Highlights
-
•
NbAGO5 expression is ubiquitously stimulated upon viral infections.
-
•
NbAGO5 confers defense against diverse viruses by binding to vsiRNAs.
-
•
CMV and TuMV counteract plant defense by degrading NbAGO5 protein.
-
•
CMV 2b and TuMV HC-Pro drive NbAGO5 degradation by 26S proteasome and autophagy.
-
•
TuMV HC-Pro provides additional counter-defense by interfering with vsiRNA loading.
Keywords: AGO5, RNA silencing, Plant antiviral defense, Viral suppressors of RNA silencing (VSR), 26S proteasome, Autophagy
Abstract
The argonaute (AGO) family proteins play a crucial role in preventing viral invasions through the plant antiviral RNA silencing pathway, with distinct AGO proteins recruited for specific antiviral mechanisms. Our previous study revealed that Nicotiana benthamiana AGO5 (NbAGO5) expression was significantly upregulated in response to bamboo mosaic virus (BaMV) infection. However, the roles of NbAGO5 in antiviral mechanisms remained to be explored. In this research, we examined the antiviral functions of NbAGO5 in the infections of different viruses. It was found that the accumulation of NbAGO5 was induced not only at the RNA but also at the protein level following the infections of BaMV, potato virus X (PVX), tobacco mosaic virus (TMV), and cucumber mosaic virus (CMV) in N. benthamiana. To explore the antiviral mechanism and regulatory function of NbAGO5, we generated NbAGO5 overexpression (OE-NbAGO5) and knockout (nbago5) transgenic N. benthamiana lines. Our findings reveal that NbAGO5 provides defense against BaMV, PVX, TMV, and a mutant CMV deficient in 2b gene, but not against the wild-type CMV and turnip mosaic virus (TuMV). Through affinity purification and small RNA northern blotting, we demonstrated that NbAGO5 exerts its antiviral function by binding to viral small interfering RNAs (vsiRNAs). Moreover, we observed that CMV 2b and TuMV HC-Pro interact with NbAGO5, triggering its degradation via the 26S proteasome and autophagy pathways, thereby allowing these viruses to overcome NbAGO5-mediated defense. In addition, TuMV HC-Pro provides another line of counter-defense by interfering with vsiRNA binding by NbAGO5. Our study provides further insights into the antiviral RNA interference mechanism and the complex interplay between NbAGO5 and plant viruses.
Graphical abstract
1. Introduction
RNA silencing is a conserved mechanism among eukaryotes, playing crucial roles in development and viral defense (Lopez-Gomollon and Baulcombe, 2022). This mechanism primarily employs small RNA molecules (sRNA) to target specific sequences and modulate gene expression. In RNA silencing pathway, Dicer-Like (DCL) enzymes identify double-stranded RNA (dsRNA) within cells and cleave them into 21- to 24-nucleotide (nt)-long microRNA (miRNA) or small interfering RNA (siRNA) molecules. In turn, argonaute (AGO) proteins carry the guide strand of the processed sRNA to form RNA-induced silencing complexes (RISCs), which were directed by the sRNA to the target RNA molecules in the cytoplasm (Ma and Zhang, 2018; Martín-Merchán et al., 2023). This interaction results in either RNA cleavage or translational inhibition. Alternatively, RISCs operating in the nucleus can mediate RNA-dependent DNA methylation at corresponding target sites, leading to gene silencing (Kørner et al., 2018; Gong et al., 2022).
Upon viral infection, plants may initiate an antiviral RNA silencing mechanism, which is triggered by dsRNA originating from the secondary structures of viral genomic RNAs or replication intermediate (Guo et al., 2019; Deng et al., 2022). The DCL enzymes in plants process the viral dsRNA into small RNA molecules referred to as virus-derived small interfering RNAs (vsiRNAs), which are subsequently recruited by AGO proteins to effectively neutralize the virus through the RNA silencing pathway. Viral single-stranded RNAs (ssRNAs) may also be amplified through the cooperative action of host RNA-dependent RNA polymerases (RDRs) and suppressor of gene silencing-3 (SGS3), synthesizing dsRNAs which were further processed by DCL to generate secondary vsiRNAs, amplifying the silencing signals and enhancing host defense against viral invasion (Zhang et al., 2015; Yoshikawa et al., 2021).
In response to this defense mechanism, many plant viruses have evolved viral suppressors of RNA silencing (VSRs), proteins that interfere with the RNA silencing pathway (Csorba et al., 2015; Li and Wang, 2019). It has been shown that VSRs can act at different stages in the RNA silencing pathway, including the biogenesis and function of miRNAs, siRNAs, and RISCs (Jin et al., 2021, 2022). For instance, turnip mosaic virus (TuMV) employs its helper component-proteinase (HC-Pro) to bind physically with Arabidopsis thaliana Hua-Enhancer 1 (AtHEN1), a key component in the methylation of sRNAs, enabling it to destabilize vsiRNAs and allowing the virus to evade host defenses and persist within the plant (Sanobar et al., 2021). In addition, TuMV HC-Pro can interact with AtAGO1 and promote its degradation through autophagy (Wei et al., 2022; Hong et al., 2023). These findings highlight the intricate strategies plant viruses employ to circumvent host defense systems and establish successful infections. However, VSR functions can vary based on the interactions of different viruses and hosts, and the mechanisms underlying RNA silencing suppression may differ even among closely related viruses. Therefore, it is vital to understand the roles of antiviral RNA silencing in various plant species and explore their interactions with distinct viral VSRs during virus infection cycles.
AGO proteins play a crucial role in antiviral RNA silencing, with distinct AGO family members performing specific functions in this pathway (Martín-Merchán et al., 2023). Previous studies on the involvement of AGOs in antiviral responses are predominantly focused on AGO1 and AGO2. For instance, in A. thaliana, studies have demonstrated that A. thaliana AGO1 (AtAGO1) plays a protective role against various viruses, such as TuMV, turnip crinkle virus (TCV), brome mosaic virus (BMV), and cucumber mosaic virus (CMV) (Qu et al., 2008; Wang et al., 2011; Dzianott et al., 2012; Garcia-Ruiz et al., 2015; Annacondia and Martinez, 2021). Similarly, AtAGO2 has been proven effective against viruses like potato virus X (PVX), tobacco rattle virus (TRV), TCV, CMV, TuMV, and bamboo mosaic virus (BaMV) (Harvey et al., 2011; Carbonell et al., 2012; Ma et al., 2015; Alazem et al., 2017; Zheng et al., 2019; Brosseau et al., 2020; Liu et al., 2022). In Nicotiana benthamiana, a widely used plant model system in virus researches (Goodin et al., 2015), related studies indicate that its AGO genes (NbAGOs) contribute to the plant's defense against viruses. NbAGO1 has been identified as essential in combating tomato ring spot virus (ToRSV), tomato bushy stunt virus (TBSV), cymbidium ringspot virus (CymRSV), BaMV, and TCV (Ghoshal and Sanfaçon, 2014; Gursinsky et al., 2015; Huang et al., 2019; Ludman and Fátyol, 2021). Likewise, NbAGO2 has been shown to participate in the defense against numerous viruses, including TBSV, carnation Italian ringspot virus (CIRV), cucumber necrosis virus (CNV), CymRSV, TCV, TuMV, ToRSV, tobacco mosaic virus (TMV), pelargonium line pattern virus (PLPV), and sweet potato mild mottle virus (SPMMV) (Scholthof et al., 2011; Odokonyero et al., 2015; Ludman et al., 2017; Paudel et al., 2018; Diao et al., 2019; Kenesi et al., 2021; Pérez-Cañamás et al., 2021). As a result, AGO1 and AGO2 are recognized as key defenders against specific viruses in antiviral RNA silencing (Silva-Martins et al., 2020), whereas the antiviral potential of other AGOs has not been fully appreciated.
While AGO1 and AGO2 attracted the most attention in the researches on plant antiviral defense mechanisms, the contributions of other AGOs are equally important. Apart from AGO1s and AGO2s, AGO5 family proteins represent one of the most important AGOs in the defense against viruses in plants. Previous studies have shown that AtAGO5 gene, primarily expressed in flowers and other reproductive tissues, becomes activated in leaves when infected by specific viruses, PVX or plantago asiatica mosaic virus (PlAMV). In the case of PVX infection, both AtAGO5 and AtAGO2 collaborate to limit the systemic spread of the virus (Brosseau and Moffett, 2015; Silva-Martins et al., 2023). Recently, our group discovered that the AGO5 family genes of Phalaenopsis aphrodite subsp. formosana (PaAGO5s) are induced by cymbidium mosaic virus (CymMV) and odontoglossum ringspot virus (ORSV) infections, playing a vital role in the defense against these viruses (Kuo et al., 2021; Kasi Viswanath et al., 2022, 2023). Additionally, we have identified the transcription factors, NbNAC42 and NbZFP3, that activate and repress, respectively, the expression of N. benthamiana AGO5 (NbAGO5) gene during BaMV infection (Ke et al., 2022). However, the underlying antiviral mechanism of NbAGO5 and its role in defense against viruses in other genera remained to be elucidated.
In this study, we investigated the antiviral properties of NbAGO5 during the infections of a variety of common viruses. We analyzed the accumulation of NbAGO5 protein following infections of different viruses, and generated NbAGO5-overexpressing and knockout transgenic lines of N. benthamiana to further verify the role of NbAGO5 in antiviral defense. It was found that NbAGO5 effectively reduced the accumulation of BaMV, PVX, and TMV, but provided little or no protection against CMV and TuMV. Our further analyses revealed that the VSRs of CMV and TuMV, 2b protein and HC-Pro, respectively, may interact with and direct the degradation of NbAGO5 through both 26S proteasome and autophagy pathways. In addition, TuMV HC-Pro may inhibit the loading of vsiRNAs into NbAGO5-associated RISC, boosting the viral arsenal for counter-defense against RNA silencing of the host. A model is proposed to illustrate the role of NbAGO5 in antiviral defense mechanism of the host and the counter-defense of specific viruses.
2. Materials and methods
2.1. Plant materials and growth conditions
Nicotiana benthamiana was used as the test subject for all experiments involving plants in this study. Wild-type (WT) and transgenic N. benthamiana plants were maintained at a constant temperature of 26 °C and a photoperiod of 16 h of light and 8 h of darkness in a growth chamber.
2.2. Construction of infectious clones and plasmids for transient overexpression of VSRs of specific viruses
To generate an infectious clone of TuMV, total RNAs were extracted from N. benthamiana leaves infected with the TuMV YC5 strain (GenBank AF530055). The viral genomic cDNAs were then amplified through reverse transcription-polymerase chain reaction (RT-PCR) using the specific primer pair, TuMV_smaIF and TuMV_smaIR, which contained flanking SmaI sites. The amplified cDNAs were subsequently ligated into the SmaI-digested pEpyon vector (Huang et al., 2019), resulting in the infectious clone designated pETuMV. To construct the infectious clone of CMV 2b-defective mutant (CMV-2bm), a two-step procedure was employed. Firstly, two megaprimers were synthesized by PCR amplification using pECMV2 (Ke et al., 2022) as the template. For megaprimer1, primer pair 2b_ATG1mF plus CMV2_KpnIR were used; whereas for megaprimer2, CMV2_BamHIF plus 2b_ATG2mR were used. In the subsequent step, the two purified megaprimers were used for the amplification through PCR. The resulting PCR products were purified and digested with BamHI and KpnI enzymes and used to replace the corresponding fragment within the pECMV2 plasmid to generate pECMV2–2bm. The CMV used in this study is the NT9 strain (CMV-NT9, GenBank accession numbers for RNA1, 2 and 3 are D28778.1, D28779.1, and D28780.1, respectively).
For the construction of transient overexpression plasmid for CMV 2b protein, the 2b open reading frame (ORF) was amplified by PCR using the primer pair, 2b_HindIIIF and 2b_BglIIR, and pECMV2 as the template. The purified PCR products were subsequently digested with HindIII and BglII, and ligated with a HindIII- and BglII-digested pKn vector (Prasanth et al., 2011) to generate pK2b. To create transient overexpression construct of TuMV HC-Pro, the corresponding region in TuMV genome was amplified through PCR using the primer pair, HC_Pro_XbaIF and HC_Pro_SacIR, and pETuMV as the template. The purified PCR products were then digested with XbaI and SacI, and ligated with an XbaI- and SacI-digested pEpyon-32 K vector, resulting in the formation of pEHC-Pro. Supplementary Table 1 provides the sequence details of primers used in this study.
2.3. Agroinfiltration for virus inoculation and transient overexpression
To infect 30-day-old N. benthamiana plants with various plant viruses, the third and fourth leaves were agroinfiltrated using Agrobacterium tumefaciens strain GV3850 harboring the corresponding infectious clones of each virus. The bacterial strain was transformed individually with different plasmids, including an empty vector and infectious constructs pKBG for BaMV-GFP (Liou et al., 2014) for BaMV, pKPG for PVX-GFP (Huang et al., 2019), pKT for TMV (Ke et al., 2022), pECMV1/pECMV2/pECMV3 for CMV (Ke et al., 2022), pECMV1/pECMV2–2bm/pECMV3 for CMV-2bm, or pETuMV for TuMV. The bacterial cells were cultured overnight at 28 °C, harvested by centrifugation, resuspended in an infiltration buffer (10 mM MES, pH 5.5, and 10 mM MgCl2) with concentration adjusted to an OD600 of 0.1, and infiltrated into the abaxial side of N. benthamiana leaves using a needleless syringe as described previously (Huang et al., 2023).
The procedure for transient overexpression of 2b, HC-Pro, or BaMV TGBp1 (Ke et al., 2022) is similar to the virus inoculation method described above, except that the bacterial cells are resuspended in infiltration buffer with concentration adjusted to an OD600 of 0.5 prior to infiltration into N. benthamiana leaves.
2.4. Construction of plasmids for overexpressing or knocking out NbAGO5 and plant transformation
To stably overexpress Flag-tagged NbAGO5 (Flag-NbAGO5) in N. benthamiana, the full-length coding DNA sequence (CDS) of NbAGO5 (GenBank accession number OQ852930) was amplified via PCR using N. benthamiana cDNA as the template and the primer pairs, Flag-NbAGO5_CDS_BamHIF and NbAGO5_CDS_SacIR. The resulting PCR product was gel-purified, digested with BamHI and SacI, and cloned into the pEpyon-32 K vector to create pE-FlagNbAGO5. To knockout NbAGO5 in N. benthamiana, the CRISPR/Cas9 technology was employed as follows. A guide RNA (gRNA) targeting NbAGO5 CDS was designed using the webtool CRISPRdirect (https://crispr.dbcls.jp/) (Naito et al., 2015) to minimize off-target effects. The specific gRNA was then amplified by PCR from the pKSE401 template (Xing et al., 2014) with the primer pair M13F and NbAGO5_gRNA_BsaIR, gel-purified, digested with AflII and BsaI, and cloned into pKSE401 to produce pKSE-NbAGO5. All constructs were verified through sequencing, and then transformed into A. tumefaciens strain GV3850 via electroporation. The A. tumefaciens harboring the desired plasmids was used for generating transgenic N. benthamiana through leaf disk transformation (Horsch et al., 1985; Jiang et al., 2020). Putative Flag-NbAGO5-overexpressing plants (OE-NbAGO5) were identified by western blot analysis, and homozygous individuals were selected on MS medium containing 100 ppm kanamycin. NbAGO5-knockout (designated as nbago5) plants were screened by PCR from genomic DNA using specific primer pairs NbA5_−20F and NbA5_322R (Bhattacharya and Van Meir, 2019). Homozygous individuals were confirmed through nucleotide sequencing to identify the edited sites. Supplementary Table 1 provides the sequence details of primers used in this study.
2.5. Quantitation of gene expression using reverse transcription and real-time quantitative PCR (RT-qPCR)
Total RNA was extracted from leaf tissue using TriPure Isolation Reagent (Roche Life Science, IN, USA) following the manufacturer's instructions. Reverse transcription was performed using the GoScript Reverse Transcriptase kit (Promega, WI, USA) and oligo(dT)18 primers following the manufacturer's instructions to synthesize the first-strand cDNA. For quantitation of target mRNA accumulation levels, RT-qPCR analyses were performed as described briefly as follows. Two-fold-diluted cDNA (2 μl) was used as a template along with specific primers (as listed in Supplementary Table 1) and KAPA SYBR® FAST qPCR master mix (Kapa Biosystems, MA, USA) according to the manufacturer's instructions. The reactions were performed using a TOptical Gradient 96 Real-Time PCR Thermocycler (Biometra, Göttingen, Germany). To standardize the mRNA levels of the target genes among the samples, the relative mRNA levels of actin gene in each sample were determined and used as the internal control. To ensure reproducibility, the experiment was performed with three biological replicates for each assay, and each biological replicate was analyzed using three technical replicates for qPCR analysis.
2.6. Protein extraction and western blot analysis
Total proteins were extracted from 0.1 g of N. benthamiana leaf tissue ground in liquid N2 using 200 μl of protein extraction buffer (20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 0.5% NP-40, and 5 mM dithiothreitol) supplemented with 1% protease inhibitor cocktail (Roche Life Science, IN, USA). The extracted proteins were separated by electrophoresis through an 8% or 12% polyacrylamide gel containing 1% sodium dodecyl sulfate (SDS-PAGE). For western blot analysis, the separated proteins in the gel were transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore, USA) and incubated with laboratory-generated primary rabbit antiserum against NbAGO5 N-terminus, BaMV- coat protein (CP), PVX-CP, TMV-CP, CMV-CP, TuMV-CP, CMV-2b, or TuMV-HC-Pro at a 1:5000 dilution in phosphate-buffered saline (PBS; 10 mM NaH2PO4, 10 mM Na2HPO4, and 8.5% NaCl). Following washing, the membranes were incubated with Alkaline Phosphatase (AP)-conjugated or Horseradish Peroxidase (HRP)-conjugated secondary antibodies (5000 × dilution) and visualized using nitro-blue tetrazolium/5‑bromo-4‑chloro-3′-indolyphosphate (NBT/BCIP) color development substrate (Thermo Scientific, Waltham, MA, USA) or HRP reagent (Merck Millipore, Darmstadt, Germany), respectively. The signals were acquired and quantified using Fujifilm LAS-4000 and Multi Gauge v.3.0 software.
2.7. Immunoprecipitation
Total proteins were extracted from virus-infected leaves at 5 dpi as described in the previous section and subjected to centrifugation twice at 1600 xg for 10 min at 4 °C. Immunoprecipitation assay was performed on the resulting supernatant by using Anti-Flag M2 magnetic beads (Sigma-Aldrich, IN, USA) for 2 h at 4 °C, followed by washing the Flag-tagged protein complex-containing beads three times with a wash buffer (20 mM Tris–HCl at pH 7.5, 5 mM MgCl2, 150 mM NaCl, and 0.5% NP40). The beads containing the protein complexes were divided into two portions to extract the associated RNAs and proteins separately. The RNAs attached to the beads were obtained using TriPure reagent (Roche Life Sciences, IN, USA) following the instructions of the manufacturer, and subjected to sRNA northern blot analysis (described in the following section). The protein complex-containing beads were suspended in 1 × protein sample buffer, and analyzed by SDS-PAGE followed by western blotting using antibodies specific to NbAGO5, CMV 2b, or TuMV HC-Pro as described in the previous section.
2.8. vsiRNA analysis by sRNA northern blot
Total RNA was extracted from leaves inoculated with the virus at 5 dpi using TriPure Isolation Reagent as described above. Low-molecular-weight RNA from each sample was separated on a 15% polyacrylamide gel containing 7 M urea and then transferred to a nylon membrane (Amersham, UK) for sRNA northern blot analysis using 32P-labeled probes to detect BaMV, PVX, or CMV as described previously (Huang et al., 2019, 2023). To detect TMV vsiRNA, a specific 32P-labeled probe for the TMV 3′untranslated region (UTR) was generated by T7 polymerase using the EcoRI-linearized pTMVEX plasmid, which was constructed as follows. TMV 3′UTR was obtained through PCR amplification using pKTMV (Ke et al., 2022) as the template and the primer pair TMV3′UTR_EcoRIF and TMV3′UTR_XbaIR. The resulting PCR product was then digested with EcoRI and XbaI enzymes and ligated into the pGEM4 vector (Promega, WI, USA) to generate pTMVEX. To detect TuMV vsiRNA, a specific 32P-labeled probe for the HC-Pro region was generated by T7 polymerase using the EcoRI-linearized plasmid pHC-ProEX, which was similarly constructed as described above for pTMVEX, except for the use of pETuMV as the template with the primer pair HCPro_EcoRIF and HCPro_HindIIIR. Signals generated in the sRNA northern blots were analyzed using the Amersham Typhoon 5 phosphorimager (GE Healthcare, IL, USA). Supplementary Table 1 provides the sequence details of primers used in this study.
2.9. Treatment with specific inhibitors of protein degradation pathways
To investigate the possible mechanisms by which CMV 2b and TuMV HC-Pro reduced the accumulation of NbAGO5 protein, the two VSRs (2b and HC-Pro) were transiently expressed in N. benthamiana leaves through agroinfiltration. Leaf samples were collected at 5 days post-infiltration. At 12 hr before harvesting, specific inhibitors of 26S proteasome or autophagy, namely carbobenzoxy-Leu-Leu-leucinal (MG132, 50 μM in DMSO) or 3-methyladenine (3-MA, 10 mM in H2O) (Sigma-Aldrich, IN, USA), respectively, was infiltrated into the abaxial side of the leaves using a syringe. DMSO or H2O was used as the negative control in the following experiments for MG132 or 3-MA, respectively.
3. Results
3.1. Accumulation of NbAGO5 protein in response to infections by different plant viruses in N. benthamiana
In the antiviral gene silencing pathway, proteins from the AGO family are crucial in the defense against viral invasions. However, distinct AGO family proteins may be expressed in response to different viruses, activating specific antiviral mechanisms (Carbonell and Carrington, 2015). The genome of N. benthamiana encodes 10 known AGO genes: NbAGO1a, NbAGO1b, NbAGO2, NbAGO4a, NbAGO4b, NbAGO5, NbAGO6, NbAGO7, NbAGO10a, and NbAGO10b. Our previous study revealed that, among all N. benthamiana-encoded AGOs, NbAGO5 mRNA expression increased most significantly under BaMV infection and was also notably induced during PVX, TMV, and CMV infections (Ke et al., 2022). However, the accumulation level of NbAGO5 protein under the infection of different plant viruses, and the underlying regulatory mechanisms, have not been studied.
In this study, we further verified the accumulation level of NbAGO5 protein following infections of different viruses via western blot using antiserum specific to the N-terminus region of NbAGO5. N. benthamiana plants at the 30-day growth stage were infected with BaMV-GFP, PVX-GFP, TMV, CMV, or TuMV through agroinfiltration. Western blot analyses of total protein extracts from mock- (empty vector) and virus-inoculated leaves at 5 dpi revealed that, relative to the mock, the endogenous NbAGO5 protein accumulation levels increased 1.8-, 2.1-, 1.8-, and 1.3-fold in response to BaMV-GFP, PVX-GFP, TMV, and CMV infections, respectively. Conversely, the NbAGO5 protein level in TuMV-infected leaves significantly decreased to 60% of that of the mock-inoculated group (Fig. 1). These findings confirmed that, except for TuMV infection, NbAGO5 gene expression was induced at both RNA and protein levels in response to general plant virus infections. The result also suggests that NbAGO5 gene might be involved in the general defense against many plant viruses, while specific viruses, such as TuMV, may launch certain counter-defense mechanisms against NbAGO5-mediated antiviral responses.
Fig. 1.
Accumulation of NbAGO5 protein in response to infections of various plant viruses in Nicotiana benthamiana.N. benthamiana leaves were inoculated with BaMV-GFP, PVX-GFP, TMV, CMV, or TuMV via agroinfiltration. Total proteins were extracted from leaves collected at 5 days post-inoculation (dpi), and analyzed by western blot using antibodies against NbAGO5 or specific viral CP. The intensity of each band was quantified and plotted, with coomassie brilliant blue (CBB)-stained RuBisCO protein present in each sample serving as the loading control. The protein accumulation levels of NbAGO5 were presented as normalized fold changes relative to that from empty vector-inoculated leaves (Mock). The values are means ± SD of three biological replicates. Statistical significance was determined by Student's t-test, with significant differences at p < 0.05, p < 0.01, and p < 0.001 denoted by *, **, and ***, respectively.
3.2. Generation and characterization of NbAGO5-overexpressing (OE-NbAGO5) and NbAGO5-knockout (nbago5) N. benthamiana plants for studying the antiviral function of NbAGO5
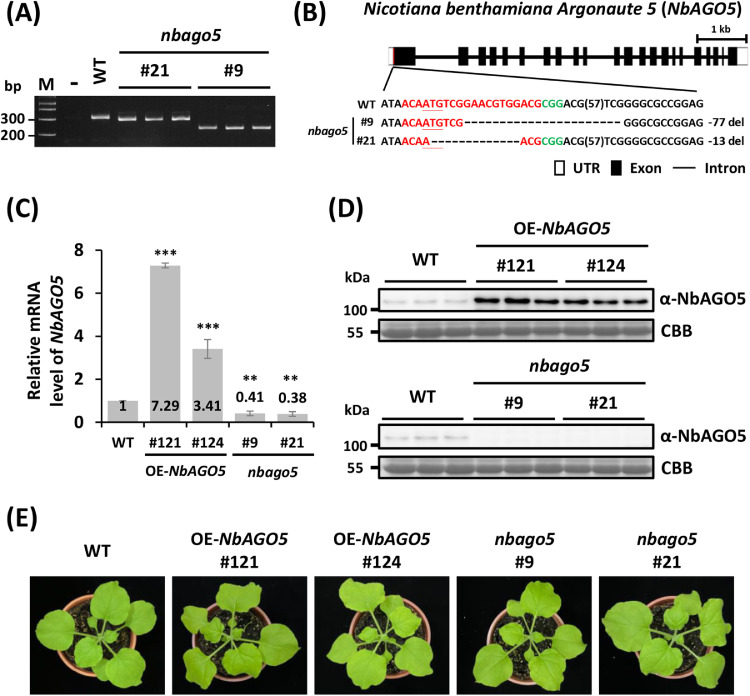
To further elucidate the antiviral function of NbAGO5, we generated NbAGO5-overexpression (OE-NbAGO5) and NbAGO5-knockout (nbago5) N. benthamiana plants for subsequent analyses. OE-NbAGO5 plants were created by introducing pEp-NbAGO5, for overexpression of Flag-tagged NbAGO5 (Flag-NbAGO5) under the control of a 35S promoter, into the N. benthamiana genome via Agrobacterium-mediated plant transformation. Western blot screening identified two independent transgenic lines (OE-NbAGO5 #121 and #124). To disrupt the expression of NbAGO5 gene, the CRISPR/Cas9 system was employed to generate nbago5. A specific target sequence on exon1 of NbAGO5, designated as CRISPRdirect (Naito et al., 2015), was cloned into pKSE401(Xing et al., 2014) to create pKSE-NbAGO5, which was introduced into N. benthamiana genome via Agrobacterium-mediated plant transformation. Genetic PCR screening yielded two independent transgenic lines (nbago5 #9 and #21). The edited target regions in two nbago5 homozygous T3 lines were PCR-amplified and sequenced (Fig. 2A). The result of sequencing analysis revealed a 77- and a 13-bp deletion in exon1 of NbAGO5 line #9 and line #21, respectively, leading to a frameshift and premature stop codon of line #9 and the loss of the start codon of the NbAGO5 open reading frame in line #21. (Fig. 2B).
Fig. 2.
Generation and characterization of NbAGO5-overexpressing (OE-NbAGO5) and NbAGO5-knockout (nbago5) N. benthamiana transgenic lines. (A) Verification of edited genome by PCR. The target regions of NbAGO5 in wild-type (WT) plant and two independent nbago5 T3 lines were amplified by PCR with specific primer pair and analyzed by electrophoresis through a 4% agarose gel. (B) Confirmation of transgenic lines by sequencing analysis. The schematic representation of the NbAGO5 gene and the nucleotide sequences of target sites for editing in nbago5 T3 lines are shown. The edited target sequences are highlighted in red, the PAM sequence is shown in green, and the start codon is underlined. (C) RT-qPCR analysis of NbAGO5 mRNA expression in WT, OE-NbAGO5, and nbago5 T3 plants. Values are means ± SD of three biological replicates. Statistical significance was denoted as described in legends to Fig. 1. (D) Western blot analysis of NbAGO5 protein accumulation levels in WT, OE-NbAGO5, and nbago5 T3 plants. CBB-stained RuBisCO protein served as the loading control. (E) Phenotypes of WT and transformed plants at the 30-day growth stage.
To validate the NbAGO5 mRNA and protein expression patterns in the transformants, RT-qPCR and western blot analyses were performed. The mRNA expression of NbAGO5 exhibited a 7.29-fold increase in T3 line #121 and a 3.41-fold increase in T3 line #124 (OE-NbAGO5 plants). In contrast, T3 lines #9 and #21 (nbago5 T3 plants) showed a 0.41-fold and 0.38-fold decrease, respectively, in comparison to that of the wild-type (WT) plants (Fig. 2C). Western blot assays also demonstrated overexpression of NbAGO5 protein in OE-NbAGO5 T3 plants (#121 and #124) and knockout of NbAGO5 in nbago5 T3 plants (#9 and #21) (Fig. 2D). Furthermore, no noticeable phenotypic differences were observed between wild-type and transformed plants under identical growth conditions (Fig. 2E). Therefore, these verified OE-NbAGO5 and nbago5 T3 plants were subjected to the following studies.
3.3. Investigation of the antiviral role of NbAGO5 in N. benthamiana plants against BaMV, PVX, TMV, CMV, and TuMV
To determine the antiviral function of NbAGO5, WT and T3 plants of OE-NbAGO5 and nbago5 were inoculated with BaMV, PVX, TMV, CMV, and TuMV, individually. N. benthamiana leaves were infiltrated with A. tumefaciens GV3850 carrying BaMV-GFP, PVX-GFP, TMV, CMV, or TuMV infectious clones. At 5 dpi, total protein extracts from infiltrated N. benthamiana leaves (local) were obtained and analyzed using western blot with antiserum specific to BaMV, PVX, TMV, CMV, or TuMV coat protein (CP). The results showed a significant decrease in BaMV, PVX, TMV, and CMV CP accumulation levels in OE-NbAGO5 leaves compared to that of WT. However, NbAGO5 overexpression did not significantly alter TuMV CP accumulation. In contrast, BaMV, PVX, TMV, and CMV CP accumulation levels increased in nbago5 compared to WT, while TuMV protein accumulation was not significantly affected by NbAGO5 knockout (Fig. 3A, B). At 12 dpi, the upper un-infiltrated leaves (designated as “systemic leaves” hereafter) of BaMV-GFP-infected N. benthamiana were collected and total protein was extracted, while those from the systemic leaves infected with other viruses were collected at 7 dpi and analyzed by western blotting. In OE-NbAGO5 plants, BaMV, PVX, and TMV CP accumulation levels significantly decreased, whereas the CP accumulation levels of CMV and TuMV remained largely unchanged compared to those from WT plants. Although no significant difference was observed in TuMV-CP accumulation between WT and nbago5 plants, the CP accumulation of BaMV, PVX, TMV, and CMV was higher in nbago5 plants (Fig. 3C, D). The phenotypes and symptoms observed after infections by various viruses on WT, OE-NbAGO5, and nbago5 plants are shown in Supplementary Fig. 1. The symptom severities were consistent with the accumulation levels of each viral CP (Fig. 3). These results suggest that overexpression of NbAGO5 may exert antiviral activities against BaMV, PVX, or TMV; however, the NbAGO5-mediated defense was subverted in TuMV-infected plants and systemic leaves of CMV-infected plants.
Fig. 3.
Effects of NbAGO5 overexpression or knockout on the accumulation of various plant viruses in N. benthamiana. (A) Analysis of NbAGO5 protein accumulation levels by western blot. Total proteins were extracted from WT, OE-NbAGO5, or nbago5 N. benthamiana leaves inoculated with BaMV-GFP, PVX-GFP, TMV, CMV, or TuMV at 5 dpi, and subjected to western blot analyses using antiserum against the CP of each virus. (B) The intensity of each band was quantified and plotted. Statistical analysis was performed as described in legends to Fig. 1. (C) The western blot analysis was conducted using total protein extracts from systemic leaves of virus-infected WT, OE-NbAGO5, or nbago5 N. benthamiana plants. Antiserum against BaMV, PVX, TMV, CMV, or TuMV CP was used, and the intensity of each band was quantified and plotted in a graph. CBB-stained RuBisCO protein served as the loading control. “Mock” or “-” represents the plant inoculated with an empty vector. (D) The protein accumulation levels of viral CP were presented as normalized fold changes relative to that from WT plants. Values are means ± SD of three biological replicates. Statistical analysis was performed as described in legends to Fig. 1. The notion “ns” indicates “non-significant”.
NbAGO1 and NbAGO2 have been reported to exhibit dominant antiviral functions against plant RNA viruses in N. benthamiana (Silva-Martins et al., 2020). To examine the impact of knocking out NbAGO5 on the expression of NbAGO1a, NbAGO1b, and NbAGO2, their mRNA levels were assessed in nbago5 T3 plants using RT-qPCR. The result revealed that the expression levels of NbAGO1 and NbAGO2 were not significantly altered in nbago5 plants (Supplementary Fig. 2), suggesting that the primary antiviral function associated with NbAGO1 and NbAGO2 is maintained in nbago5 plants. This observation may explain why NbAGO5 knockdown had relatively less effect on plant virus invasion.
3.4. The role of NbAGO5 in antiviral silencing through binding to vsiRNAs
It is known that AGO proteins may bind to the guide RNAs for directing the degradation of the target RNA (Ding, 2023). To test the hypothesis that NbAGO5 exerts its antiviral function through binding to vsiRNAs, immunoprecipitation assay and sRNA northern blot analysis were performed. WT and OE-NbAGO5 plants were inoculated with various viruses, and total proteins from the inoculated leaves were extracted at 5 dpi, and the NbAGO5-containing complexes were captured using anti-Flag M2 magnetic beads. Subsequently, a sRNA northern blot was performed using virus-specific probes to examine the binding of NbAGO5 to vsiRNAs. The results suggested that the NbAGO5 complex could bind to vsiRNAs from various viruses, except for that from TuMV (Fig. 4). Previous research noted that during TuMV infection, antiviral RNA silencing is inhibited due to the reason that HC-Pro may dissociate virus-derived siRNAs from antiviral AGO proteins (Garcia-Ruiz et al., 2015). This observation suggests that TuMV HC-Pro may interfere with the interaction between vsiRNAs and NbAGO5 during TuMV infection, thereby preventing NbAGO5 from executing the downstream antiviral silencing function.
Fig. 4.
Interactions between NbAGO5 and virus-derived small RNAs (vsiRNAs) of various viruses. WT or OE-NbAGO5 leaves were agroinfiltrated with BaMV-GFP (A), PVX-GFP (B), TMV (C), CMV (D), or TuMV (E) as indicated. Total protein and RNA (input) were extracted from WT or OE-NbAGO5 leaves at 5 dpi and subjected to analyses by immunoblotting (IB) with NbAGO5 antibody and small RNA blotting (sRNA blot), respectively. NbAGO5-containing complexes were further immunoprecipitated with anti-Flag antibody, separated into protein and RNA fractions, and subjected to IB and sRNA blot, respectively. The sRNA blot was hybridized with 32P-labeled RNA probes complementary to the 3′ end of individual viral RNA. Ethidium Bromide-stained tRNAs were used as the loading control.
3.5. CMV 2b protein interferes with the stability of NbAGO5 and inhibits its antiviral function in systemic leaves
The above experiment showed that overexpression of NbAGO5 led to reduced CMV-CP accumulation in local leaves (Fig. 3A, B); however, there was no significant difference in CMV-CP accumulation between those in WT and OE-NbAGO5 plants in systemic leaves (Fig. 3C, D). This observation suggested that the antiviral function of NbAGO5 might be compromised in CMV-infected systemic leaves. To investigate the interference on NbAGO5 caused by CMV, the accumulation of NbAGO5 protein was monitored at various CMV infection stages. The results demonstrated that NbAGO5 protein was induced during CMV infection in local leaves, but was decreased with CMV infection in systemic leaves (Fig. 5A). The observation suggested CMV might cause the instability of NbAGO5 protein in systemic leaves of CMV-infected plants. It has been reported that CMV 2b protein, encoded by the second open reading frame of RNA2, exhibits RNA silencing suppressor activity (Diaz-Pendon et al., 2007). To test whether CMV 2b protein is involved in the instability of NbAGO5 protein in systemic leaves, the accumulation of 2b protein was examined using western blot analysis. The results revealed that the 2b protein was barely detectable in local leaves, but highly expressed in systemic leaves (Fig. 5A). The result is in agreement with the observation that the accumulation of CMV CP was decreased with the induction of NbAGO5 protein in local leaves, in which little or no CMV 2b was detectable (Fig. 5A). These findings suggested that the 2b protein may cause the instability of NbAGO5 protein in the systemic leaves of CMV-infected plants, and consequently, the antiviral activity of NbAGO5 may not be sufficient to defend against the CMV infection in these leaves.
Fig. 5.
Effect of CMV 2b protein on the antiviral function of NbAGO5. (A) Protein accumulations of NbAGO5, CMV 2b, and CMV CP in CMV-infected local and systemic N. benthamiana leaves at 3, 5, 7, and 10 dpi. Total protein extracts were obtained and subjected to western blotting using antibodies specific to NbAGO5, CMV 2b, CMV CP, or ACTIN. The intensities of the target bands were quantified, and the results are shown below the panel, with the first band as the basal level. (B) Western blot analysis of CMV 2b in the CMV- or CMV-2bm-infected local (L) and systemic (S) N. benthamiana leaves at 5 and 10 dpi. (C) Western blot analysis of NbAGO5 and CMV CP in the CMV-2bm-infected leaves at 5 dpi. The intensity of the target band was quantified and plotted, with the protein accumulation levels of NbAGO5 presented as normalized fold changes relative to Mock-infected leaves. (D) CMV CP accumulation in CMV-2bm-infected local leaves of WT, OE-NbAGO5, or nbago5 plants at 5 dpi, as detected by western blotting using antiserum specific to CMV CP. The intensity of the target band was quantified and plotted. (E) Western blotting analysis of CMV CP accumulation in CMV-2bm-infected systemic leaves of WT, OE-NbAGO5, or nbago5 plants at 10 dpi. The intensity of the target band was quantified and plotted. The RuBisCO protein stained with CBB was used as the loading control. The results are presented as means ± SD of three biological replicates. Statistical analysis was performed as described in legends to Fig. 1.
To further determine whether CMV 2b is involved in the inhibition of NbAGO5 antiviral function, a CMV 2b-defective mutant (CMV-2bm) was generated and analyzed. The CMV-2bm mutant was designed with nucleotide substitutions in both the first and second start codons of the CMV 2b open reading frame, disrupting the translation initiation of CMV 2b while retaining the codon of CMV 2a. Western blot analysis confirmed that 2b protein was not expressed in CMV-2bm infected leaves (Fig. 5B). To examine the effect of CMV-2bm on NbAGO5 protein accumulation, total proteins extracted from leaf samples inoculated with empty vector (mock) or CMV-2bm collected at 5 dpi were analyzed by western blot using antiserum specific to NbAGO5. The results revealed a 2.3-fold increase of NbAGO5 protein levels in CMV-2bm infected leaves compared to that of the mock (Fig. 5C). In addition, CMV-2bm infection resulted in a higher fold change in NbAGO5 protein accumulation than that in wild-type CMV infection (Fig. 1 and Fig. 5C). These results further supported the notion that the 2b protein influenced the stability of the NbAGO5 protein.
To investigate if NbAGO5 exhibits antiviral activity against CMV in the absence of 2b, WT and transgenic plants (OE-NbAGO5 or nbago5) were inoculated with CMV-2bm. The infected local and systemic leaves were collected at 5 dpi and 10 dpi for viral accumulation by western blot analysis. The results showed that in transgenic plants overexpressing NbAGO5, CMV-2bm CP accumulation was significantly reduced compared to that in WT plants, both in local and systemic leaf samples. In contrast, CMV-2bm CP accumulation was higher in transgenic plants lacking NbAGO5 compared to that in WT plants (Fig. 5D, E). These results showed that, in the absence of CMV 2b, NbAGO5 maintains its ability to restrict CMV invasion, demonstrating that CMV 2b is required for the suppression of the antiviral activity of NbAGO5.
3.6. The involvement of CMV 2b or TuMV HC-Pro in the degradation of NbAGO5 protein via the 26S proteasome and autophagy pathways
The findings above showed that the presence of 2b encoded by CMV interferes with the accumulation of NbAGO5. Similarly, infection by TuMV leads to reduced NbAGO5 protein levels (Fig. 1), which could be due to the destabilization of either the NbAGO5 mRNA or protein. It has been shown that certain VSRs can impair AGO antiviral function by sequestering vsiRNA or degrading AGO proteins (Pumplin and Voinnet, 2013). Previous studies have reported that CMV-encoded 2b and TuMV-encoded HC-Pro possess RNA silencing suppressor activity (Zhao et al., 2016; Carr et al., 2019). Given that the protein accumulation level of NbAGO5 was impaired following infection with CMV and TuMV, we hypothesized that 2b and HC-Pro are involved in the degradation of NbAGO5. To investigate this hypothesis, 2b and HC-Pro were transiently expressed in N. benthamiana leaves through agroinfiltration and the effects of over-expression on NbAGO5 protein accumulation levels were examined at 5 dpi by western blot analysis. The result showed that, upon transient overexpression of either 2b or HC-Pro, NbAGO5 protein accumulation decreased to 0.3-fold of that of the mock group (Fig. 6A), demonstrating that both 2b and HC-Pro were capable of reducing NbAGO5 protein accumulation.
Fig. 6.
Analysis of the underlying mechanisms for down-regulation of NbAGO5 accumulation by CMV 2b and TuMV HC-Pro. (A) Western blot analysis of the accumulation levels of NbAGO5 in N. benthamiana plants infiltrated with Agrobacterium carrying plasmids for the over-expression of either CMV 2b or TuMV HC-Pro. The control plants were infiltrated with an empty vector (Mock). Total protein extracts were obtained from the infiltrated leaves at 5 dpi, and the protein levels of NbAGO5, CMV 2b, and TuMV HC-Pro were analyzed by western blotting using specific antibodies. The intensities of the target band were quantified and normalized to those from the Mock treatment. The protein levels of NbAGO5 were presented as fold changes relative to those of the mock treatment. (B) Co-immunoprecipitation analysis of the interaction between NbAGO5 and CMV 2b or HC-Pro in WT and OE-NbAGO5 plants. Total protein extracts (input) from systemic leaves of CMV- or TuMV-infected N. benthamiana plants at 10 dpi were separated by electrophoresis followed by examination with CBB staining and western blotting using specific antibodies against NbAGO5, 2b, and HC-Pro. NbAGO5-associated complexes were further immunoprecipitated from total protein extracts using an anti-Flag antibody, followed by immunoblot detection of co-immunoprecipitated proteins. (C) The effect of various proteasome inhibitors on the accumulation of NbAGO5 in 2b- or HC-Pro-overexpressing N. benthamiana. At 5 dpi following infiltration with plasmids for overexpression of empty vector (mock), CMV 2b or TuMV HC-Pro, N. benthamiana leaves were further infiltrated with MG132 (50 µM) or 3-MA (10 mM) for 12 h before harvesting. Total proteins were then extracted from the infiltrated leaves and subjected to western blotting using antibodies against NbAGO5, CMV 2b, or TuMV HC-Pro to determine the protein accumulation levels of NbAGO5, 2b, or HC-Pro. Samples from treatments with DMSO or H2O served as a solvent control, and the intensity of target band was quantified and plotted. The protein accumulation levels of NbAGO5 were presented as normalized fold changes relative to the solvent control, with the CBB-stained RuBisCO protein as the loading control. The results were based on three biological replicates, and statistical analysis was performed as described in legends to Fig. 1. The notion “ns” indicates “non-significant”.
To determine whether the reduction in NbAGO5 protein levels was a direct or indirect effect of 2b and HC-Pro, we used immunoprecipitation to analyze the interaction between NbAGO5 and these VSRs. Systemic leaves from CMV- or TuMV-infected WT or OE-NbAGO5 plants were harvested at 7 dpi. Protein extracts from the leaves were immunoprecipitated using anti-Flag M2 magnetic beads to capture the Flag-tagged NbAGO5 protein complexes, which were subsequently analyzed by western blot with specific antiserum to detect the presence of 2b and HC-Pro in these complexes. The results revealed that CMV 2b and TuMV HC-Pro were present in the Flag-NbAGO5-associated complex, with 2b displaying a higher affinity for Flag-NbAGO5 compared to that of HC-Pro (Fig. 6B). To investigate whether other VSRs could also degrade NbAGO5, we transiently expressed BaMV VSR, TGBp1 (Ke et al., 2022), and analyzed its effect on the NbAGO5 protein level. The results showed that NbAGO5 protein accumulation increased by 1.63-fold after transient expression of BaMV TGBp1 compared to that of the mock group, suggesting that BaMV TGBp1 might not function through degrading NbAGO5 protein (Supplementary Fig. 3A). These findings are consistent with a previous report that the expression of TGBp1 of BaMV is sufficient to enhance the NbAGO5 accumulation level (Ke et al., 2022). Furthermore, immunoprecipitation analysis did not detect any interaction between BaMV TGBp1 and NbAGO5 protein (Supplementary Fig. 3B). This observation highlights the unique NbAGO5-binding ability of 2b or HC-Pro. These findings demonstrated that 2b or HC-Pro interacted with NbAGO5 protein, suggesting that 2b and HC-Pro directly drive the degradation of NbAGO5 protein.
In plant cells, protein degradation is primarily governed by two pathways: the 26S proteasome and autophagy pathways. To investigate the degradation pathway of NbAGO5, inhibitors of 26S proteasome or autophagy pathway, MG132 or 3-MA, respectively, were employed for analysis. In N. benthamiana leaves with transient overexpression of CMV 2b, the NbAGO5 protein level significantly increased to 1.51- and 1.68-fold upon treatment with inhibitors MG132 or 3-MA compared to that of the control group (Fig. 6C). Likewise, in leaves with transient overexpression of TuMV HC-Pro, NbAGO5 protein accumulation in MG132 and 3-MA treated groups significantly increased to 1.48- and 1.62-fold of that of the control group (Fig. 6C). On the contrary, the treatment of inhibitors did not result in any significant increase in NbAGO5 protein in the mock group (Fig. 6C). These results indicated that treatment with MG132 or 3-MA could counteract the degradation of NbAGO5 caused by CMV 2b and TuMV HC-Pro, suggesting that 2b and HC-Pro could utilize both the 26S proteasome and autophagy pathways in cells to disrupt NbAGO5 in defense response against viruses and thereby enhance viral infection in plants.
Apart from the interference of NbAGO5 antiviral function through protein degradation pathways, the other possible counter-defense route is the inhibition of NbAGO5 mRNA accumulation level by the VSRs. It has been shown in our previous study that NbAGO5 mRNA expression actually increased significantly in CMV-infected plants (Ke et al., 2022). However, the influence of TuMV on NbAGO5 mRNA accumulation level has not been investigated. To test whether the accumulation level of NbAGO5 mRNA was also affected by TuMV infection, RT-qPCR was performed in TuMV-infected leaves. The results revealed that the NbAGO5 mRNA expression level was significantly upregulated by 7.5-fold during TuMV infection compared to the control group (Supplementary Fig. 4). Additionally, a remarkable 11.2-fold increase in the NbNAC42 gene expression level was observed following TuMV infection compared to the control group (Supplementary Fig. 4). These findings align with previous reports and demonstrate that upon viral invasion, plants rapidly activate defense mechanisms through the transcription factor NbNAC42, subsequently inducing an increase in NbAGO5 gene expression to combat the viral infection (Ke et al., 2022). Nonetheless, the increase in NbAGO5 mRNA accumulation level did not result in a higher level of NbAGO5 protein in TuMV-infected plants or the systemic leaves of CMV-infected plants (Fig. 1 and Fig. 5A). Based on the above observations, we hypothesize that the decrease in NbAGO5 protein accumulation following CMV or TuMV infection can be solely attributed to the degradation of the NbAGO5 protein through both the 26S proteasome and autophagy pathways.
4. Discussion
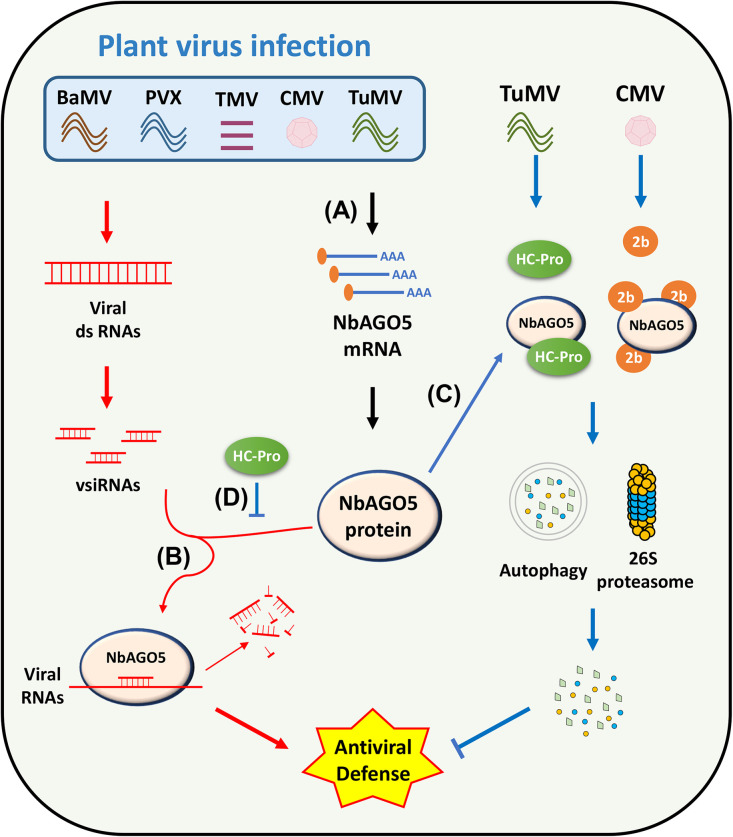
AGO proteins are central players in RNA silencing pathways, which are essential for gene regulation and defense against viruses in eukaryotes (Carbonell and Carrington, 2015; Fang and Qi, 2016; Ma and Zhang, 2018; Jin et al., 2021; Li et al., 2022). Our current research highlights the crucial role of NbAGO5 in the RNA silencing mechanism and its involvement in the complex interactions between plants and various viruses. As illustrated in Fig. 7, the results in this study revealed that the mRNA accumulation level of NbAGO5 is generally upregulated in response to the infections of various viruses (Fig. 7, step A), signifying the important role of NbAGO5 gene in the fundamental antiviral defense mechanism(s) of N. benthamian. The translated NbAGO5 protein then exerts its antiviral activity by binding to vsiRNAs and directing the degradation of the target complementary viral RNA (Fig. 7, step B). For counter-defense against NbAGO5-mediated antiviral mechanism, specific viruses have evolved different VSRs. In this study, the results revealed that CMV 2b and TuMV HC-Pro may interact with NbAGO5 (Fig. 6B), leading to degradation of NbAGO5 through the 26S proteasome and autophagy pathways (Fig. 6C), as depicted in Fig. 7, step C. In addition, TuMV HC-Pro was shown to contribute a novel route for counter-defense by interfering with the loading of the vsiRNAs into the NbAGO5-associated complexes (Fig. 4E), thereby inhibiting the antiviral activity of NbAGO5 (Fig. 7, step D). Collectively, the findings in this study provide further insights into the complicated interplay between NbAGO5 and various plant viruses.
Fig. 7.
Schematic representation of the proposed mechanisms for the antiviral defense of NbAGO5 and the counter-defense of the VSRs of specific viruses. Upon virus infection, NbAGO5 expression is induced in response to diverse viral factors, such as the TGBp1 protein of BaMV (A). NbAGO5 plays a critical role in antiviral RNA silencing by associating with vsiRNAs and targeting complementary viral RNAs for degradation (B). In counter-defense, specific viruses such as CMV and TuMV produce 2b and HC-Pro proteins, respectively, as viral suppressors of RNA silencing (VSRs). These VSRs may interact with NbAGO5, causing the degradation of NbAGO5 through cellular protein degradation pathways, including 26S proteasome and the autophagy system, thereby subverting the antiviral RNA silencing mechanism of the host plants (C). In addition, TuMV HC-Pro may also prevent the loading of vsiRNAs into the NbAGO5-associated complexes and the degradation of viral RNAs, providing another line of counter-defense against the RNA silencing mechanism (D).
4.1. Functional diversification of AGO5 in regulating plant physiology across species
AGO proteins display functional diversification across different species, regulating various biological processes based on their unique biochemical properties, such as binding affinities for sRNAs, and interactions with other proteins (Fang and Qi, 2016; Silva-Martins et al., 2020). AGO5 proteins are no exception in this regard, exhibiting diverse physiological functions in different organisms. For instance, in A. thaliana, AtAGO5 is highly expressed in the shoot apical meristem, floral organs, and seeds, influencing mega-gametogenesis and pollen development (Borges et al., 2011; Tucker et al., 2012). AtAGO5 is also involved in regulating the transcription factors squamosa-promoter binding protein-like (SPL) genes by binding with miR156, thereby controlling the flowering time of A. thaliana (Roussin-Léveillée et al., 2020). In rice (Oryza sativa), OsAGO5, also known as MEIOSIS ARRESTED AT LEPTOTENE1 (OsMEL1), plays a crucial role in the pre-meiotic stage of sporophytic germ cell formation (Nonomura et al., 2007). It operates by binding with specific phased secondary small interfering RNAs (phasiRNAs) in the cytoplasm, eliminating unnecessary or harmful specific mRNAs during meiosis and spore development (Komiya et al., 2014; Zhang et al., 2020; Lian et al., 2021; Tamotsu et al., 2023). The OsMEL1/ phasiRNAs complex may also regulate downstream transcription-related genes, leading to large-scale meiotic chromosome reprogramming, and promoting normal meiotic division (Liu and Nonomura, 2016; Zhang et al., 2020). In soybean (Glycine max), GmAGO5 influences seed pigment accumulation by regulating the expression of the chalcone synthase (CHS) gene (Cho et al., 2017). Moreover, AGO5 in legumes has been implicated in the establishment of symbiotic relationships with nitrogen-fixing rhizobia (Reyero-Saavedra et al., 2017; Sánchez-Correa et al., 2022). Although our previous study found that endogenous NbAGO5 is mainly expressed in seeds and flowers (Ke et al., 2022), we did not identify significant phenotypic differences between transgenic plants overexpressing or lacking NbAGO5 and wild-type plants in the current study (Fig. 2E). The observation suggests that, apart from the role in antiviral defense, NbAGO5 protein may participate in the regulation of other physiological functions involving other redundant or compensating components, and that AGO5 proteins in different organisms may exhibit varying binding affinities to different miRNAs. The distinct effects of AGO5 proteins on plant physiology regulation in different species also highlight the complexity and species-specific nature of AGO5 function. Further research should focus on elucidating the molecular mechanisms of the interaction between miRNAs and NbAGO5 to better understand its roles in sRNA-mediated gene silencing and antiviral RNA silencing mechanism.
4.2. The induction of AGO5 gene expression as a common response to viral infections in plants
The antiviral gene silencing pathway in plants relies on AGO family proteins as a key defense mechanism against various viral invasions. Our previous research revealed that infections by BaMV, foxtail mosaic virus (FoMV), PVX, CymMV, TMV, and CMV in N. benthamiana plants stimulate the expression of NbNAC42 transcription factors, which subsequently bind to the NbAGO5 gene promoter and upregulate NbAGO5 mRNA expression (Ke et al., 2022). This upregulation leads to the increase in accumulation levels of NbAGO5 protein, which is demonstrated in this study for many of these viruses (Fig. 1). In addition to the aforementioned viruses, we found that TuMV infection also elevates the expression levels of both NbNAC42 and NbAGO5 genes, suggesting a broad-spectrum response of NbAGO5 to viral infections (Supplementary Fig. 4). This notion is further strengthened by recent findings showing the upregulation of NbAGO5 expression in response to grapevine pinot gris virus (GPGV) infection (Tarquini et al., 2021, 2023).
Apart from N. benthamiana, research in other plant species has also demonstrated that viral infections can trigger increased AGO5 gene expression. For instance, in P. aphrodite, PaAGO5b expression is significantly induced after CymMV and ORSV infections, regulated by MYB (myeloblastosis-related) transcription factors (Kuo et al., 2021; Kasi Viswanath et al., 2022, 2023). In A. thaliana, AtAGO5 expression is elevated in systemic leaves upon PVX or PlAMV infection, with a consequence of a decrease in jasmonic acid (JA)-related signaling (Brosseau and Moffett, 2015; Silva-Martins et al., 2023). In Citrullus lanatus, cucumber green mottle mosaic virus (CGMMV) infection highly induces the expression level of ClAGO5, and ethylene (ETH) may regulate the expression of ClAGO5 through ethylene response factor (ERF) (Liu et al., 2023a). Collectively, these findings suggest that AGO5 gene expression induction is a common defense response to diverse viral infections in plants, including N. benthamiana, P. aphrodite, A. thaliana, and C. lanatus. AGO5 upregulation is mediated by a variety of transcription factors, such as NbNAC42 in N. benthamiana, MYB transcription factors in P. aphrodite, and ERF in C. lanatus, and can be influenced by negative regulation via JA-related mechanisms in A. thaliana. These results highlight the intricate and diverse mechanisms by which plants regulate AGO5 expression, providing deeper insights into the plant-induced gene silencing pathways for antiviral defense.
4.3. Diverse mechanisms and functions of antiviral AGOs in plant defense
Antiviral AGOs loaded with vsiRNAs typically recognize and suppress the functions of viral RNA or DNA that matches their programmed sequence. Upon forming complexes with vsiRNAs, AGOs assemble into antiviral RISCs that target complementary viral RNAs, leading to their degradation or translational arrest (Szittya and Burgyán, 2013; Carbonell and Carrington, 2015; Di Serio et al., 2022). Interestingly, some AGOs can adopt alternative strategies to combat or assist viruses. For example, in O. sativa, OsAGO18 does not directly bind to vsiRNA but competes with OsAGO1 for association with miR168. This competition increases OsAGO1 protein levels that are essential for the antiviral RNA silencing response in rice (Wu et al., 2015). Additionally, OsAGO18 sequesters miR528, indirectly elevating reactive oxygen species production and enhancing virus resistance by increasing L-ascorbate oxidase expression (Wu et al., 2017). In our study, we found that NbAGO5 overexpression in N. benthamiana plants conferred resistance to BaMV, PVX, TMV, and CMV, while NbAGO5 knockout plants displayed increased susceptibility to these viruses (Fig. 3). We also observed that the purified Flag-NbAGO5 complex contained virus-derived vsiRNAs, suggesting that NbAGO5 exerts its antiviral effects by binding to vsiRNAs (Fig. 4), leading to the decrease in viral RNA accumulations. This finding aligns with previous research demonstrating that HA-AtAGO5 binds to PVX-derived vsiRNAs, thereby enhancing resistance against PVX (Brosseau and Moffett, 2015). However, whether native NbAGO5 binds to vsiRNAs requires further corroboration.
4.4. The role of NbAGO5 in antiviral defense in N. benthamiana
Although transcriptional analysis of AGO gene expression in N. benthamiana revealed that AGO5 expression levels were comparatively lower than other AGOs in various tissues under both unchallenged and stressed conditions (Nakasugi et al., 2013), it was found that NbAGO5 mRNA levels increased in NbAGO1a-silenced plants. This implies that NbAGO5 may act as a supportive defense mechanism to compensate for the loss of antiviral function when NbAGO1a is absent (Huang et al., 2019). Under normal conditions, AGO1 inhibits AGO2 mRNA accumulation by targeting it with miR403. However, when AGO1 is suppressed by VSRs, plants activate a second defense pathway in which AGO2 expression increases to suppress the virus since AGO1 is no longer able to inhibit its accumulation (Harvey et al., 2011; Diao et al., 2019). Our study revealed that the expression levels of NbAGO1a, NbAGO1b, and NbAGO2 were not significantly altered in the absence of NbAGO5 (Supplementary Fig. 2). This finding suggests that when NbAGO5 is blocked, the antiviral capabilities of the primary defense components NbAGO1a, NbAGO1b, and NbAGO2 maintain adequate function to counteract viral infections.
These results demonstrate that NbAGO5 provides an additional line of defense in the antiviral mechanism of N. benthamiana. Even when AGO1 is present, AGO5 is highly induced to strengthen the defense against a broad range of viruses. This underlines the significance of NbAGO5 as a crucial player in the plant's antiviral defense system, ensuring that an alternative defense pathway remains active to protect the plant against viral invasions.
4.5. Plant viruses develop VSRs and utilize 26S proteasome and autophagy pathways to counteract RNA silencing defense
Plant viruses have evolved diverse strategies to counteract the antiviral RNA silencing response of the hosts. These strategies include the development of VSRs that interfere with the RNA silencing pathway at different stages, such as inhibiting vsiRNA biogenesis and blocking RISCs silencing functions (Giner et al., 2010; Várallyay and Havelda, 2013; Yang and Li, 2018; Jin et al., 2021; Sanobar et al., 2021; Liu et al., 2023b; Sehki et al., 2023). In this study, we found that NbAGO5 displays diverse affinities for vsiRNAs originating from different viruses (Fig. 4), possibly due to the varying abilities of these viruses to counteract the antiviral silencing.
The 26S proteasome and autophagy pathways are typically involved in plant defense mechanisms against viruses, functioning by degrading viral proteins (Verchot, 2016; Li et al., 2020; Yang et al., 2020; Lobaina et al., 2022; Yang and Liu, 2022). However, some viruses have evolved to exploit these host mechanisms to degrade proteins involved in the RNA silencing pathway. For example, P0 proteins of viruses in the genera Polerovirus and Enamovirus may target and degrade AGO1 through the autophagy pathway (Derrien et al., 2012; Fusaro et al., 2012; Michaeli et al., 2019; Barrios Barón et al., 2021), while PVX P25 destabilizes AGO1 via the 26S proteasome pathway (Chiu et al., 2010). TuMV P1/HC-Pro degrades AGO1 through autophagy (Hu et al., 2020; Hong et al., 2023), and TuMV VPg interacts with, and induces the degradation of SGS3 through both proteasome and autophagy pathways (Cheng and Wang, 2017). Additionally, CMV-induced small peptide 1 inhibits antiviral RNA silencing defense by promoting autophagic degradation of SGS3 and RDR6 bodies (Tong et al., 2021).
In A. thaliana, autophagy is induced during CMV and TuMV infection, promoting the turnover of RNA silencing suppressor 2b protein and HC-Pro protein (Hafrén et al., 2018; Shukla et al., 2022). Our findings reveal that the CMV 2b and TuMV HC-Pro protein can mediate the degradation of NbAGO5, possibly by delivering it to either the proteasome or autophagy degradation pathway (Fig. 6), thus disrupting its proper function and producing antagonistic effects. Although proteasome and autophagosome are involved in plant defense against viruses by degrading viral proteins, they can also be exploited by viruses, acting as a double-edged sword in such situations (Clavel et al., 2017; Yang et al., 2019). Our results emphasize the importance of NbAGO5 for antiviral defense in N. benthamiana, and its degradation by viral proteins can significantly compromise the ability of plants to resist against viral infection.
Understanding the molecular mechanisms of AGO5-mediated antiviral defense may also have practical implications for the development of virus-resistant crops. Genetic manipulation of AGO5 expression or the introduction of AGO5 genes from virus-resistant plant species into susceptible crops could potentially enhance their resistance to viral infections. Furthermore, the identification of viral suppressors of AGO5 function or the characterization of AGO5-interacting proteins could provide novel targets for the development of antiviral strategies based on the disruption of viral-host interactions or the enhancement of AGO5-mediated antiviral defense.
Funding
This work was funded by the National Science and Technology Council, Taiwan (NSTC- 111-2313-B-005-051 and NSTC- 110-2313-B-005-021-MY3), and the Advanced Plant Biotechnology Center from the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE).
CRediT authorship contribution statement
Chin-Wei Tu: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft. Ying-Wen Huang: Conceptualization, Methodology, Validation, Writing – review & editing. Chin-Wei Lee: Resources, Validation. Song-Yi Kuo: Methodology, Resources. Na-Sheng Lin: Conceptualization, Writing – review & editing. Yau-Heiu Hsu: Conceptualization, Writing – review & editing, Supervision, Funding acquisition. Chung-Chi Hu: Conceptualization, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to express our sincere gratitude to Dr. Shih-Shun Lin from the Institute of Biotechnology, National Taiwan University, for kindly providing us with the TuMV YC5 strain as a gift. We also extend our thanks to Dr. Ching-Hsiu Tsai's lab from the Graduate Institute of Biotechnology, National Chung Hsing University, for providing us with the experimental materials and the experimental environment for handling radioactive substances.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.virusres.2023.199179.
Appendix. Supplementary materials
Data availability
No data was used for the research described in the article.
References
- Alazem M., He M.H., Moffett P., Lin N.S. Abscisic acid induces resistance against bamboo mosaic virus through argonaute2 and 3. Plant Physiol. 2017;174:339–355. doi: 10.1104/pp.16.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annacondia M.L., Martinez G. Reprogramming of RNA silencing triggered by cucumber mosaic virus infection in Arabidopsis. Genome Biol. 2021;22:340. doi: 10.1186/s13059-021-02564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios Barón M.P., Delfosse V.C., Agrofoglio Y.C., Nahirñak V., Almasia N.I., Vazquez Rovere C., Distéfano A.J. Argentinian potato leafroll virus P0 protein: novel activities for a previously known suppressor. Plant Pathol. 2021;70:259–274. [Google Scholar]
- Bhattacharya D., Van Meir E.G. A simple genotyping method to detect small CRISPR-Cas9 induced indels by agarose gel electrophoresis. Sci. Rep. 2019;9:4437. doi: 10.1038/s41598-019-39950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges F., Pereira P.A., Slotkin R.K., Martienssen R.A., Becker J.D. MicroRNA activity in the Arabidopsis male germline. J. Exp. Bot. 2011;62:1611–1620. doi: 10.1093/jxb/erq452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosseau C., Bolaji A., Roussin-Léveillée C., Zhao Z., Biga S., Moffett P. Natural variation in the Arabidopsis AGO2 gene is associated with susceptibility to potato virus X. New Phytol. 2020;226:866–878. doi: 10.1111/nph.16397. [DOI] [PubMed] [Google Scholar]
- Brosseau C., Moffett P. Functional and genetic analysis identify a role for Arabidopsis ARGONAUTE5 in antiviral RNA silencing. Plant Cell. 2015;27:1742–1754. doi: 10.1105/tpc.15.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell A., Carrington J.C. Antiviral roles of plant ARGONAUTES. Curr. Opin. Plant Biol. 2015;27:111–117. doi: 10.1016/j.pbi.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell A., Fahlgren N., Garcia-Ruiz H., Gilbert K.B., Montgomery T.A., Nguyen T., Cuperus J.T., Carrington J.C. Functional analysis of three Arabidopsis ARGONAUTES using slicer-defective mutants. Plant Cell. 2012;24:3613–3629. doi: 10.1105/tpc.112.099945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr J.P., Murphy A.M., Tungadi T., Yoon J.Y. Plant defense signals: players and pawns in plant-virus-vector interactions. Plant Sci. 2019;279:87–95. doi: 10.1016/j.plantsci.2018.04.011. [DOI] [PubMed] [Google Scholar]
- Cheng X., Wang A. The potyvirus silencing suppressor protein VPg mediates degradation of SGS3 via ubiquitination and autophagy pathways. J. Virol. 2017:91. doi: 10.1128/JVI.01478-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu M.H., Chen I.H., Baulcombe D.C., Tsai C.H. The silencing suppressor P25 of potato virus X interacts with Argonaute1 and mediates its degradation through the proteasome pathway. Mol. Plant Pathol. 2010;11:641–649. doi: 10.1111/j.1364-3703.2010.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y.B., Jones S.I., Vodkin L.O. Mutations in argonaute5 illuminate epistatic interactions of the K1 and I loci leading to saddle seed color patterns in glycine max. Plant Cell. 2017;29:708–725. doi: 10.1105/tpc.17.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel M., Michaeli S., Genschik P. Autophagy: a double-edged sword to fight plant viruses. Trends Plant Sci. 2017;22:646–648. doi: 10.1016/j.tplants.2017.06.007. [DOI] [PubMed] [Google Scholar]
- Csorba T., Kontra L., Burgyán J. viral silencing suppressors: tools forged to fine-tune host-pathogen coexistence. VirologyVirology. 2015;479-480:85–103. doi: 10.1016/j.virol.2015.02.028. [DOI] [PubMed] [Google Scholar]
- Deng Z., Ma L., Zhang P., Zhu H. Small RNAs participate in plant-virus interaction and their application in plant viral defense. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23020696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien B., Baumberger N., Schepetilnikov M., Viotti C., De Cillia J., Ziegler-Graff V., Isono E., Schumacher K., Genschik P. Degradation of the antiviral component ARGONAUTE1 by the autophagy pathway. Proc. Natl. Acad. Sci. U. S. A. 2012;109:15942–15946. doi: 10.1073/pnas.1209487109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Serio F., Owens R.A., Navarro B., Serra P., Martínez De Alba Ñ., .E.., Delgado S., Carbonell A., Gago-Zachert S. Role of RNA silencing in plant-viroid interactions and in viroid pathogenesis. Virus Res. 2022;323 doi: 10.1016/j.virusres.2022.198964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao P., Zhang Q., Sun H., Ma W., Cao A., Yu R., Wang J., Niu Y., Wuriyanghan H. miR403a and SA are involved in NbAGO2 mediated antiviral defenses against TMV infection in nicotiana benthamiana. Genes (Basel) 2019:10. doi: 10.3390/genes10070526. Basel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Pendon J.A., Li F., Li W.X., Ding S.W. Suppression of antiviral silencing by cucumber mosaic virus 2b protein in Arabidopsis is associated with drastically reduced accumulation of three classes of viral small interfering RNAs. Plant Cell. 2007;19:2053–2063. doi: 10.1105/tpc.106.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S.W. Transgene silencing, RNA interference, and the antiviral defense mechanism directed by small interfering RNAs. Phytopathology. 2023 doi: 10.1094/PHYTO-10-22-0358-IA. [DOI] [PubMed] [Google Scholar]
- Dzianott A., Sztuba-Solińska J., Bujarski J.J. Mutations in the antiviral RNAi defense pathway modify Brome mosaic virus RNA recombinant profiles. Mol. Plant Microbe Interact. 2012;25:97–106. doi: 10.1094/MPMI-05-11-0137. [DOI] [PubMed] [Google Scholar]
- Fang X., Qi Y. RNAi in plants: an argonaute-centered view. Plant Cell. 2016;28:272–285. doi: 10.1105/tpc.15.00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusaro A.F., Correa R.L., Nakasugi K., Jackson C., Kawchuk L., Vaslin M.F., Waterhouse P.M. The Enamovirus P0 protein is a silencing suppressor which inhibits local and systemic RNA silencing through AGO1 degradation. VirologyVirology. 2012;426:178–187. doi: 10.1016/j.virol.2012.01.026. [DOI] [PubMed] [Google Scholar]
- Garcia-Ruiz H., Carbonell A., Hoyer J.S., Fahlgren N., Gilbert K.B., Takeda A., Giampetruzzi A., Garcia Ruiz M.T., Mcginn M.G., Lowery N., Martinez Baladejo M.T., Carrington J.C. Roles and programming of Arabidopsis ARGONAUTE proteins during Turnip mosaic virus infection. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1004755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal B., Sanfaçon H. Temperature-dependent symptom recovery in Nicotiana benthamiana plants infected with tomato ringspot virus is associated with reduced translation of viral RNA2 and requires ARGONAUTE 1. Virology. 2014;456-457:188–197. doi: 10.1016/j.virol.2014.03.026. [DOI] [PubMed] [Google Scholar]
- Giner A., Lakatos L., García-Chapa M., López-Moya J.J., Burgyán J. Viral protein inhibits RISC activity by argonaute binding through conserved WG/GW motifs. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q., Wang Y., Jin Z., Hong Y., Liu Y. Transcriptional and post-transcriptional regulation of RNAi-related gene expression during plant-virus interactions. Stress Biol. 2022;2:33. doi: 10.1007/s44154-022-00057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodin M.M., Zaitlin D., Naidu R.A., Lommel S.A. Nicotiana benthamiana: its history and future as a model for plant-pathogen interactions. Mol. Plant Microbe Interact. 2015;2015:28–39. doi: 10.1094/MPMI-00-00-1015-REV.testissue. [DOI] [PubMed] [Google Scholar]
- Guo Z., Li Y., Ding S.W. Small RNA-based antimicrobial immunity. Nat. Rev. Immunol. 2019;19:31–44. doi: 10.1038/s41577-018-0071-x. [DOI] [PubMed] [Google Scholar]
- Gursinsky T., Pirovano W., Gambino G., Friedrich S., Behrens S.E., Pantaleo V. Homeologs of the nicotiana benthamiana antiviral ARGONAUTE1 show different susceptibilities to microRNA168-mediated control. Plant Physiol. 2015;168:938–952. doi: 10.1104/pp.15.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafrén A., üstün S., Hochmuth A., Svenning S., Johansen T., Hofius D. Turnip mosaic virus counteracts selective autophagy of the viral silencing suppressor HCpro. Plant Physiol. 2018;176:649–662. doi: 10.1104/pp.17.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J.J., Lewsey M.G., Patel K., Westwood J., Heimstädt S., Carr J.P., Baulcombe D.C. An antiviral defense role of AGO2 in plants. PLoS ONE. 2011;6:e14639. doi: 10.1371/journal.pone.0014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S.F., Fang R.Y., Wei W.L., Jirawitchalert S., Pan Z.J., Hung Y.L., Pham T.H., Chiu Y.H., Shen T.L., Huang C.K., Lin S.S. Development of an assay system for the analysis of host RISC activity in the presence of a potyvirus RNA silencing suppressor, HC-Pro. Virol J. 2023;20:10. doi: 10.1186/s12985-022-01956-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch R.B., Fry J.E., Hoffmann N.L., Wallroth M., Eichholtz D., Rogers S.G., Fraley R.T. A simple and general method for transferring genes into plants. ScienceScience. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Hu S.F., Wei W.L., Hong S.F., Fang R.Y., Wu H.Y., Lin P.C., Sanobar N., Wang H.P., Sulistio M., Wu C.T., Lo H.F., Lin S.S. Investigation of the effects of P1 on HC-pro-mediated gene silencing suppression through genetics and omics approaches. Bot. Stud. 2020;61:22. doi: 10.1186/s40529-020-00299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.W., Hu C.C., Tsai C.H., Lin N.S., Hsu Y.H. Nicotiana benthamiana Argonaute10 plays a pro-viral role in Bamboo mosaic virus infection. New Phytol. 2019;224:804–817. doi: 10.1111/nph.16048. [DOI] [PubMed] [Google Scholar]
- Huang Y.W., Sun C.I., Hu C.C., Tsai C.H., Meng M., Lin N.S., Dinesh-Kumar S.P., Hsu Y.H. A viral movement protein co-opts endoplasmic reticulum luminal-binding protein and calreticulin to promote intracellular movement. Plant Physiol. 2023;191:904–924. doi: 10.1093/plphys/kiac547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M.C., Hu C.C., Hsu W.L., Hsu T.L., Lin N.S., Hsu Y.H. Fusion of a novel native signal peptide enhanced the secretion and solubility of bioactive human interferon gamma glycoproteins in nicotiana Benthamiana using the bamboo mosaic virus-based expression system. Front. Plant Sci. 2020;11 doi: 10.3389/fpls.2020.594758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L., Chen M., Xiang M., Guo Z. RNAi-based antiviral innate immunity in plants. VirusesViruses. 2022;14:432. doi: 10.3390/v14020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Zhao J.H., Guo H.S. Recent advances in understanding plant antiviral RNAi and viral suppressors of RNAi. Curr. Opin. Virol. 2021;46:65–72. doi: 10.1016/j.coviro.2020.12.001. [DOI] [PubMed] [Google Scholar]
- Kørner C.J., Pitzalis N., Peña E.J., Erhardt M., Vazquez F., Heinlein M. Crosstalk between PTGS and TGS pathways in natural antiviral immunity and disease recovery. Nat. Plants. 2018;4:157–164. doi: 10.1038/s41477-018-0117-x. [DOI] [PubMed] [Google Scholar]
- Kasi Viswanath K., Kuo S.-Y., Huang Y.-W., Tsao N.-W., Hu C.-C., Lin N.-S., Wang S.-Y., Hsu Y.-H. Characterization of virus-inducible orchid argonaute 5b promoter and its functional characterization in nicotiana benthamiana during virus infection. Int. J. Mol. Sci. 2022;23:9825. doi: 10.3390/ijms23179825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasi Viswanath K., Kuo S.Y., Tu C.W., Hsu Y.H., Huang Y.W., Hu C.C. The role of plant transcription factors in the fight against plant viruses. Int. J. Mol. Sci. 2023;24:8433. doi: 10.3390/ijms24098433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y.D., Huang Y.W., Viswanath K.K., Hu C.C., Yeh C.M., Mitsuda N., Lin N.S., Hsu Y.H. NbNAC42 and NbZFP3 transcription factors regulate the virus inducible NbAGO5 promoter in Nicotiana benthamiana. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.924482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenesi E., Lopez-Moya J.J., Orosz L., Burgyán J., Lakatos L. Argonaute 2 controls antiviral activity against sweet potato mild mottle virus in nicotiana benthamiana. Plants. 2021:10. doi: 10.3390/plants10050867. Basel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya R., Ohyanagi H., Niihama M., Watanabe T., Nakano M., Kurata N., Nonomura K. Rice germline-specific Argonaute MEL1 protein binds to phasiRNAs generated from more than 700 lincRNAs. Plant J. 2014;78:385–397. doi: 10.1111/tpj.12483. [DOI] [PubMed] [Google Scholar]
- Kuo S.Y., Hu C.C., Huang Y.W., Lee C.W., Luo M.J., Tu C.W., Lee S.C., Lin N.S., Hsu Y.H. Argonaute 5 family proteins play crucial roles in the defence against Cymbidium mosaic virus and Odontoglossum ringspot virus in Phalaenopsis aphrodite subsp. formosana. Mol. Plant Pathol. 2021;22:627–643. doi: 10.1111/mpp.13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Wang A. RNA-targeted antiviral immunity: more than just RNA silencing. Trends Microbiol. 2019;27:792–805. doi: 10.1016/j.tim.2019.05.007. [DOI] [PubMed] [Google Scholar]
- Li F., Zhang C., Tang Z., Zhang L., Dai Z., Lyu S., Li Y., Hou X., Bernards M., Wang A. A plant RNA virus activates selective autophagy in a UPR-dependent manner to promote virus infection. New Phytol. 2020;228:622–639. doi: 10.1111/nph.16716. [DOI] [PubMed] [Google Scholar]
- Li Z., Li W., Guo M., Liu S., Liu L., Yu Y., Mo B., Chen X., Gao L. Origin, evolution and diversification of plant ARGONAUTE proteins. Plant J. 2022;109:1086–1097. doi: 10.1111/tpj.15615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J.P., Yang Y.W., He R.R., Yang L., Zhou Y.F., Lei M.Q., Zhang Z., Huang J.H., Cheng Y., Liu Y.W., Zhang Y.C., Chen Y.Q. Ubiquitin-dependent Argonauteprotein MEL1 degradation is essential for rice sporogenesis and phasiRNA target regulation. Plant Cell. 2021;33:2685–2700. doi: 10.1093/plcell/koab138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou M.R., Huang Y.W., Hu C.C., Lin N.S., Hsu Y.H. A dual gene-silencing vector system for monocot and dicot plants. Plant Biotechnol. J. 2014;12:330–343. doi: 10.1111/pbi.12140. [DOI] [PubMed] [Google Scholar]
- Liu H., Nonomura K.I. A wide reprogramming of histone H3 modifications during male meiosis I in rice is dependent on the Argonaute protein MEL1. J. Cell Sci. 2016;129:3553–3561. doi: 10.1242/jcs.184937. [DOI] [PubMed] [Google Scholar]
- Liu M., Wu H., Hong N., Kang B., Peng B., Liu L., Gu Q. Argonaute 1 and 5 proteins play crucial roles in the defence against cucumber green mottle mosaic virus in watermelon. Mol. Plant Pathol. 2023 doi: 10.1111/mpp.13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Chen M., Li R., Li W.X., Gal-On A., Jia Z., Ding S.W. Identification of positive and negative regulators of antiviral RNA interference in Arabidopsis thaliana. Nat. Commun. 2022;13:2994. doi: 10.1038/s41467-022-30771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Han Y., Li W.X., Ding S.W. Infection defects of RNA and DNA viruses induced by antiviral RNA interference. Microbiol. Mol. Biol. Rev. 2023 doi: 10.1128/mmbr.00035-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobaina D.P., Tarazi R., Castorino T., Vaslin M.F.S. The ubiquitin-proteasome system (UPS) and viral infection in plants. Plants (Basel) 2022;11:2476. doi: 10.3390/plants11192476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gomollon S., Baulcombe D.C. Roles of RNA silencing in viral and non-viral plant immunity and in the crosstalk between disease resistance systems. Nat. Rev. Mol. Cell Biol. 2022;23:645–662. doi: 10.1038/s41580-022-00496-5. [DOI] [PubMed] [Google Scholar]
- Ludman M., Burgyán J., Fátyol K. Crispr/Cas9 mediated inactivation of argonaute 2 reveals its differential involvement in antiviral responses. Sci. Rep. 2017;7:1010. doi: 10.1038/s41598-017-01050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludman M., Fátyol K. Targeted inactivation of the AGO1 homeologues of Nicotiana benthamiana reveals their distinct roles in development and antiviral defence. New Phytol. 2021;229:1289–1297. doi: 10.1111/nph.16992. [DOI] [PubMed] [Google Scholar]
- Ma X., Nicole M.C., Meteignier L.V., Hong N., Wang G., Moffett P. Different roles for RNA silencing and RNA processing components in virus recovery and virus-induced gene silencing in plants. J. Exp. Bot. 2015;66:919–932. doi: 10.1093/jxb/eru447. [DOI] [PubMed] [Google Scholar]
- Ma Z., Zhang X. Actions of plant Argonautes: predictable or unpredictable? Curr. Opin. Plant Biol. 2018;45:59–67. doi: 10.1016/j.pbi.2018.05.007. [DOI] [PubMed] [Google Scholar]
- Martín-Merchán A., Moro B., Bouet A., Bologna N.G. Domain organization, expression, subcellular localization, and biological roles of ARGONAUTE proteins in Arabidopsis. J. Exp. Bot. 2023;74:2374–2388. doi: 10.1093/jxb/erad030. [DOI] [PubMed] [Google Scholar]
- Michaeli S., Clavel M., Lechner E., Viotti C., Wu J., Dubois M., Hacquard T., Derrien B., Izquierdo E., Lecorbeiller M., Bouteiller N., De Cilia J., Ziegler-Graff V., Vaucheret H., Galili G., Genschik P. The viral F-box protein P0 induces an ER-derived autophagy degradation pathway for the clearance of membrane-bound AGO1. Proc. Natl. Acad. Sci. U. S. A. 2019;116:22872–22883. doi: 10.1073/pnas.1912222116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y., Hino K., Bono H., Ui-Tei K. CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics. 2015;31:1120–1123. doi: 10.1093/bioinformatics/btu743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakasugi K., Crowhurst R.N., Bally J., Wood C.C., Hellens R.P., Waterhouse P.M. De novo transcriptome sequence assembly and analysis of RNA silencing genes of Nicotiana benthamiana. PLoS ONE. 2013;8:e59534. doi: 10.1371/journal.pone.0059534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonomura K., Morohoshi A., Nakano M., Eiguchi M., Miyao A., Hirochika H., Kurata N. A germ cell specific gene of the ARGONAUTE family is essential for the progression of premeiotic mitosis and meiosis during sporogenesis in rice. Plant Cell. 2007;19:2583–2594. doi: 10.1105/tpc.107.053199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odokonyero D., Mendoza M.R., Alvarado V.Y., Zhang J., Wang X., Scholthof H.B. Transgenic down-regulation of ARGONAUTE2 expression in Nicotiana benthamiana interferes with several layers of antiviral defenses. Virology. 2015;486:209–218. doi: 10.1016/j.virol.2015.09.008. [DOI] [PubMed] [Google Scholar]
- Pérez-Cañamás M., Hevia E., Katsarou K., Hernández C. Genetic evidence for the involvement of Dicer-like 2 and 4 as well as Argonaute 2 in the Nicotiana benthamiana response against Pelargonium line pattern virus. J. Gen. Virol. 2021;102 doi: 10.1099/jgv.0.001656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudel D.B., Ghoshal B., Jossey S., Ludman M., Fatyol K., Sanfaçon H. Expression and antiviral function of ARGONAUTE 2 in Nicotiana benthamiana plants infected with two isolates of tomato ringspot virus with varying degrees of virulence. Virology. 2018;524:127–139. doi: 10.1016/j.virol.2018.08.016. [DOI] [PubMed] [Google Scholar]
- Prasanth K.R., Huang Y.W., Liou M.R., Wang R.Y., Hu C.C., Tsai C.H., Meng M., Lin N.S., Hsu Y.H. Glyceraldehyde 3-phosphate dehydrogenase negatively regulates the replication of Bamboo mosaic virus and its associated satellite RNA. J. Virol. 2011;85:8829–8840. doi: 10.1128/JVI.00556-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin N., Voinnet O. RNA silencing suppression by plant pathogens: defence, counter-defence and counter-counter-defence. Nat. Rev. Microbiol. 2013;11:745–760. doi: 10.1038/nrmicro3120. [DOI] [PubMed] [Google Scholar]
- Qu F., Ye X., Morris T.J. Arabidopsis DRB4, AGO1, AGO7, and RDR6 participate in a DCL4-initiated antiviral RNA silencing pathway negatively regulated by DCL1. Proc. Natl. Acad. Sci. U. S. A. 2008;105:14732–14737. doi: 10.1073/pnas.0805760105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyero-Saavedra M.D.R., Qiao Z., Sánchez-Correa M.D.S., Díaz-Pineda M.E., Reyes J.L., Covarrubias A.A., Libault M., Valdés-López O. Gene silencing of argonaute5 negatively affects the establishment of the legume-rhizobia symbiosis. Genes (Basel) 2017:8. doi: 10.3390/genes8120352. Basel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussin-Léveillée C., Silva-Martins G., Moffett P. ARGONAUTE5 represses age-dependent induction of flowering through physical and functional interaction with miR156 in Arabidopsis. Plant Cell Physiol. 2020;61:957–966. doi: 10.1093/pcp/pcaa022. [DOI] [PubMed] [Google Scholar]
- Sanobar N., Lin P.C., Pan Z.J., Fang R.Y., Tjita V., Chen F.F., Wang H.C., Tsai H.L., Wu S.H., Shen T.L., Chen Y.H., Lin S.S. Investigating the viral suppressor HC-pro inhibiting small RNA methylation through functional comparison of HEN1 in angiosperm and bryophyte. Viruses. 2021;13 doi: 10.3390/v13091837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholthof H.B., Alvarado V.Y., Vega-Arreguin J.C., Ciomperlik J., Odokonyero D., Brosseau C., Jaubert M., Zamora A., Moffett P. Identification of an ARGONAUTE for antiviral RNA silencing in Nicotiana benthamiana. Plant Physiol. 2011;156:1548–1555. doi: 10.1104/pp.111.178764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehki H., Yu A., Elmayan T., Vaucheret H. TYMV and TRV infect Arabidopsis thaliana by expressing weak suppressors of RNA silencing and inducing host RNASE THREE LIKE1. PLoS Pathog. 2023;19 doi: 10.1371/journal.ppat.1010482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla A., Hoffmann G., Kushwaha N.K., López-González S., Hofius D., Hafrén A. Salicylic acid and the viral virulence factor 2b regulate the divergent roles of autophagy during cucumber mosaic virus infection. Autophagy. 2022;18:1450–1462. doi: 10.1080/15548627.2021.1987674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Martins G., Bolaji A., Moffett P. What does it take to be antiviral? An Argonaute-centered perspective on plant antiviral defense. J. Exp. Bot. 2020;71:6197–6210. doi: 10.1093/jxb/eraa377. [DOI] [PubMed] [Google Scholar]
- Silva-Martins G., Roussin-Léveillée C., Bolaji A., Pillay Veerapen V., Moffett P. A jasmonic acid-related mechanism affects ARGONAUTE5 expression and antiviral defense against PVX in Arabidopsis thaliana. Mol. Plant Microbe Interact. 2023 doi: 10.1094/MPMI-11-22-0224-R. [DOI] [PubMed] [Google Scholar]
- Sánchez-Correa M.D.S., Isidra-Arellano M.C., Pozas-Rodríguez E.A., Reyero-Saavedra M.D.R., Morales-Salazar A., Del Castillo S.M.L., Sanchez-Flores A., Jiménez-Jacinto V., Reyes J.L., Formey D., Valdés-López O. Argonaute5 and its associated small RNAs modulate the transcriptional response during the rhizobia-Phaseolus vulgaris symbiosis. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.1034419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szittya G., Burgyán J. RNA interference-mediated intrinsic antiviral immunity in plants. Curr. Top. Microbiol. Immunol. 2013;371:153–181. doi: 10.1007/978-3-642-37765-5_6. [DOI] [PubMed] [Google Scholar]
- Tamotsu H., Koizumi K., Briones A.V., Komiya R. Spatial distribution of three ARGONAUTEs regulates the anther phasiRNA pathway. Nat. Commun. 2023;14:3333. doi: 10.1038/s41467-023-38881-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarquini G., Ermacora P., Martini M., Firrao G. The conundrum of the connection of grapevine Pinot gris virus with the grapevine leaf mottling and deformation syndrome. Plant Pathol. 2023;72:209–217. [Google Scholar]
- Tarquini G., Pagliari L., Ermacora P., Musetti R., Firrao G. Trigger and suppression of antiviral defenses by grapevine pinot gris virus (GPGV): novel insights into virus-host interaction. Mol. Plant Microbe Interact. 2021;34:1010–1023. doi: 10.1094/MPMI-04-21-0078-R. [DOI] [PubMed] [Google Scholar]
- Tong X., Liu S.Y., Zou J.Z., Zhao J.J., Zhu F.F., Chai L.X., Wang Y., Han C., Wang X.B. A small peptide inhibits siRNA amplification in plants by mediating autophagic degradation of SGS3/RDR6 bodies. EMBO J. 2021;40 doi: 10.15252/embj.2021108050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker M.R., Okada T., Hu Y., Scholefield A., Taylor J.M., Koltunow A.M. Somatic small RNA pathways promote the mitotic events of megagametogenesis during female reproductive development in Arabidopsis. Development. 2012;139:1399–1404. doi: 10.1242/dev.075390. [DOI] [PubMed] [Google Scholar]
- Verchot J. Plant virus infection and the ubiquitin proteasome machinery: arms race along the endoplasmic reticulum. VirusesViruses. 2016;8 doi: 10.3390/v8110314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Várallyay E., Havelda Z. Unrelated viral suppressors of RNA silencing mediate the control of ARGONAUTE1 level. Mol. Plant Pathol. 2013;14:567–575. doi: 10.1111/mpp.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.B., Jovel J., Udomporn P., Wang Y., Wu Q., Li W.X., Gasciolli V., Vaucheret H., Ding S.W. The 21-nucleotide, but not 22-nucleotide, viral secondary small interfering RNAs direct potent antiviral defense by two cooperative argonautes in Arabidopsis thaliana. Plant Cell. 2011;23:1625–1638. doi: 10.1105/tpc.110.082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W.-L., Tran P.-A., Fang R.-Y., Pham T.H., Bowman J., Hong S.-F., Pan Z.-J., Shang Q.-W., Lin P.-C., Shen B.-N., Wu F.-H., Lin C.-S., Shen T.-L., Lin S.-S. The additional unique ability of TuMV HC-Pro in inhibiting HEN1 activity for enhancing the autophagic AGO1 degradation in RNA silencing suppression. Res. Sq. 2022 [Google Scholar]
- Wu J., Yang R., Yang Z., Yao S., Zhao S., Wang Y., Li P., Song X., Jin L., Zhou T., Lan Y., Xie L., Zhou X., Chu C., Qi Y., Cao X., Li Y. ROS accumulation and antiviral defence control by microRNA528 in rice. Nat. Plants. 2017;3:16203. doi: 10.1038/nplants.2016.203. [DOI] [PubMed] [Google Scholar]
- Wu J., Yang Z., Wang Y., Zheng L., Ye R., Ji Y., Zhao S., Ji S., Liu R., Xu L., Zheng H., Zhou Y., Zhang X., Cao X., Xie L., Wu Z., Qi Y., Li Y. Viral-inducible Argonaute18 confers broad-spectrum virus resistance in rice by sequestering a host microRNA. Elife. 2015:4. doi: 10.7554/eLife.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing H.L., Dong L., Wang Z.P., Zhang H.Y., Han C.Y., Liu B., Wang X.C., Chen Q.J. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 2014;14:327. doi: 10.1186/s12870-014-0327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Ismayil A., Liu Y. Autophagy in plant-virus interactions. Annu. Rev. Virol. 2020;7:403–419. doi: 10.1146/annurev-virology-010220-054709. [DOI] [PubMed] [Google Scholar]
- Yang M., Liu Y. Autophagy in plant viral infection. FEBS Lett. 2022;596:2152–2162. doi: 10.1002/1873-3468.14349. [DOI] [PubMed] [Google Scholar]
- Yang X., Guo W., Li F., Sunter G., Zhou X. Geminivirus-associated betasatellites: exploiting chinks in the antiviral arsenal of plants. Trends Plant Sci. 2019;24:519–529. doi: 10.1016/j.tplants.2019.03.010. [DOI] [PubMed] [Google Scholar]
- Yang Z., Li Y. Dissection of RNAi-based antiviral immunity in plants. Curr. Opin. Virol. 2018;32:88–99. doi: 10.1016/j.coviro.2018.08.003. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M., Han Y.W., Fujii H., Aizawa S., Nishino T., Ishikawa M. Cooperative recruitment of RDR6 by SGS3 and SDE5 during small interfering RNA amplification in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2102885118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Wu Z., Li Y., Wu J. Biogenesis, function, and applications of virus-derived small RNAs in plants. Front. Microbiol. 2015;6:1237. doi: 10.3389/fmicb.2015.01237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.C., Lei M.Q., Zhou Y.F., Yang Y.W., Lian J.P., Yu Y., Feng Y.Z., Zhou K.R., He R.R., He H., Zhang Z., Yang J.H., Chen Y.Q. Reproductive phasiRNAs regulate reprogramming of gene expression and meiotic progression in rice. Nat. Commun. 2020;11:6031. doi: 10.1038/s41467-020-19922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J.H., Hua C.L., Fang Y.Y., Guo H.S. The dual edge of RNA silencing suppressors in the virus-host interactions. Curr. Opin. Virol. 2016;17:39–44. doi: 10.1016/j.coviro.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Zheng X., Fahlgren N., Abbasi A., Berry J.C., Carrington J.C. Antiviral ARGONAUTEs against turnip crinkle virus revealed by image-based trait analysis. Plant Physiol. 2019;180:1418–1435. doi: 10.1104/pp.19.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.