Abstract
The bromodomain and extra-terminal (BET) proteins act as “readers” for lysine acetylation and facilitate the recruitment of transcriptional elongation complexes. BET protein is associated with transcriptional elongation of genes such as c-MYC and BCL-2, and is involved in the regulation of cell cycle and apoptosis. Meanwhile, BET inhibitors (BETi) have regulatory effects on immune checkpoints, immune cells, and cytokine expression. The role of BET proteins and BETi in a variety of tumors has been studied. This paper reviews the recent research progress of BET and BETi in hematologic tumors (mainly leukemia, lymphoma and multiple myeloma) from cellular level studies, animal studies, clinical trials, drug combination, etc. BETi has a promising future in hematologic tumors, and future research directions may focus on the combination with other drugs to improve the efficacy.
Keywords: BET, BRD4, Clinical trials, Hematologic tumors, Immunity, MYC
Introduction
The bromodomains are amino acid modules of approximately 110 found in chromatin-associated proteins.1 Almost all histone acetyltransferase (HAT)-associated transcriptional co-activators are known contain bromodomains, and histone acetylation is critical in chromatin remodeling and gene activation.1 The bromodomain and extra-terminal (BET) proteins possess two highly conserved N-terminal bromodomains, BD1 and BD2, and an extra-terminal (ET) region with additional functions, two of which (BRD4 and BRDT) have a carboxy-terminal domain (CTD) (Fig. 1A).2 The BET family includes four subtypes, bromodomain-containing protein 2 (BRD2), BRD3, BRD4 and the testis-ovary specific BRDT form, of which BRD4 is the most studied subtype.3 There are two isoforms of BRD4, long and short, which have different relative abundance in different cell types. The long isoform of BRD4 (BRD4L) is a transcriptional coactivator and the short isoform of BRD4 (BRD4S) corresponds to an alternative splice variant lacking exons 12–20.4 BRD4 functions as a scaffold for transcription factors at promoters and super-enhancers, and plays an essential role in mediating the expression of genes involved in cancer and non-cancer diseases (e.g., inflammatory diseases).5,6 It is closely associated with gene transcription, replication, epigenetic regulation and DNA repair, and it also serves as a mitotic bookmark for transcriptional reactivation of MYC, FOS and other genes in early G1 phase.6 BRD4 is widely distributed and has an important role in the development of many cancers, and cancer-related genes (e.g., MYC) are selectively dependent on BRD4.7,8 Studies have shown that BRD4 has an important role in the development of cancers such as gastric cancer, breast cancer and small cell lung cancer, and inactivating or down-regulating the expression of BRD4 can inhibit the development of cancer, and BRD4 is a promising new anti-cancer target.5,9, 10, 11
Figure 1.
Structural description of human BET proteins and BRD4 regulates the process of transcription. (A) Structural description of human BET proteins BRD2, BRD3, BRD4, and BRDT. Among them, BRD4 is divided into two subtypes, BRDL and BRDS. (B) BRD4 regulates the process of transcription. BRD4 binds to hyperacetylated chromatin regions, recruits proteins in a positive transcription elongation factor b (P-TEFb)-dependent manner, and regulates transcriptional processes by coupling to RNA polymerase II (Pol II).
Common hematologic tumors include non-Hodgkin's lymphoma, leukemia, multiple myeloma, and Hodgkin's lymphoma, and they ranked 13th, 15th, 24th, and 28th in global cancer incidence in 2018, respectively.12 Hematologic tumor development is closely linked to epigenetics and cancer genes such as MYC and B-cell leukemia/lymphoma-2 (BCL-2).13,14 BET is important in the pathogenesis of hematologic malignant diseases, and BET inhibitors are also effective in a variety of hematologic tumors. This article provides a review of the progress of research on BET and BET inhibitors in hematologic tumors.
The main biological functions of BET
The basic function of BET proteins
BET proteins are important regulators of epigenetics
Epigenetics refers to changes in gene expression levels based on non-genetic sequence alterations, including DNA methylation, histone modifications, chromatin remodeling and non-coding RNA regulation.15,16 Epigenetics plays an important role in the replication, modification, and transcription of DNA, and its abnormal regulation may lead to the development and maintenance of various cancers.17 Epigenetic pathways associated with cancer include DNA methylation, histone modifications, non-coding RNAs, and so on.17 Histone acetylation is particularly important in histone modifications and is regulated by three main types of epigenetic regulatory proteins, which are ''writers'', ''erasers'' and ''readers''.5,18,19 “Writers” refer to HATs, methyltransferases, kinases, and ubiquitinases, whose function is to mediate histone modifications, such as the acetylation of lysine in the protein tails by HATs.5,18,19 “Erasers” include deacetylases, phosphatases, demethylases, and deubiquitinases, which act to remove enzymes that modify proteins, such as histone deacetylases (HDACs) that remove acetyl groups from acetylated lysines.5,18,19 Proteins of the bromodomain family act as “readers” of lysine acetylation and facilitate the recruitment of transcriptional elongation complexes (e.g., positive transcription elongation factor b (P-TEFb)) (Fig. 1B).2 BET is found in different types of nuclear proteins, including helicases, transcriptional co-activators, methyltransferases, and nuclear scaffolding proteins, and the BET family proteins selectively bind to acetylated lysine, which explains why protein acetylation can produce a large number of functions.5,18,19 As a member of the BET family, BRD4 usually functions as an epigenetic “reader”, for example, it is involved in osteoblast differentiation and regulates the pluripotency of embryonic stem cells.20,21 Interestingly, recent studies have confirmed that BRD4 is not only an epigenetic reader but also an epigenetic writer, a novel HAT.6,8 BRD4 acetylates H3K122, a residue essential for nucleosome stability that leads to nucleosome expulsion and chromatin decompression.6,22
BRD4 regulates the expression of MYC
BRD4 of the BET family is closely related to the expression of the proto-oncogene MYC, which is intimately involved in the development of hematologic tumors such as diffuse large B-cell lymphoma (DLBCL), leukemia and multiple myeloma (MM).23, 24, 25 MYC expression is mainly regulated by phosphorylation, with phosphorylation of Thr58 leading to MYC degradation and phosphorylation of Ser62 resulting in MYC stabilization and activation. BRD4 phosphorylates MYC at Thr58, leading to MYC degradation, but this effect can be counteracted by activation of ERK1, a kinase that stabilizes MYC.8 Degradation of BRD4 causes a reduction in the prolongation of RNA polymerase II (Pol II) and thus reduces all transcripts, but inhibition of BRD4 produces different effects depending on the dose of the BET inhibitor (BETi) JQ1, with high doses of JQ1 inhibiting all transcripts and low doses of JQ1 having limited effects.26 JQ1 can downregulate MYC transcription, leading to a decrease in MYC-dependent target gene expression, and the inhibitory effect of JQ1 on MYC expression affects the sensitivity of tumor cells to JQ1.23,26
Common BET-related signaling pathways
BET also regulates tumor progression through multiple signaling pathways, one of which is the NF-κB pathway. BETi inhibits melanoma progression through the NF-κB pathway,27 and NF-κB inhibitors have synergistic effects with BETi in in vivo and in vitro models to overcome resistance to BETi in uveal melanoma.28 In colorectal cancer (CRC), BETi decreased c-MYC expression and NF-κB activity in BETi-sensitive CRC cells.29 In addition, BET protein inhibits human T cell leukemia virus 1-mediated adult T-cell leukemia by suppressing the NF-κB pathway.30 Another common signaling pathway associated with BET is the PI3K/AKT pathway, and in a model of metastatic breast cancer, BETi decreased PI3K signaling, and combined inhibition of PI3K and BET induced tumor cell death and tumor regression.31 BETi has synergistic effects with PI3K/AKT inhibitors in ovarian clear cell carcinoma, and targeting the IGF1R/PI3K/AKT pathway increased the sensitivity of Ewing's sarcoma to BETi.32,33 BET proteins effectively block the adaptive signaling response of cancer cells to PI3K pathway inhibitors, and BET inhibition improves the clinical efficacy of PI3K inhibitors, as detailed in a review by Stratikopoulos et al.34 BET proteins also play an important role in the MAPK and JAK/STAT pathways.35,36
Biomarkers of BETi treatment
BETi is a strategy for treating a wide range of tumors,29, 30, 31, 32, 33, 34, 35, 36 but not all types of tumor cells respond well to BETi. Shorstova et al described three different types of biomarkers for BETi treatment, namely predictive biomarkers, resistance biomarkers and pharmacodynamic biomarkers.37 Predictive biomarkers of BETi include MYC amplification, and dual SMARCA4/A2 loss.26,38 Knockdown of SMARCA4/A2 sensitizes resistant cells to BETi, while restoration of SMARCA4/A2 promotes tumor cells resistance to BETi. Biomarkers of resistance mainly include PI3K-AKT pathway activation, RAS-MAPK pathway activation and speckle-type POZ protein (SPOP) mutation. SPOP mutation is responsible for ubiquitination and degradation of BET proteins, and the mechanism of resistance of BETi in SPOP mutated cells is the reduction of BET protein degradation rate, leading to the accumulation of BET proteins.37 c-MYC mRNA is useful as a biomarker for hematological tumors, but is not ideal as a whole blood biomarker, whereas CCR2 and CD180 mRNA can be applied as whole blood pharmacodynamic biomarkers for BETi.39 Another pharmacodynamic biomarker is HEXIM1, the only gene that exhibits robust and consistent BETi regulation across multiple cancer indications and surrogate tissues.40 Biomarkers of BETi treatment can predict the efficacy of BETi and guide antitumor therapy.
Brief introduction about inhibitors of BET proteins
JQ1, a selective and potent inhibitor of BET proteins, was reported in 2010, and competitive binding of JQ1 displaces BRD4 fusion oncoprotein in chromatin, promoting squamous differentiation and exerting specific anti-proliferative effects in BRD4-dependent cell lines and patient-derived xenograft models.41 OTX015 is an oral agent with a structure similar to that of JQ1. OTX015 is effective against a variety of hematologic malignancies, including b-cell lymphoma and multiple myeloma.37 In 2014, Chaidos et al reported that I-BET151 and I-BET762, which represent the quinoline and benzodiazepine of BETi, respectively, induce cell cycle arrest in myeloma cells, resulting in potent antiproliferative effects, and proapoptotic effects are also observed.42 For multi-domain protein targets, the pharmacological outcome of small molecule antagonists is limited selective disruption of a domain-specific activity, and treatment with BETi leads to the accumulation of BET proteins associated with reversible binding and incomplete inhibition of BRD4, which may impair the antitumor activity of BETi. Winter et al converted JQ1 to a phthalimide conjugated ligand that immediately promotes cereblon-dependent BET protein degradation (dBET1).43 In 2015, Lu et al reported that ARV-825, a BET proteolysis-targeting chimera (PROTAC), recruits BRD4 to the E3 ubiquitin ligase cereblon, leading to fast, efficient, and prolonged degradation of BRD4 in all burkitt's lymphoma (BL) cell lines tested.44 BET-PROTAC (e.g., dBET1 with ARV-825) have better efficacy in treating hematologic malignancies compared to BETi. We summarize the information of common BETi and BET-PROTAC45 (Table 1).
Table 1.
The information of common BETi and BET-PROTAC.
| Compound | Classification | Molecular weight | Formula/E3 ubiquitin ligase recognition motif | PubChem Compound ID number |
|---|---|---|---|---|
| JQ1 | BETi | 400.88 | C₁₉H₁₇ClN₄O₂S | 46,907,787 |
| I-BET151 | BETi | 415.44 | C₂₃H₂₁N₅O₃ | 52,912,189 |
| I-BET762 | BETi | 396.83 | C₂₀H₁₇ClN₄O₃ | 46,943,432 |
| OTX015 | BETi | 383.42 | C₁₈H₁₇N₅O₃S | 9,936,746 |
| PLX51107 | BETi | 438.48 | C₂₆H₂₂N₄O₃ | 90,448,953 |
| RO6870810 | BETi | 540.13 | C27H34ClN7OS | 54,670,351 |
| Mivebresib, ABBV-075 | BETi | 459.47 | C₂₂H₁₉F₂N₃O₄S | 71,600,087 |
| GS-5829 | BETi | 437.49 | C₂₆H₂₃N₅O₂ | 86,281,210 |
| ABBV-744 | BETi | 491.55 | C₂₈H₃₀FN₃O₄ | 132,010,322 |
| ARV-771 | BET-PROTAC | 986.64 | VHL ligand | 126,619,980 |
| dBET1 | BET-PROTAC | 785.3 | Thalidomide | 91,799,313 |
| ARV-825 | BET-PROTAC | 923.43 | Thalidomide | 92,044,400 |
BET in immune regulation
Role of BET in immune checkpoints
Blocking PD-L1 signaling can restore anti-tumor immunity of T cells. PD-L1 is a direct target of BRD4-mediated gene transcription, and in a mouse model, JQ1 inhibits BRD4, thereby reducing PD-L1 expression on tumor cells, dendritic cells, and macrophages.46 The mechanism of action of JQ1 on PD-L1 has been described in two ways: one is that JQ1 suppresses the MYC gene, which binds directly to the promoters of CD47 and PD-L1 genes thereby regulating the anti-tumor immune response47; the other is a genome-wide analysis of BETi-induced transcriptional responses, which found that PD-L1 is a MYC-independent BETi target-gene, and BETi directly inhibits interferon-γ (IFN-γ)-induced CD274 expression in tumor cell lines and primary patient samples.48 In a study of triple-negative breast cancer, researchers found that BET proteins control PD-1 expression in T cells and that BTEi reduces IFN-γ production and signaling, thereby inhibiting PD-L1 expression in breast cancer cells.49 BETi also reduces PD-L1 mRNA and protein levels in melanoma, prostate cancer, liver cancer, lung cancer and other cell lines.50,51 CTLA-4 is also an immune checkpoint expressed on T cells, which inhibits T cell activity upon binding to ligand B7 on dendritic cells. A mathematical model of the combination of BETi and CTLA-4 inhibitors in the treatment of breast cancer showed that the two drugs had a synergistic effect, that is, the tumor volume decreased with increasing drug dose.52
BET and BETi in immune cells
BETi affects the function of a variety of immune cells. BETi increases the sensitivity of tumor cells to the antitumor activity of CD8+ T cells, and its disruption of the NF-κB signaling pathway leads to the activation of the tumor necrosis factor (TNF)-mediated extrinsic apoptotic cascade and the death of tumor cells.53 BETi PLX51107 increases activated, proliferating and functional CD8+ T cells in melanoma tumors, leading to delayed CD8+ T cell-mediated tumor growth.54 In addition to affecting CD8+ T cells, BETi can also affect the function of regulatory T cells (Treg). Treg play an important role in the immune system by maintaining immune homeostasis and preventing the development of autoimmune diseases, and Treg has emerged as a promising treatment for graft-versus-host disease (GVHD).55 JQ1 interfered with Treg expansion and altered subset distribution and phenotype, whereas BETi EP11313 did not interfere with Treg expansion, subset distribution and in vitro function, and a low level of EP11313 given at the time of allogeneic HSCT, combined with expanded Treg adoptive transfer, could further reduce GVHD.55
Regulation of cytokines by BET and BETi
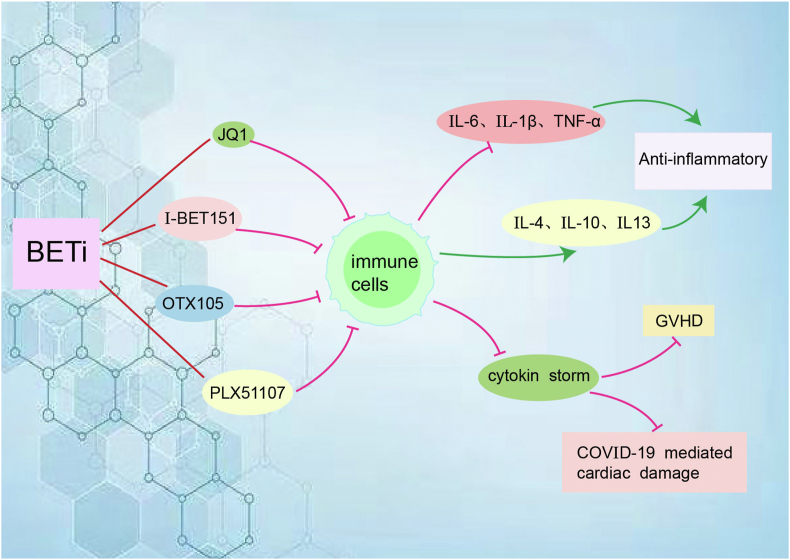
The disorder of immune function is the basis of the occurrence of many tumors. BET also has a regulatory effect on various cytokines. In spinal cord injury, JQ1 decreased the levels of pro-inflammatory cytokines interleukin-6 (IL-6), IL-1β and TNF-α, and increased the expression of anti-inflammatory cytokines IL-4, IL-10 and IL-13.56 JQ1 also significantly inhibited IL-1β, IL-6 and TNF-α in periodontitis.57 BETi OTX015 has anti-inflammatory effects by inhibiting proliferation and pro-inflammatory cytokine expression in mouse and human CD4+ T cells, including selective inhibition of IL-17 in human memory CD4+ T cells.58 Similarly, BET is essential for TH17 differentiation in mice, and IL-17 secretion is inhibited by JQ1 in a dose-dependent manner.59 BET is also involved in the epigenetic regulation of immune responses by macrophages and dendritic cells (DCs), suggesting that treatment of cancer with BETi promotes immunosuppressive effects, with JQ1-treated DCs showing a decreased capacity to induce antigen-specific T cell proliferation and antigen-specific T cells co-cultured with JQ1-treated DCs exhibiting an underactive phenotype with decreased cytokine production.60 We already know that BETi alters cytokine expression in DCs and T cells and that BETi has promising applications in GVHD.55,61 Another BETi I-BET151 also alters cytokine expression in DCs and T cells, including the secretion of surface stimulatory molecules and cytokines in vitro and in vivo. Short-term administration early during bone marrow transplantation (BMT) reduces GVHD severity and improves mortality in allogeneic BMT models, but preserves sufficient graft antitumor effects.61 BETi in combination with T-cell bispecific antibodies or immune checkpoint blockade enhances tumor growth inhibition in a TNF-dependent manner.53 The inflammatory “cytokine storm” that occurs after COVID-19 infection induces cardiac damage and dysfunction in patients, such as decreased myocardial strength due to TNF and diastolic dysfunction due to IL-1β, IFN-γ, poly (I:C), and LPS, whereas the use of BETi in a mouse cytokine storm model restores dysfunction in human cardiac organoids and completely prevents cardiac dysfunction and death (Fig. 2).62
Figure 2.
BETi affects the expression of cytokines by acting on immune cells such as CD4+ T cells and DCs. BETi decreases the levels of pro-inflammatory cytokines IL-6, IL-1β and TNF-α, and increases the expression of anti-inflammatory cytokines IL-4, IL-10 and IL-13, resulting in anti-inflammatory effects. BETi inhibits cytokine storms, thereby reducing graft-versus-host disease (GVHD) and COVID-19 infection induces cardiac damage.
The status of BET proteins and BETi in the development and progression of hematologic tumors
Study of BET proteins and BETi in leukemia
The study by Zuber et al identified the BRD4 protein as a key factor in acute myeloid leukemia (AML) maintenance.63 Inhibition of BRD4 using small hairpin RNAs (shRNAs) or JQ1 produced potent anti-leukemic effects in vitro and in vivo, along with terminal myeloid differentiation and elimination of leukemic stem cells. Similar sensitivities have been observed in various human AML cell lines and primary patient samples.63 BETi has shown good results in many types of AML.
In MLL-fusion leukemia, many common MLL fusion partners play a role in regulating transcriptional elongation through their interaction with P-TEFb, where abnormal function of the SEC/P–TEFb complex formed by superelongation complex (SEC) and P-TEFb leads to abnormal transcriptional elongation as a central mechanism in the pathogenesis of MLL-fusion leukemia.64 BRD3 and BRD4 are essential components of SEC, and BET has an important role in the pathogenesis of MLL-fusion leukemia.64 A novel small molecule inhibitor of BET, GSK1210151A (I-BET151), induces early cell cycle arrest and apoptosis in MLL-fusion leukemia cells, and the mechanism of action of I-BET151 is the inhibition of transcription of key genes BCL-2, c-MYC and CDK6.65
BETi is also efficacious in a wide range of AML cells with different oncogenic mutations. Dawson et al evaluated the role of I-BET151 in common recurrent AML mutations and found that several other AML cell lines, including those containing Cytoplasmic NPM1 mutations (NPM1c), FGFR1OP2-FGFR1 rearrangement, the EZH2 Y641C mutation, AML1-ETO rearrangement, and CBFβ-MYH11 rearrangement, all showed sensitivity to I-BET151 treatment.66 Key regulators of myelopoiesis and leukemia, such as BCL-2, c-MYC and IRF8, were generally downregulated after I-BET151 treatment, suggesting that BET proteins regulate the expression of “core” transcriptional programs in AML.66 Nuclear NPM1 exerts a suppressive effect on BRD4, while NPM1c mutation relieves the repression of BET proteins and promotes the upregulation of the “core” AML transcriptional program.66 Another BETi, JQ1, increased the percentage of G1-phase cells and decreased the percentage of S-phase cells, and induced apoptosis in AML with NPM1c mutation, and JQ1 was also lethal to primary AML cells of patient origin with NPM1 and/or FLT3-ITD mutations.67 JQ1 inhibited the binding of BRD4 and RNA Pol II and reduced the mRNA expression of c-MYC and BCL-2 in AML cells.68 Combined treatment with JQ1 and pan-HDAC inhibitor panobinostat also induced greater loss of clonal survival of AML cells. In primary AML expressing mutant NPM1c+ with or without concomitant expression of FLT3-ITD, combined treatment with panobinostat and JQ1 caused more downregulation of c-MYC and BCL-2, induced significantly more apoptosis, and increased PARP cleavage, but did not produce significantly greater lethal activity against normal CD34+ hematopoietic progenitor cells.67
BETi also plays an important role in the treatment of early thymic progenitors (ETP) leukemias. JQ1 and I-BET151 reduced tumor burden in ETP leukemic mice, significantly reducing leukemic cells in the peripheral blood, spleen, and bone marrow of mice.68 Investigators transplanted ETP leukemia cells with EZH2 and RUNX1 mutant into immunodeficient NSG mice, and treatment with JQ1 significantly inhibited the progression of EZH2 and RUNX1 mutant ETP leukemia and reduced leukemic cells, but normal hematopoietic function of bone marrow and spleen in mice was relatively preserved.68
The risk stratification of FLT3-ITD-positive AML is high risk with an extremely poor prognosis. The study by Fiskus et al found that JQ1 and FLT3 tyrosine kinase inhibitor (FLT3-TKI) quizartinib or ponatinib were synergistically lethal to AML cells expressing FLT3-ITD and induced apoptosis in AML primary cells expressing FLT3-ITD.69 Knockdown of BRD4 by shRNA also sensitized AML cells to FLT3-TKI, and the study confirmed the promising therapeutic effect of BETi in FLT3-ITD-positive AML.69 The research described above was performed in suspension cells and did not address the issue of drug resistance of tumor cells to FLT3 inhibition mediated by tumor microenvironment. In fact, JQ1 is not suitable for in vivo application. PLX51107 is a structurally unique BETi that inhibits the growth of AML cell lines carrying the FLT3-ITD mutation in vitro. In the in vivo experiments, the combination of PLX51107 and quizartinib induced a synergistic anti-leukemic effect, with PLX51107 enhancing the response of AML cells to sustain and low-dose quizartinib treatment without concomitant toxicity.70
BETi interacts with p53 in AML. JQ1 inhibited the proliferation of AML cells carrying NPM1 and DNMT3A mutations and induced apoptosis via caspase 3/7.71 BRD4-mediated recruitment of p53 to chromatin was blocked by JQ1, resulting in the inability of the DNA damage repair response to activate proper and ultimate apoptosis.71 MDM2 inhibitors (MDM2i) are drugs that activate p53 in cells expressing wild-type genes, thereby releasing the tumor suppressive function of p53, but MDM2i alone is less effective in treating tumors.72 BETi enhances the activation of p53 by MDM2i and alleviates BRD4-mediated suppression of p53 target genes. BETi enhances the killing of human AML cells by MDM2i, and the combination of BETi and MDM2i eliminates AML in mouse models.72
Myeloproliferative neoplasms with myelofibrosis can progress to secondary acute myeloid leukemia (sAML), and standard induction chemotherapy is ineffective in sAML. JQ1 treatment reduced protein expression of c-MYC, p-STAT5, Bcl-xL, CDK4/6, PIM1 and IL-7R in sAML cells, and combined treatment with BETi and ruxolitinib synergistically induced apoptosis in cultured and patient-derived sAML cells, and significantly increased the survival of immune-depleted mice engrafted with human sAML cells.73 Meanwhile, the co-treatment of BETi and HSP90i had a synergistic lethal effect on ruxolitinib-persister or ruxolitinib-resistant sAML cells.73
We already know that BETi has a good effect on AML at the cellular level. Here we look at the results of clinical trials of BETi for AML. OTX015 is a novel BETi that binds BRD2, BRD3 and BRD4 and suppresses gene transcription by preventing BET proteins from binding to chromatin.74 A dose-escalation phase 1 trial assessed efficacy and side effects in 41 patients with relapsed/refractory acute leukemia, 36 of whom had AML, with a median age of 70 years.74 OTX015 is given orally once daily at a dose of 10 mg/day gradually increasing to 160 mg/day, and common side effects are fatigue and increased bilirubin. Three patients achieved complete remission and lasted 2–5 months.74 OTX015 single-agent oral administration is effective in AML, but not ideal. RO6870810 (RO), a novel small molecule BETi, was tested in a clinical trial (NCT02308761) in 32 patients with relapsed/refractory AML and hypomethylating agent-refractory myelodysplastic syndrome, and the most common adverse reactions were fatigue, injection site reactions, diarrhea, loss of appetite, and nausea.75 One patient with AML achieved complete remission and 11 patients with AML developed stable disease (SD) and the median overall survival (OS) for AML patients was 72.0 days. For myelodysplastic syndrome (MDS), two patients developed SD.75 Because the patients included in the clinical trials were all with relapsed/refractory AML (R/R AML), these studies do not suggest that BETi has no future in the treatment of AML. We should consider BETi in combination with other drugs more often than monotherapy. A phase 1 trial evaluated the efficacy and adverse events of BETi mivebresib (ABBV-075) alone or in combination with venetoclax (ABT199) in the treatment of R/R AML. Only 1 of 19 patients in ABBV-075 monotherapy group (5 went on to receive combination treatment after disease progression) could achieve complete remission.76 The combined therapy group consisted of 30 patients with complete remission in 2 patients, partial remission in 2 patients and morphologic leukemia-free state in 2 patients. The common adverse events were loss of appetite, vomiting and nausea.76 Another new BETi, ABBV-744, was comparable to ABBV-075 in its anti-AML effect, but with an improved therapeutic index.77 A clinical trial evaluating the safety and pharmacokinetics of ABBV-744 in patients with R/R AML has been completed and we look forward to the publication of the results of this study (NCT03360006).
The resistance of leukemic stem cells to BETi is due to the activation of Wnt/β-catenin.78 Specific antagonism of the Wnt/β-catenin pathway in BETi-resistant leukemia cells recovers the sensitivity of leukemia cells to BETi in vivo and in vitro, while stimulation of Wnt/β-catenin pathway expression in BETi-sensitive leukemic cells can rapidly achieve I-BET resistance.78 Concomitant use of BETi and Wnt/β-catenin inhibitors is a promising therapeutic strategy for AML. The combination of JQ1 and azacitidine decreased the proliferation of leukemic cells and increased the apoptotic effect of azacitidine on leukemia cells.79 JQ1 also enhances the effect of ATR inhibitor (AZD6738) on apoptosis in leukemia cells.79 The anti-leukemic effect of BETi can be enhanced by combining BETi with other drugs.
In primary human leukemia cells, dBET1 had a more rapid and powerful apoptotic response compared to BETi, and in vitro study in a mouse xenograft model of human MV4-11 leukemia cells, treatment with dBET1 attenuated tumor development and reduced tumor weight, and dBET1 was well tolerated in mice after 2 weeks of treatment, and had no significant effect on body weight, white blood cell count, and platelet count.43,80 In a study of T-cell acute lymphoblastic leukemia (T-ALL), BRD4 expression was found to be higher in T-ALL samples compared to T cells from healthy donors, and BRD4 was associated with poor prognosis.81 ARV-825 is a novel BET-degrading drug conjugated with cereblon ligand that inhibits the cell cycle and promotes apoptosis, with a lower IC50 in T-ALL cells compared to JQ1, dBET1 and OTX015. In a T-ALL xenograft model, ARV-825 significantly decreased tumor growth, effectively inhibited T-ALL cell proliferation and promoted apoptosis through BET protein depletion and c-MYC inhibition.81 JQ1 has synergistic activity with venetoclax in patients at high risk of relapse or in relapsed T-ALL cell lines by a mechanism of action mediated by acute induction of the pro-apoptotic factor BCL2L11 and reduction of BCL-2 due to BETi.82 Another BET protein degrader BETd-260 is highly potent in degrading BRD2/3/4 and inhibiting the growth of RS4; 11 B-cell acute lymphoblastic leukemia (B- ALL) cells in vitro and in a xenograft mouse model.83
A novel BETi GS-5829 inhibits chronic lymphocytic leukemia (CLL) cell proliferation and induces apoptosis by regulating key signaling pathways such as BLK, AKT, ERK1/2, MYC and NF-κB.84 GS-5829 synergistically increases anti-leukemic activity when combined with the bruton's tyrosine kinase inhibitors (BTKi) ibrutinib, PI3K inhibitor idelalisib, and other drugs. BETi can also target the supportive CLL microenvironment.84
BETi has shown good efficacy in vitro and in vivo against a variety of leukemias, and resistance to it is associated with activation of the Wnt/β-catenin pathway. BETi combined with drugs such as azacitidine and AZD6738 increases the efficacy. BET-degrading drugs conjugated with cereblon ligand (e.g., dBET1 with ARV-825) have better efficacy in treating leukemia compared to BETi.
BET protein and BETi show great promise in lymphoma
World Health Organization defines diffuse large B-cell lymphomas (DLBCL) with concurrent rearrangements of c-MYC, BCL-2 and/or BCL-6 as “double/triple hit lymphomas (DHL/THL)”, accounting for approximately 5–15% of all DLBCL.85 If high expression of c-MYC, BCL-2 and/or BCL-6 is present only at the protein level, without gene rearrangements, then the lymphoma is called a “double expressor lymphoma/triple expressor lymphoma (DEL/TEL)” and accounts for about 20–30% of all DLBCL.85,86 The prognosis of DEL/TEL is worse than other DLBCLs, but it is less aggressive than DHL/THL.85,86 Because DHL/THL is positive for MYC and BETi inhibits MYC expression, the efficacy of BETi in DHL/THL is promising. In a trial evaluating the efficacy of BETi in DHL/THL, researchers found that BETi (JQ1, I-BET and OTX015) targeted MYC in DHL/THL DLBCLs and significantly reduced proliferation, accompanied by a reduction in MYC, but no significant changes in BCL-2 protein.85 The combination of BETi with Pan-HDAC inhibitor had a limited effect on cell survival in DHL/THL, whereas the combination of BETi and BCL-2 antagonist ABT-199 had a significant inhibitory effect on cell survival. The combination of JQ1 and ABT-199 reduced cell survival by over 90% in DHL and 50% in THL, and the combination of I-BET and ABT-199 resulted in almost complete cell death in DHL and THL cells.85
A phase 1b dose-escalation study (NCT03255096) evaluated the efficacy and adverse effects of the novel subcutaneous BETi RO combined with the BCL-2 inhibitors venetoclax and rituximab in relapsed/refractory DLBCL. Thirty-nine patients received a median of 2.8 cycles of treatment, with the most common adverse event being haemocytopenia.87 The overall remission rate was 38.5%, with 8 patients (20.5%) achieving complete remission.87 The objective response rate for RO monotherapy for advanced DLBCL was 11% (2/19), with fatigue, decreased appetite and injection site erythema being the most common treatment-related adverse events.88 In a dose-escalating, open-label phase 1 study, 33 patients with lymphoma and 12 patients with MM received oral OTX015 once daily, with a median age of 66 years and four prior treatments, and the common toxicities were thrombocytopenia, anaemia, neutropenia, diarrhoea, fatigue and nausea. Three patients with DLBCL achieved durable objective responses, and six others (two with DLBCL and four with indolent lymphomas) did not reach objective response criteria but had evidence of clinical activity.89
The researchers found high protein expression of interferon regulatory factor 4 (IRF4), TCF4 and BCL-2 in richter transformation DLBCL (RT-DLBCL), and that the combination of BETi or BET-PROTAC with ibrutinib or venetoclax exerted a synergistic in vitro lethal effect in RT-DLBCL cells.90 Combined treatment with BET-PROTAC and venetoclax significantly reduces lymphoma burden in mice and improves survival of immune-depleted mice engrafted with RT-DLBCL.90 RhoA belongs to a family of small GTPases that play an integral role in DLBCL migration. JQ1 inhibits migration of DLBCL cells by suppressing RAS signaling and inhibiting MYC-mediated RhoA activity.91 These cellular studies and clinical trials proved that BETi or BET-PROTAC has a promising application prospect in DLBCL. In the future, more studies could focus on the combination of BETi and other drugs to further improve its efficacy in DLBCL.
BRD4 is crucial for the transcriptional activity of NF-κB, and mantle cell lymphoma (MCL) cells show increased NF-κB activity. A study by Sun et al found that the use of JQ1 reduced the expression of NF-κB target genes, such as BTK, in MCL cells.92 Co-treatment of JQ1 with ibrutinib synergistically induced apoptosis in MCL cells, while JQ1 in combination with the histone deacetylase inhibitors panobinostat or BCL-2 antagonist ABT199 induced apoptosis in ibrutinib-resistant MCL cells.92 And compared to BETi, BET-PROTAC induced apoptosis in MCL cells more powerfully.93 This is a promising treatment modality for patients with ibrutinib-resistant MCL. The exacerbation of IRF4/MYC signalling is closely associated with the development of bortezomib resistance in MCL cells, and lenalidomide was able to act against IRF4 expression and plasma cell differentiation programs, thereby overcoming bortezomib resistance.94 The use of BETi CPI203 in combination with lenalidomide downregulated both MYC and IRF4 and induced apoptosis in bortezomib-resistant MCL cells.94
In cutaneous T-cell lymphoma (CTCL), miR-214 levels in purified CD4+ neoplastic T cells from CTCL patients were markedly higher than in healthy donors, and aberrant expression of TWIST1 and BRD4 cooperatively drove miR-214 expression in CTCL cell lines and CTCL patient samples, whereas treatment with JQ1 resulted in downregulation of miR-214. TWIST1/BRD4/miR-214 regulatory loop is an essential oncogenic pathway in CTCL.95 JQ1 down-regulated c-MYC expression and dose-dependently induced G1 phase arrest in CTCL cells. JQ1 down-regulated CD30 and CCR4 expression on both the CTCL cell surface and at the mRNA level.96 The combination of BETi and HDAC inhibitor (HDACi) has a synergistic effect on CTCL cell lines, inducing G0/G1 cell cycle arrest and increasing apoptosis.97 The mechanism of action is to reduce the proliferation drivers c-MYC, Cyclin D1, NF-κB and IL-15Rα, and to enhance the expression of extrinsic apoptotic pathway death receptors and ligands (FasL, DR4, DR5, TRAIL and TNFR1).97 BETi and JAK pathway inhibitors also have synergistic effects in CTCL.98 BETi has excellent efficacy in the treatment of CTCL.
The PI3K/AKT/mTOR signalling pathway and MYC gene are essential for the survival of burkitt's lymphoma (BL) cells,99 and the PI3K inhibitors idelalisib and IPI-145 (duvelisib), the allogenic AKT inhibitor MK-2206, and the mTOR inhibitors everolimus and deforolimus were all synergistic with OTX015.100 The combination of the CDK inhibitor SNS-032 with OTX015 also had a synergistic effect.100 The combination of HDACi romidepsin and BETi JQ1 in BL cells at concentrations close to the IC50 values (5 nM romidepsin and 1 μM JQ1) not only synergistically inhibited proliferation but also synergistically promoted apoptosis, with a significant increase in cleaved PARP1 and a decrease in the level of the anti-apoptotic protein BCL-xL after 48 h of action of the two drugs.101
The BET proteins and BETi have also been studied in other rare lymphomas. IBET and JQ1 induce cell cycle arrest in the G0/G1 phase in Waldenström macroglobulinemia (WM).102 IBET/JQ1 causes a reduction in IgM expression and secretion, BETi is synergistic with HDACi and BCL-2 antagonists in combination, but not with BTKi.102 In primary effusion lymphoma (PEL), lenalidomide showed synergistic cytotoxicity with JQ1, IBET151 and PFI-1, and the combination of lenalidomide and JQ1 significantly improved survival in a mouse transplantation tumor model of PEL compared with either agent alone.103 In anaplastic large cell lymphomas (ALCL), the combination of OTX015 with ibrutinib leads to cell cycle arrest followed by cell death, as can the combination with the ALK inhibitor CEP28122.104 BET and BETi play an important role in the pathogenesis and treatment of a variety of lymphomas, and their efficacy in DLBCL and MCL is particularly promising.
BET in multiple myeloma: current opinions
Multiple myeloma (MM) is a malignant disease of plasma cell origin, for which significant advances have been made in recent years, but for which there is no cure. Expression of BRD4 was increased in monoclonal gammopathy of undetermined significance (MGUS) and smoldering MM (SMM) compared to bone marrow plasma cells from healthy donors.105 Expression of BRD4 was significantly higher in plasma cell leukemia (PCL) compared to MM or MGUS samples.106 Thus, BRD4 expression was positively correlated with MM progression. In MM, JQ1 causes depletion of c-MYC and downregulates the c-MYC-coordinated transcriptional program, contributing to cell cycle arrest and cellular senescence.107 The binding of BRD4 to the IgH enhancer in MM.1S cells was significantly reduced after 24 h of JQ1 treatment (500 nM), and the cell cycle arrest observed after JQ1 treatment was partially rescued by overexpression of c-MYC. In a mouse model, JQ1 reduced disease burden and prolonged overall survival.107 A novel BETi CG13250 inhibited ligand binding to BRD4 in a dose-dependent manner, thereby inhibiting MM proliferation and arresting cells at G1, and thus inducing apoptosis through caspase activation, and in vivo administration of CG13250 significantly prolonged the survival of MM-bearing mice.108 Another BETi I-BET151 can dose-dependently inhibit osteoclast formation and inflammatory cytokine secretion by targeting the BRD4-mediated RANKL–NF–κB signaling pathway,109 and I-BET151 also induces apoptosis in MM cells and exerts potent anti-proliferative effects in vitro and in vivo.42 OTX015 regulated NF-κB, EGFR, cell cycle regulation and cancer proliferation signalling pathways in MM,110 and it showed synergistic effects with proteasome inhibitors, immunomodulators and alkylating agents in MM cells.111 Oral administration of OTX015 showed significant anti-tumor activity in a mouse model, and OTX015 promoted osteoblast differentiation from mesenchymal stem cells (MSCs) and inhibited osteoclast formation and resorption in in vivo experiments.110
BET-PROTACs reduce the viability of myeloma cell lines in a time- and concentration-dependent manner through cell cycle G0/G1 phase arrest, reduced levels of CDKs 4 and 6, increased levels of p21 and induction of apoptosis, and can overcome resistance to bortezomib, dexamethasone, lenalidomide and pomalidomide.112 BET-PROTACs function in synergy with dexamethasone, BH3 mimetics and AKT pathway inhibitors in a synergistic manner.112 Lim et al used a combination of cyclin dependent kinase 9 (CDK9) inhibitor and BET-PROTAC ARV825 to treat MM.113 The combination significantly reduced protein expression of BRD 2, BRD 4, MYC and phosphorylated RNA Pol II, and significantly increased apoptosis in MM cells compared to each drug alone.113
A multicentre phase 1 trial of RO in patients with advanced MM included 24 patients with a median age of 65.6 years (range: 46–82 years), all of whom received at least one dose of RO.114 The most common adverse events were injection site reactions, fatigue, anemia, thrombocytopenia, and nausea. This study confirmed 0.65 mg/kg as the recommended monotherapy dose for relapsed/refractory MM.114 Four of the 24 patients treated in the study achieved partial remission, and clinical benefit rate (minimal response or better) was 20.8% and 70.8% achieved stable disease or better.114 The best response achieved in the phase 1 trial of OTX015 in 12 patients with MM was stable disease (17%) or disease progression (83%).89
BETi or BET-PROTAC alone has been shown to inhibit proliferation and promote apoptosis in MM cell lines, and has been shown to be effective in animal studies, and in combination with proteasome inhibitors and immunomodulators. Clinical trials with single agents (RO6870810, OTX015) have been less than satisfactory and combinations with other drugs may be tried in the future.
BETi and BET-PROTAC have promising therapeutic effects in many hematologic tumors, which have been summarized in detail in this paper at the cellular level, in animal studies, and in concluded clinical trials. We next summarized the ongoing clinical trials of BETi and BET-PROTAC in hematologic tumors (Table 2).
Table 2.
Ongoing clinical trials of BETi and BET-PROTAC in hematologic tumors (from www.clinicaltrials.gov, last update).
| Compound | Sponsor | Status | Clinical phase | Diseases | Estimated Enrollment | Identifier | Estimated completion date |
|---|---|---|---|---|---|---|---|
| PLX51107 | Hannah Choe | Not yet recruiting | Phase 1 Phase 2 |
Steroid Refractory GVHD | 34 | NCT04910152 | December 31, 2024 |
| CPI-0610 | Constellation Pharmaceuticals | Recruiting | Phase 1 Phase 2 |
Hematological Malignancies, Myelofibrosis | 341 | NCT02158858 | December 31, 2022 |
| INCB057643 | Incyte Corporation | Recruiting | Phase 1 | Myelofibrosis and Other Advanced Myeloid Neoplasms | 39 | NCT04279847 | November 11, 2024 |
| BMS-986158 | Dana-Farber Cancer Institute | Recruiting | Phase 1 | Childhood Lymphoma | 34 | NCT03936465 | July 10, 2024 |
| ZEN003694 | National Cancer Institute (NCI) | Recruiting | Phase 1 Phase 2 |
Advanced Lymphoma | 30 | NCT05053971 | May 1, 2023 |
| CC-95775 | Celgene | Active, not recruiting | Phase 1 | Non-Hodgkin Lymphoma | 24 | NCT04089527 | October 31, 2021 |
| CPI-0610 | Constellation Pharmaceuticals | Recruiting | Phase 3 | Myelofibrosis | 310 | NCT04603495 | September 2023 |
| PLX51107 | M.D. Anderson Cancer Center | Recruiting | Phase 1 | AML, MDS, MPN | 32 | NCT04022785 | December 31, 2022 |
BETi combined with other drugs is more effective than monotherapy
Histone deacetylases inhibitor
It is already known that BET acts as a “reader” of lysine acetylation and binds to acetylated lysine on histones to regulate the expression of important carcinogens, while HDAC acts as an “erasers” to remove enzymes that modify proteins, and the combined application of the two inhibitors has been studied in depth.5,18,19 The combination of HDACi and BETi was investigated in AML and various lymphomas, and the combination had a synergistic effect, mainly by further promoting apoptosis and inhibiting cell proliferation, reducing tumor burden and improving overall survival in animal studies (Table 3).
Table 3.
Combined use of BETi and other drugs in hematologic tumors.
| Hematological malignancy | BETi or BET-PROTAC | Type of combination agent | References |
|---|---|---|---|
| AML | JQ1 | Pan-HADCi, panobinostat | 67 |
| FLT3-ITD + AML | JQ1 | FLT3-TKI, AC220 (quizartinib) | 69 |
| FLT3-ITD + AML | PLX51107 | FLT3-TKI, quizartinib | 70 |
| AML | CPI203 | MDM2i, Idasanutlin/RG7388 | 72 |
| sAML | JQ1 | JAKi, ruxolitinib. HSP90i, AUY922 | 73 |
| AML | ABBV-744 | BCL-2 antagonists, venetoclax | 77 |
| AML/MDS | JQ1 | Hypomethylating agents, azacitidine | 79 |
| T-ALL | JQ1 | BCL-2 antagonists, venetoclax | 82 |
| CLL | GS-5829 | BTKi, ibrutinib; PI3Ki idelalisib | 84 |
| DHL/THL | I-BET | Pan-HDACi, vorinostat | 85 |
| RT-DLBCL | ARV-771 | BTKi, ibrutinib | 90 |
| MCL | JQ1 | BTKi, ibrutinib; HDACi, Panobinostat | 92 |
| MCL | CPI203 | immunomodulators, lenalidomide | 94 |
| CTCL | OTX015 | HDACi, Romidepsin | 97 |
| CTCL | mivebresib | JAKi, ruxolitinib | 98 |
| BL | OTX015 | PI3Ki idelalisib, CDKi SNS-032 and mTORi everolimus | 100 |
| BL | JQ1 | HDACi, Romidepsin | 101 |
| WM | JQ1 | Pan-HDACi, Panobinostat; BTKi, ibrutinib | 102 |
| PEL | JQ1 | immunomodulators, lenalidomide | 103 |
| MM | OTX015 | proteasome inhibitors, lenalidomide, and melphalan | 111 |
| MM | ARV-825 and ARV-763 | dexamethasone, mTORi everolimus and Akti afuresertib | 112 |
| Myc-induced lymphoma | RVX2135 | HDACi, Vorinostat and RVX2135 | 115 |
| DLBCL | PLX51107 and PLX2853 | BCL-2 antagonists, venetoclax | 121 |
| AML | ABBV-075 | BCL-2 antagonists, venetoclax | 122 |
| DLBCL | OTX015 | BTKi, ibrutinib | 125 |
| DLBCL, BL | JQ1, CPI-203 | mTORi everolimus, AKTi MK-2206, PI3Ki CAL-101. | 126 |
| Myc-induced lymphoma | JQ1, RVX2135 | ATRi, AZ20 | 128 |
BCL-2 antagonists
Members of the BCL-2 protein family are key regulators of the intrinsic apoptotic pathway, the dysregulation of which leads to pathological survival of cancer cells.116 BCL-2 antagonist (venetoclax), which is widely used in the treatment of haematological tumors, has been approved for use or entered clinical trials in lymphomas such as MCL, DLBCL and WM and have shown good efficacy, and venetoclax has also been used in combination with demethylating agents to achieve significant results in the treatment of elderly AML.116, 117, 118, 119, 120 The combination of BETi and venetoclax has also been studied in hematologic tumors, which we summarize in Table 3.
Bruton's tyrosine kinase inhibitors
BTK is a key component of B-cell receptor signaling and functions as an essential regulator of cell proliferation and survival in B-cell malignancies.123 BTKi ibrutinib has a high response rate in patients with R/R CLL and MCL, but resistance may develop with monotherapy.124 The combination of BTKi with other drugs is the best way to improve the efficacy, and we summarize the combination of BTKi and BETi in hematologic tumors (Table 3).
Signaling pathways inhibitors
BET proteins interact with a variety of signaling pathways, such as the NF-κB pathway, PI3K/AKT pathway, and JAK/STAT pathway.27,31,35 Moreover, the resistance of leukemic stem cells to BETi is due to the activation of Wnt/β-catenin.78 Here, we summarize the studies related to the combination of BETi and signaling pathway inhibitors in hematologic tumors (Table 3).
Other drugs
The combination of BETi with other drugs has also been studied, and a study by Vangala et al found synergistic effects of JQ1 with the proteasome inhibitor carfilzomib in a variety of cancers, including lung, breast, and prostate cancers.127 In hematologic tumors, the combination of BETi with proteasome inhibitors, immunomodulators, and many other drugs have been studied, which we summarize in Table 3.
Please refer to supplemental tables for more detailed information on combination of drugs.
Conclusion
BET proteins act as “readers” for lysine acetylation and facilitate the recruitment of transcriptional elongation complexes (e.g., P-TEFb), which are closely associated with cellular modifications, transcription, chromatin remodeling, and many other physiological functions. BET plays an important role in many cancers by regulating the cell cycle, apoptosis and aggressiveness of tumor cells. BRD4 is the most studied BET protein, which is closely related to the expression of the proto-oncogene MYC, which is closely involved in the development of hematological tumors. BET is also closely related to the NF-κB pathway, PI3K/AKT pathway, and JAK/STAT pathway. BETi has regulatory effects on immune mechanisms including checkpoint inhibitors, immune cells and cytokines. BET is involved in the development of many hematologic tumors such as AML, DLBCL, MCL, and MM. BETi can inhibit hematologic tumor cell proliferation and promote apoptosis, and many clinical trials of BETi are already in order, with encouraging efficacy of monotherapy. The strong synergistic effect of BETi with other anti-cancer drugs has been confirmed at the cellular level, in animal studies and clinical trials, and the future direction lies in the development of new potent BETi and the study of combination drugs.
Author contributions
TM and YC wrote the manuscript. ZGY drew the picture. ZGY, YHL and JB completed the tables. LJL and LSZ wrote and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interests
The authors declare that the work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
The present study was supported by grants from Cuiying Technology Innovation Project of Lanzhou University Second Hospital (China) (No. CY2017-ZD04 and CY2019-MS14); Commissioned Project of National Clinical Medicine Research Center for Hematological System Diseases (China) (No. 2021WWA01) and Talent Innovation and Entrepreneurship Project of Lanzhou, China (No. 2020-RC-48).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2022.03.004.
Contributor Information
Li-Juan Li, Email: doctorjuan@sina.com.
Lian-Sheng Zhang, Email: doctorzhanglsh@sina.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Dhalluin C., Carlson J.E., Zeng L., et al. Structure and ligand of a histone acetyltransferase bromodomain. Nature. 1999;399(6735):491–496. doi: 10.1038/20974. [DOI] [PubMed] [Google Scholar]
- 2.Filippakopoulos P., Knapp S. Targeting bromodomains: epigenetic readers of lysine acetylation. Nat Rev Drug Discov. 2014;13(5):337–356. doi: 10.1038/nrd4286. [DOI] [PubMed] [Google Scholar]
- 3.Shi J., Vakoc C.R. The mechanisms behind the therapeutic activity of BET bromodomain inhibition. Mol Cell. 2014;54(5):728–736. doi: 10.1016/j.molcel.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang N., Wu R., Tang D., Kang R. The BET family in immunity and disease. Signal Transduct Target Ther. 2021;6(1):23. doi: 10.1038/s41392-020-00384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duan Y., Guan Y., Qin W., Zhai X., Yu B., Liu H. Targeting Brd4 for cancer therapy: inhibitors and degraders. Medchemcomm. 2018;9(11):1779–1802. doi: 10.1039/c8md00198g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devaiah B.N., Case-Borden C., Gegonne A., et al. BRD4 is a histone acetyltransferase that evicts nucleosomes from chromatin. Nat Struct Mol Biol. 2016;23(6):540–548. doi: 10.1038/nsmb.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donati B., Lorenzini E., Ciarrocchi A. BRD4 and cancer: going beyond transcriptional regulation. Mol Cancer. 2018;17(1):164. doi: 10.1186/s12943-018-0915-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devaiah B.N., Mu J., Akman B., et al. MYC protein stability is negatively regulated by BRD4. Proc Natl Acad Sci USA. 2020;117(24):13457–13467. doi: 10.1073/pnas.1919507117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin Z.Y., Wang T., Su S., et al. BRD4 promotes gastric cancer progression and metastasis through acetylation-dependent stabilization of snail. Cancer Res. 2019;79(19):4869–4881. doi: 10.1158/0008-5472.CAN-19-0442. [DOI] [PubMed] [Google Scholar]
- 10.Wu S.Y., Lee C.F., Lai H.T., et al. Opposing functions of BRD4 isoforms in breast cancer. Mol Cell. 2020;78(6):1114–1132. doi: 10.1016/j.molcel.2020.04.034. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szczepanski A.P., Zhao Z., Sosnowski T., Goo Y.A., Bartom E.T., Wang L. ASXL3 bridges BRD4 to BAP1 complex and governs enhancer activity in small cell lung cancer. Genome Med. 2020;12(1):63. doi: 10.1186/s13073-020-00760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 13.Nordlund J., Syvänen A.C. Epigenetics in pediatric acute lymphoblastic leukemia. Semin Cancer Biol. 2018;51:129–138. doi: 10.1016/j.semcancer.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Jovanović K.K., Roche-Lestienne C., Ghobrial I.M., Facon T., Quesnel B., Manier S. Targeting MYC in multiple myeloma. Leukemia. 2018;32(6):1295–1306. doi: 10.1038/s41375-018-0036-x. [DOI] [PubMed] [Google Scholar]
- 15.Jones P.A., Ohtani H., Chakravarthy A., De Carvalho D.D. Epigenetic therapy in immune-oncology. Nat Rev Cancer. 2019;19(3):151–161. doi: 10.1038/s41568-019-0109-9. [DOI] [PubMed] [Google Scholar]
- 16.Nebbioso A., Tambaro F.P., Dell’Aversana C., Altucci L. Cancer epigenetics: moving forward. PLoS Genet. 2018;14(6):e1007362. doi: 10.1371/journal.pgen.1007362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawson M.A., Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150(1):12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Verdin E., Ott M. 50 years of protein acetylation: from gene regulation to epigenetics, metabolism and beyond. Nat Rev Mol Cell Biol. 2015;16(4):258–264. doi: 10.1038/nrm3931. [DOI] [PubMed] [Google Scholar]
- 19.Marmorstein R., Zhou M.M. Writers and readers of histone acetylation: structure, mechanism, and inhibition. Cold Spring Harbor Perspect Biol. 2014;6(7):a018762. doi: 10.1101/cshperspect.a018762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paradise C.R., Galvan M.L., Kubrova E., et al. The epigenetic reader Brd4 is required for osteoblast differentiation. J Cell Physiol. 2020;235(6):5293–5304. doi: 10.1002/jcp.29415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzales-Cope M., Sidoli S., Bhanu N.V., Won K.J., Garcia B.A. Histone H4 acetylation and the epigenetic reader Brd4 are critical regulators of pluripotency in embryonic stem cells. BMC Genom. 2016;17:95. doi: 10.1186/s12864-016-2414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strzyz P. Chromatin: BRD4 kicks out nucleosomes with its HAT. Nat Rev Mol Cell Biol. 2016;17(7):396–397. doi: 10.1038/nrm.2016.72. [DOI] [PubMed] [Google Scholar]
- 23.Lovén J., Hoke H.A., Lin C.Y., et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153(2):320–334. doi: 10.1016/j.cell.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ceribelli M., Kelly P.N., Shaffer A.L., et al. Blockade of oncogenic IκB kinase activity in diffuse large B-cell lymphoma by bromodomain and extraterminal domain protein inhibitors. Proc Natl Acad Sci USA. 2014;111(31):11365–11370. doi: 10.1073/pnas.1411701111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roderick J.E., Tesell J., Shultz L.D., et al. c-Myc inhibition prevents leukemia initiation in mice and impairs the growth of relapsed and induction failure pediatric T-ALL cells. Blood. 2014;123(7):1040–1050. doi: 10.1182/blood-2013-08-522698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabò A., Amati B. BRD4 and MYC—clarifying regulatory specificity. Science. 2018;360(6390):713–714. doi: 10.1126/science.aat6664. [DOI] [PubMed] [Google Scholar]
- 27.Deng G., Zeng F., Su J., et al. BET inhibitor suppresses melanoma progression via the noncanonical NF-κB/SPP1 pathway. Theranostics. 2020;10(25):11428–11443. doi: 10.7150/thno.47432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ambrosini G., Do C., Tycko B., et al. Inhibition of NF-κB-dependent signaling enhances sensitivity and overcomes resistance to BET inhibition in uveal melanoma. Cancer Res. 2019;79(9):2415–2425. doi: 10.1158/0008-5472.CAN-18-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu T., Wang G., Chen W., et al. Co-inhibition of BET proteins and NF-κB as a potential therapy for colorectal cancer through synergistic inhibiting MYC and FOXM1 expressions. Cell Death Dis. 2018;9(3):315. doi: 10.1038/s41419-018-0354-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu X., Qi J., Bradner J.E., Xiao G., Chen L.F. Bromodomain and extraterminal (BET) protein inhibition suppresses human T cell leukemia virus 1 (HTLV-1) Tax protein-mediated tumorigenesis by inhibiting nuclear factor κB (NF-κB) signaling. J Biol Chem. 2013;288(50):36094–36105. doi: 10.1074/jbc.M113.485029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stratikopoulos E.E., Dendy M., Szabolcs M., et al. Kinase and BET inhibitors together clamp inhibition of PI3K signaling and overcome resistance to therapy. Cancer Cell. 2015;27(6):837–851. doi: 10.1016/j.ccell.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shigeta S., Lui G.Y.L., Shaw R., et al. Targeting BET proteins BRD2 and BRD3 in combination with PI3K-AKT inhibition as a therapeutic strategy for ovarian clear cell carcinoma. Mol Cancer Therapeut. 2021;20(4):691–703. doi: 10.1158/1535-7163.MCT-20-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loganathan S.N., Tang N., Holler A.E., Wang N., Wang J. Targeting the IGF1R/PI3K/AKT pathway sensitizes ewing sarcoma to BET bromodomain inhibitors. Mol Cancer Therapeut. 2019;18(5):929–936. doi: 10.1158/1535-7163.MCT-18-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stratikopoulos E.E., Parsons R.E. Molecular pathways: targeting the PI3K pathway in cancer-BET inhibitors to the rescue. Clin Cancer Res. 2016;22(11):2605–2610. doi: 10.1158/1078-0432.CCR-15-2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bagratuni T., Mavrianou N., Gavalas N.G., et al. JQ1 inhibits tumour growth in combination with cisplatin and suppresses JAK/STAT signalling pathway in ovarian cancer. Eur J Cancer. 2020;126:125–135. doi: 10.1016/j.ejca.2019.11.017. [DOI] [PubMed] [Google Scholar]
- 36.Ma Y., Wang L., Neitzel L.R., et al. The MAPK pathway regulates intrinsic resistance to BET inhibitors in colorectal cancer. Clin Cancer Res. 2017;23(8):2027–2037. doi: 10.1158/1078-0432.CCR-16-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shorstova T., Foulkes W.D., Witcher M. Achieving clinical success with BET inhibitors as anti-cancer agents. Br J Cancer. 2021;124(9):1478–1490. doi: 10.1038/s41416-021-01321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shorstova T., Marques M., Su J., et al. SWI/SNF-compromised cancers are susceptible to bromodomain inhibitors. Cancer Res. 2019;79(10):2761–2774. doi: 10.1158/0008-5472.CAN-18-1545. [DOI] [PubMed] [Google Scholar]
- 39.Yeh T.C., O'Connor G., Petteruti P., et al. Identification of CCR2 and CD180 as robust pharmacodynamic tumor and blood biomarkers for clinical use with BRD4/BET inhibitors. Clin Cancer Res. 2017;23(4):1025–1035. doi: 10.1158/1078-0432.CCR-16-1658. [DOI] [PubMed] [Google Scholar]
- 40.Lin X., Huang X., Uziel T., et al. HEXIM1 as a robust pharmacodynamic marker for monitoring target engagement of BET family bromodomain inhibitors in tumors and surrogate tissues. Mol Cancer Therapeut. 2017;16(2):388–396. doi: 10.1158/1535-7163.MCT-16-0475. [DOI] [PubMed] [Google Scholar]
- 41.Filippakopoulos P., Qi J., Picaud S., et al. Selective inhibition of BET bromodomains. Nature. 2010;468(7327):1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaidos A., Caputo V., Gouvedenou K., et al. Potent antimyeloma activity of the novel bromodomain inhibitors I-BET151 and I-BET762. Blood. 2014;123(5):697–705. doi: 10.1182/blood-2013-01-478420. [DOI] [PubMed] [Google Scholar]
- 43.Winter G.E., Buckley D.L., Paulk J., et al. DRUG DEVELOPMENT. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science. 2015;348(6241):1376–1381. doi: 10.1126/science.aab1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu J., Qian Y., Altieri M., et al. Hijacking the E3 ubiquitin ligase cereblon to efficiently target BRD4. Chem Biol. 2015;22(6):755–763. doi: 10.1016/j.chembiol.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spriano F., Stathis A., Bertoni F. Targeting BET bromodomain proteins in cancer: the example of lymphomas. Pharmacol Ther. 2020;215:107631. doi: 10.1016/j.pharmthera.2020.107631. [DOI] [PubMed] [Google Scholar]
- 46.Zhu H., Bengsch F., Svoronos N., et al. BET bromodomain inhibition promotes anti-tumor immunity by suppressing PD-L1 expression. Cell Rep. 2016;16(11):2829–2837. doi: 10.1016/j.celrep.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casey S.C., Tong L., Li Y., et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352(6282):227–231. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hogg S.J., Vervoort S.J., Deswal S., et al. BET-bromodomain inhibitors engage the host immune system and regulate expression of the immune checkpoint ligand PD-L1. Cell Rep. 2017;18(9):2162–2174. doi: 10.1016/j.celrep.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andrieu G.P., Shafran J.S., Smith C.L., et al. BET protein targeting suppresses the PD-1/PD-L1 pathway in triple-negative breast cancer and elicits anti-tumor immune response. Cancer Lett. 2019;465:45–58. doi: 10.1016/j.canlet.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu K., Zhou Z., Gao H., et al. JQ1, a BET-bromodomain inhibitor, inhibits human cancer growth and suppresses PD-L1 expression. Cell Biol Int. 2019;43(6):642–650. doi: 10.1002/cbin.11139. [DOI] [PubMed] [Google Scholar]
- 51.Nikbakht N., Tiago M., Erkes D.A., Chervoneva I., Aplin A.E. BET inhibition modifies melanoma infiltrating T cells and enhances response to PD-L1 blockade. J Invest Dermatol. 2019;139(7):1612–1615. doi: 10.1016/j.jid.2018.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lai X., Stiff A., Duggan M., Wesolowski R., Carson W.E., 3rd, Friedman A. Modeling combination therapy for breast cancer with BET and immune checkpoint inhibitors. Proc Natl Acad Sci USA. 2018;115(21):5534–5539. doi: 10.1073/pnas.1721559115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wellinger L.C., Hogg S.J., Newman D.M., et al. BET inhibition enhances TNF-mediated antitumor immunity. Cancer Immunol Res. 2022;10(1):87–107. doi: 10.1158/2326-6066.CIR-21-0224. [DOI] [PubMed] [Google Scholar]
- 54.Erkes D.A., Field C.O., Capparelli C., et al. The next-generation BET inhibitor, PLX51107, delays melanoma growth in a CD8-mediated manner. Pigment Cell Melanoma Res. 2019;32(5):687–696. doi: 10.1111/pcmr.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Copsel S.N., Lightbourn C.O., Barreras H., et al. BET bromodomain inhibitors which permit treg function enable a combinatorial strategy to suppress GVHD in pre-clinical allogeneic HSCT. Front Immunol. 2019;9:3104. doi: 10.3389/fimmu.2018.03104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sánchez-Ventura J., Amo-Aparicio J., Navarro X., Penas C. BET protein inhibition regulates cytokine production and promotes neuroprotection after spinal cord injury. J Neuroinflammation. 2019;16(1):124. doi: 10.1186/s12974-019-1511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meng S., Zhang L., Tang Y., et al. BET inhibitor JQ1 blocks inflammation and bone destruction. J Dent Res. 2014;93(7):657–662. doi: 10.1177/0022034514534261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu X., Schewitz-Bowers L.P., Lait P.J.P., et al. The bromodomain and extra-terminal protein inhibitor OTX015 suppresses T helper cell proliferation and differentiation. Curr Mol Med. 2018;18(9):594–601. doi: 10.2174/1566524019666190126112238. [DOI] [PubMed] [Google Scholar]
- 59.Mele D.A., Salmeron A., Ghosh S., Huang H.R., Bryant B.M., Lora J.M. BET bromodomain inhibition suppresses TH17-mediated pathology. J Exp Med. 2013;210(11):2181–2190. doi: 10.1084/jem.20130376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Remke N., Bisht S., Oberbeck S., Nolting J., Brossart P. Selective BET-bromodomain inhibition by JQ1 suppresses dendritic cell maturation and antigen-specific T-cell responses. Cancer Immunol Immunother. 2021;70(1):107–121. doi: 10.1007/s00262-020-02665-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun Y., Wang Y., Toubai T., et al. BET bromodomain inhibition suppresses graft-versus-host disease after allogeneic bone marrow transplantation in mice. Blood. 2015;125(17):2724–2728. doi: 10.1182/blood-2014-08-598037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mills R.J., Humphrey S.J., Fortuna P.R.J., et al. BET inhibition blocks inflammation-induced cardiac dysfunction and SARS-CoV-2 infection. Cell. 2021;184(8):2167–2182. doi: 10.1016/j.cell.2021.03.026. e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zuber J., Shi J., Wang E., et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478(7370):524–528. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Basheer F., Huntly B.J. BET bromodomain inhibitors in leukemia. Exp Hematol. 2015;43(8):718–731. doi: 10.1016/j.exphem.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 65.Dawson M.A., Prinjha R.K., Dittmann A., et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478(7370):529–533. doi: 10.1038/nature10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dawson M.A., Gudgin E.J., Horton S.J., et al. Recurrent mutations, including NPM1c, activate a BRD4-dependent core transcriptional program in acute myeloid leukemia. Leukemia. 2014;28(2):311–320. doi: 10.1038/leu.2013.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fiskus W., Sharma S., Qi J., et al. Highly active combination of BRD4 antagonist and histone deacetylase inhibitor against human acute myelogenous leukemia cells. Mol Cancer Therapeut. 2014;13(5):1142–1154. doi: 10.1158/1535-7163.MCT-13-0770. [DOI] [PubMed] [Google Scholar]
- 68.Booth C.A.G., Barkas N., Neo W.H., et al. Ezh2 and Runx1 mutations collaborate to initiate lympho-myeloid leukemia in early thymic progenitors. Cancer Cell. 2018;33(2):274–291. doi: 10.1016/j.ccell.2018.01.006. e8. [DOI] [PubMed] [Google Scholar]
- 69.Fiskus W., Sharma S., Qi J., et al. BET protein antagonist JQ1 is synergistically lethal with FLT3 tyrosine kinase inhibitor (TKI) and overcomes resistance to FLT3-TKI in AML cells expressing FLT-ITD. Mol Cancer Therapeut. 2014;13(10):2315–2327. doi: 10.1158/1535-7163.MCT-14-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee L., Hizukuri Y., Severson P., et al. A novel combination regimen of BET and FLT3 inhibition for FLT3-ITD acute myeloid leukemia. Haematologica. 2021;106(4):1022–1033. doi: 10.3324/haematol.2020.247346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stewart H.J., Horne G.A., Bastow S., Chevassut T.J. BRD4 associates with p53 in DNMT3A-mutated leukemia cells and is implicated in apoptosis by the bromodomain inhibitor JQ1. Cancer Med. 2013;2(6):826–835. doi: 10.1002/cam4.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Latif A.L., Newcombe A., Li S., et al. BRD4-mediated repression of p53 is a target for combination therapy in AML. Nat Commun. 2021;12(1):241. doi: 10.1038/s41467-020-20378-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saenz D.T., Fiskus W., Manshouri T., et al. BET protein bromodomain inhibitor-based combinations are highly active against post-myeloproliferative neoplasm secondary AML cells. Leukemia. 2017;31(3):678–687. doi: 10.1038/leu.2016.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berthon C., Raffoux E., Thomas X., et al. Bromodomain inhibitor OTX015 in patients with acute leukaemia: a dose-escalation, phase 1 study. Lancet Haematol. 2016;3(4):e186–e195. doi: 10.1016/S2352-3026(15)00247-1. [DOI] [PubMed] [Google Scholar]
- 75.Roboz G.J., Desai P., Lee S., et al. A dose escalation study of RO6870810/TEN-10 in patients with acute myeloid leukemia and myelodysplastic syndrome. Leuk Lymphoma. 2021;62(7):1740–1748. doi: 10.1080/10428194.2021.1881509. [DOI] [PubMed] [Google Scholar]
- 76.Borthakur G., Odenike O., Aldoss I., et al. A phase 1 study of the pan-bromodomain and extraterminal inhibitor mivebresib (ABBV-075) alone or in combination with venetoclax in patients with relapsed/refractory acute myeloid leukemia. Cancer. 2021;127(16):2943–2953. doi: 10.1002/cncr.33590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang L., Cai T., Lin X., et al. Selective inhibition of the second bromodomain of BET family proteins results in robust antitumor activity in preclinical models of acute myeloid leukemia. Mol Cancer Therapeut. 2021;20(10):1809–1819. doi: 10.1158/1535-7163.MCT-21-0029. [DOI] [PubMed] [Google Scholar]
- 78.Fong C.Y., Gilan O., Lam E.Y., et al. BET inhibitor resistance emerges from leukaemia stem cells. Nature. 2015;525(7570):538–542. doi: 10.1038/nature14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pericole F.V., Lazarini M., de Paiva L.B., et al. BRD4 inhibition enhances azacitidine efficacy in acute myeloid leukemia and myelodysplastic syndromes. Front Oncol. 2019;9:16. doi: 10.3389/fonc.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mertz J.A., Conery A.R., Bryant B.M., et al. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc Natl Acad Sci USA. 2011;108(40):16669–16674. doi: 10.1073/pnas.1108190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu S., Jiang Y., Hong Y., et al. BRD4 PROTAC degrader ARV-825 inhibits T-cell acute lymphoblastic leukemia by targeting ‘Undruggable’ Myc-pathway genes. Cancer Cell Int. 2021;21(1):230. doi: 10.1186/s12935-021-01908-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peirs S., Frismantas V., Matthijssens F., et al. Targeting BET proteins improves the therapeutic efficacy of BCL-2 inhibition in T-cell acute lymphoblastic leukemia. Leukemia. 2017;31(10):2037–2047. doi: 10.1038/leu.2017.10. [DOI] [PubMed] [Google Scholar]
- 83.Zhou B., Hu J., Xu F., et al. Discovery of a small-molecule degrader of bromodomain and extra-terminal (BET) proteins with picomolar cellular potencies and capable of achieving tumor regression. J Med Chem. 2018;61(2):462–481. doi: 10.1021/acs.jmedchem.6b01816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim E., Ten Hacken E., Sivina M., et al. The BET inhibitor GS-5829 targets chronic lymphocytic leukemia cells and their supportive microenvironment. Leukemia. 2020;34(6):1588–1598. doi: 10.1038/s41375-019-0682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li W., Gupta S.K., Han W., et al. Targeting MYC activity in double-hit lymphoma with MYC and BCL2 and/or BCL6 rearrangements with epigenetic bromodomain inhibitors. J Hematol Oncol. 2019;12(1):73. doi: 10.1186/s13045-019-0761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Riedell P.A., Smith S.M. Double hit and double expressors in lymphoma: definition and treatment. Cancer. 2018;124(24):4622–4632. doi: 10.1002/cncr.31646. [DOI] [PubMed] [Google Scholar]
- 87.Dickinson M., Briones J., Herrera A.F., et al. Phase Ib study of the BET protein inhibitor RO6870810 with venetoclax and rituximab in patients with diffuse large B-cell lymphoma. Blood Adv. 2021;5(22):4762–4770. doi: 10.1182/bloodadvances.2021004619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shapiro G.I., LoRusso P., Dowlati A., et al. A Phase 1 study of RO6870810, a novel bromodomain and extra-terminal protein inhibitor, in patients with NUT carcinoma, other solid tumours, or diffuse large B-cell lymphoma. Br J Cancer. 2021;124(4):744–753. doi: 10.1038/s41416-020-01180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Amorim S., Stathis A., Gleeson M., et al. Bromodomain inhibitor OTX015 in patients with lymphoma or multiple myeloma: a dose-escalation, open-label, pharmacokinetic, phase 1 study. Lancet Haematol. 2016;3(4):e196–e204. doi: 10.1016/S2352-3026(16)00021-1. [DOI] [PubMed] [Google Scholar]
- 90.Fiskus W., Mill C.P., Perera D., et al. BET proteolysis targeted chimera-based therapy of novel models of Richter Transformation-diffuse large B-cell lymphoma. Leukemia. 2021;35(9):2621–2634. doi: 10.1038/s41375-021-01181-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen C.C., Hsu C.C., Chen S.L., et al. RAS mediates BET inhibitor-endued repression of lymphoma migration and prognosticates a novel proteomics-based subgroup of DLBCL through its negative regulator IQGAP3. Cancers. 2021;13(19):5024. doi: 10.3390/cancers13195024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sun B., Shah B., Fiskus W., et al. Synergistic activity of BET protein antagonist-based combinations in mantle cell lymphoma cells sensitive or resistant to ibrutinib. Blood. 2015;126(13):1565–1574. doi: 10.1182/blood-2015-04-639542. Blood. 2015;126(13):1565–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun B., Fiskus W., Qian Y., et al. BET protein proteolysis targeting chimera (PROTAC) exerts potent lethal activity against mantle cell lymphoma cells. Leukemia. 2018;32(2):343–352. doi: 10.1038/leu.2017.207. [DOI] [PubMed] [Google Scholar]
- 94.Moros A., Rodríguez V., Saborit-Villarroya I., et al. Synergistic antitumor activity of lenalidomide with the BET bromodomain inhibitor CPI203 in bortezomib-resistant mantle cell lymphoma. Leukemia. 2014;28(10):2049–2059. doi: 10.1038/leu.2014.106. [DOI] [PubMed] [Google Scholar]
- 95.Kohnken R., McNeil B., Wen J., et al. Preclinical targeting of microRNA-214 in cutaneous T-cell lymphoma. J Invest Dermatol. 2019;139(9):1966–1974. doi: 10.1016/j.jid.2019.01.033. e3. [DOI] [PubMed] [Google Scholar]
- 96.Kamijo H., Sugaya M., Takahashi N., et al. BET bromodomain inhibitor JQ1 decreases CD30 and CCR4 expression and proliferation of cutaneous T-cell lymphoma cell lines. Arch Dermatol Res. 2017;309(6):491–497. doi: 10.1007/s00403-017-1749-9. [DOI] [PubMed] [Google Scholar]
- 97.Zhao L., Okhovat J.P., Hong E.K., Kim Y.H., Wood G.S. Preclinical studies support combined inhibition of BET family proteins and histone deacetylases as epigenetic therapy for cutaneous T-cell lymphoma. Neoplasia. 2019;21(1):82–92. doi: 10.1016/j.neo.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yumeen S., Mirza F.N., Lewis J.M., et al. JAK inhibition synergistically potentiates BCL2, BET, HDAC, and proteasome inhibition in advanced CTCL. Blood Adv. 2020;4(10):2213–2226. doi: 10.1182/bloodadvances.2020001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schmitz R., Young R.M., Ceribelli M., et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012;490(7418):116–120. doi: 10.1038/nature11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tomska K., Kurilov R., Lee K.S., et al. Drug-based perturbation screen uncovers synergistic drug combinations in Burkitt lymphoma. Sci Rep. 2018;8(1):12046. doi: 10.1038/s41598-018-30509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cortiguera M.G., García-Gaipo L., Wagner S.D., León J., Batlle-López A., Delgado M.D. Suppression of BCL6 function by HDAC inhibitor mediated acetylation and chromatin modification enhances BET inhibitor effects in B-cell lymphoma cells. Sci Rep. 2019;9(1):16495. doi: 10.1038/s41598-019-52714-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Matissek S.J., Han W., Karbalivand M., et al. Epigenetic targeting of Waldenström macroglobulinemia cells with BET inhibitors synergizes with BCL2 or histone deacetylase inhibition. Epigenomics. 2021;13(2):129–144. doi: 10.2217/epi-2020-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gopalakrishnan R., Matta H., Tolani B., Triche T., Jr., Chaudhary P.M. Immunomodulatory drugs target IKZF1-IRF4-MYC axis in primary effusion lymphoma in a cereblon-dependent manner and display synergistic cytotoxicity with BRD4 inhibitors. Oncogene. 2016;35(14):1797–1810. doi: 10.1038/onc.2015.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boi M., Todaro M., Vurchio V., et al. Therapeutic efficacy of the bromodomain inhibitor OTX015/MK-8628 in ALK-positive anaplastic large cell lymphoma: an alternative modality to overcome resistant phenotypes. Oncotarget. 2016;7(48):79637–79653. doi: 10.18632/oncotarget.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhan F., Barlogie B., Arzoumanian V., et al. Gene-expression signature of benign monoclonal gammopathy evident in multiple myeloma is linked to good prognosis. Blood. 2007;109(4):1692–1700. doi: 10.1182/blood-2006-07-037077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mattioli M., Agnelli L., Fabris S., et al. Gene expression profiling of plasma cell dyscrasias reveals molecular patterns associated with distinct IGH translocations in multiple myeloma. Oncogene. 2005;24(15):2461–2473. doi: 10.1038/sj.onc.1208447. [DOI] [PubMed] [Google Scholar]
- 107.Delmore J.E., Issa G.C., Lemieux M.E., et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146(6):904–917. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Imayoshi N., Yoshioka M., Chauhan J., et al. CG13250, a novel bromodomain inhibitor, suppresses proliferation of multiple myeloma cells in an orthotopic mouse model. Biochem Biophys Res Commun. 2017;484(2):262–268. doi: 10.1016/j.bbrc.2017.01.088. [DOI] [PubMed] [Google Scholar]
- 109.Guo N.H., Zheng J.F., Zi F.M., Cheng J. I-BET151 suppresses osteoclast formation and inflammatory cytokines secretion by targetting BRD4 in multiple myeloma. Biosci Rep. 2019;39(5):1245. doi: 10.1042/BSR20181245. BSR20181245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shi J., Song S., Han H., et al. Potent activity of the bromodomain inhibitor OTX015 in multiple myeloma. Mol Pharm. 2018;15(9):4139–4147. doi: 10.1021/acs.molpharmaceut.8b00554. [DOI] [PubMed] [Google Scholar]
- 111.Gu J., Song S., Han H., et al. The BET bromodomain inhibitor OTX015 synergizes with targeted agents in multiple myeloma. Mol Pharm. 2018;15(11):5387–5396. doi: 10.1021/acs.molpharmaceut.8b00880. [DOI] [PubMed] [Google Scholar]
- 112.Zhang X., Lee H.C., Shirazi F., et al. Protein targeting chimeric molecules specific for bromodomain and extra-terminal motif family proteins are active against pre-clinical models of multiple myeloma. Leukemia. 2018;32(10):2224–2239. doi: 10.1038/s41375-018-0044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lim S.L., Xu L., Han B.C., Shyamsunder P., Chng W.J., Koeffler H.P. Multiple myeloma: combination therapy of BET proteolysis targeting chimeric molecule with CDK9 inhibitor. PLoS One. 2020;15(6) doi: 10.1371/journal.pone.0232068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ramasamy K., Nooka A., Quach H., et al. A phase 1b dose-escalation/expansion study of BET inhibitor RO6870810 in patients with advanced multiple myeloma. Blood Cancer J. 2021;11(9):149. doi: 10.1038/s41408-021-00545-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bhadury J., Nilsson L.M., Muralidharan S.V., et al. BET and HDAC inhibitors induce similar genes and biological effects and synergize to kill in Myc-induced murine lymphoma. Proc Natl Acad Sci USA. 2014;111(26):E2721–E2730. doi: 10.1073/pnas.1406722111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ryan C.E., Davids M.S. BCL-2 inhibitors, present and future. Cancer J. 2019;25(6):401–409. doi: 10.1097/PPO.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 117.Zelenetz A.D., Salles G., Mason K.D., et al. Venetoclax plus R- or G-CHOP in non-Hodgkin lymphoma: results from the CAVALLI phase 1b trial. Blood. 2019;133(18):1964–1976. doi: 10.1182/blood-2018-11-880526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jain P., Wang M. Mantle cell lymphoma: 2019 update on the diagnosis, pathogenesis, prognostication, and management. Am J Hematol. 2019;94(6):710–725. doi: 10.1002/ajh.25487. [DOI] [PubMed] [Google Scholar]
- 119.Castillo J.J., Treon S.P. Management of Waldenström macroglobulinemia in 2020. Hematology Am Soc Hematol Educ Program. 2020;2020(1):372–379. doi: 10.1182/hematology.2020000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Konopleva M., Letai A. BCL-2 inhibition in AML: an unexpected bonus? Blood. 2018;132(10):1007–1012. doi: 10.1182/blood-2018-03-828269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cummin T.E.C., Cox K.L., Murray T.D., et al. BET inhibitors synergize with venetoclax to induce apoptosis in MYC-driven lymphomas with high BCL-2 expression. Blood Adv. 2020;4(14):3316–3328. doi: 10.1182/bloodadvances.2020002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bui M.H., Lin X., Albert D.H., et al. Preclinical characterization of BET family bromodomain inhibitor ABBV-075 suggests combination therapeutic strategies. Cancer Res. 2017;77(11):2976–2989. doi: 10.1158/0008-5472.CAN-16-1793. [DOI] [PubMed] [Google Scholar]
- 123.Gabizon R., London N. A fast and clean BTK inhibitor. J Med Chem. 2020;63(10):5100–5101. doi: 10.1021/acs.jmedchem.0c00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pal Singh S., Dammeijer F., Hendriks R.W. Role of bruton's tyrosine kinase in B cells and malignancies. Mol Cancer. 2018;17(1):57. doi: 10.1186/s12943-018-0779-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Boi M., Gaudio E., Bonetti P., et al. The BET bromodomain inhibitor OTX015 affects pathogenetic pathways in preclinical B-cell tumor models and synergizes with targeted drugs. Clin Cancer Res. 2015;21(7):1628–1638. doi: 10.1158/1078-0432.CCR-14-1561. [DOI] [PubMed] [Google Scholar]
- 126.Derenzini E., Mondello P., Erazo T., et al. BET inhibition-induced GSK3β feedback enhances lymphoma vulnerability to PI3K inhibitors. Cell Rep. 2018;24(8):2155–2166. doi: 10.1016/j.celrep.2018.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vangala J.R., Potluri A., Radhakrishnan S.K. BET inhibitors synergize with carfilzomib to induce cell death in cancer cells via impairing Nrf1 transcriptional activity and exacerbating the unfolded protein response. Biomolecules. 2020;10(4):501. doi: 10.3390/biom10040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Muralidharan S.V., Bhadury J., Nilsson L.M., Green L.C., McLure K.G., Nilsson J.A. BET bromodomain inhibitors synergize with ATR inhibitors to induce DNA damage, apoptosis, senescence-associated secretory pathway and ER stress in Myc-induced lymphoma cells. Oncogene. 2016;35(36):4689–4697. doi: 10.1038/onc.2015.521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.