Abstract
Dendrites are specialized neuronal compartments that sense, integrate and transfer information in the neural network. Their development is tightly controlled and abnormal dendrite morphogenesis is strongly linked to neurological disorders. While dendritic morphology ranges from relatively simple to extremely complex for a specified neuron, either requires a functional secretory pathway to continually replenish proteins and lipids to meet dendritic growth demands. The Golgi apparatus occupies the center of the secretory pathway and is regulating posttranslational modifications, sorting, transport, and signal transduction, as well as acting as a non-centrosomal microtubule organization center. The neuronal Golgi apparatus shares common features with Golgi in other eukaryotic cell types but also forms distinct structures known as Golgi outposts that specifically localize in dendrites. However, the organization and function of Golgi in dendrite development and its impact on neurological disorders is just emerging and so far lacks a systematic summary. We describe the organization of the Golgi apparatus in neurons, review the current understanding of Golgi function in dendritic morphogenesis, and discuss the current challenges and future directions.
Keywords: Dendrite, Golgi, Golgi outposts, Microtubule, Neurodevelopmental disorders, Secretory pathway
Introduction
Neurons are highly polarized cells typically with two distinct compartments, a single axon, and several highly branched dendrites. The dendritic morphology of individual neurons is cell type-specific and ranges from relatively simple (e.g. pyramidal cells in vertebrates and C1da sensory neurons in Drosophila) to extremely complex (e.g. purkinje cells and retinal ganglion cells in vertebrates and C4da sensory neurons in Drosophila). During development, dendrites continuously undergo dynamic changes1 that require a certain abundance of newly synthesized proteins and lipids. The steady supply of these cellular building blocks is regulated by the secretory pathway, which ensures proper protein and lipid transport.2 The Golgi apparatus (including somatic Golgi and specified dendritic Golgi outposts (GOPs); see following text) is at the center of the secretory pathway and essential for post-translational modification, processing and sorting of proteins and lipids, ion homeostasis, and signal transduction.3, 4, 5 In addition, the Golgi apparatus also serves as a non-centrosomal microtubule (MT) organizing center (MTOC) that nucleates and stabilizes MTs,6,7 which is critical for the homeostasis of the secretory pathway and cargo transport.8 Thus, the Golgi apparatus plays a central role in protein and lipid trafficking events and perturbations of its organization have devastating effects on dendritic development.7 Previous studies showed that more than 40% of the Golgi-related genes are known to be associated with multiple diseases affecting the central or peripheral nervous system,9 implying the importance of Golgi for neurogenesis, migration, maturation of postmitotic neurons, and myelination.9, 10, 11, 12, 13, 14, 15 Thus, the emerging evidence highlights the role and potential mechanisms of Golgi organization and function in dendrite development.
Here, we will introduce the Golgi apparatus in general, discuss the progress in the molecular control of Golgi organization and function in both vertebrate and Drosophila neurons, summarize its underlying mechanisms in dendritic morphogenesis, and highlight how Golgi function is contributing to neurological disorders and diseases.
The Golgi apparatus in general
Organization of the Golgi apparatus
In all cells, the Golgi apparatus consists of flattened membrane compartments called cisternae. Under physiological conditions, 4–8 tightly aligned cisternae form a Golgi stack.16 These stacks of cisternae are polarized and classified into three separate modules: the cis-Golgi, which directly receives cargo from the endoplasmatic reticulum (ER), the medial-Golgi cisternae transferring and further modifying cargos, and the trans-Golgi network (TGN) facing the plasma membrane and sorting cargo molecules for delivery to different destinations.17, 18, 19 Each of these three separate modules is present in a single somatic Golgi apparatus2 and contains specific resident proteins marking them as functional cis-Golgi (GMAP-210 and GM130), medial-Golgi (α-mannosidase II (ManII)) or trans-Golgi (galactosyltransferase (GalT) and N-acetyl-galactosaminyltransferase 2 (GalNacT2)) compartments.20,21 It should be noted that the contents of each cisterna vary depending on the state of the cell.22 For example, the Golgi enzyme thiamine pyrophosphatase (TPPase) is present in one or two cisternae of the TGN in supraoptic neurons that synthesize vasopressin, while it spreads to all Golgi components following hyperosmotic shock.23 This dynamic relocalization of Golgi proteins may enable cells to functionally adapt to environmental challenges.24 In addition, this is likely the reason for distinct Golgi morphology in different species and cell types.24, 25, 26 In mammalian cells, multiple Golgi stacks are often laterally linked by tubular structures to form a Golgi ribbon,27 which may facilitate anterograde transport of large cargos.28 In Drosophila, however, the Golgi apparatus typically consists of single stacks, which are only occasionally paired forming a minimal ribbon.27,29
General functions of the Golgi apparatus
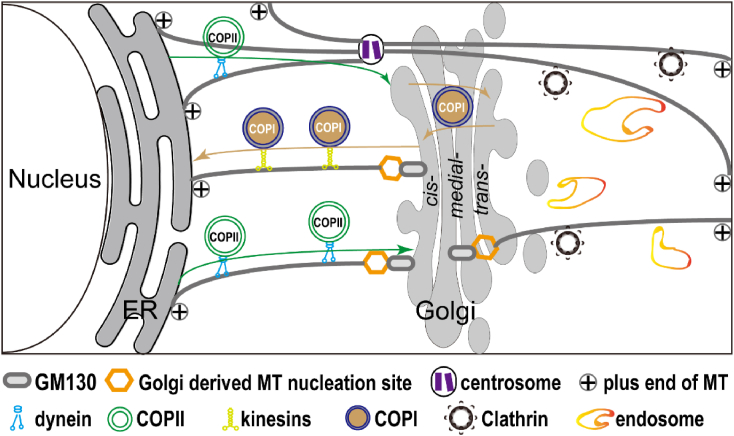
The Golgi is a multifunctional organelle (Fig. 1). It is the central station for intracellular membrane trafficking and plays a key role in processing and sorting newly synthesized and recycled proteins and lipids toward their final destinations.9 The cis-Golgi compartment receives cargo from the ER via coated protein complex II (COPII) transport vesicles.30, 31, 32, 33 Once the cargo reaches the Golgi apparatus, it undergoes post-translational modifications including O-glycosylation,34,35 sulfation,36,37 palmitoylation,38 and proteolytic processing.39 These modified and/or unmodified proteins will be returned to the ER40,41 or transported between different Golgi cisternae41,42 via COPI-mediated trafficking. When proteins or lipids finally arrive at the TGN, they will be sorted and delivered to the appropriate subcellular address within mobile clathrin-coated vesicles.43,44 In addition, the Golgi also receives recycled vesicles from the plasma membrane via endosomal pathways,45, 46, 47, 48, 49, 50 which is essential for protein recycling.
Figure 1.
Golgi-centric organization of the secretory pathway. The cis-Golgi receives cargo from the endoplasmatic reticulum (ER) via coated protein complex II (COPII)-mediated transport. Delivered cargo undergoes post-translational modification and the modified and/or unmodified proteins are either returned to the ER or transported between different Golgi cisternae via COPI-mediated transport. Proteins and lipids gradually reach the trans-Golgi network (TGN) and get sorted and delivered to the appropriate subcellular address via mobile clathrin coated vesicles. Golgi is also receiving recycled endosomal vesicles. The molecular motors dynein and kinesin move cargos along the MT tracks by either directly binding to cargo proteins or associating with adaptor or scaffold proteins. In addition, the Golgi resident protein GM130 is a key molecule for anchoring Golgi-derived MT nucleation sites.
The Golgi complex is also involved in ion homeostasis.51,52 As the main site of protein glycosylation, Golgi is found to contain multiple ions, such as Ca2+, Mn2+, Zn2+, and Cu2+ that are crucial cofactors of Golgi-resident glycosylation enzymes and are also involved in controlling luminal pH, redox homeostasis of Golgi52 and affect other organelle functions.53 It was reported that sarco-endoplasmic reticulum Ca2+-ATPase (SERCA),54 secretory pathway Ca2+-ATPase (SPCA),55 inositol 1,4,5-triphosphate (IP3) receptor and ryanodine receptor (Ryr)56 are the major types of Ca2+ regulators in the Golgi apparatus, Mn2+ is largely transported by TEME16557 and SLC30A10,58 Zn2+ is mainly mediated by ZRT1-and IRT1-like protein (ZIP) family (e.g. ZIP7, ZIP13),59, 60, 61 and Cu2+ transport is mediated by ATP7 proteins.53,62,63 Thus, mutations in these regulators can disrupt cellular physiological functions53,55,59,64,65 and have been linked to multiple diseases.58,60, 61, 62, 63,66
In addition, accumulating evidence highlights the Golgi apparatus as a signaling platform. An abundance of signaling molecules was identified on Golgi membranes, including phosphoinositides,67 small GTPases,68 kinases,69 phosphatases, lipid acylation,70 and trimeric G proteins,71 implying the presence of a diverse range of signaling networks.9 Screening for Golgi defects in human72 and Drosophila73 cell lines identified a variety of signaling molecules that not only regulate local trafficking pathways but also influence a broad range of cellular processes, including autophagy.46,74
Lastly, the Golgi is intimately linked to the cellular MT network. For one, MTs are critical for trafficking cargo to and from the Golgi via directional transport executed by the molecular motor proteins dynein and kinesin.8,75 Dynein and kinesin move Golgi-derived or aimed cargo proteins along MT tracks by either directly binding to cargo or associating with adaptor or scaffold proteins (Fig. 1). For another, the Golgi apparatus colocalizes with MT minus ends and controls the formation of non-centrosomal MT arrays,14,76, 77, 78 which in turn regulates Golgi organization.75,79 In vertebrates, the Golgi matrix protein GM130 bound to AKAP450 recruits CEP215 (also known as CDK5RAP2) and myomegalin (MMG).80, 81, 82, 83, 84 The γ-Tubulin ring complex (γ-TuRC) subsequently binds to CEP215 and MMG induces MT nucleation at the surface of the Golgi apparatus.6 The other player in Golgi-dependent MT organization is CLASP, which interacts with Golgi membranes through the trans-Golgi protein GCC185,85 but may also promote γ-TuRC-mediated nucleation and stabilization of CAMSAP2-bound MT minus-ends directly.86 In Drosophila, γ-tubulin was shown to be present in close proximity to the Golgi but its localization does not rely on the orthologues of pericentrin, AKAP450, or CDK5RAP2.87 Additionally, in Drosophila neurons the centrosome is not required for the maintenance of neuronal microtubule organization,88 adding importance to the Golgi as the major somatic MTOC. Identifying the mechanisms of how the Golgi-derived MT network is organized and in turn organizes Golgi and neuronal function is therefore of high interest.
The Golgi apparatus in neurons
The somatic Golgi apparatus in neurons is similar to other eukaryotic cells in overall organization and function, except it usually invades into the initial segment of 1–2 main dendrites89 (Fig. 2). In addition, in highly polarized cells like neurons, Golgi localization depends on the subtype and developmental stage. For example, in pyramidal neurons, the position of the somatic Golgi apparatus correlates with the position of the longest dendrite in vitro and the apical dendrite in vivo.90 However, in radial glia the Golgi is positioned non-pericentrosomal; while the centrosome localizes close to the ventral side, the Golgi apparatus is shifted toward the basal lamina close to the nucleus.7
Figure 2.
Golgi apparatus in vertebrate and Drosophila neurons. In mice (hippocampal neurons), somatic Golgi shows a ribbon structure and appears to face and extend into primary dendrite(s). Multi-compartment GOPs are largely restricted to one primary dendrite and are often found in the proximal region. Smaller Golgi structures that lack many protein components for sorting and organization of Golgi cisternae are named Golgi satellites, which were identified in distal dendritic arbors in cultured rat neurons. In Drosophila (da sensory neurons), the somatic Golgi apparatus contains cis-, medial- and trans-Golgi components as in vertebrate neurons, but lacks ribbon structure; instead, Golgi forms mini-stacks or “ring”-like stacks. In contrast to vertebrate GOPs, Drosophila GOPs are widespread in the dendritic arbors including distal dendrites, and are enriched at branch points. Both single- and multi-compartment GOPs are present in Drosophila neurons, and most of them are located in the dendritic shaft and branching points, respectively. Somatic Golgi and GOPs are considered (central) stations of the secretory pathway and MT nucleation sites. In addition, Golgi/GOP structures are excluded from the axon, indicating a critical role for the Golgi apparatus in dendritic morphogenesis.
Neurons feature long axons and extensive dendritic trees which require a fast turnover of proteins and lipids for proper information transmission. Thus, it has been intensively discussed for decades whether functional Golgi is present in the axon and/or dendrites. So far, the presence of Golgi in axons is controversial. The Golgi apparatus in neurons has been studied by detecting the activity of TTPase that localizes to Golgi and ER.91 TTPase activity is detected in axons and presynaptic axon terminals suggesting ER is present there. This finding also suggested that synaptic vesicles in axonal terminals could be partially derived from membranes of the Golgi apparatus,92 implying the possibility of Golgi being present in axons. Consistently, GM130, a typical Golgi component, was detected in cultured rat DRG neurons and Xenopus retinal neuron axons by immunostaining and Western blot.93 However, other studies failed to detect Golgi structures/compartments in rat or rabbit neurons by electron microscopy (EM) or immunostaining.94, 95, 96 Similarly, in Drosophila, Golgi markers in sensory neurons are localized specifically to dendrites but not axons.97,98 Although the evidence so far on whether axons contain Golgi compartments is inconclusive, the widely accepted view is that Golgi is absent from axons,4 or at least not functionally present there.
In contrast to axons, Golgi components are relatively abundant in dendrites in both vertebrate and Drosophila neurons, which are known as GOPs. The first evidence for GOPs in vertebrate neurons came from the distribution of temperature-sensitive mutants of the vesicular stomatitis virus glycoprotein (VSVG).94 Shifting the temperature from nonpermissive (39.5°C) to permissive (32°C) in live neurons allowed the visualization of somatic and local dendritic transport of fluorescently labeled VSVG from ER to dendritic Golgi structures. GOPs are discrete compartments that are discontinuous with somatic Golgi but share the same molecular markers and function similarly to somatic Golgi.94,99 In addition, the size of GOPs is much smaller than somatic Golgi, which indicates that dendritic GOPs likely process only a subset of locally synthesized proteins. However, the significance of GOPs in local protein processing is yet to be determined although some evidence suggests their ability to glycosylate newly synthesized membrane proteins100 or to synthesize and express exogenous membrane proteins at the plasma membrane after dendrite transsection.101,102 It should be noted that GOPs are not present in all dendrites in vertebrate neurons. Both in vivo and in vitro studies indicate that GOPs are found in about 5% of dendrites, typically within the first 30 μm of the apical dendrite. In hippocampal neurons, around one-fifth of the neurons contain GOPs in one of their 4–7 primary dendrites94,103,104 (Fig. 2).
Recently, additional Golgi structures termed Golgi satellites (GS) were identified (Fig. 2) by exploiting the Golgi-targeting property of the TGN-resident neuronal EF-hand calcium sensor protein Calneuron-2 to study Golgi organelles in neurons.105, 106, 107 The designed fluorescent probe (termed pGolt) labeled widespread GS located in close proximity to ERGIC and retromer establishing a microscretory system.107 In contrast to vertebrate GOPs, GS are dispersed throughout basal and apical dendrites of all pyramidal neurons and contain at least part of the cellular glycosylation machinery.107 In addition, GS lacks many protein components for sorting and the compartmental organization of Golgi cisternae found in GOPs.107 Furthermore, while GOPs appear to be mainly generated from the somatic Golgi apparatus,108 GS might either form locally in close proximity to ER exit sites in an activity-dependent manner109 or bud off from somatic Golgi.107 Although GS biogenesis is not yet fully understood, their function might enable local protein glycosylation in confined dendritic segments in response to local activity.107,110
In Drosophila, overexpression of ManII-GFP in sensory neurons allowed visualizing GOPs in vivo, which appear as punctate structures in both major and fine terminal dendritic branches but rarely in the axon.20 Unlike somatic Golgi that contains all cis-, medial- and trans-structures in one single Golgi unit, only half of the ManII-GFP positive GOPs also contained the trans-Golgi marker GalT-TagRFP in dendritic shafts,20 suggesting somatic Golgi and GOPs are not structurally identical. However, single- and multi-compartment GOPs are present in both Drosophila and mouse cortical neurons, indicating the heterogenous organization of GOPs is evolutionarily conserved.20 GOPs in Drosophila sensory neurons are highly dynamic and different compartments exhibit temporary interactions,20 yet the biological meaning of such contacts is still unknown. Interestingly, overexpression of GM130, a structural protein likely involved in the formation of stacks and/or ribbons in mammalian cells,21,111, 112, 113 induces the formation of multicompartment GOPs in flies in vivo,20 suggesting this process is tightly regulated. It should be noted that a major caveat of the above-mentioned observation of GOPs in both vertebrate and Drosophila neurons is that exogenous proteins may localize to dendritic organelles due to fluorescent protein tags interfering with normal trafficking or high expression levels exceeding the capacity of the target organelle.2,107 Thus, the distribution of GOPs in distal dendrites is under debate since immunostaining with antibodies to endogenous Golgi organizing proteins showed little localization in distal dendrites.94,103,104,107
Golgi in vertebrate neuronal dendrite development
In vertebrate neurons, the somatic Golgi apparatus generally exists as a ribbon forming a single stack consisting of cis-, medial- and trans-Golgi compartments oriented towards the main dendritic branches, with GOPs being exclusively present in dendrites, suggesting they play a pivotal role in dendritic development.90 The vertebrate Golgi is not only crucial for the proper sorting, trafficking, and modification of ion channels, receptors, and other signaling molecules but also mediates the transport of exogenous molecules via retrograde and trans-synaptic pathways. Although some candidates have been identified to regulate Golgi organization in different cell types,72,73 their functions in dendritic development are largely unknown. Since GOPs in vertebrate neurons are positioned close to somatic Golgi, it is difficult to distinguish Golgi fragmentation from GOP organization. Thus, most studies in vertebrate neurons are focusing on somatic Golgi and its potential role in dendritic development (Fig. 3). The function of GOPs in vertebrate neurons, however, is less well understood.108
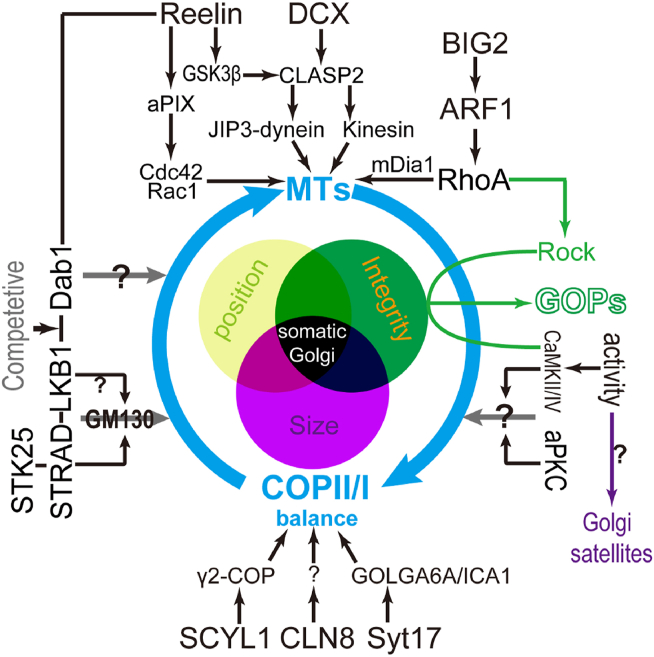
Figure 3.
Vertebrate Golgi apparatus in dendrite development. Most of the proteins that affect somatic Golgi organization will regulate both Golgi integrity and position (only CLN8 was reported to regulate Golgi size). These candidate effectors can either affect MT dynamics or control COPII and COPI transport balance. In addition, some Golgins, like GM130, may directly connect different Golgi compartments. Neuronal activity and additional kinases might regulate Golgi organization by unknown mechanisms and link Golgi function to dendrite development. While vertebrate GOPs are generated from the somatic Golgi upon enhancing neuronal excitation and/or RhoA-Rock signaling, the biogenesis of Golgi satellites is elevated upon neuronal activity.
Doublecortin (DCX)
As discussed above, the somatic Golgi usually invades into 1–2 proximal dendrites in vertebrate neurons. In cultured hippocampal neurons, knockout of doublecortin (DCX), a microtubule (MT) binding protein that links to neuronal migration and process outgrowth,114, 115, 116 limits the elongation of somatic Golgi into proximal dendrites.89 This defect is possibly mediated by multiple factors working in a synergistic manner that affect the balance of anterograde and retrograde transport in the absence of DCX. DCX associates with kinesin-3 to facilitate anterograde transport114 and inhibit dynein binding to JIP3 to negatively regulate dynein-mediated retrograde transports. Thus, the combined effect of DCX on retrograde and anterograde transport balances the extension of somatic Golgi into dendrites.89 In DCX deficient neurons, however, more JIP3 molecules bind dynein via unknown mechanisms/adaptor(s) which affects retrograde transport. In addition, loss of DCX restricts Kinesin-3114 and CLASP289 to the neuronal soma, which decreases anterograde transport. The imbalance of anterograde and retrograde transports in DXC-deficient neurons results in improper Golgi localization.
CLASP2
CLASP2, a protein that directly links to plus-ends of growing MTs and actin filaments,117 is critical for establishing continuity and proper morphology of Golgi by arranging Golgi-derived MTs in fibroblasts.79,85,118 Similarly, knocking down CLASP2 in cultured hippocampal neurons led to Golgi fragmentation, which could be fully rescued by overexpression of human CLASP2.119 Overexpression of CLASP2 alone resulted in more stacked Golgi that highly correlates with increased dendritic branching. This implies that CLASP2 action on Golgi morphology plays an important role in dendritic development and branching.119
CLN8
Ceroid-Lipofuscinosis, Neuronal 8 (CLN8) is a transmembrane ER-resident protein that shuttles between the ER and the ER–Golgi intermediate complex (ERGIC) via two C-terminal signal peptides that mediate recognition by COPII and COPI,120,121 respectively. Both COPII and COPI are essential components for the secretory pathway that shuttle the cargos between ER and Golgi and/or inter-Golgi trafficking to maintain Golgi's normal structure.33,122, 123, 124 Thus, disrupting the balance of COPII- and COPI-mediated transport will likely lead to abnormal Golgi morphology and function. Recently, it was reported that CLN8 knockdown increased the size of the Golgi apparatus, but decreased the complexity and size of the somatodendritic compartment in primary rat hippocampal neurons.125
Previous studies suggested that CLN8 modulates vesicular trafficking by physically interacting with proteins such as VAPA, syntaxin 8, and GATE16.126 Interestingly, these factors are implicated in the regulation of Golgi morphology.127,128 It was proposed that CLN8 affects Golgi morphology by controlling vesicular trafficking. Thus, expression of Rab7a-Q66L, a constitutively active GTP-bound Rab7a that mediates transport from early to late endosome/lysosome in mammalian cells,129 or silencing of TMEM106b, a protein involved in anterograde vesicular traffic,130 resulted in altered size and/or fragmentation of Golgi in HeLa cells. Although the direct mechanisms of how CLN8 affects Golgi morphology in neurons are unknown, these results suggest that the increase of Golgi size observed upon CLN8 knockdown can be explained (at least in part) by the alteration of vesicular traffic.125
SCYL1
SCY1-like pseudokinase 1 (SCYL1) is a member of the SCY1-like family of catalytically inactive protein kinases that regulates Golgi morphology.131, 132, 133 SCYL1 is prominently expressed in central nervous system (CNS) neurons and its pathological variants cause delayed brain development.134,135 SCYL1 modulates COPI-mediated retrograde transport by interacting with γ2-COP, a subunit of COPI coatomer, to affect Golgi apparatus organization.136 In addition, SCYL1 also localizes to the cis-Golgi where it co-localizes with GM130 and 58K/formiminotransferase cyclodeaminase (FTCD). Knockdown of SCYL1 disrupts COPI-mediated retrograde trafficking of the KDEL receptor to the ER without affecting anterograde traffic from the ER,132 indicating that an imbalance of the secretory pathway affects Golgi morphology.
Recently, it was reported that SCYL1 colocalizes with protein arginine methyltransferase 1 (PRMT1) in the Golgi. Upon arginine-methylation by PRMT1, SCYL1 could efficiently interact with γ2-COP. Inhibition of PRMT1 suppressed axon outgrowth and knockdown of SCYL1 inhibited axon outgrowth and dendrite complexity with abnormal Golgi morphology. The inhibitory effect was rescued by siRNA-resistant SCYL1, but not a SCYL1 mutant in which the arginine methylation site was replaced. Thus, SCYL1 arginine methylation by PRMT1 facilitates COPI transport to maintain Golgi organization, which likely contributes to axon and dendrite morphogenesis in neurons.137
Synaptotagmin 17
Synaptotagmin 17 (Syt-17), a structurally atypical Syt protein family member, plays no role in exocytosis but is highly expressed in the brain and kidney, especially in hippocampal pyramidal cell layers and in cultured hippocampal neurons.138, 139, 140, 141 Knockout of Syt-17 in mice results in defective novel object recognition.142 Unlike other Syt family members, Syt-17 lacks an N-terminal transmembrane domain; rather, it associates with the membrane via a string of fatty-acylated cysteine residues (palmitoylation or S-acylation) near the N-terminus of the protein.143 The subcellular localization of Syt-17 is not fully elucidated due to the lack of specific antibodies and reported differences depending on the detection method.140,142,144,145 Recently, Syt-17 was shown to localize to the Golgi complex in the soma of hippocampal neurons, where it coordinates the import of vesicles from the endoplasmic reticulum to support neurite outgrowth.142 Yeast two-hybrid screening identified two resident Golgi proteins, GOLGA6A and ICA1, as Syt-17 interactors that are putatively involved in importing vesicles from the ER and tethering them to the Golgi. Syt-17 thus appears to be a member of a cargo import complex on the surface of the neuronal Golgi.142 In addition, Syt-17 was occasionally (<1%) detected to colocalize with remote ManII puncta at apparent GOPs in neuronal dendrites.142 The possible function of Syt-17 on GOPs is still unknown.
GM130
GM130 was originally identified in the rat liver112 and belongs to the golgin family, which encompasses large (>500 residues) proteins localizing predominantly to the Golgi and forming a coiled-coil structure over most of their length.146 So far, 11 mammalian golgins have been identified, localizing to distinct regions of the Golgi stack. Most golgins are attached to the membrane at their carboxyl-termini via a transmembrane domain or a domain that binds a small GTPase of the Rab or Arf family.146 Together with the golgins p115, giantin, and GRASP65, GM130 facilitates vesicle fusion to the Golgi membrane as a vesicle “tethering factor”.147 In addition, it is also involved in the maintenance of the Golgi structure and plays a major role in the disassembly and reassembly of the Golgi apparatus during mitosis.148, 149, 150 GM130 function is reportedly involved in multiple biological processes including glycosylation, cell cycle progression, cell polarization, and directed cell migration.147 However, the functions of GM130 in Golgi organization and dendritic morphogenesis in developing neurons are not well characterized. Global knockout of GM130 in mice revealed that it is not essential for embryonic development, but results in developmental delay, severe ataxia, and postnatal death.151 Neuronal-specific deficiency of GM130 (GM130-nKO) in mice causes similar developmental phenotypes as the global knockout including growth retardation and striking ataxia.151 Intensive analysis of brain architecture of the forebrain or midbrain of GM130-nKO mice showed no gross changes; however, cerebellar size was dramatically reduced. By examining Purkinje cells, Liu et al found that selective deletion of GM130 in neurons causes fragmentation and defective positioning of the Golgi apparatus, impaired secretory trafficking, and dendritic atrophy in Purkinje cells.151 In addition, those phenotypes worsen with age.
It should be noted that RNAi-mediated depletion of GM130 in hippocampal neurons results in mild impairment of dendritic initiation.152 However, dendritic initiation in Purkinje neurons still occurs in the absence of GM130, but dendritic maintenance is impaired in vivo. A reasonable explanation for this discrepancy is likely due to redundancy in the golgin function, which may vary between different types of neurons.146 The other possibility is that Purkinje cells are particularly susceptible to perturbations of secretory trafficking because of their extremely large dendritic tree, which requires a significant amount of material for both its growth and its maintenance.151 Interestingly, loss of the GM130 binding partner GRASP65 in mice fails to elicit a phenotype.153 This could be attributed to a compensatory role by the related protein GRASP55.154 However, GRASP55 interaction with GM130 could not be shown in vivo,155 suggesting the phenotypes of Purkinje cells in GM130 knockout mice are likely independent of GRASP65.151
Atypical protein kinase C
Dendrite morphogenesis can be divided into three major stages including initiation to establish the primary branch(es), outgrowth, and branching.156 The number of primary dendrites may affect various cellular functions and dendritic computation.157 Cerebellar Purkinje cells extend a single primary dendrite from the soma that ramifies into a highly branched dendritic arbor. However, in vivo time-lapse imaging of zebrafish Purkinje cells revealed that they initially extend multiple neurites from the soma and subsequently retract all but one, which becomes the primary dendrite.158 This system provides an excellent model to investigate how a primary dendrite(s) gets selected. Interestingly, the Golgi apparatus is specifically located at the root of the primary dendrite, and its localization is already established in immature Purkinje cells that have multiple neurites.157 In addition, inhibiting secretory trafficking through the Golgi apparatus reduces dendritic growth, suggesting that the Golgi apparatus is involved in dendritic morphogenesis.157
Atypical protein kinase C (aPKC), together with Par3 and Par6, forms a complex that is important for neuronal polarity establishment, although aPKC function in axon or dendrite polarization is still controversial.159 Upon loss of aPKC, zebrafish Purkinje cells retain multiple primary dendrites and show disrupted localization of the Golgi apparatus.158 Importantly, a mosaic inhibition of aPKC in Purkinje cells recapitulates the whole larval mutant phenotype, suggesting that aPKC cell autonomously controls Golgi localization and thereby regulates the specification of the primary dendrite of Purkinje cells.
STK25
Adult-born dentate granule cells (DGCs) establish a DGC-like morphology at ∼7 d after birth, with a primary dendrite pointing to the molecular layer and several neurites in the neurogenic zone.160 However, the events leading to the transition from simple spindle-like DGCs to their mature dendrite morphology are largely unknown. Rao et al found that the Golgi apparatus was dynamically repositioned in the soma of adult-born DGCs during the initial integration stage. Two weeks after birth, by which time most neurites in the neurogenic zone were eliminated, a compact Golgi apparatus was positioned exclusively at the base of the primary dendrite.160 Previous single-cell transcriptome analysis in adult-born DGCs identified several Golgi-associated genes including STK25 and STRAD161 as regulators of neuronal development. Knocking down either of these two proteins showed Golgi mislocalization and extensive aberrant dendrite formation in adult-born DGCs. In addition, overexpression of STRAD-PMSE-Δ180, a mutated form causing polyhydramnios, megalencephaly, and symptomatic epilepsy,162 resulted in similar defects in Golgi localization and dendrite formation in adult-born DGCs.
Further studies showed that physical interaction between STK25 and STRAD resulted in significant stabilization of STK25 protein in HEK293 cells.160 In addition, STK25 was shown to regulate Golgi localization through the recruitment of GM130.160,163 GM130 can also associate with STRAD and coexpression of GM130 with either STRAD or STK25 in HEK-293 cells resulted in significant stabilization of the GM130 protein. The association of STK25 with STRAD might stabilize a Golgi signaling complex via interaction with GM130, which is necessary for the polarized positioning of Golgi in newborn DGCs. In support, STK25 was predominantly found in distinct somatic structures at the base of the apical dendrite of adult-born DGCs and it colocalizes with STRAD and the Golgin GRASP65.160 Overall, STK25 and STRAD are critical for polarized Golgi localization and dendrite establishment of adult-born DGCs, likely by directly interacting with GM130.
LKB1
The serine/threonine protein kinase liver kinase B1 (LKB1) is a well-known regulator of cell polarity. LKB1 expression in the dentate gyrus of 8-week-old adult mice is elevated in adult-born DGCs.152 Detailed in vivo analysis showed that LKB1 is critical for the polarized initiation and extension of the single primary dendrite in adult-born DGCs. Interestingly, although the Golgi apparatus accumulated at the base and within the initial segment of the primary dendrite in wild-type granule cells, it was dispersed over the entire soma in LKB1 knockout neurons, with no clear GOPs in any dendrite.152 Furthermore, quantitative analysis showed that Golgi distribution exhibited a clear preference toward the molecular layer in wild-type neurons, and this preference was largely lost in LKB1 knockout neurons. Thus, LKB1 is required for the polarized Golgi distribution in adult-born DGCs. Importantly, disrupting Golgi distribution by either down-regulating the Golgi matrix protein GM130 or overexpressing GRASP65 in the adult hippocampus led to significant defects in both the initiation and extension of dendrites, which is consistent with the dendritic defects in LKB1-knockdown neurons. These results confirm the idea that Golgi polarity is important for dendrite morphogenesis in adult-born DGCs and suggest that the function of LKB1 in dendrite development is linked to its role in regulating Golgi organization.
Reelin
Reelin, a secreted ligand that is produced in discreet layers in the developing brain, was originally identified to be crucial for neuronal positioning and laminar organization.164, 165, 166 Following genetic and biochemical studies showed that it also regulates dendritogenesis via multiple mechanisms.167, 168, 169, 170, 171, 172 Among these, the cytoplasmic adaptor protein Dab1,173 a core effector of the Reelin signaling pathway,174,175 affects dendritic initiation and elaboration,171,176,177 for example, by maintaining the normal extension of the Golgi apparatus into the apical dendrites in hippocampal, pyramidal and L6 cortical neurons.176,177 Interestingly, Matsuki et al further found that STK25, a modifier of Reelin-Dab1 signaling, regulates Golgi morphology and neuronal polarization as part of an LKB1-STK25-GM130 signaling pathway.177 Overexpression of STK25 induces Golgi condensation and multiple axons, both of which are rescued by Reelin treatment. Conversely, Reelin-induced extension of the Golgi into dendrites could be suppressed by STK25 overexpression.177 Thus, this elegant study highlights the importance of balance between Reelin-Dab1 and LKB1-Stk25-GM130 signaling in controlling Golgi dispersion, axon specification, and dendrite growth.
Recently, CLASP2 was also identified as a key cytoskeletal effector in the Reelin signaling pathway to regulate neocortical development.178 It was reported that the proper movement of the centrosome and Golgi apparatus is a key step in selecting the migratory direction, and their translocation into the leading neurite precedes cell movement during neuronal migration.179,180 Dillon and colleagues found that downregulation of CLASP2 in migrating neurons causes mislocalization of neurons to deeper cortical layers, abnormal positioning of the centrosome-Golgi complex, and aberrant length/orientation of the leading process.178 They further discovered Reelin could regulate several phosphorylation sites within the positively charged serine/arginine-rich region that constitute consensus GSK3β phosphorylation motifs of CLASP2. Furthermore, phosphorylation of CLASP2 regulates its interaction with the Reelin adaptor Dab1, which is required for CLASP2's effect on neurite extension and motility.178
Rho GTPases
Around 20 canonical Rho GTPases have so far been characterized in mammals that act as molecular switches by cycling between a GDP-bound, inactive state and a GTP-bound, active state, to control a wide variety of signaling pathways for dendritogenesis.181, 182, 183 The cellular regulation of this cycle is finely tuned by over 60 activators (guanine nucleotide exchange factors, GEFs), which accelerate intrinsic GDP/GTP exchange, and over 70 inactivators (GTPase-activating proteins, GAPs), which stimulate intrinsic GTP hydrolysis activity.181,182 Of these intricately regulated Rho GTPases, Rho, Rac, and Cdc42 are the three best-characterized members.181
It was reported that TRIO,184 an essential Rho GEF, localizes to the Golgi apparatus (especially the trans-Golgi)185 and is required for neurite growth186 in cerebellar granule neurons. Although it is still unknown whether Golgi morphology is altered in TRIO knockout mice, Golgi function is largely disrupted since TRIO affects membrane vesicle trafficking.185 Direct evidence for Rho GTPases controlling Golgi morphology and dendrite development stems from the observation that the Cdc42/Rac1 GEF aPIX/Arhgef6 could promote translocation of Golgi cisternae into developing dendrites of hippocampal neurons.187 Interestingly, this effect is further increased upon Reelin treatment. Importantly, overexpression of exchange activity-deficient aPIX/Arhgef6 or dominant-negative Cdc42 or Rac1 impaired dendritic Golgi positioning, an effect that was not compensated by Reelin treatment. Thus, this study implies an unexpected role of Reelin in Golgi morphology to control dendrite development that relies on the activation of Cdc42 or Rac1.187
Recently, RhoA signaling was also shown to be critical for Golgi arrangement to control dendrite development.188 Hong et al identified Brefeldin A-inhibited GEF 2 (BIG2) as a novel Rho GTPase and knockdown of BIG2 significantly reduced dendritic complexity.188 Expression of the constitutively active ARF1 could rescue the defective dendrite morphogenesis of BIG2-deficient neurons. Importantly, BIG2 co-localizes with the Golgi apparatus and is required for Golgi deployment into major dendrites in cultured hippocampal neurons. Simultaneous overexpression of BIG2 and ARF1-activated RhoA, or treatment with the RhoA activator lysophosphatidic acid in neurons lacking BIG2 or ARF1 increased the number of cells with dendritic Golgi. Finally, they characterized that mDia1, a well-known regulator of MT and actin dynamics,189,190 acts as a downstream effector of the BIG2-ARF1-RhoA axis to mediate Golgi polarization and dendritic morphogenesis.188
Although GOPs were observed in vertebrate neurons for over a decade,90 how GOPs are generated remained unanswered until a recent study showed a crucial role of RhoA in the generation of vertebrate neuronal GOPs.108 With time-lapse imaging in cultured hippocampal neurons, the authors could show that GOPs destined to major “apical” dendrites are generated from the somatic Golgi apparatus by a sequence of events involving (i) generation of a Golgi-derived tubule, (ii) tubule elongation and deployment into the dendrite, (iii) tubule fission, and (iv) transport and condensation of the fissioned tubule.108 A RhoA-Rock signaling pathway involving LIMK1, PKD1, slingshot, cofilin, and dynamin regulates polarized GOP formation by controlling the tubule fission.108 Thus, this study provided new insights regarding the involvement of RhoA in GOP biogenesis to regulate dendritic development and polarization.
Golgi in Drosophila neuronal dendrite development
Drosophila and vertebrate Golgi share a high degree of similarity.27 GOPs and Golgi function are best studied in Drosophila dendritic arborization (da) sensory neurons in the larval peripheral nervous system. Da neurons are placed into four distinct morphological classes based on their dendritic complexity named Class I to Class IV da (C1da–C4da) neurons.191 While the secretory pathway was found to be highly essential for neuronal dendrite morphogenesis in this system,98 nearly all studies are focused on GOPs instead of somatic Golgi. This is likely due to a widely accepted concept that GOPs are important for dendritic dynamics in Drosophila neurons, which is based on the following observations98: (i) directional movement of dendritic GOPs correlates with the extension and retraction of dendritic branches; (ii) focally disrupting GOPs by intense laser light limits dendritic dynamics98; and (iii) blocking the interaction of the Drosophila golgin Lava lamp (Lva) with the dynein/dynactin complex192 caused redistribution of GOPs and dendritic branching defects.
Dendritic GOPs likely serve as local secretory stations94,193 and/or non-centrosomal MT organization centers.78 Both in vitro and in vivo observations showed that GOPs could directly nucleate and orientate dendritic MTs to regulate dendrite development.78,83 However, an independent study did not support this finding since dragging GOPs out of dendrites does not affect γ-tubulin distribution.97 In addition, it was also found that GOPs may locally regulate MT orientation, but are not required for overall polarity in Drosophila neurons.194 Thus, the role of GOPs in non-centrosomal MT nucleation is controversial. While pinning down the functions of GOPs in dendrite development requires further studies, the molecular mechanisms of how GOPs are organized in dendrites are gradually emerging.
Transcription factors
In general, the wide variety of dendritic arbors from distinct neurons is mainly determined by the combinatorial expression of transcription factors. In Drosophila da neurons, the transcription factor Abrupt (Ab) is essential for controlling simple dendritic morphology in C1da neurons, while Cut (Ct) promotes dendritic filopodia/spikes in C3da and C4da neurons.195,196
A previous study showed that Cut initiates a gene expression cascade through CrebA that coordinately affects Sec31, an important component of COPII secretory transport, to bi-directionally regulate dendrite elaboration.197 Importantly, overexpression of Cut increased the number of satellite secretory endoplasmic reticulum (ER) and GOPs primarily localized to dendritic branch points, consistent with upregulation of the COPII machinery.197
In simple C1da neurons, it was found that Ab-mediated branch repression is facilitated by centrosomin (cnn), a homologue of CDK5RAP2 that localizes to the centrosome and Golgi in HeLa cells.82 While complex C4da neurons have been shown to contain a mix of single and multicomponent outposts,20 Yalgin et al predominantly detected ManII-positive outposts in C1da neuron arbors, while GalT-positive outposts were rare.83 Interestingly, half of the fluorescently labeled Cnn foci colocalized with ManII-positive GOPs. In addition, the Cnn signal was distributed only to the cis-face of GOPs, with nearly all outposts showing asymmetric Cnn localization. Cnn acts in conjunction with the Pericentrin-like protein (Plp),198,199 and loss of Plp increased clustering of Cnn foci along dendrite branches and branch points and tips, indicating that proper dendritic localization of Cnn to GOPs requires Plp. Furthermore, it was shown that Cnn recruits microtubule nucleation to GOPs to counteract wee Augmin (Wac)-driven anterograde microtubule growth.83 Thus, polarized targeting of Cnn to GOPs during dendritic development creates a local system for guiding microtubule polymerization.83
MT-based motor proteins
The microtubule-based molecular motors dynein and kinesin are essential for proper neuronal morphogenesis.200, 201, 202 Loss of dynein function alters GOP distribution,201 and ManII-positive GOPs were decreased in number and size in distal dendritic arbors. Conversely, an increased number and size of GOPs were observed in proximal dendrites. This change in GOP distribution thus parallels the change in branch distribution (more branches in proximal and reduced branches in distal regions). In addition, abnormal axonal distribution of GOPs, usually excluded from axons, was found in dynein mutants,98,201 suggesting dynein actively controls GOP distribution during neuronal development. Interestingly, one of the dynein cofactors, Nuclear distribution E (NudE), has been implicated in the regulation of early neurite extension in vertebrate neurons.203 Similarly, the only conserved homologue of the NudE family in Drosophila is also essential for dendrite development and its deficiency mimics the dendritic phenotypes in dynein mutants.204 Importantly, Drosophila NudE is colocalized with GOPs and prevents GOPs from entering the axon.204 Interestingly, overexpression of Lis, a critical interactor of dynein,205 could rescue the abnormal dendritic arborization that is caused by loss of NudE.204 Although it was reported that GOPs are involved in dendritic microtubule nucleation and orientation, NudE mutants showed only slightly increased levels of dynamic microtubules without affecting their polarity in dendrites.204
Recent in vivo analysis of endogenous kinesin-1 showed that a critical Glutamate residue within β5-loop 8 (E177) is required for dendrite-specific localization of GOPs.202 KhcE177K, but not KhcE177A, resulted in GOPs mislocalizing to axons, similarly to KhcRNAi. KhcE177K also phenocopied KhcRNAi showing decreased dendrite arborization during aging.202 Since this particular glutamate, E177, mediates contact with the C-terminal tail when kinesin-1 is in an autoinhibited conformation,206 autoinhibition of kinesin-1 seems to be essential for dendrite-specific localization of GOPs and dendrite development.
While GOPs are transported into dendrites by dynein, the dendrite-specific localization of GOPs may depend on a regulated balance of dynein and kinesin-1 activity. Consistent with this idea, reducing kinesin-1 levels in dendrites causes a distal shift of GOPs in the arbor by increasing outposts distally and reducing them proximally.202 Overall, our current understanding suggests that dynein transports outposts into dendrites,98,201,204 and selective localization of GOPs to dendrites relies on balanced autoinhibition of the anterograde motor kinesin-1.202
Golgi and neurological disorders
Abnormal dendritic morphogenesis is accompanying multiple neurological disorders including autism spectrum disorders (ASD), Alzheimer's disease (AD), Parkinson's disease (PD), spinocerebellar ataxia, and dementia.207 Although the potential roles of the Golgi apparatus in neurological disorders have been intensively reviewed,9, 10, 11, 12, 13 the involvement of abnormal dendrite development/maintenance in these diseases is rarely discussed. Recent studies showed that several proteins related to neurological disorders are involved in re-organizing the Golgi to regulate dendrite morphogenesis, including Lrrk2,208 polyglutamine (polyQ) repeat proteins,209 C9orf72,210 and APP211 (Fig. 4).
Figure 4.
The role of GOPs in dendrite development and neurological disorders. (i) The transcription factor Cut, pathogenic PolyQ proteins, and C9orf72 affect GOPs by controlling CrebA-dependent transcription. Sec31, an essential component for COPII, is one of the effectors. (ii) The transcription factor Ab regulates cnn (homologue to CDK5RAP2) that interacts with plp and wac to link GOP organization and MT dynamics. (iii) NduE promotes Lis binding to dynein to regulate GOP transport. (iv) Lrrk interacts with Lva and inhibits the interaction between Lva and dynein, disrupting the recruitment of dynein to GOPs. (v) The adaptor protein Syd mediates APP-induced alteration of multi-compartment GOP movements and dendrite development. (vi) GM130 could directly connect different Golgi compartments via an unknown mechanism.
LRRK2
Leucine-rich repeat kinase 2 (LRRK2) is a member of the ROCO family with a Ser/Thr kinase domain.212 Dominant mutations in human LRRK2 are prevalent in both familial and sporadic PD and are located in the catalytic domain.212, 213, 214 The Drosophila genome encodes a single Lrrk orthologue with conserved functional domains and is present in cell bodies, dendrites, and axons of larval da neurons.208 Interestingly, overexpressed fluorescently labeled Lrrk prominently colocalized with the Golgi marker ManII in dendrites when compared to endosomes, mitochondria, and lysosomes.208 Importantly, Lrrk2 maintains stationary GOPs and suppresses their anterograde movement. Lack of Lrrk activity resulted in dendritic overbranching indicating that GOP motility and dendrite outgrowth are correlated as previously suggested.98 In addition, the biochemical analysis showed that Lrrk2 kinase activity is critical for Lva phosphorylation to prevent Lva–Dynein interaction, thus keeping GOPs static. Overexpression of human kinase-dead LRRK2 (LRRK2G2019S) in turn seems to promote kinesin-based transport facilitating GOP movement toward cell bodies.208 Previous studies also showed that human LRRK2 interacts with ArfGAP1 at Golgi membranes and overexpression of LRRK2G2019S results in somatic Golgi fragmentation.215 These studies highlight the role of LRRK2 in regulating Golgi organization, which might directly or indirectly affect dendrite development.
Polyglutamine (polyQ) disease proteins
Polyglutamine (polyQ) diseases including spinocerebellar ataxia/Machado-Joseph disease (SCA/MJD) and Huntington disease (HD) are inherited neurodegenerative diseases caused by expansion of trinucleotide CAG repeats within the protein-coding region of the disease gene.216 Expanded/pathogenic polyQ proteins form aggregates together with many other molecules in afflicted neurons, which disrupts multiple cellular processes. Ectopic expression of pathogenic MJDtr-78Q resulted in defective terminal dendrite elongation in C4da neurons.209,217 Chung et al found that overexpression of MJDtr-78Q caused a loss of GOPs in main dendritic branches.209 mRNA sequencing of fly heads with or without MJDtr-78Q expression identified many deregulated secretory pathway-related genes, including altered expression of COPII genes regulating GOP synthesis. In addition, the transcription factor CREB3L1/CrebA, a known regulator of COPII gene expression, was found to be downregulated. Conversely, CrebA overexpression could restore the dysregulation of COPII genes and the abundance of GOPs despite polyQ expression. Furthermore, the authors performed chromatin immunoprecipitation (ChIP)-PCR and found that CrebA expression is regulated by CREB-binding protein (CBP), which is sequestered by polyQ proteins. Thus, these findings suggest that impairment of GOPs by polyQ proteins contributes to dendrite pathology through the CBP-CrebA-COPII pathway.209
C9orf72
The pathological hallmark of Amyotrophic lateral sclerosis (ALS) is motor neuron axon degeneration.218 However, several studies also identified abnormal dendritic arborization.219, 220, 221 Similarly, frontotemporal dementia (FTD), which is considered to be in the same disease spectrum as ALS due to a shared set of genetic factors and symptoms, also manifests dendritic abnormalities; pyramidal and non-pyramidal neurons from FTD postmortem brain samples display reduced complexity and number of dendritic branches.222 Although altered dendritic morphology is frequently observed in ALS and FTD, the cellular and molecular basis underlying these potentially pathogenic abnormalities remains largely unclear. The most common genetic cause of both ALS and FTD is the intronic hexanucleotide (G4C2) repeat expansion mutation in C9orf72.223,224 A fraction of the pathogenic C9orf72 RNA exits the nucleus and undergoes repeat-associated non-ATG (RAN) translation into five dipeptide repeat proteins (DPRs) including glycine–arginine (GR), proline–arginine (PR), glycine–alanine (GA), glycine–proline (GP) and proline–alanine (PA).225 Overexpression of four of the DPRs (except GP which is known to be benign in various models) in Drosophila C4da neurons resulted in a significant reduction of dendritic branching at both larval and adult stages.210 Among these, PR and GR showed the most robust reduction of dendrites. Furthermore, the expression of PR and GR reduced the number of GOPs in dendrites. Interestingly, the expression of PR, but not GR, led to a significant reduction in the mRNA level of CrebA, which transcriptionally regulates the formation of GOPs.209 Overexpressing CrebA in PR-expressing C4da neurons restored the number of GOPs, but the number of dendritic branches remained unchanged,210 suggesting that other molecules besides CrebA may be involved in PR-mediated GOP disorganization and dendritic branching defects.
Amyloid precursor protein (APP)
Fragmentation and atrophy of the Golgi apparatus have been observed in AD patients.226,227 In addition, abnormalities of protein glycosylation and neural sialyltransferase activity have also been reported in AD brain tissues, such as an enhancement of glycosylation of β-amyloid (Aβ) peptides,228,229 suggesting that defects in the Golgi apparatus are pathological features of AD. Overexpression of human APP in Drosophila sensory neurons caused developmental dendrite defects.211 However, whether and how amyloid precursor protein (APP) regulates GOPs to affect dendrite development remains unclear. Recently, Du et al found that APP resides in and affects the movements of GOPs. In particular, it reverses the distribution of multi-compartment GOPs without affecting single-compartment GOPs in sensory C3da neurons.211 Although it was shown that the bidirectional movement of multi-compartment GOPs was cooperatively controlled by dynein and kinesin, only dynein seems to mediate APP-dependent regulation of multi-compartment GOP movement. Furthermore, by loss-of-function screening and genetic interaction analysis, the adaptor protein sunday driver (Syd) was identified to mediate APP-induced movement of multi-compartment GOPs and dendritic defects.211 Thus, APP might regulate dendritic development by directing the movement and function of multi-compartment GOPs.
Prenatal alcohol exposure (PAE)
Prenatal alcohol exposure (PAE) disrupts brain development and function, and the severity depends on sex, age, and consumption.230, 231, 232 It was found that PAE causes dendritic developmental defects in the hippocampus and cortex.233,234 In a whole hemisphere explant model, acute responses to ethanol exposure included compaction of the Golgi apparatus accompanied by elaboration of supernumerary primary apical neurites, as well as a modest increase in higher order apical neurite length.234 Longer ethanol exposure leads to misorientation of the soma, the primary apical neurite, and Golgi with respect to the pial surface.234 In addition, cellular orientation defects were accompanied by decreased abundance of the microtubule-associated protein MAP2 and F-actin, critical cytoskeletal components required for dendrite development. Thus, these findings indicate that upon exposure to ethanol, developing cortical neurons manifest abnormal Golgi size and location as well as cytoskeletal regulation, which may in turn result in significant perturbation of primary neurite formation and neuronal polarity.234
PAE-induced abnormal Golgi organization is likely due to the alteration of neuronal activity since it changes the composition of glutamate receptors.235, 236, 237 Previous studies showed that neuronal hyperexcitation resulted in reversible fragmentation of the Golgi complex and biogenesis of GOPs and Golgi satellites in cultured neurons.109,238 Prolonged blockade of GABA-A-mediated inhibition or withdrawal of NMDA receptor antagonists caused Golgi fragmentation, likely by CaMKII/CaMKIV activation,238 which eventually might affect dendritic development.
Conclusions and perspectives
Unlike most other somatic cells, neurons have particularly unique demands to develop and maintain their polarized axonal and dendritic compartments. To establish and maintain complex and dynamic dendritic arbors, neurons must continually and actively transport newly synthesized proteins and lipids to their distant destinations. These finely tuned processes rely on proper Golgi organization due to its central role in the secretory pathway and emerging role as an MTOC in neurons.5,6 Although the importance of the Golgi apparatus in dendritic morphogenesis has been proposed for decades,90,94,98,99,128 the specific mechanisms of somatic Golgi and GOP organization in neurons and their roles in dendrite development are just starting to be unraveled.
Based on previous studies, the principal organization mechanisms of both somatic Golgi and GOPs involve proper MT dynamics and COPII/I transport balance. It should be noted that MT dynamics and COPII/I transport can affect each other,8 making it difficult to distinguish cause and effect in Golgi organization. GM130 was reported as a key connector for assembling Golgi stacks, yet the mechanism is still unknown. While specific functions and defects have been attributed to the somatic Golgi or GOPs, they are interconnected but have barely been investigated in parallel. Thus, challenging questions remain in this emerging field that require further studies. Firstly, how are somatic Golgi and GOP functions dynamically regulated to satisfy dendrite development? Virtually all studies so far were focused on a specific time point, but lack systematic exploration during all stages of dendrite development. In addition, dendrite pruning is also a critical developmental process but the Golgi function is even less well investigated.239 Secondly, mechanisms of neuronal Golgi organization remain to be intensively investigated. Besides Golgi-resident proteins, other associated molecules involved in signal transduction and MTOC organization are not fully understood. Thirdly, so far, we have mostly correlative data linking GOP presence and motility to dendritic morphogenesis, which demands further studies into a causal functional link. In this respect, the proposed role of GOPs as local MTOCs might be the key to gaining further understanding. Lastly, the widespread distribution of Golgi satellites compared to GOPs in mammalian neurons begs the question of whether they differ only in size or also in function.107 It further remains to be elucidated if and how Golgi satellites and GOPs might support local protein synthesis and post-translational modification of secretory pathway proteins. This will require functional analyses of Golgi/GOPs/GS in future studies to unravel their impact on dendritic spine development and synaptic plasticity.
Many candidate proteins affecting Golgi organization have been identified by large-scale screening in both human72 and Drosophila cells.73 However, these screens were performed in proliferating cell lines, which likely have different functional requirements than postmitotic neurons. Thus, systematic genetic analyses of neuronal Golgi organization and its impact on dendritic morphogenesis are needed. Deciphering novel molecular regulators and their role in Golgi organization may provide us a more complete view of its link with dendritic morphogenesis and lead to possible treatments for neurological disorders and Golgipathies.11,240,241
Author contributions
CH and PS conceived the idea. MLC, LX, YW and CH wrote the draft. MLC and LX prepared the figures. CH and PS revised and finalized the manuscript.
Conflict of interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interests.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 32000690 to CH), the Key-Area Research and Development Program of Guangdong Province, China (No. 2019B030335001 to CH), the National Social Science Foundation of China (No. 20&ZD296 to CH), the Deutsche Forschungsgemeinschaft (DFG; No. SO1337/4-1, SO1337/2-2, and SO1337/7-1 to PS), and the DFG Heisenberg program (No. SO1337/6-1 to PS) and ERA-NET NEURON (BMBF; No. 01EW1910 and 01EW2108 to PS).
Acknowledgements
We would like to apologize to our colleagues whose work could not be cited here due to space restrictions.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Peter Soba, Email: peter.soba@fau.de.
Chun Hu, Email: chun.hu@scnu.edu.cn.
References
- 1.Koleske A.J. Molecular mechanisms of dendrite stability. Nat Rev Neurosci. 2013;14(8):536–550. doi: 10.1038/nrn3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy M.J., Hanus C. Architecture and dynamics of the neuronal secretory network. Annu Rev Cell Dev Biol. 2019;35:543–566. doi: 10.1146/annurev-cellbio-100818-125418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang S., Wang Y. Golgi structure formation, function, and post-translational modifications in mammalian cells. F1000Res. 2017;6:2050. doi: 10.12688/f1000research.11900.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhong W. Golgi during development. Cold Spring Harbor Perspect Biol. 2011;3(9):a005363. doi: 10.1101/cshperspect.a005363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park K., Ju S., Kim N., et al. The Golgi complex: a hub of the secretory pathway. BMB Rep. 2021;54(5):246–252. doi: 10.5483/BMBRep.2021.54.5.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders A.A.W.M., Kaverina I. Nucleation and dynamics of Golgi-derived microtubules. Front Neurosci. 2015;9:431. doi: 10.3389/fnins.2015.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravichandran Y., Goud B., Manneville J.B. The Golgi apparatus and cell polarity: roles of the cytoskeleton, the Golgi matrix, and Golgi membranes. Curr Opin Cell Biol. 2020;62:104–113. doi: 10.1016/j.ceb.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Fourriere L., Jimenez A.J., Perez F., et al. The role of microtubules in secretory protein transport. J Cell Sci. 2020;133(2):jcs237016. doi: 10.1242/jcs.237016. [DOI] [PubMed] [Google Scholar]
- 9.Zappa F., Failli M., De Matteis M.A. The Golgi complex in disease and therapy. Curr Opin Cell Biol. 2018;50:102–116. doi: 10.1016/j.ceb.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Passemard S., Perez F., Colin-Lemesre E., et al. Golgi trafficking defects in postnatal microcephaly: the evidence for “Golgipathies”. Prog Neurobiol. 2017;153:46–63. doi: 10.1016/j.pneurobio.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Rasika S., Passemard S., Verloes A., et al. Golgipathies in neurodevelopment: a new view of old defects. Dev Neurosci. 2018;40(5–6):396–416. doi: 10.1159/000497035. [DOI] [PubMed] [Google Scholar]
- 12.Martínez-Menárguez J.Á., Tomás M., Martínez-Martínez N., et al. Golgi fragmentation in neurodegenerative diseases: is there a common cause? Cells. 2019;8(7):748. doi: 10.3390/cells8070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bexiga M.G., Simpson J.C. Human diseases associated with form and function of the Golgi complex. Int J Mol Sci. 2013;14(9):18670–18681. doi: 10.3390/ijms140918670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu M.M., McAlear T.S., Nguyen H., et al. The Golgi outpost protein TPPP nucleates microtubules and is critical for myelination. Cell. 2019;179(1):132–146. doi: 10.1016/j.cell.2019.08.025. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z., Yan M., Lei W., et al. Sec13 promotes oligodendrocyte differentiation and myelin repair through autocrine pleiotrophin signaling. J Clin Invest. 2022;132(7) doi: 10.1172/JCI155096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burke B., Griffiths G., Reggio H., et al. A monoclonal antibody against a 135-K Golgi membrane protein. EMBO J. 1982;1(12):1621–1628. doi: 10.1002/j.1460-2075.1982.tb01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothman J.E. The Golgi apparatus: two organelles in tandem. Science. 1981;213(4513):1212–1219. doi: 10.1126/science.7268428. [DOI] [PubMed] [Google Scholar]
- 18.Ladinsky M.S., Mastronarde D.N., McIntosh J.R., et al. Golgi structure in three dimensions: functional insights from the normal rat kidney cell. J Cell Biol. 1999;144(6):1135–1149. doi: 10.1083/jcb.144.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ladinsky M.S., Kremer J.R., Furcinitti P.S., et al. HVEM tomography of the trans-Golgi network: structural insights and identification of a lace-like vesicle coat. J Cell Biol. 1994;127(1):29–38. doi: 10.1083/jcb.127.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou W., Chang J., Wang X., et al. GM130 is required for compartmental organization of dendritic Golgi outposts. Curr Biol. 2014;24(11):1227–1233. doi: 10.1016/j.cub.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barr F.A., Nakamura N., Warren G. Mapping the interaction between GRASP65 and GM130, components of a protein complex involved in the stacking of Golgi cisternae. EMBO J. 1998;17(12):3258–3268. doi: 10.1093/emboj/17.12.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldfischer S. The internal reticular apparatus of Camillo Golgi: a complex, heterogeneous organelle, enriched in acid, neutral, and alkaline phosphatases, and involved in glycosylation, secretion, membrane flow, lysosome formation, and intracellular digestion. J Histochem Cytochem. 1982;30(7):717–733. doi: 10.1177/30.7.6286754. [DOI] [PubMed] [Google Scholar]
- 23.Broadwell R.D., Oliver C. Golgi apparatus, GERL, and secretory granule formation within neurons of the hypothalamo-neurohypophysial system of control and hyperosmotically stressed mice. J Cell Biol. 1981;90(2):474–484. doi: 10.1083/jcb.90.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Climer L.K., Hendrix R.D., Lupashin V.V. Conserved oligomeric Golgi and neuronal vesicular trafficking. Handb Exp Pharmacol. 2018;245:227–247. doi: 10.1007/164_2017_65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mollenhauer H.H., James Morré D. The tubular network of the Golgi apparatus. Histochem Cell Biol. 1998;109(5–6):533–543. doi: 10.1007/s004180050253. [DOI] [PubMed] [Google Scholar]
- 26.Mironov A.A., Sesorova I.S., Seliverstova E.V., et al. Different Golgi ultrastructure across species and tissues: implications under functional and pathological conditions, and an attempt at classification. Tissue Cell. 2017;49(2 Pt A):186–201. doi: 10.1016/j.tice.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Kondylis V., Rabouille C. The Golgi apparatus: lessons from Drosophila. FEBS Lett. 2009;583(23):3827–3838. doi: 10.1016/j.febslet.2009.09.048. [DOI] [PubMed] [Google Scholar]
- 28.Lavieu G., Dunlop M.H., Lerich A., et al. The Golgi ribbon structure facilitates anterograde transport of large cargoes. Mol Biol Cell. 2014;25(19):3028–3036. doi: 10.1091/mbc.E14-04-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wendler F., Gillingham A.K., Sinka R., et al. A genome-wide RNA interference screen identifies two novel components of the metazoan secretory pathway. EMBO J. 2010;29(2):304–314. doi: 10.1038/emboj.2009.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Springer S., Schekman R. Nucleation of COPII vesicular coat complex by endoplasmic reticulum to Golgi vesicle SNAREs. Science. 1998;281(5377):698–700. doi: 10.1126/science.281.5377.698. [DOI] [PubMed] [Google Scholar]
- 31.Scales S.J., Pepperkok R., Kreis T.E. Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell. 1997;90(6):1137–1148. doi: 10.1016/s0092-8674(00)80379-7. [DOI] [PubMed] [Google Scholar]
- 32.Presley J.F., Cole N.B., Schroer T.A., et al. ER-to-Golgi transport visualized in living cells. Nature. 1997;389(6646):81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- 33.Barlowe C., Orci L., Yeung T., et al. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77(6):895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 34.Soulier S., Gaye P. Enzymatic O-glycosylation of ℵ-caseinomacropeptide by ovine mammary Golgi membranes. Biochimie. 1981;63(7):619–628. doi: 10.1016/s0300-9084(81)80060-0. [DOI] [PubMed] [Google Scholar]
- 35.Hellicar J., Stevenson N.L., Stephens D.J., et al. Supply chain logistics - the role of the Golgi complex in extracellular matrix production and maintenance. J Cell Sci. 2022;135(1):jcs258879. doi: 10.1242/jcs.258879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young R.W. The role of the Golgi complex in sulfate metabolism. J Cell Biol. 1973;57(1):175–189. doi: 10.1083/jcb.57.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lane N., Caro L., Otero-Vilardebó L.R., et al. On the site of sulfation in colonic goblet cells. J Cell Biol. 1964;21(3):339–351. doi: 10.1083/jcb.21.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ernst A.M., Syed S.A., Zaki O., et al. S-palmitoylation sorts membrane cargo for anterograde transport in the Golgi. Dev Cell. 2018;47(4):479–493. doi: 10.1016/j.devcel.2018.10.024. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steiner D.F., Docherty K., Carroll R. Golgi/granule processing of peptide hormone and neuropeptide precursors: a minireview. J Cell Biochem. 1984;24(2):121–130. doi: 10.1002/jcb.240240204. [DOI] [PubMed] [Google Scholar]
- 40.Lewis M.J., Pelham H.R.B. SNARE-mediated retrograde traffic from the Golgi complex to the endoplasmic reticulum. Cell. 1996;85(2):205–215. doi: 10.1016/s0092-8674(00)81097-1. [DOI] [PubMed] [Google Scholar]
- 41.Letourneur F., Gaynor E.C., Hennecke S., et al. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79(7):1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 42.Orci L., Stamnes M., Ravazzola M., et al. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell. 1997;90(2):335–349. doi: 10.1016/s0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- 43.Orci L., Ravazzola M., Storch M.J., et al. Proteolytic maturation of insulin is a post-Golgi event which occurs in acidifying clathrin-coated secretory vesicles. Cell. 1987;49(6):865–868. doi: 10.1016/0092-8674(87)90624-6. [DOI] [PubMed] [Google Scholar]
- 44.Payne G.S., Schekman R. A test of clathrin function in protein secretion and cell growth. Science. 1985;230(4729):1009–1014. doi: 10.1126/science.2865811. [DOI] [PubMed] [Google Scholar]
- 45.Tu Y., Zhao L., Billadeau D.D., et al. Endosome-to-TGN trafficking: organelle-vesicle and organelle-organelle interactions. Front Cell Dev Biol. 2020;8:163. doi: 10.3389/fcell.2020.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Tito S., Hervás J.H., van Vliet A.R., et al. The Golgi as an assembly line to the autophagosome. Trends Biochem Sci. 2020;45(6):484–496. doi: 10.1016/j.tibs.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 47.Mattera R., Park S.Y., De Pace R., et al. AP-4 mediates export of ATG9A from the trans-Golgi network to promote autophagosome formation. Proc Natl Acad Sci U S A. 2017;114(50):E10697–E10706. doi: 10.1073/pnas.1717327114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young A.R.J., Chan E.Y.W., Hu X.W., et al. Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. J Cell Sci. 2006;119(18):3888–3900. doi: 10.1242/jcs.03172. [DOI] [PubMed] [Google Scholar]
- 49.Seaman M.N., Marcusson E.G., Cereghino J.L., et al. Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J Cell Biol. 1997;137(1):79–92. doi: 10.1083/jcb.137.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghosh R.N., Mallet W.G., Soe T.T., et al. An endocytosed TGN38 chimeric protein is delivered to the TGN after trafficking through the endocytic recycling compartment in CHO cells. J Cell Biol. 1998;142(4):923–936. doi: 10.1083/jcb.142.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao J., Gao A., Zhou H., et al. The role of metal ions in the Golgi apparatus. Cell Biol Int. 2022;46(9):1309–1319. doi: 10.1002/cbin.11848. [DOI] [PubMed] [Google Scholar]
- 52.Li J., Wang Y. Golgi metal ion homeostasis in human health and diseases. Cells. 2022;11(2):289. doi: 10.3390/cells11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hartwig C., Méndez G.M., Bhattacharjee S., et al. Golgi-dependent copper homeostasis sustains synaptic development and mitochondrial content. J Neurosci. 2021;41(2):215–233. doi: 10.1523/JNEUROSCI.1284-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lissandron V., Podini P., Pizzo P., et al. Unique characteristics of Ca2+ homeostasis of the trans-Golgi compartment. Proc Natl Acad Sci U S A. 2010;107(20):9198–9203. doi: 10.1073/pnas.1004702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steimle B.L., Bailey D.K., Smith F.M., et al. Calcium and the Ca-ATPase SPCA1 modulate plasma membrane abundance of ZIP8 and ZIP14 to regulate Mn(II) uptake in brain microvascular endothelial cells. J Biol Chem. 2022;298(8) doi: 10.1016/j.jbc.2022.102211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.George C.H., Higgs G.V., Lai F.A. Ryanodine receptor mutations associated with stress-induced ventricular tachycardia mediate increased calcium release in stimulated cardiomyocytes. Circ Res. 2003;93(6):531–540. doi: 10.1161/01.RES.0000091335.07574.86. [DOI] [PubMed] [Google Scholar]
- 57.Potelle S., Morelle W., Dulary E., et al. Glycosylation abnormalities in Gdt1p/TMEM165 deficient cells result from a defect in Golgi manganese homeostasis. Hum Mol Genet. 2016;25(8):1489–1500. doi: 10.1093/hmg/ddw026. [DOI] [PubMed] [Google Scholar]
- 58.Carmona A., Zogzas C.E., Roudeau S., et al. SLC30A10 mutation involved in Parkinsonism results in Manganese accumulation within nanovesicles of the Golgi apparatus. ACS Chem Neurosci. 2019;10(1):599–609. doi: 10.1021/acschemneuro.8b00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang L., Kirschke C.P., Zhang Y., et al. The ZIP7 gene (Slc39a7) encodes a zinc transporter involved in zinc homeostasis of the Golgi apparatus. J Biol Chem. 2005;280(15):15456–15463. doi: 10.1074/jbc.M412188200. [DOI] [PubMed] [Google Scholar]
- 60.Grubman A., Lidgerwood G.E., Duncan C., et al. Deregulation of subcellular biometal homeostasis through loss of the metal transporter, Zip7, in a childhood neurodegenerative disorder. Acta Neuropathol Commun. 2014;2:25. doi: 10.1186/2051-5960-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bin B.H., Fukada T., Hosaka T., et al. Biochemical characterization of human ZIP13 protein: a homo-dimerized zinc transporter involved in the spondylocheiro dysplastic Ehlers-Danlos syndrome. J Biol Chem. 2011;286(46):40255–40265. doi: 10.1074/jbc.M111.256784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kennerson M.L., Nicholson G.A., Kaler S.G., et al. Missense mutations in the copper transporter gene ATP7A cause X-linked distal hereditary motor neuropathy. Am J Hum Genet. 2010;86(3):343–352. doi: 10.1016/j.ajhg.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bakkar N., Starr A., Rabichow B.E., et al. The M1311V variant of ATP7A is associated with impaired trafficking and copper homeostasis in models of motor neuron disease. Neurobiol Dis. 2021;149 doi: 10.1016/j.nbd.2020.105228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watanabe S., Amagai Y., Sannino S., et al. Zinc regulates ERp44-dependent protein quality control in the early secretory pathway. Nat Commun. 2019;10:603. doi: 10.1038/s41467-019-08429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee M.G., Bin B.H. Different actions of intracellular zinc transporters ZIP7 and ZIP13 are essential for dermal development. Int J Mol Sci. 2019;20(16):3941. doi: 10.3390/ijms20163941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van de Leemput J., Chandran J., Knight M.A., et al. Deletion at ITPR1 underlies ataxia in mice and spinocerebellar ataxia 15 in humans. PLoS Genet. 2007;3(6):e108. doi: 10.1371/journal.pgen.0030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Y.J., Wang J., Sun H.Q., et al. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell. 2003;114(3):299–310. doi: 10.1016/s0092-8674(03)00603-2. [DOI] [PubMed] [Google Scholar]
- 68.Itoh T., Fujita N., Kanno E., et al. Golgi-resident small GTPase Rab33B interacts with Atg16L and modulates autophagosome formation. Mol Biol Cell. 2008;19(7):2916–2925. doi: 10.1091/mbc.E07-12-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guinea B., Ligos J.M., Laín de Lera T., et al. Nucleocytoplasmic shuttling of STK16 (PKL12), a Golgi-resident serine/threonine kinase involved in VEGF expression regulation. Exp Cell Res. 2006;312(2):135–144. doi: 10.1016/j.yexcr.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 70.Fernández-Hernando C., Fukata M., Bernatchez P.N., et al. Identification of Golgi-localized acyl transferases that palmitoylate and regulate endothelial nitric oxide synthase. J Cell Biol. 2006;174(3):369–377. doi: 10.1083/jcb.200601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Donaldson J.G., Kahn R.A., Lippincott-Schwartz J., et al. Binding of ARF and beta-COP to Golgi membranes: possible regulation by a trimeric G protein. Science. 1991;254(5035):1197–1199. doi: 10.1126/science.1957170. [DOI] [PubMed] [Google Scholar]
- 72.Chia J., Goh G., Racine V., et al. RNAi screening reveals a large signaling network controlling the Golgi apparatus in human cells. Mol Syst Biol. 2012;8:629. doi: 10.1038/msb.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bard F., Casano L., Mallabiabarrena A., et al. Functional genomics reveals genes involved in protein secretion and Golgi organization. Nature. 2006;439(7076):604–607. doi: 10.1038/nature04377. [DOI] [PubMed] [Google Scholar]
- 74.Rahman A., Lőrincz P., Gohel R., et al. GMAP is an Atg8a-interacting protein that regulates Golgi turnover in Drosophila. Cell Rep. 2022;39(9) doi: 10.1016/j.celrep.2022.110903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yadav S., Puthenveedu M.A., Linstedt A.D. Golgin160 recruits the dynein motor to position the Golgi apparatus. Dev Cell. 2012;23(1):153–165. doi: 10.1016/j.devcel.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chabin-Brion K., Marceiller J., Perez F., et al. The Golgi complex is a microtubule-organizing organelle. Mol Biol Cell. 2001;12(7):2047–2060. doi: 10.1091/mbc.12.7.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Infante C., Ramos-Morales F., Fedriani C., et al. GMAP-210, a cis-Golgi network-associated protein, is a minus end microtubule-binding protein. J Cell Biol. 1999;145(1):83–98. doi: 10.1083/jcb.145.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ori-McKenney K.M., Jan L.Y., Jan Y.N. Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron. 2012;76(5):921–930. doi: 10.1016/j.neuron.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miller P.M., Folkmann A.W., Maia A.R.R., et al. Golgi-derived CLASP-dependent microtubules control Golgi organization and polarized trafficking in motile cells. Nat Cell Biol. 2009;11(9):1069–1080. doi: 10.1038/ncb1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rivero S., Cardenas J., Bornens M., et al. Microtubule nucleation at the cis-side of the Golgi apparatus requires AKAP450 and GM130. EMBO J. 2009;28(8):1016–1028. doi: 10.1038/emboj.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choi Y.K., Liu P., Sze S.K., et al. CDK5RAP2 stimulates microtubule nucleation by the γ-tubulin ring complex. J Cell Biol. 2010;191(6):1089–1095. doi: 10.1083/jcb.201007030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Z., Wu T., Shi L., et al. Conserved motif of CDK5RAP2 mediates its localization to centrosomes and the Golgi complex. J Biol Chem. 2010;285(29):22658–22665. doi: 10.1074/jbc.M110.105965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yalgin C., Ebrahimi S., Delandre C., et al. Centrosomin represses dendrite branching by orienting microtubule nucleation. Nat Neurosci. 2015;18(10):1437–1445. doi: 10.1038/nn.4099. [DOI] [PubMed] [Google Scholar]
- 84.Roubin R., Acquaviva C., Chevrier V., et al. Myomegalin is necessary for the formation of centrosomal and Golgi-derived microtubules. Biol Open. 2013;2(2):238–250. doi: 10.1242/bio.20123392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Efimov A., Kharitonov A., Efimova N., et al. Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev Cell. 2007;12(6):917–930. doi: 10.1016/j.devcel.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu J., de Heus C., Liu Q., et al. Molecular pathway of microtubule organization at the Golgi apparatus. Dev Cell. 2016;39(1):44–60. doi: 10.1016/j.devcel.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 87.Mukherjee A., Brooks P.S., Bernard F., et al. Microtubules originate asymmetrically at the somatic Golgi and are guided via Kinesin2 to maintain polarity within neurons. Elife. 2020;9 doi: 10.7554/eLife.58943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nguyen M.M., Stone M.C., Rolls M.M. Microtubules are organized independently of the centrosome in Drosophila neurons. Neural Dev. 2011;6:38. doi: 10.1186/1749-8104-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li P., Li L., Yu B., et al. Doublecortin facilitates the elongation of the somatic Golgi apparatus into proximal dendrites. Mol Biol Cell. 2021;32(5):422–434. doi: 10.1091/mbc.E19-09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Horton A.C., Rácz B., Monson E.E., et al. Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron. 2005;48(5):757–771. doi: 10.1016/j.neuron.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 91.Castellano B., Gonzalez B., Palacios G. Cytochemical demonstration of TPPase in myelinated fibers in the central and peripheral nervous system of the rat. Brain Res. 1989;492(1–2):203–210. doi: 10.1016/0006-8993(89)90902-5. [DOI] [PubMed] [Google Scholar]
- 92.Griffith D.L., Bondareff W. Localization of thiamine pyrophosphatase in synaptic vesicles. Am J Anat. 1973;136(4):549–556. doi: 10.1002/aja.1001360412. [DOI] [PubMed] [Google Scholar]
- 93.Merianda T.T., Lin A.C., Lam J.S.Y., et al. A functional equivalent of endoplasmic reticulum and Golgi in axons for secretion of locally synthesized proteins. Mol Cell Neurosci. 2009;40(2):128–142. doi: 10.1016/j.mcn.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Horton A.C., Ehlers M.D. Dual modes of endoplasmic reticulum-to-Golgi transport in dendrites revealed by live-cell imaging. J Neurosci. 2003;23(15):6188–6199. doi: 10.1523/JNEUROSCI.23-15-06188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tennyson V.M. The fine structure of the axon and growth cone of the dorsal root neuroblast of the rabbit embryo. J Cell Biol. 1970;44(1):62–79. doi: 10.1083/jcb.44.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bunge M.B. Fine structure of nerve fibers and growth cones of isolated sympathetic neurons in culture. J Cell Biol. 1973;56(3):713–735. doi: 10.1083/jcb.56.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nguyen M.M., McCracken C.J., Milner E.S., et al. γ-Tubulin controls neuronal microtubule polarity independently of Golgi outposts. Mol Biol Cell. 2014;25(13):2039–2050. doi: 10.1091/mbc.E13-09-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ye B., Zhang Y., Song W., et al. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell. 2007;130(4):717–729. doi: 10.1016/j.cell.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Horton A.C., Ehlers M.D. Secretory trafficking in neuronal dendrites. Nat Cell Biol. 2004;6(7):585–591. doi: 10.1038/ncb0704-585. [DOI] [PubMed] [Google Scholar]
- 100.Torre E.R., Steward O. Protein synthesis within dendrites: glycosylation of newly synthesized proteins in dendrites of hippocampal neurons in culture. J Neurosci. 1996;16(19):5967–5978. doi: 10.1523/JNEUROSCI.16-19-05967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kacharmina J.E., Job C., Crino P., et al. Stimulation of glutamate receptor protein synthesis and membrane insertion within isolated neuronal dendrites. Proc Natl Acad Sci U S A. 2000;97(21):11545–11550. doi: 10.1073/pnas.97.21.11545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ju W., Morishita W., Tsui J., et al. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci. 2004;7(3):244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- 103.Bowen A.B., Bourke A.M., Hiester B.G., et al. Golgi-independent secretory trafficking through recycling endosomes in neuronal dendrites and spines. Elife. 2017;6 doi: 10.7554/eLife.27362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hanus C., Kochen L., Tom Dieck S., et al. Synaptic control of secretory trafficking in dendrites. Cell Rep. 2014;7(6):1771–1778. doi: 10.1016/j.celrep.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mikhaylova M., Reddy P.P., Munsch T., et al. Calneurons provide a calcium threshold for trans-Golgi network to plasma membrane trafficking. Proc Natl Acad Sci U S A. 2009;106(22):9093–9098. doi: 10.1073/pnas.0903001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bera S., Raghuram V., Mikhaylova M., et al. A plasmid-based expression system to study protein-protein interactions at the Golgi in vivo. Anal Biochem. 2016;502:50–52. doi: 10.1016/j.ab.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 107.Mikhaylova M., Bera S., Kobler O., et al. A dendritic Golgi satellite between ERGIC and retromer. Cell Rep. 2016;14(2):189–199. doi: 10.1016/j.celrep.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 108.Quassollo G., Wojnacki J., Salas D.A., et al. A RhoA signaling pathway regulates dendritic Golgi outpost formation. Curr Biol. 2015;25(8):971–982. doi: 10.1016/j.cub.2015.01.075. [DOI] [PubMed] [Google Scholar]
- 109.Govind A.P., Jeyifous O., Russell T.A., et al. Activity-dependent Golgi satellite formation in dendrites reshapes the neuronal surface glycoproteome. Elife. 2021;10 doi: 10.7554/eLife.68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grochowska K.M., Andres-Alonso M., Karpova A., et al. The needs of a synapse-How local organelles serve synaptic proteostasis. EMBO J. 2022;41(7) doi: 10.15252/embj.2021110057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Puthenveedu M.A., Bachert C., Puri S., et al. GM130 and GRASP65-dependent lateral cisternal fusion allows uniform Golgi-enzyme distribution. Nat Cell Biol. 2006;8(3):238–248. doi: 10.1038/ncb1366. [DOI] [PubMed] [Google Scholar]
- 112.Nakamura N., Rabouille C., Watson R., et al. Characterization of a cis-Golgi matrix protein, GM130. J Cell Biol. 1995;131(6 Pt 2):1715–1726. doi: 10.1083/jcb.131.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ríos R.M., Sanchís A., Tassin A.M., et al. GMAP-210 recruits gamma-tubulin complexes to cis-Golgi membranes and is required for Golgi ribbon formation. Cell. 2004;118(3):323–335. doi: 10.1016/j.cell.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 114.Liu J.S., Schubert C.R., Fu X., et al. Molecular basis for specific regulation of neuronal kinesin-3 motors by doublecortin family proteins. Mol Cell. 2012;47(5):707–721. doi: 10.1016/j.molcel.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.des Portes V., Pinard J.M., Billuart P., et al. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell. 1998;92(1):51–61. doi: 10.1016/s0092-8674(00)80898-3. [DOI] [PubMed] [Google Scholar]
- 116.Gleeson J.G., Allen K.M., Fox J.W., et al. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92(1):63–72. doi: 10.1016/s0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- 117.Tsvetkov A.S., Samsonov A., Akhmanova A., et al. Microtubule-binding proteins CLASP1 and CLASP2 interact with actin filaments. Cell Motil Cytoskeleton. 2007;64(7):519–530. doi: 10.1002/cm.20201. [DOI] [PubMed] [Google Scholar]
- 118.Akhmanova A., Hoogenraad C.C., Drabek K., et al. CLASPs are CLIP-115 and-170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell. 2001;104(6):923–935. doi: 10.1016/s0092-8674(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 119.Beffert U., Dillon G.M., Sullivan J.M., et al. Microtubule plus-end tracking protein CLASP2 regulates neuronal polarity and synaptic function. J Neurosci. 2012;32(40):13906–13916. doi: 10.1523/JNEUROSCI.2108-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.di Ronza A., Bajaj L., Sharma J., et al. CLN8 is an endoplasmic reticulum cargo receptor that regulates lysosome biogenesis. Nat Cell Biol. 2018;20(12):1370–1377. doi: 10.1038/s41556-018-0228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lonka L., Kyttälä A., Ranta S., et al. The neuronal ceroid lipofuscinosis CLN8 membrane protein is a resident of the endoplasmic reticulum. Hum Mol Genet. 2000;9(11):1691–1697. doi: 10.1093/hmg/9.11.1691. [DOI] [PubMed] [Google Scholar]
- 122.Jensen D., Schekman R. COPII-mediated vesicle formation at a glance. J Cell Sci. 2011;124(1):1–4. doi: 10.1242/jcs.069773. [DOI] [PubMed] [Google Scholar]
- 123.Arakel E.C., Schwappach B. Formation of COPI-coated vesicles at a glance. J Cell Sci. 2018;131(5) doi: 10.1242/jcs.209890. [DOI] [PubMed] [Google Scholar]
- 124.Duden R. ER-to-Golgi transport: COP I and COP II function (review) Mol Membr Biol. 2003;20(3):197–207. doi: 10.1080/0968768031000122548. [DOI] [PubMed] [Google Scholar]
- 125.Pesaola F., Quassollo G., Venier A.C., et al. The neuronal ceroid lipofuscinosis-related protein CLN8 regulates endo-lysosomal dynamics and dendritic morphology. Biol Cell. 2021;113(10):419–437. doi: 10.1111/boc.202000016. [DOI] [PubMed] [Google Scholar]
- 126.Passantino R., Cascio C., Deidda I., et al. Identifying protein partners of CLN8, an ER-resident protein involved in neuronal ceroid lipofuscinosis. Biochim Biophys Acta. 2013;1833(3):529–540. doi: 10.1016/j.bbamcr.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 127.Haase G., Rabouille C. Golgi fragmentation in ALS motor neurons. new mechanisms targeting microtubules, tethers, and transport vesicles. Front Neurosci. 2015;9:448. doi: 10.3389/fnins.2015.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sengupta D., Linstedt A.D. Control of organelle size: the Golgi complex. Annu Rev Cell Dev Biol. 2011;27:57–77. doi: 10.1146/annurev-cellbio-100109-104003. [DOI] [PubMed] [Google Scholar]
- 129.Mukhopadhyay A., Funato K., Stahl P.D. Rab7 regulates transport from early to late endocytic compartments in Xenopus oocytes. J Biol Chem. 1997;272(20):13055–13059. doi: 10.1074/jbc.272.20.13055. [DOI] [PubMed] [Google Scholar]
- 130.Stagi M., Klein Z.A., Gould T.J., et al. Lysosome size, motility and stress response regulated by fronto-temporal dementia modifier TMEM106B. Mol Cell Neurosci. 2014;61:226–240. doi: 10.1016/j.mcn.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pelletier S. SCYL pseudokinases in neuronal function and survival. Neural Regen Res. 2016;11(1):42–44. doi: 10.4103/1673-5374.175040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Burman J.L., Bourbonniere L., Philie J., et al. Scyl1, mutated in a recessive form of spinocerebellar neurodegeneration, regulates COPI-mediated retrograde traffic. J Biol Chem. 2008;283(33):22774–22786. doi: 10.1074/jbc.M801869200. [DOI] [PubMed] [Google Scholar]
- 133.Burman J.L., Hamlin J.N.R., McPherson P.S. Scyl1 regulates Golgi morphology. PLoS One. 2010;5(3) doi: 10.1371/journal.pone.0009537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schmidt W.M., Kraus C., Höger H., et al. Mutation in the Scyl1 gene encoding amino-terminal kinase-like protein causes a recessive form of spinocerebellar neurodegeneration. EMBO Rep. 2007;8(7):691–697. doi: 10.1038/sj.embor.7401001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lenz D., McClean P., Kansu A., et al. SCYL1 variants cause a syndrome with lowγ-glutamyl-transferase cholestasis, acute liver failure, and neurodegeneration (CALFAN) Genet Med. 2018;20(10):1255–1265. doi: 10.1038/gim.2017.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hamlin J.N.R., Schroeder L.K., Fotouhi M., et al. Scyl1 scaffolds class II Arfs to specific subcomplexes of coatomer through the γ-COP appendage domain. J Cell Sci. 2014;127(Pt 7):1454–1463. doi: 10.1242/jcs.136481. [DOI] [PubMed] [Google Scholar]
- 137.Amano G., Matsuzaki S., Mori Y., et al. SCYL1 arginine methylation by PRMT1 is essential for neurite outgrowth via Golgi morphogenesis. Mol Biol Cell. 2020;31(18):1963–1973. doi: 10.1091/mbc.E20-02-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wolfes A.C., Dean C. The diversity of synaptotagmin isoforms. Curr Opin Neurobiol. 2020;63:198–209. doi: 10.1016/j.conb.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 139.Kwon O.J., Gainer H., Wray S., et al. Identification of a novel protein containing two C2 domains selectively expressed in the rat brain and kidney. FEBS Lett. 1996;378(2):135–139. doi: 10.1016/0014-5793(95)01430-6. [DOI] [PubMed] [Google Scholar]
- 140.Lee M.Y., Choi S.H., Shin S.L., et al. Distribution of B/K protein in rat brain. Cell Tissue Res. 2001;303(1):47–56. doi: 10.1007/s004410000221. [DOI] [PubMed] [Google Scholar]
- 141.Sharma K., Schmitt S., Bergner C.G., et al. Cell type–and brain region–resolved mouse brain proteome. Nat Neurosci. 2015;18(12):1819–1831. doi: 10.1038/nn.4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ruhl D.A., Bomba-Warczak E., Watson E.T., et al. Synaptotagmin 17 controls neurite outgrowth and synaptic physiology via distinct cellular pathways. Nat Commun. 2019;10(1):3532. doi: 10.1038/s41467-019-11459-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fukuda M., Mikoshiba K. The N-terminal cysteine cluster is essential for membrane targeting of B/K protein. Biochem J. 2001;360(Pt 2):441–448. doi: 10.1042/0264-6021:3600441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dean C., Dunning F.M., Liu H., et al. Axonal and dendritic synaptotagmin isoforms revealed by a pHluorin-syt functional screen. Mol Biol Cell. 2012;23(9):1715–1727. doi: 10.1091/mbc.E11-08-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jang Y.S., Lee M.Y., Choi S.H., et al. Expression of B/K protein in the hippocampus of kainate-induced rat seizure model. Brain Res. 2004;999(2):203–211. doi: 10.1016/j.brainres.2003.11.047. [DOI] [PubMed] [Google Scholar]
- 146.Muschalik N., Munro S. Golgins. Curr Biol. 2018;28(8):R374–R376. doi: 10.1016/j.cub.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 147.Nakamura N. Emerging new roles of GM130, a cis-Golgi matrix protein, in higher order cell functions. J Pharmacol Sci. 2010;112(3):255–264. doi: 10.1254/jphs.09r03cr. [DOI] [PubMed] [Google Scholar]
- 148.Lowe M., Rabouille C., Nakamura N., et al. Cdc2 kinase directly phosphorylates the cis-Golgi matrix protein GM130 and is required for Golgi fragmentation in mitosis. Cell. 1998;94(6):783–793. doi: 10.1016/s0092-8674(00)81737-7. [DOI] [PubMed] [Google Scholar]
- 149.Lowe M., Gonatas N.K., Warren G. The mitotic phosphorylation cycle of the cis-Golgi matrix protein GM130. J Cell Biol. 2000;149(2):341–356. doi: 10.1083/jcb.149.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sundaramoorthy S., Goh J.B., Rafee S., et al. Mitotic Golgi vesiculation involves mechanisms independent of Ser25 phosphorylation of GM130. Cell Cycle. 2010;9(15):3100–3105. doi: 10.4161/cc.9.15.12522. [DOI] [PubMed] [Google Scholar]
- 151.Liu C., Mei M., Li Q., et al. Loss of the golgin GM130 causes Golgi disruption, Purkinje neuron loss, and ataxia in mice. Proc Natl Acad Sci U S A. 2017;114(2):346–351. doi: 10.1073/pnas.1608576114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Huang W., She L., Chang X.Y., et al. Protein kinase LKB1 regulates polarized dendrite formation of adult hippocampal newborn neurons. Proc Natl Acad Sci U S A. 2014;111(1):469–474. doi: 10.1073/pnas.1321454111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Veenendaal T., Jarvela T., Grieve A.G., et al. GRASP65 controls the cis Golgi integrity in vivo. Biol Open. 2014;3(6):431–443. doi: 10.1242/bio.20147757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xiang Y., Wang Y. GRASP55 and GRASP65 play complementary and essential roles in Golgi cisternal stacking. J Cell Biol. 2010;188(2):237–251. doi: 10.1083/jcb.200907132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shorter J., Watson R., Giannakou M.E., et al. GRASP55, a second mammalian GRASP protein involved in the stacking of Golgi cisternae in a cell-free system. EMBO J. 1999;18(18):4949–4960. doi: 10.1093/emboj/18.18.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Scott E.K., Luo L. How do dendrites take their shape? Nat Neurosci. 2001;4(4):359–365. doi: 10.1038/86006. [DOI] [PubMed] [Google Scholar]
- 157.Wang X., Kim J.H., Bazzi M., et al. Bimodal control of dendritic and axonal growth by the dual leucine zipper kinase pathway. PLoS Biol. 2013;11(6) doi: 10.1371/journal.pbio.1001572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tanabe K., Kani S., Shimizu T., et al. Atypical protein kinase C regulates primary dendrite specification of cerebellar Purkinje cells by localizing Golgi apparatus. J Neurosci. 2010;30(50):16983–16992. doi: 10.1523/JNEUROSCI.3352-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hapak S.M., Rothlin C.V., Ghosh S. PAR3-PAR6-atypical PKC polarity complex proteins in neuronal polarization. Cell Mol Life Sci. 2018;75(15):2735–2761. doi: 10.1007/s00018-018-2828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rao S., Kirschen G.W., Szczurkowska J., et al. Repositioning of somatic Golgi apparatus is essential for the dendritic establishment of adult-born hippocampal neurons. J Neurosci. 2018;38(3):631–647. doi: 10.1523/JNEUROSCI.1217-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gao Y., Wang F., Eisinger B.E., et al. Integrative single-cell transcriptomics reveals molecular networks defining neuronal maturation during postnatal neurogenesis. Cerebr Cortex. 2017;27(3):2064–2077. doi: 10.1093/cercor/bhw040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Orlova K.A., Parker W.E., Heuer G.G., et al. STRADα deficiency results in aberrant mTORC1 signaling during corticogenesis in humans and mice. J Clin Invest. 2010;120(5):1591–1602. doi: 10.1172/JCI41592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Preisinger C., Short B., De Corte V., et al. YSK1 is activated by the Golgi matrix protein GM130 and plays a role in cell migration through its substrate 14-3-3zeta. J Cell Biol. 2004;164(7):1009–1020. doi: 10.1083/jcb.200310061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.D'Arcangelo G., Miao G.G., Chen S.C., et al. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374(6524):719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- 165.Hirotsune S., Takahara T., Sasaki N., et al. The reeler gene encodes a protein with an EGF-like motif expressed by pioneer neurons. Nat Genet. 1995;10(1):77–83. doi: 10.1038/ng0595-77. [DOI] [PubMed] [Google Scholar]
- 166.Ogawa M., Miyata T., Nakajimat K., et al. The reeler gene-associated antigen on Cajal-retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 1995;14(5):899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- 167.Ampuero E., Jury N., Härtel S., et al. Interfering of the Reelin/ApoER2/PSD95 signaling axis reactivates dendritogenesis of mature hippocampal neurons. J Cell Physiol. 2017;232(5):1187–1199. doi: 10.1002/jcp.25605. [DOI] [PubMed] [Google Scholar]
- 168.O'Dell R.S., Cameron D.A., Zipfel W.R., et al. Reelin prevents apical neurite retraction during terminal translocation and dendrite initiation. J Neurosci. 2015;35(30):10659–10674. doi: 10.1523/JNEUROSCI.1629-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kohno T., Ishii K., Hirota Y., et al. Reelin-Nrp1 interaction regulates neocortical dendrite development in a context-specific manner. J Neurosci. 2020;40(43):8248–8261. doi: 10.1523/JNEUROSCI.1907-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hamad M.I.K., Petrova P., Daoud S., et al. Reelin restricts dendritic growth of interneurons in the neocortex. Development. 2021;148(17) doi: 10.1242/dev.199718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Niu S., Renfro A., Quattrocchi C.C., et al. Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway. Neuron. 2004;41(1):71–84. doi: 10.1016/s0896-6273(03)00819-5. [DOI] [PubMed] [Google Scholar]
- 172.Rice D.S., Nusinowitz S., Azimi A.M., et al. The Reelin pathway modulates the structure and function of retinal synaptic circuitry. Neuron. 2001;31(6):929–941. doi: 10.1016/s0896-6273(01)00436-6. [DOI] [PubMed] [Google Scholar]
- 173.Howell B.W., Hawkes R., Soriano P., et al. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature. 1997;389(6652):733–737. doi: 10.1038/39607. [DOI] [PubMed] [Google Scholar]
- 174.Rice D.S., Sheldon M., D'Arcangelo G., et al. Disabled-1 acts downstream of Reelin in a signaling pathway that controls laminar organization in the mammalian brain. Development. 1998;125(18):3719–3729. doi: 10.1242/dev.125.18.3719. [DOI] [PubMed] [Google Scholar]
- 175.Howell B.W., Herrick T.M., Hildebrand J.D., et al. Dab1 tyrosine phosphorylation sites relay positional signals during mouse brain development. Curr Biol. 2000;10(15):877–885. doi: 10.1016/s0960-9822(00)00608-4. [DOI] [PubMed] [Google Scholar]
- 176.O'Dell R.S., Ustine C.J.M., Cameron D.A., et al. Layer 6 cortical neurons require Reelin-Dab1 signaling for cellular orientation, Golgi deployment, and directed neurite growth into the marginal zone. Neural Dev. 2012;7:25. doi: 10.1186/1749-8104-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Matsuki T., Matthews R.T., Cooper J.A., et al. Reelin and Stk25 have opposing roles in neuronal polarization and dendritic Golgi deployment. Cell. 2010;143(5):826–836. doi: 10.1016/j.cell.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dillon G.M., Tyler W.A., Omuro K.C., et al. CLASP2 links Reelin to the cytoskeleton during neocortical development. Neuron. 2017;93(6):1344–1358. doi: 10.1016/j.neuron.2017.02.039. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yanagida M., Miyoshi R., Toyokuni R., et al. Dynamics of the leading process, nucleus, and Golgi apparatus of migrating cortical interneurons in living mouse embryos. Proc Natl Acad Sci U S A. 2012;109(41):16737–16742. doi: 10.1073/pnas.1209166109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sakakibara A., Ando R., Sapir T., et al. Microtubule dynamics in neuronal morphogenesis. Open Biol. 2013;3(7) doi: 10.1098/rsob.130061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Etienne-Manneville S., Hall A. Rho GTPases in cell biology. Nature. 2002;420(6916):629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 182.Mosaddeghzadeh N., Ahmadian M.R. The RHO family GTPases: mechanisms of regulation and signaling. Cells. 2021;10(7):1831. doi: 10.3390/cells10071831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Auer M., Hausott B., Klimaschewski L. Rho GTPases as regulators of morphological neuroplasticity. Ann Anat. 2011;193(4):259–266. doi: 10.1016/j.aanat.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Debant A., Serra-Pagès C., Seipel K., et al. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc Natl Acad Sci U S A. 1996;93(11):5466–5471. doi: 10.1073/pnas.93.11.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tao T., Sun J., Peng Y., et al. Golgi-resident TRIO regulates membrane trafficking during neurite outgrowth. J Biol Chem. 2019;294(28):10954–10968. doi: 10.1074/jbc.RA118.007318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Peng Y.J., He W.Q., Tang J., et al. Trio is a key guanine nucleotide exchange factor coordinating regulation of the migration and morphogenesis of granule cells in the developing cerebellum. J Biol Chem. 2010;285(32):24834–24844. doi: 10.1074/jbc.M109.096537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Meseke M., Rosenberger G., Förster E. Reelin and the Cdc42/Rac1 guanine nucleotide exchange factor αPIX/Arhgef6 promote dendritic Golgi translocation in hippocampal neurons. Eur J Neurosci. 2013;37(9):1404–1412. doi: 10.1111/ejn.12153. [DOI] [PubMed] [Google Scholar]
- 188.Hong E.H., Kim J.Y., Kim J.H., et al. BIG2-ARF1-RhoA-mDia1 signaling regulates dendritic Golgi polarization in hippocampal neurons. Mol Neurobiol. 2018;55(10):7701–7716. doi: 10.1007/s12035-018-0954-7. [DOI] [PubMed] [Google Scholar]
- 189.Watanabe N., Kato T., Fujita A., et al. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol. 1999;1(3):136–143. doi: 10.1038/11056. [DOI] [PubMed] [Google Scholar]
- 190.Ishizaki T., Morishima Y., Okamoto M., et al. Coordination of microtubules and the actin cytoskeleton by the Rho effector mDia1. Nat Cell Biol. 2001;3(1):8–14. doi: 10.1038/35050598. [DOI] [PubMed] [Google Scholar]
- 191.Grueber W.B., Jan L.Y., Jan Y.N. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129(12):2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- 192.Papoulas O., Hays T.S., Sisson J.C. The golgin Lava lamp mediates dynein-based Golgi movements during Drosophila cellularization. Nat Cell Biol. 2005;7(6):612–618. doi: 10.1038/ncb1264. [DOI] [PubMed] [Google Scholar]
- 193.Jeyifous O., Waites C.L., Specht C.G., et al. SAP97 and CASK mediate sorting of NMDA receptors through a previously unknown secretory pathway. Nat Neurosci. 2009;12(8):1011–1019. doi: 10.1038/nn.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yang S.Z., Wildonger J. Golgi outposts locally regulate microtubule orientation in neurons but are not required for the overall polarity of the dendritic cytoskeleton. Genetics. 2020;215(2):435–447. doi: 10.1534/genetics.119.302979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Blochlinger K., Bodmer R., Jan L.Y., et al. Patterns of expression of cut, a protein required for external sensory organ development in wild-type and cut mutant Drosophila embryos. Genes Dev. 1990;4(8):1322–1331. doi: 10.1101/gad.4.8.1322. [DOI] [PubMed] [Google Scholar]
- 196.Jinushi-Nakao S., Arvind R., Amikura R., et al. Knot/Collier and cut control different aspects of dendrite cytoskeleton and synergize to define final Arbor shape. Neuron. 2007;56(6):963–978. doi: 10.1016/j.neuron.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 197.Iyer S.C., Ramachandran Iyer E.P., Meduri R., et al. Cut, via CrebA, transcriptionally regulates the COPII secretory pathway to direct dendrite development in Drosophila. J Cell Sci. 2013;126(20):4732–4745. doi: 10.1242/jcs.131144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Martinez-Campos M., Basto R., Baker J., et al. The Drosophila pericentrin-like protein is essential for cilia/flagella function, but appears to be dispensable for mitosis. J Cell Biol. 2004;165(5):673–683. doi: 10.1083/jcb.200402130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Megraw T.L., Kilaru S., Turner F.R., et al. The centrosome is a dynamic structure that ejects PCM flares. J Cell Sci. 2002;115(Pt 23):4707–4718. doi: 10.1242/jcs.00134. [DOI] [PubMed] [Google Scholar]
- 200.Satoh D., Sato D., Tsuyama T., et al. Spatial control of branching within dendritic arbors by dynein-dependent transport of Rab5-endosomes. Nat Cell Biol. 2008;10(10):1164–1171. doi: 10.1038/ncb1776. [DOI] [PubMed] [Google Scholar]
- 201.Zheng Y., Wildonger J., Ye B., et al. Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nat Cell Biol. 2008;10(10):1172–1180. doi: 10.1038/ncb1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kelliher M.T., Yue Y., Ng A., et al. Autoinhibition of kinesin-1 is essential to the dendrite-specific localization of Golgi outposts. J Cell Biol. 2018;217(7):2531–2547. doi: 10.1083/jcb.201708096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mori D., Yamada M., Mimori-Kiyosue Y., et al. An essential role of the aPKC-Aurora A-NDEL1 pathway in neurite elongation by modulation of microtubule dynamics. Nat Cell Biol. 2009;11(9):1057–1068. doi: 10.1038/ncb1919. [DOI] [PubMed] [Google Scholar]
- 204.Arthur A.L., Yang S.Z., Abellaneda A.M., et al. Dendrite arborization requires the dynein cofactor NudE. J Cell Sci. 2015;128(11):2191–2201. doi: 10.1242/jcs.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jaarsma D., Hoogenraad C.C. Cytoplasmic dynein and its regulatory proteins in Golgi pathology in nervous system disorders. Front Neurosci. 2015;9:397. doi: 10.3389/fnins.2015.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kaan H.Y.K., Hackney D.D., Kozielski F. The structure of the kinesin-1 motor-tail complex reveals the mechanism of autoinhibition. Science. 2011;333(6044):883–885. doi: 10.1126/science.1204824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kulkarni V.A., Firestein B.L. The dendritic tree and brain disorders. Mol Cell Neurosci. 2012;50(1):10–20. doi: 10.1016/j.mcn.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 208.Lin C.H., Li H., Lee Y.N., et al. Lrrk regulates the dynamic profile of dendritic Golgi outposts through the golgin Lava lamp. J Cell Biol. 2015;210(3):471–483. doi: 10.1083/jcb.201411033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chung C.G., Kwon M.J., Jeon K.H., et al. Golgi outpost synthesis impaired by toxic polyglutamine proteins contributes to dendritic pathology in neurons. Cell Rep. 2017;20(2):356–369. doi: 10.1016/j.celrep.2017.06.059. [DOI] [PubMed] [Google Scholar]
- 210.Park J.H., Chung C.G., Seo J., et al. C9orf72-associated arginine-rich dipeptide repeat proteins reduce the number of Golgi outposts and dendritic branches in Drosophila neurons. Mol Cell. 2020;43(9):821–830. doi: 10.14348/molcells.2020.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Du Q., Chang J., Cheng G., et al. Sunday driver mediates multi-compartment Golgi outposts defects induced by amyloid precursor protein. Front Neurosci. 2021;15 doi: 10.3389/fnins.2021.673684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.De Wit T., Baekelandt V., Lobbestael E. LRRK2 phosphorylation: behind the scenes. Neuroscientist. 2018;24(5):486–500. doi: 10.1177/1073858418756309. [DOI] [PubMed] [Google Scholar]
- 213.Tolosa E., Vila M., Klein C., et al. LRRK2 in Parkinson disease: challenges of clinical trials. Nat Rev Neurol. 2020;16(2):97–107. doi: 10.1038/s41582-019-0301-2. [DOI] [PubMed] [Google Scholar]
- 214.Healy D.G., Falchi M., O'Sullivan S.S., et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study. Lancet Neurol. 2008;7(7):583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Stafa K., Trancikova A., Webber P.J., et al. GTPase activity and neuronal toxicity of Parkinson's disease-associated LRRK2 is regulated by ArfGAP1. PLoS Genet. 2012;8(2) doi: 10.1371/journal.pgen.1002526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sambataro F., Pennuto M. Cell-autonomous and non-cell-autonomous toxicity in polyglutamine diseases. Prog Neurobiol. 2012;97(2):152–172. doi: 10.1016/j.pneurobio.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 217.Lee S.B., Bagley J.A., Lee H.Y., et al. Pathogenic polyglutamine proteins cause dendrite defects associated with specific actin cytoskeletal alterations in Drosophila. Proc Natl Acad Sci U S A. 2011;108(40):16795–16800. doi: 10.1073/pnas.1113573108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Taylor J.P., Brown R.H., Jr., Cleveland D.W. Decoding ALS: from genes to mechanism. Nature. 2016;539(7628):197–206. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Takeda T., Uchihara T., Nakayama Y., et al. Dendritic retraction, but not atrophy, is consistent in amyotrophic lateral sclerosis-comparison between Onuf's neurons and other sacral motor neurons. Acta Neuropathol Commun. 2014;2:11. doi: 10.1186/2051-5960-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tibshirani M., Zhao B., Gentil B.J., et al. Dysregulation of chromatin remodelling complexes in amyotrophic lateral sclerosis. Hum Mol Genet. 2017;26(21):4142–4152. doi: 10.1093/hmg/ddx301. [DOI] [PubMed] [Google Scholar]
- 221.Herzog J.J., Deshpande M., Shapiro L., et al. TDP-43 misexpression causes defects in dendritic growth. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-15914-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ferrer I. Neurons and their dendrites in frontotemporal dementia. Dement Geriatr Cognit Disord. 1999;10(Suppl 1):55–60. doi: 10.1159/000051214. [DOI] [PubMed] [Google Scholar]
- 223.DeJesus-Hernandez M., Mackenzie I.R., Boeve B.F., et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Renton A.E., Majounie E., Waite A., et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72(2):257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Freibaum B.D., Taylor J.P. The role of dipeptide repeats in C9ORF72-related ALS-FTD. Front Mol Neurosci. 2017;10:35. doi: 10.3389/fnmol.2017.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Stieber A., Mourelatos Z., Gonatas N.K. In Alzheimer's disease the Golgi apparatus of a population of neurons without neurofibrillary tangles is fragmented and atrophic. Am J Pathol. 1996;148(2):415–426. [PMC free article] [PubMed] [Google Scholar]
- 227.Baloyannis S.J., Manolidis S.L., Manolidis L.S. Synaptic alterations in the vestibulocerebellar system in Alzheimer's disease: a Golgi and electron microscope study. Acta Otolaryngol. 2000;120(2):247–250. doi: 10.1080/000164800750001026. [DOI] [PubMed] [Google Scholar]
- 228.Maguire T.M., Breen K.C. A decrease in neural sialyltransferase activity in Alzheimer's disease. Dementia. 1995;6(4):185–190. doi: 10.1159/000106944. [DOI] [PubMed] [Google Scholar]
- 229.Halim A., Brinkmalm G., Rüetschi U., et al. Site-specific characterization of threonine, serine, and tyrosine glycosylations of amyloid precursor protein/amyloid beta-peptides in human cerebrospinal fluid. Proc Natl Acad Sci U S A. 2011;108(29):11848–11853. doi: 10.1073/pnas.1102664108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Fontaine C.J., Patten A.R., Sickmann H.M., et al. Effects of pre-natal alcohol exposure on hippocampal synaptic plasticity: sex, age and methodological considerations. Neurosci Biobehav Rev. 2016;64:12–34. doi: 10.1016/j.neubiorev.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 231.Hwang H.M., Ku R.Y., Hashimoto-Torii K. Prenatal environment that affects neuronal migration. Front Cell Dev Biol. 2019;7:138. doi: 10.3389/fcell.2019.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wang D., Howell B.W., Olson E.C. Maternal ethanol exposure acutely elevates src family kinase activity in the fetal cortex. Mol Neurobiol. 2021;58(10):5210–5223. doi: 10.1007/s12035-021-02467-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lee J., Naik V., Orzabal M., et al. Morphological alteration in rat hippocampal neuronal dendrites following chronic binge prenatal alcohol exposure. Brain Res. 2021;1768 doi: 10.1016/j.brainres.2021.147587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Powrozek T.A., Olson E.C. Ethanol-induced disruption of Golgi apparatus morphology, primary neurite number and cellular orientation in developing cortical neurons. Alcohol. 2012;46(7):619–627. doi: 10.1016/j.alcohol.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Bird C.W., Candelaria-Cook F.T., Magcalas C.M., et al. Moderate prenatal alcohol exposure enhances GluN2B containing NMDA receptor binding and ifenprodil sensitivity in rat agranular insular cortex. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0118721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Brady M.L., Diaz M.R., Iuso A., et al. Moderate prenatal alcohol exposure reduces plasticity and alters NMDA receptor subunit composition in the dentate gyrus. J Neurosci. 2013;33(3):1062–1067. doi: 10.1523/JNEUROSCI.1217-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Honse Y., Nixon K.M., Browning M.D., et al. Cell surface expression of NR1 splice variants and NR2 subunits is modified by prenatal ethanol exposure. Neuroscience. 2003;122(3):689–698. doi: 10.1016/s0306-4522(03)00603-1. [DOI] [PubMed] [Google Scholar]
- 238.Thayer D.A., Jan Y.N., Jan L.Y. Increased neuronal activity fragments the Golgi complex. Proc Natl Acad Sci U S A. 2013;110(4):1482–1487. doi: 10.1073/pnas.1220978110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wang Q., Wang Y., Yu F. Yif1 associates with Yip1 on Golgi and regulates dendrite pruning in sensory neurons during Drosophila metamorphosis. Development. 2018;145(12):dev164475. doi: 10.1242/dev.164475. [DOI] [PubMed] [Google Scholar]
- 240.He Q., Liu H., Deng S., et al. The Golgi apparatus may be a potential therapeutic target for apoptosis-related neurological diseases. Front Cell Dev Biol. 2020;8:830. doi: 10.3389/fcell.2020.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Caracci M.O., Fuentealba L.M., Marzolo M.P. Golgi complex dynamics and its implication in prevalent neurological disorders. Front Cell Dev Biol. 2019;7:75. doi: 10.3389/fcell.2019.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]