Abstract
This study aimed to investigate the level of lipid and protein oxidation in poultry breasts with severe white striping (WS; striation thickness > 1 mm) and nonaffected meats (N; normal breast) during storage under refrigeration (1°C for 14 d) and freezing (–18°C for 90 d). WS presented higher lipid content, although no difference in protein content was detected, compared to normal broiler breast (N). Regarding oxidative damages, a reduction in malondialdehyde and carbonyl protein, hexanal, octanal and nonanal levels, alongside the interaction of these compounds with other compounds in raw, roasted, and reheated breasts was observed under refrigerated storage (14 d). Freezing storage promotes an increase in carbonyls proteins, hexanal, octanal and nonanal levels at 45 d of storage in poultry meats and subsequent decrease, indicating the evolution of oxidative reactions. Regardless of the type of storage, in general, breasts with WS myopathy have higher levels of lipid and protein oxidation.
Key words: Myopathy, carbonyls protein, malondialdehyde, volatile compound
INTRODUCTION
The world broiler meat production between 2000 and 2020 increased by 104%, from 58.6 million to 119.5 million tons, respectively. Brazilian production increased 2.3 times in the same period, from 6 million to 14 million tons, accounting for ∼11.5% of world production in 2020 (Faostat, 2021). In this context, Brazil has been highlighted as one of the largest world producers and exporters of broiler meat (ABPA, 2020). In the last decade, even more, the poultry industry has been facing the growth of muscle abnormalities related to the development of the Pectoralis major muscle of the fast growing genotypes, which were selected for their productive performances (high growth rate and breast yield). These myopathies are called white striping, wooden breast and spaguet meat, exhibiting distinct phenotypes (Soglia et al., 2021).
The increase in production and consumption of broiler meat was made possible due to the advances in broiler genetic selection and animal feed programs (Al-Dawood and Al-Atiyat, 2022). However, these advances are associated with the occurrence of myopathies in broiler chickens, studies suggest that there is a multifactorial combination of genetic, nutritional, environmental and management variables that affect the development of myopathies (Che et al., 2022). Amongst them, white striping (WS) myopathy is easily recognized by the occurrence of white striations on the surface of the breast, which may lead to a product rejection by the consumers (Carvalho et al., 2021a; Pereira et al., 2022).
Meyer et al. (2023) brings a series of information in its text characterizing this type of myopathy. All the characteristics mentioned and cited below are commonly found in the literature, so they can be considered classic for this type of pectoral abnormality. The effects of WS are noticeable in the breast muscle pectoralis major (PM) by altering histopathological conditions when the breasts are affected mainly by severe myopathy, which leads to the installation of necrosis, lysis of fibers, infiltration of inflammatory cells, gradual replacement of muscles by connective tissue (fibrosis) and deposition of adipose tissue. Additionally, the nutritional and technological value of WS meat decreases compared to N meats
In the literature, there are few studies that compare the quality of broiler breasts affected by WS myopathy during storage under different conditions (refrigeration or freezing). Beltrán and Bellés (2019) reported that the storage of raw broiler meat under refrigeration or freezing temperatures could be considered one of the best conservation techniques since the cold chain is respected. Ali et al. (2015), investigating several cycles of freezing and thawing on the quality of broiler breasts, identified an increased lipid and protein oxidation, alongside reduced color stability and pH. Pereira et al. (2022) worked with the evaluation of chicken breasts affected with WS frozen for 12 mo and analyzed possible changes in their quality, however the analyzed parameters were different from those listed in this study. In any case, they concluded that myopathy significantly affects meat quality both sensorially and physically and chemically.
Therefore, this study is aimed to investigate the oxidation levels of lipids and proteins in broiler breasts affected by WS when stored under refrigeration and freezing conditions.
MATERIALS AND METHODS
Selection of Broiler Breasts
Broiler breasts (Cobb) were collected in a commercial slaughterhouse (Pernambuco, Brazil) under Brazil's Federal Inspection Service regulations. After slaughter and deboning, the broiler breasts (Pectoralis major muscle) were selected and classified into Severe WS (WS; striation thickness > 1 mm) and N (N; absence of WS) based on the visual appearance of the muscle. A total of 30 poultry breasts (15 N and 15 WS) were selected. After classification, the breasts were individually packed in Zip Lock bags and taken to the laboratory under refrigeration (< 4°C).
Following that, the excess fat, bone fragments, and connective tissue were removed from the broiler breasts. Then the chicken breasts (3 N and 3 WS) were analyzed for physicalchemical characterization and oxidation levels (d 0). The remaining broiler breasts were divided as followns: 1) refrigerated storage (1°C): 3 N and 3 WS breasts were stored for 11 d, and 3 N and 3 WS for 14 d, 2) freezing storage (-18°C): 3 N and 3 WS breasts were stored for 45 d, and 3 N and 3 WS were stored for 90 d. Once achieved the storage period for each temperature condition, the broiler breasts were analyzed for oxidative damage.
Physicochemical Characterization
The chemical composition was determined in the WS and N breasts according to AOAC methods for moisture content (no. 950.46.41) and proteins (no. 928.08) (AOAC, 2000). Lipid content was evaluated according to Folch et al. (1957). The pH was determined using a pH meter Model Q400 AS (Quimis Aparelhos Científicos Ltda., Diadema, Brazil) according to method no. 981.12 (AOAC, 2000). The protein and lipid contents were only analyzed on d 0; the other parameters were measured throughout storage.
The color was measured at 2 points of the breast muscle's dorsal surface (inner surface and side of the bone) in 3 different points using the Konica Minolta colorimeter (Chroma Meter CR-400, Minolta Co., Osaka, Japan). Shear force (SF) was determined in the cranial region of raw breast muscles, cut in pieces of 10 × 10 × 30 mm (width, height, and length) with the length parallel to the direction of the fiber. The SF was measured using a Texturometer TA-TXplus (Stable Micro Systems, Godalming, Surrey, UK) with a load cell of 50 kg, equipped with a Warner-Bratzler blade (HDP/WBV) and regulated with a crosshead speed of 100 mm·min−1, the penetration depth of 20 mm and a contact force of 10 g. Shear force was expressed in Newton (N).
Oxidative Damages
The a*/b* ratio was used to establish the proportion between oxymyoglobin (MbO2) and metmyoglobin (MMb) (Olivo et al., 2001). Its determination aims to evaluate the susceptibility of heme group to oxidation. Lipid oxidation was carried out through the spectrophotometric measurement of thiobarbituric acid reactive substances (TBARS) at 532 nm, according to Rosmini et al. (1996), in WS and N breasts (raw and roasted); where the roasted breasts were prepared in an oven (180°C) until they reached the internal temperature of 75°C. To quantify the malondialdehyde (MDA) content, a standard curve of 1,1,3,3-tetramethoxypropane (ranging from 2 × 10−9 to 6 × 10−8 mol) was used. The results were expressed as mg of MDA·kg−1 of meat. The warmed-over flavor (WOF) of WS and N breasts was determined as described by Soares et al. (2004), with modifications. WS and N breasts were roasted and stored at 4°C for 48 h under fluorescent light. Then, they were warmed in a water bath at 85°C for 15 min, cooled at room temperature, and analyzed for lipid oxidation (Rosmini et al., 1996). Protein oxidation was measured according to the dinitrophenylhydrazine (DNPH) method described by Ganhão et al. (2010). A standard bovine serum albumin curve (ranging from 167 to 1500 μg·mL−1) was used to calculate protein concentration. Protein oxidation was expressed in nmoles of carbonyls·mg−1 of proteins.
Hexanal, octanal and nonanal volatiles in raw and roasted WS and N broiler breast samples stored under refrigeration or freezing conditions were quantified by Headspace Solid-Phase Micro-Extraction technique (HS-SPME). A 2 g sample of ground chicken meat was weighed into a 20 mL flask. A 1.0 μL aliquot of the internal standard 1,2-dichlorobenzene in methanol (50 μg·mL−1) was added to the sample before the collection of volatile compounds. An SPME fibre (50/30 μm DVB/CAR/PDMS - Divinylbenzene/Carboxen/Polydimethylsiloxane) was inserted through the septum and left exposed in the upper space of the flask. The SPME fibre was preconditioned for analysis at 270°C for 60 min. The sample remained in equilibrium at 60°C for 5 min and volatile compounds extraction was performed at 60°C for 60 min. After extraction, the SPME fibre was immediately transferred to a 7890B gas chromatograph injector coupled to a mass spectrometer (Agilent Technologies 5977B, Little Falls, DE), for separation and identification of the volatile compounds. The separation was carried out using a VF-5MS capillary column (30 m × 0.25 mm × 0.25 μm) and the following GC/MS analytical conditions were used: initial oven temperature of 40°C for 2 min, then increased to 250°C at 4°C·min−1, remaining at 250°C for 10 min, totalling 64.5 min of running time. The injector temperature was set at 250°C and the GC was operated in the split-less mode. Helium was used as make-up gas at a flow rate of 1.0 mL·min−1. The temperature of the transfer line was 250°C. The mass spectrometer was operated in electron impact mode (70 eV), with a mass scanning range from 35 to 350 m/z at 3.33 scans/s. Identification of hexanal, octanal and nonanal volatiles was performed by analysing the fragmentation patterns displayed in the mass spectra, compared with the mass spectra available from a data library provided by the NIST 2014 equipment (National Institute of Standards & Technology, Gaithersburg, MD), as well as through the linear retention index compared to that of authentic compounds analysed under similar conditions. The linear retention index of each compound was calculated using the retention times of a homologous series of n-alkanes C8 to C20. Hexanal, octanal and nonanal volatiles were semiquantified by extrapolation of the integrated area of the internal standard of 1,2-dichlorobenzene obtained from the chromatograms of total ions (added to each analysis) to the integrated area of each compound, using a response factor of 1. The results were expressed as ng·100 g−1 of chicken breast. Each sample was analysed in triplicate. The methodology followed the procedures reported by Madruga et al. (2010).
Statistical Analysis
The Shapiro-Wilk test was used for testing the normality (α = 0.05). WS and N mean values were compared using Student's t test (P < 0.05). A one-way ANOVA was carried out to evaluate the changes during storage, and post-hoc Tukey's test (P < 0.05) was applied to compare the means. Data were analyzed using XLSTAT (version 2014.5.03, Addinsoft, New York, NY).
RESULTS AND DISCUSSION
Physicochemical Characterization
The WS breasts presented a lipid content of 2.20 g.100−1, which was 1.5x higher than that of N (1.48 g.100−1) (Table 1). According to Petracci et al. (2019), the accumulation of lipids in the Pectoralis major muscle affected by WS causes important modifications; this phenomenon is called lipidosis. The protein content of WS (21.31 g·100−1) was similar to that of N (21.69 g·100−1) (P > 0.005). No significant difference (P > 0.05) in the moisture content between WS and N meats was observed (Table 1). The moisture content of WS and N broiler breasts ranged from 70.59 to 76.2%, matching the values observed in the literature (Baldi et al., 2018; Giampietro-Ganeco et al., 2021, Pereira et al., 2022).
Table 1.
Physical an chemical properties of WS and N broiler breasts.
| Samples |
|||
|---|---|---|---|
| Parameters | N (n = 3) | WS (n = 3) | P-value |
| Chemical properties | |||
| Moisture¹ | 75.37 ± 0.61 | 75.88 ± 1.67 | 0.6625 |
| Protein¹ | 21.59 ± 0.51 | 21.38 ± 0.97 | 0.7701 |
| Lipid¹ | 1.48 ± 0.08b | 2.20 ± 0.02a | 0.0003 |
| Physical properties | |||
| pH | 5.95 ± 0.02 | 5.88 ± 0.04 | 0.0813 |
| Shear force² | 18.59 ± 0.36 | 18.63 ± 0.44 | 0.9339 |
| L* | 60.05 ± 1.11 | 60.90 ± 2.13 | 0.5723 |
| a* | 2.09 ± 0.08 | 1.92 ± 0.18 | 0.1857 |
| b* | 4.29 ± 1.25a | 7.93 ± 0.22b | 0.0076 |
Abbreviations: N, Normal breast meat.WS, White stripping breast meat.
L*: color parameter that indicates lightness.
a*: color parameter that indicates redness.
b*: color parameter that indicates yellowness.
Different lowercase letters in the same row indicates significant differences between the samples by Student t test (P < 0.05).
Results expressed as g.100g-1.
Result expressed as N (Newton).
In this study, the WS and N broiler breasts did not differ (P > 0.05) in relation to pH (Table 1). There is no unanimity on the difference in pH between WS and N broiler chicken breasts in the literature. Lee at al. (2021) observed that, regardless of the degree of WS, the pH of the broiler breast did not present a significant difference compared to N broiler breast. This result differed from Petracci et al. (2013), who observed that the pH of broiler breasts affected by WS disorder is usually several decimal cases (from 0.2 to 0.4) higher than N ones.
The shear force for WS and N showed no significant difference (P > 0.05) (Table 1). Studies have reported that the texture properties of broiler breasts are not overly influenced by the presence of WS myopathy (Tasoniero et al., 2016; Baldi et al., 2018; Petracci et al., 2019). In addition, some authors found nonsignificant effects of the WS disorder on broiler meat texture (Kuttappan et al., 2012). On the other hand, several studies reported significant differences only when the most severe degrees of WS were evaluated (Giampietro-Ganeco et al., 2021; Mello et al., 2021).
Regarding the color parameters of the breasts (Table 1), no significant difference (P > 0.05) was observed in the lightness (L*) and redness (a*) between WS and N breasts. In studies comparing WS and N breasts, Petracci et al. (2013), Trocino et al. (2015), and Giampietro-Ganeco et al. (2021), although not use L* as a discriminating factor to assess the presence of WS, reported increased redness (a*) in WS samples compared to N. For color coordinate b*, higher intensity was observed in WS (7.93) when compared to N (4.29). Corroborating with these results, Petracci et al. (2013), Kuttappan et al. (2017), Carvalho et al. (2021b) observed that the breasts affected by WS were more yellowish in comparison to nonaffected meat. Pereira et al. (2022) L* values obtained that WS samples are lighter than the N samples, however freezing affects them in such a way that these values decrease significantly over the 12 mo of freezing, falling from 63.5 to 56.7, being accompanied by the values a*. For the values b* an inversion occurs, that is, they increase significantly during freezing, agreeing with what was obtained in this study
Oxidative Damages
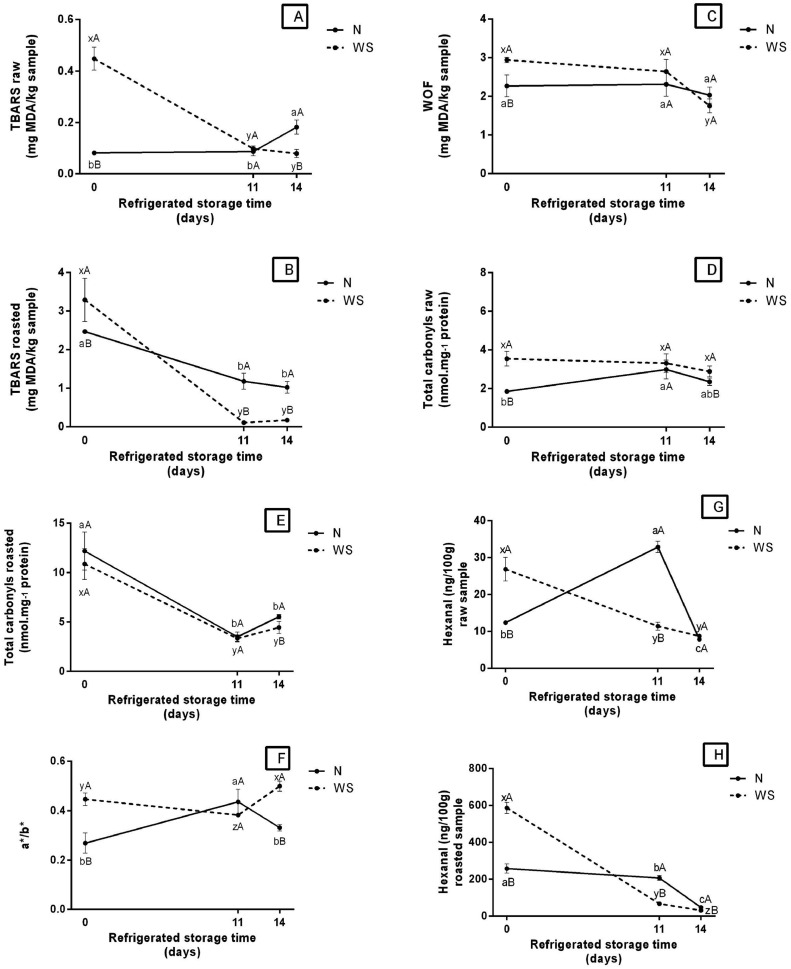
Lipid oxidation levels decreased in WS raw (Figure 1A, Supplementary Table A), roasted (Figure 1B, Supplementary Table A), and reheated (Figure 1C, Supplementary Table A) breasts under refrigerated storage. For N, a slight increase was observed on 14 d for raw breasts, and a reduction after 11 d was noticed for roasted breasts. In the refrigerated storage period, a reduction in MDAformation was observed in the first 11 d, from 0.45 to 0.1 mg MDA·kg−¹ for WS raw breasts and from 3.3 to ∼0 mg MDA·kg−¹ for WS roasted breasts. For the reheated WS, the lipid oxidation reduction occurred after 14 d, reaching levels close to 1.8 mg MDA·kg−1. It was possible to observe differences in lipid oxidation between the WS and N, regardless of the preparation condition (raw, roasted, or reheated). It is worth mentioning that on d 0, the WS samples presented a higher level of lipid oxidation compared to N breasts. However, it is also important to highlight that although the level of lipid oxidation of WS is higher at the beginning of storage, on d 14 for raw breasts and d 11 and 14 for roasted breasts, this effect was contrary, in which WS presented a lower level of lipid oxidation (Figures 1A and 1B, Supplementary Table A) compared to N.
Figure 1.
Oxidative stress in WS and N broiler breasts during refrigerated storage for 11 and 14 d. Abbreviations: N, Normal breast meat; WS, White stripping breast meat.
a,b,c Different lowercase letters in —N— indicate significant differences over storage time by Tukey test (P < 0.05).
x,y,z Different lowercase letters in —WS— indicate significant differences over storage time by Tukey test (P < 0.05).
A,B Different uppercase letters indicate significant differences between N and WS over the storage time by Student t test (P < 0.05).
The differences between WS and N corroborate the findings of Salles et al. (2019) and Carvalho et al. (2021a)., who reported higher initial levels of MDA for WS compared to N. In addition, these authors observed that WS myopathy affected endogenous antioxidant defense (i.e., impaired activity of catalase, glutathione peroxidade and superoxide dismutase enzymes). During the refrigerated storage, WS raw and roasted samples, except on d 0, presented values for lipid oxidation below 0.5 mg MDA·kg−¹. According to Abeyrathne et al. (2021), values above that are considered critical since they indicate a high level of lipid oxidation, producing a rancid flavor. Other authors also reported that TBARS values between 1 and 2 mg MDA·kg−1 are in the sensory detected range (Baldi et al., 2020; Carvalho et al., 2021a,b; Pereira et al., 2022). According to Papastergiadis et al. (2012), the reduction of MDA formation during storage may be associated with the occurrence of other reactions among secondary lipid oxidation products or the formation of other oxidation products that have not been detected (other than MDA compound) as well as the reaction of MDA with other compounds. Salles et al. (2019) observed higher lipid oxidation in breasts affected by severe WS disorder. In addition, they verified higher formation of free radicals in breasts with moderate to severe myopathy. Leite et al. (2020) observed in WS an increase in susceptibility to lipid oxidation after cooking, detected from the increase in the warmed-over flavor and suggested that this increase may occur due to the higher content of polyunsaturated fatty acids, compromising its quality due to the formation of unpleasant odors detected by consumers.
Regarding carbonylation level, WS meats only presented differences in protein oxidation compared to N breasts on d 0 for raw breasts (higher level compared to N) (Figure 1D, Supplementary Table A) and on d 14 for roasted breasts (lower level compared to N) (Figure 1E, Supplementary Table A). Higher protein oxidation levels in WS meat were also observed by Carvalho et al. (2021a), who reported an increase in the concentrations of allysine and Schiff base, which are protein oxidation products. These authors also highlight an impairment in the proteasome of WS muscles, whose upregulated proteins RNH1 and kelch-like protein famil member 41 are directly related to the occurrence of oxidative stress and accumulation of oxidized proteins. In addition, Salles et al. (2019) verified higher advanced oxidation protein products levels in breasts with moderate to severe myopathy. Evaluating the effect of storage under refrigeration on WS and N, significant differences were observed between the roasted samples on d 0, 11, and 14. Regarding carbonylation level for raw breasts, no abrupt changes occurred as it was observed for roasted breasts from d 0 to d 11, showing a high decreasing rate varying from 11.0 to approximately 3.00 nmol·mg−1 protein for both WS and N.
The oxidation data observed and those described in the literature, it was found that lipid and protein oxidation is closely associated with deterioration processes that can affect all quality characteristics of meat and meat products (Nawaz and Zhang, 2021, Nawas et al., 2022). Moreover, a correlation between the levels of oxidative indicators for lipids (TBARS) and those for proteins (carbonyl groups) was observed. This fact can be noticed when comparing the data for oxidative rancidity and protein denaturation of WS breasts after 11 d of refrigerated storage, showing a simultaneous reduction in MDA and carbonyls levels (Ganhão et al., 2010; Ferreira et al., 2016; Rocha et al., 2020; Carvalho et al., 2021b).
The a*/b* ratio (Figure 1F, Supplementary Table A) in WB sample showed an decrease at 11 d, followed by a increase at 14 d. The behavior of N sample was the opposite, showing a increase in the intermediate time and an decrease in the final time of storage under refrigeration. The reduction in the a*/b* value may be indicative of greater meat discoloration or lipid oxidation.
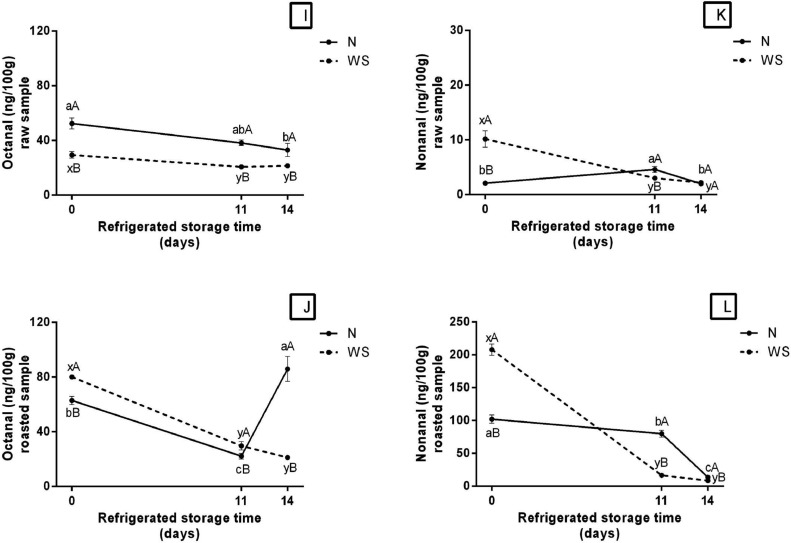
Hexanal concentration of raw and cooked meat stored under refrigeration are presented in Figures 1G and 1H (Supplementary Table A), respectively. At time 0, the WS meat had a higher concentration of hexanal compared to sample N. On the other hand, at times 11 and 14 the opposite occurred. This fact has already been described in publication by Shahidi and Hossain (2022) who describe the presence of hexanal in roasting, cooking and frozen store chicken meat. In this study, it was observed in both raw and cooked meats hexanal presence. The maximun reduction of hexanal values for raw WS meat was observed at time 14, whose value reduced about 70% in relation to its initial concentration (26.90 ng/100 g). Similar result was observed for roasted WS. Whose WS value reduced from 586.93 to 31.35 ng.100−1, and N reduced from 258.29 to 47.65 ng.100−1, throughout storage.
The octanal concentration of WS and N raw meats showed a slight decline during refrigerated storage (Figure 1I, Supplementary Table A). The concentration of this volatile in raw N remained higher compared to WS (almost double), during the 14 d of storage. As for roasted WS meat (Figure 1J, Supplementary Table A), the decline in octanal concentration during refrigerated storage was more intense than in raw meat (around 73%), whose concentration in the initial time was 80.12 ng.100−1 and in the final time of 21.26 ng.100−1. While roasted N meat showed a reduction in octanal concentration in time 11 d (22.20 ng.100−1) compared to the initial time (62.95 ng.100−1), followed by an increase in time 14 d (85.99 ng.100−1).
The initial nonanal concentration was 5 times higher in raw WS meat (10.18 ng.100−1) thas in N (2.11 ng.100−1) (Figure 1K, Supplementary Table A). In the case of roasted meat, these values were 208.03 and 102.27, respectively (Figure 1L, Supplementary Table A), that is, roasted WS presented twice the initial concentration of nonanal compared to N. During storage under refrigeration, the concentration of nonanal in raw WS reduced by about 80%. Meanwhile, raw N meat at 11 d (4.62 ng.100−1) showed an increase in the nonanal content compared to its initial concentration (Figure 1K, Supplementary Table A). In addition, roasted WS showed a significant reduction in nonanal during storage, reaching a lower concentration (8.61 ng.100−1) at time 14 d, a reduction approximately 95%. A similar behavior was observed in roasted N, however with a reduction of 87% of nonanal concentration (14 d) compared to the initial time (Figure 1L, Supplementary Table A).
Between the most important volatile compounds of cooked poultry meat include hexanal and nonenal that during oxidation of poultry fat, are formed from linoleic acids that are typical of the chicken meat flavor (Kosowska et al., 2017). Hexanal is the most abundant secondary lipid oxidation product, being the main oxidation product of omega-6 fatty acids, such as linoleic, linolenic and arachodonic acids (Shahidi, 2001). The reduction of hexanal concentration in raw and roasted WS meat during refrigerated storage (Figures 1G and 1H, Supplementary Table A) coincides with the reduction of TBARS levels (Figures 1A and 1B, Supplementary Table A), which is also a secondary product of lipid oxidation. This reduction may be indicative of strongly advanced lipid oxidation. During meat storage, the level of hexanal decreases markedly, due to its interaction with other products (Shahidi and Pegg, 1994). According to Grebenteuch et al. (2021), hexanal decresed indicates subsequent degradation reaction leading to tertiary products formation. In addition, the increase of free radicals, less sensitive fatty acids, such as oleic acid, are degraded resulting in the production of pentanal, octanal and nonanal (Wang et al., 2022). Moderate and severe WS meat has a higher content of oleic, linoleic and linolenic fatty acids compared to N (Adabi and Soncu, 2019), which may explain the higher levels of hexanal and nonanal in WS meats (initial time) observed in the present study.
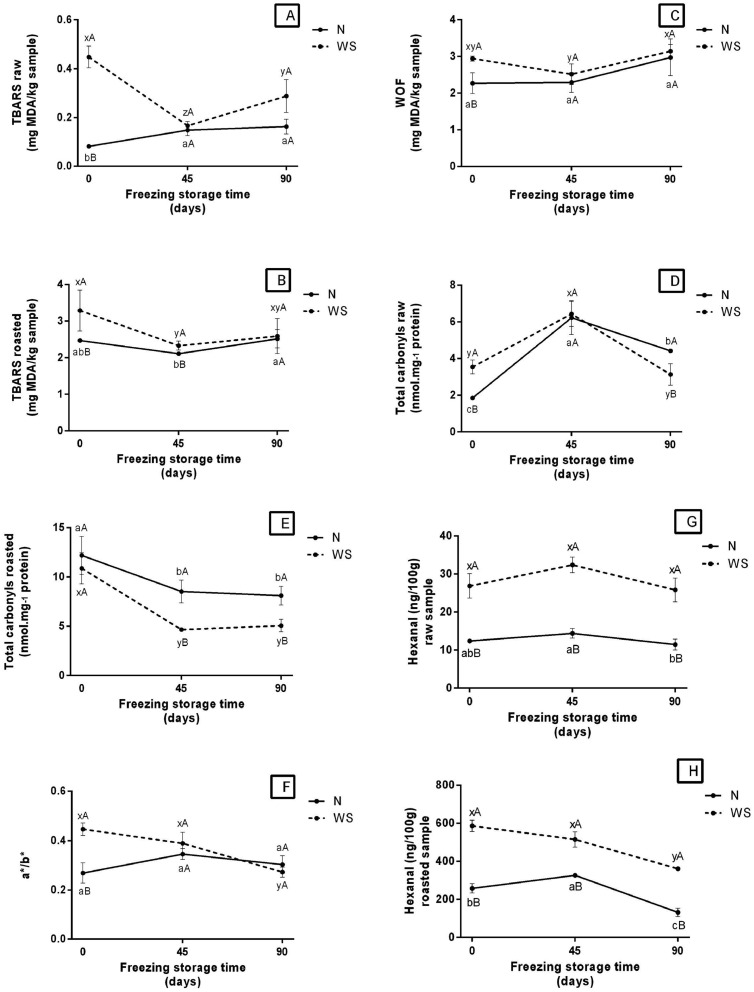
During the storage period under freezing, the levels of lipid oxidation in WS raw (Figure 2A, Supplementary Table B), roasted (Figure 2B, Supplementary Table B), and reheated (Figure 2C, Supplementary Table B) samples showed a significant reduction after 45 d. In this storage period, a downward slope for MDA formation was observed, decreasing from 0.45 to 0.18 mg MDA·kg−¹ for WS raw breasts; from 3.4 to 2.5 mg MDA·kg−¹ for WS roasted, and from 3.0 to 2.5 mg MDA·kg−¹ for WS reheated breasts. WS raw breasts (Figure 2D, Supplementary Table B) obtained significantly higher levels of protein carbonyls compared to N raw (P < 0.05) on d 0, and lower on d 90. For both affected and unaffected raw meat, the peak of protein oxidation was observed at 45 d. While evaluating the level of carbonylation in the roasted samples (Figure 2E, Supplementary Table B), it was observed that there were significant differences (P < 0.05) between WS and N samples after 45 d, where WS samples presented lower levels compared to N. In addition, it was observed that freezing had an effect on both WS and N broiler breasts after 45 d, reducing carbonyl levels and hence protein oxidation. The a*/b* ratio in the WS meat stored under freezing (Figure 2F, Supplementary Table B), showed a decrease in the last storage time, while the N meat in the same period showed a constant value. This may indicate a greater susceptibility to lipid oxidation of WS meat, at 90 d.
Figure 2.
Oxidative stress in WS and N broiler breasts during frozen storage for 45 and 90 d. Abbreviations: N, Normal breast meat; WS, White stripping breast meat.
a,b,c Different lowercase letters in —N— indicate significant differences over storage time by Tukey test (P < 0.05).
x,y,z Different lowercase letters in —WS— indicate significant differences over storage time by Tukey test (P < 0.05).
A,B Different uppercase letters indicate significant differences between N and WS over the storage time by Student t test (P < 0.05).
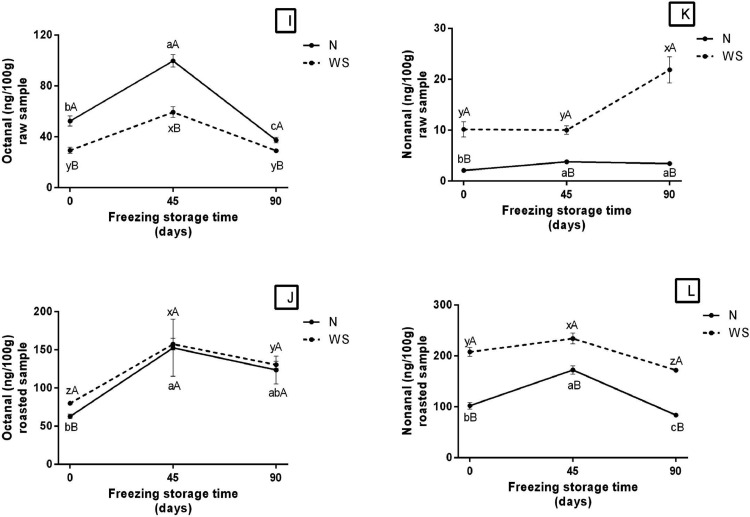
Hexanal concetration in both raw and cooked WS samples (Figures 2G and 2H, respectively, Supplementary Table B) was higher than in N samples, during frozen storage. In addition, in raw WS meat the concentration was kept stable (around 28 ng.100−1), while in the cooked WS ones there was a decrease during storage (586.93–361.43 ng.100−1). The concentration of octanal aldehyde was lower in raw meat WS compared to N, throughout the entire frozen storage (Figure 2I, Supplementary Table B). Furthermore, both WS and N showed a significant increase in concentration at 45 d of storage. Roasted WS meat showed a higher octanal concentration than N meat, only in the initial time, not directing each other throughout storage (Figure 2J, Supplementary Table B). It is also possible to observe a significant increase in the concentration of this aldehyde in both samples at 45 d, followed by a decrease at 90 d. In relation to nonanal, raw WS meat showed an increase in concentration at 90 d of storage (Figure 2K, Supplementary Table B). While roasted WS showed an increase in time 45 d, followed by a decrease in time 90 d (the same was observed in meat N) (Figure 2L, Supplementary Table B). In both raw and cooked meat, the concentration of nonanal remained always higher in the affected meats. It is also important to highlight that roasted meats have higher concentrations of hexanal, octanal and nonanal compared to raw meat.
In general, roasted and reheated N and WB stored under freezing showed an increase and/or stagnation in MDA levels at the final storage time, while samples stored under refrigeration showed a marked reduction in MDA levels. Additionally, it can be assumed that freezing did not have the same preservation effect when compared to refrigeration; and this result can be associated with disruption of muscle cells caused by ice crystals that may have exposed the intracellular material, which contributes to oxidative reactions (Ganhão et al., 2010; Leygonie et al., 2012; Leite et al., 2020; Carvalho et al., 2021a). Therefore, these above-mentioned results explain the warmed-over flavor characteristic of poultry meat, which can cause rejection by the consumer and significant economic losses to the meat industry (Wood et al., 2008; Ferreira et al., 2016; Rocha et al., 2020; Lee et al., 2021). The increase in the concentration of aldehydes during frozen storage, mainly at 45 d, may indicate an increase in oxidative reactions. Added to this, the decrease in hexanal, octanal and nonanal, mainly at 90 d, may indicate the oxidation of these compounds, or their interaction with other compounds produced by oxidative reactions (Yang et al., 2021; Grebenteuch et al., 2021).
CONCLUSIONS
The analysis of oxidative damage revealed a reduction in the levels of malondialdehyde, carbonyls, hexanal, octanal and nonanal, possibly interconnected, and the interaction of these compounds with other compounds of raw, roasted breasts stored under refrigerated conditions. In general, freezing storage promotes an increase in carbonyls proteins, hexanal, octanal nonanal levels at 45 d of storage in poultry meats, and these tend to decrease with advancing storage, indicating a gradual increase in the level of oxidation, possibly culminating in the formation of other compounds from these. In addition, lipid oxidation of cooked meats was more pronounced than that of raw meat. Although both affected and nonaffected meats are penalized by the increase in oxidation levels in both storage processes, in meats with WS myopathy this effect is more pronounced.mmc1.docx
ACKNOWLEDGMENTS
The authors are thankful to the Federal University of Paraíba (project number: PIF 13530-2020 and PVF 14858-2021), and the Federal Institute of Pernambuco for supporting this research. CNPq for granting a Productivity Scholarship in Technological Extension Level II to Dr MRP.
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.102826.
Appendix. Supplementary materials
REFERENCES
- Abeyrathne E.D.N.S., Nam K., Ahn D.U. Analytical methods for lipid oxidation and antioxidant capacity in food systems. Antioxidants (Basel) 2021;10:1587. doi: 10.3390/antiox10101587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ABPA - Associação Brasileira de Proteína Animal. 2020. Relatório Anual da Associação Brasileira de Proteína Animal. 160. Accessed Feb. 2021. https://abpa-br.org/wp-content/uploads/2020/05/abpa_relatorio_anual_2020_portugues_web.pdf
- Adabi S.G., Soncu E.D. White striping prevalence and its effect on meat quality of broiler breast fillets under commercial conditions. J. Anim. Physiol. Anim. Nutr. (Berl.) 2019;103:1060–1069. doi: 10.1111/jpn.13092. [DOI] [PubMed] [Google Scholar]
- Ali S., Zhang W., Rajput N., Khan M.A., Li C.B., Zhou G.H. Effect of multiple freeze-thaw cycles on the quality of chicken breast meat. Food Chem. 2015;173:808–814. doi: 10.1016/j.foodchem.2014.09.095. [DOI] [PubMed] [Google Scholar]
- Al-Dawood A., Al-Atiyat R. A comparative study on growth parameters of three broiler chicken strains from Jordan. Braz. J. Poult. Sci. 2022;24:1–8. [Google Scholar]
- AOAC - Association of Official Analytical Chemists. 2000. Official Methods of Analysis the of AOAC International. 17th ed. Washington, DC.
- Baldi G., Soglia F., Mazzoni M., Sirri F., Canonico L., Babini E., Laghi L., Cavani C., Petracci M. Implications of white striping and spaghetti meat abnormalities on meat quality and histological features in broilers. Animal. 2018;12:164–173. doi: 10.1017/S1751731117001069. [DOI] [PubMed] [Google Scholar]
- Baldi G., Soglia F., Petracci M. Current status of poultry meat abnormalities. Meat Muscle Biol. 2020;4:1–7. [Google Scholar]
- Beltrán J.A., Bellés M. In: Pages 493-497 in Encyclopedia of Food Security and Sustainability. Ferranti P., Berry E.M., Anderson J.R., editors. Elsevier; Cambridge, MA: 2019. Effect of freezing on the quality of meat. [Google Scholar]
- Carvalho L.M., Delgado J., Madruga M.S., Estévez M. Pinpointing oxidative stress behind the white striping myopathy: depletion of antioxidant defenses, accretion of oxidised proteins and impaired proteostasis. J. Sci. Food Agric. 2021;101:1364–1371. doi: 10.1002/jsfa.10747. [DOI] [PubMed] [Google Scholar]
- Carvalho L.T., Owens C.M., Giampietro-Ganeco A., Malagoli de Mello J.L., Ferrari F.B., de Carvalho F.A.L., Alves de Souza R., Amoroso L., Alves de Souza P., Borba H., Trindade M.A. Quality of turkeys breast meat affected by white striping myopathy. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha T.C., Carvalho L.M., Soares A.J., Coutinho D.G., Olegario L.S., de Sousa Galvão M., Estévez M., Madruga M.S. Impact of chicken wooden breast on quality and oxidative stability of raw and cooked sausages subjected to frozen storage. J. Sci. Food Agric. 2020;100:2630–2637. doi: 10.1002/jsfa.10292. [DOI] [PubMed] [Google Scholar]
- Che S., Wang C., Varga C., Barbut S., Susta L. Prevalence of breast muscle myopathies (spaghetti meat, woody breast, white striping) and associated risk factors in broiler chickens from Ontario Canada. PLoS ONE. 2022;17 doi: 10.1371/journal.pone.0267019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAOSTAT. 2021. New food balances. FAOSTAT. Available via FAO. Accessed Dec. 2022. https://www.fao.org/faostat
- Folch J., Less M., Stanley S.G.H.A. Simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Ferreira V.C.S., Morcuende D., Madruga M.S., Hernández-López S.H., Silva F.A.P., Ventanas S., Estévez M. Effect of pre-cooking methods on the chemical and sensory deterioration of ready-to-eat chicken patties during chilled storage and microwave reheating. J. Food Sci. Technol. 2016;53:2760–2769. doi: 10.1007/s13197-016-2248-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganhão R., Morcuende D., Estévez M. Protein oxidation in emulsified cooked burger patties with added fruit extracts: influence on colour and texture deterioration during chill storage. Meat Sci. 2010;85:402–409. doi: 10.1016/j.meatsci.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Giampietro-Ganeco A., Owens C.M., Borba H., de Mello J.L.M., de Souza R.A., Ferrari F.B., Cavalcanti E.N., de Oliveira R.F., Carvalho L.T., Sun X., Trindade M.A. Impact of deep pectoral myopathy on chemical composition and quality parameters of chicken breast fillet. Poult. Sci. 2021;100:1–7. doi: 10.1016/j.psj.2021.101377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebenteuch S., Kroh L.W., Drusch S., Rohn S. Formation of secondary and tertiary volatile compounds resulting from the lipid oxidation of rapeseed oil. Foods. 2021;10:2417. doi: 10.3390/foods10102417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosowska M.A., Majcher M., Fortuna T. Volatile compounds in meat and meat products. Food Sci. Technol. 2017;37:1–7. [Google Scholar]
- Kuttappan V.A., Brewer V.B., Apple J.K., Waldroup P.W., Owens C.M. Influence of growth rate on the occurrence of white striping in broiler breast fillets. Poult. Sci. 2012;91:2677–2685. doi: 10.3382/ps.2012-02259. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Owens C.M., Coon C., Hargis B.M., Vazquez-Anon M. Incidence of broiler breast myopathies at 2 different ages and its impact on selected raw meat quality parameters. Poult. Sci. 2017;96:3005–3009. doi: 10.3382/ps/pex072. [DOI] [PubMed] [Google Scholar]
- Leite N., Pedrão M.R., Kato T., Inoue J.N., Hasunuma I.L.W., Dias L.F., Souza R.B., Coró F.A.G. Poultry breasts with white striping meat x impacts on technological properties. Res. Soc. Dev. 2020;9 [Google Scholar]
- Lee B., Park C.H., Kong C., Kim Y.S., Choi Y.M. Muscle fiber and fresh meat characteristics of white-striping chicken breasts, and its effects on palatability of sous-vide cooked meat. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leygonie C., Britz T.J., Hoffman L.C. Impact of freezing and thawing on the quality of meat. Meat Sci. 2012;91:93–98. doi: 10.1016/j.meatsci.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Madruga M.S., Elmore J.S., Oruna-Concha M.J., Balagiannis D., Mottran D.S. Determination of some water-soluble aroma precursors in goat meat and their enrolment on flavour profile of goat meat. Food Chem. 2010;123:513–520. [Google Scholar]
- Mello J.L.M., Souza R.A., Ferrari F.B., Cavalcanti E.N.F., Oliveira R.F., Fidelis H.A., Pereira M.R., Villegas-Cayllahua E.A., Giampietro-Ganeco A., Dutra D.R., Souza P.A., Borba And.H. Quality of breast meat from broiler chickens raised in Brazil affected by white striping myopathy. Res. Soc. Dev. 2021;10 [Google Scholar]
- Meyer M.M., Johnson A.K., Bobeck E.A. Breast muscle white striping and serum corticosterone reduced in broilers exposed to laser environmental enrichment. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz A., Irshad S., Khan I.A., Khalifa I., Walayat N., Aadil R.M., Kumar M., Wang M., Chen F., Cheng K.-W., Lorenzo J.M. Protein oxidation in muscle-based products: effects on physicochemical properties, quality concerns, and challenges to food industry. Food Res. Int. 2022;157 doi: 10.1016/j.foodres.2022.111322. [DOI] [PubMed] [Google Scholar]
- Nawaz A.H., Zhang L. Oxidative stress in broiler chicken and its consequences on meat quality. Int. J. Life Sci. 2021;1:45–54. [Google Scholar]
- Olivo R., Soares A.L., Ida E.I., Shimokomaki M. Dietary vitamin E inhibits poultry PSE and improves meat functional properties. J. Food Biochem. 2001;25:271–283. [Google Scholar]
- Papastergiadis A.E.Mubiru, Langenhove H.V., Meulenaer B. Malondialdehyde measurement in oxidized foods: Evaluation of the spectrophotometric thiobarbituric acid reactive substances (TBARS) test in various foods. J. Agric. Food Chem. 2012;60:9589–9594. doi: 10.1021/jf302451c. [DOI] [PubMed] [Google Scholar]
- Pereira M.R., Mello J.L.M., Oliveira R.F., Villegas-Cayllahua E.A., Cavalcanti E.N.F., Fidelis H.A., Ferrari F.B., Giampietro-Ganeco A., Souza P.A., Borba H. Effect of freezing on the quality of breast meat from broilers affected by White Striping myopathy. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracci M., Mudalal S., Bonfiglio A., Cavani C. Occurrence of white striping under commercial conditions and its impact on breast meat quality in broiler chickens. Poult. Sci. 2013;92:1670–1675. doi: 10.3382/ps.2012-03001. [DOI] [PubMed] [Google Scholar]
- Petracci M., Soglia F., Madruga M., Carvalho L., Ida E., Estévez M. Wooden-breast, white striping, and spaghetti meat: causes, consequences and consumer perception of emerging broiler meat abnormalities. Compr. Rev. Food Sci. Food Saf. 2019;18:565–583. doi: 10.1111/1541-4337.12431. [DOI] [PubMed] [Google Scholar]
- Rosmini M.R., Perlo F., Pérez-Alvarez J.A., Pagán-Moreno M.J., Gago-Gago A., López-Santovena F., Aranda-Catalá V. TBA test by an extractive method applied to ‘paté. Meat Sci. 1996;42:103–110. doi: 10.1016/0309-1740(95)00010-0. [DOI] [PubMed] [Google Scholar]
- Salles G.B., Boiago M.M., Silva A.D., Morsch V.M., Gris A., Mendes R.E., Baldissera M.D., Silva A.S. Lipid peroxidation and protein oxidation in broiler breast fillets with white striping myopathy. J. Food Biochem. 2019;43:e12792. doi: 10.1111/jfbc.12792. [DOI] [PubMed] [Google Scholar]
- Shahidi, F., and Pegg, R.B. 1994. Hexanal as an Indicator of the flavor deterioration of meat and meat products. Pages 256-279 in Lipids in Food Flavors. C.-T. Ho, and T.G. Hartman, ed. American Chemical Society (ACS) Publication: Washington DC, 558.
- Shahid, F. 2001. Headspace volatile aldehydes as indicators of lipid oxidation in foods. In: Rouseff, R.L., and Cadwallader, K.R. (eds.), Headspace Analysis of Foods and Flavors: Theory and Practice. Springer: New York, NY, 488:113-123. [DOI] [PubMed]
- Shahidi F., Hossain A. Role of lipids in food flavor generation. Molecules. 2022;27:5014. doi: 10.3390/molecules27155014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares A.L., Olivo R., Shimokomaki M., Ida E.I. Synergism between dietary vitamin E and exogenous phytic acid in prevention of warmed-over flavour development in chicken breast meat, Pectoralis major M. Braz. Arch. Biol. Technol. 2004;47:57–62. [Google Scholar]
- Soglia F., Petracci M., Davoli R., Zappaterra M. A critical review of the mechanisms involved in the occurrence of growth-related abnormalities affecting broiler chicken breast muscles. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasoniero G., Cullere M., Cecchinato M., Puolanne E., Dalle Zotte A. Technological quality, mineral profile, and sensory attributes of broiler chicken breasts affected by White Striping and Wooden Breast myopathies. Poult. Sci. 2016;95:2707–2714. doi: 10.3382/ps/pew215. [DOI] [PubMed] [Google Scholar]
- Trocino A., Piccirillo A., Birolo M., Radaelli G., Bertotto D., Filiou E., Petracci M., Xiccato G. Effect of genotype, gender and feed restriction on restriction on growth, meat quality and the occurrence of white striping and wooden breast in broiler chickens. Poult. Sci. 2015;94:2996–3004. doi: 10.3382/ps/pev296. [DOI] [PubMed] [Google Scholar]
- Yang P., Douthwaite M., Pan J., Zheng L., Hong S., Morgan D.J., Gao M., Li D., Feng J., Hutchings G.J. Coordinately unsaturated O2c–Ti5c–O2c sites promote the reactivity of Pt/TiO2 catalysts in the solvent-free oxidation of n-octanol. Catal. Sci. Technol. 2021;11:4898. [Google Scholar]
- Wang Y., Wang H., Wu Y., Xiang H., Zhao Y., Chen S., Qi B., Li L. Insights into lipid oxidation and free fatty acid profiles to the development of volatile organic compounds in traditional fermented golden pomfret based on multivariate analysis. LWT. 2022;171 [Google Scholar]
- Wood J.D., Enser M., Fisher A.V., Nute G.R., Sheard P.R., Richardson R.I., Hughes S.I., Whittington F.M. Fat deposition, fatty acid composition and meat quality: a review. Meat Sci. 2008;78:343–358. doi: 10.1016/j.meatsci.2007.07.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.