Summary
Antibody–drug conjugates (ADCs) represent a novel and evolving class of antineoplastic agents, constituted by monoclonal antibody linked to biologically active drugs, delivering cytotoxic compounds at the tumor site, reducing the likelihood of systemic exposure and toxicity. They are generally well tolerated, nevertheless some predictable adverse reactions need careful monitoring and timely approach. These include neutropenia, nausea and vomiting, alopecia, diarrhea, left ventricular dysfunction, ILD/pneumonitis. The mechanisms leading to drug-associated toxicities are summarized, and prophylaxis protocols and appropriate management strategies are proposed, based on current literature. This review aims to collect the most updated evidence on toxicities potentially occurring during breast cancer treatment with approved or under clinical investigation (advanced stage) ADCs. A focus is dedicated to monitoring protocols and clinical management, aimed at preventing and/or promptly address relevant problems, in order to avoid premature discontinuation or improper dose reduction.
Keywords: Antibody drug conjugates, Breast cancer, Toxicity management
Introduction
Conventional chemotherapy has been the milestone in cancer therapy for decades. While cytotoxic agents may differ greatly in their mechanism of action, their therapeutic activity results from a balance between cell damage and repair, which is usually more favorable in normal cells than in cancer. However, dose-limiting toxicities on some normal tissues, like the bone marrow or the mucosae, have represented a major obstacle to the use of otherwise lethal doses of several cytostatic drugs in vitro. Among the resulting implications in medical oncology, is the artificial concept of the disease-specificity of different classes of drugs. This disease-specificity rather reflects the contingency of using the optimally tolerated dose of different drugs, rather than the lethal dose for cancer cells.
Since Paul Enrlich theories on the “perfect bullet”, the concept of the cancer-cell specificity of chemotherapy has been pursued, leading to the development of the first antibody-drug conjugate (ADC) gemtuzumab ozogamicin, targeting CD33 in acute myeloid leukemia therapy. ADCs represent cutting-edge therapeutic compounds in continuous implementation for cancer treatment. The most innovative aspect is represented by their complex structure, consisting of a target-specific monoclonal antibody connected to cytotoxic molecules (payload) through a cleavable/non-cleavable linker (Supplementary Table S1). In recent years, the engineering of the linker has contributed to the diversity and improved efficacy of these drugs.
Recently, several ADCs have been tested in breast cancer (BC), mostly directed against either the human epidermal growth factor receptor-2 (HER2), a tyrosine kinase receptor overexpressed in BC, whose activation through autophosphorylation results in downstream signaling pathways initiation, or Trophoblast cell surface antigen 2 (Trop2), a cell surface glycoprotein expressed in 50–90% of BC cells.1
The first ADC approved by the United States Food and Drug Administration (FDA) for the treatment of HER2-positive metastatic BC (mBC) was trastuzumab emtansine (T-DM1) in 2013. After several clinical trials with exceptional results, this compound was introduced in 2019 in the treatment of patients with early-stage BC. In the same year, a second anti-HER2 ADC, trastuzumab deruxtecan (T-DXd), was approved for the treatment of HER2-positive mBC. Moreover, its use was extended to HER2-low advanced BC in 2022. To date, the only anti-Trop2 ADC available is sacituzumab govitecan (SG), approved by FDA for both triple negative BC (TNBC) (2021) and hormone receptor (HR) positive BC (2023) treatment. Furthermore, several ADCs are currently in advanced stages of investigation, such as trastuzumab duocarmazine (SYD985), datopotamab deruxtecan (Dato-DXd), patritumab deruxtecan (HER3-DXd).
Even though ADCs were designed with high selectivity for their target, with the ultimate goal of limiting their toxicities, treatment-related adverse events (TRAEs) have been reported, nonetheless. On this matter, a recent meta-analysis of 169 trials, including different tumor types, described how the overall incidence of adverse events (AEs) reaches 91.2% during treatment with ADCs, while the rates of grade ≥3 events and AEs leading to treatment discontinuation were 46.1% and 13.2%, respectively. In addition, 1.3% of adverse reactions led to patient death.2
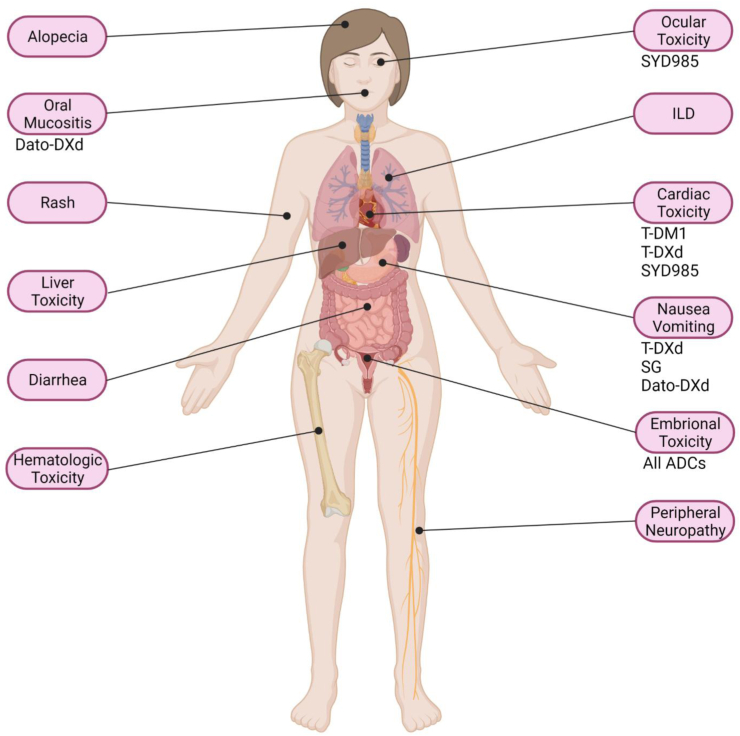
The mechanisms underlying ADC toxicity are related to the release of the payload, the stability of the linker and the internalization of the ADC. When the payload is released in the absence of the target but still in the tumor microenvironment, as it often is the case with SG it will still have on-tumor effect. However, its release in normal tissues (off-tumor) explains some of the toxicities of these compounds. Oftentimes, this is related to normal tissues expressing the target of the ADC (on-target), but not always (off-target). The present manuscript offers an overview of the toxicities that may occur during BC treatment with ADCs (Fig. 1) and represents a quick supportive care guide, to help in their assessment and management.
Fig. 1.
Main AEs described with ADCs for BC treatment. Drugs needing prophylaxis use or medical/laboratory screening are indicated. Created with BioRender.com.
Hematological adverse events
Hematological AEs have been frequently reported in patients treated with ADCs, and include pancytopenia, neutropenia, anemia, and thrombocytopenia (Supplementary Table S2). These AEs are graded according to the Common Terminology Criteria for Adverse Events (CTCAE) (Supplementary Table S3, that is reference for all the toxicities described in this manuscript.3 Microtubule inhibitors, such as DM1, the maytansine derivative part of T-DM1, induce apoptosis in cells undergoing mitosis, by causing cell cycle arrest at G2/M phase. Conversely, T-DXd, SG, Dato-DXd, and HER3-DXd have topoisomerase inhibiting payloads, leading to DNA double-strand breaks, and apoptosis of rapidly proliferating cells, including hematopoietic cell progenitors.
Neutropenia
If not properly treated, severe hematological adverse reactions may lead to complications such as bleeding, febrile neutropenia, and potential subsequent infection up to sepsis. Therefore, early assessment and management, including dose reduction (Supplementary Table S4) are of utmost importance.
In patients receiving T-DM1, the incidence of all grade neutropenia ranged from 5% to 11% across trials, while severe neutropenia (grade ≥ 3, including febrile neutropenia) was reported in up to 6% of patients, a lower rate compared to other cytotoxic agents.4, 5, 6 Indeed, neutropenia was a common AE in patients treated with T-DXd, occurring in about 70% of patients,7, 8, 9 being the main reason for dose reduction and drug interruption. Interestingly, febrile neutropenia was a rare event (only 1.6% in DESTINY-Breast01 and 0.3% in DESTINY- Breast04 trials).7,8 Conversely, severe or life-threatening neutropenia cases were frequently reported with SG: indeed, among the 64% of patients reporting this AE, 13% suffered from a grade 4 event. Febrile neutropenia occurred in 6% of cases, reaching 8% in pretreated metastatic TNBC patients. Nevertheless, less than 1% of patients with febrile neutropenia discontinued the treatment permanently.10,11 In the phase 1/2 ongoing study U31402-A-J101 (NCT02980341; JapicCTI-163401), hematological toxicities are the most reported AEs with HER3-DXd, with grade ≥3 neutropenia occurring in 39.6% of patients,12 while the reported rate after a single dose in the SOLTI TOT-HER3 trial is 19%.13 Conversely, in the TROPION-PanTumor01 study, no cases of neutropenia grade ≥3 were described with Dato-DXd, and the frequency of this toxicity was low.14
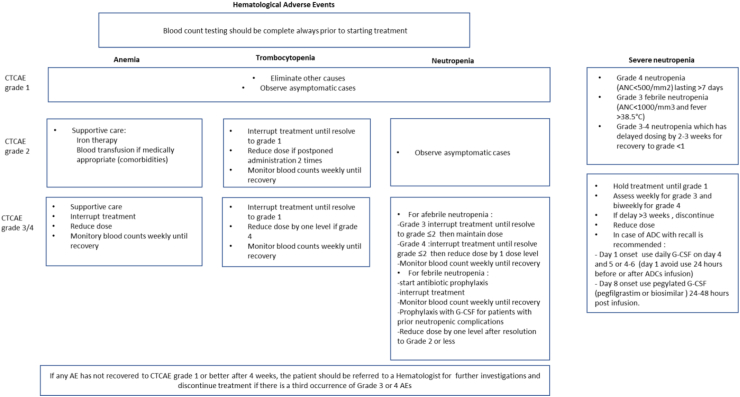
In case of neutropenia (Fig. 2), granulocyte colony-stimulating factor (G-CSF) prophylaxis may be given to patients at high risk for febrile neutropenia or at moderate risk but with risk factors. The use of a short-acting growth factor (eg, filgrastim 5 μg/kg once a day subcutaneously) for 2–3 days can be effective in increasing the absolute neutrophil count (ANC) count. In addition, a long-acting growth factor (e.g., pegfilgrastim 6 mg subcutaneously) may be administered on day 2 to avoid delays of the following treatment cycles. In patients who have experienced unpleasant AEs (e.g., headaches, muscle/joint aches) with growth factor use, the ADCs dose reduction should be considered.15 Prophylactic antibiotic therapy may be considered when the absolute neutrophil count is reported <100 cells/mm3 and lasts for more than 1 week. If the body temperature rises above 38 °C, empirical antibiotic treatment should be given promptly and indicated tests should be performed. G-CSF may be administered in patients with prior neutropenic complications, but it should not be routinely used in patients with afebrile neutropenia. Patients suffering from lower-grade neutropenia events (grade ≤ 2) do not require any dose adjustments.16 In case of grade 3 neutropenia in patients treated with SG, the management changes based on the day the AE was registered. Indeed, SG treatment is administered on day 1 and day 8 of a 21-day schedule. Therefore, if neutropenia is recorded on day 1, daily G-CSF (filgrastim or biosimilars) should be considered, starting on day 4 through day 6 of the treatment cycle. It’s important to avoid its administration during the 24 h before or after SG infusion. Whereas, if the neutropenia is recorded on day 8, long-acting pegylated G-CSF (pegfilgrastim or biosimilars) may be considered 24–48 h post-infusion.
Fig. 2.
Hematological AEs monitoring and management. Hematological toxicities may have life-threatening consequences, therefore complete blood count (CBC) assessment is always recommended before ADC administration. Blood counts should be also monitored periodically during the treatment, in addition to evaluating the possible interactions with other medication. As described in the present figure, in case of adverse events, dose adjustment or discontinuation may be needed.
Anemia
The incidence of severe anemia in patients treated with T-DM1 was as low as 2.7%,4,5 even though the population enrolled was pretreated with other chemotherapies. This rate was similar in patients treated with T-DM1 (1.1%) as first line for mBC.17 Conversely, patients treated with ADCs such as T-DXd, SG, Dato-DXd, and HER3-DXd reported higher incidence of grade 3–4 anemia (10% in DESTINY-Breast04; 8% in ASCENT, 4% in TROPION-PanTumor01, and 18.7% in U31402-A-J101 trials, respectively).7,11,12,14 As shown in Fig. 2, for grade 3 and 4 anemia, treatment should be withheld until improvement to grade <2 and resumed at a lower dose level. Packed red blood cell transfusion should be administered per institutional protocol, but it is necessary in case of grade 4 anemia or symptomatic grade 3 (tachycardia, shortness of breath, chest pain, exertional dyspnea, mild headache, syncope, and severe fatigue affecting work and daily activities). In case of grade 2 anemia, blood iron level testing is crucial to evaluate a functional iron deficiency (serum ferritin ≤800 ng/mL or transferrin saturation <20%), where iron supplementation should be considered. Intravenously administered iron supplements are preferred for their better efficacy, oral supplementation is more common, although less effective.18
Unfortunately, the use of erythropoiesis-stimulating agents has not been investigated for ADCs and their use is not mentioned in the different trials.
Thrombocytopenia
Contrariwise, thrombocytopenia was a common hematological AE in patients receiving T-DM1, occurring in 28% of patients.4,5 Indeed, it has been demonstrated that T-DM1 does not have direct effects on platelet function, but it does impair megakaryocytes’ production in the bone marrow.19 Interestingly, this effect is similar for both T-DM1 and the unconjugated DM1, therefore supporting the hypothesis of target-independent toxicity.19 Severe thrombocytopenia (grade ≥ 3) was reported in 12% of treated patients,4 and in most patients, it was observed during the first two cycles of treatment.4 Epistaxis is a frequent consequence of thrombocytopenia, reported in up to 36% of patients treated with T-DM1, while severe hemorrhagic events were rare.17 Notably, a safety analysis including six studies has shown differences in AE rates between Asian and non-Asian populations, highlighting a higher incidence of grade ≥3 thrombocytopenia in Asian patients.20 Indeed, the KAMILLA study confirmed that the Asian cohort had higher rates of grade ≥3 thrombocytopenia than the global population (51.4% vs 23.1%). However, most events were managed by interruption and dose modifications.21 The higher incidence of thrombocytopenia in Asian patients is potentially due to polymorphisms of the Fc receptors, which are involved in the T-DM1 internalization,20 although this is still matter of debate, and further studies are warranted. In addition, thrombocytopenia occurred with an incidence of 60.4% at 4.8 mg/kg and 71.4% at 6.4 mg/kg with HER3-DXd, although no bleeding events were registered.12 This toxicity has been related mainly to the unconjugated payload.22 Instead, with T-DXd the incidence of thrombocytopenia grade 3–4 events registered was 7%,8,9 and even lower with SG (3% in ASCENT trial),11 while it was not reported in the trial investigating Dato-DXd.14 In case of patients with grade 2 or grade 3 thrombocytopenia, ADC withholding is recommended until recovery to grade ≤1 (Fig. 2). After recovery, the treatment may be resumed at the same dose. Dose reduction is considered if the treatment is delayed twice due to grade 2–3 thrombocytopenia in naïve patients. In case of grade 4 thrombocytopenia, a dose reduction is required when the drug is restarted. If grade 3 or 4 thrombocytopenia occurs in patients with advanced disease and does not resolve to grade ≤1 within 42 days, treatment discontinuation should be considered. In addition, patients on anti-coagulant treatment should be closely monitored during treatment with ADCs, because hemorrhagic events could occur.23 In addition, platelet transfusions are indicated if the patient is bleeding, or to prevent major bleeding if platelet counts are less than 10 × 109/L (<20 × 109/L if febrile).24 In the event that patients are poorly responsive to conventional platelet-elevating therapy, hematologists should be consulted and corresponding examinations and management are recommended. Despite the lack of FDA approval for platelet receptor agonists (such as eltrombopag), these agents might be considered in patients who cannot be supported by platelet transfusions. However, the National Comprehensive Cancer Network (NCCN) endorsed the use of platelet receptor agonist, romiplostim, for the treatment of thrombocytopenia when platelet counts 30–50 × 109/L with discontinuation when the platelet count recovers to 50–100 × 109/L.25
Cardiotoxicity
Cardiotoxicity is a known potential AE of HER2-targeting agents. HER2 receptors, expressed on cardiomyocytes, regulate cell growth, homeostasis, and oxidative stress. Also, they play a critical role in the development of the fetal heart. The mechanisms underlying cardiotoxicity of HER2 targeting agents have been identified in many pre-clinical studies.26 Studies conducted on mice with a ventricular-restricted deletion of ERBB2 gene revealed the onset of multiple alterations of cardiac parameters including chamber dilatation, wall thinning and decreased contractility up to dilated cardiomyopathy and systolic dysfunction.26 ADC-related cardiotoxicity is an example of on-target off-tumor effect of drugs using the HER2 antibody. Therefore, based on the knowledge acquired from previous studies, HER2-targeting anticancer drugs may cause both irreversible and reversible cardiac damage. Potential toxicities include QT prolongation, arrhythmias, myocardial ischemia, hypertension, left ventricular dysfunction (LVD) and heart failure (HF). It is crucial to perform a baseline assessment of the cardiovascular toxicity risk factors, including evaluation of previous cardiotoxic therapies, lifestyle risk factors, cardiovascular history, physical examination and complementary tests, such as B-type natriuretic peptide (BNP) or its N-terminal prohormone (NT-proBNP), cardiac troponin, electrocardiogram, transthoracic echocardiography (TTE). TTE with Global Longitudinal Strain (GLS) is the recommended modality to assess the cardiac function in patients with cancer.27 Patients may present with symptomatic or asymptomatic cancer therapy-related cardiac disfunction (CTRCD) (Supplementary Table S5). European Society of Cardiology (ESC) Guidelines on cardio-oncology recommend anti-HER2 treatment interruption for symptomatic-moderate/severe and asymptomatic-severe CTRCD. In patients with asymptomatic-mild and -moderate CTRCD, treatment should be continued with the addition of cardioprotective therapy (ACE-I/ARB and beta-blocker).27
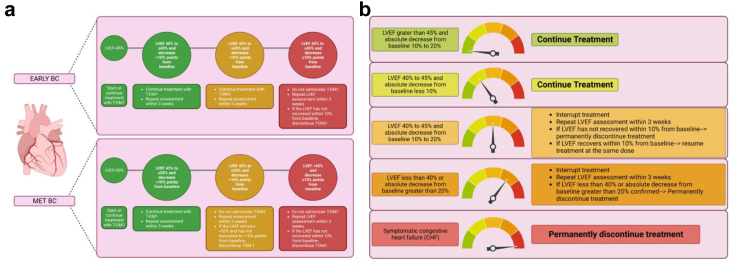
T-DM1 is associated with low risk of developing LVD. Indeed, in the EMILIA trial, 1.7% of patients in the T-DM1 group and 1.6% in the control group had a left ventricular ejection fraction (LVEF) < 50% and at least 15% below the baseline value.5 Similarly, in the TH3RESA trial, although heavily pretreated, a reduction in LVEF < 50% was reported only in 1.5% of patients in T-DM1, and 1.1% in control arm.4 In the MARIANNE trial 0.8% of patients treated with TDM-1 developed LVEF < 50% with a ≥15% decrease from baseline, compared with 4.5% of patients with trastuzumab plus taxane and 2.5% with TDM-1 plus pertuzumab.6 In addition, the SAFE-HEaRt study demonstrated that trastuzumab, pertuzumab and T-DM1 can safely be administered in patients with LVEF between 40 and 49% along with cardiac monitoring and treatment with cardioprotective agents.28 Monitoring and management of T-DM1 related cardiovascular toxicity is shown in Fig. 3a.
Fig. 3.
Monitoring and management of T-DM1 (a) and T-DXd (b) related cardiovascular toxicity. In metastatic HER2-positive disease, TTE is recommended every 3 months during the first year and, in absence of cardiovascular toxicity, every 6 months. In early-stage BC, it is recommended to perform TTE every 3 months during anti-HER2 treatment and within 12 months after completion. Created with BioRender.com.
Regarding T-DXd, in DESTINY-Breast01 trial decrease in the LVEF occurred in 3 patients (1 grade 3 event), all recovered after interruption of treatment.8 No associated events of HF were reported, and no patients discontinued treatment because of a decrease in the LVEF. Any-grade QT interval prolongation was described in 9 patients (4.9%), 2 of them grade 3 (1.1%).8 Similarly, in DESTINY-Breast03 study, 2.3% of patients in T-DXd arm reported grade 2 asymptomatic reduction in LVEF, mostly resolved without any intervention.9 Conversely, in the DESTINY-Breast04 trial, 11.9% of patients in T-DXd arm had a decrease of LVEF between 10% and 19% from baseline and 1.5% of >20%.7 Notably, this study population had not previously experienced anti-HER2 treatments. Monitoring and management of T-DXd related cardiotoxicity is shown in Fig. 3b.
In the phase I trial investigating SYD985, 5% of patients in the dose-escalation and 8% in dose-expansion cohort suffered a reversible decrease in LVEF.29 However, in the primary results of phase III TULIP trial, no cardiovascular events were reported in the SYD985 arm.30
Regarding SG, no patients experienced severe cardiac TRAEs in IMMU-132-01 and ASCENT trials.31,32
To date, no cardiovascular events have been reported for Dato-DXd33 and no data on cardiotoxicity of HER3-DXd are published.
Gastrointestinal (GI) toxicity
GI toxicity is frequent in patients treated with ADCs, mostly related to the effects of the cytotoxic payload on the mucosal cells. Depending on the location of the gastrointestinal tract damage, a different AE can be recorded.
Nausea and vomiting (NV)
NV significantly impairs patient’s quality of life and compliance to treatment. Also, they may lead to systemic complications, like metabolic imbalances and nutrient depletion. Emesis related to cancer therapy has been classically divided into acute or delayed-onset (developed respectively before or after 24 h from the infusion), and also anticipatory and breakthrough or refractory.34 While the psychological component is a recognized underlying cause of anticipatory NV, both acute and delayed-onset NV have complex biological pathophysiology. ADCs’ payloads, active chemotherapeutic agents, can kill intestinal enterochromaffin cells leading to the release of serotonin (5-hydroxytryptamine 3, 5-HT3), which therefore binds its receptor and activate vagal fibers causing acute emesis. Conversely, delayed-onset NV depends on the release of substance P, which binds the neurokinin-1 (NK-1) receptor localized both peripherally and in central nervous system. Therefore, acute emesis depends on a peripheral pathway, while delayed emesis on a central pathway, more specifically on the vomiting center (chemoreceptor trigger zone).
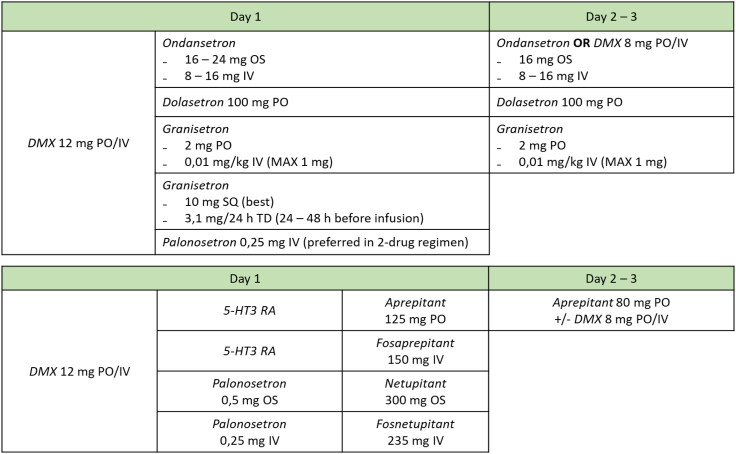
Among the ADCs considered in this review, T-DXd has the most emetogenic potential: the patients enrolled in the main clinical trials investigating this drug suffered from nausea in approximately 78% of cases, vomiting was reported by 48% of patients, while grade ≥3 nausea and vomiting were 6.6–7.6% and 1.6–4.3%, respectively.7,8 Thus, T-DXd can be considered an agent with moderate-high emetogenic potential, according to the NCCN guidelines classification.34 NV are, together with hematologic side effects, the most common grade 3 AEs associated with T-DXd treatment, and are the most common cause of dose reductions. HER3-DXd, Dato-DXd, and SG are moderate emetogenic agents, accounting for nausea rates of 55–67%, and vomiting incidence of 20–40%; whereas grade ≥3 NV occurs in <3% of patients.11,13,14,35, 36, 37 Conversely, T-DM1 and SYD985 are low emetogenic drugs (<30% for nausea and 20% for vomiting, mostly grade 1–2).30,34 For T-DXd and SG, the median onset of nausea is about one week after infusion.32 This timing is important especially for SG, which is administered on days 1 and 8 of each cycle. Thus, an optimal management of acute-onset for SG and delayed-onset for T-DXd is required. Fig. 4 shows NV prophylactic regimens according to an Italian expert consensus and NCCN guidelines.34,38
Fig. 4.
Nausea and vomiting prophylaxis regimens. For moderate-emetogenic agents, a 2-drugs prophylactic regimen can be administered on day 1 of each cycle. It should include a 5-HT3 receptor antagonist (RA) (e.g., ondansetron, palonosetron, granisetron) and dexamethasone (DMX). For high-risk patients (e.g., young women with little use of alcohol or who previously experienced NV), or in case of refractory emesis, a NK1 RA can be added (e.g., aprepitant, fosaprepitant, netupitant) in a 3-drug regimen. For delayed-onset emesis, single agent 5-HT3 RA or DMX can be offered on day 2 and 3 after the standard prophylactic regimen administered on day 1; alternatively, aprepitant (with or without DMX) can be employed if the NK-1 RA had been administered on day 1. Importantly, given their long duration of action, palonosetron and NK1 RAs other then aprepitant must not be continued at day 2–3 as well as granisetron subcutaneous or transdermal. For low emetogenic agents NCCN guidelines recommend single agent therapy with DMX 8–12 mg or single dose metoclopramide 10–20 mg or prochlorperzane 10 mg or single dose 5-HT3 RA.
Breakthrough NV occurs despite prophylaxis and needs a rescue therapy with metoclopramide 10 mg (3/die), in addition or not to dexamethasone (DMX). We refer to refractory NV when prophylactic and rescue therapies are not effective. In this case, olanzapine 5–10 mg is recommended.34,38 Anticipatory NV occurs before administration of therapy in patients who experienced these symptoms in earlier cycles. Anxiolytic drugs (such as lorazepam), relaxing measures (yoga or acupuncture) and optimal management of acute and delayed emesis can implement the anticipatory NV.34,38
Diarrhea
Incidence of approximately 12–30% of any grade diarrhea and about 1–3% of grade ≥3 events is reported for the above-described ADCs in clinical trials. However, SG causes diarrhea in about 60% of patients and about 10% of them experience grade 3 events.11,36 Median time to onset is 12–14 days (19 days for grade 3 events), and the median duration of each episode is 5–7 days.32 Indeed, diarrhea is a toxicity of topoisomerase I inhibitors class, including SN-38 and DXd.39 The main mechanism behind this toxicity is thought to be related to the early dissociation of the drug from its antibody, which leads to the diffusion of the active compound in normal tissues (off-target off-tumor toxicity).
Interestingly, the differences in the incidence of diarrhea in patients treated with SG depend on the metabolism of SN-38. This is the active metabolite of irinotecan, which is further metabolized, and thus deactivated, through glucuronidation by the enzyme uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) in the liver cells. However, the gut microbiota is able to reactivate it by the enzyme β-glucuronidase, thus concentrating the active metabolite in the gut mucosa causing cell damage.39 DXd is also metabolized in the liver end excreted in bile, however it is not affected either by glucuronidation or de-glucuronidation. Thus, the unique metabolism of SN-38 is the cause of the increased rates of diarrhea.40 To note, safety analysis from ASCENT and IMMU-132-01 trials revealed slightly increased incidence and severity of diarrhea, in terms of grade ≥3 events, in patients with UGT1A1∗28 homozygosis.11,32 Patients treated with Dato-DXd experience diarrhea infrequently: a possible explanation could involve the stable linker of Dato-DXd,41 which reduce the systemic exposure to DXd and thus AE like diarrhea or neutropenia. Another key factor is the metabolism of the payload: DXd is not exposed to glucuronidation cycle described before, that is the base of SN-38 induced diarrhea. Appropriate studies are necessary to prove this hypothesis.
Diarrhea can be divided into early and late onset: early-onset diarrhea is secondary to hyperactivation of cholinergic response elicited by the damage of enteric nervous system. Characteristic accompanying symptoms are abdominal cramps, sweating and salivation. Therefore, it can be managed with atropine administration (0.4 mg IV every 15 min for two doses, eventually followed by further 0.2 mg dose for a maximum of total 1 mg). Atropine-based prophylaxis can be considered for subsequent administration of SG.11 Late-onset diarrhea is due to gut mucositis associated to inflammation, secretions, and microbiota impairment. Standard therapy, according to both guidelines and clinical trial protocols,11,42 is based on loperamide (initial dose of 4 mg p.o., followed by 2 mg every additional episode until 16 mg of maximum dose); if resolved, loperamide should be discontinued 12 h after last diarrhea episode. Otherwise, if not resolved in 48 h from loperamide assumption, recommended drugs are octreotide (100–150 μg subcutaneously 3 times/day) or tincture of opium.42 In case of diarrhea associated with fever for more than 24 h or with absolute neutrophil count <500, antibiotic therapy (e.g., ciprofloxacin or metronidazole) is recommended, as in case of neutropenic colitis, a rare serious AE (about 1%).11,42 In addition, exclusion of other potential causes of diarrhea (infective, inflammatory, metabolic) should be carried out.42 In addition to the pharmacological therapy, a soft diet with adequate fluid intake, avoiding lactose-containing food and alcohol, should be recommended to patients suffering from diarrhea.42
Oral mucositis (OM)
Stomatitis (or OM) is, together with nausea, the most frequent AE of Dato-DXd. Preliminary results from TROPION-PanTumor01 and BEGONIA trial reveal rates of 50–70% for any grade stomatitis and 10–15% of grade ≥3 events.14,33,37 Frequency of OM for the other ADCs is about 10–20%, almost all grade 1 or 2.5,7,8,11,17,29,43,44 The mechanism underlying the high frequency of this AE associated with Dato-DXd has not been evaluated in any clinical or preclinical trial. The target protein, Trop2 may have a crucial role. According to The Human Protein Atlas, Trop2 is highly expressed in skin and proximal digestive tract cells, such as oral mucosa and esophagus. The damage of these cells induced by the payload could elicit nausea, stomatitis and alopecia. Therefore, OM is an example of on-target off-tumor toxicity. Risk factors for stomatitis include smoking, poor oral hygiene, younger age, female sex, and genetic factors.45 International guidelines recommend some preventive measures which are shared by clinical trial’s protocols involving Dato-DXd.46 The main strategy consists in improving oral hygiene: brush teeth twice a day, floss daily, rinse mouth with bland solutions (normal saline or sodium bicarbonate) at least 4 times a day.45 Steroid-containing mouthwashes (e.g., oral dexamethasone solutions), oral topical antifungal agents and pain management (e.g., 2% viscous lidocaine solution or 2% morphine mouthwash) can be added according to clinician preference, symptoms and clinical signs. In case of pain and/or dryness of mouth, holding ice chips in the mouth is an efficient solution, known as cryotherapy or ice chip therapy.47 It is also important to give patients some dietary attention: limit incidental trauma of rough, sharp or acid foods, avoiding tobacco and alcohol, maintain adequate hydration. For high-risk patients, an early dental assessment for aggressive prophylactic care (treatment of caries and extraction of compromised teeth) should be considered.45,48 Uncomplicated stomatitis is generally self-limiting, adequate symptom management and non-invasive interventions should be sufficient. Typical complications of OM are bleeding, malnutrition, uncontrolled pain and superinfections, especially in neutropenic patients. Hospitalization and nutritional status evaluation are indicated both in case of these conditions and in case of severe mucositis (grade ≥ 3).45,48
Liver toxicity
Another remarkable GI side effect is the elevation of liver enzymes, especially aspartate or alanine aminotransferase (AST or ALT) and bilirubin. The highest any grade transaminases elevation rate is observed in about 30% of patients treated with T-DM1, with about 10% of grade ≥3 events. Furthermore, together with thrombocytopenia, AST/ALT increases are the most common grade 3 events associated to T-DM1 therapy and the most common cause of dose delaying or reduction.5
As discussed above, one of the mechanisms underlying ADCs toxicity is the internalization of the drug even in absence of the target receptors (off-target off-tumor toxicity). Mannose receptors and Fcɣ receptors are frequently involved in this phenomenon. In addition to the HER2-independent uptake by the megakaryocytes described above, it has also been demonstrated an HER2-independent uptake of T-DM1 by the Kupffer cells, located in the liver, via Fcɣ receptor, which can result in cell growth inhibition. This mechanism, together with an HER2-dependent internalization of the drug, can lead to hepatotoxicity.49 Aminotransferases levels elevation usually occur on day 8 and tends to recover on day 21 without medical treatment.5 There are no guidelines about the management of liver enzymes alterations. However, FDA label of T-DM1 recommends for grade 3 AST/ALT elevation to reduce the drug dose after the recovery to a toxicity grade ≤2. A grade 3 increasing of bilirubin also requires a dose reduction of T-DM1 after the recovery to grade ≤1. In presence of grade 4 elevation of liver enzymes it is mandatory to discontinue T-DM1, after excluding other causes of liver toxicity.
Hepatotoxicity has been included in the black box warning of T-DM1 by FDA.
A rare complication of T-DM1 treatment is Nodular Regenerative Hyperplasia (NRH) of the liver. It was reported in two patients in EMILIA trial (0.4%) and two patients in KATHERINE trial (0.3%). Typical presentation of this condition is a modest elevation of liver enzymes associated with signs of portal hypertension (ascites, splenomegaly, varices). A CT scan of the abdomen should be performed in this case and can rule out other conditions like cirrhosis or hepatocellular carcinoma. However, the diagnosis of NRH can be confirmed only by liver biopsy. Pathogenesis of this condition is not fully understood. Hepatotoxicity induced by T-DM1 is mainly due to inflammation of liver tissue and could potentially lead to proliferation of hepatocytes (which form the regenerative nodules) and sinusoidal obstruction. Histologically, there is no cirrhosis but moderate fibrosis is sometimes present.50 There are no international guidelines for NRH management. This condition is described only in case reports in which the latency of insurgence of NRH was 12–27 months and it was always associated with thrombocytopenia and portal hypertension.50 However, according to FDA label of T-DM1, treatment must be permanently discontinued upon diagnosis of NRH.
Peripheral neuropathy
One of the most common adverse events related to ADCs treatment is peripheral neuropathy (PN).2 Dose-depending and often dose-limiting side effect, patients generally never completely recover.51 The most common subtype of PN ADC-related involves the peripheral sensory, clinically associated with either the so-called “plus” symptoms (e.g., paraesthesia, hyperalgesia, pain) or “minus” symptoms (e.g., hypoesthesia, numbness of hands and feet).51 In patients treated with ADCs, PN occurs in ⁓40% of cases, and leads to treatment discontinuation in 11%. Also, hypoesthesia is the second most common grade ≥3 AE in these patients (23.3%).2
According to EudraVigilance database, nervous system disorders represent the 16.2% of AEs reported during treatment with T-DM1 and about 6.5% for T-DXd and SG.52
In particular, the payload is considered the main responsible: as seen in mice, DM1 may cause axonal degeneration, secondary to the reduction of microtubule polymerization in peripheral neurons. However, an additional antigen-dependent mechanism underlying PN may exist and this observation is supported by the correlation of low-levels of HER2 expression and axonal degeneration identified in monkey and human peripheral nerve spindle cells.19
It must be considered that peripheral sensory and motor neuropathy is a known toxicity of microtubule-inhibiting chemotherapeutic agents, including taxanes, extensively used in the (neo)adjuvant setting. Indeed, in the KATHERINE trial PN was reported in 18.6% of patients treated with T-DM1 versus 6.9% for the trastuzumab arm, with grade ≥3 in 1.4% for T-DM1 and 0% for the control arm. An exploratory analysis revealed that peripheral neuropathy was slightly more common (36.3% vs 31.3%) and more severe (14.3% vs 7.0% for G2 and 3.6% vs 1.0% for G3 events) in patients with a baseline PN who received T-DM1. Furthermore, in these patients, the symptoms resolve slowly (about 100 days later in case of baseline PN) and less frequently. The type of taxane used in the neoadjuvant setting did not significantly increase the rate of any-grade PN in patients treated with T-DM1. However, prior paclitaxel was associated with more G2 events than docetaxel (9.4% vs 8.0%), while the latter with more G3 events (2.0% vs 0.9%). Moreover, an increase in any-grade PN was noted in patients who received >12 weeks of taxane. Lastly, a non-anthracycline (AC) neoadjuvant regimen increases the rate of any-grade PN in the T-DM1 arm (4.5% vs 0.8% in AC treated). It should be noted that the most common non-AC regimen was carboplatin + paclitaxel, a combination of two neurotoxic chemotherapeutics.53
Conversely, clinical trials involving T-DXd, HER3-DX, Dato-DXd and SYD985 do not mention PN as an AE.
In ASCENT trial no patients treated with SG had grade ≥3 peripheral neuropathy (vs 1% of patients in the chemotherapy arm),32 and in the phase I/II IMMU-132-01 trial, 19% of patients reported neuropathy, none of grade >2.31
There are no specific recommendations for the prevention and treatment of ADC-induced peripheral neuropathy. European guidelines indicate measures based on platinum or taxanes-induced neuropathy.51 No preventive strategies, pharmacological or not, had proven effective in reducing PN incidence. The treatment of this AE focuses on reducing pain in patients with chronic PN. Duloxetine has the highest level of evidence in reducing pain in patients with chemotherapy-induced PN.51 Anticonvulsants (pregabalin, gabapentin) or tricyclic antidepressants (amitriptyline) can be considered in case of duloxetine failure or contraindication (e.g., hypersensitivity reactions, hepatic failure, hypertension). These drugs have proven efficacy in reducing neuropathic cancer pain, although non-specifically studied for PN.51 Topical agents such as menthol cream or capsaicin-containing patch can also be considered. Growing evidence shows that physical exercise, functional pain (e.g., vibration training), cryotherapy or compression therapy may reduce PN symptoms. They should be started before or at the first manifestation of PN.51
FDA T-DM1 label recommend temporally discontinuation of therapy in case of grade 3 or 4 peripheral neuropathy until a grade ≤2 toxicity.
Ocular toxicity
The eye is a structurally complex organ consisting of actively proliferating cells, highly dense blood vessels and many epithelial receptors. Ocular AEs reported with ADCs were related with either the target or the payload, thus, although not fully understood, the mechanisms may be both on- and off-target. In the latter case, ocular toxicities are more frequently described with the delivery of unconjugated cytotoxic maytansinoids and auristatin derivatives, as in T-DM1.54
Reported ocular toxicities with T-DM1 were most frequently related to the presence of the HER2 receptor on the cells of the ocular epithelia, especially those more differentiated on the surface.55 Dry eye, conjunctivitis, blurred vision and increased lacrimation are common AEs, each reported in 3–6% of cases.56 In addition, rare cases of cataract and punctate keratitis have been reported.57 Alterations of the corneal epithelium have been described in all patients undergoing T-DM1 treatment, mostly asymptomatic and resolving upon discontinuation of the drug.58 This is probably linked to the corneal epithelium stem cells in the limbus (the border between cornea and sclera) being highly proliferating and differentiating as they proceed towards the center of the cornea. Therefore, the peripheral cells are the most affected by cytotoxicity, without a significant impact on vision. Tear duct stenosis/swelling was also described with T-DM1 treatment,59,60 probably due to drug excretion in the tears, causing inflammation of the lacrimal ducts, which led to stenosis and epiphora in the long run. This effect was also observed in patients undergoing docetaxel treatment, suggesting that it is related to the DM1 payload.
Blurred vision and visual impairment are also common AEs with T-DXd. The registration trials’ protocols recommended performing ophthalmologic assessment, including fundoscopy, visual acuity test and slit lamp examination, at screening, end-of-treatment and as clinically indicated.61 Ocular toxicity was not reported with HER3-DXd12,35 and was not described with Dato-DXd neither when administered alone in TROPION-PanTumor01 trial,62,63 nor when associated with immunotherapy in BEGONIA trial.33 To date, ocular toxicity reported only with T-DXd can lead to hypothesizing it may be linked to the HER2 targeting antibody rather than the payload. Less than 5% ocular AEs were reported in the ASCENT study,32 whereas in the IMMU-132-0110 and TROPiCS-0264 trials the same toxicity was not described.
Of note, toxicity involving the eye appeared to be prominent with SYD985. In the phase III TULIP trial, this AE was reported in 78.1% of patients, vs 29.2% of events in the control arm (physician’s choice). Grade ≥3 AEs were reported in 21.2% of patients, with 20.8% of patients discontinuing treatment and 22.9% reducing doses due to eye toxicity.30 Ocular AEs included conjunctivitis (all grades 38.2% vs 2.2%, grade ≥ 3 5.6%), keratitis (38.2% vs 8.0%, grade ≥ 3 12.2%), dry eye (30.2% vs 10.2%, grade ≥ 3 4.2%), increased lacrimation (18.4% vs 1.5%). At the time of enrolment, patients with previous diagnoses of keratitis were excluded, and for all the patients enrolled, regular ophthalmologic assessment was recommended, together with prophylactic use of lubricating eye drops. Protocol also recommended to discontinue treatment with grade ≥3 keratitis, and to delay drug administration until conjunctivitis reduced to grade ≤2.30 Symptoms improvement was obtained with topical ocular therapies in addition to dose reduction and delayed administration of SYD985, while prophylaxis with eye drops had little impact and complete recovery was reached after months.29 The differences observed with the HER2-targeting ADCs demonstrate the importance of the role played by the payload and the cleavability of the linker: SYD985 has an alkylating agent as a payload and a highly cleavable linker.
Physicians must be aware of the possibility of ocular AEs, although infrequent with some ADCs. Close monitoring and prompt management of these AEs, including an ophthalmologist in the comprehensive multidisciplinary care, is encouraged. The toxicity described with the different ADCs is often reversible and manageable with adequate ocular care. Treatment frequently consists in the use of local therapy with artificial tears, steroids or antibiotics, but interventions may be indicated in case of lacrimal duct stenosis or cataract (Supplementary Table S6). Primary or secondary prophylaxis proved to be effective in mitigating ocular toxicity due to ADCs.65 Treatment discontinuation or dose reduction may be indicated in patients not-responding to supportive care.
Dermatologic adverse events
Patients treated with ADCs may suffer from dermatologic toxicities, affecting hair and skin.
Alopecia
Alopecia is among the most frequent AE of this class (Supplementary Table S7) and is assessed as grade 1 or grade 2, as per the CTCAE grading system, which differentiates hair loss less versus more than 50%, respectively.
Alopecia is a rather uncommon AE in patients treated with T-DM1. Indeed, the highest incidence was recorded in pretreated BC patients, occurring in 2% of cases in the TH3RESA trial.4 Therefore, a markedly lower rate compared to the chemotherapy-containing arms (as well as anti-HER2 agents) of the T-DM1 investigating trials.6,66
Conversely, the number of events registered with T-DXd is much higher. This is evident in the head-to-head comparison DESTINY-Breast03 trial: while only 2.3% of patients treated with T-DM1 suffered a grade 1 alopecia, this AE occurred in 36.2% of patients treated with T-DXd, of which 9.3% were grade 2.9 The frequency of alopecia associated with T-DXd treatment varies from 36.2% to 49.8% among trials,8,9 similar to the rates registered with the conventional chemotherapy, as shown in DESTINY-Breast04 trial, where the patients in the control (chemo) arm reported 32.6% of events.7 The different incidence of alopecia events registered with these two compounds is clearly not correlated with the target, as both ADCs target HER2, but most probably related to the type of payload and linker stability. Indeed, these chemotherapeutic agents are both associated with high incidence of alopecia (microtubule inhibitors (as DM1) >80%, and topoisomerase type 1 with 60–100%), but there is an important difference in linker stability and DAR (higher in T-DXd, where the linker is less stable).
For the same reasons, in the ASCENT trial, assessing SG in patients with metastatic TNBC, alopecia has been reported at high frequencies: 47% of patients (any grade), versus only 16% of cases in the chemotherapy arm.11 This rate was higher than the 38% of patients reporting alopecia in the previous BC trial, IMMU-132-01 trial,10 although similar to the rates registered in the IMMU-132-06 trial (49%, any grade), testing SG in metastatic urothelial cancer.67 Similar are the incidence rates of HER3-DXd-related alopecia (27–36% in the SOLTI-TOT-HER3 trial), and interestingly, this AE was less frequent in the lower dose arm of the study.13
Conversely, in the phase I dose-escalation and dose-expansion study investigating SYD985, 21% and 18% of patients enrolled reported alopecia grade 1 and 2, respectively.29
The assessment of alopecia as TRAE is currently ongoing for Dato-DXd in BC. Nevertheless, we can derive some experience from the HER2-positive BC patients subgroup enrolled in the phase I study TROPION-PanTumor01, where 35% of patients treated with Dato-DXd reported this AE.14
Alopecia has a significant impact on patients’ quality of life: indeed, patients suffering from grade 2 alopecia need wigs or other types of hair pieces to obtain a complete camouflage of their hair loss, and this carries a higher psychological burden than grade 1. Nevertheless, managing this AE is still a challenge. In order to reduce the incidence of chemotherapy-induced alopecia, scalp-cooling methods have been developed.68 By reducing the temperature of the scalp, this system induces vasoconstriction and decreases the effect of the drug on hair follicles.68, 69, 70 However, there are no data available on this method being tested in patients treated with ADCs.
Cutaneous rash
Cutaneous rashes may appear as part of allergic and/or infusion-related reactions, or later during the treatment. The first type is an acute hypersensitivity reaction, that may occur within minutes to hours of the drug administration. The manifestation may include pruritus, flushing, urticaria, hypotension, anaphylaxis. Hereon, we are going to focus on cutaneous rashes as TRAEs. Moreover, it is important to clarify that often, in trials, the term rash is used as a “grouped term”, namely including several subtypes, such as generalized rash, rash pustular, rash maculo-papular, etc.
T-DM1-related rash was reported in up to ⁓12% of patients4,5,17 (Supplementary Table S6), while there are no reports of this AE in the trials investigating T-DXd, HER3-DXd, and Dato-DXd, although, for these last two compounds, we only have some preliminary data available. In the study investigating SYD985, rash maculopapular was reported in only 3% of patients in the dose-expansion cohort.29
As previously mentioned, Trop2 is highly expressed in skin, and indeed the frequency of cutaneous rash with SG varied from 12% to 30% among trials10,11 (Supplementary Table S8), and we are waiting for the reports on the cutaneous AEs for the other anti-Trop2 ADC, Dato-DXd.
To correctly assess cutaneous eruptions, and in order to understand the correlation to the treatment, it is always important to review the patient’s concomitant medication list, including prescription and over-the-counter drugs. The rash needs to be analyzed in terms of body distribution (skin, mucous membranes), types of alterations (blister, purpura, skin necrosis, etc), and the co-occurrence of symptoms such as lymphadenopathy, fever, dyspnea, hypotension, among others.71
After excluding alternative etiologies, such as viral or bacterial infections, treatment interruption would be a means to both confirm the origin and treat the eruption. Sometimes, it is possible to continue the treatment of patients with morbilliform rash, if not severe, although under strict monitoring. If the rash is accompanied by pruritus, oral antihistamines may be helpful. Topical steroids have been demonstrated to be effective in maculopapular rashes.71 Cleansing with warm water, gentle soap, avoiding sun exposure, and applying protective cream SPF 30 or higher may be helpful in the management of skin eruptions.72 Alterations of the skin impact quality of life of patients, creating discomfort in daily functioning and ultimately, emotional distress, which can lead to disorders such as depression and anxiety. Therefore, proactive management approaches to anticipate and control cutaneous AEs are strongly recommended.
Embryo-fetal toxicity
Although ADCs now represent the cornerstone of therapy for HER2-positive BC, we lack evidence on their safety in pregnant women. Pregnancy-associated BC is defined as BC diagnosed during pregnancy or within a year after delivery. Physiological changes such as breast enlargement, changes in texture and nipple discharge, may blur those of BC and thus delay the diagnosis.73 HER2-positive tumors are relatively more frequent in pregnant patients than HR-positive tumors,74 which may potentially contribute to poorer prognosis. ADCs are large molecules, thus requiring active transportation through the placenta: this mechanism is absent until week 14 of pregnancy, suggesting that a high fetal exposure is unlikely during the first trimester.75 It must be noted that ADCs target specific tumor-related mechanisms that also have a physiological role in fetal growth and development. The main AEs reported with trastuzumab during pregnancy are oligo/anhydramnios and the abnormal development of fetal kidney and lung as a result of highly expressed EGFR blockade in renal epithelium.76 T-DXd is expected to cause embryofetal damage due to the payload’s inhibition of topoisomerase I. SN-38 is a genotoxic compound, thus SG can cause teratogenicity and/or embryo-fetal mortality. Preclinical evidences of trastuzumab and maytansine suggest that T-DM1 could be teratogenic and embryotoxic.19 In the post-marketing setting, cases of oligohydramnios, of which some associated with fatal pulmonary hypoplasia, have been reported in pregnant women treated with trastuzumab.52 A step forward in understanding the risks of embryo-fetal toxicity will be the results of the MoTHER study, a prospective observational study of women with BC treated with a trastuzumab-containing regimen with or without pertuzumab or T-DM1 during pregnancy, or within 7 months before conception.
Interstitial lung disease (ILD)
ILD comprises a heterogeneous group of over 200 pulmonary disorders that occur with inflammation and/or fibrosis of the lungs at the interstitium, small airways and/or alveolar level.77
Dyspnea is the most common symptom; other symptoms may include cough, discomfort, chest pain, hypoxemia and fever.78 The clinical manifestations of ILD are associated with radiological findings of unilateral or bilateral pulmonary infiltrates on thoracic imaging and with abnormal pulmonary physiology determined by aberrant gas transfer, evidenced by respiratory function tests. Potential complications of ILD include the development and/or progression of pulmonary fibrosis, pulmonary hypertension, small airways disease and pulmonary embolism leading to respiratory failure, hypoxemia and congestive heart failure.77,78
ILD may be idiopathic (for about two-thirds of cases), or it may be triggered (for about one-third of cases) by a known cause, such as adverse drug reactions in addition to viral infections, environmental exposure, inhaled toxins or antigens, chest radiation.78,79
In the context of drug-related lung damage in cancer patients, the term ILD is often used interchangeably with the term pneumonia, which refers to disorders characterized by lung inflammation.3 Drug-induced ILD accounts for 3–5% of common cases of ILD/pneumonitis80 and represents a major problem in clinical practice. Some drug-induced ILD can be fulminant, resulting in diffuse alveolar damage (DAD) and acute respiratory distress syndrome, sometimes with fatal outcomes.81 As drug-induced ILD/pneumonitis is a diagnosis of exclusion, it is important to evaluate symptoms, collect careful clinical history (including drugs, radiation exposure, environmental/occupational exposures), perform physical examination, chest imaging, pulmonary function and clinical laboratory tests, to rule out other potential causes of lung damage78, 79, 80 ]. The trigger for the onset of drug-induced ILD is unknown and several mechanisms are likely to underlie its development. Currently, two categories of mechanisms potentially involved in drug-induced ILD/pneumonitis have been identified: direct cytotoxic lung damage and immune-mediated lung damage.81
Major risk factors associated with drug-induced ILD include: advanced age (≥60 years), pre-existing lung disease, concomitant radiation, smoking, renal failure, and genetic susceptibility, in particular, an increased risk of ILD has been observed among patients of East Asian ethnicity (Japan).79,80
The severity of drug-related ILD/pneumonitis is graded from 1 to 5, namely, asymptomatic drug-related ILD/pneumonitis is assessed as grade 1, while grades ≥2 define symptomatic cases with increased severity, and grade 5 identifies death related to AE.3
ADC-related ILD/pneumonitis was first noted in patients treated with T-DXd: in the phase 2 study DESTINY-Breast01, T-DXd was associated with ILD in 13.6% of patients, with four deaths (2.2%).82 According to the latest results of the randomized phase 3 DESTINY-Breast03 study, drug-related ILD events occurred in 10.5% of patients in the T-DXd group, of which 9.7% were grade 1 or 2, 0.8% grade 3 and none grade 4 or 5.9 Interestingly, after 12 months the risk of drug-related ILD appears to be decreased, suggesting that the risk of developing ILD is not influenced by the cumulative dose of T-DXd.82,83 In the latest trial, the frequency of ILD (all grades) was lower than that observed in previous studies with patients more heavily pretreated.83 This may be due to a higher frequency of screening, rapid dose adjustment and/or discontinuation if ILD is suspected, and possibly less pulmonary toxicity with T-DXd when administered in earlier settings.
Conversely, ILD was a rare event reported in clinical trials investigating T-DM1 and SG,32,36 although often fatal.4 Notably, a higher incidence of ILD (2.6% of patients) was observed when concomitant radiotherapy was administered with T-DM1.17
In the phase I/II study U31402-A-J101investigating HER3-DXd, ILD was observed in 6.6% of patients, mostly grade 1–2, with a single grade 5 event.12 No cases of ILD were reported in the SOLTI TOT-HER3 trial.35
There were no reported cases of ILD/pneumonitis due to Dato-DXd in TROPION-PanTumor01 and BEGONIA trials.14,33,62
Notably, most of the cited studies excluded patients with pre-existing or concomitant lung disease, and although lymphangitic carcinomatosis was not correlated with ILD onset in a recent pooled analysis,84 it still represents a pathologic condition potentially leading to high concentration of payload in the lung interstitium. Thus, we would recommend caution in these patients.
The mechanism underlying this peculiar toxicity seems to be related to the uptake of the ADCs by alveolar macrophages, through FcɣR. Indeed, ILD has been reported with all ADCs, but not with the unconjugated payload, suggesting that this event is both target- and payload-independent.
Guidelines for the management and monitoring of T-DXd-related ILD/pneumonitis were published in 2019 and updated in 2021. The guidelines emphasize the importance of proactive management and monitoring to help identify and treat ILD/pneumonitis effectively.
ILD is usually suspected because of the appearance or worsening of clinical respiratory symptoms and/or suggestive radiological findings. Patients should be educated about signs and symptoms of ILD/pneumonitis to immediately report to healthcare providers any changes or onset of symptoms such as cough, shortness of breath, fever or any other new or worsening respiratory symptoms.
Progression of underlying oncological disease must always be considered in the differential diagnosis, particularly when neoplastic lung involvement is known. Furthermore, due to the pandemic SARS-CoV-2-related disease (COVID-19), which can mimic the radiological and clinical85 features of drug-induced ILD, COVID-19 should be included in the differential diagnosis.
CT scans should be performed before starting T-DXd treatment and at least every 9–12 weeks during treatment (concurrently with tumor assessment).86 For patients experiencing ILD/pneumonitis, a follow-up high-resolution computed tomography (HR-CT) scan is recommended every 1–2 weeks or as clinically indicated.8,86 If T-DXd-induced ILD/pneumonitis is suspected, a pulmonary specialist should be consulted for monitoring and therapeutic recommendations.
Current guidelines for T-DXd-induced ILD/pneumonitis recommend promptly starting steroids upon detection of grade ≥2 ILD/pneumonitis and suggest considering steroid treatment for grade 1 cases,8,86 including prednisone and methylprednisolone. Currently, rechallenge is only recommended for patients with resolving grade 1 ILD/pneumonitis; patients with grade 2 or higher ILD/pneumonitis should discontinue T-DXd permanently.
Discussion
ADCs have been designed as a platform for the targeted delivery of cytotoxic agents directly into cancer cells. The objective was to increase their activity, with a significant reduction in side effects and treatment-related complications. Indeed, most of the approved ADCs have a better overall safety profile than chemotherapy, in head-to-head comparisons. However, as we have seen summarized in this review, some “chemotherapy-related” side effects (such as hematological toxicity, nausea/vomiting, diarrhea, etc.) have been reported, together with new uncommon toxicities (ILD, ocular disorders). In particular, ADCs may cause toxicities through different mechanisms, depending on the chemical properties of the payload (i.e., hydrophilic), the drug-to-antibody ratio (DAR), as well as the stability of the linker (cleavable or not) and the expression of the target in non-cancer tissues. Payloads of the currently available ADCs are drugs initially developed for intravenous use and some of them have been known for decades (Supplementary Table S9). Interestingly, some of the systemic AEs described here are similar to those reported in the older trials with the free respective payloads or parent compounds, clearly suggesting that significant amounts of payload may recirculate in the bloodstream.
In fact, due to the proteolytic-rich tumor microenvironment,87 a cleavable linker may release unpredictable amounts of its payload at the extracellular level. While this property is considered a strength in terms of antitumor activity (the bystander effect), on the other hand, it allows the free payload to re-enter the bloodstream, causing toxicities in other sites (off-tumor, off-target). On the other hand, it has been noted that ADCs with more stable linkers may be able to interact with albumin as a carrier, prolonging the patients’ exposition to the drug.88 In addition, ADCs have also peculiar toxicities related to antibody/epitope recognition, as in case of the ADCs targeting the Trop2 receptor, widely expressed on skin and mucosae. Other toxicities still need to be clearly investigated, as in the case of ILD/pneumonitis, or other rare occurrences, as the benign liver nodular hyperplasia described with T-DM1. Current research is aiming at establishing new payloads and new ADC-like platforms to minimize toxicity and increase potency, as well as identifying predictive biomarkers and optimizing doses and treatment schedules. However, as this review is more clinically oriented, we suggest another recent publication22 for a more detailed appraisal of this topic.
Outstanding questions
ADCs are major players in the treatment of solid and hematologic tumors, and the number of compounds and clinical indications is rapidly expanding, with transformative results on disease outcomes. Awareness and proper education are key to guide clinicians in timely identification and proper management of AEs to preserve patients’ safety, adherence to therapy and to avoid premature discontinuation of treatment or inappropriate dose reductions.
Indeed, anticancer therapy goal is to provide patients better survival outcome, while preserving quality of life (QoL). Reducing the AEs caused by the therapy will have a double positive effect on patients: giving them the best therapeutic strategy, coupled with survival benefit, and preserving and improving their QoL. While patients and healthcare professionals’ awareness and education are pivotal, research efforts are also needed. Furthermore, better understanding of the biology behind some toxicities of special interest (e.g., ILD), may help prevention and effective management. In addition, side-effect mitigation strategies, as for example schedule manipulation, need to be explored for the potential of preserving efficacy and reducing the burden of toxicity, as recently demonstrated for the ADC gemtuzumab ozogamicin.
To note, the scientific community is still improving in collection and reporting of QoL data from patients enrolled in the pivotal trials, as well as those treated in daily practice. An interesting option will be the results of the trials investigating the use of electronic reports and easy-to-use phone applications for patients.
Moreover, special clinical conditions, such as pregnancy, outlined in this manuscript, warrant further investigation.
Finally, it is important to remark the significance of continued reporting of the AEs to the pharmacovigilance registers (see Supplementary material), from which we derive additional information in terms of frequency and grade of AEs in the real-world population, as well as identifying rare occurrences (as for hepatic NRH).
Ultimately, this review aims to give to clinicians prescribing ADCs the means to ensure that the patients, while receiving the most effective treatment, keep on preserving their QoL, and this is obtained through prevention, early identification, and intervention on ADCs-related toxicities.
Search strategy.
References for this paper were identified through searches of PubMed and Embase with the terms: “breast cancer” or “breast neoplasm”, “toxicity” or “adverse events”, “hematological” or “nausea” or “vomiting” or “CINV” or “gastrointestinal” or “lung disease” or “cardiotoxicity” or “cardiovascular” or “cardiac” or “ocular” or “optic” or “ophthalmic” or “eye disease” or “cornea” or “dermatotoxicity” or “cutaneous” or “skin” or “hepatic” or “embrio” or “neuro”, “trastuzumab deruxtecan” or “T-DXd” or “trastuzumab emtansine” or “T-DM1” or “trastuzumab duocarmazine” or “SYD985” or “datopotamab deruxtecan” or “Dato-DXd” or “sacituzumab govitecan” or “patritumab deruxtecan” or “HER3-DXd” or “antibody drug conjucagte” or “ADC” or “immunoconjugate”, “antineoplastic” or “anticancer”, and “drug” or “agent”, from inception until March, 2023. Resources were also identified through searches of the material from the main international congresses in Oncology (ASCO, ESMO, San Antonio Breast Cancer Symposium, ESMO Breast Cancer). Only papers and abstracts published in English were reviewed. The final reference list was generated on the basis of originality and relevance to the broad scope of this review.
Contributors
ADA and AV: Conceptualization; Resources; Writing–Original Draft; Writing - Review & Editing. MP and FN: Conceptualization; Investigation; Visualization; Supervision; Writing - Original Draft; Writing - Review & Editing. SP and MV: Writing - Original Draft; Writing - Review & Editing; Resources. RC, FP, MG, LDM, and GA: Writing - Review & Editing. MDL: Writing - Review & Editing; Funding acquisition; Project administration. FM: Conceptualization; Methodology; Project administration.
Declaration of interests
AV has received travel grants from Lilly, Novartis, Pierre Fabre, and Gilead. MP has received travel grants from Pfizer and Gilead. RC declares honoraria from Novartis, Lilly, Gilead, Seagen, Veracyte, Daichii Sankyo, Pierre-Fabre; she is advisory board member for Novartis, Lilly, Gilead, Seagen, Daichii Sankyo, Pierre-Fabre, Roche, Astra Zeneca, and MSD; she has received travel grants from Gilead, Lilly and Novartis. FP received honoraria for advisory boards, activities as a speaker, travel grants, research grants from AstraZeneca, Daichii Sankyo, Eisai, Lilly, Gilead, MSD, Novartis, Exact Sciences, Menarini, Pierre Fabre, Pfizer, Roche, and Seagen. He has received research funding from AstraZeneca, Eisai and Roche. MG is a consultant/advisory board member for AstraZeneca, Daichii Sankyo, Eisai, Gilead, Lilly, MSD, Novartis, Pfizer, and Seagen; he has received travel grants from AstraZeneca, Pfizer and research funding (to institution) from AstraZeneca. LDM is a consultant/advisory board member for Lilly, Novartis, Roche, Pfizer, Daiichi Sankyo, Exact science, Gilead, Pierre Fabre, Eisai, AstraZeneca, GSK, Seagen, and Agendia; she has received research support from Roche, Lilly, Seagen, Daiichi Sankyo and Novartis (to institution); she declares honoraria from Roche, Pfizer, Lilly, MSD, Seagen, Gilead, Pierre Fabre, Eisai, Ipsen, Exact science, AstraZeneca and Novartis; has received travel grants from Roche, Pfizer, Eisai, AstraZeneca, and Daiichi Sankyo. GA is a consultant/advisory board member for Roche, Lilly, AstraZeneca, Novartis, Seagen, Daiichi Sankyo, Eisai, and Gilead; she has received research support from AstraZeneca (to institution); declares honoraria from Roche, Pfizer, Lilly, Eisai, AstraZeneca, Gilead, Seagen, Viatris, Exact Sciences, Daiichi Sankyo, and Novartis; has received travel grants from Roche, Daiichi Sankyo, and Novartis. MDL is a consultant/advisory board member for Pfizer, AstraZeneca, Sanofi, Seagen, Novartis, Ipsen, Roche, Pierre Fabre, Daiichi Sankyo, and GSK; he declares honoraria from Lilly, Novartis, Seagen, Takeda, Roche, Daiichi Sankyo, Tomalab, Gilead, Genetic, Menarini, and Sophos; has received travel grants from Roche, AstraZeneca. FM is a consultant/advisory board member for Roche, Daiichi Sankyo, Seagen; he received honoraria from Roche, AstraZeneca, Daiichi Sankyo, Seagen Pierre Fabre, MSD, Novartis, and Pfizer; he has received travel grants from AstraZeneca. AD, FN, SP, and MV have no conflict of interest to declare.
Acknowledgements
Funding from Istituto Nazionale Tumori IRCCS “Fondazione G. Pascale”, Via Mariano Semmola 52, 80131 Naples, Italy.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102113.
Contributor Information
Martina Pagliuca, Email: Martina.PAGLIUCA@gustaveroussy.fr.
Fabiana Napolitano, Email: fabiana.napolitano@unina.it.
Michelino De Laurentiis, Email: m.delaurentiis@istitutotumori.na.it.
Appendix A. Supplementary data
References
- 1.Sakach E., Sacks R., Kalinsky K. Trop-2 as a therapeutic target in breast cancer. Cancers. 2022;14:5936. doi: 10.3390/cancers14235936. https://www.mdpi.com/2072-6694/14/23/5936/htm [cited 2023 Mar 17]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu Y., Liu K., Wang K., Zhu H. Treatment-related adverse events of antibody–drug conjugates in clinical trials: a systematic review and meta-analysis. Cancer. 2023;129(2):283–295. doi: 10.1002/cncr.34507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Serivices, National Institutes of Health National Cancer Institute, Common Terminology Criteria for Adverse Events (CTCAE) V. 5.0. 2017 [Google Scholar]
- 4.Krop I.E., Kim S.-B.B., González-Martín A., et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(7):689–699. doi: 10.1016/S1470-2045(14)70178-0. [DOI] [PubMed] [Google Scholar]
- 5.Verma S., Miles D., Gianni L., et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez E.A., Barrios C., Eiermann W., et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: primary results from the phase III MARIANNE study. J Clin Oncol. 2017;35(2):141–148. doi: 10.1200/JCO.2016.67.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modi S., Jacot W., Yamashita T., et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9–20. doi: 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Modi S., Saura C., Yamashita T., et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortés J., Kim S.-B., Chung W.-P., et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386(12):1143–1154. doi: 10.1056/NEJMoa2115022. [cited 2023 Jan 22]. [DOI] [PubMed] [Google Scholar]
- 10.Bardia A., Mayer I.A., Vahdat L.T., et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380(8):741–751. doi: 10.1056/NEJMoa1814213. [cited 2023 Mar 15] [DOI] [PubMed] [Google Scholar]
- 11.Bardia A., Hurvitz S.A., Tolaney S.M., et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384(16):1529–1541. doi: 10.1056/NEJMoa2028485. [cited 2023 Jan 15] [DOI] [PubMed] [Google Scholar]
- 12.Krop I.E., Masuda N., Mukohara T., et al. Results from the phase 1/2 study of patritumab deruxtecan, a HER3-directed antibody-drug conjugate (ADC), in patients with HER3-expressing metastatic breast cancer (MBC) J Clin Oncol. 2022;40(16_suppl):1002. doi: 10.1200/JCO.23.00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliveira M., Pascual T., Ortega P.T., et al. 124O Patritumab deruxtecan (HER3-DXd) in hormonal receptor-positive/HER2-negative (HR+/HER2-) and triple-negative breast cancer (TNBC): results of part B of SOLTI TOT-HER3 window of opportunity trial. ESMO Open. 2023;8(1) doi: 10.1016/j.esmoop.2023.101463. [DOI] [Google Scholar]
- 14.Krop I., Juric D., Shimizu T., et al. Abstract GS1-05: Datopotamab deruxtecan in advanced/metastatic HER2- breast cancer: Results from the phase 1 TROPION-PanTumor01 study. Cancer Res. 2022;82(4_Supplement) doi: 10.1200/JCO.23.01909. https://aacrjournals.org/cancerres/article/82/4_Supplement/GS1-05/680217/Abstract-GS1-05-Datopotamab-deruxtecan-in-advanced [cited 2023 Jan 29];Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bardia A., Harnden K., Mauro L., Pennisi A., Armitage M., Soliman H. Clinical practices and institutional protocols on prophylaxis, monitoring, and management of selected adverse events associated with trastuzumab deruxtecan. Oncologist. 2022;27(8):637–645. doi: 10.1093/oncolo/oyac107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klastersky J., de Naurois J., Rolston K., et al. Management of febrile neutropaenia: ESMO clinical practice guidelines. Ann Oncol. 2016;27:v111–v118. doi: 10.1093/annonc/mdw325. [DOI] [PubMed] [Google Scholar]
- 17.von Minckwitz G., Huang C.-S., Mano M.S., et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 18.Experts Committee on Cancer -Related Anemia. Chinese Society of Clinical Oncology (CSCO) Clinical practice guidelines on cancer-related anemia (2012-2013 Edition) Chin Clin Oncol. 2012;1(2):18. doi: 10.3978/j.issn.2304-3865.2012.10.01. [DOI] [PubMed] [Google Scholar]
- 19.Poon K.A., Flagella K., Beyer J., et al. Preclinical safety profile of trastuzumab emtansine (T-DM1): mechanism of action of its cytotoxic component retained with improved tolerability. Toxicol Appl Pharmacol. 2013;273(2):298–313. doi: 10.1016/j.taap.2013.09.003. https://www.sciencedirect.com/science/article/pii/S0041008X13003955 Available from: [DOI] [PubMed] [Google Scholar]
- 20.Diéras V., Harbeck N., Budd G.T., et al. Trastuzumab emtansine in human epidermal growth factor receptor 2-positive metastatic breast cancer: an integrated safety analysis. J Clin Oncol. 2014;32(25):2750–2757. doi: 10.1200/JCO.2013.54.4999. [DOI] [PubMed] [Google Scholar]
- 21.Wuerstlein R., Ellis P., Montemurro F., et al. Final results of the global and Asia cohorts of KAMILLA, a phase IIIB safety trial of trastuzumab emtansine in patients with HER2-positive advanced breast cancer. ESMO Open. 2022;7(5) doi: 10.1016/j.esmoop.2022.100561. http://www.esmoopen.com/article/S2059702922001892/fulltext [cited 2023 Apr 22]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarantino P., Ricciuti B., Pradhan S.M., Tolaney S.M. Optimizing the safety of antibody–drug conjugates for patients with solid tumours. Nat Rev Clin Oncol. 2023 doi: 10.1038/s41571-023-00783-w. [DOI] [PubMed] [Google Scholar]
- 23.Krop I.E.E., Lin N.U.U., Blackwell K., et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol Off J Eur Soc Med Oncol. 2015;26(1):113–119. doi: 10.1093/annonc/mdu486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slichter S.J., Kaufman R.M., Assmann S.F., et al. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med. 2010;362(7):600–613. doi: 10.1056/NEJMoa0904084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffiths E.A., Alwan L.M., Bachiashvili K., et al. Considerations for use of hematopoietic growth factors in patients with cancer related to the COVID-19 pandemic. J Natl Compr Cancer Netw. 2020;19(13):1–4. doi: 10.6004/jnccn.2020.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crone S.A., Zhao Y.-Y., Fan L., et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8(5):459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 27.Lyon A.R., López-Fernández T., Couch L.S., et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Eur Heart J. 2022;43(41):4229–4361. doi: 10.1093/eurheartj/ehac244. [DOI] [PubMed] [Google Scholar]
- 28.Lynce F., Barac A., Geng X., et al. Prospective evaluation of the cardiac safety of HER2-targeted therapies in patients with HER2-positive breast cancer and compromised heart function: the SAFE-HEaRt study. Breast Cancer Res Treat. 2019;175(3):595–603. doi: 10.1007/s10549-019-05191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banerji U., van Herpen C.M.L.L., Saura C., et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20(8):1124–1135. doi: 10.1016/S1470-2045(19)30328-6. http://www.ncbi.nlm.nih.gov/pubmed/31257177 [cited 2019 Nov 19].Available from: [DOI] [PubMed] [Google Scholar]
- 30.Saura Manich C., O’Shaughnessy J., Aftimos P.G., et al. LBA15 - Primary outcome of the phase III SYD985.002/TULIP trial comparing [vic-]trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann Oncol. 2021;32(suppl_5):S1283–S1346. https://oncologypro.esmo.org/meeting-resources/esmo-congress-2021/primary-outcome-of-the-phase-iii-syd985.002-tulip-trial-comparing-vic-trastuzumab-duocarmazine-to-physician-s-choice-treatment-in-patients-with-p [cited 2023 Feb 5].Available from: [Google Scholar]
- 31.Bardia A., Messersmith W.A., Kio E.A., et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol. 2021;32(6):746–756. doi: 10.1016/j.annonc.2021.03.005. http://www.annalsofoncology.org/article/S0923753421008838/fulltext [cited 2023 Jan 21]Available from: [DOI] [PubMed] [Google Scholar]
- 32.Rugo H.S., Tolaney S.M., Loirat D., et al. Safety analyses from the phase 3 ASCENT trial of sacituzumab govitecan in metastatic triple-negative breast cancer. npj Breast Cancer. 2022;8(1):1–10. doi: 10.1038/s41523-022-00467-1. https://www.nature.com/articles/s41523-022-00467-1 [cited 2023 Jan 15].Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmid P., Jung K.H., Wysocki P.J., et al. 166MO Datopotamab deruxtecan (Dato-DXd)+ durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic triple-negative breast cancer (a/mTNBC): initial results from BEGONIA, a phase Ib/II study. Ann Oncol. 2022;33:S199. [Google Scholar]
- 34.Berger M.J., Agarwal R., Anand S., et al. 2023. NCCN guidelines version 1.2023 antiemesis continue.https://www.nccn [cited 2023 Feb 11]. Available from: [Google Scholar]
- 35.Prat A., Falato C., Pare Brunet L., et al. LBA3 Patritumab deruxtecan (HER3-DXd) in early-stage HR+/HER2- breast cancer: final results of the SOLTI TOT-HER3 window of opportunity trial. Ann Oncol. 2022;33:S164. [Google Scholar]
- 36.Rugo H.S., Bardia A., Marmé F., et al. Sacituzumab govitecan in hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2022;40(29):3365–3376. doi: 10.1200/JCO.22.01002. [DOI] [PubMed] [Google Scholar]
- 37.Bardia A., Juric D., Shimizu T., et al. LBA4 Datopotamab deruxtecan (Dato-DXd), a TROP2-directed antibody-drug conjugate (ADC), for triple-negative breast cancer (TNBC): preliminary results from an ongoing phase I trial. Ann Oncol. 2021;32:S60. [Google Scholar]
- 38.Bianchini G., Arpino G., Biganzoli L., et al. Emetogenicity of antibody-drug conjugates (ADCs) in solid tumors with a focus on trastuzumab deruxtecan: insights from an Italian expert panel [internet] Cancers. 2022;14:1022. doi: 10.3390/cancers14041022. https://www.mdpi.com/2072-6694/14/4/1022/htm Multidisciplinary Digital Publishing Institute; Feb 17, 2022 p. 1022. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pavone G., Motta L., Martorana F., Motta G., Vigneri P. A new kid on the block: sacituzumab govitecan for the treatment of breast cancer and other solid tumors. Molecules. 2021;26(23):7294. doi: 10.3390/molecules26237294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagai Y., Oitate M., Shiozawa H., Ando O. Comprehensive preclinical pharmacokinetic evaluations of trastuzumab deruxtecan (DS-8201a), a HER2-targeting antibody-drug conjugate, in cynomolgus monkeys. Xenobiotica. 2019;49(9):1086–1096. doi: 10.1080/00498254.2018.1531158. [cited 2023 Mar 8] [DOI] [PubMed] [Google Scholar]
- 41.Okajima D., Yasuda S., Maejima T., et al. Datopotamab deruxtecan, a novel TROP2-directed antibody-drug conjugate, demonstrates potent antitumor activity by efficient drug delivery to tumor cells. Mol Cancer Ther. 2021;20(12):2329–2340. doi: 10.1158/1535-7163.MCT-21-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bossi P., Antonuzzo A., Cherny N.I., et al. Diarrhoea in adult cancer patients: ESMO clinical practice guidelines. Ann Oncol. 2018;29:iv126–iv142. doi: 10.1093/annonc/mdy145. http://www.annalsofoncology.org/article/S0923753419316941/fulltext [cited 2023 Mar 9];Available from: [DOI] [PubMed] [Google Scholar]
- 43.Pascual T., Oliveira M., Ciruelos E., et al. SOLTI-1805 TOT-HER3 study concept: a window-of-opportunity trial of patritumab deruxtecan, a HER3 directed antibody drug conjugate, in patients with early breast cancer. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.638482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montemurro F., Ellis P., Anton A., et al. Safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive advanced breast cancer: primary results from the KAMILLA study cohort 1. Eur J Cancer. 2019;109:92–102. doi: 10.1016/j.ejca.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 45.Brown T.J., Gupta A. Management of cancer therapy-associated oral mucositis. JCO Oncol Practice. 2020;16(3):103–109. doi: 10.1200/JOP.19.00652. [cited 2023 Feb 16] [DOI] [PubMed] [Google Scholar]
- 46.Protocol C.S., Intervention S., Code S. 2022. A phase 3, open-label, randomised study of datopotamab deruxtecan (Dato-DXd) versus investigator’ s choice of chemotherapy in patients who are not candidates for PD-1/PD-L1 inhibitor therapy in first-line locally recurrent inoperable or metastatic. 0(Amendment 1) [Google Scholar]
- 47.Bensinger W., Schubert M., Ang K.-K., et al. NCCN task force report: prevention and management of mucositis in cancer care. J Natl Compr Cancer Netw. 2008;6(S1):S1–S21. [PubMed] [Google Scholar]
- 48.Peterson D.E., Boers-Doets C.B., Bensadoun R.J., Herrstedt J. Management of oral and gastrointestinal mucosal injury: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann Oncol. 2015;26:v139–v151. doi: 10.1093/annonc/mdv202. http://www.annalsofoncology.org/article/S0923753419471731/fulltext [cited 2023 Mar 11]. Available from: [DOI] [PubMed] [Google Scholar]
- 49.Yan H., Endo Y., Shen Y., et al. Ado-trastuzumab emtansine targets hepatocytes via human epidermal growth factor receptor 2 to induce hepatotoxicity. Mol Cancer Ther. 2016;15(3):480–490. doi: 10.1158/1535-7163.MCT-15-0580. https://aacrjournals.org/mct/article/15/3/480/92064/Ado-Trastuzumab-Emtansine-Targets-Hepatocytes-Via [cited 2023 Jan 22]. Available from: [DOI] [PubMed] [Google Scholar]
- 50.Garrido I., Magalhães A., Lopes J., MacEdo G. Trastuzumab emtansine-induced nodular regenerative hyperplasia: is dose reduction enough as a preventable measure? Dig Dis. 2022;40(6):787–792. doi: 10.1159/000521933. https://www.karger.com/Article/FullText/521933 [cited 2023 Apr 13]. Available from: [DOI] [PubMed] [Google Scholar]
- 51.Jordan B., Margulies A., Cardoso F., et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO–EONS–EANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up. Ann Oncol. 2020;31(10):1306–1319. doi: 10.1016/j.annonc.2020.07.003. http://www.annalsofoncology.org/article/S0923753420399385/fulltext [cited 2023 Mar 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 52.EudraVigilance - European database of suspected adverse drug reaction reports [Internet] https://www.adrreports.eu/en/search.html# Available from:
- 53.Mamounas E.P., Untch M., Mano M.S., et al. Adjuvant T-DM1 versus trastuzumab in patients with residual invasive disease after neoadjuvant therapy for HER2-positive breast cancer: subgroup analyses from KATHERINE. Ann Oncol. 2021;32(8):1005–1014. doi: 10.1016/j.annonc.2021.04.011. http://www.annalsofoncology.org/article/S0923753421011686/fulltext [cited 2023 Mar 22]. Available from: [DOI] [PubMed] [Google Scholar]
- 54.Donaghy H., Wade H. Effects of antibody, drug and linker on the preclinical and clinical toxicities of antibody-drug conjugates. MAbs. 2016;8(4):659–671. doi: 10.1080/19420862.2016.1156829. [cited 2023 Mar 5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Z., Carvajal M., Carraway C.A., Carraway K., Pflugfelder S.C. Expression of the receptor tyrosine kinases, epidermal growth factor receptor, ErbB2, and ErbB3, in human ocular surface epithelia. Cornea. 2001;20(1):81–85. doi: 10.1097/00003226-200101000-00016. [DOI] [PubMed] [Google Scholar]
- 56.Burris H.A., III, Rugo H.S., Vukelja S.J., et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-Positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 2010;29(4):398–405. doi: 10.1200/JCO.2010.29.5865. www.jco.org [cited 2023 Mar 12]. Available from: [DOI] [PubMed] [Google Scholar]
- 57.Beeram M., Krop I.E., Burris H.A., et al. A phase 1 study of weekly dosing of trastuzumab emtansine (T-DM1) in patients with advanced human epidermal growth factor 2–positive breast cancer. Cancer. 2012;118(23):5733–5740. doi: 10.1002/cncr.27622. [cited 2023 Mar 12] [DOI] [PubMed] [Google Scholar]
- 58.Deklerck E., Denys H., Kreps E.O. Corneal features in trastuzumab emtansine treatment: not a rare occurrence. Breast Cancer Res Treat. 2019;175(2):525–530. doi: 10.1007/s10549-019-05179-y. [cited 2023 Mar 12] [DOI] [PubMed] [Google Scholar]
- 59.Krop I.E., Beeram M., Modi S., et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(16):2698–2704. doi: 10.1200/JCO.2009.26.2071. [DOI] [PubMed] [Google Scholar]
- 60.Kim C.Y., Kim N., Choung H.K., In Khwarg S. Lacrimal drainage system stenosis associated with Trastuzumab emtansine (Kadcyla®, T-DM1) administration: a case report. BMC Cancer. 2019;19(1):1–4. doi: 10.1186/s12885-019-5986-5. https://bmccancer.biomedcentral.com/articles/10.1186/s12885-019-5986-5 [cited 2023 Mar 12]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hurvitz S.A., Hegg R., Chung W.-P.P., et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023;401(10371):105–117. doi: 10.1016/S0140-6736(22)02420-5. http://www.thelancet.com/article/S0140673622024205/fulltext [cited 2023 Feb 19]. Available from: [DOI] [PubMed] [Google Scholar]
- 62.Shimizu T., Lisberg A.E., Sands J.M., et al. O2-1 datopotamab deruxtecan (Dato-DXd; DS-1062), a TROP2 ADC, in patients with advanced NSCLC: Updated results of TROPION-PanTumor01 phase 1 study∗. Ann Oncol. 2021;32:S285. http://www.annalsofoncology.org/article/S0923753421017245/fulltext [cited 2023 Jan 28]. Available from: [Google Scholar]
- 63.Spira A., Lisberg A., Sands J., et al. Datopotamab deruxtecan (Dato-DXd; DS-1062), a TROP2 ADC, in Patients with advanced NSCLC: updated results of TROPION-PanTumor01 phase 1 study. J Thoracic Oncol. 2021;16:S106–S107. [Google Scholar]
- 64.Rugo H.S., Bardia A., Marmé F., et al. LBA76 Overall survival (OS) results from the phase III TROPiCS-02 study of sacituzumab govitecan (SG) vs treatment of physician’s choice (TPC) in patients (pts) with HR+/HER2- metastatic breast cancer (mBC) Ann Oncol. 2022;33:S1386. [Google Scholar]
- 65.Chandran K., Grochot R., Lai S., et al. 522P Risk mitigation of ocular toxicities due to antibody drug conjugates (ADCs) in novel early-phase clinical trials. Ann Oncol. 2021;32:S590. doi: 10.1016/j.annonc.2021.08.1044. [DOI] [Google Scholar]
- 66.Tolaney S.M., Tayob N., Dang C., et al. Adjuvant trastuzumab emtansine versus paclitaxel in combination with trastuzumab for stage I HER2-positive breast cancer (ATEMPT): a randomized clinical trial. J Clin Oncol. 2021;39(21):2375–2385. doi: 10.1200/JCO.20.03398. [DOI] [PubMed] [Google Scholar]
- 67.Tagawa S.T., Balar A.V., Petrylak D.P., et al. TROPHY-U-01: a phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol. 2021;39(22):2474–2485. doi: 10.1200/JCO.20.03489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silva G.B., Ciccolini K., Donati A., Hurk C.V.D. Scalp cooling to prevent chemotherapy-induced alopecia. An Bras Dermatol. 2020;95:631–637. doi: 10.1016/j.abd.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lacouture M.E., Sibaud V., Gerber P.A., et al. Prevention and management of dermatological toxicities related to anticancer agents: ESMO Clinical Practice Guidelines☆. Ann Oncol. 2021;32(2):157–170. doi: 10.1016/j.annonc.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 70.Rugo H.S., Bianchini G., Cortes J., Henning J.-W.W., Untch M. Optimizing treatment management of trastuzumab deruxtecan in clinical practice of breast cancer. ESMO Open. 2022;7 doi: 10.1016/j.esmoop.2022.100553. Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sibaud V., Lebœuf N.R., Roche H., et al. Dermatological adverse events with taxane chemotherapy. Eur J Dermatol. 2016;26(5):427–443. doi: 10.1684/ejd.2016.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bensadoun R.J., Humbert P., Krutmann J., et al. Daily baseline skin care in the prevention, treatment, and supportive care of skin toxicity in oncology patients: recommendations from a multinational expert panel. Cancer Manag Res. 2013;5:401–408. doi: 10.2147/CMAR.S52256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Amant F., Loibl S., Neven P., Van Calsteren K. Breast cancer in pregnancy. Lancet. 2012;379(9815):570–579. doi: 10.1016/S0140-6736(11)61092-1. http://www.thelancet.com/article/S0140673611610921/fulltext [cited 2023 Apr 22]. Available from: [DOI] [PubMed] [Google Scholar]
- 74.Aziz S., Pervez S., Khan S., et al. Case control study of novel prognostic markers and disease outcome in pregnancy/lactation-associated breast carcinoma. Pathol Res Pract. 2003;199(1):15–21. doi: 10.1078/0344-0338-00347. [DOI] [PubMed] [Google Scholar]
- 75.Azim H.A., Azim H., Peccatori F.A. Treatment of cancer during pregnancy with monoclonal antibodies: a real challenge. Expet Rev Clin Immunol. 2010;6:821–826. doi: 10.1586/eci.10.77. [DOI] [PubMed] [Google Scholar]
- 76.Lambertini M., Peccatori F.A., Azim H.A. Targeted agents for cancer treatment during pregnancy. Cancer Treat Rev. 2015;41:301–309. doi: 10.1016/j.ctrv.2015.03.001. W.B. Saunders Ltd. [DOI] [PubMed] [Google Scholar]
- 77.Faverio P., De Giacomi F., Bonaiti G., et al. Management of chronic respiratory failure in interstitial lung diseases: overview and clinical insights. Int J Med Sci. 2019;16(7):967. doi: 10.7150/ijms.32752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Antoine M., Mlika M. StatPearls [internet] StatPearls Publishing; 2021. Interstitial lung disease. [PubMed] [Google Scholar]
- 79.Johkoh T., Lee K.S., Nishino M., et al. Chest CT diagnosis and clinical management of drug-related pneumonitis in patients receiving molecular targeting agents and immune checkpoint inhibitors: a position paper from the Fleischner Society. Radiology. 2021;298(3):550–566. doi: 10.1148/radiol.2021203427. [DOI] [PubMed] [Google Scholar]
- 80.Skeoch S., Weatherley N., Swift A.J., et al. Drug-induced interstitial lung disease: a systematic review. J Clin Med. 2018;7(10):356. doi: 10.3390/jcm7100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matsuno O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir Res. 2012;13(1):1–9. doi: 10.1186/1465-9921-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Modi S., Saura C., Yamashita T., et al. San Antonio breast cancer Symposium. 2020. Updated results from DESTINY-breast01, a phase 2 trial of trastuzumab deruxtecan (T-DXd) in HER2 positive metastatic breast cancer; pp. 8–11. [Google Scholar]
- 83.Saura Manich C., Modi S., Krop I., et al. Trastuzumab deruxtecan (T-DXd) in patients with HER2-positive metastatic breast cancer (MBC): updated survival results from a phase II trial (DESTINY-Breast01) Ann Oncol. 2021;32:S485–S486. doi: 10.1016/j.annonc.2023.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Powell C.A., Modi S., Iwata H., et al. Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO Open. 2022;7(4) doi: 10.1016/j.esmoop.2022.100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Struyf T., Deeks J.J., Dinnes J., et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19. Cochrane Database Syst Rev. 2022;5(5) doi: 10.1002/14651858.CD013665.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Swain S.M., Nishino M., Lancaster L.H., et al. Multidisciplinary clinical guidance on trastuzumab deruxtecan (T-DXd)–related interstitial lung disease—focus on proactive monitoring, diagnosis, and management. Cancer Treat Rev. 2022;106 doi: 10.1016/j.ctrv.2022.102378. [DOI] [PubMed] [Google Scholar]
- 87.Fan J., Ning B., Lyon C.J., Hu T.Y. Circulating peptidome and tumor-resident proteolysis. Enzymes. 2017;42:1–25. doi: 10.1016/bs.enz.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 88.Wei C., Zhang G., Clark T., et al. Where did the linker-payload go? A quantitative investigation on the destination of the released linker-payload from an antibody-drug conjugate with a maleimide linker in plasma. Anal Chem. 2016;88(9):4979–4986. doi: 10.1021/acs.analchem.6b00976. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.