Abstract
Adeno-associated viruses (AAVs) are nonautonomous human parvoviruses in that they are dependent on helper functions supplied by other viruses or on genotoxic stimuli for conditions permissive for replication. In the absence of helper, AAV type 2 enters latency by integration into a specific site on human chromosome 19. This feature of AAV, in combination with a lack of pathogenicity, makes AAV an attractive candidate vector for human gene therapy. Goose parvovirus (GPV) is both autonomous and pathogenic yet is highly homologous to AAV. To address the molecular bases for the different viral lifestyles, we compare the AAV and GPV nonstructural proteins, Rep78 and Rep1, respectively. We find that Rep78 and Rep1 possess several biochemical activities in common, including (i) high-affinity DNA binding for sequences that constitute the minimal DNA replication origin; (ii) nucleoside triphosphate-dependent DNA helicase activity; and (iii) origin-specific replication of double-stranded linear DNA. These experiments also establish a specific 38-bp DNA sequence as the minimal GPV DNA replication origin. It is noteworthy that although the proposed Rep binding sites of GPV and AAV are highly similar, Rep1 and Rep78 show a high degree of specificity for their respective origins, in both binding and replication assays. One significant difference was observed; with the minimal replication origin in adenovirus-uninfected extracts, Rep78-mediated replication exhibited low processivity, as previously reported. In contrast, Rep1 efficiently replicated full-length template. Overall, our studies indicate that GPV Rep1 and AAV Rep78 support a comparable mode of replication. Thus, a comparison of the two proteins provides a model system with which to determine the contribution of Rep in the regulation of dependence and autonomy at the level of DNA replication.
Adeno-associated viruses (AAVs) are nonpathogenic human viruses with the ability to integrate site-specifically into the human genome (13, 25–28, 34, 35, 48). These features make them of great interest as potential vectors for human gene therapy.
AAV is a member of the genus Dependovirus of the subfamily Parvovirinae within the Parvoviridae family. Parvovirinae are divided into three genera, Dependovirus, Parvovirus, and Erythrovirus (2). The dependoviruses (AAVs) are unique within the parvovirus family in requiring helper functions supplied by coinfecting virus (reviewed in reference 2). These helper functions are needed for productive infection and rescue of integrated virus. Limited replication can also be induced by genotoxic stimuli (59–61). Unlike AAV, all of the members of the genus Parvovirus are capable of autonomous replication and can have pathogenic effects. Surprisingly, the closest known relatives to AAV as determined by DNA sequence comparison are three highly homologous avian viruses from the genus Parvovirus (62), namely Muscovy duck parvovirus, Barbarie duck parvovirus, and goose parvovirus (GPV). The close genetic relationship of AAV and the avian parvoviruses allows the examination of the structural and mechanistic features which differentiate a nonpathogenic, dependent virus from a potentially pathogenic, autonomous virus. Since most aspects of the viral life cycle after entry into the cell are regulated by the nonstructural proteins encoded by the virus, we have begun this analysis by comparing the in vitro activities of Rep78 of AAV and the comparable protein, Rep1, of GPV.
The products of the AAV rep genes, four nonstructural proteins, Rep78, -68, -52, and -40, are encoded within a single open reading frame in the left half of the genome (15, 36, 38). Rep78 and Rep68 are required for virtually every aspect of the viral life cycle, including replication (17, 52), repression and activation of viral and cellular promoters (16, 19, 30–32, 37, 43), and the generation of single-stranded viral genomes for packaging into virions (5, 18). Although Rep78 and -68 are not expressed at detectable levels at early stages of AAV infection in the absence of helper, genetic studies have shown that Rep78 and/or Rep68 are also required for site-specific integration (1, 35, 51). In accordance with their in vivo functions, the in vitro activities of Rep78 and -68 include specific DNA binding at the Rep binding site (RBS) (7, 21, 47, 58), site- and strand-specific endonuclease activity at the terminal resolution site (trs) (20), nucleoside triphosphate (NTP)-dependent helicase activity (20, 21), and AAV origin-dependent DNA replication (40, 57). The characterization of these proteins has been greatly facilitated by recent advances that have allowed the purification of active viral fusion proteins from Escherichia coli (6).
Much less is known about GPV, an autonomous parvovirus that causes a lethal infection in geese. The entire virus has been sequenced, revealing two open reading frames (3, 62). The first is predicted to encode at least two nonstructural proteins, Rep1 and a smaller protein that could be initiated from an internal ATG, Rep2. The other is predicted to encode capsid proteins. The larger nonstructural protein, Rep1, is most similar in size, sequence, and functional motifs to Rep78 from AAV (Fig. 1A). The highest degree of sequence identity (67%) of Rep78 and Rep1 is found in the central core, the sequence shared by all four AAV Rep proteins. If conservative amino acid replacements are included, the similarity is 83%. Furthermore, this region contains four highly conserved NTP binding-helicase motifs (14, 23). The amino-terminal sequences are 37% identical (58% similar), and they also share at least one structural motif in the vicinity of a tyrosine residue that has been implicated in the covalent attachment of vertebrate parvovirus nonstructural proteins to viral DNA (i.e., tyr152 in Rep68) (12, 50, 54). The lowest sequence identity, 22% (32% similarity), is observed at the carboxy terminus. Despite this low degree of identity, both proteins have potential Zn finger motifs in this region.
FIG. 1.
(A) Diagram of Rep1 and Rep78 showing the extent of sequence identity and similar functional motifs. Y indicates the tyrosine residue that, in Rep78, is believed to form a covalent attachment to viral DNA. Zn finger indicates clusters of cysteine and histidine residues (three in Rep78 and two in Rep1) which could form Zn fingers. (B) Synthetic oligonucleotides used as DNA substrates. The sequences, termed AAV ori and GPV ori, correspond to the putative minimal AAV or GPV origins, respectively. RS1 is an unrelated sequence used as a negative control. The number of base pairs derived from AAV or GPV viral DNA are indicated in parentheses. The duplex oligonucleotides are flanked by single-stranded tails useful for labeling with Klenow polymerase. Underlined nucleotides are those added during the labeling reaction as described in Materials and Methods. Sequences corresponding to the RBS for AAV and the putative RBS for GPV are overlined. The locations of the single-stranded cleavage site (trs) in AAV and the putative trs in GPV are indicated by slashes.
In contrast to that of GPV, the life cycle of AAV has been studied extensively. The block to replication of AAV in the absence of helper occurs at a minimum of three discrete stages in the AAV life cycle: (i) efficient second-strand synthesis (44, 45); (ii) efficient transcription of the AAV genome (4, 22, 46), leading to production of the Rep proteins; and (iii) the process of replication itself. Replication assays have been performed in extracts from both uninfected and adenovirus (Ad)-infected cells which were supplemented with exogenous Rep68MBP (6), a fusion protein of Rep68 and maltose binding protein (MBP) (55, 57). Using linear duplex AAV DNA as a template, the uninfected extracts produce a larger proportion of replication products which are correctly initiated but prematurely terminated, suggesting that one Ad helper function is to increase the processivity of AAV DNA replication (55). The increased processivity found with Ad-infected extracts has recently been shown to be due to the presence of Ad DNA binding protein (DBP) (56). Addition of DBP to uninfected extracts results in increased processivity of Rep-mediated origin-dependent replication. Replication is also partially enhanced in extracts made from HeLa cells grown continuously at high density (39). In this case, it is possible that increased activity of replication protein A (RPA), a cellular single-stranded DNA binding protein, partially alleviates the block in replication, as RPA is also able to increase the processivity of the AAV replication complex (56).
In order to establish a model system for the comparison of Rep1 and Rep78, each protein was produced in E. coli as a His-tagged fusion protein and analyzed in vitro for activities associated with replication. In order to assay GPV replication, we identify a 38-bp sequence from GPV by analogy with the known AAV origin and show that it is necessary and sufficient to direct Rep1-mediated replication in a cell-free system. We also show that, like Rep78, Rep1 binds to sequences within its origin of replication and exhibits NTP-dependent helicase activity. Overall, our findings support a close functional relatedness between Rep1 and Rep78. A significant difference between the two proteins is the ability of Rep1 acting on the GPV origin to support a more processive replication in uninfected cell extracts in comparison to Rep78 acting on the AAV origin. Based on the biochemical similarities between these two proteins, we expect that further studies of Rep78 and Rep1 will help to dissect the molecular bases underlying dependence versus autonomy.
MATERIALS AND METHODS
Materials.
All restriction enzymes were obtained from New England Biolabs, Inc. (NEB), except for BpuAI, which was from Boehringer Mannheim. Klenow and Klenow fragment (3′ to 5′ exo-) were from NEB. All NTP, dNTP, and poly(dIdC) were obtained from Pharmacia.
Cloning of GPV Rep1.
The sequence encoding GPV Rep1 was amplified with Vent DNA polymerase (NEB) by using the primers 5′-CCCGGGCATGGCACTTTCTAGGCCTCTTC-3′ and 5′-CCCGGGTCAGATTCCGCCACGAGGCG-3′ and viral DNA as a template. The product was cloned directly into the vector pCR 2.1 from Invitrogen. A SmaI fragment encoding the intact Rep1 was ligated to the expression vector pET16b (Novagen) which had been cleaved with XhoI and made blunt with Klenow. In the resulting construct, pHisRep1, the coding region of Rep1 was in frame with and carboxy terminal to the 10 histidine residues from the pET16b vector. The integrity of the rep1 gene was verified by sequencing. The coding sequences for Rep78 were subcloned into the pET16b vector to produce pHisRep78.
Mutation of Rep78 and Rep1.
Overlapping PCR mutagenesis was used to introduce single amino acid changes into Rep78 (K340H) and Rep1 (K342H). For Rep78, two separate products were amplified by using primers A (CATCATCATCATCACAGCAGCG) and A′ (GATGTTGGTGTGCCCGGTAGT) or B (ACTACCGGGCACACCAACATC) and B′ (CAGAGAGAGTGTCCTCGAGCC) using pHisRep78 as template and Pfu DNA polymerase (Stratagene). The resulting products of 1,083 and 883 nucleotides (nt), respectively, were gel purified, combined, and reamplified in the presence of primers A and B′. The final product of 2 kb was cut with SacII and DraIII to yield a 684-nt fragment. pHisRep78 was also cleaved with SacII and DraIII, gel purified, and ligated to the cleaved PCR product. Rep1M was constructed by a similar strategy using primers C (ATTGTGAGCGGATAACAATTCCC) and C′ (CAATGTTGGTGTGCCCGGTGG) or D (CCACCGGGCACACCAACATTG) and D′ (CCTCAAGACCCGTTTAGAGGC) on pHisRep1 as template. The final PCR product of 2.2 kb was cleaved with BamHI and NsiI and ligated to pHisRep1 cleaved with the same enzymes. In both cases, the introduced mutations changed the codon AAG (lysine) to CAC (histidine) and resulted in the loss of a cleavage site for BpuAI, providing a simple screen for minipreps. In the selected clones, the nucleotides generated by PCR were verified by sequencing to exclude extraneous mutations introduced by Pfu DNA polymerase.
Protein purification.
The pET16b vector (Novagen, Inc.) was used to express each of the proteins, Rep78, Rep78M, Rep1, and Rep1M in the BL21(DE3) strain of E. coli carrying pLysS. The resulting fusion proteins were purified according to the manufacturer’s instructions (Novagen, Inc.). Briefly, protein expression was induced by culture in 1.0 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 1.5 h at 37°C (both Rep78 variants) or at 30°C (both Rep1 variants). Cell lysate was loaded on a 1-ml HiTrap chelating column (Pharmacia) charged with Ni2+ and washed with binding buffer (20 mM Tris-HCl [pH 7.9], 0.5 M NaCl) containing 100 mM imidazole. Protein was eluted with binding buffer containing 1 M imidazole (Rep78 variants) or 500 mM imidazole (Rep1 variants). Glycerol was added to a final concentration of 15%, and aliquots were frozen in liquid N2 and stored at −135°C. Before use in an electrophoretic mobility shift assay (EMSA), Rep78 proteins were desalted on a Biogel 30 column (Bio-Rad) equilibrated with 34 mM Tris-HCl (pH 7.5), 0.34 M NaCl, 0.13 mM EDTA, 0.01% Nonidet P-40 (NP-40), and 1.3 mM dithiothreitol (DTT). Rep1 proteins were desalted on Sephadex G-50 columns (Boehringer Mannheim Biochemicals) equilibrated with 34 mM Tris-HCl (pH 7.5), 67 mM NaCl, 0.13 mM EDTA, 0.01% NP-40, and 1.3 mM DTT.
Substrates for DNA helicase and EMSA.
The oligomers corresponding to the substrates depicted in Fig. 1B were annealed and extended by Klenow fragment at 25°C for 15 min in the presence of 50 mM dTTP and dGTP and 0.09 mM [α-32P]dCTP (6,000 Ci/mmol; Amersham).
Electrophoretic mobility gel shift assay.
A total of 200 ng of protein was incubated with 30 fmol of labeled DNA in a 20-μl reaction mixture containing 10 mM HEPES-KOH (pH 7.5), 10 mM KCl, 3.3 mM MgCl2, 1 mM EDTA, 2.5 mM DTT, and 400 ng of poly(dI-dC). After incubation for 10 to 20 min at room temperature, 3 μl of loading buffer (40% sucrose, 1% bromophenol blue, 1% xylene cyanol) was added, and 10 μl of the total was analyzed on 6% polyacrylamide gel in 0.25× Tris-borate-EDTA (TBE), run at 18 V/cm. The gel was treated as for the DNA helicase assay.
DNA helicase assay.
Except where indicated, the DNA helicase assay was performed in a reaction mixture of 10 μl containing 20 mM Tris-HCl (pH 7.5), 1 mM MgCl2, 4% sucrose, 8 mg of bovine serum albumin, 8 mM DTT, 1 mM ATP, and the indicated amounts of substrate and protein. The reaction was incubated for 30 min at 37°C and stopped by chilling on ice water. After addition of 3 μl of gel loading buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA, 20% Ficoll 70, 0.4% sodium dodecyl sulfate, 1.2% bromophenol blue), a total of 10 μl was analyzed on an 8% polyacrylamide gel in 1× TBE. Following electrophoresis, gels were fixed in 10% trichloroacetic acid, dried, and either autoradiographed or visualized on a STORM860 PhosphorImager (Molecular Dynamics). The relative amounts of product and substrate were quantitated from the PhosphorImager data with Imagequant version 1.11 software (Molecular Dynamics).
Replication assay.
The replications were performed as described previously (55). The reactions contained 0.1 μg of template DNA and 160 ng of Rep78 or Rep1, and the reaction mixtures were preincubated for 3 h prior to the addition of [α-32P]dCTP and Rep protein and for 16 h afterwards. The latter modification reduces the labeling from the putative repair activities to negligible levels. Reactions were terminated by passage over a Sephadex G-50 spin column and digestion with proteinase K (BMB). Products were analyzed by electrophoresis on 0.8% agarose gels in 1× TBE.
RESULTS
Here, we focus on a biochemical comparison of Rep1 and Rep78 in order to study the molecular bases for the differences in viral lifestyles of AAV and GPV. We have isolated Rep78, Rep1, and their respective null mutants (constructed by mutation of a lysine from the nucleotide binding motif; see Materials and Methods) as His-tagged fusion proteins after expression in E. coli. The addition of a short histidine tag to the proteins avoids problems of steric hindrance potentially associated with bulkier fusion partners, such as MBP.
Rep78 and Rep1 exhibit specific DNA binding activity.
To establish assays for DNA replication and related functions, we first identified sequences from AAV and GPV which might function as origins of replication. The actual minimal AAV origin has not been identified but would be expected to include the RBS and trs from the inverted terminal repeats (ITRs) of the AAV genome. This is supported by the finding that nearly identical sequences derived from human chromosome 19 (AAVS1) have been shown to support replication mediated by MBP-Rep68 and MBP-Rep78 (53). Accordingly, synthetic oligonucleotides which contain 45 bp from the AAV genome and include the RBS and trs were synthesized (AAV ori; Fig. 1B). The corresponding sequences from the GPV genome, containing the proposed GPV RBS and trs (62), were also synthesized and are shown in Fig. 1B as GPV ori. A synthetic oligonucleotide of unrelated sequence (RS1) was used as a control.
In an EMSA, Rep1 was found to bind to the oligonucleotide which we have termed GPV ori, resulting in a mobility shift of 87% of the labeled DNA (Fig. 2B). The binding was specific as it was competed by a 50-fold excess of cold GPV ori (to 14% shifted) but not by an unrelated oligonucleotide, RS1 (82% shifted). Rep1 binding to GPV ori was reduced to 53% by a 50-fold excess of cold AAV ori. Surprisingly, Rep1 was able to bind weakly to the AAV ori, resulting in 29% of the probe shifting. This interaction is specific as it was efficiently competed by a 50-fold excess of cold GPV ori (no probe shifted) or AAV ori (3% shifted) but not by RS1 (18% shifted).
FIG. 2.
Rep1 and Rep78 bind specifically to viral DNA sequences. Labeled substrates, AAV ori, GPV ori, or RS1 (1.5 nM, shown in Fig. 1B) were incubated with 200 ng of Rep78 or Rep1 and 400 ng of poly(dI-dC) as nonspecific DNA in a total of 20 μl as described in Materials and Methods. Products were analyzed on an 8% polyacrylamide gel. Cold competitor DNAs were present at a 50-fold molar excess.
Similarly, Rep78 binding to the AAV ori resulted in a mobility shift of 79% of the probe (Fig. 2A). This was reduced to 6% with excess cold AAV ori but not with RS1 (81% shifted). Cold GPV ori led to a modest decrease in shifted probe (49%) indicating that Rep78 may bind the GPV ori with lower affinity. In agreement with this, Rep78 bound to the GPV ori, shifting 32% of the probe. This binding was efficiently competed by cold AAV ori (no probe shifted) and GPV ori (5% shifted) but not RS1 (24% shifted). Together, these data indicate that both Rep78 and Rep1 bind preferentially to sequences from their own origins and less well to sequences derived from other viral origins.
Rep1 and Rep78 have comparable DNA helicase activity.
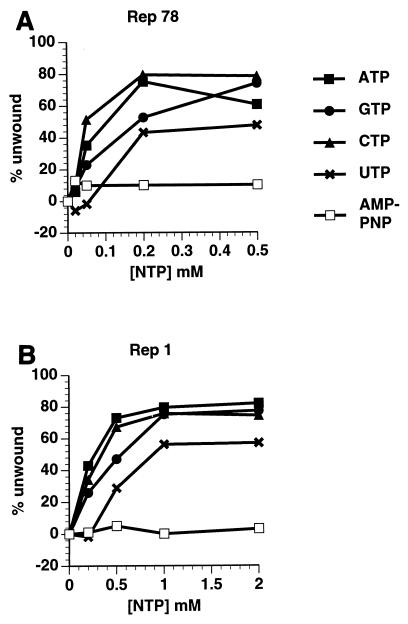
Both Rep78 and Rep1 have helicase activity on the three linear duplex DNA substrates shown in Fig. 1B, AAV ori, GPV ori, and RS1, an unrelated DNA sequence (Fig. 3). Under the conditions of our in vitro assay, Rep78 is more active than Rep1 at low enzyme concentrations. At saturating levels of protein, helicase activities are comparable for both proteins. The NTP requirements for Rep helicase activity were assayed on RS1 (Fig. 4). For both Rep78 and Rep1, no helicase activity was detected in the presence of 5′-adenylimidodiphosphate, a nonhydrolyzable analogue of ATP, indicating a dependence of helicase activity on metabolic energy. In addition to ATP, helicase activity was supported by GTP, CTP, and UTP, in that order of efficacy. A minor difference was observed in the concentration of NTP necessary for maximal helicase activity by Rep78 and Rep1. Rep78 helicase activity reached saturation at 0.2 mM NTP while Rep1 activity was not saturated until 0.5 mM ATP or CTP and 1.0 mM GTP or UTP. This may reflect different binding affinities of the two proteins for the different NTPs.
FIG. 3.
Rep1 and Rep78 have helicase activity on duplex DNA substrates. Rep78 and Rep1, in amounts indicated in nanograms, were titrated for helicase activity on three substrates, AAV ori, GPV ori, and RS1 (5 nM each; shown in Fig. 1B), in a volume of 10 μl as described in Materials and Methods. Products of the reaction were analyzed on an 8% polyacrylamide gel which was dried and exposed to a PhosphorImager screen. The percent substrate unwound was measured by using Imagequant 1.1 software and is displayed graphically. The negative control (C−) was incubated without added protein. The positive control (C+) was heat denatured and resulted in 96% AAV ori unwound, 100% GPV ori unwound, and 97% RS1 unwound. Symbols: ■, Rep 78; ●, Rep 1; ▴, C+.
FIG. 4.
Rep helicase activity requires metabolic energy. The standard helicase assay as described for Fig. 3 was performed on RS1 by using 50 ng of Rep78 (A) or Rep1 (B) and the indicated concentrations of NTP.
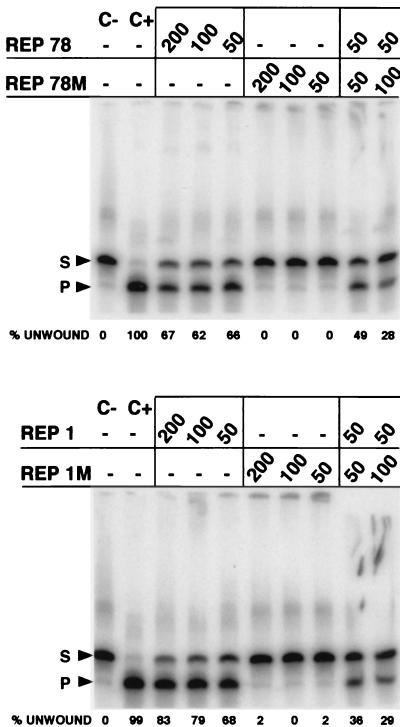
Helicase activity of Rep78 is completely abrogated by a mutation in the nucleotide binding motif of lysine 340 to histidine (6, 29). This Rep78 variant (Rep78M) has a dominant-negative phenotype (29, 41, 42), consistent with the finding that the active form of Rep78 is a hexamer (49). To test whether helicase activity of Rep1 also requires multimerization, we replaced the corresponding lysine in Rep1 (K342) with a histidine residue to produce Rep1M. As expected, both Rep variants were devoid of helicase activity (Fig. 5). Furthermore, just as Rep78 activity was reduced in the presence of Rep78M (from 66 to 49% unwinding), Rep1 activity was decreased from 68 to 36% unwinding in the presence of equimolar Rep1M. A 2:1 ratio of variant to wild-type protein resulted in a further decrease in helicase activity. The possibility that wild-type Rep activity was inhibited by a nonspecific factor is not likely since Rep1M did not inhibit Rep78 and Rep78M did not inhibit Rep1, even at a threefold molar excess (data not shown).
FIG. 5.
Rep1 mutated in the nucleotide binding motif has a dominant-negative phenotype with respect to helicase activity. The standard helicase assay described in the legend to Fig. 3 was performed on RS1 by using Rep78, Rep78M, Rep1, or Rep1M, with amounts given in nanograms. S, substrate; P, product.
Rep78 and Rep1 mediate origin-specific DNA replication.
The templates for the in vitro replication assay were constructed by cloning the AAV origin and putative GPV origin (sequences shown in Fig. 1B) into a pBluescript vector. The resulting construct was linearized by cleaving with NaeI 300 bp 5′ to the inserted sequences. Thus, replication originating at the trs site in the origin would have to proceed for 2.5 kb to produce full-length product. Figure 6 shows the products of a replication assay obtained by using uninfected cell extracts and analyzed on an agarose gel. Rep1 clearly mediates replication from a template containing the GPV origin (Fig. 6A, lane 6), but not significantly from a template which contains the AAV origin (Fig. 6A, lane 4) or from plasmid vector alone (data not shown). Similarly, Rep78 mediates replication from the AAV origin (Fig. 6A, lane 3). Rep78 incubated with other templates stimulates very little incorporation of radioactivity (Fig. 6A, lane 1, and data not shown). Additionally, GPV and AAV origin templates incubated in cell extracts in the absence of added Rep protein are not replicated. Although both Rep1 and Rep78 lead to replication of full-length product, the majority of label incorporated with Rep78 leads to the synthesis of shorter products. This is clearly seen in Fig. 6C, which shows the radioactive profile of lane 6 from Fig. 6A. For Rep78, these shorter products were previously demonstrated to be derived from the template proximal to the origin and 3′ to the trs, indicating that they are correctly initiated but prematurely terminated (55). In contrast, Rep1-mediated replication from the GPV ori leads primarily to full-length product (Fig. 6B).
FIG. 6.
Rep1 and Rep78 mediate origin-specific replication in a cell-free assay. Rep1 or Rep78 (160 ng) or buffer (−) was added to uninfected HeLa cell extracts with 100 ng of linear duplex templates containing either AAV ori or GPV ori sequences (shown in Fig. 1B) in a volume of 15 μl. The products were analyzed on a 0.8% agarose gel which was dried and exposed to film (A). The arrow indicates the full-length replication product (2.7 kb). PhosphorImager tracings of lane 3 (B) and lane 6 (C) are shown. The area under the curve is proportional to the radioactivity in that part of the lane.
DISCUSSION
The human parvovirus AAV has evolved a successful relationship with its host, being nonpathogenic while maintaining the capacity to propagate. As shown in tissue culture, AAV has adopted the strategy of two alternate pathways. First, AAV replicates only in the presence of helper factors, which are by themselves deleterious to the host cell. Among those identified to date are superinfection or coinfection with viruses like Ad and herpesviruses. In the absence of helper functions, AAV enters a latent pathway, integrating its DNA into the human genome. In contrast to randomly integrating viruses which can damage the host cell through insertional mutagenesis, AAV integrates into a specific locus of human chromosome 19q13.3-qter (27, 48). When the host cell is superinfected by a helper virus, AAV can be rescued from its latent state, resulting in the efficient propagation of the virus.
In contrast, GPV has adopted a simpler strategy in which infection results in the rapid propagation of the virus. Nevertheless, the full DNA sequence of GPV (62) has revealed a surprisingly close structural relationship between GPV and AAV. This allows us to address the molecular bases for the different viral lifestyles by a comparison of the AAV and GPV Rep proteins (Rep78 and Rep1), focusing in these initial experiments on biochemical activities related to viral DNA replication.
To study replication-related Rep activities, it was necessary to identify viral DNA sequences capable of supporting replication. For AAV, the ITR has been shown to act as an origin of DNA replication. Due to structural differences between the AAV ITR (T-shaped hairpin) and GPV ITR (simple hairpin), we decided to use a minimal origin for DNA replication containing only the RBS and trs and omitting the hairpin regions. The corresponding sequence derived from AAVS1 of ca. 40 bp has previously been shown to act as a Rep-dependent origin of replication (53) as well as constitute the minimal requirement for AAV site-specific integration (35). This data suggested that the analogous sequences from the viral ITRs might act as minimal replication origins as well. As a first experiment, we determined the relative binding affinities of the Rep78 and Rep1 proteins to their respective origins. The AAV RBS consists of three tetramer repeats (GCTC) plus GCGC. Within the ITRs of GPV a similar series of three tetramer repeats (GTTC) plus GAAC can be found. Consequently, Zadori et al. (62) proposed this motif to be the GPV RBS. The probes used in our assays extend beyond the putative RBS for 19 to 25 nt to include sequences at the end of the viral genome which, for AAV, constitute the trs. As shown in Fig. 2, Rep1 binds with high specificity to this probe while Rep78 binds with high specificity to the AAV ori probe. It is interesting to note that although the core AAV and GPV RBS motifs differ by only 1 nt (C to T), the Rep proteins exhibit a clear preference for binding to their cognate viral origins. With respect to Rep78, this finding is in agreement with a report published by Chiorini et al. (8) proposing a consensus sequence for high-affinity binding by MBP-Rep78, (GCTC)2, based on random oligonucleotide selection. Note that we report the consensus sequence of the opposite strand, GCTC, rather than GAGC. The high specificity of origin binding for Rep78 and Rep1 agrees with the relatively lower level of sequence identity in the amino-terminal region of the Rep proteins (Fig. 1A), the proposed site of the DNA binding domain (42). These observations raise the intriguing possibility that domain exchanges between Rep78 and Rep1 may allow a refined identification of the functional Rep78 (and Rep1) DNA binding domains.
Helicase activity of Rep1 and Rep78 was tested on linear duplex DNA substrates of ca. 40 bp in length. On all substrates tested, both Rep proteins, when used at optimal concentrations, unwound duplex DNA with equal efficiencies (Fig. 3). In order to ensure that the observed activities were not due to E. coli helicases copurifying with our Rep proteins, we generated the previously characterized NTP binding-site mutant which replaces lysine 340 with histidine in Rep78 (resulting in Rep78M). We also replaced the corresponding amino acid (lysine 342) in Rep1 to produce Rep1M. As expected, both variants were devoid of helicase activity (Fig. 4). Furthermore, in agreement with the observation that this AAV Rep mutant has a dominant-negative phenotype over Rep78 (29, 41, 42), the activities of the wild-type proteins (Rep78 and Rep1) were considerably reduced upon the addition of the respective mutant proteins (Fig. 4). Inhibition of wild-type Rep by a mutant which lacks activity can occur if the protein normally assembles into a multimeric structure. These results suggest that Rep1, like Rep78, may function as a multimer.
Most significantly, Rep1 is able to mediate origin-dependent replication in uninfected cell extracts. This assay also identifies a 38-nt sequence from the GPV genome, similar in structure to the AAV origin, that can function as an origin of replication. Replication mediated by either Rep1 or Rep78 is highly origin specific. Rep1 does not efficiently replicate the AAV origin and vice versa. This is despite the fact that Rep1 binds to the AAV ori with moderate affinity. Conversely, Rep78 binds to the GPV ori and to AAVS1 with similar affinity (Fig. 2 and data not shown) yet does not mediate significant replication from the former while it does from the latter. These observations might be explained if Rep1 and Rep78 are highly specific for cleavage at their own respective trs.
By analogy to AAV, the GPV origin is likely to contain a trs. This can be inferred from the ability of Rep1 to initiate replication on linear duplex DNA. Replication of this substrate can proceed only if Rep1, by making a single-stranded cut at the trs, creates a 3′ end to serve as primer for the polymerase. However, we were not able to directly detect endonuclease activity for Rep1 by using the minimal assay system developed for Rep78 (data not shown). It may be that Rep1 requires buffer conditions which are different from those identified for Rep78 (20, 21). A more intriguing possibility is that Rep1, like NS1 protein from the autonomous parvovirus MVM, requires one or more factors present in the cell to acquire endonuclease activity (9, 10). These questions are currently under investigation in our laboratory.
The cell-free DNA replication assay revealed one particularly significant difference between Rep78- and Rep1-mediated replication. It was previously shown that AAV Rep-mediated DNA replication in cell extracts devoid of helper factors demonstrated lower processivity than replication in Ad-infected cell extracts. These experiments employed linear duplex AAV templates from which one of the ITRs was deleted in order to assess the length of replication product produced from a single initiation site (55). Here, we have used a similar template but substituted all but ca. 40 bp of viral sequence with plasmid DNA. In uninfected extracts, Rep1-mediated replication from the GPV origin produces primarily full-length product whereas Rep78 acting on the AAV origin produces mostly short fragments (Fig. 6B and C). Note that for both the AAV and GPV templates, all sequences outside of the short (45 to 38 bp) viral origins are derived from plasmid and are therefore identical. Thus the short fragments produced by Rep78 are not likely to be due to impassable secondary structure on the template.
We conclude that Rep1 and Rep78 mediate highly similar modes of replication, requiring binding to an RBS, cleavage at a trs, and helicase activity. Thus, they provide a model system for the future identification of specific activities required for autonomous versus dependent lifestyles. We report here that the replication complex assembled by Rep1 on the GPV origin has a greater processivity than the one assembled by Rep78 on the AAV origin. Since this processivity problem in AAV has been shown to be abrogated by the presence of the Ad-DBP (56), the processivity difference between replication mediated by the two proteins probably reflects the respective dependence and nondependence on an Ad coinfection for productive replication. In addition, since it has been suggested that a lack of processivity in replication may be a part of the mechanism by which AAV integrates (24, 34), this difference between the two proteins may also be related to the tendency of AAV to establish latency by integration into the human genome.
Furthermore, this cross-genus comparison may serve as a tool to identify functional domains within the Rep78 protein. AAV Rep-mediated site-specific integration represents a unique activity. The prospect of altering target specificity in order to achieve targeted integration into other selected loci within the human genome has been suggested (11, 33). While this approach would be useful (e.g., in the study of chromosome position effects, in the generation of transgenic knockins and knockouts, in the establishment of gene replacement therapy, etc.) its development relies on the precise characterization of the Rep DNA binding domain presumably responsible for mediating the target binding. We characterize here an additional Rep protein which, while active in a comparable manner to Rep78, recognizes a different DNA motif. In this case, domain exchanges may well result in a functional protein, in contrast to simple mutagenesis which is likely to abolish several functions simultaneously. This approach may allow the refined localization and characterization of this potentially useful protein domain.
ACKNOWLEDGMENTS
We gratefully acknowledge Mertyn Malkinson for help in isolating the rep1 gene and Miroslav Djokic for generation of pHisRep1. We thank Aneel Aggarwal, Natalie Dutheil, Peter Palese, Aurelian Radu, and Martin Wiedmann for critical readings of the manuscript.
REFERENCES
- 1.Balague C, Kalla M, Zhang W W. Adeno-associated virus Rep78 protein and terminal repeats enhance integration of DNA sequences into the cellular genome. J Virol. 1997;71:3299–3306. doi: 10.1128/jvi.71.4.3299-3306.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berns K I. Parvoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2173–2197. [Google Scholar]
- 3.Brown K E, Green S W, Young N S. Goose parvovirus—an autonomous member of the dependovirus genus? Virology. 1995;210:283–291. doi: 10.1006/viro.1995.1345. [DOI] [PubMed] [Google Scholar]
- 4.Carter B J, Antoni B A, Klessig D F. Adenovirus containing a deletion of the early region 2A gene allows growth of adeno-associated virus with decreased efficiency. Virology. 1992;191:473–476. doi: 10.1016/0042-6822(92)90213-9. [DOI] [PubMed] [Google Scholar]
- 5.Chejanovsky N, Carter B J. Mutation of a consensus purine nucleotide binding site in the adeno-associated virus rep gene generates a dominant negative phenotype for DNA replication. J Virol. 1990;64:1764–1770. doi: 10.1128/jvi.64.4.1764-1770.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiorini J A, Weitzman M D, Owens R A, Urcelay E, Safer B, Kotin R M. Biologically active Rep proteins of adeno-associated virus type 2 produced as fusion proteins in Escherichia coli. J Virol. 1994;68:797–804. doi: 10.1128/jvi.68.2.797-804.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiorini J A, Wiener S M, Owens R A, Kyöstiö S R, Kotin R M, Safer B. Sequence requirements for stable binding and function of Rep68 on the adeno-associated virus type 2 inverted terminal repeats. J Virol. 1994;68:7448–7457. doi: 10.1128/jvi.68.11.7448-7457.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiorini J A, Yang L, Safer B, Kotin R M. Determination of adeno-associated virus Rep68 and Rep78 binding sites by random sequence oligonucleotide selection. J Virol. 1995;69:7334–7338. doi: 10.1128/jvi.69.11.7334-7338.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen J, Cotmore S F, Tattersall P. A novel cellular site-specific DNA-binding protein cooperates with the viral NS1 polypeptide to initiate parvovirus DNA replication. J Virol. 1997;71:1405–1416. doi: 10.1128/jvi.71.2.1405-1416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen J, Cotmore S F, Tattersall P. Parvovirus initiation factor PIF: a novel human DNA-binding factor which coordinately recognizes two ACGT motifs. J Virol. 1997;71:5733–5741. doi: 10.1128/jvi.71.8.5733-5741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corsini J, Tal J, Winocour E. Directed integration of minute virus of mice DNA into episomes. J Virol. 1997;71:9008–9015. doi: 10.1128/jvi.71.12.9008-9015.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotmore S F, Tattersall P. An asymmetric nucleotide in the parvoviral 3′ hairpin directs segregation of a single active origin of DNA replication. EMBO J. 1994;13:4145–4152. doi: 10.1002/j.1460-2075.1994.tb06732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giraud C, Winocour E, Berns K I. Site-specific integration by adeno-associated virus is directed by a cellular DNA sequence. Proc Natl Acad Sci USA. 1994;91:10039–10043. doi: 10.1073/pnas.91.21.10039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorbalenya A E, Koonin E V, Wolf Y I. A new superfamily of putative NTP-binding domains encoded by genomes of small DNA and RNA viruses. FEBS Lett. 1990;262:145–148. doi: 10.1016/0014-5793(90)80175-i. [DOI] [PubMed] [Google Scholar]
- 15.Green M R, Roeder R G. Transcripts of the adeno-associated virus genome: mapping of the major RNAs. J Virol. 1980;36:79–92. doi: 10.1128/jvi.36.1.79-92.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermonat P L. Down-regulation of the human c-fos and c-myc proto-oncogene promoters by adeno-associated virus Rep78. Cancer Lett. 1994;81:129–136. doi: 10.1016/0304-3835(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 17.Hermonat P L, Labow M A, Wright R, Berns K I, Muzyczka N. Genetics of adeno-associated virus: isolation and preliminary characterization of adeno-associated virus type 2 mutants. J Virol. 1984;51:329–339. doi: 10.1128/jvi.51.2.329-339.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holscher C, Kleinschmidt J A, Burkle A. High-level expression of adeno-associated virus (AAV) Rep78 or Rep68 protein is sufficient for infectious-particle formation by a rep-negative AAV mutant. J Virol. 1995;69:6880–6885. doi: 10.1128/jvi.69.11.6880-6885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horer M, Weger S, Butz K, Hoppe-Seyler F, Geisen C, Kleinschmidt J A. Mutational analysis of adeno-associated virus Rep protein-mediated inhibition of heterologous and homologous promoters. J Virol. 1995;69:5485–5496. doi: 10.1128/jvi.69.9.5485-5496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Im D S, Muzyczka N. The AAV origin binding protein Rep68 is an ATP-dependent site-specific endonuclease with DNA helicase activity. Cell. 1990;61:447–457. doi: 10.1016/0092-8674(90)90526-k. [DOI] [PubMed] [Google Scholar]
- 21.Im D S, Muzyczka N. Partial purification of adeno-associated virus Rep78, Rep52, and Rep40 and their biochemical characterization. J Virol. 1992;66:1119–1128. doi: 10.1128/jvi.66.2.1119-1128.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janik J E, Huston M M, Rose J A. Locations of adenovirus genes required for the replication of adenovirus-associated virus. Proc Natl Acad Sci USA. 1981;78:1925–1929. doi: 10.1073/pnas.78.3.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koonin E V. A common set of conserved motifs in a vast variety of putative nucleic acid-dependent ATPases including MCM proteins involved in the initiation of eukaryotic DNA replication. Nucleic Acids Res. 1993;21:2541–2547. doi: 10.1093/nar/21.11.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotin R M. Prospects for the use of adeno-associated virus as a vector for human gene therapy. Hum Gene Ther. 1994;5:793–801. doi: 10.1089/hum.1994.5.7-793. [DOI] [PubMed] [Google Scholar]
- 25.Kotin R M, Berns K I. Organization of adeno-associated virus DNA in latently infected Detroit 6 cells. Virology. 1989;170:460–467. doi: 10.1016/0042-6822(89)90437-6. [DOI] [PubMed] [Google Scholar]
- 26.Kotin R M, Linden R M, Berns K I. Characterization of a preferred site on human chromosome 19q for integration of adeno-associated virus DNA by non-homologous recombination. EMBO J. 1992;11:5071–5078. doi: 10.1002/j.1460-2075.1992.tb05614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotin R M, Menninger J C, Ward D C, Berns K I. Mapping and direct visualization of a region-specific viral DNA integration site on chromosome 19q13-qter. Genomics. 1991;10:831–834. doi: 10.1016/0888-7543(91)90470-y. [DOI] [PubMed] [Google Scholar]
- 28.Kotin R M, Siniscalco M, Samulski R J, Zhu X D, Hunter L, Laughlin C A, McLaughlin S, Muzyczka N, Rocchi M, Berns K I. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA. 1990;87:2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kyöstiö S R, Owens R A. Identification of mutant adeno-associated virus Rep proteins which are dominant-negative for DNA helicase activity. Biochem Biophys Res Commun. 1996;220:294–299. doi: 10.1006/bbrc.1996.0399. [DOI] [PubMed] [Google Scholar]
- 30.Kyöstiö S R M, Owens R A, Weitzman M D, Antoni B A, Chejanovsky N, Carter B J. Analysis of adeno-associated virus (AAV) wild-type and mutant Rep proteins for their abilities to negatively regulate AAV p5 and p19 mRNA levels. J Virol. 1994;68:2947–2957. doi: 10.1128/jvi.68.5.2947-2957.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kyöstiö S R M, Wonderling R S, Owens R A. Negative regulation of the adeno-associated virus (AAV) P5 promoter involves both the P5 Rep binding site and the consensus ATP-binding motif of the AAV Rep68 protein. J Virol. 1995;69:6787–6796. doi: 10.1128/jvi.69.11.6787-6796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labow M A, Hermonat P L, Berns K I. Positive and negative autoregulation of the adeno-associated virus type 2 genome. J Virol. 1986;60:251–258. doi: 10.1128/jvi.60.1.251-258.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linden R M, Berns K I. Site-specific integration by adeno-associated virus: a basis for a potential gene therapy vector. Gene Ther. 1997;4:4–5. doi: 10.1038/sj.gt.3300357. [DOI] [PubMed] [Google Scholar]
- 34.Linden R M, Ward P, Giraud C, Winocour E, Berns K I. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA. 1996;93:11288–11294. doi: 10.1073/pnas.93.21.11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linden R M, Winocour E, Berns K I. The recombination signals for adeno-associated virus site-specific integration. Proc Natl Acad Sci USA. 1996;93:7966–7972. doi: 10.1073/pnas.93.15.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lusby E W, Berns K I. Mapping of the 5′ termini of two adeno-associated virus 2 RNAs in the left half of the genome. J Virol. 1982;41:518–526. doi: 10.1128/jvi.41.2.518-526.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarty D M, Ni T H, Muzyczka N. Analysis of mutations in adeno-associated virus Rep protein in vivo and in vitro. J Virol. 1992;66:4050–4057. doi: 10.1128/jvi.66.7.4050-4057.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendelson E, Trempe J P, Carter B J. Identification of the trans-acting Rep proteins of adeno-associated virus by antibodies to a synthetic oligopeptide. J Virol. 1986;60:823–832. doi: 10.1128/jvi.60.3.823-832.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ni T H, McDonald W F, Zolotukhin I, Melendy T, Waga S, Stillman B, Muzyczka N. Cellular proteins required for adeno-associated virus DNA replication in the absence of adenovirus coinfection. J Virol. 1998;72:2777–2787. doi: 10.1128/jvi.72.4.2777-2787.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ni T H, Zhou X, McCarty D M, Zolotukhin I, Muzyczka N. In vitro replication of adeno-associated virus DNA. J Virol. 1994;68:1128–1138. doi: 10.1128/jvi.68.2.1128-1138.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Owens R A, Trempe J P, Chejanovsky N, Carter B J. Adeno-associated virus Rep proteins produced in insect and mammalian expression systems: wild-type and dominant-negative mutant proteins bind to the viral replication origin. Virology. 1991;184:14–22. doi: 10.1016/0042-6822(91)90817-u. [DOI] [PubMed] [Google Scholar]
- 42.Owens R A, Weitzman M D, Kyöstiö S R M, Carter B J. Identification of a DNA-binding domain in the amino terminus of adeno-associated virus Rep proteins. J Virol. 1993;67:997–1005. doi: 10.1128/jvi.67.2.997-1005.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pereira D J, McCarty D M, Muzyczka N. The adeno-associated virus (AAV) Rep protein acts as both a repressor and an activator to regulate AAV transcription during a productive infection. J Virol. 1997;71:1079–1088. doi: 10.1128/jvi.71.2.1079-1088.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qing K, Khuntirat B, Mah C, Kube D M, Wang X-S, Ponnazhagan S, Zhou S, Dwarki V J, Yoder M C, Srivastava A. Adeno-associated virus type 2-mediated gene transfer: correlation of tyrosine phosphorylation of the cellular single-stranded D sequence-binding protein with transgene expression in human cells in vitro and murine tissues in vivo. J Virol. 1998;72:1593–1599. doi: 10.1128/jvi.72.2.1593-1599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qing K, Wang X S, Kube D M, Ponnazhagan S, Bajpai A, Srivastava A. Role of tyrosine phosphorylation of a cellular protein in adeno-associated virus 2-mediated transgene expression. Proc Natl Acad Sci USA. 1997;94:10879–10884. doi: 10.1073/pnas.94.20.10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richardson W D, Westphal H. Requirement for either early region 1a or early region 1b adenovirus gene products in the helper effect for adeno-associated virus. J Virol. 1984;51:404–410. doi: 10.1128/jvi.51.2.404-410.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryan J H, Zolotukhin S, Muzyczka N. Sequence requirements for binding of Rep68 to the adeno-associated virus terminal repeats. J Virol. 1996;70:1542–1553. doi: 10.1128/jvi.70.3.1542-1553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samulski R J, Zhu X, Xiao X, Brook J D, Housman D E, Epstein N, Hunter L A. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 1991;10:3941–3950. doi: 10.1002/j.1460-2075.1991.tb04964.x. . (Erratum, 11:1228, 1992.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith R H, Spano A J, Kotin R M. The Rep78 gene product of adeno-associated virus (AAV) self-associates to form a hexameric complex in the presence of AAV ori sequences. J Virol. 1997;71:4461–4471. doi: 10.1128/jvi.71.6.4461-4471.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snyder R O, Im D S, Muzyczka N. Evidence for covalent attachment of the adeno-associated virus (AAV) rep protein to the ends of the AAV genome. J Virol. 1990;64:6204–6213. doi: 10.1128/jvi.64.12.6204-6213.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Surosky R T, Urabe M, Godwin S G, McQuiston S A, Kurtzman G J, Ozawa K, Natsoulis G. Adeno-associated virus Rep proteins target DNA sequences to a unique locus in the human genome. J Virol. 1997;71:7951–7959. doi: 10.1128/jvi.71.10.7951-7959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tratschin J D, Miller I L, Carter B J. Genetic analysis of adeno-associated virus: properties of deletion mutants constructed in vitro and evidence for an adeno-associated virus replication function. J Virol. 1984;51:611–619. doi: 10.1128/jvi.51.3.611-619.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Urcelay E, Ward P, Wiener S M, Safer B, Kotin R M. Asymmetric replication in vitro from a human sequence element is dependent on adeno-associated virus Rep protein. J Virol. 1995;69:2038–2046. doi: 10.1128/jvi.69.4.2038-2046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker S L, Wonderling R S, Owens R A. Mutational analysis of the adeno-associated virus type 2 Rep68 protein helicase motifs. J Virol. 1997;71:6996–7004. doi: 10.1128/jvi.71.9.6996-7004.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ward P, Berns K I. In vitro replication of adeno-associated virus DNA: enhancement by extracts from adenovirus-infected HeLa cells. J Virol. 1996;70:4495–4501. doi: 10.1128/jvi.70.7.4495-4501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ward P, Dean F B, O’Donnell M E, Berns K I. Role of the adenovirus DNA-binding protein in in vitro adeno-associated virus DNA replication. J Virol. 1998;72:420–427. doi: 10.1128/jvi.72.1.420-427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ward P, Urcelay E, Kotin R, Safer B, Berns K I. Adeno-associated virus DNA replication in vitro: activation by a maltose binding protein/Rep 68 fusion protein. J Virol. 1994;68:6029–6037. doi: 10.1128/jvi.68.9.6029-6037.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weitzman M D, Kyöstiö S R M, Carter B J, Owens R A. Interaction of wild-type and mutant adeno-associated virus (AAV) Rep proteins on AAV hairpin DNA. J Virol. 1996;70:2440–2448. doi: 10.1128/jvi.70.4.2440-2448.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yakinoglu A O, Heilbronn R, Burkle A, Schlehofer J R, zur Hausen H. DNA amplification of adeno-associated virus as a response to cellular genotoxic stress. Cancer Res. 1988;48:3123–3129. [PubMed] [Google Scholar]
- 60.Yakobson B, Hrynko T A, Peak M J, Winocour E. Replication of adeno-associated virus in cells irradiated with UV light at 254 nm. J Virol. 1989;63:1023–1030. doi: 10.1128/jvi.63.3.1023-1030.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yakobson B, Koch T, Winocour E. Replication of adeno-associated virus in synchronized cells without the addition of a helper virus. J Virol. 1987;61:972–981. doi: 10.1128/jvi.61.4.972-981.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zadori Z, Stefancsik R, Rauch T, Kisary J. Analysis of the complete nucleotide sequences of goose and muscovy duck parvoviruses indicates common ancestral origin with adeno-associated virus 2. Virology. 1995;212:562–573. doi: 10.1006/viro.1995.1514. [DOI] [PubMed] [Google Scholar]