Abstract
Galactomannans are abundant nonstarch polysaccharides in broiler feed ingredients. In broilers, diets with high levels of galactomannans have been associated with innate immune response stimulation, poor zootechnical performance, nutrient and lipid absorption, and excessive digesta viscosity. However, data about its effects on the gut microbiome are scarce. β-Mannanases are enzymes that can hydrolyze β-mannans, resulting in better nutrient utilization. In the current study, we have evaluated the effect of guar gum, a source of galactomannans, supplemented to broiler diets, either with or without β-mannanase supplementation, on the microbiota composition, in an attempt to describe the potential role of the intestinal microbiota in β-mannanase-induced gut health and performance improvements. One-day-old broiler chickens (n = 756) were randomly divided into 3 treatments: control diet, guar gum-supplemented diet (1.7%), or guar gum-supplemented diet + β-mannanase (Hemicell 330 g/ton). The zootechnical performance, gut morphometry, ileal and cecal microbiome, and short-chain fatty acid concentrations were evaluated at different time points. The guar gum supplementation decreased the zootechnical performance, and the β-mannanase supplementation restored performance to control levels. The mannan-rich diet-induced dysbiosis, with marked effects on the cecal microbiota composition. The guar gum-supplemented diet increased the cecal abundance of the genera Lactobacillus, Roseburia, Clostridium sensu stricto 1, and Escherichia-Shigella, and decreased Intestinimonas, Alistipes, Butyricicoccus, and Faecalibacterium. In general, dietary β-mannanase supplementation restored the main microbial shifts induced by guar gum to levels of the control group. In addition, the β-mannanase supplementation reduced cecal isobutyric, isovaleric, valeric acid, and branched-chain fatty acid concentrations as compared to the guar gum-supplemented diet group, suggesting improved protein digestion and reduced cecal protein fermentation. In conclusion, a galactomannan-rich diet impairs zootechnical performance in broilers and results in a diet-induced dysbiosis. β-Mannanase supplementation restored the gut microbiota composition and zootechnical performance to control levels.
Key words: beta-mannanase, guar gum, microbiota, mannan, gut health
INTRODUCTION
It is estimated that by 2030, poultry meat consumption will represent 41% of all meat protein sources worldwide, driven by the efficient production and the lack of cultural and religious hurdles (OECD and FAO, 2021). One of the major potential problems for the future is to keep the production cost at low levels. Currently, feed represents >60% of the final production cost (Noblet et al., 2022), so dietary additives that can improve feed utilization are of great value for the poultry industry. One of the most widely used dietary additives to enhance digestibility are carbohydrate-degrading enzymes, such as xylanases (Zhang et al., 2014). These enzymes are targeting plant cell wall components, more specifically soluble nondigestible nonstarch polysaccharides (NSP), considered to be antinutritional factors (Jezierny et al., 2010; Amerah, 2015). NSP have β-glycosidic bonds that cannot be digested by monogastric animals, and data clearly show negative correlations between digestibility and dietary NSP concentrations (Jaworski et al., 2015). High concentrations of soluble NSP increase small intestinal viscosity, decrease passage rate, favor the expansion of harmful microbiota, such as Escherichia coli and Clostridium perfringens, reduce animal performance, and affect the intestinal microbiota composition (Hussain et al., 2012; Shojadoost et al., 2012; Latorre et al., 2015; Jha et al., 2019; Bushra et al., 2020).
While arabinoxylans are the most well-known NSP, there are a variety of other NSPs that can negatively affect animal performance (Căpriţă et al., 2010; Kermanshahi et al., 2018). As an example, β-mannans (galactomannans and glucomannans) are present in different concentrations in many feed ingredients. Dehulled soy bean meal (48% crude protein), one of the most important ingredients in poultry diets, has about 2.8 to 10 g/kg of β-mannans (Hove et al., 2018). Guar gum meal typically contains between 20 and 80 g/kg of galactomannans (mannose backbone with galactose side-chains), and is often used as β-mannan-rich source to evaluate the effect of β-mannans and β-mannan-degrading enzymes in experimental trials (Hussainet al., 2012; Saeed et al., 2019).
In humans, galactomannan ingestion has been related to glycemic index reduction in diabetics, alleviation of the irritable bowel syndrome symptoms, prebiotic effects, and improvement of short-chain fatty acid (SCFA) production (Singh et al., 2018; Rao and Quartarone, 2019; Miao et al., 2021). However, in broilers, diets with high concentrations of plant-derived galactomannans are associated with poor performance, reduced feed intake, nutrient and lipid absorption, innate immune response stimulation, and excessive digesta viscosity (Lee et al., 2003; Shirouchi et al., 2011; Shastak et al., 2015).
β-Mannanases are enzymes synthesized by strains of Aspergillus niger, Paenibacillus lentus, Bacillus subtilis, or Trichoderma longibrachiatum (Li et al., 2014; Saeed et al., 2019). These enzymes can hydrolyze β-1,4-glycosidic linkages in β-mannans resulting in a better nutrient utilization, improvement of innate immune responses, reduced intestinal viscosity and lower pathogen proliferation (Hussainet al., 2012). The effects of β-mannans and β-mannanase feed supplementation on performance and gut histology in chickens have been described previously (Maisonnier et al., 2003; Zou et al., 2006; Caldas et al., 2018; Latham et al., 2018). As NSP-degrading enzymes typically cause release of smaller oligosaccharides that can be used by the intestinal microbiota, it might be that shifts in microbial composition contribute to the observed health effects when β-mannanases are added to poultry diets (Hussainet al., 2012; Saeed et al., 2019). However, data on the effect of β-mannans and β-mannanase on the intestinal microbiome are lacking. In the current study, we evaluate the effect of guar gum (GG) supplementation to the diet of broilers, either or without β-mannanase, on the microbiota composition, in an attempt to describe the potential role of the intestinal microbiota in β-mannanase-induced gut health and performance improvements.
MATERIALS AND METHODS
Animal Trial
A total of 756 one-day-old Ross-308 broiler chicks were randomly divided in 3 treatments (12 pens per treatment with 21 animals each): control diet; GG diet; GG diet + β-mannanase (GG + E) (Hemicell 330 g/ton of feed), and housed on solid floors covered with wood shavings following European Union Directive 2007/43/EC (EU, 2007). Water, feed (Table 1), and heating were provided according to broiler guidelines (Aviagen, 2018). At 1, 14, 21, 28, and 35 d of age the animals and feed leftovers were weighed per pen to calculate the feed conversion ratio (FCR), body weight (BW), daily feed intake (DFI), and average daily gain (ADG). At 14 and 28 d of age, 1 animal per pen (n = 12 birds/treatment) was euthanized by an intravenous overdose of 20% sodium pentobarbital (Kela, Hoogstraten, Belgium), according to Annex I to the Council Regulation (EC) No 1099/2009 (EC, 2009), and content from ileum and cecum was collected and stored at −20°C, while part of the duodenum and ileum were fixed in 4% buffered formalin for 24 h.
Table 1.
Composition of the experimental diets.
| Ingredients (g/kg) | Control diet |
GG diet |
||
|---|---|---|---|---|
| Starter | Grower | Starter | Grower | |
| Maize | 610.40 | 625.85 | 588.60 | 606.40 |
| Guar gum | - | - | 17.90 | 16.50 |
| Soya bean meal | 310 | 300 | 314 | 303 |
| Full fat soya bean | 2 | - | 2 | - |
| Animal fat | 25 | 25 | 25 | 25 |
| Soybean oil | 10 | 12.50 | 10 | 12.50 |
| Premix | 5 | 5 | 5 | 5 |
| Lime fine (38% Ca) | 15 | 13 | 15 | 13 |
| Dicalcium phosphate | 10.10 | 6.90 | 10.10 | 6.90 |
| Sodium bicarbonate | 2.70 | 2.20 | 2.70 | 2.20 |
| L-lysine HCL | 3.20 | 2.90 | 3.10 | 2.85 |
| DL-methionine | 3.25 | 2.90 | 3.25 | 2.90 |
| L-threonine | 1.25 | 1.10 | 1.25 | 1.10 |
| Calculate composition (g/kg) | ||||
| ME (MJ/kg) | 12.86 | 13.03 | 12.59 | 12.79 |
| Crude protein | 203.9 | 199.4 | 204 | 199.2 |
| Crude fat | 67.8 | 70.4 | 66.9 | 69.6 |
| Starch | 402.4 | 412.4 | 388.2 | 399.7 |
| Sugars | 43.7 | 42.9 | 43.7 | 42.8 |
| Ca | 9.0 | 7.8 | 9 | 7.8 |
| Available P | 4.8 | 4.2 | 4.8 | 4.2 |
The starter diet was provided from d 1 until d 14, followed by grower diet until d 35. ME, metabolizable energy. Hemicell 330 g/ton of feed was added only to the group that received the GG diet supplement with β-mannanase. Premix composition per kg of product: vitamin A 10,000 IU; vitamin D3 2,500 IU; vitamin E 50 mg; vitamin K3 1.5 mg; vitamin B1 2.0 mg; vitamin B2 7.5 mg; vitamin B6 3.5 mg; vitamin B12 20 µg; niacin 35 mg; D-pantothenic acid 12 mg; choline chloride 460 mg; folic acid 1.0 mg; biotin 0.2 mg; iron 80 mg; copper 12 mg; manganese 85 mg; zinc 60 mg; iodate 0.8 mg; selenium 0.15 mg. GG, guar gum.
Histological Analysis
The formalin-fixed tissue segments (n = 12/treatment/intestinal segment) were embedded in paraffin, 5 µm sections obtained, deparaffinized, and stained with hematoxylin and eosin (H&E). Duodenal and ileal villus length and crypt depth were assessed by random measurement of 15 villi and crypts using a PC-based image analysis system (Leica Application Suite V4.1, Leica, Diegem, Belgium). Afterward the villus to crypt ratio was calculated.
Molecular Analysis
DNA Extraction
DNA was extracted from 100 mg cecal and ileal content of 1 bird per pen (12 birds/treatment), using the hexadecyltrimethylammonium bromide (CTAB) method according to Kowalchuk et al. (1998) with small modifications. Briefly the intestinal content was suspended in 0.5 mL CTAB (Sigma Aldrich, St. Louis, MO) buffer 5% (w/v), 0.35 M NaCl, 120 mM K2HPO4) and 0.5 mL phenol-chloroform-isoamyl alcohol (25:24:1). The mixture was homogenized by grinding with 0.5 g unwashed glass beads (Sigma-Aldrich) in a bead beater (2 × 3 min 30 Hz for ileal content and 2 × 2 min, 22.5 Hz for cecal content; TissueLyser II; Qiagen, Hilden, Germany) with a 30 s interval between shakings. Samples were centrifuged for 10 min at 8,000 rpm and 300 µL of the supernatant was transferred to a new tube. A re-extraction from the remaining content was performed by adding 0.25 mL CTAB buffer and homogenizing and centrifuging the sample as described above. An equal volume (0.6 mL) of chloroform-isoamyl alcohol (24:1) was added to the supernatant collected in order to remove the phenol from the samples. The mixture was further centrifuged at 16,000 × g for 10 s. Nucleic acids were precipitated with 1.2 mL of polyethyleenglycol-6000 solution (30% w/v; 1.6 M NaCl) for 2 h at room temperature. Samples were centrifuged (13,000 × g, 20 min, 4°C) and the pellet was washed twice with 1 mL of ice-cold ethanol (70% v/v). The obtained pellet was dried and resuspended in 100 µL deionized water (LiChrosolv Water, Merck, Darmstadt, Germany). The quality and the concentration of the DNA were examined spectrophotometrically using NanoDrop (Thermo Scientific, Waltham, MA). Only samples with a 260/280 purity value above 1.7 were selected.
16S rRNA Sequencing and Bioinformatics
The extracted DNA was diluted to 20 ng/µL and the V3 to V4 hypervariable region of the 16S rRNA gene was amplified using the gene-specific primers (Table 2), as described by Aguirre et al. (2019). The final barcoded libraries were pooled at an equimolar concentration of 5 nM and sequenced with 30% PhiX spike-in using the Illumina MiSeq v3 technology (2 × 300 bp, paired-end) by the Ghent University next generation sequencing facility NXTGNT. After demultiplexing of the amplicon dataset and deletion of the barcodes, optimal trimming parameters were determined using the python-based application FIGARO (Weinstein et al., 2019). All further processing was performed in R (v4.1.2) (Bunn and Korpela, 2013). Raw sequence reads were trimmed, quality-filtered and dereplicated using the DADA2 algorithm (v1.14.0) (Callahan et al., 2016). An initial amplicon sequence variant (ASV) table was constructed before chimeras were identified using the removeBimeraDenovo function. Finally, taxonomy was assigned using DADA2’s native naïve Bayesian classifier against the Silva database (v138) (Quast et al., 2013). To construct a phylogenetic tree, multiple sequence alignment was performed using the DECIPHER (v2.14.0) algorithm (Wright, 2015), after which a neighbor-joining tree was constructed using PHANGORN (v2.7.0) (Schliep, 2011). This neighbor-joining tree was used as the starting point to fit the final GTR + G + I (generalized time-reversible with gamma rate variation) maximum likelihood tree. The resulting phylogenetic tree and ASV table were loaded into Phyloseq (v1.28.0) (McMurdie and Holmes, 2013), after which potential contaminant chloroplastic and mitochondrial ASVs were removed from the dataset. Potential contaminant DNA reads originating from the DNA extraction or library preparation buffers were identified based on both the DNA concentration and prevalence of the ASVs in the negative control samples (DNA extraction controls) using decontam (v1.14.0) (Davis et al., 2018) and removed from the final dataset.
Table 2.
Primer sequences and annealing temperatures used for quantification of the respective taxa and the butyryl-CoA CoA-transferase gene, in qPCR reactions.
| Target | Primer | Sequence | Annealing temperature and time | Reference |
|---|---|---|---|---|
| V3–V4 region of the 16s rRNA gene |
S-D-Bact-0341-b-S-17 S-D-Bact-0785-a-A-21 |
Fw—5′ TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCT ACGGGNGGCWGCAG 3′ Rv—3′ GTCTCGTGGGCTCGGAGATGTGTATAAGAG ACAGGACTACHVGGGTATCTAATCC 5′ |
55°C, 30” | Klindworth et al. (2013) |
| Enterobacteriaceae |
Eco1457F Eco1652R |
Fw—5′ CATTGACGTTACCCGCAGAAGAAGC 3′ Rv—3′ CTCTACGAGACTCAAGCTTGC 5′ |
55°C, 1’ | Bartosch et al. (2004) |
| Lactobacillus spp. |
Lacto-16S-F Lacto-16S-R |
Fw—5′ GGA ATC TTC CAC AAT GGA CG 3′ Rv—3′ CGC TTT ACG CCC AAT AAA TCC GG 5′ |
60°C, 1’ | Abdulamir et al. (2010) |
| Ruminococcaceae |
sg-Clept-F sg-Clept-R3 |
Fw—5′ GCACAAGCAGTGGAGT 3′ Rv—3′ CTTCCTCCGTTTTGTCAA 5′ |
60°C, 1’ | Matsuki et al. (2004) |
| Butyryl-CoA:acetate CoA-transferase |
BCoATscrF BCoATscrR |
Fw—5′ GCIGAICATTTCACITGGAAYWS ITGGCAYATG 3′ Rv—3′ CCTGCCTTTGCAATR TCIACRAANGC 5′ |
53°C, 30” | Louis and Flint (2007) |
Fw, forward; Rv, reverse.
Quantitative PCR
To confirm the main microbial shifts that were identified using 16S rRNA gene analysis, a qPCR for families Enterobacteriaceae and Ruminococcaceae and genus Lactobacillus was performed. Additionally, a qPCR quantifying the number of genes encoding the butyryl-CoA:acetate CoA-transferase was performed using the CFX384 BioRad detection system (BioRad, Nazareth-Eke, Belgium). Each reaction was done in triplicate in a 12 μL total reaction mixture using 1× SensiMix SYBR No-ROX mix (Bioline, Kampenhout, Belgium), 0.5 μM final primer concentration (2.5 μM for butyryl-CoA: acetate-CoA transferase enzyme), 2 μL of (20 ng/μL) DNA, and deionized water to complete the reaction volume. A standard curve was included in triplicate for each primerset. The amplification program consisted of 1 cycle at 95°C for 10 min, 40 cycles of 30 s at 95°C, followed by the annealing temperature, described in Table 2 for each primerset, for quantifying the number of gene copies encoding butyryl-CoA: acetate CoA-transferase a 3-steps protocol was used 1 cycle at 95°C for 10 min, 40 cycles of 30 s at 95°C, 30 s at 53°C, and 30 s at 72°C. The fluorescent products were detected at the last step of each cycle. A melting curve analysis was done after amplification and was obtained by slow heating from 60°C to 95°C at a rate of 0.5°C/5 s to confirm the specificity of the reaction. The primer sequences are described in Table 2.
Short-Chain Fatty Acid and Branched-Chain Fatty Acid Analysis
SCFA and branched-chain fatty acid (BCFA) were extracted from 200 mg of cecal content with diethyl ether and measured using a GC-2014 gas chromatograph (Shimadzu, ‘s-Hertogenbosch, the Netherlands) (Eaton et al., 1998; De Weirdt et al., 2010; Boesmans et al., 2018). The results are expressed as µmol of SCFA or BCFA per gram of cecal content.
Statistical Analysis
Statistical analysis of zootechnical performance parameters, intestinal morphology, qPCR, and SCFA data was performed using GraphPad Prism (version 7.04, San Diego, CA). Assumption of homoscedasticity (Bartlett's test and Brown-Forsythe test) was evaluated and when this assumption was met (P > 0.05), ANOVA, at a 5% of significance level, followed by Tukey's test, was performed. Due to the lack of homoscedasticity, the SCFA, BCFA, and qPCR values were log transformed and further subjected to ANOVA.
Statistical analysis of the gut microbiota results was performed using R (version 4.2.1). The microbial alpha diversity (number of observed ASVs and the Shannon diversity index) was calculated using phyloseq (v1.18.0). The effect of the dietary treatment on the microbial alpha diversity was assessed using a Kruskal-Wallis test, followed by a Dunn's post hoc test. Prior to beta diversity analysis, the 16S sequencing data were transformed to portions. The Bray-Curtis distance was used as a measure for the microbial beta diversity. The dispersion (variance) in the beta diversity was calculated using the betadisper function in the vegan package (Dixon, 2003). ANOVA showed no difference in variances between the groups. Significant differences in the community composition between the groups were determined through a permutational multivariate analysis of variance using distance matrices (PERMANOVA), using the adonis2 function in vegan. In case a significant effect of the diet was observed, pairwise comparison between the diets was performed using the function pairwise.perm.manova from the RVAideMemoire package and Bonferroni corrected P values were reported (Hervé, 2022). Differentially abundant taxa (phyla, families, or genera) in the ileal or cecal microbiome at the different sampling days were identified by applying DESeq2 on the nonrarefied community composition data (Love et al., 2014). Significant differences were obtained using a Wald test followed by a Benjamini-Hochberg multiple hypothesis correction.
RESULTS
Guar Gum Reduces Animal Performance and Dietary β-Mannanase Supplementation Restores Performance to Control Group Level
Through the experimental period GG supplementation impaired broiler performance (Table 3). The BW of the animals fed a GG-supplemented diet was significantly lower at all ages as compared to the animals fed the control diet, and β-mannanase supplementation restored the BW to control levels. Overall from 0 to 35 d, GG supplementation significantly reduced the ADG with 7.31 g/d and increased FCR with 0.14, relative to the control group (P < 0.0001), while the animals that received dietary β-mannanase as an additive to the GG diet, had an ADG and FCR that was not different from the animals fed the control diet (Table 3).
Table 3.
The body weight in grams (BW) at d 1, 14, 28, 35, and 42, and feed conversion ratio (FCR), daily feed intake (DFI), and daily weight gain (DWG) measured at 4 time intervals, for animals fed a control diet or a diet supplemented with guar gum, either with or without β-mannanase supplementation at 330 g/ton feed.
| Parameter and period | Control |
GG |
GG + E |
Control vs. GG |
Control vs. GG + E |
GG vs. GG + E |
|---|---|---|---|---|---|---|
| Mean ± standard deviation | Adjusted P value | |||||
| BW 1 d | 42.04 ± 1.10 | 42.31 ± 0.85 | 42.07 ± 0.35 | 0.8103 | 0.9978 | 0.8441 |
| BW 14 d | 490.80 ± 13.23 | 457.60 ± 15.09 | 497.90 ± 12.34 | <0.0001 | 0.4186 | <0.0001 |
| BW 21 d | 961.50 ± 27.68 | 901.20 ± 22.38 | 960.60 ± 29.12 | <0.0001 | 0.9967 | <0.0001 |
| BW 28 d | 1,551 ± 54.27 | 1,428 ± 51.13 | 1,544 ± 12.20 | <0.0001 | 0.9364 | <0.0001 |
| BW 35 d | 2,320 ± 138.30 | 2,038 ± 75.62 | 2,302 ± 112.30 | <0.0001 | 0.9203 | <0.0001 |
| Period 1–14 d | ||||||
| DFI (g/bird) | 35.74 ± 0.96 | 34.66 ± 1.20 | 36.06 ± 1.51 | 0.0997 | 0.8081 | 0.0252 |
| ADG (g/d/bird) | 31.86 ± 0.89 | 29.35 ± 1.22 | 32.43 ± 0.88 | <0.0001 | 0.3721 | <0.0001 |
| FCR | 1.12 ± 0.02 | 1.18 ± 0.03 | 1.11 ± 0.03 | 0.0001 | 0.7025 | <0.0001 |
| Period 15–21 d | ||||||
| DFI (g/bird) | 86.97 ± 3.08 | 85.34 ± 3.07 | 86.09 ± 3.38 | 0.4319 | 0.7808 | 0.8323 |
| ADG (g/d/bird) | 67.16 ± 2.80 | 63.01 ± 2.17 | 65.46 ± 4.76 | 0.0150 | 0.4519 | 0.2028 |
| FCR | 1.29 ± 0.02 | 1.35 ± 0.03 | 1.32 ± 0.05 | 0.0020 | 0.3052 | 0.0782 |
| Period 22–27 d | ||||||
| DFI (g/bird) | 123.80 ± 3.45 | 123.20 ± 7.33 | 124.80 ± 3.68 | 0.9616 | 0.8805 | 0.7368 |
| ADG (g/d/bird) | 84.13 ± 5.17 | 74.90 ± 4.48 | 81.86 ± 3.29 | <0.0001 | 0.4237 | 0.0013 |
| FCR | 1.47 ± 0.06 | 1.64 ± 0.08 | 1.52 ± 0.05 | <0.0001 | 0.1504 | 0.0003 |
| Period 28–35 d | ||||||
| DFI (g/bird) | 165.90 ± 8.06 | 157.30 ± 5.32 | 168.90 ± 8.50 | 0.0202 | 0.5812 | 0.0015 |
| ADG (g/d/bird) | 108.10 ± 12.81 | 85.29 ± 9.53 | 106.10 ± 11.95 | <0.0001 | 0.9115 | 0.0003 |
| FCR | 1.55 ± 0.15 | 1.86 ± 0.160 | 1.60 ± 0.14 | <0.0001 | 0.6454 | 0.0006 |
| Overall 0–35 d | ||||||
| DFI (g/bird) | 83.63 ± 2.46 | 81.24 ± 2.54 | 84.10 ± 2.69 | 0.0720 | 0.8960 | 0.0264 |
| ADG (g/d/bird) | 61.15 ± 3.35 | 53.84 ± 2.51 | 60.22 ± 3.01 | <0.0001 | 0.7248 | <0.0001 |
| FCR | 1.37 ± 0.04 | 1.51 ± 0.04 | 1.39 ± 0.03 | <0.0001 | 0.2643 | <0.0001 |
Values are the means for 12 pens of 21 chickens ± standard deviation of the mean. ANOVA, followed by Tukey multiple comparison test, was used to determine statistical differences among groups.
GG, guar gum; GG + E, guar gum + β-mannanase.
Effects of Guar Gum and β-Mannanase Supplementation on Gut Morphometry
The intestinal morphometry was evaluated at 14 and 28 d in both the duodenum and ileum segments (Table 4). At the duodenum level, no significant changes in villus height or crypt depth were observed between groups at both ages. In the ileum, GG ingestion significantly increased the villus height and the villus:crypt ratio as compared to the control group at 14 and 28 d, while both parameters did not differ between the enzyme-supplemented group and the control group.
Table 4.
Duodenal and ileal villus height, crypt depth and villus:crypt ratio, at 2 time points, for animals fed either a control diet or a diet supplemented with guar gum, either with or without β-mannanase supplementation at 330 g/ton feed.
| Parameter and period | Control |
GG |
GG + E |
Control vs. GG |
Control vs. GG + E |
GG vs. GG + E |
|---|---|---|---|---|---|---|
| Mean ± standard deviation | Adjusted P value | |||||
| Duodenum 14 d | ||||||
| Villus height, µm | 1,470 ± 267.60 | 1,419 ± 143.20 | 1,484 ± 125.20 | 0.7848 | 0.9836 | 0.6817 |
| Crypt depth, µm | 184.80 ± 33.15 | 195.00 ± 24.59 | 179.90 ± 32.23 | 0.6871 | 0.9174 | 0.4464 |
| Villi: crypt ratio | 8.20 ± 2.01 | 7.34 ± 0.87 | 8.46 ± 1.47 | 0.3593 | 0.9089 | 0.1841 |
| Duodenum 28 d | ||||||
| Villus height, µm | 1,874 ± 215.20 | 1,860 ± 219.60 | 1,720 ± 307.80 | 0.9898 | 0.3050 | 0.3723 |
| Crypt depth, µm | 180.60 ± 34.57 | 180.80 ± 22.57 | 176.10 ± 42.90 | >0.9999 | 0.9455 | 0.9410 |
| Villi: crypt ratio | 10.71 ± 2.16 | 10.38 ± 1.39 | 10.12 ± 2.26 | 0.9138 | 0.7521 | 0.9461 |
| Ileum 14 d | ||||||
| Villus height, µm | 389.20 ± 72.86 | 509.90 ± 117.40 | 416.70 ± 77.53 | 0.0076 | 0.7439 | 0.0455 |
| Crypt depth, µm | 119.70 ± 38.66 | 110.90 ± 29.47 | 122.50 ± 27.87 | 0.7848 | 0.9768 | 0.6613 |
| Villi: crypt ratio | 3.22 ± 0.70 | 4.69 ± 0.827 | 3.51 ± 0.860 | 0.0003 | 0.6553 | 0.0031 |
| Ileum 28 d | ||||||
| Villus height, µm | 489 ± 124.40 | 640 ± 167 | 615.80 ± 130.50 | 0.0354 | 0.0880 | 0.9084 |
| Crypt depth, µm | 112.20 ± 36.18 | 114.60 ± 26.68 | 116.70 ± 28.57 | 0.9793 | 0.9323 | 0.9856 |
| Villi: crypt ratio | 4.51 ± 0.85 | 5.60 ± 0.76 | 5.42 ± 0.95 | 0.0103 | 0.0364 | 0.8611 |
Values are the means of 12 animals (1/pen/treatment) ± standard deviation. ANOVA, followed by Tukey multiple comparison test, was used to determine statistical differences among groups.
GG, guar gum; GG + E, guar gum + β-mannanase.
Effects of Guar Gum and β-Mannanase Supplementation on the Ileal and Cecal Microbiota Composition
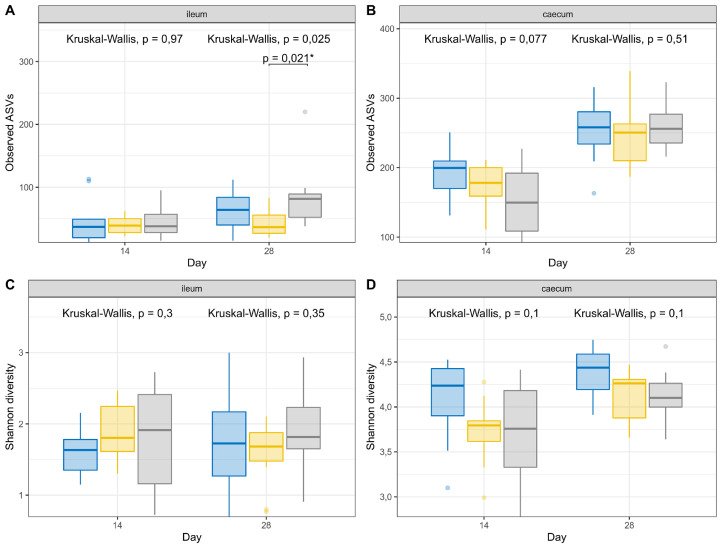
The composition of the ileal and cecal microbiome was evaluated at 14 and 28 d of age. The microbial richness was evaluated through the number of observed ASVs and the estimated community diversity (Shannon index) in each sample (Figure 1). In the ileum, no significant changes were observed at 14 d. At 28 d GG ingestion reduced the microbial richness (P = 0.021) as compared to GG + E. No differences in α-diversity metrices were seen between groups in the ceca (Figure 1B and D).
Figure 1.
Ileal and cecal bacterial α-diversity measurements (A, B: observed ASVs; C, D: Shannon diversity) at 2 time points (14 or 28 d of age) among groups of animals fed a control diet or a diet supplemented with guar gum, either or not supplemented with β-mannanase at 330 g/ton feed. Values are the means of 12 animals (1/pen/treatment)  Control;
Control;  Guar gum;
Guar gum;  Guar gum + β-mannanase.
Guar gum + β-mannanase.
Bray-Curtis dissimilarity metric was used to investigate β-diversity in the ileum and cecum between the treatment groups. In the ileum at 14 d a significant shift in the microbial communities was observed, in which 18% of the variation was due to the treatment. The β-diversity of the GG (P = 0.0033) and GG + E (P = 0.0291) groups were significantly different from the control group (Figure 2A). β-Mannanase supplementation of the GG diet did not affect the ileal microbial composition, as no difference between the GG and GG + E group could be observed (P = 0.394). No differences were observed in the ileum at 28 d. In the ceca, a significant difference in the microbial communities was observed at 14 d, in which 17% of the variation could be justified by the treatment, and all groups differed from each other (Figure 2B). At 28 d 22% of the difference in cecal microbial community composition could be attributed to the dietary treatment, and all groups differed from each other (Figure 2D).
Figure 2.
Principal coordinate analysis plot of bacterial β-diversity based on Bray-Curtis dissimilarities. Ileal and cecal bacterial β-diversity measurements at 2 time points among groups of animals fed a control diet or a diet supplemented with guar gum, either or not supplemented with β-mannanase at 330 g/ton feed. Values are the means of 12 animals (1/pen/treatment)  Control;
Control;  Guar gum;
Guar gum;  Guar gum + β-mannanase. Each dot represents an individual chicken microbiome. β-Diversity. Bray-Curtis dissimilarity metric. (A) Ileum 14 d: significant separation in the microbial communities (P = 0.002), 18% of the difference is attributed to the treatments, GG (P = 0.0033) and GG + E (P = 0.0291) differed from control. (B) Cecum 14 d: a significant difference of the microbial communities was observed (P = 0.001), 17% of the difference can be attributed to the treatments, all groups differ from each other (control vs. GG P = 0.0003; control vs. GG + E P = 0.0006; GG + E vs. GG P = 0.0003). (C) Ileum 28 d: there was no statically difference among the microbial communities (P = 0.219). (D) Cecum 28 d: a significant difference (P = 0.001) among the groups was observed. The treatments explain 22% of the variation between the samples, all groups differ from each other (control vs. GG P = 0.0003; control vs. GG + E P = 0.0006; GG + E vs. GG P = 0.0003).
Guar gum + β-mannanase. Each dot represents an individual chicken microbiome. β-Diversity. Bray-Curtis dissimilarity metric. (A) Ileum 14 d: significant separation in the microbial communities (P = 0.002), 18% of the difference is attributed to the treatments, GG (P = 0.0033) and GG + E (P = 0.0291) differed from control. (B) Cecum 14 d: a significant difference of the microbial communities was observed (P = 0.001), 17% of the difference can be attributed to the treatments, all groups differ from each other (control vs. GG P = 0.0003; control vs. GG + E P = 0.0006; GG + E vs. GG P = 0.0003). (C) Ileum 28 d: there was no statically difference among the microbial communities (P = 0.219). (D) Cecum 28 d: a significant difference (P = 0.001) among the groups was observed. The treatments explain 22% of the variation between the samples, all groups differ from each other (control vs. GG P = 0.0003; control vs. GG + E P = 0.0006; GG + E vs. GG P = 0.0003).
The phylum Firmicutes was the most abundant phylum in both intestinal segments at both ages (>90%). In the ileum on 14 d, GG group had a significantly higher relative abundance of the phylum Proteobacteria as compared to the control group (6.2% vs. 0.08%, P < 0.0001). Adding β-mannanase to the GG group (GG + E) resulted in a significantly lower relative Proteobacteria abundance as compared to the GG group (1.7 vs. 6.2%, P = 0.0264) (Table 5). No significant differences were observed in the ileum at 28 d, at phylum level.
Table 5.
Mean abundance of ileal and cecal phyla in the microbiota at 2 time points among groups of animals fed a control diet or a diet supplemented with guar gum, either or not supplemented with β-mannanase at 330 g/ton feed.
| Phylum | CTR |
GG |
GG + E |
CTR vs. GG |
CTR vs. GG + E |
GG vs. GG + E |
|---|---|---|---|---|---|---|
| Mean abundance (%) | Adjusted P value | |||||
| Ileum 14 d | ||||||
| Actinobacteriota | 2.69 | 3.78 | 6.16 | >0.05 | >0.05 | >0.05 |
| Bacteroidota | 0.01 | 0 | 0.02 | >0.05 | >0.05 | >0.05 |
| Firmicutes | 97.21 | 90.01 | 92.07 | >0.05 | >0.05 | >0.05 |
| Proteobacteria | 0.09 | 6.21 | 1.75 | < 0.0001 | 0.0316 | 0.0264 |
| Ileum 28 d | ||||||
| Actinobacteriota | 4.33 | 5.94 | 5.03 | >0.05 | >0.05 | >0.05 |
| Bacteroidota | 0.04 | 0 | 0.16 | >0.05 | >0.05 | >0.05 |
| Firmicutes | 95.10 | 92.30 | 92.87 | >0.05 | >0.05 | >0.05 |
| Proteobacteria | 0.52 | 1.76 | 1.92 | >0.05 | >0.05 | >0.05 |
| Cecum 14 d | ||||||
| Actinobacteriota | 0.25 | 0.67 | 0.33 | <0.0001 | >0.05 | <0.0001 |
| Bacteroidota | 6.04 | 1.00 | 4.65 | <0.0001 | >0.05 | <0.0001 |
| Firmicutes | 91.73 | 92.29 | 92.06 | >0.05 | >0.05 | >0.05 |
| Proteobacteria | 1.96 | 6.02 | 2.94 | 0.0163 | >0.05 | >0.05 |
| Cecum 28 d | ||||||
| Actinobacteriota | 0.17 | 1.14 | 0.26 | <0.0001 | >0.05 | 0.0001 |
| Bacteroidota | 8.86 | 3.95 | 8.28 | <0.0001 | >0.05 | 0.0001 |
| Firmicutes | 90.25 | 92.30 | 90.51 | <0.0001 | >0.05 | 0.0001 |
| Proteobacteria | 0.71 | 2.59 | 0.94 | 0.0047 | >0.05 | 0.0071 |
Values are the means of 12 animals (1/pen/treatment). DESeq2 analysis at 5% of significance level, was used to determine statistical differences among groups.
In the cecum at both 14 and 28 d, GG significantly increased the relative abundance of Actinobacteriota and Proteobacteria and reduced Bacteroidota as compared to the control group. β-Mannanase supplementation (GG + E) resulted in a significant decrease in Actinobacteriota and Proteobacteria and an increase in Bacteroidota relative abundance as compared to the GG group. No significant differences were observed between the control and GG + β-mannanase groups at phylum level.
At genus level in the ileum at 14 d, GG supplementation increased the relative abundance of Bifidobacterium, Streptococcus, UCG-008, Tyzzerella, Escherichia-Shigella, and reduced Enterococcus, Family_Peptostreptococcaceae, and Romboutsia, as compared to the control group (Table 6). The β-mannanase supplementation was able to restore all these shifts to control levels. In the ileum at 28 d GG supplementation increased Tyzzerella abundance and decreased Erysipelatoclostridium, Butyricicoccus, Faecalibacterium, Family_Ruminococcaceae, and Romboutsia, and again β-mannanase supplementation restored these changes to control levels (Table 6).
Table 6.
Differentially abundant genera in the ileal microbiota at 2 time points among groups of animals fed a control diet or a diet supplemented with guar gum, either or not supplemented with β-mannanase at 330 g/ton feed.
| Phylum | Class | Family | Genus | CTR |
GG |
GG + E |
CTR vs. GG |
CTR vs. GG + E |
GG vs. GG + E |
|---|---|---|---|---|---|---|---|---|---|
| Mean abundance (%) | Adjusted P value | ||||||||
| ILEUM 14 d | |||||||||
| Actinobacteriota | Actinobacteria | Bifidobacteriaceae | Bifidobacterium | 0.0004 | 0.0427 | 0.0086 | 0.0384 | 0.3273 | 1 |
| Firmicutes | Bacilli | Enterococcaceae | Enterococcus | 13.2080 | 0.9023 | 6.5246 | 0.0034 | 1 | 0.0073 |
| Firmicutes | Bacilli | Erysipelatoclostridiaceae | Erysipelatoclostridium | 0.0719 | 0.0104 | 0.0319 | 0.0571 | 1 | 0.6122 |
| Firmicutes | Bacilli | Lactobacillaceae | Lactobacillus | 66.8353 | 47.8278 | 49.2388 | 0.0571 | 1 | 0.4713 |
| Firmicutes | Bacilli | Lactobacillaceae | Weissella | 0 | 0.0082 | 0.0574 | 0.2450 | 0.0104 | 0.3590 |
| Firmicutes | Bacilli | Staphylococcaceae | Staphylococcus | 0.1599 | 0.0837 | 0.3014 | 0.4290 | 0.0104 | 0.1463 |
| Firmicutes | Bacilli | Streptococcaceae | Streptococcus | 2.8872 | 19.8697 | 7.4034 | 0.0226 | 0.3273 | 1 |
| Firmicutes | Clostridia | Butyricicoccaceae | UCG-008 | 0 | 0.1665 | 0 | <0.0001 | 1 | <0.0001 |
| Firmicutes | Clostridia | Clostridiaceae | Candidatus Arthromitus | 2.5497 | 1.1646 | 9.3110 | 0.1829 | 1 | 0.0192 |
| Firmicutes | Clostridia | Lachnospiraceae | Tyzzerella | 0.0028 | 1.3470 | 0.0176 | 0.0046 | 1 | 0.4713 |
| Firmicutes | Clostridia | Peptostreptococcaceae | Family_Peptostreptococcaceae | 0.5989 | 0.0160 | 0.1894 | 0.0125 | 1 | 0.0046 |
| Firmicutes | Clostridia | Peptostreptococcaceae | Romboutsia | 0.2374 | 0 | 0.0415 | <0.0001 | 1 | <0.0001 |
| Proteobacteria | Gammaproteobacteria | Enterobacteriaceae | Escherichia-Shigella | 0.0879 | 6.2105 | 1.7457 | 0.0034 | 0.1327 | 0.9838 |
| ILEUM 28 d | |||||||||
| Firmicutes | Bacilli | Erysipelatoclostridiaceae | Erysipelatoclostridium | 0.0124 | 0.0003 | 0.1467 | 0.0210 | 1 | 0.0104 |
| Firmicutes | Clostridia | Butyricicoccaceae | Butyricicoccus | 0.0389 | 0 | 0.1264 | 0.0011 | 1 | 0.0104 |
| Firmicutes | Clostridia | Clostridiaceae | Clostridium sensu stricto 1 | 4.0782 | 5.0610 | 0.0358 | 0.9294 | 0.2173 | 0.0104 |
| Firmicutes | Clostridia | Lachnospiraceae | [Ruminococcus] torques group | 0.2051 | 0.0134 | 0.6417 | 0.0987 | 1 | 0.0347 |
| Firmicutes | Clostridia | Lachnospiraceae | Tyzzerella | 0 | 0.3303 | 0 | <0.0001 | 1 | <0.0001 |
| Firmicutes | Clostridia | Ruminococcaceae | Faecalibacterium | 0.2367 | 0.0050 | 0.6200 | 0.0347 | 1 | 0.0347 |
| Firmicutes | Clostridia | Ruminococcaceae | Family_Ruminococcaceae | 0.0563 | 0.0025 | 0.2794 | 0.0443 | 1 | 0.0735 |
| Firmicutes | Clostridia | Peptostreptococcaceae | Romboutsia | 4.2001 | 0.1062 | 1.0038 | 0.0009 | 1 | 0.0735 |
Values are the means of 12 animals (1/pen/treatment). DESeq2 analysis at 5% of significance level was used to determine statistical differences among groups.
Only bacterial families with minimum relative abundance >0.05% in at least 1 group are reported. The taxonomic classification and relative abundance of each family are shown.
In the cecum, at 14 d of age, GG supplementation caused significant changes in 24 bacterial genera as compared to the control group, and dietary β-mannanase was effective to restore 20 (83.33%) of these to control levels (Table 7). The main changes induced by GG were an increase in genera belonging to the families Lactobacillaceae (HT002, Lactobacillus, and Limasilobacillus), Streptococcacea (Streptococcus), Lachnospiraceae (Marvinbryantia and Roseburia), and Enterobacteriaceae (Escherichia-Shigella) and a decrease in some genera from the families Oscillospiraceae (Intestinimonas and Family_oscillapiraceae), Ruminococcaceae (Anaerotruncus, Caproiciproducens, DTU089, Family_Ruminococcaceae, Incertae Sedis, and Negativibacillus) and Peptostreptococcaceae (Family_Peptostreptococcaceae).
Table 7.
Differentially abundant genera in the cecal microbiota at 2 time points among groups of animals fed a control diet or a diet supplemented with guar gum, either or not supplemented with β-mannanase at 330 g/ton feed.
| Phylum | Class | Family | Genus | CTR |
GG |
GG + E |
CTR vs. GG |
CTR vs. GG + E |
GG vs. GG + E |
|---|---|---|---|---|---|---|---|---|---|
| Mean abundance (%) | Adjusted P value | ||||||||
| Cecum 14 d | |||||||||
| Actinobacteriota | Actinobacteria | Bifidobacteriaceae | Bifidobacterium | 0 | 0.2836 | 0.0008 | <0.0001 | <0.0001 | 0.0220 |
| Actinobacteriota | Actinobacteria | Corynebacteriaceae | Corynebacterium | 0.0075 | 0.0189 | 0 | 0.5852 | NA | 0.0220 |
| Actinobacteriota | Coriobacteriia | Eggerthellaceae | Eggerthella | 0.0520 | 0.1896 | 0.0235 | 0.0069 | 0.5446 | 0.0002 |
| Firmicutes | Bacteroidia | Bacteroidaceae | Bacteroides | 1.7703 | 0.3534 | 1.0694 | 0.4688 | 0.3784 | 0.0353 |
| Bacteroidota | Bacteroidia | Rikenellaceae | Alistipes | 4.2728 | 0.6545 | 3.5875 | <0.0001 | 0.5750 | <0.0001 |
| Firmicutes | Bacilli | Bacillaceae | Bacillus | 0.7770 | 0.0806 | 0.2986 | 0.0170 | 0.2633 | 0.4078 |
| Firmicutes | Bacilli | Lactobacillaceae | HT002 | 1.0569 | 2.7934 | 0.7001 | 0.0069 | 0.5446 | 0.0002 |
| Firmicutes | Bacilli | Lactobacillaceae | Lactobacillus | 3.9770 | 11.3182 | 3.8157 | <0.0001 | 0.7878 | <0.0001 |
| Firmicutes | Bacilli | Lactobacillaceae | Limosilactobacillus | 0.0547 | 0.4844 | 0.0984 | 0.0006 | 0.7842 | 0.0220 |
| Firmicutes | Bacilli | Streptococcaceae | Streptococcus | 0.0830 | 7.7199 | 0.4476 | <0.0001 | 0.3490 | <0.0001 |
| Firmicutes | Clostridia | Defluviitaleaceae | Defluviitaleaceae UCG-011 | 0.1487 | 0.4659 | 0.0962 | 0.1933 | 0.7045 | 0.0415 |
| Firmicutes | Clostridia | [Eubacterium] coprostanoligenes group | Family_[Eubacterium] coprostanoligenes group | 1.8682 | 5.6657 | 3.4489 | 0.0170 | 0.4980 | 0.1555 |
| Firmicutes | Clostridia | Lachnospiraceae | Family_Lachnospiraceae | 2.6678 | 2.5480 | 2.2075 | 0.4118 | 0.4546 | 0.0460 |
| Firmicutes | Clostridia | Lachnospiraceae | Frisingicoccus | 0.0296 | 0.2682 | 0 | 0.1802 | 0.0093 | <0.0001 |
| Firmicutes | Clostridia | Lachnospiraceae | Marvinbryantia | 0.4971 | 2.3199 | 0.4942 | 0.0245 | 0.6922 | 0.0566 |
| Firmicutes | Clostridia | Lachnospiraceae | Roseburia | 0.0059 | 0.6579 | 0.0477 | 0.0418 | 0.4546 | 0.3246 |
| Firmicutes | Clostridia | Lachnospiraceae | Tyzzerella | 3.1932 | 2.4835 | 1.0642 | 0.9897 | 0.0093 | 0.0087 |
| Firmicutes | Clostridia | Monoglobaceae | Monoglobus | 1.3950 | 3.5698 | 1.5500 | 0.0457 | 0.9020 | 0.0307 |
| Firmicutes | Clostridia | Oscillospiraceae | Family_Oscillospiraceae | 2.5308 | 0.7232 | 0.9058 | 0.0457 | 0.0086 | 0.5377 |
| Firmicutes | Clostridia | Oscillospiraceae | Flavonifractor | 0.4751 | 0.2950 | 1.4531 | 0.8243 | 0.0043 | 0.0003 |
| Firmicutes | Clostridia | Oscillospiraceae | Intestinimonas | 5.9153 | 0.4555 | 1.7173 | <0.0001 | 0.0026 | 0.0566 |
| Firmicutes | Clostridia | Oscillospiraceae | Oscillibacter | 0.6241 | 0.3149 | 3.3616 | 0.3680 | 0.0577 | 0.0003 |
| Firmicutes | Clostridia | Ruminococcaceae | Anaerotruncus | 2.8432 | 0.0803 | 0.5591 | 0.0009 | 0.3936 | 0.0410 |
| Firmicutes | Clostridia | Ruminococcaceae | CAG-352 | 0.0016 | 0.3842 | 0.0591 | 0.0032 | 0.2258 | 0.2259 |
| Firmicutes | Clostridia | Ruminococcaceae | Caproiciproducens | 0.0241 | 0 | 0.0941 | 0.0221 | 0.2939 | 0.0007 |
| Firmicutes | Clostridia | Ruminococcaceae | DTU089 | 0.6647 | 0.1800 | 1.8231 | 0.0992 | 0.2633 | 0.0003 |
| Firmicutes | Clostridia | Ruminococcaceae | Family_Ruminococcaceae | 6.0167 | 2.0304 | 5.1728 | 0.0244 | 0.3936 | 0.2973 |
| Firmicutes | Clostridia | Ruminococcaceae | Fournierella | 0.0950 | 0.0411 | 0.0013 | 0.7692 | 0.0093 | 0.0410 |
| Firmicutes | Clostridia | Ruminococcaceae | Incertae Sedis | 4.7382 | 1.7320 | 12.2317 | 0.0244 | 0.0382 | <0.0001 |
| Firmicutes | Clostridia | Ruminococcaceae | Negativibacillus | 0.9024 | 0.2068 | 0.8357 | 0.0049 | 0.5767 | 0.0401 |
| Firmicutes | Clostridia | Ruminococcaceae | Subdoligranulum | 1.2818 | 5.5883 | 0.6227 | 0.0020 | 0.3784 | <0.0001 |
| Firmicutes | Clostridia | Peptostreptococcaceae | Family_Peptostreptococcaceae | 0.0419 | 0 | 0.0905 | <0.0001 | 0.8898 | 0.0003 |
| Proteobacteria | Gammaproteobacteria | Enterobacteriaceae | Escherichia-Shigella | 1.9624 | 6.0210 | 2.8657 | 0.0049 | 0.8246 | 0.0120 |
| Cecum 28 d | |||||||||
| Actinobacteriota | Actinobacteria | Bifidobacteriaceae | Bifidobacterium | 0 | 0.6695 | 0 | <0.0001 | 1 | <0.0001 |
| Actinobacteriota | Coriobacteriia | Eggerthellaceae | CHKCI002 | 0.1540 | 0.3567 | 0.2271 | 0.0385 | 0.6846 | 0.2387 |
| Bacteroidota | Bacteroidia | Rikenellaceae | Alistipes | 4.9440 | 2.0440 | 3.4186 | 0.0039 | 0.6444 | 0.0665 |
| Firmicutes | Bacilli | Bacillaceae | Bacillus | 1.1595 | 0.2377 | 0.8679 | 0.0417 | 0.9439 | 0.1153 |
| Firmicutes | Bacilli | Bacillaceae | Family_Bacillaceae | 0.1304 | 0 | 0.0796 | <0.0001 | 0.9687 | <0.0001 |
| Firmicutes | Bacilli | Lactobacillaceae | HT002 | 0.3678 | 1.9823 | 0.1494 | 0.0105 | 0.6651 | 0.0001 |
| Firmicutes | Bacilli | Lactobacillaceae | Lactobacillus | 0.9118 | 4.8281 | 0.8689 | 0.0003 | 1 | 0.0004 |
| Firmicutes | Bacilli | Streptococcaceae | Streptococcus | 7.9427 | 16.0682 | 6.0690 | 0.0982 | 0.9326 | 0.0186 |
| Firmicutes | Clostridia | Butyricicoccaceae | Butyricicoccus | 6.5845 | 2.0123 | 4.3069 | 0.0003 | 0.5928 | 0.0186 |
| Firmicutes | Clostridia | Butyricicoccaceae | UCG-008 | 0.0397 | 0 | 0.0046 | 0.0288 | 0.6444 | 0.2325 |
| Firmicutes | Clostridia | Butyricicoccaceae | UCG-009 | 0.1720 | 0.0328 | 0.1117 | 0.0026 | 0.7532 | 0.0253 |
| Firmicutes | Clostridia | Christensenellaceae | Christensenellaceae R-7 group | 4.2309 | 4.1057 | 2.3629 | 0.9887 | 0.1443 | 0.0349 |
| Firmicutes | Clostridia | Christensenellaceae | Family_Christensenellaceae | 0.0459 | 0.0094 | 0.0363 | 0.0480 | 1 | 0.0665 |
| Firmicutes | Clostridia | Clostridiaceae | Clostridium sensu stricto 1 | 0.0062 | 0.2236 | 0.0026 | 0.0367 | 0.7863 | 0.0023 |
| Firmicutes | Clostridia | Defluviitaleaceae | Defluviitaleaceae UCG-011 | 0.3430 | 0.4338 | 0.1992 | 0.3254 | 0.5929 | 0.0186 |
| Firmicutes | Clostridia | [Eubacterium] coprostanoligenes group | Family_[Eubacterium] coprostanoligenes group | 2.1012 | 4.6901 | 3.1611 | 0.0039 | 1 | 0.0031 |
| Firmicutes | Clostridia | Hydrogenoanaerobacterium | Family_Hydrogenoanaerobacterium | 0.0119 | 0.0020 | 0.0427 | 0.2526 | 0.6142 | 0.0167 |
| Firmicutes | Clostridia | Lachnospiraceae | [Eubacterium] hallii group | 0.1646 | 0.4483 | 0.2148 | 0.0155 | 0.9326 | 0.0599 |
| Firmicutes | Clostridia | Lachnospiraceae | [Eubacterium] ventriosum group | 0.0997 | 0.0135 | 0.0038 | 0.0340 | 0.0006 | 0.1633 |
| Firmicutes | Clostridia | Lachnospiraceae | Blautia | 1.7790 | 4.3392 | 0.8162 | 0.0059 | 0.3236 | <0.0001 |
| Firmicutes | Clostridia | Lachnospiraceae | Dorea | 0.3769 | 0.2576 | 0.0072 | 0.6096 | <0.0001 | <0.0001 |
| Firmicutes | Clostridia | Lachnospiraceae | Family_Lachnospiraceae | 1.9547 | 2.6942 | 2.3210 | 0.0043 | 0.3236 | 0.2824 |
| Firmicutes | Clostridia | Lachnospiraceae | Frisingicoccus | 0.1599 | 0.6978 | 0.0476 | 0.0518 | 0.5180 | 0.0004 |
| Firmicutes | Clostridia | Lachnospiraceae | Lachnoclostridium | 1.4047 | 0.9023 | 1.5288 | 0.2635 | 0.7863 | 0.0498 |
| Firmicutes | Clostridia | Lachnospiraceae | Lachnospira | 0.0110 | 0.3710 | 0.0267 | <0.0001 | 0.4807 | <0.0001 |
| Firmicutes | Clostridia | Lachnospiraceae | Lachnospiraceae NK4A136 group | 0.6699 | 0.8944 | 0.1580 | 0.7390 | 0.1044 | 0.0047 |
| Firmicutes | Clostridia | Lachnospiraceae | Marvinbryantia | 0.8277 | 1.5084 | 0.4420 | 0.1196 | 0.5928 | 0.0031 |
| Firmicutes | Clostridia | Lachnospiraceae | Roseburia | 0.0045 | 0.2859 | 0.0218 | 0.0043 | 0.6142 | 0.0835 |
| Firmicutes | Clostridia | Lachnospiraceae | Sellimonas | 1.0880 | 1.1354 | 0.4360 | 0.6137 | 0.0006 | <0.0001 |
| Firmicutes | Clostridia | Lachnospiraceae | Tyzzerella | 2.9827 | 1.1992 | 1.5099 | 0.0315 | 0.3886 | 0.5791 |
| Firmicutes | Clostridia | Lachnospiraceae | UC5-1-2E3 | 0.1178 | 0.4007 | 0.1730 | 0.0253 | 0.6611 | 0.1791 |
| Firmicutes | Clostridia | Monoglobaceae | Monoglobus | 0.7497 | 4.2514 | 0.5809 | <0.0001 | 0.9439 | <0.0001 |
| Firmicutes | Clostridia | Oscillospiraceae | Family_Oscillospiraceae | 2.5731 | 1.0761 | 2.9011 | 0.0182 | 0.5787 | 0.0023 |
| Firmicutes | Clostridia | Oscillospiraceae | Flavonifractor | 0.6582 | 0.2162 | 0.6112 | 0.0203 | 1 | 0.0090 |
| Firmicutes | Clostridia | Oscillospiraceae | Intestinimonas | 2.8395 | 0.5787 | 2.7148 | <0.0001 | 1 | <0.0001 |
| Firmicutes | Clostridia | Oscillospiraceae | Oscillibacter | 0.5280 | 0.3254 | 0.7632 | 0.4564 | 0.3973 | 0.0186 |
| Firmicutes | Clostridia | Oscillospiraceae | Oscillospira | 0.2566 | 0.0622 | 0.1776 | 0.0315 | 0.9439 | 0.0319 |
| Firmicutes | Clostridia | Oscillospiraceae | Pseudoflavonifractor | 0.1228 | 0.0291 | 0.1126 | 0.0658 | 1 | 0.0399 |
| Firmicutes | Clostridia | Oscillospiraceae | UCG-005 | 5.2923 | 2.4787 | 5.0788 | 0.0431 | 0.9687 | 0.0845 |
| Firmicutes | Clostridia | Peptococcaceae | Family_Peptococcaceae | 0.4498 | 0.7504 | 0.5115 | 0.0431 | 0.9326 | 0.1516 |
| Firmicutes | Clostridia | Ruminococcaceae | Anaerofilum | 0.2865 | 0.0282 | 0.0984 | 0.0272 | 0.6444 | 0.2236 |
| Firmicutes | Clostridia | Ruminococcaceae | Anaerotruncus | 0.8918 | 0.0214 | 0.4581 | <0.0001 | 0.6444 | <0.0001 |
| Firmicutes | Clostridia | Ruminococcaceae | Angelakisella | 0.0094 | 0.0013 | 0.0526 | 0.2987 | 0.6533 | 0.0348 |
| Firmicutes | Clostridia | Ruminococcaceae | CAG-352 | 0 | 0.1907 | 0 | <0.0001 | 1 | <0.0001 |
| Firmicutes | Clostridia | Ruminococcaceae | Caproiciproducens | 0.0172 | 0.0025 | 0.0372 | 0.0550 | 0.7296 | 0.0032 |
| Firmicutes | Clostridia | Ruminococcaceae | DTU089 | 0.4591 | 0.3197 | 0.6368 | 0.5802 | 0.3973 | 0.0265 |
| Firmicutes | Clostridia | Ruminococcaceae | Faecalibacterium | 12.9897 | 7.6660 | 21.6605 | 0.2828 | 0.2132 | 0.0007 |
| Firmicutes | Clostridia | Ruminococcaceae | Family_Ruminococcaceae | 5.0240 | 2.6720 | 5.4050 | 0.0059 | 0.9439 | 0.0006 |
| Firmicutes | Clostridia | Ruminococcaceae | Fournierella | 0.9912 | 0.1160 | 0.1417 | <0.0001 | 0.0006 | 0.7409 |
| Firmicutes | Clostridia | Ruminococcaceae | Incertae Sedis | 1.7218 | 1.7541 | 4.2044 | 0.6137 | 0.0006 | 0.0017 |
| Firmicutes | Clostridia | Ruminococcaceae | Paludicola | 0.4965 | 0.1426 | 0.3843 | 0.0368 | 0.9687 | 0.0665 |
| Firmicutes | Clostridia | Ruminococcaceae | Subdoligranulum | 3.0761 | 6.0777 | 1.3110 | 0.1316 | 0.3236 | 0.0004 |
| Proteobacteria | Gammaproteobacteria | Enterobacteriaceae | Escherichia-Shigella | 0.7102 | 2.5917 | 0.9393 | 0.0182 | 0.7865 | 0.0938 |
Values are the means of 12 animals (1/pen/treatment). DESeq2 analysis at 5% of significance level was used to determine statistical differences among groups.
Only bacterial families with minimum relative abundance >0.05% in at least 1 group are reported. The taxonomic classification and relative abundance of each family are shown.
At 28 d, in the cecum, GG supplementation caused significant changes in 38 bacterial genera as compared to the control group, and dietary β-mannanase restored 36 to control levels (Table 7). Relative to the control group, GG significantly increased the relative abundance of genera belonging to the families Clostridiaceae (Clostridium sensu stricto 1), Lactobacillaceae (HT002 and Lactobacillus), Lachnospiraceae ([Eubacterium] hallii group, Blautia, Family_Lachnospiraceae, Frisingicoccus, Lachnospira, Roseburia, and UC5-1-2E3) and Enterobacteriaceae (Escherichia-Shigella), and reduced genera from the families Bacillaceae (Bacillus and Family_Bacillaceae), Butyricoccaceae (Butyricicoccus, UCG-008, and UCG-009), Oscillospiraceae (Family_Oscillospiraceae, Flavonifractor, Intestinimonas, Oscillospira, and UCG-005), and Ruminococcaceae (Anaerofilum, Anaerotruncus, Family_Ruminococcaceae, Fournierella, and Paludicola).
In general, β-mannanase dietary supplementation was able to restore the main microbial shifts induced by GG and restore the gut microbiome to levels of the control group.
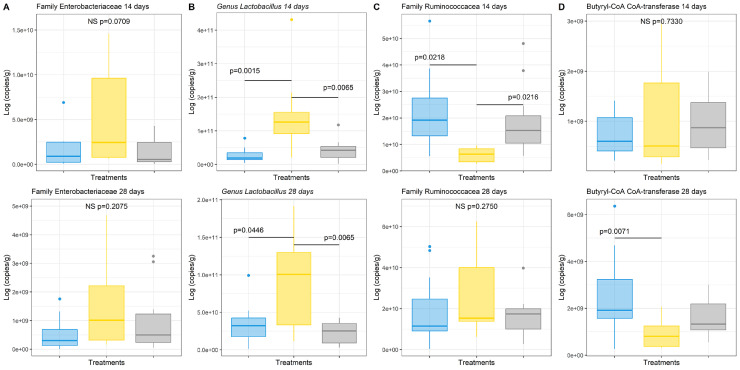
To confirm specific microbial changes induced by the diets, qPCR analysis was done to quantify the abundance of specific families and genera in cecal content. Regarding the family Enterobacteriaceae no significance was observed at both ages, however, at 14 d the GG group had a trend toward higher levels (P = 0.0709) (Figure 3). The family Ruminococcaceae was significantly reduced in the GG group at 14 d relative to the control. The abundance of the Lactobacillus genus was significantly higher in the GG group as compared to the control group at 14 and 28 d, which is in accordance with the 16SrRNA gene data.
Figure 3.
qPCR analysis from cecal content at d 14 (first line) and d 28 (second line) for animals fed a control diet or a diet supplemented with guar gum, either or not supplemented with β-mannanase at 330 g/ton feed. Values are the means of 12 animals (1/pen/treatment) ± standard deviation of the mean. ANOVA, followed by Tukey multiple comparison test, was used to determine statistical differences among groups Y axis—results are expressed as log of copies per gram of cecal content. Treatments:  Control;
Control;  Guar gum;
Guar gum;  Guar gum + β-mannanase. NS, nonsignificant.
Guar gum + β-mannanase. NS, nonsignificant.
The gene encoding butyryl-CoA: acetate CoA-transferase, which estimates the butyrate-producing ability of the microbiota (Louis and Flint, 2007), was also quantified. At 28 d a significant decrease was found in the GG group relative to the control group.
Guar Gum and β-Mannanase Supplementation Affect SCFA and BCFA Concentrations in the Cecal
SCFA and BCFA concentrations in cecal content were analyzed at 14 and 28 d of age (Table 8). At 14 d of age β-mannanase supplementation reduced propionate, valeric, isovaleric, isobutyric and BCFA levels, as compared to the GG group. At 28 d GG increased acetic and isocaproic acid levels relative to the control group, and again a reduction in propionate, caproic, valeric, isovaleric, isobutyric, isocaproic, and BCFA levels were induced by the enzyme, relative to the GG group levels.
Table 8.
SCFA concentrations in cecal content at 2 time points among groups of animals fed a control diet or a diet supplemented with guar gum, either or not supplemented with β-mannanase at 330 g/ton feed.
| SCFA | CTR |
GG |
GG + E |
CTR vs. GG |
CTR vs. GG + E |
GG vs. GG + E |
|---|---|---|---|---|---|---|
| Mean ± SD (µmol/g) | Adjusted P value | |||||
| Cecum 14 d | ||||||
| Acetic acid | 64.78 ± 22.60 | 66.12 ± 15.05 | 69.15 ± 21.53 | 0.9860 | 0.8560 | 0.9310 |
| Butyric acid | 10.37 ± 4.99 | 10.04 ± 4.67 | 10.65 ± 5.97 | 0.9871 | 0.9909 | 0.9579 |
| Propionic acid | 8.59 ± 5.22 | 7.68 ± 2.97 | 2.90 ± 0.29 | 0.9943 | 0.0001 | 0.0001 |
| Caproic acid | 0.31 ± 0.02 | 0.32 ± 0.02 | 0.31 ± 0.01 | 0.8284 | 0.9943 | 0.8669 |
| Isobutyric acid | 0.79 ± 0.19 | 0.92 ± 0.22 | 0.67 ± 0.14 | 0.2028 | 0.2943 | 0.0077 |
| Isovaleric acid | 0.81 ± 0.24 | 1.00 ± 0.22 | 0.64 ± 0.12 | 0.0726 | 0.1486 | 0.0006 |
| Isocaproic acid | 0.25 ± 0.01 | 0.25 ± 0.01 | 0.25 ± 0.01 | 0.9655 | 0.9977 | 0.9774 |
| Valeric acid | 1.14 ± 0.31 | 1.22 ± 0.30 | 0.76 ± 0.25 | 0.8024 | 0.0073 | 0.0016 |
| Total SCFA | 90.33 ±31.53 | 87.66 ± 20.3 | 85.99 ± 27.36 | 0.9695 | 0.9180 | 0.9879 |
| BCFA | 1.91 ± 0.52 | 2.19 ± 0.42 | 1.57 ± 0.26 | 0.2713 | 0.1206 | 0.0033 |
| Cecum 28 d | ||||||
| Acetic acid | 58.25 ± 9.62 | 65.01 ± 17.51 | 41.41 ± 17.32 | 0.5426 | 0.0504 | 0.0040 |
| Butyric acid | 8.49 ± 2.81 | 14.62 ± 9.53 | 14.58 ± 12.36 | 0.2677 | 0.2723 | >0.9999 |
| Propionic acid | 9.93 ± 2.73 | 11.94 ± 4.92 | 7.50 ± 4.17 | 0.4963 | 0.3656 | 0.0334 |
| Caproic acid | 0.30 ± 0.01 | 0.32 ± 0.01 | 0.30 ± 0.01 | 0.0579 | 0.9652 | 0.0292 |
| Isobutyric acid | 0.88 ± 0.17 | 0.93 ± 0.32 | 0.61 ± 0.11 | 0.9865 | 0.0064 | 0.0035 |
| Isovaleric acid | 0.91 ± 0.26 | 1.07 ± 0.49 | 0.67 ± 0.25 | 0.7524 | 0.1175 | 0.0211 |
| Isocaproic acid | 0.24 ± 0.01 | 0.26 ± 0.02 | 0.25 ± 0.01 | 0.0242 | 0.9679 | 0.0421 |
| Valeric acid | 1.06 ± 0.18 | 1.19 ± 0.16 | 0.87 ± 0.29 | 0.3485 | 0.1311 | 0.0052 |
| Total SCFA | 80.12 ± 10.74 | 95.55 ± 28.97 | 67.75 ± 37.17 | 0.3847 | 0.5659 | 0.0635 |
| BCFA | 2.04 ± 0.43 | 2.29 ± 0.78 | 1.48 ± 0.25 | 0.7004 | 0.0175 | 0.0019 |
Values are the means of 12 animals (1/pen/treatment) ± standard deviation. ANOVA, followed by Tukey multiple comparison test, was used to determine statistical differences among groups.
GG, guar gum; GG + E, guar gum + β-mannanase; SCFA, short-chain fatty acid; BCFA, branched-chain fatty acid. Quantification limit: acetic acid, 18.43 µmol/g; butyric acid, 1.45 µmol/g; propionic acid, 1.47 µmol/g; caproic acid, 0.29 µmol/g; isobutyric acid, 0.43 µmol/g; isovaleric acid, 0.42 µmol/g; isocaproic acid, 0.24 µmol/g; valeric acid, 0.43 µmol/g; total SCFA, 23.73 µmol/g; BCFA, 0.88 µmol/g.
DISCUSSION
Guar gum is produced from guar, a drought resistant legume. The byproduct guar meal may contain up to 45% protein and is used as a feed ingredient, but it contains a high concentration of β-mannans, considered to be an antinutritional factor, that leads to animal performance losses (Saeed et al., 2017). This was confirmed in our trial, and β-mannanase supplementation was able to restore the BW and FCR to control levels. The negative effects of GG on zootechnical performance was already observed in previous studies (Leeet al., 2003; Mishra et al., 2013; Rama Rao et al., 2014). The poor performance is attributed to the high intestinal viscosity induced by GG, that reduces the passage rate, increases the satiety, reduces the feed intake (also observed in this study), and also impairs the nutrient absorption (Maisonnier et al., 2003; Owusu-Asiedu et al., 2006). According to previous studies the positive effects of dietary β-mannanase supplementation on broiler performance is a consequence of a higher ileal digestibility of carbohydrates and amino acids (Caldas et al., 2018; Gomez-Osorio et al., 2021; Latham et al., 2018; White et al., 2021).
Villus morphology was not negatively affected by GG supplementation to the diets. GG increased the ileal villus height as compared to the control group. Diets containing high levels of NSP significantly increase the length and weight of the gastrointestinal tract (Jorgensen et al., 1996). Maisonnier et al. (2003) also reported an increase in the villus height as a consequence of dietary GG supplementation and suggested that this finding is an adaptative process counteracting the negative effects of the diet, attempting to improve nutrient uptake.
Dysbiosis of the gut microbiome is a disruption in the balance, diversity, and function of intestinal microbial communities (Perez et al., 2019). In the current study guar gum ingestion caused changes in microbial diversity and composition in the cecal and ileal microbiome. Guar gum ingestion increased the relative abundance of the Proteobacteria phylum (ileum 14 d, cecum 14 and 28 d). A high abundance of phylum Proteobacteria is a hallmark of dysbiosis and poor chicken performance (Shin et al., 2015; Kollarcikova et al., 2019). The poor performance observed in the GG group in this study, might thus be associated with a diet-induced dysbiosis. The GG supplementation increased the relative abundance of bacterial families and genera known to contain opportunistic pathogens. Escherichia-Shigella (ileum 14 d, cecum 14 and 28 d) and Clostridium sensu stricto 1 (encompassing C. perfringens) (cecum 28 d) are the most well-known examples of bacterial members that were increased by dietary GG supplementation. The β-mannanase supplementation restored this shift to control levels. High levels of Enterobacteriaceae may increase intestinal lipopolysaccharide concentrations, often associated with increases in gut permeability, triggering inflammation (Bibbò et al., 2016). Guar gum supplementation also increased enterotoxigenic Escherichia coli proliferation in postweaning piglets (McDonald et al., 1999). In chickens, an increase of Escherichia-Shigella has been associated with a poor zootechnical performance (Rubio et al., 2015; Han et al., 2016). Similarly, in the present study GG supplementation impaired the zootechnical performance. Another important change was observed in the genus Lactobacillus that was increased in GG group as compared to the others, a finding that was confirmed by qPCR. β-Mannanase supplementation restored the levels to control values. Lactobacillus is a galactomannan fermenter and lactate producer (Ali et al., 2022). When high quantities of nondigestible carbohydrates reach the large intestine, they will be fermented and unusual high amounts of organic acids (lactic acid and SCFA) can be produced, resulting in a lower luminal pH, affecting the microbial composition (Petersen, 2005). While lactic acid bacteria are known for their prebiotic properties, intestinal overgrowth has been related to poor intestinal health in multiple studies (Park et al., 2021; Dey and Ray Chaudhuri, 2022; Slanzon et al., 2022).
Our data show that in the cecum, the mannan-rich diet increased the relative abundance of the genus Bifidobacterium and Roseburia, known by their capacity to ferment complex polysaccharides that have escaped proximal digestion (Jandhyala et al., 2015). This result supports our hypothesis of dysbiosis as consequence of poor ileal digestibility. The relative abundance of specific members of the Ruminococcaceae and Lachnospiraceae was reduced when GG was added to the broiler diets. These families contain butyrate-producing bacteria, of which a subset use lactate, and are shown to be of crucial importance to maintain gut health (Parada Venegas et al., 2019). Examples of beneficial bacterial genera from the Ruminococcaceae family that are negatively affected by GG supplementation (and restored to normal levels after supplementation with the β-mannanase) are Butyricicoccus and Faecalibacterium. These are known as butyrate-producing microorganisms that are highly anti-inflammatory, improve intestinal integrity because of effects on tight junction protein expression, and associated with optimal animal performance (Onrust et al., 2015; Bedford et al., 2017; Sikandar et al., 2017). As an example, Butyricicoccus supplementation to broiler diets has been shown to improve animal performance in a diet-induced dysbiosis models and was shown to reduce necrotic enteritis caused by C. perfringens (Eeckhaut et al., 2016). Also, it reduced the cecal abundance of Escherichia, an association seen in our study as well. While butyrate production has been hypothesized as the main driver for beneficial effects related to these bacterial genera, we did not observe effects on SCFA production, although a lower cecal (28 d) abundance of the gene encoding butyryl-CoA acetate CoA-transferase was observed in the GG-supplemented group.
Isobutyric, isovaleric, valeric acids, and BCFA are the result of protein fermentation (Fan et al., 2015). They were increased in the group that received the GG diet as compared to the group receiving the GG + β-mannanase. Probably the higher abundance of protein-derived BCFAs in the cecal content of the GG group is a consequence of poor protein absorption in the upper intestine.
In conclusion, guar gum diet supplementation impairs the broilers’ zootechnical performance and for the first time a detailed effect of a mannan-rich diet on the broiler's gut microbiome has been described. GG causes a shift toward increases in opportunistic pathogens and reductions in beneficial microbiota and β-mannanase supplementation was able to restore the effects on the microbiota composition to control levels. Our results indicate that β-mannanase is a viable feed additive that can be used to counteract negative effects of mannan-rich feed ingredients on the microbiota composition.
ACKNOWLEDGMENTS
The authors sincerely acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) for the fellowship (88881.624517/2021-01) granted to Marielen de Souza, and to Elanco Animal Health for sponsoring this research project.
DISCLOSURES
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Marielen de Souza reports financial support was provided by National Council for Scientific and Technological Development (CAPES).
REFERENCES
- Abdulamir A.S., Yoke T.S., Nordin N., Bakar F.A. Detection and quantification of probiotic bacteria using optimized DNA extraction, traditional and real-time PCR methods in complex microbial communities. Afr. J. Biotechnol. 2010;9:1481–1492. [Google Scholar]
- Aguirre M., Vuorenmaa J., Valkonen E., Kettunen H., Callens C., Haesebrouck F., Ducatelle R., Van Immerseel F., Goossens E. In-feed resin acids reduce matrix metalloproteinase activity in the ileal mucosa of healthy broilers without inducing major effects on the gut microbiota. Vet. Res. 2019;50:1–15. doi: 10.1186/s13567-019-0633-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali Q., Ma S., La S., Guo Z., Liu B., Gao Z., Farooq U., Wang Z., Zhu X., Cui Y., Li D., Shi Y. Microbial short-chain fatty acids: a bridge between dietary fibers and poultry gut health — a review. Anim. Biosci. 2022;35:1461–1478. doi: 10.5713/ab.21.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amerah A.M. Interactions between wheat characteristics and feed enzyme supplementation in broiler diets. Anim. Feed Sci. Technol. 2015;199:1–9. [Google Scholar]
- Aviagen . Ross manual de manejo de frangos de corte 2018. Aviagen; Huntsville, AL: 2018. [Google Scholar]
- Bartosch S., Fite A., Macfarlane G.T., McMurdo M.E.T. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl. Environ. Microbiol. 2004;70:3575–3581. doi: 10.1128/AEM.70.6.3575-3581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford A., Yu H., Squires E.J., Leeson S., Gong J. Effects of supplementation level and feeding schedule of butyrate glycerides on the growth performance and carcass composition of broiler chickens. Poult. Sci. 2017;96:3221–3228. doi: 10.3382/ps/pex098. [DOI] [PubMed] [Google Scholar]
- Bibbò S., Ianiro G., Giorgio V., Scaldaferri F., Masucci L., Gasbarrini A., Cammarota GJERMPS. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016;20:4742–4749. [PubMed] [Google Scholar]
- Boesmans L., Valles-Colomer M., Wang J., Eeckhaut V., Falony G., Ducatelle R., Van Immerseel F., Raes J., Verbeke K., Cotter Paul D. Butyrate producers as potential next-generation probiotics: safety assessment of the administration of Butyricicoccus pullicaecorum to healthy volunteers. mSystems. 2018;3 doi: 10.1128/mSystems.00094-18. e00094-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn A., Korpela M. Team R Core; Vienna, Austria: 2013. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Bushra M., Al-Obaidi B., Mohammed H., Dakheel K. The potential influence of fractional substitute of soybean meal with guar meal supplement of salinomycine and mycofixe on performance and GUT ecosystem of broiler. Int. J. Pharm. Res. 2020;12:848–854. [Google Scholar]
- Caldas J.V., Vignale K., Boonsinchai N., Wang J., Putsakum M., England J.A., Coon C.N. The effect of β-mannanase on nutrient utilization and blood parameters in chicks fed diets containing soybean meal and guar gum. Poult. Sci. 2018;97:2807–2817. doi: 10.3382/ps/pey099. [DOI] [PubMed] [Google Scholar]
- Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J.A., Holmes S.P. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Căpriţă R., Căpriţă A., Julean C. Biochemical aspects of non-starch polysaccharides. Sci. Plant Anim. Sci. Biotechnol. 2010;43:368–374. [Google Scholar]
- Davis N.M., Proctor D.M., Holmes S.P., Relman D.A., Callahan B.J. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome. 2018;6:1–14. doi: 10.1186/s40168-018-0605-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weirdt R., Possemiers S., Vermeulen G., Moerdijk-Poortvliet T.C., Boschker H.T., Verstraete W., Van de Wiele T. Human faecal microbiota display variable patterns of glycerol metabolism. FEMS Microbiol. Ecol. 2010;74:601–611. doi: 10.1111/j.1574-6941.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- Dey P., Ray Chaudhuri S. The opportunistic nature of gut commensal microbiota. Crit. Rev. Microbiol. 2022:1–25. doi: 10.1080/1040841X.2022.2133987. [DOI] [PubMed] [Google Scholar]
- Dixon P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003;14:927–930. [Google Scholar]
- Eaton A.D., Clesceri L.S., Greenberg A.E., Franson M.A.H. 18th ed. APHA; Washington, DC: 1998. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- European Union (EU) Council directive 2007/43/EC of 28 June 2007 laying down minimum rules for the protection of chickens kept for meat production. Off. J. Eur. Union. 2007;182:19–28. [Google Scholar]
- Eeckhaut V., Wang J., Van Parys A., Haesebrouck F., Joossens M., Falony G., Raes J., Ducatelle R., Immerseel Van F. The probiotic Butyricicoccus pullicaecorum reduces feed conversion and protects from potentially harmful intestinal microorganisms and necrotic enteritis in broilers. Front. Microbiol. 2016;7:1–9. doi: 10.3389/fmicb.2016.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Comission (EC) Council Regulation (EC) No 1099/2009 of 24 September 2009 on the protection of animals at the time of killing (text with EEA relevance). Annex I - list of stunning methods and related specifications. Off. J. Eur. Union. 2009;303:1–30. [Google Scholar]
- Fan P., Li L., Rezaei A., Eslamfam S., Che D., Ma X.J.C.P., Science P. Metabolites of dietary protein and peptides by intestinal microbes and their impacts on gut. Protein Pept. Sci. 2015;16:646–654. doi: 10.2174/1389203716666150630133657. [DOI] [PubMed] [Google Scholar]
- Gomez-Osorio L., Oh H.G., Lee J.J. Confirmation of cage effect and prebiotic production potential of a β-mannanase, with SBM as substrate using microscopy and wet chemistry. J. Agric. Sci. 2021;13:23–31. [Google Scholar]
- Han G.G., Kim E.B., Lee J., Lee J.-Y., Jin G., Park J., Huh C.-S., Kwon I.-K., Kil D.Y., Choi Y.-J., Kong C. Relationship between the microbiota in different sections of the gastrointestinal tract, and the body weight of broiler chickens. Springerplus. 2016;5:1–9. doi: 10.1186/s40064-016-2604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervé, M. 2022. RVAideMemoire: Testing and Plotting Procedures for Biostatistics. Accessed April 2022. https://cran.r-project.org/web/packages/RVAideMemoire/index.html.
- Hove K.V., Kilroy S., Smid G., Bolderman M., Wolfs S. Elanco Animal Health; Antwerpen, Belgium: 2018. Pages 1–4 in Research Update on β-Mannanan Content in Common Feed Ingredients. [Google Scholar]
- Hussain M., Rehman A.U., Khalid M.F. Feeding value of guar meal and the application of enzymes in improving nutritive value for broilers. Worlds Poult. Sci. J. 2012;68:253–268. [Google Scholar]
- Jandhyala S.M., Talukdar R., Subramanyam C., Vuyyuru H., Sasikala M., Reddy D.Nageshwar. Role of the normal gut microbiota. World J. Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski N.W., Lærke H.N., Bach Knudsen K.E., Stein H.H. Carbohydrate composition and in vitro digestibility of dry matter and nonstarch polysaccharides in corn, sorghum, and wheat and coproducts from these grains1. J. Anim. Sci. 2015;93:1103–1113. doi: 10.2527/jas.2014-8147. [DOI] [PubMed] [Google Scholar]
- Jezierny D., Mosenthin R., Bauer E. The use of grain legumes as a protein source in pig nutrition: A review. Anim. Feed Sci. Technol. 2010;157:111–128. [Google Scholar]
- Jha R., Fouhse J.M., Tiwari U.P., Li L., Willing B.P. Dietary fiber and intestinal health of monogastric animals. Front. Vet. Sci. 2019;6:1–12. doi: 10.3389/fvets.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen H., Zhao X.-Q., Knudsen K.E.B., Eggum B.O. The influence of dietary fibre source and level on the development of the gastrointestinal tract, digestibility and energy metabolism in broiler chickens. Br. J. Nutr. 1996;75:379–395. doi: 10.1079/bjn19960141. [DOI] [PubMed] [Google Scholar]
- Kermanshahi H., Shakouri M.D., Daneshmand A. Effects of non-starch polysaccharides in semi-purified diets on performance, serum metabolites, gastrointestinal morphology, and microbial population of male broiler chickens. Livest. Sci. 2018;214:93–97. [Google Scholar]
- Klindworth A., Pruesse E., Schweer T., Peplies J., Quast C., Horn M. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic. Acids Res. 2013;41 doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollarcikova M., Kubasova T., Karasova D., Crhanova M., Cejkova D., Sisak F., Rychlik I. Use of 16S rRNA gene sequencing for prediction of new opportunistic pathogens in chicken ileal and cecal microbiota. Poult. Sci. 2019;98:2347–2353. doi: 10.3382/ps/pey594. [DOI] [PubMed] [Google Scholar]
- Kowalchuk G.A., Bodelier P.L.E., Heilig G.H.J., Stephen J.R., Laanbroek H.J. Community analysis of ammonia-oxidising bacteria, in relation to oxygen availability in soils and root-oxygenated sediments, using PCR, DGGE and oligonucleotide probe hybridisation. FEMS Microbiol. Ecol. 1998;27:339–350. [Google Scholar]
- Latham R.E., Williams M.P., Walters H.G., Carter B., Lee J.T. Efficacy of β-mannanase on broiler growth performance and energy utilization in the presence of increasing dietary galactomannan. Poult. Sci. 2018;97:549–556. doi: 10.3382/ps/pex309. [DOI] [PubMed] [Google Scholar]
- Latorre J.D., Hernandez-Velasco X., Bielke L.R., Vicente J.L., Wolfenden R., Menconi A., Hargis B.M., Tellez G. Evaluation of a Bacillus direct-fed microbial candidate on digesta viscosity, bacterial translocation, microbiota composition and bone mineralisation in broiler chickens fed on a rye-based diet. Br. Poult. Sci. 2015;56:723–732. doi: 10.1080/00071668.2015.1101053. [DOI] [PubMed] [Google Scholar]
- Lee J.T., Bailey C.A., Cartwright A.L. Guar meal germ and hull fractions differently affect growth performance and intestinal viscosity of broiler chickens. Poult. Sci. 2003;82:1589–1595. doi: 10.1093/ps/82.10.1589. [DOI] [PubMed] [Google Scholar]
- Li Y.-F., Calley J.N., Ebert P.J., Helmes E.B. Paenibacillus lentus spp. nov., a β-mannanolytic bacterium isolated from mixed soil samples in a selective enrichment using guar gum as the sole carbon source. Int. J. Syst. Evol. Microbiol. 2014;64:1166–1172. doi: 10.1099/ijs.0.054726-0. [DOI] [PubMed] [Google Scholar]
- Louis P., Flint H.J. Development of a semiquantitative degenerate real-time PCR-based assay for estimation of numbers of butyryl-coenzyme A (CoA) CoA transferase genes in complex bacterial samples. Appl. Environ. Microbiol. 2007;73:2009–2012. doi: 10.1128/AEM.02561-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonnier S., Gomez J., Brée A., Berri C., Baéza E., Carré B. Effects of microflora status, dietary bile salts and guar gum on lipid digestibility, intestinal bile salts, and histomorphology in broiler chickens. Poult. Sci. 2003;82:805–814. doi: 10.1093/ps/82.5.805. [DOI] [PubMed] [Google Scholar]
- Matsuki T., Watanabe K., Fujimoto J., Takada T., Tanaka R. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl. Environ. Microbiol. 2004;70:7220–7228. doi: 10.1128/AEM.70.12.7220-7228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D.E., Pethick D.W., Pluske J.R., Hampson D.J. Adverse effects of soluble non-starch polysaccharide (guar gum) on piglet growth and experimental colibacillosis immediately after weaning. Res. Vet. Sci. 1999;67:245–250. doi: 10.1053/rvsc.1999.0315. [DOI] [PubMed] [Google Scholar]
- McMurdie P.J., Holmes S. Phyloseq: a R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao M., Shi Y., Li Y., Jiang Z., Liu J., Yang S. Non-digestible galactomannan oligosaccharides from Cassia seed gum modulate microbiota composition and metabolites of human fecal inoculum. J. Funct. Foods. 2021;86 [Google Scholar]
- Mishra A., Sarkar S.K., Ray S., Haldar S. Effects of partial replacement of soybean meal with roasted guar korma and supplementation of mannanase on performance and carcass traits of commercial broiler chickens. Vet. World. 2013;6:693–697. [Google Scholar]
- Noblet J., Wu S.-B., Choct M. Methodologies for energy evaluation of pig and poultry feeds: a review. Anim. Nutr. 2022;8:185–203. doi: 10.1016/j.aninu.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD, and FAO . OECD Publishing; Paris, France: 2021. Pages 15 in OECD-FAO Agricultural Outlook 2021-2030 ©. [Google Scholar]
- Onrust L., Ducatelle R., Van Driessche K., De Maesschalck C., Vermeulen K., Haesebrouck F., Eeckhaut V., Immerseel F.V. Steering endogenous butyrate production in the intestinal tract of broilers as a tool to improve gut health. Front. Vet. Sci. 2015;2:1–8. doi: 10.3389/fvets.2015.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Asiedu A., Patience J.F., Laarveld B., Kessel A.G.V., Simmins P.H., Zijlstra R.T. Effects of guar gum and cellulose on digesta passage rate, ileal microbial populations, energy and protein digestibility, and performance of grower pigs. J. Anim. Sci. 2006;84:843–852. doi: 10.2527/2006.844843x. [DOI] [PubMed] [Google Scholar]
- Parada Venegas D., De la Fuente M.K., Landskron G., González M.J., Quera R., Dijkstra G., Harmsen H.J.M., Faber K.N., Hermoso M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019;10:1–16. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park T., Cheong H., Yoon J., Kim A., Yun Y., Unno T. Comparison of the fecal microbiota of horses with intestinal disease and their healthy counterparts. Vet. Sci. 2021;8:1–10. doi: 10.3390/vetsci8060113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez N.B., Dorsen C., Squires A. Dysbiosis of the gut microbiome: a concept analysis. J. Holist. Nurs. 2019;38:223–232. doi: 10.1177/0898010119879527. [DOI] [PubMed] [Google Scholar]
- Petersen C. D-lactic acidosis. Nutr. Clin. Pract. 2005;20:634–645. doi: 10.1177/0115426505020006634. [DOI] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rama Rao S.V., Prakash B., Raju M.V.L.N., Panda A.K., Murthy O.K. Effect of supplementing non-starch polysaccharide hydrolyzing enzymes in guar meal based diets on performance, carcass variables and bone mineralization in Vanaraja chicken. Anim. Feed Sci. Technol. 2014;188:85–91. [Google Scholar]
- Rao T.P., Quartarone G. Role of guar fiber in improving digestive health and function. Nutrition. 2019;59:158–169. doi: 10.1016/j.nut.2018.07.109. [DOI] [PubMed] [Google Scholar]
- Rubio L.A., Peinado M.J., Ruiz R., Suárez-Pereira E., Mellet C.O., García Fernández J.M. Correlations between changes in intestinal microbiota composition and performance parameters in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2015;99:418–423. doi: 10.1111/jpn.12256. [DOI] [PubMed] [Google Scholar]
- Saeed M., Ayaşan T., Alagawany M., El-Hack M., Abdel-Latif M., Patra A.K. The Role of ß-Mannanase (Hemicell®) in Improving Poultry Productivity, Health and Environment. Braz. J. Poult. Sci. 2019;21:1–8. [Google Scholar]
- Saeed M., Hassan F., Shah Q., Arain M., El-Hack M., Alagawany M., Dhama KJAAVS. Practical application of guar (Cyamopsis tetragonoloba L. Taub) meal in poultry nutrition. Adv. Anim. Vet. Sci. 2017;5:491–499. [Google Scholar]
- Schliep K.P. Phangorn: phylogenetic analysis in R. Bioinformatics. 2011;27:592–593. doi: 10.1093/bioinformatics/btq706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shastak Y., Ader P., Feuerstein D., Ruehle R., Matuschek M. ß-Mannan and mannanase in poultry nutrition. Worlds Poult. Sci. J. 2015;71:161–174. [Google Scholar]
- Shin N.-R., Whon T.W., Bae J.-W. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Shirouchi B., Kawamura S., Matsuoka R., Baba S., Nagata K., Shiratake S., Tomoyori H., Imaizumi K., Sato M. Dietary guar gum reduces lymph flow and diminishes lipid transport in thoracic duct-cannulated rats. Lipids. 2011;46:789–793. doi: 10.1007/s11745-011-3570-0. [DOI] [PubMed] [Google Scholar]
- Shojadoost B., Vince A.R., Prescott J.F. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review. Vet. Res. 2012;43:74. doi: 10.1186/1297-9716-43-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikandar A., Zaneb H., Younus M., Masood S., Aslam A., Khattak F., Ashraf S., Yousaf M.S., Rehman HJA-Ajoas. Effect of sodium butyrate on performance, immune status, microarchitecture of small intestinal mucosa and lymphoid organs in broiler chickens. Asian-Australas. J. Anim. Sci. 2017;30:690–699. doi: 10.5713/ajas.16.0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Singh G., Arya S.K. Mannans: an overview of properties and application in food products. Int. J. Biol. Macromol. 2018;119:79–95. doi: 10.1016/j.ijbiomac.2018.07.130. [DOI] [PubMed] [Google Scholar]
- Slanzon G.S., Ridenhour B.J., Moore D.A., Sischo W.M., Parrish L.M., Trombetta S.C., McConnel C.S. Fecal microbiome profiles of neonatal dairy calves with varying severities of gastrointestinal disease. PLoS One. 2022;17 doi: 10.1371/journal.pone.0262317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein, M. M., A. Prem, M. Jin, S. Tang, and J. M. Bhasin. 2019. FIGARO: an efficient and objective tool for optimizing microbiome rRNA gene trimming parameters. bioRxiv:610394.
- White D., Adhikari R., Wang J., Chen C., Lee J.H., Kim W.K. Effects of dietary protein, energy and β-mannanase on laying performance, egg quality, and ileal amino acid digestibility in laying hens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E.S. DECIPHER: harnessing local sequence context to improve protein multiple sequence alignment. BMC Bioinform. 2015;16:322. doi: 10.1186/s12859-015-0749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Xu J., Lei L., Jiang Y., Gao F., Zhou G.H. Effects of xylanase supplementation on growth performance, nutrient digestibility and non-starch polysaccharide degradation in different sections of the gastrointestinal tract of broilers fed wheat-based diets. Asian-Australas. J. Anim. Sci. 2014;27:855–861. doi: 10.5713/ajas.2014.14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X.T., Qiao X.J., Xu Z.R. Effect of β-mannanase (Hemicell®) on growth performance and immunity of broilers. Poult. Sci. 2006;85:2176–2179. doi: 10.1093/ps/85.12.2176. [DOI] [PubMed] [Google Scholar]