Abstract
Lysine succinylation is a naturally occurring post-translational modification (PTM) that regulates the stability and function of proteins. It can be regulated by enzymes such as SIRT5 and SIRT7. Recently, the effect and significance of lysine succinylation in cancer and its implication in immunity have been extensively explored. Lysine succinylation is involved in the malignant phenotype of cancer cells. Abnormal regulation of lysine succinylation occurs in different cancers, and inhibitors targeting lysine succinylation regulatory enzymes can be used as potential anti-cancer strategies. Therefore, this review focused on the target protein lysine succinylation and its functions in cancer and immunity, in order to provide a reference for finding more potential clinical cancer targets in the future.
Keywords: Immunity, Mitochondria, SIRT5, Succinyl-CoA, Succinylation
Introduction
Lysine succinylation is the addition of a succinyl group to a lysine residue, which belongs to chemical group modification. It may have a greater impact on the structure and function of the protein due to its greater mass transfer.1 Many other PTMs such as methylation, acetylation, and ubiquitination are present in histones.2 Later, scholars also identified sites of lysine succinylation in histones.3 Zhang et al identified 69 succinyllysine sites in 14 Escherichia coli proteins, indicating that lysine succinylation is naturally occurring.4 Lysine succinylation mainly occurs in mitochondria. HeLa cells and mouse liver are rich in mitochondrial proteins, where a large number of succinylation sites can be found.5
With the development of high-resolution mass spectrometry and affinity enrichment techniques, the research progress of lysine succinylation has been greatly promoted. Weinert et al made use of affinity enrichment and strong cation exchange chromatography to identify thousands of lysine succinylation sites in bacteria (E. coli), yeast (S. cerevisiae), human (HeLa) cells, and mouse liver tissue, suggesting that lysine succinylation is a modification that occurs frequently in both prokaryotes and eukaryotes.5 Experimental results showed that the succinylation peptide was further enriched by low pH elution. In recent years, the role of lysine succinylation in cancer has been well studied. Many experiments have shown that lysine succinylation can regulate the growth and metastasis of tumor cells, and is related to DNA damage response (DDR) and immunity.
Mechanisms of succinylation
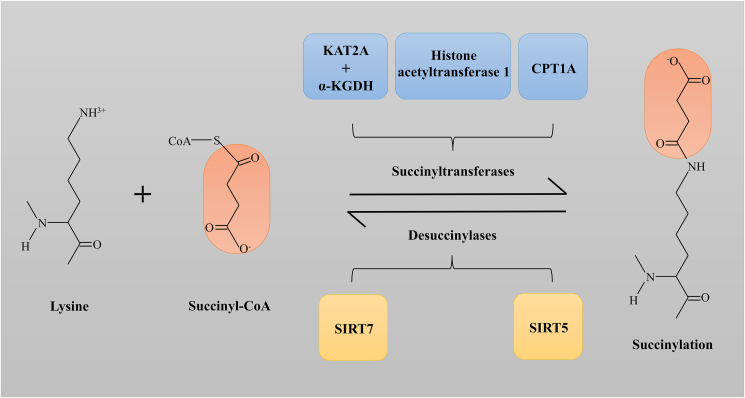
Succinylation is the covalent binding of a succinyl group to a lysine residue of a substrate protein by a succinyl group donor, either enzymatically or non-enzymatically. Gozde et al discovered the first prokaryotic desuccinylase CobB and the major substrate for lysine succinylation. In the hyperglycemia reaction, the abundance of 90% of the 324 quantifiable succinylation sites increased at least fourfold, experimentally showing that lysine succinylation was significantly enhanced by glucose.6 GAS41, reader of lysine succinylation, is a dimeric protein containing a YEATS domain that binds significantly to H3K122succ whose recognition function is influenced by pH.7,8 Apart from this, the level of succinylation modification is currently regulated mainly by succinyl donors, succinyltransferases, and desuccinylases (Fig. 1).3
Figure 1.
All known succinyl-transferases and desuccinylases associated with the regulation of succinylation modifications.
Succinyl-CoA metabolism
Succinyl-CoA is the succinyl group donor for which levels regulate the occurrence of non-enzymatic lysine succinylation.1 In a yeast study, Succinyl-CoA, in the tricarboxylic acid (TCA) cycle, has a comprehensive regulation on the level of lysine succinylation.5 The TCA cycle is the main source of energy for cells. In the TCA cycle, succinyl-CoA is both a reactant and a reaction product. In addition, succinate coenzyme A synthase binds exclusively to succinate.9 Dysregulation of these enzymes in the TCA cycle may lead to cancer.10 Succinyl-CoA is not only produced from the TCA cycle, but also from the oxidation of fatty acids and the metabolic process of degradation of amino acids.11
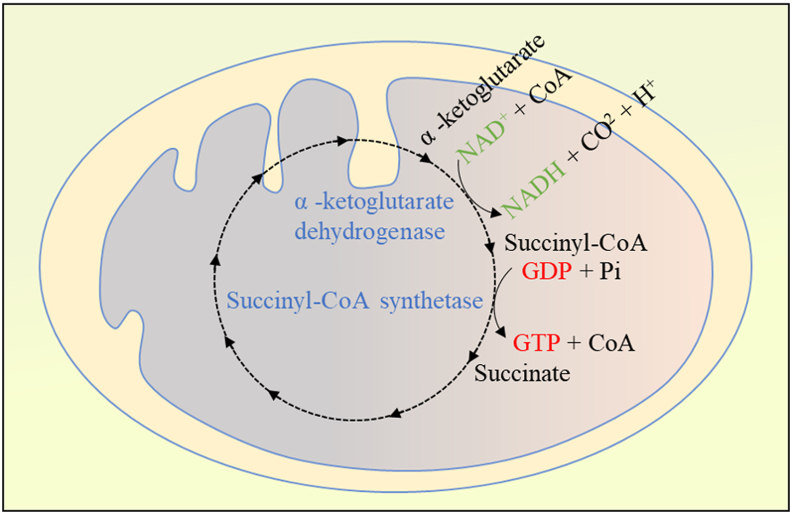
The TCA cycle consists of ten steps, two of which are directly related to succinyl-CoA. α-ketoglutarate dehydrogenase complex catalyzes the conversion of α-ketoglutarate, CoASH, and NAD+ to succinyl-CoA, NADH, and CO2. This step is an irreversible reaction, and the succinyl-CoA generated in this step can be used as a substrate to generate succinate and CoA and generate energy under the action of succinyl-CoA synthetase (Fig. 2). Succinyl-CoA and succinate are kept in equilibrium in the TCA. Evidence showed that proteins involved in metabolism are targets of succinylation such as glyceraldehyde 3-phosphate dehydrogenase, isocitrate dehydrogenase, and pyruvate dehydrogenase (PDH).4,12
Figure 2.
Succinyl-CoA metabolism in TCA cycle. α-ketoglutarate dehydrogenase complex and succinyl-CoA synthetase are involved in the metabolism of the succinyl-CoA with production of NADH and GTP.
Succinyltransferase
Studies have shown that lysine acetyltransferase 2A (KAT2A) plays an important role in histone succinylation and gene expression regulation. Wang et al found that KAT2A mediates the succinylation of Lysine 79 of histone H3, which binds to the α-ketoglutarate dehydrogenase (α-KGDH) complex to obtain succinyl-CoA, thereby promoting histone succinylation. KAT2A and α-KGDH complexes are closely related to gene expression and tumor cell proliferation.13 In another study, Yang et al found that histone acetyltransferase 1 regulates both histone and non-histone proteins. On the one hand, it succinylated H3 at the K122 site, which promotes gene expression in tumor cells; on the other hand, it can promote the succinylation of itself to increase its activity and thus accelerate the progression of the tumor.14
Desuccinylase
SIRT715 and SIRT516 are both NAD + -dependent histone desuccinylases. Studies have shown that Sirt7-catalyzed desuccinylation of H3K122succ plays an important role in chromatin condensation and double-strand break repair.15 Defective TCA cycle metabolism leads to defective DNA repair and sensitivity to genotoxic agents, similar to the effect of chromatin hypersuccinylation observed with SIRT7 deletion. Chromatin succinylation is associated with metabolic regulation of genome-wide transcription and DNA repair activities.17 Studies have shown that there are arginine and tyrosine residues in the acyl bags of SIRT5,16 so SIRT5 prefers short-chain carboxyl groups, such as malonyl and succinyl.18 SIRT5 is a highly efficient protein lysine desuccinylase. It exists in both mitochondria and outside mitochondria. The high enrichment of lysine succinylation here may affect mitochondrial metabolism.12 Succinyl-coenzyme also allows proteins to be succinylated without the enzyme.19 SIRT5 also regulates key enzymes in ketone body synthesis, which are highly succinylated, and inhibits ketone production through rate-limiting ketogenic enzyme 3-hydroxy-3-methylglutaryl-CoA synthase 2.20 Superoxide dismutase succinylation at the K123 site can reduce its activity and inhibit the proliferation of lung tumor cells, which can be regulated by SIRT5.21 SIRT5 can also desuccinate glutaminase (GLS), thereby reducing its ubiquitination and degradation. GLS can hydrolyze glutamine and provide a sufficient carbon source for the TCA cycle.22
Protein lysine succinylation is associated with cancer through a number of mechanisms, and the replacement of core histones with specifically modified histone variants has been shown to be carcinogenic.23 Dichloroacetate (DCA), a PDH kinase inhibitor, is an anti-tumor drug with a remarkable anti-tumor effect.24 Its anti-tumor mechanism is associated with succinylation. In a study of colon cancer cells, DCA-responsive succinylation has a great influence on HCT116 cells which can increase the transport of pyruvate to mitochondria, contribute to mitochondrial glucose oxidation and depolarization, and restore membrane potential, thus playing an anti-cancer role.25 Pyruvate kinase M2 (PKM2) is specifically expressed in tumor cells,26 and catalyzes the transfer of phosphoric acid from phosphoenolpyruvate to ADP, leading to pyruvate and ATP formation. Ye et al reported that PKM2 is succinylated at lysine 498 (K498).27 The combination of PKM2 and SIRT5, a desuccinylase, reduces the succinylation and activity of PKM2. Inhibition of SIRT5 and increased PKM2 succinylation at K498 inhibited cell proliferation and tumor growth.27
Studies have shown that succinate dehydrogenase (SDH) is expressed in many tumors. Succinate is a substrate for SDH, and they compete with each other. Down-regulation of the SDH gene leads to the accumulation of succinate and succinyl-CoA, resulting in global lysine hyper-succinylation, a set of changes that may promote tumor growth.17,28
Cellular roles of lysine succinylation
In an experiment involving succinylation of Vibrio alginolyticus, Zeng et al identified 2082 succinylation sites that matched 671 proteins where they found a large number of overlapped acetylated proteins involved in glycolysis/gluconeogenesis, the TCA cycle, and pyruvate metabolism. The 169 specific succinylated proteins were mainly concentrated in five KEGG pathways, including amino acid biosynthesis, ABC transporter, and cationic antimicrobial peptide resistance.29 Lysine succinylation affects a variety of cellular functions, including mitochondrial metabolism, transcription, and DNA damage repair. Lysine succinylation plays an important role in the complex metabolic process of tumor cells.
Mitochondrial metabolism
Research has shown that lysine succinylation can regulate mitochondrial function, and plays an important role in oxoacid metabolism and coenzyme metabolism.12 In addition, it is highly enriched in purine/pyridine metabolism, glycolysis/gluconeogenesis, pyruvate metabolism, TCA cycle, and fatty acid synthesis.6 In cancer cells, mitochondria aerobic respiration is restrained. It is concluded that lysine succinylation may have a negative impact on the respiration-related pathways according to the levels of the enzyme in the TCA cycle.30
Uncoupling protein 1 (UCP1), regulated by SIRT5, is a thermogenic protein in brown adipose tissue (BAT). Studies have shown that the succinylation of UCP1 reduces its stability and affects energy homeostasis regulation. Increased succinylation of mitochondrial protein in SIRT5-deficient BAT resulted in impaired mitochondrial enzyme activity and respiratory function.31
Transcription
Histone succinylation plays an important role in gene expression by reducing the affinity between DNA and histones, thereby reducing the stability of nucleosomes and chromosomes. It is well known that the separation of DNA and proteins facilitates the binding of transcription factors to DNA, thereby facilitating transcription.32 In an experiment on the stability of nucleosomes, Li et al generated flag-tagged constructs of wild-type H3 or H3 mutants H3K122E and non-succinylated H3K122R. Compared with the control group, the NaCl solubility of histone H3 in stably expressed H3K122E cells was significantly increased, while that in H3K122R cells was the opposite, indicating that the succinylation of H3K122 caused the decrease of histone-DNA interactions. Desuccinylation of H3K122succ stabilized nucleosomes.15 In another experiment,33 Zorro et al performed in vitro transcription on unmodified chromatin in the presence of Suc-CoA. The results showed that Suc-CoA stimulates transcription. Following this, in vitro transcription was performed on unmodified and H3K122succ chromatin. Interestingly, H3K122succ caused about 1.6 times more transcription than unmodified chromatin. The Succinylation of H3K122 enhances transcription.33
Smestad et al first demonstrated that SDH-deficient TCA cycle defects are associated with several human malignancies, resulting in increased succinyl-CoA and high-volume succinylated protein concentrations.17 Succinyllysine is enriched in gene promoters and has a great relationship with gene expression, which provides a basis for the idea that the chromatin of gene promoters is succinylated to activate transcription.17
In the first succinylproteomic analysis of extensively drug-resistant-tuberculosis, Xie et al34 identified 686 succinylated proteins and 1739 succinylated sites, which were shown to be involved in a variety of cellular functions, such as metabolic processes, transcription, translation, and stress response. Interestingly, lysine succinylation preferentially selects proteins involved in protein biosynthesis and carbon metabolism.34
DNA repair
P53 is a tumor suppressor that regulates complex metabolic pathways.35 P53 is also a transcription factor and activated P53 plays an important role in regulating DNA repair and apoptosis.36 PTM can regulate the activity of P53, and different modifications have different effects on P53.37 Recent studies have found that P53 is succinylated at K120, and SIRT5 can mediate the desuccinylation of this site, thus inhibiting the activation of P53.38
In breast cancer studies, it is known that lysine succinylation is enriched in H2A. H2A.X complex is associated with breast cancer DDR and is enriched in DDR-related cellular processes, such as nucleosome assembly, DNA repair, cell senescence, etc. Acetylation and succinylation of proteins in the H2A.X complex can affect DDR repair.39
The function of lysine succinylation in cancer
Dysregulation of lysine succinylation may lead to tumor formation. With the extensive application of advanced technology and people's unremitting efforts in research, increasingly succinylation sites on protein and sequence variation have been found in cancer. Abnormal succinylation of lysine residues plays different roles in different cancers as shown in Table 1.
Table 1.
The role of lysine succinylation in cancer.
| Tissue/cell | Targets | Function | Content | Reference |
|---|---|---|---|---|
| Gastric cancer tissue | CPT1A | Increase the K47 succinylation of S100A10 | Increase gastric cancer invasion | 40 |
| Gastric cancer tissue | CPT1A | Succinylate lactate dehydrogenase on K222 | Promote the invasion and proliferation of gastric cancer | 41 |
| ESCC cell | DMS | Increase the level of lysine succinylation | Inhibit the cell growth and migration of esophageal squamous cell carcinoma | 42 |
| Breast cancer tissue | SIRT5 | Inhibit the succinylation of metabolic enzymes | Promote breast cancer proliferation | 43 |
| Breast cancer tissue | H2A.X complexes | Associated with the DNA damage response | Affect DNA damage repair by regulating the formation of protein foci | 39 |
Gastric cancer
In gastric cancer tissues, the level of S100A10 mRNA in gastric cancer tissues was higher than that in the control group, and it was overexpressed in metastatic lymph nodes. A total of 503 lysine succinylation sites of 303 proteins were identified after mass spectrometry analysis. S100A10 was succinylated only at lysine 47 (K47). Antibody tests showed that lysine succinylation of S100A10 was more common in the experimental group than in the control group. S100A10 is highly succinylated at K47 mediated by carnitine palmitoyltransferase 1A (CPT1A), which enhances its expression in gastric cancer and may affect the degradation of S100A10 protease, thereby promoting invasion and metastasis of gastric cancer cells.40 Lactate dehydrogenase (LDHA), a key enzyme in aerobic glycolysis, is highly expressed in a variety of tumors. The binding of K63-ubiquitinated LDHA to sequestosome 1 (SQSTM1) can mediate the lysosomal pathway of LDHA degradation. Another experiment found that LDHA was highly succinylated at K222, which reduces the combination of ubiquitinated LDHA and SQSTM1, resulting in reduced LDHA dissolution. This overexpression also promoted the growth and migration of gastric cancer cells. It has a serious negative impact on patients with gastric cancer.41
Esophageal squamous cell carcinoma
In these experiments, upregulation of the target lysine succinylation site promoted the proliferation and diffusion of cancer cells, but in some cancers, increased target lysine succinylation inhibited tumor growth and migration. Guo et al. treated SHEEC cells with dimethyl succinate (DMS) or diluent for 48 h as the experimental group and then transplanted them into the right clawed pad of nude mice. After 30 days of inoculation, all of the mice in the control group showed tumor infiltration from the foot pad to the ankle, compared with 40 percent of the mice in the experimental group. DMS can increase the level of lysine succinylation in cancer cells which is inhibited in esophageal squamous cell carcinoma (ESCC). It was shown that the up-regulation of lysine succinylation sites in cancer cells inhibited the growth and migration of ESCC.42
Breast cancer
In one study of breast cancer, Yashira et al disrupted SIRT5 in a mouse model of breast cancer, resulting in increased succinylation of isocitrate dehydrogenase 2 and other metabolic enzymes, as well as increased oxidative stress, inhibiting the growth of breast cancer. These results suggest that inhibiting desuccinylase and increasing the succinylation of metabolic enzymes have adverse effects on breast cancer growth.43
Studies have shown that most proteins in breast cancer have a high degree of succinylation and are enriched in three histones H2A, while the modification of the H2A.X complex is related to DDR in breast cancer.39 Ye et al injected 5 million cells subcutaneously into nude mice, expressing the wild type of PKM2 and the K498E mutant, and resected the tumor about seven weeks later. Based on the volume and tumor weight, it was found that cells expressing wild-type PKM2 developed more rapidly than cells expressing PKM2-K498E. Succinylation of PKM2 at K498 increased its activity and inhibited cell proliferation and tumor growth.27 Lysine succinylation is not only associated with the proliferation of breast cancer cells, but also with DDR of breast cancer.
Crosstalk with other PTMs
In addition to lysine succinylation, other PTM modifications such as methylation, acetylation, ubiquitination, malondialylation, and crotonylation are also important for the development of cancer. Succinylation functions in concert with other PTMs to regulate protein at multiple facets.
Methylation
Succinylation of lysine has two negative charges, which are larger than the charge caused by monomethylation. The methylation of lysine residues on histones is involved in the regulation of DNA transcription, replication, and repair. In recent years, it has been found that many lysine methylations occur in non-histone proteins and regulate major cancer-related cellular pathways, such as growth signaling and DDR.44 Lysine methylation levels are regulated by lysine methyltransferases and demethylases, which may serve as targets for the study of cancer development.45 Several high-quality small molecule inhibitors that target methyltransferases or demethylases have been shown to be effective against cancer.46 Protein methylation or RNA methylation may coordinate with succinylation to regulate tumor growth and metastasis.47,48
Acetylation
Lysine acetylation and succinylation have differences and similarities. Acetylation, which adds two carbons to lysine, has a less steric effect on the protein than succinylation. However, these two modifications have been found to play a synergistic role in the regulation of cancerous diseases such as breast cancer and colon cancer.25,39 Glucose-stimulated lysine succinylation sites were more significant than lysine acetylation sites.6 Acetylase inhibitors have been shown as promising anticancer drugs. At present, many new clinical anticancer drugs such as vorinostat and chidamide have been developed.49,50
Ubiquitination
Cell homeostasis is closely related to the balance between ubiquitination and deubiquitination, and the imbalance may be carcinogenic.51 E3 ubiquitin ligase regulates a variety of biological processes, including cell growth and apoptosis, and is important for cell homeostasis. Abnormal expression of E3 ubiquitin ligase has been shown to be associated with the development of cancer.52 Both lysine succinylation and ubiquitination are involved in the regulation of innate immunity.53 Ubiquitination of key proteins in tumor metabolism can regulate signaling pathways.54
Malonylation
Both malonylation and succinylation are acid-lysine modifiers. Although they are similar in structure, there are differences in the modulations and regulated objects.55 The order of charge changes in lysine succinylation and malonylation is the same, resulting in double charge transfer of substrate residues.3 In addition, both lysine demalonylation and lysine desuccinylation can be regulated by SIRT5.56
Crotonylation
Lysine crotonylation is a recently discovered PTM and is enriched in many nuclear-related cellular processes.57 The expression of lysine crotonylation is down-regulated in the liver, stomach, and kidney cancers, and increased crotonylation inhibits cell migration and proliferation of hepatocellular carcinoma.58 The histone crotonylation in prostate cancer was higher in cancer tissue than in normal tissue and increased with the degree of malignancy of the tumor.59
Succinate and succinylation in immunity
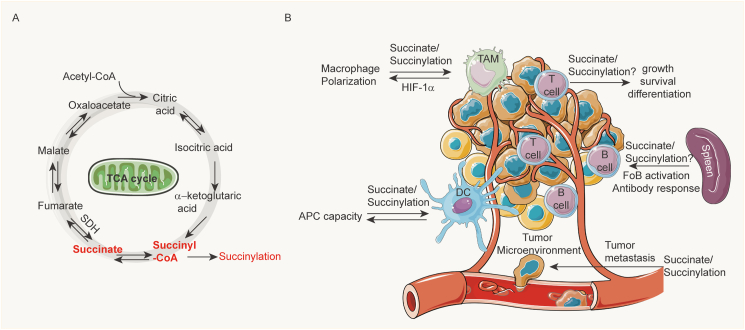
Mitochondria are the power plant for cells. The TCA cycle is one of the most prominent cellular activities for both energy and metabolism. Succinyl-CoA synthetase catalyzes the reaction from succinyl-CoA to succinate. SDH, also known as mitochondrial Complex II, functions in both the TCA cycle and electron transport chain (ETC). SDH transforms succinate to fumarate. Increasing evidence has shown that mitochondria and metabolism play crucial roles in both innate and adaptive immunity (Fig. 3). SDH deficiency or aberrant TCA cycle can cause succinate accumulation and increased succinylation.60
Figure 3.
Succinate and succinylation in immunity. (A) The scheme of the TCA cycle. (B) Succinate and succinylation play multi-faceted roles in the tumor microenvironment including macrophage (TAM, tumor-associated macrophage) polarization, T cell growth and differentiation, dendritic cell antigen processing capacity, follicular B cell activation and B cell antibody response, and tumor metastasis.
Several studies indicate that succinate activates the HIFα and induces the expression of IL-1β in M1 macrophage cells. Lipopolysaccharide stimulation can increase both succinate and succinylation, which thereby function in the inflammation–cancer cycle. Extracellular succinate also hyperpolarizes M2 macrophages.61 In the tumor microenvironment, macrophages can be activated and polarized into tumor-associated macrophages by tumor-derived succinate. Furthermore, the secreted succinate promotes cancer metastasis.62 Succinate also increased the capacity of dendritic cells to act as antigen-presenting cells, which induced adaptive immunity. The adaptive response further kills cancer cells and inhibits tumorigenesis.60
Proper mitochondrial function is also critical for T cell-mediated immune responses including growth, proliferation, production of cytokines, and sustained killing. The metabolic process always coordinates with epigenetic machinery to modify the chromatin, which affects the T cell transcriptome, lineage commitment, and maintenance. SDH/complex II deficiency in T cells caused impaired proliferation and survival. It also induced the proinflammatory gene expression in T cells and promoted T helper 1 and T helper 17 lineage differentiation.63 The underlying mechanisms are likely mediated by succinylation modification on chromatin.
B cell is the essential component of adaptive immunity, which produce antibodies in humoral immunity. Studies showed that glutathione regulates the TCA cycle and mitochondrial ETC in B cells. Abrogation of glutathione synthesis in B cells through knockout of the glutamate-cysteine ligase catalytic subunit affects complex I and II activity and leads to accumulation of succinate. Finally, glutathione deficiency blocks follicular B cell activation and antibody responses.64
Conclusions
With the deep understanding of protein PTMs in cancer research, PTM-based targeted therapy has become a hot research direction in cancer therapy.65,66 This paper reviews the recent progress of succinylation in promoting or inhibiting the growth and diffusion of tumor cells, with emphasis on the mechanism and regulatory enzymes of succinylation.
Lysine succinylation of proteins occurs widely in vivo and can coexist with other forms of PTMs. Its synergistic effect can be modified and the regulatory scope can be narrowed. For example, protein acetylation and succinylation are highly overlapped, and they have similar effects and common regulators. These two modifications play a synergistic role in the regulation of cancerous diseases such as breast cancer and colon cancer. Co-modification can narrow the regulatory scope. The mechanism of action between the succinylation of proteins and other forms of modification is still unclear, and research in this area may accelerate the development of potential anticancer drugs. In addition, the abnormal regulation of lysine succinylation by regulatory enzymes may be a carcinogenic factor, and regulatory enzyme inhibitors can be used as an anticancer treatment in the future. With the further study of protein lysine succinylation, its anticancer role will be further discovered.
Author contributions
H.S., L.L., and T.M. conceived this review and critically revised the manuscript. R.S., H.R., and S.L. drafted the manuscript. R.S. H.R., S.L., and B.L. drew the figures and collected the related references. T.M. and H.S. supervised and revised the manuscript. All authors read and approved the final version of the manuscript.
Conflict of interests
The authors declare that they have no competing interests.
Funding
This work was supported by Youth Wan Jiang Scholar of Anhui Province, China (No. DT2100001172) and Beijing Xisike Clinical Oncology Research Foundation (China) (No. Y-HR2020MS-0156).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Hang Song, Email: hangsong@ahtcm.edu.cn.
Lu Li, Email: deerlyee@hotmail.com.
Teng Ma, Email: mateng82913@163.com.
Abbreviations
- CPT1A
carnitine palmitoyltransferase 1A
- DCA
dichloroacetate
- DDR
DNA damage response
- DMS
dimethyl succinate
- ESCC
esophageal squamous cell carcinoma
- ETC
electron transport chain
- GLS
glutaminase
- KAT2A
lysine acetyltransferase 2A
- LDHA
lactate dehydrogenase
- PDH
pyruvate dehydrogenase
- PKM2
pyruvate kinase M2
- PTM
post-translational modification
- SDH
succinate dehydrogenase
- SQSTM1
sequestosome1
- TCA
tricarboxylic acid cycle
- UCP1
uncoupling protein 1
- α-KGDH
α-ketoglutarate dehydrogenase
References
- 1.Sreedhar A., Wiese E.K., Hitosugi T. Enzymatic and metabolic regulation of lysine succinylation. Genes Dis. 2020;7(2):166–171. doi: 10.1016/j.gendis.2019.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischle W., Wang Y., Allis C.D. Histone and chromatin cross-talk. Curr Opin Cell Biol. 2003;15(2):172–183. doi: 10.1016/s0955-0674(03)00013-9. [DOI] [PubMed] [Google Scholar]
- 3.Xie Z., Dai J., Dai L., et al. Lysine succinylation and lysine malonylation in histones. Mol Cell Proteomics. 2012;11(5):100–107. doi: 10.1074/mcp.M111.015875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Z., Tan M., Xie Z., et al. Identification of lysine succinylation as a new post-translational modification. Nat Chem Biol. 2011;7(1):58–63. doi: 10.1038/nchembio.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinert B.T., Schölz C., Wagner S.A., et al. Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep. 2013;4(4):842–851. doi: 10.1016/j.celrep.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Colak G., Xie Z., Zhu A.Y., et al. Identification of lysine succinylation substrates and the succinylation regulatory enzyme CobB in Escherichia coli. Mol Cell Proteomics. 2013;12(12):3509–3520. doi: 10.1074/mcp.M113.031567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Listunov D., Linhares B.M., Kim E., et al. Development of potent dimeric inhibitors of GAS41 YEATS domain. Cell Chem Biol. 2021;28(12):1716–1727.e6. doi: 10.1016/j.chembiol.2021.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y., Jin J., Chung M.W.H., et al. Identification of the YEATS domain of GAS41 as a pH-dependent reader of histone succinylation. Proc Natl Acad Sci U S A. 2018;115(10):2365–2370. doi: 10.1073/pnas.1717664115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J., Fraser M.E. Structural basis for the binding of succinate to succinyl-CoA synthetase. Acta Crystallogr D Struct Biol. 2016;72(Pt 8):912–921. doi: 10.1107/S2059798316010044. [DOI] [PubMed] [Google Scholar]
- 10.Kang W., Suzuki M., Saito T., et al. Emerging role of TCA cycle-related enzymes in human diseases. Int J Mol Sci. 2021;22(23) doi: 10.3390/ijms222313057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burch J.S., Marcero J.R., Maschek J.A., et al. Glutamine via α-ketoglutarate dehydrogenase provides succinyl-CoA for heme synthesis during erythropoiesis. Blood. 2018;132(10):987–998. doi: 10.1182/blood-2018-01-829036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park J., Chen Y., Tishkoff D.X., et al. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol Cell. 2013;50(6):919–930. doi: 10.1016/j.molcel.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y., Guo Y.R., Liu K., et al. KAT2A coupled with the α-KGDH complex acts as a histone H3 succinyltransferase. Nature. 2017;552(7684):273–277. doi: 10.1038/nature25003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang G., Yuan Y., Yuan H., et al. Histone acetyltransferase 1 is a succinyltransferase for histones and non-histones and promotes tumorigenesis. EMBO Rep. 2021;22(2) doi: 10.15252/embr.202050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L., Shi L., Yang S., et al. SIRT7 is a histone desuccinylase that functionally links to chromatin compaction and genome stability. Nat Commun. 2016;7 doi: 10.1038/ncomms12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du J., Zhou Y., Su X., et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334(6057):806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smestad J., Erber L., Chen Y., et al. Chromatin succinylation correlates with active gene expression and is perturbed by defective TCA cycle metabolism. iScience. 2018;2:63–75. doi: 10.1016/j.isci.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan M., Peng C., Anderson K.A., et al. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metabol. 2014;19(4):605–617. doi: 10.1016/j.cmet.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trefely S., Lovell C.D., Snyder N.W., et al. Compartmentalised acyl-CoA metabolism and roles in chromatin regulation. Mol Metabol. 2020;38 doi: 10.1016/j.molmet.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rardin M.J., He W., Nishida Y., et al. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metabol. 2013;18(6):920–933. doi: 10.1016/j.cmet.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Z.F., Xu H.B., Wang J.Y., et al. SIRT5 desuccinylates and activates SOD1 to eliminate ROS. Biochem Biophys Res Commun. 2013;441(1):191–195. doi: 10.1016/j.bbrc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 22.Lukey M.J., Greene K.S., Cerione R.A. Lysine succinylation and SIRT5 couple nutritional status to glutamine catabolism. Mol Cell Oncol. 2020;7(3) doi: 10.1080/23723556.2020.1735284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hake S.B., Xiao A., Allis C.D. Linking the epigenetic 'language' of covalent histone modifications to cancer. Br J Cancer. 2004;90(4):761–769. doi: 10.1038/sj.bjc.6601575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kankotia S., Stacpoole P.W. Dichloroacetate and cancer: new home for an orphan drug? Biochim Biophys Acta. 2014;1846(2):617–629. doi: 10.1016/j.bbcan.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Zhu D., Hou L., Hu B., et al. Crosstalk among proteome, acetylome and succinylome in colon cancer HCT116 cell treated with sodium dichloroacetate. Sci Rep. 2016;6 doi: 10.1038/srep37478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazurek S., Boschek C.B., Hugo F., et al. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15(4):300–308. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Ye X., Niu X., Gu L., et al. Desuccinylation of pyruvate kinase M2 by SIRT5 contributes to antioxidant response and tumor growth. Oncotarget. 2017;8(4):6984–6993. doi: 10.18632/oncotarget.14346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao M., Yang H., Xu W., et al. Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012;26(12):1326–1338. doi: 10.1101/gad.191056.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng F., Pang H., Chen Y., et al. First succinylome profiling of Vibrio alginolyticus reveals key role of lysine succinylation in cellular metabolism and virulence. Front Cell Infect Microbiol. 2020;10 doi: 10.3389/fcimb.2020.626574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song Y., Wang J., Cheng Z., et al. Quantitative global proteome and lysine succinylome analyses provide insights into metabolic regulation and lymph node metastasis in gastric cancer. Sci Rep. 2017;7 doi: 10.1038/srep42053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang G., Meyer J.G., Cai W., et al. Regulation of UCP1 and mitochondrial metabolism in brown adipose tissue by reversible succinylation. Mol Cell. 2019;74(4):844–857.e7. doi: 10.1016/j.molcel.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J., Shangguan Y., Tang D., et al. Histone succinylation and its function on the nucleosome. J Cell Mol Med. 2021;25(15):7101–7109. doi: 10.1111/jcmm.16676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zorro Shahidian L., Haas M., Le Gras S., et al. Succinylation of H3K122 destabilizes nucleosomes and enhances transcription. EMBO Rep. 2021;22(3) doi: 10.15252/embr.202051009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie L., Liu W., Li Q., et al. First succinyl-proteome profiling of extensively drug-resistant Mycobacterium tuberculosis revealed involvement of succinylation in cellular physiology. J Proteome Res. 2015;14(1):107–119. doi: 10.1021/pr500859a. [DOI] [PubMed] [Google Scholar]
- 35.Lacroix M., Riscal R., Arena G., et al. Metabolic functions of the tumor suppressor p53:implications in normal physiology, metabolic disorders, and cancer. Mol Metabol. 2020;33:2–22. doi: 10.1016/j.molmet.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kruiswijk F., Labuschagne C.F., Vousden K.H. p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat Rev Mol Cell Biol. 2015;16(7):393–405. doi: 10.1038/nrm4007. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y., Tavana O., Gu W. p53 modifications: exquisite decorations of the powerful guardian. J Mol Cell Biol. 2019;11(7):564–577. doi: 10.1093/jmcb/mjz060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X., Rong F., Tang J., et al. Repression of p53 function by SIRT5-mediated desuccinylation at Lysine 120 in response to DNA damage. Cell Death Differ. 2022;29(4):722–736. doi: 10.1038/s41418-021-00886-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao X., Bao H., Liu L., et al. Systematic analysis of lysine acetylome and succinylome reveals the correlation between modification of H2A.X complexes and DNA damage response in breast cancer. Oncol Rep. 2020;43(6):1819–1830. doi: 10.3892/or.2020.7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C., Zhang C., Li X., et al. CPT1A-mediated succinylation of S100A10 increases human gastric cancer invasion. J Cell Mol Med. 2019;23(1):293–305. doi: 10.1111/jcmm.13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X., Zhang C., Zhao T., et al. Lysine-222 succinylation reduces lysosomal degradation of lactate dehydrogenase a and is increased in gastric cancer. J Exp Clin Cancer Res. 2020;39(1):172. doi: 10.1186/s13046-020-01681-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo Z., Pan F., Peng L., et al. Systematic proteome and lysine succinylome analysis reveals enhanced cell migration by hyposuccinylation in esophageal squamous cell carcinoma. Mol Cell Proteomics. 2021;20 doi: 10.1074/mcp.RA120.002150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Negrón Abril Y.L., Fernandez I.R., Hong J.Y., et al. Pharmacological and genetic perturbation establish SIRT5 as a promising target in breast cancer. Oncogene. 2021;40(9):1644–1658. doi: 10.1038/s41388-020-01637-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carlson S.M., Gozani O. Nonhistone lysine methylation in the regulation of cancer pathways. Cold Spring Harb Perspect Med. 2016;6(11) doi: 10.1101/cshperspect.a026435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tian X., Zhang S., Liu H.M., et al. Histone lysine-specific methyltransferases and demethylases in carcinogenesis: new targets for cancer therapy and prevention. Curr Cancer Drug Targets. 2013;13(5):558–579. doi: 10.2174/1568009611313050007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGrath J., Trojer P. Targeting histone lysine methylation in cancer. Pharmacol Ther. 2015;150:1–22. doi: 10.1016/j.pharmthera.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Yuan H.F., Zhao M., Zhao L.N., et al. PRMT5 confers lipid metabolism reprogramming, tumour growth and metastasis depending on the SIRT7-mediated desuccinylation of PRMT5 K387 in tumours. Acta Pharmacol Sin. 2022;43(9):2373–2385. doi: 10.1038/s41401-021-00841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu W., Che X., Qu X., et al. Succinylation regulators promote clear cell renal cell carcinoma by immune regulation and RNA N6-methyladenosine methylation. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.622198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marks P.A., Breslow R. Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol. 2007;25(1):84–90. doi: 10.1038/nbt1272. [DOI] [PubMed] [Google Scholar]
- 50.Yang W., Sun Z., Hua C., et al. Chidamide, a histone deacetylase inhibitor-based anticancer drug, effectively reactivates latent HIV-1 provirus. Microb Infect. 2018;20(9–10):626–634. doi: 10.1016/j.micinf.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Shanmugam M.K., Arfuso F., Arumugam S., et al. Role of novel histone modifications in cancer. Oncotarget. 2017;9(13):11414–11426. doi: 10.18632/oncotarget.23356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun Y. E3 ubiquitin ligases as cancer targets and biomarkers. Neoplasia. 2006;8(8):645–654. doi: 10.1593/neo.06376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu J., Qian C., Cao X., et al. Post-translational modification control of innate immunity. Immunity. 2016;45(1):15–30. doi: 10.1016/j.immuni.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 54.Deng L., Meng T., Chen L., et al. The role of ubiquitination in tumorigenesis and targeted drug discovery. Signal Transduct Targeted Ther. 2020;5(1):11. doi: 10.1038/s41392-020-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirschey M.D., Zhao Y. Metabolic regulation by lysine malonylation, succinylation, and glutarylation. Mol Cell Proteomics. 2015;14(9):2308–2315. doi: 10.1074/mcp.R114.046664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peng C., Lu Z., Xie Z., et al. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol Cell Proteomics. 2011;10(12) doi: 10.1074/mcp.M111.012658. M111.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei W., Mao A., Tang B., et al. Large-scale identification of protein crotonylation reveals its role in multiple cellular functions. J Proteome Res. 2017;16(4):1743–1752. doi: 10.1021/acs.jproteome.7b00012. [DOI] [PubMed] [Google Scholar]
- 58.Wan J., Liu H., Ming L. Lysine crotonylation is involved in hepatocellular carcinoma progression. Biomed Pharmacother. 2019;111:976–982. doi: 10.1016/j.biopha.2018.12.148. [DOI] [PubMed] [Google Scholar]
- 59.Xu X., Zhu X., Liu F., et al. The effects of histone crotonylation and bromodomain protein 4 on prostate cancer cell lines. Transl Androl Urol. 2021;10(2):900–914. doi: 10.21037/tau-21-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang S., Yan W. Succinate in the cancer-immune cycle. Cancer Lett. 2017;390:45–47. doi: 10.1016/j.canlet.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 61.Trauelsen M., Hiron T.K., Lin D., et al. Extracellular succinate hyperpolarizes M2 macrophages through SUCNR1/GPR91-mediated gq signaling. Cell Rep. 2021;35(11) doi: 10.1016/j.celrep.2021.109246. [DOI] [PubMed] [Google Scholar]
- 62.Wu J.Y., Huang T.W., Hsieh Y.T., et al. Cancer-derived succinate promotes macrophage polarization and cancer metastasis via succinate receptor. Mol Cell. 2020;77(2):213–227.e5. doi: 10.1016/j.molcel.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 63.Chen X., Sunkel B., Wang M., et al. Succinate dehydrogenase/complex II is critical for metabolic and epigenetic regulation of T cell proliferation and inflammation. Sci Immunol. 2022;7(70) doi: 10.1126/sciimmunol.abm8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Franchina D.G., Kurniawan H., Grusdat M., et al. Glutathione-dependent redox balance characterizes the distinct metabolic properties of follicular and marginal zone B cells. Nat Commun. 2022;13(1):1789. doi: 10.1038/s41467-022-29426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krueger K.E., Srivastava S. Posttranslational protein modifications: current implications for cancer detection, prevention, and therapeutics. Mol Cell Proteomics. 2006;5(10):1799–1810. doi: 10.1074/mcp.R600009-MCP200. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y., Zhang J., Li B., et al. Advances of proteomics in novel PTM discovery: applications in cancer therapy. Small Methods. 2019;3(5) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.