Summary
Neurological diseases are one of the most pressing issues in modern times worldwide. It thus possesses explicit attention from researchers and medical health providers to guard public health against such an expanding threat. Various treatment modalities have been developed in a remarkably short time but, unfortunately, have yet to lead to the wished-for efficacy or the sought-after clinical improvement. The main hurdle in delivering therapeutics to the brain has always been the blood-brain barrier which still represents an elusive area with lots of mysteries yet to be solved. Meanwhile, nanotechnology has emerged as an optimistic platform that is potentially holding the answer to many of our questions on how to deliver drugs and treat CNS disorders using novel technologies rather than the unsatisfying conventional old methods. Nanocarriers can be engineered in a way that is capable of delivering a certain therapeutic cargo to a specific target tissue. Adding to this mind-blowing nanotechnology, the revolutionizing gene-altering biologics can have the best of both worlds, and pave the way for the long-awaited cure to many diseases, among those diseases thus far are Alzheimer’s disease (AD), brain tumors (glioma and glioblastoma), Down syndrome, stroke, and even cases with HIV. The review herein collects the studies that tested the mixture of both sciences, nanotechnology, and epigenetics, in the context of brain therapeutics using three main categories of gene-altering molecules (siRNA, miRNA, and CRISPR) with a special focus on the advancements regarding the new favorite, intranasal route of administration.
Subject areas: Pharmacology, Genetics
Pharmacology; Genetics
Introduction
Central nervous system (CNS) disorders are gaining colossal scientific attention lately, considering their widespread shooting prevalence as well as the novel relevant technologies that can aid in mitigating their heavy burden. Neurological diseases were recorded globally between the years 1990 and 2016 to be the number one cause of DALYs (disability-adjusted life years) at 276 million (95% UI) and the number two reason of mortality at 9.0 million. The total number of mortalities and DALYs combined from all neurological disorders grew while their age-standardized rates reduced. The foremost four causes of DALYs were stroke, migraine, Alzheimer’s disease (AD), and meningitis sharing by 42.2%, 16.3%, 10.4%, and 7.9%, respectively.1,2
Nowadays, nanotechnology redefines our approach toward different areas, from industry to health. Nanomedicine is defined as any therapeutic or imaging agent that includes a nanoparticle to cog the biodistribution, facilitate the efficacy, or else mitigate the toxicity of a drug. A recent snapshot of nanomedicines, whether presently approved by the Food and Drug Administration (FDA) or still in the clinical trial phase, found that the total number reaches 51 FDA-approved nanomedicines and 77 biologics still in clinical trials, including around 40% of trials initiated in 2014 or 2015 which confirms a spike in approvals and clinical trials harnessing nanoparticles. It is only reasonable to expect that this trend will continue to display a fruitful and defying domain not only for scholars but also for manufacturers, physicians, and administrators. The total approvals will keep rising, knowing that there has been a 3-fold increment in separately registered trials during 2014, 2015, and 2016.3 The main hurdle now is assorting novel biologics and deciding what further experimentation standards for integrity are needed prior to giving the public access to those medications in clinical settings.
Eloquent advancement has occurred in harnessing gene silencing, particularly in small interfering RNA (siRNA) therapeutics, since the outstanding detection of RNAi with a myriad of potential benefits. Unfortunately, major hurdles still stand in the way of most siRNA applications as researchers look for methods of safe target-oriented delivery. However, following the discovery of RNAi for almost two decades, siRNA therapeutics finally found their way to patients in need.4,5,6 For instance, patisiran, an RNAi curative factor that can selectively inhibit the synthesis of transthyretin in liver tissue, was successfully proven to achieve remarkable improvements in cases diagnosed with hereditary transthyretin amyloidosis. The assigned patients received intravenous patisiran versus a placebo once every 3 weeks. They were assessed using a modified Neuropathy Impairment Score at 18 months, Norfolk Quality of Life-Diabetic Neuropathy questionnaire, 10-m walk test, and body mass indices measurement. This clinical trial manifested that patisiran significantly ameliorated multiple clinical symptoms of hereditary transthyretin amyloidosis, including neurological symptoms such as polyneuropathy.7
Nano-formulations in vivo journey is majorly affected by their physicochemical properties, such as their size, shape, hydrophobicity, elasticity, and surface charge/chemistry/morphology, which represent the interface with the brain microenvironment. Understanding protein corona formation is primarily essential when characterizing nanocarriers and evaluating their interactions with the blood-brain barrier (BBB).8 BBB is a double-edged weapon; on the one hand, it represents a crucial anatomical part of the central nervous system keeping the essential functional integrity of the BBB and a state of homeostasis within the microenvironment of the brain. On the other hand, the BBB is considered a chief hurdle when delivering various drugs to the CNS; it limits the efficient passage of most drugs from the bloodstream peripherally to the brain parenchyma. Thus, the need for discovering new approaches allowing for the entrance of biomacromolecules to the CNS is of utmost importance for managing various neurological disorders.9,10 Pericytes are exclusively situated within the neurovascular unit among endothelial cells, astrocytes, and neurons, which are all essential for the integrity of the BBB. They coordinate and process key signaling pathways to generate different responses that control both pathological as well as physiological CNS functions, including, most importantly, regulating the permeability of the BBB, capillary hemodynamic responses, and neuroinflammation.11,12,13 Consequently, they play a major role in complex neurological disorders such as AD and brain tumors.14,15
Diverse targeting modalities have been investigated for the mechanisms of transcytosis concerning nano-formulations and therapeutic cargo passing through the BBB. The strategy of engineering named “molecular Trojan horses” (MTH) has been utilized as a mode for delivering cargos through the BBB since large constructs cannot penetrate it. An MTH system usually constitutes a monoclonal antibody that can cross the BBB fused to a therapeutic cargo through a negotiation mechanism. In principle, an MTH system can be employed for any therapeutic cargo that successfully reaches the brain from the blood using an endogenous mechanism of a negotiation moiety. The final goal is to let both the vector and the cargo keep their integrity to allow effective recognition and passage.16 Together, MTH strategy and the advancement in nanotechnology has opened ways for effective and safe delivery of the diagnostic and therapeutic substances to the brain. The development of nanoparticle-based delivery system has led to the development of numerous delivery agents with varying capabilities. Therefore, in this review, we are going to analyze some research advancements of brain therapeutics that have forged in the field of nanotechnology as it currently stands, with a special focus on gene-targeting therapy as well as direct nose-to-brain drug delivery system.
Types and advantages of nanodrug delivery systems
Nanotechnology is currently developing rapidly, offering new opportunities for discovering new therapies and improving the effectiveness of current clinical treatments. In particular, nanodrug delivery systems (NDDS) can achieve target-specific selective delivery, increase the bioavailability of drugs, improve the controlled release and stability of drugs, and can reduce the toxic side effects of drugs.17 A wide variety of carriers are available for NDDS, and both organic and inorganic materials can be used to construct them. Organic carriers include but are not limited to polymers (micelles or dendrimers), liposomes, nanoemulsions, proteins (albumin, transferrin), solid lipid nanoparticles (SLNs), nanostructured lipid carriers, etc. Inorganic nanocarriers include silicon, gold, and iron nanoparticles and so on.18,19
The nanocarriers of NDDS have unique advantages that cannot be replicated; for example, the nanoscale particle size allows it to have a large specific surface area, so it can assume a large drug loading capacity; secondly, the nanoparticles can actively target specific tissues and organs after the ligands are attached to their surfaces, thus release drugs with precise targeting, and in addition, nanomaterials have the advantages of increasing the solubility of hydrophobic drugs, extending the half-life of some drugs in vivo, and improving the duration of action.
Polymer carriers
Polymers are now widely used as drug delivery vehicles due to their good biocompatibility and rich structural variability. The known polymers can be divided into natural polymers (chitosan, starch, and cellulose) and synthetic polymers (e.g., poly(lactic-co-glycolic acid) (PLGA), poly(lactic acid), and poly (glycolic acid)), and researchers have also used different methods to mix polymers for better drug delivery, such as hydrogels and cross-linked micelles.20,21 The general polymeric carrier structure is a core-shell structure, where the hydrophobic core binds the drug through chemical bonding, and the hydrophilic nanoshell allows for the next step of modification to more intelligently target the drug pathway while also reducing the adverse effects of the drug on the outside world.22 Hydrophilic drugs are usually attached to the core-shell or inner-shell layers of nanopolymer carriers and can be released directly from the surface of the carrier by breaking chemical bonds. Hydrophobic drugs are mainly solubilized in the core of the polymer and are generally released by diffusion from the micropores or through carrier degradation. Although polymers can enhance the bioavailability of drugs and reduce their toxic effects, there are still problems that need to be overcome, such as some carriers degrading too fast or too slow and modifying the binding sites not much.
Liposomes
Liposomes are miniature vesicles consisting of a phospholipid bilayer and an encapsulated aqueous core region. Phospholipids and cholesterol are the common building blocks of liposomes and cell membranes, which help liposomes to fuse with cell membranes and deliver hydrophilic or hydrophobic drugs into the cell.23 At the same time, they confer excellent biocompatibility, biodegradability, and safety to liposomes.24 These unique advantages have made liposomes a common nanocarrier in NDDS, but their widespread application still needs to be improved. For example, on the one hand, the phospholipid structure of liposomes is unstable and may undergo dynamic dissociation in biological media, further leading to off-target drug distribution, altering efficacy, or even producing greater damage.25 On the other hand, the lack of structural diversity of liposomes, along with their high cost of preparation, is a major challenge.
Nanoemulsions
In recent years, the research on nanoemulsion technology has developed rapidly. Nanoemulsions are generally dispersed systems formed spontaneously by water, oil, surfactants, co-surfactants, etc., and can be divided into water-in-oil emulsions and oil-in-water (O/W) emulsions according to their different dispersed phases.26 Nanoemulsions can be prepared by various methods, such as high-pressure homogenization, ultrasonication, inverse phase method, and self-nanoemulsification.27 It has been found that nanoemulsions have many advantages as drug delivery vehicles, including the ability to bypass the first-pass effect of the liver and improve drug solubility and bioavailability.28 However, some unavoidable drawbacks in the clinical application of nanoemulsions, such as high preparation cost and poor stability compared with solid carriers, have created certain obstacles to the promotion and application.
SLNs
SLNs are the first generation of lipid nanoparticles derived from O/W nanoemulsions with a particle size range of 10–1000 nm.29 In recent years, SLNs have received a lot of attention from the scientific community because of the versatility of their carriers and the simplicity of their processes. In general, the smaller the particle size of SLNs, the more drug loading they will have, and the more stable and better targeting they will be. Compared with other carriers, SLNs have many advantages. On the one hand, they can load hydrophilic and lipophilic drugs, are biodegradable, have lower toxicity, and are biocompatible. On the other hand, these nanoparticles can improve the dissolution rate of drugs and achieve slow and controlled release of drugs.30
Nanostructured lipid carrier (NLC)
As the second generation of lipid nanoparticles, NLCs are made by mixing multiple lipids. After adding liquid lipids to solid lipids, the lattice structure of solid lipids will be disrupted, and the irregular lattice structure will be increased, which in turn will increase the drug loading capacity of the carrier.31 Compared with SLNs, NLC not only has all its advantages but also can better reduce drug precipitation and increase drug loading capacity. Compared to nanoemulsions, NLC prevents particle aggregation, better immobilizes the drug, and has a better stability. Compared with other nanocarriers, NLC is less toxic and can reduce side effects.
Inorganic nanoparticles
Inorganic nanoparticle carriers have good physicochemical properties and excellent functions, such as physical stability, good catalytic function, targeted delivery, and drug-controlled release capability. Inorganic nanocarriers used in NDDS include gold nanocarriers, silver nanocarriers, silica nanocarriers, magnetic nanocarriers, iron oxide nanocarriers, carbon nanotubes, etc.32,33 Gold nanoparticles, for example, are the most abundantly studied and are used in various forms, such as core-shell nanoparticles, nanocages, nanorods, etc.34,35 Stable gold nanoparticles can be used to encapsulate and slow-release drugs and/or be able to target specific tissues or organs.36 However, the choice of ligands for gold nanoparticles is particularly important, especially in terms of stability and toxicity.
Gene targeting as the future of therapeutics
Early successes in clinical trials of gene therapies are highly promising, with cumulative evidence of increasing regulatory approval for licensing. Scientists have wary optimism that safe, stable, and efficient therapeutics will offer a major advantage to patients with single-gene diseases as well as those with complex acquired disorders.37 In these next three sections (siRNA, miRNA, and CRISPR), we will go through some brain therapeutics that mainly work through altering the genetic expression within the brain cell, with all examples simplified in Table 1.
Table 1.
Therapeutic gene-targeting nanoparticles to the brain (siRNA, miRNA, and CRISPR)
| Nano-vectors | Therapeutic cargos | Targets | Advantages | Reference |
|---|---|---|---|---|
| siRNA | ||||
| Dual-modified cationic liposomes | Paclitaxel and survivin-loaded siRNA | CD133+ glioma cells | Safe, targeted, and low cytotoxicity | Sun et al.40 |
| Magneto-electric nanoparticles | Beclin1 siRNA | Microglial cells infected with HIV-1 | Low cytotoxicity | Rodriguez et al.41 |
| Two peptides: (intracellular trafficking+ targeting) | siRNA | Tissue adjacent to traumatic brain injury | Significant accumulation around injured brain tissue | Kwon et al.42 |
| Proton-ionizable amino lipid (Apo-E) | siRNA | MBEC4 cells (in vivo endothelial cells) | Endogenous gene silencing | Tamaru et al.43 |
| Bioreducible poly β-amino esters |
siRNA | Glioblastoma | Low cytotoxicity | Kozielski et al.44 |
| MPEG-PCL-Tat | siRNA | Brain cells | High permeability across the nasal mucosa | Musumeci et al.45 |
| Surfactant-like peptide nanofiber | BCL2 siRNA | Subthalamic nucleus | Increases siRNA intracranial residence time | Mazza et al.46 |
| Gold nanorods | MMP-9 siRNA | BMVECs | Modulating tight junction proteins and thus cellular traffic into CNS | Mahajan et al.47 |
| CGN peptide (BBB penetration) Tet1 peptide (neuron binding) |
BACE1 siRNA | Alzheimer’s (amyloid precursor protein) | Promoting hippocampal neurogenesis | Wang et al.48 |
| Intranasal cationic nanoemulsions | Anti-TNFα siRNA | LPS-induced inflammation | Reduced neuronal inflammation | Yadav et al.49 |
| miRNA | ||||
| mPEG-g-PEI | miR-135a | Glioma | Selectively induce apoptosis in tumor cells only | Liang et al.54 |
| Bioreducible poly β-amino ester | miR-148a miR-296-5 |
Glioblastoma (tumor-propagating human cancer stem cells) | High intracellular delivery, low cytotoxicity, and promotes cytosolic cargo release | Lopez-Bertoni et al.55 |
| Polymeric nanogel | miR-34a | Glioblastoma | Inhibited tumor growth | Shatsberg et al.56 |
| RVG fused to exosomal protein (Lamp2b) | miR-124 | Infarcted brain cells | Protection against ischemia | Yang et al.57 |
| CRISPR-Cas9 | ||||
| Poly-D L-lactide co-glycolide |
CRISPR-CAS9 | Down syndrome | Improved significantly the intellectual disability | Tafazoli et al.61 |
| dNP2 peptide | Two HypaRNP molecules | Glioblastoma | Efficient delivery, simplicity, and controllability | Thach et al.62 |
| Magneto-electric nanoparticles | Cas9/gRNA | Latent HIV-1-infected microglial cells | Reduced HIV-LTR expression | Kaushik et al.63 |
Abbreviations: HIV = human immunodeficiency virus, BBB = blood brain barrier, Apo = apolipoprotein, MBEC4 = mouse brain capillary endothelial cell line, BCL2 = B-cell lymphoma 2, MMP-9 = Matrix metallopeptidase 9, BMVECs = brain microvascular endothelial cell, BACE1 = β-site amyloid precursor protein cleaving enzyme 1, PEG-PDMAEMA = PEGylated poly 2-(N,N-dimethylamino) ethyl methacrylate, RVG = Rabies virus glycoprotein, lamp2b = lysosome-associated membrane glycoprotein 2b, hypaRNP = hyper-accurate Cas9 ribonucleoprotein complex.
siRNA
siRNA-induced RNAi responses are highly promising for treating a broad set of human diseases, from viral outbreaks to tumors to neurodegenerative diseases. Immense effort has been made in developing convenient delivery methods to apply this technology on a wide scale. A premier effort has been the development of N-acetylgalactosamine (GalNAc) siRNA conjugates targeting hepatic tissue. Tris-GalNAc attaches to the asialoglycoprotein receptor, which is mainly expressed in liver cells. Sufficient amounts of siRNAs can reach the cytoplasm to initiate certain selective reactions in vivo. Several clinical trials, including phase III trials, are presently on the way to assist in managing a broad set of other health problems. GalNAc-siRNA conjugates represent a feasible solution to the issue of siRNA-targeted delivery and have shown the ability to target tissue types other than hepatocytes.38 Currently, more than 73 siRNA-based clinical trials are in progress for a broad set of diseases comprising cancers, genetic disorders, and viral infections as shown by (clinicaltrials.gov). To maximize siRNA therapeutic potentials, many chemical strategies have been applied to tackle issues associated with efficacy, specificity, and safety, including nanoparticle delivery systems using nucleotides, lipids, and polymers.39
A designed dual-modified cationic liposome, packed with paclitaxel and survivin siRNA nanocomplex, combines chemotherapy and the effect of small RNA that can selectively induce cell apoptosis of CD133+ glioma cancer cells in vitro as well as in vivo. It is a highly promising new horizon in targeted therapy of glioma stem cells. This approach proves to be relatively safe, remarkably targeted and can penetrate the BBB with very low cytotoxicity to brain capillary endothelial cells (BCECs). Noticeably, it does not only improve the differentiation of CD133+ glioma stem cells into non-stem-cell lineages but also additionally improves the overall survival rates.40 Another nanocomplex constituting siRNA that can effectively inhibit the BECN1 gene (Beclin-1), a key factor in regulating autophagy, has proven to reduce HIV-1 replication and viral-induced inflammation in microglial cells with preserved stability after crossing the BBB using MENP (electro-magnetic nanoparticles). Moreover, the cytotoxic effects of those MENP-siBeclin1 were minimal. This study has shown that silencing autophagy can be a promising therapeutic interference to ameliorate the neurodegenerative impacts of HIV-1 infection affecting microglial cells.41 A parallel study utilized two peptides (an intracellular trafficking peptide and a targeting peptide) joined together with siRNA as a small-size nanometer structure to modulate the destructive pathways using nucleic acids, which can inhibit difficult targets using small molecules.
It has been found that nanoparticles in the bloodstream can infiltrate brain tissue shortly after an injury in some areas with compromised BBB. Systemic administration into animals with brain injuries has proven to accumulate in tissues adjacent to injury to inhibit a therapeutic target. A significantly increased accumulation of nano-vectors around injured brain tissue was noticed compared to uninjured brain tissue. Applying this to an in vivo model of traumatic brain injury transiently allowed a significant accumulation of nanoparticles if administered within a 6-h window after the onset of the injury.42 An apolipoprotein E (ApoE)-modified model has also been found to be a potential vector for delivering nucleic acids to endothelial cells within the brain. A liposomal nano-vector comprising a proton-ionizable amino lipid has been designed as a vector of siRNA. They used ApoE as a targeting ligand for in vivo mouse brain capillary endothelial cells (MBEC4). They observed that the cellular uptake of the ApoE-modified structures increased steadily in a density-dependent pattern. The ApoE-modified nanoparticles were uptaken into brain cells through both clathrin and caveolae-mediated endocytosis, allowing them to escape lysosomal degradation. Eventually, endogenous gene silencing was accomplished counting on the ApoE-modification.43 An array of bioreducible PBAEs (poly beta-amino esters) was assembled with siRNA to form another nano-vector that is capable of siRNA release upon being reduced by the cytosolic environment.
PBAEs generally cannot deliver siRNA in their native form; it requires some alteration to enable its delivery. An optimal nanomaterial essentially needs to bind with siRNA to form a stable structure that promotes release upon entering the cytoplasm. A polymeric nanoparticle was designed to inhibit the gene in glioblastoma cells without causing significant cytotoxicity and achieve substantial inhibition utilizing polymeric nanoparticles with a minimal dose of siRNA, demonstrating their convenience for siRNA-based nanomedicine.44 To improve siRNA transmission to the central nervous system, a direct nose-to-brain delivery system was designed. A group of researchers described a nose-to-brain delivery system of siRNA or dextran as a model siRNA, employing MPEG-PCL-Tat, which is a copolymer conjugated with a peptide. The nasal route of dextran with MPEG-PCL-Tat increased delivery rates in contrast with intravenous administration. In addition, they investigated the intranasal transmission of MPEG-PCL-Tat to the brain through the olfactory and trigeminal nerves, the presumptive pathways to the brain from the nasal cavity. They concluded that MPEG-PCL-Tat enhanced transmission along the nerve pathways owing to its high permeability through the nasal mucosa.45 Peptide nanofibers (PNF) can conjugate to the positively charged amino acids, allowing for electrostatic binding to the concerned siRNA, causing gene inhibition. A study designed a surfactant-like peptide that can self-assemble into peptide nanofibers. The activity of the PNF:siRNA complexes was studied both in vivo and in vitro. In vitro analysis was performed by downregulation of B cell lymphoma 2-targeted protein, in addition to induction of apoptosis. In vivo analysis was done by using the relative gene expression following stereotactic administration into the subthalamic nucleus. The results have shown that PNF:siRNA complexes are not only uptaken inside the cells but also added to the original residence time of siRNA inside the brain upon intracranial administration.
PNF:siRNA complexes have also been reported to be a potential novel treatment strategy for CNS disorders.46 A group of researchers suggested that BBB disruption due to loss of function of tight junction (TJ) is caused by the matrix metalloproteinase 9 (MMP-9) gene activation during neuroinflammation and hence can be avoided by inhibiting the expression of this gene. Accordingly, gold nanorods (GNR) were found to be capable of electrostatic binding with MMP-9 siRNA to formulate a nanocomplex whose uptake by BCECs is capable of suppressing MMP-9 expression. This investigation explored if GNR-MMP-9 siRNA nanocomplex gene inhibition controls TJ proteins expression in the BMVECs (brain microvascular endothelial cells). They altered the expression of TJ proteins (ZO-1, occludin, and claudin-5) within BMVECs which were initially transfected with the GNRs-siRNA-MMP-9. Silencing of the MMP-9 gene expression increased the expression of TJ proteins, thus decreasing endothelial permeability.47 Wang et al. used the CGN peptide for penetrating the BBB, and the Tet1 peptide for specific binding to neurons, to formulate the CT/siRNA nanoformulations with fairly well stability within the blood. CT/siRNA nanoformulations are capable of specifically directing the β-site amyloid precursor protein-cleaving enzyme (BACE) which is a main enzyme for cleaving the amyloid precursor protein responsible for AD that is highly promising of a potential treatment strategy. These nanoformulations have successfully escaped lysosomal enzymes and entered the cytoplasm of the neurons inducing effective gene silencing. Using transgenic mice, the nanoformulations substantially decreased BACE1 mRNA (about 50%), amyloid plaques, and subsequently, levels of phosphorylated tau protein as well as promoting neurogenesis within the hippocampus. Eventually, it restored the cognitive function of AD in transgenic mice up to the wild-type control without a noticeable unfavorable influence on myelination.48
Tumor necrosis factor alpha (TNFα) is a key mediator of neuroinflammation potentially causing neuronal dysregulation. A study evaluated nasal administration of cationic nanoemulsions encasing an anti-TNFα siRNA, for probable anti-inflammatory effect, experimented in a lipopolysaccharide (LPS)-induced model of neuroinflammation. The target inhibited the TNFα gene, thus providing neuroprotection against inflammatory reactions. TNFα siRNA nanoemulsions exhibited significantly lower levels of TNFα in LPS-stimulated cells. Following intranasal administration of cationic nanoemulsions, an almost 5-fold increase in uptake was noticed compared to non-encapsulated siRNA. Moreover, in vivo studies have demonstrated that nasal administration of TNFα siRNA nanoemulsions markedly reduced TNFα in an LPS-induced model of neuroinflammation. Therefore, intranasal administration of cationic nanoemulsions encasing TNFα siRNA can efficiently cause gene knockdown, consequently preventing neuroinflammation.49
miRNA
A study recognized regulatory targets of microRNAs (miRNAs) by detecting mRNAs employing conserved complementarity to the seed of the miRNA. An overrepresentation of conserved adenosines bounding the complementary sites of the seed in mRNAs suggested that primary sequence determinants are capable of base pairing to designate miRNA target recognition. Around 13,000 regulatory reactions were detected above the estimate of false-positive predictions in a four-genome analysis of 3′ UTRs; thus, miRNA is predicted to target more than 5300 human genes, which is almost 30% of the human genome. Overall, more than one-third of the human genome seems to be conserved miRNA targets.50 More compiling data suggest that miRNA-based therapies, either increasing or decreasing miRNA expression, are a huge potential. A major obstacle is the successful delivery of miRNAs to target specific sites or organs in the transition of miRNA therapy to actual clinical usage. Viral vectors and cationic polymers are considered effective options, but their immunogenicity and adverse effects limit their applicability. Luckily, specific targeting and prolonged release of miRNAs/anti-miRNAs using nanoparticles attached to peptides and antibodies are highly promising for reducing the essential therapeutic dosage as well as cellular toxicity.51 The importance of miRNA in brain development and neuroplasticity has been well established during the past few decades. Abnormal expression of miRNAs is renowned for implicating in the pathophysiology of many neuropsychiatric disorders such as schizophrenia.52 Another study has shown that mesenchymal stromal cell-derived exosomes containing miRNAs can be used for treating the high-burden disease neurovascular stroke.53
A novel nano-vector (mPEG-g-PEI) is foreseen to display an efficient intracellular miRNA delivery system for treating glioma cases that are based upon polyethylene glycol methyl ether (mPEG) and hyper-branched polyethyleneimine (hy-PEI) to increase the miRNA delivery rates. miR-135a is considered a selective inducer of apoptosis in glioma tumor cells rather than normal glial or neuronal cells. An mPEG-g-PEI/miR-135a nanocomplex was then designed to show low cytotoxicity. In vitro observations indicated that it could substantially modify miR-135a transfection by facilitating its uptake by normal glial cells as well as glioma tumor cells. Using the C6-implanted in situ models, the nanoplex could significantly improve the survival rates and mitigate the growth of glioma tumor cells as shown by MRI and immunohistochemistry. Besides, the expression of the targeted proteins of miR-135a confirmed the same results.54 A bioreducible PBAE nanoparticle that demonstrated sufficient delivery into the cells, low toxicity, and promoted cytosolic release has been found to efficiently deliver miRNA to tumor-propagating human cancer stem cells. They merged this technology with cancer stem cells inhibiting miRNAs to formulate miRNA-containing polymeric nanoparticles. Delivery of miR-148a and miR-296-5 within the bioreducible nanoparticles improved the survival of glioblastoma cells in vivo on the long run. After direct infusion within the tumor, these nano-miRNAs have proven to spread and decrease the growth of established orthotopic human glioblastoma xenografts.55
A polymeric nanogel was similarly developed based upon a polyglycerol scaffold, as a novel approach to miRNA delivery for treating glioblastoma cases. The researchers utilized miR-34a, characterized by its tumor suppression ability in different cancers including glioblastoma in both in vivo and in vitro settings. U-87 MG glioblastoma cells processed with NG-miR-34a nano-polyplexes demonstrated significant inhibition of miR-34a target genes that regulate apoptosis. Also, nano-polyplexes administration to U-87 MG glioblastoma-bearing severe combined immunodeficient mice substantially mitigated tumor growth in contrast with the control group.56 Parallel recent findings suggest that several microRNAs are involved in the CNS remodeling process. Targeted miRNAs transport for modifying neurogenesis is promising, specifically in improving the prognosis following ischemia. A study proved that modified exosomes, with rabies virus glycoprotein (RVG), fused to exosomal protein lysosome-associated membrane glycoprotein 2b, can effectively deliver miR-124 to the infarcted site. Systemic administration of miR-124 using RVG-exosomes stimulates cortical neural progenitors and protects against ischemic injury. Thus, RVG-exosomes are capable of selective delivery of epigenetic drugs to the CNS.57
CRISPR-Cas9
CRISPR-Cas9, the RNA-guided genome editing tool, which was initially inserted into mammalian organisms in 2013, offers innumerable privileges over mainstream therapies, including easy use, and design, besides multiplexing, meaning that it is capable of editing multiple genes at the same time. Thus, it has become a suitable agent for several genome editing purposes comprising primarily gene therapy research. CRISPR-Cas9 can be utilized in therapeutic objectives in various methods using in vivo and in vitro experiments. It can fix the occasional mutations in monogenic diseases, which presently represent the most applicable field in CRISPR-Cas9-mediated gene therapeutics. CRISPR-Cas9 is also capable of engineering pathogen genome such as HIV as well as inducing protective mutations. Furthermore, it has shown potential ability in cancer gene therapy, such as attenuating oncogenic viruses as well as inducing onco-suppressor expressions.58
A fundamental goal when dealing with CRISPR-based genome editing technology is to formulate a successful and safe delivery of a single-guide RNA and a CRISPR-associated protein. An applicable translational approach is utilizing viral vectors, for instance an adeno-associated virus. Nonetheless, those vectors cause long-term in vivo exposure that can possibly cause immunogenicity and possible off-target effects. Thus, constraints to clinical applications are tackled through alternative delivery systems other than viral vectors including nanotechnology-based delivery modalities. Nano-formulation-based delivery is becoming more attainable in gene therapeutics owing to specificity, efficiency, customization, minimal immunogenicity, and the ability to escape nucleases.59 To address the wide gap between research and clinical settings when facing Parkinson’s disease (PD), tools for CRISPR-based genome editing in CNS have been developed to help recognize cellular factors dominating the process of neurodegeneration causing PD. Integration of CRISPR-Cas9, genome-wide association study, and technology to generate induced pluripotent stem cells, all focus on the crucial factors behind PD pathogenesis. This can potentially blaze a trail to develop possible beneficial therapeutic targets. To modify the limitations of CRISPR-Cas9, utilizing other types of CRISPR systems, such as Cas12a, was recommended due to its ability to target T-rich motifs—but with no transactivation of CRISPR-RNA and induction of a staggered double-strand break in addition to the potential for RNA processing and DNA nuclease activity. This nuclease modality may become the main compass we use in the soon future to navigate among PD, as well as other neurodegenerative diseases.60
A study considered a pair of CRISPR-Cas9 systems to cut a particular area from the short arm of chromosome 21 and then replace it with a newly developed DNA construct that contains the required genes for inactivating an extra chromosome 21. This modality is needed especially in mimicking the native pattern taking place in normal female cells to inactivate the extra X chromosome. They used a certain dose of a suitable nanocarrier which was a surface-engineered PLGA, for introducing the construct into Trisomy21 brain cell culture media and a Down syndrome mouse model. They found that it significantly reduced the criteria of the disease, including but not limited to intellectual disabilities.61 Another study has reported a lipopeptide-based nanocomplex derived from the BBB-permeable dNP2 peptide, which is capable of delivering a hyper-accurate Cas9 ribonucleoprotein complex inside human cells with the aim of gene editing. A nanosome was able to deliver about two hyper-accurate CRISPR-Cas9 ribonucleoprotein (HypaRNP) molecules into the cytoplasm without substantial cytotoxicity. The HypaRNP thus enacted endogenous editing and silencing in glioblastoma cells (up to 19.7%). The lipopeptide-based nanosome system demonstrated high delivery efficiency, simplicity, and controllability.62 For the first time, Kaushik et al. demonstrated magnetically guided noninvasive transport of a nano-vector comprised of Cas9/gRNA attached to MENPs across the BBB to mitigate latent infection of HIV-1 in HC69 microglial cells. The outcomes have concluded that the developed nano-formulation reduced HIV-long terminal repeat expression substantially in contrast to free Cas9/gRNA in HIV latent HC69 cells. The same results were confirmed qualitatively using fluorescence microscopy, affirming that delivery of (CRISPR-Cas9-gRNA-MENPs) to the CNS across the BBB can potentially be used in clinical settings in the near future.63
Intranasal route of administration
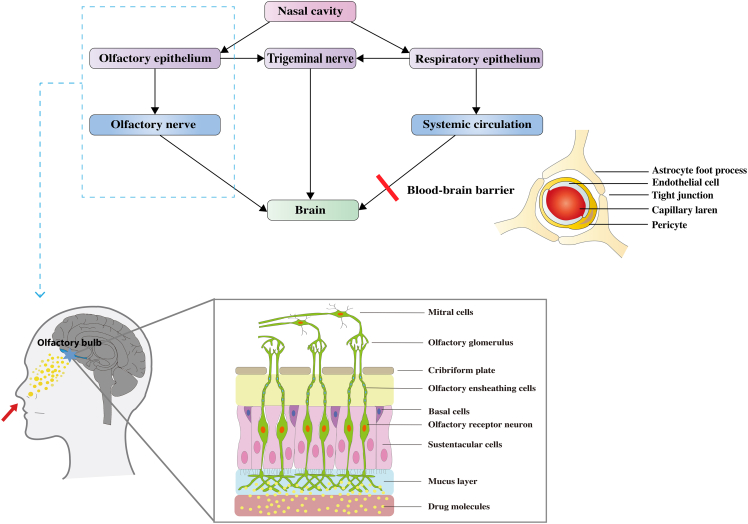
BBB is a major obstacle to the action of drug-active molecules in the CNS. Many researchers are now trying to overcome BBB by intranasal administration so that drugs can reach the CNS to act.64 The nasal cavity is composed of the vestibular area, the respiratory area, and the olfactory area. The vestibular area is the entrance to the nasal cavity, which communicates with the external environment and is not involved in the absorption function.65 The respiratory area, which accounts for the largest proportion of the nasal cavity, is mainly involved in the absorption of drugs and contains abundant blood vessels and nerves. The olfactory area is the third area, composed of olfactory epithelial cells, which has perforated capillaries that connect brain tissue to the external environment and is considered the weakest part of the BBB.66 Intranasally administered drugs can reach the brain through one of three linking pathways; the olfactory nerve, the trigeminal nerve, and the systemic circulation. The olfactory and trigeminal pathways avert the BBB and deliver therapies from the nasal cavity to the brain tissue through both passive and active transport along the nerve branches which supply different sensations to the nasal cavity. In case of the systemic circulation, the drug material must diffuse through the mucus layer, the nasal epithelium, and the venous blood flow, and then penetrate the BBB. The olfactory and trigeminal pathways represent the direct nose-to-brain transport routes.67 Refer to Figure 1 for the detailed olfactory pathway.
Figure 1.
The potential drug transport routes leading to brain uptake after intranasal administration and the structure of the blood-brain barrier
The olfactory route originates from nasal mucus layer and transports through different stations of the olfactory nerve till reaching the olfactory bulb in the brain. (Copyrights have been obtained by ELSEVIER BV).
Drug delivery via the intranasal route has several advantages; on the one hand, it is a noninvasive delivery method, safer, and more convenient, with better patient compliance.68 On the other hand, it can avoid the first-pass effect, increase drug distribution in the brain, improve bioavailability, and ensure efficacy compared with oral administration. However, the nasal-brain route of drug delivery also has some limitations, such as poor drug penetration in the nasal mucosa and easy removal by cilia.69 Many researchers have attempted to develop drug delivery systems based on nanotechnology with the intention of overcoming these limitations. Through the next sections, we are going to highlight some characterized biologics targeting brain diseases via nasal administration.
Brain tumors
Considering that liposomes have an inherent capability to penetrate the BBB and that Angiopep-2, a ligand for LRP1, has a promising potential to be used as a targeting ligand with the purpose of CNS delivery, a study aimed at evaluating the ability of Angiopep-conjugated Polysorbate 80-Coated Liposomes (T80-An2-Lps) in delivering cyclovirobuxine-D (CVB-D), a known autophagy inducer with antitumor activity, through the BBB in vitro as well as in vivo. T80-An2-Lps have been found to enhance BBB permeability and improve CVB-D transport to the brain shown by both in vivo and in vitro experiments. Rats were treated with free liposomes, free CVB-D, and CVB-D liposomes by both intranasal and intravenous routes for comparative purposes. The results have depicted that T80-An2-CVB-D-Lps were homogeneously dispersed with a high encapsulation efficiency, which is desirable when targeting the brain through the nose. This co-administrative approach of a drug on a liposome can be potentially applied to enhance brain drug accumulation in brain tumors.70
Temozolomide is an apoptotic inducer that is widely used as an antitumor drug. A group of researchers optimized Temozolomide nanolipid chitosan hydrogel formulation to be utilized as a direct nose-to-brain therapeutic. The results have shown an enhancement ratio for the new formulation of more than 2-folds higher than the control (without the nanolipid part), suggesting the role of lipid chitosan as a permeability enhancer. Also, no structural damage was observed upon performing a histopathological examination of nasal mucosa. Overall, this study produced encouraging findings in formulating an intranasal route for the antitumor drug temozolomide.71 MLT-loaded polycaprolactone nanoparticles (MLT-NP) have been studied for nose-to-brain delivery in treating glioblastoma. The formulation demonstrated better solubility and activity against glioblastoma cells sparing normal ones. The direct translocation of nanoparticles from the nose to the brain was visualized using Fluorescence tomography. Intranasal administration of MLT-NP resulted in higher brain uptake. In vivo tests confirmed the same results. Thus, the nanoencapsulation of MLT is a promising approach for selective antitumoral activity against glioblastoma.72
Brain infections
Cryptococcus neoformans-mediated meningoencephalitis is a critical CNS disorder with limited treatment options due to poor drug delivery across the BBB. Newly designed NLCs were used to develop a nose-to-brain drug delivery system for patients suffering from this condition. NLCs were specifically uptaken by the C. neoformans-infected cells. Ketoconazole-loaded NLCs have shown significantly higher antifungal activity in vivo. NLCs reached the brain via the olfactory bulb while bypassing the BBB and also maintained longer residence time within infected tissues, significantly decreasing the C. neoformans burden in infected mice brains, meaning that NLCs not only improve penetration efficiency but also increase the efficacy of antifungal drugs such as ketoconazole.73 A powder formulation comprising agglomerates of micronized ribavirin with spray-dried microparticles of permeation enhancers such as α-cyclodextrin has been developed for nasal administration. Ex vivo ribavirin permeation was tested across rabbit nasal mucosa. In vivo nasal administration to rats has shown a higher accumulation in the brain in case of the prepared formula with excipient microparticle α-cyclodextrin. Thus, powder agglomerates are a promising approach to achieving a nose-to-brain delivery system owing to their penetration-enhancing properties.74
Dementias
Along with lowering serum cholesterol, statins are known for a wide range of beneficial effects for neurodegenerative diseases.75 A group of researchers characterized simvastatin-loaded lecithin/chitosan nanoparticles (SVT-LCNs) for direct nose-to-brain administration. The developed SVT-LCNs have a number of the most advantageous features suitable for efficient mucosal delivery, such as minute particle size, long-term stability, positive surface charge, and mucoadhesion. Their capability to facilitate the nose-to-brain delivery was proven by gamma scintigraphy in rats. Moreover, their biodegradability by mucosal lysozymes has indicated a novel Trojan horse strategy that can potentially improve drug release within the nasal mucosa.76 Another study was carried out to improve the galantamine bioavailability in the brain using thiolated chitosan nanoparticles via intranasal drug delivery. Significant recovery in mice model with induced amnesia by intranasal galantamine-loaded thiolated chitosan nanoparticles found the suitability of nose-to-brain delivery rather than the conventional enteral therapeutics for the treatment of AD.77 A study evaluated a rasagiline-loaded chitosan glutamate nanoparticle (RAS-CG-NP). Biodistribution of this formulation in mice’s brain tissue and peripheral blood following intranasal and intravenous administration was assessed. Intranasal RAS-CG-NPs have been found to be substantially higher at all time intervals compared to all other counterparts. The maximum concentration and direct transport percentage were significantly higher as well. In conclusion, a noticeable enhancement of drug bioavailability in the brain has been observed following the administration of the RAS-CG-NPs representing a new promising approach to the direct nose-to-brain route in PD.78
Selegiline hydrochloride (SL) is a known anti-Parkinson’s agent but, unfortunately, has a reduced bioavailability when used orally owing to its remarkable first-pass metabolism and very little enteral absorption. A group of researchers prepared SL mucoadhesive nasal thermosensitive gel (SNT-gel) to improve its bioavailability. Behavioral studies have shown a better score of photo-actometer with a decreased motor deficit using a catalepsy score in the SNT-gel treatment group as compared to the control group. Other evidence of better drug delivery included increased brain dopamine and catalase activity with reduced monoamine oxidase B and glutathione. Results were also supported by histopathology. Thus, the nasal administration of SNT gel offers a potentially better and easier choice for Parkinson’s patients.79
A cubosomal mucoadhesive in situ nasal gel has been developed to facilitate the donepezil HCl delivery to the brain using Poloxamer 407 and glycerol monooleate. In vivo biodistribution studies have been performed using rats. It has shown substantially higher nasal permeability with better brain tissue uptake. Hence, the cubosomal mucoadhesive in situ nasal gel can potentially be used as a drug transporter targeting CNS.80 Another study has similarly developed a mucoadhesive huperzine A-loaded PLGA nanoparticle with surface modification by lactoferrin-conjugated N-trimethylated chitosan (Lf-TMC) for intranasal delivery to the brain. The role of (Lf-TMC) is to facilitate the distribution of huperzine A inside the brain. Lf-TMC nanoparticles were proven to have good adhesion, sustained-release effect, and brain-targeting ability. Thus, they are highly promising as an efficient carrier for nose-to-brain drug delivery.81
Niosomes as nanocarriers
Buspirone hydrochloride is a known anxiolytic agent with a short half-life and minimal oral bioavailability (around 4%) owing to its extensive first-pass metabolism. Consequently, a study has attempted to develop Buspirone hydrochloride-loaded niosomal in situ nasal gel, which was eventually proven overall better than the conventional gel formulations.82 Astroglial S100B protein is a main factor in neuroinflammation of PD whose inhibition has been suggested as an optimistic targeted therapy for PD. Pentamidine is known to be a very potent S100B inhibitor but the issue is its limited capacity to penetrate the BBB. An intranasal delivery system called inPentasomes has been developed using chitosan-coated niosomes with encased pentamidine to provide a new approach for treating PD. InPentasomes substantially reduced the extent of glial-related neuroinflammation detected by specific gliotic markers with a parallel reduction in releasing NO2 and PGE2 in the nigrostriatal neurons. The inPentasomes are therefore offering a likely anti-PD drug.83 Nanoliposome-encapsulated ferric ammonium citrate offered an effective iron drug delivery system to the CNS with even low cytotoxicity shown in rats. It suggests a safe, simple modality for treating iron deficiency anemia using niosomal nasal delivery of liposomal iron formulations. It increased not only the total iron content but also the iron storage protein expression in rat olfactory bulb and some other brain areas.84
Nanogels
Recent evidence suggests that insulin delivery to the CNS may potentially become a therapeutic option for AD.85 But a suitable carrier is needed to improve the efficacy of this treatment. Poly (N-vinyl pyrrolidone)-based nanogels (NG) with covalently attached insulin (NG-In) have been tested for bioavailability and CNS delivery in mice models. After the administration of (NG-In) intranasally, the formulation has proven to be well tolerated. Furthermore, enhanced delivery to the brain in contrast to free insulin was observed.86 Intranasal mice co-administration of CNS-related model peptide insulin with cell-penetrating peptide (CPP) penetratin has been investigated. The results obtained have concluded that CPPs are promising tools for CNS delivery of protein-based drugs directly through the nose. D-penetratin specifically showed less hazard of systemic insulin exposure when introduced intranasally.87 Likewise, another study has investigated exendin-4 as a potential alternative compound acting as a glucagon-like peptide-1 receptor agonist. They have found that the distribution of exendin-4 all over the brain was significantly improved by intranasal co-administration with penetratin. Moreover, spatial learning ability after administration of exendin-4 along with penetratin and insulin has suggested a therapeutic efficacy against severe cognitive dysfunction. Thus, nose-to-brain delivery exhibits a promising approach for treating cases with progressive cognitive dysfunction.88
Nanoemulsions
A study has evaluated improved drug delivery of quetiapine fumarate (QTP), an antipsychotic drug, using a nanoemulsion system for intranasal administration. In vitro studies have shown a more than 2-fold increment in drug release as compared to the original drug per se. In vivo studies using rats have shown that intranasal administration of QTP-loaded nanoemulsion had shorter T max compared to that of systemic administration but a higher drug transport efficiency. The nanoemulsion system, therefore, represents an optimistic approach for the brain-targeted delivery of QTP.89
Ziprasidone hydrochloride, a fifth-generation antipsychotic, has also been evaluated for an effective nose-to-brain delivery in animal models. Pharmacodynamic tests confirmed a significant superiority of the mucoadhesive nanoemulsion over the nanoemulsion per se using both the locomotor activity test and paw test. Moreover, a nasal ciliotoxicity study has proven the optimized formulation to have no toxic effect. Thus, a stable, effective, and buffered mucoadhesive nanoemulsion of ziprasidone hydrochloride can be safely used intranasally while targeting the brain.90
Thymoquinone possesses a strong antioxidant effect with a promising contribution to the mitigation of stroke but, unfortunately, the bioavailability of the drug is low. Consequently, a study aimed to prepare thymoquinone nanoemulsion to enhance the bioavailability and target the CNS through the noninvasive nasal route. Studies for post-intranasal administration of thymoquinone mucoadhesive nanoemulsion (TMNE) have revealed increased bioavailability in the brain compared to the intravenous route. Furthermore, the improved neurobehavioral activity shown by locomotor and grip strength was assessed in rats with middle cerebral artery occlusion-induced cerebral ischemia after the intranasal administration of TMNE. Thus, intranasal thymoquinone can be a treatment option for stroke patients.91
Zolmitriptan is a drug used in migraine but known for low bioavailability after both oral and nasal administration. A study attempted to enhance zolmitriptan bioavailability by developing a mucoadhesive nanoemulsion for the drug to attain both rapid onset and high drug levels reaching brain tissue to allow its usage in treating acute migraine attacks. The optimum formula was experimented in vivo using mice to compare it with intravenous and intranasal solutions of zolmitriptan. Outcomes have demonstrated that the addition of chitosan as a mucoadhesive agent to the nanoemulsion facilitated its tissue residence time. In vivo studies demonstrated that the mucoadhesive nanoemulsion formulation of zolmitriptan has better brain uptake as well as higher permeability through the nasal mucosa than the solution.92
Kaempferol (KPF) is a drug that is known for inducing glioma cell death. A study attempted to prepare nanoemulsions containing KPF with and without chitosan to evaluate their ability for brain targeting through intranasal administration as well as their antitumor activity against glioma cells. KPF-loaded nanoemulsion (KPF-NE) and KPF-loaded mucoadhesive nanoemulsion (KPF-MNE) were both prepared and characterized for comparative purposes. KPF-MNE has shown substantially improved permeability through nasal mucosa in ex vivo diffusion studies. Histopathological examination suggested both nanoemulsions to be non-toxic and capable of maintaining the KPF antioxidant effect. KPF-MNE and KPF-NE both significantly enhanced the amount of drug delivered into rat’s brain following intranasal administration (5- and 4.5-fold higher than free drug, respectively). Moreover, in vitro experiments have shown that KPF-MNE reduced C6 glioma cell viability through induction of apoptosis more than the other counterpart. Thus, KPF-MNE is considered a promising therapeutic alternative for patients suffering from glioma.93
A mucoadhesive lipidic nanoemulsion using hyaluronic acid has been developed while encasing the two polyphenols, resveratrol and curcumin, to prepare an intranasal drug for cases with neurodegenerative disease. The formula has shown significantly higher mucoadhesive durability compared to its non-mucoadhesive match that the diffusion-controlled release was achieved up to 6 h. Moreover, the nanoemulsion was found to be able to preserve the antioxidant effect of curcumin and resveratrol. Furthermore, the mucoadhesive nanoemulsion was proven safe when applied on sheep nasal mucosa and increased the amounts of the two encapsulated polyphenols in the brain, about 7-folds increase in AUC 0–7 h for resveratrol and 9-folds for curcumin. Therefore, hyaluronic acid-based lipidic nanoemulsion represents a potential vector improving the stability and specificity of polyphenols.94
Lipid microparticles (LMs), whether coated with chitosan or uncoated, containing the neuroprotective polyphenol resveratrol were developed in a similar investigation for targeting the brain through the nose. An aqueous suspension of LMs-Res-Ch-plus achieved a better cerebrospinal fluid (CSF) bioavailability with Cmax at 60 min. This significant increment in the CSF bioavailability without any parallel distribution in the systemic circulation suggests a promising direct nose-to-brain pathway using LMs.95
The minimal penetration of anti-HIV drugs through the BBB leads to insufficient delivery and thus insufficient treatment knowing that the CNS represents a major reservoir for HIV-1 virus or cases with neuro-AIDS. A study aimed to develop an intranasal nanoemulsion of saquinavir mesylate (SQVM) which is commonly utilized as an antiretroviral drug but with a very low enteral bioavailability (4%). Ex vivo permeation studies were applied on sheep nasal mucosa showing a significant increment in drug permeation rate with SQVM-loaded NE. Cilia toxicity study has shown no significant adverse effect. Moreover, outcomes of in vivo studies testing bioavailability in rats have shown higher drug levels in the brain after intranasal rather than intravenous administration.96 Biodistribution and pharmacokinetics of cyclosporine-A (CsA) upon intranasal administration have been evaluated in rats using an O/W nanoemulsion vector. CsA, a substrate for P-glycoprotein, is an immunosuppressive agent whose entry is restricted via the BBB. Besides, CsA has proven to be a strong anti-inflammatory and neuroprotective agent in the brain. CsA-NE administered intranasally resulted in higher bioavailability for all three regions evaluated in the brain (olfactory bulb, midbrain, and hindbrain). The brain/blood exposure ratios indicated that CsA-NE can bypass the blood-brain barrier through a direct nose-to-brain pathway mitigating untargeted tissue exposure.97
Paroxetine is used for cases suffering from depression and anxiety, but oral administration is characterized by low bioavailability of less than 50% due to remarkable first-pass metabolism. A study developed a paroxetine-loaded O/W nanoemulsion for intranasal administration. Behavioral tests such as forced swimming tests and locomotor activity tests were performed using rats to study the antidepressant effect of the newly optimized formulation. Treatment of depressed rats with paroxetine nanoemulsion administered through the nose substantially modified the behavioral activities compared to paroxetine suspension when administered orally. The biochemical evaluation has shown that the characterized formula enhanced the low levels of glutathione, which partly suggests the involved mechanism.98 Amyotrophic lateral sclerosis is a progressive neurodegenerative disease in which both upper and lower motor neuronal functions deteriorate. Riluzole is one of only four approved drugs for such cases. Riluzole bioavailability is only limited to 60% because it acts as a substrate of P-glycoprotein whose entry is restricted via the BBB. A study has developed an O/W nanoemulsion system of riluzole aiming to enhance its BBB permeability. Riluzole-loaded nanoemulsion tested free from nasal ciliotoxicity, with brain uptake significantly higher when administered nasally. Thus, intranasal riluzole nanoemulsion could be new hope for patients suffering from ALS to avert systemic side effects of oral administration.99 Shapes and structures of different nanocarriers are illustrated in Figure 2.
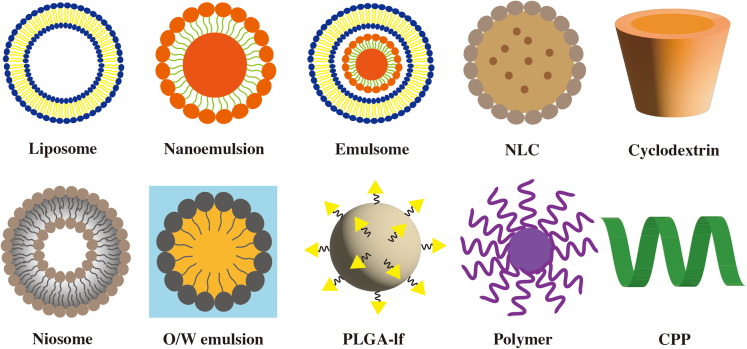
Figure 2.
Diagrammatic illustration of different shapes and structures of nanocarriers
Liposome: phospholipid bilayer, Niosome: liposome with non-ionic surfactant, O/W: oil-in-water, PLGA-Lf: PLGA surface modified with lactoferrin, NLC (nanostructured lipid carrier): solid lipid core containing oil, Cyclodextrin: hydrophilic surface with lipophilic cavity, CPP: cell-penetrating peptide: 5–30 amino acids. (Copyrights have been obtained by ELSEVIER BV).
Miscellaneous nanocarriers
A study developed a multifunctional liposome to deliver tacrine hydrochloride via the nose. Liposomes were enriched with α-tocopherol and Omega3 to enhance permeation with a concomitant neuroprotective effect. The liposomal enhancement over drug permeation, effect on neuronal viability, cytoprotective effect, and reactive oxygen species production was evaluated. Along with desirable mucoadhesive properties, multifunctional liposomes demonstrated a significant increase in tacrine permeability shown by cellular uptake studies. Moreover, the α-tocopherol solely was found to increase the neuroprotective and antioxidant effect of liposomes.100 Alpha-asarone is characterized by low bioavailability. Consequently, a study aimed at formulating mPEG-polylactic acid (mPEG-PLA) nanoparticles (NPs) surface-modified by lactoferrin (Lf) which acts as a brain targeting enhancer to deliver the drug to the brain via the nose. Ex vivo pharmacokinetics showed that Lf-NPs have a better permeability when administered intranasally. Moreover, lactoferrin helps with decreasing toxicity.101
Lamotrigine-PLGA nanoparticles were constructed to be used in treating neuropathic pain. Dissolution profiles suggested complete release within 5 h in CSF. In vitro analysis demonstrated an additive pro-inflammatory suppressor effect. In vivo studies were done using radiolabeled formulations of the drug, comparing several routes of administration (oral, intranasal, and intravenous). For the intranasal route, pharmacokinetic variables such as drug targeting efficiency, drug target organ transport, Cmax, and Tmax, all have shown that the developed intranasal PLGA nanoparticles provide a feasible option for managing neuropathic pain.102
Diazepam-loaded poly (lactic-co-glycolic acid) nanoparticles (DNP) were optimized to attain nose-to-brain delivery. An ex vivo drug release study using sheep demonstrated a high controlled release for as long as 24 h. Performing MTT assay showed less toxicity in the case of DNP in comparison with conventional diazepam suspension. Biodistribution tests and scintigraphy images suggested that the developed DNP could potentially carry diazepam in a nose-to-brain delivery fashion that can be of utmost importance while dealing with status epilepticus patients in outpatient settings.103 Polymeric nanoparticles (PNPs) of frovatriptan succinate were studied for targeting the central nervous system via nasal route. PNPs have shown sustained release of up to 72 h. Ex vivo study performed on goat nasal mucosa has shown that PNPs permeability was about 3 times higher than the pure drug. PNPs also demonstrated fast delivery to the brain using fluorescence microscopic evaluation in rats. Moreover, histopathology studies have shown minimal toxicity of nasal mucosa. The study has concluded that frovatriptan succinate, which is a hydrophilic drug, can efficiently be carried in PNPs targeting the brain via nasal administration.104
Another work investigated a new nasal carrier called Phospholipid Magnesome. The system constitutes multilamellar nanosized vesicles with the capability of carrying lipophilic as well as hydrophilic drugs. The characterized system contains phospholipid, water, propylene glycol, magnesium salt, and alginate. Mucoadhesiveness has been tested in vitro by using porcine nasal mucosa, indicating a prolonged contact time. The delivery of rhodamine 6G, insulin, and epidermal growth factor was evaluated by multiphoton microscopy and near-infrared imaging showing effective delivery. The pharmacodynamic effects of nasal delivery of oxytocin and insulin were tested in animal models and have proven efficient with an even prolonged drug effect. Moreover, histopathological tests indicated that the vector is safe for nasal administration.105
A phospholipid-based colloidal nanocubic vesicle encapsulating olanzapine was designed to target the brain via the nose. The nanocubic vesicles were developed by integrating non-ionic copolymers, poloxamer 188 or 407, in the lipid bilayer. The in vivo behavior and brain-targeting tests were evaluated in rats. The intranasal nanocubic vesicles were found substantially more efficient in delivering olanzapine to the CNS compared to the liposomal vesicles with drug targeting efficiency values of 100% and 80%, respectively, and absolute bioavailability of 37.9% and 14.9%, respectively.106
Emulsomes are structures sharing characters of both liposomes and emulsions. They are nano-triglyceride carriers formed of lipid cores with at least one outer phospholipid layer. They enable the loaded drug to benefit from more bioavailability and stability. Emulsomes of oxcarbazepine were prepared and characterized in a study to offer nanocarriers for nose-to-brain delivery. Nanocarriers were developed with suitable size, charge, encapsulation efficiency, and sustained release. MTT assay has proven less drug toxicity in the case of emulsomal encapsulation. Customizing emulsomes properties by modifying the charge and size of the nanocarrier gave rise to a more stable system for a lipophilic drug with an even prolonged release and residence time in addition to successful nose-to-brain transport.107 Various brain therapeutics characterized for nasal administration are summarized in Table 2.
Table 2.
Characterized formulations for direct nose-to-brain transport
| Vector | Therapeutic cargo | Study type | Pathology | Reference |
|---|---|---|---|---|
| Liposomes (Angiopep-conjugated Polysorbate 80-Coated) | Cyclovirobuxine D | Rats | Brain tumors | Wei et al.70 |
| Chitosan and vitamin E | Temozolomide | Animal models | Brain tumors | Khan,et al.71 |
| PCL | Melatonin | In vitro | Glioblastoma | de Oliveira Junior et al.72 |
| Nanostructured lipid carriers | Ketoconazole | Mice | Meningo-encephalitis (Cryptococcus neoformans) | Du et al.73 |
| α-cyclodextrin | Ribavirin | Rabbits and rats | Antiviral agent | Giuliani et al.74 |
| Lecithin chitosan nanoparticle | Statins | Rats | Alzheimer’s disease | Clementino et al.76 |
| Thiolated chitosan | Galantamine | Mice | Alzheimer’s disease | Sunena et al.77 |
| Chitosan glutamate | Rasagiline | Mice | Parkinson’s disease | Mittal et al.78 |
| Poloxamer 407-Chitosan | Selegiline hydrochloride | Rats | Parkinson’s disease | Sridhar et al.79 |
| Poloxamer 407- GMO | Donepezil HCl | Rats | Alzheimer’s disease | Patil et al.80 |
| PLGA, (Lf-TMC) modified | Huperzine-A | Mice | Alzheimer’s disease | Meng et al.81 |
| Niosomes | Buspirone hydrochloride | Sheep | Anxiety | Mathure et al.82 |
| Pentamidine | Mice | MPTP Parkinson’s disease | Rinaldi et al.83 | |
| Ferric ammonium citrate | Rats | Iron deficiency anemia | Guo et al.84 | |
| PVP | Insulin | Mice | Alzheimer’s disease | Picone et al.86 |
| Penetratin | Insulin | Mice | Diabetes | Kamei et al.87 |
| Penetratin and exendin-4 | Insulin | Mice | Severe cognitive dysfunction | Kamei et al.88 |
| Nanoemulsion | Quetiapine fumarate | Rats | Schizophrenia | Boche et al.89 |
| Mucoadhesive nanoemulsion | Ziprasidone | Animal models | Schizophrenia | Bahadur et al.90 |
| Thymoquinone | Rats | Stroke | Ahmad et al.91 | |
| Zolmitriptan | Mice | Acute migraine | Abdou et al.92 | |
| Kaempferol | Rats and cell line | Glioma | Colombo et al.93 | |
| Hyaluronic acid-based nanoemulsion | Polyphenols (curcumin, resveratrol) | Sheep and rats | Neurodegenerative diseases | Nasr et al.94 |
| O/W Nanoemulsion | saquinavir mesylate | Sheep and rats | Neuro-AIDS (HIV-1 infection) | Trotta et al.96 |
| Cyclosporine-A | Animal models | Neuroprotection and anti-inflammatory | Yadav et al.97 | |
| Paroxetine | Rats | Depression and anxiety | Pandey et al.98 | |
| Riluzole | Rats | Amyotrophic lateral sclerosis | Parikh et al.99 | |
| Multifunctional liposome (α-tocopherol, omega-3) | Tacrine hydrochloride | In vitro | Alzheimer’s disease | Corace et al.100 |
| (mPEG-PLA), (Lf) modified |
α-asarone | Rats and rabbits | Neuroprotection | Pan et al.101 |
| PLGA | Lamotrigine | Rats | Neuropathic pain | Nigam et al.102 |
| Diazepam | Sheep | Status epilepticus | Sharma et al.103 | |
| Polymers | Frovatriptan succinate | Goat | Migraine | Deepika et al.104 |
| Phospholipid magnesome | Oxytocin, insulin, and EGF | Animal models | Pain, AD, and spinal cord injury | Natsheh et al.105 |
| Nanocubic vesicle | Olanzapine | Rats | Schizophrenia | Salama et al.106 |
| Emulsomes | Oxcarbazepine | Rats | Epilepsy | El-Zaafarany et al.107 |
Abbreviations: GMO = glyceryl mono-oleate, MPTP = 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, PCL = polycaprolactone, mPEG = methoxy polyethylene glycol, PLA = polylactic acid, Lf = lactoferrin, TMC = trimethylated chitosan, PLGA = polylactic glycolic acid, AD = Alzheimer’s disease, EGF = epidermal growth factor, O/W = oil in water.
Current challenges
In vitro modeling
There are various hindering factors to be addressed while developing a clinically relevant therapeutic delivery system to the brain. One issue to be tackled is the species specificity of utilized antibodies, in other words, the simultaneous expression of the major part of BBB receptors elsewhere in the body creating the problem of specific targeting and interfering with other non-targeted physiological pathways. Another issue is safety considerations upon chronic use due to nonspecific body distribution. Also, the behavior of newly constructed drugs in biological media should be thoroughly tested because experiments thus far have concluded that in vitro success does not necessarily translate to in vivo efficacy all the time.108 Different models have been developed recently to mimic the complex detailed structure of the BBB as well as the characteristics of the brain microcapillaries which are of prior importance, especially in translational research. Efficient modeling of the neurovascular unit (NVU) will offer a priceless tool that can help with understanding the pathological parameters and pathophysiology prior to the onset of a certain neurological disease which will consequently enhance managing the root cause. The recent development of CNS organoids, organ-on-chip, spheroids, 3D printed microfluidics, and other emerging technologies have promising prospects to better the research area of NVU modeling.109 Recent findings made it controversial to start translating those results of animal experiments into actual human clinical trials. Unfortunately, a widespread failure in clinical trials of neurological disorders has raised questions on whether the experimented animal models are in earnest representative of the human brain microenvironment.110
In vivo imaging
Theranostic modalities based upon nanotechnology have been a research hotspot for more than a decade now. Almost all nano-formulation delivery modalities have some boundaries, whether therapeutic or diagnostic. Thus, merging nanodrug delivery approaches with diagnostic modalities using nanoparticles for modifying imaging techniques has been the key to stock up the gaps. Among those limitations is low stability in systemic circulation and high cost in case of liposomes, large molecular size in case of polysaccharide-coated NP, low drug loading capacity in case of synthetic carbon dots, and high cost with high retention in case of the metal-polymer complex.111
Off-target distribution
The random uptake of transporter-mediated transcytosis therapeutics was investigated more than once in pre-clinical trials to test further than the constituents of the peripheral blood to include main body organs, for example the spleen and liver, which is highly potential due to the pervasive expression of the same protein transporters in several untargeted tissues other than CNS.109,110 But this issue is majorly mitigated when administering drugs intranasally instead of enteral or intravenous routes.
Occasional insufficient endothelial parenchymal transport
It has been documented that intense affinity toward certain protein transporters, such as TfR, that cannot readily be turned back may obscure the transport of the therapeutic cargo from the abluminal membrane which is the last step of transcytosis. A similar preclinical study concluded that intense binding to TfR was proven via in vivo models to stimulate lysosomal sorting which subsequently causes obstructed transcellular trafficking.112,113
Clinical practice
There are still many challenges to the real application of nanotechnology in clinical treatment. Firstly, it is very costly. Secondly, for inorganic mineral-based drug particles, the physical and chemical properties of the drug will change drastically, the probability of side effects increases, the preparation process is complicated and requires reasonable ratios and precise calculations, and there are also social ethical issues involved. In addition, when people inhale nanoparticles, unexplained and incurable diseases may occur.114 There is no guarantee that more advances will not lead to new diseases that will be more difficult to treat and control in the future.
In addition, the clinical application of gene-targeted therapy for brain diseases faces many difficulties. One of the main problems is the concomitant central nervous system toxicity. Although the adverse effects of most targeted therapy drugs are generally less than those of chemotherapy, they can often cause different types of adverse effects than those of chemotherapy drugs, for example, some targeted drugs can cause severe rash, diarrhea, hypertension, and other adverse effects.115
In the clinical setting, nose-to-brain administration currently has a number of limitations that need to be addressed. Sometimes, some drugs can cause irritation and irreversible damage to the cilia on the nasal mucosa. And defense mechanisms such as mucociliary clearance can affect the permeability of the drug. If the patient has a pathological condition such as a cold or allergy, drug permeability will be even lower.116
Conclusions
Several neurological disorders, including AD, neuroinflammation, brain tumors, and stroke, have recently become a pressing matter that urges an effective solution to ameliorate the detrimental effects impacting the lifestyle of numerous patients. The unique microenvironment of the CNS has not been yet fully understood by scientists considering its complex structure, endless details, and intricating signaling pathways. Studying the physiology of the BBB represents the cornerstone of studying brain therapeutics. In parallel, nanomedicine was initially developed to address the problems related to drug delivery to the brain and to have a cutting edge over the old therapeutic methods. Nanotechnology has authorized the targeted delivery of many biologics, including gene-modifying therapy that seems to be a game-changer in therapeutics, ensuing in obvious improvements observed through in vitro and in vivo experiments but still more data are needed to study their adverse reactions and approve their usage along with their optimum route of administration that ought to be targeted with minimal systemic cytotoxicity as in the noninvasive intranasal route. Prospects in this context are then highly propitious and offer multiple novel avenues to renew the way we handle the snowball of CNS diseases.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81670088), the Training Program for Young Backbone Teachers of Institutions of Higher Learning in Henan Province, China (No. 2020GGJS038), the Foundation of Science & Technology Department of Henan Province, China (No. 192102310151), and the Science Foundation for Young Talents of Henan University College of Medicine, China (No. 2019013).
Author contributions
Conceptualization: Y.M.W., X.Y.J.; Data curation: D.D.W., Y.A.S., E.E.N., Y.X.Z., S.K., N.H.K., Y.W., T.L., Z.H.G.; Writing-original draft: D.D.W., Y.A.S.; Writing-review and editing: Y.A.S., E.E.N., Y.X.Z.; Visualization and supervision: Y.M.W., D.D.W., X.Y.J.
Declaration of interests
The authors declare no conflict of interest.
Contributor Information
Yan-Mei Wang, Email: 10210002@vip.henu.edu.cn.
Xin-Ying Ji, Email: 10190096@vip.henu.edu.cn.
References
- 1.GBD 2016 Neurology Collaborators Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 US Neurological Disorders Collaborators. Feigin V.L., Vos T., Alahdab F., Amit A.M.L., Bärnighausen T.W., Beghi E., Beheshti M., Chavan P.P., Criqui M.H., et al. Burden of Neurological Disorders Across the US From 1990-2017: A Global Burden of Disease Study. JAMA Neurol. 2021;78:165–176. doi: 10.1001/jamaneurol.2020.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bobo D., Robinson K.J., Islam J., Thurecht K.J., Corrie S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. (N. Y.) 2016;33:2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 4.Saw P.E., Song E.-W. siRNA therapeutics: a clinical reality. Sci. China Life Sci. 2020;63:485–500. doi: 10.1007/s11427-018-9438-y. [DOI] [PubMed] [Google Scholar]
- 5.Hu B., Weng Y., Xia X.-H., Liang X.-J., Huang Y. Clinical advances of siRNA therapeutics. J. Gene Med. 2019;21 doi: 10.1002/jgm.3097. [DOI] [PubMed] [Google Scholar]
- 6.Zhang M.M., Bahal R., Rasmussen T.P., Manautou J.E., Zhong X.-B. The growth of siRNA-based therapeutics: Updated clinical studies. Biochem. Pharmacol. 2021;189 doi: 10.1016/j.bcp.2021.114432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams D., Gonzalez-Duarte A., O’Riordan W.D., Yang C.-C., Ueda M., Kristen A.V., Tournev I., Schmidt H.H., Coelho T., Berk J.L., et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018;379:11–21. doi: 10.1056/NEJMoa1716153. [DOI] [PubMed] [Google Scholar]
- 8.Agrahari V., Burnouf P.-A., Burnouf T., Agrahari V. Nanoformulation properties, characterization, and behavior in complex biological matrices: Challenges and opportunities for brain-targeted drug delivery applications and enhanced translational potential. Adv. Drug Deliv. Rev. 2019;148:146–180. doi: 10.1016/j.addr.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Kabanov A.V., Batrakova E.V. New technologies for drug delivery across the blood brain barrier. Curr. Pharm. Des. 2004;10:1355–1363. doi: 10.2174/1381612043384826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie J., Shen Z., Anraku Y., Kataoka K., Chen X. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials. 2019;224 doi: 10.1016/j.biomaterials.2019.119491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armulik A., Genové G., Mäe M., Nisancioglu M.H., Wallgard E., Niaudet C., He L., Norlin J., Lindblom P., Strittmatter K., et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 12.Daneman R., Zhou L., Kebede A.A., Barres B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramsauer M., Krause D., Dermietzel R. Angiogenesis of the blood-brain barrier in vitro and the function of cerebral pericytes. FASEB J. 2002;16:1274–1276. doi: 10.1096/fj.01-0814fje. [DOI] [PubMed] [Google Scholar]
- 14.Sweeney M.D., Ayyadurai S., Zlokovic B.V. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat. Neurosci. 2016;19:771–783. doi: 10.1038/nn.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalkara T., Gursoy-Ozdemir Y., Yemisci M. Brain microvascular pericytes in health and disease. Acta Neuropathol. 2011;122:1–9. doi: 10.1007/s00401-011-0847-6. [DOI] [PubMed] [Google Scholar]
- 16.Pardridge W.M. Delivery of Biologics Across the Blood-Brain Barrier with Molecular Trojan Horse Technology. BioDrugs. 2017;31:503–519. doi: 10.1007/s40259-017-0248-z. [DOI] [PubMed] [Google Scholar]
- 17.Zhou F., Teng F., Deng P., Meng N., Song Z., Feng R. Recent Progress of Nano-drug Delivery System for Liver Cancer Treatment. Anti Cancer Agents Med. Chem. 2018;17:1884–1897. doi: 10.2174/1871520617666170713151149. [DOI] [PubMed] [Google Scholar]
- 18.Qamar Z., Qizilbash F.F., Iqubal M.K., Ali A., Narang J.K., Ali J., Baboota S. Nano-Based Drug Delivery System: Recent Strategies for the Treatment of Ocular Disease and Future Perspective. Recent Pat. Drug Deliv. Formul. 2019;13:246–254. doi: 10.2174/1872211314666191224115211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S., Sun J. Nano-drug delivery system for the treatment of acute myelogenous leukemia. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2022;51:233–240. doi: 10.3724/zdxbyxb-2022-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maghsoudi S., Taghavi Shahraki B., Rabiee N., Fatahi Y., Dinarvand R., Tavakolizadeh M., Ahmadi S., Rabiee M., Bagherzadeh M., Pourjavadi A., et al. Burgeoning Polymer Nano Blends for Improved Controlled Drug Release: A Review. Int. J. Nanomedicine. 2020;15:4363–4392. doi: 10.2147/IJN.S252237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J., Mooney D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016;1 doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vilar G., Tulla-Puche J., Albericio F. Polymers and drug delivery systems. Curr. Drug Deliv. 2012;9:367–394. doi: 10.2174/156720112801323053. [DOI] [PubMed] [Google Scholar]
- 23.Bardania H., Tarvirdipour S., Dorkoosh F. Liposome-targeted delivery for highly potent drugs. Artif. Cells, Nanomed. Biotechnol. 2017;45:1478–1489. doi: 10.1080/21691401.2017.1290647. [DOI] [PubMed] [Google Scholar]
- 24.Guimarães D., Cavaco-Paulo A., Nogueira E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021;601 doi: 10.1016/j.ijpharm.2021.120571. [DOI] [PubMed] [Google Scholar]
- 25.Münter R., Kristensen K., Pedersbæk D., Larsen J.B., Simonsen J.B., Andresen T.L. Dissociation of fluorescently labeled lipids from liposomes in biological environments challenges the interpretation of uptake studies. Nanoscale. 2018;10:22720–22724. doi: 10.1039/c8nr07755j. [DOI] [PubMed] [Google Scholar]
- 26.Singh Y., Meher J.G., Raval K., Khan F.A., Chaurasia M., Jain N.K., Chourasia M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release. 2017;252:28–49. doi: 10.1016/j.jconrel.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Kumar M., Bishnoi R.S., Shukla A.K., Jain C.P. Techniques for Formulation of Nanoemulsion Drug Delivery System: A Review. Prev. Nutr. Food Sci. 2019;24:225–234. doi: 10.3746/pnf.2019.24.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandey P., Gulati N., Makhija M., Purohit D., Dureja H. Nanoemulsion: A Novel Drug Delivery Approach for Enhancement of Bioavailability. Recent Pat. Nanotechnol. 2020;14:276–293. doi: 10.2174/1872210514666200604145755. [DOI] [PubMed] [Google Scholar]
- 29.Mukherjee S., Ray S., Thakur R.S. Solid lipid nanoparticles: a modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009;71:349–358. doi: 10.4103/0250-474X.57282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rostami E., Kashanian S., Azandaryani A.H., Faramarzi H., Dolatabadi J.E.N., Omidfar K. Drug targeting using solid lipid nanoparticles. Chem. Phys. Lipids. 2014;181:56–61. doi: 10.1016/j.chemphyslip.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Bhise K., Kashaw S.K., Sau S., Iyer A.K. Nanostructured lipid carriers employing polyphenols as promising anticancer agents: Quality by design (QbD) approach. Int. J. Pharm. 2017;526:506–515. doi: 10.1016/j.ijpharm.2017.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang F., Li C., Cheng J., Yuan Z. Recent Advances on Inorganic Nanoparticle-Based Cancer Therapeutic Agents. Int. J. Environ. Res. Public Health. 2016;13:1182. doi: 10.3390/ijerph13121182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohammadpour R., Dobrovolskaia M.A., Cheney D.L., Greish K.F., Ghandehari H. Subchronic and chronic toxicity evaluation of inorganic nanoparticles for delivery applications. Adv. Drug Deliv. Rev. 2019;144:112–132. doi: 10.1016/j.addr.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J., Saeki F., Wiley B.J., Cang H., Cobb M.J., Li Z.-Y., Au L., Zhang H., Kimmey M.B., Li X., Xia Y. Gold nanocages: bioconjugation and their potential use as optical imaging contrast agents. Nano Lett. 2005;5:473–477. doi: 10.1021/nl047950t. [DOI] [PubMed] [Google Scholar]
- 35.de la Zerda A., Prabhulkar S., Perez V.L., Ruggeri M., Paranjape A.S., Habte F., Gambhir S.S., Awdeh R.M. Optical coherence contrast imaging using gold nanorods in living mice eyes. Clin. Exp. Ophthalmol. 2015;43:358–366. doi: 10.1111/ceo.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Oliveira R., Zhao P., Li N., de Santa Maria L.C., Vergnaud J., Ruiz J., Astruc D., Barratt G. Synthesis and in vitro studies of gold nanoparticles loaded with docetaxel. Int. J. Pharm. 2013;454:703–711. doi: 10.1016/j.ijpharm.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 37.Anguela X.M., High K.A. Entering the Modern Era of Gene Therapy. Annu. Rev. Med. 2019;70:273–288. doi: 10.1146/annurev-med-012017-043332. [DOI] [PubMed] [Google Scholar]
- 38.Springer A.D., Dowdy S.F. GalNAc-siRNA Conjugates: Leading the Way for Delivery of RNAi Therapeutics. Nucleic Acid Ther. 2018;28:109–118. doi: 10.1089/nat.2018.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong Y., Siegwart D.J., Anderson D.G. Strategies, design, and chemistry in siRNA delivery systems. Adv. Drug Deliv. Rev. 2019;144:133–147. doi: 10.1016/j.addr.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun X., Chen Y., Zhao H., Qiao G., Liu M., Zhang C., Cui D., Ma L. Dual-modified cationic liposomes loaded with paclitaxel and survivin siRNA for targeted imaging and therapy of cancer stem cells in brain glioma. Drug Deliv. 2018;25:1718–1727. doi: 10.1080/10717544.2018.1494225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez M., Kaushik A., Lapierre J., Dever S.M., El-Hage N., Nair M. Electro-Magnetic Nano-Particle Bound Beclin1 siRNA Crosses the Blood-Brain Barrier to Attenuate the Inflammatory Effects of HIV-1 Infection in Vitro. J. Neuroimmune Pharmacol. 2017;12:120–132. doi: 10.1007/s11481-016-9688-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwon E.J., Skalak M., Lo Bu R., Bhatia S.N. Neuron-Targeted Nanoparticle for siRNA Delivery to Traumatic Brain Injuries. ACS Nano. 2016;10:7926–7933. doi: 10.1021/acsnano.6b03858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamaru M., Akita H., Kajimoto K., Sato Y., Hatakeyama H., Harashima H. An apolipoprotein E modified liposomal nanoparticle: ligand dependent efficiency as a siRNA delivery carrier for mouse-derived brain endothelial cells. Int. J. Pharm. 2014;465:77–82. doi: 10.1016/j.ijpharm.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 44.Kozielski K.L., Tzeng S.Y., De Mendoza B.A.H., Green J.J. Bioreducible cationic polymer-based nanoparticles for efficient and environmentally triggered cytoplasmic siRNA delivery to primary human brain cancer cells. ACS Nano. 2014;8:3232–3241. doi: 10.1021/nn500704t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Musumeci T., Bonaccorso A., Puglisi G. Epilepsy Disease and Nose-to-Brain Delivery of Polymeric Nanoparticles: An Overview. Pharmaceutics. 2019;11:118. doi: 10.3390/pharmaceutics11030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mazza M., Hadjidemetriou M., de Lázaro I., Bussy C., Kostarelos K. Peptide nanofiber complexes with siRNA for deep brain gene silencing by stereotactic neurosurgery. ACS Nano. 2015;9:1137–1149. doi: 10.1021/nn5044838. [DOI] [PubMed] [Google Scholar]
- 47.Mahajan S.D., Aalinkeel R., Reynolds J.L., Nair B., Sykes D.E., Bonoiu A., Roy I., Yong K.-T., Law W.-C., Bergey E.J., et al. Suppression of MMP-9 expression in brain microvascular endothelial cells (BMVEC) using a gold nanorod (GNR)-siRNA nanoplex. Immunol. Invest. 2012;41:337–355. doi: 10.3109/08820139.2011.604863. [DOI] [PubMed] [Google Scholar]
- 48.Wang P., Zheng X., Guo Q., Yang P., Pang X., Qian K., Lu W., Zhang Q., Jiang X. Systemic delivery of BACE1 siRNA through neuron-targeted nanocomplexes for treatment of Alzheimer’s disease. J. Control. Release. 2018;279:220–233. doi: 10.1016/j.jconrel.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 49.Yadav S., Gandham S.K., Panicucci R., Amiji M.M. Intranasal brain delivery of cationic nanoemulsion-encapsulated TNFα siRNA in prevention of experimental neuroinflammation. Nanomedicine. 2016;12:987–1002. doi: 10.1016/j.nano.2015.12.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis B.P., Burge C.B., Bartel D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 51.Ganju A., Khan S., Hafeez B.B., Behrman S.W., Yallapu M.M., Chauhan S.C., Jaggi M. miRNA nanotherapeutics for cancer. Drug Discov. Today. 2017;22:424–432. doi: 10.1016/j.drudis.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao T., Zhen X.-C. Dysregulation of miRNA and its potential therapeutic application in schizophrenia. CNS Neurosci. Ther. 2018;24:586–597. doi: 10.1111/cns.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mirzaei H., Momeni F., Saadatpour L., Sahebkar A., Goodarzi M., Masoudifar A., Kouhpayeh S., Salehi H., Mirzaei H.R., Jaafari M.R. MicroRNA: Relevance to stroke diagnosis, prognosis, and therapy. J. Cell. Physiol. 2018;233:856–865. doi: 10.1002/jcp.25787. [DOI] [PubMed] [Google Scholar]
- 54.Liang C., Sun W., He H., Zhang B., Ling C., Wang B., Huang T., Hou B., Guo Y. Antitumor effect of a new nano-vector with miRNA-135a on malignant glioma. Int. J. Nanomedicine. 2018;13:209–220. doi: 10.2147/IJN.S148142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lopez-Bertoni H., Kozielski K.L., Rui Y., Lal B., Vaughan H., Wilson D.R., Mihelson N., Eberhart C.G., Laterra J., Green J.J. Bioreducible Polymeric Nanoparticles Containing Multiplexed Cancer Stem Cell Regulating miRNAs Inhibit Glioblastoma Growth and Prolong Survival. Nano Lett. 2018;18:4086–4094. doi: 10.1021/acs.nanolett.8b00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shatsberg Z., Zhang X., Ofek P., Malhotra S., Krivitsky A., Scomparin A., Tiram G., Calderón M., Haag R., Satchi-Fainaro R. Functionalized nanogels carrying an anticancer microRNA for glioblastoma therapy. J. Control. Release. 2016;239:159–168. doi: 10.1016/j.jconrel.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 57.Yang J., Zhang X., Chen X., Wang L., Yang G. Exosome Mediated Delivery of miR-124 Promotes Neurogenesis after Ischemia. Mol. Ther. Nucleic Acids. 2017;7:278–287. doi: 10.1016/j.omtn.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiao-Jie L., Hui-Ying X., Zun-Ping K., Jin-Lian C., Li-Juan J. CRISPR-Cas9: a new and promising player in gene therapy. J. Med. Genet. 2015;52:289–296. doi: 10.1136/jmedgenet-2014-102968. [DOI] [PubMed] [Google Scholar]
- 59.Chen F., Alphonse M., Liu Q. Strategies for nonviral nanoparticle-based delivery of CRISPR/Cas9 therapeutics. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12 doi: 10.1002/wnan.1609. [DOI] [PubMed] [Google Scholar]
- 60.Safari F., Hatam G., Behbahani A.B., Rezaei V., Barekati-Mowahed M., Petramfar P., Khademi F. CRISPR System: A High-throughput Toolbox for Research and Treatment of Parkinson’s Disease. Cell. Mol. Neurobiol. 2020;40:477–493. doi: 10.1007/s10571-019-00761-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tafazoli A., Behjati F., Farhud D.D., Abbaszadegan M.R. Combination of Genetics and Nanotechnology for Down Syndrome Modification: A Potential Hypothesis and Review of the Literature. Iran. J. Public Health. 2019;48:371–378. [PMC free article] [PubMed] [Google Scholar]
- 62.Thach T.T., Bae D.H., Kim N.H., Kang E.S., Lee B.S., Han K., Kwak M., Choi H., Nam J., Bae T., et al. Lipopeptide-Based Nanosome-Mediated Delivery of Hyperaccurate CRISPR/Cas9 Ribonucleoprotein for Gene Editing. Small. 2019;15 doi: 10.1002/smll.201903172. [DOI] [PubMed] [Google Scholar]
- 63.Kaushik A., Yndart A., Atluri V., Tiwari S., Tomitaka A., Gupta P., Jayant R.D., Alvarez-Carbonell D., Khalili K., Nair M. Magnetically guided non-invasive CRISPR-Cas9/gRNA delivery across blood-brain barrier to eradicate latent HIV-1 infection. Sci. Rep. 2019;9:3928. doi: 10.1038/s41598-019-40222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur. J. Pharm. Sci. 2000;11:1–18. doi: 10.1016/s0928-0987(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 65.Erdő F., Bors L.A., Farkas D., Bajza Á., Gizurarson S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018;143:155–170. doi: 10.1016/j.brainresbull.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 66.Gizurarson S. Anatomical and histological factors affecting intranasal drug and vaccine delivery. Curr. Drug Deliv. 2012;9:566–582. doi: 10.2174/156720112803529828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ruigrok M.J.R., de Lange E.C.M. Emerging Insights for Translational Pharmacokinetic and Pharmacokinetic-Pharmacodynamic Studies: Towards Prediction of Nose-to-Brain Transport in Humans. AAPS J. 2015;17:493–505. doi: 10.1208/s12248-015-9724-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Colombo G., Lorenzini L., Zironi E., Galligioni V., Sonvico F., Balducci A.G., Pagliuca G., Giuliani A., Calzà L., Scagliarini A. Brain distribution of ribavirin after intranasal administration. Antiviral Res. 2011;92:408–414. doi: 10.1016/j.antiviral.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 69.Agrawal M., Saraf S., Saraf S., Antimisiaris S.G., Chougule M.B., Shoyele S.A., Alexander A. Nose-to-brain drug delivery: An update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J. Control. Release. 2018;281:139–177. doi: 10.1016/j.jconrel.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 70.Wei H., Liu T., Jiang N., Zhou K., Yang K., Ning W., Yu Y. A Novel Delivery System of Cyclovirobuxine D for Brain Targeting: Angiopep-Conjugated Polysorbate 80-Coated Liposomes via Intranasal Administration. J. Biomed. Nanotechnol. 2018;14:1252–1262. doi: 10.1166/jbn.2018.2581. [DOI] [PubMed] [Google Scholar]
- 71.Khan A., Aqil M., Imam S.S., Ahad A., Sultana Y., Ali A., Khan K. Temozolomide loaded nano lipid based chitosan hydrogel for nose to brain delivery: Characterization, nasal absorption, histopathology and cell line study. Int. J. Biol. Macromol. 2018;116:1260–1267. doi: 10.1016/j.ijbiomac.2018.05.079. [DOI] [PubMed] [Google Scholar]
- 72.de Oliveira Junior E.R., Nascimento T.L., Salomão M.A., da Silva A.C.G., Valadares M.C., Lima E.M. Increased Nose-to-Brain Delivery of Melatonin Mediated by Polycaprolactone Nanoparticles for the Treatment of Glioblastoma. Pharm. Res. (N. Y.) 2019;36:131. doi: 10.1007/s11095-019-2662-z. [DOI] [PubMed] [Google Scholar]
- 73.Du W., Li H., Tian B., Sai S., Gao Y., Lan T., Meng Y., Ding C. Development of nose-to-brain delivery of ketoconazole by nanostructured lipid carriers against cryptococcal meningoencephalitis in mice. Colloids Surf. B Biointerfaces. 2019;183 doi: 10.1016/j.colsurfb.2019.110446. [DOI] [PubMed] [Google Scholar]
- 74.Giuliani A., Balducci A.G., Zironi E., Colombo G., Bortolotti F., Lorenzini L., Galligioni V., Pagliuca G., Scagliarini A., Calzà L., Sonvico F. In vivo nose-to-brain delivery of the hydrophilic antiviral ribavirin by microparticle agglomerates. Drug Deliv. 2018;25:376–387. doi: 10.1080/10717544.2018.1428242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deck B.L., Rick J., Xie S.X., Chen-Plotkin A., Duda J.E., Morley J.F., Chahine L.M., Dahodwala N., Trojanowski J.Q., Weintraub D. Statins and Cognition in Parkinson’s Disease. J. Parkinsons Dis. 2017;7:661–667. doi: 10.3233/JPD-171113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clementino A., Batger M., Garrastazu G., Pozzoli M., Del Favero E., Rondelli V., Gutfilen B., Barboza T., Sukkar M.B., Souza S.A.L., et al. The nasal delivery of nanoencapsulated statins - an approach for brain delivery. Int. J. Nanomedicine. 2016;11:6575–6590. doi: 10.2147/IJN.S119033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sunena, Singh S.K., Mishra D.N. Nose to Brain Delivery of Galantamine Loaded Nanoparticles: In-vivo Pharmacodynamic and Biochemical Study in Mice. Curr. Drug Deliv. 2019;16:51–58. doi: 10.2174/1567201815666181004094707. [DOI] [PubMed] [Google Scholar]
- 78.Mittal D., Md S., Hasan Q., Fazil M., Ali A., Baboota S., Ali J. Brain targeted nanoparticulate drug delivery system of rasagiline via intranasal route. Drug Deliv. 2016;23:130–139. doi: 10.3109/10717544.2014.907372. [DOI] [PubMed] [Google Scholar]
- 79.Sridhar V., Wairkar S., Gaud R., Bajaj A., Meshram P. Brain targeted delivery of mucoadhesive thermosensitive nasal gel of selegiline hydrochloride for treatment of Parkinson’s disease. J. Drug Target. 2018;26:150–161. doi: 10.1080/1061186X.2017.1350858. [DOI] [PubMed] [Google Scholar]
- 80.Patil R.P., Pawara D.D., Gudewar C.S., Tekade A.R. Nanostructured cubosomes in an in situ nasal gel system: an alternative approach for the controlled delivery of donepezil HCl to brain. J. Liposome Res. 2019;29:264–273. doi: 10.1080/08982104.2018.1552703. [DOI] [PubMed] [Google Scholar]
- 81.Meng Q., Wang A., Hua H., Jiang Y., Wang Y., Mu H., Wu Z., Sun K. Intranasal delivery of Huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA nanoparticles for treatment of Alzheimer’s disease. Int. J. Nanomedicine. 2018;13:705–718. doi: 10.2147/IJN.S151474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mathure D., Madan J.R., Gujar K.N., Tupsamundre A., Ranpise H.A., Dua K. Formulation and Evaluation of Niosomal in situ Nasal Gel of a Serotonin Receptor Agonist, Buspirone Hydrochloride for the Brain Delivery via Intranasal Route. Pharm. Nanotechnol. 2018;6:69–78. doi: 10.2174/2211738506666180130105919. [DOI] [PubMed] [Google Scholar]
- 83.Rinaldi F., Seguella L., Gigli S., Hanieh P.N., Del Favero E., Cantù L., Pesce M., Sarnelli G., Marianecci C., Esposito G., Carafa M. inPentasomes: An innovative nose-to-brain pentamidine delivery blunts MPTP parkinsonism in mice. J. Control. Release. 2019;294:17–26. doi: 10.1016/j.jconrel.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 84.Guo X., Zheng H., Guo Y., Wang Y., Anderson G.J., Ci Y., Yu P., Geng L., Chang Y.-Z. Nasal delivery of nanoliposome-encapsulated ferric ammonium citrate can increase the iron content of rat brain. J. Nanobiotechnology. 2017;15:42. doi: 10.1186/s12951-017-0277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tumminia A., Vinciguerra F., Parisi M., Frittitta L. Type 2 Diabetes Mellitus and Alzheimer’s Disease: Role of Insulin Signalling and Therapeutic Implications. Int. J. Mol. Sci. 2018;19:3306. doi: 10.3390/ijms19113306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Picone P., Sabatino M.A., Ditta L.A., Amato A., San Biagio P.L., Mulè F., Giacomazza D., Dispenza C., Di Carlo M. Nose-to-brain delivery of insulin enhanced by a nanogel carrier. J. Control. Release. 2018;270:23–36. doi: 10.1016/j.jconrel.2017.11.040. [DOI] [PubMed] [Google Scholar]
- 87.Kamei N., Takeda-Morishita M. Brain delivery of insulin boosted by intranasal coadministration with cell-penetrating peptides. J. Control. Release. 2015;197:105–110. doi: 10.1016/j.jconrel.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 88.Kamei N., Okada N., Ikeda T., Choi H., Fujiwara Y., Okumura H., Takeda-Morishita M. Effective nose-to-brain delivery of exendin-4 via coadministration with cell-penetrating peptides for improving progressive cognitive dysfunction. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-36210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boche M., Pokharkar V. Quetiapine Nanoemulsion for Intranasal Drug Delivery: Evaluation of Brain-Targeting Efficiency. AAPS PharmSciTech. 2017;18:686–696. doi: 10.1208/s12249-016-0552-9. [DOI] [PubMed] [Google Scholar]
- 90.Bahadur S., Pathak K. Buffered nanoemulsion for nose to brain delivery of ziprasidone hydrochloride: preformulation and pharmacodynamic evaluation. Curr. Drug Deliv. 2012;9:596–607. doi: 10.2174/156720112803529792. [DOI] [PubMed] [Google Scholar]
- 91.Ahmad N., Ahmad R., Alam M.A., Samim M., Iqbal Z., Ahmad F.J. Quantification and evaluation of thymoquinone loaded mucoadhesive nanoemulsion for treatment of cerebral ischemia. Int. J. Biol. Macromol. 2016;88:320–332. doi: 10.1016/j.ijbiomac.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 92.Abdou E.M., Kandil S.M., Miniawy H.M.F.E. Brain targeting efficiency of antimigrain drug loaded mucoadhesive intranasal nanoemulsion. Int. J. Pharm. 2017;529:667–677. doi: 10.1016/j.ijpharm.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 93.Colombo M., Figueiró F., de Fraga Dias A., Teixeira H.F., Battastini A.M.O., Koester L.S. Kaempferol-loaded mucoadhesive nanoemulsion for intranasal administration reduces glioma growth in vitro. Int. J. Pharm. 2018;543:214–223. doi: 10.1016/j.ijpharm.2018.03.055. [DOI] [PubMed] [Google Scholar]
- 94.Nasr M. Development of an optimized hyaluronic acid-based lipidic nanoemulsion co-encapsulating two polyphenols for nose to brain delivery. Drug Deliv. 2016;23:1444–1452. doi: 10.3109/10717544.2015.1092619. [DOI] [PubMed] [Google Scholar]
- 95.Trotta V., Pavan B., Ferraro L., Beggiato S., Traini D., Des Reis L.G., Scalia S., Dalpiaz A. Brain targeting of resveratrol by nasal administration of chitosan-coated lipid microparticles. Eur. J. Pharm. Biopharm. 2018;127:250–259. doi: 10.1016/j.ejpb.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 96.Mahajan H.S., Mahajan M.S., Nerkar P.P., Agrawal A. Nanoemulsion-based intranasal drug delivery system of saquinavir mesylate for brain targeting. Drug Deliv. 2014;21:148–154. doi: 10.3109/10717544.2013.838014. [DOI] [PubMed] [Google Scholar]
- 97.Yadav S., Gattacceca F., Panicucci R., Amiji M.M. Comparative Biodistribution and Pharmacokinetic Analysis of Cyclosporine-A in the Brain upon Intranasal or Intravenous Administration in an Oil-in-Water Nanoemulsion Formulation. Mol. Pharm. 2015;12:1523–1533. doi: 10.1021/mp5008376. [DOI] [PubMed] [Google Scholar]
- 98.Pandey Y.R., Kumar S., Gupta B.K., Ali J., Baboota S. Intranasal delivery of paroxetine nanoemulsion via the olfactory region for the management of depression: formulation, behavioural and biochemical estimation. Nanotechnology. 2016;27 doi: 10.1088/0957-4484/27/2/025102. [DOI] [PubMed] [Google Scholar]
- 99.Parikh R.H., Patel R.J. Nanoemulsions for Intranasal Delivery of Riluzole to Improve Brain Bioavailability: Formulation Development and Pharmacokinetic Studies. Curr. Drug Deliv. 2016;13:1130–1143. doi: 10.2174/1567201813666151202195729. [DOI] [PubMed] [Google Scholar]
- 100.Corace G., Angeloni C., Malaguti M., Hrelia S., Stein P.C., Brandl M., Gotti R., Luppi B. Multifunctional liposomes for nasal delivery of the anti-Alzheimer drug tacrine hydrochloride. J. Liposome Res. 2014;24:323–335. doi: 10.3109/08982104.2014.899369. [DOI] [PubMed] [Google Scholar]
- 101.Pan L., Zhou J., Ju F., Zhu H. Intranasal delivery of α-asarone to the brain with lactoferrin-modified mPEG-PLA nanoparticles prepared by premix membrane emulsification. Drug Deliv. Transl. Res. 2018;8:83–96. doi: 10.1007/s13346-017-0438-8. [DOI] [PubMed] [Google Scholar]
- 102.Nigam K., Kaur A., Tyagi A., Nematullah M., Khan F., Gabrani R., Dang S. Nose-to-brain delivery of lamotrigine-loaded PLGA nanoparticles. Drug Deliv. Transl. Res. 2019;9:879–890. doi: 10.1007/s13346-019-00622-5. [DOI] [PubMed] [Google Scholar]
- 103.Sharma D., Sharma R.K., Sharma N., Gabrani R., Sharma S.K., Ali J., Dang S. Nose-To-Brain Delivery of PLGA-Diazepam Nanoparticles. AAPS PharmSciTech. 2015;16:1108–1121. doi: 10.1208/s12249-015-0294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Deepika D., Dewangan H.K., Maurya L., Singh S. Intranasal Drug Delivery of Frovatriptan Succinate-Loaded Polymeric Nanoparticles for Brain Targeting. J. Pharm. Sci. 2019;108:851–859. doi: 10.1016/j.xphs.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 105.Natsheh H., Touitou E. Phospholipid Magnesome-a nasal vesicular carrier for delivery of drugs to brain. Drug Deliv. Transl. Res. 2018;8:806–819. doi: 10.1007/s13346-018-0503-y. [DOI] [PubMed] [Google Scholar]
- 106.Salama H.A., Mahmoud A.A., Kamel A.O., Abdel Hady M., Awad G.A.S. Phospholipid based colloidal poloxamer-nanocubic vesicles for brain targeting via the nasal route. Colloids Surf. B Biointerfaces. 2012;100:146–154. doi: 10.1016/j.colsurfb.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 107.El-Zaafarany G.M., Soliman M.E., Mansour S., Awad G.A.S. Identifying lipidic emulsomes for improved oxcarbazepine brain targeting: In vitro and rat in vivo studies. Int. J. Pharm. 2016;503:127–140. doi: 10.1016/j.ijpharm.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 108.Herda L.M., Polo E., Kelly P.M., Rocks L., Hudecz D., Dawson K.A. Designing the future of nanomedicine: current barriers to targeted brain therapeutics. Eur. J. Nanomed. 2014;6 doi: 10.1515/ejnm-2014-0022. [DOI] [Google Scholar]
- 109.Bhalerao A., Sivandzade F., Archie S.R., Chowdhury E.A., Noorani B., Cucullo L. In vitro modeling of the neurovascular unit: advances in the field. Fluids Barriers CNS. 2020;17:22. doi: 10.1186/s12987-020-00183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van der Worp H.B., Howells D.W., Sena E.S., Porritt M.J., Rewell S., O’Collins V., Macleod M.R. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Parekh G., Shi Y., Zheng J., Zhang X., Leporatti S. Nano-carriers for targeted delivery and biomedical imaging enhancement. Ther. Deliv. 2018;9:451–468. doi: 10.4155/tde-2018-0013. [DOI] [PubMed] [Google Scholar]
- 112.Moos T., Morgan E.H. Restricted transport of anti-transferrin receptor antibody (OX26) through the blood-brain barrier in the rat. J. Neurochem. 2001;79:119–129. doi: 10.1046/j.1471-4159.2001.00541.x. [DOI] [PubMed] [Google Scholar]
- 113.Yu Y.J., Zhang Y., Kenrick M., Hoyte K., Luk W., Lu Y., Atwal J., Elliott J.M., Prabhu S., Watts R.J., Dennis M.S. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci. Transl. Med. 2011;3:84ra44. doi: 10.1126/scitranslmed.3002230. [DOI] [PubMed] [Google Scholar]
- 114.Reischl D., Zimmer A. Drug delivery of siRNA therapeutics: potentials and limits of nanosystems. Nanomedicine. 2009;5:8–20. doi: 10.1016/j.nano.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 115.Rivera-Concepcion J., Uprety D., Adjei A.A. Challenges in the Use of Targeted Therapies in Non-Small Cell Lung Cancer. Cancer Res. Treat. 2022;54:315–329. doi: 10.4143/crt.2022.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sabale A.S., Kulkarni A.D., Sabale A.S. Nasal In Situ Gel: Novel Approach for Nasal Drug Delivery. J. Drug Delivery Ther. 2020;10:183–197. doi: 10.22270/jddt.v10i2-s.4029. [DOI] [Google Scholar]