Abstract
Atherosclerosis is one of the leading causes of disease and death worldwide. The identification of new therapeutic targets and agents is critical. JAZF1 is expressed in many tissues and is found at particularly high levels in adipose tissue (AT). JAZF1 suppresses inflammation (including IL-1β, IL-4, IL-6, IL-8, IL-10, TNFα, IFN-γ, IAR-20, COL3A1, laminin, and MCP-1) by reducing NF-κB pathway activation and AT immune cell infiltration. JAZF1 reduces lipid accumulation by regulating the liver X receptor response element (LXRE) of the SREBP-1c promoter, the cAMP-response element (CRE) of HMGCR, and the TR4 axis. LXRE and CRE sites are present in many cytokine and lipid metabolism gene promoters, which suggests that JAZF1 regulates these genes through these sites. NF-κB is the center of the JAZF1-mediated inhibition of the inflammatory response. JAZF1 suppresses NF-κB expression by suppressing TAK1 expression. Interestingly, TAK1 inhibition also decreases lipid accumulation. A dual-targeting strategy of NF-κB and TAK1 could inhibit both inflammation and lipid accumulation. Dual-target compounds (including prodrugs) 1–5 exhibit nanomolar inhibition by targeting NF-κB and TAK1, EGFR, or COX-2. However, the NF-κB suppressing activity of these compounds is relatively low (IC50 > 300 nM). Compounds 6–14 suppress NF-κB expression with IC50 values ranging from 1.8 nM to 38.6 nM. HS-276 is a highly selective, orally bioavailable TAK1 inhibitor. Combined structural modifications of compounds using a prodrug strategy may enhance NF-κB inhibition. This review focused on the role and mechanism of JAZF1 in inflammation and lipid accumulation for the identification of new anti-atherosclerotic targets.
Keywords: Atherosclerosis, CRE, JAZF1, LXRE, NF-κB, TAK1
Introduction
Atherosclerosis is a common risk factor for the occurrence and development of many diseases, such as coronary heart disease (CHD), and a key problem threatening the health and life expectancy of the elderly population.1,2 Atherosclerosis, which is characterized by the formation of fat-laden plaques in large and medium-sized vessels, has been identified as a chronic inflammatory disease of the artery wall. Atherosclerosis occurs during foam cell generation. Oxidative stress and intracellular reactive oxygen species (ROS) production cause vascular aging and induce LDL to oxidize and form ox-LDL. Previous studies from our laboratory and others have demonstrated that the uncontrolled uptake of ox-LDL by lectin-like oxidized low-density-lipoprotein receptor-1 (LOX-1), class A1 scavenger receptor (SR-A1), and cluster of differentiation 36 (CD36), and the impaired cholesterol efflux by ATP-binding cassette transporter A1 (ABCA1) and ABCG1 result in cholesterol accumulation and subsequently trigger macrophages and VSMCs to become foam cells.3, 4, 5, 6, 7 Lipid accumulation in macrophages could induce NLRP3 inflammasome activation. Ox-LDL could activate the NLRP3 inflammasomes (IL-1β and IL-18) in atherosclerosis by binding to the CD36–TLR4–TLR6 signaling complex. Ox-LDL also triggers inflammatory responses, such as interleukin-6 (IL-6), IL-7, IL-1β, and IL-15, to accelerate atherosclerosis development.8 Therefore, controlling lipid metabolism dysfunction and the inflammatory response is an essential measure for preventing atherosclerosis.
Juxtaposed with another zinc finger gene 1 (JAZF1, also named Tip27 and ZNF802) is a cysteine–histidine structured zinc finger protein. This gene encodes a 27-kDa nuclear protein containing 3 putative zinc finger motifs. JAZF1 is the corepressor of orphan nuclear receptor TR4 (also named NR2C2), which is a member of the nuclear orphan receptor family. Recent studies have demonstrated that JAZF1 plays an important role in atherosclerosis progression.9 To our knowledge, JAZF1 is expressed in a variety of tissues in humans and mice and is most highly expressed in adipose tissue (AT).10 However, JAZF1 is a relatively new gene with many unknown functions and mechanisms. The main aims of this review are to describe the role and mechanism of JAZF1 in immune and inflammatory responses and lipid metabolism and to discuss its contributions to atherosclerosis and its potential role in atherosclerosis treatment.
Role of JAZF1 in atherosclerosis
Many studies have shown that JAZF1 is associated with atherosclerosis. JAZF1 is downregulated in atherosclerosis development in prediabetes patients.11 The rs864745 single nucleotide polymorphism (SNP) of JAZF1 is associated with atherosclerosis, which suggests that JAZF1 may be a potential biomarker.12 Indeed, JAZF1 has been patented by several companies and scientists as a biomarker for a variety of diseases, including atherosclerosis (US20190078093A1 and WO2015073531A1), diabetes (EP2215266B1 and US20160138103A1), aging (US20140079836A1), lupus (JP2016095330A), and cancer (US20140357516A1, US20180045727A1, and EP3179393B1). In addition, JAZF1 also suppresses atherosclerosis development. The downregulation of JAZF1 expression leads to dysfunction in lipid metabolism and the inflammatory response.13,14 JAZF1 overexpression by an adenoviral plasmid containing JAZF1 reduces the entire aorta's en face plaque area and the aortic sinus's cross-sectional plaque area in apoE−/− mice by increasing JAZF1 expression in various tissues, including the liver and AT. JAZF1 reduces the blood TC (29%) and LDL-C (30%) levels and the hepatic TC levels (65%) in apoE−/− mice. However, JAZF1 does not change the TG levels in the blood or hepatic tissue in apoE−/− mice, which suggests that JAZF1 mainly regulates cholesterol metabolism.15 JAZF1 overexpression in transgenic JAZF1 mice also reduces the risk of atherosclerosis by increasing JAZF1 expression in various tissues, including the heart, liver, lung, testis, spleen, kidney, and pancreas.16 These transgenic mice show low inflammatory reaction, body weight, and abdominal fat, low levels of plasma parameters (including TG, TC, LDL-C, glucose, FBG, ALT, and AST), and low hepatic lipid accumulation (TG and TC).13,14 However, these mice also exhibit heart failure symptoms (such as high blood pressure, cardiomyocyte apoptosis, absence of ventricular contractions, and mitochondrial defects) and enhanced expression of proapoptotic genes, including caspase-8/9, Bim, Apaf1, and Aifm1, which suggests that JAZF1 overexpression may have many side effects.16
Many studies have shown that aging, hepatic steatosis, obesity, and type 2 diabetes mellitus (T2DM) are risk factors for atherosclerosis.17,18 The expression of JAZF1 is gradually downregulated in aging mice. JAZF1 overexpression steadily decreases the serum TG, TC, and LDL-C levels in aging mice.16 JAZF1 ameliorates aging and hepatic steatosis. The role of JAZF1 in obesity and T2DM has been reviewed.19 Overall, JAZF1 inhibits atherosclerosis development by suppressing the inflammatory response and lipid metabolism dysfunction.
New insights into the mechanism of JAZF1 in suppressing inflammation and lipid accumulation
Systemic immune and inflammatory responses of JAZF1 involving reducing NF-κB expression
The inflammatory response could induce atherosclerosis. JAZF1 reduces the levels of the inflammatory markers, including IL-4, IL-6, IL-10, interferon γ (IFN-γ), tumor necrosis factor-α (TNFα), fibrotic gene collagen type III alpha 1 (COL3A1) and Lamin, in mice.13 JAZF1 decreases the inflammatory state under high-fat diet (HFD) conditions. JAZF1 inhibits the expression of proinflammatory cytokines (such as TNFα, MCP-1, IAR-20, and IL-8) by reducing the NF-κB pathway in vitro and in vivo.14,20,21 Thus, the NF-κB pathway plays key roles in suppressing the inflammatory response mediated by JAZF1.
Immune responses also regulate atherosclerosis development.22 JAZF1 suppresses chronic inflammation by inhibiting mouse peritoneal macrophage differentiation toward the CD11c+ M phenotype. In addition, JAZF1 suppresses chronic inflammation by increasing the number of Tregs and restrictive T cells and the secretion of IL-10 and IL-4. JAZF1 also reduces the number of CD4+ T cells, active T cells, and memory T cells and the secretion of IL-1β, TNF-α, and IL-6 in mice.23, 24, 25 Overall, JAZF1 suppresses atherosclerosis development by regulating immune and inflammatory responses and cytokines (including IL-1β, IL-4, IL-6, IL-8, IL-10, TNFα, IFN-γ, IAR-20, COL3A1, laminin, MCP-1, CD4+ T cells, active T cells, memory T cells, CD11c+ macrophages, and CD206+ macrophages) by reducing the activation of the NF-κB pathway.
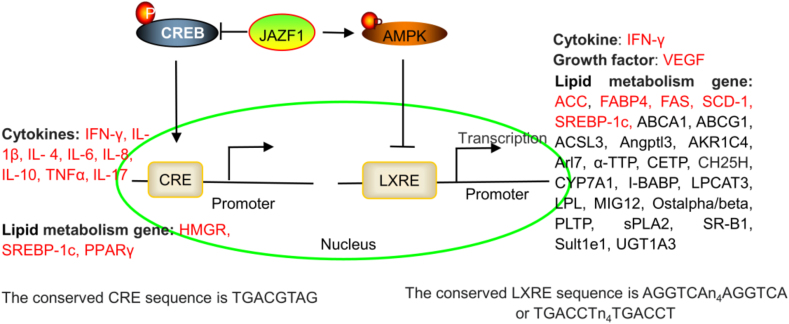
Interestingly, the JAZF1-induced reduction in gene expression depends on the LXR-responsive element (LXRE) and the cAMP-response element (CRE) site in the gene promoter.13 The promoter of IFN-γ contains LXRE and CRE sites,26,27 which suggests that JAZF1 reduces IFN-γ expression by regulating LXRE and CRE in the IFN-γ promoter. The promoters of many cytokines, including IL-1β,28 IL-4,29 IL-6,30 IL-8,31 IL-10,32 TNFα,33 and IL-17,34,35, contain a CRE site, which suggests that JAZF1 reduces the expression of these cytokines by regulating the CRE in the promoters of the genes encoding these cytokines (Fig. 1).
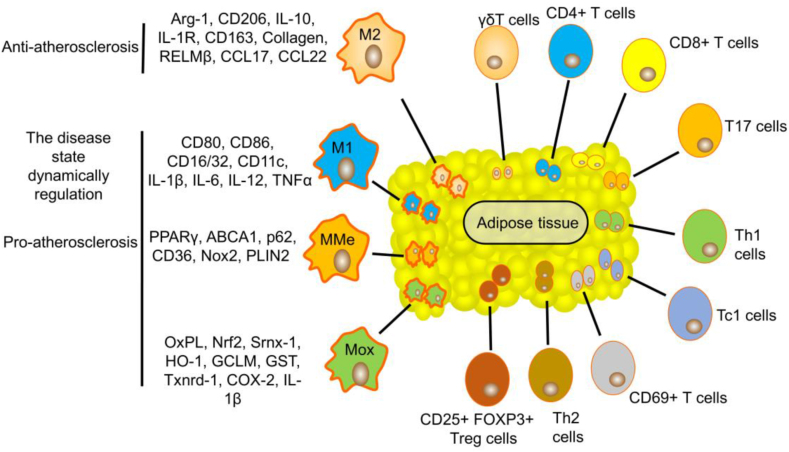
Figure 1.
Role of AT macrophages, T cells, and their markers or cytokine profiles in atherosclerosis.
Immune and inflammatory responses of JAZF1 involving the regulation of at macrophages and T cells
AT macrophages
AT is a connective tissue that is extensively found in the body and contains adipocytes, fibroblasts, vascular endothelial cells, adipocyte stem/progenitor cells, stromal cells, autonomic nerves, numerous immune cells (including mast cells, eosinophils, leukocytes, T cells, B cells, and macrophages), collagen and elastic fibers, nerve bundles, and vasa vasorum.36,37 Macrophages play various roles at all stages of atherosclerosis development by secreting proinflammatory and anti-inflammatory cytokines. The subtypes of AT macrophages include M1 macrophages, M2 macrophages, oxidized macrophages (Mox macrophages), and metabolically activated macrophages (MMe macrophages). M1 macrophages are induced by proinflammatory cytokines and secrete proinflammatory factors, including CD80, CD86, CD16/32, CD11c, IL-1β, IL-6, IL-12, and TNFα, whereas M2 macrophages inhibit inflammation and repair tissue by overexpressing anti-inflammatory and tissue repair factors, including arginase-1 (Arg-1), CD206, IL-10, IL-1 receptor (IL-1R), CD163, resistin-like molecule β (RELMβ), CCL17 and CCL22.38 In general, M2 macrophages tend to transform into M1 macrophages in AT. AT contributes to atherosclerosis development by increasing the number of M1 macrophages and releasing proinflammatory factors. Mox macrophages account for 30% of plaque macrophages, and M1 and M2 subsets comprise 40% and 20% of the remaining population, respectively. The molecules oxidized phospholipid (Ox-PL), NF-E2-related factor 2 (Nrf2), sulfiredoxin-1 (Srnx-1), heme oxygenase-1 (HO-1), glutamate-cysteine ligase modifier subunit (GCLM), glutathione S-transferase (GST), and thioredoxin reductase-1 (Txnrd-1) are markers of the Mox cell surface. Mox cells are a proatherogenic subset based on their production of proinflammatory cytokines (COX-2 and IL-1β). Genes, including peroxisome proliferator-activated receptor γ (PPARγ), ABCA1, p62, CD36, NADPH oxidase-2 (Nox2), and Perilipin 2 (PLIN2), are markers of MMe cells. Under basal conditions, MMe cells in AT could balance the progression of inflammation. CD36-mediated cholesterol uptake is in balance with ABCA1-mediated cholesterol efflux in MMe cells. However, the state of MMe cells in AT is dynamically regulated by disease states, which can promote a proinflammatory response and cholesterol accumulation and potentially lead to atherosclerosis development.39,40 Therefore, Mox and MMe cells are the proatherogenic subsets in AT (Fig. 2).
Figure 2.
JAZF1 regulates many cytokines and lipid metabolism genes by regulating the LXRE and CRE sites of the gene promoter. The JAZF1-mediated regulation of SREBP-1c and HMGCR expression via LXRE and CRE sites has been investigated. JAZF1 also regulates IFN-γ, IL-1β, IL-4, IL-6, IL-8, IL-10, TNFα, IL-17, VEGF, SCD-1, FAS, ACC, and FABP4 expression (red). However, the regulatory mechanisms have not been investigated.
As mentioned previously, JAZF1 is most highly expressed in AT. Importantly, in mouse AT, JAZF1 overexpression decreases the number of total AT macrophages (ATMs) and CD11c+ ATMs and proinflammatory cytokine secretion (including TNFα and IL-1β) and increases the number of CD206+ ATMs.24,25 JAZF1 deficiency increases the CD68-positive macrophage and monocyte numbers in mouse AT.10 CD206 is a marker of anti-inflammatory M2 ATMs, whereas CD11c, TNFα, and IL-1β are markers of proinflammatory M1 ATMs, which suggests that JAZF1 suppresses inflammation and atherosclerosis development by promoting M1 macrophage transformation into M2 macrophages in AT.
AT T cells
AT T cells are also associated with atherosclerosis, including γδ T cell, CD4+ T cell, CD8+ T cell, CD69+ T cell, T cytotoxic 1 (Tc1) cell, Th17 cell, Th1 cell, Th2 cell, and CD25+FOXP3+ Treg cell (Fig. 2).41 CD4+ T cells are involved in the initial stage of AT inflammation during the progression of atherosclerosis. Th1, Tc1, and T17 cells are proatherogenic cells that release pro-inflammatory factors, including IFN-γ, TNFα, and IL-17. Treg and Th2 cells are atheroprotective cells that release IL-10. An HFD promotes lipid accumulation and chronic inflammation in mouse AT by recruiting immune cells (such as CD4+ T cell, CD8+ T cell, CD69+ T cell, and CD44+ T cell) and promoting the production of pro-inflammatory cytokines (such as IFN-γ and IL-17), which leads to atherosclerosis development.42, 43, 44 The Th1:Th2 balance in AT is highly associated with systemic inflammation and atherosclerosis development. Overall, AT T cells are extensively involved in the development of atherosclerosis.
JAZF1 also regulates the AT T-cell numbers in vivo. Specifically, JAZF1 increases the number of total T cells and CD25+FOXP3+ Treg cells but decreases the number of total CD4+ T cells, CD69+ active T cells, and CD44+ memory T cells in the AT of mice. JAZF1 decreases the IFN-γ and IL-17 levels but increases the IL-4 levels in AT CD4+ T cells. Notably, ATMs promote AT CD4+ T-cell activation by releasing MHCII, CD86, and CD40. JAZF1 decreases CD4+ T-cell activation by reducing ATM MHCII, CD86, and CD40 secretion, which suggests that JAZF1 regulates T-cell subtype differentiation by regulating ATM cytokine secretion.24,25 Overall, JAZF1 suppresses AT inflammation and atherosclerosis development by limiting the macrophage and T-cell populations and their antigen presentation functions.
Lipid metabolism of JAZF1 involving binding to LXREs and serving as a corepressor of TR4
Binding to LXREs
Lipid metabolism dysfunction induces atherosclerosis. Many genes, including acetyl–coenzyme A carboxylase (ACC), adipose triglyceride lipase (ATGL), CCAAT/enhancer binding protein α (C/EBPα), fatty acid synthetase (FAS), fatty acid binding protein 4 (FABP4), stearoyl CoA desaturase-1 (SCD-1), sterol regulatory element–binding protein 1c (SREBP-1c) and hormone-sensitive lipase (HSL), play key roles in lipid metabolism. ACC and FAS are master enzymes in lipid synthesis that are linked to the development of atherosclerosis.45,46 ATGL is the rate-limiting enzyme that catalyzes the first step of the breakdown of TGs into glycerol and fatty acids.47 C/EBPα plays an important role in lipogenesis, VLDL secretion, and lipolysis.48 FABP4 is a lipid chaperone that binds fatty acid precursors. FABP4 also promotes macrophage foaming and inflammation by enhancing IL-1β and MMP-9 secretion and CD36 expression, which suggests that FABP4 plays a key role in lipid uptake and the inflammatory response.49,50 SCD-1 and SREBP-1c have been shown to be the principal regulatory transcription factors for lipid synthesis.51,52 HSL is the key rate-limiting enzyme that catalyzes AT lipolysis.53
JAZF1 suppresses lipid accumulation and decreases the droplet size by reducing ACC, FAS, SCD-1, and SREBP-1c expression and increasing HSL and ATGL expression in vitro and in vivo.54, 55, 56 JAZF1 deficiency in JAZF1+/− and JAZF1−/− mice decrease lipid accumulation by decreasing FABP4 and C/EBPα expression.10 Thus, JAZF1 decreases lipid accumulation by regulating ACC, FAS, SCD-1, SREBP-1c, HSL, ATGL, FABP4, and C/EBPα expression.
LXREs are necessary for SREBP-1c promoter activities. Interestingly, JAZF1 inhibits SREBP-1c transcription by increasing AMPK phosphorylation and binding to LXREs in the SREBP-1c promoter, which suggests that LXREs play a key role in JAZF1-mediated gene expression.13 JAZF1 also regulates SCD-1, FAS, ACC, and FABP4 expression.10,19,57 However, the mechanism is unclear. Interestingly, the promoters of ACC,58 FAS,59,60 FABP4,61 and SCD-162 also contain LXREs. Other lipid metabolism genes also contain LXREs, including ABCA1,63,64 ABCG1,65,66 long-chain acyl-CoA synthetases 3 (ACSL3),67 angiopoietin-like protein 3 (Angptl3),68 3alpha-hydroxysteroid dehydrogenase (AKR1C4),69 ADP ribosylation factor like GTPase 4C (Arl4c, also named Arl7),70 α-tocopherol transfer protein (α-TTP),71 cholesteryl ester transfer protein (CETP),72 cholesterol 25-hydroxylase (CH25H),73 cholesterol 7-alpha-hydroxylase (CYP7A1),74 ileal bile acid-binding protein (I-BABP),75 lysophosphatidylcholine acyltransferase 3 (LPCAT3),76 lipoprotein lipase (LPL),77 midline-1-interacting G12-like protein (MIG12),78 organic solute transporter alpha/beta (Ostalpha/beta),79 phospholipid transfer protein (PLTP),80 group IIA secretory phospholipase A2 (sPLA2),81 SR-BI,82 sulfotransferase family 1E (Sult1e1),83 and UDP-glucuronosyltransferase 1A3 (UGT1A3).84 Because JAZF1 regulates SREBP-1c expression via LXREs, we hypothesized that JAZF1 could regulate lipid metabolism by binding to the LXREs of these genes (Fig. 1). However, further studies are needed.
Serving as a corepressor of TR4
JAZF1 is a corepressor of TR4, which inhibits PPARα/β/δ expression by competitively binding to PPREs. PPARα/β/δ promote the transcription and activation of visfatin by binding to the PPRE of the visfatin promoter region.85 JAZF1 promotes visfatin promoter activity by upregulating PPARα/β/δ expression in adipocytes. Interestingly, visfatin promotes TG accumulation. However, JAZF1 inhibits lipid accumulation in adipocytes. Indeed, JAZF1 also inhibits PPARγ expression, which is mainly expressed in AT and is an essential regulatory factor associated with lipid accumulation.86,87 This finding suggests that PPARγ plays a key role in JAZF1-mediated reductions in lipid accumulation. However, JAZF1 deficiency decreases PPARγ expression in JAZF1+/− and JAZF1−/− mice, which suggests that JAZF1 may increase PPARγ expression.10 The effect of JAZF1 on PPARγ may be cell-specific, and more studies are needed to confirm the effect of JAZF1 on PPARγ.
HMGCR is the key rate-limiting enzyme of cholesterol synthesis.86 JAZF1 reduces the serum cholesterol levels and hepatic cholesterol synthesis by inhibiting HMGCR expression.15 The HMGCR promoter has a CRE site (TGACGTAG), which is necessary for CREB activities. JAZF1 suppresses CREB activation by decreasing the phosphorylation of CREB at the Ser133 site. Furthermore, JAZF1 decreases HMGCR expression by suppressing the phosphorylation of CREB.15 TR4 promotes the phosphorylation of CREB. TR4 also directly promotes lipid accumulation by activating the Fatty acid transport protein 1 (FATP1) and CD36 promoters, which suggests that FATP1 and CD36 are target genes of TR4.88,89 As mentioned previously, PPARs are also target genes of TR4. JAZF1 is a corepressor of TR4, which suggests that JAZF1 regulates lipid accumulation by regulating the TR4-PPAR, TR4-CREB-HMGCR, TR4-FATP1, and TR4-CD36 axes. In addition, the promoters of SREBP-1c90 and PPARγ91 also contain a CRE site, which suggests that JAZF1 regulates the expression of these genes by regulating the TR4-CREB pathway. Overall, JAZF1 suppresses lipid accumulation by regulating the expression of ACC, ATGL, C/EBPα, FAS, FABP4, HMGCR, HSL, PPARα/β/δ/γ, SCD-1, SREBP-1c, and visfatin, and this regulation mainly depends on LXREs and TR4.
Development of a dual-target compound targeting NF-κB and TAK1 (downstream of JAZF1)
The antiatherosclerotic therapeutic strategies targeting NF-κB were reviewed in 2012.92 However, the activity of these drugs (such as aspirin and tepoxalin) in suppressing NF-κB is low. As mentioned previously, NF-κB is the center of the JAZF1-mediated inhibition of the inflammatory response. Transforming growth factor β (TGF-β)-activated kinase 1 (TAK1, also named MAP3K7) is a pivotal kinase upstream of NF-κB. JAZF1 suppresses NF-κB expression by inhibiting TAK1 expression.93 Interestingly, TAK1 inhibition also decreases lipid accumulation.94 A positive feedback regulation exists between proinflammatory NF-κB signaling and lipid accumulation.95 The design strategy of NF-κB and JAZF1 or TAK1 dual-target compounds could inhibit both inflammation and lipid accumulation. There are no dual-target JAZF1 and NF-κB compounds because JAZF1 is a relatively new target, but dual-target TAK1 and NF-κB compounds exist. Indeed, many dual compounds target NF-κB (Table 1). For example, the dual-target compounds 1–5 exhibit nanomolar inhibition by targeting NF-κB and TAK1, EGFR, or COX-2.96, 97, 98 However, the activity of these compounds in suppressing NF-κB is relatively low (IC50 > 300 nM). Interestingly, compounds 6–14 suppress NF-κB expression with IC50 values ranging from 1.8 nM to 38.6 nM.99 Compounds 3–4 are prodrugs that target NF-κB and COX-2. Combined structural modification using a prodrug strategy may enhance the inhibition of NF-κB. However, many studies are needed. In addition, many TAK1 inhibitors have been developed, but their clinical advancement has been limited by a lack of oral bioavailability. HS-276 is a highly selective orally bioavailable TAK1 inhibitor (IC50 = 2.5 nM). HS-276 suppresses the levels of proinflammatory cytokines, such as TNFα, IL-6, and IL-1β.100 However, the role of HS-276 in NF-κB has not been investigated.
Table 1.
Structure and activities of compounds targeting NF-κB.
| Number | Structure | Activity | Reference |
|---|---|---|---|
| Compound 1 |  |
NF-κB IC50: 840 nM; TAK1 IC50: 580 nM | 96 |
| Compound 2 | 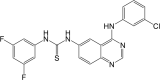 |
NF-κB IC50: 300 nM; EGFR IC50: 60.1 nM | 97 |
| Compound 3 |  |
NF-κB IC50: 600 nM; EGFR IC50: 137 nM | 97 |
| Compound 4 (Prodrug) | 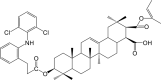 |
NF-κB IC50: 620 nM; COX-2 IC50: 680 nM | 98 |
| Compound 5 (Prodrug) | 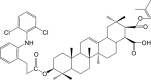 |
NF-κB IC50: 890 nM; COX-2 IC50: 840 nM | 98 |
| Compound 6 |  |
NF-κB IC50: 4.9 nM | 99 |
| Compound 7 | 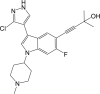 |
NF-κB IC50: 21 nM | 99 |
| Compound 8 | 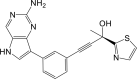 |
NF-κB IC50: 9.1 nM | 99 |
| Compound 9 |  |
NF-κB IC50: 9.87 nM | 99 |
| Compound 10 |  |
NF-κB IC50: 1.8 nM | 99 |
| Compound 11 |  |
NF-κB IC50: 38.6 nM | 99 |
| Compound 12 | 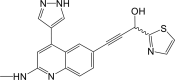 |
NF-κB IC50: 26 nM | 99 |
| Compound 13 | 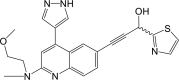 |
NF-κB IC50: 5.2 nM | 99 |
| Compound 14 |  |
NF-κB IC50: 22.1 nM | 99 |
| HS-276 |  |
TAK1 IC50: 2.5 nM | 100 |
Future directions and challenges
Atherosclerosis is the presumed cause of 40% of all deaths and the leading cause of death in elderly populations. Patients who are actively being treated with statins still have a residual cardiovascular risk even when LDL-C is controlled or decreases below 70 mg/dL.101 Therefore, the identification of new therapeutic targets and the development of new antiatherosclerotic agents are critical. JAZF1 reduces atherosclerosis development by reducing immune and inflammatory responses and lipid metabolism dysfunction (Fig. 3). Thus, JAZF1 may be a new target for the prevention and treatment of atherosclerosis. However, there remain many problems to be investigated. (i) JAZF1 is most highly expressed in AT. JAZF1 also suppresses atherosclerosis development by reducing AT inflammation via limiting the macrophage and T-cell populations and their antigen presentation functions. However, strategies for targeting JAZF1 expression in AT need to be investigated. (ii) LXRE and CRE sites are present in many cytokine and lipid metabolism gene promoters (Table 2). JAZF1 may regulate the expression of these genes by regulating LXRE and CRE sites. However, only SREBP-1c and HMGCR have been investigated. (iii) JAZF1 is a corepressor of TR4. The TR4-FATP1 and TR4-CD36 axes reduce lipid accumulation, but the role of JAZF1 in these axes has not been investigated. (iv) JAZF1 SNPs are associated with T2DM and insulin resistance (IR)-related diseases in humans.102 The rs864745 SNP of JAZF1 is associated with atherosclerosis. However, the role of other JAZF1 SNPs in atherosclerosis has not been investigated. (v) The noncoding RNA circPTK2 can promote lipolysis and decrease adipogenesis by regulating the miR-182-5p-JAZF1 axis and may be used as a diagnostic marker of cachexia.103 However, the role and mechanism of circPTK2 in atherosclerosis have not been investigated. (vi) miR-19b-3p is transferred by macrophage-derived extracellular vesicles (M-EVs) into VSMCs and then promotes VSMC migration and proliferation to induce atherosclerosis development by targeting JAZF1, which suggests that JAZF1 is expressed in M-EVs and is a target gene of miR-19b-3p.104 However, the roles of JAZF1 in M-EVs in AT, lipid metabolism, and immune and inflammatory responses have not been investigated. (vii) JAZF1-AS1, which is a long noncoding RNA, is located upstream of JAZF1 in overlapping head-to-head gene pairs. JAZF1-AS1 promotes JAZF1 expression by forming double-stranded RNAs. However, JAZF1 does not affect JAZF1-AS1.105 The role and mechanism of JAZF1-AS1 in atherosclerosis have not been investigated. (viii) JAZF1-overexpressing transgenic mice exhibit reduced atherosclerosis risk factors and atherosclerosis development. However, these mice also show heart failure symptoms. The safety of JAZF1 overexpression in vivo requires extensive evaluation. (viiii) JAZF1 exerts antiatherosclerotic effects in vitro and in animal studies. However, human studies are relatively lacking, and an evaluation of the effects on humans is needed. We thus hope that more scientists will focus on the potential roles of JAZF1 in AT and atherosclerosis and identify new therapeutic targets for this disease.
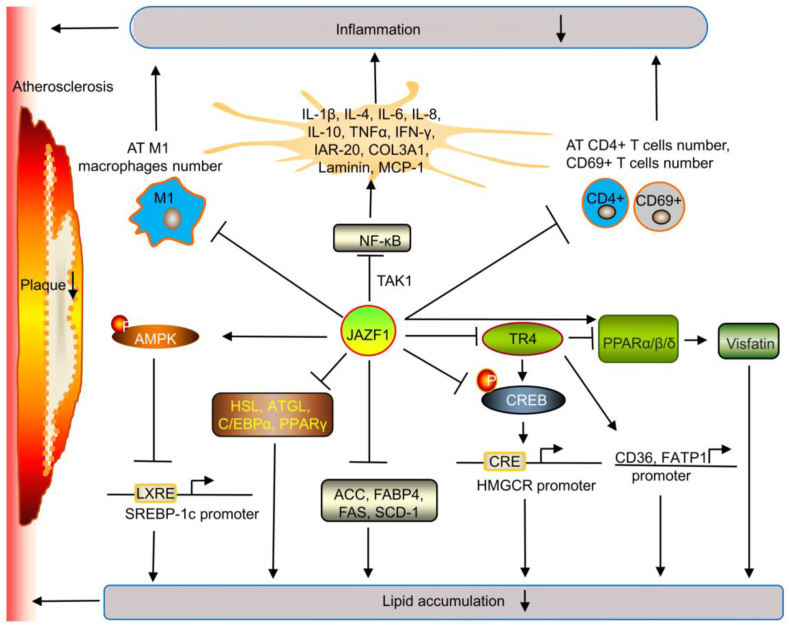
Figure 3.
Potential mechanism of JAZF1 in atherosclerosis. JAZF1 suppresses inflammation by reducing the NF-κB-mediated secretion of cytokines (including IL-1β, IL-4, IL-6, IL-8, IL-10, TNFα, IFN-γ, IAR-20, COL3A1, laminin, and MCP-1) via the JNK, MAPK, and TAK1 pathways and reducing the AT cell numbers (including M1 macrophages, CD4+, and CD69+). JAZF1 suppresses lipid accumulation by suppressing SREBP-1c transcription through increasing AMPK phosphorylation and binding to LXREs in the SREBP-1c promoter, suppressing HMGCR transcription by decreasing CREB phosphorylation and binding to CRE in the HMGCR promoter, and suppressing ACC, FAS, SCD-1, HSL, ATGL, FABP4, and C/EBPα expression. JAZF1 may also suppress the TR4-FATP1 and TR4-CD36 axes to reduce lipid accumulation. JAZF1 promotes visfatin expression and accumulation by regulating the TR4-PPARα/β/δ axis. However, this effectiveness is reduced. JAZF1 generally reduces lipid accumulation.
Table 2.
Main physiological functions of genes containing LXRE and CRE sites.
| Gene | Function | References |
|---|---|---|
| ABCA1 | Cholesterol efflux | 63,64 |
| ABCG1 | Cholesterol efflux | 65,66 |
| ACC | Fatty acid synthesis | 58 |
| ACSL3 | Fatty acid metabolism | 67 |
| Angptl3 | Triglyceride metabolism | 68 |
| AKR1C4 | Bile acid biosynthesis, steroid and hormone metabolism | 69 |
| Arl7 | Cholesterol efflux | 70 |
| α-TTP | Vitamin E regulatory protein | 71 |
| CETP | Cholesterol metabolism and transportation | 72 |
| CH25H | 25-Hydroxycholesterol synthesis | 73 |
| CYP7A1 | Conversion of cholesterol to bile acids | 74 |
| FABP4 | Fatty acid metabolism and adipocyte differentiation | 75 |
| FAS | Fatty acid synthesis | 59,60 |
| I-BABP | Enterohepatic circulation of bile acids | 75 |
| IFN-γ | Immune and inflammatory responses | 26,27 |
| IL-1β | Immune and inflammatory responses | 28 |
| IL-4 | Immune and inflammatory responses | 29 |
| IL-6 | Immune and inflammatory responses | 30 |
| IL-8 | Immune and inflammatory responses | 31 |
| IL-10 | Immune and inflammatory responses | 32 |
| IL-17 | Immune and inflammatory responses | 34,35 |
| LPCAT3 | Phospholipid metabolism | 76 |
| LPL | Hydrolysis of circulating triglyceride-rich lipoproteins | 77 |
| MIG12 | Fatty acid synthesis and TG accumulation | 78 |
| Ostalpha/beta | Bile acid absorption | 79 |
| PLTP | Phospholipid metabolism | 80 |
| PPARγ | Lipid metabolism | 91 |
| SCD-1 | Monounsaturated fatty acid synthesis | 62 |
| sPLA2 | Phospholipid metabolism | 81 |
| SR-BI | Cholesterol uptake and efflux | 82 |
| SREBP-1c | Lipid synthesis | 13,90 |
| Sult1e1 | Sulfation and inactivation of estrogen | 83 |
| TNFα | Immune and inflammatory responses | 33 |
| UGT1A3 | Bile acid glucuronidation | 84 |
| VEGF | Angiogenesis | 106 |
Author contributions
WC and YZ participated in writing-original draft, supervision, and resources. YY, MZ, and WH participated in formal analysis, and investigation. NL and DX participated in conceptualization, writing-review & editing, project administration, and funding acquisition. All authors read and approved the final version of the manuscript.
Conflict of interests
The authors declare that they have no competing interests.
Funding
The authors are grateful for the financial support provided by the Qingdao Major Scientific and Technological Project for Distinguished Scholars (China) (No. 20170103), the Laoshan Major Scientific and Technological Project for Distinguished Scholars (China) (No. 20181030), and the Natural Science Foundation of Shandong Province, China (No. ZR2020MH369).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Ning Liu, Email: jinzhancaoliuning@126.com.
Dongming Xing, Email: xdm_tsinghua@163.com.
Abbreviations
- ABCA1
ATP-binding cassette transporter A1
- ACC
acetyl–coenzyme A carboxylase
- ACSL3
long-chain acyl-CoA synthetases 3
- AKR1C4
3alpha-hydroxysteroid dehydrogenase
- Angptl3
angiopoietin-like protein 3
- Arl4c
ADP ribosylation factor-like GTPase 4C
- aP2
adipocyte fatty acid-binding protein
- Arg-1
arginase-1
- AT
adipose tissue
- ATGL
adipose triglyceride lipase
- ATM
adipose tissue macrophage
- CD206
mannose receptor
- CETP
cholesteryl ester transfer protein
- CHD
coronary heart disease
- CH25H
cholesterol 25-hydroxylase
- COL3A1
fibrotic gene collagen type III alpha 1
- CRE
cAMP responsive element
- CYP7A1
cholesterol 7-alpha-hydroxylase
- C/EBPα
CCAAT/enhancer-binding protein α
- FABP4
fatty acid-binding protein 4
- FAs
fatty acids
- FAS
fatty acid synthetase
- FATP1
fatty acid transport protein 1
- GCLM
glutamate-cysteine ligase modifier subunit
- GST
glutathione S-transferase
- HFD
high-fat diet
- HO-1
heme oxygenase-1
- HSL
hormone-sensitive lipase
- IFN-γ
interferon γ
- IL-1R
IL-1 receptor
- IL-6
interleukin-6
- I-BABP
ileal bile acid-binding protein
- JAZF1
juxtapose with another zinc finger gene 1
- LPCAT3
lysophosphatidylcholine acyltransferase 3
- LPL
lipoprotein lipase
- LXREs
liver X receptor response elements
- MCP-1
monocyte chemotactic protein 1
- MIG12
midline-1-interacting G12-like protein
- MMe
metabolically activated macrophage
- Mox
oxidized macrophage
- Nox2
NADPH oxidase-2
- Nrf2
NF-E2-related factor 2
- NR2C2
nuclear receptor subfamily 2, group C, member 2
- Ostalpha/beta
organic solute transporter alpha/beta
- Ox-PL
oxidized phospholipid
- PLIN2
perilipin 2
- PLTP
phospholipid transfer protein
- RELMβ
collagen, resistin-like molecule β
- ROS
reactive oxygen species
- SCD-1
stearoyl CoA desaturase-1
- SNP
single nucleotide polymorphism
- sPLA2
group IIA secretory phospholipase A2
- SREBP-1c
sterol regulatory element-binding protein 1c
- Srnx-1
sulfiredoxin-1
- Sult1e1
sulfotransferase family 1E
- TAK1
transforming growth factor β-activated kinase 1
- Tc1
T cytotoxic 1
- TNF-α
tumor necrosis factor-α
- TR4
testicular orphan nuclear receptor-4
- Txnrd-1
thioredoxin reductase-1
- T2DM
type 2 diabetes mellitus
- UGT1A3
UDP-glucuronosyltransferase 1A3
- VEGF
vascular endothelial growth factor
- α-TTP
α-tocopherol transfer protein
References
- 1.Yang Z., Yu G.L., Zhu X., et al. Critical roles of FTO-mediated mRNA m6A demethylation in regulating adipogenesis and lipid metabolism: implications in lipid metabolic disorders. Genes Dis. 2022;9(1):51–61. doi: 10.1016/j.gendis.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang Q., Li Y., Wu Q., et al. Pathogenic role of microRNAs in atherosclerotic ischemic stroke: implications for diagnosis and therapy. Genes Dis. 2022;9(3):682–696. doi: 10.1016/j.gendis.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W., Li L., Wang J., et al. The ABCA1-efferocytosis axis: a new strategy to protect against atherosclerosis. Clin Chim Acta. 2021;518:1–8. doi: 10.1016/j.cca.2021.02.025. [DOI] [PubMed] [Google Scholar]
- 4.Chen W., Wang S., Xing D. New horizons for the roles and association of APE1/Ref-1 and ABCA1 in atherosclerosis. J Inflamm Res. 2021;14:5251–5271. doi: 10.2147/JIR.S330147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W., Xing J., Liu X., et al. The role and transformative potential of IL-19 in atherosclerosis. Cytokine Growth Factor Rev. 2021;62:70–82. doi: 10.1016/j.cytogfr.2021.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Chen W., Zhong Y., Feng N., et al. New horizons in the roles and associations of COX-2 and novel natural inhibitors in cardiovascular diseases. Mol Med. 2021;27(1):123. doi: 10.1186/s10020-021-00358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang S., Li L., Wang J., et al. Recent advances in the regulation of ABCA1 and ABCG1 by lncRNAs. Clin Chim Acta. 2021;516:100–110. doi: 10.1016/j.cca.2021.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Bäck M., Yurdagul A., Jr., Tabas I., et al. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol. 2019;16(7):389–406. doi: 10.1038/s41569-019-0169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu Y., Wu J., Zeng G. Current research progress of JAZF1 in atherosclerosis. J Mil Surgeon Southwest China. 2021;23(2):131–133. [Google Scholar]
- 10.Jeong J., Jang S., Park S., et al. JAZF1 heterozygous knockout mice show altered adipose development and metabolism. Cell Biosci. 2021;11(1):161. doi: 10.1186/s13578-021-00625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang Y., Wang M., Wang C., et al. The mechanisms of the development of atherosclerosis in prediabetes. Int J Mol Sci. 2021;22(8):4108. doi: 10.3390/ijms22084108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou S.H., Shulman J.M., Keenan B.T., et al. Genetic susceptibility for ischemic infarction and arteriolosclerosis based on neuropathologic evaluations. Cerebrovasc Dis. 2013;36(3):181–188. doi: 10.1159/000352054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei Q., Zhou B., Yang G., et al. JAZF1 ameliorates age and diet-associated hepatic steatosis through SREBP-1c-dependent mechanism. Cell Death Dis. 2018;9(9):859. doi: 10.1038/s41419-018-0923-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang M., Dai J., Jia Y., et al. Overexpression of juxtaposed with another zinc finger gene 1 reduces proinflammatory cytokine release via inhibition of stress-activated protein kinases and nuclear factor-κB. FEBS J. 2014;281(14):3193–3205. doi: 10.1111/febs.12853. [DOI] [PubMed] [Google Scholar]
- 15.Li X., Yang M., Wang H., et al. Overexpression of JAZF1 protected ApoE-deficient mice from atherosclerosis by inhibiting hepatic cholesterol synthesis via CREB-dependent mechanisms. Int J Cardiol. 2014;177(1):100–110. doi: 10.1016/j.ijcard.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Bae K.B., Kim M.O., Yu D.H., et al. Overexpression of Jazf1 induces cardiac malformation through the upregulation of pro-apoptotic genes in mice. Transgenic Res. 2011;20(5):1019–1031. doi: 10.1007/s11248-010-9476-4. [DOI] [PubMed] [Google Scholar]
- 17.Dai W., Liu X., Su H., et al. Influence of adipose tissue immune dysfunction on childhood obesity. Cytokine Growth Factor Rev. 2022;65:27–38. doi: 10.1016/j.cytogfr.2022.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Shabir K., Brown J.E., Afzal I., et al. Asprosin, a novel pleiotropic adipokine implicated in fasting and obesity-related cardio-metabolic disease: comprehensive review of preclinical and clinical evidence. Cytokine Growth Factor Rev. 2021;60:120–132. doi: 10.1016/j.cytogfr.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Liao Z.Z., Wang Y.D., Qi X.Y., et al. JAZF1, a relevant metabolic regulator in type 2 diabetes. Diabetes Metab Res Rev. 2019;35(5):e3148. doi: 10.1002/dmrr.3148. [DOI] [PubMed] [Google Scholar]
- 20.Liu R., Lin Z., Jia Y., et al. Effects of JAZF1 overexpression on proinflammatory cytokines in hepatocytes induced by palmitic acid. Chin J Hepatol. 2015;23(12):950–954. doi: 10.3760/cma.j.issn.1007-3418.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Hu W.J., Shen W.W., Li X.J., et al. Effect of JAZF1 over-expression on high-fat diet-induced non-alcoholic fatty liver disease. Chin J Hepatol. 2016;24(8):596–600. doi: 10.3760/cma.j.issn.1007-3418.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Martynova E., Rizvanov A., Urbanowicz R.A., et al. Inflammasome contribution to the activation of Th1, Th2, and Th17 immune responses. Front Microbiol. 2022;13 doi: 10.3389/fmicb.2022.851835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thalayasingam N., Nair N., Skelton A.J., et al. CD4+ and B lymphocyte expression quantitative traits at rheumatoid arthritis risk loci in patients with untreated early arthritis: implications for causal gene identification. Arthritis Rheumatol. 2018;70(3):361–370. doi: 10.1002/art.40393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng F., Lin Y., Yang M., et al. JAZF1 inhibits adipose tissue macrophages and adipose tissue inflammation in diet-induced diabetic mice. BioMed Res Int. 2018;2018 doi: 10.1155/2018/4507659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng F., Hao P., Du H., et al. Effects of adenovirus-mediated overexpression of JAZF1 on chronic inflammation: an in vitro and in vivo study. Med Sci Monit Basic Res. 2020;26 doi: 10.12659/MSMBR.924124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma X., Wang Q., Liu Y., et al. Inhibition of tumor growth by U0126 is associated with induction of interferon-γ production. Int J Cancer. 2015;136(4):771–783. doi: 10.1002/ijc.29038. [DOI] [PubMed] [Google Scholar]
- 27.Samten B., Townsend J.C., Weis S.E., et al. CREB, ATF, and AP-1 transcription factors regulate IFN-gamma secretion by human T cells in response to mycobacterial antigen. J Immunol. 2008;181(3):2056–2064. doi: 10.4049/jimmunol.181.3.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu C.H., Yeh C.T., Shih P.H., et al. Dietary phenolic acids attenuate multiple stages of protein glycation and high-glucose-stimulated proinflammatory IL-1beta activation by interfering with chromatin remodeling and transcription in monocytes. Mol Nutr Food Res. 2010;54(Suppl 2):S127–S140. doi: 10.1002/mnfr.200900395. [DOI] [PubMed] [Google Scholar]
- 29.Sisk T.J., Gourley T., Roys S., et al. MHC class II transactivator inhibits IL-4 gene transcription by competing with NF-AT to bind the coactivator CREB binding protein (CBP)/p300. J Immunol. 2000;165(5):2511–2517. doi: 10.4049/jimmunol.165.5.2511. [DOI] [PubMed] [Google Scholar]
- 30.Lee G.L., Chang Y.W., Wu J.Y., et al. TLR 2 induces vascular smooth muscle cell migration through cAMP response element-binding protein-mediated interleukin-6 production. Arterioscler Thromb Vasc Biol. 2012;32(11):2751–2760. doi: 10.1161/ATVBAHA.112.300302. [DOI] [PubMed] [Google Scholar]
- 31.Iourgenko V., Zhang W., Mickanin C., et al. Identification of a family of cAMP response element-binding protein coactivators by genome-scale functional analysis in mammalian cells. Proc Natl Acad Sci U S A. 2003;100(21):12147–12152. doi: 10.1073/pnas.1932773100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ernst O., Glucksam-Galnoy Y., Bhatta B., et al. Exclusive temporal stimulation of IL-10 expression in LPS-stimulated mouse macrophages by cAMP inducers and type I interferons. Front Immunol. 2019;10:1788. doi: 10.3389/fimmu.2019.01788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brinkman B.M., Telliez J.B., Schievella A.R., et al. Engagement of tumor necrosis factor (TNF) receptor 1 leads to ATF-2- and p38 mitogen-activated protein kinase-dependent TNF-alpha gene expression. J Biol Chem. 1999;274(43):30882–30886. doi: 10.1074/jbc.274.43.30882. [DOI] [PubMed] [Google Scholar]
- 34.Tsai H.C., Velichko S., Lee S., et al. Cholera toxin enhances interleukin-17A production in both CD4+ and CD8+ cells via a cAMP/protein kinase A-mediated interleukin-17A promoter activation. Immunology. 2018;154(3):500–509. doi: 10.1111/imm.12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar P., Natarajan K., Shanmugam N. High glucose driven expression of pro-inflammatory cytokine and chemokine genes in lymphocytes: molecular mechanisms of IL-17 family gene expression. Cell Signal. 2014;26(3):528–539. doi: 10.1016/j.cellsig.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 36.Nakladal D., Sijbesma J.W.A., Visser L.M., et al. Perivascular adipose tissue-derived nitric oxide compensates endothelial dysfunction in aged pre-atherosclerotic apolipoprotein E-deficient rats. Vasc Pharmacol. 2022;142 doi: 10.1016/j.vph.2021.106945. [DOI] [PubMed] [Google Scholar]
- 37.Poledne R., Králová Lesná I. Adipose tissue macrophages and atherogenesis - a synergy with cholesterolaemia. Physiol Res. 2021;70(Suppl4):S535–S549. doi: 10.33549/physiolres.934745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai C.F., Chen G.W., Chen Y.C., et al. Regulatory effects of quercetin on M1/M2 macrophage polarization and oxidative/antioxidative balance. Nutrients. 2021;14(1):67. doi: 10.3390/nu14010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kauerova S., Bartuskova H., Muffova B., et al. Statins directly influence the polarization of adipose tissue macrophages: a role in chronic inflammation. Biomedicines. 2021;9(2):211. doi: 10.3390/biomedicines9020211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ivanov S., Merlin J., Lee M.K.S., et al. Biology and function of adipose tissue macrophages, dendritic cells and B cells. Atherosclerosis. 2018;271:102–110. doi: 10.1016/j.atherosclerosis.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 41.Wang L., Sun P., Wu Y., et al. Metabolic tissue-resident CD8+ T cells: a key player in obesity-related diseases. Obes Rev. 2021;22(3) doi: 10.1111/obr.13133. [DOI] [PubMed] [Google Scholar]
- 42.Peng X., Feng C., Wang X., et al. Chemical composition and antioxidant activity of essential oils from barks of Pinus pumila using microwave-assisted hydrodistillation after screw extrusion treatment. Ind Crop Prod. 2021;166 [Google Scholar]
- 43.Harford K.A., Reynolds C.M., McGillicuddy F.C., et al. Fats, inflammation and insulin resistance: insights to the role of macrophage and T-cell accumulation in adipose tissue. Proc Nutr Soc. 2011;70(4):408–417. doi: 10.1017/S0029665111000565. [DOI] [PubMed] [Google Scholar]
- 44.Wang Q., Wang Y., Xu D. The roles of T cells in obese adipose tissue inflammation. Adipocyte. 2021;10(1):435–445. doi: 10.1080/21623945.2021.1965314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alkhouri N., Lawitz E., Noureddin M., et al. GS-0976 (Firsocostat):an investigational liver-directed acetyl-CoA carboxylase (ACC) inhibitor for the treatment of non-alcoholic steatohepatitis (NASH) Expet Opin Invest Drugs. 2020;29(2):135–141. doi: 10.1080/13543784.2020.1668374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ó Monroig, Shu-Chien A.C., Kabeya N., et al. Desaturases and elongases involved in long-chain polyunsaturated fatty acid biosynthesis in aquatic animals: from genes to functions. Prog Lipid Res. 2022;86 doi: 10.1016/j.plipres.2022.101157. [DOI] [PubMed] [Google Scholar]
- 47.Schreiber R., Xie H., Schweiger M. Of mice and men: the physiological role of adipose triglyceride lipase (ATGL) Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(6):880–899. doi: 10.1016/j.bbalip.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jadhav K.S., Bauer R.C. Trouble with tribbles-1. Arterioscler Thromb Vasc Biol. 2019;39(6):998–1005. doi: 10.1161/ATVBAHA.118.311573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haider M., Al-Rashed F., Albaqsumi Z., et al. Candida albicans induces foaming and inflammation in macrophages through FABP4:its implication for atherosclerosis. Biomedicines. 2021;9(11):1567. doi: 10.3390/biomedicines9111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu L., Zhang H., Wang Y., et al. FABP4 activates the JAK2/STAT2 pathway via Rap1a in the homocysteine-induced macrophage inflammatory response in ApoE-/- mice atherosclerosis. Lab Invest. 2022;102(1):25–37. doi: 10.1038/s41374-021-00679-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferré P., Foufelle F. SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res. 2007;68(2):72–82. doi: 10.1159/000100426. [DOI] [PubMed] [Google Scholar]
- 52.Cohen P., Friedman J.M. Leptin and the control of metabolism: role for stearoyl-CoA desaturase-1 (SCD-1) J Nutr. 2004;134(9):2455S–2463S. doi: 10.1093/jn/134.9.2455S. [DOI] [PubMed] [Google Scholar]
- 53.Lampidonis A.D., Rogdakis E., Voutsinas G.E., et al. The resurgence of Hormone-Sensitive Lipase (HSL) in mammalian lipolysis. Gene. 2011;477(1–2):1–11. doi: 10.1016/j.gene.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 54.Ming G.F., Xiao D., Gong W.J., et al. JAZF1 can regulate the expression of lipid metabolic genes and inhibit lipid accumulation in adipocytes. Biochem Biophys Res Commun. 2014;445(3):673–680. doi: 10.1016/j.bbrc.2014.02.088. [DOI] [PubMed] [Google Scholar]
- 55.Li L., Yang Y., Yang G., et al. The role of JAZF1 on lipid metabolism and related genes in vitro. Metabolism. 2011;60(4):523–530. doi: 10.1016/j.metabol.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 56.Jang W.Y., Bae K.B., Kim S.H., et al. Overexpression of Jazf1 reduces body weight gain and regulates lipid metabolism in high fat diet. Biochem Biophys Res Commun. 2014;444(3):296–301. doi: 10.1016/j.bbrc.2013.12.094. [DOI] [PubMed] [Google Scholar]
- 57.Shang J., Gao Z.Y., Zhang L.Y., et al. Over-expression of JAZF1 promotes cardiac microvascular endothelial cell proliferation and angiogenesis via activation of the Akt signaling pathway in rats with myocardial ischemia-reperfusion. Cell Cycle. 2019;18(14):1619–1634. doi: 10.1080/15384101.2019.1629774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Talukdar S., Hillgartner F.B. The mechanism mediating the activation of acetyl-coenzyme A carboxylase-alpha gene transcription by the liver X receptor agonist T0-901317. J Lipid Res. 2006;47(11):2451–2461. doi: 10.1194/jlr.M600276-JLR200. [DOI] [PubMed] [Google Scholar]
- 59.Serviddio G., Bellanti F., Vendemiale G., et al. Oxysterols in the orchestra of liver cell metabolism. Free Radic Biol Med. 2014;75(Suppl 1):S6. doi: 10.1016/j.freeradbiomed.2014.10.838. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y., Fan X., Qiu L., et al. Liver X receptor α promotes milk fat synthesis in buffalo mammary epithelial cells by regulating the expression of FASN. J Dairy Sci. 2021;104(12):12980–12993. doi: 10.3168/jds.2021-20596. [DOI] [PubMed] [Google Scholar]
- 61.Liu Q.Y., Quinet E., Nambi P. Adipocyte fatty acid-binding protein (aP2), a newly identified LXR target gene, is induced by LXR agonists in human THP-1 cells. Mol Cell Biochem. 2007;302(1–2):203–213. doi: 10.1007/s11010-007-9442-5. [DOI] [PubMed] [Google Scholar]
- 62.Yao D.W., Luo J., He Q.Y., et al. Liver X receptor α promotes the synthesis of monounsaturated fatty acids in goat mammary epithelial cells via the control of stearoyl-coenzyme A desaturase 1 in an SREBP-1-dependent manner. J Dairy Sci. 2016;99(8):6391–6402. doi: 10.3168/jds.2016-10990. [DOI] [PubMed] [Google Scholar]
- 63.Nishimaki-Mogami T., Tamehiro N., Sato Y., et al. The RXR agonists PA024 and HX630 have different abilities to activate LXR/RXR and to induce ABCA1 expression in macrophage cell lines. Biochem Pharmacol. 2008;76(8):1006–1013. doi: 10.1016/j.bcp.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 64.Zhou X., He W., Huang Z., et al. Genetic deletion of low density lipoprotein receptor impairs sterol-induced mouse macrophage ABCA1 expression. A new SREBP1-dependent mechanism. J Biol Chem. 2008;283(4):2129–2138. doi: 10.1074/jbc.M706636200. [DOI] [PubMed] [Google Scholar]
- 65.Ayaori M., Yakushiji E., Ogura M., et al. Retinoic acid receptor agonists regulate expression of ATP-binding cassette transporter G1 in macrophages. Biochim Biophys Acta. 2012;1821(4):561–572. doi: 10.1016/j.bbalip.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 66.Wang H.J., Zhao X.S., Sun H.Y., et al. Decline of ATP-binding cassette transporter G1 expressions with a liver X receptor-independent pathway in patients with type 2 diabetes. J Peking Univ (Heal Sci) 2014;46(6):899–905. [PubMed] [Google Scholar]
- 67.Dong B., Kan C.F., Singh A.B., et al. High-fructose diet downregulates long-chain acyl-CoA synthetase 3 expression in liver of hamsters via impairing LXR/RXR signaling pathway. J Lipid Res. 2013;54(5):1241–1254. doi: 10.1194/jlr.M032599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Inaba T., Matsuda M., Shimamura M., et al. Angiopoietin-like protein 3 mediates hypertriglyceridemia induced by the liver X receptor. J Biol Chem. 2003;278(24):21344–21351. doi: 10.1074/jbc.M213202200. [DOI] [PubMed] [Google Scholar]
- 69.Stayrook K.R., Rogers P.M., Savkur R.S., et al. Regulation of human 3 alpha-hydroxysteroid dehydrogenase (AKR1C4) expression by the liver X receptor alpha. Mol Pharmacol. 2008;73(2):607–612. doi: 10.1124/mol.107.039099. [DOI] [PubMed] [Google Scholar]
- 70.Yin Y., Zeng S., Li Y., et al. Macrophage Lxrα reduces atherosclerosis in Ldlr-/- mice independent of Arl7 transactivation. Biochem Biophys Res Commun. 2020;529(3):540–547. doi: 10.1016/j.bbrc.2020.06.071. [DOI] [PubMed] [Google Scholar]
- 71.Koh M., Takitani K., Miyazaki H., et al. Liver X receptor up-regulates α-tocopherol transfer protein expression and α-tocopherol status. J Nutr Biochem. 2013;24(12):2158–2167. doi: 10.1016/j.jnutbio.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 72.Park S.S., Choi H., Kim S.J., et al. FXRalpha down-regulates LXRalpha signaling at the CETP promoter via a common element. Mol Cell. 2008;26(4):409–414. [PubMed] [Google Scholar]
- 73.Liu Y., Wei Z., Ma X., et al. 25-Hydroxycholesterol activates the expression of cholesterol 25-hydroxylase in an LXR-dependent mechanism. J Lipid Res. 2018;59(3):439–451. doi: 10.1194/jlr.M080440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou T., Cong S., Sun S., et al. Identification of endocrine disrupting chemicals activating SXR-mediated transactivation of CYP3A and CYP7A1. Mol Cell Endocrinol. 2013;365(1):36–43. doi: 10.1016/j.mce.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 75.Landrier J.F., Grober J., Demydchuk J., et al. FXRE can function as an LXRE in the promoter of human ileal bile acid-binding protein (I-BABP) gene. FEBS Lett. 2003;553(3):299–303. doi: 10.1016/s0014-5793(03)01033-0. [DOI] [PubMed] [Google Scholar]
- 76.Demeure O., Lecerf F., Duby C., et al. Regulation of LPCAT3 by LXR. Gene. 2011;470(1–2):7–11. doi: 10.1016/j.gene.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 77.Zhu W.F., Zhu J.F., Liang L., et al. Maternal undernutrition leads to elevated hepatic triglycerides in male rat offspring due to increased expression of lipoprotein lipase. Mol Med Rep. 2016;13(5):4487–4493. doi: 10.3892/mmr.2016.5040. [DOI] [PubMed] [Google Scholar]
- 78.Inoue J., Yamasaki K., Ikeuchi E., et al. Identification of MIG12 as a mediator for stimulation of lipogenesis by LXR activation. Mol Endocrinol. 2011;25(6):995–1005. doi: 10.1210/me.2011-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Okuwaki M., Takada T., Iwayanagi Y., et al. LXR alpha transactivates mouse organic solute transporter alpha and beta via IR-1 elements shared with FXR. Pharm Res (N Y) 2007;24(2):390–398. doi: 10.1007/s11095-006-9163-6. [DOI] [PubMed] [Google Scholar]
- 80.Mak P.A., Kast-Woelbern H.R., Anisfeld A.M., et al. Identification of PLTP as an LXR target gene and apoE as an FXR target gene reveals overlapping targets for the two nuclear receptors. J Lipid Res. 2002;43(12):2037–2041. doi: 10.1194/jlr.c200014-jlr200. [DOI] [PubMed] [Google Scholar]
- 81.Antonio V., Janvier B., Brouillet A., et al. Oxysterol and 9-cis-retinoic acid stimulate the group IIA secretory phospholipase A2 gene in rat smooth-muscle cells. Biochem J. 2003;376(Pt 2):351–360. doi: 10.1042/BJ20030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dong B., Singh A.B., Guo G.L., et al. Activation of FXR by obeticholic acid induces hepatic gene expression of SR-BI through a novel mechanism of transcriptional synergy with the nuclear receptor LXR. Int J Mol Med. 2019;43(5):1927–1938. doi: 10.3892/ijmm.2019.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matsushita N., Hassanein M.T., Martinez-Clemente M., et al. Gender difference in NASH susceptibility: roles of hepatocyte Ikkβ and Sult1e1. PLoS One. 2017;12(8) doi: 10.1371/journal.pone.0181052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Verreault M., Senekeo-Effenberger K., Trottier J., et al. The liver X-receptor alpha controls hepatic expression of the human bile acid-glucuronidating UGT1A3 enzyme in human cells and transgenic mice. Hepatology. 2006;44(2):368–378. doi: 10.1002/hep.21259. [DOI] [PubMed] [Google Scholar]
- 85.Erten M. Visfatin as a promising marker of cardiometabolic risk. Acta Cardiol Sin. 2021;37(5):464–472. doi: 10.6515/ACS.202109_37(5).20210323B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ming G.F., Li X., Yin J.Y., et al. JAZF1 regulates visfatin expression in adipocytes via PPARα and PPARβ/δ signaling. Metabolism. 2014;63(8):1012–1021. doi: 10.1016/j.metabol.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 87.Janani C., Ranjitha Kumari B.D. PPAR gamma gene: a review. Diabetes Metabol Syndr. 2015;9(1):46–50. doi: 10.1016/j.dsx.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 88.Ilias A.N., Ismail I.S., Hamzah H., et al. Rebaudioside A enhances LDL cholesterol uptake in HepG2 cells via suppression of HMGCR expression. Rep Biochem Mol Biol. 2021;10(3):477–487. doi: 10.52547/rbmb.10.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xie S., Lee Y.F., Kim E., et al. TR4 nuclear receptor functions as a fatty acid sensor to modulate CD36 expression and foam cell formation. Proc Natl Acad Sci U S A. 2009;106(32):13353–13358. doi: 10.1073/pnas.0905724106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Halder S.K., Fink M., Waterman M.R., et al. A cAMP-responsive element binding site is essential for sterol regulation of the human lanosterol 14ɑ-demethylase gene (CYP51) Mol Endocrinol. 2002;16(8):1853–1863. doi: 10.1210/me.2001-0262. [DOI] [PubMed] [Google Scholar]
- 91.He X., Hu J.L., Li J., et al. A feedback loop in PPARγ-adenosine A2A receptor signaling inhibits inflammation and attenuates lung damages in a mouse model of LPS-induced acute lung injury. Cell Signal. 2013;25(9):1913–1923. doi: 10.1016/j.cellsig.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 92.Madonna R., R D.C. Relevance of new drug discovery to reduce NF-κB activation in cardiovascular disease. Vasc Pharmacol. 2012;57(1):41–47. doi: 10.1016/j.vph.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 93.Huang L., Cai Y., Luo Y., et al. JAZF1 suppresses papillary thyroid carcinoma cell proliferation and facilitates apoptosis via regulating TAK1/NF-κB pathways. OncoTargets Ther. 2019;12:10501–10514. doi: 10.2147/OTT.S230597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qian Q., Li Y., Fu J., et al. Switch-associated protein 70 protects against nonalcoholic fatty liver disease through suppression of TAK1. Hepatology. 2022;75(6):1507–1522. doi: 10.1002/hep.32213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.He M., Zhang W., Dong Y., et al. Pro-inflammation NF-κB signaling triggers a positive feedback via enhancing cholesterol accumulation in liver cancer cells. J Exp Clin Cancer Res. 2017;36:15. doi: 10.1186/s13046-017-0490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang L., Zhang Q., Wang Z., et al. Design, synthesis, docking, molecular dynamics and bioevaluation studies on novel N-methylpicolinamide and thienopyrimidine derivatives with inhibiting NF-κB and TAK1 activities: cheminformatics tools RDKit applied in drug design. Eur J Med Chem. 2021;223 doi: 10.1016/j.ejmech.2021.113576. [DOI] [PubMed] [Google Scholar]
- 97.Tan L., Zhang J., Wang Y., et al. Development of dual inhibitors targeting epidermal growth factor receptor in cancer therapy. J Med Chem. 2022;65(7):5149–5183. doi: 10.1021/acs.jmedchem.1c01714. [DOI] [PubMed] [Google Scholar]
- 98.Suthar S.K., Sharma N., Lee H.B., et al. Novel dual inhibitors of nuclear factor-kappa B (NF-κB) and cyclooxygenase- 2 (COX-2):synthesis, in vitro anticancer activity and stability studies of lantadene-non steroidal anti-inflammatory drug (NSAID) conjugates. Curr Top Med Chem. 2014;14(8):991–1004. doi: 10.2174/1568026614666140324120503. [DOI] [PubMed] [Google Scholar]
- 99.Song J., Zhu Y., Zu W., et al. The discovery of quinoline derivatives, as NF-κB inducing kinase (NIK) inhibitors with anti-inflammatory effects in vitro, low toxicities against T cell growth. Bioorg Med Chem. 2021;29 doi: 10.1016/j.bmc.2020.115856. [DOI] [PubMed] [Google Scholar]
- 100.Scarneo S., Hughes P., Freeze R., et al. Development and efficacy of an orally bioavailable selective TAK1 inhibitor for the treatment of inflammatory arthritis. ACS Chem Biol. 2022;17(3):536–544. doi: 10.1021/acschembio.1c00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang C., Zhang Y., Wu Y., Xing D. Developments of CRBN-based PROTACs as potential therapeutic agents. Eur J Med Chem. 2021;225 doi: 10.1016/j.ejmech.2021.113749. [DOI] [PubMed] [Google Scholar]
- 102.Ustianowski P., Malinowski D., Kopytko P., et al. ADCY5, CAPN10 and JAZF1 gene polymorphisms and placental expression in women with gestational diabetes. Life. 2021;11(8):806. doi: 10.3390/life11080806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ding Z., Sun D., Han J., et al. Novel noncoding RNA CircPTK2 regulates lipolysis and adipogenesis in cachexia. Mol Metabol. 2021;53 doi: 10.1016/j.molmet.2021.101310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang Q., Dong Y., Wang H. microRNA-19b-3p-containing extracellular vesicles derived from macrophages promote the development of atherosclerosis by targeting JAZF1. J Cell Mol Med. 2022;26(1):48–59. doi: 10.1111/jcmm.16938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ding Q., Zhao W., Long J., et al. Cis-regulation of antisense non-coding RNA at the JAZF1 locus in type 2 diabetes. J Gene Med. 2022;24(4) doi: 10.1002/jgm.3407. [DOI] [PubMed] [Google Scholar]
- 106.Walczak R., Joseph S.B., Laffitte B.A., et al. Transcription of the vascular endothelial growth factor gene in macrophages is regulated by liver X receptors. J Biol Chem. 2004;279(11):9905–9911. doi: 10.1074/jbc.M310587200. [DOI] [PubMed] [Google Scholar]