Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by cartilage and bone damage with the presence of pannus formation which causes uncontrollable proliferation and invasion of fibroblast-like synoviocytes (FLS). Since rheumatoid arthritis is a chronic disorder, the patients normally need to undergo prolonged antirheumatic treatment with the use of disease-modifying antirheumatic drugs (DMARDs), steroids, and nonsteroidal anti-inflammatory drugs (NSAIDs). The efficacy of such long-term pharmaceutical intervention can be significantly affected by the development of drug resistance. The pathological relationship between rheumatoid arthritis and cancer hinted that some chemotherapeutic drugs, such as methotrexate (MTX), could be used for RA treatment. This idea was reinforced by the analysis of mutations in p53 tumor suppressor gene. Around 50% of p53 somatic mutations observed from various cancers are also identified in the synovium of RA patients1 (Fig. 1A). Of note, the hyperplastic synovium in RA with overexpressed p53 mutants contributed to the destruction of joints in patients with erosive RA. Amongst the different cellular components in the synovium of RA patients, p53 mutants are overexpressed in RA synovial fibroblasts (RASFs), which is the pathological phenotype of the FLS.2.
Figure 1.
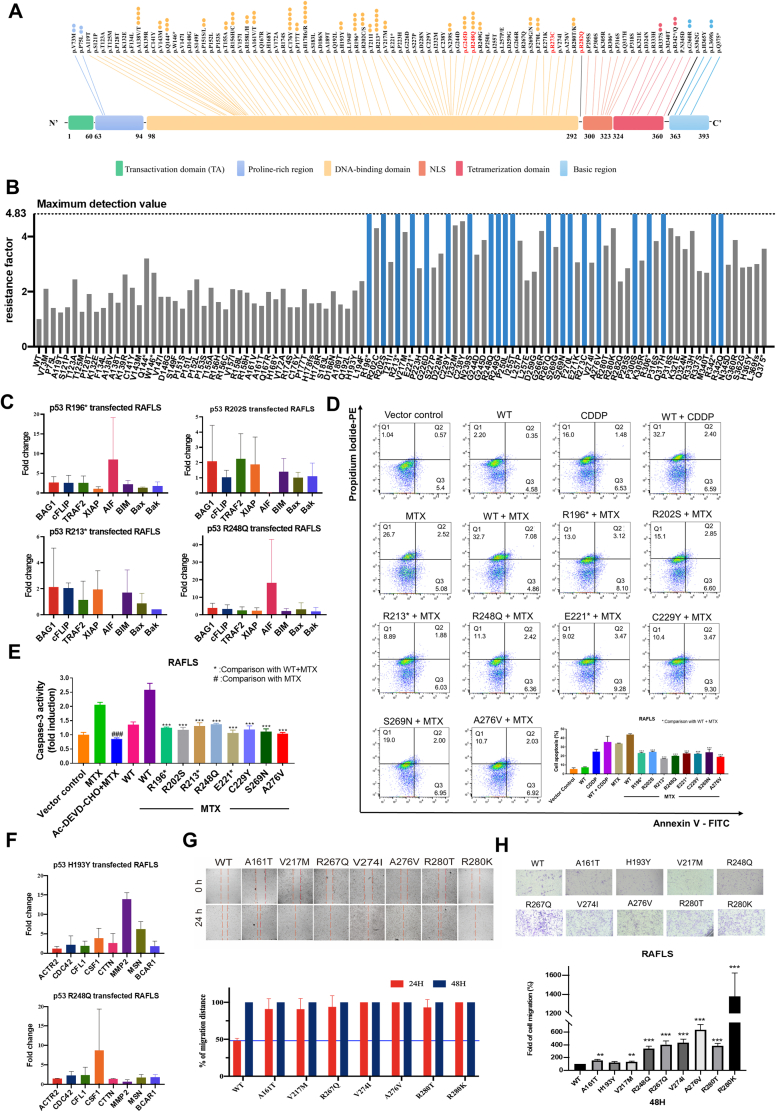
Celastrol circumvents p53 mutation-mediated methotrexate resistance in RA. (A) Hotspots map of p53 mutations in RA patients. Spots represent mutations rate, and mutation sites of protein sequences were shown on top. p53 mutation hotspots reported in cancer patients were highlighted in red and bold. (B) MTT assay of MTX in p53 mutant-transfected RAFLS. The maximum detection value of the MTT method was 4.83, blue represents those p53 mutant-transfected RAFLS MTX resistance factor > 4.83, and gray represents those p53 mutant-transfected RAFLS MTX resistance factor < 4.83. (C) Real-time PCR analysis of apoptosis-related markers' expression levels in p53 mutant-transfected RAFLS, including pro-apoptosis markers (BAG1, cFLIP, TRAF2, and XIAP) and pro-apoptosis markers (AIF, BIM, BAX, and BAK). (D, E) Measurement of p53 mutant-transfected RAFLS under MTX treatment anti-apoptosis phenotype by Annexin V-FITC/PI-PE staining and caspase-3 activity assay. Bar charts represent the anti-apoptosis activity of p53 mutant-transfected RAFLS. Data shown in the bar chart were normalized with WT + MTX or MTX. ###P < 0.005, ∗∗∗P < 0.005. (F) Real-time PCR analysis of migration-related markers' expression level in p53 mutant-transfected RAFLS, including pro-migration markers (ACTR2, CDC42, CFL1, CSF1, CTTN, MMP2, and MSN) and anti-migration marker (BCAR1). (G) p53 mutation promotes RAFLS migration in 24 h in wound healing assay. Bar charts represent the migratory activity of p53 mutant-transfected RAFLS in 24 and 48 h. (H) p53 mutation promotes RAFLS migration in 48 h in the transwell migration assay. Bar charts represent the migratory activity of p53 mutant-transfected RAFLS in 48 h. Data shown in the bar chart were normalized with WT. ∗∗P < 0.01 ∗∗∗P < 0.005.
RAFLS exhibit cancer-like characteristics such as uncontrolled cell proliferation and increased invasiveness, and such cellular characteristics may result in the development of drug-resistant/refractory RA. To investigate whether the use of MTX for RA treatment will become ineffective driven by p53 mutations, we transfected RAFLS with the plasmids overexpressing all RA-associated p53 mutants and measured their survival rates to assess the cytotoxicity of MTX. Here, our data showed that the RAFLS transfected with p53 mutants developed increased MTX-resistance phenotypes (Fig. 1B and Table S1), suggesting that some p53 mutations may affect the drug efficacy of MTX treatment in RA patients.
To investigate whether any identified p53 mutants with MTX-resistance phenotype drive apoptosis-resistant phenotype in RAFLS, the above-mentioned transfected RAFLS were used again for assessing the expression of some prominent apoptotic markers associated with RA, including anti-apoptotic BAG1 (Bcl-2-associated athanogene 1), cFLIP (cellular FLICE-like inhibitory protein), TRAF2 (tumor necrosis factor receptor-associated factor 2), and XIAP (X-linked inhibitor of apoptosis protein), as well as pro-apoptotic AIF (apoptosis-inducing factor), BIM (BCL-2-interacting mediator of cell death), BAX (Bcl-2-associated X protein), and BAK (Bcl-2 homologous antagonist killer). Our results revealed that some p53 mutations cause the up-regulation of anti-apoptotic proteins and down-regulation of pro-apoptotic proteins (Fig. 1C and Table S2), suggesting the development of apoptosis-resistant phenotype (defined by at least three apoptotic markers affected) in RAFLS is driven by these mutations. Of note, among all these mutations with the MTX-resistance phenotype, R213∗ and R248Q simultaneously up-regulated anti-apoptotic proteins and down-regulated pro-apoptotic proteins.
To further analyze whether those p53 mutants (R196∗, R202S, R213∗, R248Q, E221∗, C229Y, S269N, and A276V) overexpressed RAFLS with extremally high MTX IC50 (>100 μM) exhibited an apoptosis-resistant phenotype, we analyzed the apoptosis marker annexin V/precursor iode and caspase-3 activity. The result demonstrated that all those p53 mutants with a lower percentage of cell apoptosis and caspase-3 activity compared with p53 WT (Fig. 1D, E; Fig. S1).
In addition, there is growing evidence that p53 mutations conferring gain–of–function activity drive the promotion of cancer cell migration. Due to the cancer-like nature of RAFLS, we believe some of the RA-associated p53 mutations may affect the cell migration of RAFLS. Therefore, we used the same set of p53-mutant-transfected RALFS to test this hypothesis. The cell migration ability of these cells was evaluated by the expression of cell migration-related genes, such as ACTR2 (Actin Related Protein 2), CDC42 (Cell division cycle 42), CFL1 (Cofilin-1), CSF1 (Colony-stimulating factor-1), CTTN (Cortactin), MMP2 (Matrix metallopeptidase 2), MSN (Moesin) and BCAR1 (BCAR1 scaffold protein, Cas family member), and wound healing and transwell migration assay (Fig. 1F–H; Fig. S2 and Table S3). Two p53 mutations, H193Y and R248Q, may contribute to the pro-migratory phenotype of RASFS (defined by at least three cell migration-related genes affected) via the up-regulation of pro-migratory proteins (>2-fold increase) and the down-regulation of anti-migratory proteins (0.5-fold decrease). Furthermore, the wound healing and transwell migration assay results demonstrated that 44 p53 mutations can promote RAFLS migration after 24 h.
The high frequency of p53 mutations makes p53 become a “rainbow of mutants” with diverse properties, apart from the loss of function and dominant-negative effect, some p53 mutants acquire new functions, termed gain-of-functions (GOF), such as chemoresistance.3 We demonstrated that different mutation sites within p53 showed various levels of anti-apoptotic effect, but those mutations in the DNA-binding domain showed relatively high anti-apoptotic activity, especially the mutant R213∗. Such mutant inhibited the expression of p53-induced apoptotic marker Bax and up-regulated inflammatory cytokine IL-6 in RAFLS.4 R213∗ also limited cell apoptosis in RAFLS by up-regulating the anti-apoptotic markers, cFLIP and XIAP, and down-regulating the pro-apoptotic markers, AIF, BIM, Bax, and Bak, which possibly initiates the formation of pannus. On the other hand, p53 mutations can induce Bax deficiency to promote chemotherapy resistance, suggesting that p53 mutations may implicate the signaling pathway underpinning anti-rheumatic agent resistance by suppressing apoptosis in RAFLS. Moreover, different hotspots of p53 mutations demonstrated various levels of pro-migratory effect on RASFs in our study, which may involve the development of anti-rheumatic agent resistance. For example, R248Q not only inhibited cell apoptosis and promoted cell migration, but also induced anti-rheumatic agents-resistance.
As expected, p53 mutations lead to the development of MTX resistance in RAFLS, which is one of the main reasons for the failure of treatment in RA patients. The p53 signaling pathway is indispensable for the activation of apoptosis in most anticancer agents, which is often disrupted by p53 mutations. RA patients with persistent MTX exposure presented a relatively high mutation rate for p53 (∼75%), which eventually leads to MTX resistance suggesting that mutations of p53 could be induced by prolonged treatment of anti-rheumatic agents. Our group showed that three identified p53 mutations (R202S, R213∗, and R248Q) from synovial tissues of RA patients induced strong anti-apoptotic activity (four apoptotic markers are affected) and developed resistant to MTX treatment (RF > 4). Besides, 72.6% of p53 mutants potentially contributed to MTX resistance in RAFLS, suggesting that p53 is the key regulatory protein in MTX-induced apoptosis. Taken together, p53 mutations (such as R248Q) could promote MTX resistance upon long-term treatment, which induces p53 mutation in RA patients.
Of note, we have previously shown that celastrol, a natural compound from Chinese herbal medicine, could be used for RA treatment by removal of RAFLS via a p53-independent pathway (Table S4). Celastrol can activate autophagy by manipulating Ca2+ signaling in RAFLS and overcome MTX resistance in cancer cells via suppression of P-glycoprotein ABCB5(unpublished data) and induction of p53-independent apoptosis.5 In the current study, our data illustrated that celastrol suppressed the proliferation of most mutant p53-transfected RAFLS. Provided that mutations of p53 could promote drug-resistant protein expression in cancer patients as previously mentioned, the use of celastrol may take benefit in overcoming the drug-resistance problem via bypassing the complex network associated with p53. As such, celastrol may be a potentially effective therapeutic agent against RA due to the same reason.
Author contributions
V.W. and L.L. designed the project. C.Q., J.C., and D.Z. performed the experiments. I.W., C.Q., and J.C. drafted the manuscript. B.L. proofread the manuscript. Y.Z., S.M., W.L., and I.D. assisted with the experiments.
Conflict of interests
The authors declare no competing interests.
Funding
This work was supported by an FDCT grant from the Macao Science and Technology Development Fund (Project code: 0003/2019/AKP) and the Joint Fund of Wuyi University-Macau (No. 2019WGALH01).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2023.02.007.
Contributor Information
Liang Liu, Email: lliu@must.edu.mo.
Vincent Kam Wai Wong, Email: kawwong@must.edu.mo.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Figure S1.
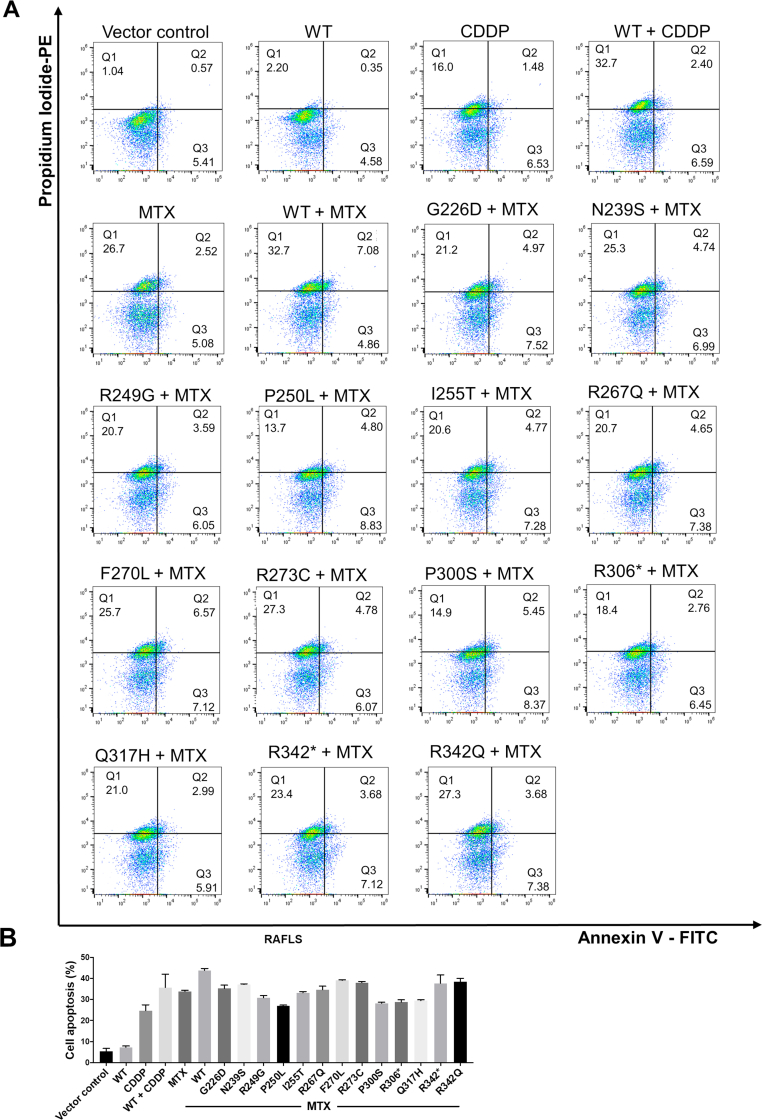
Gene mutation of p53 induces cell apoptosis in RAFLS. (A) Measurement of p53 mutant-transfected RAFLS under MTX treatment anti-apoptosis phenotype by Annexin V-FITC/PI-PE staining. (B) Bar charts represent the anti-apoptosis activity of p53 mutant-transfected RAFLS. Data shown in the bar chart were normalized with WT + MTX or MTX. ###P < 0.005, ∗∗∗P < 0.005.
Figure S2.
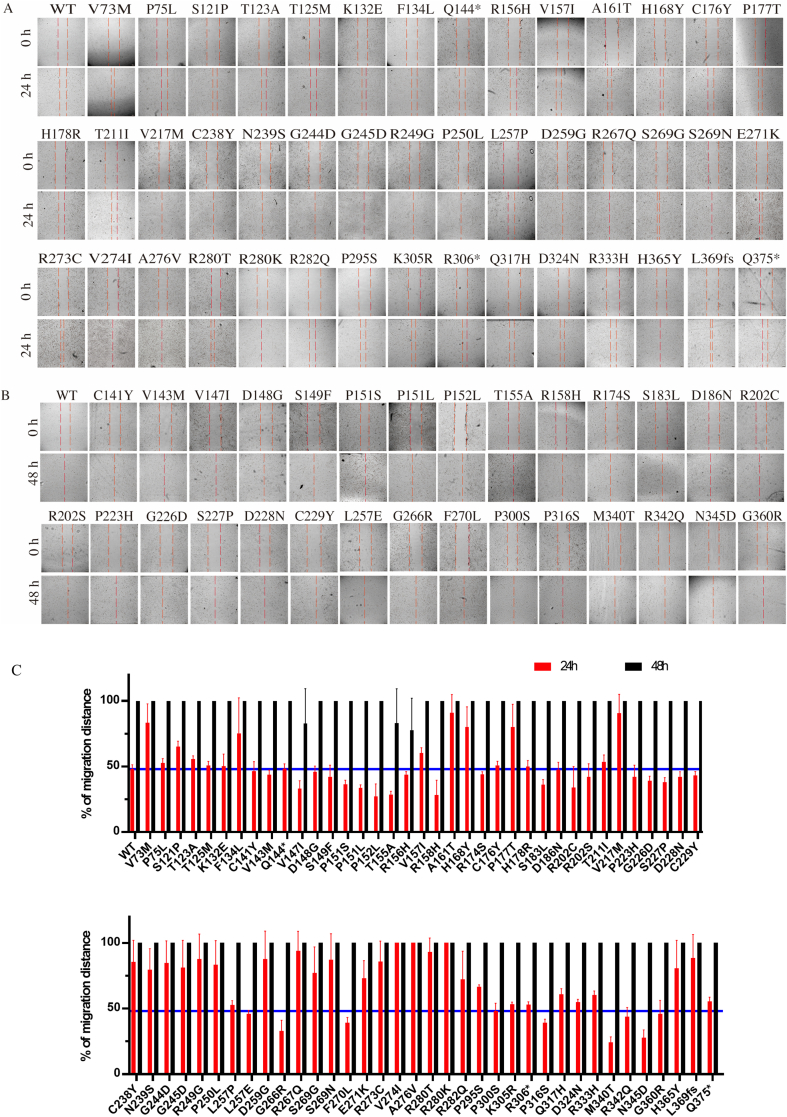
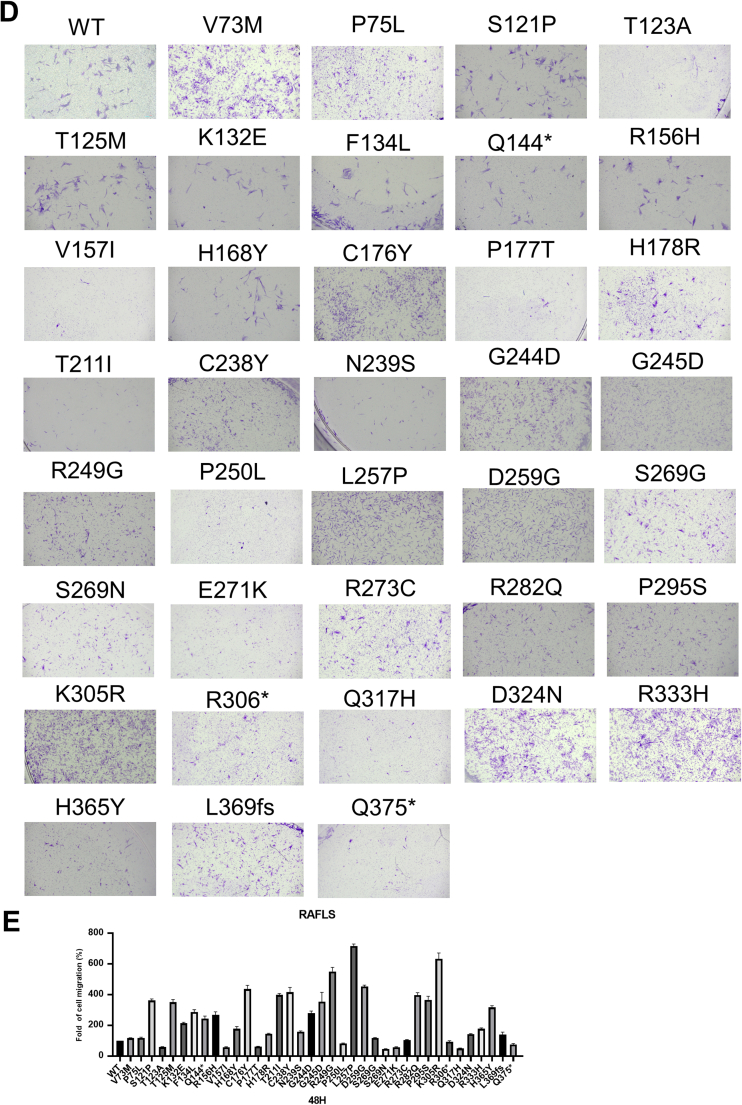
Gene mutation of p53 induces cell migration in RAFLS. (A, B) p53 mutation promotes RAFLS migration in 24 or 48 h in wound healing assay. (C) Bar charts represent the migratory activity of p53 mutant-transfected RAFLS in 24 and 48 h. (D) p53 mutation promotes RAFLS migration in 48 h in the transwell migration assay. (E) Bar charts represent the migratory activity of p53 mutant-transfected RAFLS in 48 h. Data shown in the bar chart were normalized with WT. ∗∗P < 0.01, ∗∗∗P < 0.005.
Figure S3.
References
- 1.Smolen J.S., Aletaha D., Barton A., et al. Rheumatoid arthritis. Nat Rev Dis Prim. 2018;4 doi: 10.1038/nrdp.2018.1. [DOI] [PubMed] [Google Scholar]
- 2.Taubert H., Thamm B., Meye A., et al. The p53 status in juvenile chronic arthritis and rheumatoid arthritis. Clin Exp Immunol. 2000;122(2):264–269. doi: 10.1046/j.1365-2249.2000.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He C., Li L., Guan X., et al. Mutant p53 gain of function and chemoresistance: the role of mutant p53 in response to clinical chemotherapy. Chemotherapy. 2017;62(1):43–53. doi: 10.1159/000446361. [DOI] [PubMed] [Google Scholar]
- 4.Han Z., Boyle D.L., Shi Y., et al. Dominant-negative p53 mutations in rheumatoid arthritis. Arthritis Rheum. 1999;42(6):1088–1092. doi: 10.1002/1529-0131(199906)42:6<1088::AID-ANR4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 5.Wong V.K.W., Qiu C., Xu S.W., et al. Ca2+ signalling plays a role in celastrol-mediated suppression of synovial fibroblasts of rheumatoid arthritis patients and experimental arthritis in rats. Br J Pharmacol. 2019;176(16):2922–2944. doi: 10.1111/bph.14718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.