Abstract
VEGF165 and its isoform VEGF165b have the same length but opposite functions in cancer. Some studies have indicated the important role of VEGF165 in osteosarcoma (OS); however, VEGF165b has not been taken into consideration. This study aims to clarify the roles of the two isoforms in OS and the mechanism controlling their formation from an alternative splicing perspective. By in vivo and in vitro experiments, we assessed the expression and function of VEGF165 and VEGF165b, screened the underlying splicing factors, and verified the regulatory function of splicing factor YBX1 on the two isoforms and its role in OS. The results showed that in OS, VEGF165 was upregulated but VEGF165b was downregulated. VEGF165 promoted the proliferation, migration and invasion of OS cells and induced angiogenesis in OS tumours; however, VEGF165b showed the opposite function. Of the four screened splicing factors, YBX1 was upregulated in OS tissues. It was positively correlated with VEGF165 but negatively correlated with VEGF165b. Further study indicated that YBX1 could upregulate VEGF165 but downregulate VEGF165b. Moreover, YBX1 promoted the proliferation, migration and invasion of OS cells and induced angiogenesis in OS tumours. OS patients with higher YBX1 had a poor prognosis within five years, but this difference disappeared in a longer follow-up. In conclusion, VEGF165b was antineoplastic and downregulated in OS, in contrast to VEGF165. YBX1 was found to be an important splicing factor that increased VEGF165 but decreased VEGF165b. Targeting YBX1 could endogenously alter the levels of VEGF165 and VEGF165b simultaneously.
Keywords: Osteosarcoma, Splicing factor, VEGF165, VEGF165b, YBX1
1. Introduction
Osteosarcoma (OS) is a highly aggressive malignant tumour of bone that occurs most commonly in children, adolescents, and young adults, with a peak incidence in the second decade. Vascular endothelial growth factor (VEGF) is highly expressed in OS cells and associated with a poor prognosis [1,2]. VEGF can induce angiogenesis in the tumour microenvironment as a paracrine factor and promote the proliferation and metastasis of tumour cells as an autocrine factor, thus contributing to the development of OS [3,4]. Considering the crucial role of VEGF, many studies have explored novel therapies targeting VEGF pathways for OS [5,6]. The benefit from anti-VEGF therapy is encouraging but appears modest and short-lived [7]. The conclusions about the prognostic value of VEGF in OS are still conflicting [8].
The VEGF family is comprised of several members, including VEGFA, VEGFB, VEGFC and VEGFD. VEGFA is the most dominant and is often referred to as VEGF [9]. Alternative splicing of mRNA results in the formation of multiple VEGFA isoforms with varying amino acid numbers in the final structure, such as VEGF121, VEGF165 and VEGF189 [10]. VEGF165 is the most widely studied isoform. In 2002, David et al. first found that alternative 3’ splice site selection in the terminal exon generates another isoform of VEGF165, termed VEGF165b, which has the same length as VEGF165 but differs by 6 amino acids in the C-terminal sequence [11]. Since then, VEGF165b has attracted much attention from researchers. It has been found that VEGF165b is expressed in a variety of normal tissues [11,12] but downregulated in malignant tumours, including prostate cancer, metastatic melanoma and renal carcinoma [[11], [12], [13]]. VEGF165b can inhibit the angiogenesis mediated by VEGF165, slow tumour cell growth, and inhibit tumour cell-mediated migration and proliferation of endothelial cells [12,14]. VEGF165b binds to VEGF receptor 2 with the same affinity as VEGF165 but fails to activate the signalling pathways [12].
Alternative splicing determines the production of VEGF165 or VEGF165b, which has opposite effects on tumour development. Taking advantage of alternative splicing to control the endogenous conversion from VEGF165 to VEGF165b might be a better therapy for cancer patients than inhibiting VEGF165 alone. However, the current studies on VEGF in OS did not take VEGF165b into consideration. In this study, we determined the expression of VEGF165 and VEGF165b in OS, assessed their effects on OS cells and angiogenesis, and explored the regulatory mechanism of the alternative splicing of VEGF165. We found that YBX1, as a splicing factor, upregulated VEGF165 but downregulated VEGF165b in OS, consequently promoting the progression of OS. These results provide clues for targeting YBX1 to suppress the protumour effect of VEGF165 while strengthening the antitumour effect of VEGF165b. Targeting alternative splicing factors might have a double antitumour effect by altering the proportion of isoforms with opposite functions in cancer.
2. Materials and methods
2.1. Datasets
Splicing factors show the characteristics of RNA-binding proteins (RBPs). Thus, we downloaded the RBPs targeting VEGF mRNA from two databases, MEME Suite (https://meme-suite.org/meme/) and RBPDB (http://rbpdb.ccbr.utoronto.ca/index.php). To improve the accuracy of prediction, the intersection of RBPs from the two databases was selected for further study (Table S1). In addition, the role of these targets as splicing factors was confirmed by a literature review.
We downloaded the clinical information of 86 patients with OS from the TCGA database, divided them into high and low YBX1 expression groups according to the median YBX1 level, and analysed the effect of YBX1 level on the overall survival of the OS patients.
2.2. Human tissues
OS tissues and matched paraneoplastic tissues (over 5 cm away from the cut edge of the tumour tissue) were taken from 18 OS patients who received surgery at the Cancer Hospital of Harbin Medical University from July 2018 to August 2020 (Table S2). All tissues were kept in liquid nitrogen. The diagnosis was confirmed by clinical pathology. None of the patients received any treatment before surgery. Among them, 12 patients were male, and 6 patients were female. The age ranged from 11 to 34 years old, with an average age of 20.1 ± 5.4 years. The Ethics Committee of Harbin Medical University approved this study, and all patients provided their informed consent before participation.
2.3. Cell culture, construction of vectors and transfection
Human osteoblast-derived SaOS2 was obtained from the Chinese Academy of Medical Sciences. Both cell lines were cultured in Dulbecco's Modified Eagle Medium (DMEM, HyClone, Logan, UT, USA) supplemented with 10% foetal bovine serum (FBS, Invitrogen, Carlsbad, CA, USA), 100 U/ml penicillin and 100 μg/ml streptomycin (Life Technologies, Cergy Pontoise, France) at 37 °C in a humidified 5% CO2 incubator.
To construct stable YBX1 overexpression or knockdown cells, the coding sequence of YBX1 was amplified using PCR along with specific primers and then cloned into PLVX vectors, while the coding sequence of shRNA (GENEWIZ, Suzhou, China) was synthesized and then inserted into the PLKO vector. The sequences of VEGF165 and VEGF165b (GENEWIZ) were synthesized and inserted into the PLVX vector. The vectors along with packing vectors were transfected into 293T cells to obtain pseudotype lentiviral particles. Then, the lentiviral particles were used to infect SaOS2 cells for 12 h. Stable cells were cultured and selected with puromycin. All the primers and related sequences are shown in Table S3.
2.4. Real-time PCR
Total RNA was extracted from frozen tissues or cultured cells using TRIzol reagent (Invitrogen) and reverse transcribed into cDNA using Easy Script Reverse Transcriptase and random primers (TransGen, Beijing, China). SYBR Green PCR Mix (Bioresearcher, Beijing, China) along with specific primers was used to determine the levels of VEGF165 and VEGF165b by real-time PCR [11]. The GAPDH mRNA level was used as the internal control. The initial denaturation at 96 °C for 5 min was followed by 40 cycles of denaturation at 96 °C for 30 s, annealing at 55 °C for 30 s, and elongation at 72 °C for 60 s. All samples were measured in triplicate.
2.5. ELISA
Cloned cells were seeded into 96-well plates at a concentration of 5 × 103 cells/well for 48 h at 37 °C. VEGF165b and panVEGF165 in the cell culture supernatant were measured by ELISA kits (R&D Systems, Minneapolis, MN, USA, cat. no. DY3045 for VEGF165b and cat. no. DVE00 for VEGF165) according to the manufacturer's instructions. The absorbance at 450 nm was measured using an ELISA reader. The concentrations were calculated by a standard curve generated with specific standards provided by the manufacturer. Each sample was measured in triplicate.
2.6. Western blot
Total protein was extracted from tumour tissues or cells using RIPA lysis buffer (Beyotime, Nanjing, China) and quantified using a Bradford dye binding assay kit (Beyotime). A total of 20 μg protein per lane was separated by SDS‒PAGE and then electrophoretically transferred onto PVDF membranes (Millipore, Bedford, MA, USA). The membranes were blocked in 5% skimmed milk for 2 h at room temperature (RT) and then incubated with primary antibodies against VEGF165 (R&D Systems, cat. no. AF-293-NA), VEGF165b (R&D Systems, cat. no. MAB3045) and YBX1 (Abcam, USA, cat. no. ab76149) overnight at 4 °C. The membranes were then incubated with the appropriate HRP-conjugated secondary antibody for 2 h at RT. Immunoreactivity was visualized using an ECL detection kit (EMD Millipore).
2.7. MTT
A 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT, Beyotime) assay was used to evaluate cell viability. Cells were seeded into 96-well plates at a concentration of 3000 cells per well in triplicate. After incubation for 6 h (used to reflect the cell number inoculated at 0 h), 24 h, 48 h and 72 h, the cells were treated with 20 μL MTT reagent at 37 °C for 4 h. Then, the formazan was lysed using 100 μL DMSO (Beyotime) per well, and its production was measured at 492 nm using an ELISA plate reader.
2.8. 5-Ethynyl-2′-deoxyuridine (EdU) assay
The proliferative capability of cells was assessed by a BeyoClick™ EdU kit (Beyotime). In brief, cells were seeded in 24-well plates in which a cover glass had been placed in advance and cultured for 24 h. Then, cells were incubated with 10 μM EdU for 2 h at 37 °C and fixed in 4% formaldehyde for 15 min at RT. Triton X-100 (0.3%) was used to permeabilize the cells for 10 min. After that, the cells were incubated with Click Reaction Mixture (100 μL/well) for 30 min at RT, and DAPI was used to stain nuclei. The percentage of positive cells was calculated under a fluorescence microscope (Nikon, Japan) to reflect cell proliferation.
2.9. Wound healing assay
Cells were seeded into 6-well plates at a density of 2 × 106/well. When the cells reached 80% confluence, they were starved for 8 h. A scratch was made on the bottom of the well using a 200 μL pipette tip. After washing with PBS twice, the cells were cultured for 24 h in complete medium. Then, photographs were taken under a reverse microscope, and the wound healing rate was calculated by the width of the scratch at 0 h and 24 h.
2.10. Transwell assay
The migration and invasion capability of cells were evaluated using a Transwell assay. Briefly, 5 × 104 cells were resuspended in 500 μL serum-free DMEM and then plated onto Transwell chambers with or without Matrigel (Millipore). Then, the Transwells were transferred to a 24-well plate containing 600 μL DMEM with 10% FBS. After incubation for 24 h, cells on the upper side of the membranes were removed using cotton swabs. The migrated or invaded cells on the lower side of the membranes were fixed and stained with 0.1% crystal violet diluted in methyl alcohol. Cells in three high-power fields (HPFs) were counted, and the mean value was calculated to reflect the migration and invasion potential.
2.11. Xenograft model
A xenograft tumour formation assay was performed in 6-week-old female nude mice (BALB/c) that were obtained from the Shanghai Experimental Animal Research Center. Six million SaOS2 cells transfected with empty vector or target genes were suspended in 100 μL sterile PBS and then injected subcutaneously into the flanks of nude mice. The width and length of tumours were measured by a calliper, and the tumour volume was calculated as length × width × (length + width)/2. The mice were humanely sacrificed 28 days after transplantation, and the tumours were collected. The tumour tissues were fixed in 4% paraformaldehyde, followed by paraffin embedding.
2.12. Immunohistochemical staining
Sections (5 μm thick) were cut from paraffin-embedded tissues. After deparaffinization and hydration, the sections were boiled in citrate buffer (10 mM, pH 6.0) for 15 min to retrieve the antigens, treated with 3% H2O2 at RT for 15 min to block endogenous peroxidase and blocked with 5% bovine serum albumin at RT for 15 min to reduce nonspecific binding. The sections were incubated with primary antibodies against CD31 (Abcam, cat. no. ab182981) and Ki67 (Abcam, cat. no. ab16667) overnight at 4 °C. After incubation with a biotinylated secondary antibody for 30 min at 37 °C, DAB was used to visualize the immunoreactivity. Vessels positive for CD31 were counted in five different fields at a 200 × magnification. Ki67 was located in the cell nucleus, and the percentage of positive cells was calculated in five different fields at a 400 × magnification. The mean was used to reflect the blood vessel density or Ki67 level.
2.13. RNA immunoprecipitation (RIP) assay
RIP assays were performed to confirm the binding of YBX1 to VEGF mRNA as previously reported [15]. In brief, cells were treated with 3% formaldehyde, and the reaction was stopped by 0.125 M glycine. Cells were resuspended in RIPA buffer and incubated with YBX1 antibody (Abcam, cat. no. ab76149) or IgG (Cell Signalling Technology, MA, USA; cat. no. 2729s) with Dynabeads protein G (Invitrogen, cat. no. 10004D) overnight at 4 °C. Samples were washed and then treated with proteinase K. RNA was purified using phenol chloroform extraction. Then, real-time PCR was used to measure VEGF transcript levels. The primers are shown in Table S3.
2.14. Statistical analysis
Statistical data were analysed using GraphPad Prism 9. All data are expressed as mean ± SD. According to the results of normality test and homogeneity test of variance, the means of two groups were compared by Student's T-Test, Mann-Whitney test or Wilcoxon paired signed rank test. The results of MTT and xenograft models were analysed by two-way ANOVA. Spearman rank correlation method was used for correlation analysis. The median value separation model based on YBX1 expression was used, and the effect of YBX1 on overall survival was analysed using the Kaplan‒Meier method through the log-rank test. P < 0.05 indicated statistical significance.
3. Results
3.1. VEGF165b was downregulated in OS and inhibited the growth, migration and invasion of OS cells
Isoform-specific PCR was used to determine the levels of VEGF165 and VEGF165b in OS and paraneoplastic tissues. As shown in Fig. 1A, the level of VEGF165 was increased, while the level of VEGF165b was decreased in OS tissues compared to paraneoplastic tissues. To determine the function of the two isoforms, cells overexpressing VEGF165 or VEGF165b were constructed (Fig. 1B and C), and their proliferative, migratory, and invasive capabilities were evaluated by in vitro and in vivo assays. The results showed that VEGF165 overexpression promoted the proliferation of SaOS2 cells, whereas VEGF165b overexpression inhibited the proliferation of SaOS2 cells (Fig. 1D and E). The results of wound healing and transwell assays showed that VEGF165 overexpression led to a significant increase in the migration and invasion of SaOS2 cells, whereas VEGF165b overexpression caused a significant decrease in the migration and invasion of SaOS2 cells (Fig. 1F–I). The subcutaneous tumour model also indicated that VEGF165 promoted the growth of SaOS2 cells, whereas VEGF165b inhibited the growth of SaOS2 cells (Fig. 1J and K). The immunohistochemical staining results showed that overexpression of VEGF165 induced the formation of blood vessels and the expression of Ki67; however, overexpression of VEGF165b decreased the formation of blood vessels and the expression of Ki67 in subcutaneous tumours (Fig. 1L and M). All these findings indicated that VEGF165 was upregulated and acted as a tumour promoter and that VEGF165b was downregulated and acted as an antitumour factor in OS development.
Fig. 1.
VEGF165bwas downregulated and had an opposite effect as VEGF165in OS. A. Real-time PCR was used to determine the mRNA levels of VEGF165 and VEGF165b in OS tissues and matched normal tissues of 18 OS patients. B. Real-time PCR was used to identify the overexpression of VEGF165 and VEGF165b in the stably transfected cells. C. The overexpression of VEGF165 and VEGF165b was confirmed by Western blotting using specific antibodies in stably transfected cells. D and E. MTT and EdU assays and quantitative bar charts were used to show the different effects of VEGF165 and VEGF165b on SaOS2 cell proliferation. F. A wound healing assay was used to reflect the effect of VEGF165 and VEGF165b on SaOS2 cell migration. G. Summary bar graph illustrating the percentage wound closure at the indicated time points during the wound healing assay. H. Transwell assays were used to assess the migration and invasive capabilities of SaOS2 cells. I. Quantification of the transwell assay is shown as bar graphs based on the cell number per high power field. J. A xenograft tumour formation assay was used to assess the effect of VEGF165 and VEGF165b on SaOS2 cell proliferation in vivo. K. The xenograft tumour growth curve was plotted by the tumour volume measured every four days. L. Immunohistochemical staining was used to determine the expression of CD31 and Ki67 in xenograft tumours. M. The expression of CD31 and Ki67 was quantified and represented by a bar diagram.
3.2. YBX1 was identified as the key splicing factor determining the VEGF isoforms
To determine the key alternative splicing factor that controls the creation of VEGF165 and VEGF165b, two databases, MEME and RBPDB, were used to screen the RNA-binding proteins targeting the VEGF165 pre-mRNA. From the overlapping of two databases, four splicing factors were obtained, and their functions as splicing factors were confirmed through a literature search (Fig. 2A). We assessed the mRNA levels of four splicing factors in OS tissues and paraneoplastic tissues of 18 patients. The results indicated that YBX1 and PABPC1 were significantly elevated in OS tissues compared to normal tissues (Fig. 2B). The results of correlation analysis indicated that the YBX1 level was positively correlated with the VEGF165 level (Fig. 2C), but negatively correlated with VEGF165b (Fig. 2D). PABPC1 had no significant correlation with VEGF165 and VEGF165b (Fig. 2C and D). Thus, YBX1 might be the key splicing factor controlling the formation of VEGF165 and VEGF165b.
Fig. 2.
YBX1 was the underlying splicing factor regulating the formation of VEGF165isoforms. A. Four splicing factors were obtained by overlapping the RNA-binding proteins targeting VEGF165 in the MEME and RBPDB datasets. B. Real-time PCR was used to determine the expression of four splicing factors in OS tissues and matched normal tissues of 18 OS patients. C. The correlation between VEGF165 and four splicing factors was analysed in OS tissues of 18 patients based on the real-time PCR results. D. The correlation between VEGF165b and four splicing factors was analysed in OS tissues of 18 patients based on the real-time PCR results.
3.3. YBX1 regulated the production of VEGF165 isoforms in OS
We constructed YBX1 overexpression or knockdown cells (Fig. 3A and B). Overexpression of YBX1 increased the expression of VEGF165 and decreased the expression of VEGF165b at both the mRNA and protein levels, whereas knockdown of YBX1 decreased the expression of VEGF165 and increased the expression of VEGF165b (Fig. 3A and B). RIP assays indicated that YBX1 could bind to VEGF mRNA (Fig. 3C). The ELISA results showed that overexpression of YBX1 increased the level of VEGF165 and decreased the level of VEGF165b in the supernatant of SaOS2 cells, while knockdown of YBX1 had the opposite effect (Fig. 3D). These results suggested that YBX1 was a key splicing factor that increased VEGF165 while decreasing VEGF165b.
Fig. 3.
YBX1 upregulated VEGF165but downregulated VEGF165bin OS cells. A and B. Real-time PCR and Western blotting were used to verify the overexpression or knockdown of YBX1 in SaOS2 cells. The levels of VEGF165 and VEGF165b were also determined in those cells to confirm the effect of YBX1 on VEGF isoforms. C. A RIP assay was used to confirm the direct binding of YBX1 to VEGF mRNA. D. VEGF165 and VEGF165b levels in the culture supernatant of SaOS2 cells with YBX1 overexpression or knockdown were determined by ELISA.
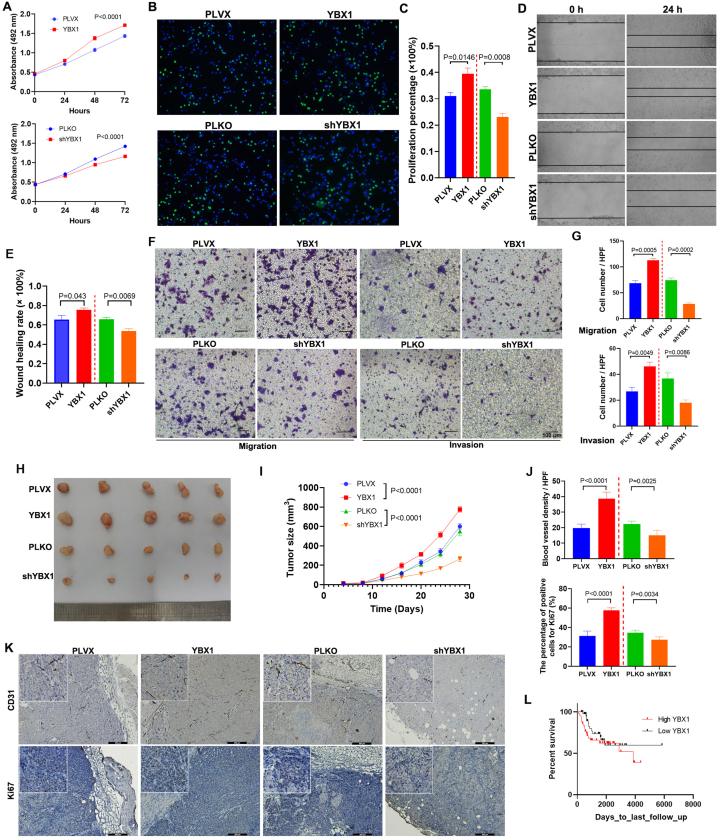
3.4. YBX1 promoted the growth, migration and invasion of SaOS2 cells
YBX1 increased VEGF165 but reduced VEGF165b; thus, we predicted that YBX1 might promote the malignant phenotype of OS by altering the ratio of VEGF165 to VEGF165b. Thus, the effect of YBX1 on SaOS2 cells was assessed by in vivo and in vitro assays. The results of MTT and EdU assays indicated that YBX1 overexpression promoted the proliferation of SaOS2 cells (Fig. 4A–C), which was consistent with the phenotype resulting from the upregulation of VEGF165 but contrary to the upregulation of VEGF165b. However, YBX1 knockdown inhibited the proliferation of SaOS2 cells (Fig. 4A–C), which was consistent with the phenotype resulting from the upregulation of VEGF165b but contrary to the upregulation of VEGF165. The migration and invasion capabilities were also elevated in YBX1-overexpressing SaOS2 cells but decreased in YBX1-knockdown SaOS2 cells (Fig. 4D–G). The subcutaneous tumour formation assay indicated that YBX1-overexpressing cells grew faster than control cells, whereas YBX1-knockdown cells grew slower than control cells in nude mice (Fig. 4H and I). Immunohistochemical staining of subcutaneous tumours revealed that YBX1 overexpression increased the number of blood vessels, whereas YBX1 knockdown decreased the number of blood vessels in the tumours (Fig. 4J and K). Ki67 expression increased in YBX1-overexpressing tumour cells but decreased in YBX1-knockdown tumour cells in nude mice (Fig. 4J and K). Additionally, we analysed the association between the level of YBX1 mRNA and overall survival of OS patients using the TCGA dataset. Although not statistically significant, there was a trend that OS patients with higher YBX1 expression had a poor prognosis within five years of follow-up, but this difference disappeared in a longer follow-up (Fig. 4L). These results showed that YBX1 acted as an oncogene in OS development at least partly by producing more VEGF165 and reducing VEGF165b as a splicing factor.
Fig. 4.
YBX1 promoted the proliferation, migration, and invasion of OS cells. A-C. MTT and EdU assays and quantitative bar charts were used to show the effect of YBX1 on SaOS2 cell proliferation. D. A wound healing assay was used to reflect the effect of YBX1 on SaOS2 cell migration. E. The quantitative bar diagram is for the wound healing assay, based on the percentage wound closure at the indicated time points. F. Transwell assays were used to assess the migratory and invasive capabilities of YBX1-overexpressing or YBX1-knockdown SaOS2 cells. G. Quantification of the transwell assay is shown as bar graphs based on the cell number per high power field. H. A xenograft tumour formation assay was used to determine the effect of YBX1 overexpression or knockdown on SaOS2 cell proliferation in vivo. I. Tumour volume was measured every 4 days, and a growth curve was plotted. J. The expression levels of CD31 and Ki67 in xenograft tumours as determined by immunohistochemical staining are shown in the bar diagram. K. Representative images of the immunohistochemical assays for CD31 and Ki67. L. The overall survival of OS patients was analysed using the TCGA database based on the YBX1 level.
4. Discussion
Alternative splicing creates different VEGF mRNA coding sequences by rearranging the pattern of intron and exon elements, resulting in the formation of different VEGF isoforms with the same or different functions [16]. Conventional VEGF isoforms, termed VEGFxxx (xxx is the amino acid number), have been described as oncogenic cytokines, promoting not only angiogenesis but also proliferation and metastasis of tumour cells [17]. However, the alternative isoforms of VEGFxxx are antiangiogenic and even antineoplastic, which have been termed VEGFxxxb. VEGF165b is the first member of the VEGFxxxb family and one of the most widely studied isoforms [11], and other members have also been identified, including VEGF121b [18], VEGF189b [19] and VEGF111b [20].
Here, we found that VEGF165b was downregulated in OS tissues and functioned as an antitumour cytokine. Similar findings have also been indicated in other types of malignancy. Bates et al. [11] first cloned the transcript of VEGF165b and demonstrated that VEGF165b plays an antiangiogenic role in renal cancer by inhibiting VEGF165-mediated endothelial cell proliferation and migration. Woolard et al. [12] confirmed the inhibitory effect of VEGF165b on angiogenesis and tumour growth in prostate cancer and indicated that VEGF165b can bind VEGF receptor 2 but not activate the downstream signalling pathways. In vivo studies also showed that VEGF165b overexpression inhibits the growth of prostate carcinoma, Ewing's sarcoma, renal cell carcinoma (RCC), and neuroblastoma in xenografted mouse tumour models [14,21]. The balance between VEGF165 and VEGF165b is an important factor in regulating the growth of colorectal cancer and the sensitivity of colon cancer to bevacizumab [22,23]. However, some researchers have suggested that VEGF165b is upregulated and weakly angiogenic in breast cancer [24]. Boudria et al. reported that VEGF165b promoted the proliferation and invasion of lung cancer cells, and the application of bevacizumab, an antiangiogenic compound, increased the expression of VEGF165b [25]. The role of VEGF in tumours remains controversial.
We tried to find the crucial splicing factor that endogenously controls the levels of VEGF165 and VEGF165b. Among four splicing factors obtained by the overlapping of two datasets, YBX1 mRNA showed a relatively higher expression in OS. The mutation frequency of YBX1 was very low in pancancer according to the IntOGen database. YBX1 upregulation in OS has been indicated by several studies [26,27]. Moreover, it has been indicated that YBX1 upregulates the level of VEGF in both epithelial cells and gastric carcinoma cells [28]. Some noncoding RNAs can positively regulate angiogenesis by binding YBX1 [29,30]. Additionally, YBX1 promoted tumour metastasis by inducing hypoxia-inducible factor 1α (HIF1α) expression in sarcoma cells [31]. YBX1 also promoted the invasion, metastasis and sunitinib resistance of clear cell RCC by regulating the EphA2 signalling pathway [32]. Thus, we explored the regulatory function of YBX1 on the production of VEGF165 isoforms. Our results first indicated that YBX1 increased VEGF165 and reduced VEGF165b in OS. Moreover, YBX1 promoted the development of OS, which was consistent with the effect of increased VEGF165 and contrary to the effect of increased VEGF165b in OS. Zhou et al. [33] assessed YBX1 expression in OS using immunohistochemical staining and found that OS patients with high YBX1 expression generally had a poor prognosis. Here, we showed a trend that YBX1 mRNA levels were associated with a poor prognosis within the first five years of follow-up.
Recent studies have also provided evidence that the dysregulation of splicing factors can alter the production of VEGF isoforms in some cancers. A switch in the expression of VEGF165 towards VEGF165b was observed in PC-3 cells with serine-arginine protein kinase 1 (SRPK1) knockdown [34]. SRPK1 can inhibit the phosphorylation of the prototypic serine-arginine protein SRSF1 and then switch VEGF splicing from the proangiogenic to antiangiogenic isoform [35]. Placental growth factor (PlGF) increases the ratio of VEGF165 to VEGF165b by inducing the expression of the splicing regulatory factor SRp40 in NSCLC [36]. Given these findings, targeting splicing factors to regulate alternative splicing is a promising approach to treat cancers. Some researchers have also used splice-switching oligonucleotides (SSOs) or CRISPR technology to interfere with the splicing process, preventing the production of a truncated or mutated protein or obtaining the preferential formation of a specific protein isoform [37].
In conclusion, this study found that VEGF165b was an antitumour factor and downregulated in OS. YBX1 could regulate the production of VEGF isoforms, increasing VEGF165 and decreasing VEGF165b in OS. These findings indicated that targeting YBX1 might endogenously alter the levels of VEGF165 and VEGF165b simultaneously. This study verified that VEGF165 was increased while VEGF165b was decreased in OS tissues. Moreover, we determined the association between YBX1 and VEGF isoforms. However, this study only used the SaOS cell line and not primary sarcoma cells. In addition, we did not explore the specific mechanism by which YBX1 regulates the alternative splicing of VEGF. In the future, we will further investigate the relevant mechanism, which will be of great significance for targeting YBX1 to improve the prognosis of OS patients.
Ethics approval and consent to participate
This study was approved by the Harbin Medical University Institutional Ethics Committee. The ethics approval number is 2021062. The procedures are in accordance with the Helsinki Declaration of 1975. Written informed consent was obtained from all participants.
Author contribution statement
Bingxuan Quan: Conceived and designed the experiments; Performed the experiments; Analysed and interpreted the data; Wrote the paper. Yansong Wang: Conceived and designed the experiments. Zhigang Li; Xiuchun Yan: Performed the experiments. Hongbo Yang: Analysed and interpreted the data. Shuo Li: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e18706.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Yu X.W., Wu T.Y., Yi X., Ren W.P., Zhou Z.B., Sun Y.Q., Zhang C.Q. Prognostic significance of VEGF expression in osteosarcoma: a meta-analysis. Tumour Biol. 2014;35(1):155–160. doi: 10.1007/s13277-013-1019-1. [DOI] [PubMed] [Google Scholar]
- 2.Yang J., Yang D., Sun Y., Sun B., Wang G., Trent J.C., Araujo D.M., Chen K., Zhang W. Genetic amplification of the vascular endothelial growth factor (VEGF) pathway genes, including VEGFA, in human osteosarcoma. Cancer. 2011;117(21):4925–4938. doi: 10.1002/cncr.26116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohba T., Cates J.M., Cole H.A., Slosky D.A., Haro H., Ando T., Schwartz H.S., Schoenecker J.G. Autocrine VEGF/VEGFR1 signaling in a subpopulation of cells associates with aggressive osteosarcoma. Mol. Cancer Res. 2014;12(8):1100–1111. doi: 10.1158/1541-7786.MCR-14-0037. [DOI] [PubMed] [Google Scholar]
- 4.Liang C., Li F., Wang L., Zhang Z.K., Wang C., He B., Li J., Chen Z., Shaikh A.B., Liu J., et al. Tumor cell-targeted delivery of CRISPR/Cas9 by aptamer-functionalized lipopolymer for therapeutic genome editing of VEGFA in osteosarcoma. Biomaterials. 2017;147:68–85. doi: 10.1016/j.biomaterials.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka T., Yui Y., Naka N., Wakamatsu T., Yoshioka K., Araki N., Yoshikawa H., Itoh K. Dynamic analysis of lung metastasis by mouse osteosarcoma LM8: VEGF is a candidate for anti-metastasis therapy. Clin. Exp. Metastasis. 2013;30(4):369–379. doi: 10.1007/s10585-012-9543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu J., Ji Z., Li D., Dong Q. Proliferation inhibition and apoptosis promotion by dual silencing of VEGF and Survivin in human osteosarcoma. Acta Biochim. Biophys. Sin. 2019;51(1):59–67. doi: 10.1093/abbs/gmy146. [DOI] [PubMed] [Google Scholar]
- 7.Assi T., Watson S., Samra B., Rassy E., Le Cesne A., Italiano A., Mir O. Targeting the VEGF pathway in osteosarcoma. Cells. 2021;10(5) doi: 10.3390/cells10051240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qu J.T., Wang M., He H.L., Tang Y., Ye X.J. The prognostic value of elevated vascular endothelial growth factor in patients with osteosarcoma: a meta-analysis and systemic review. J. Cancer Res. Clin. Oncol. 2012;138(5):819–825. doi: 10.1007/s00432-012-1149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho Q.T., Kuo C.J. Vascular endothelial growth factor: biology and therapeutic applications. Int. J. Biochem. Cell Biol. 2007;39(7–8):1349–1357. doi: 10.1016/j.biocel.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrara N., Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997;18(1):4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 11.Bates D.O., Cui T.G., Doughty J.M., Winkler M., Sugiono M., Shields J.D., Peat D., Gillatt D., Harper S.J. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62(14):4123–4131. [PubMed] [Google Scholar]
- 12.Woolard J., Wang W.Y., Bevan H.S., Qiu Y., Morbidelli L., Pritchard-Jones R.O., Cui T.G., Sugiono M., Waine E., Perrin R., et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64(21):7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- 13.Pritchard-Jones R.O., Dunn D.B., Qiu Y., Varey A.H., Orlando A., Rigby H., Harper S.J., Bates D.O. Expression of VEGF(xxx)b, the inhibitory isoforms of VEGF, in malignant melanoma. Br. J. Cancer. 2007;97(2):223–230. doi: 10.1038/sj.bjc.6603839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rennel E., Waine E., Guan H., Schuler Y., Leenders W., Woolard J., Sugiono M., Gillatt D., Kleinerman E., Bates D., et al. The endogenous anti-angiogenic VEGF isoform, VEGF165b inhibits human tumour growth in mice. Br. J. Cancer. 2008;98(7):1250–1257. doi: 10.1038/sj.bjc.6604309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai M.C., Manor O., Wan Y., Mosammaparast N., Wang J.K., Lan F., Shi Y., Segal E., Chang H.Y. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329(5992):689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowler E., Oltean S. Alternative splicing in angiogenesis. Int. J. Mol. Sci. 2019;20(9) doi: 10.3390/ijms20092067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rapisarda A., Melillo G. Role of the VEGF/VEGFR axis in cancer biology and therapy. Adv. Cancer Res. 2012;114:237–267. doi: 10.1016/B978-0-12-386503-8.00006-5. [DOI] [PubMed] [Google Scholar]
- 18.Rennel E.S., Varey A.H., Churchill A.J., Wheatley E.R., Stewart L., Mather S., Bates D.O., Harper S.J. VEGF(121)b, a new member of the VEGF(xxx)b family of VEGF-A splice isoforms, inhibits neovascularisation and tumour growth in vivo. Br. J. Cancer. 2009;101(7):1183–1193. doi: 10.1038/sj.bjc.6605249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller-Kasprzak E., Jagodzinski P.P. 5-Aza-2'-deoxycytidine increases the expression of anti-angiogenic vascular endothelial growth factor 189b variant in human lung microvascular endothelial cells. Biomed. Pharmacother. 2008;62(3):158–163. doi: 10.1016/j.biopha.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Gu F., Li X., Kong J., Pan B., Sun M., Zheng L., Yao Y. VEGF111b, a new member of VEGFxxxb isoforms and induced by mitomycin C, inhibits angiogenesis. Biochem. Biophys. Res. Commun. 2013;441(1):18–24. doi: 10.1016/j.bbrc.2013.09.144. [DOI] [PubMed] [Google Scholar]
- 21.Peiris-Pages M., Harper S.J., Bates D.O., Ramani P. Balance of pro- versus anti-angiogenic splice isoforms of vascular endothelial growth factor as a regulator of neuroblastoma growth. J. Pathol. 2010;222(2):138–147. doi: 10.1002/path.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varey A.H., Rennel E.S., Qiu Y., Bevan H.S., Perrin R.M., Raffy S., Dixon A.R., Paraskeva C., Zaccheo O., Hassan A.B., et al. VEGF 165 b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br. J. Cancer. 2008;98(8):1366–1379. doi: 10.1038/sj.bjc.6604308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bates D.O., Catalano P.J., Symonds K.E., Varey A.H., Ramani P., O'Dwyer P.J., Giantonio B.J., Meropol N.J., Benson A.B., Harper S.J. Association between VEGF splice isoforms and progression-free survival in metastatic colorectal cancer patients treated with bevacizumab. Clin. Cancer Res. 2012;18(22):6384–6391. doi: 10.1158/1078-0432.CCR-12-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catena R., Larzabal L., Larrayoz M., Molina E., Hermida J., Agorreta J., Montes R., Pio R., Montuenga L.M., Calvo A. VEGF(1)(2)(1)b and VEGF(1)(6)(5)b are weakly angiogenic isoforms of VEGF-A. Mol. Cancer. 2010;9:320. doi: 10.1186/1476-4598-9-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boudria A., Abou Faycal C., Jia T., Gout S., Keramidas M., Didier C., Lemaitre N., Manet S., Coll J.L., Toffart A.C., et al. VEGF165b, a splice variant of VEGF-A, promotes lung tumor progression and escape from anti-angiogenic therapies through a beta1 integrin/VEGFR autocrine loop. Oncogene. 2019;38(7):1050–1066. doi: 10.1038/s41388-018-0486-7. [DOI] [PubMed] [Google Scholar]
- 26.Xu M., Jin H., Xu C.X., Sun B., Song Z.G., Bi W.Z., Wang Y. miR-382 inhibits osteosarcoma metastasis and relapse by targeting Y box-binding protein 1. Mol. Ther. 2015;23(1):89–98. doi: 10.1038/mt.2014.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L., Wang P., Su X., Zhao B. Circ_0001658 promotes the proliferation and metastasis of osteosarcoma cells via regulating miR-382-5p/YB-1 axis. Cell Biochem. Funct. 2020;38(1):77–86. doi: 10.1002/cbf.3452. [DOI] [PubMed] [Google Scholar]
- 28.Xue X., Huang J., Yu K., Chen X., He Y., Qi D., Wu Y. YB-1 transferred by gastric cancer exosomes promotes angiogenesis via enhancing the expression of angiogenic factors in vascular endothelial cells. BMC Cancer. 2020;20(1):996. doi: 10.1186/s12885-020-07509-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong X., Li J., Li Y., Duan W., Qi Q., Wang T., Yang Q., Du L., Mao H., Wang C. A novel long non-coding RNA AC073352.1 promotes metastasis and angiogenesis via interacting with YBX1 in breast cancer. Cell Death Dis. 2021;12(7):670. doi: 10.1038/s41419-021-03943-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pham T.P., Bink D.I., Stanicek L., van Bergen A., van Leeuwen E., Tran Y., Matic L., Hedin U., Wittig I., Dimmeler S., et al. Long non-coding RNA aerrie controls DNA damage repair via YBX1 to maintain endothelial cell function. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.619079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Naggar A.M., Veinotte C.J., Cheng H., Grunewald T.G., Negri G.L., Somasekharan S.P., Corkery D.P., Tirode F., Mathers J., Khan D., et al. Translational activation of HIF1alpha by YB-1 promotes sarcoma metastasis. Cancer Cell. 2015;27(5):682–697. doi: 10.1016/j.ccell.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Ruan H., Li S., Bao L., Zhang X. Enhanced YB1/EphA2 axis signaling promotes acquired resistance to sunitinib and metastatic potential in renal cell carcinoma. Oncogene. 2020;39(38):6113–6128. doi: 10.1038/s41388-020-01409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou W.P., Liu X., Yang Y., Liu Y.F. The influence and regulatory mechanism of Y-box binding protein 1 in osteosarcoma and its significance. J. Biol. Regul. Homeost. Agents. 2015;29(2):485–491. [PubMed] [Google Scholar]
- 34.Mavrou A., Brakspear K., Hamdollah-Zadeh M., Damodaran G., Babaei-Jadidi R., Oxley J., Gillatt D.A., Ladomery M.R., Harper S.J., Bates D.O., et al. Serine-arginine protein kinase 1 (SRPK1) inhibition as a potential novel targeted therapeutic strategy in prostate cancer. Oncogene. 2015;34(33):4311–4319. doi: 10.1038/onc.2014.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Q., Zeng C., Liu H., Yung K.W.Y., Chen C., Xie Q., Zhang Y., Wan S.W.C., Mak B.S.W., Xia J., et al. Protein-protein interaction inhibitor of SRPKs alters the splicing isoforms of VEGF and inhibits angiogenesis. iScience. 2021;24(5) doi: 10.1016/j.isci.2021.102423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z., Liu T. Placental growth factor signaling regulates isoform splicing of vascular endothelial growth factor A in the control of lung cancer cell metastasis. Mol. Cell. Biochem. 2018;439(1–2):163–169. doi: 10.1007/s11010-017-3145-3. [DOI] [PubMed] [Google Scholar]
- 37.Jbara A., Siegfried Z., Karni R. Splice-switching as cancer therapy. Curr. Opin. Pharmacol. 2021;59:140–148. doi: 10.1016/j.coph.2021.05.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.