Abstract
Aims
To assess the trends in calcific aortic valve disease (CAVD) epidemiology, with an emphasis on CAVD mortality, leading risk factors, and their associations with age, period, and birth cohort.
Methods and results
Prevalence, disability-adjusted life years, and mortality were derived from the Global Burden of Disease Study 2019. The age–period–cohort model was employed to study the detailed trends of CAVD mortality and its leading risk factors. Globally, CAVD showed unsatisfactory results from 1990 to 2019, with the CAVD deaths of 127 000 in 2019. CAVD mortality was substantially reduced in high socio-demographic index (SDI) countries [−1.45%, 95% confidence interval (CI) (−1.61 to −1.30)], mildly increased in high-middle SDI countries [0.22%, 95% CI (0.06–0.37)], and unchanged in other SDI quintiles. There was a noticeable transition in CAVD deaths from younger to older populations globally. The CAVD mortality increased exponentially with age, and the male had higher mortality than the female before 80 years old. Favourable period [0.69, 95% CI (0.66–0.72)] and birth effects [0.30, 95% CI (0.22–0.43)] were mainly observed in high SDI countries, while unfavourable effects were mostly noticed in high-middle SDI countries. High systolic blood pressure was the leading risk factor of CAVD deaths globally, and it showed favourable trends in high SDI regions.
Conclusion
Although CAVD mortality reduction was observed globally, unfavourable period, and cohort effects were found in many countries. Increase of mortality rate among the population ≥85 years was the common challenge across all SDI quintiles, stressing the necessity to further improve health care for CAVD patients worldwide.
Keywords: Calcific aortic valve disease, Mortality, Age–period–cohort, Risk factor
Introduction
Valvular heart diseases (VHDs) are caused by structural or functional abnormalities, leading to arrhythmia, heart failure, and even death.1 VHDs are categorized as rheumatic heart diseases (RHDs) and non-rheumatic heart diseases (NRHD). The latter is further classified into calcific aortic valve disease (CAVD), degenerative mitral valve disease (DMVD), and others.2 Published epidemiologic studies suggest that VHDs have caused a heavy burden on several countries3–5 and pose an increasingly great threat to public health with the global population ageing. Thus, they have been described as ‘the next cardiac epidemic’.6
There have been country-dependent changes in the prevalence of different VHD categories in the past decades, with NRHD and RHD being dominant in developed and developing countries, respectively.7 Notably, CAVD is the most common VHD in Western countries. It is estimated to affect more people worldwide; however, it has no approved medical therapy.8 Hence, determining the epidemiological trends of CAVD is necessary because it can aid in adjusting future healthcare budgets and policies and identifying treatment gaps. The CAVD burden and risk factors are not only affected by physiological age9 (age effect) but also by related health policies or technological advances10 (period effect) and early diagnosis or treatment11 (cohort effect). Although published literature briefly describes the current situation and changing landscape, it neglects age, period, and birth cohort disparities.12–15 Therefore, this study aimed to comprehensively describe the temporal trends of disease burden and CAVD risk factors by (1) collecting disease data from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019, which covers all available population-level data; and (2) analysing the temporal trends of disease burden and leading CAVD risk factors in depth using an age–period–cohort (APC) model, which is necessary to uncover the independent effects of age, period, and birth cohort.
Methods
Overview
The GBD is a publicly available dataset, and information can be extracted from it using the Global Health Data Exchange query tool (http://ghdx.healthdata.org/gbd-results-tool). The GBD 2019 provides a comprehensive and updated estimation of the descriptive epidemiological data of 369 diseases and injuries and 87 risk factors. Additionally, it contains comparative statistics from 1990 to 2019, including 204 countries and territories worldwide. Detailed GBD information on the study design and method has been fully introduced previously.16,17 VHDs were mapped on the GBD cause list based on the International Classification of Diseases and Injuries 10th Revision (ICD-10). Additionally, the prevalence rate, disability-adjusted life years (DALYs) rate, mortality, all-age rate, age-standardized rate (ASR per 100 000 population), and risk factors were analysed using online public data.
Data sources
Following the ICD-10 codes, data related to RHD, NRHD, CAVD, DMVD, and other NRHD were mapped to the GBD cause list with I01–I09.9, I34–I37.9, I35–I35.9, I34–I34.9, and I36–I37.9, respectively.18 The epidemiological quantity of interest includes the number or rate of prevalence/DALYs/deaths across age groups, males, females, both sexes combined, and in 204 countries and territories.9 Additionally, all estimates from the GBD are presented with 95% uncertainty intervals (UIs). The 95% UIs are based on the 25th and 975th ordered values of 1000 draws of the posterior distribution.9
Risk factors attributable to CAVD mortality
The 87 risk factors included in the GBD 2019 are broadly categorized into three groups: environmental and occupational, behavioural, and metabolic. The GBD risk factor estimation approach is based on a comparative risk assessment framework and involves six steps, which are available in the GBD 2019 risk factor summary paper.10 We assessed the percentage contribution of the top three risk factors attributable to CAVD deaths in 1990 and 2019 and their percentage change in the all-age/age-standardized mortality rate from 1990 to 2019.
Socio-demographic index and geographic regions
The socio-demographic index (SDI) is measured using the average income per person, average educational attainment (age ≥15 years), and total fertility rate (age <25 years). It is a comprehensive indicator of a country or region's development status.17 The SDI ranges from 0 to 1, with a higher figure supporting a more advanced socioeconomic status. According to the 2019 SDI values, countries or territories are divided into five categories: high, high-middle, middle, low-middle, and low SDI. Furthermore, the 204 countries and territories globally are grouped into 21 regions based on geographical areas.
World Bank's classification of countries by income
Based on the gross national per capita income, as classified by the World Bank, countries were classified into four levels, namely low-income, lower-middle-income, upper-middle-income, and higher-income countries.19
Analysis of overall temporal trends in valvular heart disease burden
Temporal trends in prevalence, DALYs, and mortality of all VHDs were assessed using all-age rate and ASR. Additionally, temporal trends were assessed during the CAVD analysis using all-age, age-standardized, and annual change rates. Age-standardized and annual change rates were calculated based on age-standard population data from the GBD 2019. Furthermore, the trends in CAVD burden across six age groups (<40, 40–49, 50–59, 60–69, 70–79, and ≥80 years) were explored.
Statistical analysis
An APC model was used to analyse the temporal trends of CAVD prevalence/DALYs/mortality rate because of its advantages over traditional epidemiological analyses, as we have previously described.20 Freely available R tools were implemented to construct the APC model according to the details described in the literature.21
The APC model was constructed using input data, including the GBD 2019 estimates of CAVD prevalence/DALYs/deaths number and population data for each region or country. Supplementary material online, Table S1 shows the details of the input data, including 12 age groups (from 40–44 to 95−99 with 5-year age group intervals) and 17 partially overlapping 10-year birth cohorts [from1891–1899 (the 1895 cohort) to 1971–1979 (the 1975 cohort)]. The APC model's outputs included (1) net drift (representing the overall temporal trend, expressed as the annual percentage change of prevalence/DALYs/death rate), (2) local drift (representing the temporal trend within each age group), (3) age effect (fitted longitudinal age-specific rates in the reference cohort adjusted for period deviations), (4) period effect (relative risk of each period compared with the reference period), and (5) cohort effect (relative risk of each cohort compared with the reference cohort). The choice of the reference period (cohort) was arbitrary; hence, it did not affect the interpretation of results. The 1990–1994 period and 1921–1929 cohort were set as the reference period and cohort in this study, respectively. All statistical analyses were performed using R (version 4.1.0), and P-values <0.05 were considered statistically significant.
Role of the funding source
The funders of this study had no role in the study design, data collection/analysis/interpretation, or manuscript writing. S.S., Y.Y., B.S., and J.S. had access to the GBD 2019 public datasets. All the authors have agreed to submit this manuscript for publication.
Results
Global and regional burden trends in CAVD from 1990 to 2019
Burden trends of different types of VHD from 1990 to 2019 are shown in Supplementary material online, Figures S1–S6, indicating CAVD should be a great concern at present and in the future. We performed an in-depth analysis using both traditional parameters and APC models in this study. In 2019, the population aged <40 years accounted for only 6.90, 2.88, and 1.70% of the global prevalence, DALYs, and deaths in CAVD, respectively (Supplementary material online, Figure S7). Hence, only those aged ≥40 years (40–45 to 95–99) were enrolled to construct the APC models.
Table 1 shows the statistical data of CAVD globally and in 21 regions, including prevalent cases, DALYs, death numbers, and their relative all-age rates and ASRs in 2019. Moreover, the net drift from 1990 to 2019 was also shown as a direct demonstration of CAVD changes. Figure 1 and Supplementary material online, Figures S8–S12 display the all-age rates and ASRs of prevalence/DALYs/deaths in 2019 among countries and their net drifts from 1990 to 2019. Globally, the all-age prevalence, DALYs, and mortality rates (per 100 000) of CAVD in 2019 were 121.54, 23.75, and 1.64, and after age standardization, figures were changed to 116.34, 23.90, and 1.76, respectively. CAVD prevalence in the past 30 years has increased {net drift = 3.69%, [95% confidence interval (CI): 3.52–3.87], equal to an 86% increase from 1990 to 2019}, followed by a slight decline in the DALYs [−0.70%, (−0.76 to −0.64), 18% reduction] and death [−0.83%, (−0.76 to −0.64), 21% reduction].
Table 1.
Prevalent cases, deaths, and disability-adjusted life years (DALYs) for CAVD disease in 2019, and net drift in age standardized rates (ASRs) per 100, 000, by Global Burden of Disease region, from 1990 to 2019
| Prevalence | DALYs | Deaths | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regions | No, in thousands (95% UI), 2019 | All-age rate per 100 000 (95% UI), 2019 | ASRs per 100 000 (95% UI), 2019 | Net drift (95% CI), % | No, in thousands (95% UI), 2019 | All-age rate per 100 000 (95% UI), 2019 | ASRs per 100 000 (95% UI), 2019 | Net drift (95% CI), % | No, in thousands (95% UI), 2019 | All-age rate per 100 000 (95% UI), 2019 | ASRs per 100 000 (95% UI), 2019 | Net drift (95% CI), % |
| Global | 9404.08 (8079.6, 10 889.73) | 121.54 (104.42, 140.74) | 116.34 (100.39, 134.5) | 3.69 (3.52, 3.87) | 1837.75 (1637.02, 2031.85) | 23.75 (21.16, 26.26) | 23.90 (21.10, 26.55) | −0.70 (−0.76, −0.64) | 126.83 (105.6, 141.39) | 1.64 (1.36, 1.83) | 1.76 (1.45, 1.97) | −0.83 (−0.91, −0.74) |
| High-income Asia Pacific | 1715.7 (1450.88, 2042.13) | 916.06 (774.67, 1090.35) | 408.4 (348.93, 479.89) | 1.66 (1.57, 1.75) | 142.13 (109.60, 165.94) | 75.89 (58.52, 88.6) | 24.46 (19.89, 28.22) | −2.10 (−2.22, −1.98) | 13.57 (9.08, 16.51) | 7.25 (4.85, 8.81) | 1.98 (1.40, 2.36) | −2.51 (−2.76, −2.26) |
| High-income North America | 1492.89 (1305.2, 1727.5) | 409.50 (358.02, 473.86) | 244.39 (214.46, 279.6) | 1.07 (0.93, 1.21) | 314.81 (274.62, 346.35) | 86.35 (75.33, 95.01) | 46.80 (41.53, 51.25) | −1.58 (−1.69, −1.48) | 27.19 (22.16, 30.24) | 7.46 (6.08, 8.29) | 3.64 (3.02, 4.02) | −1.62 (−1.78, −1.46) |
| Western Europe | 1862.79 (1577.56, 2209.09) | 426.94 (361.57, 506.31) | 204.84 (174.62, 240.59) | 6.58 (6.33, 6.82) | 543.78 (471.46, 598.09) | 124.63 (108.06, 137.08) | 51.94 (45.69, 56.95) | −0.83 (−0.96, −0.70) | 47.89 (39.52, 53.66) | 10.98 (9.06, 12.3) | 4.05 (3.40, 4.51) | −0.95 (−1.17, −0.72) |
| Australasia | 320.82 (272.23, 381.06) | 1103.86 (936.68, 1311.11) | 649.5 (552, 772.74) | 10.12 (9.85, 10.38) | 23.97 (20.44, 27.5) | 82.49 (70.34, 94.62) | 44.54 (38.48, 51.02) | −1.12 (−1.29, −0.96) | 1.87 (1.51, 2.11) | 6.42 (5.21, 7.25) | 3.18 (2.62, 3.58) | −1.66 (−2.16, −1.16) |
| Andean Latin America | 33.35 (28.25, 39.02) | 52.44 (44.42, 61.35) | 59.04 (50.07, 69.05) | 8.76 (8.37, 9.16) | 7.26 (5.88, 8.87) | 11.42 (9.24, 13.95) | 12.54 (10.21, 15.28) | −0.11 (−0.35, 0.13) | 0.29 (0.24, 0.35) | 0.46 (0.37, 0.56) | 0.53 (0.43, 0.64) | −0.33 (−1.16, 0.50) |
| Tropical Latin America | 58.6 (47.23, 72.05) | 26.21 (21.12, 32.23) | 23.73 (19.16, 29.01) | 3.67 (3.44, 3.90) | 70.73 (63.57, 80.92) | 31.63 (28.43, 36.19) | 29.50 (26.5, 33.74) | −0.64 (−0.74, −0.54) | 3.58 (3.11, 4.14) | 1.60 (1.39, 1.85) | 1.56 (1.34, 1.80) | −0.65 (−0.88, −0.42) |
| Central Latin America | 76.96 (63.86, 91.38) | 30.78 (25.54, 36.55) | 31.82 (26.45, 37.75) | 6.55 (6.29, 6.81) | 44.87 (36.84, 55.56) | 17.95 (14.74, 22.22) | 18.72 (15.40, 23.13) | 0.22 (0.11, 0.32) | 1.96 (1.61, 2.43) | 0.78 (0.64, 0.97) | 0.85 (0.70, 1.06) | 0.14 (−0.20, 0.49) |
| Southern Latin America | 101.89 (87.12, 120.8) | 152.64 (130.52, 180.96) | 122.72 (104.78, 145.67) | 7.39 (7.23, 7.55) | 42.48 (38.29, 46.44) | 63.64 (57.36, 69.57) | 50.54 (45.69, 55.27) | −0.41 (−0.49, −0.33) | 2.84 (2.46, 3.16) | 4.26 (3.68, 4.73) | 3.3 (2.85, 3.66) | −0.49 (−0.78, −0.20) |
| Caribbean | 45.47 (37.69, 54.85) | 96.40 (79.92, 116.29) | 87.51 (72.62, 105.56) | 7.32 (6.95, 7.70) | 11.32 (9.16, 14.16) | 24.01 (19.42, 30.01) | 22.09 (17.87, 27.65) | 0.22 (0.07, 0.37) | 0.52 (0.42, 0.63) | 1.09 (0.89, 1.33) | 1.00 (0.82, 1.22) | 0.08 (−0.49, 0.65) |
| Central Europe | 1260.56 (1067.65, 1479.62) | 1103.59 (934.7, 1295.37) | 608.31 (517.94, 713.51) | 6.96 (6.72, 7.21) | 84.11 (69.74, 99.02) | 73.63 (61.06, 86.69) | 39.15 (32.37, 46.15) | 4.11 (3.91, 4.31) | 4.68 (3.77, 5.58) | 4.09 (3.30, 4.89) | 2.08 (1.68, 2.49) | 3.9 (3.61, 4.2) |
| Eastern Europe | 1328.69 (1065.7, 1605.21) | 632.80 (507.55, 764.49) | 395.8 (319.64, 477) | 8.59 (8.33, 8.85) | 46.12 (37.77, 56.57) | 21.97 (17.99, 26.94) | 14.17 (11.57, 17.34) | 4.15 (3.74, 4.56) | 1.64 (1.37, 1.93) | 0.78 (0.65, 0.92) | 0.48 (0.40, 0.57) | 3.39 (3.00, 3.77) |
| Central Asia | 41.06 (33.49, 48.81) | 43.90 (35.81, 52.19) | 53.22 (43.89, 62.92) | 7.24 (7.01, 7.48) | 5.54 (4.67, 6.97) | 5.92 (4.99, 7.45) | 7.45 (6.27, 9.38) | 3.77 (3.44, 4.1) | 0.21 (0.17, 0.26) | 0.22 (0.18, 0.28) | 0.33 (0.27, 0.43) | 3.63 (2.50, 4.76) |
| North Africa and Middle East | 54.3 (43.23, 66.78) | 8.92 (7.10, 10.97) | 10.83 (8.67, 13.35) | 5.92 (5.45, 6.39) | 100.97 (84.2, 119.65) | 16.59 (13.83, 19.66) | 21.11 (17.59, 24.72) | 2.81 (2.69, 2.94) | 3.82 (3.19, 4.44) | 0.63 (0.52, 0.73) | 0.97 (0.81, 1.12) | 2.81 (2.59, 3.04) |
| South Asia | 30.19 (23.68, 37.63) | 1.67 (1.31, 2.08) | 2.03 (1.6, 2.52) | 5.37 (5.07, 5.68) | 190.68 (144.06, 248.6) | 10.56 (7.98, 13.77) | 13.83 (10.63, 17.66) | 3.77 (3.57, 3.97) | 8.75 (6.85, 11.05) | 0.48 (0.38, 0.61) | 0.76 (0.6, 0.95) | 3.77 (3.58, 3.96) |
| South East Asia | 44.73 (35.66, 55.42) | 3.56 (2.78, 4.49) | 2.56 (2.05, 3.15) | 6.35 (5.98, 6.73) | 198.71 (159.21, 254.56) | 5.84 (4.78, 7.45) | 11.82 (9.52, 14.86) | 0.17 (0.05, 0.29) | 9.18 (7.44, 11.5) | 0.25 (0.21, 0.32) | 0.64 (0.52, 0.79) | 0.15 (−0.19, 0.49) |
| East Asia | 891.02 (707.25, 1093.31) | 60.52 (48.04, 74.26) | 42.41 (33.87, 51.68) | 11.96 (11.62, 12.31) | 86.82 (70.96, 103.74) | 5.90 (4.82, 7.05) | 4.36 (3.59, 5.18) | −0.08 (−0.30, 0.14) | 3.23 (2.62, 3.85) | 0.22 (0.18, 0.26) | 0.17 (0.14, 0.20) | −0.53 (−0.79, −0.26) |
| Oceania | 1.13 (0.9, 1.37) | 8.51 (6.81, 10.35) | 18.61 (14.95, 22.75) | 3.62 (1.40, 5.89) | 1.27 (0.82, 1.89) | 9.60 (6.17, 14.2) | 15.58 (10.60, 22.16) | −0.22 (−1.15, 0.72) | 0.04 (0.03, 0.06) | 0.31 (0.21, 0.44) | 0.75 (0.54, 1.04) | −0.27 (−3.03, 2.58) |
| Western sub- Saharan Africa | 3.87 (3, 4.88) | 0.85 (0.66, 1.07) | 1.65 (1.3, 2.04) | 0.82 (−0.13, 1.78) | 31.35 (21.26, 45.26) | 6.87 (4.66, 9.92) | 13.17 (9.11, 18.49) | −0.44 (−0.62, −0.27) | 1.02 (0.71, 1.42) | 0.22 (0.16, 0.31) | 0.55 (0.39, 0.76) | −0.43 (−0.94, 0.09) |
| Eastern sub- Saharan Africa | 2.6 (2, 3.27) | 0.63 (0.49, 0.79) | 1.4 (1.1, 1.74) | 1.37 (0.61, 2.15) | 29.97 (22.89, 39.84) | 7.28 (5.56, 9.67) | 16.91 (13.45, 21.67) | −0.37 (−0.50, −0.23) | 1.20 (0.95, 1.53) | 0.29 (0.23, 0.37) | 0.92 (0.72, 1.21) | −0.36 (−0.79, 0.07) |
| Central sub- Saharan Africa | 0.8 (0.62, 1.01) | 0.61 (0.47, 0.77) | 1.36 (1.08, 1.69) | 1.10 (−0.54, 2.77) | 10.43 (6.84, 15.6) | 7.93 (5.20, 11.86) | 18.97 (13.23, 26.91) | −0.02 (−0.31, 0.27) | 0.41 (0.29, 0.58) | 0.31 (0.22, 0.44) | 1.01 (0.74, 1.36) | −0.01 (−0.86, 0.84) |
| Southern sub- Saharan Africa | 57.4 (44.28, 73.39) | 73.06 (56.35, 93.4) | 94.86 (72.97, 120.7) | 8.87 (8.42, 9.32) | 9.74 (8.36, 11.08) | 12.40 (10.65, 14.1) | 17.24 (14.86, 19.32) | 0.02 (−0.13, 0.17) | 0.43 (0.37, 0.48) | 0.55 (0.47, 0.61) | 0.96 (0.82, 1.07) | −0.14 (−0.76, 0.48) |
CAVD, calcific aortic valve disease; 95% UI, 95% uncertainty intervals; 95% CI, 95% confidence interval.
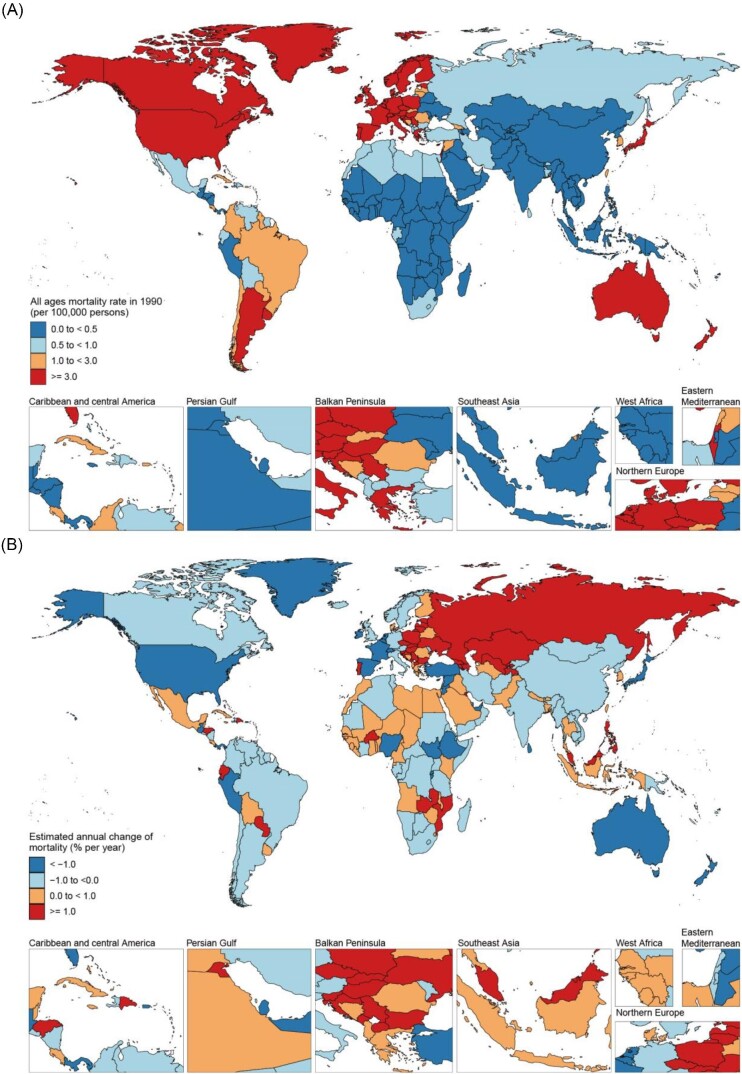
Figure 1.
All-age mortality in 2019 (A) and net drift of mortality during 1990−2019 (B) for CAVD in 204 countries and territories. (A) World map of all-age mortality for CAVD in 2019, with the global all-age mortality rate of 1.64 (95% UI 1.36−1.83) per 100 000 population. (B) World map of net drifts (estimated annual percentage change of mortality from the age–period–cohort model) for CAVD mortality, with the global net drift of CAVD mortality of −0.83% [95% CI −0.91 to −0.741]. CAVD, calcific aortic valve disease; UI, uncertainty interval; CI, confidence interval.
Consistent with the global trend, the CAVD prevalence in most regions increased substantially, except in Western and Central sub-Saharan Africa. Briefly, East Asia was the region with the highest increase in prevalence [11.96%, (11.62–12.31)], followed by Australasia [10.12%, (9.85–10.38)] and Eastern Europe [8.59%, (8.33–8.85)]. Furthermore, except for five regions (high-income Asia Pacific, high-income North America, Western Europe, Australasia, and Tropical Latin America), the DALYs rates in other regions were either increased (net drifts >0.0% per year) or modestly reduced (−0.5 to 0.0%). Despite the decreased global trend of mortality, Central Europe [3.90%, (3.61–4.20)], Eastern Europe [3.39%, (3.00–3.77)], Central Asia [3.63%, (2.5–4.76)], North Africa and Middle East [2.81%, (2.59–3.04)], and South Asia [3.77%, (3.58–3.96)] showed opposite trends. Furthermore, no favourable outcome was observed in regions, including Andean Latin America, Central Latin America, the Caribbean, South East Asia, Oceania, Western sub-Saharan Africa, Eastern sub-Saharan Africa, Central sub-Saharan Africa, and Southern sub-Saharan Africa, which required more attention on CAVD management. Trends in CAVD mortality across the SDI quintiles from 1990 to 2019 are shown in Table 2. Globally, there were 53 000 deaths in 1990 and 127 000 deaths in 2019, an 138.0% increase (113.4 to 159.3). All-age mortality increased from 1990 to 2019 [% change = 64.5%, (7.5–79.3)], while the ASR of mortality did not change. Importantly, the all-age mortality was generally higher than the age-standardized mortality in high and high-middle SDI countries, while it was opposite in other countries. These results indicate that it is more appropriate to use all-age mortality to capture the true burden of CAVD mortality in higher SDI regions. Except for the unchanged situation in the low SDI regions, all-age mortality in the other regions increased. The APC model revealed a decreasing trend globally, with a net drift of −0.83% (−0.91 to −0.74). However, significant differences were observed across the SDI quintiles, with a substantial decrease in high SDI countries [−1.45% (−1.61 to −1.30)], a slight increase in high-middle SDI countries [0.22% (0.06–0.37)], and no obvious change in other countries.
Table 2.
Trends in CAVD mortality across socio-demographic index quintiles, 1990−2019
| Global (N = 204) | Low SDI (N = 41) | Low-middle SDI (N = 41) | Middle SDI (N = 40) | High-middle SDI (N = 41) | High SDI (N = 41) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1990 | 2019 | 1990 | 2019 | 1990 | 2019 | 1990 | 2019 | 1990 | 2019 | 1990 | 2019 | |
| Population | ||||||||||||
| Number, n × 1000 000 | 5350 (5460,5239) | 7737 (7993,7483) | 528 | 1129 | 1130 | 1764 | 1717 | 2397 | 1150 | 1430 | 822 | 1013 |
| Percentage of global, % | 100.0 | 100.0 | 9.9 | 14.6 | 21.1 | 22.8 | 32.1 | 31.0 | 21.5 | 18.5 | 15.4 | 13.1 |
| Deaths | ||||||||||||
| Number, n × 1000 | 53 (48, 60) | 127 (106, 141) | 2 (1, 2) | 3 (3, 4) | 3 (2, 4) | 8 (7, 10) | 4 (4, 5) | 10 (9, 12) | 8 (7, 9) | 24 (21, 27) | 36 (32, 40) | 80 (64, 90) |
| Percentage of global, % | 100.0 | 100.0 | 2.8 | 2.7 | 5.9 | 6.4 | 8.0 | 8.3 | 15.7 | 19.3 | 67.6 | 63.2 |
| Percent change of deaths 1990–2019, % | 138 (113.4, 159.3) | 130.5 (80.9, 215.6) | 161.7 (115.6, 238.1) | 145.4 (109.5, 204.8) | 192.3 (156.7, 225.7) | 112.7 (96.9, 140.1) | ||||||
| APC model estimates | ||||||||||||
| Net drift of mortality, % per pear | −0.83 (−0.91, −0.74) | −0.03 (−0.29, 0.24) | 0.13 (−0.04, 0.30) | −0.10 (−0.24, 0.05) | 0.22 (0.06, 0.37) | −1.45 (−1.61, −1.30) | ||||||
| All-age mortality rate | ||||||||||||
| Rate per 100 000 | 1.00 (0.89, 1.12) | 1.64 (1.36, 1.83) | 0.28 (0.17, 0.42) | 0.31 (0.23, 0.39) | 0.28 (0.19, 0.37) | 0.46 (0.38, 0.57) | 0.25 (0.20, 0.30) | 0.44 (0.39, 0.49) | 0.73 (0.65, 0.80) | 1.71 (1.46, 1.91) | 4.38 (3.91, 4.85) | 7.92 (6.35, 8.89) |
| Per cent change of rate 1990–2019, % | 64.5 (47.5, 79.3) | 7.8 (−15.3, 47.7) | 67.6 (38.1, 116.5) | 75.8 (50.1, 118.3) | 135.1 (106.4, 162.0) | 80.7 (59.7, 94.8) | ||||||
| Age-standardized mortality rate | ||||||||||||
| Rate per 100 000 | 1.75 (1.55, 1.96) | 1.76 (1.45, 1.97) | 0.77 (0.47, 1.09) | 0.80 (0.61, 0.99) | 0.65 (0.44, 0.85) | 0.70 (0.57, 0.84) | 0.47 (0.39, 0.57) | 0.48 (0.43, 0.53) | 0.93 (0.81, 1.04) | 1.28 (1.08, 1.43) | 3.46 (3.06, 3.84) | 3.35 (2.75, 3.74) |
| Per cent change of rate 1990–2019, % | 0.4 (−8.9, 8.0) | 3.7 (−18.4, 38.7) | 7.5 (−10.5, 36.8) | 1.1 (−13.3, 24.6) | 36.9 (21.2, 53.6) | −3.2 (−11.9, 3.5) | ||||||
CAVD, calcific aortic valve disease; 95% UI, 95% uncertainty intervals; 95% CI, 95% confidence interval; SDI, socio-demographic index; APC, age–period–cohort.
National burden trends in CAVD mortality
Among the 204 countries and territories, 99 had ≥50 deaths due to CAVD in 2019. Supplementary material online, Table S2 shows detailed information for these 99 countries and territories, including population, the total number of deaths, all-age mortality, age-standardized mortality, and APC model-derived net drift of mortality. The top three countries were the USA [(death number = 24 826, (95% UI, 20 354–27 718)], Germany [13 154, (11 099–15 132)], and Japan [12 868, (8512–15 732)], accounting for 40.1% of CAVD deaths globally. In 2019, 30 of these 99 countries, almost all were categorized as high or high-middle SDI countries, had all-age mortality more than three-fold higher than the global average. In addition, 47 countries showed an increasing trend (net drifts >0.0% per year), and 21 countries showed a substantial increase (net drifts ≥1.0% per year) in mortality. Poland showed the highest net drift [8.46% (7.66–9.27)], with an all-age mortality rate change of 1476.4% (784.3–2521.8) from 1990 to 2019. In contrast, some countries, such as the Syrian Arab Republic, Japan, and France, have shown a substantial decrease (net drifts ≤ −1.0% per year) in mortality.
Time trends in CAVD mortality across different age groups
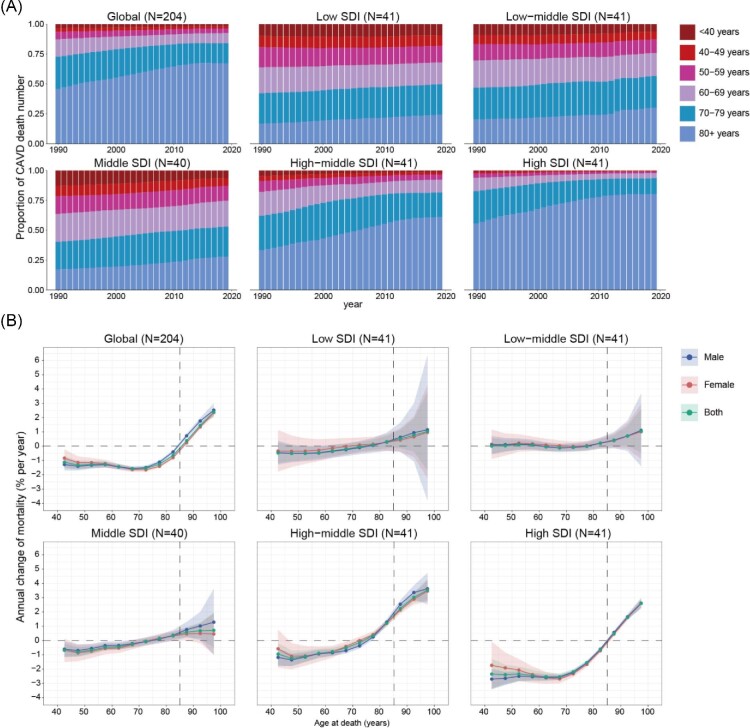
The proportion of CAVD deaths across the six age groups over the past 30 years is summarized to clearly present the temporal changes in the age distribution of CAVD deaths (Figure 2A). Globally from 1900 to 2019, there was a noticeable transition in CAVD deaths from younger to older populations (especially in the population aged ≥80 years), which was more obvious in high and high-middle SDI countries. In 2019, people aged ≥60 years accounted for the largest population of CAVD deaths across all SDI countries. However, CAVD mortality in those aged <60 years could not be ignored in low, low-middle, and middle SDI regions. The details of the age distribution of CAVD deaths in each country are summarized in Supplementary material online, Figures S13–S17.
Figure 2.
Age distribution of CAVD deaths and local drift of CAVD mortality by SDI quintiles, 1990−2019. (A) Temporal change in the relative proportion of CAVD deaths across six age groups (<40, 40–49, 50−59, 60−69, 70−79, and ≥80 years), 1990−2019. (B) Age–period–cohort model-derived estimates of local drifts of CAVD mortality for 12 age groups (40−44 to 95−99 years), 1990−2019. The dots indicate the annual percentage change of CAVD mortality (% per year), and the shaded areas indicate the corresponding 95% CIs. CAVD, calcific aortic valve disease; SDI, socio-demographic index; CI, confidence interval.
Next, the annual percentage change in the CAVD mortality rate for each age group was explored using the local drift derived from the APC model (Figure 2B). Globally, CAVD mortality showed a decreasing trend in age groups ≤84 years (P < 0.0001), while it was significantly increased in those aged ≥85 years (P < 0.0001). The groups aged 65–70 and 95–99 years showed the steepest decrease [local drift = −1.57% (−1.71 to −1.43)] and increase [2.38% (2.12–2.65)], respectively. Populations aged ≥85 years showed increased CAVD mortality across all SDI regions, especially in high-middle and high SDI countries. Interestingly, high and high-middle SDI countries showed decreased and increased CAVD mortality in the population aged 70–79 years, respectively. Generally, the high SDI regions exhibited the greatest reduction in CAVD mortality in age groups ≤84 years, from −2.54% (−2.77 to −2.31) in the 65–69 years age group to −0.64% (−0.76 to −0.52) in the 80–84 years age group. Supplementary material online, Figures S18–S22 show the local drift in CAVD mortality for each country.
Age, period, and cohort effects on CAVD mortality
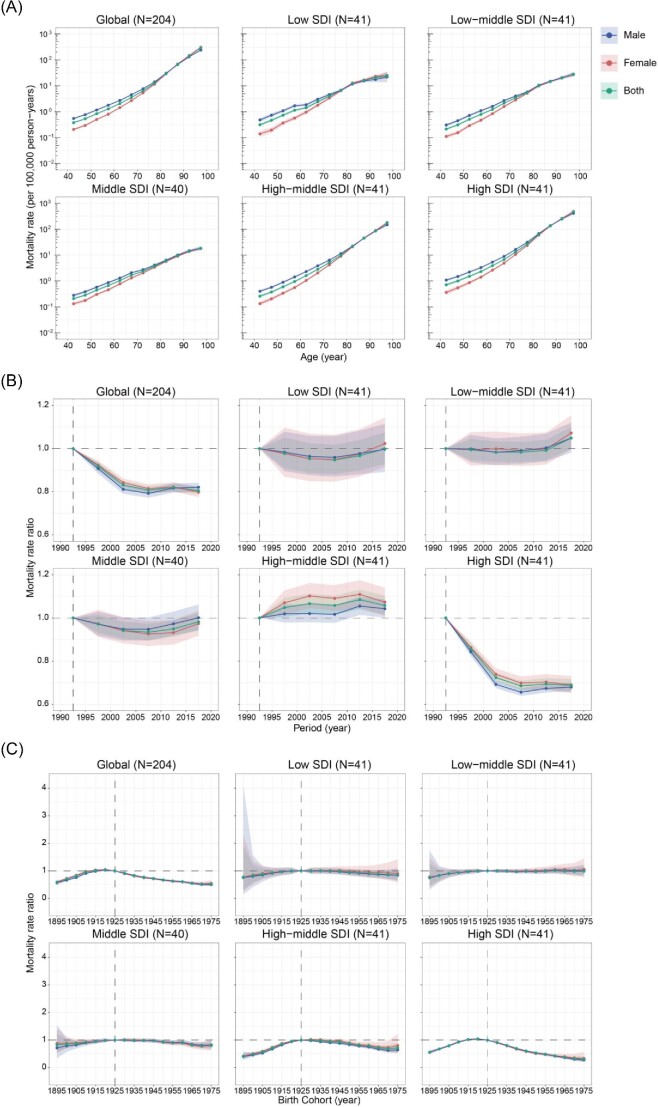
Figure 3 shows the APC model-derived estimates of the APC effects by the SDI quintiles. Age effects represent the age-associated natural history of CAVD mortality. Period and cohort effects were used to demonstrate the progress of CAVD in different periods and birth cohorts, respectively.20 Similar trends in age effects were explored in both global and different SDI quintiles, suggesting that the risk of CAVD mortality increased with age. Notably, compared with other SDI countries, high SDI regions had the highest CAVD mortality rates across all age groups. Furthermore, female mortality was lower than male mortality in those aged <80 years, while no sex difference was found in age effects after 80 years of age (Figure 3A).
Figure 3.
Parameter estimates of age, period, and cohort effects on CAVD mortality by SDI quintiles. (A) Age effects are represented by the fitted longitudinal age curves of CAVD mortality (per 100 000 person-years) and the corresponding 95% CIs. (B) Period effects are represented by the relative risk of mortality of each period compared with the reference (period 1990–1994) adjusted for age and nonlinear cohort effects and the corresponding 95% CI. (C) Cohort effects are represented by the relative risk of mortality of each cohort compared with the reference (cohort 1921–1929) adjusted for age and nonlinear period effects and the corresponding 95% CI. Age, period, and cohort effects are stratified by sex. CAVD, calcific aortic valve disease; SDI, socio-demographic index; CI, confidence interval.
Global period effects showed a declining mortality risk before 2010, but the reduction was attenuated in the past decade. However, the patterns varied in different SDI regions. For countries with low, low-middle, and middle SDI, over the past three decades, period effects remained nearly constant, suggesting an unfavourable improvement in CAVD mortality. A significant reduction in the death rate was found in high SDI regions with a relative period risk of 0.69 (95% CI, 0.66–0.72) in 2015–2019, while the opposite trend was observed in high-middle SDI countries [1.06 (1.01–1.10)], especially in females (Figure 3B).
Globally, an increasing CAVD mortality trend was observed in those born before 1925, while patterns were reversed after this cohort. This observation suggested an improvement in CAVD control. Similar situations were also shown in countries with high-middle and high SDI, with relative cohort risks for individuals born in the 1975 cohort of 0.72 (0.56–0.92) and 0.30 (0.22–0.43), respectively. However, it was disappointing that in low, low-middle, and middle SDI regions, no progress was made in disease control, indicating that more efforts should be made in those countries (Figure 3C). The age, period, and cohort effects on CAVD mortality in each country are shown in Supplementary material online, Figures S23–S27, S28–S32, and S33–S37, respectively.
Age, period, and cohort effects in exemplary countries
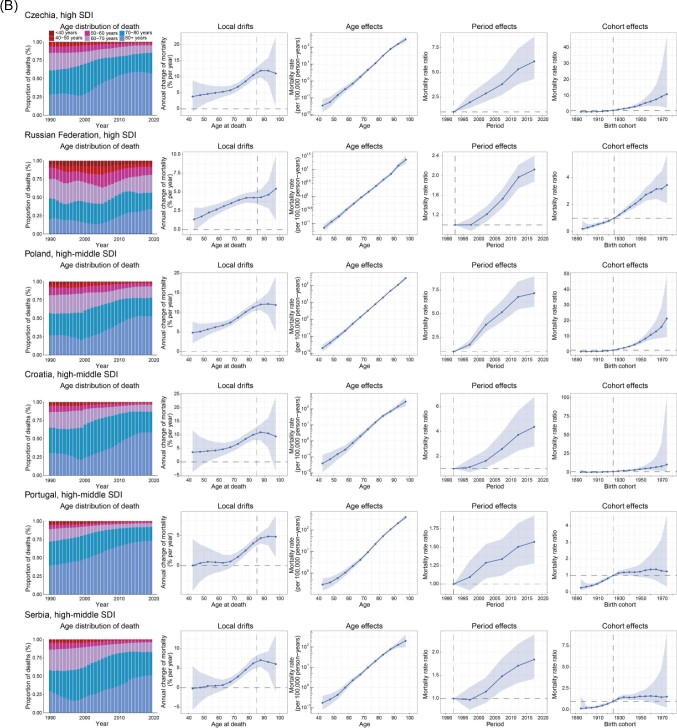
To better characterize the trends in CAVD mortality, several exemplary countries were selected across SDI quintiles, and age distribution of CAVD deaths, local drifts, and age/period/cohort effects for each country was analysed (Figure 4).
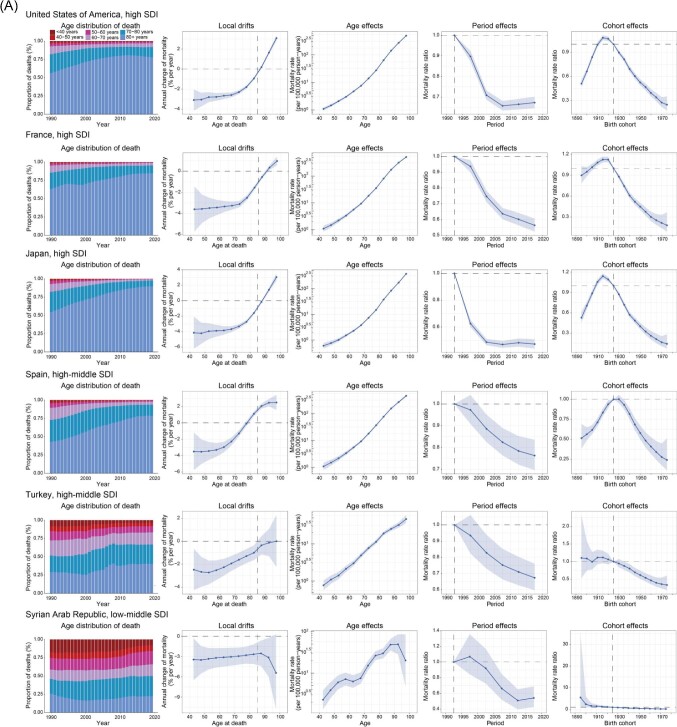
Figure 4.
Exemplar countries across SDI quintiles showing favourable (A) and unfavourable (B) age–period–cohort effects. Age distribution of deaths from 1990 to 2019 shows the relative proportion of CAVD deaths across six age groups (<40, 40–49, 50−59, 60−69, 70−79, and ≥80 years). Local drifts show the fitted longitudinal age curves of CAVD mortality (per 100 000 person-years) across 12 five-year age groups (40−44 to 95−99 years) and the corresponding 95% CIs. Age effects show the fitted longitudinal age curves of CAVD mortality (per 100 000 person-years) and the corresponding 95% CIs. Period effects show the relative risk of mortality of each period compared with the reference (period 1990–1994) adjusted for age and nonlinear cohort effects and the corresponding 95% CI. Cohort effects show the relative risk of mortality of each cohort compared with the reference (cohort 1921–1929) adjusted for age and nonlinear period effects and the corresponding 95% CI. CAVD, calcific aortic valve disease; SDI, socio-demographic index; CI, confidence interval.
Six countries from the high, high-middle, and low-middle SDI regions were chosen to characterize the favourable APC effects (Figure 4A). The USA contributed to most CAVD deaths in 2019 (Supplementary material online, Table S2). However, it showed improved mortality in the past 30 years, indicated by substantially reduced local drifts in the population aged <80 years, reduced period risks before 2010, and declining cohort risk in those born after 1915. France ranked fourth in CAVD deaths and sixth in CAVD all-age mortality in 2019 globally. Nonetheless, it achieved the greatest improvement in CAVD mortality in Europe, with a net drift of −2.46% (−2.74 to −2.18) (Supplementary material online, Table S2). Mortality reduction was found in all age groups except for those >90 years of age. Additionally, period risks showed a declining trend, and cohort risks began to decline significantly after 1920. Japan ranked first in CAVD deaths in Asia in 2019. However, favourable results have been achieved over the past three decades. Japan demonstrated a transition in the age distribution of CAVD deaths, with those aged >80 years accounting for the largest population. Similar to Japan, Spain experienced a transition in the age distribution of CAVD deaths. However, Spain showed declining risks over the entire study period, while such improvements disappeared in Japan after 2005. Turkey is the only high-middle SDI country with reduced mortality (local drifts <0) in all age groups and a notably declining risk over the periods and in successive birth cohorts. The Syrian Arab Republic, a low-middle SDI country, stood out for its highest reduction in CAVD mortality, revealed by a net drift of −3.16% (−3.98 to −2.33), equal to a 61% reduction in the past 30 years. For the Syrian Arab Republic, mortality reduction was observed in all age groups and successive birth cohorts.
Unfavourable APC effects were clearly illustrated in two high-SDI and four high-middle SDI countries (Figure 4B). Czechia and the Russian Federation were atypical high SDI countries with significantly increased CAVD mortality, while most high SDI countries showed unimproved or reduced mortality according to estimates of net drifts (Supplementary material online, Table S2). The APC results were similar between these two countries, with notable increases in mortality found in all age groups except 40–44 years old and worsening risks over the periods and in successive birth cohorts. Poland exhibited the worst trends in CAVD mortality globally, with a net drift of 8.46% (7.66–9.27), equal to a 955% increase from 1990 to 2019. Poland showed a transition in the age distribution of CAVD deaths, especially after 2000. Poland was the only country with a substantial increase (local drift >1.0%) in all age groups, consistent with the worsening period and cohort risks over the entire study period. The other three high-middle SDI countries (Croatia, Portugal, and Serbia) showed similar transitions in the age distribution of CAVD deaths and unfavourable APC effects. The population aged >70 years old showed a notable increase in CAVD mortality in the three countries. Of note, all six selected countries with unfavourable APC effects were located in Europe, indicating the necessity to strengthen the management of CAVD in European countries.
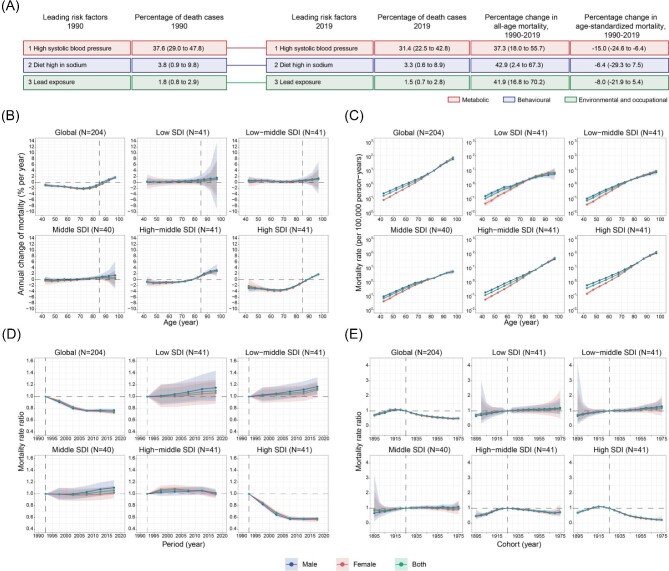
Top leading risk factor and its age, period, and cohort effects
The three leading risk factors in 1990 and 2019 were ranked to explore the top leading risk factors of CAVD deaths globally. This study found that high systolic blood pressure (HSBP), a metabolic risk factor, ranked first in both 1990 and 2019. Interestingly, the all-age mortality rate attributable to HSBP increased by 37.3% (18.0–55.7), while the age-standardized mortality rate decreased by 15.0 (−24.6 to −6.4) from 1990 to 2019 (Figure 5A). This inconsistency may be related to the change in the population age structure along the calendar time.
Figure 5.
Leading three risk factors for global CAVD deaths and APC-derived parameters for CAVD mortality attributable to high systolic blood pressure. (A) Leading three risk factors for global CAVD deaths and percentage of total deaths (1990 and 2019), and percentage change in all-age and age-standardized mortality from 1990 to 2019 for both sexes combined. Risk factors are connected by lines between time periods. (B) APC model-derived estimates of local drifts of CAVD mortality for 12 age groups (40−44 to 95−99 years), 1990−2019. The dots indicate the annual percentage change of CAVD mortality (% per year), and the shaded areas indicate the corresponding 95% CIs. (C) Age effects are represented by the fitted longitudinal age curves of CAVD mortality (per 100 000 person-years) and the corresponding 95% CIs. (D) Period effects are represented by the relative risk of mortality of each period compared with the reference (period 1990–1994) adjusted for age and nonlinear cohort effects and the corresponding 95% CI. (E) Cohort effects are represented by the relative risk of mortality of each cohort compared with the reference (cohort 1921–1929) adjusted for age and nonlinear period effects and the corresponding 95% CI. Age, period, and cohort effects are stratified by sex. CAVD, calcific aortic valve disease; APC, age–period–cohort; SDI, socio-demographic index; CI, confidence interval.
Next, the APC model was used to explore the annual percentage change in the CAVD mortality rate attributable to HSBP for each age group and the age, period, and cohort effects. Globally, noticeable improvements have been made in those aged <85 years. Notably, the mortality rate attributable to HSBP was substantially reduced (local drift ≤1.0%) in most age groups in high SDI regions. In contrast, such improvements were not observed in other SDI regions (Figure 5B). CAVD mortality caused by HSBP increased with age, both globally and across SDI quintiles, and was higher in males than in females aged <70 years (Figure 5C). Favourable period and cohort effects were only observed in high SDI regions. Contrastingly, no noticeable improvement was observed in other SDI quintiles, indicating the importance of controlling HSBP in these regions (Figure 5D, E).
Discussion
VHDs have attracted global attention as the next cardiac epidemic. The disease burden of RHD has been well controlled. On the other hand, the management effects of NRHD, especially CAVD, are less satisfactory. Our analysis showed that the CAVD prevalence rate is still increasing globally and in most regions, partly because of improvements in diagnostic workflow.22 Moreover, CAVD DALYs and mortality rates were unimproved or even worse in some countries, especially in European countries. We also revealed an obvious transition of CAVD deaths from the younger to the older population, which was more noticeable in countries with a higher SDI. Importantly, this study's novel approach of using the APC model for CAVD mortality revealed several key findings: (1) Traditional all-age rates/ASRs might not fully agree with the net drift derived from the APC model; (2) males with CAVD had a higher mortality rate than females of the same age before 80 years of age; (3) generally, CAVD mortality increased with age in an exponential mode; (4) the local drift, period effects, and cohort effects exhibited distinct differences (favourable or unfavourable) across different SDI regions and among different countries; and (5) HSBP was the leading risk factor for CAVD mortality, and it showed favourable APC effects in high SDI regions.
This study compared several parameters, including traditional all-age rates/ASRs and APC-derived estimates, to comprehensively describe the disease burden of CAVD. Interestingly, the all-age rates of CAVD prevalence/DALYs/death are generally higher than ASRs in economically advantaged regions and countries (Tables 1, 2, and Supplementary material online, Table S2), which account for most CAVD cases. Such results might be attributable to the older population and relatively easy access to essential interventions for survival.18 Therefore, it is more reliable to use the all-age rate to describe CAVD in economically advantaged regions and countries, while relying on ASRs could be misleading. Furthermore, we observed that the change in the rate of CAVD deaths from 1990 to 2019 using conventional parameters (all-age/age-standardized mortality) might not fully agree with that observed using the net drift derived from the APC model in some countries (such as the USA, Germany, the UK, Spain, Canada, the Netherlands, and Belgium) (Supplementary material online, Table S2). This indicates the necessity of differentiating period, and cohort trends in CAVD mortality.
The present study showed that males with CAVD had a higher mortality rate than females of the same age before 80 years of age (Figure 3A). This result is consistent with those of several reported studies, both in the clinical and basic sciences. Cardiac imaging revealed that males with CAVD had more severe calcium accumulation in aortic valves,23 more obvious remodelling of the left ventricle,24 and worse ventricular function and hemodynamics,25 compared to females with CAVD. Pathological studies have confirmed severe calcific remodelling of the aortic valves in males compared to females.26,27 These pathologic differences might be related to testosterone,28 immune cells,29 and other factors. Nevertheless, more studies on sexual differences in CAVD are required in terms of pathophysiology, management, and outcomes.
The CAVD mortality increased exponentially with age (Figure 3A), indicating that a country's mortality is closely related to its degree of ageing. Generally, the degree of ageing in economically advantaged countries is higher than that of economically disadvantaged countries, which might be one of the key reasons for higher CAVD mortality (Supplementary material online, Figures S4C and S5C) and a higher proportion of deaths in the ageing population (Figure 2A) in higher SDI regions. However, CAVD would pose a great health burden in lower SDI countries with an obvious ageing trend, especially in countries with large populations such as China. As the world's most populous country (1.42 billion), China will face the severe challenge of population ageing considering that the number of older adults is increasing exponentially, and by 2050, there will be 400 million Chinese citizens aged >65 years, 150 million of whom will be aged >80 years.30
The USA, France, Japan, Spain, Turkey, and the Syrian Arab Republic are representative countries that exhibit favourable APC effects on CAVD mortality. Favourable gains may be multifactorial. Historically, symptomatic CAVD has been treated by surgical aortic valve replacement (SAVR),31,32 balloon aortic valvuloplasty,33 transcatheter AV implantation (TAVI),34 and medical treatments.35 However, only SAVR and TAVI are regarded as definitive treatments, while balloon aortic valvuloplasty mainly acts as a bridge therapy to definitive treatments, and no available medical therapy influences the natural history of CAVD.36,37 It should be noted that TAVI reduces mortality in the highest-risk patients, and eligibility is expanding to moderate- and low-surgical-risk patients.38 The USA maintained a stable number of SAVR procedures and decreased in-hospital mortality before 2011. After FDA approval of TAVI in the USA in 2011, the total number of aortic valve replacement surgeries surged,39 and the all-age mortality rate of CAVD further decreased.10 France showed the greatest improvement in CAVD mortality in Europe, which might be partly attributable to the increasing number of SAVR40 and the introduction and optimization of TAVI.41,42 According to the data from French TAVI registries, mortality after TAVI declined dramatically, with in-hospital mortality decreased from 10.1% in 2010 to 2.2% in 2019 and 1-year mortality decreased from 22.0% in 2010 to 11.0% in 2018.43 Japan had the largest absolute number of CAVD deaths in Asia in 2019. However, noticeable improvements have been made in CAVD mortality, which might partly be the result of TAVI implementation, such as the OCEAN-TAVI44 and LAPLACE-TAVI45 registry studies. Spain has achieved an increasing number of SAVR cases with decreasing in-hospital mortality in the past two decades.46 Furthermore, CAVD management in Spain has been further optimized by introducing TAVI since 2014.47 There is still room for improvement despite the great gains of the above countries considering that their all-age mortality remains 4.5–7.5 times higher than that of the average level globally. Turkey and the Syrian Arab Republic are two relatively income-disadvantaged members of the European Society of Cardiology.48 CAVD mortality in these two countries decreased to the average level globally, but relevant national data were scarce and will be needed in the future.49
In contrast, six European countries with high or high-middle SDI, including the Czech Republic, Russia, Poland, Croatia, Portugal, and Serbia, stood out for their unsatisfactory CAVD mortality rate, indicating a failure in CAVD control. The mismatch between increasing population ageing and the health medical system, and therapy practices in these countries might contribute to the disappointing results of CAVD management. In Russia, lower health expenditures were shown compared with other European regions, and a continuously descending trend of expenditures as a proportion of the gross domestic product was revealed.50,51 Additionally, cardiac procedures performed annually in Russia could not cover the needs of the Russian population,51 which may have caused the failure of disease management. Poland, a European country with a huge burden on medical security, has been criticized for its inadequate level and allocation of funds and staff shortages in the health system.52,53 Compared with other European countries, the slow increase in the TAVI adoption rate in Poland and the significant variations among national TAVI centres suggest that the limited progress in CAVD treatment was not proportionate to the increasing prevalence of CAVD.54 Similarly, Croatia and Portugal had increasingly elevated CAVD prevalence, while TAVI promotion was insufficient, characterized by low numbers of TAVI procedures and deficient treatment strategy.55,56 This elevated trend of CAVD prevalence in most European countries is obvious. With an ageing population, the medical burden of CAVD in these countries will become increasingly huge, which appeals to further attention on disease control in Europe.
HSBP was recently shown to be a risk factor for developing various cardiovascular diseases, including CAVD.57,58 Hence, the awareness of blood pressure control was widely raised. Furthermore, measures were taken by countries and individuals, contributing to the increasing control rate of hypertension globally, especially in high SDI countries (control rate up to 60% in 2019),59 which was consistent with the favourable effects in high SDI regions in the present study. However, the control rate in other regions, especially in low- and middle-income countries (LMICs), is relatively disappointing and should be tightly managed.60
This study had several limitations. First, primary data were limited in LMICs, resulting in wide uncertainty bounds and affecting the accuracy of the APC-derived estimates. Hence, primary data collection in LMICs should be strengthened to improve research accuracy. Second, the data were analysed at the national level, and subnational differences were not further explored. Therefore, subnational data should be analysed to understand detailed differences in the future, especially in high-burdened countries. Third, limited to the 5-year age group data in the GBD 2019, our APC analysis was performed using data with 5-year intervals, which might omit some subtle variations in age, period, and cohort effects.
This study involved an in-depth analysis of the CAVD mortality rate, an increasingly important global player in the ageing population. Many countries showed unimproved or worsened mortality, and CAVD mortality in the aged population showed an increasing trend. CAVD prevalence and mortality are of concern with increasing global ageing; hence, more effective and timely strategies are needed to prevent the enormous burden of this disease.
Supplementary Material
Acknowledgement
We highly appreciate the works by the Global Burden of Diseases, Injuries, and Risk Factors Study 2019 collaborators.
Contributor Information
Songren Shu, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China; The Cardiomyopathy Research Group, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Yicheng Yang, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China; Center of Respiratory and Pulmonary Vascular Disease, Fuwai Hospital, National Center for Cardiovascular Disease, Chinese Academy of Medical.
Bo Sun, Research Unit of Virtual Body and Virtual Surgery Technologies, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China.
Zhanhao Su, Department of Cardiovascular Surgery, Guangdong Cardiovascular Institute, Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China.
Mengxia Fu, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China; The Cardiomyopathy Research Group, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Changming Xiong, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China; Center of Respiratory and Pulmonary Vascular Disease, Fuwai Hospital, National Center for Cardiovascular Disease, Chinese Academy of Medical.
Xueyi Zhang, Medical Science Research Center, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China.
Shengshou Hu, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China; The Cardiomyopathy Research Group, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China; Department of Cardiovascular Surgery, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China; Shenzhen Key Laboratory of Cardiovascular Disease, Fuwai Hospital Chinese Academy of Medical Sciences, Shenzhen, China.
Jiangping Song, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China; The Cardiomyopathy Research Group, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China; Department of Cardiovascular Surgery, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China; Shenzhen Key Laboratory of Cardiovascular Disease, Fuwai Hospital Chinese Academy of Medical Sciences, Shenzhen, China.
Funding
This study was funded by the National Natural Science Foundation of China (82125004 to J.S.); the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2019ZT08Y481 to J.S.); the National High Level Hospital Clinical Research Funding (GSP-TS-6 to C.X.); and the Fundamental Research Funds for the Central Universities (3332022125 to S.S.).
Conflict of interest: We declare that we have no conflicts of interest.
Data availability
The data underlying this article were derived from sources in the public domain: Institute for Health Metrics and Evaluation (IHME), at http://ghdx.healthdata.org/gbd-results-tool, accessed 1 October 2022.
References
- 1. Gill H, Chehab O, Allen C, Patterson, T, Redwood, S, Rajani, Ret al. . The advantages, pitfalls and limitations of guideline-directed medical therapy in patients with valvular heart disease. Eur J Heart Fail 2021;23:1325–1333. [DOI] [PubMed] [Google Scholar]
- 2. Coffey S, Roberts-Thomson R, Brown A, Carapetis, J, Chen, M, Enriquez-Sarano, Met al. . Global epidemiology of valvular heart disease. Nat Rev Cardiol 2021;18:853–864. [DOI] [PubMed] [Google Scholar]
- 3. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M.. Burden of valvular heart diseases: a population-based study. Lancet North Am Ed 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 4. Andell P, Li X, Martinsson A, Andersson, C, Stagmo, M, Zöller, Bet al. . Epidemiology of valvular heart disease in a Swedish nationwide hospital-based register study. Heart 2017;103:1696–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. d'Arcy JL, Coffey S, Loudon MA, Kennedy, A, Pearson-Stuttard, J, Birks, Jet al. . Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. Eur Heart J 2016;37:3515–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. d'Arcy JL, Prendergast BD, Chambers JB, Ray SG, Bridgewater B.. Valvular heart disease: the next cardiac epidemic. Heart 2011;97:91–93. [DOI] [PubMed] [Google Scholar]
- 7. Ghamari S-H, Abbasi-Kangevari M, Saeedi Moghaddam S, Aminorroaya A, Rezaei N, Shobeiri Pet al. . Rheumatic heart disease is a neglected disease relative to its burden worldwide: findings from Global Burden of Disease 2019. J Am Heart Assoc 2022;11:e025284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blaser MC, Kraler S, Lüscher TF, Aikawa E.. Multi-omics approaches to define calcific aortic valve disease pathogenesis. Circ Res 2021;128:1371–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kraler S, Blaser MC, Aikawa E, Camici GG, Lüscher TF.. Calcific aortic valve disease: from molecular and cellular mechanisms to medical therapy. Eur Heart J 2022;43:683–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bevan GH, Zidar DA, Josephson RA, SG Al-Kindi. Mortality due to aortic stenosis in the United States, 2008–2017. JAMA 2019;321:2236–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doris MK, Everett RJ, Shun-Shin M, Clavel M-A, Dweck MR.. The role of imaging in measuring disease progression and assessing novel therapies in aortic stenosis. JACC: Cardiovasc Imaging 2019;12:185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yi B, Zeng W, Lv L, Hua P.. Changing epidemiology of calcific aortic valve disease: 30-year trends of incidence, prevalence, and deaths across 204 countries and territories. Aging 2021;13:12710–12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang C, Xu H, Jia R, Jin Z, Zhang C, Yuan J. Global burden and improvement gap of non-rheumatic calcific aortic valve disease: 1990–2019 findings from Global Burden of Disease Study 2019. J Clin Med 2022;11:6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yadgir S, Johnson CO, Aboyans V, Adebayo OM, Adedoyin RA, Afarideh Met al. . Global, regional, and national burden of calcific aortic valve and degenerative mitral valve diseases, 1990–2017. Circulation 2020;141:1670–1680. [DOI] [PubMed] [Google Scholar]
- 15. Yu J, Wang Z, Bao Q, Lei S, You Y, Yin Zet al. . Global burden of calcific aortic valve disease and attributable risk factors from 1990 to 2019. Front Cardiovasc Med 2022;9:1003233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet North Am Ed 2020;396:1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. GBD 2019 Risk Factors Collaborators . Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet North Am Ed 2020;396:1223–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LMet al. . Global burden of cardiovascular diseases and risk factors, 1990–2019: update From the GBD 2019 study. J Am Coll Cardiol 2020;76:2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Bank Country and Lending Groups . https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups(accessed 1 Octomber 2022).
- 20. Su Z, Zou Z, Hay SI, Liu Y, Li S, Chen Het al. . Global, regional, and national time trends in mortality for congenital heart disease, 1990–2019: an age–period–cohort analysis for the Global Burden of Disease 2019 study. EClinicalMedicine 2022;43:101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosenberg PS, Check DP, Anderson WF.. A web tool for age–period–cohort analysis of cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev 2014;23:2296–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Michalski B, Dweck MR, Marsan NA, Cameli M, D'Andrea A, Carvalho RFet al. . The evaluation of aortic stenosis, how the new guidelines are implemented across Europe: a survey by EACVI. Eur Heart J Cardiovasc Imaging 2020;21:357–362. [DOI] [PubMed] [Google Scholar]
- 23. Aggarwal SR, Clavel M-A, Messika-Zeitoun D, Cueff C, Malouf J, Araoz PAet al. . Sex differences in aortic valve calcification measured by multidetector computed tomography in aortic stenosis. Circ Cardiovasc Imaging 2013;6:40–47. [DOI] [PubMed] [Google Scholar]
- 24. Singh A, Chan DCS, Greenwood JP, Dawson DK, Sonecki P, Hogrefe Ket al. . Symptom onset in aortic stenosis: relation to sex differences in left ventricular remodeling. JACC: Cardiovasc Imaging 2019;12: 96–105. [DOI] [PubMed] [Google Scholar]
- 25. Ito S, Miranda WR, Nkomo VT, Lewis BR, Oh JK.. Sex differences in LV remodeling and hemodynamics in aortic stenosis: sex-specific criteria for severe stenosis? JACC: Cardiovasc Imaging 2022;15:1175–1189. [DOI] [PubMed] [Google Scholar]
- 26. Simard L, Côté N, Dagenais F, Mathieu P, Couture C, Trahan Set al. . Sex-related discordance between aortic valve calcification and hemodynamic severity of aortic stenosis: is valvular fibrosis the explanation? Circ Res 2017;120:681–691. [DOI] [PubMed] [Google Scholar]
- 27. Voisine M, Hervault M, Shen M, Boilard A-J, Filion B, Rosa Met al. . Age, sex, and valve phenotype differences in fibro-calcific remodeling of calcified aortic valve. J Am Heart Assoc 2020;9:e015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhu D, Hadoke PWF, Wu J, Vesey AT, Lerman DA, Dweck MRet al. . Ablation of the androgen receptor from vascular smooth muscle cells demonstrates a role for testosterone in vascular calcification. Sci Rep 2016;6:24807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jover E, Matilla L, Martín-Núñez E, Garaikoetxea M, Navarro A, Fernández-Celis Aet al. . Sex-dependent expression of neutrophil gelatinase-associated lipocalin in aortic stenosis. Biol Sex Differ 2022;13:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fang EF, Scheibye-Knudsen M, Jahn HJ, Li J, Ling L, Guo Het al. . A research agenda for aging in China in the 21st century. Ageing Res Rev. 2015;24:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schwarz F, Baumann P, Manthey J, Hoffmann M, Schuler G, Mehmel HCet al. . The effect of aortic valve replacement on survival. Circulation 1982;66:1105–1110. [DOI] [PubMed] [Google Scholar]
- 32. Harken DE, Soroff HS, Taylor WJ, Lefemine AA, Gupta SK, Lunzer S.. Partial and complete prostheses in aortic insufficiency. J Thorac Cardiovasc Surg 1960;40:744–762. [PubMed] [Google Scholar]
- 33. Lababidi Z, Wu JR, Walls JT.. Percutaneous balloon aortic valvuloplasty: results in 23 patients. Am J Cardiol 1984;53:194–197. [DOI] [PubMed] [Google Scholar]
- 34. Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer Fet al. . Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002;106:3006–3008. [DOI] [PubMed] [Google Scholar]
- 35. Rossebø AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup Ket al. . Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008;359:1343–1356. [DOI] [PubMed] [Google Scholar]
- 36. Williams T, Hildick-Smith DJR.. Balloon aortic valvuloplasty: indications, patient eligibility, technique and contemporary outcomes. Heart 2020;106:1102–1110. [DOI] [PubMed] [Google Scholar]
- 37. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs Jet al. . 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561–632. [DOI] [PubMed] [Google Scholar]
- 38. Siontis GCM, Overtchouk P, Cahill TJ, Modine T, Prendergast B, Praz Fet al. . Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: an updated meta-analysis. Eur Heart J 2019;40:3143–3153. [DOI] [PubMed] [Google Scholar]
- 39. Akintoye E, Ando T, Sandio A, Adegbala O, Salih M, Zubairu Jet al. . Aortic valve replacement for severe aortic stenosis before and during the era of transcatheter aortic valve implantation. Am J Cardiol 2020;126:73–81. [DOI] [PubMed] [Google Scholar]
- 40. Nguyen V, Willner N, Eltchaninoff H, Burwash IG, Michel M, Durand Eet al. . Trends in aortic valve replacement for aortic stenosis: a French nationwide study. Eur Heart J 2022;43:666–679. [DOI] [PubMed] [Google Scholar]
- 41. Nguyen V, Michel M, Eltchaninoff H, Gilard M, Dindorf C, Iung Bet al. . Implementation of transcatheter aortic valve replacement in France. J Am Coll Cardiol 2018;71:1614–1627. [DOI] [PubMed] [Google Scholar]
- 42. Montalescot G, Redheuil A, Vincent F, Desch S, De Benedictis M, Eltchaninoff Het al. . Apixaban and valve thrombosis after transcatheter aortic valve replacement: the ATLANTIS-4D-CT randomized clinical trial substudy. JACC: Cardiovasc Interv 2022;15:1794–1804. [DOI] [PubMed] [Google Scholar]
- 43. Didier R, Le Breton H, Eltchaninoff H, Cayla G, Commeau P, Collet J-Pet al. . Evolution of TAVI patients and techniques over the past decade: the French TAVI registries. Arch Cardiovasc Dis 2022;115:206–213. [DOI] [PubMed] [Google Scholar]
- 44. Miyasaka M, Tada N, Taguri M, Kato S, Enta Y, Otomo Tet al. . Incidence, predictors, and clinical impact of prosthesis-patient mismatch following transcatheter aortic valve replacement in Asian patients: the OCEAN-TAVI Registry. JACC: Cardiovasc Interv 2018;11:771–780. [DOI] [PubMed] [Google Scholar]
- 45. Tezuka T, Higuchi R, Hagiya K, Saji M, Takamisawa I, Nanasato Met al. . Midterm outcomes of underweight patients undergoing transcatheter aortic valve implantation: insight from the LAPLACE-TAVI Registry. JACC: Asia 2022;3:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carnero-Alcázar M, Maroto-Castellanos LC, Hernández-Vaquero D, López-Menéndez J, Hornero-Sos F, Silva-Guisasola Jet al. . Isolated aortic valve replacement in Spain: national trends in risks, valve types, and mortality from 1998 to 2017. Rev Esp Cardiol 2021;74:700–707. [DOI] [PubMed] [Google Scholar]
- 47. Rosillo N, Vicent L, Martín de la Mota Sanz D, Elola FJ, Moreno G, Bueno H. Time trends in the epidemiology of nonrheumatic aortic valve disease in Spain, 2003–2018. Rev Esp Cardiol 2022;75:1020–1028. [DOI] [PubMed] [Google Scholar]
- 48. Timmis A, Vardas P, Townsend N, Torbica A, Katus H, De Smedt Det al. . European Society of Cardiology: cardiovascular disease statistics 2021. Eur Heart J 2022;43:716–799. [DOI] [PubMed] [Google Scholar]
- 49. Barçın C. Editorial: we need more national data on transcatheter aortic valve implantation. Anatol J Cardiol 2020;23:297–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Younger DS. Health care in the Russian Federation. Neurol Clin 2016;34:1085–1102. [DOI] [PubMed] [Google Scholar]
- 51. Lomivorotov VV, Efremov SM, Kirov MY, Guvakov DV, Kozlov IA, Lomivorotov VNet al. . History and current status of cardiac anesthesiology in Russia. J Cardiothorac Vasc Anesth 2019;33:3358–3365. [DOI] [PubMed] [Google Scholar]
- 52. Miszczyńska K, Miszczyński P.. Debt, ownership, and size: the case of hospitals in Poland. Int J Environ Res Public Health 2021;18: 4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Smarżewska D, Wereda WS, Jończyk JA.. Assessment of the health care system in Poland and other OECD countries using the Hellwig method. Int J Environ Res Public Health 2022;19:16733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dąbrowski M, Parma R, Huczek Z, Jagielak D, Grygier M, Trębacz Jet al. . The Polish Interventional Cardiology TAVI Survey (PICTS): 10 years of transcatheter aortic valve implantation in Poland. The landscape after the first stage of the Valve for Life Initiative. Pol Arch Intern Med. 2021;131:413–420. [DOI] [PubMed] [Google Scholar]
- 55. Hartley A, Hammond-Haley M, Marshall DC, Salciccioli JD, Malik IS, Khamis RYet al. . Trends in mortality from aortic stenosis in Europe: 2000–2017. Front Cardiovasc Med 2021;8:748137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Campante Teles R, Gama Ribeiro V, Patrício L, Neves JP, Vouga L, Fragata Jet al. . Position statement on transcatheter aortic valve implantation in Portugal. Rev Port Cardiol 2013;32:801–805. [DOI] [PubMed] [Google Scholar]
- 57. Rahimi K, Mohseni H, Kiran A, Tran J, Nazarzadeh M, Rahimian Fet al. . Elevated blood pressure and risk of aortic valve disease: a cohort analysis of 5.4 million UK adults. Eur Heart J 2018;39:3596–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nazarzadeh M, Pinho-Gomes A-C, Smith Byrne K, Canoy D, Raimondi F, Ayala Solares JRet al. . Systolic blood pressure and risk of valvular heart disease: a mendelian randomization study. JAMA Cardiol 2019;4:788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. NCD Risk Factor Collaboration . Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet North Am Ed 2021;398:957–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Geldsetzer P, Manne-Goehler J, Marcus M-E, Ebert C, Zhumadilov Z, Wesseh CSet al. . The state of hypertension care in 44 low-income and middle-income countries: a cross-sectional study of nationally representative individual-level data from 1·1 million adults. Lancet North Am Ed 2019;394:652–662. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were derived from sources in the public domain: Institute for Health Metrics and Evaluation (IHME), at http://ghdx.healthdata.org/gbd-results-tool, accessed 1 October 2022.