Abstract
Siewert–Kartagener’s syndrome, a type of primary ciliary dyskinesia, is a complex disease comprising situs inversus, rhinosinusitis, and bronchiectasis. Situs inversus totalis is a condition in which all organs in the thoracic and abdominal cavities are reversed. Furthermore, primary ciliary dyskinesia, an autosomal genetic disease, may coexist with situs inversus totalis. Reports on Siewert–Kartagener’s syndrome in veterinary medicine are limited. We report a rare case of primary ciliary dyskinesia with Siewert-Kartagener’s syndrome in a dog, concurrently infected with canine distemper virus and type-2 adenovirus. This case highlights that situs inversus totalis can cause primary ciliary dyskinesia, and concurrent infections are possible.
Keywords: Adenovirus E3 proteins; ciliary motility disorders; distemper virus, canine; kartagener syndrome; situs inversus
INTRODUCTION
Siewert–Kartagener’s syndrome is a triad of diseases comprising situs inversus, rhinosinusitis, and bronchiectasis [1]. Situs inversus is a rare congenital disorder in humans and veterinary medicine in which the visceral organs are transposed to the opposite body side [2]. Particularly, situs inversus totalis (SIT; also called situs inversus with dextrocardia), a subcategory of situs inversus, is the complete left-to-right transposition of the thoracic and abdominal organs with only a few reported cases in dogs [2,3,4].
Primary ciliary dyskinesia (PCD), an autosomal recessive genetic disease, is a heterogeneous medical condition of ciliary movement [5]. PCD may be accompanied by structural or functional abnormalities of the cilia and cause disturbed mucociliary clearance, increasing vulnerability to upper and lower respiratory infections [6]. Other cilia-related disorders, such as polycystic kidney disease, male infertility by immobile or hypomobile spermatozoa or dysfunction of the epididymal duct, female hypofertility by ciliary function abnormalities of the fallopian tube, and SIT can co-exist with PCD [7,8,9]. However, the etiology and epidemiology of SIT and PCD have not yet been determined in veterinary medicine.
Herein, we present a case of Siewert–Kartagener’s syndrome with SIT in an Italian hound showing respiratory signs.
CASE PRESENTATION
A three-month-old, intact female Italian hound presented with lethargy, productive cough, respiratory distress, and seizures. The dog consistently showed naso-ocular purulent discharge with cachexia and dehydration since its adoption. Given the respiratory and neurologic clinical signs in an unvaccinated young puppy, canine distemper virus (CDV) infection was the primary differential diagnosis. A complete blood-count showed mild anemia (hematocrit, 24.1%, [reference interval (RI), 37.3–61.7%]), lymphopenia (1.03 × 109/L [RI, 1.05–5.1 × 109/L]), monocytosis (1.49 × 109/L [RI, 0.16–1.12 × 109/L]), and a normal total white blood cell count (12.94 × 109/L [RI, 5.05-16.76 × 109/L]). Neutrophil count was within normal range with moderate (12%) toxic change. A serum biochemistry analysis showed mild hypoalbuminemia (2.2 g/dL, [RI, 2.3–4.0 g/dL]). CDV antigen rapid test (RapiGEN, Korea) was positive.
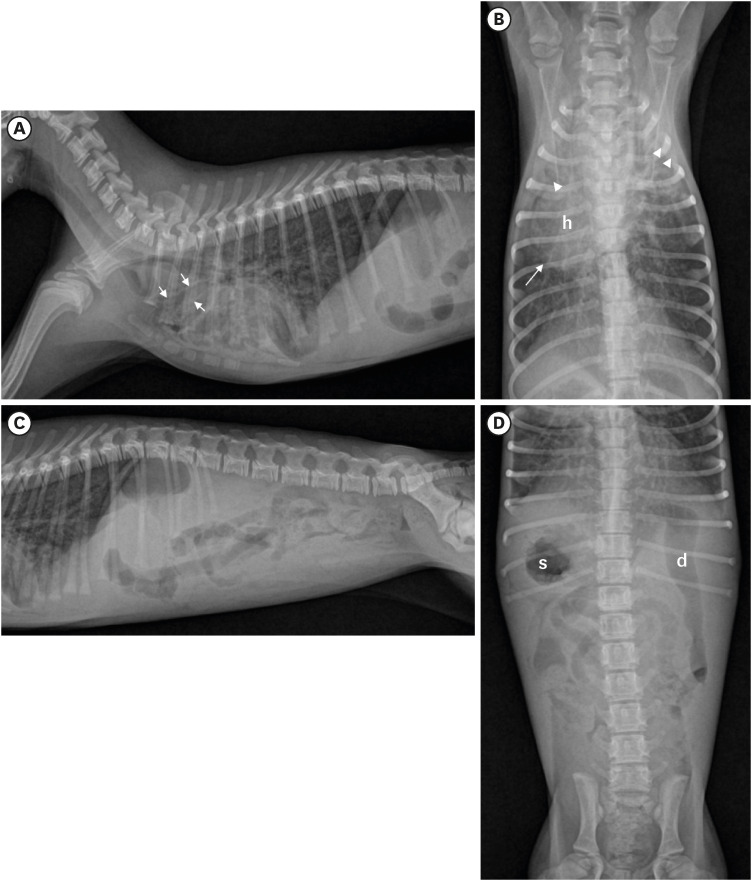
Thoracic and abdominal radiographs (Fig. 1) showed irregular bilateral alveolar and bronchial-interstitial infiltration and dilated bronchial lumen in the consolidated cranial lung lobes, indicating chronic bronchitis with bronchiectasis and bronchopneumonia. The organs in the thoracic and abdominal cavities were inverted as in a mirror-image. Specifically, the apex of the heart was placed towards the right side of the thorax, with right-axis deviation, the stomach body was situated in the right cranial abdomen, whereas the duodenum was observed in the left abdomen. There was no evidence of the gas- or fluid-distended “double-bubble” appearance or abnormal displacement of pylorus, fundus, duodenum, and spleen due to twisting, which are indicative of gastric torsion.
Fig. 1. Radiography of the dog with situs inversus totalis and bronchiectasis. On the right lateral (A) and ventrodorsal (B) views of thoracic radiograph, the heart (h) is located on the right side of the chest and the apex points to the right, indicating dextrocardia. Note an irregular alveolar and bronchointerstitial pattern, cranial lung field consolidation, and bronchodilation (arrow), indicating chronic bronchitis with bronchiectasis and bronchopneumonia. Right lateral (C) and ventrodorsal (D) views of abdominal radiography show the stomach (s) situated in the right cranial abdomen and duodenum (d) in the left cranial abdomen. All thoracic and abdominal organs are completely reversed (mirror-image), indicating situs inversus totalis. Left side of the lateral views (A, C) is the cranial part of the patient and left side of the VD views (B, D) is the right side of the patient.
Consequently, the dog was diagnosed as having SIT with CDV infection. The owner opted for humane euthanasia because of the poor prognosis and agreed to a computed tomography (CT) scan, electrocardiogram, and cerebrospinal fluid (CSF) analysis under general anesthesia before euthanasia for research purposes.
CSF obtained via the cerebellomedullary-cisternal puncture method using a 22-gauge needle was visually colorless and clear. The total nucleated cell count determined via unstained direct smear using a standard hemocytometer was 2 cells/μL (RI: 0–5 cells/μL); no red blood cells were observed. Cytological examination with cytocentrifugation (Cytospin4, Thermo Shandon, UK) and Diff-Quik staining revealed a few lymphocytes.
Standard 6-lead electrocardiogram (cardiofaxVET, Nihon-Koden Corp, Japan) revealed a marked right-axis deviation, and all electrode directions were in a left-right switched direction: positive P, QRS complex, and T waves in the aVR and all negative depolarizations in the aVL were observed; the aVR lead was in the opposite direction of the aVL lead, and lead I showed global negativity, meaning all depolarizations were inversely negative (Supplementary Fig. 1).
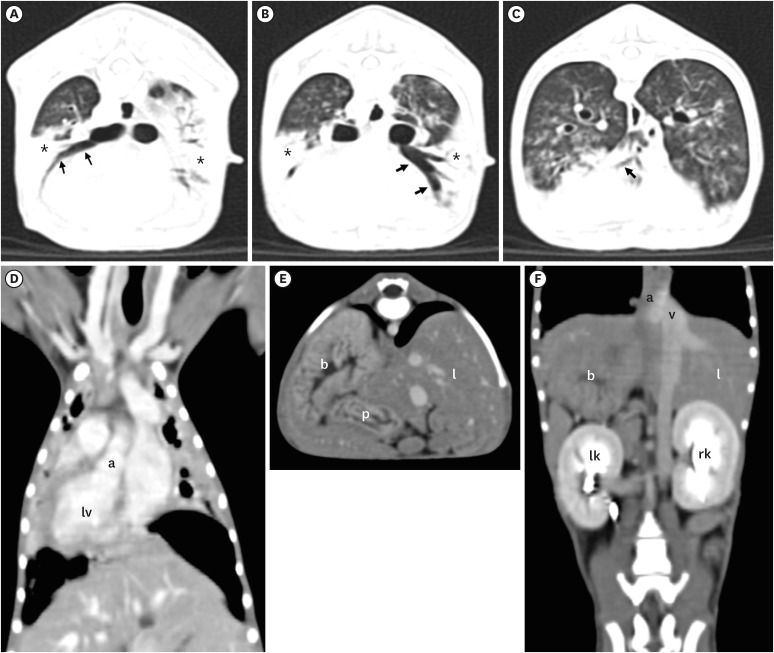
CT findings confirmed SIT, evidenced by the transposed cardiac chambers and large vessels, such as aorta and caudal vena cava (Fig. 2), and revealed consolidation of the cranial lung lobe with a clear ventral part on both sides of the lung, accompanied by bronchointerstitial infiltration throughout the remaining lung fields. Furthermore, increased fluid and secretions in the sinuses, indicative of sinusitis, were noted.
Fig. 2. Computed tomography images of the thorax (A-D) and abdomen (E, F) of the dog. On the transverse plane of CT images with lung window (A-C), dilated bronchial lumen (arrows) without tapering in the severely infiltrated bilateral lung lobes (*) indicated bronchopneumonia and bronchiectasis. Note situs inversus (dextrocardia), in which the heart, lung lobe, and blood vessels are completely reversed (a: aorta, lv: left ventricle) on the dorsal plane of the post-contrast CT image with mediastinal window (D). Transverse (E) and dorsal (F) planes of the post-contrast CT images with mediastinal window, all abdominal organs such as liver (l), stomach (s), pancreas (p), left kidney (lk), right kidney (rk), aorta (a), and caudal vena cava (v) appear completely left-right inverted, indicating situs inversus totalis.
CT, computed tomography.
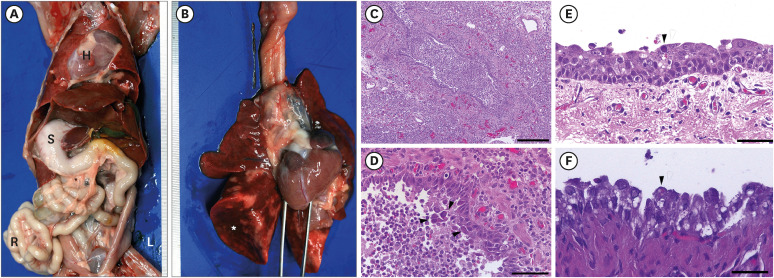
Necropsy confirmed complete transposition of the thoracic and abdominal organs (Fig. 3A). Severe lung collapse with acute multiple locally extensive purulent bronchopneumonia was observed (Fig. 3B). Histopathologically, whole lung lobe tissue exhibited diffuse thickening of the alveolar wall with hyperplasia of type II cells, fibroblast proliferation, and lymphocyte and interstitial macrophage infiltration (Fig. 3C). Additionally, purulent bronchopneumonia characterized by neutrophil infiltration in the lumen of bronchi, bronchioles, and alveoli was observed throughout the lobes (Fig. 3D). Nasal turbinate showed moderate diffuse catarrhal rhinitis with hyperemia. The trachea and nasal turbinate were severely deciliated with mild multiple locally-extensive desquamation of the epithelial cells and lymphoid cell infiltrations (Fig. 3E). Interestingly, attached and detached epithelial cells in the upper and lower respiratory tract, including the nasal turbinate, trachea, and lungs, contained large basophilic intranuclear inclusion body, pathognomonic to infection with canine adenovirus type 2 (CAV-2) (Fig. 3D and E). Moreover, small-to-medium sized eosinophilic intracytoplasmic inclusion bodies were observed in the epithelial cells in the upper and lower respiratory tracts and urinary bladder (Fig. 3F).
Fig. 3. Necropsy and histologic findings. (A) Gross findings of the abdominal organs. All internal organs show complete left-to-right transposition of the thoracic and abdominal organs. (B) Gross findings of the lungs. Severe diffuse collapse, congestion, and hemorrhages of lung lobes. (C) Severe diffuse purulent bronchopneumonia of the lung. (D) Large eosinophilic and basophilic intranuclear inclusion bodies are observed in the detached epithelial cells in the bronchiolar lumen (arrow heads). (E) Severe diffuse deciliation, mild diffuse desquamation of the epithelial cells, and mild diffuse lymphoid cell infiltrations in the submucosa. Note: basophilic intranuclear inclusion body (arrowhead). (F) Transitional epithelial cells in the urinary bladder contain small eosinophilic intracytoplasmic inclusion bodies. Staining: hematoxylin and eosin. Scale bar: (C) 200 μm, (D) 50 μm, (E, F) 25 μm.
L, left; R, right; S, stomach; H, heart.
Based on the history, physical examination, diagnostic images, necropsy, and histopathologic findings, the dog was diagnosed with canine SIT with bronchiectasis and PCD that lead to purulent bronchointerstitial pneumonia and concurrent CDV and CAV-2 infections.
DISCUSSION
This is a case of Siewert-Kartagener’s syndrome in an Italian hound co-infected with CDV and CAV-2. PCD contributed to SIT and respiratory signs in this case. Ciliary dysfunction attributed to impaired mucociliary clearance in the respiratory tract, as well as right-to-left transposition of the organs due to dysfunction of embryonic nodal cilia.
Siewert–Kartagener’s syndrome is a rare complex disease, a subset of PCD, comprising SIT, bronchiectasis, and sinusitis [9,10,11]. Normally, cilia at the apex of respiratory epithelial cells and mucin secreted by goblet cells are important components of the mucociliary apparatus. When pathogens are inhaled, they bind to mucin, and are discharged into the oral cavity by the escalator action of the underlying cilia. Therefore, immotile or structural cilia defects caused by PCD increase the incidence of infectious respiratory diseases and their recurrence. PCD without Kartagener’s syndrome is also known as immotile cilia syndrome. It is associated with wider spectrum of diseases including complex congenital heart diseases, polycystic kidney and liver disease, hydrocephalus, biliary atresia, severe esophageal diseases, retinal degeneration including retinitis pigmentosa, renal fibrosis, dilated renal tubules, otitis media and hearing loss, male infertility, recurrent pneumonia, as well as skeletal abnormalities in sternum, vertebrae, and ribs [9,10].
Approximately 50% of human PCD patients have SIT [9]. SIT, a rare autosomal recessive genetic disorder, results in a complete right-to-left transposition of all thoracic and abdominal organs. It is caused by the dysfunction of embryonic nodal cilia, which play an important role in directing the normal rotation and positioning of organs during embryonic development [2,12]. It is explained by the “morphogen hypothesis,” or the Nodal signaling cascade. Motile monocilia at the node generate a leftward flow leading to the concentration of a secreted factor on the node’s left side, triggering a cascade of left-defining genes [13,14,15,16]. Thus, failure to efficiently and directly affect nodal flow generated by motile monocilia at the node can result in left-right asymmetry [17,18,19].
In humans, 17%–25% of SIT patients have Siewert–Kartagener’s syndrome. Although Siewert-Kartagener’s syndrome is rare in veterinary medicine, there have been a few reported cases. The most common clinical manifestation of Kartagener’s syndrome in dogs is respiratory signs due to the impairment of mucociliary clearance [20]; additionally, there have been reports of communicating hydrocephalus, otitis media, renal diseases such as renal amyloidosis, and hepatoencephalopathy caused by vascular abnormalities [2,3,4]. This case was similar to other case reports where respiratory signs were the main symptoms. While there were a few reports of coexisting microorganism infections, such as Streptococcus and Mycoplasma spp. [10], there have been no reports of viral infections such as CDV or CAV-2. Herein, coinfection of CDV and CAV-2 was diagnosed by antigen test and pathognomonic histopathological findings. To our knowledge, this is the first case of Kartagener’s syndrome in a dog with concurrent CDV and CAV-2 infections.
In this case, the main clinical signs were lethargy, respiratory distress with productive cough, and seizures. The respiratory signs were consistent since the time the dog was adopted. This is consistent with previous reports indicating that respiratory symptoms typically manifest from birth in most dogs diagnosed with PCD [21]. Bronchiectasis and sinusitis were found in diagnostic imaging analyses, and ultimately severe diffuse deciliation of the nasal turbinate and trachea on histopathological examination supported the PCD diagnosis; thus, the diagnostic triads indicating Siewert–Kartagener’s syndrome were observed. CDV was confirmed by characteristic clinical symptoms, including neurological signs, histopathologic findings (eosinophilic intracytoplasmic inclusion bodies), and a CDV rapid antigen test [22]. CAV-2 was diagnosed based on pathognomonic characteristics such as large basophilic intranuclear inclusion bodies in the epithelial cells of the upper and lower respiratory tracts [23]. Consequently, we speculate that mucociliary apparatus failure since birth predisposes to viral epithelial cell infections because the viruses attach to mucous after inhalation and are not removed by the ciliary escalator and lead to secondary bacterial infections in the respiratory tract. Furthermore, concurrent infections with CDV and CAV-2 might have triggered other respiratory signs, potentially worsening the secondary bacterial infection owing to PCD.
There is no gold standard for diagnosing PCD in veterinary or human medicine. When dextrocardia and situs inversus or unexplained sinopulmonary infections are detected, diagnostic PCD algorithms are recommended. Consequently, the diagnosis is made by sequentially evaluating ciliary abnormalities, such as dynein arm, tubular defects, ciliary aplasia, and the orientation by electron microscopy [5]. Herein, only histopathology after the necropsy was performed, which is a limitation of our PCD diagnosis. Moreover, ciliary deciliation may not be congenitally formed due to PCD, but ciliary loss may have occurred due to canine distemper infection; thus, additional PCD verifications might be required.
In conclusion, this report highlights to clinicians the vulnerability to viral and secondary bacterial infections in Kartagener’s syndrome. Though SIT may be asymptomatic, it may also be associated with PCD, and recognizing an abnormal situs can indicate underlying PCD. Clinicians should consider the possibility of and vulnerability to infections if situs inversus is observed, even incidentally during a screening test.
Footnotes
Funding: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2020R1C1C1008675).
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Choi J, Cho KO, Yu D.
- Data curation: Bae H.
- Formal analysis: Choi J, Cho KO, Yu D.
- Funding acquisition: Cho KO.
- Investigation: Cho KO, Jung R.
- Methodology: Choi J, Jung R.
- Project administration: Yu D.
- Supervision: Choi J, Cho KO, Yu D.
- Validation: Choi J, Cho KO, Yu D.
- Visualization: Choi J, Cho KO.
- Writing - original draft: Jung R, Bae H, Jung DI.
- Writing - review & editing: Choi J, Cho KO, Yu D.
SUPPLEMENTARY MATERIAL
Electrocardiogram of the dog showing dextrocardia characteristics. All depolarizations are negative (i.e., global negativity) in leads I and aVL. Positive P, QRS complex, and T waves are visible in the aVR lead.
References
- 1.Witsberger TH, Dismukes DI, Kelmer EY. Situs inversus totalis in a dog with a chronic diaphragmatic hernia. J Am Anim Hosp Assoc. 2009;45(5):245–248. doi: 10.5326/0450245. [DOI] [PubMed] [Google Scholar]
- 2.Cahua J, Dias D, Gonzales-Viera O. Complete situs inversus in 2 asymptomatic dogs. Top Companion Anim Med. 2015;30(2):68–71. doi: 10.1053/j.tcam.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Cavrenne R, De Busscher V, Bolen G, Billen F, Clercx C, Snaps F. Primary ciliary dyskinesia and situs inversus in a young dog. Vet Rec. 2008;163(2):54–55. doi: 10.1136/vr.163.2.54. [DOI] [PubMed] [Google Scholar]
- 4.Neil JA, Canapp SO, Jr, Cook CR, Lattimer JC. Kartagener’s syndrome in a Dachshund dog. J Am Anim Hosp Assoc. 2002;38(1):45–49. doi: 10.5326/0380045. [DOI] [PubMed] [Google Scholar]
- 5.Bush A, Cole P, Hariri M, Mackay I, Phillips G, O’Callaghan C, et al. Primary ciliary dyskinesia: diagnosis and standards of care. Eur Respir J. 1998;12(4):982–988. doi: 10.1183/09031936.98.12040982. [DOI] [PubMed] [Google Scholar]
- 6.Damseh N, Quercia N, Rumman N, Dell SD, Kim RH. Primary ciliary dyskinesia: mechanisms and management. Appl Clin Genet. 2017;10:67–74. doi: 10.2147/TACG.S127129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goutaki M, Meier AB, Halbeisen FS, Lucas JS, Dell SD, Maurer E, et al. Clinical manifestations in primary ciliary dyskinesia: systematic review and meta-analysis. Eur Respir J. 2016;48(4):1081–1095. doi: 10.1183/13993003.00736-2016. [DOI] [PubMed] [Google Scholar]
- 8.Leigh MW, Pittman JE, Carson JL, Ferkol TW, Dell SD, Davis SD, et al. Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome. Genet Med. 2009;11(7):473–487. doi: 10.1097/GIM.0b013e3181a53562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bush A, Chodhari R, Collins N, Copeland F, Hall P, Harcourt J, et al. Primary ciliary dyskinesia: current state of the art. Arch Dis Child. 2007;92(12):1136–1140. doi: 10.1136/adc.2006.096958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison WB, Wilsman NJ, Fox LE, Farnum CE. Primary ciliary dyskinesia in the dog. J Vet Intern Med. 1987;1(2):67–74. doi: 10.1111/j.1939-1676.1987.tb01989.x. [DOI] [PubMed] [Google Scholar]
- 11.Knowles MR, Daniels LA, Davis SD, Zariwala MA, Leigh MW. Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med. 2013;188(8):913–922. doi: 10.1164/rccm.201301-0059CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilmott RW, Bush A, Boat TF, Deterding RR, Ratjen F, Chernick V. Kendig and Chernick’s Disorders of the Respiratory Tract in Children. 8th ed. Philadelphia: Elsevier Saunders; 2012. pp. 995–1002. [Google Scholar]
- 13.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, et al. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95(6):829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 14.Pennekamp P, Menchen T, Dworniczak B, Hamada H. Situs inversus and ciliary abnormalities: 20 years later, what is the connection? Cilia. 2015;4(1):1–12. doi: 10.1186/s13630-014-0010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Knowles HJ, Hebert JL, Hackett BP. Mutation of the mouse hepatocyte nuclear factor/forkhead homologue 4 gene results in an absence of cilia and random left-right asymmetry. J Clin Invest. 1998;102(6):1077–1082. doi: 10.1172/JCI4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Supp DM, Witte DP, Potter SS, Brueckner M. Mutation of an axonemal dynein affects left-right asymmetry in inversus viscerum mice. Nature. 1997;389(6654):963–966. doi: 10.1038/40140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner MK, Yost HJ. Left-right development: the roles of nodal cilia. Curr Biol. 2000;10(4):R149–R151. doi: 10.1016/s0960-9822(00)00328-6. [DOI] [PubMed] [Google Scholar]
- 18.Supp DM, Potter SS, Brueckner M. Molecular motors: the driving force behind mammalian left-right development. Trends Cell Biol. 2000;10(2):41–45. doi: 10.1016/s0962-8924(99)01701-8. [DOI] [PubMed] [Google Scholar]
- 19.Brueckner M. Cilia propel the embryo in the right direction. Am J Med Genet. 2001;101(4):339–344. doi: 10.1002/1096-8628(20010715)101:4<339::aid-ajmg1442>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 20.Celona B, Crinò C, Bruno C, Di Pietro S, Giudice E. Renal amyloidosis associated with Kartagener syndrome in a dog. Top Companion Anim Med. 2017;32(2):61–65. doi: 10.1053/j.tcam.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Merveille AC, Battaille G, Billen F, Deleuze S, Fredholm M, Thomas A, et al. Clinical findings and prevalence of the mutation associated with primary ciliary dyskinesia in Old English Sheepdogs. J Vet Intern Med. 2014;28(3):771–778. doi: 10.1111/jvim.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubo T, Kagawa Y, Taniyama H, Hasegawa A. Distribution of inclusion bodies in tissues from 100 dogs infected with canine distemper virus. J Vet Med Sci. 2007;69(5):527–529. doi: 10.1292/jvms.69.527. [DOI] [PubMed] [Google Scholar]
- 23.Yoon SS, Byun JW, Park YI, Kim MJ, Bae YC, Song JY. Comparison of the diagnostic methods on the canine adenovirus type 2 infection. Basic Appl Pathol. 2010;3(2):52–56. doi: 10.1111/j.1755-9294.2010.01073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electrocardiogram of the dog showing dextrocardia characteristics. All depolarizations are negative (i.e., global negativity) in leads I and aVL. Positive P, QRS complex, and T waves are visible in the aVR lead.