Abstract
This study aimed to determine the mechanisms of heat-induced oxidative stress in the thymus and spleen of broilers. After 28 d, 30 broilers were randomly divided into the control (25°C ± 2°C; 24 h/d) and heat-stressed (36°C ± 2°C; 8 h/d) groups; the experiment lasted for 1 wk. The broilers in each group were euthanized, and some samples were collected and analyzed at 35 d. The results showed that the birds subjected to heat stress reduced the weight (P < 0.01) and the indices of thymus (P < 0.01), the activities of T-AOC (P < 0.01) and SOD (P < 0.05) of spleen, and levels of IL-10 (P < 0.05) and the GSH-PX (P < 0.05) in thymus and spleen, and increased the IL-6 content of thymus (P < 0.05), the MDA content (P < 0.01), and the reactive oxygen species (ROS) levels (P < 0.01) in thymus and spleen. Moreover, the expression of the IgG gene in the thymus and spleen of heat-stressed broilers was increased (P < 0.05); however, the expression of the IgM gene in the spleen was increased (P < 0.05), with no difference (P > 0.05) in the thymus of heat-stressed broilers compared with the control. Furthermore, the relative expression of adenosine triphosphate-binding cassette subfamily G member 2 (ABCG2) in the thymus and spleen both increased (P < 0.05). The sodium-dependent vitamin C transporter-2 (SVCT-2) (P < 0.01) and mitochondrial calcium uniporter (MCU) (P < 0.01) mRNA levels in the thymus of heat-stressed broilers increased, and the expression of ABCG2 (P < 0.05), SVCT-2 (P < 0.01), and MCU (P < 0.01) proteins in the thymus and spleen of heat-stressed broilers increased compared with the control group. This study confirmed that heat stress-induced oxidative stress in the immune organs of broilers, further reducing immune function.
Key words: broiler, heat stress, oxidative stress, immune
INTRODUCTION
Heat stress has increased mortality and reduced feed efficiency, body weight, feed intake, and immunity in the poultry industry. Immune system is one of the main targets of heat stress-induced negative effects on the organism (Starkie et al., 2005). The cause of heat-induced oxidative stress is mainly intracellular reactive oxygen species (ROS) production changes leading to the modification of the enzyme activity (Akbarian et al., 2016). However, the underlying mechanism of the regulation of ROS production in the thymus and spleen of heat-stressed broilers remains unclear.
Previous studies have shown that the downregulation of ATP-binding cassette subfamily G member 2 (ABCG2) induces the overproduction of ROS to inhibit the production of antioxidants (Kurokawa et al., 2019). Moreover, the sodium-dependent Vc transporter-2 (SVCT-2) mainly participates in the absorption of Vc to protect the tissues from oxidative damage (Harrison et al., 2010). While the change of the mitochondrial calcium uniporter (MCU) activity is closely related to ROS production and oxidative stress (Zhang et al., 2020). Therefore, this study aimed to investigate that the underlying mechanism of the regulation of ROS production in the thymus and spleen of heat-stressed broilers. This will provide a therapeutic target for the prevention of heat stress in broilers.
MATERIALS AND METHODS
Broilers and Experimental Design
This work was approved by the Animal Care and Use Committee of the Anhui Agricultural University. One-day-old Arbor Acres broilers were kept in cages with wood-shaving littered floor reared under routine commercial management practices. After 28 d, 30 chickens were randomly divided into 2 groups (the control and heat stress groups, 15 chickens per group), with 3 replicates per group and 5 birds per replicate. The control group was kept at normal temperature conditions (25°C ± 2°C; 42–66% RH for 1 wk), whereas the heat stress group was exposed daily to high ambient temperature (36°C ± 2°C; 33–38% RH, 8 h/d for 1 wk) to mimic an environmental heat wave in an environmentally controlled chamber. The heat exposure protocol was conducted for 1 wk (from 28 to 35 d). The chickens were fed the same basal diets to meet the standard requirements of broilers according to NRC (1994), and kept under constant light throughout the experiment with feed and water provided ad libitum. The ingredients and chemical composition of the basal diet are described in our previous published paper (Wang et al., 2019). The experiment was repeated 3 times.
Sample Collection and Preservation
After 1 wk of heat stress, the broilers in the control and heat stress groups were slaughtered. The thymus and spleen were collected, weighed, and divided into 2 parts. Part of the sample was quickly placed in liquid nitrogen and stored at −80°C for RNA isolation. The other part of the sample was immediately homogenized to determine oxidative stress and immune indices.
Measurement of Oxidative Stress and Immune Indices of the Thymus and Spleen
The thymus and spleen were immediately collected and homogenized for 2 min in 9 mL ice-cold saline solution, and the homogenates were centrifuged at 3,000 rpm for 15 min at 4°C. The supernatant was collected, and the malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-PX), total antioxidant capacity (T-AOC), H2O2, interleukin-6 (IL-6), interleukin-10 (IL-10), and tumor necrosis factor-alpha (TNF-α) level in the supernatant of the thymus and spleen were determined using the corresponding diagnostic kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions.
Relative Quantitative Real-Time Polymerase Chain Reaction Analysis
Reverse transcription polymerase chain reaction (RT-PCR) and reverse transcription-qualitative polymerase chain reaction (RT-qPCR) were performed by referring to previously described methods (Wang et al., 2019). Total RNA was extracted from the thymus and spleen of the broilers using the OMEGA Total RNA kit II (SPECTRIS CO., Egham, Surrey, UK) according to the manufacturer's instructions, and then reverse transcribed into cDNA using a Takara prime scripter kit (Takara Bio, Kusatsu, Shiga, Japan) following the manufacturer's instructions. The cDNA was diluted and stored at −30°C until further analysis. The PCR products were examined using 1.5% agarose gel electrophoresis, and β-actin was used as the internal standard. The relative levels of immunoglobulin G (IgG), immunoglobulin M (IgM), ABCG2, SVCT-2, and MCU mRNA were determined using the CFX Connect Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The amplification conditions were as follows: 95°C for 10 min, followed 40 cycles (95°C for 15 s, 60°C for 1 min, 72°C for 15 s, respectively). The melting curve showed a single peak for each PCR product. The relative levels of IgG, IgM, ABCG2, SVCT-2, and MCU mRNA were calculated using the 2−ΔΔCT method. The β-actin, IgG, IgM, ABCG2, SVCT-2, MCU-specific primers were respectively 5′-AGACATCAGGGTGTGATGGTTGGT-3′ and 5′-TCCCAGTTGGTGACAATACCGTGT-3′, 5′-ATCACGTCAAGGGATGCCCG-3′ and 5′-ACCAGGCACCTCAGTTTGG-3′, 5′-CAATGGGATGATGGTGAGG-3′ and 5′-TGAGTGGGACAATGATACG-3′, 5′-ATTTTCATTGCTCGCTTCTTT-3′ and 5′-GACAGTCTGACATTACTAGCTTTGG-3′, 5′-GATTGTCTTGTGCTCCTCCTC-3′ and 5′-GGCTGCTCCATACTGAATAACC-3′, 5′-CCTATCTCAGACTCCGTTGG-3′ and 5′-CATCATTCAGCGTGGTTGC-3′.
Protein Extraction and Western Blotting
The thymus and spleen were cut into small pieces in the homogenate tube, and homogenized for 2 min in 10 times the tissue volume of the lysate. After the homogenates were thawed on ice for 30 min, they were centrifuged at 12,000 rpm for 10 min at 4°C. The supernatant was collected as the total protein solution. The protein content of the tissue homogenate was measured using Coomassie Brilliant Blue (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) staining following the manufactures’ instructions.
Tissue protein samples of the thymus and spleen were separated by a sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA) by electroblotting. After blocking with 5% skimmed milk for 1 h at room temperature, the proteins were labeled with primary antibodies overnight at 4°C, and incubated with horseradish peroxidase (HRP)-conjugated anti-mouse IgG secondary antibody at room temperature for 30 min, followed by detection with electrochemical luminescence Western blot detection reagent (GE Healthcare, Pittsburgh, USA) and quantified with Fiji/Image J. Relative signal intensities were normalized to β-actin. All the primary antibodies and secondary antibodies were purchased from Santa Cruz (Santa Cruz Biotechnology, Santa Cruz, CA).
Statistical Analysis
All data were analyzed using the Statistic Package for Social Science (SPSS) software (version 21; IBM, Chicago, IL) and expressed as mean ± SEM from 9 replicates. Statistical comparisons were performed using the independent-sample T test. Statistical significance was set at a P < 0.05.
RESULTS AND DISCUSSION
Changes in the Development and Immune Indices of the Thymus and Spleen Under Heat Stress
The spleen is the largest peripheral immune organ, and the thymus is the central lymphoid organ in the immune system of poultry (He et al., 2019). Hence, we selected the spleen and thymus as target tissues to analyze the mechanism of change in ROS production in heat-stressed birds. In our previous study, the broilers in experiment have been showed a typical heat stress response (Wang et al., 2019). In this study, the absolute weight and indices of the thymus in the heat stress group (3.47 ± 0.43; 3.12 ± 0.29) were lower than those in the control group (5.33 ± 0.32; 4.89 ± 0.41) (P < 0.01). There was no significant difference in absolute weight and indices of the spleen between the heat stress (1.09 ± 0.09; 0.75 ± 0.07) and control groups (1.69 ± 0.16; 1.29 ± 0.13) (P > 0.05). The results showed that heat stress could inhibit thymus and spleen development to some extent and may cause damage to the immune organs.
The TNF-α and IL-6 are important proinflammatory factors in both immune responses and inflammation (Rancourt et al., 2020), while IL-10 is an important anti-inflammatory cytokine (Laffer et al., 2019). In the study, compared with the control group (48.43 ± 2.58), the IL-6 levels in the thymus in the heat stress group (64.65 ± 1.93) were increased (P < 0.05) and no significant change between the control group (63.35 ± 3.31) and the heat stress group (58.75 ± 11.62) in the spleen (P > 0.05); whereas IL-10 levels in the thymus and spleen of the heat stress group were both decreased (P < 0.05), and TNF-α levels were not significantly different in the thymus and spleen (P > 0.05). These results confirmed that heat stress can increase the levels of inflammatory cytokines and decrease the levels of anti-inflammatory cytokines in thymus and spleen of broilers.
Oxidative Stress and Immune Response Indices in the Thymus and Spleen of Broilers Under Heat Stress
Studies have shown that heat stress primarily affects poultry performance by inducing oxidative stress (Maibam et al., 2018). In the study, the MDA and ROS levels (measured as H2O2) of the thymus and spleen in the heat stress group were significantly higher than that in the control (P < 0.01; Figure 1A1 and A2). Compared with the control group, the T-AOC (P < 0.01) and SOD (P < 0.05) levels in the spleen for the heat stress group were lower; however, there was no significant change in the thymus (P > 0.05; Figure 1A3 and A4). Moreover, the GSH-PX level in the thymus (P < 0.05) and spleen (P < 0.01) in the heat stress group was lower than that in control group (Figure 1A5). The results showed that heat stress caused an increase in lipid peroxides and ROS, and a decrease in antioxidant enzymes in the thymus and spleen of broilers. Moreover, in the study, the expression of IgG and IgM genes in the thymus and spleen of broilers in the heat stress group increased compared with the control group (Figure 1A6), and the relative expression of IgG and IgM mRNA in the spleen of broilers in the heat stress group significantly increased compared with the control group (P < 0.05; Figure 1A7 and A8). In the thymus, the expression of IgG mRNA in the heat stress group was higher than that of the control group (P < 0.05); however, the expression of IgM mRNA was not significantly different (P > 0.05) (Figure 1A7 and A8). This indicates that heat stress can lead to an inflammatory response and immunoglobulin expression increases in the thymus and spleen.
Figure 1.
Effects of heat stress on the oxidative stress and immune indices in the thymus and spleen of broilers. A1: Effects of heat stress on MDA level; A2: Effects of heat stress on ROS level; A3: Effects of heat stress on T-AOC level; A4: Effects of heat stress on SOD level; A5: Effects of heat stress on GSH-PX level; A6: Expression of IgG and IgM genes using RT-PCR analysis; A7: Relative mRNA expression levels of IgG in the thymus and spleen; A8: Relative mRNA expression levels of IgM in the thymus and spleen. CG, the control group; HS, heat stress group. Note: Compared with the control group, *P < 0.05, ** P < 0.01. Error bars, SEM.
Expression of ABCG2, SVCT-2, and MCU Genes and Proteins in Thymus and Spleen of Broilers Under Heat Stress
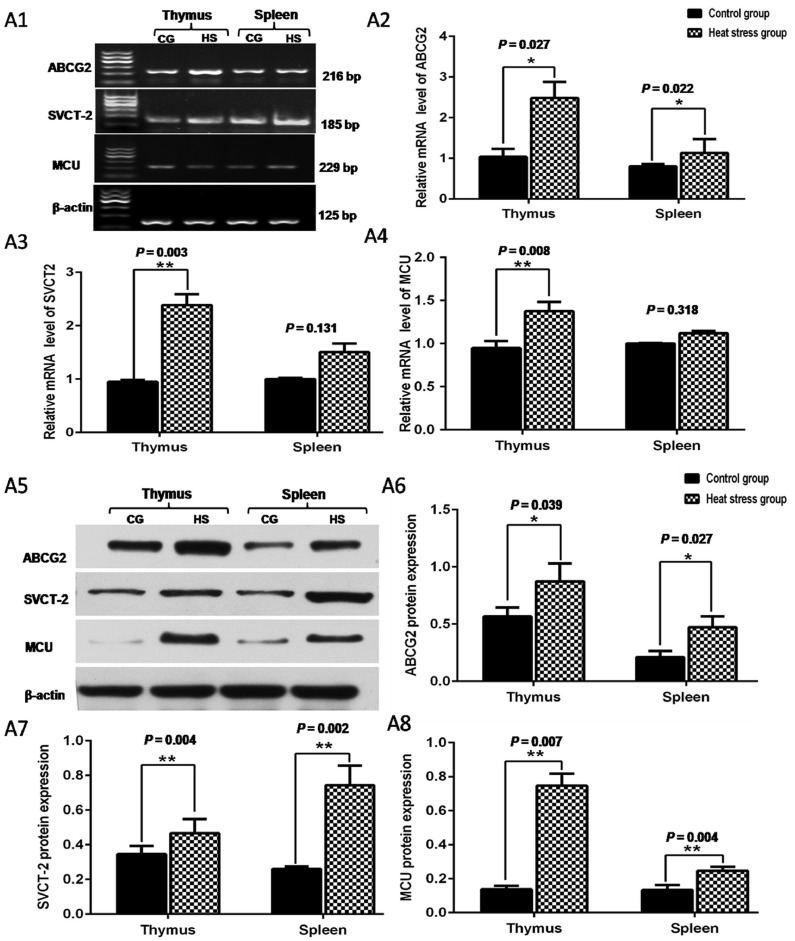
To explore the mechanism of the change in ROS levels in the thymus and spleen of heat-stressed broilers, the expression of several transporters in the thymus and spleen associated with ROS production was evaluated. Studies have shown that ABCG2 is capable of protecting cells from ROS-mediated cell damage (Kurokawa et al., 2019). Moreover, SVCT-2 gene knockout can increase cell oxidative damage and death (Harrison et al., 2010). The MCU is associated with Ca2+ concentration, and mitochondrial Ca2+ overload induces a large amount of ROS production in cells (Zhang et al., 2020). In the study, the broilers exposed to heat stress had higher expression of ABCG2, SVCT-2, and MCU mRNA in the thymus and spleen compared with broilers in the control group using RT-PCR analysis (Figure 2A1). The relative expression of ABCG2 mRNA in the heat stress group increased in the thymus and spleen (P < 0.05; Figure 2A2). Furthermore, the relative expression of SVCT-2 and MCU mRNA in the thymus of heat-stressed broilers was significantly higher than that of broilers in the control group (P < 0.01; Figure 2A3 and A4); however, there was no significant change in the spleen (P > 0.05; Figure 2A3 and A4). Moreover, the results showed that the expression of ABCG2, SVCT-2, and MCU proteins dramatically increased in the thymus and spleen after exposure to heat stress (Figure 2A5). The expression of ABCG2 (P < 0.05; Figure 2A6), SVCT-2 (P < 0.01; Figure 2A7), and MCU (P < 0.01; Figure 2A8) proteins in the thymus and spleen of broilers in the heat stress group was significantly higher than that in the control group. These results showed that heat stress can cause oxidative stress in the thymus and spleen of broiler chickens by regulating the expression of the ABCG2, SVCT-2, and MCU proteins, and further affects the immune function of broilers, ultimately reduces the performance of broilers.
Figure 2.
The mRNA and protein expression of ABCG2, SVCT-2 and MCU in the thymus and spleen. A1: Expression of ABCG2, SVCT-2 and MCU genes using RT-PCR analysis; A2: Relative expression levels of ABCG2 mRNA in the thymus and spleen; A3: Relative expression levels of SVCT-2 mRNA in the thymus and spleen. A4: Relative expression levels of MCU mRNA in the thymus and spleen. A5: Protein levels of ABCG2, SVCT-2 and MCU in the thymus and spleen by Western blots analysis. A6: Expression of ABCG2 protein in the thymus and spleen; A7: Expression of SVCT-2 protein in the thymus and spleen; A8: Expression of MCU protein in the thymus and spleen.CG, the control group; HS, heat stress group. Note: Compared with the control group, *P < 0.05, ** P < 0.01. Error bars, SEM.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.cn) for English language editing.
Compliance With Ethical Standards: All experiments were compliant with the ethical standards of Anhui Agricultural University.
Author Contributions: Yong Wang and Xinmei Yang conceived of the study, carried out the experiment, and drafted the manuscript. Shuyan Li and Qifei Wu participated in the data collection and analysis. Hao Guo, Hongyan Wang, and Pin Su participated in statistical analysis. Juhua Wang conceived of the study, revising the manuscript critically. All authors have read and approved the final manuscript.
DISCLOSURES
The authors declare that they have no conflicts of interest.
REFERENCES
- Akbarian A., Michiels J., Degroote J., Majdeddin M., Golian A., De Smet S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016;7:37. doi: 10.1186/s40104-016-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison F.E., Dawes S.M., Meredith M.E., Babaev V.R., Li L., May J.M. Low vitamin C and increased oxidative stress and cell death in mice that lack the sodium-dependent vitamin C transporter SVCT2. Free Radic. Biol. Med. 2010;49:821–829. doi: 10.1016/j.freeradbiomed.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Yu Q., He Y., Hu R., Xia S., He J. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers. Poult. Sci. 2019;98:6378–6387. doi: 10.3382/ps/pez471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa H., Ito H., Terasaki M., Matsui H. Hyperthermia enhances photodynamic therapy by regulation of HCP1 and ABCG2 expressions via high level ROS generation. Sci. Rep. UK. 2019;9:1638. doi: 10.1038/s41598-018-38460-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffer B., Bauer D., Wasmuth S., Busch M., Jalilvand T.V., Thanos S., Meyer Zu Horste G., Loser K., Langmann T., Heiligenhaus A., Kasper M. Loss of IL-10 promotes differentiation of microglia to a M1 phenotype. Front. Cell Neurosci. 2019;13:430. doi: 10.3389/fncel.2019.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maibam U., Hooda O.K., Sharma P.S., Upadhyay R.C., Mohanty A.K. Differential level of oxidative stress markers in skin tissue of zebu and crossbreed cattle during thermal stress. Livest. Sci. 2018;207:45–50. [Google Scholar]
- NRC. 1994. Nutrient Requirements of Poultry, 9th rev. ed., 1994, National Research Council, National Academy Press; Washington, DC.
- Rancourt R.C., Ott R., Ziska T., Schellong K., Melchior K., Henrich W., Plagemann A. Visceral adipose tissue inflammatory factors (TNF-alpha, SOCS3) in gestational diabetes (GDM): epigenetics as a clue in GDM pathophysiology. Int. J. Mol. Sci. 2020;21:479. doi: 10.3390/ijms21020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkie R.L., Hargreaves M., Rolland J., Febbraio M.A. Heat stress, cytokines, and the immune response to exercise. Brain Behav. Immun. 2005;19:404–412. doi: 10.1016/j.bbi.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Wang J., Xue X., Liu Q., Zhang S., Peng M., Zhou J., Chen L., Fang F. Effects of duration of thermal stress on growth performance, serum oxidative stress indices, the expression and localization of ABCG2 and mitochondria ROS production of skeletal muscle, small intestine and immune organs in broilers. J. Therm. Biol. 2019;85 doi: 10.1016/j.jtherbio.2019.102420. [DOI] [PubMed] [Google Scholar]
- Zhang L., Wang Z., Lu T., Meng L., Luo Y., Fu X., Hou Y. Mitochondrial Ca(2+) overload leads to mitochondrial oxidative stress and delayed meiotic resumption in mouse oocytes. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.580876. [DOI] [PMC free article] [PubMed] [Google Scholar]