Abstract
Background
Mesenchymal stem cells (MSCs) have been investigated as therapeutic agents for inflammatory bowel disease (IBD). Stimulation of MSCs with pro-inflammatory cytokines is an approach to enhance their immunomodulatory effects. However, further investigation is required to support their application in immune-mediated disorders and companion animals.
Objectives
This study aimed to assess the therapeutic effect of tumor necrosis factor (TNF)-α-stimulated feline adipose tissue-derived MSCs (fAT-MSCs) in a dextran sulfate sodium (DSS)-induced colitis mouse model.
Methods
Colitis mice was made by drinking water with 3% DSS and fAT-MSCs were injected intraperitoneally. Colons were collected on day 10. The severity of the disease was evaluated and compared. Raw 264.7 cells were cultured with the conditioned medium to determine the mechanism, using quantitative real-time polymerase chain reaction and enzyme-linked immunosorbent assay.
Results
TNF-α-stimulated fAT-MSCs more improved severity of DSS-induced colitis in disease activity, colon length, histologic score, and inflammatory cytokine. In sectionized colon tissues, the group comprising TNF-α-stimulated fAT-MSCs had higher proportion of CD11b+CD206+ macrophages than in the other groups. In vitro, TNF-α-stimulation increased cyclooxygenase-2 (COX-2) expression and prostaglandin E2 (PGE2) secretion from fAT-MSCs. The conditioned medium from TNF-α-stimulated fAT-MSCs enhanced the expression of interleukin-10 and arginase-1 in LPS-activated Raw 264.7 cells.
Conclusions
These results represent that TNF-α-stimulated fat-mscs ameliorate the inflamed colon more effectively. Furthermore, we demonstrated that the effectiveness was interlinked with the COX-2/PGE2 pathway.
Keywords: Colitis, mesenchymal stem cell, Inflammatory bowel disease, tumor necrosis factor (TNF)-α
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic enteropathy characterized by persistent or recurrent gastrointestinal signs, such as vomiting, diarrhea, anorexia, and weight loss. The exact cause of IBD remains unknown; however, various factors, including genetic, environmental, and microbiome, are suspected to be involved [1,2]. Idiopathic IBD is a common histopathologic diagnosis in cats with chronic enteropathy [3]; however, its prevalence remains undetermined. Chronic enteropathy in cats requires medication such as antibiotics, glucocorticoid and immunosuppressant, diet control, and frequent visit, and it is difficult to treat even in these trials. One study suggested that cats with chronic enteropathy partly benefited from the injection of allogenic adipose tissue-derived feline adipose tissue-derived mesenchymal stem cells (fAT-MSCs). However, the small population limited the evaluation of the therapeutic effect of mesenchymal stem cells (MSCs) [4]. Despite the constantly developing treatments for IBD, further investigations are required to identify an effective treatment regimen [5,6].
Macrophages play vital role in regulating inflammatory response and protecting the host. By secreting cytokines, such as interleukin (IL)-1β and tumor necrosis factor (TNF)-α, M1 macrophages induce inflammation, by contrast, M2 macrophages promote anti-inflammation by secreting IL-10 [7]. Thus, the role of M2 macrophages in inflammatory diseases is gaining interest.
For more than 40 years, MSCs have been investigated, and strategies to enhance their effectiveness have also been investigated. Notably, MSCs have been studied in various immune-mediated inflammatory diseases, including IBD, osteoarthritis, atopic dermatitis, and encephalomyelitis [8,9,10,11]. The anti-inflammatory function of MSCs is attributed to their immunomodulatory effects from secreting molecules [12]. Inflamed tissue is characterized by hypoxia, a high concentration of inflammatory cytokines, and bacteria. Transplanted MSCs can modulate inflammatory responses and promote regeneration in such environments. Consequently, methods to enhance the therapeutic effect of stem cells using pre-conditioning with hypoxia, pro-inflammatory cytokines, and pattern recognition receptor ligands have been investigated. Among these, pro-inflammatory cytokines have been proven to be convenient [13]. TNF-α is the most commonly used pro-inflammatory cytokine for pre-conditioning MSCs [14,15,16]. However, we lack studies on the priming of fAT-MSCs with TNF-α.
MSCs could be treatment option for colitis [6,11,12,13] owing to their ability to modulate inflammatory response and repair the impaired tissue. Therefore, we aimed to demonstrate the anti-inflammatory ability and therapeutic potential of fAT-MSCs by pre-treating them with TNF-α and injecting them into the dextran sulfate sodium (DSS)-induced colitis mouse model.
MATERIALS AND METHODS
Isolation and characterization of fAT-MSCs
During ovariohysterectomy, feline adipose tissues from healthy cats were harvested at the Seoul National University Veterinary Medical Teaching Hospital. All procedures were approved by the Institutional Animal Care and Use Committee of Seoul National University (protocol No. SNU-190411-10). Adipose tissue was washed thrice in Dulbecco’s phosphate-buffered saline (DPBS) (PAN-Biotech, Aidenbach, Germany) with 1% penicillin-streptomycin (PS; PAN-Biotech), cut into small pieces, and digested with collagenase type 1A (1 mg/mL; Sigma-Aldrich, USA) for 1 h at 37°C. The enzyme was inactivated by Dulbecco’s modified Eagle’s medium (DMEM; PAN-Biotech) containing 20% fetal bovine serum (FBS; PAN-Biotech). The mixture was centrifugated at 1,200 rpm for 5 min. The pellets were filtered using a 70-μm Falcon cell strainer (Fisher Scientific, USA) and centrifugated at 1,200 rpm for 5 min. By using red blood cell lysis buffer (Sigma-Aldrich), red blood cells in the pellets were lysed for 5 min at 25°C and centrifugated at 1,200 rpm for 5 min. The pellets were resuspended in DMEM containing 20% FBS and 1% PS, and transferred to 150-mm dishes at a density of 3,500 cells/cm2. Transferred cells were incubated in DMEM containing 20% FBS at 37°C in a humidified atmosphere of 5% CO2, and the medium was changed every 2–3 days until it reached 70% confluency. In this experiment, isolated fAT-MSCs at passage 3–4 were used.
Cells were characterized using flow cytometry using antibodies against several stem cell markers; CD29-FITC (BD Biosciences, USA), CD34-PE (BD Biosciences), CD44-FITC (Invitrogen, USA), CD45-FITC (BD Biosciences), and CD90-PE (BD Biosciences). Cellular differentiation was confirmed by the StemPro Adipogenesis, Chondrogenesis, and Osteogenesis Differentiation Kits (Gibco/Life Technologies, USA) in accordance with manufacturer’s instructions.
TNF-α-stimulation
The fAT-MSCs were spread in six-well plates (5 × 105 cells/well) and cultured in DMEM medium supplemented with 20% FBS and 1% PS. After 24 h, the cells were stimulated with 50 ng/mL feline TNF-α (Kingfisher Biotech, USA) for 24 h. The vitality of the cells was evaluated using the D-Plus CCK Cell vitality Assay Kit (DonginLS, Korea) after three DPBS washes.
RNA extraction, cDNA synthesis, and quantitative reverse transcription polymerase chain reaction (qRT-PCR)
RNA was collected from fAT-MSCs and RAW 264.7 cells using the Easy-BLUE Total RNA Extraction Kit (iNtRON Biotechnology, Korea). Using the LaboPass M-MuLV Reverse Transcriptase Kit (Cosmogenetech, Korea), cDNA was synthesized. qRT-PCR was used to examine the cDNA samples using AMPIGENE qPCR Green Mix Hi-ROX with SYBR Green Dye (Enzo Life Sciences, USA). The mRNA expression levels were quantified using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the positive control. Table 1 contains a list of the primer sequences utilized in this study.
Table 1. Sequences of PCR primers used in this study.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| mGAPDH | AGTATGTCGTGGAGTCTACTGGTGT | AGTGAGTTGTCATATTTCTCGTGGT |
| mArg1 | AACACGGCAGTGGCTTTAAC | GTCAGTCCCTGGCTTATGGTT |
| mIL-10 | TGGCCCAGAAATCAAGGAGC | CAGCAGACTCAATACACACT |
| mIL-6 | AGTTGCCTTCTTGGGACTGA | TCCACGATTTCCCAGAGAAC |
| mIL-1β | TGGACCTTCCAGGATGAGGACA | GTTCATCTCGGAGCCTGTAGTG |
| fGAPDH | AGGTCGGTGTGAACGGATTT | GCCGTGGGTGGAATCATACT |
| fCOX-2 | CGATTCAGTCTCTCATCTGCAATAA | TCAGTTGAACGTTCTTTTAGCAGTA |
| fTGF-β | CCAACAAAATCTATGAGAAAGTCCA | TATTGCTGTATTTCTGGTACAGCTC |
| fHGF | ATTCCATGGGATTATTGTCCTATTT | TTCAAACTAACCATCCATCCTACAT |
PCR, polymerase chain reaction; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; Arg1, arginase 1; IL, interleukin; COX-2, cyclooxygenase-2; TGF, transforming growth factor; HGF, hepatocyte growth factor.
Enzyme-linked immunosorbent assay (ELISA)
The cell-conditioned medium was obtained from fAT-MSCs, TNF-α-stimulated fAT-MSC (24 h), and TNF-α- and NS-398-stimulated (5 µM; Enzo Life Sciences) fAT-MSC (48 h) incubated in six-well plates at a density of 5 × 104 cells/well. Following the manufacturer’s instructions, a prostaglandin E2 (PGE2) ELISA kit (Enzo Life Sciences) was used to determine the concentration of PGE2 present in the cell-conditioned medium.
Culturing RAW 264.7 cell with conditioned media from fAT-MSCs
RAW 264.7 cells obtained from Korean Cell Line Bank were seeded in six-well plates (5 × 105 cells/well) for 24 h and then stimulated with lipopolysaccharide (LPS) for 6 h. Cells were washed thrice with DPBS and incubated with the conditioned medium supplemented with 10% FBS for 48 h.
DSS-induced colitis mice
Six-week-old male C57BL/6J mice were acquired from Nara Biotech (Korea) and maintained in regulated temperature, humidity, and light cycle conditions. Mice were randomly divided into four groups (n = 4 for the control group and n = 6 for the DSS, DSS with fAT-MSC, and DSS with TNF-α primed fAT-MSC). Colitis was induced by adding 3% DSS (36–50 kDa; MP Biomedicals, USA) to drinking water ad libitum from days 0–7. On day 1, mice were intraperitoneally injected with 200 µL DPBS, 2 × 106 fAT-MSCs, or TNF-α-primed fAT-MSCs diluted with 200 µL DPBS. The water was changed on day 8, and the mice were euthanized on day 10. All procedures were approved by the Institutional Animal Care and Use Committee of Seoul National University, and the protocols were carried out in accordance with approved guidelines (protocol No. SNU-211207-5).
Evaluating colitis severity
The disease activity index was evaluated daily by scoring the body weight loss (scores 0–4: 0, none; 1, < 10% loss of the initial body weight; 2, 10%–15% loss of the initial body weight; 3, 15%–20% loss of the initial body weight; 4, > 20% loss of the initial body weight), stool consistency (scores 0–4: 0, none; 2, mild diarrhea; 4, moderate to severe diarrhea), rectal bleeding (scores 0–4: 0, none; 2, mild bleeding; 4, moderate to severe bleeding), and general activity (scores 0–4: 0, none; 2, mildly depressed; 4, moderately to severely depressed).
Histological analysis
Colon tissues were fixed in 10% formalin, embedded in paraffin, and sectionized at 4 µm. The sections were stained with hematoxylin and eosin. A total of 20 fields per group were selected randomly and evaluated in blind manner. The histologic score was calculated based on the infiltration of inflammatory cells (scores 0–4: 0, no infiltration; 1, infiltration around crypt basis; 2, infiltration reaching to lamina muscularis mucosa layer; 3, extensive infiltration reaching the muscularis mucosa with abundant edema; 4, infiltration of the submucosa layer) and damage to the epithelium (scores 0–4: 0, normal; 1, loss of goblet cells; 2, loss of goblet cells in large areas; 3, loss of crypts; 4, loss of crypts in large areas).
Western blot analysis
Using the PRO-PREP Protein Extraction Kit (iNtRON Biotechnology), total proteins were isolated from colon tissues. The proteins were quantified with the BCA assay. Approximately 20 µg of samples were loaded onto SDS-PAGE and immunoblotted with antibodies against beta-actin (1:500; Santa Cruz Biotechnology, USA) and IL-1β (1:100; LSBio, USA).
Immunofluorescence analysis
Paraffin-embedded colon tissues were cut into 4-µm thick sections. Sections were deparaffinized, rehydrated, and antigen retrieval was done in 10 mM citrate buffer. Sections were washed and blocked with a buffer comprising 5% bovine serum albumin and 0.3% Triton X-100 for 1 h. The sections were incubated with CD11b-PE (1:100; Santa Cruz Biotechnology) and CD206-FITC (1:100; Biolegend, USA) antibodies at 4°C overnight. The sections were washed thrice and mounted in Vectashield mounting medium containing 4′, 6-diamidino-2-phenylindole (DAPI; Vector Laboratories, USA). The sections were examined under an EVOS FL microscope (Life Technologies, Germany), and the number of immunoreactive cells was counted in 20 random fields per group.
Statistical analyses
Data are presented as the mean ± SD. Mean values from each group were compared using the t-test and one-way analysis of variance (ANOVA) using GraphPad Prism v.7.01 software (GraphPad Software, USA). p values < 0.5 were considered statistically significant.
RESULTS
Characterization of fAT-MSCs
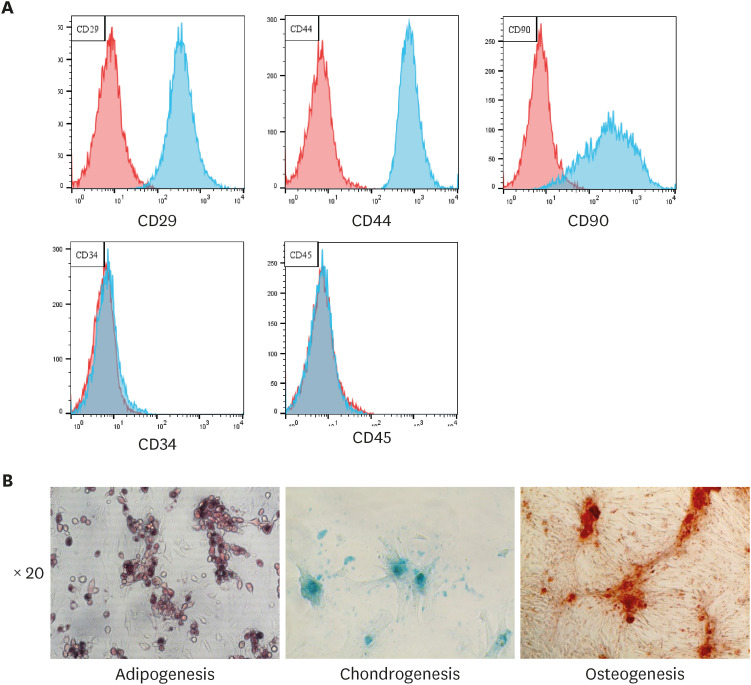
The cells isolated from feline adipose tissue displayed a fibroblast-like morphology. Flow cytometric analysis revealed that cells had a high expression of MSC markers, such as CD29, CD44, and CD90, and low expression of hematopoietic markers, including CD34 and CD45 (Fig. 1A). Subsequently, cells were differentiated in to adipocytes, chondrocytes, and osteocytes (Fig. 1B).
Fig. 1. Characterization of fAT-MSCs. (A) Analysis of surface markers of fAT-MSCs using flow cytometry. (B) Adipogenesis; intracellular lipid vacuoles were stained pink with Oil Red O. Chondrogenesis; proteoglycans were stained with Alcian Blue. Osteogenesis; fAT-MSCs were stained positive for calcium deposits using 1% Alizarin red.
fAT-MSC, feline adipose tissue-derived mesenchymal stem cell.
Immunomodulatory factors expressed by TNF-α-stimulated fAT-MSCs
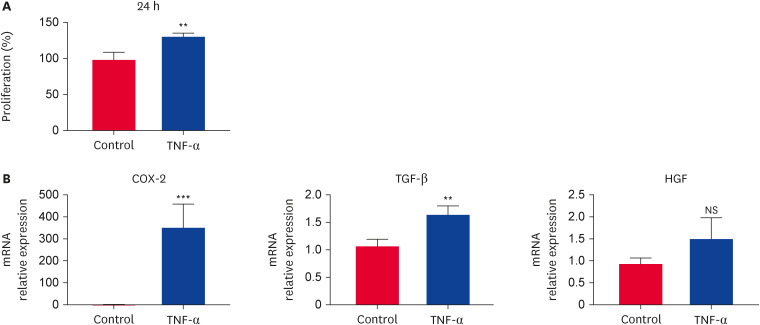
TNF-α-stimulation for 24 h significantly increased the degree of cell proliferation compared with that in the non-stimulating group (Fig. 2A). The TNF-α pre-treated fAT-MSCs exhibited a significantly higher expression of TGF-β and cyclooxygenase-2 (COX-2) genes than that of non-treated fAT-MSCs. Contrary to the non-significant increase in HGF expression in fAT-MSCs, these levels were increased when fAT-MSCs were pre-treated with TNF-α (Fig. 2B).
Fig. 2. Immunomodulatory effects of TNF-α-stimulated fAT-MSCs. (A) Cell viability of non-stimulated and TNF-α stimulated fAT-MSCs. (B) Increased gene expression of COX-2 and TGF-β. Results are presented as means ± SD.
TNF, tumor necrosis factor; fAT-MSC, feline adipose tissue-derived mesenchymal stem cell; COX-2, cyclooxygenase-2; TGF, transforming growth factor; NS, not significant.
**p < 0.01, ***p < 0.001. NS indicates not statistically significant.
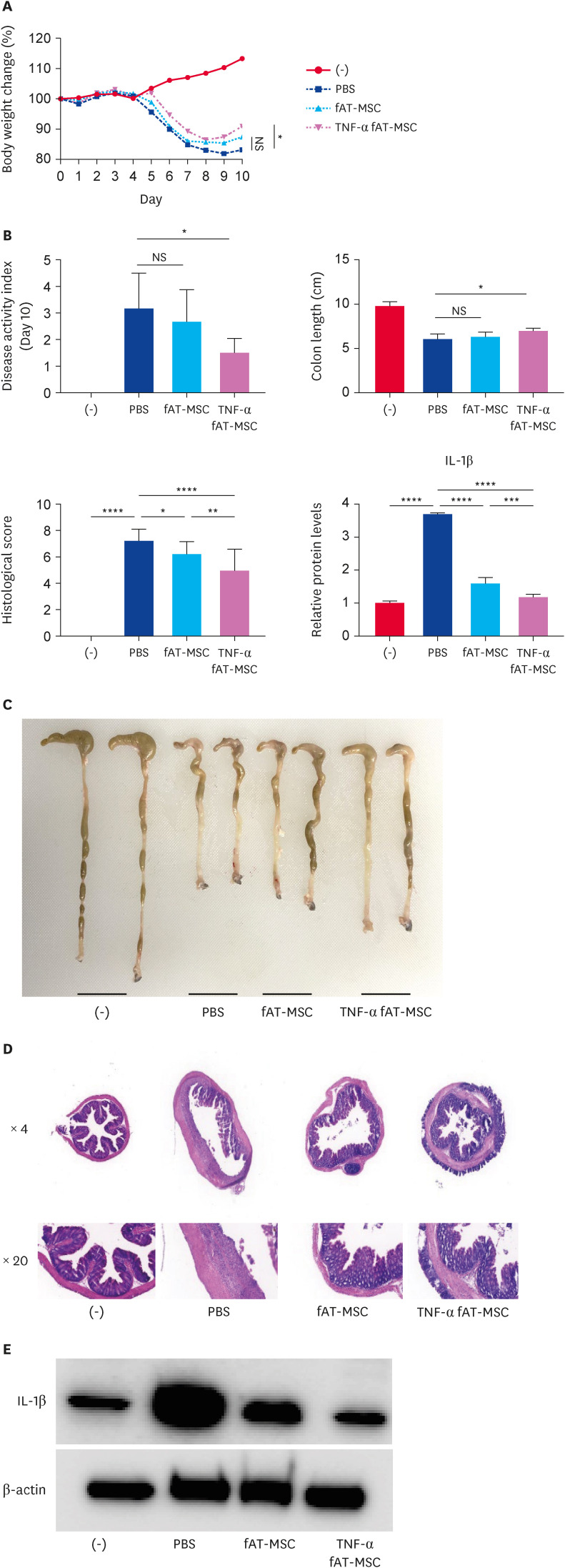
TNF-α-stimulated fAT-MSCs ameliorate DSS-induced colitis
TNF-α-stimulated fAT-MSCs administered intraperitoneally reduced body weight loss and disease activity index over 10 days compared with that in phosphate-buffered saline (PBS)-treated mice. On the other hand, there was no significant difference between PBS-treated group and fAT-MSCs group (Fig. 3A and B). On day 10, the mice were euthanized, and their colons were collected for analysis. Unlike fAT-MSCs group, TNF-α-stimulated fAT-MSCs group showed improvement in colon length shortening when compared to the PBS-treated group (Fig. 3C). Histological examination revealed that both fAT-MSCs and TNF-α-stimulated fAT-MSCs groups had lower histological scores than that of the PBS-treated group. Furthermore, the TNF-α-stimulated fAT-MSCs group revealed a significant reduction in the histological score compared with that in the fAT-MSC group (Fig. 3D). TNF-α-stimulated fAT-MSCs group exhibited a decreased inflammatory response by reducing IL-1β levels in the colon compared with that in the PBS- and fAT-MSC treated groups (Fig. 3E).
Fig. 3. TNF-α stimulated fAT-MSCs ameliorate DSS-induced colitis mice. PBS, fAT-MSCs, or TNF-α stimulated fAT-MSCs were injected intraperitoneally one day after 3% DSS supplement. (A) Body weight was measured every day. Body weight change was calculated relative to day 0. (B) The DAPI was monitored for 10 days. (C) Colon length was evaluated on day 10. (D) H&E staining of the colon section and the histological score was calculated. (E) IL-1β levels in the colon were evaluated using western blot analysis. Results are presented as means ± SD.
fAT-MSC, feline adipose tissue-derived mesenchymal stem cell; DSS, dextran sulfate sodium; PBS, phosphate-buffered saline; TNF, tumor necrosis factor; DAPI, 4′, 6-diamidino-2-phenylindole; H&E, hematoxylin and eosin; IL, interleukin; NS, not significant.
*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
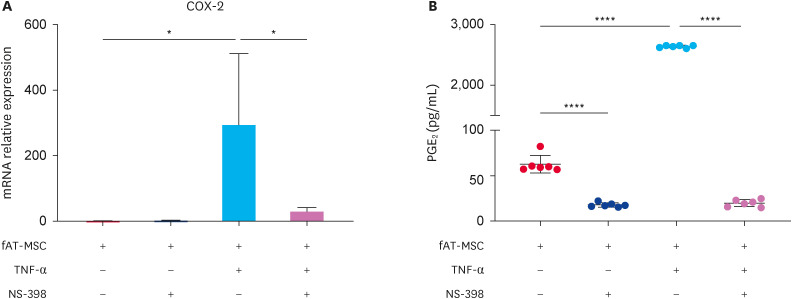
Alteration in PGE2 levels, secreted by TNF-α-stimulated fAT-MSCs with and without NS-398 treatment
The efficacy of COX inhibitor, NS-398, in inhibiting COX-2 expression was monitored by measuring COX-2 gene expression in fAT-MSCs and PGE2 levels in the conditioned medium derived from fAT-MSCs, fAT-MSCs with NS-398, TNF-α-stimulated fAT-MSCs, and TNF-α-stimulated fAT-MSCs with NS-398 using PGE2 ELISA kit. In TNF-α-stimulated fAT-MSCs, COX-2 gene expression was increased, COX-2 expression was markedly decreased in the additional NS-398 treatment groups (Fig. 4A). PGE2 levels were considerably increased in TNF-α-stimulated fAT-MSCs conditioned medium. Additional NS-398 treatment reduced PGE2 levels in the fAT-MSCs and TNF-α-stimulated fAT-MSCs groups (Fig. 4B).
Fig. 4. NS-398 decreased COX-2 expression and PGE2 secretion in TNF-α stimulated fAT-MSCs. NS-398 was treated to fAT-MSCs for confirming its effectiveness of inhibiting COX-2 expression. (A) TNF-α-stimulated fAT-MSCs highly expressed COX-2 gene. NS-398 treatment effectively inhibited COX-2 gene expression in fAT-MSCs. (B) PGE2 levels in the conditioned medium of non-stimulated, TNF-α-stimulated, and NS-398-treated fAT-MSCs. Results are presented as means ± SD.
COX-2, cyclooxygenase-2; PGE2, prostaglandin E2; TNF, tumor necrosis factor; fAT-MSC, feline adipose tissue-derived mesenchymal stem cell; ns, not significant.
*p < 0.05, ****p < 0.0001.
TNF-α stimulated fAT-MSCs induce M2 macrophage polarization via the COX-2/ PGE2 pathway
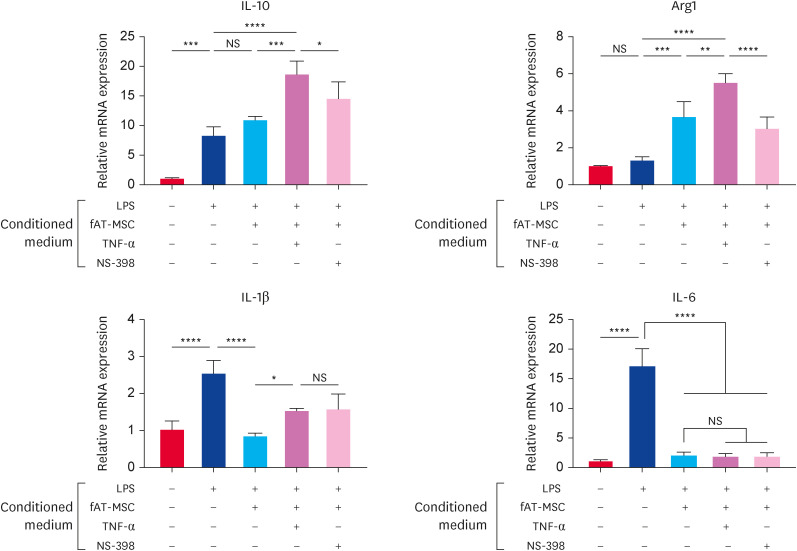
To identify the role of PGE2, Raw 264.7 cells were incubated in the fAT-MSCs conditioned medium pretreated with TNF-α or NS-398 after LPS stimulation for 6 h (200 ng/mL). The levels of M2 macrophage-associated factors, IL-10 and arginase-1, were elevated in the TNF-α-stimulated fAT-MSCs-derived conditioned medium compared with other groups. However, the additional NS-398 treatment decreased the levels of these factors when compared to the only TNF-α-treated group. Conversely, the levels of IL-1β, related with M1 macrophage-associated factors, were rather increased in TNF-α-treated group compared with naïve fAT-MSCs group. IL-6 levels were not significantly altered in all fAT-MSCs-treated groups (Fig. 5).
Fig. 5. Conditioned medium from TNF-α stimulated fAT-MSCs increased M2 macrophage-related factors. Changes in the expression of M1 and M2 macrophage-related factors in LPS-activated Raw 264.7 cells incubated with the conditioned medium. Results are presented as means ± SD.
TNF, tumor necrosis factor; fAT-MSC, feline adipose tissue-derived mesenchymal stem cell; LPS, lipopolysaccharide; IL, interleukin; Arg1, arginase 1; ns, not significant.
*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. NS indicates not statistically significant.
TNF-α stimulated fAT-MSCs increased the number of M2 macrophages in the inflamed colon
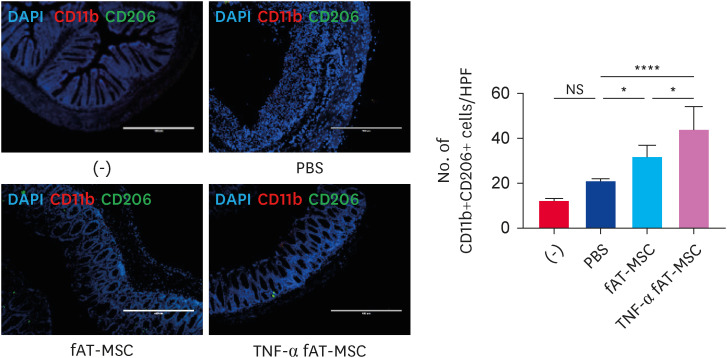
Given that the cytokines (arginase 1 [Arg1], IL-6, IL-1β, and IL-10) modulated in the above results are mainly secreted from macrophages, we further investigated whether fAT-MSCs or TNF-α-stimulated fAT-MSCs could alter macrophage phenotypes in inflamed colon tissue. Immunofluorescence analysis revealed that the number of CD11b+CD206+ M2 macrophage was increased in both fAT-MSCs and TNF-α-stimulated fAT-MSCs groups; however, the numbers in the TNF-α-stimulated group were significantly increased (Fig. 6).
Fig. 6. TNF-α stimulated fAT-MSCs induced M2 macrophage polarization in the inflamed colon. Immunofluorescence staining of colon tissue sections using CD11b- and CD206-specific antibodies, and numbers of CD11b- and CD206-positive cells were calculated (scale bar = 400 µm). Results are presented as means ± SD.
TNF, tumor necrosis factor; fAT-MSC, feline adipose tissue-derived mesenchymal stem cell; DAPI, 4′, 6-diamidino-2-phenylindole; PBS, phosphate-buffered saline; ns, not significant.
*p < 0.05, ****p < 0.0001. NS indicates not statistically significant.
DISCUSSION
In this study, we enhanced the effectiveness of fAT-MSCs by stimulating with TNF-α, and elucidated the associated mechanisms in DSS-induced colitis mice.
We demonstrated that TNF-α-stimulation increased the proliferation of fAT-MSCs and the immunomodulatory factors, such as TGF-β and COX-2. TGF-β has an immunosuppressive effect, polarizes M2 macrophages, and aids in the homeostasis of peripheral CD4+CD25+ regulatory T cells [17,18,19]. COX-2 directs anti-inflammatory response by increasing regulatory T cells [20].
In colitis mouse model, the naïve fAT-MSCs group had no significant effect. However, the TNF-α-stimulated fAT-MSCs group ameliorated colitis by altering the body weight, colon length, and DAI. The naïve fAT-MSCs group also improved histological scores compared with that of the PBS group, but the scores of the TNF-α-stimulated fAT-MSCs group were relatively higher. TNF-α-stimulated fAT-MSCs group also had a lower concentration of IL-1β in the colon than that in the naïve fAT-MSCs group. To determine the underlying mechanisms, we focused on the significantly increased expression of COX-2, which regulates the immune response. The COX-2/PGE2 pathway is associated with reducing the inflammatory environment by down-regulating pro-inflammatory cytokines and increasing regulatory T cells [20,21]. COX-2 inhibition blocks M2 macrophage differentiation [22]. Furthermore, PGE2 inhibits pro-inflammatory secretion by LPS-activated macrophages [23]. Therefore, we evaluated whether TNF-α-stimulated fAT-MSCs affect M2 macrophages polarization in vitro. We confirmed that TNF-α-stimulated fAT-MSCs secrete more PGE2 than naïve fAT-MSCs, and NS-398 inhibit COX-2 gene expression and PGE2 secretion in the conditioned medium using qRT-PCR and ELISA. M2 macrophage-related factors, IL-10 and arginase-1, were significantly increased in TNF-α-stimulated fAT-MSCs group when compared to the naïve fAT-MSCs group. Their expression was decreased in TNF-α-stimulated fAT-MSCs group with NS-398 treatment. M1 macrophage-related factors, IL-6 and IL-1β, were not decreased, instead IL-1β was increased in TNF-α-stimulated fAT-MSCs group when compared to the naïve fAT-MSCs group. These results suggest that PGE2 secreted by TNF-α-stimulated fAT-MSCs is at least partly involved in inducing M2 macrophage differentiation.
Macrophages are present throughout the gastrointestinal mucosa and smooth muscle layer of the intestine [24,25]. Eissa et al. [26] demonstrated that macrophages play a major role in the treatment of colitis. Macrophages are classified into M1 and M2 macrophages based on their activation state, and studies revealed that M2 macrophages are closely related to improving colitis [8,27,28]. Our immunofluorescence findings revealed that markers of M2 macrophages in the colon tissue sections from DSS-induced colitis mice were highly expressed in both naïve fAT-MSCs and TNF-α-stimulated fAT-MSCs groups compared with that in the PBS group; notably the increase was prominent in the TNF-α-stimulated fAT-MSCs group.
Our study has the following limitation: macrophage related other factors, such as Ym1 and FIZZ 1 [29], secreted from TNF-α-stimulated fAT-MSCs may also direct polarization of M2 macrophage in DSS-induced colitis mice. Further research is required to verify the effects on M2 macrophage polarization. Nonetheless, these findings provide ground to enhance stem cell therapy for feline IBD patients through cytokine pre-conditioning.
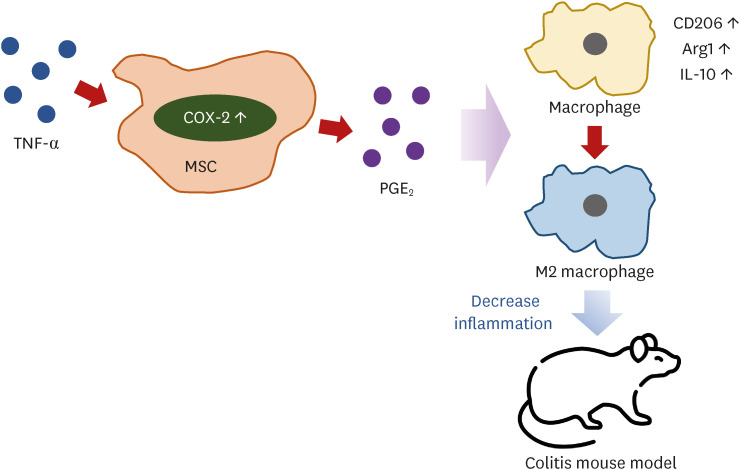
We demonstrated that TNF-α-stimulation ameliorates inflammation in the colon more effectively than naïve fAT-MSCs. Our findings suggest that TNF-α-stimulation activates the COX-2/PGE2 pathway and plays a crucial role in promoting M2 macrophage polarization (Fig. 7). As we know, therapeutic effect of TNF-α-stimulated fAT-MSCs in a DSS-induced colitis mouse model has been confirmed in this report for the first time.
Fig. 7. Schema of TNF-α stimulated fAT-MSCs action in this study. TNF-α stimulation increased COX-2/PGE2 pathway in the fAT-MSCs. Secreted PGE2 induced polarization of macrophage into M2 phenotype and decreased inflammation in DSS-induced colitis mouse model.
TNF, tumor necrosis factor; fAT-MSC, feline adipose tissue-derived mesenchymal stem cell; COX-2, cyclooxygenase-2; PGE2, prostaglandin E2; DSS, dextran sulfate sodium; Arg1, arginase 1; IL, interleukin.
ACKNOWLEDGEMENTS
We are very thankful to the Research Institute for Veterinary Science of Seoul National University and the BK21 PLUS Program for Creative Veterinary Science Research.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Kim K, An JH.
- Data curation: Kim K.
- Formal analysis: Kim K.
- Funding acquisition: Seo KW, Youn HY.
- Investigation: Kim K, An JH, Lim G.
- Methodology: Kim K, An JH, Park SM.
- Project administration: Seo KW, Youn HY.
- Resources: Seo KW, Youn HY.
- Supervision: Seo KW, Youn HY.
- Validation: An JH, Park SM, Lim G.
- Visualization: Kim K.
- Writing - original draft: Kim K.
- Writing - review & editing: Seo KW, Youn HY.
References
- 1.Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9(10):599–608. doi: 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- 2.Knights D, Lassen KG, Xavier RJ. Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome. Gut. 2013;62(10):1505–1510. doi: 10.1136/gutjnl-2012-303954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trepanier L. Idiopathic inflammatory bowel disease in cats. Rational treatment selection. J Feline Med Surg. 2009;11(1):32–38. doi: 10.1016/j.jfms.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webb TL, Webb CB. Stem cell therapy in cats with chronic enteropathy: a proof-of-concept study. J Feline Med Surg. 2015;17(10):901–908. doi: 10.1177/1098612X14561105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isaacs KL, Lewis JD, Sandborn WJ, Sands BE, Targan SR. State of the art: IBD therapy and clinical trials in IBD. Inflamm Bowel Dis. 2005;11(Suppl 1):S3–SS12. doi: 10.1097/01.mib.0000184852.84558.b2. [DOI] [PubMed] [Google Scholar]
- 6.Sandborn WJ. Current directions in IBD therapy: what goals are feasible with biological modifiers? Gastroenterology. 2008;135(5):1442–1447. doi: 10.1053/j.gastro.2008.09.053. [DOI] [PubMed] [Google Scholar]
- 7.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song WJ, Li Q, Ryu MO, Ahn JO, Bhang DH, Jung YC, et al. TSG-6 released from intraperitoneally injected canine adipose tissue-derived mesenchymal stem cells ameliorate inflammatory bowel disease by inducing M2 macrophage switch in mice. Stem Cell Res Ther. 2018;9(1):91. doi: 10.1186/s13287-018-0841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koh YG, Jo SB, Kwon OR, Suh DS, Lee SW, Park SH, et al. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29(4):748–755. doi: 10.1016/j.arthro.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Kim HS, Yun JW, Shin TH, Lee SH, Lee BC, Yu KR, et al. Human umbilical cord blood mesenchymal stem cell-derived PGE2 and TGF-β1 alleviate atopic dermatitis by reducing mast cell degranulation. Stem Cells. 2015;33(4):1254–1266. doi: 10.1002/stem.1913. [DOI] [PubMed] [Google Scholar]
- 11.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106(5):1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 12.Legaki E, Roubelakis MG, Theodoropoulos GE, Lazaris A, Kollia A, Karamanolis G, et al. Therapeutic potential of secreted molecules derived from human amniotic fluid mesenchymal stem/stroma cells in a mice model of colitis. Stem Cell Rev Rep. 2016;12(5):604–612. doi: 10.1007/s12015-016-9677-1. [DOI] [PubMed] [Google Scholar]
- 13.Uberti B, Plaza A, Henríquez C. Pre-conditioning strategies for mesenchymal stromal/stem cells in inflammatory conditions of livestock species. Front Vet Sci. 2022;9:806069. doi: 10.3389/fvets.2022.806069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Putra A, Ridwan FB, Putridewi AI, Kustiyah AR, Wirastuti K, Sadyah NA, et al. The role of TNF-α induced MSCs on suppressive inflammation by increasing TGF-β and IL-10. Open Access Maced J Med Sci. 2018;6(10):1779–1783. doi: 10.3889/oamjms.2018.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon YW, Heo SC, Jeong GO, Yoon JW, Mo WM, Lee MJ, et al. Tumor necrosis factor-α-activated mesenchymal stem cells promote endothelial progenitor cell homing and angiogenesis. Biochim Biophys Acta. 2013;1832(12):2136–2144. doi: 10.1016/j.bbadis.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Min XH, Wang QY, Leung FW, Shi L, Zhou Y, et al. Pre-activation of mesenchymal stem cells with TNF-α, IL-1β and nitric oxide enhances its paracrine effects on radiation-induced intestinal injury. Sci Rep. 2015;5:8718. doi: 10.1038/srep08718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang F, Wang H, Wang X, Jiang G, Liu H, Zhang G, et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget. 2016;7(32):52294–52306. doi: 10.18632/oncotarget.10561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong D, Shi W, Yi SJ, Chen H, Groffen J, Heisterkamp N. TGFβ signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. 2012;13(1):31. doi: 10.1186/1471-2172-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201(7):1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.An JH, Song WJ, Li Q, Kim SM, Yang JI, Ryu MO, et al. Prostaglandin E2 secreted from feline adipose tissue-derived mesenchymal stem cells alleviate DSS-induced colitis by increasing regulatory T cells in mice. BMC Vet Res. 2018;14(1):354. doi: 10.1186/s12917-018-1684-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang HM, Song WJ, Li Q, Kim SY, Kim HJ, Ryu MO, et al. Canine mesenchymal stem cells treated with TNF-α and IFN-γ enhance anti-inflammatory effects through the COX-2/PGE2 pathway. Res Vet Sci. 2018;119:19–26. doi: 10.1016/j.rvsc.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Na YR, Yoon YN, Son DI, Seok SH. Cyclooxygenase-2 inhibition blocks M2 macrophage differentiation and suppresses metastasis in murine breast cancer model. PLoS One. 2013;8(5):e63451. doi: 10.1371/journal.pone.0063451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang T, Scambler TE, Smallie T, Cunliffe HE, Ross EA, Rosner DR, et al. Macrophage responses to lipopolysaccharide are modulated by a feedback loop involving prostaglandin E2, dual specificity phosphatase 1 and tristetraprolin. Sci Rep. 2017;7(1):4350. doi: 10.1038/s41598-017-04100-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tajima T, Murata T, Aritake K, Urade Y, Michishita M, Matsuoka T, et al. EP2 and EP4 receptors on muscularis resident macrophages mediate LPS-induced intestinal dysmotility via iNOS upregulation through cAMP/ERK signals. Am J Physiol Gastrointest Liver Physiol. 2012;302(5):G524–G534. doi: 10.1152/ajpgi.00264.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, Mucida D. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell. 2016;164(3):378–391. doi: 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eissa N, Hussein H, Kermarrec L, Grover J, Metz-Boutigue ME, Bernstein CN, et al. Chromofungin ameliorates the progression of colitis by regulating alternatively activated macrophages. Front Immunol. 2017;8:1131. doi: 10.3389/fimmu.2017.01131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.An JH, Li Q, Bhang DH, Song WJ, Youn HY. TNF-α and INF-γ primed canine stem cell-derived extracellular vesicles alleviate experimental murine colitis. Sci Rep. 2020;10(1):2115. doi: 10.1038/s41598-020-58909-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Zhang J, Guo H, Yang S, Fan W, Ye N, et al. Critical role of alternative M2 skewing in miR-155 deletion-mediated protection of colitis. Front Immunol. 2018;9:904. doi: 10.3389/fimmu.2018.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raes G, Van den Bergh R, De Baetselier P, Ghassabeh GH, Scotton C, Locati M, et al. Arginase-1 and Ym1 are markers for murine, but not human, alternatively activated myeloid cells. J Immunol. 2005;174(11):6561–6562. doi: 10.4049/jimmunol.174.11.6561. [DOI] [PubMed] [Google Scholar]