Abstract
The neuroepithelial cell transforming gene 1 (Net1) is a guanine nucleotide exchange factor for the small GTPase RhoA that promotes cancer cell motility and metastasis. Two isoforms of Net1 exist, Net1 and Net1A, both of which are sequestered in the nucleus in quiescent cells to prevent aberrant RhoA activation. Many cell motility stimuli drive cytosolic relocalization of Net1A, but mechanisms controlling this event are not fully understood. Here, we demonstrate that epithelial growth factor stimulates protein kinase Src- and Abl1-dependent phosphorylation of Net1A to promote its cytosolic localization. We show that Abl1 efficiently phosphorylates Net1A on Y373, and that phenylalanine substitution of Y373 prevents Net1A cytosolic localization. Furthermore, we found that Abl1-driven cytosolic localization of Net1A does not require S52, which is a phosphorylation site of a different kinase, c-Jun N-terminal kinase, that inhibits nuclear import of Net1A. However, we did find that MKK7-stimulated cytosolic localization of Net1A does require Y373. We also demonstrate that aspartate substitution at Y373 is sufficient to promote Net1A cytosolic accumulation, and expression of Net1A Y373D potentiates epithelial growth factor–stimulated RhoA activation, downstream myosin light chain 2 phosphorylation, and F-actin accumulation. Moreover, we show that expression of Net1A Y373D in breast cancer cells also significantly increases cell motility and Matrigel invasion. Finally, we show that Net1A is required for Abl1-stimulated cell motility, which is rescued by expression of Net1A Y373D, but not Net1A Y373F. Taken together, this work demonstrates a novel mechanism controlling Net1A subcellular localization to regulate RhoA-dependent cell motility and invasion.
Keywords: Net1, Src, Abl1, RhoA, phosphorylation, motility
Rho GTPases regulate many basic cellular functions that impact tumor progression, including cell migration, extracellular matrix (ECM) invasion, and cell proliferation (1, 2). Within this gene family, Rac1, Cdc42, and RhoA have been studied the most intensely. Like Rac1 and Cdc42, RhoA makes essential contributions to cell motility and invasion that are controlled by tight spatiotemporal regulation. Although wildtype RhoA is often overexpressed in cancers, including breast cancers, its overexpression alone does not confer increased activity, as wildtype RhoA still requires activation by upstream guanine exchange factors (GEFs) (3, 4, 5). GEFs promote the release of GDP by Rho proteins, thereby allowing GTP binding (6). Thus, it is important to identify RhoA GEFs that drive cancer progression.
The neuroepithelial transforming gene 1 (Net1) is a RhoA/RhoB-specific GEF that is overexpressed in multiple cancers, including breast cancer (7, 8, 9, 10, 11, 12). This is consequential, as both overexpression of Net1 and expression of a Net1-dependent gene expression signature are associated with reduced survival in breast cancer patients (9, 13). Furthermore, coexpression of Net1 and β4 integrin proteins is correlated with reduced distant metastasis–free survival and worse overall survival in treatment-resistant estrogen receptor–positive breast cancer patients (14). Because Net1 overexpression is associated with more aggressive disease in breast cancer, understanding the mechanisms by which Net1 is regulated is important.
A critical function of Net1 is to control cancer cell motility. Our laboratory and others have shown that Net1 expression is required for cancer cell motility and invasion in vitro (7, 15, 16). Net1 is also important for metastasis in mouse models of disease, including breast cancer (13). Two isoforms of Net1 exist in most cells, called Net1 and Net1A, which are identical except in their N-terminal regulatory domains (17). Both Net1 isoforms demonstrate unusual subcellular localization for RhoGEFs, as they are sequestered in the nucleus to prevent aberrant RhoA activation at the plasma membrane (17, 18). This occurs because of the presence of multiple nuclear localization signal (NLS) sequences in each Net1 isoform. In this regard, Net1 contains four NLS sequences, two of which are located in its unique N terminus, whereas Net1A only contains two NLS sequences, which are present in a region common to both Net1 isoforms (19). Cells generally express both Net1 isoforms, with the shorter Net1A isoform being less abundant (15, 20). Importantly, we have shown that growth factors such as lysophosphatidic acid and epidermal growth factor (EGF) that stimulate breast cancer cell motility, as well as integrin engagement, cause relocalization of the Net1A isoform to the cytosol to drive RhoA-dependent signaling, actin cytoskeletal rearrangement, and cell motility. Under these conditions, the longer Net1 isoform largely remains in the nucleus and does not contribute to cell motility and invasion (15, 19, 20).
The mechanisms controlling the subcellular localization of Net1 isoforms are only partly understood. For example, we have shown that EGF stimulation promotes Net1A acetylation within one of its two NLS sequences (19). EGF also stimulates phosphorylation of Net1A by the c-Jun N-terminal kinase (JNK) family of mitogen-activated protein kinases (21, 22), and both these modifications slow the rate of Net1A reimport into the nucleus, thereby causing cytoplasmic accumulation (19, 21). We have also shown that Net1A nuclear export is chromosome region maintenance 1 dependent, and that proteasomal degradation of Net1A limits its cytosolic accumulation after integrin engagement (20, 21). However, it is unknown whether additional mechanisms exist to control the subcellular localization of Net1A.
When considering additional Net1A regulatory mechanisms, we focused on the Src and Abl families of cytosolic tyrosine kinases. This was because many cell motility stimuli activate Src and Abl, and these kinases, in turn, regulate the spatial and temporal localization and activity of RhoGEFs and RhoGAPs to ensure precise control of Rho family member activity (23, 24, 25, 26). Both Src and Abl1/2 function downstream of integrins and growth factor receptors and are required for efficient cell motility and invasion (24, 25, 27, 28). Because they often function in the same signaling pathways, Src and Abl1/2 interact with many of the same molecules, including paxillin and focal adhesion kinase (FAK). Hyperactivation of either Src or Abl1 can result in oncogenic transformation and can increase cancer cell motility and invasion in breast cancer cells (26, 28, 29). Moreover, patients with elevated Src kinase activity have reduced distant metastasis–free survival (28).
In this work, we examined whether Src also controlled the subcellular localization and function of Net1 isoforms. We found that Src promotes Abl-dependent phosphorylation of Net1A on Y373 to control Net1A cytosolic accumulation and signaling to RhoA. In addition, substitution of this site with aspartate causes Net1A to accumulate in the cytosol without ligand stimulation and drives breast cancer cell motility and invasion. We also found that Net1A activity is necessary for Abl1-stimulated cell motility. These data indicate that Net1A function is precisely regulated by Src and Abl1 and suggest that targeting of Abl kinases in Net1-overexpressing cancers might have therapeutic value.
Results
Constitutively active Src stimulates cytosolic localization of the Net1A isoform
Src is well known to control RhoA activation following receptor tyrosine kinase activation and integrin engagement (27, 30). Because Net1A cytoplasmic accumulation is stimulated by both EGF receptor (EGFR) and integrin activation, we tested whether expression of constitutively active Src could stimulate relocalization of Net1A to the cytoplasm. MCF7 cells were transfected with hemagglutinin (HA)-epitope tagged wildtype Net1 or wildtype Net1A, plus or minus constitutively active Src (Src∗), and then serum starved overnight. Epitope-tagged Net1 isoforms were transfected because antibodies capable of distinguishing between Net1 isoforms do not exist (21). We observed that coexpression of active Src was ineffective at driving cytoplasmic localization of the longer Net1 isoform (Fig. 1A) but significantly elevated the cytoplasmic localization of the Net1A isoform (Fig. 1B). To quantify this effect, we calculated the ratio of cytosolic to nuclear staining. Cells were costained with phalloidin to define cell boundaries and 4′,6-diamidino-2-phenylindole (DAPI) to define nuclei, and identical camera settings were used for all images to enable quantification of fluorescent signals. We have shown previously that assessing Net1 isoform localization in this manner accounts for the inherent variation in transgene expression in transiently transfected cells and is linear over a wide range of expression (19, 21). As shown in Figure 1C, although there was significant variability between cells, expression of constitutively active Src caused a fourfold increase in the median ratio of cytoplasmic to nuclear staining for Net1A. On the other hand, active Src did not significantly change the localization of the Net1 isoform. These observations are consistent with previous observations indicating that EGFR and integrin activation stimulates cytosolic localization of Net1A but not Net1 (20, 21).
Figure 1.
Constitutively active Src stimulates cytosolic localization of the Net1A isoform. MCF7 cells were transfected with expression plasmids for FLAG-Myc-Net1 or FLAG-Myc-Net1A with or without constitutively active Src (Src∗). Cells were serum-starved overnight, fixed, and stained for Myc-epitope (green), Src (magenta), and DNA (blue). Images for each Net1 isoform were acquired with identical camera settings. Representative images of Net1 (A) or Net1A (B) are shown. Scale bars represent 10 μm. C, quantification of Net1 and Net1A localization. Data were obtained from three independent experiments. In each experiment, at least 20 cells per condition were quantified. Lines indicate the median values. Significance determined by one-way ANOVA with Tukey’s post hoc test. ∗∗∗∗p < 0.0001. Net1, neuroepithelial cell transforming gene 1; ns, not significant.
Phosphorylation of Net1A on Y373 is necessary for Src-stimulated cytosolic localization
To identify the mechanism by which Src controls Net1A subcellular localization, we assessed whether Src stimulates tyrosine phosphorylation of Net1A. Thus, constitutively active Src was coexpressed with either HA-Net1 or HA-Net1A in MCF7 cells. The cells were then serum-starved overnight, and Net1 isoforms were immunoprecipitated using an anti-HA antibody. Immunoprecipitates were resolved by SDS-PAGE and tested for tyrosine phosphorylation by Western blotting with a phospho-tyrosine-specific antibody. Surprisingly, we observed that both Net1 and Net1A were tyrosine phosphorylated in response to active Src expression (Fig. 2A). Although both Net1 isoforms appeared equally well tyrosine phosphorylated, we decided to identify the Src-stimulated tyrosine phosphorylation sites within Net1A in order to assess whether they were important for Net1A cytosolic localization. HeLa cells were transfected with active Src and HA-Net1A, serum-starved, and the HA-Net1A was immunoprecipitated and resolved by SDS-PAGE. The HA-Net1A band was visualized by Coomassie staining, excised, and subjected to phospho-peptide analysis using LC–MS/MS (Taplin Mass Spectrometry Facility). This analysis showed that expression of active Src stimulated the phosphorylation of four residues within Net1A, namely Y126, Y310, Y370, and Y373 (Table 1; Fig. 2B). To determine whether these sites were necessary for Net1A relocalization, we used site-directed mutagenesis to generate a 5YF mutant, where each tyrosine phosphorylated residue, plus Y311, was mutated to phenylalanine. Y311 was included in this analysis to preclude its phosphorylation when Y310 was mutated. Constitutively active Src was then coexpressed with wildtype Net1A or Net1A-5YF in MCF7 cells, and the cells were serum-starved. We observed that active Src was unable to stimulate cytosolic localization of Net1A-5YF, suggesting that one or more of the tyrosine phosphorylation sites played an important role in mediating Src-stimulated Net1A relocalization (Fig. 2, C–E). To determine which phosphorylation sites were most important for Net1A relocalization, we created Net1A constructs that contained single or double mutations of the identified phosphorylation sites and coexpressed these mutants with constitutively active Src. We observed that mutation of Y126 was without effect. Mutation of Y310/Y311 decreased Src-stimulated cytosolic localization, but this was not statistically significant. On the other hand, mutation of Y370/Y373 together or Y373 alone essentially nullified the effect of active Src on Net1A localization (Fig. 2F). These data indicate that phosphorylation of Net1A on Y373 is necessary for Src-stimulated cytosolic relocalization.
Figure 2.
Phosphorylation of Net1A on Y373 is necessary for Src-stimulated cytosolic localization.A, MCF7 cells were transfected with HA-Net1 or HA-Net1A with or without constitutively active Src (Src∗). Cells were serum-starved, and Net1 isoforms were immunoprecipitated using an anti-HA antibody. The presence of phospho-tyrosine and HA was detected by Western blotting. Shown is a representative experiment from three independent experiments. B, schematic of Net1A showing Src-stimulated phosphorylation sites (arrows) and locations of nuclear localization signal sequence (NLS), Dbl homology, and Pleckstrin homology domains (DH, PH, respectively), and PDZ-binding domain. Numbers refer to the amino acid residues of wildtype mouse Net1A. C and D, MCF7 cells transfected with expression plasmids for wildtype Net1A (WT) or Net1A 5YF (5YF) with or without constitutively active Src. Cells were serum starved, fixed, and stained for Myc-epitope (green), Src (magenta), and DNA (blue). Representative images of wildtype Net1A (C) or Net1A 5YF (D) are shown. Scale bars represent 10 μm. E, quantification of wildtype Net1A or Net1A 5YF localization. Data were obtained from four independent experiments. At least 20 cells per condition per experiment were quantified. Lines indicate median values. F, quantification of the localization of wildtype Net1A and the Net1A mutants shown, in the presence of active Src. G, MCF7 cells were transfected with FLAG-Myc-Net1A or FLAG-Myc-Net1A Y373F plus or minus active Src. Cells were serum-starved, and Net1A proteins were immunoprecipitated using an anti-FLAG antibody. pY373, FLAG-epitope, and Src were detected by Western blotting. H, HA-Net1A was immunoprecipitated from active Src-transfected MCF7 cells and incubated without or with λ-phosphatase. Immunoprecipitates and lysates were then resolved by SDS-PAGE and tested for pY373, HA-Net1A, and Src by Western blotting. Shown is a representative experiment from three independent experiments. I, phosphorylation of endogenous Net1 on Y373 in active Src–transfected cells. Cells were preincubated with DMSO or the Src inhibitor bosutinib (10 μM) and lysed in RIPA buffer. Phosphorylation of Y373 was assessed by Western blotting of the RIPA insoluble pellets. Shown is a representative experiment from three independent experiments. J, quantification of pY373 phosphorylation of endogenous Net1. Values were normalized to total Net1 expression. Significance in (E) and (F) was assessed with one-way ANOVA with Tukey’s post hoc test. Significance in (J) was assessed by unpaired Student’s t test. ∗∗∗∗p < 0.0001. DMSO, dimethyl sulfoxide; HA, hemagglutinin; Net1, neuroepithelial cell transforming gene 1; ns, not significant; RIPA, radioimmunoprecipitation assay.
Table 1.
Identification of tyrosine phosphorylated Net1A peptides in active Src–transfected cells
| Peptide | Intensity | % | A-score | Localization | Most likely sites if not localized | Residue number |
|---|---|---|---|---|---|---|
| R.HLYQVYR.Q | 2.04e+07 | — | ||||
| R.HLpYQVYR.Q | 3.17e+05 | 1.6 | 60.66 | Yes | 370 | |
| R.HLYQVpYR.Q | 1.42e+05 | 0.7 | 64.14 | Yes | 373 | |
| R.HLpYQVpYR.Q | 5.84e+04 | 0.3 | — | Yes | 370/373 | |
| K.KGESECQYYINKLEYLDEK.Q | 3.91e+05 | — | ||||
| K.KGESECQpYYINKLEYLDEK.Q | 1.08e+05 | 27.6 | 8.94 | No | K.KGESECQpYpYINKLEYLDEK.Q | 310/311 |
| K.RQEAIYELSR.G | 5.75e+06 | — | ||||
| K.RQEAIpYELSR.G | 4.49e+05 | 7.8 | 53.2 | Yes | 126 |
Shown are the unphosphorylated and tyrosine phosphorylated (pY) Net1A peptides. Intensity refers to the mass spectrum peak area/height. Percent (%) refers to the amount of phosphorylation signal relative to total signal for that peptide. The Ambiguity score (A-score) reflects the likelihood of a particular residue being phosphorylated within a given peptide (64). Localization refers to whether the phosphorylation site was unambiguously identified. Residue number refers to the amino acid number in mouse Net1A.
To confirm that Src stimulated Net1A phosphorylation on Y373, we created an antibody specific for phosphorylated Y373. Using this antibody, we were able to demonstrate that Net1A was phosphorylated on Y373 in response to active Src coexpression. The antibody was specific for phosphotyrosine Y373 (pY373), as mutation of this site to phenylalanine eliminated detection of phosphorylated Net1A by Western blotting (Fig. 2G). In addition, treatment of Net1A immunoprecipitated from active Src–transfected cells with λ phosphatase substantially reduced the pY373 signal (Fig. 2H). Thus, these data indicate that the pY373 antibody is specific and confirm that Src can stimulate Net1A phosphorylation on this site. To determine whether Src stimulates the phosphorylation of endogenous Net1 proteins on Y373, MCF7 cells were transfected with constitutively active Src and tested for pY373 Net1 by Western blotting. We were unable to observe that endogenous Net1 phosphorylated on Y373 in 2% SDS-containing whole-cell lysates. As an alternative approach, we lysed active Src–transfected cells in radioimmunoprecipitation assay (RIPA) buffer and tested for endogenous Net1 Y373 phosphorylation in the soluble and insoluble fractions. Using this approach, we were able to detect endogenous Net1 phosphorylated on Y373 in the RIPA insoluble fraction (Fig. 2I) but not the RIPA soluble fraction (not shown). As the RIPA insoluble fraction contains pelleted membranes and polymerized cytoskeletal elements, we hypothesize that this approach concentrated the phosphorylated Net1 within a small volume that allowed us to detect it, as neither the total Net1 nor the Net1 pY373 antibodies were very sensitive. Notably, endogenous Net1 Y373 phosphorylation was sensitive to the Src inhibitor bosutinib, indicating that Src was driving this modification (Fig. 2, I and J). Treatment with bosutinib also reduced the amount of total Net1 in the RIPA insoluble fraction.
Abl inhibition blocks EGF-stimulated Net1A relocalization
EGFR stimulation activates Src, which can phosphorylate and activate three downstream nonreceptor tyrosine kinases in epithelial cells: FAK, Abelson nonreceptor kinase 1 (Abl1), and Abl2 (31, 32, 33, 34) (Fig. 3A). To determine which kinase mediates Net1A cytosolic localization downstream of EGF, we inhibited their activities using small-molecule inhibitors. MCF7 cells were transfected with HA-Net1A and serum-starved overnight. During this starvation period, the cells were also incubated with dimethyl sulfoxide (DMSO) (control) or small-molecule inhibitors of Src, FAK, or Abl1/2. The next day, the cells were stimulated with EGF for 15 min, which we have shown previously to be the length of time required to maximally stimulate Net1A cytosolic localization in MCF7 cells (19, 21). Interestingly, inhibitors of Src (bosutinib), FAK (PF-562271), and Abl1/2 (imatinib) were all effective at blocking EGF-stimulated Net1A cytosolic localization (Fig. 3B). These data suggest that Net1A relocalization was ultimately dependent on Abl1/2 activity, which function downstream of Src and FAK (31, 32, 33). In support of this, the region surrounding Y373 (LYQ-Y373-RQPIP) correlates most closely with a consensus sequence for Abl substrates (AEVIV-Y-AAPF) (35). Thus, we tested whether GNF5, which is an allosteric inhibitor of Abl1 and to a lesser extent Abl2 (36), was capable of blocking EGF-stimulated cytosolic localization of Net1A. We observed that GNF5 pretreatment significantly decreased EGF-stimulated Net1A cytosolic relocalization. Interestingly, GNF5 was less effective than imatinib at blocking Net1A relocalization, suggesting that both Abl isoforms may regulate Net1A (Fig. 3, C and D).
Figure 3.
Abl1 inhibition prevents cytosolic accumulation of Net1A.A, schematic of nonreceptor tyrosine kinase cascade activated by ligand stimulation of epidermal growth factor receptor (EGFR). Direct activation of Src by EGFR leads to focal adhesion kinase (FAK) activation. Increased FAK activity drives increased Src activity and, subsequently, activation of Abelson nonreceptor tyrosine kinases (Abl1/2). B, MCF7 cells were transfected with HA-Net1A, serum-starved overnight, stimulated with EGF (100 ng/ml) for 15 min, fixed, and stained for HA-Net1A localization. Cells were treated with DMSO (control), the Src inhibitor bosutinib (10 μM), the FAK inhibitor PF-582271 (10 μM), or the Abl inhibitor imatinib (10 μM) overnight. Data were obtained from three independent experiments with at least 20 cells quantified per condition per experiment. C, representative images of MCF7 cells transfected with HA-Net1A, serum-starved, and treated with or without EGF (100 ng/ml) for 15 min. Where noted, cells were incubated with GNF5 (10 μM) overnight, prior to EGF treatment. D, quantification of Net1A localization. Data were obtained from three independent experiments. At least 20 cells per condition per experiment were quantified. E and F, subcellular fractionation of MDA-MB-231 cells. Proteins were analyzed by Western blotting with the indicated antibodies. HSP90 and lamin A/C were monitored as controls for cytosolic and nuclear fractions, respectively. Endogenous Net1 expression in each fraction was quantified from X-ray films using ImageJ. Shown are representative Western blots (E) and quantification from three independent experiments (F). Lines indicate median values. Significance was assessed by one-way ANOVA with Tukey’s post hoc test. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001. Scale bars represent 10 μm. HA, hemagglutinin; Net1A, neuroepithelial cell transforming gene 1; ns, not significant.
To confirm that endogenous Net1A localization is also regulated by Abl1/2, we tested whether Abl1/2 inhibition altered the steady-state localization of Net1 proteins in the invasive breast cancer cell line MDA-MB-231. We have shown previously that MDA-MB-231 cells express a significant amount of endogenous Net1A, which is required for cell motility and invasion (13). We used subcellular fractionation followed by Western blotting for these experiments, as Net1 isoform–specific antibodies suitable for immunofluorescence do not exist. The presence of Net1 in each fraction was quantified by densitometry and normalized to the cytosolic and nuclear markers HSP90 and lamin A/C, respectively. We observed that overnight treatment with GNF5 significantly reduced Net1A localization in the cytosol (Fig. 3, E and F). There was also a trend toward reduced expression of Net1 isoforms in the nucleus, but this was not significant. These data indicate that cytosolic localization of endogenous Net1 proteins is also controlled by Abl kinases.
Abl1 phosphorylates Net1A on Y373 and stimulates Net1A cytosolic accumulation
To assess whether Abl1 was capable of directly phosphorylating Net1A on Y373, we performed in vitro kinase assays with purified, recombinant Src, Abl1, FAK, and Net1A. Net1A phosphorylation was assessed by Western blotting using the pY373 antibody that we created, and the activity of each kinase was confirmed by Western blotting for phosphotyrosine of the autophosphorylated kinases as well as cortactin, which is phosphorylated to some extent by each of these kinases (34, 37, 38, 39). Although all three kinases exhibited significant autophosphorylation and cortactin phosphorylation activities, only Abl1 was capable of phosphorylating Net1A on Y373 in vitro (Fig. 4, A and B).
Figure 4.
Abl1 directly phosphorylates Net1A on Y373 and stimulates Net1A cytosolic accumulation.A, representative Western blots from in vitro kinase assays to detect Net1A pY373 in the presence of recombinant, active focal adhesion kinase (FAK), Src, or Abl1. Shown is a representative experiment from five independent experiments. B, quantification of Net1A phosphorylation on pY373 after in vitro phosphorylation from three independent experiments. Bars are median values. C, MCF7 cells were transfected with expression plasmids for HA-Net1A, alone, or with wildtype FLAG-Abl1 or FLAG Abl1 I/E (SH2 domain mutant). Cells were serum-starved overnight. Where noted, cells were stimulated with EGF (100 ng/ml) for 15 min. All cells were fixed and stained for HA-Net1A (green), Abl1 (magenta), and DNA (blue). Scale bar represents 10 μm. Representative images from three independent experiments are shown. D, quantification of Net1A localization in the presence of wildtype Abl1 or Abl1 I/E. Data were obtained from at least three independent experiments. At least 20 cells per condition per experiment were quantified. E, representative Western blots of phosphotyrosine and FLAG-epitopes for the Abl1 proteins are shown. F, quantification of Net1A localization in the presence of wildtype or constitutively active Abl1 (PP/EE). Data were obtained from three independent experiments. At least 20 cells per condition per experiment were quantified. G, representative Western blots of phosphotyrosine and FLAG-epitopes for the Abl1 proteins are shown. H, MCF7 cells were transfected with HA-Net1A alone or with wildtype FLAG-Abl1, FLAG-Abl1 PP/EE, or FLAG-Abl1 I/E. Cells were serum-starved overnight, and HA-Net1A was immunoprecipitated using an anti-HA antibody. The presence of pY373, HA, or Abl1 was detected by Western blotting. Representative Western blots from three independent experiments are shown. For graphs, lines indicate median values. Significance in all experiments was assessed using one-way ANOVA with Tukey’s post hoc test. ∗p < 0.05; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001. EGF, epidermal growth factor; HA, hemagglutinin; Net1A, neuroepithelial cell transforming gene 1; ns, not significant.
To determine whether Abl1 overexpression could drive Net1A cytosolic accumulation in the absence of EGF stimulation, we coexpressed wildtype Abl1 with Net1A in MCF7 cells. We also tested whether the SH2 domain of Abl1 was important for Net1A relocalization by coexpressing Abl1 I164E. Abl1 often requires binding of its SH2 domain to substrates for efficient phosphorylation (35, 40). We observed that coexpression of wildtype Abl1 drove Net1A accumulation in the cytosol to a similar extent as EGF stimulation (Fig. 4, C and D). On the other hand, the SH2 domain–inactive Abl1 I164E was incapable of stimulating Net1A cytosolic localization (Fig. 4, C and D), consistent with the idea that Abl1 phosphorylates Net1A to drive its cytosolic localization. We confirmed by Western blotting that wildtype Abl1 and Abl1 I164E were expressed at similar levels, and that Abl1 I164E was impaired in its autophosphorylation activity (Fig. 4E). We then tested whether constitutively active Abl1 P242E/P249E (Abl1 PP/EE) was better at stimulating Net1A cytosolic localization than wildtype Abl1. In these experiments, we transfected substantially less wildtype Abl1 than used for panels C–E, as the cells would not tolerate high expression of Abl1 PP/EE. We found that constitutively active Abl1 PP/EE was better than wildtype Abl1 at stimulating Net1A cytosolic localization (Fig. 4F). Western blotting showed that wildtype Abl1 and Abl1 PP/EE were expressed at similar levels, but that Abl1 PP/EE exhibited a greatly elevated autophosphorylation activity (Fig. 4G).
We then tested whether Abl1 stimulated Net1A phosphorylation on Y373 in cells. MCF7 cells were cotransfected with HA-Net1A and equal amounts of either wildtype, constitutively active, or SH2 domain mutant Abl1. The cells were then serum-starved overnight, and the HA-Net1A was immunoprecipitated and tested for Y373 phosphorylation by Western blotting. These experiments showed that wildtype Abl1 stimulated a modest degree of Net1A pY373 phosphorylation, whereas constitutively active Abl1 PP/EE was much more efficient at doing so. The SH2 domain–inactive Abl1 I164E did not stimulate appreciable phosphorylation of Net1A on Y373 (Fig. 4H).
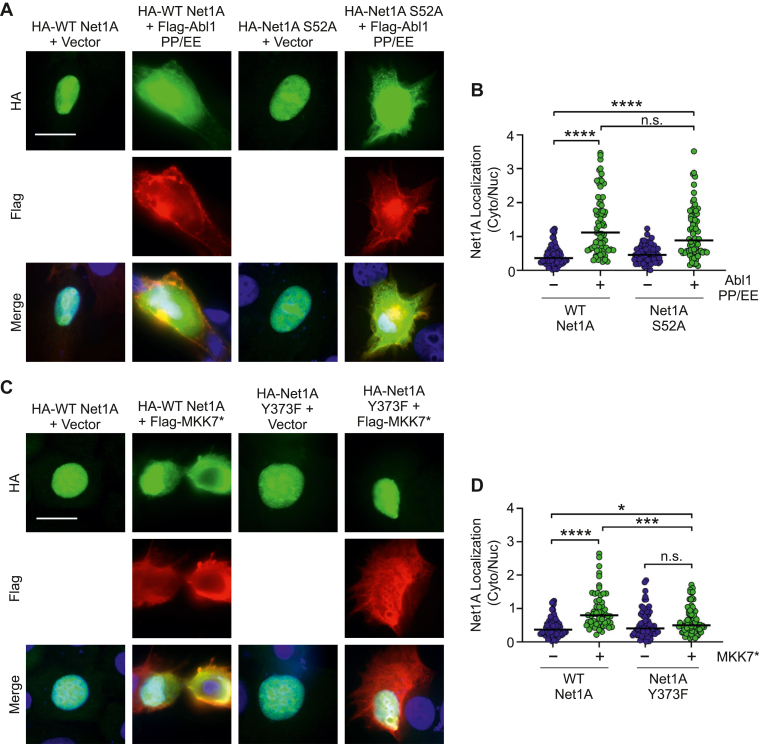
Net1A cytosolic localization stimulated by MKK7 requires Y373
We have shown previously that EGF stimulates JNK-dependent phosphorylation of Net1A on S52, which causes Net1A accumulation in the cytosol by slowing its rate of nuclear reimport (21). To begin to understand the relationship between JNK and Abl1 in controlling Net1A subcellular localization, we assessed the requirement for S52 and Y373 in Abl1- and MKK7-stimulated cytosolic localization of Net1A. MCF7 cells were transfected with wildtype or S52A Net1A, minus or plus constitutively active Abl1 PP/EE. After serum starvation, the cells were fixed and stained for HA-Net1A and FLAG-tagged Abl1. We observed that constitutively active Abl1 stimulated the same degree of cytosolic localization of both wildtype and Net1A S52A (Fig. 5, A and B). Thus, Abl1 does not require S52 to promote Net1A cytosolic localization.
Figure 5.
MKK7 requires Y373 to stimulate Net1A cytosolic localization.A, MCF7 cells were transfected with wildtype HA-Net1A or HA-Net1A S52A, minus or plus FLAG-Abl1 PP/EE. The cells were serum-starved overnight and fixed and stained for HA-Net1A (green), FLAG-Abl1 (red), and DNA (blue). Shown are representative micrographs from three independent experiments. Scale bar represents 20 μm. B, quantification of HA-Net1A localization. Data are from three independent experiments. Lines indicate median values. C, MCF7 cells were transfected with wildtype HA-Net1A or HA-Net1A Y373F minus or plus FLAG-tagged constitutively active MKK7 (MKK7∗). Cells were serum-starved overnight and fixed and stained for HA-Net1A (green), FLAG-MKK7 (red), and DNA (blue). Shown are representative micrographs from three independent experiments. Scale bar represents 20 μm. D, quantification of HA-Net1A localization. Data were obtained from three independent experiments. At least 20 cells per condition per experiment were quantified. Lines indicate median values. Significance was assessed using one-way ANOVA with Tukey’s post hoc test. ∗p < 0.05; ∗∗∗∗p < 0.0001. HA, hemagglutinin; Net1A, neuroepithelial cell transforming gene 1; ns, not significant.
We then tested whether JNK-stimulated Net1A cytosolic localization required Y373. To stimulate JNK signaling, we expressed constitutively active MKK7, which is an upstream activator of JNK (21). MCF7 cells were transfected with wildtype or Y373F Net1A, plus or minus constitutively active MKK7. After serum starvation, the cells were fixed and stained for HA-Net1A and FLAG-tagged MKK7. Contrary to what we observed with Abl1 and Net1A S52A, we found that MKK7 was unable to stimulate cytosolic localization of Net1A Y373F (Fig. 5, C and D). These data indicate that Y373 is essential for MKK7-stimulated Net1A cytosolic localization and suggest that JNK requires Abl signaling to promote Net1A cytosolic localization.
Substitution of an aspartate for tyrosine 373 results in constitutive cytosolic localization of Net1A and potentiates EGF-stimulated RhoA signaling
To determine whether Net1A phosphorylation on Y373 was sufficient for cytosolic localization and downstream signaling, we tested the effect of an aspartate substitution at Y373, which can mimic the negative charge of phosphorylation. When expressed in serum-starved MCF7 cells, Net1A Y373D exhibited cytosolic localization. Stimulation with EGF slightly increased cytosolic localization of this mutant, but this was not statistically significant (Fig. 6, A and B). These data suggest that phosphorylation of Y373 is sufficient for cytosolic localization of Net1A and suggest that the Y373D mutant may promote elevated signaling to RhoA.
Figure 6.
Substitution of aspartate for tyrosine 373 results in cytosolic localization of Net1A.A, MCF7 cells were transfected with expression vectors for wildtype FLAG-Myc-Net1A or FLAG-Myc-Net1A Y373D. Cells were serum-starved overnight. Where noted, cells were stimulated with EGF (100 ng/ml) for 15 min. All cells were fixed and stained for Myc-Net1A (green) and DNA (blue). Scale bar represents 10 μm. Shown are representative images from three independent experiments. B, quantification of Net1A localization. Data were obtained from three independent experiments. At least 20 cells per condition per experiment were quantified. C, RhoA activity assays. MCF7 cells were transfected with empty vector, wildtype FLAG-Myc-Net1A, FLAG-Myc-Net1A Y373D, or FLAG-Myc-Net1A Y373F and then starved in serum-free media overnight. Cell lysates were collected, incubated with GST-Rhotekin RBD beads for 30 min, washed and RhoA activation (RhoA), Net1A expression (Myc), and GST were evaluated by Western blotting. Representative Western blots from three independent experiments are shown. D, quantification of RhoA activation. RhoA bound to GST-RBD was detected by Western blotting and divided by the amount of RhoA in the cell lysates. Vector-transfected values were set to 1 to allow comparison between experiments. E, MCF7 cells were transfected with wildtype FLAG-Myc-Net1A, FLAG-Myc-Net1A Y373D, FLAG-Myc-Net1A Y373F, or Myc-tagged-NLS-β-galactosidase (β-GAL). Cells were then serum-starved overnight. Cells were fixed and stained for phosphorylated MLC2 (green), F-actin (red), DNA (blue), and the transfected proteins (magenta). Scale bar represents 10 μm. Representative images from three independent experiments are shown. F and G, quantification of pMLC2 (F) and F-actin (G). Data were obtained from three independent experiments. For each experiment, at least 20 cells per condition per experiment were quantified. Lines indicate median values. Significance was assessed using one-way ANOVA with Tukey’s post hoc test. ∗p < 0.05; ∗∗∗∗p < 0.0001. EGF, epidermal growth factor; GST, glutathione-S-transferase; Net1, neuroepithelial cell transforming gene 1; ns, not significant.
To determine whether Net1A Y373D stimulated RhoA activation, we measured endogenous RhoA activity in serum-starved cells transfected with Net1A constructs. RhoA activation was measured using glutathione-S-transferase (GST)-RBD pulldown assays, which measure the amount of GTP-bound RhoA present in cell lysates that binds to the recombinant RhoA-binding domain from Rhotekin. RhoA bound to GST-RBD and RhoA in cell lysates was detected by Western blotting. For quantification, values for GST-RBD-bound RhoA were divided by the amount of RhoA in the cell lysates, and vector transfected values were set to 1 to allow comparison between experiments. In these assays, we observed that cells expressing Net1A Y373D had significantly more active RhoA than cells expressing either wildtype Net1A or empty vector–transfected cells (Fig. 6, C and D). Surprisingly, Net1A Y373F transfection also stimulated a modest degree of RhoA activation relative to vector-transfected cells. These assays suggest that Net1A Y373D as well as Net1A Y373F promote RhoA activation. However, it should be noted that the amount of RhoA in the lysate was difficult to detect, making quantification somewhat variable.
To support these results, we measured effects on myosin light chain phosphorylation and filamentous actin accumulation in Net1A-transfected cells. When in the cytosol, Net1A promotes RhoA-dependent phosphorylation of myosin light chain 2 (pMLC2) and stabilization of F-actin filaments (15, 19, 20, 41, 42). We observed that Net1A Y373D expression stimulated MLC2 phosphorylation slightly better than wildtype Net1A but was not more effective than wildtype Net1A at promoting F-actin accumulation. Expression of the Net1A Y373F mutant also stimulated MLC2 phosphorylation but was relatively ineffective at promoting F-actin accumulation (Fig. 6, E–G). These data suggest that Net1A Y373D is not significantly more active than wildtype Net1A at promoting actin cytoskeletal reorganization, even though it exhibits enhanced cytosolic localization and seems to promote a low degree of RhoA activation.
To determine whether Net1A Y373D required an additional signal to promote actin cytoskeletal reorganization, we stimulated transfected cells with EGF for 15 min prior to fixation and staining. In these experiments, we found that Net1A Y373D was significantly more effective than wildtype Net1A or Net1A Y373F at stimulating MLC2 phosphorylation and F-actin accumulation (Fig. 7, A–C). We then measured the activation state of wildtype Net1A and Net1A Y373D in serum-starved and EGF-stimulated cells using GST-A17RhoA pull-down assays. A17RhoA is a nucleotide-free mutant of RhoA that binds tightly to activated RhoGEFs (21, 43). We found that the activation state of Net1A Y373D was equivalent to wildtype Net1A, regardless of whether cells were stimulated or not with EGF (Fig. 7, D and E). These data indicate that EGF does not stimulate Net1A Y373D activity directly but instead must modify its ability to promote actin cytoskeletal reorganization in another manner. Taken together, these data indicate that aspartate substitution of Y373 promotes Net1A cytosolic localization but does not represent at gain-of-function mutant toward the actin cytoskeleton in the absence of an added stimulus.
Figure 7.
Net1A Y373D potentiates EGF-stimulated signaling to the actin cytoskeleton.A, MCF7 cells were transfected with wildtype FLAG-Myc-Net1A, FLAG-Myc-Net1A Y373D, FLAG-Myc-Net1A Y373F, or Myc-tagged-NLS-β-galactosidase (β-GAL). Cells were then serum-starved overnight. Where noted, cells were stimulated with EGF (100 ng/ml) for 15 min. All cells were fixed and stained for phosphorylated MLC2 (green), F-actin (red), DNA (blue), and the transfected proteins (magenta). Scale bar represents 10 μm. Representative images from three independent experiments are shown. B and C, quantification of cytosolic pMLC2 (B) and cytosolic F-actin (C). Data were obtained from three independent experiments. For each experiment, at least 20 cells per condition per experiment were quantified. Lines indicate median values. Significance was assessed using one-way ANOVA with Tukey’s post hoc test. D, MCF7 cells were transfected with wildtype Net1A or Net1A Y373D and then serum-starved overnight. Where noted, cells were stimulated with EGF (100 ng/ml) for 15 min. Cells were lysed, incubated with GST-A17RhoA, and precipitated with glutathione–agarose. Proteins bound to GST-A17RhoA and in the lysates were assessed by Western blotting. Shown is a representative experiment from three independent experiments. E, quantification of GST-A17RhoA pulldowns. Data are from three independent experiments. Lines indicate median values. Significance in all experiments was assessed with one-way ANOVA with Tukey’s post hoc test. ∗p < 0.05; ∗∗∗p < 0.001. EGF, epidermal growth factor; GST, glutathione-S-transferase; Net1, neuroepithelial cell transforming gene 1; ns, not significant.
Cytosolic Net1A is required for Abl1-stimulated cell motility and invasion
Previous work from our laboratory has shown that cytosolic localization of Net1A is required to drive RhoA-mediated cell migration and invasion (19, 20, 21). Thus, we examined whether expression of Net1A Y373D promoted migration and invasion in MCF7 cells, which are a minimally invasive breast cancer cell line (44). MCF7 cells were transfected with a β-galactosidase control vector, wildtype Net1A, Net1A Y373D, or Net1A Y373F. To enrich for Net1A-transfected cells, we also cotransfected an empty vector containing a puromycin selection cassette. After 48 h, transiently transfected cells were selected by puromycin treatment overnight, which consistently resulted in an expression efficiency of 50% (not shown). After puromycin selection, the cells were allowed to recover in full-serum media for 2 h and then trypsinized and replated in a transwell apparatus without serum. The bottom well contained serum-free media supplemented with EGF. After 6 h, the transwell membranes were fixed and stained with DAPI to evaluate cell motility. We observed that overexpression of wildtype Net1A significantly elevated cell migration, and that expression of Net1A Y373D was even more effective. On the other hand, Net1A Y373F was completely unable to promote MCF7 cell motility (Fig. 8A). We also assessed the ability of Net1A to stimulate ECM invasion by replating cells in transwells coated with Matrigel. In these experiments, only Net1A Y373D was able to drive ECM invasion (Fig. 8B). In all experiments, we confirmed that Net1A proteins were expressed at similar levels by Western blotting (Fig. 8C). Taken together, these data suggest that phosphorylation of Net1A on Y373 is sufficient to stimulate cell motility and ECM invasion in otherwise weakly invasive breast cancer cells.
Figure 8.
Net1A is required for Abl1-stimulated cell motility and invasion.A and B, MCF7 cells were transfected with wildtype FLAG-Myc-Net1A, FLAG-Myc-Net1A Y373D, or FLAG-Myc-Net1A Y373F. All cells were also transfected with an empty vector containing a puromycin selection cassette. Cells were serum-starved and selected with 1 μg/ml puromycin overnight and then allowed to recover in full-serum media for 2 h. Cells were then placed in the upper chamber of a transwell apparatus and allowed to migrate toward serum-free media containing EGF (100 ng/ml) for 6 h (migration, A) or 20 h (invasion, B). Data were obtained from three independent experiments. C, Western blots showing expression of Myc-tagged Net1A proteins and GAPDH. Shown are representative blots. D, MCF7 cells were transfected with control siRNA or an siRNA targeting both Net1 isoforms. Twenty-four hours later, the cells were transfected with expression vectors for wildtype Abl1 plus Net1A Y373D or Net1A Y373F. After 48 h, the cells were serum-starved overnight, placed in the upper chamber of a transwell apparatus, and allowed to migrate toward EGF (100 ng/ml) in serum-free media for 6 h. Data are from three independent experiments. E, representative Western blots to show knockdown of endogenous Net1 and expression of Abl1, Net1A Y373D, and Net1A Y373F. In all graphs, lines indicate median values. Significance was assessed using one-way ANOVA with Tukey’s post hoc test. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001. EGF, epidermal growth factor; Net1, neuroepithelial cell transforming gene 1; ns, not significant.
We then examined whether Net1A is needed for Abl1-stimulated cell motility and if this requires Net1A phosphorylation on Y373. MCF7 cells were transfected with control or Net1-specific siRNAs. For this experiment, we chose to knockdown both Net1 isoforms, as we had done previously (15). One day later, the cells were transfected with expression vectors for wildtype Abl1, plus Net1A Y373D, or Net1A Y373F. The cells were then assessed for EGF-stimulated cell motility, as described previously. We observed that wildtype Abl1 overexpression stimulated MCF7 cell motility, and that this was abrogated by Net1 knockdown (Fig. 8D). Importantly, expression of Net1A Y373D completely rescued Abl1-stimulated cell motility, whereas Net1A Y373F was unable to do so (Fig. 8D). We confirmed Net1 knockdown and overexpression of Abl1 and Net1A mutants by Western blotting (Fig. 8E). These data show that endogenous Net1 expression is required for Abl1-driven cell migration and indicate that Net1A phosphorylation on Y373 is essential for rescue of Net1 function downstream of Abl1.
Discussion
In the present work, we have shown that active Src stimulates Net1A tyrosine phosphorylation and accumulation in the cytosol, and that phosphorylation of one of these sites, Y373, is primarily responsible for this effect. In addition, we found that Src-dependent Y373 phosphorylation occurs via Abl kinases, and that Abl1 phosphorylates this site in vitro and in cells. Importantly, the ability of EGF to stimulate Net1A cytosolic accumulation requires Abl-dependent phosphorylation of Y373, and substitution of Y373 with the acidic amino acid aspartate promotes Net1A cytosolic accumulation in the absence of EGF stimulation. Overexpression of Net1A Y373D potentiates EGF-stimulated RhoA signaling to the actin cytoskeleton and enhances MCF7 cell motility and invasive capacity. Moreover, we found that knockdown of Net1 isoforms inhibits Abl1-stimulated motility in MCF7 cells, and that this can be rescued by expression of Net1A Y373D but not Net1A Y373F. These data indicate that Src and downstream Abl kinases are important regulators of Net1A cytosolic localization and signaling to RhoA.
Our data reinforce the notion that Src is an important regulator of Rho GTPase signaling that either directly phosphorylates or stimulates the phosphorylation of many RhoGEFs, RhoGAPs, and Rho proteins themselves. For example, the Vav1 guanine nucleotide exchange factor is phosphorylated by Src family kinases on multiple sites within its regulatory acidic, zinc finger, and C-terminal SH3 domains, and this directly controls its activation and signaling potential (45, 46, 47). Similarly, the RhoGEFs FRG, ASEF, βPIX, ARHGEF5, Trio, and GEF-H1 are regulated by Src-dependent phosphorylation (48, 49, 50, 51, 52). Abl proteins, on the other hand, have far fewer substrates in the Rho pathway. Notable exceptions are BCR1 (Rac GAP), p190RhoGAP (RhoA GAP), and SOS2 (Rac GEF) (33). Instead, Abl proteins tend to regulate actin cytoskeletal organization more directly through phosphorylation of a myriad of linker proteins and actin regulatory proteins (24, 25). Thus, Net1A is somewhat unusual in being directly regulated by Abl proteins instead of Src. In this regard, we should note that active Src promoted the phosphorylation of multiple tyrosines within Net1A (Fig. 2B). Although we showed that Y373 phosphorylation was dependent on Abl function, it is an open question as to whether phosphorylation of the other tyrosines within Net1A is directly catalyzed by Abl kinases. In addition, although we showed that Abl1 directly phosphorylated Y373 in vitro, it is possible that Abl2 may also phosphorylate this site in cells.
Our data clearly show that phosphorylation of Y373 was necessary for EGF-stimulated cytosolic accumulation of Net1A, but they do not demonstrate how phosphorylation of this site controls Net1A relocalization. Our previous studies indicate that Net1A continuously cycles between the nucleus and cytoplasm, and that cytosolic accumulation of Net1A can result from mechanisms that prevent its cytosolic degradation or that slow its rate of nuclear import (19, 20, 21, 53, 54, 55). In this regard, it was interesting that MKK7-stimulated cytosolic accumulation of Net1A, which slows nuclear import of Net1A via phosphorylation of S52 (21), was dependent on Y373 phosphorylation (Fig. 5, C and D). This suggests that Y373 phosphorylation may either increase the rate of Net1A nuclear export or enhance its residence in the cytosol by preventing Net1A degradation or promoting interaction with a cytosolic anchor. In this regard, phosphorylated Y373 is predicted to create an SH2 domain–binding site. Thus, it is possible that one or more SH2 domain–containing proteins may function as an extranuclear anchor for Net1A. One of these proteins could be Abl1 itself, which contains an SH2 domain and also interacts with many cytosolic and actin regulatory proteins (24, 25). In support of this, we found that an Abl1 mutant with an inactive SH2 domain was unable to stimulate Net1A Y373 phosphorylation and cytosolic localization (Fig. 4).
The ability of Abl kinases to regulate Net1A Y373 phosphorylation may have significant implications for cancer progression. We have shown previously that Net1 is required for efficient tumorigenesis and metastasis in the polyoma virus middle T-antigen (PyMT) mouse genetic model of breast cancer (13). PyMT functions as a plasma membrane scaffold to initiate Src and PI3K signaling, both of which are necessary for mammary gland tumorigenesis (56, 57, 58). Hence, our current data may provide an important indication as to how Net1 contributes to cancer in this model. In addition, we have found that Net1 and β4 integrin coexpression correlate with poor distant metastasis–free survival in estrogen receptor–positive breast cancer patients (14). We have also found that breast cancer patients with a Net1-dependent gene expression signature exhibit worse overall survival (13). These data agree with the findings of others indicating that high Net1 expression correlates with worse outcome in breast cancer patients (9, 12). How Net1 contributes to breast cancer progression is an open question. Net1 undoubtedly promotes breast cancer cell migration and invasion (Fig. 8) (15, 21), and Net1 deletion significantly reduced RhoA activation within mammary gland tumors in the PyMT mouse model (13). However, Net1 may make important contributions in addition to controlling cell motility. Even with a strong cytoplasmic localization stimulus, a significant fraction of Net1A remained in the nucleus, and in fact, the Net1 isoform essentially never relocalized to the cytosol (Fig. 1). Thus, Net1 may have an important nuclear role in breast cancer. In this regard, others have shown that nuclear catalytically inactive Net1 controls Smad2-dependent transcription downstream of the transforming growth factor beta family member Nodal (59). Thus, it may be that transcriptional regulation is an important aspect of Net1 function that contributes to cancer progression.
A role for Net1A in Abl1-regulated events may also be relevant to other cancers. For example, BCR-Abl is the transforming oncogene in chronic myeloid leukemia, and Abl1 is important in many tumors, including lung, breast, and gastric tumors (25, 26). In support of this, Net1 has been shown to play significant roles in small cell lung cancer, chronic myeloid leukemia, acute myeloid leukemia, and gastric cancer (12, 60, 61, 62). Our data indicating that Net1 expression is necessary for Abl1-stimulated MCF7 cell motility support a role for Net1 in Abl1-regulated events. Future work will be required to confirm whether Abl1-driven cancer progression requires Net1 function.
Experimental procedures
Cell lines, plasmids, reagents, and pY373 Net1A antibody
MCF7, MDA-MB-231, and HeLa cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with high glucose and glutamine or DMEM nutrient mixture F12 (DMEM/F12), respectively (Hyclone). All media contained 10% fetal bovine serum (FBS) (Invitrogen), 100 μg/ml penicillin, and 100 μg/ml streptomycin (HyClone). MCF7 cells were grown in 10% CO2. MDA-MB-231 cells were grown in 5% CO2. MCF7 cells were transfected using Lipofectamine Plus or Lipofectamine 2000 reagents (Invitrogen) according to the manufacturer’s instructions. siRNA transfection was performed using INTERFERin reagent (PolyPlus Transfection). Control and Net1-specific siRNAs were as described (15). After plasmid or siRNA transfection, cells were allowed to grow for an additional 48 h or 72 h, respectively. As indicated, cells were serum-starved overnight in DMEM plus 0.5% FBS and 1% penicillin–streptomycin. Recombinant human EGF (R&D Systems) was used at 100 ng/ml for 15 min. Inhibitors of Abl1/2 (imatinib; R&D Systems); GNF5 (SelleckChem), Src (bosutinib; R&D Systems), and FAK (PF-582271; AdipoGen Life Sciences) were dissolved in DMSO. Cells were treated with inhibitors overnight at 10 μM, while in starvation media.
HA-epitope tagged mouse Net1 and HA-Net1A was as previously described (17). pcDNA3-FLAG-Myc-Net1A 5YF (Y126F/Y310F/Y311F/Y370F/Y373F), Y126F, Y310/311F, Y370/373F, Y373F, and Y373D were generated by site-directed mutagenesis using Phusion polymerase (Thermo Scientific). pcDNA3-Abl1-His-FLAG (catalog no.: 52684) was purchased from Addgene (63). Abl1 P242E/P249E, and I164E were generated by site-directed mutagenesis. Complementary DNA (cDNA) inserts for all point mutants were fully sequenced to confirm correct DNA sequence. Wildtype RhoA cDNA from pGEXKG-RhoA was PCR amplified and subcloned into the pCMV5M vector to generate Myc epitope–tagged pCMV5M-RhoA. FLAG-tagged constitutively active MKK7 was as described (21). A cDNA for constitutively active chicken c-Src was a kind gift of Jeffrey Rosen (Baylor College of Medicine). The active c-Src cDNA was subcloned into pCMV5.
To produce a polyclonal antibody to phosphorylated Y373 of Net1A, a mouse pY373 peptide (RHSYQV-pY-RQPIPC) was conjugated to keyhole limpet hemocyanin and used to immunize two New Zealand white rabbits, according to the service provider’s protocol (Pocono Rabbit Farm and Laboratory). Unpurified rabbit serum from one of the two rabbits was characterized for specificity toward pY373 Net1A and used for all Western blotting experiments.
Immunofluorescence microscopy
MCF7 cells were plated on acid-washed glass coverslips 24 h prior to transfection. For immunofluorescent detection of proteins, cells on coverslips were fixed with 4% paraformaldehyde in PBS for 5 min at room temperature (ThermoScientific), washed with PBS, and transferred to a humidified chamber. Cells were permeabilized with 0.2% Triton X-100 in PBS and blocked with 2.5% normal goat serum (NGS; Vector Labs) for 30 min at room temperature. Cells were incubated with the relevant primary antibodies diluted in 2.5% NGS in PBS + 0.2% Tween-20 (PBST) for 1 h at 37 °C. For detecting pMLC2, cells were incubated with primary antibody diluted in 2.5% NGS in PBS (no Tween-20), overnight at 4 °C. After washing three times in PBST, cells were incubated with secondary antibodies diluted in 2.5% NGS plus DAPI (1 μg/ml) (Sigma–Aldrich) and AlexaFluor 647-phalloidin (Invitrogen) for 30 min at 37 °C. Secondary antibodies were antimouse-immunoglobulin G (IgG) or anti-rabbit-IgG conjugated to AlexaFluor 488 and antimouse-IgG or anti-rabbit-IgG conjugated to AlexaFluor 594 (Invitrogen). F-actin was visualized with Acti-Stain 670 Phalloidin (PHDN1-A; Cytoskeleton, Inc). Cells were then washed three times with PBST, rinsed with water, and mounted on glass slides with Fluoromount reagent (Invitrogen). Fluorescent staining was visualized using a Zeiss Axiophot epifluorescence microscope, and image acquisition was performed using Axiovision software. Identical camera settings were used for each Net1 isoform when assessing each subcellular localization. To evaluate Net1A localization, cytosolic-to-nuclear ratios were calculated as previously described in detail (19). Antibodies used for immunofluorescence were as follows: mouse anti-HA tag (1:100 dilution, sc-7392; Santa Cruz Biotechnology), rabbit anti-HA (1:1000 dilution, C29F4; Cell Signaling), mouse anti-Myc epitope 9E10 (1:100 dilution; National Cell Culture Center), rabbit anti-Myc epitope (1:100 dilution, 71D10; Cell Signaling), rabbit anti-pMLC2 (1:100 dilution, 3671; Cell Signaling), and mouse anti-FLAG M2 (1:250 dilution, F3165; Sigma).
Immunoprecipitation and Western blotting
To assess Src-stimulated tyrosine phosphorylation of Net1 isoforms (Fig. 2A), Src and HA-Net1 or HA-Net1A-transfected cells were washed with PBS and harvested in Triton lysis buffer (0.5% Triton X-100, 20 mM Tris–HCl [pH 8.0], 100 mM NaCl, 1 mM EDTA, 50 mM NaF, 80 mM β-glycerophosphate, 1 mM Na2VO3, 10 μg/ml pepstatin A, 10 μg/ml leupeptin, 2 μg/ml aprotinin, and 1 mM PMSF). Cell lysates were incubated with 2 μg mouse anti-HA antibody and 40 μl of a Protein A-Sepharose (Rockland, Inc) slurry for 2 h at 4 °C. Sepharose beads were pelleted by centrifugation and washed with wash buffer (20 mM Tris [pH 8.0], 150 mM NaCl, and 0.5% Triton X-100). Beads were resuspended in equal volumes of water and 2× Laemmli sample buffer and boiled for 5 min in the presence of 1 mM DTT. Samples were resolved by SDS-PAGE, transferred to 0.45 μm pore polyvinylidene difluoride (PVDF) membranes, and blocked in 5% nonfat dry milk, or 1% bovine serum albumin) for phospho-tyrosine blots, prior to incubation with relevant antibodies overnight at 4 °C. Membranes were then washed three times with PBST, incubated with the relevant horseradish peroxidase–linked secondary antibodies for 30 min at room temperature, washed three times with PBST, and immunoreactive bands were detected by enhanced chemiluminescence using either X-ray film or an Azure C280 Chemiluminescent Western Blot Imager, according to the manufacturer’s instructions. The following antibodies were used: mouse anti-phospho-tyrosine (1:1000 dilution, 9411S; Cell Signaling) and rabbit anti-HA (1:1000 dilution, C29F4; Cell Signaling).
To determine if Net1A was phosphorylated on Y373 (Figs. 2G, 4I and 5A), transfected cells were washed with PBS and lysed in RIPA lysis buffer (50 mM Tris–HCl [pH 8.0], 150 mM NaCl, 1% Triton X-100, 0.5% deoxycholate [DOC], 0.1% SDS, 80 mM β-glycerophosphate, 1 mM Na2VO3, 10 μg/ml pepstatin A, 10 μg/ml leupeptin, 2 μg/ml aprotinin, 1 mM PMSF, 25 mM N-ethylmaleimide, and 10 μM MG132). Samples were passed 10 times through a 20-gauge needle and 10 times through a 27-gauge needle to shear DNA and clarified by centrifugation at 16,100g for 10 min at 4 °C. Immunoprecipitation of HA-tagged proteins and Western blotting were done as described previously. The following antibodies were used: rabbit anti-FLAG (F2555; Sigma, 1:1000 dilution), mouse anti-Src B12 (SC-8056; Santa Cruz Biotechnology, 1:1000 dilution), rabbit anti-HA (3724; Cell Signaling, 1:1000 dilution), rabbit anti-c-Abl1 (2862; Cell Signaling, 1:100 dilution), and rabbit anti-pY373 (described previously). To demonstrate hosphorspecificity of the anti-Y373 antibody, immunoprecipitated HA-Net1A was incubated in the presence or the absence of 2000 U of lambda phosphatase (Santa Cruz Biotechnology; sc-200312) for 1 h at 30 °C according to the manufacturer’s instructions.
For Western blotting of whole-cell lysates, samples were lysed in either SDS—urea lysis buffer (Tris–HCl [pH 6.8], 2% SDS, 20% glycerol, 6 M urea) (Figs. 4, E–H and 7C) or 2% SDS lysis buffer (2.0% SDS, 20 mM Tris–HCl [pH 8.0], 100 mM NaCl, 80 mM β-glycerophosphate, 1 mM Na2VO3, 20 mM NaF, 10 μg/ml pepstatin A, 10 μg/ml leupeptin, 2 μg/ml aprotinin, and 1 PMSF) for the detection of endogenous Net1 (Fig. 8E). Harvested cells were sonicated for 10 to 30 s and boiled for 5 min in Laemmli sample buffer plus 1 mM DTT. Western blotting was performed as described previously. The following antibodies were used: mouse anti-phosphotyrosine (1:1000 dilution, 9411S; Cell Signaling), mouse anti-FLAG M2 (1:100 dilution, F3165; Sigma), mouse anti-GAPDH (1:1000 dilution, sc-47724; Santa Cruz Biotechnology), and mouse anti-Myc epitope 9E10 (1:100 dilution; National Cell Culture Center). All Western blots were quantified using ImageJ software (NIH).
For Western blotting of endogenous Net1 pY373, cells were transfected with 100 ng empty vector or constitutively active Src and allowed to recover for 24 h and then starved overnight in DMEM containing 0.5% FBS in the presence or the absence of 10 μM bosutinib. Cells were harvested with cold PBS and lysed with RIPA buffer. The RIPA-insoluble fraction was extracted in SDS–urea buffer, and Western blotting was performed as described previously. Additional antibodies utilized were mouse anti-Net1 (sc-271941) and rabbit anti-Net1 (Abcam; catalog no.: 113202).
Identification of Src-stimulated tyrosine phosphorylation sites in Net1A
Ten, 10-cm plates of HeLa cells were cotransfected with HA-Net1A and constitutively active Src and allowed to grow for 48 h. Cells were then lysed in RIPA buffer (50 mM Tris–HCl [pH 7.5], 150 mM NaCl, 1% Triton X-100, 0.5% DOC, 0.1% SDS, 1 mM DTT, 1 mM EDTA, 50 mM NaF, 80 mM β-glycerophosphate, 1 mM sodium orthovanadate, 10 μg/ml aprotinin, 10 μg/ml pepstatin A, 2 μg/ml aprotinin, and 1 mM PMSF) and sonicated for 15 to 30 s to fragment DNA. The insoluble portion was pelleted by centrifugation at 16,100g for 10 min at 4 °C. The soluble fraction was precleared by incubating with 2 μg normal IgG + 40 μl of a 50% slurry of Protein A-Sepharose at 4 °C with gentle rocking for 30 min, followed by centrifugation to pellet beads. Once pelleted, the supernatant was transferred to a new tube and incubated with 2 μg of mouse anti-HA antibody (SC-7392; Santa Cruz Biotechnology) at 4 °C for 1 h with gentle rocking, followed by the addition of Protein A-Sepharose beads and further incubation for an additional hour. The beads were repelleted, the supernatant was aspirated, and the pellet was washed three times with wash buffer (20 mM Tris [pH 8], 250 mM NaCl, and 0.5% Triton X-100). The beads were then suspended in 40 μl of 2× Laemmli sample buffer with DTT, boiled for 5 min, and resolved on a 10% SDS-PAGE gel. The appropriate band was excised from a Coomassie-stained gel and sent for mass spectrometry analysis (LC–MS/MS) at the Taplin Mass Spectrometry Facility (Harvard Medical School).
Subcellular fractionation
MDA-MB-231 cells were plated in 10 cm dishes, allowed to grow to 85% confluence, and subsequently treated with either DMSO or GNF5 (10 μM) overnight. Cells were harvested and fractionated using the Subcellular Protein Fractionation Kit for Cultured Cells (Thermo Scientific) according to the manufacturer’s instructions. The protein concentration of each sample was quantified using the Pierce BCA Protein Assay kit (ThermoFisher), and equal amounts of protein for each fraction were resolved by SDS-PAGE, transferred to PVDF, blocked with 5% milk, and probed with mouse anti-Net1 (1:50 dilution, sc-271941; Santa Cruz Biotechnology), rabbit anti-HSP90 (1:500 dilution, SPA-830; Enzo Life Sciences), mouse anti-lamin A/C (1:1000 dilution; sc-376248, Santa Cruz Biotechnology). Blots were incubated with the appropriate horseradish peroxidase–conjugated antibodies and developed using enhanced chemiluminescence reagent and X-ray film. Intensities of the relevant protein bands were determined in scanned films using ImageJ.
In vitro kinase assays
GST-Net1A was expressed in BL21DE3 Escherichia coli and affinity purified using glutathione–agarose (Pierce), as previously described (17). pGEXKG-Net1A plasmid was transformed into BL21(DE3) E. coli (Stratagene), and protein expression was induced by 400 μM IPTG once the culture had reached an absorbance of 0.8 at 600 nm. The culture was further incubated with IPTG for 4 h at 37 °C. Cells were pelleted by centrifugation, lysed by addition of lysozyme, and then sonicated. Insoluble proteins were pelleted by centrifugation at 20,000g, and GST-Net1A in the soluble fraction was purified using glutathione–agarose affinity chromatography. Purified proteins were dialyzed in buffer A (20 mM Tris–HCl [pH 8.0], 100 mM NaCl, 1 mM DTT, 1 mM EDTA, and 10% glycerol) and stored in aliquots at −80 °C. In vitro kinase assays were performed with recombinant Abl1 (Fisher), Src (R&D Systems), or FAK catalytic domain (R&D Systems). For kinase reactions, GST-Net1A (2 μg), GST (0.5 μg), or purified cortactin (LSBio) (0.5 μg) was mixed with Src (0.8 μg), Abl1 (0.2 μg), or FAK (0.1 μg) in the presence of 2 μM ATP, 1 mM DTT, and kinase buffer (20 mM Hepes, pH 7.5, 100 mM NaCl, 0.05% NP-40, and 10 mM MgCl2). Reactions were incubated for 30 min at 30 °C and terminated by adding Laemmli sample buffer. Samples were resolved by SDS-PAGE, transferred to PVDF membrane, blocked with 5% bovine serum albumin, and probed with the rabbit anti-pY373 sera (1:500 dilution), mouse anti-phospho-tyrosine (1:1000 dilution, 9411S; Cell Signaling), and rabbit anti-Net1 antibody (1:500 dilution, HPA020068; Sigma).
GST-RBD and GST-A17RhoA pull-down assays
For GST-RBD assays, MCF7 cells were transfected with plasmids expressing FLAG-Myc-epitope–tagged wildtype Net1A, Net1A Y373D, or Net1A Y373F, or control vector pcDNA3.1. Twenty-four hours after transfection, cells were starved in DMEM containing 0.5% FBS overnight, then lysed in RBD lysis buffer (50 mM Tris [pH 7.5], 1% Triton X-100, 0.5% DOC, 0.1% SDS, 500 mM NaCl, 10 mM MgCl2, 5 μg/ml leupeptin, 5 μg/ml pepstatin A, and 50 μg/ml PMSF), and clarified by centrifugation at 16,100g at 4 °C. Equal amounts of lysate were incubated with 10 μl of Rhotekin-RBD Beads (Cytoskeleton) at 4 °C. After 1 h, the beads were pelleted by centrifugation, the supernatant was removed, and beads were washed three times each with 1 ml of ice-cold wash buffer (50 mM Tris [pH 7.5], 1% Triton X-100, 150 mM NaCl, 10 mM MgCl2, 5 μg/ml leupeptin, 5 μg/ml pepstatin A, and 50 μg/ml PMSF). Beads were resuspended in 45 μl of water and 15 μl 4× Laemmli sample buffer. Samples were resolved by SDS-PAGE, transferred to PVDF, blocked in 5% milk, and probed with mouse anti-RhoA (sc-418; Santa Cruz Biotechnology), mouse anti-Myc epitope 9E10 (1:100 dilution; National Cell Culture Center), and mouse anti-GST (1:1000 dilution, Cell Signaling Technologies).
GST-A17RhoA was expressed in BL21DE3 E. coli and purified using glutathione–agarose (Pierce), as described (19). For pull-down assays, 10 cm plates of MCF7 cells were transfected with empty vector or Myc epitope–tagged Net1A plasmids. Cells were washed with Hepes-buffered saline and harvested in Hepes lysis buffer (20 mM Hepes [pH 7.5], 150 mM NaCl, 5 mM MgCl2, 1% Triton X-100, 1 mM DTT, 1 mM PMSF, and 10 μg/ml each of aprotinin, leupeptin, and pepstatin A). After 10 min incubation on ice, cells were centrifuged for 10 min at 16,100g, 4 °C. Equal amounts of the supernatant were incubated for 1 h at 4 °C with 40 μg GST-A17RhoA immobilized on glutathione–agarose. Proteins bound to GST-A17RhoA were precipitated by centrifugation and washed three times with the Hepes lysis buffer. After washing, beads were reconstituted in water plus 5× Laemmli sample buffer and boiled for 5 min. Equal amounts of pulldown and input were resolved by SDS-PAGE, transferred to PVDF membranes, and processed for Western blotting using mouse anti-Myc epitope 9E10 (1:200 dilution; National Cell Culture Center) to detect FLAG-Myc-Net1A and a mouse anti-RhoA antibody (SC-418; Santa Cruz Biotechnology) to detect GST-A17RhoA.
Cell motility and invasion assays
MCF7 cells were transfected with empty pBabepuro vector and Myc-epitope–tagged β-galactosidase, FLAG-Myc-epitope–tagged wildtype Net1A, Net1A Y373F, or Net1A Y373D. Twenty-four hours later, the cells were treated with 10 μg/ml puromycin overnight. The following morning, the selection media were replaced with full-serum media for 2 h to allow for recovery. The cells were then trypsinized, and 250,000 cells were seeded in 500 μl DMEM without serum in the upper chamber of an 8 μm pore transwell. For invasion assays, the transwells were coated with Matrigel by the manufacturer (Corning Life Sciences). The bottom well contained DMEM plus EGF (100 ng/ml). Cells were allowed to migrate for 6 h for migration assays and 20 h for invasion assays. At the end of the incubation period, cells were removed from the upper surface of the membrane by scrubbing with a cotton-tipped swab, twice. Membranes were fixed with methanol for 5 min at room temperature, rinsed with distilled water, stained with 1 μg/ml DAPI in PBST for 20 min at room temperature in the dark, rinsed with water, and inverted to dry in the dark. The membranes were excised and mounted on a microscope slide with Fluoromount reagent. Cells were imaged at 20× magnification. Ten fields were imaged per membrane. The number of cells were counted in each field and averaged for quantification.
Statistical analysis
Comparisons among different treatment groups for Western blot and immunofluorescence experiments were examined using unpaired Student’s t tests or one-way ANOVA with Tukey’s post hoc test, as appropriate. p < 0.05 was considered statistically significant. GraphPad Prism software (GraphPad Software, Inc) was employed for statistical analyses. All experiments were repeated at least three times.
Data availability
All reported data are contained within the article.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We acknowledge helpful comments and support by members of the Frost laboratory and advice from the Cheng, Cunha, and Dessauer laboratories. We thank Jeffrey Rosen (Baylor College of Medicine) for the kind gift of the constitutively active chicken c-Src plasmid.
Author contributions
A. S., H. S. C., and A. U. investigation; A. S., H. S. C., and J. A. F. writing–review & editing; A. S. and H. S. C. visualization; J. A. F. project administration; J. A. F. funding acquisition.
Funding and additional information
This work was funded by grants CA232634 to A. S., and CA172129, NETRF 720469, and DOD BCRP W81XWH-20-1-0004 to J. A. F.
Reviewed by members of the JBC Editorial Board. Edited by Enrique De La Cruz
Footnotes
Present address for Arzu Ulu: Division of Biomedical Sciences, School of Medicine, University of California, Riverside, California, USA.
References
- 1.Raftopoulou M., Hall A. Cell migration: rho GTPases lead the way. Dev. Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Wheeler A.P., Ridley A.J. Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp. Cell Res. 2004;301:43–49. doi: 10.1016/j.yexcr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Fritz G., Just I., Kaina B. Rho GTPases are over-expressed in human tumors. Int. J. Cancer. 1999;81:682–687. doi: 10.1002/(sici)1097-0215(19990531)81:5<682::aid-ijc2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 4.Fritz G., Brachetti C., Bahlmann F., Schmidt M., Kaina B. Rho GTPases in human breast tumours: expression and mutation analyses and correlation with clinical parameters. Br. J. Cancer. 2002;87:635–644. doi: 10.1038/sj.bjc.6600510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orgaz J.L., Herraiz C., Sanz-Moreno V. Rho GTPases modulate malignant transformation of tumor cells. Small GTPases. 2014;5 doi: 10.4161/sgtp.29019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossman K.L., Der C.J., Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 7.Leyden J., Murray D., Moss A., Arumuguma M., Doyle E., McEntee G., et al. Net1 and Myeov: computationally identified mediators of gastric cancer. Br. J. Cancer. 2006;94:1204–1212. doi: 10.1038/sj.bjc.6603054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen S.Q., Li K., Zhu N., Nakao A. Expression and clinical significance of NET-1 and PCNA in hepatocellular carcinoma. Med. Oncol. 2008;25:341–345. doi: 10.1007/s12032-008-9042-6. [DOI] [PubMed] [Google Scholar]
- 9.Dutertre M., Gratadou L., Dardenne E., Germann S., Samaan S., Lidereau R., et al. Estrogen regulation and physiopathologic significance of alternative promoters in breast cancer. Cancer Res. 2010;70:3760–3770. doi: 10.1158/0008-5472.CAN-09-3988. [DOI] [PubMed] [Google Scholar]
- 10.Tu Y., Lu J., Fu J., Cao Y., Fu G., Kang R., et al. Over-expression of neuroepithelial-transforming protein 1 confers poor prognosis of patients with gliomas. Jpn. J. Clin. Oncol. 2010;40:388–394. doi: 10.1093/jjco/hyp186. [DOI] [PubMed] [Google Scholar]
- 11.Kawata H., Shimada N., Kamiakito T., Komatsu K., Morita T., Ota T., et al. RhoC and guanine nucleotide exchange factor Net1 in androgen-unresponsive mouse mammary carcinoma SC-4 cells and human prostate cancer after short-term endocrine therapy. Prostate. 2012;72:1071–1079. doi: 10.1002/pros.21511. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad H.M., Muiwo P., Ramachandran S.S., Pandey P., Gupta Y.K., Kumar L., et al. miR-22 regulates expression of oncogenic neuro-epithelial transforming gene 1, NET1. FEBS J. 2014;281:3904–3919. doi: 10.1111/febs.12926. [DOI] [PubMed] [Google Scholar]
- 13.Zuo Y., Ulu A., Chang J.T., Frost J.A. Contributions of the RhoA guanine nucleotide exchange factor Net1 to polyoma middle T antigen-mediated mammary gland tumorigenesis and metastasis. Breast Cancer Res. 2018;20:41. doi: 10.1186/s13058-018-0966-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilcrease M.Z., Kilpatrick S.K., Woodward W.A., Zhou X., Nicolas M.M., Corley L.J., et al. Coexpression of alpha6beta4 integrin and guanine nucleotide exchange factor Net1 identifies node-positive breast cancer patients at high risk for distant metastasis. Cancer Epidemiol. Biomarkers Prev. 2009;18:80–86. doi: 10.1158/1055-9965.EPI-08-0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carr H.S., Zuo Y., Oh W., Frost J.A. Regulation of focal adhesion kinase activation, breast cancer cell motility, and amoeboid invasion by the RhoA guanine nucleotide exchange factor Net1. Mol. Cell. Biol. 2013;33:2773–2786. doi: 10.1128/MCB.00175-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z.H., Ni Q.Z., Zhang X.P., Ma N., Feng J.K., Wang K., et al. NET1 promotes HCC growth and metastasis in vitro and in vivo via activating the Akt signaling pathway. Aging. 2021;13:10672–10687. doi: 10.18632/aging.202845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin H., Carr H.S., Wu X., Muallem D., Tran N.H., Frost J.A. Characterization of the biochemical and transforming properties of the neuroepithelial transforming protein 1. J. Biol. Chem. 2005;280:7603–7613. doi: 10.1074/jbc.M412141200. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt A., Hall A. The Rho exchange factor Net1 is regulated by nuclear sequestration. J. Biol. Chem. 2002;277:14581–14588. doi: 10.1074/jbc.M111108200. [DOI] [PubMed] [Google Scholar]
- 19.Song E.H., Oh W., Ulu A., Carr H.S., Zuo Y., Frost J.A. Acetylation of the RhoA GEF Net1A controls its subcellular localization and activity. J. Cell Sci. 2015;128:913–922. doi: 10.1242/jcs.158121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carr H.S., Morris C.A., Menon S., Song E.H., Frost J.A. Rac1 controls the subcellular localization of the Rho guanine nucleotide exchange factor Net1A to regulate focal adhesion formation and cell spreading. Mol. Cell. Biol. 2013;33:622–634. doi: 10.1128/MCB.00980-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ulu A., Oh W., Zuo Y., Frost J.A. Stress-activated MAPKs and CRM1 regulate the subcellular localization of Net1A to control cell motility and invasion. J. Cell Sci. 2018;131 doi: 10.1242/jcs.204644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ulu A., Frost J.A. Regulation of RhoA activation and cell motility by c-Jun N-terminal kinases and Net1. Small GTPases. 2020;11:385–391. doi: 10.1080/21541248.2018.1536638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timpson P., Jones G.E., Frame M.C., Brunton V.G. Coordination of cell polarization and migration by the Rho family GTPases requires Src tyrosine kinase activity. Curr. Biol. 2001;11:1836–1846. doi: 10.1016/s0960-9822(01)00583-8. [DOI] [PubMed] [Google Scholar]
- 24.Bradley W.D., Koleske A.J. Regulation of cell migration and morphogenesis by Abl-family kinases: emerging mechanisms and physiological contexts. J. Cell Sci. 2009;122:3441–3454. doi: 10.1242/jcs.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J.Y. The capable ABL: what is its biological function? Mol. Cell. Biol. 2014;34:1188–1197. doi: 10.1128/MCB.01454-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J., Pendergast A.M. The emerging role of ABL kinases in solid tumors. Trends Cancer. 2015;1:110–123. doi: 10.1016/j.trecan.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huveneers S., Danen E.H. Adhesion signaling - crosstalk between integrins, Src and Rho. J. Cell Sci. 2009;122:1059–1069. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- 28.Elsberger B. Translational evidence on the role of Src kinase and activated Src kinase in invasive breast cancer. Crit. Rev. Oncol. Hematol. 2014;89:343–351. doi: 10.1016/j.critrevonc.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Finn R.S. Targeting Src in breast cancer. Ann. Oncol. 2008;19:1379–1386. doi: 10.1093/annonc/mdn291. [DOI] [PubMed] [Google Scholar]
- 30.Playford M.P., Schaller M.D. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23:7928–7946. doi: 10.1038/sj.onc.1208080. [DOI] [PubMed] [Google Scholar]
- 31.Plattner R., Kadlec L., DeMali K.A., Kazlauskas A., Pendergast A.M. c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 1999;13:2400–2411. doi: 10.1101/gad.13.18.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitra S.K., Schlaepfer D.D. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Colicelli J. ABL tyrosine kinases: evolution of function, regulation, and specificity. Sci. Signal. 2010;3:re6. doi: 10.1126/scisignal.3139re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mader C.C., Oser M., Magalhaes M.A., Bravo-Cordero J.J., Condeelis J., Koleske A.J., et al. An EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion. Cancer Res. 2011;71:1730–1741. doi: 10.1158/0008-5472.CAN-10-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Songyang Z., Carraway K.L., 3rd, Eck M.J., Harrison S.C., Feldman R.A., Mohammadi M., et al. Catalytic specificity of protein-tyrosine kinases is critical for selective signalling. Nature. 1995;373:536–539. doi: 10.1038/373536a0. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J., Adrián F.J., Jahnke W., Cowan-Jacob S.W., Li A.G., Iacob R.E., et al. Targeting Bcr-Abl by combining allosteric with ATP-binding-site inhibitors. Nature. 2010;463:501–506. doi: 10.1038/nature08675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomar A., Lim S.T., Lim Y., Schlaepfer D.D. A FAK-p120RasGAP-p190RhoGAP complex regulates polarity in migrating cells. J. Cell Sci. 2009;122:1852–1862. doi: 10.1242/jcs.046870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boateng L.R., Huttenlocher A. Spatiotemporal regulation of Src and its substrates at invadosomes. Eur. J. Cell Biol. 2012;91:878–888. doi: 10.1016/j.ejcb.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cleary R.A., Wang R., Waqar O., Singer H.A., Tang D.D. Role of c-Abl tyrosine kinase in smooth muscle cell migration. Am. J. Physiol. Cell Physiol. 2014;306:C753–C761. doi: 10.1152/ajpcell.00327.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamontanara A.J., Georgeon S., Tria G., Svergun D.I., Hantschel O. The SH2 domain of Abl kinases regulates kinase autophosphorylation by controlling activation loop accessibility. Nat. Commun. 2014;5:5470. doi: 10.1038/ncomms6470. [DOI] [PubMed] [Google Scholar]
- 41.Amano M., Ito M., Kimura K., Fukata Y., Chihara K., Nakano T., et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J. Biol. Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 42.Kimura K., Ito M., Amano M., Chihara K., Fukata Y., Nakafuku M., et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Mata R., Wennerberg K., Arthur W.T., Noren N.K., Ellerbroek S.M., Burridge K. Analysis of activated GAPs and GEFs in cell lysates. Methods Enzymol. 2006;406:425–437. doi: 10.1016/S0076-6879(06)06031-9. [DOI] [PubMed] [Google Scholar]
- 44.Holliday D.L., Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011;13:215. doi: 10.1186/bcr2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bustelo X.R., Barbacid M. Tyrosine phosphorylation of the vav proto-oncogene product in activated B cells. Science. 1992;256:1196–1199. doi: 10.1126/science.256.5060.1196. [DOI] [PubMed] [Google Scholar]
- 46.Margolis B., Hu P., Katzav S., Li W., Oliver J.M., Ullrich A., et al. Tyrosine phosphorylation of vav proto-oncogene product containing SH2 domain and transcription factor motifs. Nature. 1992;356:71–74. doi: 10.1038/356071a0. [DOI] [PubMed] [Google Scholar]
- 47.Rodríguez-Fdez S., Bustelo X.R. The Vav GEF Family: an evolutionary and functional perspective. Cells. 2019;8:465. doi: 10.3390/cells8050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyamoto Y., Yamauchi J., Itoh H. Src kinase regulates the activation of a novel FGD-1-related Cdc42 guanine nucleotide exchange factor in the signaling pathway from the endothelin A receptor to JNK. J. Biol. Chem. 2003;278:29890–29900. doi: 10.1074/jbc.M301559200. [DOI] [PubMed] [Google Scholar]
- 49.Itoh R.E., Kiyokawa E., Aoki K., Nishioka T., Akiyama T., Matsuda M. Phosphorylation and activation of the Rac1 and Cdc42 GEF Asef in A431 cells stimulated by EGF. J. Cell Sci. 2008;121:2635–2642. doi: 10.1242/jcs.028647. [DOI] [PubMed] [Google Scholar]
- 50.Kuroiwa M., Oneyama C., Nada S., Okada M. The guanine nucleotide exchange factor Arhgef5 plays crucial roles in Src-induced podosome formation. J. Cell Sci. 2011;124:1726–1738. doi: 10.1242/jcs.080291. [DOI] [PubMed] [Google Scholar]
- 51.DeGeer J., Boudeau J., Schmidt S., Bedford F., Lamarche-Vane N., Debant A. Tyrosine phosphorylation of the Rho guanine nucleotide exchange factor Trio regulates netrin-1/DCC-mediated cortical axon outgrowth. Mol. Cell. Biol. 2013;33:739–751. doi: 10.1128/MCB.01264-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azoitei M.L., Noh J., Marston D.J., Roudot P., Marshall C.B., Daugird T.A., et al. Spatiotemporal dynamics of GEF-H1 activation controlled by microtubule- and Src-mediated pathways. J. Cell Biol. 2019;218:3077–3097. doi: 10.1083/jcb.201812073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carr H.S., Cai C., Keinanen K., Frost J.A. Interaction of the RhoA exchange factor Net1 with discs large homolog 1 protects it from proteasome-mediated degradation and potentiates Net1 activity. J. Biol. Chem. 2009;284:24269–24280. doi: 10.1074/jbc.M109.029439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Papadimitriou E., Vasilaki E., Vorvis C., Iliopoulos D., Moustakas A., Kardassis D., et al. Differential regulation of the two RhoA-specific GEF isoforms Net1/Net1A by TGF-beta and miR-24: role in epithelial-to-mesenchymal transition. Oncogene. 2012;31:2862–2875. doi: 10.1038/onc.2011.457. [DOI] [PubMed] [Google Scholar]
- 55.Ye K., Chang S., Li J., Li X., Zhou Y., Wang Z. A functional and protein-protein interaction analysis of neuroepithelial cell transforming gene 1 in hepatocellular carcinoma. Tumour Biol. 2014;35:11219–11227. doi: 10.1007/s13277-014-2454-3. [DOI] [PubMed] [Google Scholar]
- 56.Guy C.T., Cardiff R.D., Muller W.J. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol. Cell. Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guy C.T., Muthuswamy S.K., Cardiff R.D., Soriano P., Muller W.J. Activation of the c-Src tyrosine kinase is required for the induction of mammary tumors in transgenic mice. Genes Dev. 1994;8:23–32. doi: 10.1101/gad.8.1.23. [DOI] [PubMed] [Google Scholar]
- 58.Hutchinson J., Jin J., Cardiff R.D., Woodgett J.R., Muller W.J. Activation of Akt (protein kinase B) in mammary epithelium provides a critical cell survival signal required for tumor progression. Mol. Cell. Biol. 2001;21:2203–2212. doi: 10.1128/MCB.21.6.2203-2212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei S., Ning G., Li L., Yan Y., Yang S., Cao Y., et al. A GEF activity-independent function for nuclear Net1 in nodal signal transduction and mesendoderm formation. J. Cell Sci. 2017;130:3072–3082. doi: 10.1242/jcs.204917. [DOI] [PubMed] [Google Scholar]
- 60.Murray D., Horgan G., Macmathuna P., Doran P. NET1-mediated RhoA activation facilitates lysophosphatidic acid-induced cell migration and invasion in gastric cancer. Br. J. Cancer. 2008;99:1322–1329. doi: 10.1038/sj.bjc.6604688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun H., Zhang Z., Luo W., Liu J., Lou Y., Xia S. NET1 Enhances Proliferation and chemoresistance in acute lymphoblastic leukemia Cells. Oncol. Res. 2019;27:935–944. doi: 10.3727/096504019X15555388198071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zong W., Feng W., Jiang Y., Cao Y., Ke Y., Shi X., et al. LncRNA CTC-497E21.4 promotes the progression of gastric cancer via modulating miR-22/NET1 axis through RhoA signaling pathway. Gastric Cancer. 2020;23:228–240. doi: 10.1007/s10120-019-00998-w. [DOI] [PubMed] [Google Scholar]
- 63.Deng Y., Couch B.A., Koleske A.J., Turk B.E. A peptide photoaffinity probe specific for the active conformation of the Abl tyrosine kinase. Chembiochem. 2012;13:2510–2512. doi: 10.1002/cbic.201200560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beausoleil S.A., Villen J., Gerber S.A., Rush J., Gygi S.P. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat. Biotechnol. 2006;24:1285–1292. doi: 10.1038/nbt1240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All reported data are contained within the article.