Abstract
The present experiment was conducted to investigate the effect of different stocking densities on the organ development, blood biochemical indices, and antioxidative status of breeder pigeons during the rearing period. A total of 280 (half male and half female) 40-day-old young pigeons were allocated into 4 groups, including 3 experimental groups (in compartments of the flying room): the high stocking density (HSD) (0.308 m3/bird), standard stocking density (SD) (0.616 m3/bird), and low stocking density (LSD) (1.232 m3/bird) and a caged control (0.04125 m3/bird). The results showed that the contents of corticosterone and heat shock protein 70 in males and the corticosterone content in females were higher in the control than in the other groups. The relative weight of liver, lung, and gizzard in males of the HSD group was the highest among the 4 treatments, whereas the abdominal fat index in the control group was higher than those in the other 3 treatments. Body weight and the relative weight of liver and abdominal fat in female pigeons in HSD group increased significantly. The levels of serum urea nitrogen and uric acid in pigeons of LSD group increased significantly, while the concentration of total cholesterol and the activity of alanine aminotransferase were higher in the control group. Ion (K+, Ca2+, and Na+) concentrations in female pigeon serum were also elevated in the control. The activity of antioxidant enzymes, including the total antioxidant capacity, superoxide dismutase, and glutathione peroxidase in pigeon breast muscle and liver had different degrees of inhibition when the space room was crowded. Moreover, the level of malondialdehyde in the liver of male caged pigeons was higher than that in the other treatments. In summary, rearing in cages or at a high density caused stress responses in the breeder pigeons. The stocking density of breeder pigeons during the rearing period should be ranged from 0.616 m3/bird to 1.232 m3/bird.
Key words: stocking density, pigeon, organ development, blood biochemical indices, antioxidation
INTRODUCTION
The growth performance of chicks is closely related to pen space, feeding management, and nutritional levels (Campbell and Marquardt, 1977; Gabanakgosi and Moreki, 2014). Obtaining high-quality young breeder poultry on the farm is an essential step for the final production, such as egg production. The key to this stage is to cultivate healthy poultry flocks with good development, reaching a certain standard of weight and uniform physical and sexual maturity (Gaweł et al., 2022). Inner organs of poultry, including gonad, muscle, and bone develop rapidly during the rearing period. Previous studies have found that the initiation of follicular activity and surge of estrogen levels in blood coincides with the cessation of formation and progressive loss of structural bone in layer-type chickens (Webster, 2004; Bain et al., 2016). Errors made during this stage would cause irreversible loss in the laying phase (Coon, 2002).
The stocking density (SD) is closely related to feed efficiency, herd uniformity, animal welfare, and even the survival rate (Narejo et al., 2010; Kim, 2011; Guo et al., 2012; Erasmus, 2017). To reduce the economic cost from the increase in space quotas in production systems, traditional poultry farming generally opts for high-density conditions. However, excessive high-density breeding can result in negative physiological and behavioral responses in animal flocks (Mendl, 1999; Marin et al., 2001; Ravindran et al., 2006), including the appearance of chaos, fierce competition, anal pecking and feather pecking (Nicol et al., 1999; Yin et al., 2017). Studies have shown that high-density feeding can lead to a decline in the final body weight (BW) of broilers and can affect carcass development in some cases (Puron et al., 1995; Tong et al., 2014). In addition, poor flock immunity and increased mortality are easily caused by this feeding strategy (Cravener et al., 1992; Martrenchar et al., 1997; Feddes et al., 2002). In contrast, the high degree of freedom feeding system expands the activity space and reduces the pressure of the environment on poultry, improving its comfort and welfare index, which is conducive to the growth and development of poultry (Lewis et al., 1997; Fanatico et al., 2006). Therefore, to improve the growth potential and health of poultry, it is necessary to provide a suitable stocking density on the premise of lowering the production cost.
Organ development in animal can be significantly affected by the nutritional supply, activity level and environment factors (Hammond et al., 2001; Bauchinger and Biebach, 2006). In meat-type broiler, previous study found that specific metabolic organs including liver and heart were prone to be influenced by SD, but carcass yield seemed to be stable (Onbaşılar et al., 2008; Wang et al., 2014; Qaid et al., 2016). Serum biochemical indices are important for assessment of organ system function. They are commonly applied in the clinical research in nutriology, pathology, and physiology. Antioxidant defense system includes dietary antioxidants, antioxidative enzymes, and micromolecular sacrificial molecules. It is essential to protect against the damage caused by the reactive oxygen species (ROS). The stress from high SD has been shown to have negative effects on antioxidant status in broilers (Cai et al., 2019). However, whether these parameters changed in response to the effect of SD on the pigeons was still unknown.
The pigeon industry has experienced the rapid development in Asian countries in recent years. However, unlike other poultry, pigeons are altricial birds, and baby squabs (Columba livia domestica) rely on the cheese-like substance (crop milk) secreted by the crop tissue of both parents until they can feed themselves (Studer-Thiersch, 1967; Kierończyk et al., 2016). Due to the rich nutrients of pigeon milk, pigeons have a much faster growth rate than other poultry. At the age of 28 d, the body size is basically the same as that of adult pigeons. In addition, pigeons are good at flying. For migratory birds, physiological changes induced by energy-intensive activities can produce carry-over effects that will impact subsequent reproductive performance (Bauchinger et al., 2009; Legagneux et al., 2012). Regular exercise training has also been proven to benefit the antioxidative system and immune function of animals (Ruzicic et al., 2016). In China, flying rooms rather than only cages are often provided for young breeder pigeons during the rearing period before laying production. The majority of producers think that enough flying space and time could guarantee the expected laying performance and limited cage space is not beneficial to the development of birds during the breeding period. However, no one has evaluated the effects of flying on pigeon production until now, and there is also no data standard for the stocking density in the practice of pigeon farming. The hypothesis put forward here is that the size of flight space had an impact on the body development and antioxidative capacity of breeder pigeons.
Therefore, the present study investigated the effects of different stocking densities on the organ development, blood biochemical indices, and antioxidative status of young pigeons. This study aims to provide theoretical guidance for the breeding pigeon industry.
MATERIALS AND METHODS
All procedures used in this study were approved by the Animal Care Advisory Committee of Huaiyin Normal University.
Birds and Housing
All pigeons were obtained from a commercial pigeon farm (Xuzhou Kunpeng Pigeon Co., Ltd., Xuzhou, China). A total of 288 young white king pigeons (288 males and 288 females, 40-days old) with an average weight of 400 ± 15 g were selected from the cages and subjected to sex identification. Then, they were put into a flying room in a shed for the rearing period, and the room made of steel wire mesh was divided into thirty-six 1.2 m × 1.4 m × 2.2 m compartments (width × length × height) with purse seine. The room was 1-m above the ground. Pigeons were allocated into 4 groups: 3 experimental groups (in compartments of the flying room), high stocking density (HSD) (12 birds/compartment, 0.308 m3/bird), standard stocking density (SD) (6 birds/compartment, 0.616 m3/bird), and low stocking density (LSD) (3 birds/compartment, 1.232 m3/bird) and a control group (3 birds/cage, 0.04125 m3/bird). Pigeons in the control group were kept in breeding cages with dimensions of 0.55 m × 0.50 m × 0.45 m. No extra items were provided in the flying room. Every group had 6 replicates with the same sex each. Each compartment or cage was taken as one replicate. The whole experiment lasted for 90 d.
A compound diet of 55% corn, 24.5% soybean meal, 11% wheat, 1.2% dicalcium phosphate, 2% limestone, 0.25% salt, 0.5% vitamin and mineral premix, 2% soybean oil, 3.42% zeolite powder, 0.07% lysine, and 0.06% methionine was provided for the parent pigeons. The nutritional levels (16.67% crude protein, 12.00 MJ/kg metabolizable energy, 1.13% calcium, 0.34% available phosphorus, 0.89% lysine, and 0.31% methionine) of the diet were referenced in a previous study (Xie et al., 2016) and were calculated from data provided by Feed Database in China (2010). The birds had free access to feed, sand and water. During the whole experiment, 16 h of light was provided every day. The mean daily temperature was 23°C ± 4°C.
Sex Identification
Pigeon belongs to the monomorphic bird, and it is hard to identify the gender only from the appearance. To determining whether a similar size of flying room has different effects on males and females, sex identification was carried out, and it was based on the amplification of the chromo-helicase-DNA-binding 1 (CHD1) gene found on the sex chromosomes. The length of the CHD1 region differs between the 2 chromosomes. Males have a single product (CHD1Z) and females have 2 (CHD1W and CHD1Z) (Ellegren, 1996; Griffiths et al., 1996; Kahn et al., 1998; Lee et al., 2010) that can be distinguished by gel electrophoresis. Therefore, the present test was performed according to the method described by Zhang et al. (2013) with some modifications. Briefly, 10 μL of blood was sampled, and genomic DNA was extracted using a Genomic DNA Purification Kit (Tiangen Biotech Co., Ltd., Beijing, China). A pair of degenerate primers was designed to amplify fragments of the CHD1 gene. The sequence of CHD1F was 5′-TGCTTGTTTCCTYARTTC-3′ and that of CHD1R was 5′-WCAGATGGTKAGGATGCT-3′. PCR was performed in a total volume of 25 μL on a Thermal Cycler (Longene, China). The PCR mixture contained 2 μL of 2.5 mM dNTP mix, 2.5 μL of 10 × reaction buffer, 2 μL of 25 mM MgCl2, 2 μL of each primer, 2 μL of genomic DNA, 0.25 μL of ExTaq DNA polymerase (5 U/μL) (TaKaRa, Dalian, China), and 12.25 μL of Mili-Q water. The PCR procedure was conducted at 95°C for 3 min followed by 30 cycles of 94°C for 30 s, 56°C for 30 s, 72°C for 1 min, and a final cycle of 72°C for 7 min. The PCR products were analyzed by electrophoresis on a 1.2% agarose gel, and sex identification was judged by the number of bands as shown in Figure 1. Due to the difference in the CHD1 region between the 2 chromosomes, males have a single product (CHD1Z) and females have 2 products (CHD1W and CHD1Z) (Figure 2).
Figure 1.
Sex determination of 22 pigeons based on the amplification of the chromo-helicase-DNA-binding 1 (CHD1) gene in blood samples. Lane 1–22: pigeon 1 to pigeon 22. Male (1 band): pigeon 4, 7, 9, 12, 17, 18, and 19. Female (2 bands): pigeon 1, 2, 3, 5, 6, 8, 10, 11, 13, 14, 15, 16, 20, 21, and 22.
Figure 2.
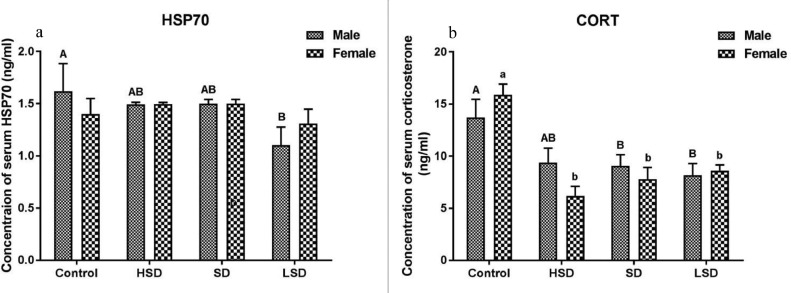
Concentrations of HSP70 (A) and corticosterone (CORT) (B) in serum of male and female pigeons in the control group, high stocking density group (HSD), standard stocking density group (SD), and low stocking density group (LSD). The graphical data represent mean ± SEM of 6 pigeons per group. The bars representing the treatment with different superscripts are significantly different at P < 0.05.
Sample Collection
After fasting for 12 h, the pigeons were weighed. One pigeon from each replicate was randomly chosen for sampling. Blood from the wing vein was collected and centrifuged at 1,000 × g for 10 min. Serum was pipetted, and then stored at −20°C for subsequent analysis. Then pigeons were euthanized by cervical dislocation to collect the organs, including heart, liver, lung, kidney, leg muscle, breast muscle, muscular stomach, glandular stomach, and abdominal fat. The ratio of organ to BW was calculated according to the percentage of BW. The liver and breast muscle tissues were stored at −80°C for the subsequent inspection.
ELISA
Heat shock protein70 (HSP70) and corticosterone (CORT) in pigeon serum were measured using commercial ELISA kits (Cusabio Biotech Co., Ltd., Wuhan, China). According to the instruction, 50 uL of standard or sample was added per well, and then 50 uL of antibody was mixed immediately. The plate was shaken for 30 s, and incubated for 30 min at 25°C. The reaction liquid was cleaned up, and each well was washed for 4 times with buffer. Horseradish peroxidase (HRP) secondary conjugate was added and incubated for 30 min followed by 4 times washing. 3,3′,5,5′-Tetramethylbenzidine (TMB) substrate was used for the color reaction, and it was terminated by the stop solution. Finally, absorbance at 450 nm was recorded by a microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA).
Biochemical Analysis
The concentrations of total protein (TP), albumin (ALB), globulin (GLO), glucose (GLU), triglyceride (TG), total cholesterol (TCHOL), high-density lipoprotein (HDL), low-density lipoprotein (LDL), urea nitrogen (UN), uric acid (UA), K+, Na+, Ca2+, and Cl− in pigeon serum were determined by an automated system (7020 analyzer, Hitachi High Technologies Co., Tokyo, Japan). The activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) in pigeon serum were measured by a microplate reader (SpectraMax M5; Molecular Devices). All these parameters were investigated according to the protocols from the commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The serum globulin (GLB) concentration was calculated by subtracting the ALB concentration from the TP concentration.
Enzyme Activity
The commercial kits for the measurement of the antioxidant parameters were also purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The liver and breast muscle samples were prepared as 10% homogenates with physiological saline. Both liver and breast muscle were analyzed for the activity of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), total antioxidant capability (T-AOC), and the content of the lipid peroxidation product malondialdehyde (MDA). The protein concentration in the samples was measured by the BCA protein assay method. The absorbance value was measured by using a microplate reader (SpectraMax M5; Molecular Devices).
Statistical Analysis
All the data are presented as the mean ± standard error of the mean (SEM). The data were statistically evaluated using SPSS 20.0 for Windows (SPSS Inc., Chicago, IL). Variables in males or in females during breeding were analyzed with 1-way analysis of variance (ANOVA) followed by Duncan's post hoc test. Differences or effects were considered significant at P < 0.05.
RESULTS
HSP70 and CORT in Serum
As shown in Figure 1, the concentrations of HSP70 and CORT in the serum of male young pigeons in the LSD group were significantly lower than those in the control group (P < 0.05) (Figure 1A and B). In females, there was no significant difference in the serum HSP70 concentration among the treatments, but the CORT content in the control group was significantly higher than that in the other groups (P < 0.001).
Body Weight and Relative Organ Weight
As shown in Table 1, there was no significant difference in the BW and relative weight (RW) of the heart, kidney, breast, thigh, and proventriculus in young male pigeons exposed to different stocking densities (P > 0.05). The RWs of lung and gizzard in males of HSD were the highest among the 4 treatments, whereas the RW of abdominal fat in control group was higher than that in the other 3 treatments (P < 0.05). In contrast to males, the BW of female young pigeons in LSD was lower (P = 0.028). The RWs of liver and abdominal fat in females of LSD were the lowest (P = 0.038; P < 0.001). In addition, no statistically differences were found among the groups in the RW of other organs in young female pigeons (P > 0.05).
Table 1.
Body weight (BW) and relative weight (RW) of organ in male and female young pigeons exposed to different stocking densities.
| Item2 | Stocking density1 |
SEM | P value | |||
|---|---|---|---|---|---|---|
| Control | HSD | SD | LSD | |||
| BW (g) | ||||||
| Male | 497.13 ± 12.28 | 498.26 ± 16.29 | 488.54 ± 6.78 | 497.46 ± 11.03 | 5.50 | 0.929 |
| Female | 448.05 ± 6.06ab | 461.49 ± 8.24a | 451.59 ± 6.35a | 430.08 ± 11.03b | 4.11 | 0.028 |
| RW (%) | ||||||
| Heart | ||||||
| Male | 1.17 ± 0.04 | 1.19 ± 0.05 | 1.09 ± 0.085 | 1.04 ± 0.03 | 0.032 | 0.276 |
| Female | 1.19 ± 0.08 | 1.18 ± 0.04 | 1.21 ± 0.083 | 1.12 ± 0.06 | 0.032 | 0.774 |
| Liver | ||||||
| Male | 1.78 ± 0.05A | 1.59 ± 0.09AB | 1.45 ± 0.076B | 1.49 ± 0.08B | 0.049 | 0.035 |
| Female | 1.65 ± 0.05a | 1.76 ± 0.14a | 1.58 ± 0.06b | 1.38 ± 0.04b | 0.051 | 0.038 |
| Lung | ||||||
| Male | 0.24 ± 0.02AB | 0.28 ± 0.02A | 0.23 ± 0.013AB | 0.21 ± 0.01B | 0.012 | 0.032 |
| Female | 0.24 ± 0.02 | 0.24 ± 0.02 | 0.25 ± 0.01 | 0.21 ± 0.01 | 0.007 | 0.278 |
| Kidney | ||||||
| Male | 0.84 ± 0.07 | 0.89 ± 0.02 | 0.83 ± 0.061 | 0.79 ± 0.04 | 0.025 | 0.582 |
| Female | 0.84 ± 0.03 | 0.99 ± 0.11 | 0.91 ± 0.02 | 0.89 ± 0.04 | 0.031 | 0.449 |
| Breast | ||||||
| Male | 11.22 ± 0.15 | 10.51 ± 0.26 | 10.29 ± 0.368 | 10.47 ± 0.31 | 0.16 | 0.238 |
| Female | 11.36 ± 0.54 | 10.73 ± 0.39 | 10.42 ± 0.26 | 10.76 ± 0.36 | 0.19 | 0.440 |
| Thigh | ||||||
| Male | 2.53 ± 0.13 | 2.41 ± 0.14 | 2.37 ± 0.14 | 2.81 ± 0.13 | 0.079 | 0.153 |
| Female | 2.41 ± 0.14 | 2.59 ± 0.12 | 2.45 ± 0.11 | 2.41 ± 0.07 | 0.053 | 0.601 |
| Gizzard | ||||||
| Male | 2.59 ± 0.08AB | 2.89 ± 0.09A | 2.48 ± 0.06B | 2.51 ± 0.21B | 0.066 | 0.041 |
| Female | 2.74 ± 0.21 | 2.84 ± 0.14 | 2.99 ± 0.25 | 2.87 ± 0.11 | 0.086 | 0.831 |
| Proventriculus | ||||||
| Male | 0.19 ± 0.01 | 0.20 ± 0.02 | 0.16 ± 0.01 | 0.15 ± 0.01 | 0.009 | 0.168 |
| Female | 0.21 ± 0.02 | 0.21 ± 0.01 | 0.18 ± 0.01 | 0.18 ± 0.01 | 0.006 | 0.136 |
| Abdominal fat | ||||||
| Male | 0.78 ± 0.04A | 0.78 ± 0.05A | 0.57 ± 0.05B | 0.39 ± 0.01C | 0.047 | <0.001 |
| Female | 0.96 ± 0.09a | 0.87 ± 0.07a | 0.56 ± 0.05b | 0.48 ± 0.02b | 0.054 | <0.001 |
HSD: high stocking density (0.308 m3/bird); SD: standard stocking density (0.616 m3/bird); LSD: low stocking density (1.232 m3/bird); Control: every 3 pigeons were kept in 1 breeding cage (0.04125 m3/bird).
BW = body weight; RW: relative weight.
Mean values within the same row not sharing a common superscript letter are significantly different (P < 0.05).
Serum Biochemical Indices
As shown in Table 2, there was no significant difference in the serum TP, ALB, and GLO concentrations of breeder pigeons exposed to different stocking densities (P > 0.05). The GLU concentration in male pigeon serum increased significantly in LSD group (P = 0.021), while the TCHOL concentration was highest in caged birds of the control group (P = 0.047) (Table 3). In females, caged birds had a lower HDL concentration than SD birds (P = 0.034). No remarkable changes were found in the TG and LDL parameters in pigeons. Both serum UN and UA increased to the maximum value in male and female breeder pigeons of the LSD group (P < 0.05) (Table 4).
Table 2.
Concentrations of serum total protein (TP), albumin (ALB), and globulin (GLO) of male and female young pigeons exposed to different stocking densities.
| Item2 | Stocking density1 |
SEM | P value | |||
|---|---|---|---|---|---|---|
| Control | HSD | SD | LSD | |||
| TP (g/L) | ||||||
| Male | 26.60 ± 0.58 | 24.85 ± 1.38 | 24.12 ± 0.85 | 27.05 ± 0.25 | 0.55 | 0.184 |
| Female | 25.16 ± 0.64 | 23.44 ± 0.9 | 25.48 ± 1.27 | 25.46 ± 0.79 | 0.47 | 0.382 |
| ALB (g/L) | ||||||
| Male | 18.30 ± 1.11 | 17.35 ± 0.61 | 16.30 ± 0.91 | 17.10 ± 0.17 | 0.40 | 0.406 |
| Female | 16.32 ± 0.45 | 15.92 ± 0.72 | 17.40 ± 0.56 | 17.18 ± 1.01 | 0.36 | 0.441 |
| GLO (g/L) | ||||||
| Male | 8.30 ± 0.56 | 7.50 ± 0.51 | 7.82 ± 0.35 | 9.95 ± 0.08 | 0.32 | 0.141 |
| Female | 8.84 ± 0.38 | 7.52 ± 0.51 | 8.08 ± 0.72 | 8.28 ± 0.43 | 0.27 | 0.397 |
HSD: high stocking density (0.308 m3/bird); SD: standard stocking density (0.616 m3/bird); LSD: low stocking density (1.232 m3/bird); Control: every 3 pigeons were kept in 1 breeding cage (0.04125 m3/bird).
TP: total protein; ALB: albumin; GLO: globulin.
Table 3.
Concentrations of serum glucose (GLU), triglyceride (TG), total cholesterol (TCHOL), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) of male and female young pigeons exposed to different stocking densities.
| Item2 | Stocking density1 |
SEM | P value | |||
|---|---|---|---|---|---|---|
| Control | HSD | SD | LSD | |||
| GLU (mmol/L) | ||||||
| Male | 17.19 ± 0.61C | 17.97 ± 0.53BC | 19.28 ± 0.17AB | 20.29 ± 0.83A | 0.421 | 0.021 |
| Female | 18.41 ± 0.46 | 18.41 ± 0.59 | 19.88 ± 0.88 | 18.31 ± 0.28 | 0.314 | 0.234 |
| TG (mmol/L) | ||||||
| Male | 1.38 ± 0.09 | 1.37 ± 0.09 | 1.21 ± 0.21 | 1.05 ± 0.01 | 0.072 | 0.393 |
| Female | 1.66 ± 0.15 | 1.27 ± 0.11 | 1.43 ± 0.25 | 1.45 ± 0.21 | 0.093 | 0.568 |
| TCHOL (mmol/L) | ||||||
| Male | 8.65 ± 0.08A | 8.11 ± 0.16A | 8.14 ± 0.02A | 7.48 ± 0.51B | 0.179 | 0.047 |
| Female | 8.59 ± 0.39a | 7.15 ± 0.63b | 7.62 ± 0.41b | 7.85 ± 0.56b | 0.264 | 0.291 |
| HDL (mmol/L) | ||||||
| Male | 4.49 ± 0.17 | 4.31 ± 0.11 | 4.07 ± 0.25 | 4.53 ± 0.16 | 0.097 | 0.353 |
| Female | 3.65 ± 0.41b | 4.05 ± 0.21ab | 4.93 ± 0.17a | 4.42 ± 0.28ab | 0.169 | 0.034 |
| LDL (mmol/L) | ||||||
| Male | 3.53 ± 0.21 | 3.18 ± 0.21 | 2.85 ± 0.21 | 3.13 ± 0.19 | 0.114 | 0.238 |
| Female | 2.81 ± 0.31 | 2.92 ± 0.28 | 3.01 ± 0.33 | 2.77 ± 0.31 | 0.145 | 0.949 |
HSD: high stocking density (0.308 m3/bird); SD: standard stocking density (0.616 m3/bird); LSD: low stocking density (1.232 m3/bird); Control: every 3 pigeons were kept in 1 breeding cage (0.04125 m3/bird).
GLU: glucose; TG: triglyceride; TCHOL: total cholesterol; HDL: high-density lipoprotein; LDL: low-density lipoprotein.
Mean values within the same row not sharing a common superscript letter are significantly different (P < 0.05).
Table 4.
Concentrations of serum urea nitrogen (UN) and uric acid (UA) of male and female young pigeons exposed to different stocking densities.
| Item2 | Stocking density1 |
SEM | P value | |||
|---|---|---|---|---|---|---|
| Control | HSD | SD | LSD | |||
| UN (mmol/L) | ||||||
| Male | 0.35 ± 0.02B | 0.37 ± 0.02AB | 0.45 ± 0.03A | 0.46 ± 0.03A | 0.018 | 0.043 |
| Female | 0.28 ± 0.02b | 0.38 ± 0.03ab | 0.47 ± 0.03a | 0.48 ± 0.08a | 0.029 | 0.031 |
| UA (Umol/L) | ||||||
| Male | 221 ± 8.35B | 227.25 ± 10.42B | 289.5 ± 39.8AB | 320.3 ± 33.57A | 16.35 | 0.044 |
| Female | 194.20 ± 14.51b | 253.4 ± 23.55ab | 245.40 ± 26.58ab | 315.20 ± 28.27a | 14.72 | 0.020 |
HSD: high stocking density (0.308 m3/bird); SD: standard stocking density (0.616 m3/bird); LSD: low stocking density (1.232 m3/bird); Control: every 3 pigeons were kept in 1 breeding cage (0.04125 m3/bird).
UN: urea nitrogen; UA: uric acid.
Mean values within the same row not sharing a common superscript letter are significantly different (P < 0.05).
Different stocking densities had effects on the ion concentrations in pigeon serum (P > 0.05) (Table 5). In male pigeons, the contents of K+ and Na+ were significantly decreased in male pigeons in LSD compared with those in the other groups (P = 0.031; P = 0.046), and the Ca2+ level in the control group was the highest (P = 0.002). In females, only the Ca2+ level was affected by the stocking density treatment, and it was also the highest in the control group (P = 0.016). No changes were found in the Cl− levels in either male or female pigeons exposed to different stocking densities (P > 0.05).
Table 5.
Concentrations of serum K+, Na+, Ca2+, and Cl− of male and female young pigeons exposed to different stocking densities.
| Item | Stocking density1 |
SEM | P value | |||
|---|---|---|---|---|---|---|
| Control | HSD | SD | LSD | |||
| K+ (mmol/L) | ||||||
| Male | 5.66 ± 0.14A | 5.63 ± 0.25A | 5.42 ± 0.28A | 4.53 ± 0.09B | 0.158 | 0.031 |
| Female | 5.48 ± 0.22 | 4.71 ± 0.21 | 5.27 ± 0.31 | 5.33 ± 0.15 | 0.124 | 0.125 |
| Na+ (mmol/L) | ||||||
| Male | 147.00 ± 1.52A | 141.00 ± 0.41B | 145.20 ± 0.47AB | 138.40 ± 1.57B | 0.468 | 0.046 |
| Female | 147.80 ± 1.2 | 144.60 ± 0.97 | 146.20 ± 0.86 | 146.40 ± 0.91 | 0.521 | 0.195 |
| Ca2+ (mmol/L) | ||||||
| Male | 2.55 ± 0.01A | 2.26 ± 0.03B | 2.31 ± 0.05B | 2.32 ± 0.01B | 0.034 | 0.002 |
| Female | 2.42 ± 0.04a | 2.22 ± 0.03b | 2.33 ± 0.03ab | 2.29 ± 0.04b | 0.024 | 0.016 |
| Cl− (mmol/L) | ||||||
| Male | 111.93 ± 2.18 | 111.30 ± 0.61 | 112.22 ± 1.09 | 110.45 ± 0.49 | 0.551 | 0.745 |
| Female | 112.90 ± 1.63 | 111.26 ± 1.31 | 112.00 ± 0.43 | 112.42 ± 0.61 | 0.529 | 0.762 |
HSD: high stocking density (0.308 m3/bird); SD: standard stocking density (0.616 m3/bird); LSD: low stocking density (1.232 m3/bird); Control: every 3 pigeons were kept in 1 breeding cage (0.04125 m3/bird).
Mean values within the same row not sharing a common superscript letter are significantly different (P < 0.05).
As shown in Table 6, in male pigeons, serum ALT activity was significantly higher than that in the other groups, but there were no significant changes in females. Different stocking densities also had no effects on the activities of AST and ALP in male and female pigeons.
Table 6.
Activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) in serum of male and female young pigeons exposed to different stocking densities.
| Item2 | Stocking density (birds/m3)1 |
SEM | P value | |||
|---|---|---|---|---|---|---|
| Control | HSD | SD | LSD | |||
| ALT (U/L) | ||||||
| Male | 34.50 ± 4.33A | 25.75 ± 0.94B | 20.50 ± 2.21B | 25.00 ± 2.51B | 1.756 | 0.021 |
| Female | 29.40 ± 2.11a | 24.40 ± 1.96ab | 22.80 ± 4.01b | 21.40 ± 3.65b | 1.448 | 0.031 |
| AST (U/L) | ||||||
| Male | 220 ± 57.73 | 150.25 ± 17.99 | 179.25 ± 22.65 | 165.33 ± 21.4 | 15.123 | 0.476 |
| Female | 173.40 ± 10.54 | 135.00 ± 20.48 | 158.60 ± 30.85 | 132.40 ± 12.73 | 10.088 | 0.446 |
| ALP (U/L) | ||||||
| Male | 269.66 ± 45.4 | 293.25 ± 31.08 | 230.00 ± 25.63 | 254.00 ± 16.16 | 15.152 | 0.505 |
| Female | 279.60 ± 18.71 | 264.80 ± 29.05 | 215.20 ± 29.33 | 206.40 ± 18.24 | 13.312 | 0.129 |
HSD: high stocking density (0.308 m3/bird); SD: standard stocking density (0.616 m3/bird); LSD: low stocking density (1.232 m3/bird); Control: every 3 pigeons were kept in 1 breeding cage (0.04125 m3/bird).
ALT: alanine aminotransferase; AST: aspartate aminotransferase; ALP: alkaline phosphatase.
, a-bMean values within the same row not sharing a common superscript letter are significantly different (P < 0.05).
Antioxidative Status in Liver and Breast Muscle
As shown in Table 7, the activities of T-AOC and SOD in male breast muscle of SD treatment group were significantly higher than those of the control group (P < 0.05). However, in females, the activities of 3 antioxidative enzymes, namely, T-AOC, SOD, and GSH-Px in breast muscle were all increased to a maximum in the LSD group. The MDA content showed no significant changes in pigeon breast muscle (P > 0.05).
Table 7.
Antioxidative status of breast muscle in male and female young pigeons exposed to different stocking densities.
| Item2 | Stocking density1 |
SEM | P value | |||
|---|---|---|---|---|---|---|
| Control | HSD | SD | LSD | |||
| T-AOC (U/mg protein) | ||||||
| Male | 92.5 ± 17.27B | 98.0 ± 7.32B | 145.1 ± 13.67A | 116.5 ± 11.86AB | 7.71 | 0.037 |
| Female | 88.85 ± 13.52b | 83.26 ± 10.27b | 96.02 ± 3.15b | 129.52 ± 10.02a | 6.15 | 0.021 |
| SOD (U/mg protein) | ||||||
| Male | 179.1 ± 15.64B | 183.90 ± 7.45B | 251.4 ± 24.69A | 210.2 ± 18.76AB | 11.53 | 0.039 |
| Female | 189.41 ± 13.74ab | 164.09 ± 16.46b | 220.94 ± 16.19a | 225.74 ± 13.62a | 8.98 | 0.035 |
| GSH-Px (U/mg protein) | ||||||
| Male | 51.85 ± 5.35 | 55.93 ± 2.94 | 44.63 ± 5.01 | 52.27 ± 1.42 | 2.12 | 0.243 |
| Female | 49.97 ± 1.58b | 42.20 ± 1.32c | 57.38 ± 3.08a | 57.43 ± 2.57a | 1.78 | <0.001 |
| MDA (nmol/mg protein) | ||||||
| Male | 1.06 ± 0.07 | 1.02 ± 0.09 | 1.06 ± 0.08 | 1.13 ± 0.17 | 0.05 | 0.916 |
| Female | 1.06 ± 0.09 | 1.39 ± 0.16 | 1.31 ± 0.07 | 1.50 ± 0.21 | 0.08 | 0.097 |
HSD: high stocking density (0.308 m3/bird); SD: standard stocking density (0.616 m3/bird); LSD: low stocking density (1.232 m3/bird); Control: every 3 pigeons were kept in 1 breeding cage (0.04125 m3/bird).
T-AOC: total antioxidant capability; SOD: superoxide dismutase; GSH-Px: glutathione peroxidase; MDA: malondialdehyde.
Mean values within the same row not sharing a common superscript letter are significantly different (P < 0.05).
As shown in Table 8, T-AOC and GSH-Px activity in the male liver and SOD activity in the female liver were the lowest in the control group (P < 0.05). The MDA content in the liver of male caged pigeons was higher than that in the other treatments (P = 0.032). In addition, no significant differences in the activity of T-AOC and GSH-Px in the liver were found among the treatments in females.
Table 8.
Antioxidative status of liver in male and female young pigeons under different stocking densities.
| Item2 | Stocking density1 |
SEM | P value | |||
|---|---|---|---|---|---|---|
| Control | HSD | SD | LSD | |||
| T-AOC (U/mg protein) | ||||||
| Male | 251.7 ± 23.8B | 409.6 ± 26.6A | 429.6 ± 34.2A | 428.4 ± 30.1A | 23.07 | 0.009 |
| Female | 345.2 ± 44.18 | 348.4 ± 43.69 | 315.2 ± 35.58 | 300.1 ± 36.45 | 19.01 | 0.795 |
| SOD (U/mg protein) | ||||||
| Male | 67.68 ± 3.54 | 81.44 ± 7.21 | 74.92 ± 7.07 | 71.34 ± 4.62 | 3.27 | 0.514 |
| Female | 52.03 ± 6.81b | 60.51 ± 6.55ab | 79.19 ± 2.49a | 64.36 ± 8.77ab | 3.83 | 0.049 |
| GSH-Px (U/mg protein) | ||||||
| Male | 41.27 ± 2.35C | 63.96 ± 4.89B | 52.79 ± 4.79BC | 97.13 ± 13.05A | 5.83 | <0.001 |
| Female | 33.71 ± 4.42 | 34.08 ± 4.17 | 32.33 ± 3.45 | 36.78 ± 5.19 | 2.03 | 0.906 |
| MDA (nmol/mg protein) | ||||||
| Male | 2.84 ± 0.14A | 2.37 ± 0.34B | 2.59 ± 0.68B | 2.45 ± 0.27B | 0.22 | 0.032 |
| Female | 2.18 ± 0.32 | 1.87 ± 0.14 | 1.92 ± 0.21 | 1.65 ± 0.26 | 0.12 | 0.063 |
HSD: high stocking density (0.308 m3/bird); SD: standard stocking density (0.616 m3/bird); LSD: low stocking density (1.232 m3/bird); Control: every 3 pigeons were kept in 1 breeding cage (0.04125 m3/bird).
T-AOC: total antioxidant capability; SOD: superoxide dismutase; GSH-Px: glutathione peroxidase; MDA: malondialdehyde.
Mean values within the same row not sharing a common superscript letter are significantly different (P < 0.05).
DISCUSSION
Under the condition of high-density feeding, birds have little space to move and cannot realize their instinctive behavior, so they may be in an irritable state and experience helplessness and even pain (Albentosa et al., 2007). HSP70 known as a stress protein, is highly conserved and widely exists in organisms. When cells are stimulated by the unfavorable factors, the production of HSP70 is induced (Zager and Johnson, 2001). Studies have shown that the concentration of serum HSP70 in chickens kept at a high density is significantly increased (Zager and Johnson, 2001; Cai et al., 2019). The serum HSP70 content of caged male pigeons was significantly higher than that in the low-density group. Physiological stress (feed deprivation, heat stress, and dexamethasone injection) or psychological stress (individual isolation) significantly induces the expression of HSP70 in serum (Greene et al., 2019). A high stocking density, as a type of stressor, may induce a stress level in the pigeon body. Moreover, HSP70 appears to be involved in the protection of cell damage associated with cytokines and energy expenditure (Kregel, 2002). This means that the high level of HSP70 may protect breeding pigeons from the crowding stress produced by high stocking density. In addition, the cortisol level of breeding pigeons in the cages was also significantly increased. Corticosterone is a kind of glucocorticoid that has a strong effect on carbohydrate metabolism. The animal is often in a state of fatigue under high pressure. To maintain normal physiological function, the body will increase the synthesis and release of cortisol (El-Tarabany, 2016; Eugen et al., 2019). Therefore, it was suggested that young pigeons under severe deprivation of activity space may be experiencing a hormone-regulated metabolic stress response.
Reducing the stocking density can promote an increase in the daily weight gain, feed conversion rates and relative liver weight of broilers, and the effect is age-dependent (Qaid et al., 2016). However, the present study showed that the SD does not appear to have a significant effect on the BW of pigeons, and even a decreasing tendency of female BW was observed in the LSD group. Correspondingly, the abdominal fat contents of male and female pigeons in the control and HSD groups were significantly higher than those in the LSD group. It was speculated that the size of the activity space was probably an important factor. The liver has antioxidant, detoxification, and anticoagulant functions, and the total serum protein synthesized by the liver can promote the growth and development of the animal body when metabolism is vigorous (Zhao et al., 2021). Abnormal hyperplasia of liver organs is a stress response and pathological phenomenon (Nakanuma et al., 1993). In our study, the liver index of pigeons increased with the reduction of activity space of single pigeon, which was consistent with the results of a study in ducks and broilers (Qaid et al., 2016; Eratalar et al., 2022). The high-density stocking system leads to long-term stress in animals. Under the continuous stimulation of adrenocorticotropic hormone (ACTH), the synthesis of fatty acids in the liver will increase, which could strengthen the fatty deposition with the result of a significant increase in the liver index (Puvadolpirod and Thaxton, 2000a,b). Interestingly, the RWs of lung and gizzard in the HSD group were higher than those in the LSD group. The effect of stocking density on the respiratory and digestive systems of pigeons needs to be further studied.
Blood biochemical indices in animals reflect the physiological metabolism from the balance between the internal and external environment (Sohail et al., 2010). Sufficient activities require more energy for birds, and serum glucose level was higher in LSD group, indicating a relatively vigorous digestion and metabolism of carbohydrates. The increased TCHOL levels in caged pigeons could be partly attributed to the deposition of abdominal fat found in the present study. In addition, HDL can mediate the reverse cholesterol transport from peripheral cells to the liver. Its concentration was lower in the female caged pigeon, but no changes were found in male pigeons. Potential differences in lipid metabolism existed between males and females, as shown in our previous study (Xie et al., 2018). The deposition of abdominal fat induced by high stocking density may lead to impaired liver function (Puvadolpirod and Thaxton, 2000a; Beg et al., 2011), which seems to be consistent with the increase of serum ALT activity in the high-density group. The synthesis of purine or the activity of purine oxidase will be inhibited when the function of hepatocytes is abnormal; thus, excessive purine cannot be oxidized into uric acid in time, and the level of uric acid in the blood will decrease (Pareek et al., 2021). A higher stocking density can induce the higher concentrations of serum Na+ and Cl− and lower concentrations of serum Ca2+ and K+ in rock partridges (Ӧzbey and Esen, 2007), which is not exactly the same as our experimental results. The ion concentrations in serum depend on the nutrition level, water consumption, and renal metabolic function. Moreover, the flying characteristics of pigeon possibly cause their metabolism to differ from that of other bird.
Several studies have confirmed that stress caused by a high stocking density inhibits the antioxidant capacity of poultry (Houshmand et al., 2012; Ma et al., 2020). A high stocking density significantly decreased T-AOC and SOD levels in the liver of broilers and had a negative effect on liver cells (Cai et al., 2019). The antioxidant capacity of breast muscle in fattening chickens decreased with increasing stocking density (Son et al., 2022). In this study, the levels of T-AOC and SOD in the breast muscle of breeding pigeons in the LSD group were significantly higher than those in the HSD and control groups. Some differences existed in that T-AOC and GSH-Px levels were higher only in the livers of male pigeons in the LSD group. MDA is the major product of lipid peroxidation, and its content can reflect the existence of lipid peroxidation free radicals and the degree of membrane lipid peroxidation (Su et al., 2019; Livingston et al., 2020). An increase in MDA level in the livers of male pigeons in the control group was found, but there was no statistical difference in MDA level in the livers of female pigeons. The mechanism of sex difference requires further research. High-density feeding will increase the concentration of harmful gases such as carbon dioxide and ammonia, which will lead to the excessive production of ROS and reactive nitrogen (RNS) in animals (Johnson et al., 2020). Excessive high-density breeding limits the activity space of breeding pigeons and probably stimulates physiological stress, resulting in excessive free radical production in the body. It could result in the destruction of the redox state of the body which probably in turn affects liver tissue function. Additionally, the increased level of corticosterone can also affect the antioxidant system in poultry (Nawaz et al., 2021).
CONCLUSIONS
Rearing in cages or at a high-density caused stress responses in breeder pigeons. It increased the relative organ weight of liver and abdominal fat and may induce changes in lipid and ion metabolism. The antioxidant capacity of breast muscle and liver was also decreased under the condition of limited activity space. Therefore, the stocking density of young pigeons during the rearing period should be ranged from 0.616 m3/bird to 1.232 m3/bird.
ACKNOWLEDGMENTS
The author thanks all the members in the school for their generous technical suggestions. This research was supported by the Key R&D Plan Project of Huai'an City (Rural Revitalization) (HAN202203) and the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (19KJA150003).
Data Availability Statement: All data included in this study are available upon request by contact with the corresponding author.
DISCLOSURES
No potential conflict of interest was reported by the author(s).
REFERENCES
- Albentosa M.J., Cooper J.J., Luddem T., Redgate S.E., Elson H.A., Walker A.W. Evaluation of the effects of cage height and stocking density on the behaviour of laying hens in furnished cages. Br. Poult. Sci. 2007;48:1–11. doi: 10.1080/00071660601156479. [DOI] [PubMed] [Google Scholar]
- Bain M.M., Nys Y., Dunn I.C. Increasing persistency in lay and stabilising egg quality in longer laying cycles. What are the challenges? Br. Poult. Sci. 2016;57:330–338. doi: 10.1080/00071668.2016.1161727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchinger U., Biebach H. Transition between moult and migration in a long distance migratory passerine: organ flexibility in the African wintering area. J. Ornithol. 2006;147:266–273. [Google Scholar]
- Bauchinger U., Van't Hof T., Biebach H. Food availability during migratory stopover affects testis growth and reproductive behaviour in a migratory passerine. Horm. Behav. 2009;55:425–433. doi: 10.1016/j.yhbeh.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Beg M., Baqui M.A., Sarker N.R., Hossain M.M. Effect of stocking density and feeding regime on performance of broiler chicken in summer season. Int. J. Poult. Sci. 2011;10:365–375. [Google Scholar]
- Cai C.H., Zhao R.X., Wang P., Wang J.S., Li K.X., Zhan X.A., Wang K.Y. Effects of different stocking densities on growth performance, antioxidant ability, and immunity of finishing broilers. Anim. Sci. J. 2019;90:583–588. doi: 10.1111/asj.13148. [DOI] [PubMed] [Google Scholar]
- Campbell L.D., Marquardt R.R. Performance of broiler chicks fed diets of varying energy density and containing varied levels of raw or heat-treated faba beans. Poult. Sci. 1977;56:442–448. [Google Scholar]
- Coon C.N. Feeding Egg-Type Replacement Pullets. Pages 267–285 in Commercial Chicken Meat and Egg Production. Bell D.D. and Weaver W.D. Springer; Berlin, Germany: 2002. [Google Scholar]
- Cravener T.L., Roush W.B., Mashaly M.M. Broiler production under varying population-densities. Poult. Sci. 1992;71:427–433. doi: 10.3382/ps.0710427. [DOI] [PubMed] [Google Scholar]
- Ellegren H. First gene on the avian W chromosome (CHD) provides a tag for universal sexing of non-ratite birds. Proc. Biol. Sci. 1996;263:1635–1641. doi: 10.1098/rspb.1996.0239. [DOI] [PubMed] [Google Scholar]
- El-Tarabany M.S. Impact of cage stocking density on egg laying characteristics and related stress and immunity parameters of Japanese quails in subtropics. J. Anim. Physiol. Anim. Nutr. 2016;100:893–901. doi: 10.1111/jpn.12404. [DOI] [PubMed] [Google Scholar]
- Erasmus M.A. A review of the effects of stocking density on turkey behavior, welfare, and productivity. Poult. Sci. 2017;96:2540–2545. doi: 10.3382/ps/pex075. [DOI] [PubMed] [Google Scholar]
- Eratalar S.A., Okur N., Yaman A. The effects of stocking density on slaughter performance and some meat quality parameters of Pekin ducks. Arch. Anim. Breed. 2022;65:199–206. doi: 10.5194/aab-65-199-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugen K.V., Nordquist R.E., Zeinstra E., Staay F.J.V. Stocking density affects stress and anxious behavior in the laying hen chick during rearing. Animals-Basel. 2019;9:53. doi: 10.3390/ani9020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanatico A.C., Pillai P.B., Cavitt L.C., Emmert J.L., Meullenet J.F., Owens C.M. Evaluation of slower growing broiler genotypes grown with and without outdoor access: sensory attributes. Poult. Sci. 2006;85:337–343. doi: 10.1093/ps/85.2.337. [DOI] [PubMed] [Google Scholar]
- Feddes J.J.R., Emmanuel E.J., Zuidhof M.J. Broiler performance, body weight variance, feed and water intake, and carcass quality at different stocking densities. Poult. Sci. 2002;81:774–779. doi: 10.1093/ps/81.6.774. [DOI] [PubMed] [Google Scholar]
- Gabanakgosi K., Moreki J.C. Influence of stocking density on growth performance of family chicks reared up to 18 weeks of age in under an intensive system. Int. J. Curr. Microbiol. App. Sci. 2014;3:291–302. [Google Scholar]
- Gaweł A., Madej J.P., Kozak B., Bobrek K. Early post-hatch nutrition influences performance and muscle growth in broiler chickens. Animals-Basel. 2022;12:3281. doi: 10.3390/ani12233281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene E.S., Rajaei-Sharifabadi H., Dridi S. Feather HSP70: a novel non-invasive molecular marker for monitoring stress induced by heat exposure in broilers. Poult. Sci. 2019;98:3400–3404. doi: 10.3382/ps/pez120. [DOI] [PubMed] [Google Scholar]
- Griffiths R., Daan S., Dijkstra C. Sex indentification in birds using two CHD genes. Proc. Biol. Sei. 1996;263:51–56. doi: 10.1098/rspb.1996.0184. [DOI] [PubMed] [Google Scholar]
- Guo Y.Y., Song Z.G., Jiao H.C., Song Q.Q., Lin H. The effect of group size and stocking density on the welfare and performance of hens housed in furnished cages during summer. Anim. Welfare. 2012;21:41–49. [Google Scholar]
- Hammond K.A., Szewczak J., Krol E. Effects of altitude and temperature on organ phenotypic plasticity along an altitudinal gradient. J. Exp. Biol. 2001;204:1991–2000. doi: 10.1242/jeb.204.11.1991. [DOI] [PubMed] [Google Scholar]
- Houshmand M., Azhar K., Zulkifli I., Bejo M.H., Kamyab A. Effects of prebiotic, protein level, and stocking density on performance, immunity, and stress indicators of broilers. Poult. Sci. 2012;91:393–401. doi: 10.3382/ps.2010-01050. [DOI] [PubMed] [Google Scholar]
- Johnson J.S., Maskal J.M., Duttlinger A.W., Kpodo K.R., McConn B.R., Byrd C.J., Richert B.T., Marchant-Forde J.N., Lay D.C., Perry S.D., Lucy M.C., Safranski T.J. In utero heat stress alters the postnatal innate immune response of pigs. J. Anim. Sci. 2020;98:skaa356. doi: 10.1093/jas/skaa356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn N.W., St John J., Quinn T.W. Chromosome-specific intron size differences in the avian CHD gene provide an efficient method for sex identification in birds. Auk. 1998;115:1074–1078. [Google Scholar]
- Kierończyk B., Rawski M., Długosz J., Świątkiewicz S., Józefiak D. Avian crop function - a review. Ann. Anim. Sci. 2016;16:653–678. [Google Scholar]
- Kim P.K. Effects of stocking density and dissolved oxygen concentration on the growth and hematology of the parrotfish oplegnathus fasciatus in a recirculating aquaculture system (RAS) Korean J. Fisheries Aquat. Sci. 2011;44:747–752. [Google Scholar]
- Kregel K.C. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J. Appl. Physiol. 2002;92:2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- Lee J.C., Tsai L.C., Hwa P.Y., Chan C.L., Hsieh H.M. A novel strategy for avian species and gender identification using the CHD gene. Mol. Cell Probes. 2010;24:27–31. doi: 10.1016/j.mcp.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Legagneux P., Fast P.L.F., Gauthier G., Bêty J. Manipulating individual state during migration provides evidence for carry-over effects modulated by environmental conditions. Proc. R. Soc. Lond. B Biol. Sci. 2012;279:876–883. doi: 10.1098/rspb.2011.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P.D., Perry G.C., Farmer L.J., Patterson R.L.S. Responses of two genotypes of chicken to the diets and stocking densities typical of UK and “label rouge” systems: I. Performance, behaviour and carcass composition. Meat Sci. 1997;45:501–516. doi: 10.1016/s0309-1740(96)00084-8. [DOI] [PubMed] [Google Scholar]
- Livingston M.L., Cowieson A.J., Crespo R., Hoang V., Nogal B., Browning M., Livingston K.A. Effect of broiler genetics, age, and gender on performance and blood chemistry. Heliyon. 2020;6:e04400. doi: 10.1016/j.heliyon.2020.e04400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Xu B., Li W., Wei F., Kim W.K., Chen C., Sun Q., Fu C., Wang G., Li S. Effects of alpha-lipoic acid on the behavior, serum indicators, and bone quality of broilers under stocking density stress. Poult. Sci. 2020;99:4653–4661. doi: 10.1016/j.psj.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin R.H., Fretes P., Gusman D., Jones R.B. Effects of an acute stressor on fear and on the social reinstatement responses of domestic chicks to cage mates and strangers. Appl. Anim. Behav. Sci. 2001;71:57–66. doi: 10.1016/s0168-1591(00)00167-2. [DOI] [PubMed] [Google Scholar]
- Martrenchar A., Morisse J.P., Huonnic D., Cotte J.P. Influence of stocking density on some behavioural, physiological, and productivity traits of broilers. Vet. Res. 1997;28:473–480. [PubMed] [Google Scholar]
- Mendl M. Performing under pressure: stress and cognitive function. Appl. Anim. Behav. Sci. 1999;65:221–224. [Google Scholar]
- Nakanuma Y., Terada T., Ueda K., Terasaki S., Nonomura A., Matsui O. Adenomatous hyperplasia of the liver as a precancerous lesion. Liver. 1993;13:1–9. doi: 10.1111/j.1600-0676.1993.tb00597.x. [DOI] [PubMed] [Google Scholar]
- Narejo N.T., Khoso H.B., Mahesar H., Laghari M.Y., Lashari P.K. Effect of stocking density and survival rate of Labeo rohita (Hamilton) fed with formulated feed. Sindh Univ. Res. J. 2010;42:35–38. [Google Scholar]
- Nawaz A.H., Amoah K., Leng Q.Y., Zheng J.H., Zhang W.L., Zhang L. Poultry response to heat stress: its physiological, metabolic, and genetic implications on meat production and quality including strategies to improve broiler production in a warming world. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.699081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol C.J., Gregory N.G., Knowles T.G., Parkman I.D., Wilkins L.J. Differential effects of increased stocking density, mediated by increased flock size, on feather pecking and aggression in laying hens. Appl. Anim. Behav. Sci. 1999;65:137–152. [Google Scholar]
- Onbaşılar E.E., Poyraz Ö., Cetin S. Effects of breeder age and stocking density on performance, carcass characteristics and some stress parameters of broilers. Asian-Aust. J. Anim. Sci. 2008;21:262–269. [Google Scholar]
- Ӧzbey O., Esen F. The effects of breeding system and stocking density on some blood parameters of rock partridges (Alectoris graeca) Poult. Sci. 2007;86:420–422. doi: 10.1093/ps/86.2.420. [DOI] [PubMed] [Google Scholar]
- Pareek V., Pedley A.M., Benkovic S.J. Human de novo purine biosynthesis. Crit. Rev. Biochem. Mol. Biol. 2021;56:1–16. doi: 10.1080/10409238.2020.1832438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puron D., Santamaria R., Segura J.C., Alamilla J.L. Broiler performance at different stocking densities. J. Appl. Poult. Res. 1995;4:55–60. [Google Scholar]
- Puvadolpirod S., Thaxton J.P. Model of physiological stress in chickens 1. Response parameters. Poult. Sci. 2000;79:363–369. doi: 10.1093/ps/79.3.363. [DOI] [PubMed] [Google Scholar]
- Puvadolpirod S., Thaxton J.P. Model of physiological stress in chickens 2. Dosimetry of adrenocorticotropin. Poult. Sci. 2000;79:370–376. doi: 10.1093/ps/79.3.370. [DOI] [PubMed] [Google Scholar]
- Qaid M., Albatshan H., Shafey T., Hussein E., Abudabos A.M. Effect of stocking density on the performance and immunity of 1- to 14-d-old broiler chicks. Braz. J. Poult. Sci. 2016;18:683–691. [Google Scholar]
- Ravindran V., Thomas D.V., Thomas D.G., Morel P.C.H. Performance and welfare of broilers as affected by stocking density and zinc bacitracin supplementation. Anim. Sci. J. 2006;77:110–116. [Google Scholar]
- Ruzicic R.D., Jakovljevic V., Djordjevic D. Oxidative stress in training, overtraining and detraining: from experimental to applied research. Ser. J. Exp. Clin. Res. 2016;17:361–367. [Google Scholar]
- Sohail M.U., Ijaz A., Yousaf M.S., Ashraf K., Zaneb H., Aleem M., Rehman H. Alleviation of cyclic heat stress in broilers by dietary supplementation of mannan-oligosaccharide and Lactobacillus-based probiotic: dynamics of cortisol, thyroid hormones, cholesterol, C-reactive protein, and humoral immunity. Poult. Sci. 2010;89:1934–1938. doi: 10.3382/ps.2010-00751. [DOI] [PubMed] [Google Scholar]
- Son J., Kim H.J., Hong E.C., Kang H.K. Effects of stocking density on growth performance, antioxidant status, and meat quality of finisher broiler chickens under high temperature. Antioxidants-Basel. 2022;11:871. doi: 10.3390/antiox11050871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer-Thiersch A. Beitrage zur brutbiologie der flamingos (Gattung phoenicopterus) Zool. Gart. 1967;34:159–229. [Google Scholar]
- Su L.J., Zhang J.H., Gomez H., Murugan R., Hong X., Xu D., Jiang F., Peng Z.Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell Longev. 2019;2019 doi: 10.1155/2019/5080843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H.B., Wang Q., Lu J., Zou J.M., Chang L.L., Fu S.Y. Effect of free-range days on a local chicken breed: growth performance, carcass yield, meat quality, and lymphoid organ index. Poult. Sci. 2014;93:1883–1889. doi: 10.3382/ps.2013-03470. [DOI] [PubMed] [Google Scholar]
- Wang B., Min Z., Yuan J., Zhang B., Guo Y. Effects of dietary tryptophan and stocking density on the performance, meat quality, and metabolic status of broilers. J. Anim. Sci. Biol. 2014;5:44. doi: 10.1186/2049-1891-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster A.B. Welfare implications of avian osteoporosis. Poult. Sci. 2004;83:184–192. doi: 10.1093/ps/83.2.184. [DOI] [PubMed] [Google Scholar]
- Xie P., Jiang X.Y., Bu Z., Fu S.Y., Zhang S.Y., Tang Q.P. Free choice feeding of whole grains in meat-type pigeons: I. Effect on performance, carcass traits, and organ development. Br. Poult. Sci. 2016;57:699–706. doi: 10.1080/00071668.2016.1206191. [DOI] [PubMed] [Google Scholar]
- Xie P., Wan X.P., Bu Z., Diao E.J., Gong D.Q., Zou X.T. Changes in hormone profiles, growth factors and mRNA expression of the related receptors in crop tissue, relative organ weight, and serum biochemical parameters in the domestic pigeon (Columba livia) during incubation and chick-rearing periods under artificial farming conditions. Poult. Sci. 2018;97:2189–2202. doi: 10.3382/ps/pey061. [DOI] [PubMed] [Google Scholar]
- Yin L., Yang H., Xu L., Zhang J., Xing H., Wang Z. Feather performance, walking ability, and behavioral changes of geese in response to different stocking densities. Appl. Anim. Behav. Sci. 2017;196:108–112. [Google Scholar]
- Zager R.A., Johnson A. Renal cortical cholesterol accumulation is an integral component of the systemic stress response. Kidney Int. 2001;60:2299–2310. doi: 10.1046/j.1523-1755.2001.00071.x. [DOI] [PubMed] [Google Scholar]
- Zhang P., Han J., Liu Q., Zhang J., Zhang X. Sex identification of four penguin species using locus-specific PCR. Zoo Biol. 2013;32:257–261. doi: 10.1002/zoo.21005. [DOI] [PubMed] [Google Scholar]
- Zhao M., Sun Q., Khogali M.K., Liu L., Geng T., Yu L., Gong D. Dietary selenized glucose increases selenium concentration and antioxidant capacity of the liver, oviduct, and spleen in laying hens. Biol. Trace Elem. Res. 2021;199:4746–4752. doi: 10.1007/s12011-021-02603-7. [DOI] [PubMed] [Google Scholar]