the percentage of SYTOX Green positive cells out of the total number of cells was depicted. F, MCF-10A cells were engineered to overexpress either EV or the oncogene ErbB2. Overexpression was validated by immunoblot. G, MCF-10A EV and ErbB2 overexpressing cells were treated with CTRL or SASP CM collected from BIS or OIS fibroblasts and imaged using immunofluorescence microscopy. Cells were stained with Hoechst and SYTOX Green. Quantification of the percentage of SYTOX Green positive cells out of the total number of cells was depicted. SYTOX data were analyzed by one-way ANOVA followed by Tukey comparison test where ns is so statistical significance, ∗∗p < 0.01 and ∗∗∗∗p < 0.0001. Data are presented as mean ± STDEV. Graphs are representative data collected from a minimum of three biological replicates. Isogenic manipulations were analyzed by two-way ANOVA followed by Tukey comparison test. Graphs are representative data collected from a minimum of three biological replicates. BIS, bleomycin-induced senescence; CM, conditioned media; OIS, oncogene-induced senescence; SASP, senescence-associated secretory phenotype.
SASP CM does not induce apoptotic cell death
We next sought to determine the molecular mechanism by which SASP CM promotes cell death in mammary epithelial cells. Due to the fact that SASP CM–mediated cell death was blocked by the pan-caspase inhibitor z-VAD-fmk (Figs. 1, E–G, and 2, E–G), we posited that these cells were dying by apoptosis. We first investigated the role of the intrinsic apoptotic pathway, which is mediated by the release of cytochrome c from the mitochondria (43, 44). To do so, we engineered MCF-10A cells to stably express Bcl-2, which blocks the release of cytochrome c from the mitochondria and thereby prevents downstream caspase activation (45). We confirmed Bcl-2 expression and function by immunoblotting for Bcl-2 and cleaved poly (ADP-ribose) polymerase (PARP) (Fig. 4A). Interestingly, while Bcl-2 expression was able to effectively inhibit staurosporine (STS)-induced apoptosis, it was unable to block cell death caused by exposure of cells to SASP CM (from either BIS or OIS) (Fig. 4B). Given these results, we next assessed the role of the extrinsic apoptotic pathway, which is characterized by ligand-mediated activation of death receptors (46, 47). To do so, we utilized lentiviral-mediated transduction of short hairpin RNA (shRNA) in MCF-10A cells to decrease the abundance of caspase-8 (Fig. 4C), a key mediator of the extrinsic apoptotic pathway. Despite a substantial (shRNA-mediated) decrease in the abundance of caspase-8 protein, MCF-10A cells were still sensitive to cell death induced by SASP CM (from either BIS or OIS) (Fig. 4D). Lastly, to rule out a role for apoptotic cell death, we measured cleaved PARP in cells that had been treated with SASP CM. While STS treatment can induce PARP cleavage, we found no evidence of cleaved PARP in cells treated with SASP CM (Fig. 4E). Collectively, these data suggest that neither the intrinsic nor extrinsic apoptotic pathways mediate cell death caused by SASP CM, indicating the cell death is likely nonapoptotic.
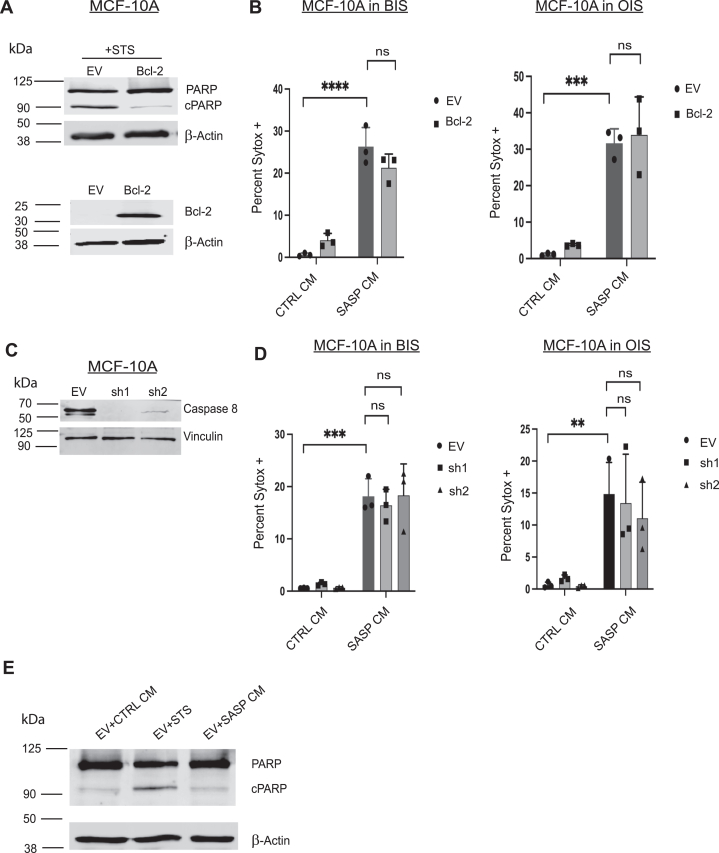
Figure 4.
SASP CM-induced cell death is independent of the intrinsic and extrinsic apoptotic pathways.A, MCF-10A cells were engineered to express EV or Bcl-2. 10A-EV and 10A-Bcl-2 cell lysates were immunoblotted to validate Bcl-2 overexpression. To confirm that the Bcl-2 overexpression is functional, both 10A-EV and 10A-Bcl-2 cells were treated with the staurosporine (STS) and probed for the cleavage of PARP, a marker of apoptosis. B, 10A-EV and 10A-Bcl-2 cells were treated with CTRL or SASP CM collected from BIS or OIS fibroblasts were imaged using immunofluorescence microscopy. Cells were stained with Hoechst and SYTOX Green. Quantification of the percentage of SYTOX Green positive cells out of the total number of cells was depicted. C, MCF-10A cells were engineered with shRNA against caspase-8 and immunoblotted to validate the reduction in caspase-8 protein. D, control or caspase-8 shRNA-transduced cells were treated with CTRL or SASP CM from BIS or OIS and imaged using immunofluorescence microscopy. Cells were stained with Hoechst and SYTOX Green. Quantification of the percentage of SYTOX Green positive cells out of the total number of cells was depicted. SYTOX data were analyzed by two-way ANOVA followed by Tukey comparison test where ns is no statistical significance, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001. Data are presented as mean ± STDEV. Graphs are representative data collected from a minimum of three biological replicates. E, MCF-10A EV cell lysates were treated with CTRL, staurosporine (STS) or SASP CM from BIS senescent fibroblasts. Lysates were immunoblotted for cleaved PARP. BIS, bleomycin-induced senescence; CM, conditioned media; EV, empty vector; OIS, oncogene-induced senescence; SASP, senescence-associated secretory phenotype.
NLRP3, caspase-1, and GSDMD–mediated pyroptosis underlies the capacity of SASP CM to induce cell death
Thus far, our data suggest that BIS and OIS in fibroblasts cause these cells to secrete factors that cause a caspase-dependent, but nonapoptotic cell death. When considering these surprising results, we hypothesized that the ability of z-VAD-fmk to block SASP CM–mediated cell death may be linked to its capacity to inhibit inflammatory, rather than apoptotic, caspases (48, 49, 50). Indeed, inflammatory caspases such as caspase-1 can cause cell death by pyroptosis, which is ultimately characterized by downstream activation of GSDMD proteins that form pores in the plasma membrane (51, 52). In addition, the composition of the SASP includes several factors that are linked to inflammatory caspase activation through regulation of (or by) inflammasome complexes (53, 54). In particular, the NLRP3 inflammasome can be primed or indirectly activated by inflammatory cytokines (e.g., IL-1β, IL-6) that are often found as components of the SASP (39, 55, 56, 57).
In order to determine if SASP CM was causing cell death by pyroptosis in noncancerous mammary epithelial cells, we first treated MCF-10A cells with MCC950, a small molecule inhibitor of NLRP3, and assessed the capacity of SASP CM to induce cell death. Indeed, MCC950 treatment abrogated SASP CM (from BIS or OIS)–induced cell death (Fig. 5A). Given these data, we next sought to ascertain if caspase-1 was required for SASP CM to induce cell death. In order to do so, we engineered MCF-10A cells to be deficient in caspase-1 levels (Fig. 5B) and assessed the capacity of SASP CM to induce cell death. Our data revealed that shRNA-mediated reduction in caspase-1 significantly attenuated the induction of cell death from exposure to SASP CM (from BIS or OIS) (Fig. 5C). Finally, we assessed the importance of GSDMD in mediating SASP CM-induced cell death. During pyroptosis, the N-terminal domain of GSDMD is separated from its C-terminal domain by caspase-1-mediated cleavage, which subsequently removes the intramolecular inhibition of the pore forming capabilities of the N-terminal domain (52). As a result, N-terminal GSDMD can permeabilize the plasma membrane to cause pyroptosis. As such, we engineered MCF-10A cells with shRNA-mediated reduction of GSDMD levels (Fig. 5D) and assessed the capacity of SASP CM to promote cell death. Indeed, SASP CM was unable to promote death in cells deficient in GSDMD (Fig. 5E). Lastly, to solidify that the cell death induced by SASP CM is pyroptotic, we investigated the cleavage of GSDMD in cells treated with SASP CM. As expected, treatment with SASP CM led to a significant elevation in GSDMD cleavage (Fig. 5F).
Figure 5.
SASP CM causes cell death by pyroptosis.A, MCF-10A cells were treated with CTRL or SASP CM collected from BIS or OIS fibroblasts with or without MCC950 (10 μM), an NLRP3 inhibitor. Cells were imaged using immunofluorescence microscopy. Images were taken at 10× and the scale bar is 100 μm. Cells were stained with Hoechst and SYTOX Green. Quantification of the percentage of SYTOX Green positive cells out of the total number of cells was depicted. B, MCF-10A cells were engineered using shRNA against caspase-1 and immunoblotted to validate the reduction in caspase-1 protein. C, EV control or caspase-1 shRNA-transduced cells were treated with CTRL or SASP CM from BIS or OIS and imaged using immunofluorescence microscopy. Cells were stained with Hoechst and SYTOX Green. Quantification of the percentage of SYTOX Green positive cells out of the total number of cells was depicted. D, MCF-10A cells were engineered using shRNA against GSDMD and immunoblotted to validate the reduction in GSDMD protein. E, EV control or GSDMD shRNA–transduced cells were treated with CTRL or SASP CM from BIS or OIS and imaged using immunofluorescence microscopy. Cells were stained with Hoechst and SYTOX Green. Quantification of the percentage of SYTOX Green positive cells out of the total number of cells was depicted. Sytox data were analyzed by two-way ANOVA followed by Tukey comparison test, where ns is no statistical significance, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001. Data are presented as mean ± STDEV. Graphs are representative data collected from a minimum of three biological replicates. F, MCF-10A cell lysates were treated with CTRL or SASP CM from BIS fibroblasts and immunoblotted for full-length (FL) and cleaved (c) GSDMD. Densitometry is represented as fold change relative to EV control. BIS, bleomycin-induced senescence; CM, conditioned media; EV, empty vector; GSDMD, gasdermin D; OIS, oncogene-induced senescence; SASP, senescence-associated secretory phenotype.
Taken together, our data demonstrate that the ability of SASP CM from senescent fibroblasts to promote cell death is dependent on NLRP3-mediated activation of caspase-1 and GSDMD-mediated pyroptosis.
Discussion
Our studies reveal that the induction of senescence in fibroblasts (by BIS and OIS) alter the fibroblast secretome in a fashion that promotes the induction of cell death in normal, nontransformed mammary epithelial cells. In contrast, SASP CM is unable to cause cell death in breast cancer cells or in mammary epithelial cells that have been engineered to express activated oncogenes. Furthermore, the observed cell death in normal mammary epithelial cells is blocked by z-VAD-fmk treatment, suggesting that it is dependent on the activation of caspases. Surprisingly, neither the intrinsic nor extrinsic apoptotic pathways are required for SASP CM-induced cell death. Instead, the induction of cell death is dependent on NLRP3-mediated activation of caspase-1 and the downstream actions of GSDMD. Taken together, these findings suggest that SASP CM is capable of inducing cell death by pyroptosis in normal mammary epithelial cells.
The fact that senescent fibroblasts can secrete factors that cause the induction of pyroptosis in nearby mammary epithelial cells is surprising and raises several important and interesting questions. Perhaps most salient is the ramifications in the tissue environment after the induction of a pyroptotic death, which is widely characterized to result in an inflammatory response (51). As such, the consequences of a potential influx of inflammatory cells on the remaining mammary epithelial cells and on the tissue microenvironment are potentially profound. Indeed, even if pyroptosis functions as potential mechanism to eliminate incipient neoplastic cells, the resulting inflammatory response may counterintuitively create a protumorigenic niche that favors oncogenic transformation (23). Relatedly, pyroptosis has perhaps been most often studied in the context of host immune response against infections (58, 59, 60, 61). The role of pyroptosis in cancer is less well understood, although evidence for pyroptosis playing a tumor suppressive role is accumulating in melanomas and breast cancers (62, 63, 64, 65, 66). Thus, it will be important to better understand the role of SASP CM–mediated pyroptosis in additional cell types and disease contexts to better appreciate resulting physiological and pathological conditions.
In addition, these surprising findings raise questions about the ramifications of therapeutic strategies that focus on the elimination of senescent cells. Given that the expression of oncogenes in normal mammary epithelial cells can prevent SASP CM–mediated cell death, it seems plausible that senescent fibroblasts are functioning in a fashion to eliminate cells that could become cancerous. Thus, senolytic agents may, in fact, function to eliminate a possible mechanism of tumor suppression. More broadly, it is unclear if senescent fibroblasts may affect adjacent epithelial cells in other tissue settings. While mammary epithelial cells respond to SASP CM by undergoing pyroptosis, it remains possible that epithelial cells from other tissues may respond in a different fashion. Moreover, additional studies investigating the anatomical location and appearance of senescent fibroblasts will help contextualize these findings to better consider the potential impacts of systemic administration of senolytic agents.
Experimental procedures
Cell culture
MCF-10A cells (ATCC) and derivatives were cultured in Dulbecco’s Modified Eagle Medium (DMEM) Nutrient Mixture F-12 Ham 1:1 powder (Gibco) supplemented with 5% horse serum (Invitrogen), 20 ng/ml epidermal growth factor, 10 μg/ml insulin, 500 μg/ml hydrocortisone, 100 ng/ml cholera toxin, 1% penicillin/streptomycin, and Plasmocin prophylactic. BJ human foreskin fibroblasts, MDA-MB-231, and MDA-MB-436 were cultured in DMEM high glucose supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. HMEC human mammary epithelial cells and derivatives (Lonza) were cultured in mammary epithelium basal medium MEBM (Lonza) plus MEBM SingleQuot Supplements. Komen Tissue bank cells (KTB-34) were cultured as previously described (67). HuVEC cells were grown in EGM endothelial cell growth medium Bulletkit (Lonza; CC-3124). Mycoplasma testing was routinely performed using mycoplasma PCR detection kit (LiliF, 102407-870) in all cell lines.
Reagents
z-VAD-fmk (ApexBio) was dissolved in DMSO and used at a final concentration of 20 μM. Staurosporine (Sigma Aldrich) was dissolved in DMSO and used at a final concentration of 1 μM. MCC950 (Sigma Aldrich) was dissolved in DMSO and used at a final concentration of 10 μM. Sytox Green (Life Technologies) was used at a final concentration of 500 nM. Hoechst 33342 was dissolved in DMSO and used at a final concentration of 0.33 μg/ml. Bleomycin sulfate (ApexBio) was dissolved in DMSO and used at a final concentration of 75 μg/ml. 5-Bromo-4-chloro-3-indolyl β-D-galactopyranoside (Sigma) was used at a final concentration of 1 mg/ml. Senescence-associated β-galactosidase staining solution was made as previously described (68). Puromycin (Invivogen) was used at a final concentration of 1 μg/ml.
Cell viability assay
Cells were plated at a density of 30,000 cells per well in triplicate in 12-well plates and allowed to acclimate for 24 h. Cells were treated with either CTRL or SASP CM for an additional 24 h prior to imaging. Cells were stained first in Hoechst 33342 in cell culture media for 15 min protected from light at a final concentration of 0.33 μg/ml. Hoechst stain was removed, and cells were washed in 1X PBS. Cells were stained in culture media plus SYTOX Green at a final concentration of 500 nM for 15 min and protected from light. The SYTOX Green stain was removed, cells were washed in 1X PBS and then placed back in normal culture medium, while images were taken using Zeiss Axio Observer.A1 inverted fluorescent microscope using ZEN 2012 software. Images were taken in brightfield using the 10× objective. Images were merged and quantified using the cell counter plug in on FIJI (National Institutes of Health). Five images were taken per well across three wells per condition. Averages, standard deviation, and SEM were calculated. Data are represented as average percentage SYTOX out of the total number of cells. Representative data from at least three biological replicates are shown.
Western blot analysis
After treatment, cells were plated in a 6-well plate at a density of 200,000 cells, and whole cell lysates were collected at 24 h (69, 70, 71). Cells were washed one time with 2 ml PBS and lysed in RIPA buffer (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate) supplemented with 1 μg/ml aprotinin, 5 μg/ml leupeptin, 20 μg/ml phenylmethylsulfonyl fluoride, and HALT phosphatase inhibitor mixture (Thermo Scientific). Lysis buffer is added directly to the wells, and the cells are lysed on the plate. Cell lysates are sonicated with 1-s pulse on and 1-s pulse off for 8 s using Branson sonifier at 10% amplitude. After sonication, cell lysates were cleared by centrifuging at 13,000 rpm for 15 min. Supernatants were quantified by BCA assay (Pierce Biotechnology) and normalized to the same protein concentrations. 6× Laemmli sample buffer was added to the cell lysates, and they were boiled on a heat block at 95 °C for 10 min. After running samples on SDS-PAGE alongside Chameleon Duo Pre-stained protein ladder (LI-COR), gels were then transferred onto Immobilon-FL PVDF membrane (Millipore) and blocked for 45 min in 5% milk. Membranes were probed in 2% milk along with primary antibodies at 4 °C overnight. The following antibodies were used for Western blotting: β-actin (Sigma-Aldrich; no. A1978), caspase 8 (Proteintech; 13423-1-AP), caspase 1 (Proteintech; 22915-1-AP), GSDMD (CST;69469) laminB1(Proteintech; 66095-1), RAS (CST; 3965S), PARP/cPARP (CST; 9542S), Bcl-2; Proteintech (12789-1-AP), vinculin (Proteintech; 66305-1-Ig), HER2 (Cell Signaling Technology; 2242S), gasdermin D (Full length) (CST; 39754S), cleaved gasdermin D (CST; 36425S), p21/Cip1 (CST; 2947T), and phosphorylated ERK 1/2 (PTG; 28733-1-AP). Donkey anti-Mouse IgG (H + L) Alexa Fluor Plus 680 Highly Cross-Adsorbed Secondary Antibody and Donkey anti-Rabbit IgG (H + L) Alexa Fluor Plus 800 Highly Cross-Adsorbed Secondary Antibody were incubated for 1 h in 2% milk protected from light. Membranes were imaged using Odyssey CLX Infared Imaging system by LI-COR Biosciences and analyzed using Image Studio Version 5.2. Where indicated, densitometry was performed using Image Studio Version 5.2 software. Values were normalized to the control to calculate fold change.
Generation of stable cell lines using retrovirus
Generation of stable cell lines was made using the pBABE-Puro-based retroviral vectors encoding the overexpression of HRAS. Bcl-2 and ErbB2 were used to generate stable cell lines. HEK293T cells were transfected with target DNA (10ug) along with the packaging vector pCLAmpho (10ug) using the calcium phosphate transduction method. This method uses 2× HBSS (280 mM NaCl, 10 mM KCl, 1.5 mM Na2HPO4, 12 mM glucose, 50 mM Hepes, pH 7.05) and 2.5 M CaCl2 and sterile water. Supernatants were collected 48-h posttransfection, filtered through a 0.45 μm filter (EMD Millipore) and used for viral transduction. Stable populations of puromycin-resistant cells were obtained by selecting in 1ug/ml puromycin (Invivogen) for at least 48 h.
Generation of stable knockdown cell lines using lentiviral delivery of shRNA
MISSION shRNA constructs against CASP8 (NM 033356 clone TRCN0000376482 and TRCN0000377309) in the puromycin-resistant pLKO.4 vector along with an empty vector control were purchased from Sigma-Aldrich. MISSION shRNA constructs against CASP1 (NM_001223 clones TRCN0000003502 and TRCN00000010796) in the puromycin-resistant pLKO.4 vector along with an empty vector control were purchased from Sigma-Aldrich. MISSION shRNA) constructs against GSDMD (NM_024736.4 clones TRCN000000179394 and TRCN000000180013) in the puromycin-resistant pLKO.4 vector along with an empty vector control were purchased from Sigma-Aldrich. For packaging of lentivirus, HEK293T cells were transfected with 2-3ug target DNA along with the packaging vectors psPAX2 (200 ng) and pCMV-VSV-G (300 ng) using Lipofectamine 2000. Virus was collected 48-h posttransfection, filtered through a 0.45 μm filter (EMD Millipore), and used for infecting MCF-10A, HMEC, and KTB-34 cells in the presence of 10 μg/ml polybrene. Stable populations of cells were selected using 1 μg/ml puromycin for at least 48 h. Immunoblots were performed to confirm success of knockdowns.
RNA isolation and quantitative real-time PCR
Total RNA was isolated with Zymo Research Quick-RNA Mini Prep RNA kit. Cells were lysed directly on the plate. Total mRNA was extracted according to manufacturer’s protocol. Reverse transcription was performed using 1 μg of RNA using an iScript Reverse Transcription Supermix (Bio-Rad, 1708840) to generate cDNA. This cDNA was utilized for qRT-PCR using SYBR green supermix (Bio-Rad, 1725271) and specific primers on a 7500 fast real-time PCR system (Applied Biosystems, Life Technologies). The relative levels of gene transcripts were normalized to GAPDH which were determined by quantitative real-time PCR. The primer sequence for specific transcripts were as followed: Human GAPDH Forward: 5′-GCATGGCCTTCGGTGTCC, Human GAPDH Reverse: 5′-AATGCCAGCCCCAGCGTC- AAA, Human IL-6 Forward: 5′ACATCCTCGACGGCATCT-CA, Human IL-6 Reverse: 5′-TCACCAGGCAAGTCTCCT-CA, Human IL-8 Forward: 5′GCTCTGTGTGAAGGTGCA-GT, Human IL-8 Reverse: 5′-TGCACCCAGTTTTCCTTG-GG, Human CCL20 Forward: 5′-CTGCGGCGAATCAGAAGC-AGC. Human CCL20 Reverse: 5′-CCTTCATTGGCCAGCTGC-CGT. Amplification was carried out at 95 °C for 12 min, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C. Standard error of the mean is represented by the error bars, and p-values were calculated using a two-tailed t test.
CM generation
Cells were seeded at 90,000 cells for DMSO treatment and 200,000 cells for Bleomycin treatment, respectively, onto a 10 cm2 plate and allowed to grow 48 h prior to senescence induction using Bleomycin or control treatment with DMSO. Cells were treated for 48 h, media containing bleomycin was removed, and cells were washed with 1X PBS two times and placed in DMEM-F12 media to condition for a period of 7 days for senescence and 1 day for control plates. For the oncogene-induced senescence model, retrovirus was used to create pBABE-EV and pBABE-HRas(G12V) virus. Fibroblasts were infected for 24 h with supernatant and selected in 1 μg/ml puromycin for 48 h. EV CTRL cells were conditioned in DMEM-F12 media for 24 h, and HRAS cells were conditioned for 7 days as described above. Media was collected and filtered through 0.2 μm PVDF filter.
SA β-gal staining
Cells were fixed using 4% PFA diluted 1:1 with 1X PBS for 5 min. Cells were then washed three times using 1X PBS and stained using the chromogenic assay as previously described (68). Images were captured on an Axio Observer Inverted microscope using Zen 2.5 (blue edition) software for image processing. All images were taken at 10× in brightfield.
Quantification and statistical analysis
As indicated in the legends, quantitative data are represented as the mean ± standard deviation (STDEV). All graphs and statistical analysis were performed using GraphPad Prism (9.0.0). Statistical significance with two groups was calculated using a student two-tailed t test. Each graph represents data collected from a minimum of three biological replicates. Group comparisons were made using one or two-way analysis of variance (ANOVA) followed by Tukey test.
Data availability
Further information and requests for resources, reagents, or data will be fulfilled by the corresponding author Zachary T. Schafer (zschafe1@nd.edu).
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We thank Veronica Schafer and all current/past Schafer lab members for helpful comments and/or valuable discussion. We thank Harikrishna Nakshatri (Indiana University School of Medicine) for the KTB-37 cell line, Ana Flores-Mireles and Felipe Santiago-Tirado (University of Notre Dame) for experimental assistance, Athanasia Panopoulos (Cedars-Sinai) for the BJ cell line, Shaun W. Lee (University of Notre Dame) for the HuVEC cell line, and the Notre Dame Integrated Imaging Facility for assistance with microscopy.
Author contributions
L. M. H. and Z. T. S. conceptualization; L. M. H. and S. S. methodology; L. M. H. and S. S. validation; L. M. H., S. S., J. C., P. L., S. C., and I. M. M. investigation; L. M. H., J. C., P. L., S. C., and I. M. M. formal analysis; L. M. H. data curation; L. M. H. and Z. T. S. writing–original draft; L. M. H., S. S., J. C., P. L., S. C., I. M. M., and Z. T. S. writing–review & editing; L. M. H., S. S., and Z. T. S. visualization; Z. T. S. supervision; Z. T. S. project administration; Z. T. S. funding acquisition.
Funding and additional information
Z. T. S. is supported by the National Institutes of Health/National Cancer Institute (R01CA262439), the Coleman Foundation, the Malanga Family Excellence Fund for Cancer Research at Notre Dame, the College of Science at Notre Dame, the Department of Biological Sciences at Notre Dame, and funds from Mr Nick L. Petroni. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reviewed by members of the JBC Editorial Board. Edited by Donita C. Brady
References
- 1.Buess M., Nuyten D.S., Hastie T., Nielsen T., Pesich R., Brown P.O. Characterization of heterotypic interaction effects in vitro to deconvolute global gene expression profiles in cancer. Genome Biol. 2007;8:R191. doi: 10.1186/gb-2007-8-9-r191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song L., Yan Y., Marzano M., Li Y. Studying Heterotypic Cell⁻Cell interactions in the human brain using pluripotent stem cell models for neurodegeneration. Cells. 2019;8:299. doi: 10.3390/cells8040299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armingol E., Officer A., Harismendy O., Lewis N.E. Deciphering cell-cell interactions and communication from gene expression. Nat. Rev. Genet. 2021;22:71–88. doi: 10.1038/s41576-020-00292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parrinello S., Noon L.A., Harrisingh M.C., Wingfield Digby P., Rosenberg L.H., Cremona C.A., et al. NF1 loss disrupts Schwann cell-axonal interactions: a novel role for semaphorin 4F. Genes Dev. 2008;22:3335–3348. doi: 10.1101/gad.490608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szekanecz Z., Koch A.E. Successes and failures of chemokine-pathway targeting in rheumatoid arthritis. Nat. Rev. Rheumatol. 2016;12:5–13. doi: 10.1038/nrrheum.2015.157. [DOI] [PubMed] [Google Scholar]
- 6.Tirziu D., Giordano F.J., Simons M. Cell communications in the heart. Circulation. 2010;122:928–937. doi: 10.1161/CIRCULATIONAHA.108.847731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bejarano L., Jordāo M.J.C., Joyce J.A. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 2021;11:933–959. doi: 10.1158/2159-8290.CD-20-1808. [DOI] [PubMed] [Google Scholar]
- 8.Sahai E., Astsaturov I., Cukierman E., DeNardo D.G., Egeblad M., Evans R.M., et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer. 2020;20:174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Place A.E., Jin Huh S., Polyak K. The microenvironment in breast cancer progression: biology and implications for treatment. Breast Cancer Res. 2011;13:227. doi: 10.1186/bcr2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weigel K.J., Jakimenko A., Conti B.A., Chapman S.E., Kaliney W.J., Leevy W.M., et al. CAF-secreted IGFBPs regulate breast cancer cell anoikis. Mol. Cancer Res. 2014;12:855–866. doi: 10.1158/1541-7786.MCR-14-0090. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y., Lazarus J., Steele N.G., Yan W., Lee H.J., Nwosu Z.C., et al. Regulatory T-cell depletion alters the tumor microenvironment and accelerates pancreatic carcinogenesis. Cancer Discov. 2020;10:422–439. doi: 10.1158/2159-8290.CD-19-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richards K.E., Zeleniak A.E., Fishel M.L., Wu J., Littlepage L.E., Hill R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 2017;36:1770–1778. doi: 10.1038/onc.2016.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costanzo-Garvey D.L., Keeley T., Case A.J., Watson G.F., Alsamraae M., Yu Y., et al. Neutrophils are mediators of metastatic prostate cancer progression in bone. Cancer Immunol. Immunother. 2020;69:1113–1130. doi: 10.1007/s00262-020-02527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allinen M., Beroukhim R., Cai L., Brennan C., Lahti-Domenici J., Huang H., et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Steele N.G., Carpenter E.S., Kemp S.B., Sirihorachai V.R., The S., Delrosario L., et al. Multimodal mapping of the tumor and peripheral blood immune landscape in human pancreatic cancer. Nat. Cancer. 2020;1:1097–1112. doi: 10.1038/s43018-020-00121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuilman T., Michaloglou C., Mooi W.J., Peeper D.S. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiley C.D., Campisi J. The metabolic roots of senescence: mechanisms and opportunities for intervention. Nat. Metab. 2021;3:1290–1301. doi: 10.1038/s42255-021-00483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campisi J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leon K.E., Buj R., Lesko E., Dahl E.S., Chen C.W., Tangudu N.K., et al. DOT1L modulates the senescence-associated secretory phenotype through epigenetic regulation of IL1A. J. Cell Biol. 2021;220 doi: 10.1083/jcb.202008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buj R., Leon K.E., Anguelov M.A., Aird K.M. Suppression of p16 alleviates the senescence-associated secretory phenotype. Aging (Albany NY) 2021;13:3290–3312. doi: 10.18632/aging.202640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faget D.V., Ren Q., Stewart S.A. Unmasking senescence: context-dependent effects of SASP in cancer. Nat. Rev. Cancer. 2019;19:439–453. doi: 10.1038/s41568-019-0156-2. [DOI] [PubMed] [Google Scholar]
- 22.Schmitt C.A., Wang B., Demaria M. Senescence and cancer - role and therapeutic opportunities. Nat. Rev. Clin. Oncol. 2022;19:619–636. doi: 10.1038/s41571-022-00668-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruhland M.K., Loza A.J., Capietto A.H., Luo X., Knolhoff B.L., Flanagan K.C., et al. Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nat. Commun. 2016;7 doi: 10.1038/ncomms11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pazolli E., Alspach E., Milczarek A., Prior J., Piwnica-Worms D., Stewart S.A. Chromatin remodeling underlies the senescence-associated secretory phenotype of tumor stromal fibroblasts that supports cancer progression. Cancer Res. 2012;72:2251–2261. doi: 10.1158/0008-5472.CAN-11-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alspach E., Flanagan K.C., Luo X., Ruhland M.K., Huang H., Pazolli E., et al. p38MAPK plays a crucial role in stromal-mediated tumorigenesis. Cancer Discov. 2014;4:716–729. doi: 10.1158/2159-8290.CD-13-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen H.A., Ho Y.J., Mezzadra R., Adrover J.M., Smolkin R., Zhu C., et al. Senescence rewires microenvironment sensing to facilitate antitumor immunity. Cancer Discov. 2023;13:432–453. doi: 10.1158/2159-8290.CD-22-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amor C., Feucht J., Leibold J., Ho Y.J., Zhu C., Alonso-Curbelo D., et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature. 2020;583:127–132. doi: 10.1038/s41586-020-2403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shafqat S., Arana Chicas E., Shafqat A., Hashmi S.K. The Achilles' heel of cancer survivors: fundamentals of accelerated cellular senescence. J. Clin. Invest. 2022;132 doi: 10.1172/JCI158452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirkland J.L., Tchkonia T. Senolytic drugs: from discovery to translation. J. Intern. Med. 2020;288:518–536. doi: 10.1111/joim.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reyes N.S., Krasilnikov M., Allen N.C., Lee J.Y., Hyams B., Zhou M., et al. Sentinel p16(INK4a+) cells in the basement membrane form a reparative niche in the lung. Science. 2022;378:192–201. doi: 10.1126/science.abf3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang C., Xu Q., Martin T.D., Li M.Z., Demaria M., Aron L., et al. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science. 2015;349 doi: 10.1126/science.aaa5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Itahana K., Zou Y., Itahana Y., Martinez J.L., Beausejour C., Jacobs J.J., et al. Control of the replicative life span of human fibroblasts by p16 and the polycomb protein Bmi-1. Mol. Cell. Biol. 2003;23:389–401. doi: 10.1128/MCB.23.1.389-401.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coppé J.P., Patil C.K., Rodier F., Sun Y., Muñoz D.P., Goldstein J., et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aird K.M., Worth A.J., Snyder N.W., Lee J.V., Sivanand S., Liu Q., et al. ATM couples replication stress and metabolic reprogramming during cellular senescence. Cell Rep. 2015;11:893–901. doi: 10.1016/j.celrep.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernandez-Segura A., de Jong T.V., Melov S., Guryev V., Campisi J., Demaria M. Unmasking transcriptional heterogeneity in senescent cells. Curr. Biol. 2017;27:2652–2660.e2654. doi: 10.1016/j.cub.2017.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pazolli E., Luo X., Brehm S., Carbery K., Chung J.J., Prior J.L., et al. Senescent stromal-derived osteopontin promotes preneoplastic cell growth. Cancer Res. 2009;69:1230–1239. doi: 10.1158/0008-5472.CAN-08-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimi T., Butin-Israeli V., Adam S.A., Hamanaka R.B., Goldman A.E., Lucas C.A., et al. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev. 2011;25:2579–2593. doi: 10.1101/gad.179515.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.González-Gualda E., Baker A.G., Fruk L., Muñoz-Espín D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J. 2021;288:56–80. doi: 10.1111/febs.15570. [DOI] [PubMed] [Google Scholar]
- 39.Coppé J.P., Desprez P.Y., Krtolica A., Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabai Y., Assouline B., Ben-Porath I. Senescent stromal cells: roles in the tumor microenvironment. Trends Cancer. 2023;9:28–41. doi: 10.1016/j.trecan.2022.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Krtolica A., Parrinello S., Lockett S., Desprez P.Y., Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl. Acad. Sci. U. S. A. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brouwers B., Fumagalli D., Brohee S., Hatse S., Govaere O., Floris G., et al. The footprint of the ageing stroma in older patients with breast cancer. Breast Cancer Res. 2017;19:78. doi: 10.1186/s13058-017-0871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carneiro B.A., El-Deiry W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020;17:395–417. doi: 10.1038/s41571-020-0341-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Letai A. Apoptosis and cancer. Annu. Rev. Cancer Biol. 2017;1:275–294. [Google Scholar]
- 45.Warren C.F.A., Wong-Brown M.W., Bowden N.A. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019;10:177. doi: 10.1038/s41419-019-1407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ashkenazi A. Targeting the extrinsic apoptotic pathway in cancer: lessons learned and future directions. J. Clin. Invest. 2015;125:487–489. doi: 10.1172/JCI80420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong R.S.Y. Apoptosis in cancer: from pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinon F., Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Jiménez Fernández D., Lamkanfi M. Inflammatory caspases: key regulators of inflammation and cell death. Biol. Chem. 2015;396:193–203. doi: 10.1515/hsz-2014-0253. [DOI] [PubMed] [Google Scholar]
- 50.Ross C., Chan A.H., Pein Von J.B., Maddugoda M.P., Boucher D., Schroder K. Inflammatory caspases: toward a unified model for caspase activation by inflammasomes. Annu. Rev. Immunol. 2022;40:249–269. doi: 10.1146/annurev-immunol-101220-030653. [DOI] [PubMed] [Google Scholar]
- 51.Yu P., Zhang X., Liu N., Tang L., Peng C., Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct. Target. Ther. 2021;6:128. doi: 10.1038/s41392-021-00507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 53.Acosta J.C., Banito A., Wuestefeld T., Georgilis A., Janich P., Morton J.P., et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013;15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Birch J., Gil J. Senescence and the SASP: many therapeutic avenues. Genes Dev. 2020;34:1565–1576. doi: 10.1101/gad.343129.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swanson K.V., Deng M., Ting J.P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiao L., Li X., Cao P., Fei W., Zhou H., Tang N., et al. Interleukin-6 mediated inflammasome activation promotes oral squamous cell carcinoma progression via JAK2/STAT3/Sox4/NLRP3 signaling pathway. J. Exp. Clin. Cancer Res. 2022;41:166. doi: 10.1186/s13046-022-02376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun Y., Wang X., Liu T., Zhu X., Pan X. The multifaceted role of the SASP in atherosclerosis: from mechanisms to therapeutic opportunities. Cell Biosci. 2022;12:74. doi: 10.1186/s13578-022-00815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bergsbaken T., Fink S.L., Cookson B.T. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brokatzky D., Mostowy S. Pyroptosis in host defence against bacterial infection. Dis. Model. Mech. 2022;15 doi: 10.1242/dmm.049414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Briard B., Malireddi R.K.S., Kanneganti T.D. Role of inflammasomes/pyroptosis and PANoptosis during fungal infection. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Byrne B.G., Dubuisson J.F., Joshi A.D., Persson J.J., Swanson M.S. Inflammasome components coordinate autophagy and pyroptosis as macrophage responses to infection. mBio. 2013;4:e00620–e00712. doi: 10.1128/mBio.00620-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou B., Sha S., Tao J., Li J., Shen C., Zhu J., et al. The expression pattern of pyroptosis-related genes predicts the prognosis and drug response of melanoma. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-24879-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vernon M., Wilski N.A., Kotas D., Cai W., Pomante D., Tiago M., et al. Raptinal Induces Gasdermin E-Dependent Pyroptosis in Naïve and Therapy-Resistant Melanoma. Mol. Cancer Res. 2022;20:1811–1821. doi: 10.1158/1541-7786.MCR-22-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Erkes D.A., Cai W., Sanchez I.M., Purwin T.J., Rogers C., Field C.O., et al. Mutant BRAF and MEK Inhibitors regulate the tumor immune microenvironment via pyroptosis. Cancer Discov. 2020;10:254–269. doi: 10.1158/2159-8290.CD-19-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu H., Fu Y., Tang Z., Jiang L., Qu C., Li H., et al. A novel pyroptosis-related signature predicts prognosis and response to treatment in breast carcinoma. Aging (Albany NY) 2022;14:989–1013. doi: 10.18632/aging.203855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Z., Zhang H., Li D., Zhou X., Qin Q., Zhang Q. Caspase-3-mediated GSDME induced Pyroptosis in breast cancer cells through the ROS/JNK signalling pathway. J. Cell. Mol. Med. 2021;25:8159–8168. doi: 10.1111/jcmm.16574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar B., Prasad M., Bhat-Nakshatri P., Anjanappa M., Kalra M., Marino N., et al. Normal breast-derived epithelial cells with luminal and intrinsic subtype-enriched gene expression document interindividual differences in their differentiation cascade. Cancer Res. 2018;78:5107–5123. doi: 10.1158/0008-5472.CAN-18-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Debacq-Chainiaux F., Erusalimsky J.D., Campisi J., Toussaint O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat. Protoc. 2009;4:1798–1806. doi: 10.1038/nprot.2009.191. [DOI] [PubMed] [Google Scholar]
- 69.Hawk M.A., Gorsuch C.L., Fagan P., Lee C., Kim S.E., Hamann J.C., et al. RIPK1-mediated induction of mitophagy compromises the viability of extracellular-matrix-detached cells. Nat. Cell Biol. 2018;20:272–284. doi: 10.1038/s41556-018-0034-2. [DOI] [PubMed] [Google Scholar]
- 70.Mason J.A., Cockfield J.A., Pape D.J., Meissner H., Sokolowski M.T., White T.C., et al. SGK1 signaling promotes glucose metabolism and survival in extracellular matrix detached cells. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2021.108821. [DOI] [PubMed] [Google Scholar]
- 71.Tsegaye M.A., He J., McGeehan K., Murphy I.M., Nemera M., Schafer Z.T. Oncogenic signaling inhibits c-FLIP(L) expression and its non-apoptotic function during ECM-detachment. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-97715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Further information and requests for resources, reagents, or data will be fulfilled by the corresponding author Zachary T. Schafer (zschafe1@nd.edu).