Abstract
Metal-organic coordination polymers (CPs) are a broad class of materials that include metal-organic frameworks (MOFs). CPs are highly ordered crystalline materials that are composed of metal ions (or metal ion clusters) and multidentate organic ligands that serve as linkers. One, two and three-dimensional CPs can be formed, with 2D and 3D structures referred to as MOFs. CPs have gained a lot of attention due to attractive structural features like structure versatility and tunability, and well-defined pores that enable the encapsulation of cargo. Further, CPs show a lot of promise for drug delivery applications, but only a very limited number of CPs are currently being evaluated in clinical trials. In this review, we outlined features that are desired for CP-based drug delivery platform, and briefly described most relevant characterization techniques. We highlighted some of the recent efforts directed towards developing CP-based drug delivery platforms with the emphasis on vaccines against cancer, infectious diseases, and viruses. We hope this review will be a helpful guide for those interested in the design and evaluation of CP-based immunological drug delivery platforms.
Keywords: MOFs, ZIF-8, OVA, CpG, antigen, adjuvant, biocompatibility, drug delivery platform, vaccine, cancer
Introduction
Traditionally, subunit vaccine delivery systems contain an adjuvant in combination with protein antigens to enhance the antigen-specific immune response. A carrier system can enhance this basic response by prolonging immune system stimulation. Metal-organic coordination polymers (CPs) are a family of carrier systems that are suitable for enabling the controlled and sustained release of antigens and adjuvants, while protecting the vaccine from degradation during storage throughout its complete lifecycle. This review will discuss CP characterization strategies for immunotherapy adjuvant delivery applications, model vaccine formulations, and its use in infectious disease and cancer vaccines.
Coordination Polymers (CPs)
CPs are highly ordered crystalline materials that are composed of metal ions (or metal ion clusters) and multidentate organic ligands that serve as linkers (Stock & Biswas, 2012; Yuan et al., 2018). These materials self-assemble from their components based on coordination chemistry interactions. A family of CPs are metal-organic frameworks (MOFs). CPs can form one-, two-, and in three-dimensional structures, with the latter two commonly referred to as MOFs (Figure 1). Additional reviews discuss CPs (mostly MOFs) that are broadly for biomedical applications (Al Sharabati et al., 2022; Cai et al., 2015; Horcajada et al., 2012; Liu et al., 2016; Simon-Yarza et al., 2018; Tibbetts & Kostakis, 2020; Yang & Yang, 2020)
Figure 1.
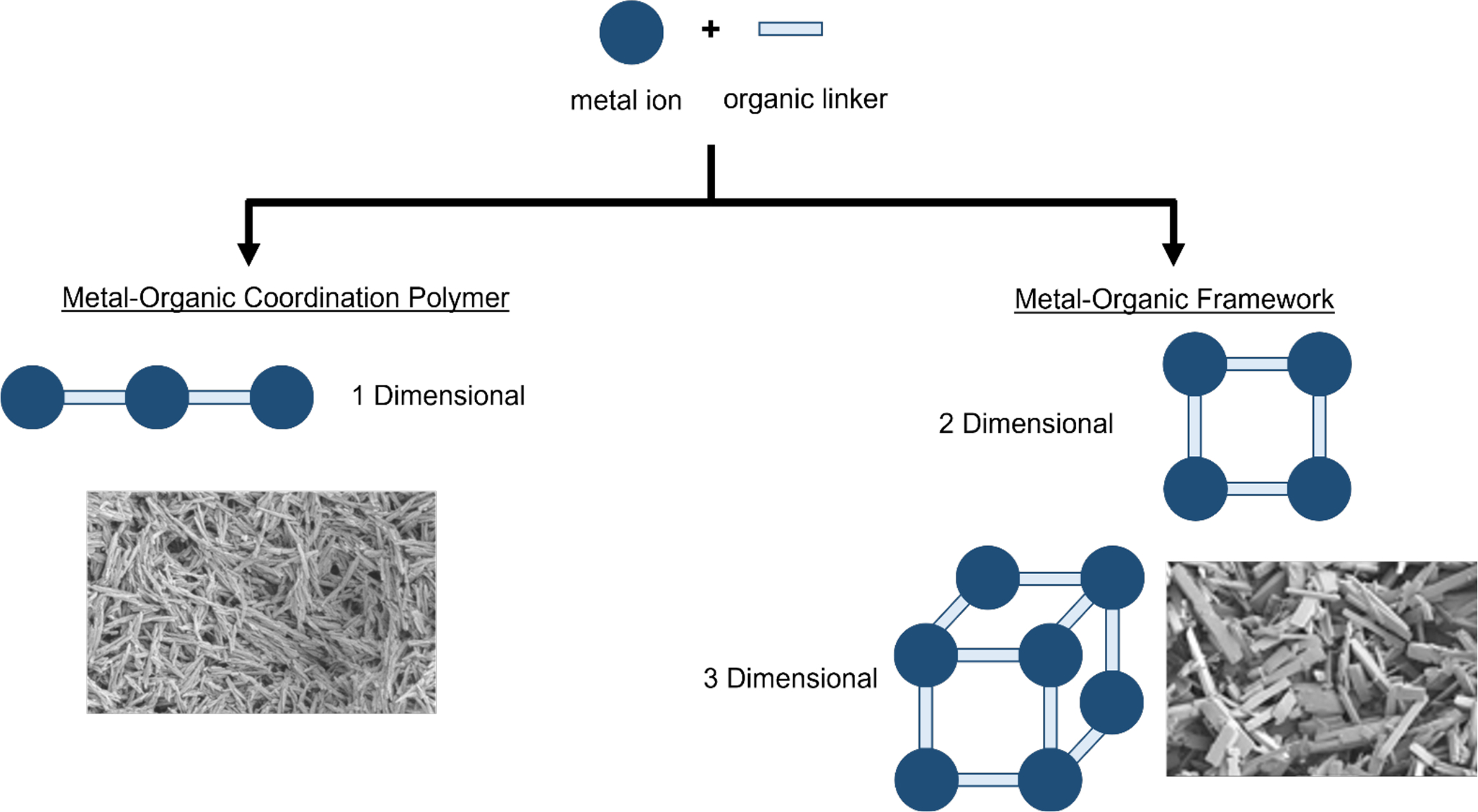
Schematic of metal organic coordination polymers (CP) in one-dimensional self-assembly and metal-organic frameworks (MOF) in their two- and three-dimensional self-assembly.(Eckshtain-Levi et al., 2022)
CPs are referred to as hybrid materials, since they have both inorganic and organic components, and exhibit a variety of features that make them very attractive for numerous applications. Since many metal ions and organic ligands are suitable for CP synthesis, one can achieve desired function of the material via selection of suitable metal and appropriate functionalization of the ligand. Further, CPs are commonly highly porous, which enables applications like gas storage and separation (Li et al., 2019), and a variety of guest-host chemistries including drug and vaccine delivery (Lawson et al., 2021). CPs applied to immunotherapeutic research is relatively new, with most of the extensive research being conducted in other non-biomedical engineering fields within the last three decades. As the field is actively growing, promising new composites are being frequently reported. Here we touch on many common and emerging CPs applied to vaccines and immunotherapy.
Synthesis Conditions and Parameters
For CPs to be suitable for biomedical applications, biologically compatible conditions for synthesis and functionalization must be considered. If not, biologic based cargo can be denatured leading to a loss of function or therapeutic effect along with the potential of increasing toxicity. For instance, in the case of protein antigen, denaturation of its tertiary or quaternary structure leads to improper B cell activation, which in turn could lead to the generation of non-neutralizing antibodies (Gallovic et al., 2016) or even a very potent aberrant immune responses (Janeway et al., 2001; Tsan & Gao, 2009). Even though T cells rely on peptide sequence for activation, they too can have altered responses due to misfolding or denaturation of proteins (Streicher et al., 1984). Parameters that can lead to protein denaturation include high energy mixing, high contact times with organic solvents, pH, salt concentration, and temperature (Genito et al., 2021; Seetharaj et al., 2019; Stock & Biswas, 2012). It is important to tune synthesis conditions in such a way that these detrimental parameters are avoided.
The temperature and pH of CP synthesis conditions greatly influence CP morphology, reaction kinetics and yield, wherein low temperatures and neutral pH are needed to prevent denaturation. Further, CP synthesis can be performed in both organic and aqueous solvents. With the incorporation of biologics in CPs, aqueous or buffer solutions with appropriate pHs should be used over organic solvents during synthesis. The CPs morphology and reaction kinetics can be also controlled with mixing. Slow gentle shaking might lead to longer reaction time with lower yield, whereas vigorous stirring might promote fast reaction rate with higher yield. Both the shear rate when mixing and the time of mixing could affect the tertiary structure of biologics in addition to CPs features.
Metal Ions and Organic Ligands Selection
A major criteria when designing CPs for vaccine drug delivery applications is to consider the toxicity of the precursors (Rojas et al., 2017; Sajid, 2016). Examples of biocompatible metals include Zn2+ and Fe2+/3+, which are endogenous elements that serve biological functions and are found in relatively significant amounts in the body. However, zinc metal ions can compete with Fe2+/3+ and Ca2+ ions in cellular function, and can lead to cellular damage (Borovanský & Riley, 1989). Even iron metal ions can generate reactive oxygen species (ROS) which can lead to cellular death or apoptosis (Tamames-Tabar et al., 2014). These factors explain the cytotoxicity observed with well-established zeolitic imidazolate frameworks (ZIF-8) and iron-based MOFs.
A wide array of organic ligands has been utilized in the formation of CPs but only a few studies have been performed to properly assess their biological applicability. It has been shown that the hydrophobic–hydrophilic balance of the organic linker (expressed via partition coefficient logP) can be correlated with MOF cytotoxicity, with MOFs built from more hydrophilic linkers exhibiting lower cytotoxicity (Tamames-Tabar et al., 2014). Further, reduced cytotoxicity was shown with ball-milling because it led to increased hydrophilicity on the external surface of ZIF-8 (Shearier et al., 2016). However, correlation of MOF cytotoxicity with linker hydrophilicity is not perfectly linear, and other important factors like specific cell line-related internalization and whether the linker is an endogenous molecule may result in deviation from this trend (Tamames-Tabar et al., 2014).
Additionally, the choice of metal ions and organic linkers have direct correlation on CP stability. In order to assemble a stable CP, hard/soft-acid/base principle should be considered as corroborated by many observations in MOFs research (Devic & Serre, 2014). For example, the carboxylate-based ligands can be regarded as hard bases, which tend to form stable MOFs together with high-valent metal ions, such as Ti4+, Zr4+, Al3+, Fe3+, and Cr3+. On the other hand, soft divalent metal ions such as Zn2+, Cu2+, Ni2+ and Mn2+, can coordinate to soft azolate ligands such as imidazolates, pyrazolates, triazolates, and tetrazolates (Yuan et al., 2018). The most representative examples are ZIFs constructed by Zn2+ and imidazolate linkers.
Incorporation of Biomolecules
CP’s unique chemical and structural features allow for adsorption or entrapment of biologics or biomolecules within their pores as well as on external surfaces (Liang et al., 2015). Illustrated in Figure 2, there are three common routes to incorporate biologics within CPs for vaccine research (An et al., 2019): (1) Incorporation within it; (2) Covalent attachment to the surface; (3) Adsorption.
Figure 2.
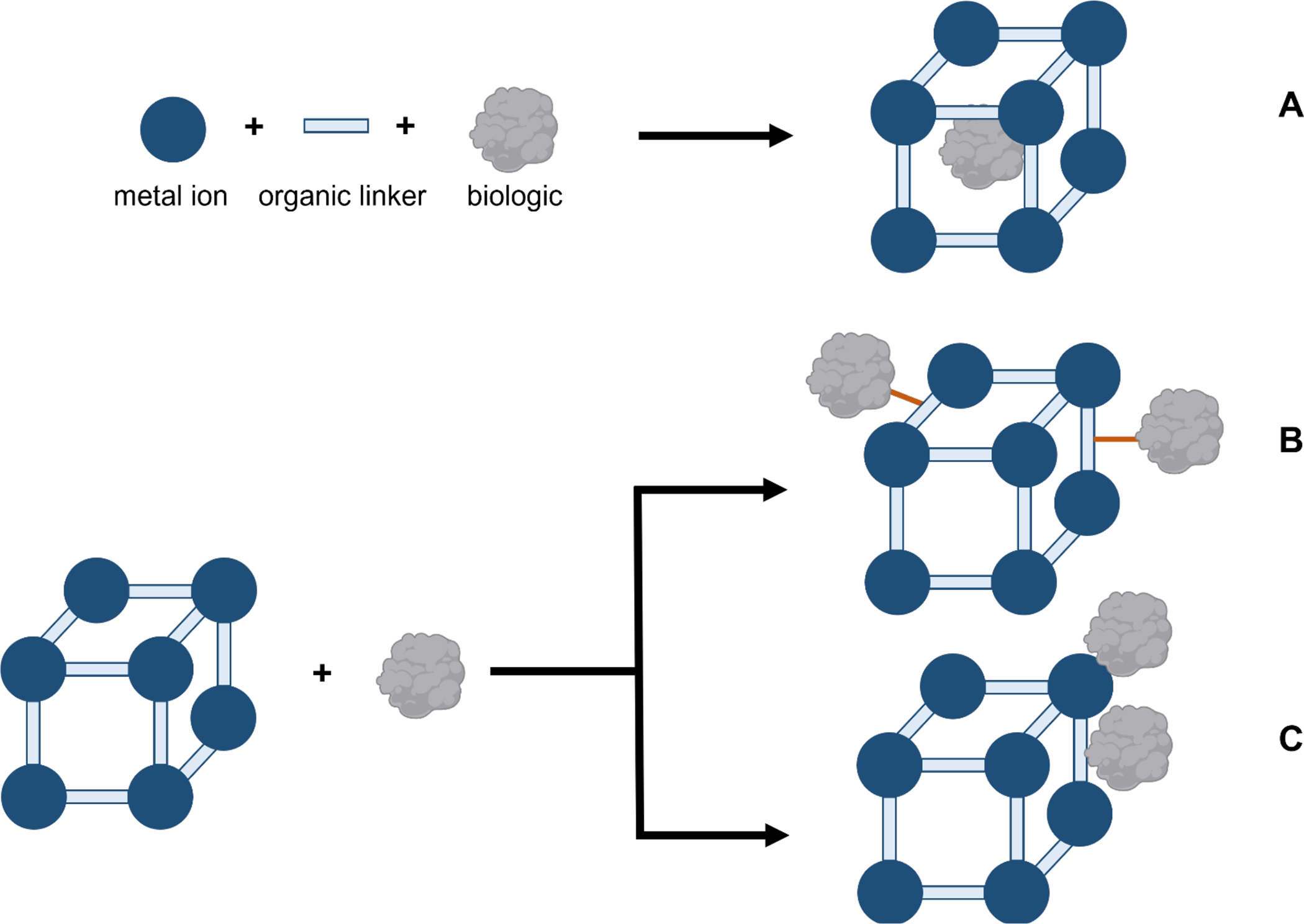
Schematic representation of different routes for incorporation of biomolecules within MOFs via (A) in situ encapsulation, (B) covalently bonding the biologic, and (C) physical adsorption through electrostatic interactions.
During in situ encapsulation (Figure 2A), CPs grow around the biomolecule in a one-pot synthesis where nucleation and immobilization occur simultaneously which can uniformly distribute the biomolecule within the structure. For example, for infectious disease vaccines, whole viruses and bacteria can be encapsulated in MOFs (Luzuriaga et al., 2021; Luzuriaga et al., 2019; Miao et al., 2019). Incorporation of the biologic can occur post-synthesis by surface attachment via a strong covalent bond, such as a disulfide bond (Yang et al., 2018), (Figure 2B) or a weak physical adsorption (Z. Wang et al., 2017) through electrostatic interactions between the phosphate groups on the biologic and the metal (Figure 2C). For vaccines and immunotherapy, electrostatic interactions are commonly used to incorporate the FDA approved vaccine adjuvant CpG. CpG can be adsorbed on to the surface of CPs through a strong metal-phosphate bond (Z. Wang et al., 2017) or by π–π interactions with an organic ligand (Y. Zhang et al., 2017). Proteins with a His-tag can allow for metal ion to protein complexation on the CP surface (Eckshtain-Levi et al., 2022). One drawback to having the biomolecule surface attached is that it is exposed to the environment which can result in denaturation. However, many CPs are synthesized under conditions that can denature the biomolecule so post-synthesis incorporation may be desirable. Interestingly, several publications report that the biologic is incorporated both within the pores of a CP, given the biologic is small enough to enter, and on its surface to enhance immune responses (Duan et al., 2017; Zhang et al., 2020; Zhang et al., 2016; Zhong et al., 2019).
CPs used in Vaccine Studies
Several CP formulations, based on a variety of metal ions have been used as an infectious disease or therapeutic cancer vaccine (Table 1). The acid sensitivity of many of the formulations facilitates triggered release upon internalization by an antigen presenting cell (APC) as well as release in the low pH environment of tumors, making these platforms advantageous for these applications.
Table 1:
Overview of CPs used for vaccine applications. Anti-PD-L1: anti-programmed death-ligand 1, COBRA: computationally optimized broadly reactive antigen for influenza, CpG: Toll-like receptor 9 agonist cytosine-phosphate-guanine oligodeoxynucleotides, IDO: indoleamine 2,3-dioxygenase, OVA: ovalbumin, R837: imiquimod, VLP: virus like particle.
| Metal | Organic Linker | Adjuvant (incorporation method) | Antigen (incorporation method) | Highlights | Ref |
|---|---|---|---|---|---|
| Zinc | 2-methylimidazole | CpG (encapsulation) | None | • Tuning metal to ligand ratios affects size. • Protects CpG and activates B cells. |
(Brohlin et al., 2022) |
| CpG (adsorption) | OVA (encapsulation) | • Incorporation of OVA & CpG reduced MOF’s cytotoxicity. • Activated immune cells in vitro and in vivo. |
(Zhang et al., 2016) | ||
| CpG (adsorption) | None | • CpG attachment reduced MOF’s cytotoxicity. • Best weight ratio for CpG loading was 5:1 MOF:CpG. • Activated immune cells in vitro and in vivo, outperformed soluble CpG. |
(H. Zhang et al., 2017) | ||
| CpG (adsorption) | OVA (encapsulation) | • Loading OVA into MOF decreased toxicity. • Demonstrated co-delivering antigens and adjuvants via MOF to treat cancer. • Also integrated aluminum into MOF, which modulated CD8+ T cell activation. |
(Zhong et al., 2019) | ||
| Tobacco Mosaic Virus (encapsulation) | • MOF provides protection against denaturation conditions (heat and organic solvents) • Encapsulated virus showed generation of humoral response and protection against chemical and heat denaturation. |
(Luzuriaga et al., 2019) | |||
| Newcastle Disease Virus (encapsulation) Influenza A (encapsulation) | • Storage study found MOFs provide thermal stability for live-virus viability. | (Singh et al., 2022) | |||
| Foot-and-Mouth Disease Virus VLPs | • MOFs enhanced heat resistance, uptake by cells, and lysosome escape. | (Teng et al., 2022) | |||
| R837 (encapsulation) | None | • Two MOFs tested with different drugs: o Inside: thermal responsive near-IR dye IR820, outside: HA (hyaluronic acid) to target tumor cells. o Inside: R837 and IDO inhibitor 1 MT. outside: mannan to enrich dendritic cells. • Tumor releases specific antigens upon photothermal ablation • Treated mice with both and found enhanced tumor clearance. |
(Zhang et al., 2020) | ||
| Poly(I:C) (encapsulation) | OVA (encapsulation) | • Loaded OVA or Poly(I:C) into mesoporous Si particles, then coated in ZIF-8 shell. • Best therapy was observed only with anti-CTLA4 co- delivery. • Subcutaneous lymphoma model was used. |
(Li et al., 2020) | ||
| E. coli (encapsulation) | • Showed that ZIF-8 coating inactivates the bacteria similarly to chemical inactivation. • ZIF-8 coated bacteria results in increased titers and pro-inflammatory cytokines with antigen restimulation. |
(Luzuriaga et al., 2021) | |||
| Carnosine | CpG (adsorption) | COBRA (adsorption) or OVA (encapsulation) | • CP composed of zinc and carnosine. • Antigen utilizes influenza hemagglutinin for use against viral strains. • Elevated levels of cellular and humoral response to that of soluble CpG and HA. |
(Eckshtain-Levi et al., 2022) | |
| Zirconium | Terephthalate | CpG (adsorption) | None | • CpG adsorbed to the MOF surface and then mineralized with calcium phosphate exoskeleton resulted in increased cytokine production in cells and mice. | (Z. Wang et al., 2017) |
| 1,4-dicarboxybenzene | None | OVA (adsorption) | • Activates the alternative complement cascade via adsorption of C3a to the MOF due to the amine groups on the surface. • Increased cellular response and in vivo serum cytokines. |
(Qi et al., 2019) | |
| Photosensitizer H2TCPP | CpG (adsorption) | None | • Loaded with HIF signaling inhibitor and coated with hyaluronic acid to target tumor cells. • Hypoxia inducible factor signaling inhibitor (ACF) and CpG were loaded into MOF followed by coating of the material with hyaluronic acid. |
(Cai et al., 2020) | |
| Iron | Terephthalic acid | CpG (adsorption) | None | • CpG delivered via MOF was shown to be more immunostimulatory in vivo and in vitro. • MOFs were also used as an MRI contrast agent. |
(Y. Zhang et al., 2017) |
| CpG (adsorption) | OVA (covalent bond) | • Vaccination with MOF illustrated significant T cell killing and CD8+ memory responses. • Compared encapsulated OVA to covalently bonded OVA MOFs and found higher levels of cytokines secreted by splenocytes. |
(Yang et al., 2018) | ||
| Hafnium | 5,10,15,20-tetra(p-benzaoto)chlorin | IDO Inhibitor (adsorption) | None | • Intratumoral injections of an IDO inhibitor with irradiation was able to illustrate abscopal effect; mediated clearance of subcutaneous colorectal tumors. | (Lu et al., 2016) |
| 2,5-di(p-benzoato)aniline | IDO inhibitor (adsorption) | None | • Displayed abscopal effect in multiple subcutaneous tumor models and memory effect by clearance of tumors with re-challenge of survivors. | (Lu et al., 2018) | |
| None | None | • Radiotherapy working in tandem with checkpoint blockade (anti-PD-L1 antibody) to eliminate primary and distant tumors. | (Ni, Lan, Chan, et al., 2018) | ||
| Aluminum | 2-aminoterephthalic acid | Aluminum is an adjuvant Yeast capsule (encapsulating the MOF) |
OVA (encapsulation) | •Formulated the antigen for oral delivery by loading empty yeast capsules with MOFs. • Generation of IgA and IgG was observed with three oral dosing of the OVA MOF loaded yeast capsules. |
(Miao et al., 2019) |
| Europium | Guanine monophosphate | CpG (adsorption) | OVA (encapsulation) | • Displayed pH sensitive release of antigen. • Inhibited tumor growth and increased survival in an OVA expressing melanoma model. |
(Duan et al., 2017) |
| Manganese | Meso-2,6-diaminopimelic acid (DAP) | DAP is an adjuvant | OVA (encapsulation) | • Nano CP (NCP) has a linker that acts as an adjuvant. • Increased levels of DC maturation, cross, presentation, and cytokine production with NCP. • Slowed the growth of tumors and increased survival. |
(Zhao et al., 2019) |
Zinc-Based CPs
Zeolitic imidazolate framework-8 (ZIF-8), constructed from Zn2+ and 2-methylimidazole (2-HMIM), is one of the most investigated MOF applied for vaccine delivery. Several different methods have been developed to fabricate ZIF-8 at temperatures varying from room temperature to 200°C in different solvents. Jian et al. and Zhang et al. showed that particle sizes and shapes of resulting ZIF-8 materials can be controlled by the 2-Hmim/Zn2+ molar ratio both in water and methanol, respectively (Jian et al., 2015)(Zhang et al., 2018). Bustamante et al. investigated the role of solvent using series of aliphatic alcohols and acetone which resulted in ultra-fast synthesis of ZIF-8 (Bustamante et al., 2014).
ZIF-8 MOFs for vaccines and immunotherapies most commonly use surface adsorption of CpG by electrostatic forces after the MOF has been formed (Figure 3) (Zhang et al., 2016; Zhong et al., 2019). It has been shown that with the incorporation of CpG, cytotoxicity decreases when incubated with cells, but this can probably be attributed to CpG increasing cell proliferation and the cellular assay used measuring both proliferation and viability (e.g. MTT). Zhang et al. reports loading CpG onto the surface of ZIF-8 MOFs (H. Zhang et al., 2017). Mice treated with the formulation intravenously had cytokine signaling above background. Furthermore, ZIF-8 MOFs can co-deliver CpG and ovalbumin (OVA) where the protein antigen is encapsulated into the MOF in situ. This co-delivery strategy has achieved an OVA specific adaptive immune response. Vaccinating mice with OVA CpG ZIF-8 MOFs had greater total IgG titers but marginal differences in splenocyte proliferation and T cell counts (H. Zhang et al., 2017; Zhang et al., 2016). Another study encapsulated adjuvant imiquimod (R837) and a near-infrared dye in ZIF-8 MOFs that were coated with hyaluronic acid to target tumor cells or mannan to target dendritic cells. This formulation could then be ‘activated’ with a laser for thermal ablation and thus releases tumor specific antigens in order to generate a therapeutic cancer vaccine (Zhang et al., 2020). It was found that there was enhanced tumor clearance when treating mice with this formulation.
Figure 3.
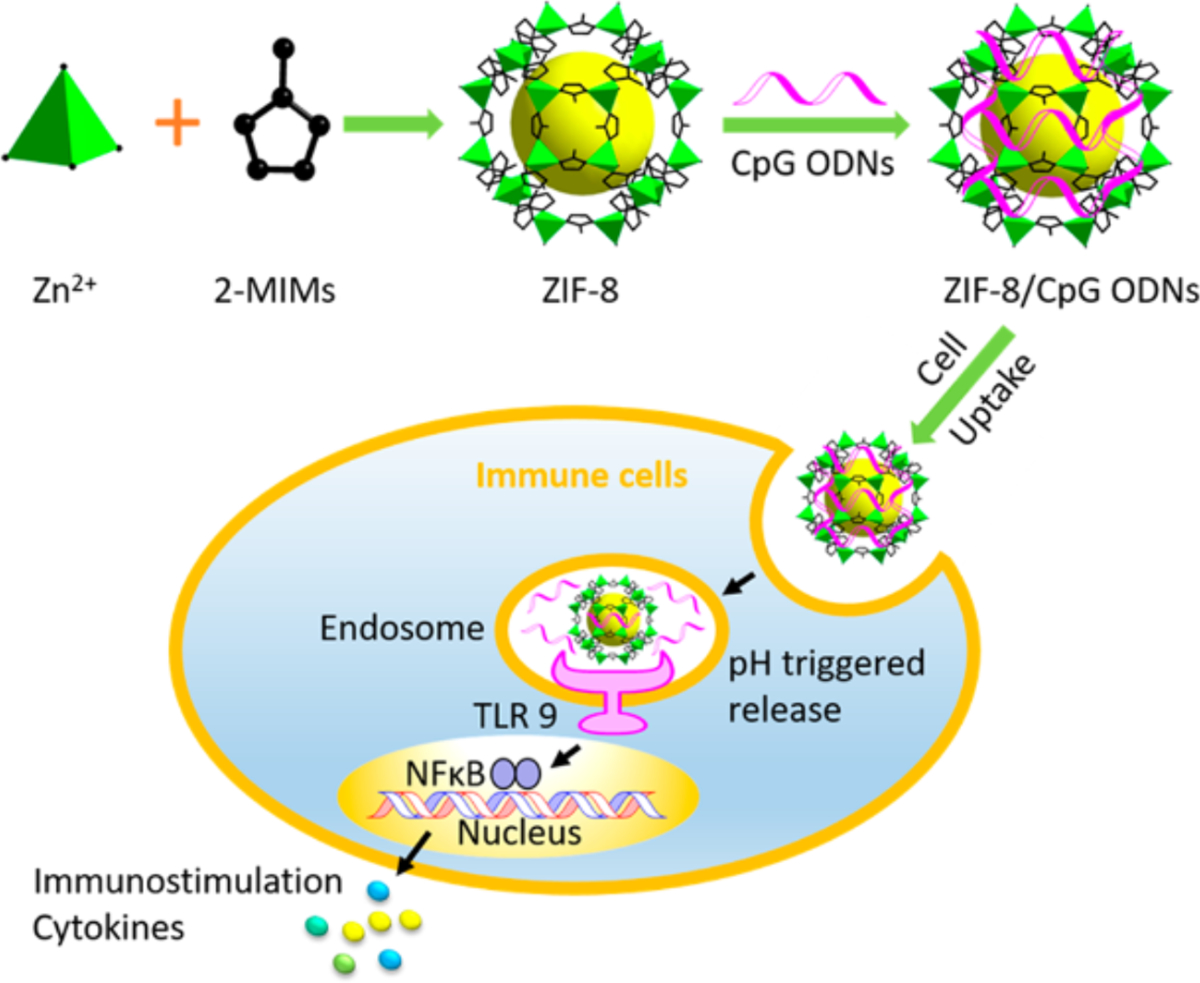
Schematic of ZIF-8 structure delivering CpG and encapsulated antigen. Reprinted (adapted) with permission from H. Zhang et al., 2017 (H. Zhang et al., 2017). Copyright 2017 American Chemical Society.
Aside from co-delivering an adjuvant and antigen, ZIF-8 MOFs have been used to encapsulate whole viruses, virus like particles (VLP), and bacteria (Luzuriaga et al., 2021; Luzuriaga et al., 2019; Singh et al., 2022; Teng et al., 2022). The primary purpose for this is to protect the cargo from outside influences that may denature or damage it, such as heat or harsh organic solvents. Tobacco mosaic virus (TMV) loaded into MOFs were subjected to 100°C for 20 min or to solvents such as, methanol, ethyl acetate, or guanidinium chloride, and it was found that there was only a 10–30% reduction in antibody binding whereas soluble TMV ranged from 30–100% reduction (Luzuriaga et al., 2019). Whole bacteria, Escherichia coli (E. coli) strain CFT073, was coated with a ZIF-8 shell and inactivated the bacteria similarly to heat or chemical inactivation (Luzuriaga et al., 2021). Mice vaccinated with the MOF showed persistence at the injection site for 120 hours compared to two hours for an uncoated virus, significantly greater total IgG titers were seen by day 21 with no sign of toxicity, and there were increased T cells, B cells, CD4+ and CD8+ T cells in the spleens and lymph nodes. Other studies have encapsulated other pathogens and have shown that MOFs are able to protect viability, indicating its feasibility in the delivery of live viruses (Singh et al., 2022; Teng et al., 2022).
(Li et al., 2020) reported the use of ZIF-8 MOF encapsulated mesoporous silica (MS) for the development of a cancer vaccine. The design is based on the idea that the MS has ample surface area for the antigen and adjuvant, whereas the ZIF-8 coating allows for more controlled release of the vaccine elements. The authors show increased thickness of coating with increased ratios of MS to Zn2+. With OVA and TLR3 agonist poly(I:C) adsorbed on the outside of MS, the ZIF-8 coating demonstrated a pH-sensitive release of the components, Zn, Si, OVA, and poly(I:C), which was faster at pH 5 than at neutral pH. Furthermore, innate immune activity in bone-marrow derived dendritic cells (BMDCs) was observed with the MOF-MS complex. This study displays that MOF can enhance the delivery of cancer vaccines antigens through extended release, and that the ZIF-8 shell can form around a metal oxide microparticle.
Eckshtain-Levi et al. reports a zinc-based CP where the organic linker is carnosine, a dipeptide that is already found in the body in high amounts in muscle tissue (Eckshtain-Levi et al., 2022). This study adsorbed CpG to the surface of the CP and OVA encapsulated or embedded between the CP structures. This CP can be formed at biocompatible conditions where the components are mixed at 37°C for 18 hours in HEPES buffer. The study also used an influenza hemagglutinin protein to illustrate its use for a subunit vaccine delivery platform where mice vaccinated with this formulation had enhanced levels of antibody production and cytokine production. Overall, these studies show that zinc-based CPs can be used as a vaccine platform especially with the ability to protect the cargo from denaturing events.
Zirconium-Based MOFs
Zr MOFs are known for their stability and robustness. It is attributed to formation of Zr6 cluster which act as a secondary-building unit within MOF structure. Most notable examples of zirconium-based MOFs include UiO-66 (UiO=University of Oslo), which is constructed from Zr4+ ions and terephthalic acid as a linker and is known for its exceptional thermal stability in addition to biocompatibility. UiO-66 also exhibit highly robust structure topology, which tolerates various modifications of its original linker. For example, one member of UiO-66 family is UiO-66-NH2 and it is based on amine-functionalized terephthalic acid linker, 2-aminoterephthalic acid (Z. Wang et al., 2017). Utilization of 2-aminoterephthalic acid and terephthalic acid at 1:1 molar ratio also led to a MOF that retains UiO-66 topology, UiO-AM (Qi et al., 2019) (Figure 4). Both UiO-66-NH2 and UiO-AM show promise for biomedical applications.
Figure 4.
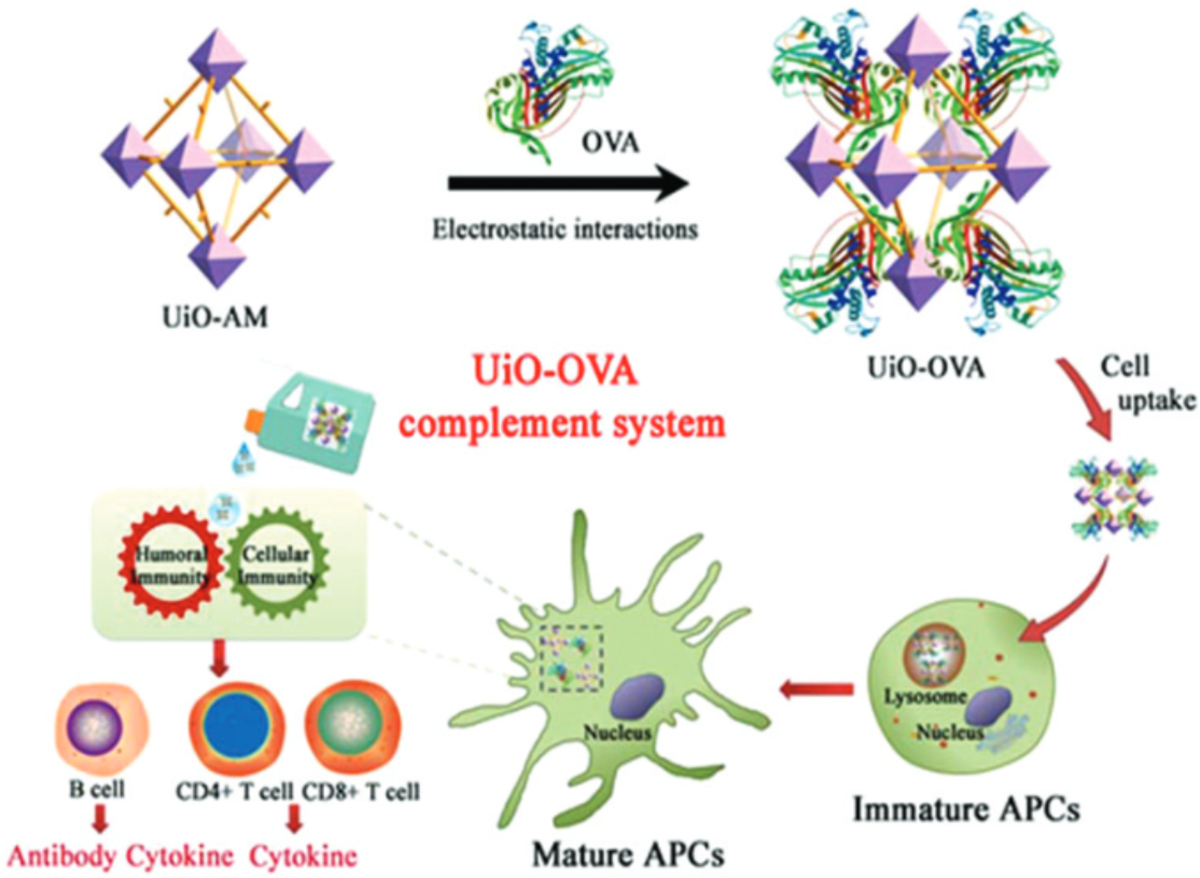
Schematic of UiO-AM taken from Qi et al., 2019 depicting the structure of the MOF and inducing an immune response to OVA (Qi et al., 2019). Used with permission of Royal Society of Chemistry, from Antigen-enabled facile preparation of MOF nanovaccine to activate the complement system for enhanced antigen-mediated immune response, Qi et al., 7, 2019; permission conveyed through Copyright Clearance Center, Inc.
A study adsorbed CpG onto UiO-66-NH2 via ultrasonic mixing and encapsulated the MOF in a calcium phosphate shell (Z. Wang et al., 2017). The formulated nanoparticle (NP) was approximately 100 nm in diameter and released CpG in a pH dependent manner. The NPs were taken up via micropinocytosis by HeLa cells and when treating RAW macrophages, IL-6 and TNF-α cytokine production was similar to that of soluble CpG. Mice treated with mineralized CpG MOFs had significantly greater TNF-α serum levels than CpG-functionalized gold NPs demonstrating that Zr-MOFs show promise as a vaccine delivery vehicle.
Qi et al. used a Zr-MOF with OVA loaded via electrostatic interactions as the antigen (Qi et al., 2019). No adjuvant was used in this formulation, but it was discussed that complement activation drove an adjuvant-like response, however, endotoxin was not evaluated. Mice were vaccinated subcutaneously, which resulted in higher anti-OVA titers, C3a (complement protein) binding, and CD8+ T cells from isolated spleens when treated with the MOF formulation versus soluble OVA. There was also an increase in pro-inflammatory cytokines observed in mice sera after vaccination with the MOF treated group. This study showed that new formulation of common elements, such as Zr4+, could modify their biological effect and give them properties similar to adjuvants, such as triggering the innate immune system to help drive an adaptive immune response.
Another study adsorbed CpG to the surface, but the organic linker was a photosensitizer that allowed for photothermal ablation of tumor cells (Cai et al., 2020). The MOF was also coated with hyaluronic acid to target tumor cells and the tumor associated antigens were released upon thermal ablation. CpG helped stimulate the immune system to mount a response against tumor antigens. This study showed that there are other means to thermally ablate tumor cells without the use of a photothermal dye but instead an organic linker and that in combination with an adjuvant can help drive tumor elimination.
Iron-Based MOFs
Most notable example of iron-based MOFs include MIL-101(Fe) (MIL = Materials of Institut Lavoisier), developed by G. Férey’s research group (Horcajada et al., 2008). MIL-101(Fe) is constructed from Fe3+ clusters and an aromatic dicarboxylic acid as a linker (terephthalic acid). It has a very porous structure and was reported to be one of the first examples of drug release from a MOF with ibuprofen (Horcajada et al., 2006).
MIL-101(Fe) was used to deliver CpG and this displayed enhanced activity compared to soluble CpG (Y. Zhang et al., 2017). Zhang et al. showed by flow cytometry and epifluorescence that there were higher association of CpG with RAW macrophages when delivered via MOF than soluble agent. There were also increased levels of IL-6 and TNF-α compared to soluble CpG. This translates to mice studies where the serum of mice treated with the MOF showed an upregulation of cytokines. These can also be used in MRI detection (due to Fe3+ ions) and display a high contrast when given subcutaneously, indicating that the MOFs can also have a theranostics effect.
OVA has also been covalently attached via disulfide bond to the surface of Fe-based MOFs with CpG adsorbed to the surface (Figure 5) (Yang et al., 2018). Treating DC2.4 cells with the OVA CpG MOFs, there was increased cellular association and uptake of the MOFs measured via flow cytometry and confocal microscopy, respectively. In a splenocyte antigen recall, it was shown that MOFs containing OVA had an increased degree of proliferation and production of pro-inflammatory cytokines IFN-γ, TNF-α, and IL-4. Serum total IgG antibody levels were also significantly greater with the OVA and CpG MOF group. Using an in vivo T cell killing assay, the cells isolated from an OVA and CpG MOF vaccinated mouse displayed significantly higher killing than the other groups. This work displays that vaccination with MOF carriers can generate strong CD8+ mediated killing.
Figure 5.
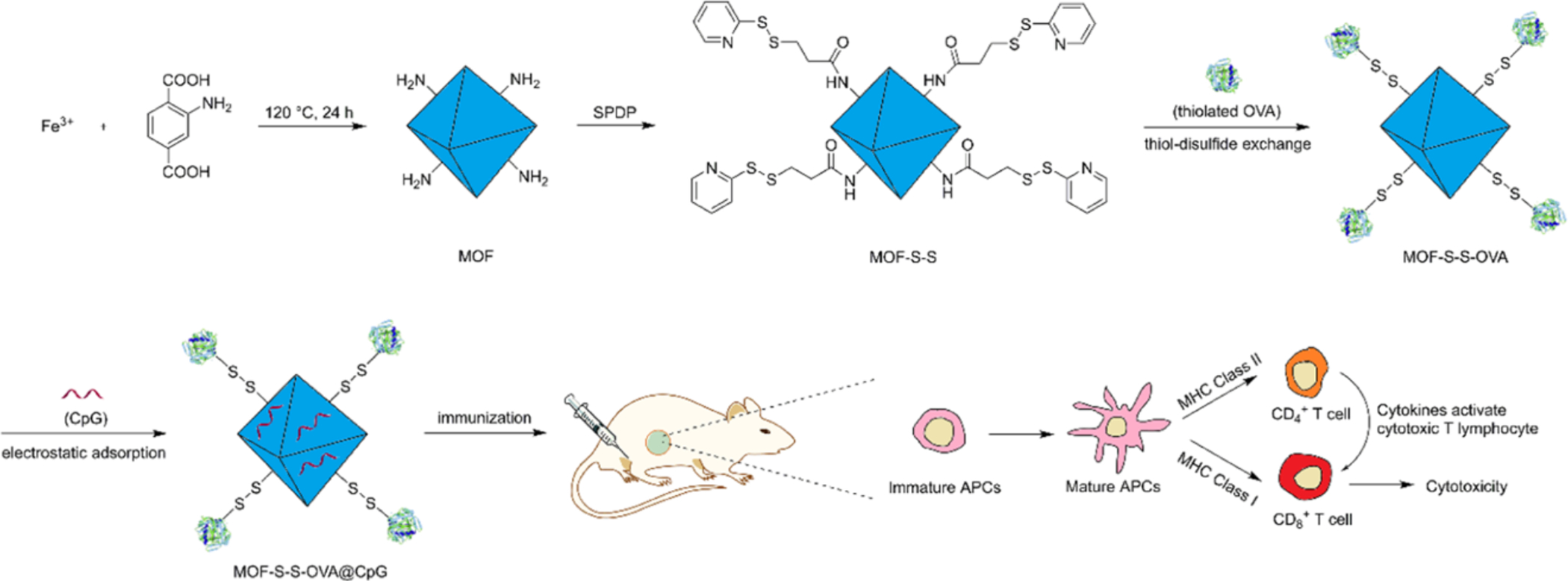
Schematic of Fe MOF with OVA conjugated to the surface and CpG adsorbed to stimulate immature APCs and induce a T cell response. Reprinted (adapted) with permission from Yang et al., 2018 (Yang et al., 2018). Copyright 2018 American Chemical Society.
Hafnium-Based MOFs
Hafnium-based MOFs are constructed from Hf4+ ions which form robust Hf6 clusters upon coordination with organic linkers leading to exceptionally stable structures. Notable examples of Hf MOFs utilized for cancer therapy applications include MOFs with photosensitizer linkers. Such MOFs are TBC-Hf, where TBC is a chlorin-based ligand 5,10,15,20-tetra(p-benzoato)chlorin (Lu et al., 2016), porphyrin-based MOFs DBP-Hf (5,15-di(p-benzoato)porphyrin linker) and TBP-Hf (5,10,15,20-tetra(p-benzoato)porphyrin linker) (Lu et al., 2018). A post-synthetic modification strategy was used to load indoleamine 2,3-dioxygenase (IDO) inhibitor into Hf-based MOFs TBC-Hf (Lu et al., 2016) and DBP-Hf (Lu et al., 2018), and the obtained materials showed high promise in cancer therapy. Hf MOFs with IDO inhibitors were cultured with colon cancer CT26 cells and induced greater than 50% cell toxicity at 20 μM (Lu et al., 2016). However, when using the same concentration against colon adenocarcinoma MC38 cells, less cytotoxicity was observed, although some variability can occur with different cell lines. With the addition of irradiation (90 J/cm2), significantly greater cell apoptosis and death was observed with MOF treatment compared to PBS. The Lin lab also tested the Hf-MOF with anti-programmed death-ligand 1 antibody (anti-PD L1) and reported reduced tumor burden (Ni, Lan, Chan, et al., 2018). The structure of their Hf6 or Hf12 cluster MOFs differ and provide difference in therapeutic results due to the difference in surface area and porosity (Figure 6). Overall, these studies show that Hf-MOFs show great promise as a cancer immunotherapeutic in combination of radiation and checkpoint blockade.
Figure 6.
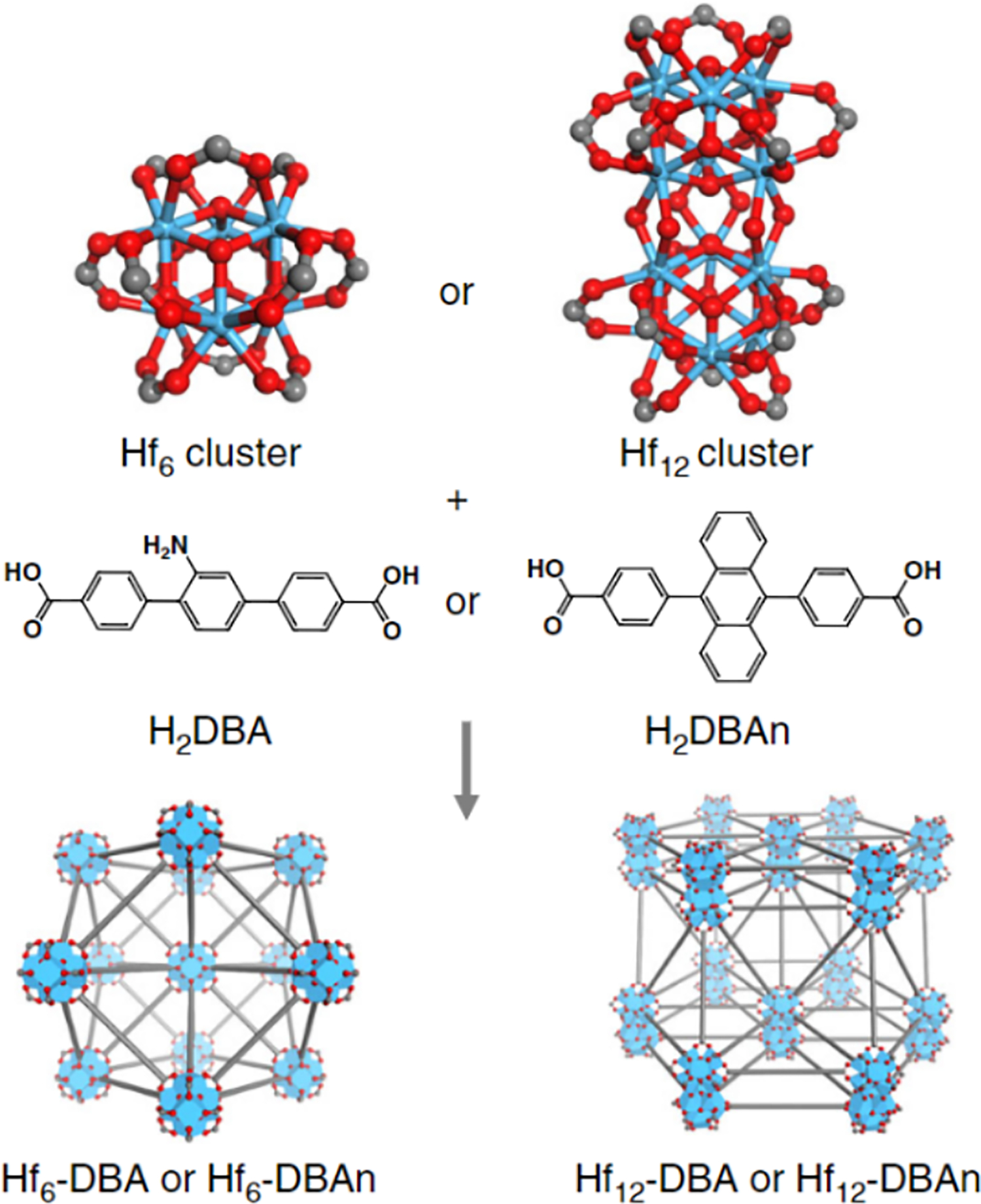
Schematic of Hf clusters synthesizing into Hf6-DBA and Hf12-DBAn provided by Chan, et al. (Ni, Lan, Chan, et al., 2018).
Aluminum-Based MOFs
Aluminum-based IL-57(Al)-NH2 MOF, constructed from Al3+ ions and 2-aminoterephthalic acid linker, has been used in vaccine delivery (Figure 7) (Miao et al., 2019). Miao et al. used this Al-MOF to load OVA in a one pot reaction under mild sonication and delivered the antigen orally (Miao et al., 2019). Using circular dichroism, FTIR and SDS-PAGE, it was confirmed that OVA was not denatured during the formation process. When encapsulating β-galactosidase, it was shown that the enzyme activity did not change at 4, 20, and 37°C, whereas the enzyme lost activity in its soluble form at those temperatures. OVA encapsulated MOFs were also incubated in hollow yeast cells to electrostatically load the MOFs into a yeast capsule so that the yeast would act as an adjuvant to stimulate multiple innate immune cell receptors (Taghavi et al., 2017). Confocal microscopy and measuring the zeta potential before and after encapsulation confirmed the MOFs were incorporated in the yeast capsule. When cultured with RAW macrophages, IL-6 and IL-1β cytokine production and MHCII and CD80 upregulation were increased compared to OVA MOFs alone. When orally delivered in mice, the MOFs were seen to traffic into the draining lymph node and resulted in increased fecal antibodies. The study illustrates that the metal can act as an adjuvant and the MOF itself can be encapsulated into another carrier which may also act as an adjuvant and assist with penetrating the mucosal layer to stimulate an immune response.
Figure 7.
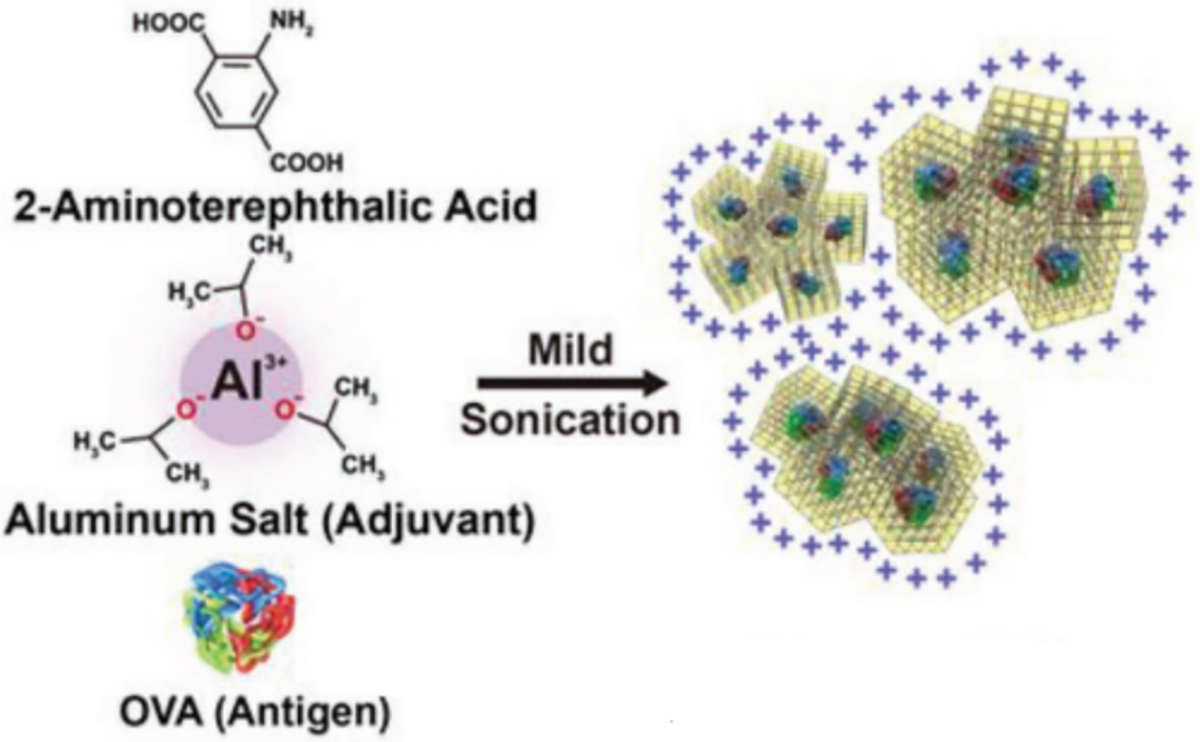
Schematic of aluminum based MOFs encapsulating OVA provided by Miao et al., 2019 (Miao et al., 2019).
Europium-Based MOFs
Several MOFs based on lanthanides have been reported as suitable for applications in drug delivery. One MOF based on Eu3+ and biomolecule guanine monophosphate (GMP) as a linker was used for immunotherapy against cancer (Figure 8) (Duan et al., 2017). Duan et al. used this Eu MOF to deliver OVA and CpG for generation of a melanoma vaccine. OVA was encapsulated into the Eu MOF during the MOF formation and CpG adsorbed post synthesis. This MOF showed acid sensitivity in vitro. When treating mice with a F16-OVA subcutaneous melanoma, the MOF with OVA with and without CpG significantly reduced tumor growth and extended survival rates compared to controls. Immunohistochemistry also showed infiltration of NK and CD8+ T cells into the tumor with the MOF treatment which led to increased survival and decrease in tumor volume. The study displays that Eu MOFs can be used to deliver and adjuvant and antigen for immunotherapy of cancer.
Figure 8.
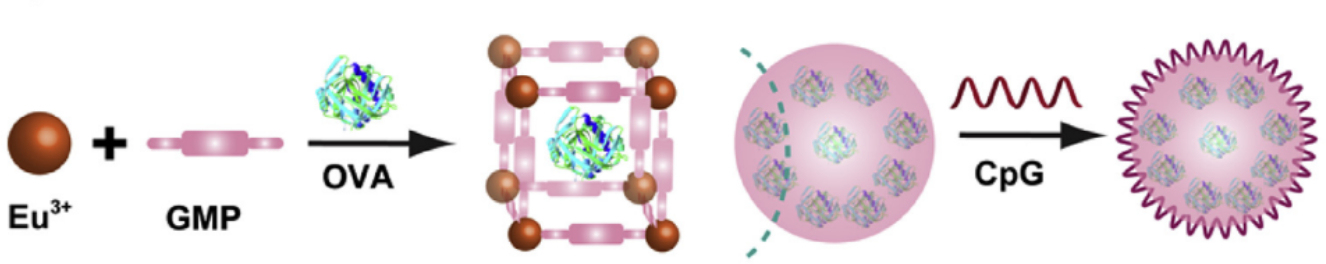
Schematic of europium coordinating with GMP and encapsulating OVA with CpG adsorbed onto the surface of the MOF. Reprinted from Biomaterials, 122, Duan et al., A simple and powerful co-delivery system based on pH-responsive metal organic frameworks for enhanced cancer immunotherapy, 22–33, 2017, with permission from Elsevier. (Duan et al., 2017).
Manganese-Based CPs
Zhao et al., reports a manganese-based CP (Mn-CP) where the Mn2+ ion forms a CP with a nucleotide oligomerization binding domain agonist, meso-2,6-diaminopimelic acid (DAP), as the organic linker (Figure 9). In this paper, DAP acts as the adjuvant itself and it can assist with antigen cross-presentation (Zhao et al., 2019). OVA is used as the model antigen and is encapsulated within the CP. The use of this CP demonstrates greater DC maturation, cross-presentation, and cytokine production with also increased survival and slowed tumor progression in a mouse cancer challenge study with B16-OVA cell lines. This paper illustrates that Mn-CPs can also be used as immunotherapeutic vehicles and that the organic linker can act as the adjuvant.
Figure 9.
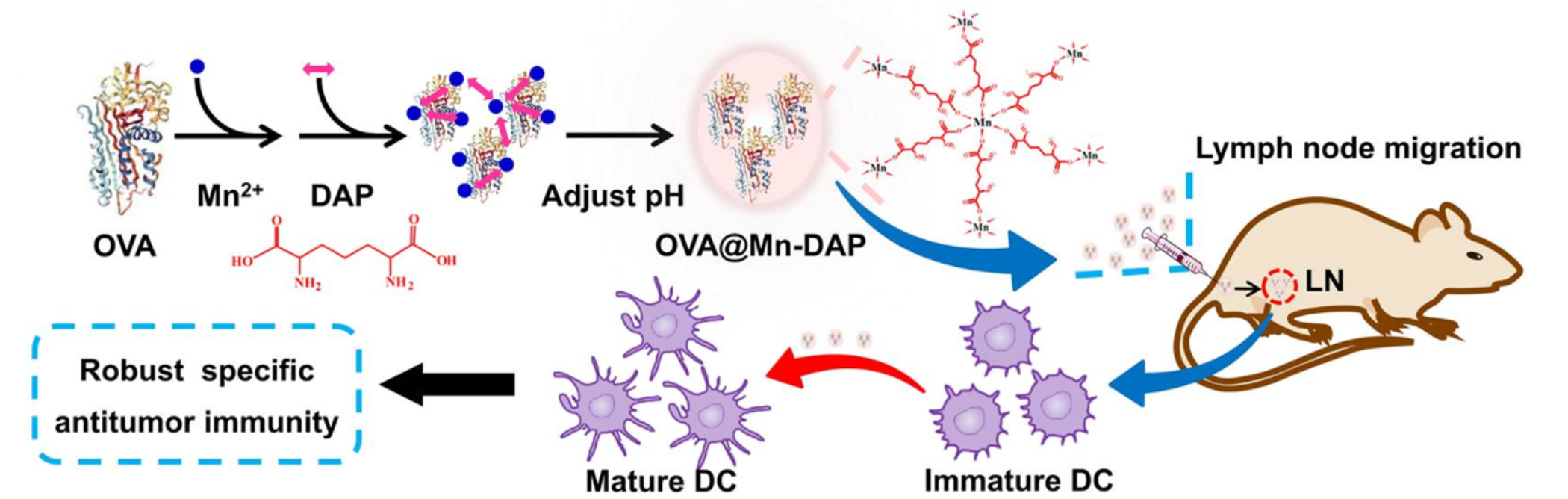
Schematic of manganese based CPs coordinating with DAP for the delivery of OVA into murine lymph nodes to stimulate dendritic cell (DC) maturation for cancer immunotherapy. Reprinted (adapted) with permission from Zhao et al., 2019 (Zhao et al., 2019). Copyright 2019 American Chemical Society.
Chemical and Physical Characterization of CPs
CPs are typically characterized with several diverse and complementary physicochemical techniques to determine their structural properties: size, charge, loading, and other properties. These characteristics are important in assessing a CPs capability as a vaccine delivery system since their morphology can change with the introduction of a biomolecule (Luzuriaga et al., 2019) or even assessing whether or not the biomolecule has been properly encapsulated via surface charge (S. Wang et al., 2017; Yang et al., 2018; Zhang et al., 2020). Table 2 lists out the various techniques used to characterize the morphology, chemical, and physical characteristics of CPs.
Table 2:
Chemical and physical characterization methods and their purpose in assessing CPs.
| Technique | Purpose |
|---|---|
| Structure and Elemental Properties | |
| Single crystal X-ray diffraction (SXRD) & powder X-ray diffraction (PXRD) | Identify crystal structure (morphology), phase purity, crystallinity, and particles size. |
| Scanning electron microscopy (SEM) & transmission electron microscopy (TEM) | Evaluate morphologies and size. |
| Fourier-Transform Infrared Spectroscopy (FTIR) | Identify functional groups and bonding type. |
| Nuclear magnetic resonance (NMR) Solid state NMR (SS-NMR) | Determine purity and linker ratios, probing the local chemical environment and identify elements. |
| Inductively Coupled Plasma Mass Spectrometry (ICP-MS) | Confirm elemental ratios, quantitatively determine cellular uptake. |
| Size and Surface | |
| Dynamic light scattering (DLS) | Determine the size distribution profile in solution. |
| Zeta potential | Measure the effective electric charge on the surface which can change based on the surface adsorption of a biomolecule. |
| Thermal Gravimetric Analysis | Assess thermal stability and pore volume. |
| Adsorption isotherm | Determine the surface area, pore size, and pore size distribution. |
Structure and Elemental Analysis
With CPs, confirmation of the structure is required to determine the dimensionality of the crystals formed (i.e., 1D, 2D, 3D) through diffraction techniques as well as imaging methods. Further, elemental analysis can be used to confirm the ratio of metal to organic linker to verify the structure as well as the purity of the CP.
The most common way to determine CP crystal structure is X-ray diffraction. Single crystal X-ray diffraction (SXRD) is a technique that requires the use of a single crystal, and it provides absolute structural information. However, obtaining a single crystal can be difficult as most SXRD-quality crystals are formed under solvothermal reactions, which limits its biomedical application. Powder X-ray diffraction (PXRD) analysis can be used when a single crystal cannot be obtained. This method, however, does not provide the absolute structure of the crystal but only PXRD patterns to determine crystallinity and phase purity. The patterns obtained by PXRD can only be elucidated when compared to a diffractogram previously reported in literature, or as a simulated model. For example, Eckshtain-Levi et al. reported a zinc-carnosine CP and confirmed the structure via predicted simulations of PXRD patterns (Eckshtain-Levi et al., 2022).
To confirm the ratio of metal to organic linker in the CP or the presence of the biologic cargo, several techniques can be used. The Fourier-transform infrared spectroscopy (FTIR) measurement is usually used as a qualitative analysis to identify functional groups on CPs (Duan et al., 2017; Ni, Lan, et al., 2020). For example, FTIR spectrum of ZIF-8 features specific peaks associated with Zn−N stretching at 420 cm−1 and several bands at 758, 995, 1307, 1583, 2856, 2927, 2959, and 3135 cm−1 corresponding to the C−H stretching of 2-methylimidazole (H. Zhang et al., 2017). Further, comparison between FTIR spectra of the starting and the final materials can revel if a biologic was successfully incorporated (Pang et al., 2020). For example, OVA incorporation within a CP surface was identified by the appearance of new bands in the FTIR spectrum of the material (Qi et al., 2019; Yang et al., 2018).
Nuclear magnetic resonance (NMR) can provide information on the CP’s purity and linker to metal ratio. Conventional NMR solvents are often not suitable due to limited MOF solubility and their digestion is needed in order to obtain a spectrum (Howarth et al., 2017). For example, DBP-Hf MOF can be digested in 85% D3PO4 in DMSO-d6 where the ratio of acetic acid (AcOH) to DBP can be determined by the ratio of the integral of the AcOH peak to the H2DBP peak (Lu et al., 2018). Solid-state NMR (SS-NMR) is another option to characterize CPs. This method probes the local chemical environment inside of the CP, making it useful to verify encapsulation of the biologic.
Inductively coupled plasma mass spectrometry (ICP-MS) can also be used to determine the purity or elemental ratios of a CP (Lu et al., 2016; Ni, Luo, et al., 2020). This technique atomizes the CP for analyte detection with mass spectrometry. Metal ions at concentration as low as parts per a trillion can be analyzed, making this a useful tool when dealing with limited sample amounts. ICP-MS can also be used to determine cellular uptake (Lu et al., 2016; Ni, Luo, et al., 2020) and determine biodistribution in organs and tissues (Lan et al., 2018; Lu et al., 2018; H. Zhang et al., 2017).
Size and Surface Analysis
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) are techniques that can provide images of the CPs for their size distribution as well as their shape and morphology. SEMs provide a 3D image of the surface of the sample whereas TEM images are 2D projections of the samples. When coupled with energy-dispersive X-ray spectroscopy (EDS), this technique can yield both quantitative and qualitative information regarding elemental composition (Miao et al., 2019; Shao et al., 2020; S. Wang et al., 2017; Yang et al., 2018; Zhang et al., 2016). TEM allows the detection of smaller particles due to its larger spatial enhancement compared to SEM. High resolution transmission electron microscopy (HRTEM) is a TEM imaging mode that allows the imaging at the atomic level, providing direct images of the atomic structure/arrangement and crystalline lattice of the samples (Ni, Lan, et al., 2020; Ni, Luo, et al., 2020; Pang et al., 2020). Furthermore, these methods can confirm morphology changes when incorporating a biologic into a MOF. The Gassensmith lab (Luzuriaga et al., 2019) encapsulated TMV within ZIF-8 and the morphology changed drastically from the common rhombic dodecahedral native ZIF-8 crystals to a rod-like morphology.
Dynamic light scattering (DLS) is one of the most frequently used techniques for measuring the hydrodynamic diameter of particles. In several publications DLS was utilized to prove that a biologic was attached to the surface by demonstrating that hydrodynamic size of the particles increased (Pang et al., 2020; S. Wang et al., 2017; Zhang et al., 2020). However, it does not describe the morphology of the particles and usually the chosen fitting model assumes a solid sphere or another ideal geometric shape (Genito et al., 2021). Therefore, the values should be compared to those obtained from an image (e.g. TEM or SEM) (Lei et al., 2020; Lu et al., 2016; Miao et al., 2019; Ni, Lan, et al., 2020; Zeng et al., 2018). It should be noted that particle uptake in cells can be associated with size where particles less than 100 nm can be internalized by most cells, but particles larger than 100nm are primarily taken up by phagocytic cells (Foged et al., 2005; Hirota et al., 2007). However, particles around one to two micrometers in diameter are 50x more likely to be taken up by dendritic cells (DC) rather than macrophages (Genito et al., 2021; Manolova et al., 2008). Particle size can range from 10s of nanometers to micrometers, especially if one is encasing biological elements such as bacteria (Luzuriaga et al., 2021; Luzuriaga et al., 2019) therefore, it is important to measure the size of the particles and their uptake in antigen presenting cells (e.g. DCs and macrophages) to ensure proper antigen processing and generation of reliable vaccine responses.
Zeta potential measurement is a technique for determining the effective electric charge on the material’s surface. The magnitude of the zeta potential combined with the size of material’s particles can provide information about sample stability, where particles with higher magnitude zeta potentials (negative or positive) usually exhibit increased stability due to a larger electrostatic repulsion between particles (Zeng et al., 2018). The zeta potential is sensitive to the sample’s surface layer, thus perfectly suited for monitoring changes in the surface charge value upon adsorption or attachment of the biomolecule (S. Wang et al., 2017; Yang et al., 2018; Zhang et al., 2020). Zhang et al. reports loading CpG onto the surface of ZIF-8 MOF where the ratio of MOF to CpG was varied (H. Zhang et al., 2017). It was shown that the inclusion of CpG alters the charge on the surface where ZIF-8 had a positive charge of ~20 mV and with CpG it was lowered to ~4 mV.
Thermal gravimetric analysis (TGA) monitors the sample’s mass loss as a function of temperature in a controlled atmosphere. Using this method, the thermal stability of a CP (Ni, Luo, et al., 2020), its solvent-accessible pore volume, and the mass loading of incorporated biomolecule can be determined. For determination of pore volume, a TGA spectrum of a solvated sample needs to be collected, and the mass loss will indicate the trapped solvent in the pores. However, weight loss does not necessarily correlate with structural changes and complementary measurements should always be taken. In the case of TBP MOF (Cu), TGA reveled weight loss that was attributed to hydroxide groups/water molecules and isolated N,N-dimethylformamide (DMF) molecules incorporated into the material’s structure, which corresponded well with the crystal structure derived from the SXRD data the authors have reported (Zeng et al., 2018).
Adsorption isotherms determine parameters such as surface areas, pore size and pore size distributions. The method relies on nonreactive gas adsorption to the surface of the studied solid at cryogenic temperatures. The resulting output is an adsorption isotherm with a specific shape, corresponding to the homogeneity of the solid. Typically, nitrogen gas adsorption at 77 K is used for these measurements and the most common approach to calculate the surface area is the Brunauer-Emmett-Teller (BET) method (Ambroz et al., 2018). This can be used in combination with PXRD to confirm the surface area of a MOF. A Cu MOF was studied using nitrogen adsorption which can be seen on a PXRD pattern was in agreement with the porosity determined using the BET method (Zeng et al., 2018).
Biological Characterization of CPs
There are several biological assays and characterizations needed to determine not only the cytotoxicity and stability, but also the efficacy of the carrier as a vaccine delivery platform (Table 3). Additionally, for the use of CPs in the biomedical field, further characterization such as in vitro release studies and endotoxin evaluation should be performed. As shown in Figure 10, the typical workflow when assessing the therapeutic efficacy of CPs preclinically starts with determining and confirming the physical and chemical properties of the CP. Then one can move to analyzing in vitro by treating cells with the CP. Finally, vaccination of mice with the CP can be done.
Table 3:
Biological characterization methods for CPs
| Technique | Purpose |
|---|---|
| Endotoxin assays (Limulus Amebocyte Lysate Assay) | Confirm absence of contamination with endotoxin. |
| In vitro release profiles | Determine pH/buffer dependence and stability. |
| In vitro cytotoxicity assay (MTT, MTS, CCK-8, LDH, TUNEL) and IC50 | Determine toxicity to most relevant cell types. |
| Confocal microscopy | Observe cellular association and uptake. |
| Flow cytometry | Observe cellular association. When paired with antigen recall, can evaluate specific cellular markers that represent long-term immunity or activation markers. |
| ELISA, Luminex®, and ELISpot | Measure cytokine production from cells treated in vitro. Determine activation of key cell populations necessary for vaccination and assessment of cellular response when paired with antigen recall. Measure antibodies from serum for humoral response. |
| Hematoxylin and eosin histology staining and hemolysis | Determine toxicity in animal models. |
| In vivo imaging systems | Tracking the biodistribution of material upon in vivo administration. |
| Challenge studies | Evaluation of the material’s ability to protect against lethal disease. |
Figure 10.
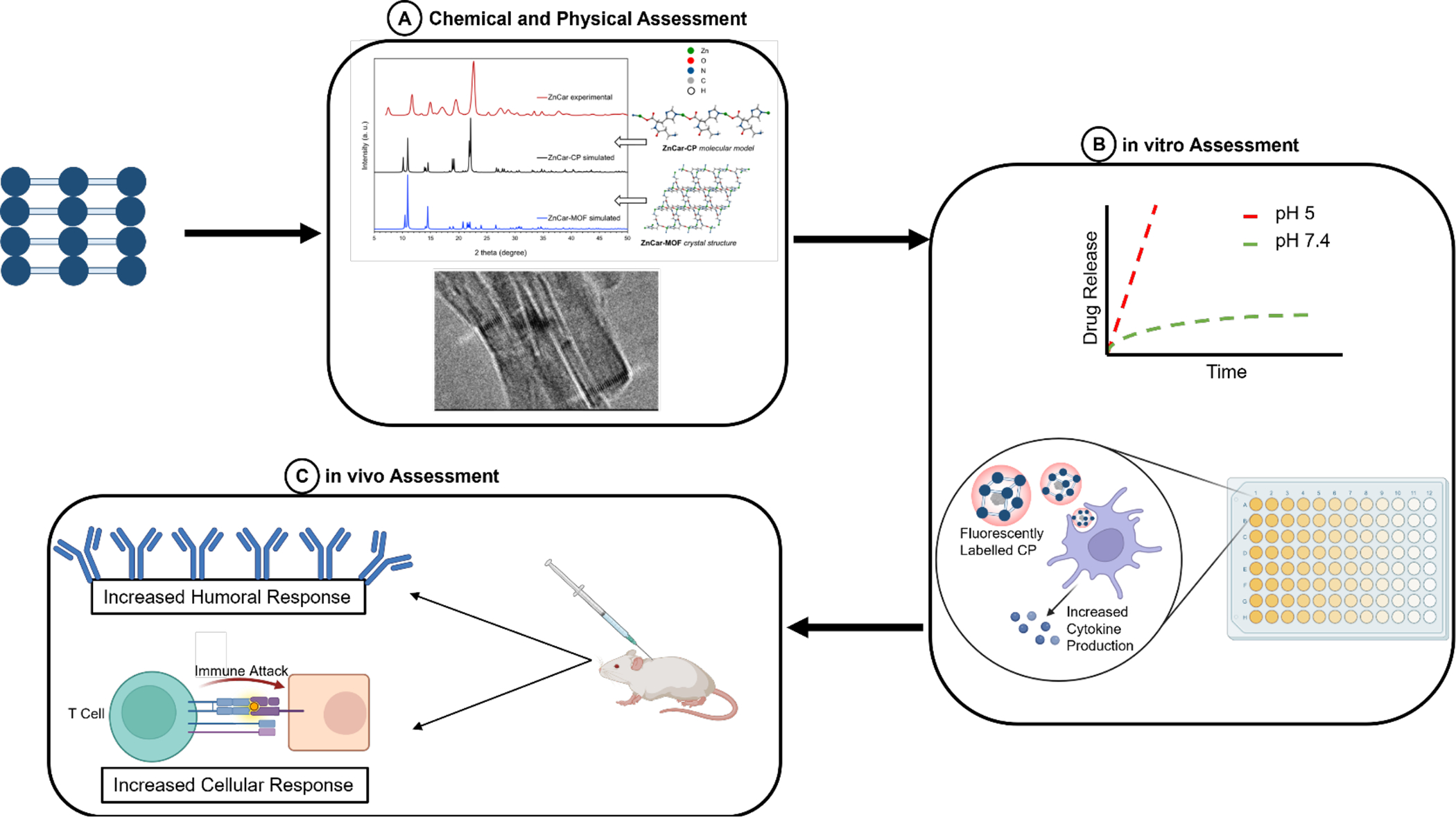
Workflow of assessing CPs starting with (A) to determine the chemical and physical properties (Eckshtain-Levi et al., 2022); (B) appraise in vitro the drug release profile, observe cellular uptake using fluorescently labelled CPs, and measure cytokine production of treated cells; and (C) evaluate the therapeutic efficacy in vivo by vaccinating mice and analyzing for humoral and cellular responses.
After characterizing the chemical and physical properties of the fabricated CP (Figure 10A), one should assess the endotoxin level of the CPs before performing in vitro or in vivo experiments, particularly if aiming to determine the immunostimulatory effect of MOFs as the presence of endotoxins will confound the data. Endotoxins are lipopolysaccharides that originate from bacteria and viruses that can activate the immune system through the toll-like receptor 4 pathway (Genito et al., 2021). These can grow primarily in aqueous solutions that are not maintained in sterile conditions. To determine the presence of endotoxin, kits can be purchased commercially (Limulus Amebocyte Lysate Assay). When determining the immunostimulatory effect of MOFs, the presence of endotoxins can confound the data.
The high porosity and tunability of MOF structures are promising for achieving controlled release, which should be evaluated in vitro. ZIF-8 and several other CPs exhibit pH-sensitive release which is relevant to triggered release in the acidic environment of a lysosome or tumor. Acid sensitive CPs are stable at neutral pH and quickly decompose at acidic conditions (Figure 10B) (Eckshtain-Levi et al., 2022; Lei et al., 2020; Zhang et al., 2020; Zhong et al., 2019). Evaluating release at two different pH’s is needed to verifying acid sensitive release. When performing release studies, it is important to consider the chemical identities of the buffers and counterions present in the solutions as these can interfere with the experiment and yield misleading results. For example, it was shown that ZIF-8 slowly degrades in 10 mM PBS buffer, due to the high affinity of the phosphate ions towards the Zn2+ ions and substitution by them of the original ZIF-8 linkers, 2-Hmim ligands (Velásquez-Hernández et al., 2019). It has been shown that drug release from ZIF-8 in ‘non-coordinating’ buffer such as HEPES is negligible (Liu et al., 2019).
In vitro Analysis
Cytotoxicity Assay
To evaluate the cytotoxicity of CPs in vitro, the most common assays used are MTT, MTS or CCK-8 assays where a tetrazolium salt is applied to cells that have been cultured with CPs to measure cell viability and proliferation. Zhang et al. incubated their ZIF-8 MOFs with RAW macrophages and found that cell viability decreases with MOF concentration (H. Zhang et al., 2017). However, when ZIF-8 was delivering CpG, cell viability increased indicating that the drug being carried can also affect cell proliferation. Eckshtain-Levi et al. measured cell viability using MTT and found that their ZnCar CP had better cytocompatibility than the commonly used ZIF-8 MOF (Eckshtain-Levi et al., 2022). Tamames-Tabar et al. assessed the cytotoxicity of multiple Fe-MOFs and ZIF-8 against HeLa and J774 cells by determining the half maximal inhibitory concentration (IC50) and found that MOFs have similar cytotoxicity capacities to clinically used nanoparticle systems showing promise as drug delivery vehicles (Tamames-Tabar et al., 2014).
Cellular Uptake and Association
Particle uptake can be measured or observed using confocal microscopy with Z-stacks. This method can incorporate multiple Z-stacks where the top and bottom of the cell can be identified with fluorescent staining to allow for visualization of fluorescently-labeled CPs within the cell as depicted in Figure 10B. Methods such as epifluorescence microscopy or flow cytometry can only determine cell association and not internalization in less a dye that responds to the internal cell environment (e.g. DQ Ovalbumin) or stimuli from the particle is used is used to activate an internal dye (e.g. FRET pairing). Yang et al. and Zhang et al. have both used confocal microscopy to image fluorescently labeled MOFs to confirm cellular uptake but also performed flow cytometry to further corroborate their findings (Yang et al., 2018; H. Zhang et al., 2017). Wang et al. tracked in real time cellular uptake of their MOFs against HeLa cells and found that MOF uptake still occurs by 34 hours (Z. Wang et al., 2017). It was also shown that the MOFs were internalized via macropinocytosis through TEM imaging, giving evidence as to how MOFs are internalized by cells. The main purpose of dendritic cells in the body is to present antigens to T-cells to propagate an immune response. Eckshtain-Levi et al. has shown that CPs carrying OVA are internalized by DC2.4s and efficiently process the antigen for cross-presentation to T-cell (Eckshtain-Levi et al., 2022).
Cytokine ELISA Assays
Cytokines play a major role in mediating the immune response and propagating a desired vaccine response (Blanco et al., 2008). Evaluating the innate immune response of the CP can establish if endotoxin is present as well as if the incorporated adjuvant is functional. Endotoxins have a non-specific cytokine secretion profile that could not be discerned from adjuvants. Measuring the concentration of cytokines expressed when CPs are cultured with immune cells is therefore critical to evaluate the innate and adaptive immune responses generated by the formulation (Figure 10B). Typically these measurements are performed after 48 hours of incubation with the CPs and in tandem with cytotoxicity measurements. Cytotoxicity measurements can be used to determine a viable range where cell death is minimal, since increased cell death can lead to elevated cytokines. Since the most common adjuvant incorporated into CPs is CpG, papers typically measure for tumor necrosis factor alpha (TNF-α) and IL-6, as different adjuvants will invoke different cytokines. Generally, it is found that there is an increase in cytokine production when delivered via CP than when its soluble form (Eckshtain-Levi et al., 2022; Z. Wang et al., 2017; H. Zhang et al., 2017), and this is called dose sparing. This dose sparing is likely observed due to the CP formulation setting to the bottom of the well where the cells are as well as more efficient delivery of the adjuvant to the cell which can occur when CP formulations with the adjuvant are internalized and/or are pH sensitive. It should be noted that endotoxin examination should be performed prior to incubating MOFs with cells as the endotoxin can lead to cytokine production via TLR4 pathway.
In vivo Analysis
Animal Toxicity
Toxicity can depend on factors like administration route, exposure time and accumulation/elimination of the CP from the body. Overall, toxicity is dose-dependent. To assess the animal toxicity of their MOFs, Qi et al. used histopathological images of the liver, spleen, kidney, and injection site (Qi et al., 2019). They saw minimal damage at the injection site and no damage to the other organs. Zhang et al. used hemolysis and hematoxylin and eosin histology (H&E) staining to discover that their MOFs did not produce any morphological or toxic effects in mice (Zhang et al., 2020). MOFs encapsulating TMV showed no toxicity when assessed via H&E staining (Luzuriaga et al., 2019). In conclusion, although it is highly dependent on the metal, many MOFs show low toxicity.
Biodistribution
Determining the biodistribution of a MOF helps assess where the MOFs are accumulating towards, and which organs are most enriched. This can be observed and measured by an in vivo imaging system, such as near infrared fluorescence imaging, using a fluorescently-labeled analyte that is incorporated in the MOF. Zhang et al. imaged tumor bearing mice after administration of their MOFs carrying a fluorescent drug both in vivo and ex vivo and found that MOFs accumulated more drug to the tumor than soluble drug due to the enhanced permeability and retention effect (Zhang et al., 2020). During ex vivo imaging it was seen that the MOFs accumulated in the liver which is typical for drug delivery vehicles.
Immune Responses
Humoral responses refer to the immune response in the ‘humors’ (e.g. blood, extracellular fluid). This includes the antibodies generated by B cells as well as complement. The most common way to measure antibodies in serum is to perform an ELISA towards IgG and its subtypes IgG1 and IgG2a or IgG2c for BALB/c or C57BL/6 mice, respectively. A number of studies have shown that the delivery of an adjuvant with a CP can increase antibody production (Figure 10C) (Eckshtain-Levi et al., 2022; Zhang et al., 2016). ZnCar CPs loaded with CpG to deliver OVA or hemagglutinin have also been reported to have increased antibody production upon vaccinating mice showing that CPs can be used for subunit viral vaccination (Eckshtain-Levi et al., 2022). OVA incorporation inside an Fe-MOF was compared to having the protein covalently bonded to the surface. It was reported that there was a significantly higher antibody production when OVA was on the surface, likely because it can then interact with B cells directly (Yang et al., 2018). OVA loaded Al-MOFs were encapsulated into yeast capsules and given orally where the yeast capsules acts to assist the MOF to penetrate through the mucosal lining and elicit an immune response (Miao et al., 2019). Through this study, it was shown that IgA and IgG antibodies can be produced using this method of delivery.
Cellular responses are more desirable for cancer vaccines and some infectious diseases. To evaluate this response, an antigen recall assay can be used where cells isolated from the spleen or lymph nodes are stimulated with the antigen and the response of those cells is monitored. Generally it is best to isolate spleens and lymph nodes 7–10 days after the last vaccine boost. Measurement of cytokines, such as IFN-γ and IL-2, via ELISA or ELISpot can determine if there is a cellular response to the vaccinated antigen (Janeway et al., 2001; Teng et al., 2022; H. Zhang et al., 2017). Cytokine specific markers and activation markers for T cells can also be stained for and analyzed by flow cytometry (Figure 10C) (Li et al., 2020; Zhong et al., 2019). A stronger cellular response has been shown by several groups using CPs to deliver adjuvant with or without antigen (Zhong et al., 2019)(Zhang et al., 2020)(Cai et al., 2020; Eckshtain-Levi et al., 2022). Teng et al., found that encapsulating virus-like particles into MOFs enhance thermal stability and allowed for similar T-cell activation whether the formulation was treated with heat or not (Teng et al., 2022). It was also shown that Zr-MOFs loaded with OVA activate the complement cascade in immunized mice to enhance T cell activation and cytokine production (Qi et al., 2019). The Zr-MOFs were shown to have amino groups that allow for complement protein C3a to adsorb onto the surface. OVA that was conjugated to the surface of an Fe-MOF had higher amounts of cytokine production from splenocytes compared to OVA just encapsulated, indicating that surface expression and conjugation of OVA can enhance cellular responses (Yang et al., 2018). Hf-MOFs with anti-PD-L1 antibodies elicited strong cellular responses where tumors had an elevated level of NK cells, T cells, and dendritic cells (Ni, Lan, Chan, et al., 2018). Overall, the delivery of a vaccine via CPs has shown promising results preclinically as both the humoral and cellular immunity can be enhanced or elevated.
Challenge Studies
Lastly, challenge studies are used to determine the overall effectiveness of the vaccine tested and give a robust evaluation of its therapeutic efficacy. One such way to do a challenge study for cancer is to use cancer that expresses OVA. ZIF-8 MOFs have been used to carry aluminum, CpG and OVA to treat EG7-OVA tumor cells implanted into mice. Median survival was increased by two days in the MOF group and tumor size was significantly reduced in the MOF without aluminum, compared to the MOF with aluminum (Mestas & Hughes, 2004). Li et al. report the use of a ZIF-8 MOF encapsulated in mesoporous silica (MS) (Li et al., 2020). When mice were prophylactically vaccinated with the vaccine prior to subcutaneous implantation of the lymphoma line EG7-OVA, the MOF-MS complex with OVA co-delivered with anti-CTLA4 increased survival and significantly reduced tumor burden compared to the various control groups. When the vaccine was given four times three days after cancer injection, the MOF-MS complex with OVA and anti-CTLA4 with and without poly(I:C) significantly increased survival and decreased tumor burden.
Hyaluronic acid (HA) can also be used to target tumors that have high expression for its receptor, CD44. ZIF-8 was modified with HA along with a photothermal agent IR820 while another ZIF-8 was formulated with imiquimod and IDO inhibitor 1-methyl-D-tryptophan and mannan to enrich DC cell populations (Zhang et al., 2020). In this study, melanoma cells were inhibited at a significantly greater effect and had a higher necrotic and apoptotic fraction when HA modified IR820 MOFs were illuminated with near-infrared light. Zr-MOFs have also been used to target tumors with HA and act as a photosensitizer (Cai et al., 2020). In a subcutaneous H22 tumor model, the photosensitizer-embedded MOF loaded with CpG and hypoxia-inducible factor signaling inhibitor coated in HA significantly reduced tumor volume out to 14 days when stimulated with laser light, compared to controls. This work helps to shine a light on the use of complex CP composites for multi-drug delivery.
To determine immunogenic cell death and if an anti-cancer vaccine response could be developed through the abscopal effect using Hf-MOFs with IDO inhibitors, mice were given subcutaneous implants of CT26 or MC38 cells on both flanks and treated in one of the tumors with MOF formulations given intratumorally (Lu et al., 2016). The MOFs given alone had direct anti-tumor responses, but clearance of the second tumor on the opposite flank of the treated tumor was only observed when mice were treated with IDO laden MOFs that were irradiated, displaying evidence of the abscopal effect. Also in the Lin lab (Lu et al., 2018), Hf-MOFs were used to deliver an IDO inhibitor and to act as a radioenhancer with its organic linker, DBA, for cancer therapy. They observed near complete inhibition of tumor growth for 10 days in all models when tumors were irradiated. When surviving mice were rechallenged with tumor, four out of the six surviving mice were able to clear the tumor, indicating that those mice had a memory response to the tumor. Another study by the Lin lab uses the Hf-MOFs with DBA to function as a radioenhancer but the mice are also treated with anti-PD L1 (Ni, Lan, Chan, et al., 2018). The study found that radiotherapy with a checkpoint blockade elicits systemic antitumor immunity and eliminates tumors in challenge studies. These studies show that Hf-MOFs can be used to treat for a number of cancers when delivering a drug and being used in tandem with irradiation to release cancer antigens.
Duan et al. used Eu-MOFs to deliver OVA and CpG for generation of a melanoma vaccine (Duan et al., 2017). OVA was encapsulated into the Eu MOF during the MOF formation and CpG surface attached post synthesis. This MOF showed acid sensitivity where if introduced to a pH of 5 had a quick release of cargo compared to neutral pH. When treating mice with a F16-OVA subcutaneous cancer, the MOF with OVA with and without CpG significantly reduced tumor growth and extended survival rates compared to controls.
For pathogen vaccines, evaluation of survival after challenge and pathogen load in relevant tissues can provide quantitative information about the vaccine efficacy. The Gassensmith lab encapsulated E. coli in a ZIF-8 and after vaccination with the platform and challenge with the bacteria, greater protection and lower bacterial burden in mice vaccinated with ZIF-8 coated bacteria was illustrated (Luzuriaga et al., 2021). These studies provide evidence that MOFs and CPs can be used as drug delivery vehicles for vaccines against cancer, pathogens, and viruses.
CPs in Clinical Trials
CPs show a lot of promise for biomedical field applications, but since they are still a relatively new type of materials only limited number have advanced to clinical trials. The Lin laboratory at the University of Chicago is the only research group that developed several CPs materials that are currently in clinical trials. Several are constructed from hafnium clusters and organic ligands that serve as photosensitizers, such as porphyrins and ruthenium-loaded organic moieties (Ni, Lan, Veroneau, et al., 2018). These CPs generate two cytotoxic species upon X-ray irradiation and are capable of targeting mitochondria, which in combination with intratumoral delivery leads to targeted tumor cell death. These CPs show high promise for improvement of X-ray radiotherapy, such as allowing to significantly lower the dose of radiation required for the treatment. One CP from this family, coined RiMO-301, is the first CP to enter clinical trials (clinical trials identifier NCT03444714). The clinical trial started in 2018 and is currently in Phase I; it is estimated to be completed by December 2022.
A different family of materials, also developed by Lin’s group as anticancer therapies, are nanoscale CPs (NCP) comprised of zinc ions and phosphate- or phosphonate-functionalized ligands to encapsulate chemotherapeutics (Duan et al., 2019; He et al., 2016; Liu et al., 2014). The NCPs are then covered in a lipid bilayer. Two materials from this family have advanced to clinical trials, namely CPI-100 (clinical trials identifier NCT03781362) and CPI-300 (clinical trials identifier NCT04808453), in 2018 and 2021, respectively. Unlike RiMO-301 which is administered intratumorally, CPI materials are being delivered intravenously. Since the trials are either still actively ongoing or have been completed very recently, results have yet to be disclosed.
Conclusions and Future Work
CPs are a promising platform for drug delivery applications as outlined by the research efforts covered in this review. Their versatility when incorporating biomolecules stemming from a variety of functional groups present within the structure and high porosity/surface area enable both encapsulation and surface attachment of cargo like antigens and adjuvants. Additionally, the acid-sensitivity of some CPs allows for triggered release at low pH, which is especially desired for delivery to tumor microenvironments. Despite the platforms promise and a vast variety of reported structures, only a few CPs are currently being evaluated in clinical trials. To advance these platforms, more emphasis needs to be put on in vitro and in vivo safety evaluation, and robust pre-clinical efforts are required to close the gap between bench work and clinic. Multidisciplinary efforts and collaborations between synthetic chemists, biochemists, biologists, oncologists, and immunologists are pivotal to capitalize on CPs promise as drug delivery platforms and enable transition of the most promising materials to the clinical trials stage.
References
- Al Sharabati M, Sabouni R, & Husseini GA (2022). Biomedical Applications of Metal−Organic Frameworks for Disease Diagnosis and Drug Delivery: A Review. Nanomaterials, 12(2), 277. 10.3390/nano12020277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambroz F, Macdonald TJ, Martis V, & Parkin IP (2018). Evaluation of the BET Theory for the Characterization of Meso and Microporous MOFs. Small Methods, 2(11), 1800173. [Google Scholar]
- An H, Li M, Gao J, Zhang Z, Ma S, & Chen Y (2019). Incorporation of biomolecules in Metal-Organic Frameworks for advanced applications. Coordination Chemistry Reviews, 384, 90–106. [Google Scholar]
- Blanco P, Palucka AK, Pascual V, & Banchereau J (2008). Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev, 19(1), 41–52. 10.1016/j.cytogfr.2007.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovanský J, & Riley PA (1989). Cytotoxicity of zinc in vitro. Chemico-Biological Interactions, 69(2–3), 279–291. [DOI] [PubMed] [Google Scholar]
- Brohlin OR, Ehrman RN, Herbert FC, Wijesundara YH, Raja A, Shahrivarkevishahi A, Diwakara SD, Smaldone RA, & Gassensmith JJ (2022). Zeolitic Imidazolate Framework Nanoencapsulation of CpG for Stabilization and Enhancement of Immunoadjuvancy. ACS Applied Nano Materials. 10.1021/acsanm.1c03555 [DOI] [Google Scholar]
- Bustamante EL, Fernández JL, & Zamaro JM (2014). Influence of the solvent in the synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals at room temperature. Journal of Colloid and Interface Science, 424, 37–43. [DOI] [PubMed] [Google Scholar]
- Cai W, Chu CC, Liu G, & Wáng YX (2015). Metal-Organic Framework-Based Nanomedicine Platforms for Drug Delivery and Molecular Imaging. Small, 11(37), 4806–4822. 10.1002/smll.201500802 [DOI] [PubMed] [Google Scholar]
- Cai Z, Xin F, Wei Z, Wu M, Lin X, Du X, Chen G, Zhang D, Zhang Z, Liu X, & Yao C (2020). Photodynamic Therapy Combined with Antihypoxic Signaling and CpG Adjuvant as an In Situ Tumor Vaccine Based on Metal-Organic Framework Nanoparticles to Boost Cancer Immunotherapy. Adv Healthc Mater, 9(1), e1900996. 10.1002/adhm.201900996 [DOI] [PubMed] [Google Scholar]
- Devic T, & Serre C (2014). High valence 3p and transition metal based MOFs. Chemical Society Reviews, 43(16), 6097–6115. [DOI] [PubMed] [Google Scholar]
- Duan F, Feng X, Yang X, Sun W, Jin Y, Liu H, Ge K, Li Z, & Zhang J (2017). A simple and powerful co-delivery system based on pH-responsive metal-organic frameworks for enhanced cancer immunotherapy. Biomaterials, 122, 23–33. [DOI] [PubMed] [Google Scholar]
- Duan X, Chan C, Han W, Guo N, Weichselbaum RR, & Lin W (2019). Immunostimulatory nanomedicines synergize with checkpoint blockade immunotherapy to eradicate colorectal tumors. Nature Communications, 10(1), 1899. 10.1038/s41467-019-09221-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckshtain-Levi M, Batty CJ, Lifshits LM, McCammitt B, Moore KM, Amouzougan EA, Stiepel RT, Duggan E, Ross TM, Bachelder EM, & Ainslie KM (2022). Metal-Organic Coordination Polymer for Delivery of a Subunit Broadly Acting Influenza Vaccine. ACS Appl Mater Interfaces, 14(25), 28548–28558. 10.1021/acsami.2c04671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foged C, Brodin B, Frokjaer S, & Sundblad A (2005). Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm, 298(2), 315–322. https://doi.org/S0378-5173(05)00244-9 [pii] 10.1016/j.ijpharm.2005.03.035 [DOI] [PubMed] [Google Scholar]
- Gallovic MD, Schully KL, Bell MG, Elberson MA, Palmer JR, Darko CA, Bachelder EM, Wyslouzil BE, Keane-Myers AM, & Ainslie KM (2016). Acetalated Dextran Microparticulate Vaccine Formulated via Coaxial Electrospray Preserves Toxin Neutralization and Enhances Murine Survival Following Inhalational Bacillus Anthracis Exposure. Adv Healthc Mater. 10.1002/adhm.201600642 [DOI] [PubMed] [Google Scholar]
- Genito C, Batty C, Bachelder E, & Ainslie K (2021). Considerations for size, surface charge, polymer degradation, co-delivery, and manufacturability in the development of polymeric particle vaccines for infectious diseases. Advanced NanoBiomed Research In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Duan X, Guo N, Chan C, Poon C, Weichselbaum RR, & Lin W (2016). Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nature Communications, 7(1), 12499. 10.1038/ncomms12499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Hasegawa T, Hinata H, Ito F, Inagawa H, Kochi C, Soma G, Makino K, & Terada H (2007). Optimum conditions for efficient phagocytosis of rifampicin-loaded PLGA microspheres by alveolar macrophages. J Control Release, 119(1), 69–76. https://doi.org/S0168-3659(07)00067-3 [pii] 10.1016/j.jconrel.2007.01.013 [DOI] [PubMed] [Google Scholar]
- Horcajada P, Gref R, Baati T, Allan PK, Maurin G, Couvreur P, Ferey G, Morris RE, & Serre C (2012). Metal-organic frameworks in biomedicine. Chem Rev, 112(2), 1232–1268. 10.1021/cr200256v [DOI] [PubMed] [Google Scholar]
- Horcajada P, Serre C, Maurin G, Ramsahye NA, Balas F, Vallet-Regí M, Sebban M, Taulelle F, & Férey G (2008). Flexible Porous Metal-Organic Frameworks for a Controlled Drug Delivery. Journal of the American Chemical Society, 130(21), 6774–6780. 10.1021/ja710973k [DOI] [PubMed] [Google Scholar]
- Horcajada P, Serre C, Vallet-Regí M, Sebban M, Taulelle F, & Férey G (2006). Metal–Organic Frameworks as Efficient Materials for Drug Delivery. Angewandte Chemie International Edition, 45(36), 5974–5978. 10.1002/anie.200601878 [DOI] [PubMed] [Google Scholar]
- Howarth AJ, Peters AW, Vermeulen NA, Wang TC, Hupp JT, & Farha OK (2017). Best Practices for the Synthesis, Activation, and Characterization of Metal–Organic Frameworks. Chemistry of Materials, 29(1), 26–39. 10.1021/acs.chemmater.6b02626 [DOI] [Google Scholar]
- Janeway C, Travers P, Walport M, & Shlomchik M (2001). ImmunoBiology (5 ed.). Garland Science. [Google Scholar]
- Jian M, Liu B, Liu R, Qu J, Wang H, & Zhang X (2015). Water-based synthesis of zeolitic imidazolate framework-8 with high morphology level at room temperature. RSC advances, 5(60), 48433–48441. [Google Scholar]
- Lan G, Ni K, Xu Z, Veroneau SS, Song Y, & Lin W (2018). Nanoscale metal–organic framework overcomes hypoxia for photodynamic therapy primed cancer immunotherapy. Journal of the American Chemical Society, 140(17), 5670–5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson HD, Walton SP, & Chan C (2021). Metal–Organic Frameworks for Drug Delivery: A Design Perspective. ACS Applied Materials & Interfaces, 13(6), 7004–7020. 10.1021/acsami.1c01089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei J, Wang H, Zhu D, Wan Y, & Yin L (2020). Combined effects of avasimibe immunotherapy, doxorubicin chemotherapy, and metal–organic frameworks nanoparticles on breast cancer [ 10.1002/jcp.29358]. Journal of Cellular Physiology, 235(5), 4814–4823. 10.1002/jcp.29358 [DOI] [PubMed] [Google Scholar]
- Li H, Li L, Lin R-B, Zhou W, Zhang Z, Xiang S, & Chen B (2019). Porous metal-organic frameworks for gas storage and separation: Status and challenges. EnergyChem, 1(1), 100006. 10.1016/j.enchem.2019.100006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang X, Ito A, & Tsuji NM (2020). A nanoscale metal organic frameworks-based vaccine synergises with PD-1 blockade to potentiate anti-tumour immunity. Nature Communications, 11(1), 3858. 10.1038/s41467-020-17637-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K, Ricco R, Doherty CM, Styles MJ, Bell S, Kirby N, Mudie S, Haylock D, Hill AJ, & Doonan CJ (2015). Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nature communications, 6, 7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Hu F, Zhang J, Wang C, & Li L (2019). A Biomimetic Coordination Nanoplatform for Controlled Encapsulation and Delivery of Drug–Gene Combinations. Angewandte Chemie, 131(26), 8896–8900. [DOI] [PubMed] [Google Scholar]
- Liu D, Poon C, Lu K, He C, & Lin W (2014). Self-assembled nanoscale coordination polymers with trigger release properties for effective anticancer therapy. Nature Communications, 5(1), 4182. 10.1038/ncomms5182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Yu T, Shi Z, & Wang Z (2016). The preparation of metal–organic frameworks and their biomedical application. International Journal of Nanomedicine, 1187. 10.2147/ijn.s100877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, He C, Guo N, Chan C, Ni K, Lan G, Tang H, Pelizzari C, Fu Y-X, Spiotto MT, Weichselbaum RR, & Lin W (2018). Low-dose X-ray radiotherapy–radiodynamic therapy via nanoscale metal–organic frameworks enhances checkpoint blockade immunotherapy. Nature Biomedical Engineering, 2(8), 600–610. 10.1038/s41551-018-0203-4 [DOI] [PubMed] [Google Scholar]
- Lu K, He C, Guo N, Chan C, Ni K, Weichselbaum RR, & Lin W (2016). Chlorin-Based Nanoscale Metal–Organic Framework Systemically Rejects Colorectal Cancers via Synergistic Photodynamic Therapy and Checkpoint Blockade Immunotherapy. Journal of the American Chemical Society, 138(38), 12502–12510. 10.1021/jacs.6b06663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzuriaga MA, Herbert FC, Brohlin OR, Gadhvi J, Howlett T, Shahrivarkevishahi A, Wijesundara YH, Venkitapathi S, Veera K, Ehrman R, Benjamin CE, Popal S, Burton MD, Ingersoll MA, De Nisco NJ, & Gassensmith JJ (2021). Metal-Organic Framework Encapsulated Whole-Cell Vaccines Enhance Humoral Immunity against Bacterial Infection. ACS Nano. 10.1021/acsnano.1c03092 [DOI] [PubMed] [Google Scholar]
- Luzuriaga MA, Welch RP, Dharmarwardana M, Benjamin CE, Li S, Shahrivarkevishahi A, Popal S, Tuong LH, Creswell CT, & Gassensmith JJ (2019). Enhanced Stability and Controlled Delivery of MOF-Encapsulated Vaccines and Their Immunogenic Response In Vivo. ACS Appl Mater Interfaces, 11(10), 9740–9746. 10.1021/acsami.8b20504 [DOI] [PubMed] [Google Scholar]
- Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, & Bachmann MF (2008). Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol, 38(5), 1404–1413. 10.1002/eji.200737984 [DOI] [PubMed] [Google Scholar]
- Mestas J, & Hughes CCW (2004). Of Mice and Not Men: Differences between Mouse and Human Immunology. The Journal of Immunology, 172(5), 2731. 10.4049/jimmunol.172.5.2731 [DOI] [PubMed] [Google Scholar]
- Miao Y-B, Pan W-Y, Chen K-H, Wei H-J, Mi F-L, Lu M-Y, Chang Y, & Sung H-W (2019). Engineering a Nanoscale Al-MOF-Armored Antigen Carried by a “Trojan Horse”-Like Platform for Oral Vaccination to Induce Potent and Long-Lasting Immunity. Advanced Functional Materials, 29(43), 1904828. 10.1002/adfm.201904828 [DOI] [Google Scholar]
- Ni K, Lan G, Chan C, Quigley B, Lu K, Aung T, Guo N, La Riviere P, Weichselbaum RR, & Lin W (2018). Nanoscale metal-organic frameworks enhance radiotherapy to potentiate checkpoint blockade immunotherapy. Nature Communications, 9(1), 2351. 10.1038/s41467-018-04703-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni K, Lan G, Song Y, Hao Z, & Lin W (2020). Biomimetic Nanoscale Metal-Organic Framework Harnesses Hypoxia for Effective Cancer Radiotherapy and Immunotherapy. Chemical Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni K, Lan G, Veroneau SS, Duan X, Song Y, & Lin W (2018). Nanoscale metal-organic frameworks for mitochondria-targeted radiotherapy-radiodynamic therapy. Nature Communications, 9(1), 4321. 10.1038/s41467-018-06655-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni K, Luo T, Lan G, Culbert A, Song Y, Wu T, Jiang X, & Lin W (2020). A nanoscale metal–organic framework to mediate photodynamic therapy and deliver CpG oligodeoxynucleotides to enhance antigen presentation and cancer immunotherapy. Angewandte Chemie, 132(3), 1124–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Fu Y, Li C, Wu Z, Cao W, Hu X, Sun X, He W, Cao X, & Ling D (2020). Metal-Organic Framework Nanoparticles for Ameliorating Breast Cancer Associated Osteolysis. Nano Letters. [DOI] [PubMed] [Google Scholar]
- Qi Y, Wang L, Guo H, Pan Y, Xie Z, Jin N, & Huang Y (2019). Antigen-enabled facile preparation of MOF nanovaccine to activate the complement system for enhanced antigen-mediated immune response [ 10.1039/C9BM01145E]. Biomaterials Science, 7(10), 4022–4026. 10.1039/C9BM01145E [DOI] [PubMed] [Google Scholar]
- Rojas S, Devic T, & Horcajada P (2017). Metal organic frameworks based on bioactive components. Journal of Materials Chemistry B, 5(14), 2560–2573. [DOI] [PubMed] [Google Scholar]
- Sajid M (2016). Toxicity of nanoscale metal organic frameworks: a perspective. Environmental Science and Pollution Research, 23(15), 14805–14807. [DOI] [PubMed] [Google Scholar]
- Seetharaj R, Vandana P, Arya P, & Mathew S (2019). Dependence of solvents, pH, molar ratio and temperature in tuning metal organic framework architecture. Arabian journal of chemistry, 12(3), 295315. [Google Scholar]
- Shao Y, Liu B, Di Z, Zhang G, Sun L-D, Li L, & Yan C-H (2020). Engineering of Upconverted Metal–Organic Frameworks for Near-Infrared Light-Triggered Combinational Photodynamic/Chemo-/Immunotherapy against Hypoxic Tumors. Journal of the American Chemical Society, 142(8), 3939–3946. [DOI] [PubMed] [Google Scholar]
- Shearier E, Cheng P, Zhu Z, Bao J, Hu YH, & Zhao F (2016). Surface defection reduces cytotoxicity of Zn (2-methylimidazole) 2 (ZIF-8) without compromising its drug delivery capacity. RSC advances, 6(5), 4128–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Yarza T, Mielcarek A, Couvreur P, & Serre C (2018). Nanoparticles of Metal-Organic Frameworks: On the Road to In Vivo Efficacy in Biomedicine. Adv Mater, 30(37), e1707365. 10.1002/adma.201707365 [DOI] [PubMed] [Google Scholar]
- Singh R, White JF, de Vries M, Beddome G, Dai M, Bean AG, Mulet X, Layton D, & Doherty CM (2022). Biomimetic metal-organic frameworks as protective scaffolds for live-virus encapsulation and vaccine stabilization. Acta Biomaterialia, 142, 320–331. 10.1016/j.actbio.2022.02.002 [DOI] [PubMed] [Google Scholar]
- Stock N, & Biswas S (2012). Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chemical Reviews, 112(2), 933–969. 10.1021/cr200304e [DOI] [PubMed] [Google Scholar]
- Streicher HZ, Berkower IJ, Busch M, Gurd FR, & Berzofsky JA (1984). Antigen conformation determines processing requirements for T-cell activation. Proc Natl Acad Sci U S A, 81(21), 6831–6835. 10.1073/pnas.81.21.6831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghavi M, Khosravi A, Mortaz E, Nikaein D, & Athari SS (2017). Role of pathogen-associated molecular patterns (PAMPS) in immune responses to fungal infections. European Journal of Pharmacology, 808, 8–13. 10.1016/j.ejphar.2016.11.013 [DOI] [PubMed] [Google Scholar]
- Tamames-Tabar C, Cunha D, Imbuluzqueta E, Ragon F, Serre C, Blanco-Prieto MJ, & Horcajada P (2014). Cytotoxicity of nanoscaled metal–organic frameworks. Journal of Materials Chemistry B, 2(3), 262–271. [DOI] [PubMed] [Google Scholar]
- Teng Z, Hou F, Bai M, Li J, Wang J, Wu J, Ru J, Ren M, Sun S, & Guo H (2022). Bio-mineralization of virus-like particles by metal–organic framework nanoparticles enhances the thermostability and immune responses of the vaccines. Journal of Materials Chemistry B, 10(15), 2853–2864. 10.1039/d1tb02719k [DOI] [PubMed] [Google Scholar]
- Tibbetts I, & Kostakis G (2020). Recent Bio-Advances in Metal-Organic Frameworks. Molecules, 25(6), 1291. 10.3390/molecules25061291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan MF, & Gao B (2009). Heat shock proteins and immune system. J Leukoc Biol, 85(6), 905–910. 10.1189/jlb.0109005 [DOI] [PubMed] [Google Scholar]
- Velásquez-Hernández M. d. J., Ricco R, Carraro F, Limpoco FT, Linares-Moreau M, Leitner E, Wiltsche H, Rattenberger J, Schröttner H, & Frühwirt P (2019). Degradation of ZIF-8 in phosphate buffered saline media. CrystEngComm, 21(31), 4538–4544. [Google Scholar]
- Wang S, McGuirk CM, Ross MB, Wang S, Chen P, Xing H, Liu Y, & Mirkin CA (2017). General and direct method for preparing oligonucleotide-functionalized metal–organic framework Nanoparticles. Journal of the American Chemical Society, 139(29), 9827–9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Fu Y, Kang Z, Liu X, Chen N, Wang Q, Tu Y, Wang L, Song S, Ling D, Song H, Kong X, & Fan C (2017). Organelle-Specific Triggered Release of Immunostimulatory Oligonucleotides from Intrinsically Coordinated DNA–Metal–Organic Frameworks with Soluble Exoskeleton. Journal of the American Chemical Society, 139(44), 15784–15791. 10.1021/jacs.7b07895 [DOI] [PubMed] [Google Scholar]
- Yang J, & Yang Y-W (2020). Metal–Organic Frameworks for Biomedical Applications. Small, 16(10), 1906846. 10.1002/smll.201906846 [DOI] [PubMed] [Google Scholar]
- Yang Y, Chen Q, Wu J-P, Kirk TB, Xu J, Liu Z, & Xue W (2018). Reduction-Responsive Codelivery System Based on a Metal–Organic Framework for Eliciting Potent Cellular Immune Response. ACS Applied Materials & Interfaces, 10(15), 12463–12473. 10.1021/acsami.8b01680 [DOI] [PubMed] [Google Scholar]
- Yuan S, Feng L, Wang K, Pang J, Bosch M, Lollar C, Sun Y, Qin J, Yang X, Zhang P, Wang Q, Zou L, Zhang Y, Zhang L, Fang Y, Li J, & Zhou H-C (2018). Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Advanced Materials, 30(37), 1704303. 10.1002/adma.201704303 [DOI] [PubMed] [Google Scholar]
- Zeng J-Y, Zou M-Z, Zhang M, Wang X-S, Zeng X, Cong H, & Zhang X-Z (2018). π-extended benzoporphyrin-based metal–organic framework for inhibition of tumor metastasis. ACS nano, 12(5), 4630–4640. [DOI] [PubMed] [Google Scholar]
- Zhang H, Chen W, Gong K, & Chen J (2017). Nanoscale Zeolitic Imidazolate Framework-8 as Efficient Vehicles for Enhanced Delivery of CpG Oligodeoxynucleotides. ACS Applied Materials & Interfaces, 9(37), 31519–31525. 10.1021/acsami.7b09583 [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang J, Li Q, Song A, Tian H, Wang J, Li Z, & Luan Y (2020). Site-specific MOF-based immunotherapeutic nanoplatforms via synergistic tumor cells-targeted treatment and dendritic cells-targeted immunomodulation. Biomaterials, 245, 119983. 10.1016/j.biomaterials.2020.119983 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jia Y, Li M, & Hou L. a. (2018). Influence of the 2-methylimidazole/zinc nitrate hexahydrate molar ratio on the synthesis of zeolitic imidazolate framework-8 crystals at room temperature. Scientific Reports, 8(1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu C, Wang F, Liu Z, Ren J, & Qu X (2017). Metal–organic-framework-supported immunostimulatory oligonucleotides for enhanced immune response and imaging [ 10.1039/C6CC09280B]. Chemical Communications, 53(11), 1840–1843. 10.1039/C6CC09280B [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang F, Ju E, Liu Z, Chen Z, Ren J, & Qu X (2016). Metal-Organic-Framework-Based Vaccine Platforms for Enhanced Systemic Immune and Memory Response. Advanced Functional Materials, 26(35), 6454–6461. 10.1002/adfm.201600650 [DOI] [Google Scholar]
- Zhao H, Xu J, Li Y, Guan X, Han X, Xu Y, Zhou H, Peng R, Wang J, & Liu Z (2019). Nanoscale Coordination Polymer Based Nanovaccine for Tumor Immunotherapy. ACS Nano, 13(11), 13127–13135. 10.1021/acsnano.9b05974 [DOI] [PubMed] [Google Scholar]
- Zhong X, Zhang Y, Tan L, Zheng T, Hou Y, Hong X, Du G, Chen X, Zhang Y, & Sun X (2019). An aluminum adjuvant-integrated nano-MOF as antigen delivery system to induce strong humoral and cellular immune responses. J Control Release, 300, 81–92. 10.1016/j.jconrel.2019.02.035 [DOI] [PubMed] [Google Scholar]


