Abstract
Essential oils (EO) are known for their antioxidant, anti-inflammatory, antimicrobial, and growth-promoting properties. However, data rgarding their impact on the intestinal health and gut microbiota of ducks remain limited. Thus, this study aimed to investigate the effects of plant EO on the growth performance, intestinal health, and gut microbiota of Muscovy ducks. A total of 360 healthy male Muscovy ducks aged 1 d were randomly divided into 4 groups with 6 replicates and 15 ducks per replicate. Ducks were fed basal diets supplemented with 0, 100, 200, or 300 mg/kg EO. The results showed that 200 mg/kg EO supplementation significantly (P < 0.05) increased the final body weight and average daily gain, while significantly (P < 0.05) decreased the feed conversion ratio during the 56-d experimental period. Furthermore, dietary 200 mg/kg EO significantly (P < 0.05) enhanced antioxidant capacity and immune function and improved the barrier function of the intestine. Additionally, 16S rDNA sequencing analysis results showed that 200 mg/kg EO favorably modulated the cecal microbial diversities and composition evidenced by the increased (P < 0.05) the abundances of short-chain fatty acid-producing bacteria (e.g., Subdoligranulum and Shuttleworthia) and decreased (P < 0.05) abundances of potential enteric pathogenic bacteria (e.g., Alistipes, Eisenbergiella, and Olsenella). The relative abundance of beneficial bacteria was positively correlated with antioxidant, immune, and barrier function biomarkers. Overall, these findings revealed that dietary supplementation with 200 mg/kg EO had several potentially beneficial effects on the growth performance of Muscovy ducks by improving antioxidant capacity, enhancing the intestinal barrier function and favorably modulating gut microbiota.
Key words: essential oils, Muscovy duck, growth performance, barrier function, gut microbiota
INTRODUCTION
Antibiotics have been widely used to promote growth and enhance the feed utilization ratio in poultry production by improving intestinal health (Zhu et al., 2021). However, the overuse of antibiotics has led to increased antibiotic resistance alongside the problem of antibiotic residues in poultry meat and waste, which can directly or indirectly threaten the health of humans and animals (Hernando-Amado et al., 2020). In animal diets, antibiotic growth promoters are being restricted or banned by an increasing number of countries due to increased concerns regarding antibiotic residues and bacterial resistance. Therefore, the search for new growth-promoting alternatives to antibiotics in poultry production has recently become a popular research subject worldwide.
Essential oils (EO) are complex mixtures of hydrophobic and volatile compounds extracted from plants, which have been regarded as possible substitutes for antibiotics due to their unique biological activities, including antioxidant, anti-inflammatory, antimicrobial, and growth-promoting attributes (Ariana et al., 2002; Quiroga et al., 2013; Huang et al., 2018; Adaszynska-Skwirzynska and Szczerbinska, 2019). The major active ingredients of EO applied in poultry diets mainly consist of carvacrol, thymol, cinnamaldehyde, and capsaicin (Burt, 2004; Lippens et al., 2005). EO have been shown to promote production parameters and nutrient digestibility in broilers and laying hens, along with improving intestinal morphology and microbiota (Migliorini et al., 2019; Xue et al., 2020; Su et al., 2021) by exhibiting antioxidant and antibacterial activity (Gao et al., 2022; Noruzi et al., 2022). In addition, carvacrol and cinnamaldehyde have been used to improve the performance and meat quality of broilers (Reis et al., 2018a; Safwat et al., 2021). A mixture of EO (e.g., carvacrol, cinnamaldehyde, and capsicum oleoresin) has also been applied in bird diets to enhance their antioxidant and immune capacities (Lillehoj et al., 2011; Karadas et al., 2014).
Although EO have been widely used as antioxidant and antibacterial additives in broilers and laying hens (Reis et al., 2018b; Safwat et al., 2021; Noruzi et al., 2022), it is important to examine their efficacy in other types of poultry, such as ducks and quails. Moreover, increasing evidence has demonstrated that the impacts of dietary EO on poultry gut microbiota may differ, or even be contradictory (Reda et al., 2020; Kurekci et al., 2021; Ruan et al., 2021). Ducks are one of the most economically significant waterfowl in the world (Yin et al., 2019). However, to date, few studies have been conducted on the impacts in meat-type ducks, particularly Muscovy ducks. Furthermore, data regarding the impacts of EO on the gut microbiota of ducks are very limited. Consequently, this study aimed to estimate the impacts of EO on the growth performance, antioxidant and anti-inflammatory capacities, and intestinal health of Muscovy ducks, with particular emphasis on the alterations of cecal microbiota.
MATERIALS AND METHODS
Plant Essential Oils
The active ingredients of the plant EO blend (Pancosma S.A., Geneva, Switzerland) used in this study were consisted of 5% carvacrol, 3% cinnamaldehyde, and 2% capsicum oleoresin.
Animal Experimental Design, Management, and Diet
In total, 720 male Muscovy ducks aged 1 d were freely assigned to 4 groups with 6 replicates each (30 ducks per replicate). Ducks in these 4 treatment groups were then fed either basal diets (control, CON) or basal diets with 100, 200, or 300 mg/kg EO (EO100, EO200, and EO300, respectively). The basal diets for the starter (1–28 d) and grower (29–56 d) ducks were formulated to meet the NRC requirements (NRC, 1994) for all nutrients (Table 1). All ducks were maintained in environmentally controlled cages with feed and water supplementation ad libitum. Feed consumption and body weight (BW) were recorded weekly, with average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR) being calculated.
Table 1.
Composition and nutrients levels of the basal diet (as-fed basis).
| Ingredient | Starter diet (1–28 d) | Grower diet (29–56 d) |
|---|---|---|
| Corn | 46.00 | 33.00 |
| Soybean meal (46% CP) | 31.00 | 20.00 |
| Barley | 10.00 | 20.00 |
| Rice bran | - | 15.00 |
| Wheat middling | 6.20 | 7.45 |
| Soybean oil | 2.00 | - |
| Limestone | 1.25 | 1.25 |
| Dicalcium phosphate | 1.10 | 0.90 |
| DL-methionine | 0.15 | 0.10 |
| Sodium chloride | 0.30 | 0.30 |
| Vitamin and trace mineral premix1 | 2.00 | 2.00 |
| Total | 100.0 | 100.0 |
| Calculated nutrients levels | ||
| Metabolizable energy, MJ/kg | 12.20 | 11.50 |
| Crude protein, % | 20.02 | 17.10 |
| Calcium, % | 0.93 | 0.85 |
| Lysine, % | 1.02 | 0.86 |
| Methionine, % | 0.45 | 0.37 |
| Methionine + cysteine, % | 0.78 | 0.66 |
| Phosphorus, % | 0.65 | 0.67 |
| Available phosphorus, % | 0.40 | 0.34 |
Vitamin and trace mineral premix provided the following per kilogram of diets: VA: 12,000 IU, VD3: 5,000 IU, VE: 130 mg, VK3: 3.6 mg, VB1: 3 mg, VB2: 8 mg, VB6: 4.95 mg, VB12: 0.17 mg, niacin: 60 mg, folic acid: 2.10 mg, biotin: 200 mg, calcium D-pantothenate: 18.3 mg, copper: 80 mg, iodine: 2 mg, selenium: 150 mg, iron: 80 mg, manganese: 100 mg, zinc: 80 mg, Se: 0.15 mg.
Sample Collection and Preparation
At 28 and 56 d of age, 1 duck with a BW close to the replicate mean (6 ducks per treatment) was picked and weighed. After collecting blood samples from the wing vein, sera were obtained via centrifugation at 5,000 × g at 4°C for 15 min, as described previously (Liu et al., 2021). Subsequently, the selected birds were euthanized via cervical dislocation, while jejunum tissues, cecal content, and breast muscle tissue were collected.
Meat Quality
To assess whether EO supplementation affected the meat quality of 56-d-old Muscovy ducks, the pH, color, and drip loss of breast muscle were measured. The pH values of breast muscle samples were evaluated at 45 min and 24 h postmortem using a pH meter (HANNA Instruments, Padova, Italy). Furthermore, the Hunter lightness (L*), redness (a*), and yellowness (b*) values of breast muscle were determined by using a Minolta chroma meter (Konica Minolta Sensing, Inc., Osaka, Japan). The breast muscle drip loss was measured as described previously (Liu et al., 2019a).
Immune Organ Index
The liver, spleen, and bursa of fabricius of ducks at 28- and 56-d old were removed and weighed in situ, while the immune organ indices were determined as follows: Immune organ index (g/kg) = Immune organ mass/Live body weight.
Antioxidant Enzyme Activity Analysis
The activities of catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px), and total antioxidant capacity (T-AOC) alongside malonaldehyde (MDA) level in the serum samples collected on 28 and 56 d of the trial were analyzed using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) in accordance with the manufacturer's instructions.
Determination of Immunoglobulins and Cytokine Levels in the Serum
Immunoglobulin A (IgA), immunoglobulin G (IgG), and immunoglobulin M (IgM) levels in the serum were determined using ELISA kits for ducks (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The inflammatory biomarkers in serum including tumor necrosis factor-α (TNF-α), interlukin-2 (IL-2), and interlukin-6 (IL-6) were all detected using ELISA kits specific for ducks (Jiangsu Meibiao Biotechnology Co., Ltd., Yancheng, China).
Jejunal Morphology Analysis
The middle jejunal segments were fixed in 10% formalin solution, then embedded in paraffin before being cut into slices and stained with hematoxylin and eosin (H&E) for mucosal morphology analysis, as described previously (Liu et al., 2020). Additionally, Olympus Microsystem (Tokyo, Japan) was used to capture digital pictures. The middle jejunum was treated in 2.5% glutaraldehyde, prior to observation using transmission electron microscopy (TEM). After being dehydrated in a graded ethanol series, ultra-thin slices were cut and stained with uranyl acetate and lead citrate. A transmission electron microscope (JEOL-JEM-1200EX, Peabody, MA) was subsequently used to obtain the micrographs.
Jejunal Tight Junction mRNA Expression
The mRNA expression of jejunal tight junction genes, including ZO-1 and claudin-1, was determined using RT-qPCR with the ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) in Applied Biosystems. Table S1 shows a list of the primer sequences for the genes used in this study. β-Actin was selected as the endogenous control while the expression of related genes was calculated using the 2−∆∆Ct method (Livak and Schmittgen, 2001).
Cecal Microbiota Analysis
Microbiota genomic DNA was extracted from the cecal contents of ducks in the CON and EO200 groups using TIANamp fecal DNA Kits (Tiangen Biotech, Beijing, China) in accordance with the manufacturer's protocol. The V3 to V4 regions of the microbiota 16S rDNA gene were amplified using 338F/806R primers, and then the purified amplicons were sequenced in high throughput using the Illumina MiSeq PE300 platform by Majorbio Bio-technology Co. Ltd. (Shanghai, China). Analysis and visualization of high-throughput sequencing data were collected via the online Majorbio Cloud Platform (www.majorbio.com).
Statistical Analysis
Data were analyzed using 1-way ANOVA procedure on IBM SPSS Statistics 19.0 software followed by Tukey multiple range tests among 4 groups. Linear and quadratic effects for the responses to EO levels were carried out using orthogonal contrasts. A 2-tailed Student t test was used to determine the significance between the 2 groups. For all tests, P < 0.05 was regarded as statistically significant. Graphics were then visualized using GraphPad prism 8.0 (GraphPad Software Inc., San Diego, CA).
RESULTS
Growth Performance
Dietary EO supplementation, regardless of the levels, had no significant (P > 0.05) effects on ADFI during the starter period (1–28 d), grower period (29–56 d), or across the whole experimental period (1–56 d; Table 2). However, the BW and ADG of ducks in the EO groups increased linearly and quadratically (P < 0.05), while a linear decrease (P < 0.05) was observed in FCR, compared with the CON group. Moreover, the administration of 200 and 300 mg/kg EO significantly increased the ADG (P < 0.05), whereas 200 mg/kg EO decreased the FCR (P < 0.05), compared to the CON during both the growing and whole periods. Overall, ducks treated with 200 mg/kg EO showed the best growth performance.
Table 2.
Effect of EO on the growth performance of Muscovy ducks (n = 6).
| Items | CON | EO100 | EO200 | EO300 |
P value |
||
|---|---|---|---|---|---|---|---|
| EO | Linear | Quadratic | |||||
| 1–28 d of age | |||||||
| BW (g) | 1172.6 ± 45.1b | 1245.9 ± 49.4ab | 1264.1 ± 60.8a | 1259.3 ± 43. 9a | 0.017 | 0.007 | 0.072 |
| ADG (g/d) | 48.92 ± 1.77b | 52.29 ± 2.09a | 53.27 ± 2.59a | 53.06 ± 1.74a | 0.006 | 0.002 | 0.047 |
| ADFI (g/d) | 130.45 ± 8.60 | 128.01 ± 20.31 | 129.05 ± 19.79 | 127.15 ± 20.51 | 0.743 | 0.475 | 0.412 |
| FCR | 2.67 ± 0.21 | 2.36 ± 0.30 | 2.30 ± 0.23 | 2.35 ± 0.31 | 0.097 | 0.049 | 0.265 |
| 28–56 d of age | |||||||
| BW (g) | 3546.2 ± 72.3c | 3649.4 ± 87.6bc | 3779.2 ± 60.6a | 3727.0 ± 59.3ab | <0.000 | <0.000 | 0.014 |
| ADG (g/d) | 84.77 ± 1.69b | 85.84 ± 1.81b | 89.83 ± 0.82a | 88.13 ± 0.91a | <0.000 | <0.000 | 0.023 |
| ADFI (g/d) | 279.59 ± 28.92 | 279.03 ± 24.23 | 282.93 ± 27.34 | 284.07 ± 27.10 | 0.252 | 0.141 | 0.196 |
| FCR | 3.46 ± 0.35b | 3.16 ± 0.20ab | 3.03 ± 0.14a | 3.10 ± 0.30ab | 0.045 | 0.019 | 0.092 |
| 1–56 d of age | |||||||
| ADG (g/d) | 69.41 ± 1.30a | 71.46 ± 1.69ab | 74.16 ± 1.11c | 73.10 ± 1.07bc | <0.000 | <0.000 | 0.009 |
| ADFI (g/d) | 216.11 ± 19.58 | 214.82 ± 12.84 | 217.47 ± 10.40 | 217.25 ± 19.49 | 0.358 | 0.198 | 0.241 |
| FCR | 3.23 ± 0.31a | 2.92 ± 0.22ab | 2.81 ± 0.17b | 2.88 ± 0.30ab | 0.047 | 0.021 | 0.089 |
Values within a row with different letters differ significantly (P < 0.05).
Meat Quality
Compared with those of the CON group, the a* values of the breast muscles of 56-d-old ducks in the EO group increased linearly (P < 0.05), whereas the b* values and drip loss decreased linearly (P < 0.05) alongside the increasing levels of EO (Table 3). Dietary EO at 200 and 300 mg/kg significantly increased the redness (a* value), whereas 200 mg/kg EO significantly decreased the yellowness (b* value) and drip loss of breast muscle compared to the CON (P < 0.05). However, 200 mg/kg EO had no impact (P > 0.05) on pH45 min, pH24 h, or lightness (L* value).
Table 3.
Effect of EO on the meat quality breast muscle of 56-days-old Muscovy ducks (n = 6).
| Items | CON | EO100 | EO200 | EO300 |
P value |
||
|---|---|---|---|---|---|---|---|
| EO | Linear | Quadratic | |||||
| pH45min | 6.22 ± 0.08 | 6.27 ± 0.08 | 6.27 ± 0.12 | 6.28 ± 0.27 | 0.896 | 0.498 | 0.800 |
| pH24h | 5.77 ± 0.05 | 5.80 ± 0.06 | 5.85 ± 0.10 | 5.82 ± 0.08 | 0.322 | 0.167 | 0.298 |
| Lightness (L*) | 53.25 ± 2.50 | 51.88 ± 4.50 | 49.95 ± 3.93 | 51.27 ± 3.68 | 0.503 | 0.261 | 0.388 |
| Redness (a*) | 8.66 ± 1.14b | 9.42 ± 1.10ab | 11.19 ± 0.97a | 10.53 ± 1.34a | 0.005 | 0.002 | 0.144 |
| Yellowness (b*) | 12.86 ± 0.99a | 12.12 ± 0.85ab | 11.15 ± 0.98b | 11.16 ± 0.81b | 0.010 | 0.002 | 0.328 |
| Drip loss (%) | 1.44 ± 0.19a | 1.57 ± 0. 24a | 1.04 ± 0.18b | 1.31 ± 0.13ab | 0.001 | 0.014 | 0.384 |
Values within a row with different letters differ significantly (P < 0.05).
Antioxidant Capacity
Ducks fed diets containing EO exhibited higher antioxidant capacity than those fed the control diet, to some extent, which was reflected by the linearly increased (P < 0.05) activities of T-AOC, CAT, SOD, and GSH-Px and linearly decreasing (P < 0.05) MDA content in response to the rising inclusion levels of EO in the sera of ducks at both ages of 28 and 56 d (Table 4). Specifically, the highest T-AOC, CAT, SOD, and GSH-Px alongside the lowest MDA values were observed in ducks supplemented with 200 mg/kg EO (P < 0.05).
Table 4.
Effect of EO on the serum antioxidative parameters of Muscovy ducks (n = 6).
| Items | CON | EO100 | EO200 | EO300 |
P value |
|||
|---|---|---|---|---|---|---|---|---|
| EO | Linear | Quadratic | ||||||
| 28 d of age | ||||||||
| T-AOC (U/mL) | 0.40 ± 0.02b | 0.42 ± 0.02ab | 0.45 ± 0.04a | 0.44 ± 0.03ab | 0.015 | 0.008 | 0.106 | |
| CAT (U/mL) | 3.25 ± 0.36b | 3.57 ± 0.43ab | 4.00 ± 0.60a | 3.70 ± 0.23ab | 0.044 | 0.032 | 0.091 | |
| GSH-Px (U/mL) | 547.45 ± 27.32b | 586.11 ± 31.13ab | 609.15 ± 30.16a | 599.33 ± 32.04a | 0.011 | 0.004 | 0.063 | |
| SOD (U/mL) | 232.07 ± 22.83b | 238.33 ± 20.72b | 287.12 ± 31.14a | 262.95 ± 34.36ab | 0.010 | 0.012 | 0.196 | |
| MDA (nmol/mL) | 6.10 ± 0.87a | 5.15 ± 0.68ab | 4.24 ± 0.75b | 4.46 ± 0. 81b | 0.002 | 0.001 | 0.079 | |
| 56 d of age | ||||||||
| T-AOC (U/mL) | 0.59 ± 0.02b | 0.63 ± 0.02a | 0.65 ± 0.02a | 0.65 ± 0.02a | <0.000 | <0.000 | 0.026 | |
| CAT (U/mL) | 5.27 ± 0.69b | 5.89 ± 0.49ab | 6.11 ± 0.31a | 6.05 ± 0.34a | 0.026 | 0.008 | 0.101 | |
| GSH-Px (U/mL) | 593.75 ± 41.57b | 644.05 ± 46.15ab | 684.63 ± 66.45a | 653.09 ± 49.32ab | 0.046 | 0.031 | 0.067 | |
| SOD (U/mL) | 259.59 ± 20.79b | 279.39 ± 24.09ab | 303.68 ± 23.29a | 294.47 ± 31.52ab | 0.035 | 0.011 | 0.287 | |
| MDA (nmol/mL) | 5.83 ± 0.73a | 4.98 ± 0.58ab | 4.58 ± 0.61b | 4.74 ± 0.74b | 0.019 | 0.007 | 0.080 | |
Values within a row with different letters differ significantly (P < 0.05).
Abbreviations: CAT, catalase; GSH-Px, glutathione peroxidase; MDA, malonaldehyde; SOD, superoxide dismutase; T-AOC, total antioxidant capacity.
Immune Function
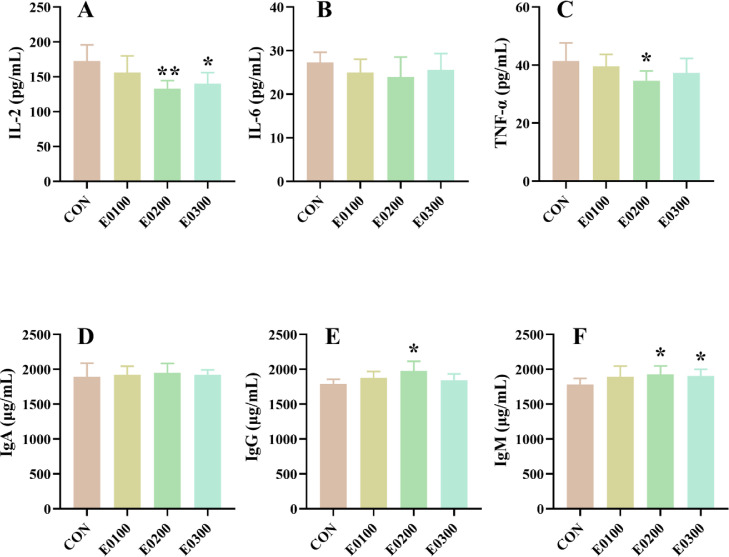
The effect of EO on the immune function of ducks was reflected by the immune organ index alongside the immunoglobulin and cytokine levels in serum. The immune organ indices are presented in Table 5. No noticeable differences were noted in the liver index, spleen index, or bursal index in 28-d-old ducks (P > 0.05), whereas 200 mg/kg EO increased the bursal index of ducks, compared to the CON group at age of 56 d (P < 0.05). Compared with the CON group, supplementing diets with 200 and 300 mg/kg EO significantly decreased the proinflammatory IL-2 levels while increased IgM levels in the serum (P < 0.05, Figure 1). In addition, ducks fed diets containing 200 mg/kg EO exhibited higher IgM contents with lower TNF-α in their serum than those in CON ducks (P < 0.05).
Table 5.
Effect of EO on the relative organ weight of Muscovy ducks (g/kg, n = 6).
| Items | CON | EO100 | EO200 | EO300 |
P value |
||||
|---|---|---|---|---|---|---|---|---|---|
| EO | Linear | Quadratic | |||||||
| 28 d of age | |||||||||
| Liver index | 27.66 ± 2.54 | 25.58 ± 4.13 | 26.78 ± 1.96 | 26.64 ± 1.19 | 0.617 | 0.708 | 0.385 | ||
| Spleen index | 1.20 ± 0.18 | 1.19 ± 0.13 | 1.34 ± 0.12 | 1.43 ± 0.22 | 0.062 | 0.012 | 0.466 | ||
| Bursal index | 1.82 ± 0.31 | 1.85 ± 0.21 | 2.19 ± 0.20 | 1.98 ± 0.27 | 0.082 | 0.099 | 0.249 | ||
| 56 d of age | |||||||||
| Liver index | 21.60 ± 3.00 | 21.48 ± 1.32 | 21.18 ± 4.51 | 21.46 ± 2.72 | 0.996 | 0.897 | 0.877 | ||
| Spleen index | 0.85 ± 0.13 | 0.95 ± 0.11 | 1.02 ± 0.13 | 0.93 ± 0.06 | 0.102 | 0.148 | 0.047 | ||
| Bursal index | 0.31 ± 0.07b | 0.35 ± 0.04ab | 0.44 ± 0.07a | 0.40 ± 0.06ab | 0.012 | 0.008 | 0.112 | ||
Values within a row with different letters differ significantly (P < 0.05).
Figure 1.
Effects of EO supplementation on serum immune factors of 56-d-old ducks. (A) Interleukin 2 (IL-2), (B) interleukin 6 (IL-6), (C) tumor necrosis factor alpha (TNF-α), (D) immunoglobulin A (IgA), (E) immunoglobulin G (IgG), and (F) immunoglobulin M (IgM). Data were showed as mean ± SD (n = 6). *P < 0.05, **P < 0.01, compared with CON.
Jejunal Morphology and Barrier Functions
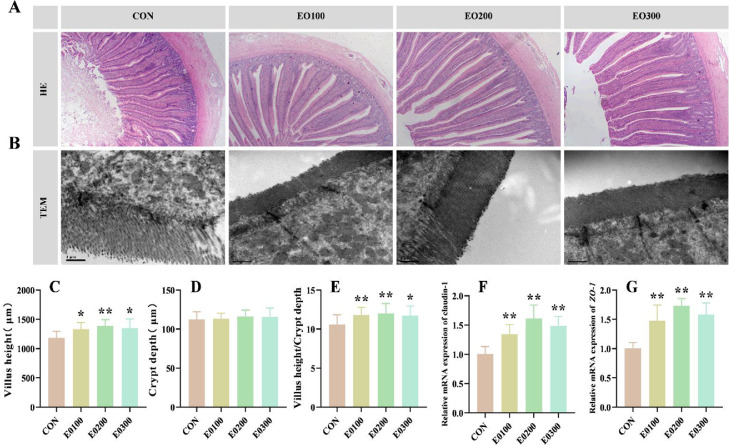
Supplementing the diet with 100 or 200 mg/kg EO considerably increased the villus height (VH) alongside the ratio of villus height to crypt depth (VH/CD) in ducks aged 56 d (P < 0.05), whereas no significant effects (P > 0.05) were observed on crypt depth (Figure 2A, C–E). The TEM results showed that ducks fed diets with EO had more microvilli and longer tight junctions in the jejunum than those in the CON group (Figure 2B). These outcomes were then confirmed by the gene expression of tight junctions in ducks fed diets containing EO, regardless of the levels, which upregulated the mRNA expression of claudin-1 and ZO-1 in the jejunum (Figure 2F and G).
Figure 2.
Effect of EO supplementation on the jejunal morphology and barrier of 56-d-old ducks. (A) The representative figures of jejunal morphologies hematoxylin and eosin (H&E) staining. (B) Transmission electron micrographs of the jejunal microvilli. (C–E) Villus height, crypt depth, and villus height to-crypt depth ratio. (F and G) Relative mRNA expression of genes related with tight junction. Abbreviations: ZO-1, zonula occludens-1. Data were showed as mean ± SD. *P < 0.05, **P < 0.01, compared with control. n = 6.
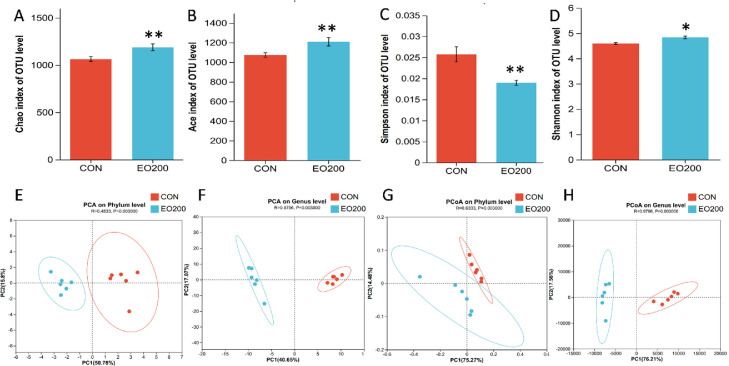
Cecum Microbiota Diversity
Ducks fed diets with 200 mg/kg EO showed the best growth performance, antioxidant capacity, immune functions, jejunal morphology, and barrier functions. Therefore, the effect of the EO200 group on gut microbiota was further studied. Alpha diversity was reflected by the richness (Chao1 and Ace indices) and diversity (Shannon and Simpson indices) of the gut microbiota. Compared with the CON group, ducks fed EO showed significantly increased Chao1, Ace, and Shannon indices alongside a decreased Simpson index (P < 0.05; Figure 3A–D), indicating that bacterial richness and diversity were improved after EO treatment. Principal component analysis (PCA) and principal coordinate analysis (PCoA), based on the Bray-Curtis distance at both the phylum and genus levels, were performed to visualize changes in the microbiota community. The samples within the same treatment clustered together and were separated from the other group at both the phylum and genus levels, suggesting a highly significant difference (P < 0.01) in the structure of gut microbes after feeding with EO (Figure 3E–H). Collectively, these results suggest that dietary EO intake could increase the variety of gut microbiota.
Figure 3.
Effects of dietary EO supplementation (200 mg/kg) on alpha and beta diversity of cecal microbiota of 56-d-old ducks. (A–D) Alpha diversity at the OTU level. (E and F) Principal component analysis (PCA) plot about the cecal microbiota composition at the phylum and genus level. (G and H) Principal coordinate analysis (PCoA) based on Bray-Curtis distance at the phylum and genus level. Data were showed as mean ± SD. *P < 0.05, **P < 0.01, compared with CON. n = 6.
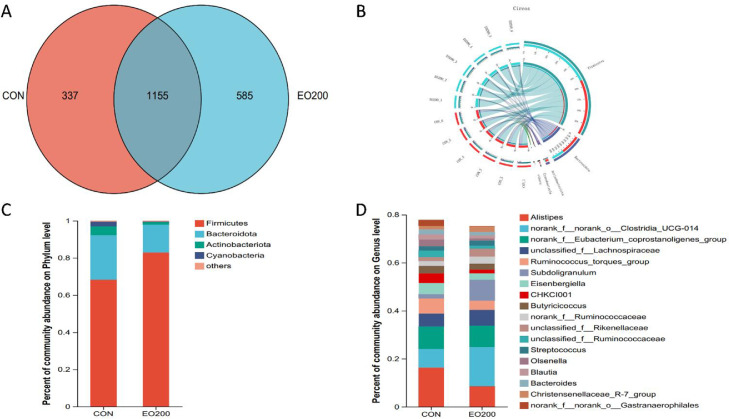
Cecum Microbiota Composition
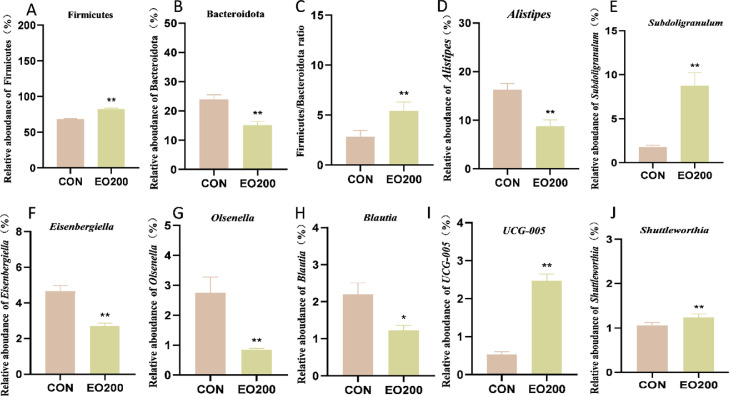
Common and unique features of the cecal samples were visualized with a Venn diagram. The total number of OTUs in the CON and EO200 groups was 1,492 and 1,740, respectively (Figure 4A). In addition, the EO200 treatment resulted in more unique OTUs (585) than the CON treatment (337). The Circos graph showed the distribution of the microbiota communities in different samples at the phylum level Compared to the CON group, the relative abundances of some phyla were influenced (Figure 4B). Firmicutes, Bacteroidetes, Actinobacteriota, and Cyanobacteria were the most abundant phyla of the cecum microbiota (Figure 4C). At the genus level, Alistipes, Subdoligranulum, Eisenbergiella, CHKCI001, Butyricicoccus, Streptococcus, Olsenella, Blautia, and Bacteroides were the dominant bacteria with these 2 groups (Figure 4D). In addition, 200 mg/kg EO significantly (P < 0.05) increased the relative abundance of Firmicutes, Subdoligranulum, UCG_005, and Shuttleworthia, while significantly decreasing the relative abundance of potential enteric pathogenic bacteria, such as Alistipes, Eisenbergiella, and Olsenella (P < 0.05; Figure 5).
Figure 4.
Effects of dietary 200 mg/kg EO supplementation on the microbial community in the cecal contents of 56-d-old ducks. (A) The Venn graph summarizing some mutual and unique observed taxonomic units (OTUs) in the microbial community in the cecal contents of ducks. (B) Circos graph showed the distribution of microbial communities in different samples at the phylum level. (C and D) Percent of community abundance of the cecal microbial community at the phylum and genus level. n = 6.
Figure 5.
Effects of dietary EO supplementation (200 mg/kg) on cecal contents specific microbiota of 56-d-old ducks. (A–J) The relative abundance of cecal microbiota at the genus or species level with significant variations and specific functions. Data were showed as mean ± SD (n = 6). *P < 0.05, **P < 0.01, compared with CON.
Analysis of Taxonomic Biomarkers
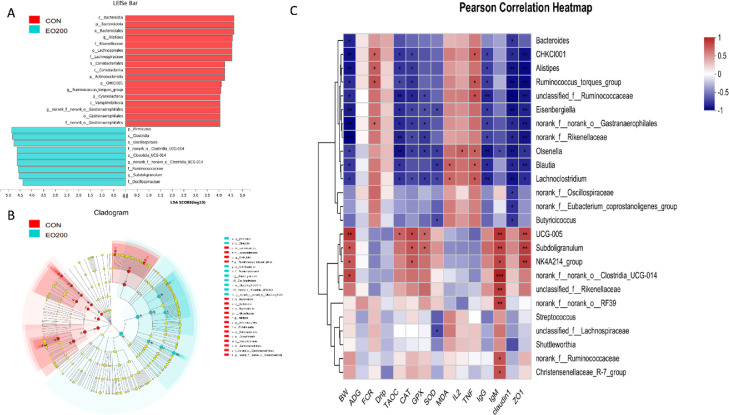
The most likely explanation for the differences between the CON and EO200 groups of microbiota taxa was determined by Linear discriminant analysis effect size (LEfSe) analysis. A total of 26 taxon biomarkers were identified in the CON and EO200 groups with a linear discriminant analysis (LDA) score >4.0 (Figure 6A and B), which belonged mainly to the phyla Firmicutes, Bacteroidetes, and Proteobacteria. Additionally, the CON group exhibited higher abundances of Bacteroidia, Alistipes, Rikenellaceae, Lachnospirales, Coriobacteriales, Actinobacteriota, CHKCI001, Cyanobacteria, Vampirivibrionia, and Gastranaerophilales. Meanwhile, the abundances of Firmicutes, Clostridia, Oscillospirales, Clostridia-UCG_014, Ruminococcaceae, Subdoligranulum, and Oscillospiraceae were enriched as biomarkers in EO-treated ducks. Pearson's correlation analysis was also executed to identify the latent connection between altered intestinal microbiota and growth performance, serum antioxidative status, immune biomarkers, and gut barrier functions. Most parameters of growth performance (e.g., BW), antioxidative and immune status (e.g., CAT and GSH-Px activity, and serum IgM content) as well as gut barrier functions (e.g., the mRNA expression of ZO-1) were positively correlated (P < 0.05) with the relative abundances of Subdoligranulum and UCG_005. In contrast, the relative abundances of Alistipes, Eisenbergiella, and Olsenella all had a negative association with BW, T-AOC, CAT, IgG, claudin-1, and ZO-1 (P < 0.05).
Figure 6.
Effects of dietary 200 mg/kg EO supplementation on taxon biomarkers in the cecal contents of 56-d-old ducks. (A and B) LDA effect size (LEfSe) analyzed (LDA score 4.0) from the phylum level to the genus level. (C) Heatmap showing Pearson's correlation analysis of performance, antioxidant and immune-related indices with intestinal bacterial genera. The red areas represent positive correlations, while the blue areas represent negative correlations. The depth of colors represents the strength of the correlation. *P < 0.05, **P < 0.01, ***P < 0.001. n = 6.
DISCUSSION
EO have been regarded as an alternative to antibiotics considering their unique biological activities, including antioxidant, anti-inflammatory, antimicrobial, and growth-promoting attributes (Ariana et al., 2002; Quiroga et al., 2013; Huang et al., 2018; Adaszynska-Skwirzynska and Szczerbinska, 2019). With the increasing prohibition of antibiotics worldwide, EO are being used more often in livestock production. Consistent with findings of previous studies (Adaszynska-Skwirzynska and Szczerbinska, 2019; Mohebodini et al., 2021; Gao et al., 2022), the addition of EO had no adverse effect on hematological parameters (Table S2)—an important index reflecting the health status of animals, indicating that EO as a dietary supplement up to 300 mg/kg did not adversely affect the health of ducks. In addition, several previous studies have shown that EO could increase production performance while improving the health status of ducks (Adaszynska-Skwirzynska and Szczerbinska, 2019; Xue et al., 2020; Kim et al., 2021). The improvement in growth performance was further verified in this study, in that, Muscovy ducks fed diets containing 200 and 300 mg/kg EO showed markedly increased ADG alongside decreased FCR, when compared to the CON throughout the entire experiment. The active components (carvacrol, cinnamaldehyde, and capsicum oleoresin) in EO could have been responsible for these positive effects, which have previously been demonstrated to have anti-inflammatory and antioxidant abilities as well as improving intestinal health (Zare Mehrjerdi et al., 2020; Firmino et al., 2021). However, according to other studies, dietary dry oregano powder had no impact on duck growth performance (Abouelezz et al., 2019). Differences in the composition of ingredients, supplemental dosages, and environmental conditions may explain the discrepancies among these studies (Abouelezz et al., 2019; Torki et al., 2021). Hence, the ingredients and supplemental dosages of EO should be considered in production practices
EO are good sources of natural antioxidants (Kong et al., 2022), which have been widely used as antioxidant feed additives in poultry production (Zhang et al., 2021; Zhao et al., 2021b). Previous studies in vivo have shown that EO could inhibit oxidative stress, especially lipid peroxidation, by preventing free radical attack on biomembranes (Misharina et al., 2014; Zhumakanova et al., 2021). In this study, EO increased the activities of T-AOC, CAT, SOD, and GSH-Px, while decreased the lipid peroxidation biomarker MDA content in the serum. Our results are partially consistent with previous findings in broilers (Zhang et al., 2021) and laying hens (Zhao et al., 2021b). In addition, owing to their free radical-scavenging ability preventing lipid oxidation, EO are also used as natural antioxidants incorporated into meat to improve the quality attributes and oxidative stability of poultry meat. The positive effect of EO on chicken meat quality and oxidative stability has been proven in several previous studies (Ipcak and Alcicek, 2018; Hao et al., 2023). However, duck meat is more susceptible to oxidative deterioration due to its relatively high fat content compared to that of broilers (Biswas et al., 2019). Although some data on the effects of EO on broiler meat quality are available, data regarding EO effects on ducks remain lacking. In this study, dietary EO supplementation improved the water-holding capacity of breast muscle meat, as evidenced by the decreased drip loss. This can be presumably explained by the fact that dietary EO supplements incorporate natural antioxidants into duck meat and thus protect the membrane (phospholipids) of myocytes against lipid oxidation and maintain the structural integrity of membrane (Liu et al., 2019a). Overall, these results confirmed that dietary EO supplementation in ducks could exert favorable effects on antioxidant status, thus improving meat quality.
EO can boost the immune systems of birds and protect them against bacterial infection (Liu et al., 2019b; Mo et al., 2021). Immunoglobulins are required to regulate the immune function (Liu et al., 2019c; Mo et al., 2021). In this study, EO significantly increased serum IgM levels in ducks. Consistent with our findings, previous studies also reported that EO exhibited immunostimulant effects by raising immunoglobulin levels in broilers (Alp et al., 2012) and quails (Alagawany et al., 2021). This increased immunoglobulin could stimulate complementary components to enhance specific immune mechanisms in birds, thus protecting them against infection (Placha et al., 2014). In addition, dietary EO supplementation also showed anti-inflammatory effects in poultry (Liu et al., 2019c; Mo et al., 2021). The proinflammatory cytokines (e.g., TNF-α, IL-2, and IL-6) are markers of inflammatory status in birds (Zuo et al., 2014; Zyla et al., 2021; Zuza et al., 2022). EO have previously been reported to restrain the excretion of TNF-α and IL-6 in broilers (Liu et al., 2019b). This anti-inflammatory effect has also been reported in ducks (Yao et al., 2023). Consistent with these results, the IL-2 and TNF-α were decreased in the serum of ducks that received 200 to 300 mg/kg EO in this study. Furthermore, the anti-inflammatory properties of EO could be attributed to their roles in promoting the nonspecific immune response of host cells by nonspecifically destroying harmful germs (Liu et al., 2019b). In conclusion, these findings imply that EO addition to the diet could modulate the immune status of ducks.
The gut is responsible for digesting and absorbing nutrients, and ultimately affects the growth performance of birds (Turner, 2009; Prakatur et al., 2019). Villus height, crypt depth, and VH/CD are all considered vital indicators of digestive and absorptive capacity (Turner, 2009; Prakatur et al., 2019). Our results found that EO increased VH and CD in the jejunum of Muscovy ducks, indicating that EO could improve intestinal morphology, thus enhancing the digestion and absorption of nutrients. These findings were agreement with those of a previous study that reported a significant increase in jejunal VH/CD of ducks fed 100 mg/kg EO (Ding et al., 2020). Tight junctions play vital roles in the maintenance of normal functions in the intestine (Liu et al., 2020), damages to which would break down the barrier structure, thereby increasing intestinal permeability (Zhao et al., 2021a). In this study, we found that EO upregulated the mRNA expression levels of tight junctions such as claudin-1 and ZO-1 in jejunum, thus improving intestinal barrier function. Additionally, the increased jejunal antioxidant enzyme activities (e.g., T-AOC, CAT, SOD, and GSH-Px; Table S3) in response to EO in the jejunum could potentially improve intestinal morphology and health. The regulation of barrier functions by EO has also been reported in numerous previous studies (Humer et al., 2015; Liu et al., 2018; Su et al., 2020). The boosted intestinal morphology and barrier function of the intestinal mucosa in reaction to dietary EO observed in the current study further support the superior performance of ducks in EO-treated groups. Overall, both EO200 and EO300 showed positive effect on these parameters on growth performance and gut health. However, no more extra positive effects on these parameters were observed in EO300 than those in EO200, namely EO200 is more economical than EO300 on improving the growth performance and gut health of ducks. Therefore, considering the cost and efficacy of EO, an inclusion level of 200 mg/kg EO in duck diets is recommended.
The gut microbiota plays vital roles in the host's physiological, immunological, nutritional, and metabolic homeostasis (Kogut, 2013). Nevertheless, data on the effects of EO on the gut flora of ducks are relatively limited. To better comprehend the beneficial impacts of EO, further investigations were carried out on the gut microbiota of ducks in the CON and EO200 groups. The variety of gut microbiota is a credible indicator of host health, while a high diversity of microbiota is thought to be favorable for host health (Chen et al., 2017). In this study, dietary EO supplementation improved the richness and variety of gut microbiota, as seen by the elevated Chao1, Ace, and Shannon indices.
As shown by the elevated Chao1, Ace, and Shannon indices, dietary EO supplementation enhanced the richness and diversity of gut microbiota. Moreover, the gut microbiota in the EO and CON groups showed evident differences in beta diversity. These observations are consistent with those of several recent studies on EO supplementation (Ceppa et al., 2018; Wang et al., 2019; Spisni et al., 2020). These results demonstrate that the community structure of the cecal microbiota could be enhanced by adding 200 mg/kg EO to diet.
Changes in the microbiota composition were also observed in EO-treated ducks. In this study, Firmicutes and Bacteroidetes were the 2 primary phyla in the cecal contents of Muscovy ducks, which is consistent with the findings previously reported for ducks (Yang et al., 2020). Various members of Firmicutes and Bacteroidetes are beneficial for host health and growth (Wexler, 2007; Wang et al., 2022). For instance, increased Firmicutes has been associated with improved nutrient absorption (Oakley et al., 2014) and BW gain (Lee et al., 2017). This study verified the beneficial impact of increased Firmicutes abundance on growth performance. The Firmicutes/Bacteroidetes ratio is an important parameter reflecting microbiota function, with a higher Firmicutes to Bacteroidota ratio being beneficial for minimizing pathogens (Hao et al., 2021). In this study, we found that diet supplementation with EO markedly increased the ratio of Firmicutes to Bacteroidota, which is agreement with the results of a previous study (Yang et al., 2022). Given the increased Firmicutes/Bacteroidetes ratio, in parallel with the increased ADG, we postulated that the alterations of Firmicutes/Bacteroidetes ratios induced by EO supplementation could be the cause of the increased growth performance.
Differential analysis results showed that EO treatment increased the abundance of several probiotics, such as Subdoligranulum, UCG_005, and Shuttleworthia, which help in maintaining the overall microbiota structure. These results are partially consistent with the findings of previous studies (Zhang et al., 2022, 2023). Subdoligranulum and UCG_005 are known butyrate-producing bacteria (Radjabzadeh et al., 2022). Microbially derived butyrate has been reported to promote intestinal epithelial barrier function in poultry (Onrust et al., 2015). In this study, the relative abundances of Subdoligranulum and UCG_005 were positively correlated with the biomarkers of barrier function such as the increased VH and mRNA expression levels of claudin-1 and ZO-1 in the jejunum. Furthermore, SCFA produced by the gut microbiota play vital roles in the antioxidant and anti-inflammatory functions of poultry (Ali et al., 2022). Our results showed that CAT and GSH-Px activities, and serum IgM levels, were all positively correlated with the relative abundances of Subdoligranulum and UCG_005 in the cecum of ducks, partly supporting the results of previous studies (Xu et al., 2022). Therefore, combined with the improvement in antioxidant and immune status, jejunal morphology, and barrier function in EO-treated ducks, it can be speculated that EO supplementation could stimulate intestinal development and improve gut health in ducks by facilitating the relative abundance of butyrate-producing Subdoligranulum and UCG_005.
Growing evidence has demonstrated that Alistipes and Olsenella are generally regarded as harmful bacteria, with their increased abundance being closely associated with inflammation (Cobo et al., 2020). Meanwhile, the increased abundance of Blautia may stimulate TNF-α secretion (Egshatyan et al., 2016; Zhu et al., 2020). Similarly, this study indicated that the relative abundances of Alistipes, Olsenella, and Blautia positively correlated with the levels of proinflammatory cytokines in duck sera. These results indicate that the improved immune status resulting from dietary supplementation of EO could be attributed to the inhibition of proinflammatory pathogen colonization in duck cecum. Overall, the suppressed abundance of pathogens and increased abundance of several health-promoting bacteria may account for higher antioxidant and immune status, jejunal morphology, and barrier function in EO-treated ducks. Therefore, dietary EO could be utilized to shape the cecal microbiota composition and structure of ducks to maintain intestinal homeostasis and improve growth performance.
CONCLUSIONS
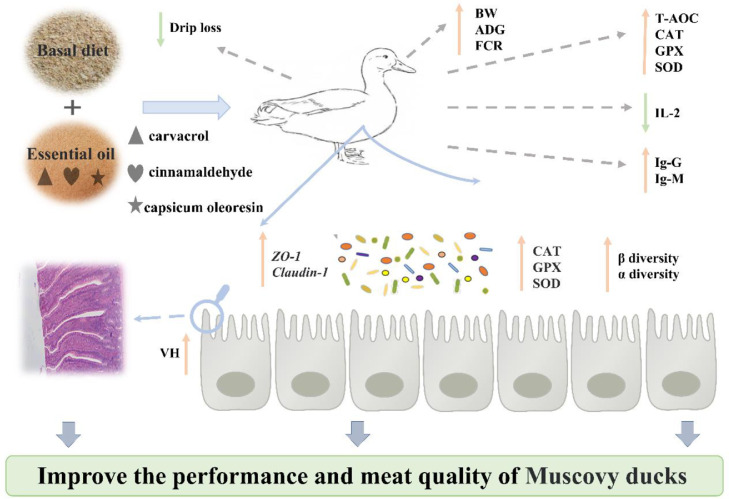
This study demonstrated that dietary supplementation with plant EO increased the growth performance and intestinal health of Muscovy ducks by enhancing antioxidative capacity, improving intestinal morphology and barrier function, increasing the abundance of SCFA-producing bacteria, and decreasing the abundance of enteric pathogens (Figure 7). Considering the cost and the efficacy of EO, an inclusion level of 200 mg/kg EO in duck diets is recommended.
Figure 7.
The schematic graphical of effects of mixed plant essential oils on performance, meat quality, and intestinal health of Muscovy ducks.
ACKNOWLEDGMENTS
This work was funded by the Collaborative Extension Plan for Major Agricultural Technologies in Zhejiang Province (grant no: 2021XTTGXM04).
Ethics Approval and Consent to Participate: Every experimental procedure was carried out in accordance with the Chinese Guidelines for Animal Welfare Act and all protocols were authorized by the Zhejiang University Institutional Animal Care and Use Committee (permission number: ZJU20220329).
Availability of Data and Materials: The datasets analyzed during the current study are available from the corresponding author upon request.
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in the present study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.102813.
Contributor Information
Dongyou Yu, Email: dyyu@zju.edu.cn.
Bing Liu, Email: bing.liu@zju.edu.cn.
Appendix. Supplementary materials
REFERENCES
- Abouelezz K., Abou-Hadied M., Yuan J., Elokil A.A., Wang G., Wang S., Wang J., Bian G. Nutritional impacts of dietary oregano and Enviva essential oils on the performance, gut microbiota and blood biochemicals of growing ducks. Animal. 2019;13:2216–2222. doi: 10.1017/S1751731119000508. [DOI] [PubMed] [Google Scholar]
- Adaszynska-Skwirzynska M., Szczerbinska D. The effect of lavender (Lavandula angustifolia) essential oil as a drinking water supplement on the production performance, blood biochemical parameters, and ileal microflora in broiler chickens. Poult. Sci. 2019;98:358–365. doi: 10.3382/ps/pey385. [DOI] [PubMed] [Google Scholar]
- Alagawany M., El-Saadony M.T., Elnesr S.S., Farahat M., Attia G., Madkour M., Reda F.M. Use of lemongrass essential oil as a feed additive in quail's nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali Q., Ma S., La S.K., Guo Z.G., Liu B.S., Gao Z.M., Farooq U., Wang Z.C., Zhu X.Y., Cui Y.L., Li D.F., Shi Y.H. Microbial short-chain fatty acids: a bridge between dietary fibers and poultry gut health – a review. Anim. Biosci. 2022;35:1461–1478. doi: 10.5713/ab.21.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alp M., Midilli M., Kocabagli N., Yilmaz H., Turan N., Gargili A., Acar N. The effects of dietary oregano essential oil on live performance, carcass yield, serum immunoglobulin G level, and oocyst count in broilers. J. Appl. Poult. Res. 2012;21:630–636. [Google Scholar]
- Ariana A., Ebadi R., Tahmasebi G. Laboratory evaluation of some plant essences to control Varroa destructor (Acari: Varroidae) Exp. Appl. Acarol. 2002;27:319–327. doi: 10.1023/a:1023342118549. [DOI] [PubMed] [Google Scholar]
- Biswas S., Banerjee R., Bhattacharyya D., Patra G., Das A.K., Das S.K. Technological investigation into duck meat and its products – a potential alternative to chicken. World Poult. Sci. J. 2019;75:609–620. [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods – a review. Int. J. Food. Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Ceppa F., Faccenda F., De Filippo C., Albanese D., Pindo M., Martelli R., Marconi P., Lunelli F., Fava F., Parisi G. Influence of essential oils in diet and life-stage on gut microbiota and fillet quality of rainbow trout (Oncorhynchus mykiss) Int. J. Food. Sci. Nutr. 2018;69:318–333. doi: 10.1080/09637486.2017.1370699. [DOI] [PubMed] [Google Scholar]
- Chen L.M., Xu Y.S., Chen X.Y., Fang C., Zhao L.P., Chen F. The maturing development of gut microbiota in commercial piglets during the weaning transition. Front. Microbiol. 2017;8:1688. doi: 10.3389/fmicb.2017.01688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobo F., Foronda C., Perez-Carrasco V., Martin-Hita L., Garcia-Salcedo J.A., Navarro-Mari J.M. First description of abdominal infection due to Alistipes onderdonkii. Anaerobe. 2020;66 doi: 10.1016/j.anaerobe.2020.102283. [DOI] [PubMed] [Google Scholar]
- Ding X., Wu X., Zhang K., Bai S., Wang J., Peng H., Xuan Y., Su Z., Zeng Q. Dietary supplement of essential oil from oregano affects growth performance, nutrient utilization, intestinal morphology and antioxidant ability in Pekin ducks. J. Anim. Physiol. Anim. Nutr. (Berl). 2020;104:1067–1074. doi: 10.1111/jpn.13311. [DOI] [PubMed] [Google Scholar]
- Egshatyan L., Kashtanova D., Popenko A., Tkacheva O., Tyakht A., Alexeev D., Karamnova N., Kostryukova E., Babenko V., Vakhitova M., Boytsov S. Gut microbiota and diet in patients with different glucose tolerance. Endocr. Connect. 2016;5:1–9. doi: 10.1530/EC-15-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firmino J.P., Vallejos-Vidal E., Balebona M.C., Ramayo-Caldas Y., Cerezo I.M., Salomon R., Tort L., Estevez A., Morinigo M.A., Reyes-Lopez F.E., Gisbert E. Diet, immunity, and microbiota interactions: an integrative analysis of the intestine transcriptional response and microbiota modulation in gilthead seabream (Sparus aurata) fed an essential oils-based functional diet. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.625297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F., Zhang L., Li H., Xia F., Bai H., Piao X., Sun Z., Cui H., Shi L. Dietary oregano essential oil supplementation influences production performance and gut microbiota in late-phase laying hens fed wheat-based diets. Animals (Basel) 2022;12:3007. doi: 10.3390/ani12213007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y., Ji Z., Shen Z., Wu Y., Zhang B., Tang J., Hou S., Xie M. Effects of total dietary fiber on cecal microbial community and intestinal morphology of growing white Pekin duck. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.727200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y., Kang J., Guo X., Sun M., Li H., Bai H., Cui H., Shi L. PH-responsive chitosan-based film containing oregano essential oil and black rice bran anthocyanin for preserving pork and monitoring freshness. Food Chem. 2023;403 doi: 10.1016/j.foodchem.2022.134393. [DOI] [PubMed] [Google Scholar]
- Hernando-Amado S., Coque T.M., Baquero F., Martinez J.L. Antibiotic resistance: moving from individual health norms to social norms in one health and global health. Front. Microbiol. 2020;11:1914. doi: 10.3389/fmicb.2020.01914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Liu X.C., Jia S.L., Zhang L.T., Luo Y.K. The effect of essential oils on microbial composition and quality of grass carp (Ctenopharyngodon idellus) fillets during chilled storage. Int. J. Food Microbiol. 2018;266:52–59. doi: 10.1016/j.ijfoodmicro.2017.11.003. [DOI] [PubMed] [Google Scholar]
- Humer E., Rohrer E., Windisch W., Wetscherek W., Schwarz C., Jungbauer L., Schedle K. Gender-specific effects of a phytogenic feed additive on performance, intestinal physiology and morphology in broiler chickens. J. Anim. Physiol. Anim. Nutr. (Berl). 2015;99:788–800. doi: 10.1111/jpn.12238. [DOI] [PubMed] [Google Scholar]
- Ipcak H.H., Alcicek A. Addition of capsicum oleoresin, carvacrol, cinnamaldehyde and their mixtures to the broiler diet II: effects on meat quality. J. Anim. Sci. Technol. 2018;60:9. doi: 10.1186/s40781-018-0165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadas F., Pirgozliev V., Rose S.P., Dimitrov D., Oduguwa O., Bravo D. Dietary essential oils improve the hepatic antioxidative status of broiler chickens. Br. Poult. Sci. 2014;55:329–334. doi: 10.1080/00071668.2014.891098. [DOI] [PubMed] [Google Scholar]
- Kim S.Y., Han S.D., Kim M., Mony T.J., Lee E.S., Kim K.M., Choi S.H., Hong S.H., Choi J.W., Park S.J. Mentha arvensis essential oil exerts anti-inflammatory in LPS-stimulated inflammatory responses via inhibition of ERK/NF-kappa B signaling pathway and anti-atopic dermatitis-like effects in 2,4-dinitrochlorobezene-induced BALB/c mice. Antioxidants Basel. 2021;10:1941. doi: 10.3390/antiox10121941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogut M.H. The gut microbiota and host innate immunity: regulators of host metabolism and metabolic diseases in poultry? J. Appl. Poult. Res. 2013;22:637–646. [Google Scholar]
- Kong A.S., Maran S., Yap P.S., Lim S.E., Yang S.K., Cheng W.H., Tan Y.H., Lai K.S. Anti- and pro-oxidant properties of essential oils against antimicrobial resistance. Antioxidants. 2022;11:1819. doi: 10.3390/antiox11091819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurekci C., Ozsoy B., Hassan E., Ozkan H., Gundogdu A., Ozsoy S.Y., Yakan A. Effect of essential oil supplementation to diet on meat quality, fatty acid composition, performance parameters and intestinal microbiota of Japanese quails. J. Anim. Physiol. Anim. Nutr. (Berl.) 2021;105:927–937. doi: 10.1111/jpn.13445. [DOI] [PubMed] [Google Scholar]
- Lee K.C., Kil D.Y., Sul W.J. Cecal microbiome divergence of broiler chickens by sex and body weight. J. Microbiol. 2017;55:939–945. doi: 10.1007/s12275-017-7202-0. [DOI] [PubMed] [Google Scholar]
- Lillehoj H.S., Kim D.K., Bravo D.M., Lee S.H. Effects of dietary plant-derived phytonutrients on the genome-wide profiles and coccidiosis resistance in the broiler chickens. BMC Proc. 2011;5(Suppl. 4):S34. doi: 10.1186/1753-6561-5-S4-S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippens M., Huyghebaert G., Cerchiari E. Effect of the use of coated plant extracts and organic acids as alternatives for antimicrobial growth promoters on the performance of broiler chickens. Arch. Geflugelkd. 2005;69:261–266. [Google Scholar]
- Liu K., Jia M., Wong E.A. Delayed access to feed affects broiler small intestinal morphology and goblet cell ontogeny. Poult. Sci. 2020;99:5275–5285. doi: 10.1016/j.psj.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Jiang J., Lin G., Yu D., Xiong Y.L. Upregulation of antioxidant enzymes by organic mineral co-factors to improve oxidative stability and quality attributes of muscle from laying hens. Food Res. Int. 2019;125 doi: 10.1016/j.foodres.2019.108575. [DOI] [PubMed] [Google Scholar]
- Liu S.D., Song M.H., Yun W., Lee J.H., Kim H.B., Cho J.H. Effect of carvacrol essential oils on immune response and inflammation-related genes expression in broilers challenged by lipopolysaccharide. Poult. Sci. 2019;98:2026–2033. doi: 10.3382/ps/pey575. [DOI] [PubMed] [Google Scholar]
- Liu S., Song M., Yun W., Lee J., Lee C., Kwak W., Han N., Kim H., Cho J. Effects of oral administration of different dosages of carvacrol essential oils on intestinal barrier function in broilers. J. Anim. Physiol. Anim. Nutr. (Berl.) 2018;102:1257–1265. doi: 10.1111/jpn.12944. [DOI] [PubMed] [Google Scholar]
- Liu B., Xiong Y.L.L., Jiang J., Yu D.Y., Lin G. Cellular antioxidant mechanism of selenium-enriched yeast diets in the protection of meat quality of heat-stressed hens. Food Biosci. 2021;39 [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Migliorini M.J., Boiago M.M., Roza L.F., Barreta M., Arno A., Robazza W.S., Galvao A.C., Galli G.M., Machado G., Baldissera M.D., Wagner R., Stefani L.C.M., Silva A.S.D. Oregano essential oil (Origanum vulgare) to feed laying hens and its effects on animal health. Ann. Acad. Bras. Cienc. 2019;91 doi: 10.1590/0001-3765201920170901. [DOI] [PubMed] [Google Scholar]
- Misharina T.A., Fatkullina L.D., Alinkina E.S., Kozachenko A.I., Nagler L.G., Medvedeva I.B., Goloshchapov A.N., Burlakova E.B. Effects of low doses of essential oil on the antioxidant state of the erythrocytes, liver, and the brains of mice. Prikl. Biokhim. Mikrobiol. 2014;50:101–107. doi: 10.7868/s0555109914010097. [DOI] [PubMed] [Google Scholar]
- Mo K., Li J., Liu F., Xu Y., Huang X., Ni H. Superiority of microencapsulated essential oils compared with common essential oils and antibiotics: effects on the intestinal health and gut microbiota of weaning piglet. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohebodini H., Jazi V., Ashayerizadeh A., Toghyani M., Tellez-Isaias G. Productive parameters, cecal microflora, nutrient digestibility, antioxidant status, and thigh muscle fatty acid profile in broiler chickens fed with Eucalyptus globulus essential oil. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . Nutrient Requirements of Poultry. 9th rev. ed. National Academies Press; Washington, DC: 1994. [Google Scholar]
- Noruzi S., Torki M., Mohammadi H. Effects of supplementing diet with Thyme (Thymuas vulgaris L.) essential oil and/or selenium yeast on production performance and blood variables of broiler chickens. Vet. Med. Sci. 2022;8:1137–1145. doi: 10.1002/vms3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B.B., Lillehoj H.S., Kogut M.H., Kim W.K., Maurer J.J., Pedroso A., Lee M.D., Collett S.R., Johnson T.J., Cox N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014;360:100–112. doi: 10.1111/1574-6968.12608. [DOI] [PubMed] [Google Scholar]
- Onrust L., Ducatelle R., Van Driessche K., De Maesschalck C., Vermeulen K., Haesebrouck F., Eeckhaut V., Van Immerseel F. Steering endogenous butyrate production in the intestinal tract of broilers as a tool to improve gut health. Front. Vet. Sci. 2015;2:75. doi: 10.3389/fvets.2015.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placha I., Takacova J., Ryzner M., Cobanova K., Laukova A., Strompfova V., Venglovska K., Faix S. Effect of thyme essential oil and selenium on intestine integrity and antioxidant status of broilers. Br. Poult. Sci. 2014;55:105–114. doi: 10.1080/00071668.2013.873772. [DOI] [PubMed] [Google Scholar]
- Prakatur I., Miskulin M., Pavic M., Marjanovic K., Blazicevic V., Miskulin I., Domacinovic M. Intestinal morphology in broiler chickens supplemented with propolis and bee pollen. Animals (Basel) 2019;9:301. doi: 10.3390/ani9060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga P.R., Grosso N.R., Lante A., Lomolino G., Zygadlo J.A., Nepote V. Chemical composition, antioxidant activity and anti-lipase activity of Origanum vulgare and Lippia turbinata essential oils. Int. J. Food Sci. Technol. 2013;48:642–649. [Google Scholar]
- Radjabzadeh D., Bosch J.A., Uitterlinden A.G., Zwinderman A.H., Ikram M.A., van Meurs J.B.J., Luik A.I., Nieuwdorp M., Lok A., van Duijn C.M., Kraaij R., Amin N. Gut microbiome-wide association study of depressive symptoms. Nat. Commun. 2022;13:7128. doi: 10.1038/s41467-022-34502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda F.M., Alagawany M., Mahmoud H.K., Mahgoub S.A., Elnesr S.S. Use of red pepper oil in quail diets and its effect on performance, carcass measurements, intestinal microbiota, antioxidant indices, immunity and blood constituents. Animal. 2020;14:1025–1033. doi: 10.1017/S1751731119002891. [DOI] [PubMed] [Google Scholar]
- Reis J.H., Gebert R.R., Barreta M., Baldissera M.D., Dos Santos I.D., Wagner R., Campigotto G., Jaguezeski A.M., Gris A., de Lima J.L.J.M.p. Effects of phytogenic feed additive based on thymol, carvacrol and cinnamic aldehyde on body weight, blood parameters and environmental bacteria in broilers chickens. Microb. Pathog. 2018;125:168–176. doi: 10.1016/j.micpath.2018.09.015. [DOI] [PubMed] [Google Scholar]
- Reis J.H., Gebert R.R., Barreta M., Baldissera M.D., Dos Santos I.D., Wagner R., Campigotto G., Jaguezeski A.M., Gris A., de Lima J.L.F., Mendes R.E., Fracasso M., Boiago M.M., Stefani L.M., Dos Santos D.S., Robazza W.S., Da Silva A.S. Effects of phytogenic feed additive based on thymol, carvacrol and cinnamic aldehyde on body weight, blood parameters and environmental bacteria in broilers chickens. Microb. Pathog. 2018;125:168–176. doi: 10.1016/j.micpath.2018.09.015. [DOI] [PubMed] [Google Scholar]
- Ruan D., Fan Q., Fouad A.M., Sun Y., Huang S., Wu A., Lin C., Kuang Z., Zhang C., Jiang S. Effects of dietary oregano essential oil supplementation on growth performance, intestinal antioxidative capacity, immunity, and intestinal microbiota in yellow-feathered chickens. J. Anim. Sci. 2021;99:skab033. doi: 10.1093/jas/skab033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safwat A.M., Taher M.O., El-Deen M.B., El-Naeem M.A. Response to dietary supplementation of mixtures of either selected synbiotic, organic acids or essential oils as growth promoters for growing Japanese quails. J. Anim. Feed Sci. 2021;30:279–287. [Google Scholar]
- Spisni E., Petrocelli G., Imbesi V., Spigarelli R., Azzinnari D., Sarti M.D., Campieri M., Valerii M.C. Antioxidant, anti-inflammatory, and microbial-modulating activities of essential oils: implications in colonic pathophysiology. Int. J. Mol. Sci. 2020;21:4152. doi: 10.3390/ijms21114152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su G.Q., Wang L., Zhou X.W., Wu X.Y., Chen D.W., Yu B., Huang Z.Q., Luo Y.H., Mao X.B., Zheng P., Yu J., Luo J.Q., He J. Effects of essential oil on growth performance, digestibility, immunity, and intestinal health in broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su G.Q., Zhou X.W., Wang Y., Chen D.W., Chen G., Li Y., He J. Dietary supplementation of plant essential oil improves growth performance, intestinal morphology and health in weaned pigs. J. Anim. Physiol. Anim. Nutr. (Berl.) 2020;104:579–589. doi: 10.1111/jpn.13271. [DOI] [PubMed] [Google Scholar]
- Torki M., Mohebbifar A., Mohammadi H. Effects of supplementing hen diet with Lavandula angustifolia and/or Mentha spicata essential oils on production performance, egg quality and blood variables of laying hens. Vet. Med. Sci. 2021;7:184–193. doi: 10.1002/vms3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- Wang C., Xiao Y., Yu L., Tian F., Zhao J., Zhang H., Chen W., Zhai Q. Protective effects of different Bacteroides vulgatus strains against lipopolysaccharide-induced acute intestinal injury, and their underlying functional genes. J. Adv. Res. 2022;36:27–37. doi: 10.1016/j.jare.2021.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zhang Y., Fan G., Ren J.N., Zhang L.L., Pan S.Y. Effects of orange essential oil on intestinal microflora in mice. J. Sci. Food Agric. 2019;99:4019–4028. doi: 10.1002/jsfa.9629. [DOI] [PubMed] [Google Scholar]
- Wexler H.M. Bacteroides: the good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007;20:593–621. doi: 10.1128/CMR.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q.L., Liu C., Mo X.J., Chen M., Zhao X.L., Liu M.Z., Wang S.B., Zhou B., Zhao C.X. Drinking water supplemented with acidifiers improves the growth performance of weaned pigs and potentially regulates antioxidant capacity, immunity, and gastrointestinal microbiota diversity. Antioxidants (Basel) 2022;11:809. doi: 10.3390/antiox11050809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue F.G., Shi L., Li Y.L., Ni A.X., Ma H., Sun Y.Y., Chen J.L. Effects of replacing dietary Aureomycin with a combination of plant essential oils on production performance and gastrointestinal health of broilers. Poult. Sci. 2020;99:4521–4529. doi: 10.1016/j.psj.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.Y., Chen S.Y., Wu Y.H., Liao Y.L., Yen G.C. Ameliorative effect of buckwheat polysaccharides on colitis via regulation of the gut microbiota. Int. J. Biol. Macromol. 2022;227:872–883. doi: 10.1016/j.ijbiomac.2022.12.155. [DOI] [PubMed] [Google Scholar]
- Yang H., Lyu W., Lu L., Shi X., Li N., Wang W., Xiao Y. Biogeography of microbiome and short-chain fatty acids in the gastrointestinal tract of duck. Poult. Sci. 2020;99:4016–4027. doi: 10.1016/j.psj.2020.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Liu Y., Li C., Huang X., Zhang X., Deng P., Jiang G., Dai Q. Effects of rosemary extract supplementation in feed on growth performance, meat quality, serum biochemistry, antioxidant capacity, and immune function of meat ducks. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z., Zhang F., Smith J., Kuo R., Hou Z.C. Full-length transcriptome sequencing from multiple tissues of duck, Anas platyrhynchos. Sci. Data. 2019;6:275. doi: 10.1038/s41597-019-0293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare Mehrjerdi F., Niknazar S., Yadegari M., Akbari F.A., Pirmoradi Z., Khaksari M. Carvacrol reduces hippocampal cell death and improves learning and memory deficits following lead-induced neurotoxicity via antioxidant activity. Naunyn. Schmiedebergs Arch. Pharmacol. 2020;393:1229–1237. doi: 10.1007/s00210-020-01866-6. [DOI] [PubMed] [Google Scholar]
- Zhang L.Y., Peng Q.Y., Liu Y.R., Ma Q.G., Zhang J.Y., Guo Y.P., Xue Z., Zhao L.H. Effects of oregano essential oil as an antibiotic growth promoter alternative on growth performance, antioxidant status, and intestinal health of broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Wang Y., Ye J., Fan Q., Lin X., Gou Z., Jiang S. Dietary supplementation of bilberry anthocyanin on growth performance, intestinal mucosal barrier and cecal microbes of chickens challenged with Salmonella Typhimurium. J. Anim. Sci. Biotechnol. 2023;14:15. doi: 10.1186/s40104-022-00799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Zhou D., Wang X., Xiao T., Wu L., Tang Q., Lu Y. Effects of Kadsura coccinea L. fruit extract on growth performance, meat quality, immunity, antioxidant, intestinal morphology and flora of white-feathered broilers. Animals (Basel) 2022;13:93. doi: 10.3390/ani13010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Xie Q., Evivie S.E., Liu D., Dong J., Ping L., Liu F., Li B., Huo G. Bifidobacterium dentium N8 with potential probiotic characteristics prevents LPS-induced intestinal barrier injury by alleviating the inflammatory response and regulating the tight junction in Caco-2 cell monolayers. Food Funct. 2021;12:7171–7184. doi: 10.1039/d1fo01164b. [DOI] [PubMed] [Google Scholar]
- Zhao P., Yan L., Zhang T., Yin H., Liu J., Wang J. Effect of 25-hydroxyvitamin D and essential oil complex on productive performance, egg quality, and uterus antioxidant capacity of laying hens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L.L., Sha L.P., Li K., Wang Z., Wang T., Li Y.W., Liu P., Dong X.Y., Dong Y.P., Zhang X.X., Wang H. Dietary flaxseed oil rich in omega-3 suppresses severity of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in rats. Lipids Health Dis. 2020;19:20. doi: 10.1186/s12944-019-1167-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q., Sun P., Zhang B., Kong L., Xiao C., Song Z. Progress on gut health maintenance and antibiotic alternatives in broiler chicken production. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.692839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhumakanova B.S., Korona-Glowniak I., Skalicka-Wozniak K., Ludwiczuk A., Baj T., Wojtanowski K.K., Jozefczyk A., Zhaparkulova K.A., Sakipova Z.B., Malm A. Phytochemical fingerprinting and in vitro antimicrobial and antioxidant activity of the aerial parts of Thymus marschallianus willd and Thymus seravschanicus klokov growing widely in southern Kazakhstan. Molecules. 2021;26:3193. doi: 10.3390/molecules26113193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L., Yuan K.T., Yu L., Meng Q.H., Chung P.C., Yang D.H. Bifidobacterium infantis attenuates colitis by regulating T cell subset responses. World J. Gastroenterol. 2014;20:18316–18329. doi: 10.3748/wjg.v20.i48.18316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuza O., Minic R., Kotur-Stevuljevic J., Zujovic D., Dordevic B., Ilic A. A combination of N-acetyl cysteine and propolis attenuates oxidative-inflammatory parameters during COPD exacerbation. Eur. Rev. Med. Pharmacol. Sci. 2022;26:2467–2477. doi: 10.26355/eurrev_202204_28481. [DOI] [PubMed] [Google Scholar]
- Zyla E., Dziendzikowska K., Kamola D., Wilczak J., Sapierzynski R., Harasym J., Gromadzka-Ostrowska J. Anti-inflammatory activity of oat beta-glucans in a Crohn's disease model: time- and molar mass-dependent effects. Int. J. Mol. Sci. 2021;22:4485. doi: 10.3390/ijms22094485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.