Abstract
The broiler industry frequently encounters 2 common problems: excessive deposition of abdominal fat and poor quality of meat. However, there are limited nutritional manipulation strategies to address these issues. While Anoectochilus roxburghii (Wall.) Lindl., a traditional Chinese herb, has been shown to have multiple beneficial effects in humans, its potential roles in broiler chickens remain unexplored. In this study, the effects of dietary supplementation with Anoectochilus roxburghii extract (ARE) on growth performance, abdominal fat deposition, meat quality, blood indices, and gut microbiota were investigated in yellow-feather broiler chickens. A total of 90 twenty-one-day-old yellow-feather broilers were randomly divided into 3 treatments, and each treatment included 5 replicates with 6 birds per replicate. Birds were fed a basal diet supplemented with 0, 0.15, or 0.30% ARE for 6 wk. The results showed that the inclusion of ARE in the diet did not have any significant effect on meat yield (P > 0.05). However, it did lead to a reduction in abdominal fat deposition and an improvement in meat quality (P < 0.05). Mechanistically, the addition of ARE inhibited lipid biosynthesis and enhanced lipid breakdown in both the liver and adipose tissue of the broilers. Furthermore, ARE supplementation increased the antioxidase activities in the muscle and serum of the broilers (P < 0.05). In addition, the supplementation of ARE optimized the diversity and composition of the cecal microbiota, particularly by lowering the ratio of Firmicutes to Bacteroidetes (P < 0.05). Moreover, the abundance of some bacteria that were positively correlated with abdominal fat deposition was reduced by ARE, and vice versa (P < 0.05). Collectively, the results suggest that ARE is a promising candidate as a feed additive for reducing abdominal fat deposition and improving meat quality in the broiler industry.
Key words: broiler chicken, fat deposition, meat quality, gut microbiota, Anoectochilus roxburghii extract
INTRODUCTION
The modern broiler industry plays a crucial role in providing animal protein for human growth and development worldwide (Wen et al., 2019). To meet the demand for the market, the industry employs energy-dense feeds and intensive genetic selection to increase carcass yield (Brito et al., 2021). These practices have led to significant improvements in economic traits such as body weight gain, feed efficiency, and breast yield (Leenstra and Cahaner, 1992; Kerr et al., 1999). However, these improvements have also caused undesirable consequences, including the excessive accumulation of abdominal fat and poor meat quality (Siegel, 2014; Sell-Kubiak et al., 2017).
Generally, excessive fat deposition in chickens is mainly caused by high dietary consumption of carbohydrates. Carbohydrates provide energy for efficient muscle growth and facilitate lipid biosynthesis (Nematbakhsh et al., 2021). In chickens, the liver is the primary site for lipid metabolism, with more than 90% of de novo fatty acids synthesized there (Ameer et al., 2014). When lipid synthesis exceeds lipid oxidation in the liver, the excess lipids are packaged into very low-density lipoproteins (VLDL) and secreted into the bloodstream, where they are taken up by fat tissues, primarily in the abdominal cavity and the ovary (Ameer et al., 2014; Alvares et al., 2019). This chronic positive energy balance ultimately leads to overdeposition of abdominal fat, which negatively impacts feed efficiency, meat production cost, and consumer health (Siegel, 2014). Therefore, it is urgent to develop strategies to address this problem.
In contrast, poor meat quality is believed to result from genetic selection for the efficient production of leaner meat (Sandercock et al., 2009). This selection has not only reduced fat levels in the whole body but also decreased intramuscular fat (IMF) levels (Hocquette et al., 2010). As IMF levels are a key factor affecting meat quality, meat with low IMF levels becomes tougher, less moist, and less flavorful (Dunshea et al., 2005). Poor meat quality adversely affects consumer preference and consumption of broiler meat. Therefore, it is imperative to increase muscle mass while concurrently improving meat quality.
Nutritional strategies have proven to be effective in regulating lipid metabolism and improving meat quality in birds. For instance, we previously found that dietary supplementation with 0.05 or 0.10% β-hydroxy-β-methylbutyrate (HMB), a derivative of leucine, significantly reduced abdominal fat deposition in layer chickens (Liao et al., 2022). Additionally, lycopene (Wan et al., 2021) and propionate (Li et al., 2021) have been reported to lower lipid levels in broiler chickens. These feed additives may reduce fat deposition by regulating hepatic lipid metabolism and modulating gut microbiota function. Furthermore, although the underlying mechanism is unclear, acidifiers (Gao et al., 2021), aluminosilicates (Banaszak et al., 2021), and conjugated linoleic acid (Du et al., 2000) have been found to positively regulate meat quality. Despite some progress, it is still uncommon for a single feed additive to reduce fat deposition while simultaneously enhancing meat quality.
Due to the diverse chemical composition, traditional Chinese medicine is increasingly being considered a treasure for exploring new feed additives (Hu et al., 2019; Zhang et al., 2021). Among them, Anoectochilus roxburghii (Wall.) Lindl., a traditional Chinese medicinal herb, has been found to contain many bioactive chemicals, such as polysaccharides, flavonoids, glycosides, organic acids, volatile compounds, steroids, triterpenes, alkaloids, and nucleosides (Ye et al., 2017). As a result, Anoectochilus roxburghii extract (ARE) has been shown to have multiple beneficial effects, such as antidiabetic, antilipemic, anti-inflammatory, antiviral, liver-protective, renal-protective, immunomodulatory, abirritant, sedative, and antineoplastic effects (Ye et al., 2017; Tian et al., 2022). However, to date, it is still unclear whether the compounds from Anoectochilus roxburghii (Wall.) Lindl. play a role in birds' lipid metabolism and meat quality.
Therefore, in this study, the effects and underlying mechanisms of dietary supplementation with ARE on abdominal fat accumulation and meat quality were evaluated in broiler chickens. Interestingly, the results showed that ARE significantly reduced abdominal fat deposition while improving meat quality. Regarding the mechanisms, it was found that ARE enhanced lipid oxidation and blocked lipid biosynthesis in both adipose tissue and liver. Furthermore, ARE increased antioxidase activities in muscle and serum. Finally, ARE optimized the composition and function of gut microbiota. Overall, the results indicate that ARE is a promising candidate as a feed additive to overcome the problems faced by the broiler industry.
MATERIALS AND METHODS
Animals and Experimental Protocol
All animal studies were conducted in compliance with the protocols approved by the Animal Ethics Committee of Guangxi University (GXU-2022-023). One hundred and twenty 1-day-old yellow-feather broilers of Guangxi Lingshan native chicken were purchased from a live-poultry market (Nanning, China) and housed in an environmentally controlled room in Guangxi University Animal Experimental Center. All birds were allowed free access to diets and water. Twenty-day later, 90 broilers with similar body weights were selected and randomly divided into 3 groups, with 5 replicates per group and 6 chickens per replicate. They were provided with either the basal diet or the basal diet supplemented with different concentrations of ARE (0, 0.15%, or 0.30%) for 6 wk. ARE, which was ordered from Xi'an Yuankui Biotechnology Co., Ltd. (Xi'an, China), was prepared as following: The dried Anoectochilus roxburghii (Wall.) Lindl. were ground into a fine powder in a grinding machine and passed through a 200-mesh sieve. The powder was then refluxed with 95% ethanol in a ratio of 1 g:5 mL for 3 h, with stirring every 30 min. This procedure was repeated 3 times, and the resulting solutions were filtered through gauze and a suction filtration device. The filtrate was combined and the filtrate residue was discarded. The concentrate was obtained by vacuum rotary evaporation at 50°C. The concentrate was then dried and powdered to obtain the crude extract of Anoectochilus roxburghii (Wall.) Lindl. The basal diet was purchased from Nanning Weimin Feed Co., Ltd. (Nanning, China) and the diets' compositions are presented in Table 1. All birds were allowed free access to diet and water. For growth performance analysis, body weight and feed intake were measured every 2 wk. Average daily feed intake (ADFI) was calculated based on the weight and feed intake data of all chickens.
Table 1.
Ingredient composition of basal diets.
| Ingredients | Percentage (%) |
|---|---|
| Corn | 67.546 |
| Soybean protein | 1.804 |
| Soybean meal | 9.500 |
| Wheat bran | 4.500 |
| Rice bran | 4.500 |
| CaCO3 | 2.255 |
| CaHPO4 | 1.804 |
| Pig tallow | 5.402 |
| NaCl | 0.363 |
| DL-methionine | 0.363 |
| Premix1 | 0.005 |
| Cholesterol | 1.961 |
| Nutrient composition2 | |
| ME (MJ/kg) | 13.052 |
| CP, % | 12.976 |
| Lys, % | 0.600 |
| Met, % | 0.590 |
Premix (per kg of diet): Vit A, 6,000 IU; Vit D3, 200 IU; Vit E, 10 IU; Vit K, 0.05 mg; Vit B12, 0.007 μg; pantothenic acid, 2.99 mg; riboflavin, 1.63 mg; Cu, 1.25 mg; Mn, 24.06 mg; Zn, 12.70 mg; Se, 0.06 mg; iodide, 0.35 mg.
Values were calculated from data provided by Feed Database in China (2020).
Slaughter Indicators and Organ Index
At the end of the trial, approximately 8 to 10 birds with weights close to the average body weight of the group were randomly selected from each group and fasted for 12 h prior to slaughter. The following slaughter indicators and organ indices were measured: slaughter rate, evisceration rate, chest muscle rate, thigh muscle rate, subcutaneous fat thickness, intramuscular fat width, and abdominal fat weight. Calculation methods were as follows: slaughter rate (%) = (weight of chicken after bleeding and depilation/fasting weight) × 100; evisceration rate (%) = (remaining weight after removal of trachea, esophagus, fibrosac, intestine, spleen, pancreas, heart, liver, glandular stomach, muscular stomach, abdominal fat, and reproductive organs)/fasting body weight × 100; abdominal fat rate (%) = (weight of abdominal fat/(abdominal fat weight + eviscerated weight)) × 100; chest/thigh muscle rate (%) = (left chest/thigh muscle weight/slaughter weight) × 2 × 100.
Measurement of Meat Quality
After slaughtering the chickens, the left chest and thigh muscles were separated to evaluate the meat quality. The following indices were measured 45 min postmortem using standardized protocols. To determine the pH value, a pH meter electrode (pH-STAR, Matthäus, Pottmes, Germany) was inserted into the muscle slit, and the pH values were measured 5 times to obtain the average value. For flesh color and electrical conductivity, a flesh color analyzer (OPTO-STAR, Matthäus, Pöttmes, Germany) and a conductivity meter (LF-STAR, Matthäus, Pöttmes, Germany) were used, respectively, and the average values were obtained after 5 measurements. To measure the shear force, the chest muscles were cooked in plastic bags in a water bath at 80°C to an internal temperature of 72°C for 30 min and then cooled to room temperature. The muscles were then cut into strips (1 cm × 1 cm × 3 cm) along the muscle fibers using a digital meat tenderness meter (Shear tester C-LM3B, Tenovo International Co., Limited, Beijing, China), and the shear force values were recorded. The tests were repeated 3 times, and the average values were taken.
Sample Collection
Before slaughter, blood samples were obtained from the pterygoid veins and stored at 4°C overnight. The next day, the samples were centrifuged at 3,000 × g for 10 min to obtain serum. At the time of slaughter, tissue samples, including abdominal fat tissues, livers, and chest muscles, were carefully isolated using forceps and scissors. The isolated samples were then wrapped in aluminum foil, placed in liquid nitrogen and stored at −80°C for subsequent analysis. To collect intestinal contents, ceca were opened, and the luminal contents were collected using sterile forceps. The collected contents were placed in a 1.5 mL Eppendorf tube on ice, frozen in liquid nitrogen for 15 s, and then stored at −80°C in the laboratory before shipment.
Serum and Tissue Biochemical Analysis
The biochemical parameters of serum, liver, and chest muscle, including triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), glutathione peroxidase (GSH-Px), and superoxide dismutase (SOD), were measured using assay kits (A110-1-1, A111-1-1, A112-1-1, A113-1-1, A006-2-1, and S0103, respectively) following the manufacturer's instructions. These kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Hematoxylin and Eosin Staining
The hematoxylin and eosin (H&E) staining procedure followed a previously described protocol (Liao et al., 2022). Liver and abdominal adipose tissues were fixed in 4% fixative (P1110, Solar Biotechnology Co., Beijing, China) and then sent to Wuhan Servicebio Biotechnology Co., Ltd. for embedding, slicing, and H&E staining. The adipocyte cell area of the tissue sections was analyzed using an optical microscope and ImageJ software (Version 1.53t).
Total RNA Extraction and Quantitative Real-Time PCR
Fifty milligrams of liver, abdominal fat, or chest muscle tissues were ground in 1 mL of Trigent (MF 034-01, Meri 5 Biotechnology Co., Ltd., Beijing, China) using a tissue grinder (KZ-III-F, Wuhan Service Biotechnology Co., Ltd., Wuhan, China), and total RNA was extracted according to the manufacturer's instructions. The all-in-one first-strand cDNA Synthesis Super Mix (AE 341-02, Transgen, Beijing, China) was used to synthesize cDNA from 1 μg of total RNA. Real-time fluorescent quantitative PCR was performed using 2 × RealStar Green Fast Mixture (GenStar, Beijing, China), with β-actin as the internal reference. The primer sequences used in this study are listed in Table 2. The analysis was conducted using the 2−ΔΔCt method.
Table 2.
Primers used for a quantitative polymerase chain reaction.
| Gene1 | Accession number | Primer (5′–3′) | Product size, bp |
|---|---|---|---|
| β-ACTIN | NM_205518.2 | F: TGCGTGACATCAAGGAGAAG | 300 |
| R: TGCCAGGGTACATTGTGGTA | |||
| SREBP-1c | NM_204126.3 | F: GCCCTCTGTGCCTTTGTCTTC | 130 |
| R: ACTCAGCCATGATGCTTCTTC | |||
| FAS | NM_205155.4 | F: TGAAGGACCTTATCGCATTGC | 96 |
| R: GCATGGGAAGCATTTTGTTGT | |||
| SCD1 | NM_204890.2 | F: GTTTCCACAACTACCACCATACATT | 175 |
| R: CCATCTCCAGTCCGCATTTT | |||
| ACC | NM_205505.2 | F: GCTTCCCATTTGCCGTCCTA | 185 |
| R: GCCATTCTCACCACCTGATTACTG | |||
| ELOVL6 | NM_001031539.2 | F: GGTGGTCGGCACCTAATGAA | 169 |
| R: TCTGGTCACACACTGACTGC | |||
| CD36 | XM_038183702.1 | F: TATCGTTTCGCAGTTCCTCGTGAAG | 94 |
| R: AGTTCTGGGATATGACCTCCTCTGTAC | |||
| FATP | XM_027470622.2 | F: GCCTGATGACGTGATGTACGACTG | 113 |
| R: AGAACTTCTTGCGGATGACGATGG | |||
| FABP | NM204290 | F: GCCTGACAAAATGTGCGACC | 130 |
| R: ATTAGGCTTGGCCACACCAG | |||
| APOA1 | NM_205525.5 | F: GTGACCCTCGCTGTGCTCTT | 217 |
| R: CACTCAGCGTGTCCAGGTTGT | |||
| APOB | NM_001044633.1 | F: GGTTACTCCCACGATGGCAA | 113 |
| R: AATGCCCTTCCTTCAGGAGC | |||
| ATGL | NM_001113291.2 | F: TCCTAGGGGCCTACCACATC | 195 |
| R: CCAGGAACCTCTTTCGTGCT | |||
| PPARα | NM_001001464.1 | F: TGCTGTGGAGATCGTCCTGGTC | 166 |
| R: CTGTGACAAGTTGCCGGAGGTC | |||
| CPTL | NM_001012898.1 | F: GGAGAACCCAAGTGAAAGTAATGAA | 135 |
| R: GAAACGACATAAAGGCAGAACAGA | |||
| PGC1a | NM_001330751.2 | F: CAAAGGATGCGCTCTCGTTC | 229 |
| R: CCTCGTAGCTGTCATACCTGG | |||
| MyHC I | NM_204587.4 | F: AACGCCGCAACAACCT | 331 |
| R: TTCTTCTTCATCCGCTCC | |||
| MyHC IIa | NM_204228.4 | F: CCACCAATCCATACGACT | 216 |
| R: CTGGCTCTGCTTGCTCT | |||
| MyHC IIb | NM_001013396.2 | F: TGTGAGTCAAGGCGAGAT | 347 |
| R: CCAGAGCACCTACAGCAT | |||
| PPARγ | NM_001001460.2 | F: TGACAGCGCCAGAGATTACA | 93 |
| R: CATCCATCGCAGACAGATCCA |
16S rDNA Sequencing of Cecal Microbiome
The microbial DNA purification, PCR amplification, and sequencing were performed by Shanghai Biozeron Biotechnology Co., Ltd. (Shanghai, China). Briefly, the cecal microbiome's total genomic DNA was extracted using the CTAB/SDS method, and the DNA concentration and purity were monitored on a 1% agarose gel. The V3 to V4 hypervariable regions of the 16S rRNA genes were then PCR amplified using primers 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACNNGGGTTAAT-3′). Sequencing was performed on the Illumina NovaSeq 175 PE250 Platform (Illumina, San Diego, CA). The sequenced raw files were submitted to the SRA (PRJNA880611).
Analysis of 16S rDNA Sequencing
The sequence files were analyzed using QIIME2 (v2021.2.0). First, the original reads of each sample were normalized to 40,000 reads. Then, the DADA2 module was used to denoise the reads and create an amplicon sequence variant (ASV) table (-p-trim-left-f15 -p-trim-left-r20 -p-trunc-len-f250 -p-trunc-len-r240). Next, the GreenGenes reference database (https://greengenes.lbl.gov, accessed on 20 July 2022) was downloaded and a naive Bayesian classifier was used to assign taxonomic labels to the ASVs. Alpha diversity indices (Shannon, Simpson, Chao1, and ACE) were calculated using the Vegan (v2.5-7, R software package) and beta diversity was assessed by Phyloseq (v1.3.20, R software package). The taxonomic abundance of specific genera was summarized at the phylum and genus levels by amplicon sequencing (v1.13.0, R software package). Linear discriminant analysis (LDA) effect size (LEfSe) method and the nonparametric Kruskal-Wallis rank sum test were used to identify different populations in multiple samples. Pearson correlation analysis was used to determine the correlation between the abundance of fecal flora and biochemical indicators of fat and muscle. All results were visualized using the ggplot2 package (v3.3.5) in R software.
Statistical Analysis
The data analysis was conducted using SPSS 20.0. One-way ANOVA followed by Duncan's multiple range tests were performed to test for significant differences between treatments. Polynomial orthogonal contrasts were used to determine the linear and quadratic responses of measured parameters to dietary ARE concentrations. The results were expressed as mean and pooled standard errors of the means (SEM). A probability value of P < 0.05 was considered statistically significant.
RESULTS
Effects of ARE on Growth Performance of Broilers
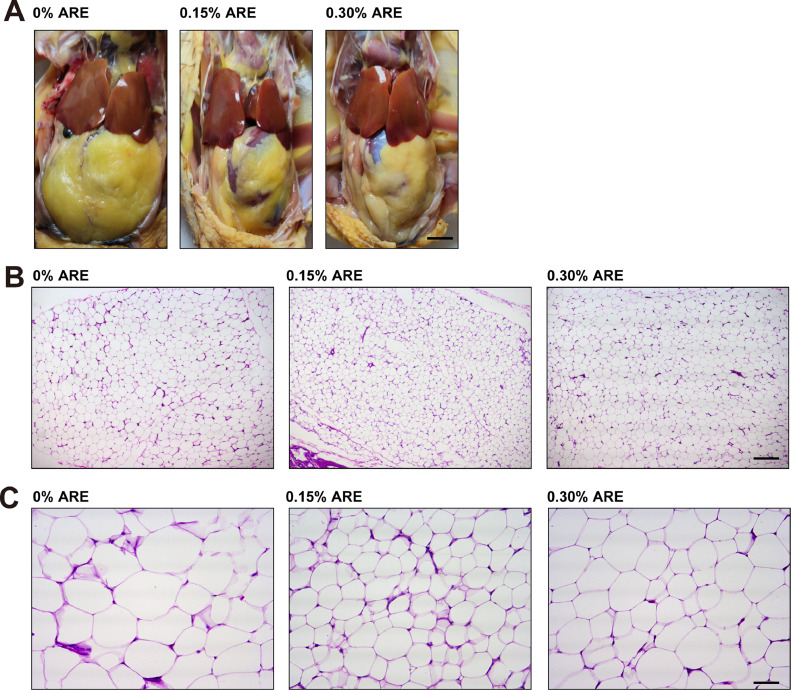
As shown in Table 3, ARE had no significant effects on feed intake, slaughtering rate, and eviscerated rate (P > 0.05). However, there was a significant reduction in body weight and abdominal fat percentage (AFP) (P < 0.05) in the ARE-supplemented group compared to the control group (Figure 1A), and significant linear (P < 0.001) and quadratic relationships (P = 0.005) were noticed between AFP and dietary ARE level. In agreement with the reduction in AFP, intermuscular fat width (IFW) and subcutaneous fat thickness (SFT) (Table 3), as well as adipocyte size (Table 3 and Figure 1B and C), were also decreased in the ARE-supplemented group (P < 0.05). In contrast, chest and thigh muscle percentages showed a slight but significant increase (P < 0.05, Table 3), while liver percentage remained unchanged in the ARE-supplemented group compared to the control group. In summary, the most prominent effect of ARE supplementation on broilers was the reduction in abdominal fat deposition.
Table 3.
Effects of dietary ARE addition on growth performance and fat deposition in broilers.
| Treatments |
Statistics |
||||||
|---|---|---|---|---|---|---|---|
| Index1 | 0% ARE | 0.15% ARE | 0.30% ARE | SEM2 | Panova | Plinear | Pquadratic |
| Initial BW, g | 242.26 | 241.98 | 238.59 | 2.04 | 0.748 | 0.501 | 0.741 |
| Final BW, g | 901.82a | 860.70b | 830.99c | 8.20 | <0.001 | <0.001 | 0.367 |
| ADFI, g | 412.79 | 403.31 | 402.26 | 2.41 | 0.145 | 0.076 | 0.388 |
| Slaughtering rate, % | 93.90 | 95.14 | 95.03 | 0.33 | 0.250 | 0.171 | 0.340 |
| Eviscerated rate, % | 54.58 | 56.60 | 55.08 | 0.62 | 0.407 | 0.748 | 0.197 |
| Chest muscle rate, % | 8.03b | 10.13a | 10.16a | 0.29 | <0.001 | <0.001 | 0.030 |
| Thigh muscle rate, % | 13.29b | 14.75a | 15.02a | 0.23 | 0.001 | 0.001 | 0.125 |
| Liver rate, % | 2.21 | 2.08 | 2.12 | 0.04 | 0.507 | 0.435 | 0.391 |
| Abdominal fat rate, % | 7.59a | 4.77b | 4.77b | 0.34 | <0.001 | <0.001 | 0.005 |
| IFW, mm | 9.77a | 7.70b | 7.79b | 0.21 | 0.011 | 0.010 | 0.088 |
| SFT, mm | 5.52a | 3.91b | 4.06b | 0.34 | 0.001 | 0.001 | 0.101 |
| Mean adipocyte size, μm² | 2700.65a | 710.68c | 1658.70b | 311.90 | 0.003 | 0.023 | 0.003 |
Means within a row with different superscripts are different at P < 0.05.
Abbreviations: ADF, average daily feed intake; BW, body weight; IFW, intermuscular fat width; SFT, subcutaneous fat thickness.
SEM = standard error of mean, n = 8–10.
Figure 1.
Effects of dietary supplementation of ARE on fat deposition in broilers. (A) Representative abdominal pictures of the broilers. (B, C) Representative images of abdominal adipose tissue with H&E staining. Scale bar, 300 μm for upper panel (B) and 75 μm for lower panel (C).
Effects of ARE on Liver and Serum Lipid Parameters in Broilers
As shown in Table 4, consistent with the AFP results, the levels of triglycerides (TG) in the liver and serum were significantly lower in the ARE addition groups (P < 0.05). Although the total cholesterol levels were not significantly different between the groups (P > 0.05), the levels of LDL-C were reduced (P < 0.05) in the ARE addition groups. In line with the improved lipid profiles, the activities of serum antioxidative enzymes (GSH-Px and SOD) were significantly increased by ARE addition (P < 0.05), and significant linear relationships (P < 0.05) were noted between GSH-Px and SOD with dietary ARE level. These data suggest that ARE supplementation improves lipid profiles in the liver and serum of broilers.
Table 4.
Effects of dietary ARE addition on biochemical parameters of serum and liver in broilers.
| Treatments |
Statistics |
||||||
|---|---|---|---|---|---|---|---|
| Index1 | 0% ARE | 0.15% ARE | 0.30% ARE | SEM2 | Panova | Plinear | Pquadratic |
| Liver TG, mmol/mg | 18.50a | 12.84b | 13.27b | 0.92 | 0.013 | 0.016 | 0.094 |
| Liver TC, mmol/mg | 11.07 | 8.02 | 8.10 | 0.77 | 0.186 | 0.152 | 0.309 |
| Serum TG, mmol/L | 0.37a | 0.31ab | 0.26b | 0.14 | 0.001 | <0.001 | 0.875 |
| Serum TC, mmol/L | 5.02 | 4.94 | 4.70 | 0.14 | 0.628 | 0.356 | 0.803 |
| Serum LDL-C, mmol/L | 2.24a | 1.54ab | 1.34b | 0.13 | 0.010 | 0.004 | 0.311 |
| Serum HDL-C, mmol/L | 2.20 | 2.26 | 2.33 | 0.05 | 0.593 | 0.312 | 0.989 |
| Serum GSH-Px, μmol/g protein | 13.26b | 21.54a | 22.55a | 1.25 | 0.001 | 0.012 | 0.0465 |
| Serum SOD, units/mg protein | 2.78b | 3.66ab | 8.86a | 1.03 | 0.026 | 0.012 | 0.273 |
Means within a row with different superscripts are different at P < 0.05.
Abbreviations: GSH-Px, glutathione peroxidase; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SOD = superoxide dismutase; TC = total cholesterol; TG = triglyceride.
SEM = standard error of mean, n = 8.
Effects of ARE on Lipid Metabolism-Related Gene Expression in Liver and Abdominal Fat of Broilers
As depicted in Table 5, ARE treatment led to downregulation of lipogenic genes including SCD1, FAS, ACC, and ELOVL6 in both liver and abdominal fat. On the other hand, the expression of genes associated with lipid uptake (CD36), lipolysis (ATGL), and lipid oxidation (PPARα and CPT1) were upregulated following ARE treatment (Table 5). Therefore, these findings suggest that dietary ARE supplementation reduces abdominal fat deposition by inhibiting lipid biosynthesis while promoting lipid breakdown, but not by inhibiting lipid uptake, in both liver and abdominal fat.
Table 5.
Effects of dietary supplementation of ARE on expression of lipid metabolism-related genes in broilers.
| Treatments |
Statistics |
|||||||
|---|---|---|---|---|---|---|---|---|
| Tissues | Gene1 | 0% ARE | 0.15% ARE | 0.30% ARE | SEM2 | Panova | Plinear | Pquadratic |
| Liver | Fatty acids synthesis | |||||||
| SREBP-1c | 1.00 | 0.90 | 0.83 | 0.06 | 0.535 | 0.275 | 0.889 | |
| SCD1 | 1.00 | 0.52 | 0.72 | 0.11 | 0.230 | 0.317 | 0.161 | |
| FAS | 1.00a | 0.34b | 0.62ab | 0.11 | 0.033 | 0.111 | 0.030 | |
| ACC | 1.00a | 0.25b | 0.36b | 0.12 | 0.012 | 0.016 | 0.050 | |
| ELOVL6 | 1.00a | 0.75ab | 0.43b | 0.10 | 0.045 | 0.014 | 0.823 | |
| Fatty acids uptake | ||||||||
| CD36 | 1.00b | 1.92a | 2.24a | 0.18 | 0.006 | 0.002 | 0.325 | |
| APOA1 | 1.00b | 2.79a | 2.54a | 0.24 | 0.001 | 0.003 | 0.017 | |
| APOB | 1.00 | 0.95 | 0.89 | 0.07 | 0.822 | 0.540 | 0.951 | |
| Fatty acids breakdown | ||||||||
| PPARα | 1.00b | 3.43a | 3.15a | 0.39 | 0.009 | 0.011 | 0.051 | |
| ATGL | 1.00c | 5.75a | 3.56b | 0.54 | <0.001 | 0.002 | 0.051 | |
| CPT1 | 1.00b | 4.91a | 2.79ab | 0.64 | 0.034 | 0.202 | 0.020 | |
| Adipose | Fatty acids synthesis | |||||||
| SREBP-1c | 1.00a | 0.31b | 0.52b | 0.10 | 0.010 | 0.027 | 0.019 | |
| SCD1 | 1.00a | 0.31b | 0.24b | 0.10 | 0.001 | 0.001 | 0.069 | |
| FAS | 1.00 | 0.56 | 0.73 | 0.09 | 0.155 | 0.235 | 0.121 | |
| ACC | 1.00a | 0.42b | 0.42b | 0.10 | 0.009 | 0.007 | 0.099 | |
| ELOVL6 | 1.00a | 0.23b | 0.41b | 0.13 | 0.035 | 0.049 | 0.069 | |
| Fatty acids uptake | ||||||||
| CD36 | 1.00b | 2.15ab | 3.89a | 0.44 | 0.017 | 0.005 | 0.714 | |
| FATP | 1.00b | 3.96a | 4.60a | 0.62 | 0.029 | 0.013 | 0.313 | |
| FABP | 1.00b | 3.41a | 3.64a | 0.39 | 0.004 | 0.003 | 0.118 | |
| Fatty acids breakdown | ||||||||
| PPARα | 1.00b | 3.43a | 3.15a | 0.39 | 0.009 | 0.011 | 0.051 | |
| ATGL | 1.00c | 3.87a | 2.46b | 0.34 | <0.001 | 0.022 | <0.001 | |
| CPT1 | 1.00b | 4.91a | 2.79ab | 0.64 | 0.034 | 0.202 | 0.020 | |
Means within a row with different superscripts are different at P < 0.05.
Abbreviations: ACC, acetyl-CoA carboxylase; APOA1, apolipoprotein A1; APOB, apolipoprotein B; ATGL, adipose triglyceride lipase; CD36, fatty acid transferase; CPT-1, carnitine palmitoyltransferase 1; ELOVL6, fatty acid elongase 6; FABP, fatty acid-binding protein; FAS, fatty acid synthase; FATP, fatty acid transporter; PPARα, peroxisome proliferator-activated receptor-α; SCD1, stearoyl-CoA desaturase-1; SREBP-1c, sterol regulatory element binding protein 1.
SEM = standard error of mean, n = 6–8.
Effect of ARE on Meat Quality of Broilers
As shown in Table 6, ARE supplementation significantly increased the pH45min, electrical conductivity, and TG levels of the chest muscle (P < 0.05). Additionally, the shear force of the chest muscle was decreased (P < 0.05), while the fresh color of the meat remained unchanged (P > 0.05). Moreover, significantly quadratic relationships were noticed between the measured muscle TG levels and dietary ARE levels (P < 0.001), and significant linear relationships was noticed between shear force in chest muscle and dietary ARE levels (P < 0.001). These findings suggest that ARE supplementation improves the quality of broiler meat.
Table 6.
Effects of dietary ARE addition on indices of meat quality in broilers.
| Treatments |
Statistics |
||||||
|---|---|---|---|---|---|---|---|
| Index1 | 0% ARE | 0.15% ARE | 0.30% ARE | SEM2 | Panova | Plinear | Pquadratic |
| Flesh color | 72.35 | 76.87 | 67.38 | 1.62 | 0.052 | 0.190 | 0.038 |
| pH45min | 5.78b | 5.84ab | 6.16a | 0.07 | 0.036 | 0.016 | 0.324 |
| EC, μS/cm | 3.38b | 7.48a | 7.76a | 0.56 | <0.001 | <0.001 | 0.046 |
| Shear force, N | 4.27a | 3.83ab | 3.05b | 0.14 | <0.001 | <0.001 | 0.458 |
| Muscle TG, mmol/g protein | 0.13b | 0.20a | 0.11b | 0.13 | 0.001 | 0.240 | <0.001 |
Means within a row with different superscripts are different at P < 0.05.
Abbreviations: EC, electrical conductivity; TG, triglyceride.
SEM = standard error of mean, n = 8.
Effects of ARE on Gene Expression and Antioxidation Activities in Muscles of Broilers
As shown in Table 7, chest muscles from broilers supplemented with ARE exhibited a significant upregulation of the mRNA expression of peroxisome proliferator-activated receptor gamma coactivator 1α (PGC1a), a transcriptional coactivator that plays a role in converting fast glycolytic fibers to slow and oxidative fibers (Shu et al., 2014). Consistent with the upregulation of PGC1a, ARE addition increased the mRNA expression levels of oxidative fiber markers such as MyHC I and MyHC IIa, while also decreasing the expression of glycolytic fiber gene MyHC IIb (Table 7). Additionally, ARE-supplemented broilers had elevated mRNA levels of peroxisome proliferator-activated receptor γ (PPARγ), a master regulator of adipogenesis (Spaas et al., 2022; Table 7). Furthermore, the activities of antioxidative enzymes (GSH-Px and SOD) were also upregulated in chest muscle by ARE addition (Table 7). Collectively, these molecular data suggest that ARE addition remodels muscle fiber types and improves antioxidative activities, contributing to the improvement of meat quality in broilers.
Table 7.
Effects of dietary supplementation of ARE on muscle fiber-related gene expression and antioxidants activities in broilers’ muscle.
| Treatments |
Statistics |
||||||
|---|---|---|---|---|---|---|---|
| Index1 | 0% ARE | 0.15% ARE | 0.30% ARE | SEM2 | Panova | Plinear | Pquadratic |
| PGC1α | 1.00b | 5.31b | 23.92a | 2.82 | <0.001 | <0.001 | 0.047 |
| MyHC I | 1.00b | 1.34ab | 2.11a | 0.19 | 0.036 | 0.013 | 0.531 |
| MyHC IIa | 1.00b | 4.15a | 1.33b | 0.48 | <0.005 | 0.715 | 0.001 |
| MyHC IIb | 1.00 | 0.82 | 0.85 | 0.13 | 0.848 | 0.650 | 0.734 |
| PPARγ | 1.00c | 4.26b | 8.11a | 0.83 | <0.001 | <0.001 | 0.767 |
| Muscle GSH-Px, μmol/g protein | 107.25b | 136.43a | 101.09b | 5.71 | 0.018 | 0.616 | 0.006 |
| Muscle SOD, units/mg protein | 13.26b | 21.54a | 22.55a | 1.25 | 0.001 | 0.001 | 0.084 |
Means within a row with different superscripts are different at P < 0.05.
Abbreviations: GSH-Px, glutathione peroxidase; MyHC, myosin heavy chain; PGC1α, peroxisome proliferator-activated receptor-gamma coactivator; PPARγ, peroxisome proliferator-activated receptor-γ; SOD, superoxide dismutase.
SEM = standard error of mean, n = 8.
Effects of ARE on Diversity and Composition of Cecal Microbiota in Broilers
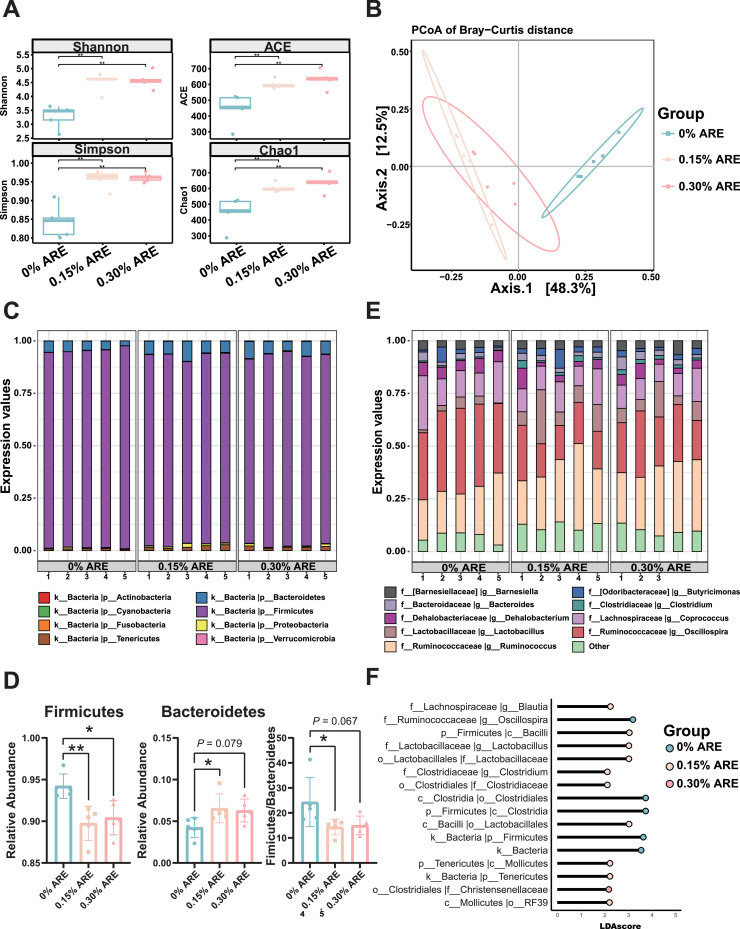
First, dietary supplementation of ARE significantly increased the alpha diversity of the intestinal flora (P < 0.01), as the indices for diversity (Shannon and Simpson) and richness (ACE and Chao1) of bacteria were all elevated by ARE addition when compared with the control group (Figure 2A). Then, the PCoA showed that the cecal microbiome from the control and experimental groups were in distinct clusters, suggesting that ARE altered the beta diversity of the intestinal flora (Figure 2B). Next, the gut microbial composition was analyzed in detail based on the abundance of bacteria. As shown in Figure 2C, Firmicutes and Bacteroidetes were the most abundant phyla, but notably the ratio of Firmicutes to Bacteroidetes was shifted in favor of the Bacteroidetes (Figure 2D, P < 0.05). At the genus level, the dominant species were Oscillospira and Ruminococcus (Figure 2E). Furthermore, LEfSe analysis was used to identify taxa that were discriminative between groups. As shown in Figure 2F, ARE altered the differentially abundant taxa between the control and ARE groups at the OUTs. For example, at the genus level, the dominant genera in the control group were Oscillospira, whereas in the ARE addition group, Blautia and Lactobacillus were more specific. Overall, these data suggest that ARE supplementation modifies the diversity and composition of the intestinal microbiota in broilers.
Figure 2.
Effects of dietary supplementation of ARE on cecal microbial diversity and composition in broilers. (A) Alpha diversity analysis. (B) Principal coordinates analysis (PCoA). (C) Microbiota composition analysis at the phylum level. (D) Relative abundance of Firmicutes and Bacteroidetes. (E) Microbiota composition analysis at the genus level. (F) LEfSe analysis (sort by P value). All the results are shown as the means ± SD; *P < 0.05, **P < 0.01 (n = 5).
Correlation Analysis Between Cecal Microbiome and the Phenotypes
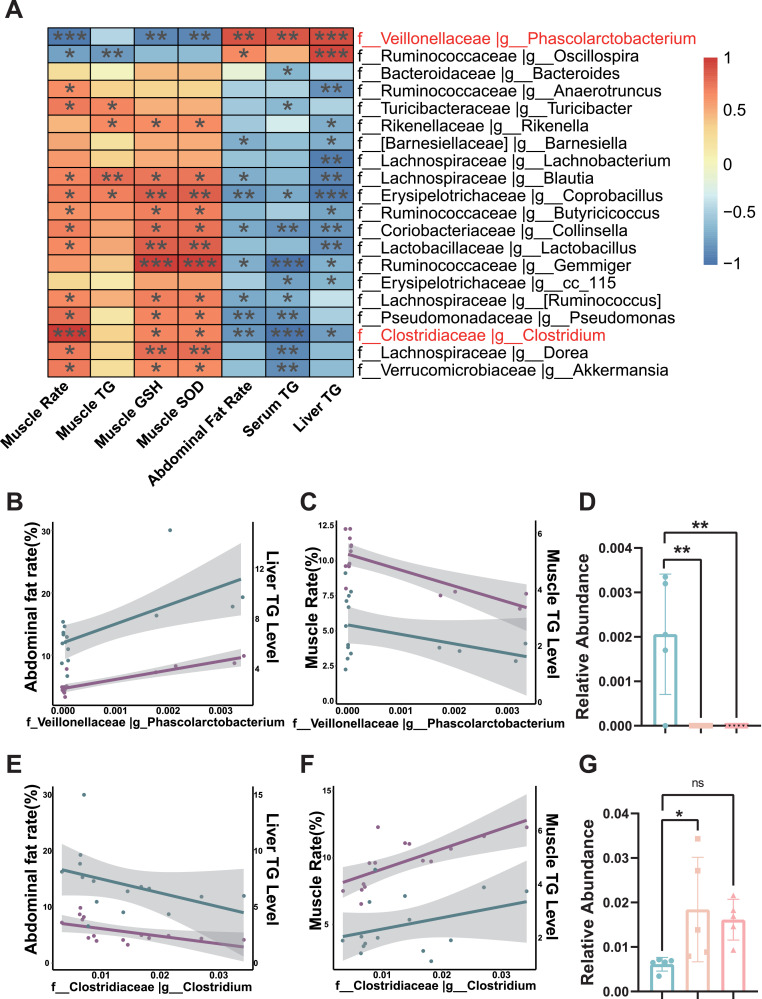
To reveal the relationship between the cecal microbiome, abdominal fat, and meat quality, Pearson correlation analysis was performed among cecal microbiota, abdominal fat rate, muscle rate, muscle TG level, and other parameters. The top 20 genus-level floras which significantly correlated with the phenotype parameters were shown in Figure 3A. Specifically, the abundance of Phascolarctobacterium, which was positively correlated with abdominal fat deposition (Figure 3B) and negatively correlated with muscle TG levels (Figure 3C), was significantly reduced by ARE addition (Figure 3D, P < 0.01). By contrast, Clostridium abundance, which was inversely correlated with abdominal fat rate (Figure 3E) but positively correlated with TG levels in muscles (Figure 3F), was significantly elevated by ARE addition (Figure 3G, P < 0.05). Collectively, these analyses indicate that the intestinal microflora is involved in the contribution of ARE in alleviating fat deposition and improving the meat quality of broilers.
Figure 3.
Combined analysis of cecal microbiota and broilers phenotypes. (A) Heatmap showing correlation analyses between muscle quality and lipid metabolism-related parameters and relative abundances of gut microbiota. Only the top 20 significant correlations are displayed. (B, E) Correlation of the relative abundances of Phascolarctobacterium (B) or Clostridium (E) with liver TG level and abdominal fat rate. (C, F) Correlation of the relative abundances of Phascolarctobacterium (C) or Clostridium (F) with chest muscle rate and chest muscle TG level. (D, G) Alterations of the relative abundances of Phascolarctobacterium (D) or Clostridium (G) in gut microbiota after ARE supplementation. All the results are shown as the means ± SD; *P < 0.05, **P < 0.01, ***P < 0.001 (n = 5).
DISCUSSION
Excessive abdominal fat deposition and poor meat quality have emerged as 2 major problems negatively affecting the modern broiler industry. This study demonstrated that dietary supplementation of ARE efficiently reduced abdominal fat deposition while improving meat quality in yellow-feather broilers. To date, only a few Chinese herb extracts have been reported to have such effects, such as curcumin (Zhang et al., 2015; Xie et al., 2019). Therefore, the findings here are significant in the development of new feed additives to address the challenges facing the broiler industry today.
Excessive accumulation of adipose tissue in broilers is mainly caused by adipocyte hypertrophy rather than hyperplasia (Everaert et al., 2022). Thus, reduction in fat mass is primarily reflected in the reduction of cell size but not cell number. Consistently, in this study it was observed that dietary supplementation of ARE efficiently reduced adipocyte size and lipid accumulation in the adipose tissue. The decrease in cell size was likely due to the reduction in stored lipid in adipocytes, which is usually determined by a complex balance of fatty acid uptake, de novo lipid biosynthesis, lipolysis, and fatty acid oxidation. In birds, the liver is the major site for de novo fatty acid synthesis, accounting for more than 90% of it (Nematbakhsh et al., 2021). Thus, the lipid metabolism in both liver and adipose tissues was investigated and was found that ARE inhibited lipid biosynthesis while promoting lipid breakdown, indicating the involvement of liver in ARE-induced fat reduction in broilers. Similarly, a recent study revealed that Anoectochilus roxburghii polysaccharide, one of the main ingredients of ARE, could protect mice against diet-induced obesity and fatty liver partially by promoting lipid mobilization in both adipose tissue and liver (Tian et al., 2022). Although the mechanisms underlying the regulation of lipid metabolism by ARE remain unclear, it is possible that the mTOR- and AMPK-mediated pathways are involved, as they act as metabolic hubs for lipid homeostasis (Lamming and Sabatini, 2013; Pang et al., 2021). Further studies are needed to confirm this hypothesis.
Regarding meat quality, it was found that ARE supplementation, especially at the low dose, significantly improved the meat quality of broilers. This conclusion is supported by several key findings: First, several important meat quality indices were improved by ARE, such as pH45min, electrical conductivity, intramuscular fat content, and shear force. Second, there was a shift in muscle fiber type from glycolytic to oxidative, which is known to have a positive effect on meat quality (Ismail and Joo, 2017; Hwang et al., 2019). Lastly, the activities of antioxidant enzymes (GSH-Px and SOD) were upregulated, indicating that ARE enhances the protection of muscle structure from damage caused by reactive oxygen species (ROS), peroxides, and oxygen free radicals produced by muscle metabolism (Weydert and Cullen, 2010). This is an intriguing finding because many nutritional strategies can either reduce abdominal fat accumulation or improve meat quality (Ji et al., 2021; Li et al., 2021). That is likely due to the fact that ARE is composed of multiple active components, such as polysaccharides, flavonoids, glycosides, organic acids, steroids, alkaloids, etc., and each component has unique biological effects (Ye et al., 2017). Future studies are needed to determine which ingredients in ARE play a predominant role in the improvement of fat deposition and meat quality.
The gut microbiota has been shown to have a variety of effects on the host, from shaping the structure and function of the gut to modulating the development, metabolism, immunity, and nervous system of the host (Nicolas and Chang, 2019). In this study, it was found that the addition of ARE increased the diversity of the microbiome while profoundly altering its composition. Notably, ARE reduced the ratio of Firmicutes to Bacteroidetes at the phylum level, which has been found to be positively related to improved lipid metabolism in the host (Fan and Pedersen, 2021). Therefore, the modified cecum microbiota may be involved in mediating ARE-induced abdominal fat reduction in broilers. Additionally, significant changes was observed in the abundance of Phascolarctobacterium and Clostridium, which have been previously reported to be positively or negatively associated with the development of obesity (Yang et al., 2010; Lecomte et al., 2015; Yang et al., 2021), respectively. Therefore, the microbiota may also be another factor shaping host lipid metabolism. Although the exact mechanism remains unknown, short-chain fatty acids (SCFAs) are expected to play a role in the link between bacteria and host lipid metabolism (He et al., 2020). SCFAs are one of the main metabolic products of bacteria and can be absorbed and rapidly transported from the intestine to the liver and other tissues (He et al., 2020). Furthermore, SCFAs, particularly butyrate, have been shown to regulate host lipid metabolism by promoting fatty acid oxidation and inhibiting lipid biosynthesis (He et al., 2020). Thus, in future studies, intestinal SCFA content should be measured to further investigate this speculation.
However, it is worth noting that there are several limitations to this study. First, the Guangxi Lingshan native chicken was used as a model breed in this investigation, rather than an internationally commercialized yellow-feather or white-feather broiler line. This slow-growing breed with high fat deposition differs from internationally fast-growing breeds. Although nutritional regulation of lipid metabolism is conserved among different breeds, it is still necessary to verify the effects of ARE on other chicken breeds before the widespread application of ARE in broiler production. Additionally, due to space constraints, only 90 broilers with similar body weights were used in this study. The small size of the birds may have unpredictable effects on the experimental results. Therefore, further research involving larger sample sizes is needed to explore the effects of ARE on fat deposition and meat quality in broilers.
CONCLUSIONS
This study aims to determine the effects of dietary ARE supplementation on fat deposition, meat quality, and cecum microbiota in broilers. The results have indicated that ARE significantly reduces the fat deposition but improves the meat quality. Mechanistically, ARE inhibits lipid biosynthesis while promoting lipid breakdown in both liver and abdominal fat. Besides, ARE enhances the activities of antioxidative enzymes in the muscle and serum. Moreover, ARE remodels the microbial diversity, composition, and function. In a word, these findings suggest that ARE is a promising candidate to be a new feed additive for reducing abdominal fat deposition and improving the meat quality in broilers.
ACKNOWLEDGMENTS
This work was supported by grants from the Project of Bama County for Talents in Science and Technology (No. 20220016), the Specific Research Project of Guangxi for Research Bases and Talents (AD22035061), the Youth Science Foundation of the National Natural Science Foundation of China (82100913), and the Natural Science Foundation of Guangxi Province (2020GXNSFAA297043).
DISCLOSURES
The authors declare that all authors have read and approved this version of the paper and that no conflict of interest, financial or otherwise, exists.
REFERENCES
- Alvares D., Hoffman S., Stankovic B., Adeli K. Gut peptide and neuroendocrine regulation of hepatic lipid and lipoprotein metabolism in health and disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2019;1864:326–334. doi: 10.1016/j.bbalip.2018.12.010. [DOI] [PubMed] [Google Scholar]
- Ameer F., Scandiuzzi L., Hasnain S., Kalbacher H., Zaidi N. De novo lipogenesis in health and disease. Metabolism. 2014;63:895–902. doi: 10.1016/j.metabol.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Banaszak M., Biesek J., Adamski M. Wheat litter and feed with aluminosilicates for improved growth and meat quality in broiler chickens. PeerJ. 2021;9:e11918. doi: 10.7717/peerj.11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito L.F., Bedere N., Douhard F., Oliveira H.R., Arnal M., Peñagaricano F., Schinckel A.P., Baes C.F., Miglior F. Review: genetic selection of high-yielding dairy cattle toward sustainable farming systems in a rapidly changing world. Animal. 2021;15 doi: 10.1016/j.animal.2021.100292. [DOI] [PubMed] [Google Scholar]
- Du M., Ahn D.U., Nam K.C., Sell J.L. Influence of dietary conjugated linoleic acid on volatile profiles, color and lipid oxidation of irradiated raw chicken meat. Meat Sci. 2000;56:387–395. doi: 10.1016/s0309-1740(00)00067-x. [DOI] [PubMed] [Google Scholar]
- Dunshea F.R., Souza D.N.D., Pethick D.W., Harper G.S., Warner R.D. Effects of dietary factors and other metabolic modifiers on quality and nutritional value of meat. Meat Sci. 2005;71:8–38. doi: 10.1016/j.meatsci.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Everaert N., E. Decuypere, and J, Buyse. 2022. Adipose tissue and lipid metabolism, In: G. Colin & D. Sami (Eds), Sturkie's Avian Physiology. Elsevier: Amsterdam, The Netherlands, 647–660.
- Fan Y., Pedersen O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021;19:55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- Gao C.Q., Shi H.Q., Xie W.Y., Zhao L.H., Zhang J.Y., Ji C., Ma Q.G. Dietary supplementation with acidifiers improves the growth performance, meat quality and intestinal health of broiler chickens. Anim. Nutr. 2021;7:762–769. doi: 10.1016/j.aninu.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Zhang P., Shen L., Niu L., Tan Y., Chen L., Zhao Y., Bai L., Hao X., Li X., Zhang S., Zhu L. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21176356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocquette J.F., Gondret F., Baéza E., Médale F., Jurie C., Pethick D.W. Intramuscular fat content in meat-producing animals: development, genetic and nutritional control, and identification of putative markers. Animal. 2010;4:303–319. doi: 10.1017/S1751731109991091. [DOI] [PubMed] [Google Scholar]
- Hu H., Shen X., Liao B., Luo L., Xu J., Chen S. Herbgenomics: a stepping stone for research into herbal medicine. Sci. China Life Sci. 2019;62:913–920. doi: 10.1007/s11427-018-9472-y. [DOI] [PubMed] [Google Scholar]
- Hwang Y.H., Bakhsh A., Lee J.G., Joo S.T. Differences in muscle fiber characteristics and meat quality by muscle type and age of Korean native black goat. Food Sci. Anim. Resour. 2019;39:988–999. doi: 10.5851/kosfa.2019.e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail I., Joo S.T. Poultry meat quality in relation to muscle growth and muscle fiber characteristics. Korean J. Food Sci. Anim. Resour. 2017;37:873–883. doi: 10.5851/kosfa.2017.37.6.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji F., Gu L., Rong G., Hu C., Sun W., Wang D., Peng W., Lin D., Liu Q., Wu H., Dai H., Zhou H., Xu T. Using extract from the stems and leaves of Yizhi (Alpiniae oxyphyllae) as feed additive increases meat quality and intestinal health in ducks. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.793698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr B.J., Kidd M.T., Halpin K.M., McWard G.W., Quarles C.L. Lysine level increases live performance and breast yield in male broilers. J. Appl. Poult. Res. 1999;8:381–390. [Google Scholar]
- Lamming D.W., Sabatini D.M. A central role for mTOR in lipid homeostasis. Cell Metab. 2013;18:465–469. doi: 10.1016/j.cmet.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecomte V., Kaakoush N.O., Maloney C.A., Raipuria M., Huinao K.D., Mitchell H.M., Morris M.J. Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenstra F., Cahaner A. Effects of low, normal, and high temperatures on slaughter yield of broilers from lines selected for high weight gain, favorable feed conversion, and high or low fat content. Poult. Sci. 1992;71:1994–2006. doi: 10.3382/ps.0711994. [DOI] [PubMed] [Google Scholar]
- Li H., Zhao L., Liu S., Zhang Z., Wang X., Lin H. Propionate inhibits fat deposition via affecting feed intake and modulating gut microbiota in broilers. Poult. Sci. 2021;100:235–245. doi: 10.1016/j.psj.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Q., Wu T., Fu Q., Wang P., Zhao Y., Li Y., Xiao H., Zhou L., Song Z. Effects of dietary inclusion of beta-hydroxy-beta-methylbutyrate on growth performance, fat deposition, bile acid metabolism, and gut microbiota function in high-fat and high-cholesterol diet-challenged layer chickens. Curr. Issues Mol. Biol. 2022;44:3413–3427. doi: 10.3390/cimb44080235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nematbakhsh S., Pei Pei C., Selamat J., Nordin N., Idris L.H., Abdull Razis A.F. Molecular regulation of lipogenesis, adipogenesis and fat deposition in chicken. Genes-Basel. 2021;12:414. doi: 10.3390/genes12030414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas G.R., Chang P.V. Deciphering the chemical lexicon of host-gut microbiota interactions. Trends Pharmacol. Sci. 2019;40:430–445. doi: 10.1016/j.tips.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y., Xu X., Xiang X., Li Y., Zhao Z., Li J., Gao S., Liu Q., Mai K., Ai Q. High fat activates O-GlcNAcylation and affects AMPK/ACC pathway to regulate lipid metabolism. Nutrients. 2021;13 doi: 10.3390/nu13061740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandercock D.A., Nute G.R., Hocking P.M. Quantifying the effects of genetic selection and genetic variation for body size, carcass composition, and meat quality in the domestic fowl (Gallus domesticus) Poult. Sci. 2009;88:923–931. doi: 10.3382/ps.2008-00376. [DOI] [PubMed] [Google Scholar]
- Sell-Kubiak E., Wimmers K., Reyer H., Szwaczkowski T. Genetic aspects of feed efficiency and reduction of environmental footprint in broilers: a review. J. Appl. Genet. 2017;58:487–498. doi: 10.1007/s13353-017-0392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu J.T., Xu W.J., Zhang M., Song W.T., Shan Y.J., Song C., Zhu W.Q., Zhang X.Y., Li H.F. Transcriptional co-activator PGC-1alpha gene is associated with chicken skeletal muscle fiber types. Genet. Mol. Res. 2014;13:895–905. doi: 10.4238/2014.February.14.19. [DOI] [PubMed] [Google Scholar]
- Siegel P.B. Evolution of the modern broiler and feed efficiency. Annu. Rev. Anim. Biosci. 2014;2:375–385. doi: 10.1146/annurev-animal-022513-114132. [DOI] [PubMed] [Google Scholar]
- Spaas J., Goulding R.P., Keytsman C., Fonteyn L., van Horssen J., Jaspers R.T., Eijnde B.O., Wust R. Altered muscle oxidative phenotype impairs exercise tolerance but does not improve after exercise training in multiple sclerosis. J. Cachex. Sarcopen. Muscle. 2022;13:2537–2550. doi: 10.1002/jcsm.13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian D., Zhong X., Fu L., Zhu W., Liu X., Wu Z., Li Y., Li X., Li X., Tao X., Wei Q., Yang X., Huang Y. Therapeutic effect and mechanism of polysaccharides from Anoectochilus roxburghii (Wall.) Lindl. in diet-induced obesity. Phytomedicine. 2022;99 doi: 10.1016/j.phymed.2022.154031. [DOI] [PubMed] [Google Scholar]
- Wan X., Yang Z., Ji H., Li N., Yang Z., Xu L., Yang H., Wang Z. Effects of lycopene on abdominal fat deposition, serum lipids levels and hepatic lipid metabolism-related enzymes in broiler chickens. Anim. Biosci. 2021;34:385–392. doi: 10.5713/ajas.20.0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C., Yan W., Sun C., Ji C., Zhou Q., Zhang D., Zheng J., Yang N. The gut microbiota is largely independent of host genetics in regulating fat deposition in chickens. Isme J. 2019;13:1422–1436. doi: 10.1038/s41396-019-0367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weydert C.J., Cullen J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010;5:51–66. doi: 10.1038/nprot.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z., Shen G., Wang Y., Wu C. Curcumin supplementation regulates lipid metabolism in broiler chickens. Poult. Sci. 2019;98:422–429. doi: 10.3382/ps/pey315. [DOI] [PubMed] [Google Scholar]
- Yang H.T., Liu J.K., Xiu W.J., Tian T.T., Yang Y., Hou X.G., Xie X. Gut microbiome-based diagnostic model to predict diabetes mellitus. Bioengineered. 2021;12:12521–12534. doi: 10.1080/21655979.2021.2009752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Zhang B., Guo Y., Jiao P., Long F. Effects of dietary lipids and Clostridium butyricum on fat deposition and meat quality of broiler chickens. Poult. Sci. 2010;89:254–260. doi: 10.3382/ps.2009-00234. [DOI] [PubMed] [Google Scholar]
- Ye S., Shao Q., Zhang A. Anoectochilus roxburghii: a review of its phytochemistry, pharmacology, and clinical applications. J. Ethnopharmacol. 2017;209:184–202. doi: 10.1016/j.jep.2017.07.032. [DOI] [PubMed] [Google Scholar]
- Zhang J., Hu Z., Lu C., Bai K., Zhang L., Wang T. Effect of various levels of dietary curcumin on meat quality and antioxidant profile of breast muscle in broilers. J. Agric. Food Chem. 2015;63:3880–3886. doi: 10.1021/jf505889b. [DOI] [PubMed] [Google Scholar]
- Zhang M., Wang C., Zhang R., Chen Y., Zhang C., Heidi H., Li M. Comparison of the guidelines on good agricultural and collection practices in herbal medicine of the European Union, China, the WHO, and the United States of America. Pharmacol. Res. 2021;167 doi: 10.1016/j.phrs.2021.105533. [DOI] [PubMed] [Google Scholar]