Abstract
Social determinants of health (SDoH) impact health and wellness. The link between SDoH and adverse health outcomes, including symptom occurrence and severity, may be explained by an individual’s physiologic response to one or more SDoH. One potential mechanism underlying this physiologic response linking SDoH and symptoms is the dynamic epigenome. The purpose of this scoping review of the literature was to examine differential susceptibility for symptoms by identifying and summarizing research linking SDoH and symptoms through epigenomic mechanisms. PubMed was searched to identify empirical research where at least one SDoH was an independent or dependent variable, at least one symptom was investigated, and the investigation included an epigenomic measure. Of the 484 articles initially retrieved, after thorough vetting, 41 articles met eligibility. The most studied symptom was depressive symptoms followed by anxiety, cognitive function, sleep dysfunction, and pain. The most frequently studied SDoH were: 1) stress, particularly early life stress and acculturative stress; and 2) trauma, predominantly childhood trauma. DNA methylation and telomere length were the most studied epigenomic measures. Four genes (SLC6A4, BDNF, NR3C1, OXTR) had evidence from multiple studies and across methodological approaches linking SDoH to symptoms. This review supports the inclusion of epigenomic approaches to better understand the link between SDoH and symptoms and provides evidence that SDoH impact telomere length and the methylation of genes involved in neurotransmitter signaling, neuronal survival, behavior, inflammation and stress response.
Keywords: social determinants of health, symptoms, epigenomic, epigenetic, DNA methylation, health inequities
Introduction
Precision health, as defined by the Centers for Disease Control and Prevention, has the goal of protecting health and wellness by measuring factors such as genes, behaviors, and the environment, and acting on those factors using interventions that are tailored rather than using the same approach for everyone (Center for Disease Control and Prevention, 2021). Precision health highlights that health and wellness are individualized and involve a complex set of health determinants (e.g., behavior and lifestyle, social and lived experiences, biological) (Dewell et al., 2020). Identifying and understanding these interconnected determinants and the resulting mechanisms that influence health has potential to yield interventions to improve health of individuals, families, and communities. Additionally, variability in physiologic responses to these determinants adds to this complexity. Nursing’s holistic approaches toward clinical practice and research present a unique opportunity to study these interconnected determinants to improve precision health care.
The comprehensive toll of symptoms across the lifespan, across patient populations, and across symptoms is something that has never been quantified. However, there are data available for specific patient populations and symptoms that support the significant negative impact of symptoms on health, wellness, and quality of life. Depressive symptoms, more prevalent in young and older adulthood (Sutin et al., 2013), are highly correlated with wellbeing across the lifespan (Baselmans et al., 2018). Greater anxiety symptom severity (Wilmer et al., 2021), chronic pain (Hadi et al., 2019), poorer sleep quality (Lee et al., 2021), subjective cognitive decline (Jenkins et al., 2021), nausea (Jung et al., 2019), and fatigue (Bouvron et al., 2022; McCabe et al., 2015) are correlated with poorer quality of life. In addition, the presence of co-occurring symptoms and their impact on quality of life is becoming more appreciated, particularly in cancer survivors (Dodd et al., 2010).
Social determinants of health (SDoH) influence health and wellness through many mechanisms including modifications to the epigenome and telomere length. For example, physical activity (Swiatowy et al., 2021) and smoking (Ambatipudi et al., 2016; Joehanes et al., 2016) are known behaviors that have an impact on DNA methylation, which in turn influences regulation of genes and levels of health influencing proteins. Social determinants of health, as defined by the World Health Organization, are “the conditions in which people are born, grow, work, live, and age, and the wider set of forces and systems shaping the conditions of daily life” (World_Health_Organization, 2010). Social determinants of health also contribute to health inequities (Crear-Perry et al., 2021; Marmot et al., 2012; Penman-Aguilar et al., 2016). The Centers for Disease Control and Prevention groups SDoH into 5 domains: economic stability, education access and quality, healthcare access and quality, neighborhood and built environment, and social and community contexts. Economic stability includes income, employment status, and assets, plus conditions that may result from economic instability such as food or housing insecurity. Related to economic stability, the education access and quality domain connects educational attainment and literacy with health and wellbeing. Healthcare access and quality include insurance status, health literacy, accessibility, and other topics specific to navigating the healthcare system. The neighborhood/built environment and social and community contexts are similar, yet distinct. The neighborhood and built environment domain captures green spaces, safe drinking water, and other tangible environmental conditions, and broader concepts such as environmental justice and neighborhood cohesion. The fifth domain, social and community contexts, includes elements of communities such as civic engagement, aspects of the social environment (e.g., racism, ageism), individual-level characteristics (e.g., social capital), and experiences (e.g., trauma) which influence health (Center for Disease Control and Prevention, 2021).
The World Health Organization (WHO) conceptual framework on Social Determinants of Health is an action-oriented framework that takes a public health perspective and presents the interactions between factors that impact health inequities. The framework has 3 elements: 1) socioeconomic and political context; 2) structural determinants and socioeconomic position; and 3) intermediary determinants (World_Health_Organization, 2010). (Figure 1) Our inquiry was conceptually grounded in portions of the WHO SDoH framework, using the CDC’s 5 domains of SDoH to build search terms, to examine differential susceptibility for symptoms by identifying and summarizing research linking structural and social determinants to symptoms through epigenomic mechanisms (intermediary determinant) and to consider future directions for nursing science.
Figure 1.
World health organization conceptual framework for action on the social determinants of health (World_Health_Organization, 2010).
Methods
Literature Review
The literature review was conducted in June - August of 2021 and updated in April 2022. A comprehensive list of search terms representing SDoH was crafted with input from the University librarian (see Figure 2). The search was built in numerous phases. PubMed was our search engine of choice given its coverage of biomedical research and epigenomic measures. Phase one created search terms in PubMed for the CDC’s 5 SDoH domains (economic stability, neighborhood/built environment, education access and quality, health care access and quality, and social and community contexts). Phase 2 created the search terms in PubMed using the epigenomic terms (DNA methylation, telomere, epigenetic, epigenomic). DNA methylation and telomere were called out because they are the most frequently used epigenomic-related measures in the literature and the terms “epigenetic” and “epigenomic” were included to capture other potential measures that may have been used and not captured by “DNA methylation” and “telomere”. Phase 3 created the search terms for the symptoms of interest (pain, sleep, fatigue, anxiety, depression, cognitive function, nausea). Symptoms were prioritized for this review because they were identified as common data elements for symptom research by the NINR supported centers of excellence (Redeker et al., 2015). The search results for the 5 SDoH factors, epigenomic terms, and symptoms were merged together into one PubMed search. Finally, we applied the PubMed filters of human, title and abstract, and English language.
Figure 2.
Social determinants of health search terms.
The resulting abstracts (n = 484) were divided among the authors for the initial review. Abstracts were reviewed according to the following criteria: 1) at least one SDoH as an independent or dependent variable, as we didn’t want to look at those that used SDoH as a co-variate or confounder but focus on those that actually looked at direct relationships; 2) at least one symptom as an independent or dependent variable; and 3) investigation of an epigenomic measure. Abstracts were excluded that 1) did not include human subjects, 2) were written in a language other than English, or 3) were reviews, conference abstracts, or editorials. Full articles were reviewed for abstracts that appeared to meet the inclusion/exclusion criteria; full articles for which eligibility was not able to be determined by abstract alone were also reviewed at this phase. Data were extracted from included articles to note the study populations, sample size, symptom(s) investigated, tool for measuring symptom(s), SDoH(s) investigated, tool for measuring SDoH(s), epigenomic measure used, and main finding(s). MR, MKW, and YPC resolved any disputes about inclusion after full article review. These data are presented in Supplemental Table 1.
Ingenuity Pathway Analysis
Realizing that a cadre of genes were targeted for DNA methylation measures or were found to explain the relationship between SDoH and symptoms during discovery-based analyses in the reviewed articles, Ingenuity Pathway Analysis (IPA) was used to investigate known relationships among the list of genes implicated in the included studies. Biological relationships among the implicated genes could aid in interpretation of these cumulative findings. Ingenuity pathway analysis provides visualization of complex biological relationships and is available from Qiagen Corp We used the Path Explorer tool within IPA to identify any direct or indirect interactions between the genes using information from the Ingenuity Knowledge Base. Next, we used the Overlay tool within IPA to investigate known disease and function pathways associated with the included genes. Specifically, we looked at disease pathways associated with depression and anxiety, because those were the 2 most commonly investigated symptoms in the included studies.
Results
Literature Review: Identification, screening, and selection of publications included for review are summarized in Figure 3. Our initial literature search identified a total of 484 abstracts for review. After omitting publications that did not include at least one symptom, one SDoH variable, and one epigenomic measurement, as well as publications that were not data-based and publications that applied animal models, 64 publications remained for review. After a secondary, deeper full-text evaluation, 22 additional publications were omitted because a SDoH term was mentioned but not actually used as a variable in the analyses, depression or anxiety being utilized as a diagnosis rather than depressive and anxiety symptoms, and use of parent/infant dyads where epigenomic analysis was conducted using samples from offspring and not parent. This selection process yielded a total of 41 eligible publications for review. The 41 eligible publications spanned varying symptoms, SDoH, and epigenomic measures. Please refer to Supplemental Table 1 for study-level findings.
Figure 3.
Literature review process showing identification, selection, and inclusion of publications. IV = independent variable; DV = dependent variable.
Symptoms: In this review of the literature, the frequencies of symptoms studied were 1) depressive symptoms, represented in 31 articles; 2) anxiety, represented in 12 articles (often investigated with depressive symptoms in the same study); 3) 9 for cognitive function; 4) 3 for sleep; and 5) 2 for pain. Depressive symptoms were investigated within the context of several SDoH including trauma, early life stressors and early life adversity, discrimination and acculturative stress, neighborhood conditions, economic hardship, education, income, religiosity, and food insecurity. Telomere length and DNA methylation of several genes including NR3C1, SLC6A4, and BDNF were significantly associated with depressive symptoms. Anxiety was investigated within the context of several SDoH including trauma, early life stressors and early life adversity, discrimination and acculturative stress, pollution, economic hardship, education and income. Telomere length and DNA methylation of several genes including NR3C1, and SLC6A4 were significantly associated with anxiety. Cognitive function was investigated within the context of several SDoH including trauma, early life stressors, housing type, poverty, education level and smoking. Telomere length was significantly associated with cognitive function as were smoking-related differentially methylated sites across the genome. Sleep was investigated within the context of several SDoH including trauma, early life stressors, and culturative stress. DNA methylation of the AVP gene was significantly associated with sleep disturbance. Pain was investigated within the context of several SDoH including trauma and early life stressors. Telomere length and DNA methylation of the TRPA1 gene were significantly associated with pain.
SDoH: In this review of the literature, 61% of the articles (25/41) focused on psychosocial stress, with many authors evaluating particular stressors as a SDoH of interest, particularly trauma and childhood maltreatment, which fall under the domain of social and community contexts. The most commonly investigated SDoH was stress, accounting for 13 publications in total (Supplemental Table 1). The majority of these publications evaluated stress levels in adulthood, with one study focused on cumulative stress in adulthood, totaling 8 publications. Three publications included assessment of early life stress, 2 measured acculturative stress, and one measured work-related stress. Authors of 5 publications conducted their studies among pregnant individuals, and there were instances where more than one type of stress was evaluated within a single publication. The second most frequently studied SDoH was emotional trauma, a stress-related exposure, which accounted for 9 publications: 7 focused on childhood trauma, one measured trauma experienced in adulthood, and one evaluated trauma related to war time prisoner status. One publication evaluated childhood trauma among pregnant individuals. Three additional publications evaluated childhood maltreatment, which is also related to both stress and trauma. Three reports published in the United States addressed discrimination by utilizing the Everyday Discrimination Scale. Socioeconomic status was not well represented in our findings. Several validated clinical instruments were utilized for the measurements of stress and trauma. The most common tool was the Childhood Trauma Questionnaire, applied by the authors of 11 reports to assess trauma or maltreatment experienced in childhood. The Perceived Stress Scale was the most frequently applied scale to assess stress in adulthood, applied by the authors of 4 reports. Additional tools that were used include: Early Trauma Inventory, Acculturative Stress Scale, Antenatal Risk Questionnaire, Pregnancy Risk Monitoring System, Post-traumatic Stress Diagnostic Scale, Trier Inventory of Chronic Stress, and Wheaton Chronic Stress Inventory. Additional SDoH within the social and community contexts domains that were evaluated included racial discrimination, early/childhood adversity, and religion.
Following social and community contexts, the remaining SDoH domains, in order of frequency of investigation, rank as follows: (2) economic stability (studies spanned income, housing, and food insecurity), (3) neighborhood and built environment (studies spanned air pollution, crime, smoking, alcohol use, and toxin exposure), and (4) education access and quality (Educational attainment). Our search did not produce any studies that evaluated SDoH that fall under the health care access and quality domain.
Epigenomic measures: The epigenomic measures retrieved through the search were DNA methylation for 23 studies and telomere length for 19 studies. Seventeen DNA methylation studies were focused on a targeted candidate gene, and 6 were epigenome-wide association studies (EWAS). Fifteen different candidate genes were investigated, with 5 investigated in more than one study. The glucocorticoid receptor (NR3C1) was the most investigated candidate gene and was the target of 6 studies. The serotonin transporter (SLC6A4; 5HTT) and brain derived neurotrophic factor (BDNF) were both the target of 4 studies; the oxytocin receptor (OXTR) was the target of 3 studies; and FK506-binding protein 5 (FKPB5) was the target of 2 studies. EWAS findings, using discovery-based analyses, found 11 genes to be important to the relationship between SDoH and symptoms, including 4 genes that were also the target of candidate gene analyses (SLC6A4, BDNF, NR3C1, OXTR). One EWAS study did not identify a gene of interest but did find that DNA methylation markers of smoking were associated with cognitive function; however, these DNA methylation markers of smoking were more strongly correlated with cognitive function scores than measures of smoking. Genes with significant findings reported are provided in Supplemental Table 2.
Ingenuity Pathway Analysis
Ingenuity Pathway Analysis (Figure 4) revealed biological pathway associations among MAOA, OXTR, FKPB5, GRIN2B, AVPR1A, GRIN1, and NR3C1. NR3C1 had the most associated pathways and had pathways connected to MAOA, FKBP5, and GRIN1. We then assessed for pathways associated with anxiety and depression, because they were the 2 most commonly investigated symptoms in the included articles. Anxiety was associated with MAOB, NR3C2, GRIN1, NR3C1, BDNF, SLC6A4, GRIN2B, FKBP5, OXTR, MAOA. Depression was associated with NR3C2, MAOB, GRIN1, AVP, NR3C1, BDNF, MAOA, SLC64A, OXTR, FKBP5, NOS1.
Figure 4.
Results of ingenuity pathway analysis.
Discussion
In our review of the literature, we found that depressive symptoms and anxiety were the most frequently studied symptoms at the intersection of SDoH and epigenomics and stress across the lifespan was the most frequently studied SDoH, followed by childhood trauma. Depression and anxiety have both been independently associated with stress, and numerous frameworks and studies linking the complex relationships between depression or anxiety and stress have been published. Over the years, countless studies have characterized deleterious physical effects of stress (e.g., early life stress, chronic stress, cumulative stress) on the body, including changes in DNA methylation (Bakusic et al., 2017; Chakravarty et al., 2014; Giurgescu et al., 2019; Kertes et al., 2016; Smith et al., 2011, 2017).
Stress-related alterations in DNA methylation may explain the deleterious downstream effects that the many forms of racism have on historically excluded and marginalized individuals and communities and the resulting disparate outcomes so commonly observed (Simons et al., 2021). Aberrant DNA methylation can alter accessibility to DNA, resulting in altered gene expression, which can lead to development of symptoms and disease. Inappropriate DNA methylation can also result in accelerated epigenetic aging and weathering. Numerous studies have demonstrated an association between chronic stress and changes in DNA methylation (Bakusic et al., 2017; Chakravarty et al., 2014; Giurgescu et al., 2019; Kertes et al., 2016; Smith et al., 2011, 2017). The weathering hypothesis is one proposed explanation of how chronic stress exposures present in an individual’s environment accelerate deterioration of health via physiologic pathways (Geronimus, 1992). Changes in DNA methylation are associated with aging, and epigenetic age is considered a type of biological age that can differ from one’s chronological age (Forde et al., 2019; Hannum et al., 2013; Heinsberg et al., 2021; Horvath & Raj, 2018; Levine et al., 2018; Simons et al., 2016; Simons et al., 2021; Simons et al., 2021). The weathering hypothesis suggests that exposure to chronic stress can result in accelerated biological aging (Noren Hooten et al., 2022; Palma-Gudiel et al., 2020; Tajuddin et al., 2019). There is no common conceptual model integrating stress as an exposure, stress as a consequence of exposure, and the impact of stress on health and behavior nor are there consistent and precise measures of stress used across the lifespan, however what is well documented is that stress is a process that has physiologic (including accelerated biologic aging) and psychologic consequences (Epel et al., 2018). Differences in SDoH, such as economic instability and social contexts, could be stressors that result in biological aging occurring at a different rate than chronological aging. Consequently, cumulative chronic stress across the lifespan can result in accelerated biological aging and the development of adverse health outcomes, including the development of symptoms.
Authors of 3 reports included in our scoping review were published in the United States and addressed discrimination by utilizing the Everyday Discrimination Scale. One of these studies (Chae et al., 2016) reported an association between racial discrimination and shorter leukocyte telomere length among participants with lower levels of depressive symptoms among N = 92 African American individuals whose sex was assigned male at birth. The additional 2 reports (Incollingo Rodriguez et al., 2022; Santos et al., 2021) were conducted by the same research group and investigated discrimination among Latinx birthing parent populations (n = 150). Santos et al. (2021) determined that discrimination was strongly associated with anxiety and depressive symptoms, particularly among participants with hypermethylation of NR3C1. A follow-up study in the same cohort (n = 15) reported that acculturative stress, but not discrimination, predicted shorter telomere length (Incollingo Rodriguez et al., 2022). Importantly, the authors discuss how the Everyday Discrimination Scale was originally developed to assess discrimination experienced in Black populations and, therefore, may not be able to accurately capture biological impact on telomere length in Latinx populations.
Notably, our review reveals a large gap in the literature around investigation of discrimination as a SDoH in the context of epigenomic changes and symptoms. A better understanding of how racism, and the resulting discrimination, change the epigenome and effects symptoms is important because of the growing evidence that links racism to physiologic stress and health inequities. Furthermore, as evidenced by a recent call for funding from the National Institutes of Health (https://grants.nih.gov/grants/guide/pa-files/PAR-22-072.html: Measures and Methods to Advance Research on Minority Health and Health Disparities-Related Constructs), development and validation of tools for accurately measuring an individual or populations’ lived experience, including racism and discrimination, are also needed. It is important to note that the weathering hypothesis was proposed as a framework to account for disparate health outcomes by the social construct of race in this call. Further, racial equity frameworks (Crear-Perry et al., 2021; Nardi et al., 2020) explain the ways in which intersecting levels of racism perpetuate inequities that drive disparate health outcomes. Few studies have begun to analyze the relationships between weathering, biological aging, and symptoms, within the context of SDoH (Forrester et al., 2019; Simons et al., 2016). However, the inclusion of SDoH in symptom science is an emerging area of science, reflected in the 2022 revised Symptom Science Model (Kurnat-Thoma et al., 2022). This change represents a growing appreciation of the influence of social determinants on every aspect of health, partly illuminated by the COVID-19 pandemic (Dennison Himmelfarb & Baptiste, 2020; Scott et al., 2021).
Another established and growing area of stress biology research applies allostatic load, an integrative framework for understanding the embodiment of cumulative lifetime chronic stress (McEwen, 2002; Seeman et al., 1997). Aggregate cycles of allostasis, the mechanism by which the body maintains homeostasis in response to stress, can result in systemic dysfunction, or “wear and tear,” termed allostatic load. Allostatic load has been evaluated extensively as a health hazard, and high allostatic load has been associated with adverse health outcomes as well as an excess of chronic health conditions (Castagne et al., 2018; Johnston-Brooks et al., 1998; Logan & Barksdale, 2008; Parker et al., 2022; Zhang et al., 2021). Given that allostatic load is cumulative in nature, it has been posited as a potential mechanism responsible for associations between adverse childhood experiences and development of disease later in life (McEwen, 2002; Misiak et al., 2022). While allostatic load was not explicitly explored in the studies included in this review, it may be a mechanism by which early life stressors and trauma are associated with epigenomic changes and depressive and anxiety symptoms, representing an additional important area for future investigations.
The search found that telomere and DNA methylation measures were the only epigenomic measures used to date to assess the relationship between SDoH and symptoms. A large number of publications that were reviewed evaluated telomere length as an epigenomic measure. Telomeres, non-coding segments of DNA with specialized chromatin structures located at the ends of chromosomes, shorten with each cell division, and telomere length is known to shorten with chronological aging (Turner et al., 2019). A large, growing body of literature contain analyses that assess the relationship between external environmental stressors and telomere length, which is also considered a metric for accelerated aging (Mathur et al., 2016; Oliveira et al., 2016). Various stressful conditions have been associated with accelerated aging measured via telomere length shortening in previous studies (Cerveira de Baumont et al., 2021; Kim et al., 2017), and several publications discuss the potential for telomere length to serve as a biomarker for stress, progression of age-related diseases, and mortality (Fasching, 2018; Kodali & Borrell, 2021; Lin & Epel, 2022; Schneider et al., 2022). Given that stress and trauma were the most frequently studied SDoH among the publications for this review, it is not surprising that telomere length was so heavily investigated. It is, however, important to note that discrepancies have been reported in the literature when evaluating the relationship between stress and telomere length (Coimbra et al., 2020; Sanders & Newman, 2013; Wang et al., 2018). These may be due to heterogeneity of telomere length across leukocytes and tissues, variations in rigor of statistical approach, limited sample sizes, the preponderance of ethnically/racially homogeneous samples including mostly white participants, and publication bias.
The most frequently evaluated epigenomic measure in the studies reviewed was DNA methylation, with 17 focusing on a priori selected candidate genes and 6 using a discovery based EWAS approach. The candidate genes selected reflected the focus on depressive symptoms and stress, particularly early life stress, representing the majority of studies captured by this review. An EWAS approach does not select a priori what genes to target but instead evaluates the entire epigenome for differentially methylated genes implicated in the relationship between SDoH and symptoms. There were 4 genes [SLC6A4, BDNF, NR3C1, OXTR] identified through the EWAS approach that overlapped with the candidate gene studies. Significant findings for the same genes using a targeted approach and an EWAS discovery-based approach provides support that these 4 genes are impacting the relationship between SDoH and symptoms.
SLC6A4, which was one of the most frequently targeted candidate gene, was also implicated in an EWAS study, where methylation of SLC6A4 mediated the relationship between neighborhood crime and occurrence of depressive symptoms (Lei et al., 2015). This gene codes for a serotonin transporter that plays an important role in the regulation of serotonin signaling and has been implicated in depression and anxiety (GeneCards, 2022). This review provides evidence that not only is SLC6A4 involved in risk of depressive symptoms, but that increased risk of depressive symptoms is mediated by methylation of SLC6A4 in response to SDoH.
BDNF was also one of the most frequently targeted candidate genes and was implicated, along with NR3C1, in and EWAS study where methylation of BDNF and NR3C1 mediated the relationship between childhood trauma and occurrence of depressive symptoms (Peng et al., 2018). BDNF codes for a nerve growth factor that promotes neuronal survival (GeneCards, 2022) and may impact risk for symptoms through regulation of stress responses. NR3C1 codes for a glucocorticoid receptor that functions as a transcription factor that plays a primary role in regulation of genes that respond to glucocorticoid, impacting inflammatory responses, and has been implicated in stress-related phenotypes such as post-traumatic stress disorder (GeneCards, 2022). This review provides evidence that not only is BDNF and NR3C1 involved in risk of developing symptoms, but that the risk is mediated by methylation of these genes in response to SDoH.
OXTR was also one of the most frequently targeted candidate genes and was also implicated in an EWAS study where methylation of OXTR interacted with history of abuse during childhood to predict occurrence of psychiatric symptoms (Smearman et al., 2016). Childhood trauma was also found to impact DNA methylation patterns for OXTR was also the target of a candidate gene DNA methylation study where childhood trauma was also investigated (Robakis et al., 2020). OXTR is the receptor for oxytocin which is a pituitary hormone found in abundance at nerve endings and has been implicated in cognition, adaptation, and behavior (GeneCards, 2022).
Our IPA evaluations confirm that these 4 genes (SLC6A4, BDNF, NR3C1, OXTR) are instrumental in the biological pathways involved with the development of depression and anxiety. We conclude that their involvement in depressive symptoms and anxiety are, at least partially, due to DNA methylation in response to exposure to SDoH. The IPA analysis also demonstrated relationships among NR3C1, MAOA GRIN1, and FKBP5; AVPR1A and OXTR; and GRIN1 and GRIN2B. These genes, except for AVPR1A, were all investigated in studies exploring childhood trauma/maltreatment and depressive symptoms (Bustamante et al., 2018; Engdahl et al., 2021; Peng et al., 2018; Smearman et al., 2016; Weder et al., 2014). Adverse experiences in childhood have previously been strongly associated with risk for developing depression in adulthood (Bustamante et al., 2018; Peng et al., 2018; Weder et al., 2014). The included studies highlight that the mechanism of developing depressive symptoms in relation to childhood trauma may be related to altered DNA methylation.
We also explored the depression and anxiety related pathways within IPA to determine if genes identified from this review were identified within these pathways. The anxiety pathway contained MAOB, NR3C2, GRIN1, NR3C1, BDNF, SLC6A4, GRIN2B, FKBP5, OXTR, MAOA. The depression pathway contained NR3C2, MAOB, GRIN1, AVP, NR3C1, BDNF, MAOA, SLC64A, OXTR, FKBP5, NOS1. Because depression and anxiety symptoms were investigated in the majority of the included articles, association of the included genes and anxiety and depression is not surprising; however, it does suggest that the mechanisms that underlie the association between genes and clinical manifestation of anxiety and depression may be modified by SDoH (Bustamante et al., 2018; Engdahl et al., 2021; Peng et al., 2018; Smearman et al., 2016; Weder et al., 2014). The IPA analyses also identified several genes that were not investigated in the publications included in this review, indicating additional candidate genes for investigation within the context of symptoms, SDoH, and epigenomics.
We identified gaps in the literature by noting what was not found during the scoping review. Symptom research with respect to SDoH and epigenomics is an understudied area but one that has potential for increasing our understanding of the relationships between SDoH and the variability observed in occurrence and severity of symptoms. Our search found that the 5 domains of SDoH from the CDC were not equally studied. Notably, our review did not produce any studies that evaluated SDoH that fall under the health care access and quality domain, indicating an area ripe for investigation, particularly as valid and reliable instruments to assess across the domains are developed and validated. As mentioned previously, investigation of how racism, and the resulting discrimination, change the epigenome and effects symptoms is currently understudied. Additionally, there is a need to increase diversity of participants in research studies focused on SDoH, symptoms, and epigenomics to extend generalizability of research findings.
This review has several strengths including the development and utility of an exhaustive list of SDoH search terms. However, there are notable weaknesses. The search was limited to PubMed and limited to publications written in English. The search was also limited to the study of DNA methylation and telomeres and was limited to symptoms prioritized as common data elements. Therefore, other omics-based mechanisms (i.e., transcriptomic, proteomic, metabolomic) that could assist in understanding the link between SDoH and symptoms were not evaluated, and this review was not exhaustive for all symptoms.
This review found that a strength of studies investigating epigenomics, symptoms and SDoH is that they were conducted globally [Germany, Finland, Israel, Brazil, Singapore, Scotland, Sweden, Australia, China, Netherlands, Canada, Japan, Columbia, South Korea, Italy and the USA] and that most of the studies conducted within the USA focused on individuals historically excluded from biomedical research. Because SDoH can differ significantly by country or regionally within a country, the findings of these studies need to be interpreted within the context of the country/region investigated. Several studies conducted across countries found that the impact of SDoH on depressive symptoms was through epigenomic changes, increasing support for this relationship.
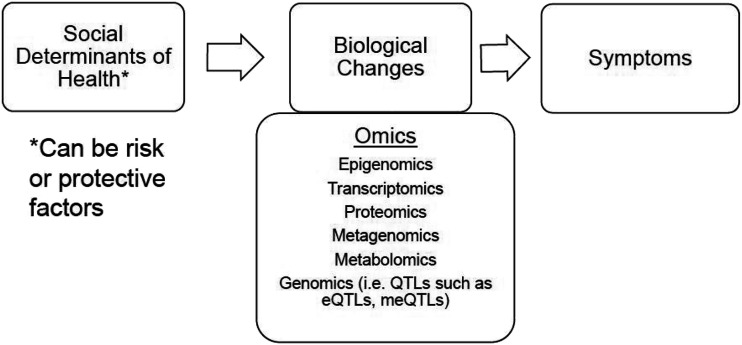
In conclusion, the biologic underpinnings that explain the relationship between SDoH and symptoms is a burgeoning area of research that requires more attention. Prioritizing the WHO framework for health and wellness requires a holistic view of patients, families, and communities. This holistic view is also an essential component of understanding how an individual’s lived experience, including SHoD, impacts one’s overall health and their symptom experiences. Nurses utilize this holistic view and therefore are well poised to take on this research and then translate it to clinical utility to impact nursing practice, health systems, and patient health outcomes. This also lends itself to the nursing discipline by capitalizing upon nursing’s diverse training and skill sets to conduct the science and implement practice-based changes. A pared-down version of the WHO framework addressed in this review that focuses on SDoH and their biological links to health and wellness, including development of symptoms, is conceptualized in Figure 5. This review supports the inclusion of epigenomic measures to better understand the link between SDoH and symptoms, and, based on the conceptualization supported by this review, we conclude that the relationship between SDoH and symptoms is at least partially due to epigenomic modification to key genes in response to SDoH.
Figure 5.
Conceptual framework linking SDoH to symptoms through biological changes. SDoH = Social Determinants of Health; eQTL = expression quantitative trait loci; meQTL = methylation quantitative trait loci.
Supplemental Material
Supplemental Material for Epigenomic Links Between Social Determinants of Health and Symptoms: A Scoping Review by Mitali Ray, McKenzie K. Wallace, Susan C. Grayson, Meredith H. Cummings, Jessica A. Davis, Jewel Scott, Sarah M. Belcher, Tara S. Davis, and Yvette P. Conley in Biological Research For Nursing
Supplemental Material for Epigenomic Links Between Social Determinants of Health and Symptoms: A Scoping Review by Mitali Ray, McKenzie K. Wallace, Susan C. Grayson, Meredith H. Cummings, Jessica A. Davis, Jewel Scott, Sarah M. Belcher, Tara S. Davis, and Yvette P. Conley in Biological Research For Nursing
Acknowledgments
We would like to acknowledge Rebekah Miller for her assistance with search terminology for this study.
Author Contributions: Yvette P Conley contributed to conception and design contributed to acquisition, analysis, and interpretation drafted manuscript critically revised manuscript gave final approval agrees to be accountable for all aspects of work ensuring integrity and accuracy. Mitali Ray contributed to conception and design contributed to acquisition, analysis, and interpretation drafted manuscript critically revised manuscript gave final approval agrees to be accountable for all aspects of work ensuring integrity and accuracy. McKenzie K. Wallace contributed to conception and design contributed to acquisition, analysis, and interpretation drafted manuscript critically revised manuscript gave final approval agrees to be accountable for all aspects of work ensuring integrity and accuracy. Susan C. Grayson contributed to conception and design contributed to acquisition, analysis, and interpretation critically revised manuscript gave final approval agrees to be accountable for all aspects of work ensuring integrity and accuracy. Meredith H. Cummings contributed to conception and design contributed to acquisition, analysis, and interpretation critically revised manuscript gave final approval agrees to be accountable for all aspects of work ensuring integrity and accuracy. Jessica A. Davis contributed to conception and design contributed to acquisition, analysis, and interpretation critically revised manuscript gave final approval agrees to be accountable for all aspects of work ensuring integrity and accuracy. Jewel Scott contributed to conception and design contributed to acquisition, analysis, and interpretation critically revised manuscript gave final approval agrees to be accountable for all aspects of work ensuring integrity and accuracy. Sarah M. Belcher contributed to conception and design contributed to acquisition, analysis, and interpretation critically revised manuscript gave final approval agrees to be accountable for all aspects of work ensuring integrity and accuracy. Tara S. Davis contributed to conception and design contributed to acquisition, analysis, and interpretation critically revised manuscript gave final approval agrees to be accountable for all aspects of work ensuring integrity and accuracy.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the study and Belcher’s scholarship have been supported, in part, by the National Institute of Nursing Research (T32NR009759 and K23NR019296, respectively).
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Mitali Ray https://orcid.org/0000-0002-7175-8287
Susan C. Grayson https://orcid.org/0000-0002-1893-603X
Jessica A. Davis https://orcid.org/0000-0002-1295-4149
Jewel Scott https://orcid.org/0000-0001-5103-6087
Sarah M. Belcher https://orcid.org/0000-0002-4095-295X
Tara S. Davis https://orcid.org/0000-0002-6293-3602
Yvette P. Conley https://orcid.org/0000-0002-1784-6067
References
- Ambatipudi S., Cuenin C., Hernandez-Vargas H., Ghantous A., Le Calvez-Kelm F., Kaaks R., Barrdahl M., Boeing H., Aleksandrova K., Trichopoulou A., Lagiou P., Naska A., Palli D., Krogh V., Polidoro S., Tumino R., Panico S., Bueno-de-Mesquita B., Peeters P. H., Herceg Z. (2016, May). Tobacco smoking-associated genome-wide DNA methylation changes in the EPIC study. Epigenomics, 8(5), 599–618. 10.2217/epi-2016-0001 [DOI] [PubMed] [Google Scholar]
- Bakusic J., Schaufeli W., Claes S., Godderis L. (2017, Jan). Stress, burnout and depression: A systematic review on DNA methylation mechanisms. Journal of Psychosomatic Research, 92, 34–44. 10.1016/j.jpsychores.2016.11.005 [DOI] [PubMed] [Google Scholar]
- Baselmans B. M. L., Willems Y. E., van Beijsterveldt C. E. M., Ligthart L., Willemsen G., Dolan C. V., Boomsma D. I., Bartels M. (2018). Unraveling the genetic and environmental relationship between well-being and depressive symptoms throughout the lifespan. Front Psychiatry, 9, 261. 10.3389/fpsyt.2018.00261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvron B., Mackin L., Kober K. M., Paul S. M., Cooper B. A., Conley Y. P., Hammer M. J., Wright F., Levine J. D., Miaskowski C. (2022, Dec). Impact of worst pain severity and morning fatigue profiles on oncology outpatients’ symptom burden and quality of life. Supportive Care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer, 30(12), 9929–9944. 10.1007/s00520-022-07431-6 [DOI] [PubMed] [Google Scholar]
- Bustamante A. C., Aiello A. E., Guffanti G., Galea S., Wildman D. E., Uddin M. (2018, Jan). FKBP5 DNA methylation does not mediate the association between childhood maltreatment and depression symptom severity in the Detroit Neighborhood Health Study. Journal of Psychiatric Research, 96, 39–48. 10.1016/j.jpsychires.2017.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagne R., Gares V., Karimi M., Chadeau-Hyam M., Vineis P., Delpierre C., Kelly-Irving M., Lifepath C. (2018, May). Allostatic load and subsequent all-cause mortality: Which biological markers drive the relationship? Findings from a UK birth cohort. European Journal of Epidemiology, 33(5), 441–458. 10.1007/s10654-018-0364-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention (2021). About social determinants of health (SDOH). https://www.cdc.gov/socialdeterminants/about.html [Google Scholar]
- Cerveira de Baumont A., Hoffmann M. S., Bortoluzzi A., Fries G. R., Lavandoski P., Grun L. K., Guimaraes L. S. P., Guma F., Salum G. A., Barbe-Tuana F. M., Manfro G. G. (2021, Apr 8). Telomere length and epigenetic age acceleration in adolescents with anxiety disorders. Scientific Reports, 11(1), 7716. 10.1038/s41598-021-87045-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae D. H., Epel E. S., Nuru-Jeter A. M., Lincoln K. D., Taylor R. J., Lin J., Blackburn E. H., Thomas S. B. (2016, Jan). Discrimination, mental health, and leukocyte telomere length among African American men. Psychoneuroendocrinology, 63, 10–16. 10.1016/j.psyneuen.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty S., Pathak S. S., Maitra S., Khandelwal N., Chandra K. B., Kumar A. (2014). Epigenetic regulatory mechanisms in stress-induced behavior. Int Rev Neurobiol, 115, 117–154. 10.1016/B978-0-12-801311-3.00004-4 [DOI] [PubMed] [Google Scholar]
- Coimbra B. M., Carvalho C. M., Ota V. K., Vieira-Fonseca T., Bugiga A., Mello A. F., Mello M. F., Belangero S. I. (2020, Oct). A systematic review on the effects of social discrimination on telomere length. Psychoneuroendocrinology, 120, 104766. 10.1016/j.psyneuen.2020.104766 [DOI] [PubMed] [Google Scholar]
- Crear-Perry J., Correa-de-Araujo R., Lewis Johnson T., McLemore M. R., Neilson E., Wallace M. (2021, Feb). Social and structural determinants of health inequities in maternal health. Journal of Women’s Health, 30(2), 230–235. 10.1089/jwh.2020.8882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison Himmelfarb C. R., Baptiste D. (2020, Jul/Aug). Coronavirus disease (COVID-19): Implications for cardiovascular and socially at-risk populations. Journal of Cardiovascular Nursing, 35(4), 318–321. 10.1097/JCN.0000000000000710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewell S., Benzies K., Ginn C. (2020, Sep). Precision health and nursing: Seeing the familiar in the foreign. Cancer Journal of Nursing Research, 52(3), 199–208. 10.1177/0844562120945159 [DOI] [PubMed] [Google Scholar]
- Dodd M. J., Cho M. H., Cooper B. A., Miaskowski C. (2010, Apr). The effect of symptom clusters on functional status and quality of life in women with breast cancer. European Journal of Oncology Nursing: the Official Journal of European Oncology Nursing Society, 14(2), 101–110. 10.1016/j.ejon.2009.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engdahl E., Alavian-Ghavanini A., Forsell Y., Lavebratt C., Rüegg J. (2021, Jan). Childhood adversity increases methylation in the GRIN2B gene. Journal of Psychiatric Research, 132, 38–43. 10.1016/j.jpsychires.2020.09.022 [DOI] [PubMed] [Google Scholar]
- Epel E. S., Crosswell A. D., Mayer S. E., Prather A. A., Slavich G. M., Puterman E., Mendes W. B. (2018, Apr). More than a feeling: A unified view of stress measurement for population science. Front Neuroendocrinol, 49, 146–169. 10.1016/j.yfrne.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasching C. L. (2018, Nov). Telomere length measurement as a clinical biomarker of aging and disease. Critical Reviews in Clinical Laboratory Sciences, 55(7), 443–465. 10.1080/10408363.2018.1504274 [DOI] [PubMed] [Google Scholar]
- Forde A. T., Crookes D. M., Suglia S. F., Demmer R. T. (2019, May). The weathering hypothesis as an explanation for racial disparities in health: A systematic review. Annals of Epidemiology, 33, 1–18, Article e13. 10.1016/j.annepidem.2019.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester S., Jacobs D., Zmora R., Schreiner P., Roger V., Kiefe C. I. (2019, Apr). Racial differences in weathering and its associations with psychosocial stress: The CARDIA study. SSM Popul Health, 7, 003. 10.1016/j.ssmph.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GeneCards . (2022). https://www.genecards.org
- Geronimus A. T. (1992, Summer). The weathering hypothesis and the health of african-American women and infants: Evidence and speculations. Ethnicity & Disease, 2(3), 207–221. https://www.ncbi.nlm.nih.gov/pubmed/1467758 [PubMed] [Google Scholar]
- Giurgescu C., Nowak A. L., Gillespie S., Nolan T. S., Anderson C. M., Ford J. L., Hood D. B., Williams K. P. (2019, Mar). Neighborhood environment and DNA methylation: Implications for cardiovascular disease risk. Journal of Urban Health: Bulletin of the New York Academy of Medicine, 96(Suppl 1), 23–34. 10.1007/s11524-018-00341-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadi M. A., McHugh G. A., Closs S. J. (2019, Jun). Impact of chronic pain on patients’ quality of life: A comparative mixed-methods study. Journal of Patient Experience, 6(2), 133–141. 10.1177/2374373518786013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum G., Guinney J., Zhao L., Zhang L., Hughes G., Sadda S., Klotzle B., Bibikova M., Fan J. B., Gao Y., Deconde R., Chen M., Rajapakse I., Friend S., Ideker T., Zhang K. (2013, Jan 24). Genome-wide methylation profiles reveal quantitative views of human aging rates. Molecular Cell, 49(2), 359–367. 10.1016/j.molcel.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsberg L. W., Ray M., Conley Y. P., Roberts J. M., Jeyabalan A., Hubel C. A., Weeks D. E., Schmella M. J. (2021, Dec). An exploratory study of epigenetic age in preeclamptic and normotensive pregnancy reveals differences by self-reported race but not pregnancy outcome. Reproductive Sciences, 28(12), 3519–3528. 10.1007/s43032-021-00575-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S., Raj K. (2018, Jun). DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nature Reviews. Genetics, 19(6), 371–384. 10.1038/s41576-018-0004-3 [DOI] [PubMed] [Google Scholar]
- Incollingo Rodriguez A. C., Polcari J. J., Nephew B. C., Harris R., Zhang C., Murgatroyd C., Santos H. P., Jr. (2022, Mar). Acculturative stress, telomere length, and postpartum depression in Latinx mothers. Journal of Psychiatric Research, 147, 301–306. 10.1016/j.jpsychires.2022.01.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins A., Tree J., Tales A. (2021). Distinct profile differences in subjective cognitive decline in the general public are associated with metacognition, negative affective symptoms, neuroticism, stress, and poor quality of life. Journal of Alzheimers Disease, 80(3), 1231–1242. 10.3233/JAD-200882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joehanes R., Just A. C., Marioni R. E., Pilling L. C., Reynolds L. M., Mandaviya P. R., Guan W., Xu T., Elks C. E., Aslibekyan S., Moreno-Macias H., Smith J. A., Brody J. A., Dhingra R., Yousefi P., Pankow J. S., Kunze S., Shah S. H., McRae A. F., London S. J. (2016, Oct). Epigenetic signatures of cigarette smoking. Circulation Cardiovascular Genetics, 9(5), 436–447. 10.1161/CIRCGENETICS.116.001506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston-Brooks C. H., Lewis M. A., Evans G. W., Whalen C. K. (1998, Sep-Oct). Chronic stress and illness in children: The role of allostatic load. Psychosomatic Medicine, 60(5), 597–603. 10.1097/00006842-199809000-00015 [DOI] [PubMed] [Google Scholar]
- Jung H. K., Tae C. H., Moon C. M., Kim S. E., Shim K. N., Jung S. A. (2019). Chronic unexplained nausea in adults: Prevalence, impact on quality of life, and underlying organic diseases in a cohort of 5096 subjects comprehensively investigated. Plos One, 14(12), Article e0225364. 10.1371/journal.pone.0225364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertes D. A., Kamin H. S., Hughes D. A., Rodney N. C., Bhatt S., Mulligan C. J. (2016, Jan-Feb). Prenatal maternal stress predicts methylation of genes regulating the hypothalamic-pituitary-adrenocortical system in mothers and newborns in the democratic republic of Congo. Child Development, 87(1), 61–72. 10.1111/cdev.12487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T. Y., Kim S. J., Choi J. R., Lee S. T., Kim J., Hwang I. S., Chung H. G., Choi J. H., Kim H. W., Kim S. H., Kang J. I. (2017, Jun 29). The effect of trauma and PTSD on telomere length: An exploratory study in people exposed to combat trauma. Scientific Reports, 7(1), 4375. 10.1038/s41598-017-04682-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali H. P., Borrell L. N. (2021, Nov). Telomere length and mortality risk among adults in the United States: The role of age and race and ethnicity. Annals of Epidemiology, 63, 68–74. 10.1016/j.annepidem.2021.07.013 [DOI] [PubMed] [Google Scholar]
- Kurnat-Thoma E. L., Graves L. Y., Billones R. R. (2022, May 18). A concept development for the symptom science model (SSM) 2.0. Nursing Research, 71(6). 10.1097/NNR.0000000000000605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Kim J. H., Chung J. H. (2021, Aug). The association between sleep quality and quality of life: A population-based study. Sleep Medicine, 84, 121–126. 10.1016/j.sleep.2021.05.022 [DOI] [PubMed] [Google Scholar]
- Lei M. K., Beach S. R., Simons R. L., Philibert R. A. (2015, Dec). Neighborhood crime and depressive symptoms among African American women: Genetic moderation and epigenetic mediation of effects. Social Science & Medicine, 146, 120–128. 10.1016/j.socscimed.2015.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. E., Lu A. T., Quach A., Chen B. H., Assimes T. L., Bandinelli S., Hou L., Baccarelli A. A., Stewart J. D., Li Y., Whitsel E. A., Wilson J. G., Reiner A. P., Aviv A., Lohman K., Liu Y., Ferrucci L., Horvath S. (2018, Apr 18). An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY), 10(4), 573–591. 10.18632/aging.101414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Epel E. (2022, Jan). Stress and telomere shortening: Insights from cellular mechanisms. Ageing Research Reviews, 73, 101507. 10.1016/j.arr.2021.101507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J. G., Barksdale D. J. (2008, Apr). Allostasis and allostatic load: Expanding the discourse on stress and cardiovascular disease. Journal of Clinical Nursing, 17(7B), 201–208. 10.1111/j.1365-2702.2008.02347.x [DOI] [PubMed] [Google Scholar]
- Marmot M., Allen J., Bell R., Bloomer E., Goldblatt P. (2012). Consortium for the European review of social determinants of, H., & the health, DSep 15). WHO European review of social determinants of health and the health divide. Lancet, 380(9846), 1011–1029. 10.1016/S0140-6736(12)61228-8 [DOI] [PubMed] [Google Scholar]
- Mathur M. B., Epel E., Kind S., Desai M., Parks C. G., Sandler D. P., Khazeni N. (2016, May). Perceived stress and telomere length: A systematic review, meta-analysis, and methodologic considerations for advancing the field. Brain, Behavior, and Immunity, 54, 158–169. 10.1016/j.bbi.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe R. M., Grutsch J. F., Braun D. P., Nutakki S. B. (2015). Fatigue as a driver of overall quality of life in cancer patients. Plos One, 10(6), Article e0130023. 10.1371/journal.pone.0130023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. S. (2002, Sep-Oct). Sex, stress and the hippocampus: Allostasis, allostatic load and the aging process. Neurobiology of Aging, 23(5), 921–939. 10.1016/s0197-4580(02)00027-1 [DOI] [PubMed] [Google Scholar]
- Misiak B., Stanczykiewicz B., Pawlak A., Szewczuk-Boguslawska M., Samochowiec J., Samochowiec A., Tyburski E., Juster R. P. (2022, Feb). Adverse childhood experiences and low socioeconomic status with respect to allostatic load in adulthood: A systematic review. Psychoneuroendocrinology, 136, 105602. 10.1016/j.psyneuen.2021.105602 [DOI] [PubMed] [Google Scholar]
- Nardi D., Waite R., Nowak M., Hatcher B., Hines-Martin V., Stacciarini J. R. (2020, Nov). Achieving health equity through eradicating structural racism in the United States: A call to action for nursing leadership. Journal of Nursing Scholarship, 52(6), 696–704. 10.1111/jnu.12602 [DOI] [PubMed] [Google Scholar]
- Noren Hooten N., Pacheco N. L., Smith J. T., Evans M. K. (2022, Jan). The accelerated aging phenotype: The role of race and social determinants of health on aging. Ageing Research Reviews, 73, 101536. 10.1016/j.arr.2021.101536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira B. S., Zunzunegui M. V., Quinlan J., Fahmi H., Tu M. T., Guerra R. O. (2016, Mar). Systematic review of the association between chronic social stress and telomere length: A life course perspective. Ageing Research Reviews, 26, 37–52. 10.1016/j.arr.2015.12.006 [DOI] [PubMed] [Google Scholar]
- Palma-Gudiel H., Fananas L., Horvath S., Zannas A. S. (2020). Psychosocial stress and epigenetic aging. International Reviews of Neurobiology, 150, 107–128. 10.1016/bs.irn.2019.10.020 [DOI] [PubMed] [Google Scholar]
- Parker H. W., Abreu A. M., Sullivan M. C., Vadiveloo M. K. (2022, Jul). Allostatic load and mortality: A systematic review and meta-analysis. American Journal of Preventive Medicine, 63(1), 131–140. 10.1016/j.amepre.2022.02.003 [DOI] [PubMed] [Google Scholar]
- Peng H., Zhu Y., Strachan E., Fowler E., Bacus T., Roy-Byrne P., Goldberg J., Vaccarino V., Zhao J. (2018, Sep). Childhood trauma, DNA methylation of stress-related genes, and depression: Findings from two monozygotic twin studies. Psychosomatic Medicine, 80(7), 599–608. 10.1097/psy.0000000000000604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman-Aguilar A., Talih M., Huang D., Moonesinghe R., Bouye K., Beckles G. (2016, Jan-Feb). Measurement of health disparities, health inequities, and social determinants of health to support the advancement of health equity. Journal of Public Health Management and Practice: JPHMP, 22(Suppl 1), S33–S42. 10.1097/PHH.0000000000000373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redeker N. S., Anderson R., Bakken S., Corwin E., Docherty S., Dorsey S. G., Heitkemper M., McCloskey D. J., Moore S., Pullen C., Rapkin B., Schiffman R., Waldrop-Valverde D., Grady P. (2015, Sep). Advancing symptom science through use of common data elements. Journal of Nursing Scholarship, 47(5), 379–388. 10.1111/jnu.12155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robakis T. K., Zhang S., Rasgon N. L., Li T., Wang T., Roth M. C., Humphreys K. L., Gotlib I. H., Ho M., Khechaduri A., Watson K., Roat-Shumway S., Budhan V. V., Davis K. N., Crowe S. D., Ellie Williams K., Urban A. E. (2020, Feb 3). Epigenetic signatures of attachment insecurity and childhood adversity provide evidence for role transition in the pathogenesis of perinatal depression. Translational Psychiatry Electronic Resource, 10(1), 48. 10.1038/s41398-020-0703-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J. L., Newman A. B. (2013). Telomere length in epidemiology: A biomarker of aging, age-related disease, both, or neither? Epidemiologic Reviews, 35(1), 112–131. 10.1093/epirev/mxs008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos H. P., Jr., Adynski H., Harris R., Bhattacharya A., Incollingo Rodriguez A. C., Cali R., Yabar A. T., Nephew B. C., Murgatroyd C. (2021, Mar 1). Biopsychosocial correlates of psychological distress in Latina mothers. Journal of Affective Disorder, 282, 617–626. 10.1016/j.jad.2020.12.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C. V., Schneider K. M., Teumer A., Rudolph K. L., Hartmann D., Rader D. J., Strnad P. (2022, Mar 1). Association of telomere length with risk of disease and mortality. JAMA International Medicine, 182(3), 291–300. 10.1001/jamainternmed.2021.7804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J., Johnson R., Ibemere S. (2021, Jan). Addressing health inequities re-illuminated by the COVID-19 pandemic: How can nursing respond? Nursing Forum, 56(1), 217–221. 10.1111/nuf.12509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T. E., Singer B. H., Rowe J. W., Horwitz R. I., McEwen B. S. (1997, Oct 27). Price of adaptation--allostatic load and its health consequences. MacArthur studies of successful aging. Archives of Internal Medicine, 157(19), 2259–2268. 10.1001/archinte.157.19.2259. https://www.ncbi.nlm.nih.gov/pubmed/9343003 [DOI] [PubMed] [Google Scholar]
- Simons R. L., Lei M. K., Beach S. R., Philibert R. A., Cutrona C. E., Gibbons F. X., Barr A. (2016, Feb). Economic hardship and biological weathering: The epigenetics of aging in a U.S. sample of black women. Social Science & Medicine, 150, 192–200. 10.1016/j.socscimed.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. L., Lei M. K., Klopack E., Beach S. R. H., Gibbons F. X., Philibert R. A. (2021, Aug). The effects of social adversity, discrimination, and health risk behaviors on the accelerated aging of African Americans: Further support for the weathering hypothesis. Social Science & Medicine, 282, 113169. 10.1016/j.socscimed.2020.113169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. L., Lei M. K., Klopack E., Zhang Y., Gibbons F. X., Beach S. R. H. (2021, Apr). Racial discrimination, inflammation, and chronic illness among african American women at midlife: Support for the weathering perspective. Journal of Racial and Ethnic Health Disparities, 8(2), 339–349. 10.1007/s40615-020-00786-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smearman E. L., Almli L. M., Conneely K. N., Brody G. H., Sales J. M., Bradley B., Ressler K. J., Smith A. K. (2016, Jan-Feb). Oxytocin receptor genetic and epigenetic variations: Association with child abuse and adult psychiatric symptoms. Child Development, 87(1), 122–134. 10.1111/cdev.12493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. K., Conneely K. N., Kilaru V., Mercer K. B., Weiss T. E., Bradley B., Tang Y., Gillespie C. F., Cubells J. F., Ressler K. J. (2011, Sep). Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics, 156B(6), 700–708. 10.1002/ajmg.b.31212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. A., Zhao W., Wang X., Ratliff S. M., Mukherjee B., Kardia S. L. R., Liu Y., Roux A. V. D., Needham B. L. (2017, Aug). Neighborhood characteristics influence DNA methylation of genes involved in stress response and inflammation: The Multi-Ethnic Study of Atherosclerosis. Epigenetics: Official Journal of the DNA Methylation Society, 12(8), 662–673. 10.1080/15592294.2017.1341026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin A. R., Terracciano A., Milaneschi Y., An Y., Ferrucci L., Zonderman A. B. (2013, Aug). The trajectory of depressive symptoms across the adult life span. JAMA Psychiatry, 70(8), 803–811. 10.1001/jamapsychiatry.2013.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiatowy W. J., Drzewiecka H., Kliber M., Sasiadek M., Karpinski P., Plawski A., Jagodzinski P. P. (2021, Nov 30). Physical activity and DNA methylation in humans. International Journal of Molecular Science, 22(23), 12989. 10.3390/ijms222312989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajuddin S. M., Hernandez D. G., Chen B. H., Noren Hooten N., Mode N. A., Nalls M. A., Singleton A. B., Ejiogu N., Chitrala K. N., Zonderman A. B., Evans M. K. (2019, Aug 19). Novel age-associated DNA methylation changes and epigenetic age acceleration in middle-aged African Americans and whites. Clinical Epigenetics, 11(1), 119. 10.1186/s13148-019-0722-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner K. J., Vasu V., Griffin D. K. (2019, Jan 19). Telomere biology and human phenotype. Cells, 8(1), 73. 10.3390/cells8010073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Zhan Y., Pedersen N. L., Fang F., Hagg S. (2018, Dec). Telomere length and all-cause mortality: A meta-analysis. Ageing Research Reviews, 48, 11–20. 10.1016/j.arr.2018.09.002 [DOI] [PubMed] [Google Scholar]
- Weder N., Zhang H., Jensen K., Yang B. Z., Simen A., Jackowski A., Lipschitz D., Douglas-Palumberi H., Ge M., Perepletchikova F., O’Loughlin K., Hudziak J. J., Gelernter J., Kaufman J. (2014, Apr). Child abuse, depression, and methylation in genes involved with stress, neural plasticity, and brain circuitry. Journal of the American Academy of Child and Adolescent Psychiatry, 53(4), 417–424, Article e415. 10.1016/j.jaac.2013.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmer M. T., Anderson K., Reynolds M. (2021, Oct 6). Correlates of quality of life in anxiety disorders: Review of recent research. Current Psychiatry Reports, 23(11), 77. 10.1007/s11920-021-01290-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World_Health_Organization (2010). A conceptual framework for action on the social determinants of health. https://www.who.int/publications/i/item/9789241500852 [Google Scholar]
- Zhang T., Yan L. L., Chen H. S., Jin H. Y., Wu C. (2021, Aug 3). Association between allostatic load and mortality among Chinese older adults: The Chinese longitudinal health and longevity study. BMJ Open, 11(8), Article e045369. 10.1136/bmjopen-2020-045369 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Epigenomic Links Between Social Determinants of Health and Symptoms: A Scoping Review by Mitali Ray, McKenzie K. Wallace, Susan C. Grayson, Meredith H. Cummings, Jessica A. Davis, Jewel Scott, Sarah M. Belcher, Tara S. Davis, and Yvette P. Conley in Biological Research For Nursing
Supplemental Material for Epigenomic Links Between Social Determinants of Health and Symptoms: A Scoping Review by Mitali Ray, McKenzie K. Wallace, Susan C. Grayson, Meredith H. Cummings, Jessica A. Davis, Jewel Scott, Sarah M. Belcher, Tara S. Davis, and Yvette P. Conley in Biological Research For Nursing