Abstract
The present study was conducted to determine the ability of multicomponent mycotoxin detoxifying agent (MMDA) in feed to prevent the gastrointestinal absorption of aflatoxin B1 (AFB1) and T2-toxin supplemented via spiked maize. For comparisons, hens were fed with uncontaminated basal diet without or with addition of MMDA at 2 g/kg feed. The trial consisted of 105 laying hens (Lohmann Brown) without obvious signs of disease allocated to 7 treatment groups in 35 pens. Responses were demonstrated on laying performance and health status throughout the 42 d experimental period. The results of laying performance indicated significantly decreased egg mass with increasing mycotoxin (AFB1 and T2-toxin) levels up to the maximum tolerated dosage, however simultaneous presence of MMDA laying performance was slightly modified linearly to increasing application. Dose-dependent pathological changes in liver and kidneys and their relative weights, changes in blood parameters and reduced eggshell weights were observed in the hens fed AFB1 and T2-toxin. The pathological changes in the hens fed with diets containing AFB1 and T2-toxin without MMDA were significantly higher as compared with the control group, but eggshell stability was not affected. The contents of AFB1, T2-toxin and their metabolites in liver and kidney tissues were significantly decreased in the hens supplemented with MMDA at 2 and 3 g/kg in feed. MMDA supplementation significantly reduced the deposition of AFB1, T2-toxin and their metabolites in liver and kidneys at the maximum tolerated dosage (2 and 3 g/kg) indicating specific binding to AFB1 and T2-toxin in the digestive tract as compared to the corresponding diets without MMDA. Exposure of AFB1 and T2-toxin indicated significantly decreased egg mass with increasing mycotoxin levels up to the maximum tolerated dosage because of the significantly reduced egg production. Therefore, in this study, MMDA could reduce negative effects of feeding AFB1 and T-2 to laying hens.
Key words: mycotoxin, laying hen, aflatoxin B1, MMDA, T2-toxin
INTRODUCTION
Mycotoxins are harmful secondary metabolites originated from filamentous fungi and poses several detrimental effects to human and animal health (Warth et al., 2016; Alshannaq and Yu, 2017; Eskola et al., 2020). The cereal crops in agricultural practices may be contaminated with fungal toxins during harvesting, transport, processing and storage and their production is influenced by several climatic factors (Coffey et al., 2009; Warth et al., 2016). Apart from the alarming deteriorative health effects produced by mycotoxins, animal derived products such as meat, milk, and eggs carry over the mycotoxins and does produce an impact on human health as well (Alshannaq and Yu, 2017). An around more than 200 mycotoxins producing species of molds are already known and the most common harmful mycotoxins contaminating the feed stuff and feed material include AFB1 (AFB1), HT-2, T-2, ochratoxin A, fumonisin B1, zearalenone, citrinin, etc. (Binder, 2007).
Aflatoxins in feed are reported to produce severe losses in terms of mortality and production losses. The metabolites of aflatoxins are very hardy and are not even destroyed after processing. There are several forms of aflatoxins produced in poultry namely AFB1, B2, G1 and G2 produced by fungi Aspergillus favus and Aspergillus parasiticus (Arafa et al., 1981). AFB1 is most potent known among these and is reported to be transferred from feedstuff given to the poultry to eggs, meat and other edible parts of the poultry carcass (Pitt and Miller, 2017; Rajput et al., 2017; Liu et al., 2018; Saminathan et al., 2018). Aflatoxicosis in layer birds is concerned with reduced egg weight and production, decline in body weight, poor feed conversion ratio (FCR), fatty liver, immunosuppression, and reduction in the levels of enzymes related with the digestion of lipids, proteins and starch (Edds and Bortell, 1983; Leeson et al., 1995; Devegowda and Murthy, 2005). T-2 is another important mycotoxin linked to declined growth, decreased egg production and quality, leucopenia, regression in bursa of Fabricius, defective blood coagulation, and immunosuppression in poultry birds (Leeson et al., 1995; Dänicke et al., 2001). T2 and ochratoxin are the commonly known mycotoxins present in poultry feed and work synergistically with aflatoxins (Huff and Doerr, 1981; Huff et al., 1988a).
To limit the effect of mycotoxins several strategies are tried to counteract the detrimental impacts of mycotoxins. The strategies to prevent the absorption of mycotoxins must be economical and should not produce any harmful effects through the deposition of their residues in the feed and tissues and simultaneously should not create any deteriorating effect on the nutritional quality of feed products (Parlat et al., 1999). Addition of clinoptilolite at the rate of 15 g/kg has shown protective effect against 2,500 μg/kg aflatoxin in feed (Oguz and Kurtoglu, 2000). According to 1 study's findings, chicks given AFB1-contaminated diets at 2,500 μg/kg can effectively boost bodyweight gain and partially recover from aflatoxicosis using hydrated sodium calcium aluminosilicate at dose of 5 g/kg (Chen et al., 2014). Absorbent bentonite (7.5 g/kg at 2,000 μg/kg aflatoxin dose) into the AFB1 diet diminished the effects of AFB1 and a substantial drop in the amount of AFB1 residues found in avian liver (Dos Anjos et al., 2015; Bhatti et al., 2018). Nano-composite magnetic grapheme oxide with chitosan (5 g/kg at 22 μg/kg aflatoxin dose) produced pronounced reduction in the aflatoxins in gastrointestinal tract of birds and effectively increased overall performance (Saminathan et al., 2018). Yeast cell wall (1.5 g/kg at 350 μg/kg aflatoxin dose) improved weight gain and feed conversion rate (Mendieta et al., 2018). Probiotic (1 g/kg at 250 μg/kg aflatoxin dose) substantially lowered liver aflatoxin concentrations and neutralized the harmful effects of AFB1 (Salem et al., 2018). Alpha-lipoic acid (300 g/kg at 300 μg/kg aflatoxin dose) reduce tissue damage brought about by AF in the chicks' kidney and liver (Karaman et al., 2010). Urtica diocia seed extract (300 g/kg at 1,000 μg/kg aflatoxin dose) exhibited a hepatorenal protective effect in birds, possibly acting via enhancing the cellular antioxidant mechanisms (Uyar et al., 2016). Grape seed proanthrocyanidin extract (250 g/kg at 1,000 μg/kg aflatoxin dose) significantly reduced AFB1 residues in liver and proacted against AF induced damage (Rajput et al., 2017). Curcuminoids (74 mg/kg at 1000 μg/kg aflatoxin dose) produced increase in feed intake and weight gain, and abated relative liver weight (Gowda et al., 2008).
Researchers have studied several mycotoxin binding materials to hamper the absorption of aflatoxins into blood circulation (Abo-Norag et al., 1995; Rosa et al., 2001). One in vivo experimental study in broiler birds has reflected the absorbent nature of smectite clay and the binding ability is attributed to the ion exchange capability, high surface area and swelling behavior in the vicinity of water (Manafi, 2012). The use of smectite- based mycotoxin binder is proven to improve the humoral immune response, growth performance and reduction in harmful toxicological effects on liver in broiler birds administered with AFB1 (Zabiulla et al., 2021). In one of the studies conducted has proven the ameliorative effect of multicomponent mycotoxin detoxifying agent (MMDA) against AFB1 and ochratoxin A in broiler birds (Tsiouris et al. 2021).
Multicomponent mycotoxin detoxifying agent a product from PATENT CO DOO. (Misicevo, Serbia), contains modified zeolite (Clinoptilolite), Bacillus subtilis, Bacillus licheniformis, Saccharomyces cerevisiae cell wall and silymarin in-feed, to reduce gastrointestinal absorption of AFB1 and T-2 toxin in broilers. The present study was performed to evaluate the effects of MMDA, on various performance parameters, egg characteristics, blood profile and gross examination of various organs in layer birds exposed to AFB1 and T-2 toxins.
RESULTS AND DISCUSSION
Clinical Symptoms and Effect on Overall Laying Performance of Hens
Laying hens were healthy during the study and conditioning scores reached a scale of 1 indicating normal activity and alertness, normal coat, and eyes. Consistency of excreta was within the physiological range; dry matter content of excreta reached approximately 30% and did not differ across treatment groups. Therefore, neither MMDA nor AFB1 and T2-toxin at 2 dose levels each did show any treatment related clinical sign of hens during the 42-d experimental feeding period.
The analyzed nutrients and mycotoxin concentrations in the experimental diets were in line with the calculated contents within the accepted tolerances. Furthermore, the reported ash contents indirectly confirm the expected dose levels of MMDA. Feeding AFB1 and T2-Toxin via spiked maize at the guidance dose level without MMDA (T3) showed significantly (overall egg production: -7.7%; overall egg mass: -6.8%) or slightly negative impacts on laying performance (overall feed intake: -2.9%; overall FCR: +4.3%) in comparison with the control group. Application of MMDA at 1 g/kg feed to diets containing AFB1 at 0.05 mg/kg feed in combination with T2-toxin at 1.5 mg/kg feed (T4) resulted in slightly positive modifications as compared with hens fed corresponding mycotoxin levels without MMDA (T3). AFB1 and T2-toxin at the maximum tolerated dose level (T5) did significantly reduce overall feed intake (-3.7%), egg production (-9.7%), and overall egg mass (-9.5%) in comparison with the control group. With presence of MMDA at 2 g /kg feed (T6) and 3 g/kg feed (T7) the negative responses of mycotoxins at the maximum tolerated dosage were slightly reduced (overall feed intake: +0.8%; overall egg production: +1.5%; overall egg mass: +1.6%; overall FCR: -1%). Moreover, results indicated that MMDA in combination with mycotoxins at the guidance level seemed to be more efficient than those at the maximum tolerated dosage. There were no consistent treatment effects in overall body weight change, mean egg weight, and overall number of broken or dirty eggs. Comparisons among the weekly feeding phases revealed that negative responses of mycotoxin application at 2 dose levels increased with from the start of the second week onwards (Table 1, Table 2, Table 3). Treatment of feed containing 70 μg AFB1/kg with fermentation liquor of B. subtilis ANSB060 has protective effect on eggshell quality and liver damage in layer birds (Salem et al., 2018). White Leghorn layer breeder hens provided with 10 μg AFB1/kg produced by Aspergillus flavus with 0.1 mg/kg vitamin E in feed has produced partial protective effect on egg quality and hatchability (Khan et al., 2014).
Table 1.
Effects of MMDA on laying performance of hens from d 15 to d 28 on trial (d 246 to d 259 of age).
| Treatment groups | T1 | T2 | T3 | T4 | T4 | T6 | T7 | Oneway ANOVA | |
|---|---|---|---|---|---|---|---|---|---|
| Hens | no | 15 | 15 | 15 | 15 | 15 | 15 | 15 | P |
| Replicates | no | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| MMDA | g/kg | 2 | 1 | 2 | 3 | ||||
| AFB1 | mg/kg | 0.05 | 0.05 | 0.50 | 0.50 | 0.50 | |||
| T2-toxin | mg/kg | 1.5 | 1.5 | 2.0 | 2.0 | 2.0 | |||
| d 15 to d 21 on trial (d 246 to d 252 of age) | |||||||||
| Body weight - end | g | 1992.4 ± 53.5 | 1967.8 ± 102.8 | 2011.8 ± 58.0 | 1963.0 ± 35.3 | 1945.8 ± 101.7 | 1968.0 ± 71.2 | 1957.2 ± 38.2 | 0.799 |
| Body weight change | g | 1.6 ± 18.2 | 4.8 ± 33.4 | 22.6 ± 13.0 | 25.6 ± 8.8 | 9.6 ± 30.7 | -2.2 ± 27.5 | 7.0 ± 28.1 | 0.497 |
| plusmn;">Feed intake | g | 866.6 ± 16.0 | 847.5 ± 53.3 | 839.5 ± 29.2 | 836.3 ± 34.6 | 828.4 ± 24.9 | 833.2 ± 15.9 | 834.2 ± 41.5 | 0.618 |
| Egg production | no | 6.9 ± 0.2c | 6.8 ± 0.4bc | 5.9 ± 0.4ab | 6.3 ± 0.4abc | 5.9 ± 0.7a | 6.1 ± 0.5abc | 6.1 ± 0.5abc | 0.008 |
| Egg weight | g | 62.9 ± 1.7a | 64.5 ± 0.9ab | 65.4 ± 1.0b | 64.5 ± 0.7ab | 64.4 ± 0.6ab | 63.9 ± 0.6ab | 64.6 ± 0.9ab | 0.028 |
| Egg mass | g | 432.0 ± 10.2ab | 438.7 ± 27.3a | 388.0 ± 29.8ab | 404.3 ± 22.3ab | 377.7 ± 43.8b | 392.1 ± 31.3ab | 395.8 ± 29.8ab | 0.020 |
| Feed conversion ratio1 | 2.007 ± 0.038 | 1.942 ± 0.226 | 2.175 ± 0.207 | 2.075 ± 0.164 | 2.214 ± 0.235 | 2.136 ± 0.171 | 2.122 ± 0.246 | 0.340 | |
| Broken eggs | no | 0.4 ± 0.5 | 0 ± 0 | 0 ± 0 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0 ± 0 | 0.516 |
| Dirty eggs | no | 0.2 ± 0.4 | 0.6 ± 0.9 | 0.4 ± 0.5 | 0.2 ± 0.4 | 0 ± 0 | 0.2 ± 0.4 | 0 ± 0 | 0.482 |
| d 22 to d 28 on trial (d 253 to d 259 of age) | |||||||||
| Body weight end | g | 1996.2 ± 61.2 | 1963.6 ± 94.7 | 1992.6 ± 58.7 | 1978.8 ± 43.6 | 1945.0 ± 73.1 | 1967.4 ± 67.8 | 1965.8 ± 57.6 | 0.903 |
| Body weight change | g | 3.8 ± 39.4 | -4.2 ± 19.0 | -19.2 ± 14.8 | 15.8 ± 25.4 | -0.8 ± 33.7 | -0.6 ± 13.1 | 8.6 ± 21.6 | 0.491 |
| Feed intake | g | 868.4 ± 11.7 | 858.2 ± 16.9 | 862.4 ± 18.1 | 869.8 ± 15.9 | 861.0 ± 45.1 | 875.6 ± 24.6 | 867.1 ± 19.3 | 0.927 |
| Egg production | no | 7.0 ± 0.2 | 6.9 ± 0.4 | 6.6 ± 0.4 | 6.7 ± 0.4 | 6.3 ± 0.6 | 6.5 ± 0.5 | 6.5 ± 0.4 | 0.143 |
| Egg weight | g | 65.1 ± 1.8 | 65.0 ± 1.0 | 65.4 ± 1.3 | 64.9 ± 1.8 | 65.4 ± 1.6 | 65.2 ± 0.7 | 65.3 ± 1.2 | 0.996 |
| Egg mass | g | 456.0 ± 19.6 | 450.7 ± 23.4 | 431.9 ± 29.2 | 437.3 ± 32.2 | 414.2 ± 36.3 | 422.1 ± 36.3 | 426.4 ± 22.8 | 0.275 |
| Feed conversion ratio1 | 1.907 ± 0.094 | 1.910 ± 0.130 | 2.006 ± 0.174 | 1.999 ± 0.173 | 2.093 ± 0.231 | 2.090 ± 0.232 | 2.038 ± 0.118 | 0.460 | |
| Broken eggs | no | 0.2 ± 0.4 | 0 ± 0 | 0.2 ± 0.4 | 0.4 ± 0.5 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.4 ± 0.5 | 0.820 |
| Dirty eggs | no | 0.4 ± 0.5 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.991 |
Abbreviations: AFB1, aflatoxin B1; MMDA, multicomponent mycotoxin detoxifying agent.
kg feed per kg egg mass.
Table 2.
Effects of MMDA on laying performance of hens from d 29 to d 42 on trial (d 260 to d 273 of age).
| Treatment groups | T1 | T2 | T3 | T4 | T4 | T6 | T7 | Oneway ANOVA | |
|---|---|---|---|---|---|---|---|---|---|
| Hens | no | 15 | 15 | 15 | 15 | 15 | 15 | 15 | P |
| Replicates | no | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| MMDA | g/kg | 2 | 1 | 2 | 3 | ||||
| AFB1 | mg/kg | 0.05 | 0.05 | 0.50 | 0.50 | 0.50 | |||
| T2-toxin | mg/kg | 1.5 | 1.5 | 2.0 | 2.0 | 2.0 | |||
| d 29 to d 35 on trial (d 260 to d 266 of age) | |||||||||
| Body weight - end | g | 2002.0 ± 68.6 | 1968.2 ± 69.9 | 2007.2 ± 57.3 | 1978.0 ± 55.1 | 1954.4 ± 62.3 | 1986.4 ± 78.6 | 1984.6 ± 62.6 | 0.876 |
| Body weight change | g | 5.8 ± 20.0 | 4.6 ± 31.5 | 14.6 ± 15.2 | -0.8 ± 17.4 | 9.4 ± 22.4 | 19.0 ± 11.9 | 18.8 ± 20.4 | 0.674 |
| Feed intake | g | 856.8 ± 28.3 | 865.2 ± 12.7 | 836.5 ± 18.5 | 843.4 ± 12.3 | 830.2 ± 17.6 | 835.5 ± 22.9 | 833.4 ± 24.2 | 0.184 |
| Egg production | no | 7.0 ± 0.2a | 6.8 ± 0.2a | 6.3 ± 0.3ab | 6.3 ± 0.5ab | 5.7 ± 0.2b | 5.8 ± 0.6b | 5.8 ± 0.4b | <0.001 |
| Egg weight | g | 65.5 ± 1.7 | 65.5 ± 1.3 | 64.8 ± 2.0 | 65.8 ± 0.9 | 65.2 ± 1.1 | 65.8 ± 2.8 | 66.1 ± 0.3 | 0.916 |
| Egg mass | g | 458.2 ± 14.2a | 445.3 ± 7.2a | 410.7 ± 28.9ab | 416.5 ± 26.3ab | 369.8 ± 19.2b | 381.3 ± 36.3b | 383.1 ± 24.8b | <0.001 |
| Feed conversion ratio1 | 1.871 ± 0.084a | 1.943 ± 0.052ab | 2.046 ± 0.165abc | 2.033 ± 0.154abc | 2.252 ± 0.157c | 2.206 ± 0.203bc | 2.184 ± 0.177bc | 0.003 | |
| Broken eggs | no | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0 ± 0 | 0.2 ± 0.4 | 0.984 |
| Dirty eggs | no | 0.4 ± 0.5 | 0.4 ± 0.9 | 0.4 ± 0.9 | 0.2 ± 0.4 | 0.4 ± 0.9 | 0.4 ± 0.5 | 0 ± 0 | 0.946 |
| d 36 to d 42 on trial (d 267 to d 273 of age) | |||||||||
| Body weight end | g | 1998.4 ± 52.4 | 1990.0 ± 75.4 | 2000.2 ± 42.0 | 1991.2 ± 30.4 | 1971.8 ± 40.8 | 2001.6 ± 89.2 | 1981.2 ± 49.4 | 0.980 |
| Body weight change | g | -3.6 ± 24.6 | 21.8 ± 14.9 | -7.0 ± 20.8 | 13.2 ± 27.9 | 17.4 ± 22.6 | 15.2 ± 11.9 | -3.4 ± 23.6 | 0.208 |
| Feed intake | g | 886.1 ± 18.9 | 882.0 ± 20.4 | 833.9 ± 33.3 | 847.0 ± 19.7 | 834.4 ± 48.1 | 831.6 ± 44.1 | 833.0 ± 11.1 | 0.136 |
| Egg production | no | 6.7 ± 0.5b | 6.7 ± 0.2b | 6.0 ± 0.3ab | 6.1 ± 0.2ab | 6.0 ± 0.5ab | 5.7 ± 0.3a | 5.9 ± 0.3a | 0.001 |
| Egg weight | g | 66.1 ± 1.3 | 66.4 ± 0.9 | 66.3 ± 1.7 | 65.9 ± 1.0 | 66.4 ± 0.6 | 65.7 ± 1.4 | 65.4 ± 1.2 | 0.820 |
| Egg mass | g | 440.5 ± 29.5bc | 442.9 ± 14.9c | 397.4 ± 18.2ab | 404.5 ± 16.0abc | 398.1 ± 33.7ab | 376.7 ± 12.7a | 383.6 ± 17.0a | <0.001 |
| Feed conversion ratio1 | 2.017 ± 0.097 | 1.993 ± 0.063 | 2.100 ± 0.080 | 2.098 ± 0.130 | 2.114 ± 0.273 | 2.210 ± 0.143 | 2.176 ± 0.122 | 0.231 | |
| Broken eggs | no | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0 ± 0 | 0 ± 0 | 0.914 |
| Dirty eggs | no | 0.4 ± 0.9 | 0.4 ± 0.5 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.4 ± 0.5 | 0.2 ± 0.4 | 0.980 |
Abbreviations: AFB1, aflatoxin B1; MMDA, multicomponent mycotoxin detoxifying agent.
Different superscripts in same row are significant (a/b: P ≤ 0.05).
kg feed per kg egg mass.
Table 3.
Effects of MMDA on overall laying performance of hens from d 1 to d 42 on trial (d 232 to d 273 of age).
| Treatment groups | T1 | T2 | T3 | T4 | T4 | T6 | T7 | Oneway ANOVA | |
|---|---|---|---|---|---|---|---|---|---|
| Hens | no | 15 | 15 | 15 | 15 | 15 | 15 | 15 | P |
| Replicates | no | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| MMDA | g/kg | 2 | 1 | 2 | 3 | ||||
| AFB1 | mg/kg | 0.05 | 0.05 | 0.50 | 0.50 | 0.50 | |||
| T2-toxin | mg/kg | 1.5 | 1.5 | 2.0 | 2.0 | 2.0 | |||
| d 1 to d 42 on trial (d 232 to d 273of age) | |||||||||
| Body weight start | 1947.2 ± 87.1 | 1946.0 ± 147.6 | 1945.4 ± 37.7 | 1946.8 ± 44.7 | 1946.2 ± 67.6 | 1947.4 ± 41.9 | 1951.6 ± 44.2 | 1.000 | |
| Body weight - end | g | 1998.4 ± 52.4 | 1990.0 ± 75.4 | 2000.2 ± 42.0 | 1991.2 ± 30.4 | 1971.8 ± 40.8 | 2001.6 ± 89.2 | 1981.2 ± 49.4 | 0.980 |
| Body weight change | g | 51.2 ± 91.4 | 44.0 ± 75.5 | 54.8 ± 24.4 | 44.4 ± 23.2 | 25.6 ± 46.2 | 54.2 ± 63.6 | 29.6 ± 54.7 | 0.975 |
| Cumulative feed intake | g | 5151.5 ± 73.0a | 5133.6 ± 102.5a | 5003.6 ± 60.7ab | 5042.5 ± 81.2ab | 4965.1 ± 91.0b | 4999.3 ± 47.6ab | 4997.8 ± 85.5ab | 0.004 |
| Daily feed intake | g | 122.7 ± 1.7a | 122.2 ± 2.4a | 119.1 ± 1.4ab | 120.1 ± 1.9ab | 118.2 ± 2.2b | 119.0 ± 1.1ab | 119.0 ± 2.0ab | 0.004 |
| Cumulative egg production | no | 40.4 ± 0.8c | 40.2 ± 0.9bc | 37.3 ± 1.1a | 38.1 ± 0.6ab | 36.5 ± 1.3a | 36.7 ± 1.3a | 36.9 ± 1.4a | <0.001 |
| Mean egg weight | g | 64.1 ± 0.6 | 64.7 ± 0.6 | 64.6 ± 0.7 | 64.5 ± 0.5 | 64.3 ± 0.6 | 64.5 ± 0.8 | 64.8 ± 0.6 | 0.636 |
| Cumulative egg mass | g | 2590.3 ± 42.8bc | 2603.0 ± 65.9c | 2413.2 ± 83.2a | 2458.7 ± 48.2ab | 2343.5 ± 68.1a | 2363.0 ± 61.9a | 2386.6 ± 83.8a | <0.001 |
| Cumulative feed conversion ratio1 | 1.989 ± 0.025ab | 1.974 ± 0.082a | 2.075 ± 0.075ab | 2.051 ± 0.052ab | 2.121 ± 0.092ab | 2.117 ± 0.073b | 2.097 ± 0.105ab | 0.020 | |
| Cumulative broken eggs | no | 1.8 ± 1.1 | 0.6 ± 0.9 | 1.0 ± 1.2 | 1.2 ± 0.8 | 1.2 ± 1.3 | 1.0 ± 0.7 | 0.8 ± 0.4 | 0.602 |
| Cumulative dirty eggs | no | 2.0 ± 1.9 | 1.8 ± 1.3 | 1.6 ± 0.9 | 1.6 ± 1.1 | 1.6 ± 1.1 | 2.0 ± 0.7 | 1.0 ± 1.0 | 0.871 |
Abbreviations: AFB1, aflatoxin B1; MMDA, multicomponent mycotoxin detoxifying agent.
Different superscripts in same row are significant (a/b: P ≤ 0.05).
kg feed per kg egg mass.
Blood Profiles
The hens fed diets containing AFB1 at 0.05 mg/kg feed in combination with T2-toxin at 1.5 mg/kg feed (T3) without inclusion of MMDA resulted in slightly or significantly enhanced means of leukocytes, hemoglobin (significant), mean corpuscular hemoglobin (MCH), MCH concentration (MCHC, significant), triglycerides and creatine as compared with the control group. The addition of MMDA at 3 g/kg feed to contaminated diets at the maximum tolerated dose level (T7) seemed to be slightly effective to counteract some of the mycotoxin promoted effects (leukocytes, calcium, cholesterol, triglycerides, creatine, glucose, albumins, globulins, and total protein) in comparison with the high mycotoxin level alone. In presence of MMDA at 2 g/kg feed (T6) changes in comparison with the high mycotoxin level alone were lower than those recorded for MMDA at 3 g/kg feed. Comparison between hens fed mycotoxins at the guidance level without or with MMDA at 1 g/kg feed showed slightly higher (potassium, aspartate-amino-transferase [AST], total cholesterol, glucose, globulins, and total protein) or reduced concentrations (hemoglobin, MCH, MCHC, calcium, triglycerides) as compared to contaminated hens fed mycotoxins at the guidance level(Table 4, Table 5, Table 6). Alkaline phosphatase and AST are important liver enzymes related to protein metabolism and cell integrity (Walzem et al., 1993; Jiang et al., 2014). The poultry blood is found to exhibit elevated levels of ALT and AST after administration of AFB1 in diet as a result of liver damage(Han et al., 2008; Diaz et al., 2009; He et al., 2013; Gómez-Espinosa et al., 2017).The plasma of the birds provided with a diet containing AFB1 show declined content of cholesterol, triglycerides, total protein, albumin and globulin (Bailey et al., 2006; Siloto et al., 2013; Gholami-Ahangaran et al., 2016; Salem et al., 2018).
Table 4.
Effects of MMDA on hematological traits at d 42 on trial (d 273 of age).
| Treatment groups | T1 | T2 | T3 | T4 | T4 | T6 | T7 | ||
|---|---|---|---|---|---|---|---|---|---|
| Hens | no | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Replicates | no | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| MMDA | g/kg | 2 | 1 | 2 | 3 | ANOVA | |||
| AFB1 | mg/kg | 0.05 | 0.05 | 0.50 | 0.50 | 0.50 | |||
| T2-toxin | mg/kg | 1.5 | 1.5 | 2.0 | 2.0 | 2.0 | |||
| Body weight | |||||||||
| Body weight | g | ||||||||
| Hematological traits | |||||||||
| Erythrocytes | T/L | 2.29 ± 0.04ab | 2.13 ± 0.12a | 2.30 ± 0.08ab | 2.19 ± 0.12ab | 2.28 ± 0.04ab | 2.32 ± 0.14b | 2.23 ± 0.16ab | 0.021 |
| Leukocytes | G/L | 7.71 ± 1.89 | 7.94 ± 1.76 | 8.77 ± 5.66 | 8.97 ± 1.23 | 7.01 ± 1.03 | 6.89 ± 1.45 | 7.14 ± 1.56 | 0.610 |
| Lymphocytes | % | 42.0 ± 6.6 | 44.9 ± 6.4 | 44.1 ± 7.7 | 47.0 ± 6.1 | 37.6 ± 6.7 | 36.6 ± 6.6 | 39.0 ± 8.5 | 0.172 |
| Heterophils | % | 55.3 ± 6.8 | 51.4 ± 6.6 | 51.3 ± 10.2 | 49.6 ± 5.9 | 58.2 ± 6.9 | 58.7 ± 6.4 | 56.7 ± 8.5 | 0.122 |
| Monocytes | % | 1.4 ± 0.8 | 2.6 ± 1.1 | 3.1 ± 2.6 | 1.7 ± 1.1 | 1.6 ± 1.5 | 1.7 ± 1.1 | 1.9 ± 0.4 | 0.253 |
| Eosinophils | % | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.4 ± 0.8 | 0.8 ± 1.1 | 0.9 ± 1.2 | 0.6 ± 0.8 | 0.119 |
| Basophils | % | 1.3 ± 0.8 | 1.1 ± 1.1 | 1.4 ± 1.1 | 1.7 ± 1.1 | 1.8 ± 1.1 | 2.1 ± 1.3 | 1.9 ± 1.2 | 0.659 |
| Hemoglobin | g/L | 72.14 ± 3.44a | 74.43 ± 3.64a | 83.14 ± 7.84bc | 73.57 ± 10.72ab | 85.00 ± 1.91c | 86.14 ± 4.49c | 84.86 ± 4.38c | <0.001 |
| Hematocrit | L/L | 0.23 ± 0.01b | 0.21 ± 0.01a | 0.22 ± 0.01ab | 0.22 ± 0.01ab | 0.22 ± 0.01ab | 0.22 ± 0.01ab | 0.21 ± 0.02ab | 0.025 |
| MCV1 | Fl | 99.41 ± 2.31c | 96.54 ± 1.15abc | 94.47 ± 2.09ab | 97.66 ± 2.22bc | 94.63 ± 2.42ab | 93.70 ± 2.10a | 94.17 ± 2.77ab | <0.001 |
| MCH2 | Pg | 31.43 ± 0.33abc | 31.32 ± 0.26ab | 38.39 ± 2.92bcd | 34.01 ± 4.03a | 39.37 ± 0.77cd | 39.66 ± 1.06cd | 40.49 ± 1.42d | <0.001 |
| MCHC3 | g/dl | 31.61 ± 0.32a | 31.33 ± 0.52a | 36.29 ± 3.07b | 29.21 ± 9.36a | 37.24 ± 0.62b | 39.66 ± 1.06b | 40.49 ± 1.42b | <0.001 |
Abbreviations: AFB1, aflatoxin B1; MCH, mean corpuscular hemoglobin, MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MMDA, multicomponent mycotoxin detoxifying agent.
Different superscripts in same row are significant (a/b: P ≤ 0.05).
Mean corpuscular volume;
Mean corpuscular hemoglobin;
Mean corpuscular hemoglobin concentration.
Table 5.
Effects of MMDA on plasma concentrations of electrolytes and enzymes at d 42 on trial (d 273 of age).
| Treatment groups | T1 | T2 | T3 | T4 | T4 | T6 | T7 | ||
|---|---|---|---|---|---|---|---|---|---|
| Hens | no | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Replicates | no | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| MMDA | g/kg | 2 | 1 | 2 | 3 | ||||
| AFB1 | mg/kg | 0.05 | 0.05 | 0.50 | 0.50 | 0.50 | |||
| T2-toxin | mg/kg | 1.5 | 1.5 | 2.0 | 2.0 | 2.0 | |||
| Body weight | |||||||||
| Body weight | g | ||||||||
| Electrolytes and enzymes | |||||||||
| Sodium | mmol/L | 150 ± 4 | 153 ± 3 | 153 ± 2 | 152 ± 4 | 150 ± 1 | 151 ± 3 | 153 ± 1 | 0.209 |
| Potassium | mmol/L | 4.2 ± 0.7a | 4.6 ± 0.5ab | 4.4 ± 0.6ab | 5.4 ± 0.9ab | 4.8 ± 1.2ab | 5.8 ± 1.2ab | 5.6 ± 1.3b | 0.015 |
| Chloride | mmol/L | 113 ± 3x | 116 ± 3xy | 117 ± 1xy | 115 ± 3xy | 115 ± 1xy | 114 ± 3xy | 116 ± 2y | 0.096 |
| Calcium | mmol/L | 6.85 ± 0.50a | 6.72 ± 0.92a | 6.80 ± 0.56a | 4.67 ± 2.85ab | 4.86 ± 0.73ab | 4.71 ± 0.81b | 5.32 ± 1.13b | 0.002 |
| Magnesium | mmol/L | 0.99 ± 0.10 | 0.97 ± 0.05 | 0.94 ± 0.06 | 0.94 ± 0.09 | 0.95 ± 0.05 | 0.95 ± 0.06 | 0.95 ± 0.06 | 0.919 |
| Phosphate | mmol/L | 2.12 ± 0.43 | 2.29 ± 0.42 | 2.13 ± 0.22 | 1.97 ± 0.47 | 2.03 ± 0.45 | 1.89 ± 0.27 | 1.96 ± 0.47 | 0.583 |
| AST1 | U/L | 216 ± 32 | 201 ± 20 | 194 ± 21 | 246 ± 73 | 220 ± 23 | 206 ± 19 | 207 ± 13 | 0.151 |
| ALT2 | U/L | <3 | <3 | <3 | <3 | <3 | <3 | <3 | |
| GLDH3 | U/L | 7 ± 10 | 9 ± 11 | 9 ± 8 | 10 ± 3 | 9 ± 14 | 10 ± 11 | 9 ± 19 | 1.000 |
| ALP4 | U/L | 647 ± 101 | 507 ± 117 | 590 ± 281 | 618 ± 285 | 656 ± 213 | 614 ± 175 | 668 ± 260 | 0.842 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate aminotransferase; GLDH, glutamate dehydrogenase.
Different superscripts in same row are significant or trending (a/b: P ≤ 0.05; x/y 0.05 < P ≤ 0.10).
Aspartate transaminase.
Alanine aminotransferase.
Glutamate dehydrogenase.
Alkaline phosphatase.
Table 6.
Effects of MMDA on biochemical parameters in blood at d 42 on trial (d 273 of age).
| Treatment groups | T1 | T2 | T3 | T4 | T4 | T6 | T7 | ||
|---|---|---|---|---|---|---|---|---|---|
| Hens | no | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Replicates | no | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| MMDA | g/kg | 2 | 1 | 2 | 3 | ANOVA | |||
| AFB1 | mg/kg | 0.05 | 0.05 | 0.50 | 0.50 | 0.50 | |||
| T2-toxin | mg/kg | 1.5 | 1.5 | 2.0 | 2.0 | 2.0 | |||
| Body weight | |||||||||
| Body weight (g) | g | ||||||||
| Biochemical parameters | |||||||||
| Total cholesterol | mmol/L | 2.80 ± 0.40ab | 2.64 ± 0.77ab | 2.70 ± 0.27ab | 3.27 ± 0.76b | 1.85 ± 0.34a | 2.22 ± 0.76ab | 2.38 ± 0.77ab | 0.005 |
| Triglycerides | mmol/L | 14.35 ± 3.35 | 14.29 ± 4.48 | 15.01 ± 2.32 | 13.31 ± 9.25 | 8.29 ± 2.24 | 10.59 ± 5.58 | 12.22 ± 4.76 | 0.178 |
| Urea | mmol/L | <0.80 | <0.80 | <0.80 | <0.80 | <0.80 | <0.80 | <0.80 | |
| Total bilirubin | µmol/L | 2.29 ± 0.30a | 2.01 ± 0.34ab | 1.79 ± 0.30b | 2.00 ± 0.40ab | 1.93 ± 0.28ab | 1.98 ± 0.19ab | 2.21 ± 0.11ab | 0.043 |
| Creatine | µmol/L | 9.24 ± 0.85 | 9.27 ± 1.61 | 11.66 ± 2.99 | 11.81 ± 3.62 | 12.16 ± 2.85 | 12.41 ± 5.29 | 11.11 ± 2.63 | 0.302 |
| Glucose | mmol/L | 7.29 ± 2.20 | 6.64 ± 1.56 | 5.52 ± 1.51 | 9.02 ± 3.64 | 8.59 ± 2.20 | 9.28 ± 3.78 | 8.22 ± 1 2.72 | 0.109 |
| Albumins | mmol/L | 17.0 ± 0.2x | 16.2 ± 1.0xy | 16.8 ± 1.0xy | 16.8 ± 0.8xy | 14.8 ± 0.7y | 15.3 ± 2.7xy | 15.8 ± 1.9y | 0.060 |
| Globulins | mmol/L | 33.0 ± 1.8b | 28.9 ± 2.5ab | 29.4 ± 2.9ab | 32.1 ± 4.7b | 25.9 ± 3.0a | 25.6 ± 4.4a | 26.1 ± 3.5a | <0.001 |
| Total protein | mmol/L | 50.0 ± 1.8c | 45.2 ± 3.4abc | 46.1 ± 3.8abc | 48.9 ± 4.0bc | 40.7 ± 3.5a | 40.8 ± 6.8a | 41.9 ± 5.3b | <0.001 |
Different superscripts in same row are significant or trending (a/b: P ≤ 0.05; x/y 0.05 <P ≤ 0.10).
Pathological Examination
Results of pathological signs are presented in Tables 6 and 7 show the effect of increasing mycotoxins in feed on pathological examination at d 42 on trial (d 273 of age). Pathological alterations of liver, kidneys, gall bladder and bile duct in hens fed AFB1 and T2-toxin via spiked maize at the guidance or maximum tolerated dosage without MMDA application increased with increasing dose level of mycotoxins as compared with the control group. Grossly, there was a pale or yellowish discoloration of liver along with its size and distension of gall bladder and swollen kidneys bulging out of their sockets with congestion. However, fatty liver syndrome was also observed in hens fed diets without spiked maize. The addition of MMDA at dosage 1 to 3 g/kg feed did slightly modify pathological alterations of liver and kidneys in comparison with hens fed the contaminated diets at both dose levels without inclusion of MMDA. The greatest response was observed with feeding MMDA at 3 g/kg feed. Furthermore, data indicated no treatment effects on intestinal and respiratory tract, heart, and bursa of Fabricius (Tables 7 and 8). The administration of 108 cfu of Berevibacillus laterosporus/mL of drinking water declined necrosis in liver, enhanced production of proteins and antibodies and increased growth rate in quails provide with 2,500 μg of AFB1/kg produced by A. parasiticus (PTCC 5286) (Bagherzadeh Kasmani et al., 2012).
Table 7.
Effect of MMDA on pathological examination of intestinal and respiratory tract in laying hens at d 42 on trial (d 27 age).
| Treatment groups | T1 | T2 | T3 | T4 | T5 | T6 | T7 | P ANOVA | |
|---|---|---|---|---|---|---|---|---|---|
| Hens | no | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Replicates | no | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| MMDA | g/kg | 2 | 1 | 2 | 3 | ||||
| AFB1 | mg/kg | 0.05 | 0.05 | 0.50 | 0.50 | 0.50 | |||
| T2-toxin | mg/kg | 1.5 | 1.5 | 2.0 | 2.0 | 2.0 | |||
| Body weight | |||||||||
| Body weight | g | 2066.3 ± 108.6a | 2050.1 ± 115.7ab | 2012.4 ± 62.6ab | 2013.6 ± 66.8ab | 1945.4 ± 56.3b | 1987.6 ± 83.2ab | 1980.0 ± 41.0ab | 0.023 |
| Intestinal tract | |||||||||
| Larynx, oesophagus, crop, proventriculus/gizzard, small intestine, large intestine, caeca, cloaca | No pathological signs | ||||||||
| Respiratory tract | |||||||||
| Trachea, bronchi, lungs | No pathological signs | ||||||||
Different superscripts in same row are significant (a/b: P ≤ 0.001).
Table 8.
Effect of MMDA on pathological examination of organs in laying hens at d 42 on trial (d 273 of age).
| Treatment groups | T1 | T2 | T3 | T4 | T5 | T6 | T7 | P ANOVA | |
|---|---|---|---|---|---|---|---|---|---|
| Total birds per group | no | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Repetitions | no | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| MMDA | g/kg | 2 | 1 | 2 | 3 | ||||
| AFB1 | mg/kg | 0.05 | 0.05 | 0.50 | 0.50 | 0.50 | |||
| T2-toxin | mg/kg | 1.5 | 1.5 | 2.0 | 2.0 | 2.0 | |||
| Body weight | |||||||||
| Body weight | g | 2066.3 ± 108.6a | 2050.1 ± 115.7ab | 2012.4 ± 62.6ab | 2013.6 ± 66.8ab | 1945.4 ± 56.3b | 1987.6 ± 83.2ab | 1980.0 ± 41.0ab | 0.023 |
| Organs | |||||||||
| Liver | Enlargement, yellowish, rounded borders, fatty: n = 2 Sporadic petechial hemorrhages n= 1 No pathological signs: n = 8 |
Enlargement, yellowish, rounded borders, fatty: n = 3 Sporadic petechial hemorrhages n= 1 No pathological signs: n = 7 |
Enlargement, yellowish, rounded borders, fatty: n = 4 Sporadic petechial hemorrhages n= 1 No pathological signs: n = 5 |
Enlargement, yellowish, rounded borders, fatty: n = 5 Sporadic petechial hemorrhages n= 2 No pathological signs: n = 3 |
Enlargement, yellowish, rounded borders, fatty: n = 4 Sporadic petechial hemorrhages n= 1 No pathological signs: n = 5 |
Enlargement, yellowish, rounded borders, fatty: n = 5 Sporadic petechial hemorrhages n= 2 No pathological signs: n = 3 |
Enlargement, yellowish, rounded borders, fatty: n = 3 Sporadic petechial hemorrhages n= 1 No pathological signs: n = 6 |
||
| Gall bladder | Distension: n = 2 No pathological signs: n = 8 |
Distension: n = 3 No pathological signs: n = 7 |
Distension: n = 3 No pathological signs: n = 7 |
Distension: n = 4 No pathological signs: n = 6 |
Distension: n = 5 No pathological signs: n = 5 |
Distension: n = 3 No pathological signs: n = 7 |
Distension: n = 4 No pathological signs: n = 6 |
||
| Bile duct | No pathological signs | No pathological signs | Distension: n = 4 No pathological signs: n = 6 |
Distension: n = 2 No pathological signs: n = 8 |
Distension: n = 3 No pathological signs: n = 7 |
Distension: n = 2 No pathological signs: n = 8 |
Distension: n = 1 No pathological signs: n = 9 |
||
| Spleen | No pathological signs | No pathological signs | No pathological signs | No pathological signs | No pathological signs | No pathological signs | No pathological signs | ||
| Kidneys | No pathological signs | No pathological signs | Enlargement and pale: n = 3 Sporadic petechial hemorrhages n = 1 No pathological signs: n = 6 |
Enlargement and pale: n= 2 No pathological signs: n = 8 |
Enlargement and pale: n = 4 Sporadic petechial hemorrhages n= 1 No pathological signs: n = 5 |
Enlargement and pale: n= 4 No pathological signs: n = 6 |
Enlargement and pale: n = 3 No pathological signs: n = 7 |
||
| Bursa of Fabricius | No pathological signs | No pathological signs | No pathological signs | No pathological signs | No pathological signs | No pathological signs | No pathological signs | ||
| Heart | No pathological signs | No pathological signs | No pathological signs | No pathological signs | No pathological signs | No pathological signs | No pathological signs | ||
Different superscripts in same row are significant (a/b: P ≤ 0.001).
Effect on Organ and Tissue Weights
In Table 9 of dietary treatments on organ weights at d 42 of on trial (d 273 of age) are summarized. Homogeneity of variances was asserted using ANOVA which showed that equal variances could be assumed. Measured values among treatment groups were normally distributed (ANOVA). Feeding mycotoxins at the guidance dosage alone (T3) caused significantly enhanced relative weights of kidneys (+13.8%) and slightly increased relative weights of liver in comparison with the control group (+8.5%). AFB1 and T2-toxin at the maximum tolerated dosage (T5) did significantly increase relative weights of liver, kidneys and spleen as compared with the control group (liver: +8.5%; kidneys: +22.4%; spleen: +9.1%). Results responded linearly to increasing dietary AFB1 and T2-toxin levels, but significant effect of dose level was only found regarding relative weight of kidneys (+7.6%). Simultaneous presence of MMDA in diets containing AFB1 and T2-toxin at both dose levels did on average slightly reduce relative organ weights in comparison to contaminated diets alone (liver: -2.1%; kidneys: -1.4%; spleen: -8.3%); effect of MMDA dose level was not evident. No treatment response on relative breast weight was observed, nor in dry matter content of organs and breast muscle. AFB1 is associated with liver damage through the suppression of the activity of anti-inflammatory cytokines and anti-oxidative enzymes, increased apoptotic activity (Liao et al., 2014; Ma et al., 2015; Muhammad et al., 2018; Wang et al., 2019) and deposition of lipid in hepatocytes (Tejada-Castañeda et al., 2008; Siloto et al., 2013).
Table 9.
Effects of MMDA on body weight, liver weight, spleen weight, kidney weight, and breast muscle weight of laying hens at d 42 on trial (d 273 of age).
| Treatment groups | T1 | T2 | T3 | T4 | T5 | T6 | T7 | P value | |
|---|---|---|---|---|---|---|---|---|---|
| Total birds per group | no | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Repetitions | no | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| MMDA | g/kg | 2 | 1 | 2 | 3 | ||||
| AFB1 | mg/kg | 0.05 | 0.05 | 0.50 | 0.50 | 0.50 | |||
| T2-toxin | mg/kg | 1.5 | 1.5 | 2.0 | 2.0 | 2.0 | |||
| Body weight at d 42 on trial (d 273 of age) | |||||||||
| Body weight | g | 2066.3 ± 108.6a | 2050.1 ± 115.7ab | 2012.4 ± 62.6ab | 2013.6 ± 66.8ab | 1945.4 ± 56.3b | 1987.6 ± 83.2ab | 1980.0 ± 41.0ab | 0.023 |
| Liver at d 42 on trial (d 273 of age) | |||||||||
| Weight (as is) | g | 41.07 ± 2.57a | 41.45 ± 3.28ab | 43.43 ± 1.73abc | 43.60 ± 2.25abc | 45.36 ± 2.41c | 45.32 ± 4.06c | 45.04 ± 2.65bc | <0.001 |
| Relative weight (as is) | % of BW | 1.99 ± 0.06a | 2.02 ± 0.13ab | 2.16 ± 0.12abc | 2.16 ± 0.07bc | 2.33 ± 0.11c | 2.28 ± 0.21c | 2.28 ± 0.15c | <0.001 |
| Dry matter | % | 28.37 ± 1.18a | 28.44 ± 0.87ab | 28.71 ± 0.73abc | 28.07 ± 1.11a | 28.93 ± 1.16abc | 30.10 ± 1.17c | 29.90 ± 1.41bc | <0.001 |
| Kidneys at d 42 on trial (d 273 of age) | |||||||||
| Weight (as is) | g | 12.00 ± 0.63a | 12.18 ± 0.59b | 13.32 ± 0.77c | 13.49 ± 0.41c | 13.80 ± 0.34c | 14.05 ± 0.60c | 13.95 ± 0.34c | <0.001 |
| Relative weight (as is) | % of BW | 0.58 ± 0.02a | 0.59 ± 0.03a | 0.66 ± 0.03b | 0.67 ± 0.01b | 0.71 ± 0.01c | 0.70 ± 0.02c | 0.70 ± 0.02c | <0.001 |
| Dry matter | % | 20.66 ± 1.34 | 20.73 ± 1.63 | 20.94 ± 1.21 | 20.77 ± 1.12 | 20.01 ± 1.93 | 20.12 ± 1.19 | 20.38 ± 1.03 | 0.685 |
| Spleen at d 42 on trial (d 273 of age) | |||||||||
| Weight (as is) | g | 2.29 ± 0.20c | 2.30 ± 0.14c | 1.97 ± 0.13a | 2.23 ± 0.23abc | 2.40 ± 0.23c | 2.26 ± 0.24bc | 2.02 ± 0.16ab | <0.001 |
| Relative weight (as is) | % of BW | 0.11 ± 0.01abc | 0.11 ± 0.01bc | 0.10 ± 0.01a | 0.11 ± 0.01abc | 0.12 ± 0.01c | 0.11 ± 0.01bc | 0.10 ± 0.01ab | <0.001 |
| Dry matter | % | 71.76 ± 1.74 | 71.14 ± 1.32 | 70.96 ± 0.97 | 71.19 ± 1.64 | 70.81 ± 3.20 | 70.36 ± 2.32 | 70.67 ± 1.76 | 0.795 |
| Breast at d 42 on trial (d 273 of age) | |||||||||
| Weight (as is) | g | 87.31 ± 4.21bc | 88.31 ± 4.58c | 85.30 ± 2.91abc | 86.15 ± 1.36abc | 82.82 ± 2.28a | 83.79 ± 2.95ab | 83.89 ± 2.75ab | 0.004 |
| Relative weight (as is) | % of BW | 4.23 ± 0.13 | 4.31 ± 0.14 | 4.24 ± 0.15 | 4.28 ± 0.17 | 4.26 ± 0.11 | 4.22 ± 0.18 | 4.24 ± 0.13 | 0.801 |
| Dry matter | % | 28.07 ± 1.30 | 27.19 ± 1.95 | 27.81 ± 2.05 | 28.10 ± 2.27 | 26.69 ± 0.81 | 27.52 ± 1.91 | 26.55 ± 1.73 | 0.289 |
Different superscripts in same row are significant (a/b: P ≤0.05).
An inclusion of 5 g of hydrated calcium aluminosilicate or 5.0 to 7.5 g of bentonite, 15 g of clinoptilolite in the diet of poultry birds containing 2,000 to 2,500 μg of AFB1 produced by A. parasiticus reduced the detrimental effect on performance (Chen et al., 2014; Dos Anjos et al., 2015; Shannon et al., 2017) and liver by declining the concentration of AFB1 from 8.3 to 1.5 μg/kg (Neeff et al., 2013). Studies have reflected the renal enlargement after administration of birds with AFB1 (Şehu et al., 2005; Uyar et al., 2016; Gómez-Espinosa et al., 2017) and may be attributed to tubular distension, increased thickness of glomerular basement membrane and mesangial cells (Karaman et al., 2005; Liang et al., 2015). Introduction of B. subtilis (ANSB060) (organic binder) at the dose of 2.0 g/kg in the diet of broiler birds containing 70 μg AFB1/kg has enhanced the FCR and declined the level of accumulation of AFB1 from 0.24 to 0.09 μg/kg in liver and 7 to 1.5 μg/kg in intestine (Fan et al., 2013; Fan et al., 2015). The relative weight of liver in the broiler birds was within normal range fed with 5.0 g of hydrated sodium aluminosilicate/kg of feed containing 2,000 μg AFB1/kg obtained from Aspergillus (Chen et al., 2014).
Egg Characteristics
Results of egg characteristics determined at d 40 and d 42 on trial (d 271 of age, d 273 of age are given in Table 10. Significant treatment effects were identified on eggshell weight. Means of hens fed diets containing AFB1 and T2-toxin at 2 dose levels each without inclusion of MMDA (T3, T5) were significantly decreased by an average of 6.2% as compared with the control group. Application of MMDA to mycotoxin contaminated diets at 2 dose levels each (T4, T6, T7) did not markedly modify eggshell weight in comparison to hens fed mycotoxin contaminated diets alone. Moreover, inclusion of MMDA at 2 g/kg feed to uncontaminated diet (T2) did slightly reduce eggshell weight as compared with the control group (-3.1%). Nevertheless, eggshell stability was nearly similar across treatment groups. Although egg yolk color among treatment groups was not significantly different, mycotoxin contamination did slightly decrease egg yolk color as compared with the control group. In presence of MMDA mycotoxin effects on egg yolk color seemed to be slightly diminished. A marked reduction in yolk color score and eggshell thickness was documented in a study after administration of layer hens with a feed containing 2,500 μg of AFB1/kg from 14 d to 280 d of age (Pandey and Chauhan, 2007).
Table 10.
Effects of MMDA on egg characteristics at d 40 and d 42 on trial (d 271 and d 273 of age).
| Treatment groups | T1 | T2 | T3 | T4 | T5 | T6 | T7 | P value | |
|---|---|---|---|---|---|---|---|---|---|
| MMDA | g/kg | 2 | 1 | 2 | 3 | ||||
| AFB1 | mg/kg | 0.05 | 0.05 | 0.50 | 0.50 | 0.50 | |||
| T2-toxin | mg/kg | 1.5 | 1.5 | 2.0 | 2.0 | 2.0 | |||
| Eggshell stability & egg yolk color (d 40 on trial) | |||||||||
| Eggs | no | 14 | 13 | 14 | 13 | 14 | 13 | 11 | |
| Egg weight (as is) | G | 65.6 ± 1.1 | 65.1 ± 1.6 | 64.8 ± 0.9 | 65.1 ± 1.1 | 65.0 ± 0.8 | 65.1 ± 1.2 | 64.9 ± 1.0 | 0.645 |
| Eggshell stability | N | 62.3 ± 1.1 | 61.9 ± 1.9 | 61.7 ± 2.7 | 61.4 ± 1.8 | 61.8 ± 2.1 | 61.0 ± 1.6 | 61.1 ± 1.8 | 0.637 |
| Egg yolk color fan 1) | 13.5 ± 0.1x | 13.4 ± 0.3xy | 13.1 ± 0.4y | 13.4 ± 0.2xy | 13.2 ± 0.5xy | 13.2 ± 0.4xy | 13.3 ± 0.3xy | 0.067 | |
| Weight of eggshell, egg mass and their dry matter contents (d 42 on trial) | |||||||||
| Eggs | no | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Egg weight (as is) | g | 66.2 ± 1.5 | 65.8 ± 1.2 | 64.2 ± 2.0 | 65.0 ± 2.1 | 64.7 ± 1.0 | 65.2 ± 1.2 | 65.2 ± 2.1 | 0.173 |
| Eggshell weight (as is) | g | 6.5 ± 0.2a | 6.3 ± 0.2ab | 6.1 ± 0.2b | 6.2 ± 0.2ab | 6.1 ± 0.3b | 6.1 ± 0.2b | 6.1 ± 0.2b | 0.001 |
| Dry matter of eggshell | % | 88.85 ± 1.65 | 88.97 ± 0.72 | 88.55 ± 1.15 | 88.96 ± 1.28 | 89.02 ± 1.28 | 88.97 ± 0.77 | 89.08 ± 0.92 | 0.964 |
| Egg mass without shell (as is) | g | 59.7 ± 1.4 | 59.5 ± 1.1 | 58.1 ± 1.8 | 58.8 ± 2.0 | 58.6 ± 0.9 | 59.1 ± 1.1 | 59.0 ± 1.9 | 0.293 |
| Dry matter of egg mass without shell | % | 24.17 ± 0.47 | 24.16 ± 0.52 | 24.10 ± 0.37 | 24.08 ± 0.25 | 24.01 ± 0.26 | 23.98 ± 0.45 | 24.05 ± 0.36 | 0.916 |
Different superscripts in same row are significant or trending (a/b: P ≤ 0.05; x/y 0.05 < P ≤ 0.10).
Residue Levels in Tissues and Eggs
Deposition of AFB1 and T2-toxin and their main metabolites aflatoxin M1 and HT-2 toxin in selected tissues and eggs (without eggshell) are given in Table 11. Tissues and eggs of hens fed diets with naturally corn without or with MMDA were tested negative (under the limit of detection: = 0) for AFB1, T2-toxin and their metabolites (aflatoxin M1, HT-2 toxin). With increasing dosage of AFB1 and HT-2 toxin, residue levels of AFB1 in liver tissues increased significantly up 0.93 µg/kg DM as compared with the control group whereas deposition of T2-toxin, aflatoxin M1 and HT-2 toxin was not detected. In kidneys retention of AFB1, T2-toxin and their metabolites increased dose dependently, whereby modifications between the low and high contamination rate were significant. With application of graded dose levels of MMDA into the high contaminated diets both mycotoxins and their metabolites decreased significantly in comparison to the corresponding mycotoxin containing diets without using MMDA. Comparisons between both dose levels of MMDA indicated the greatest response at 3 g/kg feed. Moreover, at the low contamination level effects of MMDA on deposition of AFB1 and T2-toxin were neglectable. Breast and eggs were always tested negative for AFB1, T2-toxin and their main metabolites (aflatoxin M1, HT-2 toxin). The chickens fed with 40 μg AFB1/kg bw obtained from A. flavus and 3.0 g of hydrated sodium calcium aluminosilicate/kg has increased the excretion and reduced the accumulation of AFB1 in liver and decreased the relative liver weight (Liu et al., 2018).
Table 11.
Effects of MMDA on residue levels of mycotoxins and their main metabolites in tissues and eggs of laying hens at d 42 on trial (d 273 of age).
| Treatment groups | T1 | T2 | T3 | T4 | T5 | T6 | T7 | P value | |
|---|---|---|---|---|---|---|---|---|---|
| Total birds per group | no | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Repetitions | no | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| MMDA | g/kg | 2 | 1 | 2 | 3 | ||||
| AFB1 | mg/kg | 0.05 | 0.05 | 0.50 | 0.50 | 0.50 | |||
| T2-toxin | mg/kg | 1.5 | 1.5 | 2.0 | 2.0 | 2.0 | |||
| Liver at d 42 on trial (d 273 of age) | |||||||||
| AFB1 | µg/kg DM | 0a | 0a | 0.19 ± 0.06a | 0.16 ± 0.03a | 0.93 ± 0.24b | 0.89 ± 0.08a | 0.77 ± 0.10a | <0.001 |
| AFM1 | µg/kg DM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.000 |
| T2-toxin | µg/kg DM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.000 |
| HT-2 toxin | µg/kg DM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.000 |
| Kidneys at d 42 on trial (d 273 of age) | |||||||||
| AFB1 | µg/kg DM | 0a | 0a | 0.12 ± 0.02b | 0.12 ± 0.02b | 0.99 ± 0.15d | 0.70 ± 0.06c | 0.71 ± 0.07c | <0.001 |
| AFM1 | µg/kg DM | 0a | 0 | 0 | 0 | 0.05 ± 0.0 | 0 | 0 | 0.872 |
| T2-toxin | µg/kg DM | 0a | 0a | 0.05 ± 0.11a | 0a | 0.84 ± 0.90b | 0.09 ± 0.11a | 0a | <0.001 |
| HT-2 toxin | µg/kg DM | 0a | 0a | 0.55 ± 0.90b | 0.62 ± 0.84b | 1.27 ± 1.35c | 0.22 ± 0.45b | 0a | <0.001 |
| Breast at d 42 on trial (d 273 of age) | |||||||||
| AFB1 | µg/kg DM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.000 |
| AFM1 | µg/kg DM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.000 |
| T2-toxin | µg/kg DM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.000 |
| HT-2 toxin | µg/kg DM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.000 |
| Egg yolk & egg albumen at d 42 on trial (d 273 of age) | |||||||||
| AFB1 | µg/kg DM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.000 |
| AFM1 | µg/kg DM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.000 |
| T2-toxin | µg/kg DM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.000 |
| HT-2 toxin | µg/kg DM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.000 |
0 = Level under detection.
Different superscripts in same row are significant (a/b: P ≤ 0.05).
CONCLUSIONS
AFB1 and T-2 exposure in hens resulted in decreased egg production, decreased eggshell weights, changes in blood parameters, and pathological alterations in liver and kidney tissues in a dose-dependent manner. The addition of MMDA to the diet reduced the changes in the aforementioned parameters as well as the buildup of metabolites in liver and kidney tissues, which indicated the specific binding to AFB1 and T-2 toxin. The current study suggests that MMDA can be utilized in laying hen feed to reduce the negative effects brought on by AFB1 and T-2 toxins.
MATERIALS AND METHODS
Animals, Identification, Standard Health Program and Husbandry
A total of 105 Lohmann Brown laying hens (age-233 d; weight-1,950 g) were purchased from a local commercial source. The bird exhibiting the signs of injury or disease was excluded from selection. At arrival selected hens were randomly assigned to 35 pens with bedding of Giant Miscanthus grass chopped into 1-inch pieces, within a climate-controlled barn for laying hens according to body weight and laying performance. Adult layers were acclimatized for 7 d and supplemented with control diet. Following acclimatization, pens were distributed to the different treatments such that there were 15 birds per treatment and 3 hens per pen (replicate). Pens were labeled with texts and colored codes (T1: white, T2: green; T3: yellow, T4: blue, T5: red; T6: grey; T7: brown) to identify treatments. Pens were measuring 2.2 × 2.0 m, providing 1.5 m2 per bird. Feeder adjustment (which controls feed flow) was checked daily and adjusted as necessary to ensure that approximately 50% of the bottom of the feed trough was visible and 50% of the feed trough was covered with feed. This was minimizing spillage of feed. Any spoiled or wet feed was removed daily and collected in buckets. After weighing aliquots, waste feed was freeze-dried and amounts were used for determination of the correct feed intake. Drinkers were checked daily to ensure adequate water flow.
Throughout the 7-d adaption period and the 42-d experimental period the poultry house was provided with controlled climate and forced ventilation (air speed about 0.5 m/s). The room temperature was kept at about 21.5°C throughout the 7-d adaption period and the following 42-d experimental period. The relative humidity was ranging between 55 and 60%. Hens were maintained on a 16 h light and an 8 h dark schedule per day with an average light intensity of about 365 lux throughout the experiment.
Measures to Avoid Cross-Contamination, Diet Composition, and Manufacture
All diets were manufactured in the institute mill (registration number: DE-BE-100001) that ensured that all stages of production, processing and distribution under their control were carried out in accordance with EU Community legislation, national law compatible therewith, and good practice. According to the REGULATION (EC) No 183/2005 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 12 January 2005 laying down requirements for feed hygiene; feed businesses was met several conditions relevant to their operations, concerning facilities, equipment, personnel, production, quality control, storage and documentation, in order to ensure both feed safety and product traceability. The application of hazard analysis and critical control points principles to the production of feed is the medium-term objective of European hygiene legislation. Moreover, the traceability of feed and feed ingredients throughout the feed chain is an essential element in ensuring feed safety. Regulation (EC) No 178/2002 contains rules to ensure the traceability of feed and feed ingredients and provides a procedure for the adoption of implementing rules applicable to specific sectors.
The basal ingredients for the diets were provided by the institute; MMDA containing modified zeolite (Clinoptilolite), B. subtilis, B. Licheniformis, S. cerevisiae cell wall and silymarin and premixtures of AFB1 and T2-toxin based on maize were supplied by PATENT CO DOO (https://global.patent-co.com/).The amounts of all the basal ingredients as well as MMDA and the mycotoxin containing premixes to produce the diets were recorded. The total amount of feed manufactured for the study was amounting to 800 kg. The batch was subsequently divided into 7 aliquots (T1: 200 kg; T2: 100 kg; T3: 100 kg; T4: 100 kg; T4: 100 kg; T6: 100 kg; T7: 100 kg) for mixing the experimental diets. Records of diet mixing were maintained. Diets were prepared without antibiotics, coccidiostats, antimicrobials, enzymes, or growth promoters (e.g., organic acids, probiotics, and other botanicals).
Dose levels of MMDA were supplemented at the expense of Tixosil (>97% silicon dioxide). In addition, AFB1 and T2-toxin via spiked maize was included to the experimental diets at the expense of uncontaminated maize. AFB1 mycotoxin was produced by infecting sterile corn with an in-house strain of Aspergillus mold, and T-2 by infecting either corn, or oat with Fusarium langsethiae Fe 2391strain. Water was added in the amount that measured 30% of the weight of the substrate for AFB1 and 20 to 30% for T-2. The Aspergillus infected corn was incubated at 25°C for 21 d. F. langsethiae infected corn was incubated for 15 d with a temperature regimen alternating between 15°C and 22.5°C. After the incubation period, the contaminated materials were dried in the drying oven at 105° for 3 d. The material was then left to cool, ground in the Perten mill and the mycotoxin content was measured with LC-MS/MS. For proper mixing premixtures (6 kg each) containing aliquots of basal diet and Tixosil & uncontaminated maize (T1), Tixosil & MMDA & uncontaminated maize (T2), Tixosil & uncontaminated/spiked maize (T3); Tixosil & MMDA & uncontaminated/spiked maize (T4), Tixosil & spiked maize (T5), Tixosil & MMDA & spiked maize (T6), and MMDA & spiked maize (T7) were produced by very gentle mixing and included to the corresponding batches of 194 kg (T1) and 94 kg each (T2–T7) under a minimum of dust formation prior mixing into the remaining feed at a minimum of dust formation. It is recommended that ventilation inside the mixing equipment was reduced to a minimum. To avoid contamination with previous productions, feed was manufactured in an appropriate rank order starting with the control diet and with a neutral meal mixing in between each of the diets. The rank of order was T1, T2, T3, T4, T5, T6, and T7, respectively. Amounts of all the basal ingredients and the required test product in form of premixes to produce the diets are recorded (Table 12) and the analysis of feed after mixing is shown in Table 13. Subsamples of each diet were collected during the run out of the feed from the feed mixer to the bagging unit. About 6 subsamples were obtained at a port in the auger line at equally spaced intervals between the beginning of run out and the end of run out. Subsamples were subsequently mixed. Representative samples of the mixture were obtained by splitting the samples from the mixer using a sample splitting device. Two samples (2 × 500 g) were taken for proximate and mycotoxin analysis and storage at the institute. Samples were labeled with the unique study code LH 5/20, the treatment code (T1, T2, T3, T4, T5, T6, and T7), the type of diet (layer), the date of manufacture and the analysis required (proximate, mycotoxins). Samples were stored in standard polyethylene bags and back up samples were frozen and stored at -18°C until further analysis.
Table 12.
Feed and test item required.
| Treatment | AFB1 mg/kg | T2-Toxin mg/kg | MMDA g/kg | No hens | Total feed/treatment (kg) | AFB1 required Mg | T2-Toxin,requried mg | MMDA, required g |
|---|---|---|---|---|---|---|---|---|
| T1 | 15 | 200 | ||||||
| T2 | 2 | 15 | 100 | 200 | ||||
| T3 | 0.05 | 1.50 | 15 | 100 | 5.0 | 150 | ||
| T4 | 0.05 | 1.50 | 1 | 15 | 100 | 5.0 | 150 | 100 |
| T5 | 0.50 | 2.00 | 15 | 100 | 50.0 | 200 | ||
| T6 | 0.50 | 2.00 | 2 | 15 | 100 | 50.0 | 200 | 200 |
| T7 | 0.50 | 2.00 | 3 | 15 | 100 | 50.0 | 200 | 300 |
| Totals | 105 | 800 | 160.0 | 900 | 800 |
Table 13.
Key proximate analysis and mycotoxin concentrations in the experimental diets (as fed).
| Treatment groups | T1 | T2 | T3 | T4 | T5 | T6 | T7 | |
|---|---|---|---|---|---|---|---|---|
| Dry matter | g/kg | 902.3 | 902.1 | 902.6 | 901.9 | 902.4 | 902.5 | 901.8 |
| Crude protein | g/kg | 168.5 | 168.7 | 168.5 | 169.1 | 168.8 | 168.6 | 169.2 |
| Crude fiber | g/kg | 27.5 | 27.2 | 27.5 | 27.8 | 27.3 | 27.7 | 27.3 |
| Crude fat | g/kg | 47.9 | 48.1 | 47.8 | 48.2 | 47.9 | 47.7 | 48.1 |
| Crude ash | g/kg | 125.5 | 126.8 | 125.7 | 128.2 | 125.8 | 128.5 | 130.4 |
| Starch | g/kg | 418.3 | 418.8 | 418.0 | 418.6 | 417.4 | 417.8 | 418.3 |
| Sugars | g/kg | 38.3 | 37.7 | 38.1 | 38.5 | 37.8 | 38.0 | 38.6 |
| Calcium | g/kg | 38.3 | 37.9 | 38.0 | 37.8 | 38.3 | 38.4 | 37.6 |
| Phosphorus (total) | g/kg | 5.6 | 5.8 | 5.7 | 5.6 | 5.9 | 5.6 | 5.9 |
| Sodium | g/kg | 1.8 | 1.8 | 1.8 | 1.9 | 1.8 | 1.8 | 1.9 |
| AFB1 | µg/kg | <0.10 | <0.10 | 53.6 | 51.7 | 497.3 | 489.9 | 492.7 |
| T2-toxin | µg/kg | 29 | 27 | 1583 | 2012 | 1993 | 1972 | 1988 |
| Ochratoxin | µg/kg | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
Body Weight Measurement and Egg Characteristics
All hens were observed twice daily for any abnormalities, abnormal behavior, and clinical signs of sickness throughout the 42-d experimental period. This included the exterior (with special emphasis on skin, feathers, eyes, and visible mucous membranes), abnormal locomotion, movements and posture, reduced movement, presence of convulsions or paralysis, stereotypes, bizarre behavior as well as autonomous activities (e.g., abnormal breathing and body surface temperature) and morbidity/mortality. Any signs of abnormal behavior or a general change in feeding habits were recorded, if necessary. The following clinical sign key was used: individual body weights were recorded every week, and the feed supplied to each hen over each preceding week. Body weight change of hens was calculated by using the body weight at the end of each period minus the body weight at the start of each period. Feed consumption per hen was estimated as the total feed supplied per pen and period corrected for dispersed/leftover feed per pen. Eggs and their weights were recorded daily per hen and were summarized in weekly periods per hen. Feed conversion ratio (g feed/g egg) was calculated from the relationship of weekly corrected feed intake and egg mass for this period.
Blood Profile
Venous blood samples were taken at the end of the 42-d feeding period from 10 hens per treatment (2 hen per pen) selected for body weights closest to the average of the corresponding treatment group. Samples were collected from the vena cutanea into plain and heparinized or EDTA containing tubes. Samples were analyzed for hematological variables (erythrocytes, leukocytes, differential hemogram: lymphocytes, monocytes, eosinophils, basophils, neutrophils, hemoglobin, hematocrit, mean corpuscular volume (MCV), MCH, and MCH concentration (MCHC)) as well as for electrolytes (sodium, potassium, chlorine, calcium, and inorganic phosphate). Additionally, biochemical parameters (total cholesterol, triglycerides, bilirubin, urea, glucose, albumin, total protein) and enzyme activities (AST, gamma-glutamyl-transferase, glutamate-dehydrogenase) were measured. Hens used for venous blood sampling before were killed by stunning and sacrificed by exsanguination for post-mortem external and internal macroscopically examination by a veterinarian in accordance with the necropsy key.
Egg Shell Stability
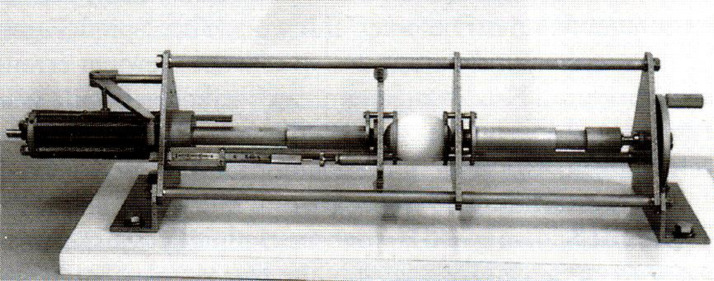
All eggs collected at d 40 on trial were used for determination of eggshell stability and egg yolk color. Egg yolk color was evaluated by the Roche Yolk Color Fan (15 = dark orange; 1 = light pale). The eggshell stability was determined in an apparatus which compresses each egg between flat plates to measure breakage force (Figure 1). This procedure was carried out on eggs with the major axis parallel to the compression surfaces (force applied at equator). The figure of the equipment is shown above.
Figure 1.
Apparatus for measuring the eggshell breaking strength.
Mycotoxins and Metabolites Analysis in Organs and Eggs
Additionally, weights of liver, spleen, breast, and kidneys were monitored from each eviscerated carcass. Afterwards the liver, breast and kidneys were packed into polyethylene bags and kept at -20°C before being freeze-dried and subjected to chemical analyses. Ten eggs per treatment (2 eggs per pen) were collected at d 42 on trial for mycotoxin analysis. After weighing eggs without eggshell were packed into polyethylene bags and kept at -20°C before being freeze-dried and subjected to chemical analyses.
AFB1 and T-2 Toxin and Their Metabolites Analysis
The analysis of AFB1, B2, G1, G2, M1, T-2 and HT-2 toxins and their metabolites in the samples was performed using 6460c MS/MS QQQ with Jet Stream electrospray ion source, Agilent Technologies. The method was developed and validated in house (in communication for publication). The LOQ (µg/kg) for AFB1, AFB2, AFG1, AFG2 was 0.1 μg/kg, 0.2 μg/kg for T-2 and 1 μg/kg for HT-2 toxin. To compensate the matrix effect in electrospray ionization, as internal standard, isotopically labeled (13C24) AFB1 CRM BiopureTM—(0.5 µg/mL) for aflatoxins and (13C24) T-2 toxin; CRM BiopureTM—(25 µg/mL) for T-2/HT-2 toxins were used. The recovery of more than 75% was recorded for all the toxins. The method was linear from 0.1 to 1.2 μg/kg for aflatoxins, 0.2 to 4.0 μg/kg for T-2 and 1 to 20 μg/kg for HT-2 toxin.
The tissue samples were finely grounded and thoroughly mixed using a blender. A 2g test portion was removed for analysis. The samples were then extracted using a 10 mL extraction mixture (80% Acetonitrile: 15% Water: 5%Formic acid) and by shaking the mixture in an orbital shaker at 200 rpm for 1 h at room temperature. After extraction, this portion was centrifuged at 4 200 × g for 5 min, 7 mL of supernatant were removed and placed into another conical tube. The samples were cleaned by adding 2.8 g MgSO4 and 0.7g NaCl to the supernatant and vortexed for 60 s. These tubes were centrifuged at 4200 × g for 5 min. A 1 mL solution was removed from the supernatant and diluted with 250 µL water. Further clean-up was performed on Captiva EMR Lipid cartridge (Agilent): 1.25 mL supernatant were passed through the cartridge (by gravity) and collected into a 15 mL centrifuge tube. When all the extract has passed through the cartridge, 400 µL of the extraction solvent was added and collected into the same centrifuge tube. The extract in the evaporator (CHRIST RVC 2-18 CD plus) was evaporated at 1500 rpm under 40°C. Then, 500 µL solvent for reconstitution (50% Acetonitrile: 50% Water, containing 0.1% Formic acid) was add to the evaporated sample and vortexed well. The prepared samples were filtered across a nylon membrane syringe (pore size 0.22 µm) into a glass vial and vortexed. The samples were run on LC-MS/MS using analytical column Agilent ZORBAX Rapid Resolution HD 2.1*50mm 1.8µm and guard column ZORBAX Eclipse Plus C18, 2.1mm, 1.8µm, UHPLC guard column. The following LC-MS/MS conditions were obtained with mobile phase A (containing 5 mM ammonium formate, 0.1%formic acid in water) and mobile phase B (containing 5 mM ammonium formate, 0.1%formic acid in 75% methanol and 25% acetonitrile)
-
-
Gradient settings:
| Time [min] | Mobil phase A [%] | Mobile phase B [%] | Flow [mL/min] |
|---|---|---|---|
| 0.00 | 88 | 12 | 0.2 |
| 5.00 | 88 | 12 | 0.2 |
| 5.01 | 50 | 50 | 0.2 |
| 16.00 | 0 | 100 | 0.2 |
| 17.00 | 0 | 100 | 0.2 |
| 17.01 | 88 | 12 | 0.2 |
-
-
Injection volume: 6 µL
-
-
Column temperature: 30°C
-
→
MS/MS parameters:
| Gas temperature [°C] | 200 |
| Gas flow [L/min] | 8 |
| Nebulizer [psi] | 40 |
| Sheath gas temp [°C] | 350 |
| Sheath gas flow [L/min] | 11 |
| Capillary [V] | 3500 |
| Nozzle voltage [V] | 500 |
-
→
Compound specific MS/MS parameters for mycotoxins, measured in ESI positive mode:
| Analyte | Precursor Ion | Product Ion | Fragmentor [V] | Collision energy [V] | Polarity |
|---|---|---|---|---|---|
| Aflatoxin B1 | 313 | 285 | 165 | 24 | + |
| 241 | 42 | ||||
| Aflatoxin B2 | 315 | 287 | 165 | 28 | + |
| 2,589 | 32 | ||||
| Aflatoxin G1 | 329 | 243 | 160 | 28 | + |
| 200 | 48 | ||||
| Aflatoxin G2 | 331 | 313 | 165 | 24 | + |
| 245 | 32 | ||||
| Aflatoxin M1 | 3,291 | 2,731 | 163 | 24 | + |
| 2,291 | 44 | ||||
| 3,182 | 46 | ||||
| HT-2 | 4,422 | 263 | 80 | 8 | + |
| 2,151 | 8 | ||||
| T-2 | 4,842 | 2,151 | 80 | 16 | + |
| 1,851 | 24 | ||||
| 1,891 | 20 | ||||
| [13C17] Aflatoxin B1 | 3,302 | 3,011 | 156 | 24 | + |
| [13C24] T-2 | 5,084 | 322 | 110 | 8 | + |
Analytical Methods
Feed samples were ground to pass through a 0.25 mm screen before analysis. Laboratory measurements were including Weender constituents (VDLUFA Section-III, 1993) and additionally starch, total sugars, calcium, phosphorus, and sodium. Analyses were in accordance with the methods issued by VDLUFA (Association of German Agricultural Inspection and research institutes) (dry matter: VDLUFA III 3.1; crude protein: VDLUFA III 4.1.1 modified according to macro-N determination (vario Max CN); crude fiber: VDLUFA III 6.1.4; crude ash: VDLUFA III 8.1; crude fat: VDLUFA III 5.1.1; starch: VDLUFA III 7.2.1; total sugars: VDLUFA III 7.1.1; calcium: VDLUFA VII 2.2.2.6). AFB1, the most common and biologically active aflatoxin, T2/HT2-toxin and ochratoxin in feeds were measured in an accredited external lab (D-PL-14016-01-00) according to FB-558-03-IAC-R7 9/17 (AFB1: LOD = 0.1 µg/kg), FB-535-04-R10 1/18 (T2/HT2: LOD = 1 µg/kg), and FB-554-78-R7 (Ochratoxin: LOD = 25 µg/kg). Blood cells were measured by flow cytometry. Blood concentrations in plasma were determined by using photometry. Sodium, potassium, and chlorides were measured by ionic liquid-polyacrylamide gel electrophoresis.
Statistical Evaluation
Results were presented according to the EFSA Guidance on Statistical reporting (EFSA Journal 2014; 12 (12):3908), descriptive statistics following Section 9.2.1 and results of statistical analyses in line with Section 9.2.2, respectively. Main analyses results were presented as point estimate and confidence interval. For all measurements taken at pen-level or at individual level, the basic statistical technique used was ANOVA with treatment as explanatory variable. After checking model assumptions, Tukey test was applied. Differences were considered significant when P < 0.05, whereas P < 0.10 were considered a near-significant trend. Analysis was performed with the software package SPSS (IBM SPSS Version 25).
Based on the supposed benefit of the test product on reducing the residue level of AFB1 in breast muscle the following power was estimated:
| Study parameter | |
|---|---|
| Residue level of AFB1 in liver tissue | µg/kg dry matter |
| Mean group 1: AFB1 | 0.50 |
| Mean group 2: AFB1 + MMDA | 0.44 |
| Sample size | 5 |
| Alpha | 0.05 |
| Post-hoc power calculation | 83.5 |
Calculation based on the formula: n = f(α/2, β) × 2 × σ2 / (μ1 − μ2)2, where μ1 and μ2 are the mean outcome in the control and experimental group respectively, σ is the standard deviation, and f(α, β) = [Φ-1(α) + Φ-1(β)]2,Φ-1 is the cumulative distribution function of a standardized normal deviate.
ACKNOWLEDGMENTS
The research was funded by PATENT CO DOO.
Ethical Statement: The trial was performed in accordance with the Animal Welfare Act of Germany approved by the local state office of occupational health and technical safety (Landesamt für Gesundheit und Soziales, LaGeSo, no. A 0439/17). Animals used in the study were raised and treated according to European Union Directive 2010/63/EU covering the protection of animals used for experimental or other purposes and according to the recommendation of Commission 2007/526/CE covering the accommodation and care of animals used for experimental and other scientific purposes. During the study, appropriate animal health and welfare inspections were carried out. The study animals were owned by Humboldt Universität zu Berlin.
Author contributions: This study was performed by Klaus Maennner and managed and designed by Jog Raj, Hunor Farkas and Marko Vasiljevic. Jog Raj, Marko Vasiljevic, Rakesh Kumar and Rajesh Asrani managed this study and wrote the scientific paper.
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: All authors agree for publication of this manuscript.
Data Availability Statement: Additional data can be provided on request.
DISCLOSURES
The authors declare no conflicts of interest.
Footnotes
Key contribution: The research was performed to assess the effect of MMDA to prevent gastrointestinal absorption of aflatoxin B1 and T2-toxin in the basal diet of laying hens.
References
- Abo-Norag M., Edrington T.S., Kubena L.F., Harvey R.B., Phillips T.D. Influence of a hydrated sodium calcium aluminosilicate and virginiamycin on aflatoxicosis in broiler chicks. Poult. Sci. 1995;74:626–632. doi: 10.3382/ps.0740626. [DOI] [PubMed] [Google Scholar]
- Alshannaq A., Yu J.H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health. 2017;14:632. doi: 10.3390/ijerph14060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arafa A.S., Bloomer R.J., Wilson H.R., Simpson C.F., Harms R.H. Susceptibility of various poultry species to dietary aflatoxin. Br. Poult. Sci. 1981;22:431–436. doi: 10.1080/00071688108447906. [DOI] [PubMed] [Google Scholar]
- Bagherzadeh Kasmani F., Karimi Torshizi M.A., Allameh A., Shariatmadari F.A. A novel aflatoxin-binding Bacillus probiotic: performance, serum biochemistry, and immunological parameters in Japanese quail. Poult. Sci. 2012;91:1846–1853. doi: 10.3382/ps.2011-01830. [DOI] [PubMed] [Google Scholar]
- Bailey C.A., Latimer G.W., Barr A.C., Wigle W.L., Haq A.U., Balthrop J.E., Kubena L.F. Efficacy of montmorillonite clay (NovaSil PLUS) for protecting full-term broilers from aflatoxicosis. J. Appl. Poult. Res. 2006;15:198–206. [Google Scholar]
- Bhatti S.A., Khan M.Z., Hassan Z.U., Saleemi M.K., Saqib M., Khatoon A., Akhter M. Comparative efficacy of bentonite clay, activated charcoal and Trichosporon mycotoxinivorans in regulating the feed-to-tissue transfer of mycotoxins. J. Sci. Food Agric. 2018;98:884–890. doi: 10.1002/jsfa.8533. [DOI] [PubMed] [Google Scholar]
- Binder E.M. Managing the risk of mycotoxins in modern feed production. Anim. Feed Sci. Technol. 2007;133:149–166. [Google Scholar]
- Chen X., Horn N., Applegate T.J. Efficiency of hydrated sodium calcium aluminosilicate to ameliorate the adverse effects of graded levels of aflatoxin B1 in broiler chicks. Poult. Sci. 2014;93:2037–2047. doi: 10.3382/ps.2014-03984. [DOI] [PubMed] [Google Scholar]
- Coffey R., Cummins E., Ward S. Exposure assessment of mycotoxins in dairy milk. Food Control. 2009;20:239–249. [Google Scholar]
- Dänicke S., Gareis M., Bauer J. Orientation values for critical concentrations of deoxynivalenol and zearalenone in diets for pigs, ruminants and gallinaceous poultry. Proc. Soc. Nutr. Physiol. 2001;10:171–174. [Google Scholar]
- Devegowda G., Murthy T.N.K. In: Pages 25–56 in The Mycotoxin Blue Book. Diaz D.E., editor. Nottingham University Press; Nottingham, UK: 2005. Mycotoxins: their effects in poultry and some practical solutions. [Google Scholar]
- Diaz G.J., Cortes A., Botero L. Evaluation of the ability of a feed additive to ameliorate the adverse effects of aflatoxins in turkey poults. Br. Poult. Sci. 2009;50:240–250. doi: 10.1080/00071660902774566. [DOI] [PubMed] [Google Scholar]
- Dos Anjos F.R., Ledoux D.R., Rottinghaus G.E., Chimonyo M. Efficacy of adsorbents (bentonite and diatomaceous earth) and turmeric (Curcuma longa) in alleviating the toxic effects of aflatoxin in chicks. Br. Poult. Sci. 2015;56:459–469. doi: 10.1080/00071668.2015.1053431. [DOI] [PubMed] [Google Scholar]
- Edds G.T., Bortell R.A. In: Pages 56–61 in Aflatoxin and Aspergillus flavus in Corn. Diener U.L., Asquith R.L., Dickens J.W., editors. Southern Cooperative Series Bulletin 279. Auburn University; Auburn, AL: 1983. Biological effects of aflatoxins: poultry. [Google Scholar]
- Eskola M., Kos G., Elliott C.T., Hajšlová J., Mayar S., Krska R. Worldwide contamination of food-crops with mycotoxins: validity of the widely cited ‘FAO estimate’ of 25% Crit. Rev. Food Sci. Nutr. 2020;60:2773–2789. doi: 10.1080/10408398.2019.1658570. [DOI] [PubMed] [Google Scholar]
- Fan Y., Zhao L., Ji C., Li X., Jia R., Xi L., Zhang J., Ma Q. Protective effects of Bacillus subtilis ANSB060 on serum biochemistry, histopathological changes and antioxidant enzyme activities of broilers fed moldy peanut meal naturally contaminated with aflatoxins. Toxins. 2015;7:3330–3343. doi: 10.3390/toxins7083330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Zhao L., Ma Q., Li X., Shi H., Zhou T., Zhang J., Ji C. Effects of Bacillus subtilis ANSB060 on growth performance, meat quality and aflatoxin residues in broilers fed moldy peanut meal naturally contaminated with aflatoxins. Food Chem. Toxicol. 2013;59:748–753. doi: 10.1016/j.fct.2013.07.010. [DOI] [PubMed] [Google Scholar]
- Gholami-Ahangaran M., Rangsaz N., Azizi S. Evaluation of turmeric (Curcuma longa) effect on biochemical and pathological parameters of liver and kidney in chicken aflatoxicosis. Pharm. Biol. 2016;54:780–787. doi: 10.3109/13880209.2015.1080731. [DOI] [PubMed] [Google Scholar]
- Gómez-Espinosa D., Cervantes-Aguilar F.J., Del Río-García J.C., Villarreal-Barajas T., Vázquez-Durán A., Méndez-Albores A. Ameliorative effects of neutral electrolyzed water on growth performance, biochemical constituents, and histopathological changes in turkey poults during aflatoxicosis. Toxins. 2017;9:104. doi: 10.3390/toxins9030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda N.K.S., Ledoux D.R., Rottinghaus G.E., Bermudez A.J., Chen Y.C. Efficacy of turmeric (Curcuma longa), containing a known level of curcumin, and a hydrated sodium calcium aluminosilicate to ameliorate the adverse effects of aflatoxin in broiler chicks. Poult. Sci. 2008;87:1125–1130. doi: 10.3382/ps.2007-00313. [DOI] [PubMed] [Google Scholar]
- Han X.Y., Huang Q.C., Li W.F., Jiang J.F., Xu Z.R. Changes in growth performance, digestive enzyme activities and nutrient digestibility of cherry valley ducks in response to aflatoxin B1 levels. Livest. Sci. 2008;9:216–220. [Google Scholar]
- He J., Zhang K.Y., Chen D.W., Ding X.M., Feng G.D., Ao X. Effects of vitamin E and selenium yeast on growth performance and immune function in ducks fed maize naturally contaminated with aflatoxin B1. Livest. Sci. 2013;152:200–207. [Google Scholar]
- Huff W.E., Doerr J.A. Synergism between aflatoxin and ochratoxin A in broiler chickens. Poult. Sci. 1981;60:550–555. doi: 10.3382/ps.0600550. [DOI] [PubMed] [Google Scholar]
- Huff W.E., Harvey R.B., Kubena L.F., Rottinghaus G.E. Toxic synergism between aflatoxin and T-2 toxin in broiler chickens. Poult. Sci. 1988;67:1418–1423. doi: 10.3382/ps.0671418. [DOI] [PubMed] [Google Scholar]
- Jiang S., Hester P.Y., Hu J.Y., Yan F.F., Dennis R.L., Cheng H.W. Effect of perches on liver health of hens. Poult. Sci. 2014;93:1618–1622. doi: 10.3382/ps.2013-03659. [DOI] [PubMed] [Google Scholar]
- Karaman M., Basmacioglu H., Ortatatli M., Oguz H. Evaluation of the detoxifying effect of yeast glucomannan on aflatoxicosis in broilers as assessed by gross examination and histopathology. Br. Poult. Sci. 2005;46:394–400. doi: 10.1080/00071660500124487. [DOI] [PubMed] [Google Scholar]
- Karaman M., Özen H., Tuzcu M., Çiğremiş Y., Önder F., Özcan K. Pathological, biochemical and haematological investigations on the protective effect of α-lipoic acid in experimental aflatoxin toxicosis in chicks. Br. Poult. Sci. 2010;51:132–141. doi: 10.1080/00071660903401839. [DOI] [PubMed] [Google Scholar]
- Khan W.A., Khan M.Z., Khan A., Hassan Z.U., Rafique S., Saleemi M.K., Ahad A. Dietary vitamin E in White Leghorn layer breeder hens: a strategy to combat aflatoxin B1-induced damage. Avian Pathol. 2014;43:389–395. doi: 10.1080/03079457.2014.943691. [DOI] [PubMed] [Google Scholar]
- Leeson S., Diaz G.J., Summers J.D. University Books; Guelph, Ontario, Canada: 1995. Poultry Metabolic Disorders and Mycotoxins. [Google Scholar]
- Liang N., Wang F., Peng X., Fang J., Cui H., Chen Z., Lai W., Zhou Y., Geng Y. Effect of sodium selenite on pathological changes and renal functions in broilers fed a diet containing aflatoxin B1. Int. J. Environ. Res. Public Health. 2015;12:11196–11208. doi: 10.3390/ijerph120911196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S., Shi D., Clemons-Chevis C.L., Guo S., Su R., Qiang P., Tang Z. Protective role of selenium on aflatoxin B1-induced hepatic dysfunction and apoptosis of liver in ducklings. Biol. Trace Elem. Res. 2014;162:296–301. doi: 10.1007/s12011-014-0131-4. [DOI] [PubMed] [Google Scholar]
- Liu N., Wang J., Deng Q., Gu K., Wang J. Detoxification of aflatoxin B1 by lactic acid bacteria and hydrated sodium calcium aluminosilicate in broiler chickens. Livest. Sci. 2018;208:28–32. [Google Scholar]
- Ma Q., Li Y., Fan Y., Zhao L., Wei H., Ji C., Zhang J. Molecular mechanisms of lipoic acid protection against aflatoxin B1-induced liver oxidative damage and inflammatory responses in broilers. Toxins. 2015;7:5435–5447. doi: 10.3390/toxins7124879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manafi M. Counteracting effect of high grade sodium bentonite during aflatoxicosis in broilers. J. Agric. Sci. Technol. 2012;14:539–547. [Google Scholar]
- Mendieta C.R., Gómez G.V., Del Río J.C.G., Cuevas A.C., Arce J.M., Ávila E.G. Effect of the addition of Saccharomyces cerevisiae yeast cell walls to diets with mycotoxins on the performance and immune responses of broilers. J. Poult. Sci. 2018;55:38–46. doi: 10.2141/jpsa.0170019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad I., Wang X., Li S., Li R., Zhang X. Curcumin confers hepatoprotection against AFB1-induced toxicity via activating autophagy and ameliorating inflammation involving Nrf2/HO-1 signaling pathway. Mol. Biol. Rep. 2018;45:1775–1785. doi: 10.1007/s11033-018-4323-4. [DOI] [PubMed] [Google Scholar]
- Neeff D.V., Ledoux D.R., Rottinghaus G.E., Bermudez A.J., Dakovic A., Murarolli R.A., Oliveira C.A.F. In vitro and in vivo efficacy of a hydrated sodium calcium aluminosilicate to bind and reduce aflatoxin residues in tissues of broiler chicks fed aflatoxin B1. Poult. Sci. 2013;92:131–137. doi: 10.3382/ps.2012-02510. [DOI] [PubMed] [Google Scholar]
- Oguz H., Kurtoglu V. Effect of clinoptilolite on performance of broiler chickens during experimental aflatoxicosis. Br. Poult. Sci. 2000;41:512–517. doi: 10.1080/713654953. [DOI] [PubMed] [Google Scholar]
- Pandey I., Chauhan S.S. Studies on production performance and toxin residues in tissues and eggs of layer chickens fed on diets with various concentrations of aflatoxin AFB1. Br. Poult. Sci. 2007;48:713–723. doi: 10.1080/00071660701713534. [DOI] [PubMed] [Google Scholar]
- Parlat S.S., Yildiz A.O., Oguz H. Effect of clinoptilolite on performance of Japanese quail (Coturnix coturnix japonica) during experimental aflatoxicosis. Br. Poult. Sci. 1999;40:495–500. doi: 10.1080/00071669987269. [DOI] [PubMed] [Google Scholar]
- Pitt J.I., Miller J.D. A concise history of mycotoxin research. J. Agric. Food Chem. 2017;65:7021–7033. doi: 10.1021/acs.jafc.6b04494. [DOI] [PubMed] [Google Scholar]
- Rajput S.A., Sun L., Zhang N., Mohamed Khalil M., Gao X., Ling Z., Zhu L., Khan F.A., Zhang J., Qi D. Ameliorative effects of grape seed proanthocyanidin extract on growth performance, immune function, antioxidant capacity, biochemical constituents, liver histopathology and aflatoxin residues in broilers exposed to aflatoxin B1. Toxins. 2017;9:371. doi: 10.3390/toxins9110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa C.A.R., Miazzo R., Magnoli C., Salvano M., Chiacchiera S.M., Ferrero S., Saenz M., Carvalho E.C.Q., Dalcero A. Evaluation of the efficacy of bentonite from the south of Argentina to ameliorate the toxin effects of aflatoxin in broilers. Poult. Sci. 2001;80:139–144. doi: 10.1093/ps/80.2.139. [DOI] [PubMed] [Google Scholar]
- Salem R., El-Habashi N., Fadl S.E., Sakr O.A., Elbialy Z.I. Effect of probiotic supplement on aflatoxicosis and gene expression in the liver of broiler chicken. Environ. Toxicol. Pharmacol. 2018;60:118–127. doi: 10.1016/j.etap.2018.04.015. [DOI] [PubMed] [Google Scholar]
- Saminathan M., Selamat J., Abbasi Pirouz A., Abdullah N., Zulkifli I. Effects of nano-composite adsorbents on the growth performance, serum biochemistry, and organ weights of broilers fed with aflatoxin-contaminated feed. Toxins. 2018;10:345. doi: 10.3390/toxins10090345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon T.A., Ledoux D.R., Rottinghaus G.E., Shaw D.P., Daković A., Marković M. The efficacy of raw and concentrated bentonite clay in reducing the toxic effects of aflatoxin in broiler chicks. Poult. Sci. 2017;96:1651–1658. doi: 10.3382/ps/pew408. [DOI] [PubMed] [Google Scholar]
- Siloto E.V., Oliveira E.F.A., Sartori J.R., Fascina V.B., Martins B.A.B., Ledoux D.R., Rottinghaus G.E., Sartori D.R.S. Lipid metabolism of commercial layers fed diets containing aflatoxin, fumonisin, and a binder. Poult. Sci. 2013;92:2077–2083. doi: 10.3382/ps.2012-02777. [DOI] [PubMed] [Google Scholar]
- Şehu A., Çakir S., Cengiz Ö., Eşsiz D. MYCOTOX® and aflatoxicosis in quails. Br. Poult. Sci. 2005;46:520–524. doi: 10.1080/00071660500181529. [DOI] [PubMed] [Google Scholar]
- Tejada-Castañeda Z.I., Ávila-Gonzalez E., Casaubon-Huguenin M.T., Cervantes-Olivares R.A., Vásquez-Peláez C., Hernández-Baumgarten E.M., Moreno-Martínez E. Biodetoxification of aflatoxin-contaminated chick feed. Poult. Sci. 2008;87:1569–1576. doi: 10.3382/ps.2007-00304. [DOI] [PubMed] [Google Scholar]
- Tsiouris V., Tassis P., Raj J., Mantzios T., Kiskinis K., Vasiljević M., Delić N., Petridou E., Brellou G.D., Polizopoulou Z., Mittas N., Georgopoulou I. Investigation of a novel multicomponent mycotoxin detoxifying agent in amelioration of mycotoxicosis induced by aflatoxin-B1 and ochratoxin A in broiler chicks. Toxins. 2021;13:367. doi: 10.3390/toxins13060367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyar A., Yener Z., Dogan A. Protective effects of Urtica dioica seed extract in aflatoxicosis: histopathological and biochemical findings. Br. Poult. Sci. 2016;57:235–245. doi: 10.1080/00071668.2015.1129664. [DOI] [PubMed] [Google Scholar]
- VDLUFA III . VDLUFA; Verlag, Speyer: 1993. The Chemical Analysis of Feedstuffs of VDLUFA (1st –8th Supplement Delivery) [Google Scholar]
- Walzem R.L., Simon C., Morishita T., Lowenstine L., Hansen R.J. Fatty liver hemorrhagic syndrome in hens overfed a purified diet. Selected enzyme activities and liver histology in relation to liver hemorrhage and reproductive performance. Poult. Sci. 1993;72:1479–1491. doi: 10.3382/ps.0721479. [DOI] [PubMed] [Google Scholar]
- Wang X.H., Li W., Wang X.H., Han M.Y., Muhammad I., Zhang X.Y., Sun X.Q., Cui X.X. Water-soluble substances of wheat: a potential preventer of aflatoxin B1-induced liver damage in broilers. Poult. Sci. 2019;98:136–149. doi: 10.3382/ps/pey358. [DOI] [PubMed] [Google Scholar]
- Warth B., Braun D., Ezekiel C.N., Turner P.C., Degen G.H., Marko D. Biomonitoring of mycotoxins in human breast milk: current state and future perspectives. Chem. Res. Toxicol. 2016;29:1087–1097. doi: 10.1021/acs.chemrestox.6b00125. [DOI] [PubMed] [Google Scholar]
- Zabiulla I., Malathi V., Swamy H.V.L.N., Naik J., Pineda L., Han Y. The efficacy of a smectite-based mycotoxin binder in reducing aflatoxin B1 toxicity on performance, health and histopathologyof broiler chickens. Toxins. 2021;13:856. doi: 10.3390/toxins13120856. [DOI] [PMC free article] [PubMed] [Google Scholar]