Abstract
Background
Post-viral olfactory dysfunction is a common cause of both short- and long-term smell alteration. The coronavirus pandemic further highlights the importance of post-viral olfactory dysfunction. Currently, a comprehensive review of the neural mechanism underpinning post-viral olfactory dysfunction is lacking.
Objectives
To synthesize the existing primary literature related to olfactory dysfunction secondary to viral infection, detail the underlying pathophysiological mechanisms, highlight relevance for the current COVID-19 pandemic, and identify high impact areas of future research.
Methods
PubMed and Embase were searched to identify studies reporting primary scientific data on post-viral olfactory dysfunction. Results were supplemented by manual searches. Studies were categorized into animal and human studies for final analysis and summary.
Results
A total of 38 animal studies and 7 human studies met inclusion criteria and were analyzed. There was significant variability in study design, experimental model, and outcome measured. Viral effects on the olfactory system varies significantly based on viral substrain but generally include damage or alteration in components of the olfactory epithelium and/or the olfactory bulb.
Conclusions
The mechanism of post-viral olfactory dysfunction is highly complex, virus-dependent, and involves a combination of insults at multiple levels of the olfactory pathway. This will have important implications for future diagnostic and therapeutic developments for patients infected with COVID-19.
Keywords: anosmia, hyposmia, smell dysfunction, olfactory dysfunction, post-viral olfactory dysfunction, coronavirus, SARS, influenza, respiratory syncytial virus, herpes simplex virus, neuropathology
Introduction
The pandemic of COVID-19, caused by a novel strain of coronavirus (SARS-CoV-2), is rapidly spreading globally. In the acute phase, symptoms commonly reported include fatigue, cough, dyspnea, myalgia, joint pain, anosmia, dysgeusia, and lack of appetite. 1 Mounting evidence suggests that isolated chemosensory dysfunction – namely hyposmia, anosmia, and dysgeusia – is frequent. 2 Recent reports indicate the incidence of anosmia in COVID-19 positive patients is as high as 80–98%, with olfactory dysfunction (OD) being the primary symptom in nearly 12% of patients.3,4
Anosmia related to viral upper respiratory tract infection (URI) is not unique to COVID-19. Post-viral olfactory dysfunction (PVOD) has long been recognized as a major cause of clinically significant olfactory loss, accounting for up to 40% of the overall incidence in OD.5,6 Nevertheless, the precise neuropathological mechanism underpinning PVOD in general is poorly understood.
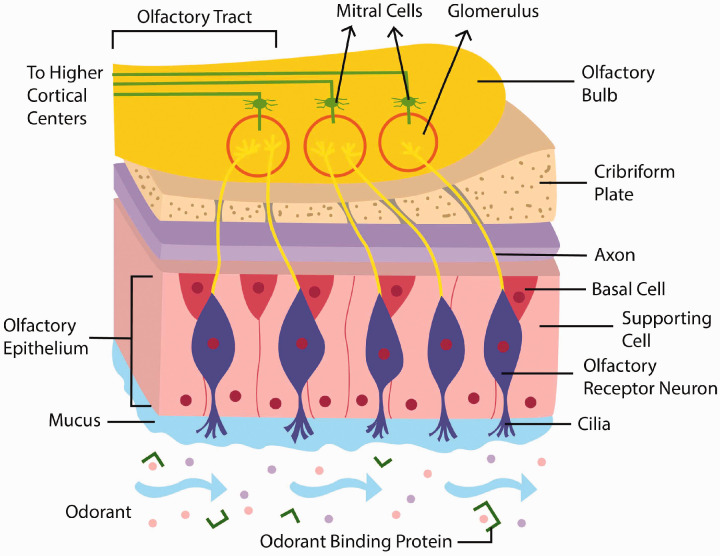
The olfactory system is complex, and its organization consists of distinct structural and functional cellular components (Figure 1). Briefly, odorants must reach the olfactory epithelium (OE) and bind to odorant binding proteins (OBPs). Activation of olfactory receptor neurons (ORNs) then transmits the resultant signal to the olfactory bulb (OB). The summation of this signal is subsequently transduced to higher olfactory cortices and central sensory areas in the brain, where olfactory processing becomes increasingly more complex. Additionally, there are multiple supporting and regenerative cells critical to olfactory function that continually replenish and maintain this system. A viral insult to any of these interconnected components may potentially result in OD.
Figure 1.
A simplified illustration of the olfactory pathway, from initial odorant binding to signal transduction to higher olfactory cortical centers.
Here, we present a systematic review that summarizes and synthesizes the current scientific literature on PVOD from both animal and human studies, with an emphasis on pathophysiologic mechanisms. Based on the available data, we propose a novel “Two-Hit Hypothesis” on the development of PVOD and highlight implications of this hypothesis for the development and deployment of novel therapeutics, especially those that may have immediate relevance during the current COVID-19 pandemic.
Methods
Literature Search
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed for this study. We conducted a broad literature search in PubMed and Embase from inception through January 31st, 2020 for studies reporting primary scientific data investigating the mechanism of PVOD. The comprehensive search terms used, including Mesh Terms, was the following: “olfaction disorders”[MeSH Terms] OR “olfactory dysfunction” OR “olfactory disorder” OR “anosmia” OR “hyposmia” OR “smell disorder” OR “olfactory loss” OR “hyposmia” OR “smell” AND “respiratory tract infections”[MeSH Terms] OR “Virus” OR “viral” OR “postviral” OR “post-viral” OR “post-infectious” OR “postinfectious” OR “URI” OR “URTI” OR “severe acute respiratory syndrome” OR “SARS” OR “covid” OR “coronavirus” OR “influenza” OR “rhinovirus” OR “respiratory syncytial virus” OR “human metapneumovirus” OR “herpes simplex virus” OR “parainfluenza virus” OR “cytomegalovirus” OR “Epstein-barr virus” OR “adenovirus”. Additional searches were conducted for each aforementioned URI virus type with AND “olfactory” OR “olfaction” OR “smell”.
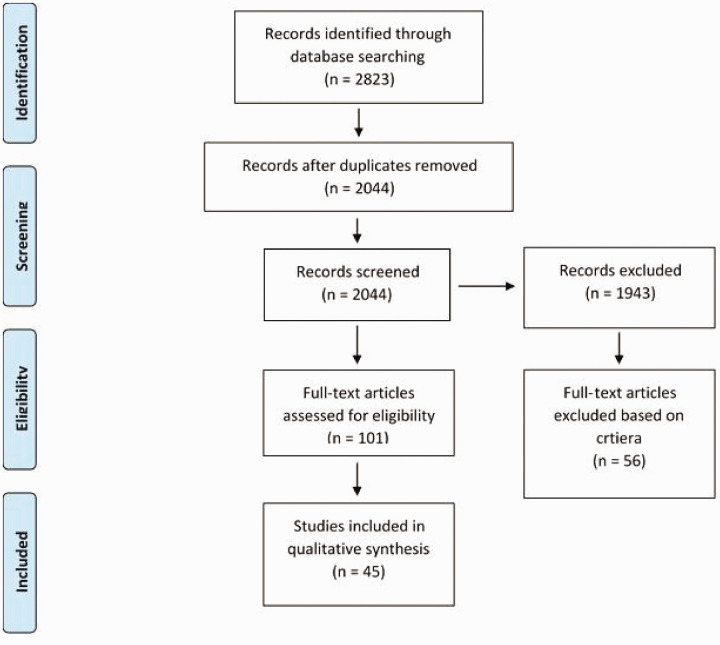
Two authors independently completed the literature search and reviewed titles and abstracts, eliminating ineligible articles. Disagreements in article selection were discussed in depth, and a third independent reviewer was involved if a tie-breaking vote was needed. Figure 2 displays initial studies identified and excluded studies at different screening points.
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flowchart based on our predefined inclusion and exclusion criteria.
Study Selection
Only primary, retrievable scientific literature available in the English language were included. Only literature examining viruses that are known to cause acute URI in humans or are analogous in the experimental species were included. Many viruses, such as vesicular stomatitis virus, rabies virus, and poliovirus, are also neurotropic and may target the olfactory system. 7 However, studies of these viruses were beyond the scope of this review focusing on viral URI-induced olfactory loss and thus were excluded.
Overall exclusion criteria for animal studies was as follows. Studies that examined only viral titers as parameters were excluded. In animal studies, experimental models utilizing viral injection directly into the olfactory bulb (OB) were excluded as such models contrast with the natural mode of URI transmission and induce cytotoxicity and inflammation beyond what would be expected. Furthermore, studies that did not examine either the olfactory epithelium (OE) or OB, or their component parts, were excluded as pathophysiologic mechanisms could not be determined.
Human studies were limited to those assessing PVOD. Studies that investigate viral detection or serology without further investigation of any mechanistic component (i.e. anatomical or physiological changes within the olfactory system) were excluded as well. Studies of sinonasal inflammatory disorders or OD secondary to head trauma were excluded. Additionally, studies examining prognosis and treatment of PVOD were also excluded from the formal systematic review. For both animal and human studies, references within the selected articles were also reviewed.
Data Extraction
A wide variety of methods were utilized in selected studies. For animal studies, data extracted included animal model, type of virus examined, structural olfactory unit examined, morphological changes, functional cellular unit examined, involvement of inflammation, activation of microglia, any role of apoptosis or programmed cell death, and any olfactory testing employed in the study. For human studies, data extraction assessed study type, sample sizes, imaging modality, anatomical site or brain region studied, brief summary of results, olfactory testing used, and any limitations of the study relevant to understanding the pathophysiology of PVOD.
Results
The systematic search resulted in a total of 45 primary scientific articles specific to the investigation of PVOD (Figure 2). A subtotal of 38 animal studies were found and are summarized. Data from animal studies are listed and summarized in Table 1. A subtotal of 7 human studies were identified and are summarized in Table 2.
Table 1.
Animal Studies.
| Virus | Model | Examined Region | Lesioned Site | Affected Cell | Microglia | Inflammation | Cytokine | Apoptosis |
|---|---|---|---|---|---|---|---|---|
| RSV 8 | Mice 8 | OE, OB | OE− | ORN+/BS+ | N/A | N/A | N/A | N/A |
| Mice 9 | OE, OB | OE+, OB− | ORN+ | N/A | Yes | IL-17C | N/A | |
| CMV | Mice 10 | OE | OE+ | ORN+/SC+ | N/A | N/A | N/A | N/A |
| Mice 11 | OE | N/A | ORN+/SC+ | N/A | N/A | N/A | N/A | |
| Mice 12 | OE | N/A | ORN+/SC+ | N/A | N/A | N/A | N/A | |
| HSV | Mice 13 | OB, HCR | OB+, HCR+ | MC+/GC+Neurons+ | Yes | Yes | TNF-α IFN-γ | Unchanged |
| Mice 14 | OB, HCR | N/A | MC+ | N/A | Yes | N/A | N/A | |
| Rats 15 | OB, HCR | OB+, HCR+ | N/A | N/A | Yes | N/A | N/A | |
| Mice 16 | OB, HCR | OB+, HCR+ | MC+/GC+ | Activated | Yes | N/A | N/A | |
| Mice 17 | OE, OB, HCR | OB+, HCR+ | MC+/GC+ | N/A | Yes | N/A | N/A | |
| Mice 18 ¥ | OE, OB | 186 - OE+/OB+/HCR+L1BR1 -OE+/OB−/HCR− | ORN+ | N/A | N/A | N/A | 186 - unchangedL1BR1 - increased | |
| Influenza | Chicken 19 | OE, OB, HCR | OE+/OB−/HCR+ | ORN−/SC+/BC+; Neurons+, glial cells+ | Activated | N/A | N/A | N/A |
| Ferrets 20 | OB, HCR | OB+, HCR+ | Neurons+ | Activated | Yes | N/A | N/A | |
| Ferrets 21 ¥ | OE | H5N1 - OE+H3N2 - OE−H1N1 - OE+ | N/A | N/A | Yes | N/A | N/A | |
| Ferrets 22 | OE, OB, HCR | OE+/OB+/HCR+ | ORN+/MC+/GC+ | N/A | Yes | N/A | N/A | |
| Ferrets 23 ¥ | OE, OB, HCR | H5N1 - OE+/OB+/HCR+MBCS -OE+/OB−/HCR− | ORN+/MC+/GC+ | Activated | Yes | N/A | N/A | |
| Mice 24 | OB, HCR | N/A | MC+/Neuron+ | Activated | N/A | N/A | Increased | |
| Ferrets 25 ¥ | OB, HCR | VN1203 - OB+/HCR+HK483 - OB+/HCR+ | N/A | N/A | Yes | N/A | N/A | |
| Ferrets 26 ¥ | OB, HCR | HK486 - OB+/HCR+HK483 - OB+/HCR+ | N/A | N/A | Yes | N/A | N/A | |
| Mice 27 | OB, HCR | OB+/HCR+ | ORN+/Neurons+ | N/A | N/A | N/A | N/A | |
| Mice 28 ¥ | OB, HCR | HK483 - OB+/HCR+PR8 - Epithelial cells | HK483 -Neurons+/Glial cellsPR8 -Epithelial cells | N/A | Yes | N/A | N/A | |
| Ferrets 29 | OE, OB | OE+/OB− | N/A | N/A | Yes | TNF-αIL-6 | N/A | |
| Mice 30 | OB | N/A | Neuron-/Glial cells+ | Activated | N/A | TNF-α IL1-β | N/A | |
| Mice 31 | OB | OB+ | N/A | N/A | N/A | TNF-α IL1-β | N/A | |
| Mice 32 | OE, OB | OE+, OB− | ORN+ | Activated | No | N/A | Increased | |
| Mice Tg 33 | OE, OB, HCR | OE+, OB+, HCR+ | ORN+/SC−/MC+/TC+/GC+ | Activated | Yes | N/A | N/A | |
| Coronavirus | Mice 34 | OB | N/A | N/A | Activated | N/A | IFN | N/A |
| Mice 35 | OB | OB+/HCR+ | MC+ | N/A | Yes | N/A | N/A | |
| Mice Tg 36 | OB, HCR | OB−/HCR+ | N/A | N/A | No | N/A | N/A | |
| Mice 37 | OB | OB+ | ORN+ | N/A | N/A | N/A | Increased | |
| Mice 38 | OE, OB, HCR | OE−/OB+/HCR+ | ORN+/MC+/GC+/TC+ | N/A | Yes | N/A | N/A | |
| Mice 39 | OE, OB | OE−/OB+ | ORN+/MC+/GC+/TC+ | N/A | N/A | N/A | N/A | |
| Mice 40 | OE | OE− | ORN+ | N/A | N/A | N/A | N/A | |
| Parainfluenza | Mice 41 | OE, OB | OE− | ORN+/BC+ | N/A | No | N/A | Decreased |
| Mice 42 | OE, OB | OE+/OB− | ORN+/MC−/TC− | N/A | N/A | N/A | N/A | |
| Dogs 43 | OE | OE− | N/A | N/A | No | N/A | N/A | |
| Mice 44 | OE, OB, HCR | OE−/OB−/HCR− | ORN+/SC+ | N/A | No | N/A | N/A | |
| Mice 45 | OE, OB | OE+/OB− | ORN+/SC−/MC−/TC− | N/A | N/A | N/A | N/A |
Abbreviations: OE, olfactory epithelium; OB, olfactory bulb; HCR, higher cortical centers; ORN, olfactory receptor neuron; SC, supporting cell; BS, basal cell; MC, mitrial cell; GC, granule cell; TC, tufted cell; Neurons, neural cells not otherwise specified in the article; +, denotes lesion or cell infected cells; − denotes no lesion or uninfected cells; Tg, transgenic; N/A, not applicable; ¥, studies that used multiple viral strains.
Table 2.
Human Studies.
| Authors | Year | Study Type | Sample Size | Imaging Modality | Anatomical Site or Brain Region Studied | Results | Olfactory Testing |
|---|---|---|---|---|---|---|---|
| Altundag et al. 46 | 2020 | Retrospective Case–Control | n = 71 | CT Scan | OC | Significantly greater OC width and volume in OD group compared to controls. No difference in olfactory fossa depth | Sniffin Sticks |
| Wolf et al. 47 | 2018 | Cross-sectional | n = 21 | N/A | OC | 1117 different proteins detected with 10 being most abundantNo differences between OD patients and healthy controls in olfactory cleft proteome | Sniffin Sticks |
| Yao et al. 48 | 2018 | Cross-sectional | n = 38 | Voxel-based MRI | OB/OFC | Significant decrease in right OFC and total OB volume in PVOD. Significant negative correlation between right OFC and OB volume and duration of olfactory loss | T&T Olfactometry & Sniffin Sticks |
| Kim et al. 49 | 2012 | Cross-sectional | n = 18 | FDG-PET | OFC | Significant hypometabolism in olfactory sensory and integration brain regions in PVOD patients compared to controlsRegional metabolism in cortical regions inversely correlated with olfactory tests | Olfactory Butanol Threshold Test & Olfactory Identification Test |
| Rombaux et al. 50 | 2009 | Review + Cross-sectional Cohort | n = 122 | MRI | OB | Reduced OB volume in all patientsOB volume significantly correlated with TDI and retronasal scores. | Sniffin SticksRetronasal Testing |
| Rombaux et al. 51 | 2006 | Retrospective Cohort | n = 26 | MRI | OB/Olfactory Sulcus | OB volume smaller in anosmic patients compared to hyposmia. No difference in olfactory sulcus depth in hyposmia vs. anosmia patients. Significant negative correlation between OB volume and duration of olfactory loss | Sniffin Sticks |
| Yamagishi et al. 52 | 1994 | Cross-sectional | n = 70 | N/A | Olfactory Epithelium | Variable damage to and degeneration of peripheral ORNs identified on mucosal samples |
T&T Olfactometry & IV Alinamin Injection Test |
Abbreviations: PVOD, post-viral olfactory dysfunction; OC, olfactory cleft; OD, olfactory dysfunction; OFC, orbitofrontal cortex; OB, olfactory bulb; T&T, odor-threshold & identification; TDI, Threshold, Discrimination, Identification; ORN, Olfactory Receptor Neuron; NSE, neuron-specific enolase.
Animal Studies
There was high variability with respect to methodology and outcome measured in experimental models of OD induced by viral infection. Of the 38 animal studies included in the review, 29 (76.3%) were conducted using mice or rats, 7 (18.5%) were done using ferrets, one (2.6%) was done using dogs, and one (2.6%) using chicken. Of the different type of viruses used, influenza was the most widely studied, followed by coronavirus, herpes simplex virus (HSV), parainfluenza virus, cytomegalovirus (CMV), and respiratory syncytial virus (RSV).
In influenza virus, all studies that examined OE demonstrated lesional changes in the OE,19,21–23,29,32,33 with exception of a non-neurovirulent subtype H3N2. 21 Olfactory bulb was examined specifically in 12 studies, with 9 studies (75%) showing histological evidence of lesional changes.20,22,23,25–28,31,33 Neural damages in higher cortical centers were also noted in 9 studies.19,20,22,23,25–28,33 Damages to various cell types within the olfactory system were observed, including ORNs,22,23,27,32,33 supporting cells (SC), 19 basal cells (BC), 19 granule cells (GC),22,23,33 and mitral cells (MC).22–24,33
In coronavirus, none of the studies demonstrated gross lesioning in the OE.38–40 However, the number or turnover of ORNs were negatively impacted.37–40 The OB was specifically examined histologically in 4 studies, with 3 studies (75%) showing some evidence of lesional changes.35,38,53 Three studies that investigated higher cortical centers also noted neuronal changes.35,36,38
In parainfluenza virus, 2 of the 5 studies noted gross changes in the OE,42,45 while 3 others did not.41,43,44 Notably, no gross changes in the olfactory bulb or higher cortical centers were demonstrated.42,44,45 The ORNs were damaged or negatively impacted in all available studies.41,42,44,45
In HSV, neuronal lesions were identified throughout the olfactory system, including the OE, OB, as well as higher cortical centers in all 6 included studies,13–18 with the exception of a neuro-attenuated L1BR1 substrain. 18
Human Studies
Human studies of PVOD also varied widely in methodology, outcomes, and approach. At the level of the OE, viral insult caused variable degeneration and death of the ORNs on histopathology with biopsy. 52 Increase in olfactory cleft (OC) width and volume was noted to be a potential risk factor for PVOD. 46 Three studies demonstrated significant reduction in OB volume in patients with PVOD,48,50,51 and that greater duration of PVOD led to greater reduction in OB volume.48,51 Finally, one study showed that metabolic activities of higher olfactory centers were reduced following PVOD. 49
Discussion
Our systematic review revealed that PVOD is attributable to disruption at many levels of the olfactory pathway by the cumulative effects of direct cell damage, inflammation, and cytokine effects. Most animal studies demonstrated that the numbers of ORNs are reduced in the OE and OB, which is likely the major cause of olfactory disruption following most types of viral infections. Importantly, our review also highlighted the fact that targets of virus-mediated cellular disruption in the olfactory system are viral substrain dependent. This has significant implications and emphasizes the need to study and direct patient care in a highly virus-specific manner following PVOD. Our review also found that human studies consistently suggest that both peripheral and central olfactory structures are negatively impacted in relation to structural integrity, volume, or metabolism. Many important studies in humans have shown putative viral agents in PVOD. For instance, high levels of parainfluenza virus 3 have been detected in patients with PVOD. 54 However, direct demonstration of specific pathophysiological changes within the olfactory system following viral subtype insults in humans has yet to be accomplished.
Viral Specific Effects on the Olfactory System
Two important aspects of the influenza virus emerged during our review: (1) histologic changes in the OE and OB are relatively small, consisting of small foci of degeneration, and (2) hemagglutinin plays a critical role in influenza neurotropism, or the ability to infect certain neural cells. The OE and OB are consistently affected by the influenza virus but exhibit only small foci of histologic abnormalities in all included studies. Involvement of higher cortical centers within the olfactory pathway appears to be subtype specific. 28 Direct comparison of H5N1 and H1N1 showed that infection with H5N1 resulted in lesion and inflammation of higher cortical centers with poor animal survival, while H1N1 infection was strictly limited to the OE. 28 Adding to the complexity, different substrains of H5N1 influenza also exhibited variable neurovirulence. H5N1 VN1203 substrain exhibited a rapid and widespread CNS transmission that resulted in more extensive neuronal death and inflammatory response, while infection with its counterpart H5N1 HK483 showed a much slower spread within the CNS with less notable cellular damage. 25 Interestingly, mutation of a multi-basic cleavage site within the hemagglutinin impaired the ability of H5N1 to spread within the olfactory system and the CNS. 23 These together suggest that hemagglutinin has a critical role in neurotropism. In general, microglial activation, inflammatory response, and cytokine production appear to be elevated following neuroinvasion of influenza, which collectively may contribute to viral clearance and cell death. A notable exception is influenza A R404BP which induces minimal inflammation in the olfactory system but significant apoptosis of the ORNs. 32
Coronaviruses similarly displayed strain specific differences in neurovirulence. For example, SARS-CoV-1 in transgenic mice expressing human angiotensin-converting enzyme 2 (ACE2) in epithelial cell lines demonstrated a high degree of neurovirulence towards higher cortical regions in the olfactory pathway while sparing much of the morphology of the OB. 36 This contrasts with the highly neurovirulent strain of MHV-JHM and MHV-OBLV—both of which are murine coronavirus counterpart—that significantly disrupt the OB and higher cortical regions with resultant cell death.35,38 Consistent with other studies, disruption of the architecture within the OB was shown to lead to olfactory impairment after coronavirus infection in vivo. 53
Parainfluenza virus had unique tropism—or the ability of specific virus to target and infect different cells—within the olfactory system. All studies to date have shown that, regardless of subtypes, the neurovirulence of parainfluenza is restricted to primary ORNs and their surrounding cells in the OE and OB. Infectivity or lesion of higher cortical lesions has yet to be demonstrated. The component that restricts parainfluenza tropism within the olfactory system remains elusive. It is also unclear if viral components are transported to higher cortical regions. Two studies objectively evaluated olfaction after parainfluenza infection and found deficits.41,43 Following infection of Sendai virus (murine parainfluenza), mice showed increased latency to find buried food pallet even in a food deprived state. 41 ORNs also showed diminished calcium signaling to odorant binding, suggesting impairment in depolarization and signal transduction on a cellular level. 41 Interestingly, parainfluenza virus also produces minimal morphologic changes or inflammatory response when compared to other viruses, such as influenza or herpes. This may account for the prolonged presence of viral protein and viral RNA within the OB.41,45
Studies demonstrated high neurovirulence of HSV for the CNS. In vivo studies of HSV-1 and HSV-2 consistently showed high degree of neuroinvasion within the olfactory pathway as well as in higher cortical centers, resulting in encephalitis and cell death.13,15,16 ORNs, mitral cells, and granule cells have all been shown to undergo cell death following HSV infection.
Implications for COVID-19
Fortunately, investigation into the pathophysiological mechanism of SARS-CoV-2-induced OD is already underway.55–57 As indicated earlier, viral tropism within the olfactory system is important in the pathogenesis of OD. According to single cell sequencing analysis of RNA profiles from existing databases, only a subset of sustentacular cells, horizontal basal cells, and Bowman’s gland cells co-express the genes encoding the ACE2 receptor and TMPRSS2 protease, both of which are essential for SARS-CoV-2 viral entry.55–57 Therefore, SARS-CoV-2 may disrupt homeostasis and regeneration of ORNs in the OE, leading to clinically observable OD. Alternatively, SARS-CoV-2 may produce extensive neuronal damage in higher cortical centers of the olfactory pathway, like its SARS-CoV-1 counterpart. 36
Filling the Knowledge Gap in PVOD
Available animal and human studies demonstrate that measurable and observable damages to the olfactory system following viral infection are apparent: reduction in progenitor cells, cell death of ORNs, damage to supporting cells, deciliation of neuroepithelium, and lesions of higher olfactory cortical centers. However, most studies also showed some degree of regeneration and repair following viral clearance. Notably, the olfactory system has a remarkable ability to regenerate. For instance, bilaterally bulbectomized rats have been shown to retain the ability to smell via newly generated connections to the forebrain. 58 Furthermore, induced chemical lesion of the OE—by as much as 95%—and the OB in rats showed little to no impairment of olfactory function and failed to produce anosmia.53,59,60 Additionally, anosmia is reported in 65% of patients who survive herpes encephalitis, which inflicts devastating neuronal damage in multiple brain areas, including the olfactory pathway. 61 This highlights the remarkable permissibility of the olfactory pathway to neuronal damage and its regenerative ability. Finally, normal and subnormal olfaction in humans has been reported in the absence of the OB.62,63 Therefore, the mechanism of how viral insults account for the lack of olfactory perception on a long-term or even permanent basis remains elusive.
For a previously normosmic individual to become truly anosmic, the damage would theoretically need to be quite extensive and long lasting, which does not appear to be the case based on the available animal literature. Yet, PVOD is consistently a major cause of long-term OD despite differences in the mechanism of initial insults. Furthermore, human studies that broadly studied PVOD consistently show volumetric changes in olfactory related brain regions.50,51 Barring complete disruption of regenerative ability and destruction of the olfactory pathway, this evidence taken together suggests a convergent mechanism for clinical manifestation of long-term OD beyond the apparent anatomical changes. We therefore postulate that the fundamental process of olfactory coding is altered following the initial viral insult. This is further supported by fMRI studies in humans that have demonstrated aberrant functional olfactory connectivity in PVOD patients compared to that of control subjects. 64 The question naturally arises, then, as to how viral infection alters olfactory coding. Here, we suggest that synaptic plasticity, or the ability to strengthen or weaken communications between neurons, is a potential target of viral disruption.
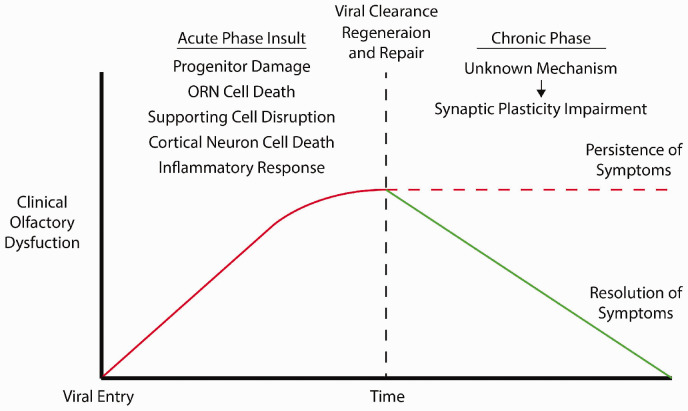
Two-Hit Hypothesis
We propose a “Two-Hit Hypothesis” which posits that secondary impairment of synaptic plasticity in combination with initial viral insults is necessary for the clinical development of persistent PVOD (Figure 3). Consistent with our hypothesis, influenza A has been shown to induce long lasting changes in synaptic plasticity in the hippocampus even after viral clearance, presumably through the action of cytokines, activated microglia, as well as altered gene expression. 65 Similarly, RSV infection has been shown to impair synaptic plasticity even after viral clearance, leading to cognitive impairment. 66 The ability of neurotropic viruses to hijack synaptic plasticity has also been demonstrated in Borna disease virus, which disrupts synaptic vesicle recycling. 67 It is conceivable that most, if not all, neurotropic viruses have the ability to alter synaptic plasticity through unknown mechanisms.
Figure 3.
Natural progression of PVOD. Each phase consists of distinctive sets of viral insults. Persistence of OD into a long-term, or permanent, basis requires impairment of synaptic plasticity in addition to cellular damage.
The hallmark of synaptic plasticity is activity-induced or learning-induced modification, 68 which may, in part, explain why olfactory training is an effective therapy for PVOD.69,70 Interestingly, older individuals appear to be more susceptible to develop PVOD. 71 Age-dependent changes in synaptic plasticity are also well observed and may, in part, account for age-related susceptibility to PVOD. 72 Similarly, synaptic plasticity is closely regulated by hormonal changes, 73 which may partly explain the preponderance of female patients affected by PVOD, especially in the elderly. 50 However, it should be noted that this novel theory is speculative and further investigation in warranted.
Novel Therapeutic Strategy
Based on the natural clinical history of PVOD (Figure 3), treatment strategy can be categorized into those that specifically target the acute phase, and those that target the chronic phase. Arguably, the best form of intervention is to prevent the initial viral entry, which may be relevant for COVID-19. SARS-CoV utilizes host ACE2 receptors for cell entry. 57 Prevention of SARS-CoV-2 viral entry has been demonstrated using specific protease inhibitors in vivo. 56 Given the current COVID-19 pandemic, a push towards viral-entry specific research and intervention may be important in the absence of effective vaccination. For substrains of viruses that induce inflammation, topical or systemic corticosteroids may prove useful in the acute phase of PVOD. Viral mediated induction of cytokines may persist for many months. 74 Elevated cytokines such as IFN-γ and TNF-α have been well demonstrated to cause OD in vivo.75,76 Therefore, selectively targeting these cytokines may provide yet another avenue for therapy. Cytokine targeted treatment may however be complicated by their critical function in viral clearance in the early phase of the infection. 74 Stem cell or gene therapy targeting basal proliferating cells in the OE or neuroblast precursors in the subventricular zone to specifically improve regenerative potential of the OE or OB, respectively, may also prove to be useful in the future.
However, patients presenting to otolaryngologists are more likely to fall in the chronic phase category; anosmia alone may not lead one to seek treatment immediately. According to our novel “Two-Hit Hypothesis”, therapies specifically targeting synaptic plasticity should be explored. Nevertheless, determining the precise mechanism by which different viruses detrimentally affect synaptic plasticity within the olfactory pathway may open a new frontier in therapeutic options.
Conclusion
The underlying pathophysiological mechanism of PVOD is complex and virus dependent. A combination of direct damage to ORNs, impairment in olfactory neuron regeneration, inflammatory response, cytokine action, as well as higher cortical damage may account for the clinical manifestation of PVOD. Permanent or long-term OD beyond the period of viral clearance and cellular regeneration likely involves additional alteration in the olfactory system.
Acknowledgment
We would like thank Teresa Lee for kindly providing the illustrations included in this article.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Jason C. Lee https://orcid.org/0000-0001-9117-4492
Rohit Nallani https://orcid.org/0000-0001-6313-205X
References
- 1.Carfi A, Bernabei R, Landi F, for the Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020; 324(6):603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaira LA, Salzano G, Deiana G, Riu GD. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020; 130(7):1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020; 277(8):2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moein ST, Hashemian SMR, Mansourafshar B, et al. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020; 10(8):944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henkin RI, Levy LM, Fordyce A. Taste and smell function in chronic disease: a review of clinical and biochemical evaluations of taste and smell dysfunction in over 5000 patients at the taste and smell clinic in Washington, DC. Am J Otolaryngol. 2013; 34(5):477–489. [DOI] [PubMed] [Google Scholar]
- 6.Seiden AM. Postviral olfactory loss. Otolaryngol Clin North Am. 2004; 37(6):1159–1166. [DOI] [PubMed] [Google Scholar]
- 7.van Riel D, Verdijk R, Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol. 2015; 235(2):277–287. [DOI] [PubMed] [Google Scholar]
- 8.Ueha R, Mukherjee S, Ueha S, et al. Viral disruption of olfactory progenitors is exacerbated in allergic mice. Int Immunopharmacol. 2014; 22(1):242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryche B, Fretaud M, Saint-Albin Deliot A, et al. Respiratory syncytial virus tropism for olfactory sensory neurons in mice. J Neurochem. 2019;e14936. [DOI] [PubMed] [Google Scholar]
- 10.Farrell HE, Lawler C, Tan CS, et al. Murine cytomegalovirus exploits olfaction to enter new hosts. mBio. 2016; 7(2):e00251–e00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiang J, Zhang S, Nauwynck H. Infections of neonatal and adult mice with murine CMV HaNa1 strain upon oronasal inoculation: new insights in the pathogenesis of natural primary CMV infections. Virus Res. 2016; 211:96–102. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, Xiang J, Van Doorsselaere J, et al. Comparison of the pathogenesis of the highly passaged MCMV smith strain with that of the low passaged MCMV HaNa1 isolate in BALB/c mice upon oronasal inoculation. Vet Res. 2015; 46:94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armien AG, Hu S, Little MR, et al. Chronic cortical and subcortical pathology with associated neurological deficits ensuing experimental herpes encephalitis. Brain Pathol. 2010; 20(4):738–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jennische E, Eriksson CE, Lange S, et al. The anterior commissure is a pathway for contralateral spread of herpes simplex virus type 1 after olfactory tract infection. J Neurovirol. 2015; 21(2):129–147. [DOI] [PubMed] [Google Scholar]
- 15.Beers DR, Henkel JS, Schaefer DC, et al. Neuropathology of herpes simplex virus encephalitis in a rat seizure model. J Neuropathol Exp Neurol. 1993; 52(3):241–252. [DOI] [PubMed] [Google Scholar]
- 16.Tomlinson AH, Esiri MM. Herpes simplex encephalitis. Immunohistological demonstration of spread of virus via olfactory pathways in mice. J Neurol Sci. 1983; 60(3):473–484. [DOI] [PubMed] [Google Scholar]
- 17.Anderson JR, Field HJ. The distribution of herpes simplex type 1 antigen in mouse Central nervous system after different routes of inoculation. J Neurol Sci. 1983; 60(2):181–195. [DOI] [PubMed] [Google Scholar]
- 18.Mori I, Goshima F, Watanabe D, et al. Herpes simplex virus US3 protein kinase regulates virus-induced apoptosis in olfactory and vomeronasal chemosensory neurons in vivo. Microbes Infect. 2006; 8(7):1806–1812. [DOI] [PubMed] [Google Scholar]
- 19.Chaves AJ, Busquets N, Valle R, et al. Neuropathogenesis of a highly pathogenic avian influenza virus (H7N1) in experimentally infected chickens. Vet Res. 2011; 42:106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodewes R, Kreijtz JH, van Amerongen G, et al. Pathogenesis of influenza a/H5N1 virus infection in ferrets differs between intranasal and intratracheal routes of inoculation. Am J Pathol. 2011; 179(1):30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Brand JM, Stittelaar KJ, van Amerongen G, et al. Comparison of temporal and spatial dynamics of seasonal H3N2, pandemic H1N1 and highly pathogenic avian influenza H5N1 virus infections in ferrets. PLoS One. 2012; 7(8):e42343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada M, Bingham J, Payne J, et al. Multiple routes of invasion of wild-type clade 1 highly pathogenic avian influenza H5N1 virus into the central nervous system (CNS) after intranasal exposure in ferrets. Acta Neuropathol. 2012; 124(4):505–516. [DOI] [PubMed] [Google Scholar]
- 23.Schrauwen EJ, Herfst S, Leijten LM, et al. The multibasic cleavage site in H5N1 virus is critical for systemic spread along the olfactory and hematogenous routes in ferrets. J Virol. 2012; 86(7):3975–3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jang H, Boltz D, Sturm-Ramirez K, et al. Highly pathogenic H5N1 influenza virus can enter the Central nervous system and induce neuroinflammation and neurodegeneration. Proc Natl Acad Sci USA. 2009; 106(33):14063–14068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plourde JR, Pyles JA, Layton RC, et al. Neurovirulence of H5N1 infection in ferrets is mediated by multifocal replication in distinct permissive neuronal cell regions. PLoS One. 2012; 7(10):e46605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shinya K, Makino A, Hatta M, et al. Subclinical brain injury caused by H5N1 influenza virus infection. J Virol. 2011; 85(10):5202–5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park CH, Ishinaka M, Takada A, et al. The invasion routes of neurovirulent a/Hong Kong/483/97 (H5N1) influenza virus into the Central nervous system after respiratory infection in mice. Arch Virol. 2002; 147(7):1425–1436. [DOI] [PubMed] [Google Scholar]
- 28.Iwasaki T, Itamura S, Nishimura H, et al. Productive infection in the murine central nervous system with avian influenza virus A (H5N1) after intranasal inoculation. Acta Neuropathol. 2004; 108(6):485–492. [DOI] [PubMed] [Google Scholar]
- 29.de Wit E, Siegers JY, Cronin JM, et al. 1918 H1N1 influenza virus replicates and induces proinflammatory cytokine responses in extrarespiratory tissues of ferrets. J Infect Dis. 2018; 217(8):1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leyva-Grado VH, Churchill L, Wu M, et al. Influenza virus- and cytokine-immunoreactive cells in the murine olfactory and central autonomic nervous systems before and after illness onset. J Neuroimmunol. 2009; 211(1–2):73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majde JA, Bohnet SG, Ellis GA, et al. Detection of mouse-adapted human influenza virus in the olfactory bulbs of mice within hours after intranasal infection. J Neurovirol. 2007; 13(5):399–409. [DOI] [PubMed] [Google Scholar]
- 32.Mori I, Goshima F, Imai Y, et al. Olfactory receptor neurons prevent dissemination of neurovirulent influenza a virus into the brain by undergoing virus-induced apoptosis. J Gen Virol. 2002; 83(Pt 9):2109–2116. [DOI] [PubMed] [Google Scholar]
- 33.Aronsson F, Robertson B, Ljunggren HG, et al. Invasion and persistence of the neuroadapted influenza virus a/WSN/33 in the mouse olfactory system. Viral Immunol. 2003; 16(3):415–423. [DOI] [PubMed] [Google Scholar]
- 34.Wheeler DL, Sariol A, Meyerholz DK, et al. Microglia are required for protection against lethal coronavirus encephalitis in mice. J Clin Invest. 2018; 128(3):931–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai JC, de Groot L, Pinon JD, et al. Amino acid substitutions within the heptad repeat domain 1 of murine coronavirus spike protein restrict viral antigen spread in the central nervous system. Virology. 2003; 312(2):369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Netland J, Meyerholz DK, Moore S, et al. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008; 82(15):7264–7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz T, Fu L, Lavi E. Differential induction of apoptosis in demyelinating and nondemyelinating infection by mouse hepatitis virus. J Neurovirol. 2002; 8(5):392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwob JE, Saha S, Youngentob SL, et al. Intranasal inoculation with the olfactory bulb line variant of mouse hepatitis virus causes extensive destruction of the olfactory bulb and accelerated turnover of neurons in the olfactory epithelium of mice. Chem Senses. 2001; 26(8):937–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Youngentob SL, Schwob JE, Saha S, et al. Functional consequences following infection of the olfactory system by intranasal infusion of the olfactory bulb line variant (OBLV) of mouse hepatitis strain JHM. Chem Senses. 2001; 26(8):953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bihun CG, Percy DH. Morphologic changes in the nasal cavity associated with sialodacryoadenitis virus infection in the wistar rat. Vet Pathol. 1995; 32(1):1–10. [DOI] [PubMed] [Google Scholar]
- 41.Tian J, Pinto JM, Cui X, et al. Sendai virus induces persistent olfactory dysfunction in a murine model of PVOD via effects on apoptosis, cell proliferation, and response to odorants. PLoS One. 2016; 11(7):e0159033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mori I, Nakakuki K, Kimura Y. Temperature-sensitive parainfluenza type 1 vaccine virus directly accesses the Central nervous system by infecting olfactory neurons. J Gen Virol. 1996; 77(9):2121–2124. [DOI] [PubMed] [Google Scholar]
- 43.Myers LJ, Nusbaum KE, Swango LJ, et al. Dysfunction of sense of smell caused by canine parainfluenza virus infection in dogs. Am J Vet Res. 1988; 49(2):188–190. [PubMed] [Google Scholar]
- 44.Lundh B, Kristensson K, Norrby E. Selective infections of olfactory and respiratory epithelium by vesicular stomatitis and sendai viruses. Neuropathol Appl Neurobiol. 1987; 13(2):111–122. [DOI] [PubMed] [Google Scholar]
- 45.Mori I, Komatsu T, Takeuchi K, et al. Parainfluenza virus type 1 infects olfactory neurons and establishes long-term persistence in the nerve tissue. J Gen Virol. 1995; 76(5):1251–1254. [DOI] [PubMed] [Google Scholar]
- 46.Altundag A, Temirbekov D, Haci C, et al. Olfactory cleft width and volume: possible risk factors for postinfectious olfactory dysfunction [published online ahead of print February 6, 2020]. Laryngoscope. doi:10.1002/lary.28524 [DOI] [PubMed]
- 47.Wolf A, Liesinger L, Spoerk S, et al. Olfactory cleft proteome does not reflect olfactory performance in patients with idiopathic and postinfectious olfactory disorder: a pilot study. Sci Rep. 2018; 8(1):17554–17512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao L, Yi X, Pinto JM, et al. Olfactory cortex and olfactory bulb volume alterations in patients with post-infectious olfactory loss. Brain Imag Behav. 2018; 12(5):1355–1362. [DOI] [PubMed] [Google Scholar]
- 49.Kim YK, Hong SL, Yoon EJ, et al. Central presentation of postviral olfactory loss evaluated by positron emission tomography scan: a pilot study. Am J Rhinol Allergy. 2012; 26(3):204–208. [DOI] [PubMed] [Google Scholar]
- 50.Rombaux P, Martinage S, Huart C, et al. Post-infectious olfactory loss: a cohort study and update. B-ENT. 2009; 5(Suppl 13):89–95. [PubMed] [Google Scholar]
- 51.Rombaux P, Mouraux A, Bertrand B, et al. Olfactory function and olfactory bulb volume in patients with postinfectious olfactory loss. Laryngoscope. 2006; 116(3):436–439. [DOI] [PubMed] [Google Scholar]
- 52.Yamagishi M, Fujiwara M, Nakamura H. Olfactory mucosal findings and clinical course in patients with olfactory disorders following upper respiratory viral infection. Rhinology. 1994; 32(3):113–118. [PubMed] [Google Scholar]
- 53.Youngentob SL, Schwob JE, Sheehe PR, et al. Odorant threshold following methyl bromide-induced lesions of the olfactory epithelium. Physiol Behav. 1997; 62(6):1241–1252. [DOI] [PubMed] [Google Scholar]
- 54.Wang JH, Kwon HJ, Jang YJ. Detection of parainfluenza virus 3 in turbinate epithelial cells of postviral olfactory dysfunction patients. Laryngoscope. 2007; 117(8):1445–1449. [DOI] [PubMed] [Google Scholar]
- 55.Brann D, Tsukahara T, Weinreb C, et al. Non-neural expression of SARS-CoV-2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia in COVID-19 patients. bioRxiv. 2020:2020.2003.2025.009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020; 181(2):271–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003; 426(6965):450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Slotnick B, Cockerham R, Pickett E. Olfaction in olfactory bulbectomized rats. J Neurosci. 2004; 24(41):9195–9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu XC, Slotnick BM. Olfaction in rats with extensive lesions of the olfactory bulbs: implications for odor coding. Neuroscience. 1998; 84(3):849–866. [DOI] [PubMed] [Google Scholar]
- 60.Slotnick BM, Gutman LA. Evaluation of intranasal zinc sulfate treatment on olfactory discrimination in rats. J Comp Physiol Psychol. 1977; 91(4):942–950. [DOI] [PubMed] [Google Scholar]
- 61.McGrath N, Anderson NE, Croxson MC, et al. Herpes simplex encephalitis treated with acyclovir: diagnosis and long term outcome. J Neurol Neurosurg Psychiatry. 1997; 63(3):321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rombaux P, Mouraux A, Bertrand B, et al. Can we smell without an olfactory bulb? Am J Rhinol. 2007; 21(5): 548–550. [DOI] [PubMed] [Google Scholar]
- 63.Weiss T, Soroka T, Gorodisky L, et al. Human olfaction without apparent olfactory bulbs. Neuron. 2020; 105(1):35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kollndorfer K, Jakab A, Mueller CA, et al. Effects of chronic peripheral olfactory loss on functional brain networks. Neuroscience. 2015; 310:589–599. [DOI] [PubMed] [Google Scholar]
- 65.Hosseini S, Wilk E, Michaelsen-Preusse K, et al. Long-Term neuroinflammation induced by influenza a virus infection and the impact on hippocampal neuron morphology and function. J Neurosci. 2018; 38(12):3060–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Espinoza JA, Bohmwald K, Cespedes PF, et al. Impaired learning resulting from respiratory syncytial virus infection. Proc Natl Acad Sci USA. 2013; 110(22):9112–9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Volmer R, Prat CM, Le Masson G, et al. Borna disease virus infection impairs synaptic plasticity. J Virol. 2007; 81(16):8833–8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000; 23:649–711. [DOI] [PubMed] [Google Scholar]
- 69.Damm M, Pikart LK, Reimann H, et al. Olfactory training is helpful in postinfectious olfactory loss: a randomized, controlled, multicenter study. Laryngoscope. 2014; 124(4):826–831. [DOI] [PubMed] [Google Scholar]
- 70.Hummel T, Rissom K, Reden J, et al. Effects of olfactory training in patients with olfactory loss. Laryngoscope. 2009; 119(3):496–499. [DOI] [PubMed] [Google Scholar]
- 71.Temmel AF, Quint C, Schickinger-Fischer B, et al. Characteristics of olfactory disorders in relation to major causes of olfactory loss. Arch Otolaryngol Head Neck Surg. 2002; 128(6):635–641. [DOI] [PubMed] [Google Scholar]
- 72.Lynch G, Rex CS, Gall CM. Synaptic plasticity in early aging. Ageing Res Rev. 2006; 5(3):255–280. [DOI] [PubMed] [Google Scholar]
- 73.Garcia-Segura LM, Chowen JA, Parducz A, et al. Gonadal hormones as promoters of structural synaptic plasticity: cellular mechanisms. Prog Neurobiol. 1994; 44(3):279–307. [DOI] [PubMed] [Google Scholar]
- 74.Mogensen TH, Paludan SR. Molecular pathways in virus-induced cytokine production. Microbiol Mol Biol Rev. 2001; 65(1):131–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sultan B, May LA, Lane AP. The role of TNF-alpha in inflammatory olfactory loss. Laryngoscope. 2011; 121(11): 2481–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pozharskaya T, Lane AP. Interferon gamma causes olfactory dysfunction without concomitant neuroepithelial damage. Int Forum Allergy Rhinol. 2013; 3(11):861–865. [DOI] [PMC free article] [PubMed] [Google Scholar]