Abstract
Cerebellar, hippocampal, and basal nuclei transient edema with restricted diffusion (CHANTER) syndrome is a constellation of specific imaging findings characterized by cytotoxic edema in the bilateral hippocampi, cerebellar cortices, and basal ganglia in patients presenting with altered mental status in the setting of substance intoxication. Previous case reports have demonstrated a strong correlation between CHANTER syndrome and polysubstance abuse, particularly with opioid intoxication. The patient we present in this case was found unresponsive following opioid use and demonstrated a constellation of findings on initial and follow-up imaging, consistent with CHANTER syndrome. While cases of irreversible brain damage or death during hospitalization have been reported in the literature, our patient demonstrated near-full recovery a few days after admission to the hospital. We aim to highlight the presentation and progression of CHANTER syndrome and alert clinicians and radiologists to include this entity in their diagnostic checklist for patients with polysubstance abuse and altered mental status.
Keywords: CHANTER, Opioid intoxication, Cerebellum, Hippocampi, Basal ganglia, Leukoencephalopathy
Introduction
Cerebellar, hippocampal, and basal nuclei transient edema with restricted diffusion (CHANTER) syndrome is a recently described constellation of imaging findings in adults with opioid neurotoxicity. It was first described by a group of clinicians in a case series in 2019 and further characterized by other case series and case reports in the following years [1]. The syndrome is characterized by transient cytotoxic edema in the bilateral cerebellar cortices, hippocampi, and basal ganglia accompanied by diffusion restriction and, in some cases, obstructive hydrocephalus secondary to the edema [1]. It is believed that CHANTER may be related to primary metabolic or mitochondrial failure with some degree of anoxic brain injury secondary to opioid use [1]. Data from postmortem analysis of heroin users demonstrated hippocampal Purkinje cell loss and evidence of micro- or astroglial reactivity which may represent a combination of hypoxic and neurotoxic effects [2]. In another study, fentanyl and other synthetic opiates have been linked to focal brain injury, including the hippocampus [3]. Although this entity shares a very similar clinical picture with other opioid-toxidromes such as heroin-associated spongiform leukoencephalopathy or opioid-associated amnestic syndrome, as well as common neurologic entities such as ischemic stroke, hypoxic injury, and metabolic leukoencephalopathies, the distinct imaging pattern in CHANTER helps radiologists and clinicians to identify this entity from other possible differential diagnoses [1,4]. In this case, we present a 47-year-old female with a history of HIV and polysubstance use who presented to the emergency department of our medical center with altered mental status and was subsequently diagnosed with CHANTER syndrome.
Case presentation
A 47-year-old African American female with a history of recreational drug use presented to the emergency department after being found unresponsive at a bus stop. Physical examination revealed a respiratory rate of 5 breaths per minute, an oxygen saturation of 77% on room air, blood pressure of 83/71 mm Hg, temperature of 92.5°F, pinpoint pupils, and minimal response to sternal rub. The patient was administered 2 mg of Naloxone without significant improvement, followed by an additional 4 mg, which resulted in slightly increased alertness. Given the low serum glucose level of 56 mg/dL, 25 mL of 50% dextrose solution was administered. The patient was intubated due to persistent reduced responsiveness and the need to protect the airway. Several etiologies were considered, including ischemic, metabolic, infectious, drug-related, and traumatic.
Owing to the wide range of potential differentials, an extensive laboratory workup was conducted, revealing leukocytosis of 18.29 K/uL (3.6-9.5 K/uL), elevated serum lactate of 3.1 mmol/L (0.5-2.2 mmol/L), elevated serum creatinine of 2.2 mg/dL (0.4 – 1.0 mg/dL), and mild hyponatremia of 132 mmol/L (135-145 mmol/L). Blood cultures were negative. Drug screening was positive for cocaine and phencyclidine, while a drug screening performed 6 months prior tested positive for amphetamines, cocaine, and phencyclidine.
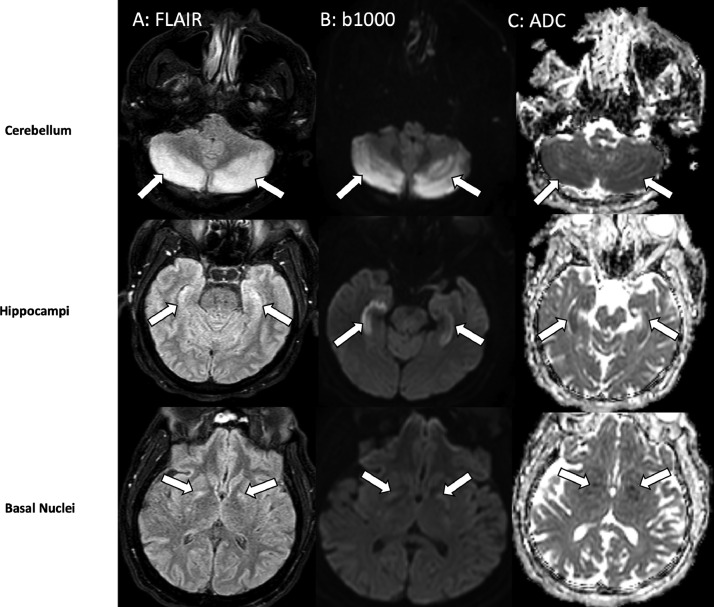
Noncontrast computed tomography (CT) of the brain revealed multiple hypodense regions in the cerebellar hemispheres (Fig. 1A), basal ganglia (Fig. 1B), and hippocampi (Fig. 1C). Subsequent magnetic resonance (MR) imaging of the brain displayed multiple areas of diffusion restriction and FLAIR hyperintensities within the cerebellar hemispheres, hippocampus, and putamen bilaterally (Fig. 2A–C). Additionally, mild diffuse cerebral edema was noted with mass effect on the fourth ventricle, without evidence of brain herniation. The bilateral imaging findings did not correspond to a specific vascular territory, so a differential of toxic or metabolic insult was considered. Furthermore, the evidence of recreational drug abuse along with the above-described characteristic imaging findings were strongly suggestive of CHANTER syndrome.
Fig. 1.
Noncontrast computed tomography images of the head from the patient at the time of arrival. Representative images from axial noncontrast CT head demonstrate hypodense areas within bilateral cerebellar hemispheres (arrows in A), bilateral basal ganglia (arrows in B) and bilateral hippocampi (arrows in C).
Fig. 2.
Axial FLAIR, (A) diffusion-weighted, (B) and ADC (C) MR images at the level of the cerebellum (top row), hippocampi (middle row) and basal nuclei (bottom row) demonstrate symmetric high signal within the cerebellar hemispheres, bilateral hippocampi and globus pallidus of basal ganglia (arrows in A, B, C). Hypointensity of the involved areas on ADC images (C) indicates restricted diffusion and cytotoxic edema.
The patient was managed conservatively with fluid resuscitation, electrolyte replenishment, antiseizure medication, and blood pressure control. Antibiotics were discontinued due to negative blood cultures. By day 4, the patient's condition improved. Upon discharge, the patient was awake and alert to self and place, but not to time. Neurological examination indicated bilateral upper extremity strength of 5/5, bilateral lower extremity strength of 4/5, and mildly reduced sensation in the lower extremities bilaterally.
Discussion
It is well known that in patients with altered mental status, differential diagnosis can be a long list of possibilities, including but not limited to acute ischemic stroke, anoxic brain injury, posterior reversible encephalopathy syndrome (PRES), and other described toxic and metabolic injuries. All these entities share similar clinical and radiologic features, which can be challenging for physicians to differentiate 1 from another and promptly make a correct diagnosis. It is crucial to determine if the underlying etiology is a reversible pathology and, most importantly, whether the outcome can drastically change positively with urgent intervention [1,[4], [5], [6]].
Given the distinct pattern of involvement in the brain on imaging and history of opioid abuse, CHANTER syndrome is an important entity that should be distinguished from other clinical entities with similar presentation and should be included in the clinical and radiological differential diagnoses in patients with cognitive decline and history of substance abuse [1,[4], [5], [6]].
Acute ischemic stroke is almost always at the top of clinical differential diagnoses in patients with altered mental status. Restricted diffusion on brain MRI is one of the radiologic features in CHANTER syndrome and might be misinterpreted for acute ischemic stroke. However, CHANTER syndrome involves particular brain regions without favoring a vascular distribution and does not have an underlying vascular occlusion on imaging [1].
PRES is another clinical entity that can share similar radiologic and clinical features with CHANTER. Although PRES might affect the posterior fossa and cause edema with mass effect, it predominantly affects the white matter, contrary to CHANTER syndrome [1].
Opioid toxidromes also demonstrate features akin to CHANTER syndrome, including opioid-associated amnestic syndrome (OAA), pediatric opioid use-associated neurotoxicity with cerebellar edema syndrome (POUNCE), and heroin-associated spongiform leukoencephalopathy, also known as “chasing the dragon leukoencephalopathy” [1,4]. In the case series reported by Mallikarjun et al. [4] in 2022, the authors mentioned the similarities between these clinical entities and alluded to the possibility of a spectrum of opioid syndromes consisting of CHANTER, OAA, and POUNCE syndrome.
OAA shares similar features with CHANTER syndrome in patients with opioid use, particularly with fentanyl [4,[7], [8], [9], [10], [11], [12]]. Patients typically present with complaints of amnesia after opioid use, and they usually have milder clinical course and better outcomes in comparison to CHANTER syndrome [4]. Imaging in OAA patients demonstrates T2/FLAIR signal hyperintensity with concurrent restricted diffusion in the hippocampi, but the cerebellum and deep nuclei are usually spared. Another defining feature of OAA is the absence of obstructive hydrocephalus, given that the cerebellum is often spared [4].
Heroin-associated spongiform leukoencephalopathy is strictly seen in patients inhaling heroin vapors. It affects predominantly white matter without significant diffusion restriction, which differs from CHANTER, where gray matter predilection and restricted diffusion are hallmarks of the entity [1].
POUNCE is another syndrome that belongs to the proposed spectrum, which is seen in the pediatric population after exposure to opioids and the patient typically presents with altered mental status [13,14]. On imaging, malignant cerebellar edema is a prominent finding which may result in obstructive hydrocephalus similar to CHANTER syndrome [13,15,16]. However, POUNCE syndrome rarely involves the hippocampi and basal ganglia, and it rather has a predilection for white matter with areas of hypoattenuation on CT, and T2 hyperintense lesions on MRI [13], [14], [15], [16], [17].
CHANTER syndrome is associated with a wide range of clinical outcomes, varying from rapid recovery to severe morbidity and death [1,4]. While some patients in the prior case series had poor prognoses or died, most had favorable outcomes with near-complete recovery or mild-to-moderate residual impairment. Like many diagnosed with CHANTER syndrome, our patient demonstrated a swift and complete recovery after presenting with severely declined cognitive function and requiring intubation [1,4,5,18]. However, our patient differed from most prior cases in that they had cerebellar edema and some degree of mass effect on the 4th ventricle, without resulting in obstructive hydrocephalus [1,4,5,18]. In our case, the patient also had cerebellar edema and some degree of mass effect on the fourth ventricle, which was not to the extent to cause obstructive hydrocephalus. For this reason, we believe that the absence of malignant mass effect and resultant obstructive hydrocephalus might have played a significant role in our patient's quick recovery without debilitating complications.
Conclusion
We present a case of CHANTER syndrome with near-complete recovery. We believe that clinicians and radiologists should be in the know about this entity and include it in their differential. Although there are some exceptions in prior case reports with poor outcomes with significant morbidity and even death, patients diagnosed with CHANTER syndrome had an overall good prognosis with early identification and intervention.
Patient consent
The authors declare that this report does not contain any personal information that could lead to the identification of the patients. Written informed consent for the publication of this case report was obtained from the patient.
Footnotes
Acknowledgments: The authors received no financial support for the research, authorship, and/or publication of this article
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Jasne AS, Alsherbini KH, Smith MS, Pandhi A, Vagal A, Kanter D. Cerebellar hippocampal and basal nuclei transient edema with restricted diffusion (CHANTER) syndrome. Neurocrit Care. 2019;31:288–296. doi: 10.1007/s12028-018-00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oehmichen M, Meissner C, Reiter A, Birkholz M. Neuropathology in non-human immunodeficiency virus-infected drug addicts: hypoxic brain damage after chronic intravenous drug abuse. Acta Neuropathol. 1996;91:642–646. doi: 10.1007/s004010050478. [DOI] [PubMed] [Google Scholar]
- 3.Kofke WA, Blissitt PA, Rao H, Wang J, Addya K, Detre J. Remifentanil-induced cerebral blood flow effects in normal humans: dose and ApoE genotype. Anesth Analg. 2007;105:167–175. doi: 10.1213/01.ane.0000266490.64814.ff. [DOI] [PubMed] [Google Scholar]
- 4.Mallikarjun KS, Parsons MS, Nigogosyan Z, Goyal MS, Eldaya RW. Neuroimaging findings in CHANTER syndrome: a case series. Am J Neuroradiol. 2022;43:1136–1141. doi: 10.3174/ajnr.A7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi N, Antimisiaris M, Lakhani S. CHANTER syndrome: a case report with near complete recovery. Neurology. 2021;96:2050. [Google Scholar]
- 6.Ahmed U, Wilson R, Hung SC. Bilateral cerebellar hemorrhagic infarcts as an early presentation following opioid-induced toxic encephalopathy in an adult patient. Radiol Case Rep. 2021;16:1207–1210. doi: 10.1016/j.radcr.2021.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barash JA, Whitledge J, Watson CJ, Boyle K, Lim C, Lev MH, et al. Opioid-associated amnestic syndrome: description of the syndrome and validation of a proposed definition. J Neurol Sci. 2020;417:117048. doi: 10.1016/j.jns.2020.117048. [DOI] [PubMed] [Google Scholar]
- 8.Small JE, Butler PM, Zabar Y, Barash JA. Complete, bilateral hippocampal ischemia: a case series. Neurocase. 2016;22:411–415. doi: 10.1080/13554794.2016.1213299. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharyya S, Gholipour T, Colorado RA, Klein JP. Bilateral hippocampal restricted diffusion: same picture many causes. J Neuroimaging. 2017;27:300–305. doi: 10.1111/jon.12420. [DOI] [PubMed] [Google Scholar]
- 10.Barash JA, Somerville N, DeMaria A. Cluster of an Unusual Amnestic Syndrome — Massachusetts, 2012–2016. MMWR Morb Mortal Wkly Rep. 2017;66:76–79. doi: 10.15585/mmwr.mm6603a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barash JA, Ganetsky M, Boyle KL, Raman V, Toce MS, Kaplan S, et al. Acute amnestic syndrome associated with fentanyl overdose. N Engl J Med. 2018;378:1157–1158. doi: 10.1056/NEJMc1716355. [DOI] [PubMed] [Google Scholar]
- 12.Taylor RG, Budhram A, Lee DH, Mirsattari SM. Opioid-associated amnestic syndrome observed with fentanyl patch use. CMAJ. 2019;191:E337–E339. doi: 10.1503/cmaj.181291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen CH, Mullen AJ, Hofstede D, Rizvi T. Malignant cerebellar edema in three-year-old girl following accidental opioid ingestion and fentanyl administration. Neuroradiol J. 2019;32:386–391. doi: 10.1177/1971400919863713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jasne AS, Alsherbini KA, Smith MS, Kuohn LR, Pandhi A, Vagal A, et al. Response to Malignant cerebella edema in three-year-old girl following accidental opioid ingestion and fentanyl administration. Neuroradiol J. 2020;33:158. doi: 10.1177/1971400920903106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duran D, Messina RD, Beslow LA, Montejo JD, Karimy JK, Gavankar Furey C, et al. Malignant cerebellar edema subsequent to accidental prescription opioid intoxication in children. Front Neurol. 2017;8:362. doi: 10.3389/fneur.2017.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shrot S, Poretti A, Tucker EW, Soares BP, Huisman TA. Acute brain injury following illicit drug abuse in adolescent and young adult patients: spectrum of neuroimaging findings. Neuroradiol J. 2017;30:144–150. doi: 10.1177/1971400917691994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wheaton T, Toll BJ, Breznak K, Da-Silva S, Melvin J, Misra A, et al. Opioid-induced toxic leukoencephalopathy: a case report and review of the literature. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e03005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou YMJ, Shah M, Fayngersh A. A case of cerebellar hippocampal and basal nuclei transient edema with restricted diffusion syndrome with poor clinical outcome. Cureus. 2022;14:e22767. doi: 10.7759/cureus.22767. [DOI] [PMC free article] [PubMed] [Google Scholar]