Abstract
Germ cell loss is a crucial biological event during germ cell development. The number of female germ cells determines the reproductive performance and egg production of hens. Various intrinsic and extrinsic factors affect germ cell loss, such as germ cell nest breakdown in early life and nutritional deficiencies during daily husbandry. Here, we examined the effect of fasting on the germ cell number of chicks. The results showed that 72 h fasting resulted in a higher germ cell loss than that by 24 h fasting in chicks. The RNA-seq analysis revealed that the genes of ribosome pathway were down-regulated and the biological processes of protein processing in endoplasmic reticulum were inhibited in starved chicks. Furthermore, in female chicks treated with 72 h fasting, the qPCR of ovaries showed down-regulation of ribosome-related genes, and transmission electron microscopy imaging of ovaries showed fewer ribosomes. The blood biochemical indices indicated that 72 h fasting reduced the liver functions and affected the glucose metabolism, lipid metabolites and ion metabolites. In summary, the present results concluded negative impacts on the germ cell pool by prolonged fasting in the early life of chicks and manifested that adequate management should be cared for fasted time for breeding.
Key words: fasting, germ cell loss, chicken, protein synthesis
INTRODUCTION
Primordial follicle assembly is one of the most important reproduction events in the early life of female animals. To some extent, the number of primordial follicles indicates female fecundity as it represents the available follicles for their entire life. Most lost primordial follicles undergo the germ cell nest breakdown, and this phenomenon remains to be elucidated (Pepling, 2006). Mammal may lose two-thirds, whereas birds may lose even more. Typically, only 5% of follicles in avian ovaries complete the development process and mature to ovulation (Hall et al., 2020). Thus, preventing substantial follicle loss in hens is important with regard to the laying performance.
In poultry production, for ease of management, the chicks remain in the incubator for 1 or 2 d after hatch, after which they are transported from an incubation base to a breeding base, which may require at least 1 d and up to 3 d, during which little chicks face water and food shortage until they arrive at their destination (Jacobs et al., 2016; de Jong et al., 2020). The reduction in the body weight of chicks has been suggested to be inversely correlated with the duration of transportation time and relative humidity experienced during transport (Yerpes et al., 2021). Additionally, it has been demonstrated that depriving chicks of food and water for a period exceeding 28 h leads to negative effects on their final body weight (özlü et al., 2020). Moreover, under these conditions, the chicks experience germ cell nest breakdown. In mice, a reduced body energy balance may negatively affect ovarian follicle development (Meng et al., 2021), and starvation after birth also reduces germ cell nest breakdown and increases apoptosis of ovarian follicles (Wang et al., 2017). Whether starvation at early life history stages affects germ cell loss in chicks is unclear. We thus tested whether the external nutritional condition of chicks after hatching would result in germ cell nest breakdown and thus affects the number of germ cells.
Protein is one of the most important organic substances, and protein synthesis is an essential organismic process. Protein is also the main dry matter required for growth of chickens and egg development (Baker, 2009). Growth of chickens does not rely exclusively on protein synthesis but also requires free amino acids (Tomonaga et al., 2004; He et al., 2021). In mice, oocytes showing perturbations in protein metabolism indicated that proteostasis may impact gamete health (Duncan et al., 2017).
This study was conducted to produce RNA sequencing data from the ovary cortex isolated from differently fasting chicks to assess the effects on germ cell loss. Here, we identified a new signature pattern indicating that protein synthesis may be the way to affect the germ cell loss of ovaries in chicks fasting after hatching.
MATERIALS AND METHODS
Animal Experiments and Ethics Statement
A DAZL-mCherry gene editing rooster (Pu et al., 2023) used in this study were homozygous, and mCherry is specifically expressed in germ cells by the DAZL endogenous promoter in DAZL-mCherry gene edited chickens. The breeding eggs were incubated under 65% humidity and at 37.8°C. After fledging, all chicks were fed and maintained at 35°C with 24 h of light during the first 3 d, which was reduced to 23 h of light and 1 h of darkness from 3 d post-hatch (dph) to 7 dph. Thereafter, the light was reduced by 3 h per week until 28 dph. The study was approved by the Animal Experiments Ethics Committee of Guangxi University. All animal experiments were conducted according to the Guidelines for the Care and Use of Laboratory Animals.
Sample Collection
The animal experiments were conducted according to the guidelines for the Care and Use of Laboratory Animals. The female chicks used in this study were selected by vent identification after hatch. Then female chicks were divided into 3 groups, ten chicks in each group. The chicks were libitum feed at 24 h post-hatch in the control group, and the chicks were libitum feed at 48 h or 72 h post-hatch in the treatment group. Finally, the number of germ cells of chicks was quantified at 7 dph, and the RNA-seq of ovaries was performed at 3 dph by using carbon dioxide to euthanize chicks. At 3 dph, blood samples were collected and stored at room temperature for 30 min, followed by centrifugation at 3,000 rpm for 30 min to harvest serum, which was stored at -80°C.
Total Germ Cell Count at 7 dph Using Acoustic Focusing Flow Cytometry
The number of germ cells in chicks at 7 dph was determined by flow cytometry of DAZL-mCherry cells. At 7 dph, the chicks were killed to remove the ovaries. The ovarian tissue was digested into single cells using collagenase and trypsin as described previously (Pu et al., 2023). The digested cells were analyzed by acoustic focusing flow cytometry (Attune NxT, Invitrogen, Singapore). Voltage parameters were used as described (Pu et al., 2023): FSC was 150, SSC was 400, YL2 was 380. A volume of 300 µL sample was loaded.
Morphological Observation and Total Follicle Number Count at 28 dph
Chicks were killed at 7 and 28 dph to remove the whole ovary to prepare paraffin sections. First, ovaries were fixed in Bouin's fixative solution (PH0976, Phygene, China) for 24 h at 4°C. Then, the ovaries were washed using phosphate-buffered saline (PBS) and stored in 70% ethanol at 4°C. Then, ovaries were dehydrated using an ethanol concentration gradient from 75 to 100% and xylene, after which they were embedded in paraffin and were sectioned at a thickness of 5-µm using an automatic rotary microtome (RM2255, Leica, Germany). Paraffin sections were stained using hematoxylin and eosin (HE) using an HE Staining Kit (G1120, Solarbio, Beijing, China), according to the manufacturer's instructions. The sections were examined using a light microscope (FV3000, Olympus, Japan). Every tenth section of the ovaries of chicks at 28 dph was analyzed to determine the follicle number. Visible nuclei were counted and then summed to produce the total follicle number (Zhao et al., 2018).
RNA-seq and Quantitative Real-Rime PCR
Total RNA was isolated from ovaries of chicks at 3 dph using an E.Z.N.A. Total RNA Kit I (R6834, Omega Bio-tek, Norcross, GA). The experimental design involved the use of a control group consisting of chicks that underwent a 24 h fasting period and were subsequently killed at 3 dph. The treatment group, on the other hand, included chicks that underwent a 72 h fasting period and were killed. Each group was subjected to 3 replicate samples for analysis.The raw sequencing data reported in this study have been deposited in NCBI Sequence Read Archive (SRA) with the accession number PRJNA984594. Gene ontology (GO) and kyoto encyclopedia of genes and genomes (KEGG) pathway analyses were used to examine functional pathway. Gene set enrichment analysis (GSEA) was used to assess the probable molecular mechanisms. Protein-protein interaction networks for target genes were retrieved from the STRING database to assess the protein relationship among the ribosome.
Reverse transcription was conducted using a TransScript Uni All-in-One First-Strand cDNA Synthesis SuperMix for qPCR (AU341, Transgen, China), according to the manufacturer's instructions. Quantitative real-time PCR was performed using TB Green Premix Ex Taq Tli RNaseH Plus (RR420A, TaKaRa Biotechnology, Beijing, China) on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad, San Jose, CA). The amplification signal of the housekeeping gene β-actin was used to normalize the results. PCR primers sequences are shown in Table 1.
Table 1.
Primers used for qRT-PCR.
| Gene name | Accession no. | Primer sequence (5′-3′) | Product length/bp |
|---|---|---|---|
| RPL31 | NM_001277755.2 | F:CGATCTGCCATCAACGAGGT | 158 |
| R:GTGTCAATGCGAACGTCAGG | |||
| RPLP2 | XM_040673100.2 | F:TTGCAGCATACCTTCTCGCA | 107 |
| R:CAGGCGTTCATCGTCTGTCT | |||
| RPL36 | NM_204140.2 | F:ACAAGGGCTACAAGGTGACG | 205 |
| R:TGAGTGCCAACCCGTTTCTT | |||
| β-actin | NM_205518.1 | F:ACACCCACACCCCTGTGATGAA | 136 |
| R:TGCTGCTGACACCTTCACCATTC |
Abbreviations: RPL31, ribosomal protein L31; RPLP2, ribosomal protein lateral stalk subunit P2; RPL36, ribosomal protein L36.
Blood Amino Acid Concentrations
Serum samples were harvested from blood from chicks at 3 dph. Total amino acids were measured using commercially available kits (AA-1-W, Cominbio, Suzhou, China). Free amino acids in the supernatants were determined using liquid mass spectrometry (AB Sciex API4000, Singapore). Before detection, 300 µL serum was added to 600 µL anhydrous ethanol, followed by vortexing for 30 s. The mixture was centrifuged for 30 min at 14,000 rpm and at 4°C, and the supernatant was separated and concentrated under vacuum until it was dry. Then, 150 µL 50% acetonitrile was added, followed by filtration through a 0.22 µm membrane. The procedure was conducted according to determination of free amino acids (GB/T 30987-2020).
Ribosome Quantification Through Transmission Electron Microscopy (TEM)
Ovaries were isolated and fixed in 2.5% glutaraldehyde (EM Grade, Solarbio) at 3 dph. Samples were double-fixed with 2.5% glutaraldehyde in PBS (0.1M, pH 7.0) for over 4 h. Then, we used phosphate buffer (0.1M, pH 7.0) to wash the samples 3 times, 15 min each time. Thereafter, they were postfixed with 1% OsO4 in PBS for 1 to 2 h and were washed 3 times using phosphate buffer (0.1M, pH 7.0) for 15 min each time. Dehydration was performed: first using gradient ethanol (30%, 50%, 70%, and 80%) for approximately 15 min at each concentration and a second dehydration step using gradient acetone (90%, and 95%) for approximately 15 min at each step. Thereafter, the samples were dehydrated twice using absolute acetone for 20 min each time. Then, infiltration was performed: Each specimen was placed in a 1:1 mixture of absolute acetone and the final Spurr resin mixture for 1 h at room temperature. After this, was transferred to a 1:3 mixture of absolute acetone and the final resin mixture for 3 h and to the final Spurr resin mixture for overnight incubation. Specimens were then placed in Eppendorf tubes containing Spurr resin and were heated to 70°C for over 9 h. Each specimen was then sectioned using a LEICA EM UC7 ultratome (Leica), and sections were stained using uranyl acetate and alkaline lead citrate for 5 and 10 min, respectively. Images were observed under an H-7800 transmission electron microscope (Hitachi, Tokyo, Japan).
Serum Biochemical Indicators
Serum biochemical indicators including alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein (TP), albumin (ALB), direct bilirubin (DBIL), total bilirubin (TBIL), alkaline phosphatase (ALP), indirect bilirubin (IBIL), globulin (GLB), urea, triglycerides (TG), cholesterol (CHOL), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), glucose (GLU), immunoglobulin (Ig) G, IgA, IgM, calcium (Ca), iron (Fe), magnesium (Mg), and phosphorus (P) were determined using commercially available kits and Biochemical analyzer (8021AVet, URIT), all obtained from Guilin URIT Medical Electronic Co., Ltd. (Guangxi, China).
Statistical Analyses
Flow Jo v10.6.2 and GraphPad Prism 8were used to analyze and visualize the data, t tests for comparisons between 2 groups and one-way analysis of variance among multiple groups. Statistical significance is reported at P < 0.05.
RESULTS
Prolonged Fasting After Hatching Induces Loss of Ovarian Germ Cells
To gain insight into the effect of time access to feed in chicks post-hatch, morphological observations of ovaries were conducted at 7 dph (Figure 1A). Fasting time affected the ovaries, as chicks fasting for 24 and 48 h showed more germ cells at the cortex than that for 72 h. The germ cell number of different fasting time treatments at 7 dph was counted through the DAZL-mCherry germ cell (Figure 1B), with the DAZL-mCherry transgenic chicken model established in a previous study (Pu et al., 2023). The number of germ cells at 7 dph was reduced after prolonged fasting, as chicks fasted for 72 h showed the smallest number, and those fasted for 48 h did not differ significantly from the other groups (Figure 1B).
Figure. 1.
Quantification of ovarian germ cells in chickens at 7 dph. (A) Ovary morphologic observation in differently fasted groups at 7 dph. Red arrow indicates primordial follicle. Scale bar: 100 μm. (B) Germ cell counts of DAZL-mCherry germ cells through flow cytometry at 7 dph. Data are shown as the means ± S.E.M. of independent triplicate experiments. *P < 0.05. Abbreviations: dph, day post-hatch.
Germ Cell Numbers in Fasted Chickens Remain Low at 28 dph
In order to confirm whether the loss of follicles will revert, the number of total follicles at 28 dph were counted and the results displayed a decrease trend with increasing fasting time (Figure 2B), and chicks fasting for 72 h had fewer follicles at cortex than the other groups (Figure 2A).
Figure. 2.
Histological analysis and total follicle quantification of chicken ovaries at 28 dph. (A) Histological analysis of ovarian tissues at 28 dph of differently fasted groups. Red arrow indicates primordial follicle. Scale bars: 1 mm, 200 μm and 100 μm, respectively. (B) Comparison of the number of total follicles in differently fasted groups at 28 dph. Data are shown as the means ± S.E.M. *P < 0.05. Abbreviations: dph, day post-hatch.
Prolonged Fasting Alters Gene Expression Profiles in Chicken Ovaries at 3 dph
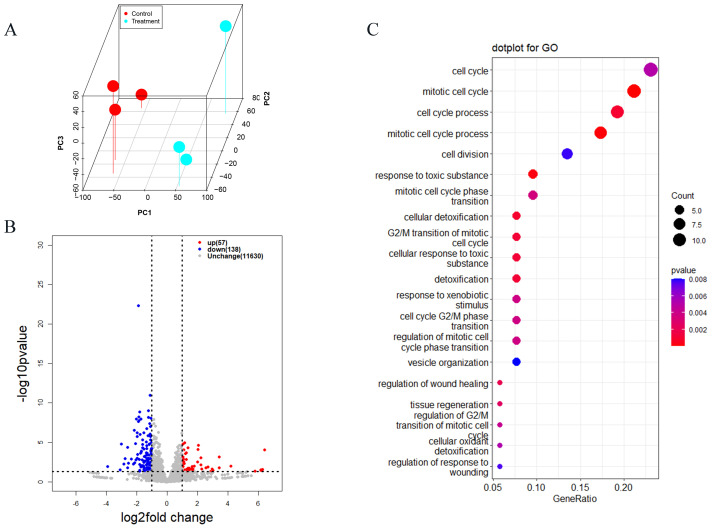
With the aim of shedding light on the underlying mechanism of germ cell loss after fasting post-hatch, 3 dph ovary cortex were used for RNA sequencing (RNA-seq). According to the principal component analysis (PCA), the RNA-seq data showed that biological replicates clustered together, which was consistent among replicates (Figure 3A). Samples from the 24 h fasted group were separate from those of 72 h fasted group. These findings suggested that the transcriptome profiles of the 2 groups differed significantly. Volcano plots of the significantly differentially expressed genes (DEGs) are shown in Figure 3B. According to the transcriptome data, 57 upregulated and 138 downregulated DEGs were identified in the ovaries of chicks fasted for 24 h, compared to those of chicks fasted for 72 h. GO analysis indicated numerous common downregulated DEGs that were significantly enriched for GO terms associated with the cell cycle, such as cell cycle, mitotic cell cycle, cell cycle process and mitotic cell cycle process (Figure 3C).
Figure. 3.
Analysis of RNA sequencing data of chicken ovaries at 3 dph. Control: chicks fasted for 24 h Treatment: chicks fasted for 72 h. (A) Principal component analysis (PCA) of the ovarian transcriptomic data from the 2 groups (n = 3). (B) Volcano plots showing the DEGs identified in chicks fasted for 24 h vs. 72 h. Red and blue dots indicate upregulated and downregulated DEGs. (C) Common upregulated and downregulated DEGs analyzed through GO enrichment analysis. Abbreviations: DEG, differentially expressed genes; dph, day post-hatch; GO, gene ontology.
Ribosome Pathway is Involved in Ovarian Germ Cell Loss in Fasted Chickens
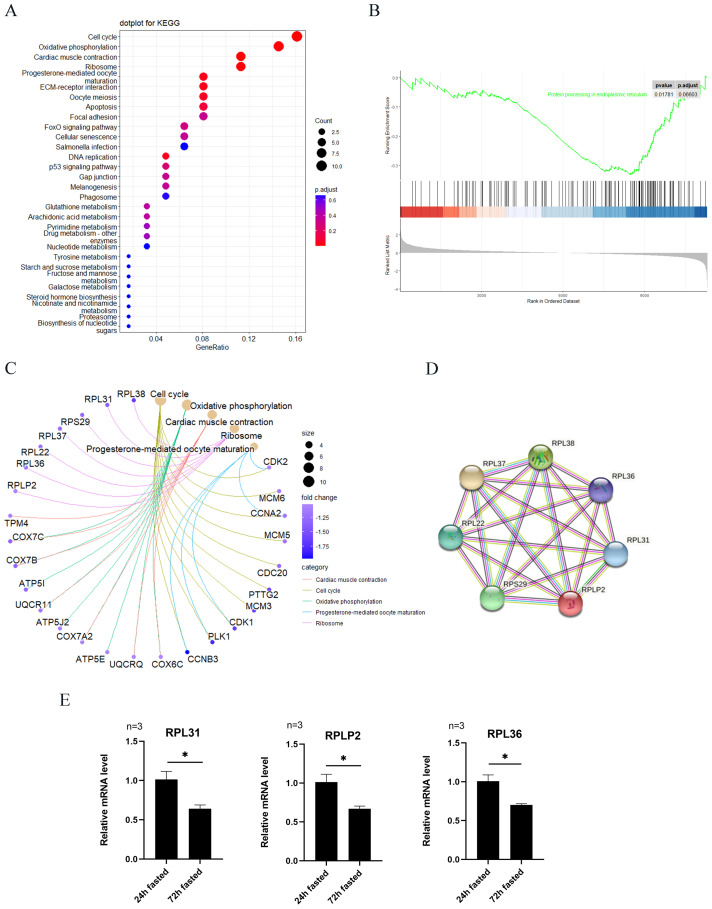
To find out which pathway is the major effector, using KEGG analysis on DEGs showed that downregulated pathways were enriched in the cell cycle, oxidative phosphorylation, cardiac muscle contraction, ribosome, progesterone-mediated oocyte maturation, extracellular matrix (ECM)-receptor interaction, and oocyte meiosis (Figure 4A). Related genes in different interaction pathways are shown in Figure 4C. The pathway categories included cardiac muscle contraction, cell cycle, oxidative phosphorylation, progesterone-mediated oocyte maturation, and ribosome pathways. GSEA analysis showed that the most probable biological process associated with the germ cell loss might be the genes downregulated in protein processing in endoplasmic reticulum (Figure 4B). The ribosome pathway is associated with protein processing, and combined with the KEGG analysis results, ribosome pathway may relate to the germ cell loss. Protein-protein interaction network for target genes were constructed that the predicted protein may be an interaction complex (Figure 4D). qRT-PCR results showed that the mRNA levels RPL31, RPLP2, and RPL36, which are associated with the ribosome, were lower after 72 h of fasting (Figure 4E).
Figure. 4.
KEGG analysis, GSEA analysis, and qRT-PCR validation. (A) Identification of target effectors of fasting in ovaries by KEGG analysis. (B) GSEA of the shared upregulated and downregulated DEGs. (C) DEGs analyzed by KEGG in significantly different pathways, respectively. (D) Retrieval of the ribosome pathway genes by protein-protein interaction network. (E) Three genes were selected to validate the relative mRNA expression by qRT-PCR (n = 3). Data are shown as the means ± SEM, and asterisks indicate significant differences (P < 0.05). Abbreviations: DEG, differentially expressed genes; KEGG, kyoto encyclopedia of genes and genome.
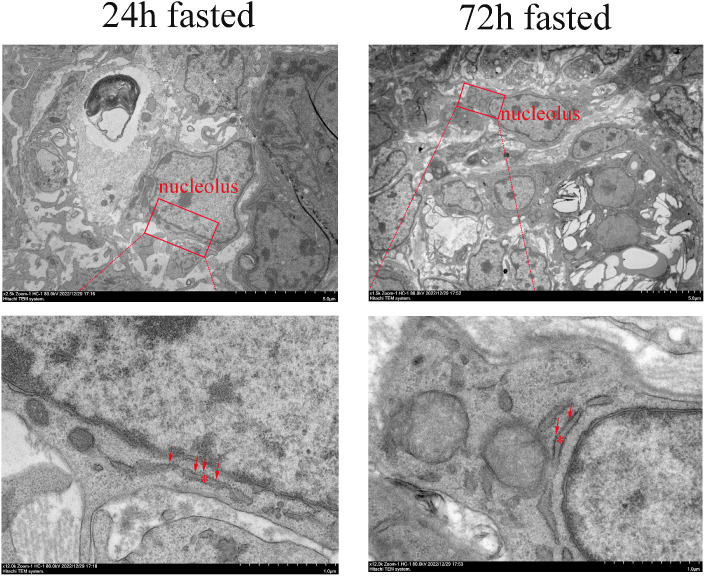
To know more about fasting acts on cells, transmission electron microscopy observations have done to find out how prolonged fasting affected germ cells inside (Figure 5). The results of different fasting times showed that germ cells in chicks fasted for 72 h had fewer ribosomes on the endoplasmic reticulum, which was in line with the RNA sequencing results.
Figure. 5.
TEM observation of the endoplasmic reticulum in germ cells of chicks 3 dph. Endoplasmic reticulum in germ cells observed by the TEM. The asterisk indicates the endoplasmic reticulum in germ cells. The red arrow indicates ribosomes on the endoplasmic reticulum. Scale bars: 5.0 μm and 1.0 μm, respectively. Abbreviations: dph, day post-hatch; TEM, transmission electron microscopy.
Prolonged Fasting Alters the Blood Biochemical Indices of Chickens
To determine whether fasting in chicks was associated with changes in processes such as enzymes, lipids, ions and immunoglobulins, we gage the blood biochemical indices. PCA confirmed high reproducibility among the replicates, and 24 h fasted as the control groups and 72 h fasted as the treatment group differed significantly (Figure 6A). ALT, AST, ALB, GLU, DBIL, IBIL, urea, and UA showed pronounced differences, indicating that long fasting reduced the liver functions and affected the glucose metabolism (Table 2). Indices of lipids (HDL-C, LDL-C) suggested that lipid metabolites may change after long fasting (Table 3). The indices of ions also showed substantial differences in ion metabolites, which were affected by the fasting time after hatching (Table 4). The immunoglobulins showed no significant difference (Table 5). AST, HDL-C, LDL-C, and urea were positively correlated with Fe levels, whereas DBIL, Mg, Ca, P, and GLU were negatively correlated with Fe (Figure 6B).
Figure. 6.
Analysis of blood biochemical indices and serum amino acids in chickens fasted for 3 dph.
Control: chicks fasted for 24 h treatment: chicks fasted for 72 h. (A) PCA of the blood biochemical indices. (B) Correlation analysis between biochemical blood indices. Red and blue arrows indicate downregulated and upregulated. (C) Detect the amino acids in the blood except for proline and hydroxyproline (n = 3). (D) Free serum amino acids detected by liquid mass spectrometry (n = 5). Shown are the means ± SEM. Abbreviations: dph, day post-hatch; PCA, Principal component analysis.
Table 2.
Effect of different starved time on serum biochemical indexes of chicks.
| Items | 24 h starved | 72 h starved | P-value |
|---|---|---|---|
| ALT, U/L | 8.10 ± 0.900a | 5.90 ± 0.526b | 0.0491 |
| AST, U/L | 187.10 ± 12.67b | 267.00 ± 16.29a | 0.0011 |
| ALB, g/L | 12.72 ± 0.7583b | 15.39 ± 0.5847a | 0.0121 |
| GLB, g/L | 15.26 ± 1.127a | 18.70 ± 2.074a | 0.1623 |
| TP, g/L | 34.27 ± 1.992a | 31.26 ± 1.965a | 0.2964 |
| ALP, U/L | 1538 ± 222.8a | 1646 ± 108.6a | 0.1903 |
| GLU, mmol/L | 7.181 ± 0.2857a | 3.301 ± 0.6602b | 0.0001 |
| DBIL, μmol/L | 21.61 ± 1.930 a | 9.690 ± 1.762b | 0.0002 |
| IBIL, μmol/L | 21.88 ± 2.068a | 10.10 ± 1.300b | 0.0001 |
| TBIL, μmol/L | 35.59 ± 4.278a | 21.97 ± 2.858a | 0.0164 |
| urea, mmol/L | 0.728 ± 0.1498b | 2.502 ± 0.1964a | 0.0001 |
Abbreviations: ALT, alanine aminotransferase;ALP, alkaline phosphatase; ALB, albumin;AST, aspartate aminotransferase;DBIL, direct bilirubin; IBIL, indirect bilirubin; GLB, globulin;GLU, glucose; TP, total protein;TBIL, total bilirubin.
Different letters on the shoulder in the same row indicated significant difference in the blood biochemical indices at the P < 0.05 significant level (n = 10).
The results are presented as means ± S.E.M.
Table 3.
Effects of different starved time on lipids in serum of chicks.
| Items | 24 h starved | 72 h starved | P-value |
|---|---|---|---|
| TG, mmol/L | 1.259 ± 0.1345a | 1.025 ± 0.0673a | 0.1371 |
| CHOL, mmol/L | 12.00 ± 1.113a | 10.42 ± 0.5121a | 0.2799 |
| HDL-C, mmol/L | 1.408 ± 0.1409b | 2.567 ± 0.2260a | 0.0004 |
| LDL-C, mmol/L | 2.163 ± 0.1659b | 3.859 ± 0.1609a | 0.0001 |
Abbreviations: CHOL, Cholesterol; HDL-C, high density liptein cholesterol; LDL-C, low density liptein cholesterol; TG, Triglyceride.
Different letters on the shoulder in the same row indicated significant difference in the lipids at the P < 0.05 significant level (n = 10).
The results are presented as mean ± S.E.M.
Table 4.
Effect of different starved time on ions in serum of chicks.
| Items | 24 h starved | 72 h starved | P-value |
|---|---|---|---|
| Ca, mmol/L | 4.995 ± 0.2283a | 1.650 ± 0.1578b | 0.0001 |
| Fe, μmol/L | 5.350 ± 0.6776b | 12.45 ± 1.076a | 0.0001 |
| Mg, mmol/L | 0.4686 ± 0.030a | 0.1420 ± 0.034b | 0.0001 |
| P, mmol/L | 1.315 ± 0.1217a | 0.161 ± 0.0810b | 0.0001 |
Abbreviations: Ca, Calcium; Fe, iron; Mg, Magnesium; P, Phosphorus.
Different letters on the shoulder in the same row indicated significant difference in the ions at the P < 0.05 significant level (n = 10).
The results are presented as mean ± S.E.M.
Table 5.
Effect of different starved time on immunoglobulins in serum of chicks.
| Items | 24 h starved | 72 h starved | P-value |
|---|---|---|---|
| IgG, g/L | 0.173 ± 0.0870a | 0.182 ± 0.0769a | 0.9391 |
| IgA, g/L | 0.042 ± 0.0067a | 0.086 ± 0.0228a | 0.3392 |
| IgM, g/L | 0.128 ± 0.0555a | 0.108 ± 0.0618a | 0.8125 |
Abbreviations: IgG, immunoglobulin G; IgA, immunoglobulin A; IgM, immunoglobulin M.
Different letters on the shoulder in the same row indicated significant difference in the immunoglobulins at the P < 0.05 significant level (n = 10). The results are presented as mean ± S.E.M.
Total amino acids in blood (except proline and hydroxyproline) produced no significant difference between groups (Figure 6C), whereas free amino acids indicated that the amino acid metabolism in chicks fasted for 72 h was altered (Figure 6D). TP detected in blood showed no difference as same as the result in total amino acids in the blood.
DISCUSSION
Newborn animals commonly experience fasting, and mice have been shown to adapt their metabolic and oxidative responses in the ovaries leading to an increase in apoptosis of somatic cells and oocytes due to nutrient deficiency at birth (Wang et al., 2017). In addition, whole body energy fluxes may have adverse effects on ovarian follicle development and ovulation (Meng et al., 2021). Conversely, chicks given early access to feed and water may compensate for the developmental disadvantage experienced at hatching (van de Ven et al., 2013). The current study, found that the weight of chicks at 7 dph was correlated with the duration of fasting (S1). Further, more, we used a DAZL-mCherry gene-edited chicken with self-emitting red fluorescence to monitor germ cells after various fasting periods. In our previous study, we found that germ cell abundance was correlated with body weight (Pu et al., 2023), and the results of the current study confirmed that fasting for 72 h may lead to decreased body weight and increased loss of germ cells.
Perinatal germ cell loss is a developmentally regulated process caused by germ cell cyst breakdown (Pepling and Spradling, 2001). There is contrasting evidence to our current research, as a recent study suggested that nutrient starvation in neonatal subjects induced autophagy (Kuma et al., 2004). Activation of autophagy promotes primordial follicles formation in mice (Watanabe and Kimura, 2018; Sun et al., 2020; Watanabe et al., 2020). Interestingly, previous studies showed opposite results, that is, starvation negatively affects mouse oocyte development (Wang et al., 2017; Meng et al., 2021). Our results showed that early-stage fasting in chickens may lead to an increase in germ cell loss. In mice, the number of follicles can be restored to a normal level after 3 wk of re-feeding (Wang et al., 2017). In contrast, follicle loss remains irreversible at 28 dph, when the primordial pool is established in chickens. The structure of the yolk sac, which is a multifunctional organ supplying nutrients, differs between chickens and mice (Wong and Uni, 2021), which may explain the discrepancy in results in starvation studies.
RNA-seq suggested the potential effectors of fasting, and GO analysis showed that starvation downregulated the expression of genes related to the cell cycle progression with regard to GO term including, cell cycle, mitotic cell cycle, cell cycle process, and mitotic cell cycle process. KEGG analysis revealed that the common downregulated DEGs were enriched with respect to the cell cycle, oxidative phosphorylation, cardiac muscle contraction, ribosome, progesterone-mediated oocyte maturation, ECM-receptor interaction, and oocyte meiosis. Previous studies showed that activation of these pathways might inhibit the maturation of oocytes, and they may be the potential therapeutic targets for ovarian cancer patients (Pomerantz and Bilello, 1987; Feng et al., 2019). Thus, fasting diets are frequently used in cancer therapy (Blazevits et al., 2023). Adequate protein synthesis is key to meiotic and cytoplasmic competence during oogenesis (Eichenlaub-Ritter and Peschke, 2002). Aging may cause protein metabolism dysregulation, leading to a decline in oocyte quality (Duncan et al., 2017). Using KEGG and GSEA analyses, we focused on the ribosome pathway correlated with protein processing in the endoplasmic reticulum, which may be the main effector of induced germ cell loss. A lack of translational fidelity in ribosomes may result in poor-quality proteins (Duncan et al., 2017). The mRNA levels of the genes RPL31, RPLP2, and RPL36 were also downregulated after 72 h fasting. Ribosome biogenesis is associated with the shape, size, and number of nucleoli (Lafita-Navarro and Conacci-Sorrell, 2023). TEM examination of germ cells showed that the nucleoli in chicks fasted for 72 h seems like smaller than those of chicks fasted for 24 h, suggesting a lower ability for protein synthesis; further, the number of ribosomes on the endoplasmic reticulum seems like lower after 72 h of fasting. The ribosomes on the endoplasmic reticulum are important structures for protein synthesis, and damage to them may result in disturbed protein metabolism.
Serum biomarkers are widely used in disease detection (Chen et al., 2022; Mirshafiei et al., 2022). Serum citrulline levels are associated with food intake in mice (Park et al., 2019). The levels of total amino acids in serum showed no significant difference between treatments. However, the abundance of free amino acids altered after fasting suggested essential amino acid supplementation through the breakdown of non-essential amino acids to maintain the balance for survival.
Taken together, we used multimodal approaches to confirm that fasting is correlated with protein metabolism during the period of chicken germ cell loss. Chicks fasting for 72 h showed significant germ cell loss, starvation for 72 h may significantly affect egg production. This study highlights the importance of proper nutrition and feeding regimens during husbandry to promote healthy germ cell development in chickens. Furthermore, this study provides insights into the biological mechanisms involved in germ cell loss, with the ribosome pathway identified as a major factor, which may aid in the development of targeted interventions to prevent germ cell loss. Despite the valuable insights gained from this study, several limitations should be considered. The study was limited by the use of a gene-edited chicken model and may not fully reflect the impact of fasting on germ cell development in other chicken populations.
ACKNOWLEDGMENTS
This study was jointly supported by the National Key R&D Program of China (2021YFD1300100), Guangxi Key R&D Program (AB21220005)and National Natural Science Foundation of China (31960157).
DISCLOSURES
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.102815.
Appendix. Supplementary materials
References
- Baker D.H. Advances in protein-amino acid nutrition of poultry. Amino Acids. 2009;37:29–41. doi: 10.1007/s00726-008-0198-3. [DOI] [PubMed] [Google Scholar]
- Blazevits O., Di Tano M., Longo V.D. Fasting and fasting mimicking diets in cancer prevention and therapy. Trends Cancer. 2023;9:212–222. doi: 10.1016/j.trecan.2022.12.006. [DOI] [PubMed] [Google Scholar]
- Chen Z., Ma Y., Cai J., Sun M., Zeng L., Wu F., Zhang Y., Hu M. Serum biomarkers for liver fibrosis. Clin. Chim. Acta. 2022;537:16–25. doi: 10.1016/j.cca.2022.09.022. [DOI] [PubMed] [Google Scholar]
- de Jong I.C., van Hattum T., van Riel J.W., De Baere K., Kempen I., Cardinaels S., Gunnink H. Effects of on-farm and traditional hatching on welfare, health, and performance of broiler chickens. Poult. Sci. 2020;99:4662–4671. doi: 10.1016/j.psj.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan F.E., Jasti S., Paulson A., Kelsh J.M., Fegley B., Gerton J.L. Age-associated dysregulation of protein metabolism in the mammalian oocyte. Aging Cell. 2017;16(6):1381–1393. doi: 10.1111/acel.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenlaub-Ritter U., Peschke M. Expression in in-vivo and in-vitro growing and maturing oocytes: focus on regulation of expression at the translational level. Hum. Reprod. Update. 2002;8:21–41. doi: 10.1093/humupd/8.1.21. [DOI] [PubMed] [Google Scholar]
- Feng H., Gu Z.Y., Li Q., Liu Q.H., Yang X.Y., Zhang J.J. Identification of significant genes with poor prognosis in ovarian cancer via bioinformatical analysis. J. Ovarian. Res. 2019;12:35. doi: 10.1186/s13048-019-0508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G.B., Long J.A., Wood B.J., Bedecarrats G.Y. Germ cell dynamics during nest breakdown and formation of the primordial follicle pool in the domestic turkey (Meleagris gallopavo) Poult. Sci. 2020;99:2746–2756. doi: 10.1016/j.psj.2019.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W., Li P., Wu G. Amino acid nutrition and metabolism in chickens. Adv. Exp. Med. Biol. 2021;1285:109–131. doi: 10.1007/978-3-030-54462-1_7. [DOI] [PubMed] [Google Scholar]
- Jacobs L., Delezie E., Duchateau L., Goethals K., Ampe B., Lambrecht E., Gellynck X., Tuyttens F.A.M. Effect of post-hatch transportation duration and parental age on broiler chicken quality, welfare, and productivity. Poult. Sci. 2016;95:1973–1979. doi: 10.3382/ps/pew155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma A., Hatano M., Matsui M., Yamamoto A., Nakaya H., Yoshimori T., Ohsumi Y., Tokuhisa T., Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- Lafita-Navarro M.C., Conacci-Sorrell M. Nucleolar stress: from development to cancer. Semin. Cell Dev. Biol. 2023;136:64–74. doi: 10.1016/j.semcdb.2022.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L., Coleman V., Zhao Y., Ost M., Voigt A., Bunschoten A., Keijer J., Teerds K., Klaus S. Pseudo-starvation driven energy expenditure negatively affects ovarian follicle development. Int. J Mole. Sci.s. 2021;22:3557. doi: 10.3390/ijms22073557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirshafiei H., Darroudi S., Ghayour-Mobarhan M., Esmaeili H., AkbariRad M., Mouhebati M., Ferns G.A. Altered triglyceride glucose index and fasted serum triglyceride high-density lipoprotein cholesterol ratio predict incidence of cardiovascular disease in the Mashhad cohort study. Biofactors. 2022;48:643–650. doi: 10.1002/biof.1816. [DOI] [PubMed] [Google Scholar]
- Özlü S., Uçar A., Romanini C.E.B., Banwell R., Elibol O. Effect of posthatch feed and water access time on residual yolk and broiler live performance1. Poult. Sci. 2020;99:6737–6744. doi: 10.1016/j.psj.2020.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.J., Shaughnessy M.P., Armenia S.J., Cowles R.A. Serum citrulline levels exhibit circadian variation and fluctuations in relation to food intake in mice. Gastroenterol. Res. 2019;12:88–92. doi: 10.14740/gr1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepling M.E. From primordial germ cell to primordial follicle: mammalian female germ cell development. Genesis. 2006;44:622–632. doi: 10.1002/dvg.20258. [DOI] [PubMed] [Google Scholar]
- Pepling M.E., Spradling A.C. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev. Biol. 2001;234:339–351. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- Pomerantz S.H., Bilello P.A. Inhibition of progesterone-mediated maturation of oocytes of Xenopus laevis by oocyte maturation inhibitor from pig follicular fluid: development of a routine assay for the inhibitor with Xenopus oocytes. Gamete Res. 1987;17:267–278. doi: 10.1002/mrd.1120170310. [DOI] [PubMed] [Google Scholar]
- Pu L., Xie L., Chen J., Sun H., Huang Z., Xu T., Tian K., Zhong J., Xu H., Liu X., Lu Y. Tracking the dynamics of female germ cell development during peri-hatch periods using a gene-edited chicken model. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Klinger F.G., Liu J., De Felici M., Shen W., Sun X. miR-378-3p maintains the size of mouse primordial follicle pool by regulating cell autophagy and apoptosis. Cell Death Dis. 2020;11:737. doi: 10.1038/s41419-020-02965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomonaga S., Tachibana T., Takagi T., Saito E.S., Zhang R., Denbow D.M., Furuse M. Effect of central administration of carnosine and its constituents on behaviors in chicks. Brain Res. Bull. 2004;63:75–82. doi: 10.1016/j.brainresbull.2004.01.002. [DOI] [PubMed] [Google Scholar]
- van de Ven L.J.F., van Wagenberg A.V., Decuypere E., Kemp B., van den Brand H. Perinatal broiler physiology between hatching and chick collection in 2 hatching systems. Poult. Sci. 2013;92:1050–1061. doi: 10.3382/ps.2012-02534. [DOI] [PubMed] [Google Scholar]
- Wang Y., Sun Y., Sun X., Cheng S., Li B., Zhang X., De Felici M., Shen W. Starvation at birth impairs germ cell cyst breakdown and increases autophagy and apoptosis in mouse oocytes. Cell Death Dis. 2017;8:e2613. doi: 10.1038/cddis.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe R., Sasaki S., Kimura N. Activation of autophagy in early neonatal mice increases primordial follicle number and improves lifelong fertility†. Biol. Reprod. 2020;102:399–411. doi: 10.1093/biolre/ioz179. [DOI] [PubMed] [Google Scholar]
- Watanabe R., Kimura N. Non-suckling starvation of neonatal mice promotes primordial follicle formation with activation of ovarian autophagy. J. Reprod. Dev. 2018;64:89–94. doi: 10.1262/jrd.2017-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E.A., Uni Z. Centennial review: the chicken yolk sac is a multifunctional organ. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerpes M., Llonch P., Manteca X. Effect of environmental conditions during transport on chick weight loss and mortality. Poult. Sci. 2021;100:129–137. doi: 10.1016/j.psj.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Zhang Y., Li J., Zheng N., Xu X., Yang J., Xia G., Zhang M. MAPK3/1 participates in the activation of primordial follicles through mTORC1-KITL signaling. J. Cell Physiol. 2018;233:226–237. doi: 10.1002/jcp.25868. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.