Summary
Background
The endothelium is supposedly activated and damaged in COVID-19 because of endothelin-1 over-secretion. This study evaluates the effect of bosentan as an endothelin receptor blocker on the progression of disease in high-risk outpatients with COVID-19 infection.
Methods
From 15 December 2021 to 15 May 2022, high-risk outpatients were randomly assigned to receive bosentan, 62.5 mg or placebo twice daily from enrollment for 30 days. Both groups received standard medical treatment too. On day 30 of the trial, the patients underwent complete doppler ultrasound of the lower extremities to detect asymptomatic thromboembolic events. The primary outcome in this study was hospitalization or death from any cause within the first 15 days. Secondary outcomes included thromboembolic events, hospital-free days and death from any cause within 30 days after randomization (IRCT.ir, IRCT20211203053263N1).
Findings
Basal characteristics of the two groups were similar. Primary outcomes occurred in 3 (2.3%) of the 129 patients in the bosentan group versus 15 (11.5%) of the 130 patients in the placebo group [risk difference: −9.2% (95% CI: −15.3 to −3.1), P = 0.006]. Median hospital-free days was significantly higher in the bosentan group (P = 0.004). A total of three deaths occurred and all were in the control group. Bosentan was associated with a nonsignificant reduction in mortality compared with placebo (P = 0.24). Thromboembolic events occurred in one (1%) of 97 patients in the bosentan group versus nine (8.7%) of 104 patients in the placebo group within 30 days after randomization [risk difference: −8.3% (95% CI: −14.4 to −2.2), P = 0.008].
Interpretation
Early administration of bosentan may prevent disease progression and thromboembolic events in high-risk outpatients with COVID-19.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Keywords: Mortality, Thromboembolic events, Hospitalization, Doppler, Adverse event
Research in context.
Evidence before this study
Endothelium is activated and damaged in COVID-19 because of endothelin-1 over-secretion. Bosentan is an orally active endothelin receptor antagonist that blocks the endothelin receptors on endothelium and vascular smooth muscles. Bosentan is used in the management of pulmonary hypertension and Raynaud's phenomenon. To date, no randomized controlled trial studies have evaluated the efficacy of bosentan administration on covid-19 infection. This study evaluated the effect of bosentan as an endothelin receptor blocker on the progression of disease in high-risk outpatients with COVID-19 infection.
Added value of this study
The early administration of bosentan initiated within three days of the onset of symptoms was found to be effective in the prevention of severe COVID-19 in high-risk non-hospitalized patients. Moreover, this intervention may be effective in the prevention of thromboembolic events.
Implications of all the available evidence
This research evaluated the efficacy of bosentan in high risk outpatients with covid-19. Larger, adequately powered clinical trials are needed to show definitive benefits of bosentan.
Introduction
A large percentage of coronavirus disease 2019 (COVID-19) patients are high-risk outpatients at risk of developing severe or critical disease. The prevention of disease progression reduces the need for hospitalization and mortality. It has been reported that the endothelium is activated and damaged in COVID-19. It seems that a hyper-inflammatory state is responsible for endothelial dysfunction with subsequent overexpression of vascular endothelin-1 (ET1), the most powerful vasopressor of endothelial cells.1 Other effects of ET1 on the vascular system include hyperplasia, hypertrophy, fibrosis, and hyperpermeability.2 ET1 is also secreted from the endothelial lining of the airways, cardiac cells, brain neurons, fibroblasts, macrophages, and vascular smooth muscle cell.2 Endothelial dysfunction leads to disintegrated vessel barrier, pro-coagulative state, inflammation, and leukocyte infiltration.3 The incidence of acute respiratory distress syndrome (ARDS), Kawasaki disease,4 pulmonary embolism, deep vein thrombosis, stroke, thrombosis in the extracorporeal circuits,5 multiple organ failure and acro-ischemic change6 in COVID-19 infection has been considered a result of endothelial cell damage. Furthermore, many high risk COVID-19 patients have underlying comorbidities (diabetes, hypertension, and cardiovascular diseases) and are older adults. These conditions are often accompanied by chronic vascular endothelial dysfunction because of the increase in the production of endothelium-derived contracting factors such as ET1.7,8
Bosentan is an orally active endothelin receptor antagonist that blocks the endothelin receptors on endothelium and vascular smooth muscles. In the lung of guinea pigs with pulmonary inflammation, bosentan reduces inflammatory reactions, prevents increase in permeability of pulmonary vessels, and prevents the development of fibrosis.9,10
In the present study, we hypothesized that bosentan can prevent the progression of disease in high-risk non-hospitalized COVID-19 patients by maintaining the integrity of endothelium. The efficacy of bosentan administration on the need for hospitalization, thromboembolic events and mortality in high-risk outpatients was thus evaluated.
Methods
Study design and oversight
This randomized double-blind, placebo-controlled, clinical trial is an ongoing phase 3 trial study with an add-on design conducted from 15 December 2021 to 15 May 2022 across two university hospitals in Ilam.
Ethics
The study protocol, protocol amendments and other relevant documents were reviewed and approved by the review board and ethics committee of Ilam University of Medical Sciences (IR.MEDILAM.REC.1400.164), and the work has been reported in line with Consolidated Standards of Reporting Trials (CONSORT) Guidelines. Also, it was carried out in accordance with the Declaration of Helsinki of the World Medical Association. This study was registered in the Iranian Registry of Clinical Trials (IRCT20211203053263N1) and full protocol available from the corresponding author on request. All participants provided written informed consent before intervention.
Participants
Consecutive high-risk, non-hospitalized, COVID-19 patients who were clinically stable and unvaccinated and who met the following criteria were recruited for the study: having at least one risk factor for severe COVID-19 illness, Age ≤ 18 years, presenting to a COVID-19 clinic with laboratory-confirmed COVID-19 illness, showing at least one sign or symptom of COVID-19, having adequately stable conditions for outpatient management without supplemental oxygen intake, and at least one risk factor for severe COVID-19 illness. High-risk patients were defined as those at high risk for progression to severe/critical illness, but clinically stable for randomized outpatient management. These risk factors included age ≥50 years, hypertension, diabetes, serious heart conditions, chronic lung disease, chronic kidney disease, immunosuppression, sickle cell disease, BMI ≥ 25 and active cancer. The patients needed to have a vigilant caregiver who could take care of them and be able to communicate well with the medical staff over video call. The exclusion criteria were as follows: More than three days elapsed since the onset of signs and symptoms, serum aminotransferase levels more than three times normal, pregnancy or lactation, glibenclamide, cyclosporine or anticoagulant consumption, SARS-CoV-2 vaccination, living alone, inability to monitor and report on one's condition by oneself or through a caregiver, inability to be contacted by video call, and enrollment in another interventional trial for COVID-19. Women of childbearing age who did not agree to use at least one primary form of contraception were also excluded from the study. Non-Iranian nationals (Iraqi or Afghan) were excluded from the study due to the possibility of loss to follow-up. All efforts were made to determine the survival status and hospitalization by day 30 for participants who terminated the trial earlier.
Randomization and intervention
In order to randomly assign the subjects to the intervention and placebo groups, stratified random blocks were used with a block size of 4, leading to six different permutations. A nurse was unblinded to the treatment assignment and preparation, whereas the patients, investigators, and all clinical personnel remained blinded to the randomization. The intervention group received bosentan tab 62.5 mg twice daily and the placebo group received identical placebo in the same manner. The intervention had to be within three days of the onset of signs and symptoms. Due to budget constraints, we placed bosentan tablets in 1-g capsules and filled them with starch for concealment. Similar capsules filled entirely with starch were given to the placebo group. The patients received symptomatic treatment, including antipyretics and analgesics for fever, myalgias, and headache, if needed. Acetaminophen was the analgesic of choice, but if no response was seen to acetaminophen, Naproxen was prescribed in its stead.
Persistent cough interfering with sleep was managed with dextromethorphan syrup. We also advised the patients to take a rest, keep well hydrated (particularly in the febrile patients), and consume a high protein diet with vegetables and fruits according to the Healthy Eating Diet.
The duration of the intervention was 30 days. In the first visit, half of the capsules (n = 30) were given to the patients. On day 15, when the patient came back for a blood test, the rest of the capsules were given to them. The number of unused capsules was also counted in this session as well as on day 30 of the intervention. In those who were initially non-hospitalized and then required hospitalization, treatment with bosentan was continued. In hospitalized patients who were fed via a feeding tube, the capsules were filled with powdered bosentan tablets mixed with starch.
The patients were assessed by video calls daily for 15 days to assess their disease progression and biweekly thereafter. If needed, the patients were allowed to make phone or video calls to the researchers at other occasions.
The questions raised by video calls assessed: (1) Dyspnea, such as any breathing problems resulting in limited activities; (2) Mental status changes, such as lethargy, confusion, change in behavior, difficulty rousing from sleep, and falls. The patients were also asked about potential decrease in urine volume, any chest pain, and any symptoms of hepatotoxicity, including vomiting, abdominal pain, or jaundice. Cyanosis was also examined by observation during the video calls. Cases of hospitalization or death were also recorded during this period. Furthermore, serum aminotransferase levels were measured on days one and 15 of the intervention.
On day 30 of the trial, the patients underwent complete Doppler ultrasound of the lower extremities, which included the proximal and distal territories, to detect asymptomatic thromboembolic events.
Endpoints
The primary outcome was disease progression, which was defined as death or hospitalization within 15 days after randomization. Secondary outcomes were thromboembolic events, hospital-free days, and death from any cause within 30 days after randomization. Adverse events were also evaluated throughout the follow-up period. All the randomized patients who had received any dose of bosentan or placebo were evaluated for adverse events.
As the number of COVID-19 patients declined, an interim analysis was performed to report the outcomes. Data were collected by the researchers and analyzed by a statistician working at the university and then interpreted by the authors. The authors guarantee the accuracy and completeness of the data and have reviewed and approved the final manuscript.
Statistics
At the time the trial was initiated, we estimated that the primary outcome would occur in 10% of the patients in the placebo group, and the trial required a sample size of 270 patients to detect an absolute between-group difference of 8 percentage points with a power of 80%. Assuming the possibility of dropouts, the total sample size for the study was taken as approximately 300. As the number of COVID-19 patients declined, an interim analysis was performed to report the outcomes.
Baseline demographic and clinical characteristics are presented as frequencies and percentages, means and 95% confidence intervals (CIs) or standard deviations, or medians and interquartile ranges (IQR), whenever appropriate. Analyses between the groups were conducted under intention-to-treat principles except for thromboembolic events which was analyzed under per protocol analysis. The Chi-square or Fisher exact tests were used to evaluate differences in the distribution of the categorical variables, and Mann–Whitney's U-test or the student t-test were used to check differences in the distribution of the continuous variables between the intervention and placebo groups.
For the primary efficacy endpoint, and the secondary outcomes of death and thromboembolic events, which had binary responses, the risk difference and relative risk for the bosentan group versus the placebo group as a reference were calculated.
A Kaplan–Meier curve was also used to summarize the time to the primary event during the first 15 days post-dose for each group. Furthermore, a Cox proportional-hazards model was used to obtain the hazard ratio (HR) and respective 95% CIs to support the primary outcome.
Hospital-free days was compared between the two groups using Mann–Whitney's U-test, given the non-normal distribution of this outcome. Data were analyzed in SPSS-21 (Chicago, IL, USA). All the hypothesis tests were two-tailed, with P < 0.05 denoting the level of statistical significance. The data monitoring committee conducted a review of masked safety data once 100 participants had been enrolled.
Role of funders
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Results
Patients
From 15 December 2021 to 15 May 2022, a total of 259 patients underwent randomization; 129 of the patients received bosentan and 130 patients received placebo (Fig. 1). All the patients were followed for 30 days to evaluate the outcomes; however, some of them did not cooperate in getting a Doppler ultrasound. The baseline characteristic of the patients was the same, as shown in Table 1.
Fig. 1.
Flow chart depicting the study design.
Table 1.
Demographic and clinical characteristics patients.
| Variable | Placebo (N = 130) | Bosentan (N = 129) | Total (N = 259) |
|---|---|---|---|
| Age, Year | 45.99 ± 13.20 | 45.73 ± 11.29 | 45.86 ± 12.26 |
| Male | 73 (56.2) | 79 (61.2) | 152 (58.7) |
| Risk factors | |||
| Age ≥ 50 years | 55 (52.4) | 50 (47.6) | 105 (40.5) |
| BMI ≥ 25 | 67 (51.5) | 63 (48.5) | 130 (50.2) |
| Diabetes Mellitus | 80 (61.5) | 82 (63.5) | 162 (62.5) |
| Hypertension | 50 (38.5) | 39 (30.2) | 89 (34.4) |
| Tobacco or Nicotine User | 19 (14.6) | 24 (18.6) | 43 (16.6) |
| Serious heart condition | 5 (3.8) | 7 (5.4) | 12 (4.6) |
| COPD | 12 (9.2) | 11 (8.5) | 23 (8.9) |
| Number of risk factors | |||
| 1 | 28 (21.5) | 37 (28.7) | 65 (25.1) |
| 2 | 58 (44.6) | 49 (38) | 107 (41.3) |
| 3 | 37 (28.5) | 39 (30.2) | 76 (29.3) |
| 4 | 7 (5.4) | 4 (3.1) | 11 (4.2) |
| Days from illness onset to admission | 3 (2–3) | 3 (2–3) | 3 (2–3) |
BMI, Body Mass Index; COPD, Chronic obstructive pulmonary disease.
Data are reported as X2 test (n, %), T test (mean, SD) or Median (Q1–Q3).
The median (Q1–Q3) time from symptom onset until enrollment was 3 (2–3) days.
The risk factors for progression to severe COVID-19 in order of prevalence included diabetes mellitus (with the rate of 62.5%), BMI ≥25 (52%), age ≥50 years (40.5%), hypertension (34.4%), smoking 43 (16.6), chronic lung disease (8.9%), and serious heart conditions (4.6%).
Overall, 85% (n = 110) of the patients were taking 62.5 mg bosentan, twice daily for 30 days, 11.5% (n = 15) were taking 50–60 capsules of bosentan for 30 days, and 3% (n = 4) were taking 62.5 mg of bosentan twice per day for 15 days.
Outcomes
The primary outcome was a combination of hospitalization or death from any cause up to day 15 of the trial. The results showed that the primary event occurred in 6.9% (18) of the total population, including three (2.3%) of the 129 patients in the bosentan group and 15 (11.5%) of the 130 patients in the placebo group [risk difference: −9.2% (95% CI: −15.3 to −3.1), P = 0.006] (Table 2).
Table 2.
Primary and secondary efficacy outcomes.
| Variable | Placebo, n/N (%) | Bosentan, n/N (%) | Risk difference, Bosentan vs. placebo (95% CI) | Relative risk, Bosentan vs. placebo (95% CI) | P value |
|---|---|---|---|---|---|
| Hospitalization or death from any cause within 15 daysa | 15/130 (11.5) | 3/129 (2.3) | −9.2% (−15.3 to −3.1) | 0.2 (0.07–0.68) | 0.006 |
| Death from any cause within 30 daysa | 3/130 (2.3) | 0/129 (0) | −2.3% (−4.7 to 0.3) | – | 0.24 |
| Thromboembolic events within 30 daysb | 9/97 (9.2) | 1/104 (0.9) | −8.3% (−14.4 to −2.2) | 0.10 (0.01–0.80) | 0.008 |
Intention to treat analysis.
Per protocol analysis.
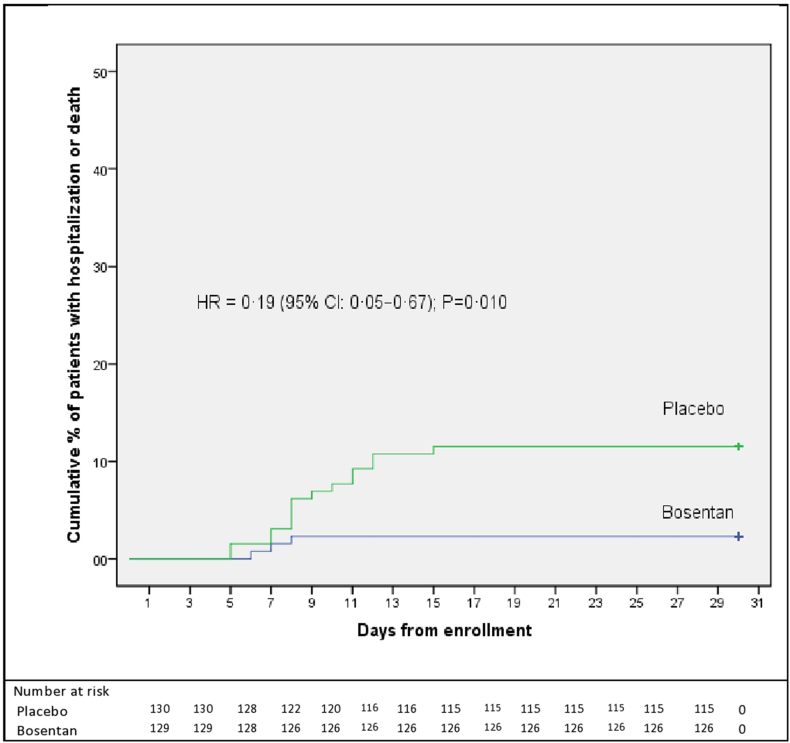
The results of the time-to-event analysis were also consistent with the primary results. The Cox regression showed 81% reduction in the risk of hospitalization or death from any cause in the bosentan group compared to the placebo group (HR = 0.19 (95% CI 0.05–0.67), P = 0.010).
Fig. 2 shows the Kaplan–Meier probability of hospitalization or death from any cause up to day 15.
Fig. 2.
Kaplan–Meier plot of time to hospitalization or death from any cause through day 30.
Due to the small sample size, subgroup analysis based on the risk factors was not conducted.
Secondary outcomes
A total of three deaths occurred and all three were in the control group. The difference in mortality risk for the bosentan group versus the placebo was −2.3% [95% CI (−4.7 to 0.3), P = 0.24] (Table 2).
The frequency of hospitalization within 30 days of randomization was 3 in the bosentan group and 15 in the placebo group. The median hospital-free days was 30 days for the entire population and significantly higher in the bosentan group (P = 0.004).
Symptomatic and asymptomatic thromboembolic events within 30 days after randomization were seen in ten (5%) patients out of 201 patients. The reason for the decrease in the sample size was that some patients did not consent to undergo doppler ultrasound. The number of missing people in each group was 24. There were no significant differences between the two missing groups in terms of gender, number of risk factors, death or hospitalization, and adverse events.
In the control group (n = 104), pulmonary embolism occurred in one patient on the eighth day and asymptomatic DVT was found in eight patients on day 30 of the study (cumulative incidence of 9.2%). In the bosentan group (n = 97), asymptomatic DVT was found in one patient (0.9%) on day 30. The difference in risk of thromboembolic events for the bosentan group versus the placebo was −8.3% [95% CI (−14.4 to −2.2), P = 0.008] (Table 2).
In addition, thromboembolic events occurred in seven of the hospitalized patients compared to three of the non-hospitalized ones (P < 0.001).
Adverse events were reported by 45 (34.9%) of 129 participants in the bosentan group and 43 (33.1%) of 130 in the placebo group (Table 3).
Table 3.
Adverse events during 30 days of intervention.a
| Event | Placebo N (%) | Bosentan N (%) |
|---|---|---|
| Headache | 7 (5) | 16 (12) |
| Dyspnea | 19 (15) | 13 (10) |
| Dizziness | 20 (15) | 11 (9) |
| Abnormal liver enzymes | 1 (0.7) | 7 (5) |
| Pneumonia | 15 (11) | 12 (9) |
| Diarrhea | 8 (6) | 9 (7) |
| Nausea/vomiting | 13 (10) | 15 (12) |
| Jaundice | 0 | 0 |
| Abdominal pain | 8 (6) | 9 (7) |
| Pulmonary Thromboembolism | 1 (0.7) | 0 |
| Cardiovascular event | 1 (0.7) | 0 |
| ARDS | 2 (1.5) | 0 |
| Asymptomatic deep vein thrombosisb | 8 (8) | 1 (1) |
ARDS; Acute Respiratory Distress Syndrome.
Some participants had multiple events.
It was 97 patients in the placebo group and 104 patients in the bosentan group.
An adverse event deemed to be associated with the intervention that could lead to the discontinuation of the intervention was rising serum aminotransferase levels ≥3 times the normal, which was observed in two patients in the bosentan group and one patient in the placebo group.
All the deaths occurring in this study were related to COVID-19 and not the trial regimens.
Discussion
Our results showed that high-risk outpatients with COVID-19 infection who received bosentan had 80% lower risk of COVID-19-related hospitalization or death from any cause in the first 15 days of the intervention and 88% lower risk of symptomatic and asymptomatic thromboembolic events within 30 days of randomization compared to the patients who received the placebo.
In addition, no deaths occurred in the bosentan group, and bosentan was associated with a nonsignificant reduction in mortality compared with placebo. Hospital-free days were significantly higher in the bosentan group compared to the placebo group and the incidence of adverse events was similar between the groups. Bosentan did not cause any severe adverse events and was well tolerated, and its consumption was stopped in only two patients because of a sharp increase in serum levels of aminotransferase.
To the researchers’ knowledge, this study is the first clinical trial conducted on the effects of early bosentan administration on disease outcomes in high-risk COVID-19 outpatients with the hypothesis of protecting the vascular endothelium.
In clinical trials published on antiviral treatments in similar populations, the incidence of hospitalization or death was reported as 0.7% in the Remdesivir group vs. 5.3% in the control group,11 7.3% in the Molnupiravir group vs. 14.1% in the control group,12 0.77% in the Nirmatrelvir group vs. 7% in the control group,13 and 4% in the Tixagevimab-Cilgavimab group vs. 9% in the control group.14 In comparison, the incidence of hospitalization or death in our trial was 2.3% in the bosentan group and 11.5% in the placebo group. The difference of 9.2% percentage points indicates the acceptable efficacy of bosentan compared to antiviral therapies.
In our study, the cumulative incidence of thromboembolic events was 8.7% in the placebo group (one symptomatic and eight asymptomatic events). In the bosentan group, only one asymptomatic DVT was found. The first experiences with COVID-19 as investigated in retrospective and then prospective studies revealed the incidence of thromboembolic events was 15%–85% in ICU or non-ICU wards.15 Later, it was shown that the risk of hospital-related venous thromboembolic events persists in COVID-19 patients from the time of admission until 90 days post-discharge.16
In a prospective and single-center study in non-ICU hospitalized patients, the incidence of asymptomatic DVT was reported as approximately 15% despite routine thromboprophylaxis.17 In another study, the prevalence of asymptomatic DVT was 19% in the first days of hospitalization in randomly selected COVID-19 patients in general wards despite the routine administration of thromboprophylaxis.18 In a brief report drafted on the findings of an observational study, 163 patients with COVID-19 hospitalized in general wards or ICUs were followed up for 30 days. The cumulative incidence of symptomatic post-discharge thrombosis (including arterial and venous events) at day 30 following discharge was 2.5% in that study.19
The association between thrombosis and coronavirus infection can be explained by several pathophysiological changes in Covid-19, such as the direct effect of the virus on endothelial cells, exaggerated inflammatory response, downregulation of angiotensin-converting enzyme 2 receptors, and activation of the coagulation system.20,21 As an endothelin receptor antagonist, bosentan blocks endothelin receptors in the endothelium and vascular smooth muscle, and thus may protect the endothelium from damage caused by the coronavirus. Of course, the earlier bosentan is administered, the more endothelial protection is guaranteed.
Importantly, thromboembolic events occur months after covid infection, and long-term anticoagulant therapy does not appear to protect against deep vein thrombosis.22 Findings from a recent cohort study showed that covid-19 is an independent risk factor for deep vein thrombosis, pulmonary embolism, and bleeding, and the risk of these outcomes increases three, six, and two months after covid-19 respectively.22 It can be concluded that endothelium damage and long-term thromboembolic events may be prevented by early administration of endothelium protective drugs. The mentioned cohort study also found a higher risk of events in patients with comorbidities, patients with more severe covid-19, and during the first pandemic wave compared with the second and third waves. In our study, participants had at least one risk factor for severe COVID-19 illness including diabetes mellitus, hypertension, age ≥50 years, hypertension, smoking, chronic lung disease, serious heart conditions or obesity. These conditions are often associated with chronic vascular endothelial dysfunction due to increased production of endothelium-derived contractile factors such as ET1.
The frequency of hospitalization within 30 days after randomization in our study was 3 in the bosentan group and 15 in the placebo group. It seems logical that we would have continued the administration of bosentan in hospitalized patients for a longer period of time, but we did not have the permission of the ethics committee. This was one of the limitations of this study.
This study had other limitations. Budget constraints did not allow the measurement of other variables such as D-dimer level, right ventricular function and pulmonary pressure in this study. It was a not a multi-center trial. Sample size was reduced due to the decrease in the prevalence of the disease.
Because the number of deaths occurred over time was relatively small, the precision with which the true probability of the event could be estimated was relatively low. However, no death in the bosentan group and 3 deaths in the placebo group can be clinically important, and therefore, larger, adequately powered clinical trials are needed to demonstrate a definitive benefit of bosentan in reducing mortality.
However, our study had some strengths. The most important strength of the study was the early administration of bosentan to ensure maximum endothelial protection. For this reason, many patients had to be excluded. Moreover, despite the budget constraints in providing the placebo, blinding was carefully carried out through alternative methods to conceal the external appearance of the medication vs. the placebo capsules and to increase the accuracy of the adverse event reports.
In summary, the early administration of bosentan initiated within three days of the onset of symptoms was found to be effective in the prevention of severe COVID-19 in high-risk non-hospitalized patients. Moreover, this intervention may be effective in the prevention of thromboembolic events.
Contributors
Shaahin Shahbazi, Zahra Vahdat Shariatpanahi, and Erfan Shahbazi contributed to the conception and design, acquisition and interpretation of data, verified the underlying data, and writing and editing of the manuscript, and all reviewed and approved the manuscript for submission.
Data sharing statements
Data will not be made available in a public repository as we have not obtained ethical clearance to share data publicly. However, on request from the corresponding author, data could be provided while maintaining anonymity, as stated in the protocol.
Declaration of interests
The authors declare that there is no conflict of interest regarding the publication of this paper. The authors confirm that the manuscript is original research that has not been published and is not under consideration elsewhere. The authors confirm that all of the authors participated in the preparation of the manuscript. The authors confirm that we have permission to reprint any figures or tables that were initially printed elsewhere.
Acknowledgements
Our appreciation goes to the Ilam University of Medical Sciences, all the patients who volunteered for this trial, and all those who gave their valuable help and support towards this study.
Footnotes
IRCT registration number: IRCT20211203053263N1.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102117.
Appendix A. Supplementary data
References
- 1.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christopher F. 6th ed. Vol. 2. W.B. Saunders; 2016. pp. 1050–1065.e5. (Barnett, Teresa de Marco, in Murray and Nadel's textbook of respiratory medicine). [Google Scholar]
- 3.Jin Y., Ji W., Yang H., Chen S., Zhang W., Duan G. Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Signal Transduct Target Ther. 2020;5(1):293. doi: 10.1038/s41392-020-00454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ouldali N., Pouletty M., Mariani P., et al. Emergence of Kawasaki disease related to SARS-CoV-2 infection in an epicentre of the French COVID-19 epidemic: a time-series analysis. Lancet Child Adolesc Health. 2020;4:662–668. doi: 10.1016/S2352-4642(20)30175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iba T., Levy J.H., Levi M., Connors J.M., Thachil J. Coagulopathy of coronavirus disease 2019. Crit Care Med. 2020;48:1358–1364. doi: 10.1097/CCM.0000000000004616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bermejo-Martin J.F., Almansa R., Torres A., Gonzalez-Rivera M., Kelvin D.J. COVID-19 as a cardiovascular disease: the potential role of chronic endothelial dysfunction. Cardiovasc Res. 2020;116:e132–e133. doi: 10.1093/cvr/cvaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hang W., Chen C., Zhang X.A., Wang D.W. Endothelial dysfunction in COVID-19 calls for immediate attention: the emerging roles of the endothelium in inflammation caused by SARS-CoV-2. Front Med. 2021;15(4):638–643. doi: 10.1007/s11684-021-0831-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filep J.G., Fournier A., Foldes-Filep E. Acute pro-inflammatory actions of endothelin-1 in the guinea-pig lung: involvement of ETA and ETB receptors. Br J Pharmacol. 1995;115:227–236. doi: 10.1007/s11684-021-0831-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park S.H., Saleh D., Giaid A., Michel R.P. Increased endothelin-1 in bleomycin-induced pulmonary fibrosis and the effect of an endothelin receptor antagonist. Am J Respir Crit Care Med. 1997;156:600–608. doi: 10.1164/ajrccm.156.2.9607123. [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb R.L., Vaca C.E., Paredes R., et al. GS-US-540-9012 (PINETREE) investigators. Early Remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022;386(4):305–315. doi: 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayk Bernal A., Gomes da Silva M.M., MOVe-OUT Study Group, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammond J., Leister-Tebbe H., EPIC-HR Investigators, et al. Oral Nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386(15):1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montgomery H., Hobbs F.D.R., TACKLE Study Group, et al. Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2022;S2213-2600(22):180–181. doi: 10.1016/S2213-2600(22)00180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribes A., Vardon-Bounes F., Mémier V., et al. Thromboembolic events and Covid-19. Adv Biol Regul. 2020;77 doi: 10.1016/j.jbior.2020.100735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuin M., Engelen M.M., Barco S., et al. Incidence of venous thromboembolic events in COVID-19 patients after hospital discharge: a systematic review and meta-analysis. Thromb Res. 2022;209:94–98. doi: 10.1016/j.thromres.2021.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demelo-Rodríguez P., Cervilla-Muñoz E., Ordieres-Ortega L., et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res. 2020;192:23–26. doi: 10.1016/j.thromres.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Jeune S., Suhl J., Benainous R., et al. High prevalence of early asymptomatic venous thromboembolism in anticoagulated COVID-19 patients hospitalized in general wards. J Thromb Thrombolysis. 2021;51(3):637–641. doi: 10.1007/s11239-020-02246-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patell R., Bogue T., Koshy A., et al. Post discharge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136(11):1342–1346. doi: 10.1182/blood.2020007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magadum A., Kishore R. Cardiovascular manifestations of COVID-19 infection. Cells. 2020;9:E2508. doi: 10.3390/cells9112508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katsoularis I., Fonseca-RodrÃguez O., Farrington P., et al. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after covid-19: nationwide self-controlled cases series and matched cohort study. BMJ. 2022;377 doi: 10.1136/bmj-2021-069590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.