Abstract
Yucca schidigera extract (YSE) is a green feed additive that is known to reduce toxic gas emissions and promote intestinal health in animal production. This study investigated the potential of dietary YSE supplementation to mitigate the negative effect of Clostridium perfringens and coccidia infection on productive performance and gut health in laying hens. A total of 48 Lohmann gray laying hens (35 wk of age) were randomly allotted to 1 of 2 groups (n = 24) fed with either a basal diet or a YSE-supplemented diet for 45 d. From d 36 to 45, half of the hens in each group were orally administrated with Clostridium perfringens type A and coccidia. This challenge impaired productive performance and egg quality (P < 0.05), destroyed jejunal morphology and functions (P < 0.05), induced jejunal epithelial cell apoptosis (P < 0.05), and downregulated the antioxidant capacity and Nrf2 pathway expression of jejunal mucosa (P < 0.05) in laying hens. Supplementing YSE in the laying hen diet, to some extents, improved productive performance and egg quality (P < 0.05), and alleviated the effect of challenge on morphology, functions, cell apoptosis, and antioxidant capacity in the jejunum (P < 0.05). Overall, the results suggested that dietary YSE supplementation might mitigate the negative effects of Clostridium perfringens and coccidia infection on gut health, and thereby improve the productive performance and egg quality of laying hens, possibly through enhancing the antioxidant capacity of the jejunum.
Key words: Yucca schidigera extract, laying hen, egg quality, gut health, Clostridium perfringens type A and coccidia challenge
INTRODUCTION
Clostridium perfringens (C. perfringens), known as the anaerobic gram-positive rod bacterium, is a common inhabitant of soil and intestinal tracts of animals and humans (Swayne, 2020). It is classified into 7 types (A–G), with type A, type C, and type G being the primary pathogenic strains of necrotic enteritis in poultry. The rapid colonization of C. perfringens leading to necrotic enteritis is associated with various risk factors, including coccidiosis induced by Eimeria (Swayne, 2020). Both C. perfringens and coccidia can cause serious intestinal inflammation, impair the structure and function of tight junction, and ultimately destroy gut barrier (Awad et al., 2017). This results in the damaged intestine health and the reduced poultry production. Therefore, in this study, we used oral administration of C. perfringens type A and coccidia to model intestinal damage.
Yucca schidigera is an herb from the Asparagaceae family, native to deserts of the southwestern United States and northern Mexico, and occasionally planted in China (Kucukkurt et al., 2016). Indigenous peoples have traditionally used this plant to relieve joint pain, treat skin ulcers, and stop diarrhea or bleeding (Patel, 2012). Yucca schidigera extract (YSE) is a green feed additive that has been shown to reduce the emission of poisonous gas in animal production (Pen et al., 2006; Liang et al., 2009). Previous studies have demonstrated that YSE (even its active contents) administration can improve various physiological functions, including lipid and protein metabolism, anti-inflammation activity, gut health, and reproductive performance in pigs, chicken, and egg layers (Cheeke et al., 2006; Espinosa Munoz et al., 2008; Valverde Piedra et al., 2009; Gurbuz et al., 2011a,b; Sun et al., 2017). Our recent study also found that dietary YSE supplementation improved digestive, absorptive, and barrier function in piglets.
Thus, the primary objective of this study was to investigate the hypothesis that dietary YSE supplementation could alleviate the negative effect of C. perfringens type A and coccidia challenge on productive performance, egg quality, and gut health in laying hens, we would also investigate the related potential mechanism.
MATERIALS AND METHODS
Birds and Diets
All animal procedures were carried out following the Guidelines for Care and Use of Laboratory Animals of Sichuan Agricultural University and were approved by the Animal Ethics Committee of Sichuan Agricultural University (Chengdu, China).
A total of 48 Lohmann gray laying hens (35 wk of age) were used in this experiment, and were provided with ad libitum access to water and diets. All hens were individually housed at approximately 22°C, and were subjected to a 16L:8D photoperiod.
The diets were formulated to meet the nutrient requirements of the National Research Council (1994), and were presented in Table 1. The basal diet served as the control diet, and the YSE-supplemented diet contained 500 mg/kg of YSE product. This YSE product was purchased from DPI GLOBAL (Porterville, CA), and had a content of active constituents (including saponins, phenolics, and polysaccharides) is 30%, with the remaining 70% comprising granules and ground limestone.
Table 1.
Composition and nutrient level of basal diet (as-fed basis).
| Ingredients | Content, % |
|---|---|
| Corn | 55.15 |
| Wheat bran | 5.00 |
| Soybean oil | 3.00 |
| Soybean meal | 25.25 |
| Stone powder (granular) | 4.50 |
| Stone powder (powder) | 4.50 |
| CaHPO4 (powder) | 1.30 |
| NaCl | 0.30 |
| L-lysine sulfate | 0.13 |
| DL-methionine | 0.20 |
| L-threonine | 0.09 |
| Choline chloride, 60% | 0.10 |
| Vitamin premix1 | 0.03 |
| Mineral premix2 | 0.45 |
| Nutrient levels3 | |
| Metabolizable energy, kcal/kg | 2690 |
| Crude protein, % | 16.50 |
| Crude fiber, % | 2.71 |
| Calcium, % | 3.86 |
| Total phosphorus, % | 0.59 |
| Lysine, % | 0.85 |
| Methionine, % | 0.42 |
Provided the following per kilogram of diet: vitamin A, 9,300 IU; vitamin D3, 3,000 IU; vitamin E, 30 IU; vitamin K3, 4.8 mg; vitamin B1 (thiamine), 3 mg; vitamin B2 (riboflavin), 9.6 mg; vitamin B6, 6 mg; vitamin B12, 0.3 mg; biotin, 1.67 mg; pantothenic acid, 18 mg; folic acid, 1.5 mg; niacin, 60 mg.
Provided the following per kilogram of diet: copper (CuSO4·5H2O), 8 mg; Iron (FeSO4·H2O), 60 mg; manganese (MnSO4·H2O), 60 mg; zinc (ZnSO4·H2O), 80 mg; Iodine (KI), 0.35 mg; selenium (Na2SeO3), 0.3 mg.
Nutrient levels represent the calculated values. Metabolizable energy was calculated according to NRC (1994).
Experimental Design and Sample Collection
A 2 × 2 factorial design, including the main effects of diets and challenge, was used in this trial. After 3 d of acclimation, 48 Lohmann gray laying hens (35 wk of age) were allotted randomly into 1 of 2 groups (24 replicates per group, and 1 laying hen per replicate) fed with the basal diet or the YSE-supplemented diet. The duration of whole trial was 45 d. At each day of d 36 to 45, half of the hens in every group were orally administrated with 80-fold coccidiosis vaccine (0.15 mL, 550,000 coccidia sporangia/mL) and 40 mL of C. perfringens type A (7.5 × 108 CFU/mL), and the other hens were orally administrated with same volume of sterile phosphate-buffered saline (PBS) (12 replicates per group, and 1 laying hen per replicate). In each laying hen, oral administration of coccidiosis vaccine and C. perfringens type A or PBS was implemented 3 times daily.
C. perfringens type A was from the China Veterinary Drug Administration (CVCC2030). Following activation, it was cultivated by using the sterile thioglycolate liquid medium at 37°C for 24 h. The avian coccidiosis quadrivalent live vaccine was purchased from Foshan Standard Biotech Co., Ltd. (Guangzhou, China).
In each day of the whole experiment, egg numbers, egg weight, and unqualified egg (egg weight <50 g or >75 g, misshaped egg, dirty egg, and sand-shelled egg) were recorded. Feed conversion ratio (FCR) was defined as the ratio of total feed intake (g) to total egg weight (g).
On d 46, 8 hens from each treatment were randomly selected. Blood samples were gathered through wing vein using vacutainer tubes (Axygen Biotechnology, Taizhou, China), and centrifuged at 3,500 × g for 10 min. Serum samples were stored at −20°C. Then, these hens were slaughtered by cervical dislocation. The jejunum was quickly removed, and flushed by using sterile ice-cold saline. Jejunal segments (about 2 cm) were fixed in 4% paraformaldehyde for mucosal morphology, histopathology, and TUNEL assay. The jejunal mucosa was also collected through scraping the jejunal wall with a sterile glass microscope slide. The jejunal digesta (about 3 g) were collected in sterile tubes. The samples of jejunal mucosa and digesta were immediately frozen in liquid nitrogen, and stored at −80°C.
Measurement of Egg Quality
For the measurement of egg quality, at d 28 and d 42, 12 eggs and 6 eggs were gathered from each treatment, respectively. Eggshell strength and thickness (blunt end, tip, and equator) were determined with the testers of eggshell strength and thickness (Robotmation Co., Ltd., Tokyo, Japan). Eggshell color is analyzed using a colorimeter (CR410, Japan), and egg internal quality (Haugh unit (HU), albumen height, and yolk color) were measured by an Egg Multi-tester (EMT-7300, Robotmation Co., Ltd., Tokyo, Japan). The index of albumen or eggshell was calculated as 100 × [albumen weight or eggshell weight (g)/egg weight (g)]. Additionally, albumen and yolk were separated with an egg separator, and then yolk weight was determined.
The Assay of Digestive Enzyme Activity in Jejunal Digesta
Following diluting with ice-cold saline (1:9, w:v), the jejunal digesta were homogenized at 4°C, and centrifuged at 2,000 × g for 10 min at 4°C. The supernatants were used to measure the digestive enzyme activity. Then, the activities of trypsin, lipase, and amylase were determined with commercial kits from Nanjing Jiancheng Biochemistry Institute (Nanjing, China) according to the manufacturer's instructions.
Morphology and Histopathology Assay in Jejunum
The morphology and histopathology assay of jejunal mucosa was measured as described previously with some modification (Mao et al., 2019, 2022). In brief, jejunal segments that were fixed in 4% paraformaldehyde were embedded in paraffin. Then, these consecutive sections (4 µm) were stained with hematoxylin and eosin (H&E). 1) Morphology. Villus height and crypt depth were determined by using a Nikon Eclipse E100 microscope. Ten intact villi and crypts were randomly selected in each sample. 2) Histopathology. The histopathology in jejunum was observed with a Nikon Eclipse E100 microscope.
TUNEL Assay in Jejunum
After fixing in 4% paraformaldehyde, jejunum segments were embedded in paraffin, and sectioned into 4 μm thick slices. The apoptosis of sections was determined by TUNEL assay (In Situ Cell Death Detection Kit) according to the manufacturer's instructions (Roche, Shanghai, China). Briefly, the jejunal sections were washed in xylene and ethanol (95, 85, and 75%), diluted in double-distilled water, and incubated for 20 min at 37°C with proteinase K (Servicebio Biotech, Wuhan, China). The slides were rinsed 3 times in PBS. Then, the TUNEL reaction mixture was added to sections, which were incubated for 60 min at 37°C in a humidified atmosphere and dark place. DAPI (Solarbio, Beijing, China) was added to sections, which were incubated for 3 min. The slides were rinsed 3 times in PBS, again. The apoptotic cell was analyzed with a fluorescent microscope (Leica DMI4000B, Wetzlar, Germany). Three images were collected for each replicate, and TUNEL-positive cells were counted in villi. Then, the average number of TUNEL-positive cells in villi was calculated.
Measurement of Antioxidant Capacity in Serum and Jejunal Mucosa
About 100 mg of jejunal mucosa was added into ice-cold PBS (1:9, w:v), homogenized at 4°C, and then centrifuged at 5,000 × g for 15 min at 4°C. The supernatants were used to measure the related indices.
Total antioxidant capacity (T-AOC) and malondialdehyde (MDA) level in serum and jejunal mucosa were measured by using commercial kits from Beyotime Biotechnology (Jiangmen, China) according to the manufacturer's instructions. Myeloperoxidase (MPO) activity was analyzed in jejunal mucosa with commercial kits from Nanjing Jiancheng Biochemistry Institute (Nanjing, China) according to the manufacturer's instructions.
The mRNA Expression of Tight-Junction-Related and Antioxidant-Related Genes in Jejunal Mucosa
The extraction of total RNA in jejunal mucosa, the synthesis of first strand of cDNA, the real-time quantitative PCR, and the calculation of relative gene expression were executed as described previously (Mao et al., 2021). The gene-specific primers of this trial were listed in Table 2, and were purchased from Sangon Biotech (Shanghai, China).
Table 2.
Primer sequences used for real-time PCR.
| Gene | Primer | Nucleotide sequences, 5′–3′ |
|---|---|---|
| ZO-1 | Forward | GGCAAGTTGAAGATGGTGGT |
| Reverse | ATGCCAGCGACTGAATTTCT | |
| ZO-2 | Forward | AGCAGACCCTGCTCAACATT |
| Reverse | GGGGAGAACGATCTGTTTGA | |
| CLDN-1 | Forward | GTCTTTGGTGGCGTGATCTT |
| Reverse | TCTGGTGTTAACGGGGTGTGA | |
| OCLN | Forward | GCTGAGATGGACAGCATCAA |
| Reverse | TGCCACATCCTGGTATTGAG | |
| NQO1 | Forward | GTTCAATGCCGTGCTCTCAC |
| Reverse | CCGCTTCAATCTTCTTCTGC | |
| Nrf2 | Forward | TGTGTGTGATTCAACCCGACT |
| Reverse | TTAATGGAAGCCGCACCACT | |
| β-actin | Forward | ATCCGGACCCTCCATTGTC |
| Reverse | AGCCATGCCAATCTCGTCTT |
Abbreviations: CLDN-1, claudin 1; NQO1, NAD(P)H:quinone oxidoreductase 1; Nrf2, nuclear factor erythroid 2-related factor 2; OCLN, occludin; ZO-1, zonula occluden 1; ZO-2, zonula occluden 2.
Statistics Analysis
Statistical analyses were executed by using SPSS 20.0 (Statistical Product and Service Solutions, NY). The data of productive performance and egg quality in laying hens before challenge were analyzed by using the unpaired t test. The other data were analyzed as a 2 × 2 factorial with the general linear model procedures. The model factors included the addition of YSE, challenge and their interaction. All data were expressed as means with their SEs. The P < 0.01, P < 0.05, and P ≤ 0.10 were deemed the statistical high significance, significance, and tendency, respectively.
RESULTS
Productive Performance and Egg Quality
Before C. perfringens and coccidia challenge, the YSE-supplemented diet only tended to increase ADFI (P = 0.076, Table 3), and did not significantly affect egg quality in laying hens (P > 0.05, Table 4). Following C. perfringens and coccidia infection, egg weight, ADFI, FCR, laying rate, albumen weight, yolk color, yolk weight, eggshell thickness, eggshell weight, and eggshell colors were impaired in laying hens (P < 0.05, Tables 3 and 4). At the 6th wk, dietary YSE supplementation increased albumen height (P = 0.055) and weight (P < 0.05) in laying hens (Table 4). And in the challenged laying hens, YSE administration improved egg weight, albumen height, and yolk weight (P < 0.05, Tables 3 and 4).
Table 3.
Effects of dietary YSE supplementation on the productive performance of laying hens with or without Clostridium perfringens and coccidia challenge.
| Items | Nonchallenge |
Challenge |
P value |
||||
|---|---|---|---|---|---|---|---|
| CON | YSE | CON | YSE | YSE | Challenge | YSE × challenge | |
| 1–35 d | |||||||
| Egg weight, g | 58.83 ± 0.53 | 59.57 ± 0.46 | 0.297 | ||||
| ADFI, g | 105.23 ± 0.62 | 106.76 ± 0.57 | 0.076 | ||||
| FCR | 1.83 ± 0.02 | 1.84 ± 0.02 | 0.657 | ||||
| Laying rate | 0.98 ± 0.01 | 0.97 ± 0.01 | 0.468 | ||||
| Unqualified egg rate, % | 1.09 ± 0.38 | 0.73 ± 0.32 | 0.476 | ||||
| 36–45 d | |||||||
| Egg weight, g | 63.09 ± 0.74a | 62.33 ± 0.85a | 57.48 ± 0.77c | 59.87 ± 0.69b | 0.295 | <0.01 | 0.046 |
| ADFI, g | 114.64 ± 1.88 | 116.46 ± 0.68 | 101.18 ± 1.51 | 102.96 ± 2.64 | 0.327 | <0.01 | 0.991 |
| FCR | 1.87 ± 0.03 | 1.90 ± 0.03 | 2.30 ± 0.13 | 2.13 ± 0.09 | 0.431 | <0.01 | 0.239 |
| Laying rate | 0.98 ± 0.01 | 0.99 ± 0.01 | 0.79 ± 0.04 | 0.82 ± 0.04 | 0.420 | <0.01 | 0.670 |
| Unqualified egg rate, % | 1.43 ± 0.98 | 0.60 ± 0.60 | 2.23 ± 1.23 | 1.07 ± 0.85 | 0.295 | 0.499 | 0.863 |
Mean values within a row with unlike superscript letters are significantly different (P < 0.05; 1–35 d, n = 24; 36–45 d, n = 12).
Abbreviations: ADFI, average daily feed intake; Challenge, infusing Clostridium perfringens and coccidian; CON, the basal diet; FCR, feed conversion ratio; Nonchallenge, infusing sterile phosphate-buffered saline; YSE, Yucca schidigera extract-supplemented diet.
Table 4.
Effects of dietary YSE supplementation on the egg quality of laying hens with or without Clostridium perfringens and coccidia challenge.
| Items | Nonchallenge |
Challenge |
P value |
||||
|---|---|---|---|---|---|---|---|
| CON | YSE | CON | YSE | YSE | Challenge | YSE × challenge | |
| The 4th wk | |||||||
| Albumen height, mm | 7.77 ± 0.26 | 8.15 ± 0.16 | 0.210 | ||||
| Albumen weight, g | 36.97 ± 0.71 | 37.64 ± 0.54 | 0.459 | ||||
| Yolk color | 6.87 ± 0.10 | 6.65 ± 0.11 | 0.167 | ||||
| Haugh unit | 87.98 ± 1.63 | 89.73 ± 0.98 | 0.360 | ||||
| Yolk weight, g | 15.26 ± 0.24 | 15.48 ± 0.24 | 0.533 | ||||
| Eggshell strength, kg/cm3 | 5.21 ± 0.20 | 5.17 ± 0.12 | 0.859 | ||||
| Eggshell thickness, mm | 0.37 ± 0.01 | 0.37 ± 0.00 | 0.629 | ||||
| Eggshell weight, g | 6.32 ± 0.11 | 6.21 ± 0.06 | 0.366 | ||||
| Eggshell color L* | 75.44 ± 0.48 | 75.31 ± 0.42 | 0.839 | ||||
| Eggshell color a* | 7.13 ± 0.28 | 7.14 ± 0.22 | 0.963 | ||||
| Eggshell color b* | 21.40 ± 0.47 | 22.05 ± 0.48 | 0.335 | ||||
| The 6th wk | |||||||
| Albumen height, mm | 8.46 ± 0.54a | 8.11 ± 0.34a | 6.40 ± 0.72b | 8.92 ± 0.55a | 0.055 | 0.251 | 0.010 |
| Albumen weight, g | 39.54 ± 1.23 | 39.90 ± 0.64 | 34.52 ± 0.98 | 38.49 ± 0.85 | 0.028 | <0.01 | 0.062 |
| Yolk color | 7.32 ± 0.21 | 7.21 ± 0.18 | 7.08 ± 0.08 | 6.68 ± 0.10 | 0.128 | 0.025 | 0.369 |
| Haugh unit | 91.18 ± 2.66 | 89.59 ± 1.67 | 87.25 ± 1.41 | 93.28 ± 2.68 | 0.346 | 0.960 | 0.114 |
| Yolk weight, g | 16.55 ± 0.55 | 15.59 ± 0.35 | 13.76 ± 0.67 | 15.70 ± 0.44 | 0.312 | 0.012 | <0.01 |
| Eggshell strength, kg/cm3 | 4.02 ± 0.57 | 4.76 ± 0.28 | 3.87 ± 0.72 | 3.44 ± 0.41 | 0.758 | 0.144 | 0.242 |
| Eggshell thickness, mm | 0.35 ± 0.01 | 0.35 ± 0.01 | 0.30 ± 0.01 | 0.31 ± 0.01 | 0.686 | <0.01 | 0.755 |
| Eggshell weight, g | 6.20 ± 0.11 | 6.11 ± 0.15 | 4.65 ± 0.38 | 5.22 ± 0.35 | 0.381 | <0.01 | 0.236 |
| Eggshell color L* | 74.81 ± 1.70 | 75.78 ± 1.05 | 83.83 ± 1.97 | 83.75 ± 1.39 | 0.768 | <0.01 | 0.728 |
| Eggshell color a* | 7.61 ± 0.74 | 8.02 ± 0.84 | 3.02 ± 1.14 | 3.07 ± 0.75 | 0.798 | <0.01 | 0.839 |
| Eggshell color b* | 17.56 ± 3.38 | 21.18 ± 0.37 | 14.62 ± 2.22 | 15.44 ± 1.31 | 0.256 | 0.033 | 0.468 |
Mean values within a row with unlike superscript letters are significantly different (P < 0.05; the 4th wk, n = 12; the 6th wk, n = 6).
Abbreviations: a*, redness; b*, yellowness; Challenge, infusing Clostridium perfringens and coccidian; CON, the basal diet; L*, lightness; Nonchallenge, infusing sterile phosphate-buffered saline; YSE, Yucca schidigera extract-supplemented diet.
Digestive Enzyme Activity in Jejunal Digesta
As shown in Table 5, the activities of trypsin, amylase, and lipase in the jejunal digesta of laying hens were inhibited by oral administration of C. perfringens and coccidia (P < 0.01). Supplementing YSE in diet promoted the activities of trypsin (P < 0.01) and lipase (P < 0.05) in the jejunal digesta of laying hens (Table 5), and further attenuated the negative effect of C. perfringens and coccidia challenge on trypsin and lipase activities in the jejunal digesta of laying hens (Table 5).
Table 5.
Effects of dietary YSE supplementation on the activity of digestive enzymes in the jejunal digesta of laying hens with or without Clostridium perfringens and coccidia challenge.
| Items | Nonchallenge |
Challenge |
P value |
|||||
|---|---|---|---|---|---|---|---|---|
| CON | YSE | CON | YSE | YSE | Challenge | YSE × challenge | ||
| Trypsin, U/mg protein | 3188.95 ± 46.15 | 3247.89 ± 27.25 | 2584.15 ± 84.51 | 2847.61 ± 41.02 | <0.01 | <0.01 | 0.073 | |
| Amylase, U/mg protein | 1142.02 ± 35.23 | 1093.19 ± 26.92 | 865.67 ± 38.07 | 928.93 ± 31.40 | 0.830 | <0.01 | 0.214 | |
| Lipase, U/g protein | 1.26 ± 0.01 | 1.22 ± 0.03 | 0.77 ± 0.03 | 0.97 ± 0.04 | 0.031 | <0.01 | 0.702 | |
Abbreviations: Challenge, infusing Clostridium perfringens and coccidian; CON, the basal diet; Nonchallenge, infusing sterile phosphate-buffered saline; YSE, Yucca schidigera extract-supplemented diet.
Morphology and Histopathology in Jejunum
C. perfringens and coccidia challenge decreased villus height and villus height-to-crypt depth ratio, and increased crypt depth in the jejunum of laying hens (P < 0.01, Table 6). Dietary YSE supplementation enhanced villus height-to-crypt depth ratio, and reduced crypt depth in the jejunum of laying hens (P < 0.01, Table 6). And in the laying hens with C. perfringens and coccidia challenge, YSE administration relieved the effect of C. perfringens and coccidia challenge on the crypt depth of jejunum (P < 0.01, Table 6).
Table 6.
Effects of dietary YSE supplementation on the jejunal morphology of laying hens with or without Clostridium perfringens and coccidia challenge.
| Items | Nonchallenge |
Challenge |
P value |
||||
|---|---|---|---|---|---|---|---|
| CON | YSE | CON | YSE | YSE | Challenge | YSE × challenge | |
| Villus height, μm | 1084.06 ± 38.56 | 1139.69 ± 27.41 | 929.11 ± 33.38 | 857.40 ± 23.96 | 0.799 | <0.01 | 0.052 |
| Crypt depth, μm | 118.65 ± 5.19c | 107.36 ± 6.22c | 227.04 ± 8.71a | 169.55 ± 9.01b | <0.01 | <0.01 | <0.01 |
| V/C | 9.40 ± 0.39 | 11.15 ± 0.54 | 4.38 ± 0.20 | 5.37 ± 0.28 | <0.01 | <0.01 | 0.317 |
Mean values within a row with unlike superscript letters are significantly different (P < 0.05; n = 8).
Abbreviations: Challenge, infusing Clostridium perfringens and coccidian; CON, the basal diet; Nonchallenge, infusing sterile phosphate-buffered saline; V/C, villus height-to-crypt depth ratio; YSE, Yucca schidigera extract-supplemented diet.
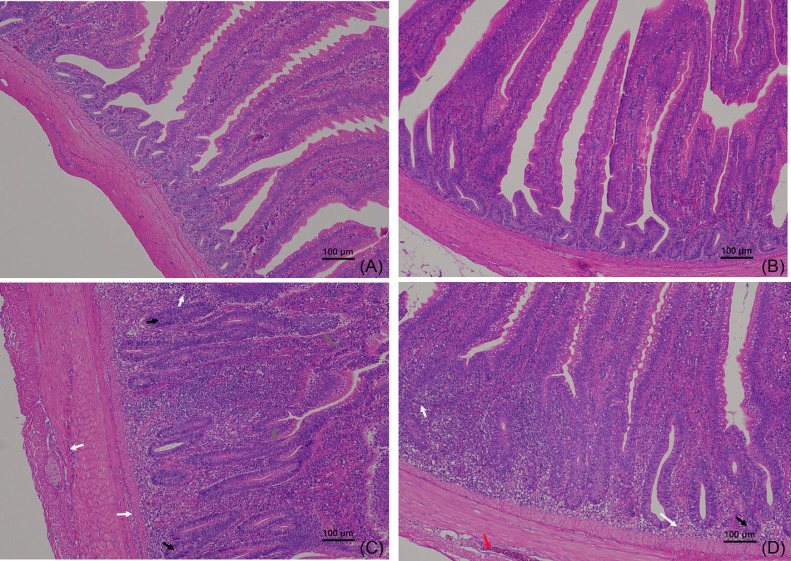
There were no pathological changes in the jejunum of nonchallenge laying hens (Figure 1). In the jejunum of laying hens feeding the basal diet and challenge of C. perfringens and coccidia, epithelial structure was damaged, intestinal glands were atrophic, and mucous and muscular layers had the extensive infiltration of neutrophils (Figure 1). In the jejunum of laying hens feeding the YSE-supplemented diet and challenge of C. perfringens and coccidia, epithelial structure was normal, some intestinal glands were atrophic, and mucous and muscular layers had the infiltration of some neutrophils, but there was a little hemorrhage in the muscular layer (Figure 1).
Figure 1.
The histopathology in the jejunum of laying hens. (A) The laying hens feeding the basal diet and infusing sterile phosphate-buffered saline; (B) the laying hens feeding the Yucca schidigera extract-supplemented diet and infusing sterile phosphate-buffered saline; (C) the laying hens feeding the basal diet and infusing Clostridium perfringens and coccidia; (D) the laying hens feeding the Yucca schidigera extract-supplemented diet and infusing Clostridium perfringens and coccidia. Gray arrow, the destruction of epithelial structure; black arrow, the atrophy of intestinal glands; white arrow, the infiltration of neutrophils; red arrow, hemorrhage.
The Apoptosis in Jejunal Mucosa
In this study, the apoptotic cell in the jejunal mucosa of laying hens was counted by TUNEL assay (Table 7 and Supplementary Figure 1). C. perfringens and coccidia challenge increased the number of apoptotic cells in the jejunal mucosa of laying hens (P < 0.01). Compared with the basal diet, the YSE-supplemented diet decreased the number of apoptotic cells in the jejunal mucosa of laying hens (P < 0.01). And in the laying hens of C. perfringens and coccidia challenge, dietary YSE supplementation alleviated the effect of challenge on apoptotic cell count in jejunal mucosa (P < 0.01).
Table 7.
Effects of dietary YSE supplementation on apoptotic cell count in the jejunal mucosa of laying hens with or without Clostridium perfringens and coccidia challenge.
| Items | Nonchallenge |
Challenge |
P value |
||||
|---|---|---|---|---|---|---|---|
| CON | YSE | CON | YSE | YSE | Challenge | YSE × challenge | |
| Apoptotic cell count (n/villi in an image) | 16.83 ± 3.24b | 19.33 ± 1.57b | 206.44 ± 30.99a | 51.44 ± 4.34b | <0.01 | <0.01 | <0.01 |
Mean values within a row with unlike superscript letters are significantly different (P < 0.05; n = 3).
Abbreviations: Challenge, infusing Clostridium perfringens and coccidian; CON, the basal diet; Nonchallenge, infusing sterile phosphate-buffered saline; YSE, Yucca schidigera extract-supplemented diet.
Antioxidant Capacity in Serum and Jejunal Mucosa
The effect of C. perfringens and coccidia challenge or dietary YSE supplementation or both on antioxidant capacity in the serum and jejunal mucosa of laying hens was shown in Table 8. Oral administration of C. perfringens and coccidia enhanced serum MDA level (P < 0.05) and the MPO activity of jejunal mucosa (P < 0.01), tended to increase the MDA concentration of jejunal mucosa (P = 0.052), and reduced the T-AOC of jejunal mucosa (P < 0.01) in laying hens. Dietary YSE supplementation increased the T-AOC of serum (P < 0.01) and jejunal mucosa (P < 0.01), and tended to decrease the MDA level of serum (P = 0.084) and jejunal mucosa (P = 0.078), and the MPO activity of jejunal mucosa (P = 0.100) in laying hens. Moreover, in the laying hens with C. perfringens and coccidia infection, YSE-supplemented diet improved serum MDA level, and T-AOC and MPO activity of jejunal mucosa (P < 0.05).
Table 8.
Effects of dietary YSE supplementation on antioxidant capacity in the serum and jejunal mucosa of laying hens with or without Clostridium perfringens and coccidia challenge.
| Items | Nonchallenge |
Challenge |
P value |
||||
|---|---|---|---|---|---|---|---|
| CON | YSE | CON | YSE | YSE | Challenge | YSE × challenge | |
| Serum | |||||||
| T-AOC, mmol/L | 3.06 ± 0.17 | 6.99 ± 0.67 | 3.28 ± 0.39 | 6.77 ± 0.55 | <0.01 | 0.993 | 0.651 |
| MDA, μmol/L | 15.00 ± 4.34b | 20.05 ± 2.70b | 51.49 ± 8.18a | 20.50 ± 4.14b | 0.084 | 0.026 | 0.028 |
| Jejunal mucosa | |||||||
| T-AOC, μmol/g protein | 13.90 ± 0.35b | 15.44 ± 0.21a | 8.20 ± 0.31d | 11.33 ± 0.47c | <0.01 | <0.01 | 0.030 |
| MDA, μmol/g protein | 3.57 ± 0.86 | 2.84 ± 0.41 | 6.18 ± 0.95 | 3.75 ± 0.73 | 0.078 | 0.052 | 0.326 |
| MPO, U/g protein | 12.29 ± 0.50c | 13.45 ± 0.49c | 29.18 ± 2.06a | 23.47 ± 1.40b | 0.100 | <0.01 | 0.016 |
Mean values within a row with unlike superscript letters are significantly different (P < 0.05; n = 6).
Abbreviations: Challenge, infusing Clostridium perfringens and coccidian; CON, the basal diet; MDA, malondialdehyde; MPO, myeloperoxidase; Nonchallenge, infusing sterile phosphate-buffered saline; T-AOC, total antioxidant capacity; YSE, Yucca schidigera extract-supplemented diet.
The mRNA Expression of Tight-Junction-Related and Antioxidant-Related Genes in Jejunal Mucosa
The effect of C. perfringens and coccidia challenge or dietary YSE supplementation or both on the mRNA expression of tight-junction-related and antioxidant-related genes in the jejunal mucosa of laying hens was shown in Table 9. The mRNA expression of zonula occluden 1 (ZO-1), ZO-2, claudin 1 (CLDN-1), occludin (OCLN), NAD(P)H:quinone oxidoreductase 1 (NQO1), and nuclear factor erythroid 2-related factor 2 (Nrf2) in the jejunal mucosa of laying hens was downregulated by oral administration of C. perfringens and coccidia (P < 0.01). Dietary YSE supplementation upregulated the mRNA expression of CLDN-1 (P < 0.05), NQO1 (P < 0.01), and Nrf2 (P < 0.01), and tended to enhance the mRNA expression of OCLN (P = 0.076) in the jejunal mucosa of laying hens. Additionally, in the laying hens with C. perfringens and coccidia infection, YSE-supplemented diet was beneficial for the mRNA expression of CLDN-1, OCLN, NQO1, and Nrf2 in jejunal mucosa.
Table 9.
Effects of dietary YSE supplementation on the expression of tight-junction-related and antioxidant-related genes in the jejunal mucosa of laying hens with or without Clostridium perfringens and coccidia challenge.
| Items | Nonchallenge |
Challenge |
P value |
||||
|---|---|---|---|---|---|---|---|
| CON | YSE | CON | YSE | YSE | Challenge | YSE × challenge | |
| ZO-1 | 1.00 ± 0.17 | 1.03 ± 0.04 | 0.37 ± 0.04 | 0.51 ± 0.08 | 0.349 | <0.01 | 0.537 |
| ZO-2 | 1.00 ± 0.13 | 1.10 ± 0.11 | 0.29 ± 0.03 | 0.40 ± 0.04 | 0.245 | <0.01 | 0.986 |
| CLDN-1 | 1.00 ± 0.16 | 1.11 ± 0.14 | 0.23 ± 0.04 | 0.54 ± 0.07 | 0.039 | <0.01 | 0.298 |
| OCLN | 1.00 ± 0.21 | 1.05 ± 0.14 | 0.28 ± 0.06 | 0.69 ± 0.09 | 0.076 | <0.01 | 0.156 |
| NQO1 | 1.00 ± 0.10 | 1.22 ± 0.05 | 0.33 ± 0.05 | 0.55 ± 0.09 | <0.01 | <0.01 | 0.909 |
| Nrf2 | 1.00 ± 0.16 | 1.50 ± 0.13 | 0.44 ± 0.05 | 0.77 ± 0.07 | <0.01 | <0.01 | 0.453 |
Abbreviations: Challenge, infusing Clostridium perfringens and coccidian; CLDN-1, claudin 1; CON, the basal diet; Nonchallenge, infusing sterile phosphate-buffered saline; NQO1, NAD(P)H:quinone oxidoreductase 1; Nrf2, nuclear factor erythroid 2-related factor 2; OCLN, occludin; YSE, Yucca schidigera extract-supplemented diet; ZO-1, zonula occluden 1; ZO-2, zonula occluden 2.
DISCUSSION
C. perfringens is widely recognized as the primary pathogenic strain responsible for inducing necrotic enteritis in poultry, and its rapid proliferation may be facilitated by various risk factors such as coccidia infection (Swayne, 2020). Numerous investigations have demonstrated that C. perfringens and coccidia infection can negatively impact growth performance and gut health, and may even lead to the development of necrotic enteritis in broilers (Quiroz-Castañeda and Dantán-González, 2015; Awad et al., 2017; Pham et al., 2020). Recently, our research has revealed that a challenge with C. perfringens and coccidia led to the decreased productive performance and egg quality, as well as damaged intestinal morphology and barrier function in laying hens (Zhang et al., 2022). In addition to these findings, our investigation has revealed that this challenge also reduced antioxidant capacity and induced the cell apoptosis in the jejunal mucosa of laying hens. Thus, based on these results, it is proposed that a model for inducing intestinal damage by C. perfringens and coccidia challenge in laying hens has been successfully established.
In animal production, YSE has gained significant attention as a green feed additive due to its ability to reduce toxic gas emissions (Pen et al., 2006; Liang et al., 2009). Numerous studies have shown that YSE administration can enhance growth and production performance in various livestock species such as pigs, broilers and layer hens (Espinosa Munoz et al., 2008; Gurbuz et al., 2011a,b; Alagawany et al., 2016; He et al., 2017; Sun et al., 2017). Notably, our recent study demonstrated that dietary YSE supplementation can promote growth performance in piglets (Fan et al., 2022). The results of this study showed that, in the laying hens challenged with C. perfringens and coccidia, the YSE-supplemented diet led to the improvement of productive performance and egg quality, including increased egg weight, albumen height and weight, and yolk weight.
The efficient digestion and absorption of nutrients are essential for promoting growth, productivity and overall health of animals, as reflected by improvements in productive performance and egg quality in laying hens. Digestive enzymes play a vital important role in facilitating nutrient digestion, and maintaining or increasing their activity in the intestine can potentially promote animal growth (Chen and Yu, 2020). Additionally, a reduction of crypt depth in mucosa indicates maturation of epithelial cells, which can promote intestinal secretion function (Chen and Yu, 2020). The increase in villus height-to-crypt depth ratio reflects the improvement of mucosa structure and villus absorptive area, leading to the enhanced nutrient digestion and absorption in intestine (Chen and Yu, 2020). Our study revealed that YSE administration increase the trypsin and lipase activities of jejunal digesta in the laying hens, as well as enhanced the jejunal villus height-to-crypt depth ratio in the laying hens, and alleviate the increasing jejunal crypt depth in the laying hens with C. perfringens and coccidia infection, which is similar with the results of piglets in our previous research on piglets (Fan et al., 2022). Thus, it is plausible that YSE improving productive performance and egg quality of the challenge hens should be related to the increase of digestive and absorptive functions.
The gut barrier is a critical component of intestinal function and overall health, encompassing some elements, such as mucosal integrity, and the intercellular junctions between epithelial cells (Mao et al., 2011). Morphological analysis is common method used to evaluate mucosal integrity, while the expression levels of transmembrane and nonmembrane proteins (such as ZOs, claudins, and occludin) determine the intercellular junctions between epithelial cells (Mao et al., 2011). Our findings indicate that YSE administration mitigated the negative effect of C. perfringens and coccidia challenge on morphology, histopathology and the tight-junction protein expression of jejunal mucosa, suggesting that YSE improved gut barrier function in laying hens. These results are consistent with previous studies that dietary YSE supplementation improved barrier function in the proximal intestine of broilers (Alagawany et al., 2016).
Cell survival is closely associated with gut functions (especially barrier function). Apoptosis is widely known as programmed cell death, and many factors (such as pathogen invasion) will induce cell apoptosis in tissues and organs (Xu et al., 2019). The cell apoptosis can be considered as the reflection of integrity and damage degree. In the current experiment, YSE administration inhibited the apoptotic cell count in the jejunal villi of laying hens with C. perfringens and coccidia infection. Thus, dietary YSE supplementation improved gut barrier function, which was derived from the protection of cell survival.
Redox balance is critical for cell survival and animal health. It involves the production of free radicals and antioxidant capacity (Zheng, 2007). T-AOC represents the total activity of all antioxidants, including antioxidant enzymes and nonenzymatic antioxidants (He et al., 2017). MDA is a type of lipid peroxide (He et al., 2017), while MPO promote the generation of reactive oxygen intermediates (Aratani, 2018). Previous studies have shown that supplementing YSE in diets can improve antioxidant capacity of the proximal intestine in broilers (Alagawany et al., 2016). Similarly, in this study, we also found that dietary YSE supplementation increased T-AOC, and decreased MDA level and MPO activity in the jejunal mucosa of laying hens challenged with C. perfringens and coccidia infection. These results suggest that YSE may alleviate cell apoptosis and morphology damage induced by challenge through improving the antioxidant capacity in the jejunal mucosa of laying hens.
MPO is mainly expressed in inflammatory cells (particularly neutrophils), and is known to play a role in tissue damage and inflammation in various inflammatory diseases (Aratani, 2018). In this study, YSE administration inhibited MPO activity in the jejunal mucosa of laying hens with challenge. This finding indirectly suggests that YSE may have contributed to the observed improvement in jejunal destruction and reduced neutrophil infiltration. The decrease in inflammatory status observed in this study is consistent with the result of histopathology in the jejunum of laying hens.
Nrf2 is a crucial transcription regulator that initiates the transcription of various antioxidant enzymes, including superoxide dismutase, glutathione peroxidase, heme oxygenase-1, glutathione reductase, thioredoxin reductase, ferritin, and NQO1, by binding to antioxidant response elements in the nucleus (Yamamoto et al., 2018). The Nrf2 signaling pathway plays a critical role in regulating antioxidant function. and animals with Nrf2 knockout display a dysfunction of their antioxidant system (Cheung et al., 2014). Recent researches have shown that some plant extract can increase antioxidant capacity through upregulating Nrf2 expression, thereby protecting cell and body from oxidative damage (Wen et al., 2019; Xue et al., 2020; Wang et al., 2022). In this study dietary YSE supplementation upregulated the expression of Nrf2 and NQO-1 in the jejunal mucosa of laying hens infected by C. perfringens and coccidia. Therefore, it is potential that YSE improved antioxidant capacity by stimulating Nrf2 pathway.
CONCLUSIONS
To conclude, we first used oral administration of C. perfringens and coccidia to successfully set up the model of gut damage in laying hens, and this led to the decrease of productive performance and egg quality. However, dietary YSE supplementation improved intestinal morphology and function, which was due to the increasing antioxidant capacity via upregulating Nrf2 pathway. As a result, YSE alleviated the negative impact of C. perfringens and coccidia infection on egg quality (including egg weight, albumen height and weight, and yolk weight) in laying hens. These findings suggest that YSE has potential as a feed additive in poultry breeding for improving gut health and productivity.
ACKNOWLEDGMENTS
This study was financially supported by the grant from the Science and Technology Support Project of Sichuan Province (2021YFYZ0008).
Ethics Approval: The animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of Sichuan Agricultural University (S2020314068). All experimental procedures followed established guidelines for the care and handling of laboratory animals.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for Publication: Not applicable.
Author Contributions: Conceptualization, X. M., B. Y., and J. H.; Funding acquisition, X. M.; Methodology, P. Z., J. Y., J. L. and Y. L.; Data analysis, H. Y. and J. W.; Project administration, Y. D., X. F., and X. M.; Writing-original draft, X. M., Y. D., and X. F.; Resources, H. W. and Q. W.; Writing-review & editing, H. Y. and X. M.
DISCLOSURES
The authors declare that there are no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.102822.
Appendix. Supplementary materials
REFERENCES
- Alagawany M., Abd El-Hack M.E., El-Kholy M.S. Productive performance, egg quality, blood constituents, immune functions, and antioxidant parameters in laying hens fed diets with different levels of Yucca schidigera extract. Environ. Sci. Pollut. Res. Int. 2016;23:6774–6782. doi: 10.1007/s11356-015-5919-z. [DOI] [PubMed] [Google Scholar]
- Aratani Y. Myeloperoxidase: its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018;640:47–52. doi: 10.1016/j.abb.2018.01.004. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Hess C., Hess M. Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins. 2017;9:1–22. doi: 10.3390/toxins9020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeke PR., Piacente S., Oleszek W. Anti-inflammatory and anti-arthritic effects of Yucca schidigera: a review. Inflammation. 2006;3:6. doi: 10.1186/1476-9255-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Yu B. 4th ed. China Agriculture Press; Beijing, China: 2020. Animal Nutrition. [Google Scholar]
- Cheung K.L., Lee J.H., Khor T.O., Wu T.Y., Li G.X., Chan J., Yang C.S., Kong A.N.T. Nrf2 knockout enhances intestinal tumorigenesis in Apc (min/+) mice due to attenuation of anti-oxidative stress pathway while potentiates inflammation. Mol. Carcinog. 2014;53:77–84. doi: 10.1002/mc.21950. [DOI] [PubMed] [Google Scholar]
- Espinosa Munoz V., Contreras A.D.G., Herrera Haro J.G., Alvarez Macias A.G., Estrada Barron S.G., Meza Cortes M. Effects of Yucca schidigera extract on biochemical and hematological profiles of growing and fattening pigs. Rev. Vet. 2008;18:51–58. [Google Scholar]
- Fan X., Xiao X., Chen D., Yu B., He J., Yu J., Luo J., Luo Y., Wang J., Yan H., Mao X. Yucca schidigera extract decreases nitrogen emission via improving nutrient utilisation and gut barrier function in weaned piglets. J. Anim. Physiol. Anim. Nutr. 2022;106:1036–1045. doi: 10.1111/jpn.13647. [DOI] [PubMed] [Google Scholar]
- Gurbuz E., Balevi T., Kurtoglu V., Oznurlu Y. Effects of adding yeast cell walls and Yucca schidigera extract to diets of layer chicks. Br. Poult. Sci. 2011;52:625–631. doi: 10.1080/00071668.2011.619517. [DOI] [PubMed] [Google Scholar]
- Gurbuz E., Balevi T., Kurtoglu V., Oznurlu Y. Use of yeast cell walls and Yucca schidigera extract in layer hens’ diets. Ital. J. Anim. Sci. 2011;10:134–138. doi: 10.1080/00071668.2011.619517. [DOI] [PubMed] [Google Scholar]
- He L., He T., Farrar S., Ji L., Liu T., Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017;44:532–553. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- Kucukkurt I., Akkol E.K., Karabag F., Ince S., Süntar I., Eryavuz A., Sözbilir N.B. Determination of the regulatory properties of Yucca schidigera extracts on the biochemical parameters and plasma hormone levels associated with obesity. Rev. Bras. Farmacogn. 2016;26:246–250. [Google Scholar]
- Liang G., Wang X., Wang X., Li C., Chen G. Effects of camphor familial plant extract and Yucca extracts on emission of NH3 and H2S in slurry of weaned pigs. Chin. J. Anim. Sci. 2009;45:22–26. [Google Scholar]
- Mao X., Ding X., Zeng Q., Bai S., Zhang K., Chen D., Yu B., He J., Yu J., Yan H., Luo J., Luo Y., Wang J. The effect of dietary pectic oligosaccharide supplementation on intestinal health of broiler breeders with different egg-laying rates. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X., Hu H., Xiao X., Chen D., Yu B., He J., Yu J., Zheng P., Luo J., Luo Y., Wang J. Lentinan administration relieves gut barrier dysfunction induced by rotavirus in a weaned piglet model. Food Funct. 2019;10:2094–2101. doi: 10.1039/c8fo01764f. [DOI] [PubMed] [Google Scholar]
- Mao X., Sun R., Wang Q., Chen D., Yu B., He J., Yu J., Luo J., Luo Y., Yan H., Wang J., Wang H., Wang Q. L-isoleucine administration alleviates DSS-induced colitis by regulating TLR4/MyD88/NF-κB pathway in rats. Front. Immunol. 2022;12 doi: 10.3389/fimmu.2021.817583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X., Zeng X., Qiao S., Wu G., Li D. Specific roles of threonine in intestinal mucosal integrity and barrier function. Front. Biosci. (Elite Ed.) 2011;3:1192–1200. doi: 10.2741/e322. [DOI] [PubMed] [Google Scholar]
- National Research Council . Nutrient Requirements of Poultry. 9th. Natl. Acad. Press; Washington, DC: 1994. [Google Scholar]
- Patel S. Yucca: a medicinally significant genus with manifold therapeutic attributes. NPB. 2012;2:231–234. [Google Scholar]
- Pen B., Sar C., Mwenya B., Kuwaki K., Morikawa R., Takahashi J. Effects of Yucca schidigera and Quillaja saponaria extracts on in vitro ruminal fermentation and methane emission. Anim. Feed Sci. Technol. 2006;129:175–186. [Google Scholar]
- Pham V.H., Kan L., Huang J., Geng Y., Zhen W., Guo Y., Abbas W., Wang Z. Dietary encapsulated essential oils and organic acids mixture improves gut health in broiler chickens challenged with necrotic enteritis. J. Anim. Sci. Biotechnol. 2020;11:18. doi: 10.1186/s40104-019-0421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz-Castañeda R.E., Dantán-González E. Control avian coccidiosis: future and present natural alternatives. BioMed. Res. Int. 2015;2015 doi: 10.1155/2015/430610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D.S., Jin X., Shi B.L., Su J.L., Tong M.M., Yan S.M. Dietary Yucca schidigera extract improved growth performance and liver antioxidative function in broilers. Ital. J. Anim. Sci. 2017;16:677–684. [Google Scholar]
- Swayne D.E. 14th ed. Wiley-Blackwell; Hoboken, NJ: 2020. Diseases of Poultry. [Google Scholar]
- Valverde Piedra J.L., Szymanczyk S.E., Kapica M., Puzio I., Pawlowska M., Michalowski P. Combined effect of butyrate and Yucca schidigera extract on the gastrointestinal tract of pigs around weaning. Krmiva. 2009;51:11–18. [Google Scholar]
- Wang Y., Li Q., Zha X., Luo J. Dendrobium fimbriatum hook polysaccharide ameliorates dextran-sodium-sulfate-induced colitis in mice via improving intestinal barrier function, modulating intestinal microbiota, and reducing oxidative stress and inflammatory responses. Food Funct. 2022;13:143–160. doi: 10.1039/d1fo03003e. [DOI] [PubMed] [Google Scholar]
- Wen Z., Xue R., Du M., Tang Z., Xiang X., Zheng B., Qu Y. Hemp seed polysaccharides protect intestinal epithelial cells from hydrogen peroxide-induced oxidative stress. Int. J. Biol. Macromol. 2019;135:203–211. doi: 10.1016/j.ijbiomac.2019.05.082. [DOI] [PubMed] [Google Scholar]
- Xu X., Lai Y., Hua Z. Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci. Rep. 2019;39 doi: 10.1042/BSR20180992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue R., Du M., Zhou T., Ai W., Zhang Z., Xiang X., Zhou Y., Wen Z. Polysaccharides from hemp seed protect against cyclophosphamide-induced intestinal oxidative damage via Nrf2-Keap1 signaling pathway in mice. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/1813798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Kensler T.W., Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018;98:1169–1203. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Ding X., Bai S., Zeng Q., Zhang K., Mao X., Chu L., Hou D., Xuan Y., Wang J. Alleviating effect of dietary supplementation of benzoic acid, Enterococcus faecium and essential oil complex on coccidia and Clostridium perfringerns challenge in laying hens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R. 3rd ed. Peking Higher Education Press; Beijing, China: 2007. Free Radical Biology. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.