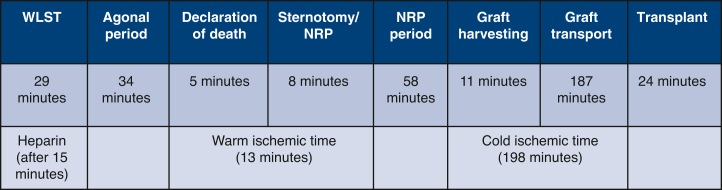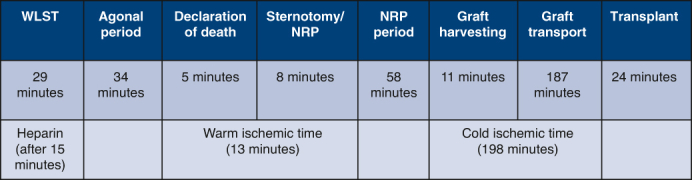
DCD timeline.
Central Message.
DCD can greatly increase the pediatric donor pool. This is the first reported case of an infant DCD heart transplantation in the US using NRP and distant transport.
Cardiac donation after circulatory death (DCD) may greatly increase the pediatric donor pool1 and decrease waitlist times. Pediatric patients <40 kg are not suitable for ex vivo organ care systems and traditional DCD procurement methods.2 We established a pediatric normothermic regional perfusion (NRP) procurement protocol using extracorporeal membrane circulation (ECMO) in attempt to mirror the excellent results with adult DCD-NRP transplantation. This is the first report of a pediatric infant DCD–heart transplantation (HT) in the United States using NRP.
The recipient was a 6-month-old female (6.2 kg) who presented to an outside hospital in cardiac arrest, requiring venoarterial ECMO support at 6 weeks of age. The patient was later weaned from ECMO, underwent permanent epicardial pacemaker implantation, and remained hospitalized with severe biventricular heart failure. The patient was transferred to our center and listed status 1A for transplantation. Our listing strategy was to accept the first-available suitable organ regardless of DCD or circulatory death, given data suggesting comparable outcomes in the adult population. Risks and benefits of DCD and alternatives were reviewed and discussed extensively with the parents. This report was published with permission from the institutional review board, and written consent was waived. Parental verbal consent for publication of study data was acquired.
After a 2-month wait, an ABO-incompatible DCD heart became available. The donor was a 2-month-old 66 cm/6.4 kg infant in a distant center (180 minutes’ travel time). The procuring team consisted of 2 surgeons and 2 perfusion specialists. The surgical back table and instruments were prepared, and an ECMO pump and circuit were blood primed. Withdrawal of life-sustaining therapy (WLST: extubation, stopping intravenous drips) was performed in the operating room, with the parents holding the baby. Heparin was administered antemortem 15 minutes after WLST. The donor was declared deceased 63 minutes after WLST, followed by a 5-minute “stand off” period (donor hospital’s policy). After this, the donor was placed on the operating room table, positioned, prepped, and draped in a sterile fashion. Median sternotomy was undertaken expeditiously, arch vessels (carotids, subclavian arteries) were ligated, and the aorta (10 F) and right atrium (24 F) were cannulated for ECMO support (150 mL/kg flow). The heart recovered normal sinus rhythm and activity a few seconds after initiation of ECMO support. Time from end of “stand-off” period until initiation of ECMO support was 8 minutes. Reintubation was undertaken, and ventilation (8 mL/kg tidal volume, rate of 24/min) was initiated.
The heart was reanimated for a total period of 40 minutes with no inotropic support, after which weaning off ECMO support was performed. Transesophageal echocardiography (TEE) demonstrated normal ventricular function. The heart was deemed suitable for transplantation, and the recipient repeat median sternotomy initiated. Next, the donor heart was preserved using cold del Nido cardioplegia solution, procured, and transported (Paragonix SherpaPak Cardiac Transport System). Upon arrival at our hospital, the heart was given a full dose (30 mL/kg) of cold del Nido cardioplegia, implanted in the standard bicaval fashion. Total cold ischemia time was 198 minutes. Key time points are summarized in Figure 1. The donor heart and postoperative patient recovery were unremarkable. Postimplant TEE showed good left ventricular and mildly reduced right ventricular function. Chest was closed on postoperative day (POD) 1. The patient was extubated on POD 4 and discharged home on POD 56. Most recent echocardiogram and catheter (POD 150) demonstrated normal biventricular function, central venous pressure 7 mm Hg, pulmonary capillary wedge pressure 10 mm Hg, and cardiac index 3.6 L/min/m2. The postoperative recovery did not deviate from that of a non-DCD.
Figure 1.
Key time points. WLST, Withdrawal of life-sustaining therapy; NRP, normothermic regional perfusion.
To the best of the authors knowledge, this report describes the first infant DCD–HT in the United States using NRP. Similar previous cases were reported from by Gil-Jaurena and colleagues3 from Spain and Tchana-Sato and colleages4 from Belgium. One benefit of NRP is the ability to reanimate the heart after WLST and perform a functional assessment before retrieval using TEE (other biochemical markers as troponin may be useful as well).
Direct aortic and right atrial cannulation via median sternotomy for donors <30 kg can be achieved expeditiously (<10 minutes) and allows for safe cardiac reperfusion. This technique avoids the need for premortem cannulation and allows arch vessel control before the initiation of ECMO support to exclude cerebral reperfusion concerns. The recipient surgical procedure should not begin before verification of donor heart reanimation and recovery, which requires careful coordination.
This case, together with positive outcomes in DCD transplantation,2,5 supports the consideration of a US clinical trial of DCD–HT with NRP in carefully selected pediatric donors and recipients.
In this first reported US case of DCD-NRP, a 2-month-old donor infant from a distant hospital underwent cardiac graft rescue on ECMO support, cold storage for 198 minutes, and implantation in an ABO-noncompatible recipient, with successful cardiac recovery and postoperative course. This approach may increase the donor pool and allow for many more infant and neonatal heart transplantations.
Footnotes
Disclosures: The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Read at the 103rd Annual Meeting of The American Association for Thoracic Surgery, Los Angeles, California, May 6-9, 2023.
References
- 1.Scheuer S.E., Jansz P.C., Macdonald P.S. Heart transplantation following donation after circulatory death: expanding the donor pool. J Heart Lung Transplant. 2021;40:882–889. doi: 10.1016/j.healun.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Andersen N.D., Bryner B.S., Aughtman S.L., Kang L., Carboni M.P., Casalinova S., et al. A report of the first pediatric heart transplant following donation after circulatory death in the United States using ex-vivo perfusion. J Heart Lung Transplant. 2022;42:287–288. doi: 10.1016/j.healun.2022.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Gil-Jaurena J.M., Pérez-Caballero R., Murgoitio U., Pardo C., Pita A., Calle C., et al. A neonatal ABO non-compatible heart transplant from a circulatory-determined death donor using NRP/cold storage. Pediatr Transplant. 2021;26 doi: 10.1111/petr.14169. [DOI] [PubMed] [Google Scholar]
- 4.Tchana-Sato V., Ledoux D., Vandendriessche K., Van Cleemput J., Hans G., Ancion A., et al. First report of a successful pediatric heart transplantation from donation after circulatory death with distant procurement using normothermic regional perfusion and cold storage. J Heart Lung Transplant. 2019;38:1112–1115. doi: 10.1016/j.healun.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Truby L.K., Casalinova S., Patel C.B., Agarwal R., Holley C.L., Mentz R.J., et al. Donation after circulatory death in heart transplantation: history, outcomes, clinical challenges, and opportunities to expand the donor pool. J Card Fail. 2022;28:1456–1463. doi: 10.1016/j.cardfail.2022.03.353. [DOI] [PubMed] [Google Scholar]