Abstract
Aims
Frailty is disproportionately prevalent in cardiovascular disease patients and exacerbated during hospital admissions, heightening the risk for adverse events and functional decline. Using the Essential Frailty Toolset (EFT) to target physical weakness, cognitive impairment, malnourishment, and anaemia, we tested a multicomponent targeted intervention to de-frail older adults with acute cardiovascular conditions during their hospital admission.
Methods and results
The TARGET-EFT trial was a single-center randomized clinical trial at the Jewish General Hospital, Montreal, Canada. We compared a multicomponent de-frailing intervention with usual clinical care. Intervention group patients received exercise, cognitive stimulation, protein supplementation, and iron replacement, as required. In this study, the primary outcome was frailty, as assessed by the SPPB score (Short Physical Performance Battery) at discharge, and the secondary outcome was the SARC-F score (Strength, Assistance walking, Rising from chair, Climbing, Falls) assessed 30 days later. The analysis consisted of 135 patients (mean age of 79.3 years; 54% female) who survived and completed the frailty assessments.
Compared with control patients, intervention group patients had a 1.52-point superior SPPB score and a 0.74-point superior SARC-F score. Subgroup analysis suggested that patients with low left ventricular ejection fraction may have attenuated benefits, and that patients who underwent invasive cardiac procedures had the greatest benefits from the intervention.
Conclusion
We achieved our objective of de-frailing older cardiac inpatients on a short-term basis by improving their physical performance and functioning using a pragmatic multicomponent intervention. This could have positive impacts on their clinical outcomes and ability to maintain independent living in the future.
One sentence summary
The multicomponent intervention targeted to the deficits of vulnerable older adults hospitalized with acute cardiovascular diseases successfully de-frailed them on a short-term basis, which can have positive implications on their post-discharge health outcomes.
Keywords: randomized clinical trial, frailty, physical performance, intervention, cardiology, geriatrics
Introduction
Frailty, a geriatric syndrome that interferes with the physiological mechanisms required for healthy homeostasis after a stressor, has been found to be disproportionately prevalent in cardiovascular disease (CVD) patients.1,2 The prevalence of frailty is estimated to be 10% in community-dwelling older adults, and up to 50% in high-risk subgroups, such as those with heart failure.3–5 In fact, frailty and CVD have been found to have a bidirectional relationship, where frailty increases risk of fatal and non-fatal CVD, CVD increases the risk of prevalent and incident frailty, and the combination increases the risk of functional decline and all-cause mortality by two- three-fold.5–8
The Essential Frailty Toolset (EFT) is a screening tool that focuses on four actionable domains: lower extremity weakness, cognitive impairment, malnourishment, and anaemia.6,9 All of these are exacerbated during a hospital admission, the reasons for which are multifactorial and include: acute illness, bedrest, undernutrition, disturbed sleep patterns, and repeated blood tests.10,11 As a result, older patients often leave the hospital frailer than they were beforehand and have difficulty regaining their physical and mental capabilities. In turn, greater degrees of frailty using the EFT have been shown to be predictive of incident disability and mortality in clinical cardiac populations.6,9
Frailty is potentially modifiable, and admission to the hospital presents an opportunistic timeframe and captive audience for initiating interventions aimed at de-frailing patients at the same time as their cardiac care. Research has shown positive impacts of in-hospital exercise programs, nutritional supplementation, cognitive stimulation, and anaemia correction in medical patients,12–16 yet there is a paucity of evidence in acute CVD patients and using multicomponent interventions. We hypothesized that a multicomponent de-frailing intervention would improve physical frailty in vulnerable older adults admitted to the hospital with acute cardiovascular conditions.
Methods
Trial design & participants
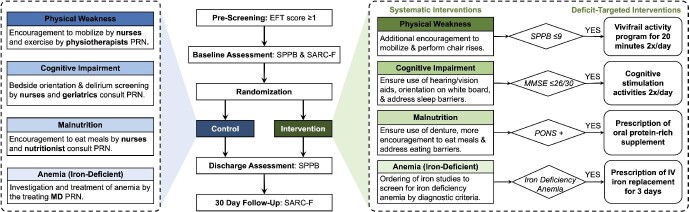
We conducted the TARGET-EFT trial (MulTicomponent Acute Intervention in FRail GEriatric PaTients with Cardiovascular Disease using the Essential Frailty Toolset) to assess the effect of a multicomponent geriatric intervention on patient-centered outcomes (ClinicalTrials.gov identifier: NCT04291690). We now report the results of a pre-planned analysis for a key secondary outcome—frailty. The methodology of the TARGET-EFT trial complied with the Declaration of Helsinki, was approved by the institutional review board of the Jewish General Hospital, and has previously been described in detail.17 In brief, TARGET-EFT was a parallel-group randomized controlled trial at the Jewish General Hospital (Montreal, Canada), an academic tertiary care center affiliated with McGill University. Consenting patients admitted to the cardiovascular ward who were aged ≥65 years with signs of frailty (EFT ≥ 1) were randomized using 1:1 block randomization stratified by sex. Detailed inclusion and exclusion criteria are listed in the supplementary material online, Table S1. After randomization, patients underwent further frailty assessments to confirm the frailty deficits identified through the EFT. These consisted of the Short Physical Performance Batter (SPPB) for physical weakness,18 the Mini-Mental State Examination (MMSE) for cognitive deficits,19 the Preoperative Nutrition Score (PONS) for malnutrition,20 and iron studies for anaemia.
Control group
Patients randomized to the control group received usual clinical care. This consisted of treatment of their cardiovascular condition by cardiologists, along with inpatient physiotherapy, nutritional support, treatment of anaemia, and consultation with healthcare specialists at the discretion of the treating team (Figure 1). Physiotherapy involvement is systematic post-cardiac surgery, but otherwise it is variable depending on the elicited needs of the patient and referral of the treating clinician. Most typically on our ward, physiotherapists visit their inpatients 2–3 times per week and focus on mobility and balance. The standard cardiac diet on our ward contains three meals per day with low salt (under 2300 mg), moderate fat, and at least one protein per meal. Breakfast contains two starches; lunch and dinner contain a soup, main meal, and dessert (usually a yogurt or fruit).
Figure 1.
Trial design snapshot. The middle section depicts the timeline of frailty assessments. The left (blue) section depicts the components of usual care received by all patients. The right (green) section depicts the components of our multicomponent intervention, subdivided by systematic interventions received by all intervention group patients and deficit-targeted interventions received by only intervention group patients who had confirmed deficits in those specific domains. EFT, essential frailty toolset; IV, intravenous; MMSE, mini mental state examination, PONS, preoperative nutrition score; PRN, as needed per clinical indication; SPPB, short physical performance battery.
Intervention group
Patients randomized to the intervention group received usual clinical care, as well as the EFT-based interventions (Figure 1). All intervention group patients received bi-daily visits from an assigned research team member who provided orientation to time and place, encouragement to mobilize and perform chair rise exercises, encouragement to wear hearing/visual aids and dentures, encouragement to eat their regular meals, encouragement to sleep without interruptions, with help to address barriers to nutrition and sleep; if anaemic, they received investigations for iron deficiency. Moreover, intervention patients received additional therapies depending on the frailty deficits identified in their individualized cases.
Specifically, patients with physical weakness received bi-daily supervised, 20-minute, multicomponent exercise sessions combining strength, flexibility, balance and gait exercises for the prevention of weakness and falls, adapted from the Vivifrail program.21 Patients were encouraged to continue these exercises along with the consumption of a healthy diet at home post-discharge. Patients with cognitive deficits received cognitive simulation twice daily consisting of activities such as reading the news, doing crossword puzzles, and playing memory games. Those who had confirmed malnourishment received MedPass supplementation; MedPass is a 60 mL calorically- and protein-dense (2 kcal/mL) oral nutritional supplement consumed between meals four times per day. Finally, those with confirmed iron deficiency anaemia (i.e., haemoglobin <130 g/L in men or <120 g/L in women, in addition to ferritin <100 ug/L, or ferritin <300 ug/L and iron saturation <20%) were prescribed intravenous iron sucrose at 300 mg/day for three consecutive days.22
Outcomes
This study sought to determine whether the intervention caused changes in physical frailty as measured by the SPPB and SARC-F scales. The primary outcome of this study was the SBBP score at the time of discharge from the cardiovascular unit. SPPB includes three physical tests scored from 0 to 4 for a total score from 0 to 12 (0 = worst, 12 = best): time to walk 5 meters at a comfortable pace (best of two trials), time to stand five times from a chair without using arms, and 10-second standing balance in three positions (feet together, semi-tandem, and full tandem).18 The secondary outcome for this study was the SARC-F score ascertained by a blinded assessor at 30 days post-discharge from the cardiovascular unit. SARC-F includes five self-reported questions scored from 0 to 2 for a total score from 0 to 10 (0 = best, 10 = worst): difficulty with transferring, walking, carrying objects, climbing stairs and history of falls.23 The main outcomes for the overarching trial, reported separately, were the EQ-5D-5L scale for health-related quality of life and the OARS (Older American Resources and Services) scale for hospital-acquired disability at 30 days.
Statistical analysis
We performed intention-to-treat analysis using multivariable linear regression to determine the effect of the intervention on the continuous physical frailty score after adjusting for the baseline score and duration of hospitalization (number of days from the date of randomization to discharge or death). We tested for effect modification for age, sex, duration of hospitalization, cardiac surgery or transcatheter valve replacement during the index hospitalization (i.e., invasive cardiac procedures), obesity, diabetes, left ventricular ejection fraction (LVEF), baseline New York Heart Association class (NYHA class), baseline Clinical Frailty Scale (CFS) score, baseline SPPB score, baseline MMSE score, and baseline PONS score. We performed randomization and data storage using REDCap electronic data capture tools hosted at the Lady Davis Institute's Centre for Clinical Epidemiology. All data analyses were performed using STATA version 17 (College Station, TX). The data underlying this article can be shared on reasonable request to the corresponding author.
Results
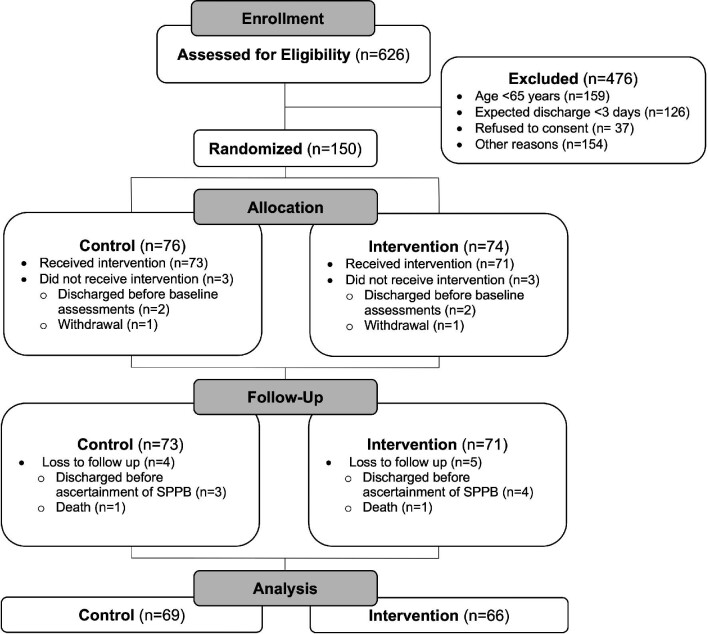
Out of 150 patients randomized between March 2020 and September 2021, this study analyzed 135 patients who completed the SPPB at discharge; all but two of which also completed the SARC-F at 30 days post-discharge. The flow diagram can be found in Figure 2. Participant baseline characteristics by allocation group can be found in Table 1 and in supplementary material online, Table S2. The mean age of all participants was 79.3 ± 7.7 years and 54% of all participants were females. The most common reasons for admission were evenly distributed between ischaemic heart disease and heart failure, followed by arrhythmia and valvular heart disease. The mean duration of all hospitalization after randomization was 11.0 ± 11.7 days. There were no reported intervention-related adverse events and no negative effects on renal function.
Figure 2.
Flow chart. Out of 150 patients randomized, 135 patients completed the primary outcome assessment at discharge (SPPB) and 133 completed the secondary outcome assessment at 30 days (SARC-F). SPPB, short physical performance battery.
Table 1.
Mean baseline characteristics of participants by group
| Intervention N = 66 | Control N = 69 | |
|---|---|---|
| Clinical characteristics | ||
| Age (years) | 78.2 ± 8.0 | 80.2 ± 7.3 |
| Female sex | 35 (53.0%) | 38 (55.1%) |
| BMI (kg/m2) | 28.3 ± 7.0 | 28.5 ± 6.3 |
| LVEF (%) | 51.7 ± 17.9 | 55.6 ± 15.6 |
| NYHA class | 2.6 ± 1.0 | 2.4 ± 1.0 |
| Heart failure | 22 (33.3%) | 19 (27.5%) |
| Diabetes | 29 (43.9%) | 42 (60.9%) |
| Percutaneous coronary intervention* | 8 (12.1%) | 88 (11.6%) |
| Cardiac surgery/transcatheter valve procedure* | 15 (22.7%) | 15 (21.7%) |
| Hospital days post-randomization | 11.5 ± 12.7 | 10.5 ± 10.7 |
| Reason for admission: | ||
| Ischaemic heart disease | 21 (31.8) | 19 (27.5) |
| Arrhythmia | 13 (19.7) | 5 (7.2) |
| Valvular heart disease | 6 (9.1) | 8 (11.6) |
| Congestive heart failure | 16 (24.2) | 23 (33.3) |
| Other | 10 (15.2) | 14 (20.3) |
| Frailty markers | ||
| EFT (out of 5) | 2.7 ± 1.1 | 2.8 ± 1.0 |
| SPPB (out of 12) | 4.4 ± 3.1 | 4.6 ± 2.9 |
| SARC-F (out of 10) | 5.4 ± 2.6 | 4.9 ± 2.6 |
| CFS (out of 9) | 4.4 ± 1.5 | 4.6 ± 1.4 |
| MMSE (out of 30) | 25.4 ± 3.6 | 25.6 ± 3.3 |
| PONS (out of 3) | 0.8 ± 0.8 | 0.6 ± 0.7 |
| Albumin (g/L) | 34.0 ± 4.5 | 34.8 ± 4.4 |
| Haemoglobin (g/L) | 105.1 ± 20.0 | 107.4 ± 19.4 |
| IDA | 28 (42.4%) | 23 (33.3%) |
*During the index hospital admission. BMI, body mass index; CFS, clinical frailty scale; EFT, essential frailty toolset; IDA, iron deficiency anaemia; LVEF, left ventricular ejection fraction; MMSE, mini mental state examination; NYHA Class, New York Heart Association Functional Classification; PONS, preoperative nutrition score; SPPB, short physical performance battery.
Interventions
The therapies received by group can be found in Table 2. All intervention patients received encouragement and support for physical activity, cognitive orientation, and eating meals; in addition, depending on the frailty deficits identified, 94% received the Vivifrail exercise program (mean of 1.0 session per weekday and planned rest onweekend days), 42% received cognitive stimulation activities (mean of 1.1 session per day), 49% received oral nutritional supplements (compared to 22% of control patients), and 35% received intravenous iron replacement therapy (compared to 16% of control patients). There were no significant between-group differences in those who received clinical consultations with geriatric medicine specialists and allied-health professionals.
Table 2.
Therapies received by group
| Intervention | Control | |
|---|---|---|
| N = 66 | N = 69 | |
| Trial interventions | ||
| Vivifrail exercise | 93.9% (1.0/day) | – |
| Cognitive stimulation | 42.4% (1.1/day) | – |
| Nutritional supplementation | 48.5% | 21.7% |
| Intravenous iron replacement | 34.8% | 15.9% |
| Non-trial intervention | ||
| Physiotherapy consult | 56.1% | 55.1% |
| Occupational therapy consult | 33.3% | 21.7% |
| Nutritionist consult | 33.3% | 37.7% |
| Geriatrics consult | 6.1% | 2.9% |
| Psychiatry consult | 10.6% | 2.9% |
Outcomes
The mean SPPB score out of 12 (higher is stronger) was 4.5 ± 3.0 at baseline, 6.5 ± 3.3 at follow-up in the intervention group, 5.1 ± 3.3 at follow-up in the control group. The mean SARC-F score out of 10 (lower is stronger) was 5.2 ± 2.6 at baseline, 3.6 ± 2.3 at follow-up in the intervention group, 4.0 ± 2.4 at follow-up in the control group. The changes in SPPB and SARC-F scores from baseline to follow-up can be found in Figure 3, showing, on average, improved scores in intervention group patients and minimally changed scores in control group patients. Change in frailty scores after adjusting for length of intervention and baseline score can be found in Table 3. Compared with the control group, the intervention led to a 1.52-point superior SPPB score (95% CI 0.75, 2.29; P < 0.001; effect size 0.5) and a 0.74-point superior SARC-F score (95% CI -1.38, -0.11; P = 0.02; effect size 0.3).
Figure 3.
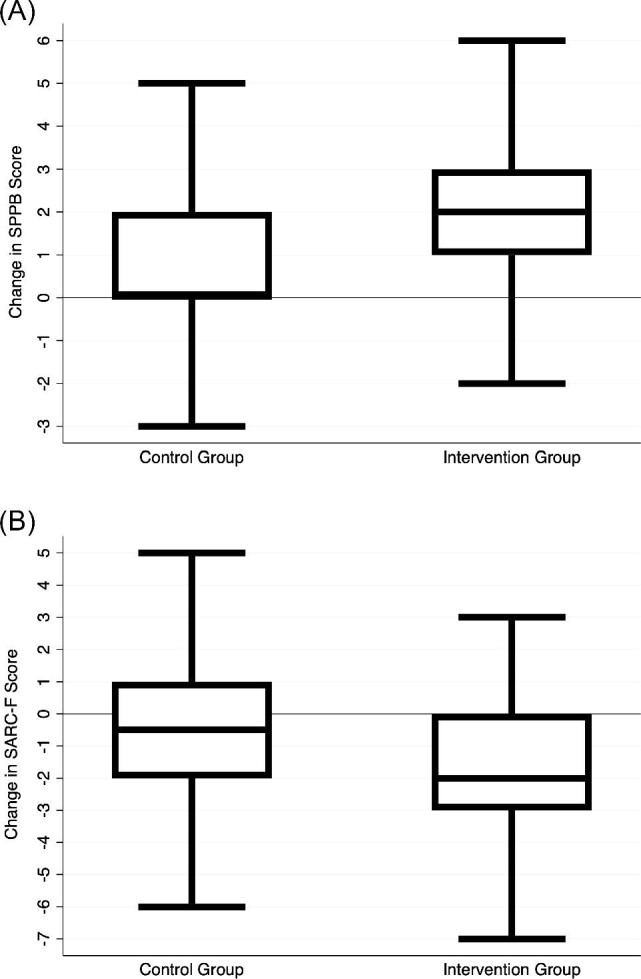
Change in frailty scores from baseline to follow-up. (A) The top panel depicts the change in SPPB score from baseline to discharge, which was favorable for the intervention group patients. (B) The bottom panel depicts the change in SARC-F score from baseline to 30 days post-discharge, which was favorable for the intervention group patients. SPPB, short physical performance battery.
Table 3.
Multivariable linear regression for frailty outcome measures
| SPPB at discharge Beta (95% CI); P-value | SARC-F at 30 days Beta (95% CI); P-value | |
|---|---|---|
| Intervention | 1.52 (0.75, 2.28); P < 0.001 | −0.74 (1.38, −0.11); P = 0.02 |
| Baseline frailty score | 0.75 (0.62, 0.89); P < 0.001 | 0.36 (0.23, 0.49); P < 0.001 |
| Hospitalization days | −0.04 (−0.07, −0.004); P = 0.03 | 0.07 (0.04, 0.10); P < 0.001 |
A positive beta denotes stronger SPPB, whereas a negative beta denotes stronger SARC-F. CI, confidence interval; SPPB, short physical performance battery.
Subgroup analysis
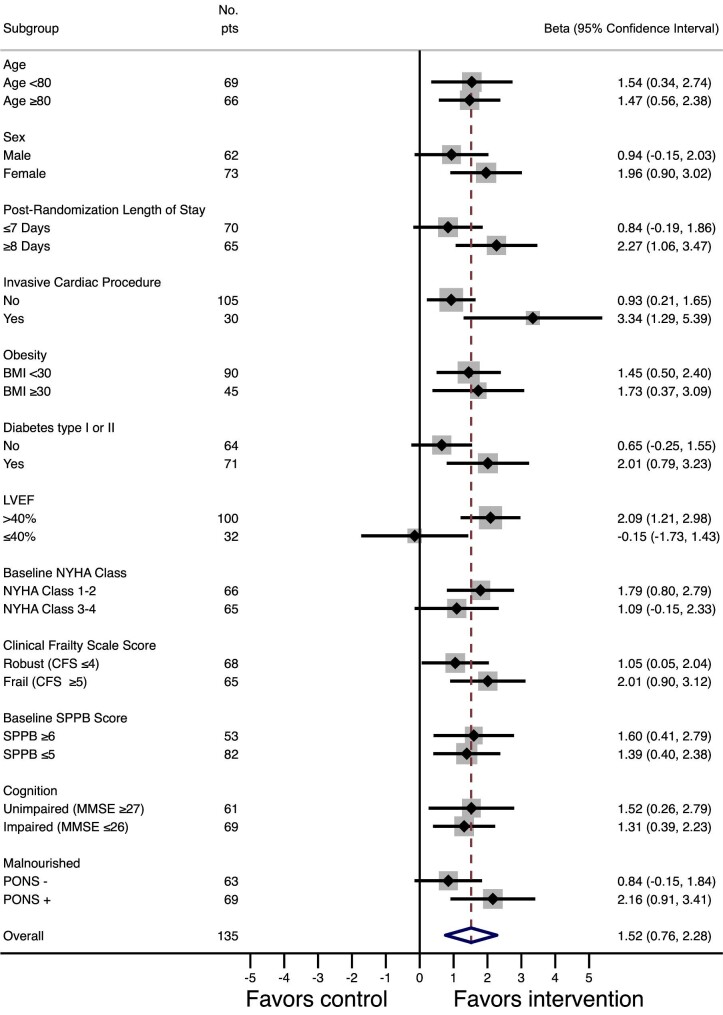
Forest plots for pre-defined subgroups can be found in Figure 4. Patients who had undergone cardiac surgery or transcatheter aortic valve replacement derived greater improvements in SPPB score with the intervention (interaction P = 0.007). Conversely, patients who had reduced LVEF ≤ 40% derived lesser improvements compared with those with LVEF > 40% (interaction P = 0.017) although this represented a small subgroup of 36 patients. Patients who did not have diabetes mellitus trended to derive lesser improvements (interaction P = 0.073) and those who were hospitalized for >7 days trended to derive greater improvements (P = 0.071). There were no significant interactions by age, sex, BMI, NYHA class, cognitive function, baseline nutritional status, or severity of frailty.
Figure 4.
Effect-modification forest plot. This subgroup analysis forest plot shows the adjusted beta coefficient effect for the intervention stratified by various subgroups of patients. There were two statistically significant interactions: patients who had invasive cardiac procedures derived greater benefits from the intervention, whereas those who had reduced left ventricular ejection fraction derived lesser benefits. There were two other trending interactions: patients who had longer length of stay and thus received a greater volume of intervention visits appeared to derive greater benefits, whereas non-diabetic patients appeared to derive lesser benefits. BMI, body mass index; CFS, clinical frailty score; MMSE, mini mental state examination; NYHA class, New York Heart Association functional classification; PONS, preoperative nutrition score; SPPB, short physical performance batter.
Discussion
In this trial, we achieved our objective of physically de-frailing older CVD inpatients through a multicomponent targeted geriatric intervention. Our intervention was safe and led to moderate improvements in frailty, as measured by the SPPB and SARC-F scales, which were clinically apparent as gains in physical performance and functioning. While community-based frailty interventions typically span 2–3 months or longer, the current trial is unique in that it spanned an average of 11 days within the hospital. Our results also demonstrate that patients undergoing invasive cardiac procedures have the greatest benefits from the intervention, rendering this a high-yield population for future implementation.
A paucity of randomized controlled trials have addressed de-frailing hospitalized patients, and none—to our knowledge—have been conducted on a CVD unit. Ekerstad et al. randomized 408 frail older inpatients to a comprehensive geriatric assessment-guided intervention and reported improvements in physical frailty and ability to perform activities of daily living 3 months post-discharge.24,25 Martínez–Velilla et al. randomized 370 frail older inpatients to a bi-daily resistance exercise intervention adapted from the Vivifrail program and reported improvements in the SPPB (2.2 points) at discharge.14,26 While they used specialized exercise equipment, we achieved similar benefits with a pragmatic bedside program. Our trial targeted patients with recently decompensated CVD, which were purposefully excluded by previous trials since they pose unique challenges such as symptomatic shortness of breath on exertion, wounds from recent cardiac interventions, and impediments from telemetry devices, oxygen tubing, and intravenous lines.
The downstream clinical impact of reducing frailty in hospitalized patients can be extrapolated from prior research. Frailty measured using the SPPB at the time of hospital discharge was associated with a three-fold increase in subsequent mortality or readmission and a 50% incidence of functional decline and disability at 1 year.27 Moreover, each 1-point improvement in the SPPB was associated with a 14% reduction in risk of mortality or readmission.27 Our intervention successfully led to a 1.5-point, superior SPPB score, which would be expected to translate to a meaningful reduction in mortality or readmission—although this remains to be proven through a dedicated randomized controlled trial. This hypothesis is supported by epidemiology data demonstrating that adverse outcomes after a CVD hospitalization are often non-cardiac in nature,28,29 and driven by comorbid diseases and geriatric issues, such as frailty. Furthermore, the observed 1.5-point superior SPPB score in the intervention group is greater than the previously defined threshold for minimal clinically meaningful change of 1 point.30 Given the number of patients enrolled, a posteriori sample size calculations showed a power of 0.82 to detect this magnitude of effect.
The patient-centered benefits of our intervention are evidenced by the superior SARC-F score at 30 days post-discharge in the intervention group, which reflects the functional consequences of physical frailty and sarcopenia.31 The observed superior SARC-F score translates to our intervention group patients feeling more capable of mobilizing, transferring, and performing physical tasks after returning to their home environment, which is critical to maintain independent living and foster rehabilitation after a CVD event. While the SARC-F has previously been used extensively to screen for frailty, including in CVD patients,32 use of the SARC-F as an outcome measure pre-post intervention is a novel aspect of this trial that appears to have empirical construct validity given the consistent effects between improving SARC-F and SPPB scales. The benefits of our intervention appeared to be less pronounced in patients with reduced LVEF, which may be driven by chance (due to the small size of this subgroup) or by the decreased volume of exercise completed during the brief 20-minute sessions (due to breathlessness, exhaustion and need for frequent pauses). Successful exercise interventions in heart failure patients have entailed longer sessions, allowing for warm up and graded intervals, over a period of at least 3 weeks.33
Limitations
A number of limitations should be acknowledged. First, the SPPB assessment at discharge was not blinded given that the trained personnel administering the assessment were also involved in delivering the intervention (or control). This potential bias is minimal given that the SPPB is a series of objectively-timed physical performance tests, with little assessor influence. Moreover, the SARC-F assessment was blinded and confirmed meaningful improvements in frailty. Secondly, the SARC-F questionnaire requires self-report of functional abilities that is susceptible to recall bias by the patient. This potential bias was mitigated by interviewer administration of the questionnaire and involvement of family members or caregivers whenever possible. Thirdly, though we aimed to deliver two exercise sessions per weekday for those who required this intervention, our achieved average was 1.0 sessions/weekday owing to the realities of a busy cardiovascular unit, wherein patients are often symptomatic or preoccupied with their cardiac tests and procedures. Despite this, we still achieved clinically meaningful improvements in physical frailty, further highlighting and strengthening the potential pragmatic nature of our intervention. Finally, the TARGET-EFT trial was a single-center trial, the first of its kind in CVD patients; multicenter trials are required to ensure the reproducibility and generalizability of our procedures and results. This is a critical issue to account for the potential variability in ‘usual care’ that may exist between centers, especially with respect to co-interventions, such as physiotherapy and nutritional support.
Conclusions
Our multicomponent intervention targeted to the deficits of older cardiac inpatients led to clinically meaningful improvements in short-term physical frailty, which has ramifications for physical functioning and health outcomes post-discharge. These findings have important clinical implications that will enable cardiovascular clinicians to reverse physical frailty in patients with CVD, thereby improving their physical function and health outcomes at discharge and post- discharge from the hospital.
Supplementary Material
Acknowledgement
The authors would like to acknowledge the doctors, nurses, allied health professionals, and clerical staff at the Jewish General Hospital Azrieli Heart Centre for supporting this trial.
Contributor Information
Fayeza Ahmad, Division of Experimental Medicine, McGill University, Montreal,QC H4A 3J1, Canada; Centre for Clinical Epidemiology, Jewish General Hospital, Montreal, QC H3T 1E2, Canada.
Rosie Fountotos, Division of Experimental Medicine, McGill University, Montreal,QC H4A 3J1, Canada; Centre for Clinical Epidemiology, Jewish General Hospital, Montreal, QC H3T 1E2, Canada.
Michael Goldfarb, Division of Experimental Medicine, McGill University, Montreal,QC H4A 3J1, Canada; Division of Cardiology, Jewish General Hospital, McGill University, Montreal, QC H3T 1E2, Canada.
Neetika Bharaj, Centre for Clinical Epidemiology, Jewish General Hospital, Montreal, QC H3T 1E2, Canada; Department of Kinesiology & Physical Education, McGill University, Montreal, QC H2W 1S4, Canada. Institution of research trial: Jewish General Hospital, Montreal, QC H3T 1E2, Canada.
Haroon Munir, Division of Experimental Medicine, McGill University, Montreal,QC H4A 3J1, Canada; Centre for Clinical Epidemiology, Jewish General Hospital, Montreal, QC H3T 1E2, Canada.
John Marsala, Division of Cardiology, Jewish General Hospital, McGill University, Montreal, QC H3T 1E2, Canada.
Lawrence G Rudski, Division of Cardiology, Jewish General Hospital, McGill University, Montreal, QC H3T 1E2, Canada.
Jonathan Afilalo, Division of Experimental Medicine, McGill University, Montreal,QC H4A 3J1, Canada; Centre for Clinical Epidemiology, Jewish General Hospital, Montreal, QC H3T 1E2, Canada; Division of Cardiology, Jewish General Hospital, McGill University, Montreal, QC H3T 1E2, Canada.
Funding
F.A. is supported by the CIHR Canada Graduate Scholarship for Master's and the Fond de Recherche en Santé du Québec Master's Scholarship. R.F. is supported by the Gordon Phillips Fellowship and Graduate Excellence Fellowship from the McGill Faculty of Medicine and Health Sciences. J.A. is supported by the Fond de Recherche en Santé du Québec Clinical Research Scholar Award.
Conflict of interest
The authors declare that there is no conflict of interest.
Author contributions
F.A. was responsible for conception and design, acquisition of data, project administration, analysis and interpretation of data, and writing of the original draft. R.F. was responsible for conception and design, acquisition of data, and project administration. N.B. was responsible for acquisition of data. H.M. was responsible for the conception and design, and acquisition of data. J.M. and L.G.R. were responsible for conception and design. M.G. was responsible for conception and design, and supervision. J.A. was responsible for the conception and design, analysis and interpretation of data, supervision, and writing. All authors critically read and reviewed the manuscript for important intellectual content and approval for publication.
Data availability
The e-data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H.. Role of frailty in patients with cardiovascular disease. Am J Cardiol 2009;103:1616–1621. [DOI] [PubMed] [Google Scholar]
- 2. Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan Set al. . Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A: Biol. Sci. Med. Sci. 2007;62:731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012;60:1487–1492. [DOI] [PubMed] [Google Scholar]
- 4. Goldfarb M, Sheppard R, Afilalo J.. Prognostic and therapeutic implications of frailty in older adults with heart failure. Curr Cardiol Rep 2015;17:92. [DOI] [PubMed] [Google Scholar]
- 5. Piankova P, Afilalo J.. Prevalence and prognostic implications of frailty in transcatheter aortic valve replacement. Cardiol Clin 2020;38:75–87. [DOI] [PubMed] [Google Scholar]
- 6. Afilalo J, Lauck S, Kim Dae H, Lefèvre T, Piazza N, Lachapelle Ket al. . Frailty in older adults undergoing aortic valve replacement. J Am Coll Cardiol 2017;70:689–700. [DOI] [PubMed] [Google Scholar]
- 7. Ijaz N, Buta B, Xue Q-L, Mohess DT, Bushan A, Tran Het al. . Interventions for frailty among older adults with cardiovascular disease. J Am Coll Cardiol 2022;79:482–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin HS, Watts JN, Peel NM, Hubbard RE.. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatrics 2016;16:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Solomon J, Moss E, Morin JF, Langlois Y, Cecere R, Varennes Bet al. . The essential frailty toolset in older adults undergoing coronary artery bypass surgery. J Am Heart Assoc 2021;10:e020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med 2013;368:100–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sourdet S, Lafont C, Rolland Y, Nourhashemi F, Andrieu S, Vellas Bet al. . Preventable iatrogenic disability in elderly patients during hospitalization. J Am Med Dir Assoc 2015;16:674–681. [DOI] [PubMed] [Google Scholar]
- 12. Avni T, Leibovici L, Gafter-Gvili A.. Iron supplementation for the treatment of chronic heart failure and iron deficiency: systematic review and meta-analysis. Eur J Heart Fail 2012;14:423–429. [DOI] [PubMed] [Google Scholar]
- 13. Deutz NE, Matheson EM, Matarese LE, Luo M, Baggs GE, Nelson JLet al. . Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: a randomized clinical trial. Clin Nutr 2016;35:18–26. [DOI] [PubMed] [Google Scholar]
- 14. Martínez-Velilla N, Casas-Herrero A, Zambom-Ferraresi F, Sáez de Asteasu ML, Lucia A, Galbete Aet al. . Effect of exercise intervention on functional decline in very elderly patients during acute hospitalization: a randomized clinical trial. JAMA lIntern. Med. 2019;179:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matheson EM, Nelson JL, Baggs GE, Luo M, Deutz NE.. Specialized oral nutritional supplement (ONS) improves handgrip strength in hospitalized, malnourished older patients with cardiovascular and pulmonary disease: a randomized clinical trial. Clin Nutr 2021;40:844–849. [DOI] [PubMed] [Google Scholar]
- 16. Noriega FJ, Vidán MT, Sánchez E, Díaz A, Serra-Rexach JA, Fernández-Avilés Fet al. . Incidence and impact of delirium on clinical and functional outcomes in older patients hospitalized for acute cardiac diseases. Am Heart J 2015;170:938–944. [DOI] [PubMed] [Google Scholar]
- 17. Fountotos R, Munir H, Ahmad F, Goldfarb M, Afilalo J.. Rationale and design of the TARGET-EFT trial: multicomponent intervention for frail and pre-frail patients hospitalized with acute cardiac conditions. J nutr. health aging 2022:3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DGet al. . A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 19. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 20. Wischmeyer PE, Carli F, Evans DC, Guilbert S, Kozar R, Pryor Ret al. . American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on nutrition screening and therapy within a surgical enhanced recovery pathway. Anesth Analg 2018;126:1883–1895. [DOI] [PubMed] [Google Scholar]
- 21. Izquierdo M, Casas-Herrero A, Zambom-Ferraresi F, Martínez-Velilla N, Alonso-Bouzón C, Rodríguez-Mañas L, et al. Multi-component physical exercise program VIVIFRAIL. http://vivifrail.com/wp-content/uploads/2019/11/VIVIFRAIL-ENG-Interactivo.pdf. [Google Scholar]
- 22. Ezekowitz JA, O'Meara E, McDonald MA, Abrams H, Chan M, Ducharme Aet al. . 2017 Comprehensive update of the Canadian cardiovascular society guidelines for the management of heart failure. Can J Cardiol 2017;33:1342–1433. [DOI] [PubMed] [Google Scholar]
- 23. Malmstrom TK, Morley JE.. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc 2013;14:531–532. [DOI] [PubMed] [Google Scholar]
- 24. Åhlund K, Bäck M, Öberg B, Ekerstad N.. Effects of comprehensive geriatric assessment on physical fitness in an acute medical setting for frail elderly patients. Clin interv aging 2017;12:1929–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ekerstad N, Dahlin Ivanoff S, Landahl S, Östberg G, Johansson M, Andersson Det al. . Acute care of severely frail elderly patients in a CGA-unit is associated with less functional decline than conventional acute care. Clin interv aging 2017;12:1239–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Asteasu MLS, Martínez-Velilla N, Zambom-Ferraresi F, Lucia A, Galbete A, Izquierdo Met al. . Physical exercise improves function in acutely hospitalized older patients: secondary analysis of a randomized clinical trial. J Am Med Dir Assoc 2019;20:866–873. [DOI] [PubMed] [Google Scholar]
- 27. Volpato S, Cavalieri M, Sioulis F, Guerra G, Maraldi C, Zulianiu Get al. . Predictive value of the short physical performance battery following hospitalization in older patients. J. Gerontol. Series A: Biol. Sci. Med. Sci. 2011;66A:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jencks SF, Williams MV, Coleman EA.. Rehospitalizations among patients in the medicare fee-for-service program. N Engl J Med 2009;360:1418–1428. [DOI] [PubMed] [Google Scholar]
- 29. Yeo I, Cheung JW, Feldman DN, Amin N, Chae J, Wong Cet al. . Assessment of hospital readmission rates, risk factors, and causes after cardiac arrest: analysis of the US Nationwide Readmissions Database. JAMA Network Open 2019;2:e1912208–e1912208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 2006;54:743–749. [DOI] [PubMed] [Google Scholar]
- 31. Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J cachexia, sarcopenia, and muscle 2016;7:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tanaka S, Kamiya K, Hamazaki N, Matsuzawa R, Nozaki K, Maekawa Eet al. . Utility of SARC-F for assessing physical function in elderly patients with cardiovascular disease. J Am Med Dir Assoc 2017;18:176–181. [DOI] [PubMed] [Google Scholar]
- 33. Piña IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BDet al. . Exercise and heart failure. Circulation 2003;107:1210–1225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The e-data underlying this article will be shared on reasonable request to the corresponding author.