Abstract
Objective.
Individuals with ankylosing spondylitis (AS) have a greater cardiovascular (CV) risk than those in the general population. The effect of tumor necrosis factor inhibitors (TNFis) on CV risk, including on the development of hypertension (HTN), remains unclear, with some data suggesting higher risk. We assessed the association of TNFi use with incident HTN in a longitudinal AS cohort.
Methods.
Adults with AS enrolled in a prospective cohort in 2002–2018 were examined every 4–6 months. TNFi use during the preceding 6 months was ascertained at each study visit. We defined HTN by patient-reported HTN, antihypertensive medication use, or, on 2 consecutive visits, systolic blood pressure (BP) ≥ 140 mmHg or diastolic BP ≥ 90 mmHg. We evaluated the association between TNFi use and the development of HTN with marginal structural models, estimated by inverse probability-of-treatment weighting, to account for time-dependent confounders and informative censoring. Potential confounders included age, sex, race, site, nonsteroidal antiinflammatory drug use, and disease activity.
Results.
We included 630 patients without baseline HTN and with at least 1 year of follow-up. Of these, 72% were male, mean age was 39 ± 13 years, and 43% used TNFi at baseline. On follow-up (median 5 yrs), 129 developed incident HTN and 163 started on TNFi during follow-up. TNFi use was not associated with incident HTN (adjusted HR 1.10, 95% CI 0.83–1.37).
Conclusion.
In our prospective AS cohort, TNFi use was not significantly associated with incident HTN.
Keywords: ankylosing spondylitis, cardiovascular diseases, spondyloarthropathy
Individuals with ankylosing spondylitis (AS) have an increased risk of cardiovascular disease (CVD) and CVD-related mortality compared to those of the same age and sex without AS.1,2 This increased cardiovascular (CV) risk in AS may be partially explained by an increased prevalence of established CV risk factors such as hypertension (HTN), as compared to the general population.3 Chronic systemic inflammation related to AS disease activity may be another contributor to the overall CV risk.4,5,6,7
Nonsteroidal antiinflammatory drugs (NSAIDs) are the first-line pharmacologic therapy in AS8; however, their potential for increasing CV risk9,10 may limit their use in this high-risk population. Emerging evidence suggests that tumor necrosis factor inhibitors (TNFi) may be cardioprotective through control of the underlying inflammatory disease, although their effect on both CV risk factors and CV events such as myocardial infarction (MI) remains unclear.11,12 High levels of the cytokine TNF decreases blood pressure (BP) through effects on renal physiology.13 The available data regarding the effect of TNFi use on BP or incident HTN in AS or, more generally, axial spondyloarthritis, are limited and mixed. Small prospective studies in AS did not demonstrate significant changes in BP with TNFi initiation, but further inference from these studies is hampered due to their small sample size and short follow-up duration.14,15
In our previous analysis of the Prospective Study of Outcomes in AS (PSOAS) cohort, as well as an analysis of another prospective AS cohort, TNFi use appeared to be independently associated with increased risk of incident HTN.16,17 However, there are 3 reasons that these associations, which were derived using conventional multivariable models, might not represent a true elevation of HTN risk caused by using TNFi. First, the models used in these studies were not constructed to address this association directly.18 Second, conventional regression models cannot account for time-dependent confounding19 such as that which is due to disease activity,20 NSAID use, adiposity, or changes thereof.21 Third, conventional regression models cannot account for study participants who were lost to follow-up due to reasons associated with TNFi use or HTN (informative censoring).22 Potential associations between TNFi and CV risk require further clarity for clinical decision making. Thus, the aim of our study was to assess the association of TNFi use with incident HTN in a prospective AS cohort, utilizing causal inference methods to account for time-varying confounders and informative censoring.
METHODS
Study population.
The PSOAS cohort has been described in detail elsewhere.23 Briefly, subjects were enrolled if they were aged ≥ 18 years and met the 1984 modified New York criteria for AS.24 The anterior posterior pelvis radiographs for radiographic sacroiliitis were centrally read by an expert musculoskeletal radiologist. Individuals were recruited from the investigators’ clinics, patient support groups, and community rheumatologists. There were 5 participating study sites: Cedars-Sinai Medical Center (Los Angeles, CA); University of Texas Health – McGovern Medical School (Houston, TX); National Institutes of Health (NIH; Bethesda, MD); University of California San Francisco (San Francisco, CA); and Princess Alexandra Hospital, Queensland University of Technology (Brisbane, Australia). Enrollment in the PSOAS cohort began in 2002 and continued through 2018.
Clinical evaluation was performed by a study site investigator using standardized protocols at study entry and at subsequent study visits every 4–6 months. At baseline, patient demographics and characteristics of AS disease status, date of symptom onset, patient-reported outcomes, extramusculoskeletal manifestations, comorbidities, and medication history were recorded. At follow-up study visits, patient-reported outcomes and medications were collected. All medications used in the preceding 6 months, including those obtained over the counter, were recorded per patient report, and/or through electronic medical record (EMR) review with investigator confirmation (where available). The number of missed doses in the past week, month, and 6 months was also documented, along with whether the patient was still taking the medication. C-reactive protein (CRP) and erythrocyte sedimentation rate levels were also determined at each study visit. Comorbid conditions, including CVD and its risk factors, were ascertained at baseline and every 2 years.
All study data were also entered into REDCap (Research Database Capture) and quality assurance of data for our study was performed by the Data Management and Statistical Core, housed in the Biostatistics/Epidemiology/Research Design component of the Center for Clinical and Translational Sciences at the McGovern Medical School at The University of Texas Health Science Center at Houston. Each institution at which the study was conducted had approval by their respective institutional review boards: Cedars-Sinai, CR00011435/Pro00010016; University of Texas — Houston, UTH-HSC-MS-07–0022; University of California — San Francisco, 1–01695, Ref #183280; NIH, #03-AR-0131; Queensland University of Technology, HREC/05/QPAH/221. All patients were provided written informed consent for enrollment into PSOAS.
Exposure of interest.
TNFi use was a binary variable indicating use in the 6 months prior to the study visit. TNFis included etanercept, infliximab, adalimumab, certolizumab pegol, and golimumab.
Outcome of interest.
Incident HTN was defined using a modified version of the standard National Heart, Lung, and Blood Institute study definition of HTN. Patients met criteria if they had any of the following: a diagnosis of HTN (patient-reported and confirmed by investigators using the EMR where available); the use of antihypertensive medication(s); or on 2 consecutive study visits, systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg.16,25 Antihypertensive medications included the categories diuretics, beta blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, and other BP medications; aspirin was excluded. Indications for use were not recorded. Subjects were considered to have a new diagnosis of HTN if they did not meet HTN criteria as defined above at the baseline visit but met those criteria at a subsequent visit. Patients who met criteria for HTN at their baseline visit were excluded from all analyses.
Other covariates.
We included age at each study visit, in years, which was derived from age at the baseline visit, as well as sex, race (White, Black, Asian, other), and smoking status (current smoker, yes/no). BMI (kg/m2, continuous) was derived from height and weight measurements taken at study visits from 2013 onwards. NSAID usage was quantified in the cohort by the NSAID index according to Assessment of Spondyloarthritis international Society recommendations.26 An individual taking the full recommended dosage of an NSAID in the 6 months preceding the study visit would receive an NSAID index of 100, whereas an individual reporting no NSAID use would receive an index of 0. For our analyses, NSAID use was dichotomized as continuous use (NSAID index ≥ 50) vs on demand/no use (NSAID index < 50).16,27 Disease activity was measured using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI; range 0–10).28 We calculated the Ankylosing Spondylitis Disease Activity Score (ASDAS), which was a continuous variable (possible range 0.6 to > 6.0), using BASDAI questions 2, 3, and 6, a patient global score, and the CRP. If the CRP was undetectable or < 2 mg/L, a constant value of 2 mg/L was utilized in the calculation.29 Separate models included either the BASDAI or the ASDAS as a measure of disease activity.
Analysis.
We included all study visits for patients who were followed longitudinally for at least 1 year in the 2003–2018 study cycles. Due to limited data collected in 2002, we excluded study visits from that year. We initially included 834 patients in our study cohort. Those patients with prevalent HTN at baseline (n = 204, 24% of the cohort) were then excluded from the analysis (Figure 1). We performed descriptive statistics for baseline demographic and clinical characteristics of patients without HTN at baseline (n = 630).
Figure 1.
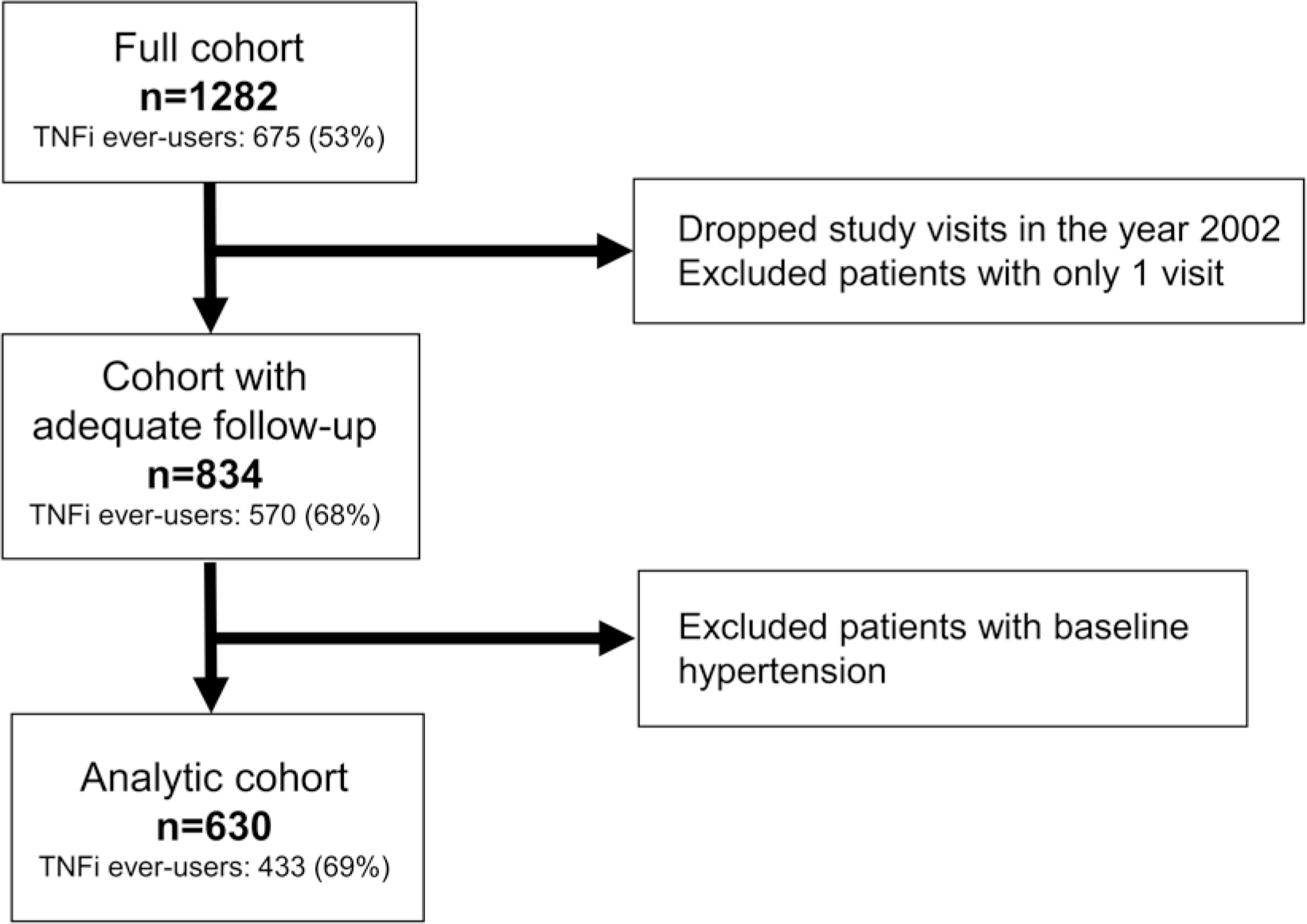
Flow chart of participant inclusion into study analyses. TNFi ever-users in each group were defined by having at least 1 study visit in which the patient indicated they were taking any TNFi. TNFi: tumor necrosis factor inhibitor.
Potential confounders were determined a priori based on the current literature and our clinical understanding of relationships30: age, sex, race, study site, BMI, disease activity, and NSAID use. Although smoking status and CV risk factors such as diabetes are associated with the outcome of interest (HTN), they are not associated with TNFi use. Thus, they were not included as confounders in our models.
We used multiple imputation with chained equations (MICE) with 5 iterations to impute missing values for TNFi use (5.8% missingness), NSAID use (5.8% missingness), disease activity (11.5% missingness for BASDAI, 20.5% missingness for ASDAS), BMI (86% missingness, due to start of standardized collection during study visits in 2013; see sensitivity analysis below), and HTN status (2.2% missingness).31,32,33 We used a random forest algorithm that provides robustness with regard to a potential misspecification of the imputation model, compared to parametric MICE techniques.34
To determine the association of TNFi use with incident HTN, we used marginal structural models (MSM) that were estimated by inverse probability-of-treatment and censoring weights (IPWs). Using MSM, we were able to account for disease activity, NSAID use, and BMI as time-dependent confounders with regard to the association between TNFi use and HTN risk.35 These methods allow for adjustment of confounders that vary over follow-up. To minimize confounding by indication for TNFi treatment, we calculated the inverse probability of TNFi treatment and censoring weights conditional on both baseline and time-dependent confounders in each interval of follow-up (i.e., at each study visit). To minimize extreme weights that could result in inflated variance and bias, we used the stabilized weights by using the marginal means of treatment and evaluated the distribution of weights for positivity violations. Stabilized weights were truncated at the 1st and 99th percentiles. We assessed summary statistics of truncated stabilized weights for the presence of extreme weights. To estimate the association between TNFi use and incident HTN to approximate HRs and 95% CIs, we used pooled logistic regression models weighted by the stabilized IPWs for each study visit as described in Robins et al.22
To assess the robustness of our results, we performed the following sensitivity analyses using MSM: (1) replacement of the BASDAI covariate with the ASDAS in the multiply imputed analyses; (2) without inclusion of the BMI covariate, due to its large degree of missingness, in the multiply imputed analyses; (3) a complete case analysis in lieu of multiple imputation, with the BASDAI covariate; and (4) a complete case analysis in lieu of multiple imputation, with the ASDAS covariate. In addition, we used a conventional regression approach, Cox proportional hazards, to model the association between TNFi use and incident HTN, with both multiply imputed and complete case analyses. Although the Cox models included time-varying exposures (i.e., allowed TNFi treatment status to vary by study visit), these models were unable to account for confounding by indication to the degree of the MSM models, nor were they able to account for informative censoring. Since older patients included in our analyses who had not developed HTN by their baseline study visit may be at lower risk for HTN overall, we performed an exploratory subanalysis restricted to participants aged < 45 years at their baseline visit. To explore an association among TNFi users with a duration ≥ 1 year, we excluded those who were recorded as TNFi users for only 1 study visit (equivalent to ≤ 6 months of use). Analyses were conducted using R, version 4.036 and Stata version 15 (StataCorp).
RESULTS
There were 834 individuals with AS from the PSOAS cohort with at least 1 year of follow-up. After excluding those with baseline HTN (n = 204), 630 individuals remained for our analyses. Baseline characteristics of the cohort entered into the main analysis, stratified by TNFi use at baseline, are shown in Table 1. Overall, the median age at study entry was 38 years (IQR 29–48 yrs), 72% were male, and 80% were White. The median symptom duration at study entry was 13 (IQR 7–23) years, and individuals had low disease activity (median BASDAI 3.2 [IQR 1.6–5.5]; median ASDAS 1.8 [IQR 1.2–2.6]). At baseline, 269 (43%) were on TNFi. Compared to those not on TNFi, a lower proportion of TNFi users had an elevated CRP or were on continuous NSAIDs. However, a higher proportion of TNFi users were obese (BMI ≥ 30 kg/m2), with a median of 26.2 (IQR 23.2–30.0) kg/m2 compared to median 25.8 (IQR 23.2–28.8) kg/m2 in TNFi nonusers. Apart from obesity, CVD and CV risk factors were low (≤ 10%) at baseline overall. The median follow-up time was 5.1 (IQR 2.7–7.8) years overall, and was similar between those who were baseline TNFi users and nonusers (5.3 [IQR 2.5–8.0] vs 5.1 [IQR 3.0–7.8] yrs). Over the follow-up period, 129 developed incident HTN, and 163 started TNFi.
Table 1.
Baseline demographic and clinical characteristics of subjects without hypertension at baseline, stratified by baseline TNFi use.
| Entire Cohort, n = 630 | TNFi Users, n = 269 | TNFi Nonusers, n = 361 | |
|---|---|---|---|
|
| |||
| Demographics | |||
| Age, yrs | 39.4 ± 12.9 | 39.1 ± 12.2 | 39.6 ± 13.3 |
| 37.5 (29–48) | 38 (29–46) | 37 (29–49) | |
| Male sex | 72 | 74 | 70 |
| White race | 80 | 80 | 80 |
| Disease characteristics | |||
| Age at symptom onset, yrsa | 23.8 ± 9.2 | 23.8 ± 9.3 | 23.9 ± 9.2 |
| 22 (18–28) | 22 (17–28) | 22 (18–28) | |
| Symptom duration, yrsa | 15.7 ± 11.9 | 15.7 ± 10.7 | 15.8 ± 12.7 |
| 13 (7–23) | 14 (7–23) | 13 (6–23) | |
| BASDAI (0–10)a | 3.7 ± 2.4 | 3.3 ± 2.4 | 3.9 ± 2.4 |
| 3.2 (1.6–5.5) | 2.9 (1.3–5.0) | 3.7 (1.8–5.9) | |
| ASDAS (0.6–6.6)a | 2.0 ± 0.9 | 1.9 ± 0.8 | 2.1 ± 0.9 |
| 1.8 (1.2–2.6) | 1.7 (1.1–2.5) | 1.9 (1.3–2.7) | |
| Elevated CRPa,b | 37 | 26 | 46 |
| Continuous NSAID usec | 32 | 25 | 37 |
| Glucocorticoid use | 7 | 7 | 6 |
| CVD and risk factors | |||
| Obese (BMI ≥ 30 kg/m2)a | 22 | 25 | 19 |
| CVDa,d | 2 | 2 | 3 |
| Diabetesa | 1 | 2 | 1 |
| Current smoking | 10 | 10 | 10 |
| Statin use | 3 | 4 | 2 |
Continuous variables are presented as mean ± SD and median (IQR). Categorical variables are presented as %.
Data were missing for age at symptom onset (n = 61), symptom duration (n = 61), BASDAI (n = 3), ASDAS (n = 7), exercise (n = 2), abnormal CRP (n = 3), obese BMI (n = 319), CVD (n = 9), and diabetes (n = 9).
CRP was elevated if above the upper limit of the reference range associated with the value.
Continuous NSAID use was defined as an NSAID index of ≥ 50.
CVD was the composite of patient-reported coronary artery disease, coronary bypass surgery, coronary angioplasty, heart attack, heart valve problems, and angina. ASDAS: Ankylosing Spondylitis Disease Activity Score; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; CRP: C-reactive protein; CVD: cardiovascular disease; NSAID: nonsteroidal antiinflammatory drug; TNFi: tumor necrosis factor inhibitor.
The results of the multivariable analyses for the association of TNFi use and incident HTN are shown in Table 2. In the main analysis, this association was not statistically significant (HR 1.10, 95% CI 0.83–1.37) after accounting for time-dependent confounders and loss to follow-up. The results did not change when ASDAS replaced BASDAI as a disease activity measure, or when BMI was excluded as a covariate in the estimation of IPW. The analyses restricted to participants < 45 years at their baseline study visit and excluding TNFi users with a duration < 1 year had findings similar to those of the main analysis (data not shown). The complete case analysis included 618 subjects, in which the association between TNFi use and incident HTN reached statistical significance (HR 1.35, 95% CI 1.09–1.69), as did results from the conventional regression models (HR 1.08, 95% CI 1.01–1.16 after multiple imputation and HR 1.09, 95% CI 1.01–1.17 for complete case analysis).
Table 2.
Results from multivariable analyses for the association of TNFi use and the development of incident hypertension in an AS cohort.
| Adjusted HR | 95% CI | |
|---|---|---|
|
| ||
| Main analysis using MSM (n = 630) | ||
| With BASDAI | 1.10 | 0.83–1.37 |
| With ASDAS | 1.09 | 0.84–1.34 |
| Without BMI | 1.10 | 0.84–1.36 |
| Complete case analysis using MSM (n = 618)a | ||
| With BASDAI | 1.35 | 1.09–1.69 |
| With ASDAS | 1.34 | 1.08–1.67 |
| Conventional regression analysisb, multiply imputed (n = 630) | ||
| Univariable | 1.04 | 0.73–1.47 |
| Multivariable | 1.08 | 1.01–1.16 |
| Conventional regression analysis, complete case (n = 618)a | ||
| Univariable | 1.03 | 0.73–1.47 |
| Multivariable | 1.09 | 1.01–1.17 |
Complete-case analysis did not include BMI as a covariate due to degree of missingness. This analysis included 618 participants with 4152 observations, compared to 5393 observations for 630 participants in the multiply imputed main analyses.
Cox proportional hazards models with time-varying exposure (TNFi use). AS: ankylosing spondylitis; ASDAS: Ankylosing Spondylitis Disease Activity Score; BASDAI: Bath Ankylosing Spondylitis Activity Index; MSM: marginal structural model; TNFi: tumor necrosis factor inhibitor.
DISCUSSION
In this prospective AS cohort, we did not find conclusive evidence to support an increased risk of incident HTN with TNFi use after accounting for important baseline and time-dependent confounding factors. Our results differ from prior studies, which utilized methods that were neither able to account for the time-varying relationships between TNFi use, disease activity, and NSAID use nor to adjust for bias caused by informative censoring due to loss to follow-up.
Population-level studies have shown that the incidence of CV outcomes such as MI, and the prevalence of CV risk factors such as HTN, are elevated among people with AS vs age- and sex-matched comparators.1,2,3,37,38 However, since the relationship between AS and CV risk is poorly understood compared to other rheumatic conditions such as rheumatoid arthritis (RA) or psoriatic arthritis, there currently are no data-driven guidelines for management of excess CVD burden in patients with AS.8,39,40,41,42,43,44
The general hypothesis is that control of systemic inflammation with antiinflammatory therapy leads to reduced CV risk in individuals with rheumatic disease.15,16,17,18,45 However, this hypothesis has not been well studied in AS. The question of whether antiinflammatory therapies attenuate CVD in AS is important not only because of the increased CV risk in general, regardless of AS treatment, but also because NSAIDs are the first-line pharmacologic therapy in AS8 and may be associated with the development of HTN. In our previous study using data from this same cohort, we found that continuous NSAID use was associated with a modestly increased risk of incident HTN in patients with AS, as compared to noncontinuous or no NSAID use.16 Concern still remains that long-term NSAID use in AS may increase risk of CV outcomes; thus, a better understanding of how second- and third-line therapeutic agents influence these outcomes is important.
TNFis are second-line pharmacologic therapies for AS, but there are limited data on their effect on CV risk factors such as HTN or CV outcomes such as MI or stroke.43,44 Several studies of TNFi use in relation to subclinical atherosclerosis in AS have suggested that CV risk is reduced alongside the reduction of inflammation.45,46,47,48 However, other studies in AS did not demonstrate significant changes in BP with TNFi use, likely due to their small sample size and short duration of follow-up.14,15 Additionally, in a prospective AS cohort based in Toronto, Fitzgerald et al found that TNFi use appeared to be an independent predictor of incident HTN in a multivariable model.17 A similar finding was seen in our prior study,16 although our multivariable models were not performed to estimate the direct effect of TNFi use on HTN. The interpretation of a confounder effect in a multivariable model may be different than that of the interpretation of the effect of the exposure of interest.18 Although both studies adjusted for disease activity and NSAID use, the time-varying nature of these covariates was not accounted for. However, the association of TNFi use with the development of HTN could be biologically plausible, as studies in animal models have shown that high levels of TNF reduce BP, while moderate levels increase BP.13 This association of TNFi use with incident HTN was also seen in a study of RA.49
The present study has the strength of using a long-standing prospective cohort of AS patients with multiple study visits. At baseline, patients were well balanced with regard to the number of TNFi users vs nonusers. Since complete case analysis of data that are not missing at random may produce biased results, we used multiple imputation methods to account for missing data. By using MSM with IPW, we were able to account for time-varying TNFi use as well as time-dependent confounders such as disease activity and NSAID use. We were also able to explicitly account for informative censoring, a major concern in observational studies with relatively long follow-up. Our findings also demonstrate that interpretations of the associations between TNFi use and incident HTN can differ depending on the type of statistical analysis that was conducted. Using more sophisticated methods, the statistically significant associations were attenuated, suggesting that these associations were due to biases inherent in the use of conventional regression models and complete case analyses.
However, we are limited by precision in this study’s main analysis to fully exclude a modestly increased risk of HTN with TNFi use. Of note, this estimated effect size is similar for high-dose NSAIDs. In the case of both medications, we are studying the outcome of HTN, a CV risk factor that serves as a surrogate for CV outcomes such as MI or stroke. We do not know whether an increase in HTN risk would translate directly to an increased risk of MI or stroke in this AS population. Prior literature in patients with AS and other rheumatic diseases has suggested that treatment with therapies that target inflammation, including NSAIDs and TNFi, may reduce the risk of CV outcomes such as MI or stroke.40,41,43 Perhaps better control of disease activity and systemic inflammation balances out, and possibly outweighs, the use of medications that increase the BP.
Other limitations of this study must be acknowledged. We used observational data from a longitudinal cohort that had a large amount of missingness (86%) for a time-varying covariate, BMI. TNFi initiation can increase BMI through changes in body composition. With this degree of missingness, both complete case analysis and imputation strategies may produce biased and thus, incorrect, results. However, the consistency of results across sensitivity analyses provides some reassurance regarding the robustness of our results to various assumptions that potentially bias our estimate of effect sizes. Additionally, the remainder of covariates in our models had much lower degrees of missingness. The ability to make causal inference for these observational data relies upon a set of assumptions.22 These assumptions require that TNFi users and nonusers are similar after accounting for measured confounders; that all study participants could have received treatment with TNFi in the clinical setting; and that the treatment of interest—TNFi—was well defined in our study. As with all observational studies, unmeasured and residual confounding may be possible, and could bias our estimates of effect sizes. To address potential confounding by indication, we used IPW. Although we are limited by BP measurements being available only from 2013 to 2018, we minimized misclassification of the outcome by using a composite definition of HTN that included BP readings and HTN diagnosis or antihypertensive medication use. TNFi use was defined by patient report and confirmation in the EMR accounting for the 6 months between each study visit, and was allowed to vary over time.
To address possible depletion of susceptibles (i.e., those who were older at baseline but did not have HTN may have been unlikely to develop HTN), we performed a subanalysis restricted to participants aged < 45 years and found results similar to those of the main analysis. However, there may be prevalent user bias or channeling bias due to inclusion of those who were TNFi users at baseline. Our use of MSM does not answer the question of whether different durations of TNFi exposure may have different effects on the development of HTN. Given the possibility that longer TNFi exposure is required to see clinical effects on BP, we explored whether restricting our analysis to include only TNFi nonusers and TNFi users ≥ 1 year would demonstrate different results; no such differences were seen. Finally, due to rare CV events such as MI in our cohort, we were unable to assess the association of TNFi use on these outcomes.
In conclusion, we did not find conclusive evidence of increased risk of HTN with TNFi use, nor was there evidence to support a cardioprotective effect among patients with AS. The current evidence base leaves room for potentially reduced CV risk (through reduction of systemic inflammation) and potentially increased CV risk (through elevation of BP) for second-line TNFi treatment in AS. Further studies with larger sample sizes and more granular data collection in terms of confounders would be needed to better answer these questions. When engaging in shared decision making with patients, clinicians should continue to address the balance of risks and benefits of the available treatments, especially regarding the burden of CV comorbidity. Beyond CV risks, considerations include other side effects, patient comorbidities, treatment costs, and patient preferences.
Supplementary Material
Acknowledgments
JWL is supported by the Spondyloarthritis Research and Treatment Network (SPARTAN). SRJ is supported by the National Institute on Aging (award number: R03AG060272) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (award number: R21AR074578) of the National Institutes of Health, Rheumatology Research Foundation (> $10,000). LSG is supported by the Russell/Engleman Rheumatology Research Center.
JWL received grant/research support from Pfizer (> $10,000) for this work. SRJ received grant/research support from Pfizer (> $10,000) unrelated to this work. MD received consulting fees for UCB (< $10,000). JDR received consulting fees for UCB (< $10,000) and research support from Lilly and Janssen unrelated to this work. MHW received consulting fees for Novartis, UCB, Gilead, and GSK (< $10,000). LSG received consulting fees for AbbVie, Eli Lilly, GSK, Gilead, Pfizer (< $10,000); and grant/research support from UCB and Novartis (> $10,000). The remaining authors declare no conflicts of interest relevant to this article.
REFERENCES
- 1.Haroon NN, Paterson JM, Li P, Inman RD, Haroon N. Patients with ankylosing spondylitis have increased cardiovascular and cerebrovascular mortality: a population-based study. Ann Intern Med 2015;163:409–16. [DOI] [PubMed] [Google Scholar]
- 2.Mathieu S, Soubrier M. Cardiovascular events in ankylosing spondylitis: a 2018 meta-analysis. Ann Rheum Dis 2019;78:e57. [DOI] [PubMed] [Google Scholar]
- 3.Bremander A, Petersson IF, Bergman S, Englund M. Population-based estimates of common comorbidities and cardiovascular disease in ankylosing spondylitis. Arthritis Care Res 2011;63:550–6. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Juanatey C, Vazquez-Rodriguez TR, Miranda-Filloy JA, et al. The high prevalence of subclinical atherosclerosis in patients with ankylosing spondylitis without clinically evident cardiovascular disease. Medicine 2009;88:358–65. [DOI] [PubMed] [Google Scholar]
- 5.Mathieu S, Joly H, Baron G, et al. Trend towards increased arterial stiffness or intima-media thickness in ankylosing spondylitis patients without clinically evident cardiovascular disease. Rheumatology 2008;47:1203–7. [DOI] [PubMed] [Google Scholar]
- 6.Sari I, Okan T, Akar S, et al. Impaired endothelial function in patients with ankylosing spondylitis. Rheumatology 2006;45:283–6. [DOI] [PubMed] [Google Scholar]
- 7.Berg IJ, Semb AG, van Der Heijde D, et al. CRP and ASDAS are associated with future elevated arterial stiffness, a risk marker of cardiovascular disease, in patients with ankylosing spondylitis: results after 5-year follow-up. Ann Rheum Dis 2015;74:1562–6. [DOI] [PubMed] [Google Scholar]
- 8.Ward MM, Deodhar A, Gensler LS, et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2019;71:1599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mcgettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA 2016;296:1633–44. [DOI] [PubMed] [Google Scholar]
- 10.Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ 2011;342:c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roubille C, Richer V, Starnino T, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh S, Fumery M, Singh AG, et al. Comparative risk of cardiovascular events with biologic and synthetic disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res 2020;72:561–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramseyer VD, Garvin JL. Tumor necrosis factor-α: regulation of renal function and blood pressure. Am J Physiol Renal Physiol 2013;304:F1231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moraes JCB, Ribeiro ACM, Saad CGS, Lianza AC, Silva CA, Bonfá E. NT-proBNP levels may be influenced by inflammation in active ankylosing spondylitis receiving TNF blockers: a pilot study. Clin Rheumatol 2013;32:879–83. [DOI] [PubMed] [Google Scholar]
- 15.Mathieu S, Pereira B, Couderc M, Rabois E, Dubost JJ, Soubrier M. No significant changes in arterial stiffness in patients with ankylosing spondylitis after tumour necrosis factor alpha blockade treatment for 6 and 12 months. Rheumatology 2013;52:204–9. [DOI] [PubMed] [Google Scholar]
- 16.Liew J, Ward M, Reveille J, et al. Nonsteroidal anti-inflammatory drug use is associated with incident hypertension in ankylosing spondylitis. Arthritis Care Res 2020;72:1645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzgerald G, Tomlinson G, Ramkissoon S, Wojcik S, Inman R, Haroon N. Time-dependent analysis of incident extra-articular manifestations and comorbidities in axial spondyloarthritis [abstract]. Arthritis Rheumatol. 2020;72 Suppl 10. [Google Scholar]
- 18.Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol 2013;177:292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daniel RM, Cousens SN, De Stavola BL, Kenward MG, Sterne JAC. Methods for dealing with time-dependent confounding. Stat Med 2013;32:1584–618. [DOI] [PubMed] [Google Scholar]
- 20.Midtbø H, Gerdts E, Kvien TK, et al. The association of hypertension with asymptomatic cardiovascular organ damage in rheumatoid arthritis. Blood Press 2016;25:298–304. [DOI] [PubMed] [Google Scholar]
- 21.Hmamouchi I, Roux C, Paternotte S, Kolta S, Dougados M, Briot K. Early increase of abdominal adiposity in patients with spondyloarthritis receiving anti-tumor necrosis factor-α treatment. J Rheumatol 2014;41:1112–7. [DOI] [PubMed] [Google Scholar]
- 22.Robins JM, Hernán MÁ, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550–60. [DOI] [PubMed] [Google Scholar]
- 23.Reveille JD, Lee MJ, Gensler LS, et al. The changing profile of ankylosing spondylitis in the biologic era. Clin Rheumatol 2020;39:2641–51. [DOI] [PubMed] [Google Scholar]
- 24.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 25.James P, Oparil S, Carter B, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–20. [DOI] [PubMed] [Google Scholar]
- 26.Dougados M, Simon P, Braun J, et al. ASAS recommendations for collecting, analysing and reporting NSAID intake in clinical trials/epidemiological studies in axial spondyloarthritis. Ann Rheum Dis 2011;70:249–51. [DOI] [PubMed] [Google Scholar]
- 27.Sieper J, Listing J, Poddubnyy D, et al. Effect of continuous versus on-demand treatment of ankylosing spondylitis with diclofenac over 2 years on radiographic progression of the spine: results from a randomised multicentre trial (ENRADAS). Ann Rheum Dis 2016;75:1438–43. [DOI] [PubMed] [Google Scholar]
- 28.Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 29.Machado P, Navarro-Compán V, Landewé R, van Gaalen FA, Roux C, van Der Heijde D. Calculating the ankylosing spondylitis disease activity score if the conventional C-reactive protein level is below the limit of detection or if high-sensitivity C-reactive protein is used: an analysis in the DESIR cohort. Arthritis Rheumatol 2015;67:408–13. [DOI] [PubMed] [Google Scholar]
- 30.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999;10:37–48. [PubMed] [Google Scholar]
- 31.Rubin D Introduction in multiple imputation for nonresponse in surveys. New York, NY: John Wiley & Sons, Inc; 1987. [Google Scholar]
- 32.van Buuren S Flexible imputation of missing data. Boca Raton, FL: Chapman & Hall/CRC; 2012. [Google Scholar]
- 33.Moons KGM, Donders RART, Stijnen T, Harrell FE Jr. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol 2006;59:1092–101. [DOI] [PubMed] [Google Scholar]
- 34.Shah AD, Bartlett JW, Carpenter J, Nicholas O, Hemingway H. Comparison of random forest and parametric imputation models for imputing missing data using MICE: a CALIBER study. Am J Epidemiol 2014;179:764–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lange T, Vansteelandt S, Bekaert M. A simple unified approach for estimating natural direct and indirect effects. Am J Epidemiol 2012;176:190–5. [DOI] [PubMed] [Google Scholar]
- 36.R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Internet. Accessed September 15, 2021.] Available from: https://www.R-project.org [Google Scholar]
- 37.Brophy S, Cooksey R, Atkinson M, et al. No increased rate of acute myocardial infarction or stroke among patients with ankylosing spondylitis – a retrospective cohort study using routine data. Semin Arthritis Rheum 2012;42:140–5. [DOI] [PubMed] [Google Scholar]
- 38.Chen HH, Yeh SY, Chen HY, Lin CL, Sung FC, Kao CH. Ankylosing spondylitis and other inflammatory spondyloarthritis increase the risk of developing type 2 diabetes in an Asian population. Rheumatol Int 2014;34:265–70. [DOI] [PubMed] [Google Scholar]
- 39.Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2016;76:17–28. [DOI] [PubMed] [Google Scholar]
- 40.Barnabe C, Martin B-J, Ghali WA. Systematic review and meta-analysis: anti-tumor necrosis factor α therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res 2010;63:522–9. [DOI] [PubMed] [Google Scholar]
- 41.Westlake SL, Colebatch AN, Baird J, et al. Tumour necrosis factor antagonists and the risk of cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology 2011;50:518–31. [DOI] [PubMed] [Google Scholar]
- 42.Sattar N, Mccarey DW, Capell H, Mcinnes IB. Explaining how “high-grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation 2003;108:2957–63. [DOI] [PubMed] [Google Scholar]
- 43.Lee J, Sinnathurai P, Buchbinder R, Hill C, Lassere M, March L. Biologics and cardiovascular events in inflammatory arthritis: a prospective national cohort study. Arthritis Res Ther 2018;20:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dubreuil M, Peloquin C, Felson D, Neogi T. Myocardial infarctions among ankylosing spondylitis patients in a large US insurance database [abstract]. Arthritis Rheumatol 2018;70 Suppl 10. [Google Scholar]
- 45.van Eijk IC, de Vries MK, Levels JHM, et al. Improvement of lipid profile is accompanied by atheroprotective alterations in high-density lipoprotein composition upon tumor necrosis factor blockade: a prospective cohort study in ankylosing spondylitis. Arthritis Rheum 2009;60:1324–30. [DOI] [PubMed] [Google Scholar]
- 46.Spanakis E, Sidiropoulos P, Papadakis J, et al. Modest but sustained increase of serum high density lipoprotein cholesterol levels in patients with inflammatory arthritides treated with infliximab. J Rheumatol 2006;33:2440–6. [PubMed] [Google Scholar]
- 47.van Sijl AM, van Eijk IC, Peters MJL, et al. Tumour necrosis factor blocking agents and progression of subclinical atherosclerosis in patients with ankylosing spondylitis. Ann Rheum Dis 2015;74:119–23. [DOI] [PubMed] [Google Scholar]
- 48.Angel K, Provan SA, Fagerhol MK, et al. Effect of 1-year Anti-TNF-α therapy on aortic stiffness, carotid atherosclerosis, and calprotectin in inflammatory arthropathies: a controlled study. Am J Hypertens 2012;25:644–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Desai R, Solomon D, Schneeweiss S, Danaei G, Liao K, Kim S. Tumor necrosis factor-α inhibitor use and the risk of incident hypertension in patients with rheumatoid arthritis. Epidemiology 2016;27:414–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


