Abstract
Between 2018 and 2020, over 100 wild turkey fecal samples were collected from the Eastern and Central thirds of the United States, where commercial turkey production is uncommon. We hypothesized that anticoccidial-sensitive Eimeria spp. would be present in wild turkey fecal samples. Samples containing Eimeria spp. oocysts were amplified in vivo. If propagation was successful, the samples were PCR-speciated and subjected to anticoccidial sensitivity testing (AST) for key members of both ionophore and chemical categories of anticoccidial drugs. The purpose of this study was to isolate Eimeria spp. relevant to commercial turkey production that possessed sensitivity to monensin, zoalene, and amprolium. Future research would evaluate the efficacy of wild turkey Eimeria spp. as vaccine candidates for reducing coccidiosis in commercial turkey flocks utilizing single oocyst-derived stocks obtained in the present study.
Key words: coccidiosis, turkey, Eimeria, anticoccidial sensitivity testing, speciation
INTRODUCTION
Intestinal coccidiosis caused by Eimeria, which are host specific and have evolved to infect different locations of the gastrointestinal tract, causes significant economic losses for the poultry industry (Chapman, 2008; Chapman and Jeffers, 2014). There has been insufficient research on the incidence and diversity of Eimeria spp. in North American wild turkey populations (MacDonald et al., 2019). Currently, PCR-based speciation is necessary to differentiate between turkey Eimeria spp. followed by sequencing of PCR products to capture false positives due to considerable sequence homology between some of the species (El-Sherry et al., 2013). Molecular detection of Eimeria spp. in turkeys has primarily been restricted to commercial turkeys in the United States or Canada (Imai and Barta, 2019; Duff et al., 2022) or a single report evaluating Eimeria spp. circulating in wild turkey populations in Canada (MacDonald et al., 2019).
Anticoccidial drugs have been used to prevent coccidiosis in commercial poultry. Alternative strategies to control coccidiosis are needed considering the evidence of multidrug resistant phenotypes affecting commercial turkeys (Rathinam and Chapman, 2009). Anticoccidial sensitivity testing (AST) can be used for monitoring drug programs to ensure the proper use of anticoccidial drugs and vaccination strategies to control coccidiosis in a commercial setting (Cervantes and McDougald, 2022). Since wild turkey populations harbor Eimeria spp. that are significant to commercial turkey production (MacDonald et al., 2019), we predicted that drug-sensitive strains would be present in wild turkey fecal samples collected from locations devout of commercial turkey facilities and could be used as potential vaccine candidates in commercial turkeys to displace drug-resistant phenotypes in the barn. Mathis and McDougald (1989) demonstrated that vaccination with drug-sensitive Eimeria spp. sufficiently improve sensitivity to zolene and amprolium in a commercial turkey flock. However, to our knowledge, there are no published studies on the restoration of anticoccidial sensitivity using drug-sensitive Eimeria spp. specifically obtained from wild turkeys. The objective of the current study was to obtain drug-sensitive strains of wild turkey-derived Eimeria adenoeides, Eimeria gallopavonis, Eimeria meleagrimitis, Eimeria meleagridis, and Eimeria dispersa. If effective, these drug-sensitive Eimeria species could be used to create a multispecies live coccidiosis vaccine with potential application in commercial turkey flocks.
MATERIALS AND METHODS
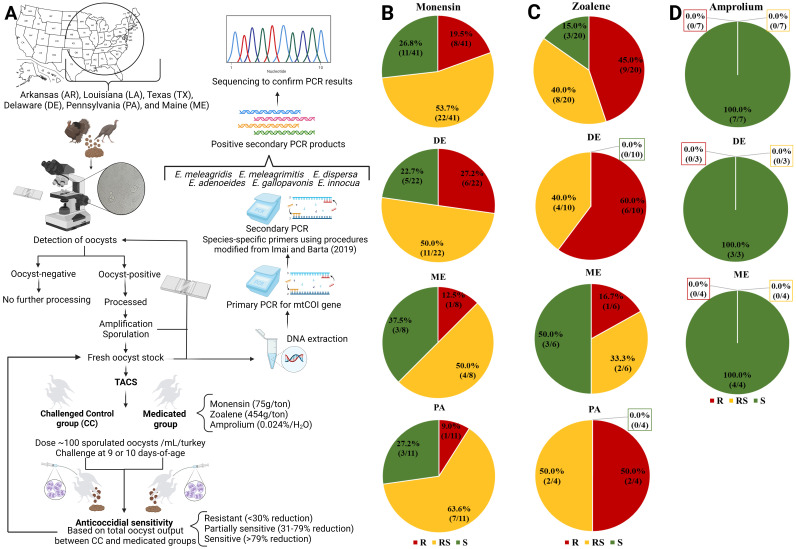
A schematic representation of the material and methods is presented in Figure 1A.
Figure 1.
(A) A schematic of the methodology used to detect, isolate, speciate, and determine anticoccidial sensitivity of Eimeria spp. isolated wild turkey feces collected from various states in the United States. Anticoccidial Sensitivity monensin (B), zoalene (C), and amprolium (D) testing (AST) of oocyst-positive samples recovered postamplification from Delaware (DE), Pennsylvania (PA), or Maine (ME). Classification: <30% reduction, R = resistant; 31 to 79% reduction, RS = reduced sensitivity; >79% reduction, S=sensitive. Created with BioRender.
Recovery of Wild Turkey Eimeria spp.: Across the Eastern and Central thirds of the country, individuals and state authorities collected fecal samples from wild turkeys. Samples were transferred to the University of Arkansas on wet ice packs. Oocyst-positive fecal samples were partially purified postamplification following previously described method (Imai and Barta, 2019). In brief, fresh fecal samples were suspended in a sterile saturated salt solution (1:3 w/v), blended for 30 to 60 sec, then sieved to remove coarse debris. The filtrate was centrifugated at 1,250 × g for 10 min to float oocysts away from fecal debris. The supernatant containing oocysts was decanted, diluted in sterile distilled water (10x), and centrifugated again at 1,250 × g for 10 min to pellet the oocyst. The pellet was resuspended in 2.5% potassium dichromate (w/v, aqueous) and transferred to Erlenmeyer flasks capped with sterile gauze at ∼100,000 oocyst/mL to permit adequate aeration. The flask was placed on a rotary platform shaker operating at ∼100 rpm at 26°C for 3 to 4 d or until sporulation was confirmed by microscopy.
Animal Source: Turkey hen poults donated by a local integrator were utilized for in vivo amplification or drug testing. Animal care and handling procedures complied with the University of Arkansas Institutional Animal Care and Use Committee (Animal Use Protocol #21026).
DNA Extraction: DNA was isolated from sporulated oocysts using methods previously described by El-Sherry et al. (2015) with slight modifications. Sporulated oocysts were transferred to a 1.5 mL microcentrifuge tube and pelleted by centrifugation 1,200 × g for 2 min. The pelleted oocysts were resuspended in 100 μL DNAzol reagent (Invitrogen Life Technologies Inc., Cat. No. 10503027, Grand Island, NY), and sterile 0.5 mm glass beads were added until a layer of dry beads was observed above the liquid surface of the sample. Contents were vortexed for approximately 60 sec. Oocyst breakage was confirmed microscopically, and additional rounds of disruption were used until most of the oocysts were lysed. Once sufficient oocyst breakage was confirmed microscopically, an additional 900 μL of DNAzol was added. The tubes were placed on a rocker at room temperature for a minimum of 18 h. Postincubation, the sample was centrifuged at 13,000 × g for 15 min at 4°C. The supernatant was transferred to a new 1.5 mL microcentrifuge tube to remove insoluble debris and glass beads. Pelleted DNA was added to 500 μL of 100% ethanol (Life Technologies, Cat. No. T038181000, Grand Island, NY) and mixed by inversion, and kept at room temperature for 5 min, followed by centrifugation at 14,000 × g for 8 min at 4°C. The pelleted DNA was washed twice with 500 μL of 70% cold ethanol at 4°C, then mixed by centrifugation at 10,000 × g for 5 min. The DNA pellet was air-dried by inversion and was then resuspended in 40 μL of EB Buffer (Qiagen, Cat. No. 19086 / 10mM Tris-Cl). A BioTek spectrophotometer (BioTek Synergy HT, Winooski, VT) was used to determine the purity and quantity of DNA. After the spectrophotometric-generated concentration (A260/A280 ratio should be 1.7–1.9) and molecular weight from 20 to 100 Kb, an aliquot of DNA was analyzed by agarose gel electrophoresis to confirm. DNA samples were stored at −20°C.
Speciation and Identification by Polymerase Chain Reaction (PCR)
Primary PCR: Purified gDNA was used for primary PCR to amplify Eimeria species-specific mitochondrial oxidase 1 (mt COI) gene, a 1,272-base pair (bp) fragment, as described by Imai and Barta (2019). LongAmp Taq PCR kit (#E5200S New England Biolabs, Ipswich, MA) was used for PCR per the manufacturer's instructions. Magnesium sulfate was not included in the reaction. Primers and nuclease-free water were obtained from Integrated DNA Technologies (Cat No. 10-05-01-14, Coralville, IA). In the present study, each primary PCR tube consisted of 2 μL purified genomic DNA (50 ng), 5X PCR Buffer, 10 mM dNTPs, 2,500 units/mL LongAmp Taq DNA Polymerase, 0.4 μM forward primer, 0.4 μM reverse primer, and nuclease-free water to make up a final volume of 50 μL, using Applied Biosystems SimpliAmp Thermal Cycler (ThermoFisher, Cat. No. A24811, Waltham, MA). The product size was estimated by electrophoresis using 1% agarose gel containing 5 μL/100 mL agarose of ethidium bromide in 1X Tris-Acetate-EDTA (TAE) buffer (40 mM Tris, 20 mM acetic acid, 1 mM EDTA) at 80 V and 150 mA for ∼60 min. Primary PCR products estimated to be ∼1,272 kb in size were purified using Pure Link PCR purification Kit (Life Technologies, Cat. No. K310001, Grand Island, NY), and electrophoresis was repeated to confirm the size of the purified primary PCR. Purified primary PCR products were diluted at a 1:10 ratio in nuclease-free water with a final volume of 20 μL.
Nested PCR. A species-specific PCR-based assay using diluted primary PCR products as templates were conducted. Briefly, 2.0 µL aliquots of each diluted (1:10) primary PCR product were used as templates for secondary PCR amplification using species-specific primers for E. adenoeides, E. gallopavonis, E. dispersa, E. innocua, E. meleagridis, or E. meleagrimitis based on Imai and Barta (2019) with minor modifications in annealing temperatures. The resultant PCR products were separated using a 1% or 2% agarose gel as described above depending on the expected size of the product. All positive-nested PCR products were sent to Eton Bioscience (Eton Bioscience, Inc., Raleigh, NC) for Sanger sequencing as secondary confirmation. Sequences were evaluated by mapping sample sequences to reference sequences obtained from the NCBI database using Geneious bioinformatics software version 2021.2.
Anticoccidial Sensitivity Testing (AST): To assess anticoccidial sensitivity, the reduction in total oocyst output on a volumetric basis for the medicated, challenged group was compared to the nonmedicated, challenged control group (CC). There were 6 turkeys utilized per sample/evaluation (n = 3 CC, n = 3 medicated). The anticoccidial drugs evaluated included monensin (75 g/ton of feed), zoalene (454 g/ton of feed), and amprolium (0.024% in drinking water). Drug concentrations aligned with manufacturer's guidelines and were administered in the diet 48 h prior to challenge similar to methods described by Cervantes and McDougald (2022). Between 9 and -10 d of age, 100 sporulated oocysts (aged <6 mo) were orally administered to respective CC and medicated groups. Total fecal output from d 5 to 9 postchallenge was collected and pooled. Based on total oocyst output between CC and medicated groups, samples were deemed resistant (<30% reduction), partially sensitive (31–79% reduction), or sensitive (>79% reduction) to monensin, zoalene, or amprolium.
Single Oocyst-Derived Stocks: A single sporulated oocyst was extracted from a mixed species sample and was administered by oral gavage to a single turkey poult between 12 and 14 d of age to obtain single oocyst-derived stocks as described by El-Sherry et al. (2015).
RESULTS AND DISCUSSION
There were 106 wild turkey fecal samples recovered across AR, LA, TX, DE, PA, and ME. Microscopic evaluation confirmed that 78.3% (83/106) of the samples were oocyst-positive, including 100% of the samples collected from DE (36/36), PA (38/38), or ME (9/9). These data suggest that Eimeria cycling is ongoing in wild turkey populations in these regions. Of the 83 oocyst-positive samples obtained in the present study, oocysts were successfully recovered from 41/83 (49.4%) samples after amplification (in vivo passage through Eimeria-free turkey poults) that was necessary to obtain a fresh oocyst stock for speciation for all samples obtained from 22 DE, 11 PA, and 8 ME. These samples were subjected to monensin AST. There were 11/41 (26.8%) samples that possessed sensitivity, 22/41 (53.7%) samples had reduced sensitivity, and 8/41 (19.5%) samples were resistant to monensin (Figure 1B). In the present study, only 20/41 (48.8%) of the wild turkey Eimeria spp. were considered to be sensitive to monensin (>70% reduction in total oocyst output) were subjected to zoalene AST. Of the 20 samples, 9/20 (45%) were resistant, 8/20 (40%) had reduced sensitivity, and 3/20 (15%) were sensitive to zoalene (Figure 1C). To our knowledge, there are no reports of zoalene sensitivity for Eimeria spp. recovered from wild turkey populations. Although only 3 Eimeria spp. samples recovered from ME exhibited true sensitivity to zoalene, the “leakiness,” similar to what is observed with ionophorous anticoccidial drugs, related to the increased excretion of oocysts after drug treatment may have skewed the total oocyst output results in the present study. Speciation results for 35/41 samples subjected to monensin and/or zoalene AST are shown in Table 1. More than 1 Eimeria spp. was detected in 25/35 (71.4%) of the samples, whereas 10/35 (28.6%) samples contained only E. meleagrimitis. The prevalence of E. meleagrimitis, E. dispersa, and E. adenoeides in wild turkey fecal samples collected in the present study was 85.7%, 54.3%, and 42.8%, respectively (Table 1). Samples with reduced or complete sensitivity to monensin and zoalene were selected as potential vaccine candidates, used to generate single oocyst-derived stocks. For candidates recovered from the cloning process, an amplification step was required to obtain enough oocysts to confirm recovery of a single Eimeria sp. by PCR and sequencing, and to re-confirm sensitivity to monensin, zoamix, and amprolium. Seven stocks were generated for amprolium AST, and 7/7 (100%) were confirmed to be sensitive to amprolium (Figure 1D).
Table 1.
Speciation of wild turkey Eimeria spp. propagated from fecal samples collected in Delaware (DE), Pennsylvania (PA), and Maine (ME).
| Location | E. adenoeides | E. dispersa | E. gallopavonis | E. innocua | E. meleagridis | E. meleagrimitis |
|---|---|---|---|---|---|---|
| DE | 10/17 (58.8) | 10/17 (58.8) | 2/17 (11.8) | 3/17 (17.6) | 2/17 (11.8) | 15/17 (88.2) |
| PA | 4/10 (40.0) | 7/10 (70.0) | 1/10 (1.0) | 5/10 (50.0) | 1/10 (1.0) | 8/10 (80.0) |
| ME | 1/8 (12.5) | 2/8 (25.0) | 0/8 (0.0) | 0/8 (0.0) | 1/8 (12.5) | 6/8 (75.0) |
| Total | 15/35 (42.8) | 19/35 (54.3) | 3/35 (8.5) | 8/35 (22.9) | 4/35 (11.4) | 30/35 (85.7) |
Data presented as positive/total (%). Positive species-specific PCR products were subjected to sequencing to confirm results.
E. meleagrimitis, E. meleagridis, E. dispersa, E. gallopavonis, E. adenoeides and E. innocua are prevalent in wild turkey populations in the Eastern and Central thirds of the United States based on our speciation results (Table 1). These findings were similar to Imai and Barta (2019) who detected the same 6 species in commercial turkey flocks in Canada. However, only 4 of the 6 Eimeria spp. were prevalent in commercial turkeys in the Midwestern United States (Duff et al., 2022). It is important to note that the PCR-positive products were not sequenced to confirm PCR results (Duff et al., 2022). In contrast, PCR-positive species-specific PCR products were sequenced in the current study and the study published by Imai and Barta (2019). The most prevalent Eimeria sp. collected in the current study was E. meleagrimitis. This aligns with others reporting a high prevalence of E. meleagrimitis detected by PCR in commercial or wild turkey populations in North America (Imai and Barta, 2019; Duff et al., 2022). The prevalence of E. meleagrimitis and E. adenoeides in the wild turkey fecal samples were similar to those published by Imai and Barta (2019) and Duff et al. (2022). E. dispersa prevalence was detected at low levels in commercial turkey flocks in Canada (Imai and Barta, 2019) or absent in commercial turkey flocks in the Midwestern United States (Duff et al., 2022), but detected frequently in the wild turkey samples collected in the current study. The low prevalence of E. meleagridis in the present study was similar to a report by Duff et al. (2022). Coinfection with more than 1 Eimeria spp. in the present study suggests that wild turkeys may be effective reservoirs potentially impacting commercial turkey operations. With help from co-operators (hunters, state wildlife departments, and wildlife refuges) in the present study, isolated 6 of the 7 described turkey Eimeria spp. suggesting that these protozoa are readily circulating in wild turkey populations. Immunogenicity studies and additional AST for single oocyst-derived stocks obtained in the present study are underway.
ACKNOWLEDGMENTS
Thank you to the hunters, state wildlife departments, and wildlife refuges that were eager to assist with sample collection in the field. This project was funded by USDA Animal Health Awards (FY2021 & FY2022), and by USDA-NIFA Sustainable Agriculture Systems, Grant No. 2019-69012-29905. Title of Project: Empowering U.S. Broiler Production for Transformation and Sustainability USDA-NIFA (Sustainable Agriculture Systems): No. 2019-69012-29905.
DISCLOSURES
The authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.102819.
Appendix. Supplementary materials
REFERENCES
- Cervantes H.M., McDougald L.R. The use of anticoccidial sensitivity tests (ASTs) by the poultry industry. Avian Dis. 2022;66:1–5. doi: 10.1637/21-00110. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. Coccidiosis in the turkey. Avian Pathol. 2008;37:205–223. doi: 10.1080/03079450802050689. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Jeffers T.K. Vaccination of chickens against coccidiosis ameliorates drug resistance in commercial poultry production. Int. J. Parasitol. Drugs Drug Resist. 2014;4:214–217. doi: 10.1016/j.ijpddr.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff A.F., Briggs W.N., Bielke J.C., McGovern K.E., Trombetta M., Abdullah H., Bielke L.R., Chasser K.M. PCR identification and prevalence of Eimeria species in commercial turkey flocks of the Midwestern United States. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sherry S., Ogedengbe M.E., Hafeez M.A., Barta J.R. Divergent nuclear 18S rDNA paralogs in a turkey coccidium, Eimeria meleagrimitis, complicate molecular systematics and identification. Int. J. Parasitol. 2013;43:679–685. doi: 10.1016/j.ijpara.2013.03.005. [DOI] [PubMed] [Google Scholar]
- El-Sherry S., Ogedengbe M.E., Hafeez M.A., Sayf-Al-Din M., Gad N., Barta J.R. Sequence-based genotyping clarifies conflicting historical morphometric and biological data for 5 Eimeria species infecting turkeys. Poult. Sci. 2015;94:262–272. doi: 10.3382/ps/peu007. [DOI] [PubMed] [Google Scholar]
- Imai R.K., Barta J.R. Distribution and abundance of Eimeria species in commercial turkey flocks across Canada. Can. Vet. J. 2019;60:153–159. [PMC free article] [PubMed] [Google Scholar]
- Mathis G.F., McDougald L.R. Restoration of drug sensitivity on turkey farms after introduction of sensitive coccidia during controlled-exposure immunization. Proc. Vth Int. Coccidiosis Conf., INRA; Tours, France; 1989. [Google Scholar]
- MacDonald A.M., Jardine C.M., Rejman E., Barta J.R., Bowman J., Cai H.Y., Susta L., Nemeth N.M. High prevalence of Mycoplasma and Eimeria species in free-ranging eastern wild turkeys (Meleagris gallopavo Silvestris) in Ontario. Canada J. Wildl. Dis. 2019;55:54–63. doi: 10.7589/2017-11-273. [DOI] [PubMed] [Google Scholar]
- Rathinam T., Chapman H.D. Sensitivity of isolates of Eimeria from turkey flocks to the anticoccidial drugs amprolium, clopidol, diclazuril, and monensin. Avian Dis. 2009;53:405–408. doi: 10.1637/8679-030509-Reg.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.