Summary
Background
Clostridioides difficile is the foremost cause of nosocomial infectious diarrhoea and one of the most prevalent healthcare associated infections (HAIs).
Aims
To investigate the impact of the coronavirus disease 2019 (COVID-19) pandemic on the incidence of healthcare associated C. difficile infection (HA-CDI).
Methods
A retrospective study was conducted from January 2019–December 2022 inclusive at a tertiary University Hospital in Dublin, Ireland. The study period was divided into COVID-19 and non-COVID-19 periods determined in tangent with the then national incidences of COVID-19 and number of hospitalized patients with COVID-19. Analyses looked at quantity of testing performed, incidence rates and antimicrobial consumption. An independent samples t-test was used to determine significance between groups.
Results
Between COVID-19 and non-COVID-19 periods, no statistically significant difference was observed among HA-CDI rates per 10,000 bed-days (2.1 cases vs 1.76 cases; P=0.34), consumption of defined daily doses per 100 bed-days of antimicrobials – all antimicrobials (83.36 vs 89.5; P=0.091), fluoroquinolones only (3.71 vs 4.46; P=0.067), third-generation cephalosporins only (4.17 vs 4.43; P=0.449), carbapenems only (3.28 vs 3.26; P=0.944) – or the number of C. difficile tests performed per 10,000 bed-days (321.81 tests vs 326.63 tests; P=0.696).
Conclusions
There was no difference in the incidence rates of HA-CDI between COVID-19 and non-COVID-19 periods at our institution.
Keywords: COVID-19, Clostridioides difficile, Infection prevention and control, Healthcare associated infections
Introduction
Clostridioides difficile is the foremost cause of nosocomial infectious diarrhoea and one of the most prevalent healthcare associated infections (HAIs) [1,2]. The Health Protection Surveillance Centre reported 1,774 enhanced cases of C. difficile infection (CDI) in 2021, with an average rate of healthcare associated CDI (HA-CDI) of 2.1 cases per 10,000 bed-days in the Republic of Ireland [3]. Predisposing factors for C. difficile colonization and infection, among others, include exposure to C. difficile spores colonizing the environment as well as previous exposure to broad-spectrum antimicrobials [4]. As such, environment decontamination, hand hygiene and antimicrobial stewardship have been widely accepted interventions for CDI prevention [5].
The coronavirus disease 2019 (COVID-19) pandemic placed significant demands on an already strained healthcare system. Hospitals experienced an extreme restructuring with a heavy reliance on robust infection prevention and control (IPC) services, including hand hygiene and personal protective equipment compliance. Theoretically, this increased focus on IPC practices may have positively impacted on HAIs, including those from multi-drug resistant organisms (MDROs) and C. difficile. On the other hand, the shift of resources from surveillance of HAIs to support the COVID-19 response, the prioritisation of limited isolation facilities for COVID-19 and the discontinuation of antimicrobial stewardship activities, could lead to an increase in HAIs [6].
The impact of COVID-19 pandemic on the incidence of CDI has been assessed by a few studies with conflicting results – most report no effect or a reduction of CDI incidence during the first wave [[7], [8], [9], [10], [11], [12], [13]]. However, as the pandemic evolved, so too could its impact on CDI incidence. The medical management of hospitalized patients with COVID-19 and the profile of these patients has greatly changed over the course of the previous years; and while aggressive lockdowns and restricted hospital activity existed during the first wave, a progressive synchronicity of COVID-19 and non-COVID-19 medical care has been established in recent times. As such, we aim to investigate the impact of the COVID-19 pandemic on the incidence of HA-CDI from January 2019–December 2022 inclusive at a tertiary hospital in the Republic of Ireland.
Materials and methods
Study design and setting
This was a retrospective study conducted from January 2019–December 2022 inclusive at a tertiary University Hospital in Dublin, Ireland. St. Vincent's University Hospital, part of the St. Vincent's Healthcare Group, is a 600-bedded academic teaching hospital providing front line, acute, chronic and emergency care across over 50 different medical specialities. The Emergency Department is a major referral centre for cerebrovascular events and major trauma; and the National Cancer Control Programme, Liver Transplant Programme, Pancreas Transplant Programme and Centre for Cystic Fibrosis all operate internally.
The study period was divided into COVID-19 and non-COVID-19 periods determined in tangent with the then national incidences of COVID-19 and number of hospitalized patients with COVID-19. Using 50 COVID-19 inpatients as a lower limit to define a “COVID-19 Period”, April and May 2020, January and February 2021, January, February and March 2022 and June and July 2022 were designated as same. The threshold of 50 patients was decided upon as an indirect indicator of a hospital monthly occupancy bed rate due to COVID-19 >5%.
Data collection and definitions
C. difficile testing is performed using the EntericBio® real-time PCR assay since 2013 at our institution according to manufacturer instructions [14]. Testing is only performed on loose stool samples from patients over the age of two-years and repeated samples are only performed after at least four-weeks from a positive PCR result, or by request of the Clinical Microbiologist. The total number of C. difficile tests performed, total number of samples where C. difficile was detected and classification of cases according to accepted surveillance definitions during the study period was extracted from weekly IPC meeting reports. CDI diagnosis was established following definitions described by the Health Protection Surveillance Centre (HPSC) – diarrhoeal stools (at least three loose bowel motions in 24 hours) or toxic megacolon; with either a positive laboratory assay for C. difficile toxin A and/or toxin B in stools or a toxin-producing C. difficile organism detected in stool via culture. Cases of pseudomembranous colitis revealed by lower gastrointestinal endoscopy or colonic histopathology characteristic of C. difficile infection (with or without diarrhoea) were also considered diagnostic of CDI for enhanced surveillance purposes [15]. According to European Centre for Disease Prevention and Control criteria, a CDI episode was classified as HA-CDI if the onset of symptoms occurred on day three or later following admission to a healthcare facility or within four weeks of discharge from a healthcare facility [16]. The rate of HA-CDI was reported as the number of cases per 10,000 bed-days (BDs).
Total antimicrobial consumption during the study period was also examined, with a focus on specific classes of antimicrobials traditionally associated with inducing CDI, namely third-generation cephalosporins, fluoroquinolones and carbapenems. Antimicrobial consumption was assessed by defined daily dose (DDD) per 100 BDs according to the Anatomical Therapeutic Chemical Classification System defined by the World Health Organisation [17]. This data was obtained from quarterly reports compiled by the local Antimicrobial Advisory Committee.
Study outcomes
The primary outcome of this investigation was to assess the impact of the COVID-19 pandemic on HA-CDI rates. Analyses looked at gross incidence rates as well as antimicrobial consumption and testing practices during the outlined period.
Statistical analysis
Data analysis was performed using IBM SPSS V.25. Continuous variables were expressed as means within each group and an independent samples t-test was used to determine significance of difference between groups. A P value of <0.05 was considered statistically significant.
Ethical approval
The project was discussed with St Vincent's University Hospital Ethics Committee. As no direct patient-level data was to be collected and the dataset was anonymised with only surveillance figures formal ethics application was waived as there were no concerning issues from an ethical point of view that needed a committee review. This article does not contain any studies with human participants or animals performed by any of the authors.
Results
Our first patient with COVID-19 was admitted on March 19th 2020. Since then, the evolution of the number of hospitalised patients with COVID-19 is presented in Figure 1.
Figure 1.
Number of Hospitalised Patients with COVID-19.
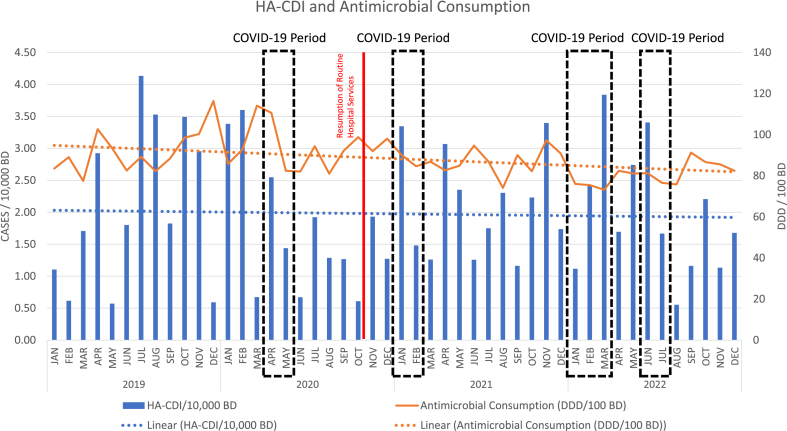
A look at the overall trend in HA-CDI shows a very marginal decrease, in tandem with a complementary overall decrease in total antimicrobial consumption over the study period (See Figure 2).
Figure 2.
HA-CDI and Antimicrobial Consumption Trend.
Comparisons between COVID-19 and non-COVID-19 periods (as shown in Table I), found no statistically significant difference in HA-CDI (P=0.34) or consumption of all antimicrobials (P=0.091), fluoroquinolones only (P=0.067), third-generation cephalosporins only (P=0.449) or carbapenems only (P=0.944). However, the overall number of bed-days was noted to be statistically less during the COVID-19 period (P=0.034), as well as the absolute number of C. difficile tests performed during this period (P=0.029); however, when expressed as a rate (per 10,000 BDs) to reflect hospital activity, this difference in the number of C. difficile tests performed was not statistically significant (P=0.696).
Table I.
Variable comparisons between COVID-19 and non-COVID-19 periods
| COVID-19 period | Non-COVID-19 period | P | |
|---|---|---|---|
| Bed Days | |||
| Mean | 15832.56 | 16874.36 | 0.034 |
| HA-CDI | |||
| Mean | 3.78 | 3.21 | 0.386 |
| Per 10,000 BD | 2.10 | 1.76 | 0.340 |
| Antimicrobial Consumption (DDD/100BD) | |||
| Overall | 83.36 | 89.50 | 0.091 |
| Fluoroquinolones Only | 3.71 | 4.46 | 0.067 |
| Third-Generation Cephalosporins Only | 4.17 | 4.43 | 0.449 |
| Carbapenems Only | 3.28 | 3.26 | 0.944 |
| C.difficile Testing | |||
| Mean | 506.22 | 549.46 | 0.029 |
| Per 10,000 BD | 321.81 | 326.63 | 0.696 |
Discussion
At the beginning of the pandemic, some experts expressed concern that COVID-19 could impact CDI rates [6]. However, initial studies conducted in Spain, Dublin, Rome and Mexico reported a lower than anticipated incidence when compared to historical data sets; and those conducted in New York and Singapore have reported no impact [6]. Our study findings support the latter with no statistically significant difference in HA-CDI rates between COVID-19 and non-COVID-19 periods found (P=0.34).
Antimicrobial exposure is one of most relevant individual factors associated with the development of CDI [18,19]. In Scotland, limiting hospital use of high-risk antimicrobials was associated with a substantial decline in CDI [20]; and studies performed in the United States of America have shown that facilities achieving significant reductions in hospital antimicrobial use experienced reductions of HA-CDI, which was more evident for quinolones, cephalosporins and carbapenems [21,22]. This trend, albeit minor and not statistically significant, is also reflected in our analysis as shown in Figure 2, with the overall consumption of antimicrobials steadily decreasing across the years – including fluoroquinolones, third-generation cephalosporins and carbapenems – with a simultaneous decreasing rate of CDI.
By contrast, the implementation of the antimicrobial stewardship program PIRASOA in Spain has led to a significant decrease in total antimicrobial consumption and incidence of infections due MDROs, but the incidence of CDI increased during the same period [23].
This reflects that the impact of hospital antimicrobial use on CDI rates is not linear but dependent on several factors, such as the degree of implementation of IPC measures and the prevalence of high-risk ribotypes [24].
Testing practices during the pandemic must also be considered as variations in CDI rates could be explained by underdiagnosis. A statistically significant difference was noted for the absolute number of C. difficile tests performed between the COVID-19 and non-COVID-19 periods (P=0.029), but when expressed as a rate i.e., the number of tests per 10,000 BDs to reflect hospital activity at the time, the difference was not significant (P=0.696).
During the pandemic, many clinical services in hospitals and the community were suspended or severely curtailed leading to a reduction in patient consultations, and hence clinical specimens received in laboratories. Furthermore, based on the knowledge that COVID-19 also produced gastrointestinal symptoms and the intense focus on COVID-19 in all arenas, C. difficile testing may not have been requested as readily by treating physicians. All in all, the actual number of CDI cases during the pandemic may be underestimated [25].
Furthermore, the organisational structure and utilisation of resources within the hospital infrastructure changed throughout the pandemic as knowledge on transmission models and mitigation measures in relation to COVID-19 was gained. There was a reduction in hospital activity during early COVID-19 periods due to suspension of all non-COVID-19 work (as reflected by the statistically significant lower number of bed-days during COVID-19 periods (P=0.034)). This meant that implementation of appropriate IPC policy with regard to isolation or accommodation of patients with transmissible infections or MDROs was more achievable compared with non-COVID-19 periods due to less pressure on limited isolation resources. With the reintroduction of full clinical services, alongside the additional COVID-19 burden, hospital resources and bed capacity came under considerable pressure, affecting the isolation facilities available for patients with potentially infectious diarrhoea (and other transmissible infections).
This theoretically suggests a potential delay in time to isolation of individuals with new diarrhoeal symptoms and a greater probability of environmental contamination and exposure risk to patients [11,26]. An in-depth review of each HA-CDI case at our institution found that all infected individuals were isolated within 24-hours of diagnosis due to robust IPC involvement, and likely contributed to our reported low rates.
However, the peaks in HA-CDI cases denoted in Figure 2 after the initial 2020 COVID-19 period probably reflects the resumption of normal services alongside COVID-19 care, with increase in patient numbers and competing demands for limited isolation beds. In fact, bed occupancy had been markedly elevated, and at times above capacity for much of 2021 and 2022. It is also important to recognise that there was minimal circulating influenza in the Republic of Ireland for the usual 2020/2021 influenza season [27], which if present would have placed heavy pressure on already scarce hospital isolation facilities and could have resulted in an increased incidence of infectious diseases, like CDI [28].
We do acknowledge other limitations in our study. Our findings are observational and retrospective from a very defined cohort thereby limiting the generalizability of results and recognising that definitive causal relationships cannot be inferred. Even though we compared antimicrobial consumption between COVID-19 and non-COVID-19 periods by means of DDD per 100 BD, we neither evaluated whether durations of regimens differed between periods nor examined the relation to other antimicrobial classes such as penicillins. Ribotyping also represents an area of further study. Isolates are only sent to the Reference Laboratory at Cherry Orchard Hospital for sequencing on request by the Clinical Microbiologist, largely in cases of suspected or confirmed outbreaks; and as there were no C. difficile outbreaks during the study period, information on circulating ribotypes is unavailable. Furthermore, our study lacks patient-level data; individual risk factors for developing HA-CDI may have differed between our non-COVID-19 and COVID-19 cohort.
Conclusion
The COVID-19 pandemic has not promoted an increase of HA-CDI. The association between antimicrobial consumption and HA-CDI appeared synchronous, but the value of strict IPC measures must not be overlooked.
Credit author statement
Saied Ali: Conceptualisation, Methodology, Formal Analysis, Investigation, Resources, Writing – Original Draft. Sinead McDermott: Writing – Review & Editing, Visualisation, Supervision, Project Administration.
Acknowledgements
We would like to thank the staff at the Department of Microbiology, St Vincent's University Hospital, for their continued support toward improving patient care.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The author have no conflict of interest to declare.
References
- 1.Lessa F.C., Mu Y., Bamberg W.M., Beldavs Z.G., Dumyati G.K., Dunn J.R., et al. Burden of Clostridium difficile Infection in the United States. N Engl J Med. 2015;372(9):825–834. doi: 10.1056/nejmoa1408913. Massachusetts Medical Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies K.A., Longshaw C.M., Davis G.L., Bouza E., Barbut F., Barna Z., et al. Underdiagnosis of Clostridium difficile across Europe: the European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID) Lancet Infect Dis. 2014;14(12):1208–1219. doi: 10.1016/s1473-3099(14)70991-0. Elsevier BV. [DOI] [PubMed] [Google Scholar]
- 3.Health Protection Surveillance Centre . 2022. “Clostridioides difficile.” clostridioides difficile - Health protection surveillance centre.https://www.hpsc.ie/a-z/microbiologyantimicrobialresistance/clostridioidesdifficile/ [Google Scholar]
- 4.Leffler D.A., Lamont J.T. Clostridium difficile Infection. Longo D.L., editor. New England J Med. 2015;372(16):1539–1548. doi: 10.1056/nejmra1403772. Massachusetts Medical Society. [DOI] [PubMed] [Google Scholar]
- 5.Tschudin-Sutter S., Kuijper E.J., Durovic A., Vehreschild M.J.G.T., Barbut F., Eckert C., et al. Guidance document for prevention of Clostridium difficile infection in acute healthcare settings. Clin Microbiol Infection. 2018;24(10):1051–1054. doi: 10.1016/j.cmi.2018.02.020. Elsevier BV. [DOI] [PubMed] [Google Scholar]
- 6.Merchante N., Chico P., Márquez-Saavedra E., Riera G., Herrero R., González-de-la-Aleja P., et al. Impact of COVID19 pandemic on the incidence of health-care associated Clostridioides difficile infection. Anaerobe. 2022;75 doi: 10.1016/j.anaerobe.2022.102579. Elsevier BV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo Y., Grinspan L.T., Fu Y., Adams-Sommer V., Willey D.K., Patel G., et al. Hospital-onset Clostridioides difficile infections during the COVID-19 pandemic. Infect Control Hosp Epidemiol. 2020;42(9):1165–1166. doi: 10.1017/ice.2020.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wee L.E.I., Conceicao E.P., Tan J.Y., Magesparan K.D., Amin I.B.M., Ismail B.B.S., et al. Unintended consequences of infection prevention and control measures during COVID-19 pandemic. Am J Infect Control. 2021;49(4):469–477. doi: 10.1016/j.ajic.2020.10.019. Elsevier BV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker M.A., Sands K.E., Huang S.S., Kleinman K., Septimus E.J., Varma N., et al. The Impact of Coronavirus Disease 2019 (COVID-19) on Healthcare-Associated Infections. Clin Infect Dis. 2021;74(10):1748–1754. doi: 10.1093/cid/ciab688. Oxford University Press (OUP). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ponce-Alonso M., Sáez de la Fuente J., Rincón-Carlavilla A., Moreno-Nunez P., Martínez-García L., Escudero-Sánchez R., et al. Impact of the coronavirus disease 2019 (COVID-19) pandemic on nosocomial Clostridioides difficile infection. Infect Control Hosp Epidemiol. 2020;42(4):406–410. doi: 10.1017/ice.2020.454. Cambridge University Press (CUP). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hazel K., Skally M., Glynn E., Foley M., Burns K., O’Toole A., et al. The other ‘C’: Hospital-acquired Clostridioides difficile infection during the coronavirus disease 2019 (COVID-19) pandemic. Infect Control Hosp Epidemiol. 2021;43(4):540–541. doi: 10.1017/ice.2021.3. Cambridge University Press (CUP). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bentivegna E., Alessio G., Spuntarelli V., Luciani M., Santino I., Simmaco M., et al. Impact of COVID-19 prevention measures on risk of health care-associated Clostridium difficile infection. Am J Infect Control. 2021;49(5):640–642. doi: 10.1016/j.ajic.2020.09.010. Elsevier BV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ochoa-Hein E., Rajme-López S., Rodríguez-Aldama J.C., Huertas-Jiménez M.A., Chávez-Ríos A.R., de Paz-García R., et al. Substantial reduction of healthcare facility-onset Clostridioides difficile infection (HO-CDI) rates after conversion of a hospital for exclusive treatment of COVID-19 patients. Am J Infect Control. 2021;49(7):966–968. doi: 10.1016/j.ajic.2020.12.008. Elsevier BV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serosep. (2023, January 9). Entericbio. Serosep. Retrieved April 15, 2023, from. https://www.serosep.com/product/entericbio/.
- 15.Difficile H.P.S.C.“C. 2011. Enhanced surveillance.” enhanced surveillance - Health protection surveillance centre.https://www.hpsc.ie/a-z/microbiologyantimicrobialresistance/clostridioidesdifficile/enhancedsurveillance/ [Google Scholar]
- 16.European Centre for Disease Prevention and Control . Publications Office; 2019. European Surveillance of clostridioides (Clostridium) difficile infections: surveillance protocol version 2.4. [DOI] [Google Scholar]
- 17.World Health Organization Collaborating Center for Drug Statistics Methodology, Norwegian Institute of Public Health, Definition and General Considerations: Defined Daily Dose (DDD), 2018. Available at:. https://www.whocc.no/ddd/definition_and_general_considera/.
- 18.Brown K.A., Langford B., Schwartz K.L., Diong C., Garber G., Daneman N. Antibiotic Prescribing Choices and Their Comparative C. Difficile Infection Risks: A Longitudinal Case-Cohort Study. Clin Infect Dis. 2020;72(5):836–844. doi: 10.1093/cid/ciaa124. Oxford University Press (OUP). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webb B.J., Subramanian A., Lopansri B., Goodman B., Jones P.B., Ferraro J., et al. Antibiotic Exposure and Risk for Hospital-Associated Clostridioides difficile Infection. Antimicrob Agents Chemother. 2020;64(4) doi: 10.1128/aac.02169-19. American Society for Microbiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawes T., Lopez-Lozano J.-M., Nebot C.A., Macartney G., Subbarao-Sharma R., Wares K.D., et al. Effect of a national 4C antibiotic stewardship intervention on the clinical and molecular epidemiology of Clostridium difficile infections in a region of Scotland: a non-linear time-series analysis. Lancet Infect Dis. 2017;17(2):194–206. doi: 10.1016/s1473-3099(16)30397-8. Elsevier BV. [DOI] [PubMed] [Google Scholar]
- 21.Kazakova S.V., Baggs J., McDonald L.C., Yi S.H., Hatfield K.M., Guh A., et al. Association Between Antibiotic Use and Hospital-onset Clostridioides difficile Infection in US Acute Care Hospitals, 2006–2012: An Ecologic Analysis. Clin Infect Dis. 2019;70(1):11–18. doi: 10.1093/cid/ciz169. Oxford University Press (OUP). [DOI] [PubMed] [Google Scholar]
- 22.Kazakova S.V., Baggs J., Yi S.H., Reddy S.C., Hatfield K.M., Guh A.Y., et al. Associations of facility-level antibiotic use and hospital-onset Clostridioides difficile infection in US acute-care hospitals, 2012–2018. Infect Control Hosp Epidemiol. 2021;43(8):1067–1069. doi: 10.1017/ice.2021.151. Cambridge University Press (CUP). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodríguez-Baño J., Pérez-Moreno M.A., Peñalva G., Garnacho-Montero J., Pinto C., Salcedo I., et al. Outcomes of the PIRASOA programme, an antimicrobial stewardship programme implemented in hospitals of the Public Health System of Andalusia, Spain: an ecologic study of time-trend analysis. Clin Microbiol Infection. 2020;26(3):358–365. doi: 10.1016/j.cmi.2019.07.009. Elsevier BV. [DOI] [PubMed] [Google Scholar]
- 24.Kim B., Kim J., Pai H. Association between Antibiotic Consumption and Incidence of Clostridioides difficile Infection in a Hospital. J Kor Med Sci. 2020;35(47) doi: 10.3346/jkms.2020.35.e407. Korean Academy of Medical Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yadlapati S., Jarrett S.A., Lo K.B., Sweet J., Judge T.A. Cureus. Cureus, Inc; 2021. Examining the Rate of Clostridioides (Formerly Clostridium) Difficile Infection Pre- and Post-COVID-19 Pandemic: An Institutional Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sipos S., Vlad C., Prejbeanu R., Haragus H., Vlad D., Cristian H., et al. Impact of COVID-19 prevention measures on Clostridioides difficile infections in a regional acute care hospital. Exper Therapeutic Med. 2021;22(5) doi: 10.3892/etm.2021.10649. Spandidos Publications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Health Protection Surveillance Centre . Health Protection Surveillance Centre; 2021. “Influenza surveillance reports.” influenza surveillance reports - Health protection surveillance centre.https://www.hpsc.ie/a-z/respiratory/influenza/seasonalinfluenza/surveillance/influenzasurveillancereports/ [Google Scholar]
- 28.Polgreen P.M., Yang M., Bohnett L.C., Cavanaugh J.E. A Time-Series Analysis of Clostridium difficile and Its Seasonal Association with Influenza. Infect Control Hosp Epidemiol. 2010;31(4):382–387. doi: 10.1086/651095. Cambridge University Press (CUP). [DOI] [PMC free article] [PubMed] [Google Scholar]