Abstract
Simian immunodeficiency virus (SIV) infection of newborn macaques is a useful animal model of human pediatric AIDS to study disease pathogenesis and to develop intervention strategies aimed at delaying disease. In the present study, we demonstrate that very early events of infection greatly determine the ultimate disease course, as short-term antiviral drug administration during the initial viremia stage significantly delayed the onset of AIDS. Fourteen newborn macaques were inoculated orally with uncloned, highly virulent SIVmac251. The four untreated control animals showed persistently high virus levels and poor antiviral immune responses; they developed fatal immunodeficiency within 15 weeks. In contrast, SIV-infected newborn macaques which were started on 9-[2-(R)-(phosphonomethoxy)propyl]adenine (PMPA) treatment at 5 days of age and continued for either 14 or 60 days showed reduced virus levels and enhanced antiviral immune responses. This short-term PMPA treatment did not induce detectable emergence of SIV mutants with reduced in vitro susceptibility to PMPA. Although viremia increased in most animals after PMPA treatment was withdrawn, all animals remained disease-free for at least 6 months. Our data suggest that short-term treatment with a potent antiviral drug regimen during the initial viremia will significantly prolong AIDS-free survival for HIV-infected infants and adults.
Although zidovudine administration to human immunodeficiency virus (HIV)-infected pregnant women and their newborns has greatly reduced the rate of perinatal HIV infection in developed countries (4, 10), it is currently estimated that every day, approximately 1,700 newborns become infected perinatally with HIV, and the majority of these infections occur in developing countries (43). HIV infection of human newborns and infants often results in a more rapid and severe disease than is seen for adults, as about one-third of these infants develop symptoms within 1 year and die early (2, 16, 39). Accordingly, there is an urgent need for aggressive but safe treatment regimens to combat pediatric HIV infection.
Although the recent development of more potent anti-HIV drugs has led to major improvements in the clinical management of HIV-infected adults in the developed countries (5), progress in the treatment of pediatric HIV infection has been slower due to several problems. First, many of the antiretroviral drugs which have been approved for adults have a complicated dosage regimen or have considerable toxicity, which limits their use in infants. In addition, there is still uncertainty among clinicians regarding when to initiate treatment for pediatric HIV patients (6). For HIV-infected adults, there is a growing body of evidence which indicates that prolonged antiviral drug treatment initiated during primary infection is beneficial (19, 20, 34). In addition, Luzuriaga and colleagues (24) have demonstrated that a prolonged combination treatment of HIV-infected infants starting at ≥2 months of age resulted in strong, long-lasting suppression of virus replication, and they are attempting to treat HIV-infected infants even earlier. Many clinicians, however, are still reluctant to initiate drug treatment during the primary viremia stage of pediatric or adult HIV infection. There are concerns that potent antiviral drug treatment during the primary stages of HIV infection might be harmful because of the risk of causing a less vigorous immune response (40), or because it might induce early emergence of drug-resistant HIV mutants, which could reduce the efficacy of drug treatment at later disease stages. In addition, some clinicians think that for drug therapy to be effective, it should, once initiated, be continued indefinitely. Fears that prolonged drug therapy will induce harmful side effects, especially for the growing infant, and concerns for the high costs of prolonged drug therapy are used as rationales to postpone drug treatment until later stages of HIV infection.
However, because the rapid development of AIDS in many infants is associated with high levels of viremia and weak antiviral immune responses (3, 15, 32, 33, 35, 37), it is possible that even short-term drug treatment during the early stages of HIV infection may permanently alter the disease course by reducing the initial viremia which is responsible for early systemic dissemination and virus-induced immunosuppression. Because it is ethically and logistically difficult to perform these kinds of studies with human newborns, an appropriate animal model can provide a scientifically solid basis to gather information regarding the role of early viremia on the subsequent disease course and regarding the effect of short-term antiviral drug treatment.
Simian immunodeficiency virus (SIV) infection of newborn and infant rhesus macaques has been shown to be a very useful animal model of pediatric AIDS to rapidly evaluate the efficacy of intervention strategies (45–47, 50, 52). We previously demonstrated that prolonged therapy with the potent reverse transcriptase (RT) inhibitor 9-[2-(R)-(phosphonomethoxy)propyl]adenine (PMPA) was highly effective in delaying the onset of AIDS in newborn macaques with established SIV infections (47). In the present study, we demonstrate that short-term PMPA treatment of newborn macaques, starting 5 days after oral SIV inoculation and continuing for either 14 or 60 days, resulted in reduced virus levels, enhanced antiviral immune responses, and a long-lasting delay in the onset of disease. These results suggest that short-term intervention with a potent antiviral drug regimen during the initial infection can dramatically improve the course of pediatric HIV infection.
MATERIALS AND METHODS
Animals, virus and PMPA administration.
Newborn rhesus macaques (Macaca mulatta) were from the type D-retrovirus- and SIV-free colony at the California Regional Primate Research Center, and were hand reared in a primate nursery in accordance with American Association for Accreditation of Laboratory Animal Care standards. We strictly adhered to the Guide for the Care and Use of Laboratory Animals prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Resources, National Resource Council (28a). When necessary, animals were immobilized with ketamine HCl (Parke-Davis, Morris Plains, N.J.) (10 mg/kg of body weight) injected intramuscularly.
Within 3 days of birth, newborn macaques were inoculated orally with 2 doses of uncloned SIVmac251 under ketamine anesthesia. Each dose consisted of 1 ml of uncloned SIVmac251, administered atraumatically by dispensing the virus slowly into the mouth. The SIVmac251 stock used in this study was propagated in rhesus peripheral blood mononuclear cells (PBMC), had a titer of 105 50% tissue culture infective doses (TCID50) per ml, and had been used previously to inoculate newborn macaques by the oral route (45, 50). The second SIVmac251 dose was given 24 h later.
To monitor the immune response to nonviral, nonreplicating antigens, all newborn rhesus macaques were immunized subcutaneously with 0.1 mg of cholera toxin B subunit (List Biological Laboratories, Campbell, Calif.) just before the first virus inoculation. A booster immunization was given at 7 weeks of age. The cholera toxin-specific immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) has been described previously (53).
PMPA (Gilead Sciences, Foster City, Calif.) was suspended in distilled water and dissolved by the addition of NaOH to a final pH of 7.0 at 60 mg/ml and was filter sterilized (0.2-μm pore size; Nalgene). Starting 5 days after the first SIV inoculation, PMPA was administered subcutaneously at a dosage regimen of 30 mg/kg of body weight (42) once daily, into the back of the animal. PMPA treatment was continued for either 14 or 60 days. The untreated control animals did not receive daily sham inoculations.
Blood samples were collected immediately before virus inoculation and regularly thereafter for monitoring viral and immunologic parameters; 0.5- to 1-ml heparinized blood samples were taken weekly for the first 5 weeks, every 2 weeks for the next 2 months, and then every 3 to 4 weeks. Complete blood cell counts were done with EDTA-anticoagulated blood samples from all animals. Samples were analyzed by using an automated electronic cell counter (Baker 9000; Serono Baker Diagnostics, Bethlehem, Pa.); differential cell counts were determined manually.
Quantitative virus isolation (cell associated and cell free).
Levels of infectious virus in cells and plasma of peripheral blood were determined regularly by a limiting dilution assay (four replicates per dilution) of PBMC and plasma, respectively, in cultures with CEM×174 cells in 24-well plates and subsequent p27 core antigen measurement in accordance with methods previously described (51–53). In addition, for animals with low or undetectable virus loads, 1 × 106 to 5 × 106 PBMC were cocultivated for 8 weeks with CEM×174 cells in tissue culture flasks (51). Virus levels in fresh lymphoid tissues (lymph nodes, spleen, thymus), collected from the animals at time of euthanasia, were determined by aseptically teasing tissues into single-cell suspensions of mononuclear cells and by a limiting dilution culture assay similar to the one described above for PBMC.
Plasma viral RNA levels.
Quantitative assays for the measurement of SIV RNA were performed by using a branched DNA signal amplification assay specific for SIV (11). This assay is similar to the Quantiplex HIV RNA assay (31) except that target probes were designed to hybridize with the pol region of the SIVmac group of strains including SIVmac251. SIV pol RNA in plasma samples was quantified by comparison with a standard curve produced using serial dilutions of cell-free SIV-infected tissue culture supernatant. The quantification of this standard curve was determined by comparison with purified, quantified, in vitro-transcribed SIVmac239 pol RNA. The lower quantification limit of this assay was 10,000 copies of SIV RNA per plasma sample. Due to the limited blood volume that can be collected from newborn macaques, plasma volumes of ≤50 μl were available during the early time points, which limited the sensitivity of this assay to ≥200,000 copies of SIV RNA per ml of plasma.
PCR amplification.
Nested PCR was carried out in a GeneAmp 9600 thermocycler (Perkin-Elmer Cetus, Emeryville, Calif.). Two rounds of 30 cycles of amplification were performed on aliquots (at 5 or 10 replicates per sample) of PBMC or mononuclear cells of lymphoid tissues using SIVmac-specific gag primers and conditions described elsewhere (23). Positive controls included PBMC lysates of known SIV-infected animals. To detect potential inhibitors of Taq polymerase in cell lysates, β-actin DNA sequences were amplified with two rounds of PCR (23).
Anti-SIV class-specific antibody determination.
The anti-SIV IgG- and IgM-specific antibody ELISAs have been described previously (30, 50).
T-lymphocyte phenotyping.
T-lymphocyte antigens were detected by direct labeling of whole blood with PerCP-conjugated anti-human CD8 (Leu-2a; Becton Dickinson Immunocytometry Inc., San Jose, Calif.), phycoerythrin-conjugated anti-human CD4 (OKT4; Ortho Diagnostic Systems Inc., Raritan, N.J.), and fluorescein-conjugated anti-human CD3 (Pharmingen, from Becton Dickinson). Erythrocytes were lysed, and the samples were fixed in paraformaldehyde by using the Coulter Q-prep system (Coulter Corporation, Hialeah, Fla.). Lymphocytes were gated by forward and side light scatter and were then analyzed with a FACScan flow cytometer (Becton Dickinson).
Drug susceptibility assay.
Phenotypic drug susceptibility was characterized by a previously described assay which is based on a dose-dependent reduction of viral infectivity (52); this assay was used previously to detect SIV mutants with fivefold-reduced susceptibility to PMPA (47).
Sequencing of viral RT region.
CEM×174 cells infected with virus isolated from the SIV-infected animals were harvested as soon as culture supernatants were positive by antigen capture ELISA (22). The genomic DNA preparation, PCR, and sequencing of the RT region were done according to methods previously described (47). This method can detect the presence of a 20% subpopulation in the PCR mixture.
Criteria for euthanasia.
Euthanasia of animals with simian AIDS was indicated by three or more of the following clinical observations: weight loss of >10% in 2 weeks or >30% in 2 months; chronic diarrhea unresponsive to treatment; infections unresponsive to treatment; inability to maintain body heat or fluids without supplementation; persistent, marked hematologic abnormalities, including lymphopenia, anemia, thrombocytopenia, or neutropenia; and persistent, marked splenomegaly or hepatomegaly (25). A complete necropsy examination was performed on all animals, and a routine histopathologic examination was done on tissues collected at necropsy.
Statistical analysis.
Statistical analysis was used to compare PMPA-treated and untreated SIV-infected animals with regard to survival and virus levels. Survival was compared by the generalized Wilcoxon test (12). Virus levels in peripheral blood and development of antibody responses were compared by calculating the area under the concentration-time curve for each animal for the first 11 weeks after SIV inoculation, followed by analysis according to the Wilcoxon rank-sum test (12). We have previously shown that these analyses can distinguish biologically relevant differences (25, 52).
RESULTS
Study design.
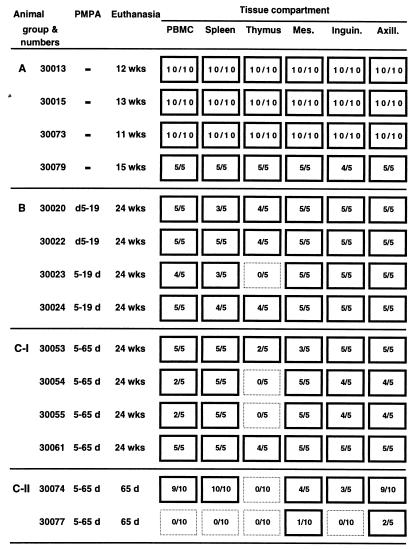
Within 3 days of birth, newborn macaques were inoculated orally with SIVmac251 (Table 1). One group of four animals consisted of untreated SIV-infected control animals (Table 1, group A). Five days after virus inoculation, the other groups were started on PMPA treatment. PMPA treatment was continued for either 14 or 60 days (Table 1, groups B and C). To monitor the immune response to nonviral, nonreplicating antigens, all newborn rhesus macaques were also immunized subcutaneously with cholera toxin B subunit prior to the SIV inoculation, and a booster immunization was given at 7 weeks of age. To determine virus levels in lymphoid tissues at the end of drug treatment, two animals (Table 1, group C-II) were euthanized at the end of the 60-day PMPA treatment period. The other SIV-infected animals were euthanized when their conditions were judged terminal (53); animals that did not develop AIDS during the course of this study were euthanized at 24 weeks of age.
TABLE 1.
Short-term PMPA treatment of early SIV infection in newborn macaques: summary of study design and outcomea
| Group (size) | Period of PMPA treatmentb | Animal no. | Clinical outcomec | Histopathology |
|---|---|---|---|---|
| A (n = 4) | None | 30013 | AIDS at 12 wks | Typhlocolitis; Cryptosporidium-positive enteritis and cholecystitis; interstitial pneumonia |
| 30015 | AIDS at 13 wks | Pancreatitis; Cryptosporidium-positive enteritis; hepatitis | ||
| 30073 | AIDS at 11 wks | Cryptosporidium-positive enteritis; mixed pattern of lymphoid hyperplasia/depletion | ||
| 30079 | AIDS at 15 wks | Cryptosporidium-positive cholecystitis, cholangitis, pancreatitis and enteritis; lymphoid depletion | ||
| B (n = 4) | Days 5–19 | 30020 | Healthy at 24 wks | Cryptosporidium infection of upper respiratory tract; lymphoid hyperplasia |
| 30022 | Healthy at 24 wks | Lymphofollicular hyperplasia of spleen; Cryptosporidium infection of upper respiratory tract; glomerulonephritis; paracortical depletion of lymph nodes | ||
| 30023 | Healthy at 24 wks | Lymphoid hyperplasia | ||
| 30024 | Healthy at 24 wks | Lymphoid hyperplasia; Cryptosporidium-positive cholecystitis; pancreatitis | ||
| C-I (n = 4) | Days 5–65 | 30053 | Healthy at 24 wks | Lymphoid hyperplasia |
| 30054 | Healthy at 24 wks | Lymphoid hyperplasia | ||
| 30055 | Healthy at 24 wks | Lymphoid hyperplasia | ||
| 30061 | Healthy at 24 wks | Lymphoid hyperplasia | ||
| C-II (n = 2) | Days 5–65 | 30074 | Healthy at day 65 | Mild lymphoid hyperplasia; mild enteritis; meningoencephalitis |
| 30077 | Healthy at day 65 | Moderate acute typhlocolitis; mild lymphoid depletion of lymph nodes |
Within 3 days of birth, all animals were inoculated orally with SIVmac251.
Groups B and C were started on short-term PMPA treatment (30 mg/kg subcutaneously once daily) 5 days after SIV inoculation.
Statistical analysis of survival showed the significant benefit of PMPA treatment on clinical outcome (group A versus B or group A versus C-I, P = 0.014; group A versus groups B and C-I, P < 0.003). Animals of groups B and C were euthanized either at the end of PMPA treatment (day 65, group C-II) or at 24 weeks of age (groups B and C-I), prior to the development of clinical simian AIDS.
Control newborn macaques infected with SIVmac251.
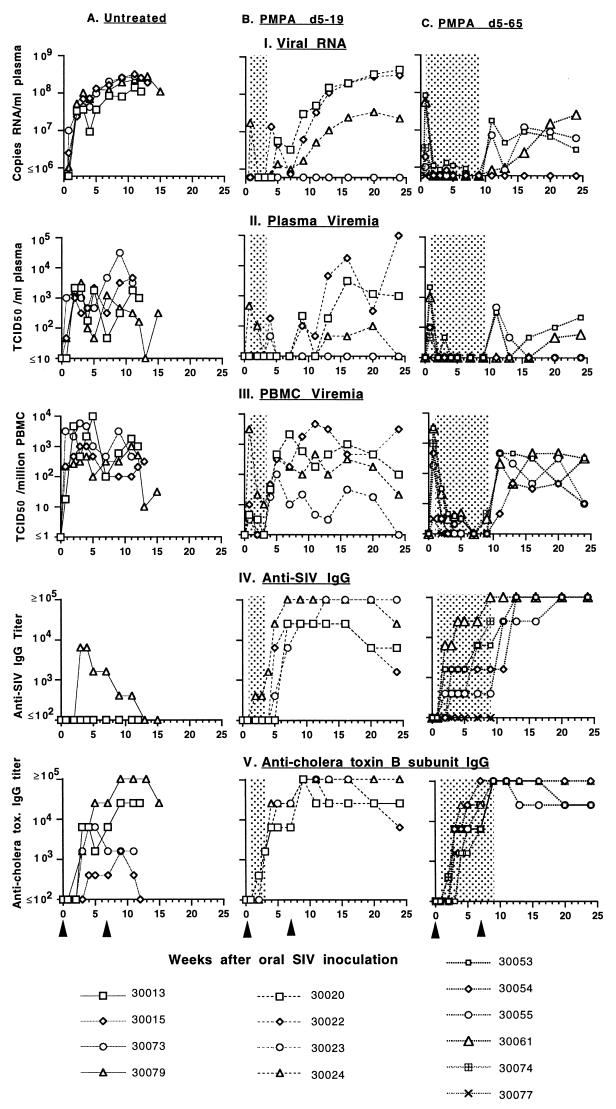
The four untreated control animals became infected and maintained persistently high virus levels in peripheral blood following infection (Fig. 1). Three of the four animals (no. 30013, 30073, and 30079) made a detectable SIV-specific IgM response between 1 and 7 weeks after SIV inoculation (data not shown). Three of these four untreated SIV-infected animals failed to make a detectable anti-SIV IgG response; the fourth infant (30079) developed an anti-SIV IgG titer of 1:6,400 at 3 to 5 weeks, but this declined rapidly to undetectable levels at time of clinical disease (Fig. 1). Two untreated animals (30013 and 30079) developed a persistent IgG response to cholera toxin B subunit following immunization, while the other two untreated animals (30015 and 30073) had weak or transient IgG responses to this antigen (Fig. 1). All four untreated control animals developed clinical signs consistent with progressive immunodeficiency and were euthanized between 11 and 15 weeks of age. These animals had widespread systemic dissemination of virus in lymphoid tissues (Table 2), and histopathological findings were consistent with terminal SIV infection in this age group (Table 1).
FIG. 1.
Time course of SIVmac251 infection of newborn macaques and the therapeutic effects of PMPA. All newborn macaques were inoculated at birth orally with uncloned SIVmac251. The left graphs show data for the untreated control animals; the middle and right graphs show data for the animals which were given PMPA treatment (shaded area) between days 5 and 19 or 5 and 65 (Table 1, groups A to C, respectively). Figure symbols for each vertical set of five graphs are indicated at the bottom. Plasma RNA levels (I) were measured by branched DNA assay; plasma (II) and PBMC-associated (III) virus levels were determined by limiting dilution culture of plasma and PBMC, respectively. IgG titers to SIV (IV) and cholera toxin B subunit (V) were determined by ELISA and are expressed as the reciprocals of the highest of fourfold dilutions (starting from 1:100 dilution, with two replicates per dilution) which gave a positive optical density above the cutoff value. Cholera toxin subunit B immunizations were given at birth and at 7 weeks of age (arrowheads); four uninfected newborn macaques from a different study which received cholera toxin B subunit immunizations had IgG titers that were ≥6,400 at 4 weeks and ≥102,400 at 12 weeks of age (45). The untreated control animals all died between 11 and 15 weeks of age. Statistical comparison of values of the area under the concentration-time curve demonstrated significant reduction of virus levels and enhanced immune responses in PMPA-treated animals versus untreated SIV-infected animals (P < 0.01).
TABLE 2.
Infectious virus levels in blood and lymphoid tissues at time of euthanasia or death, and CD4+/CD8+ T-cell ratio in thymus cells
| Animal group and numbers | Period of PMPA treatmenta | Time of euthanasiab | TCID50c
|
CD4+/CD8+ thymus cellsd | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PBMC | Plasma | Spleen | Mes. LN | Ing. LN | Ax. LN | Thymus | ||||
| A | ||||||||||
| 30013 | None | 12 wks | 1,000 | 1,000 | 2,153 | 464 | 4,640 | 215 | 1,778 | 0.60 |
| 30015 | 13 wks | 316 | 1,000 | 2,153 | 2,153 | 1,000 | 100 | 316 | 0.36 | |
| 30073 | 11 wks | 464 | 3,160 | 3,160 | 3,160 | 3,160 | 32 | 316 | 0.38 | |
| 30079 | 15 wks | 32 | 316 | 100 | 316 | 32 | 22 | 316 | 0.51 | |
| B | ||||||||||
| 30020 | Days 5–19 | 24 wks | 100 | 1,000 | 100 | 1,000 | 46 | 316 | 3 | 5.96 |
| 30022 | 24 wks | 3,160 | 100,000 | 10,000 | 10,000 | 178 | 3,160 | ≤3 | 0.85 | |
| 30023 | 24 wks | ≤1 | ≤3 | 100 | 3 | ≤1 | 5 | ≤0.3 | 9.69 | |
| 30024 | 24 wks | 22 | ≤3 | 100 | 100 | 316 | 32 | 22 | 1.09 | |
| C-I | ||||||||||
| 30053 | Days 5–65 | 24 wks | 316 | 215 | 3,160 | 10,000 | 2,153 | 10,000 | ≤1 | 1.82 |
| 30054 | 24 wks | 10 | <3 | 588 | 178 | 46 | 178 | ≤3 | 1.08 | |
| 30055 | 24 wks | 10 | ≤3 | 46 | 100 | 100 | 100 | ≤0.3 | 1.79 | |
| 30061 | 24 wks | 316 | 59 | 3,160 | 1,778 | 215 | 588 | ≤3 | 1.79 | |
| C-II | ||||||||||
| 30074 | Days 5–65 | 65 days | 5 | ≤3 | 46 | 5 | 3 | ≤3 | ≤0.1 | 2.11 |
| 30077e | 65 days | ≤0.1 | ≤3 | ≤0.1 | ≤0.1 | ≤0.1 | ≤0.1 | ≤0.1 | 2.44 | |
Within 3 days of birth, all animals were inoculated orally with SIVmac251; groups B and C were started on PMPA treatment at 5 days of age for 14 days (group B) or 60 days (group C).
Animals of group A were euthanized at time of clinical immunodeficiency (simian AIDS), while animals of groups B and C were euthanized prior to development of simian AIDS, either at the end of PMPA treatment (group C-II) or at 24 weeks of age (group B and C-I).
Cell-associated and cell-free virus levels in peripheral blood and fresh lymphoid tissues collected at time of euthanasia were determined by limiting dilution culture of single-cell suspensions or plasma and are expressed as the numbers of TCID50s per 106 mononuclear cells or per milliliter of plasma. When no virus could be detected, the detection limit is given, based on the number of mononuclear cells or the amount of plasma available for virus isolation. Abbreviations: Mes. LN, Ing. LN and Ax. LN indicate mesenteric, inguineal and axillary lymph node, respectively.
Ratio of CD4+CD3+ over CD8+CD3+ thymus cells was determined by flow cytometry.
Virus could not be isolated from animal 30077 from 0.5 ml of plasma or from 107 cells of each lymphoid tissue at time of euthanasia.
Early short-term PMPA treatment benefits SIVmac251-infected newborn macaques.
Virus was isolated from PBMC of all animals 5 days after oral SIVmac251 inoculation, just prior to the start of PMPA treatment. Some animals demonstrated very high titers in peripheral blood at this very early time point; animal 30053 had 3,160 TCID50 per million PBMC, 2,150 TCID50 per ml of plasma, and 8.4 × 107 RNA copies/ml of plasma (Fig. 1).
As expected, the 14 or 60 days of PMPA treatment resulted in a dramatic decrease (100- to 1,000-fold) in virus levels in peripheral blood, and in some instances no virus was detected during PMPA treatment (Fig. 1; P < 0.01). Different patterns of SIV replication were observed following withdrawal of PMPA treatment. For each of the two PMPA-treated groups, there was one animal (30023 and 30054; Fig. 1) for which PBMC-associated virus became detectable following the discontinuation of PMPA treatment, but viral RNA and infectious virus levels in plasma remained below the limit of detection. The other three animals in each group showed an increase in both PBMC-associated and plasma viremia as well as viral RNA levels following PMPA withdrawal (Fig. 1). Overall, virus levels in the animals which had received 60 days of PMPA treatment remained approximately 10- to 100-fold lower than those in the animals that had only received 14 days of PMPA treatment (Fig. 1).
To monitor whether this short course of PMPA treatment resulted in the rapid development of PMPA-resistant viral mutants, we tested virus isolates obtained from all PMPA-treated animals at the end of PMPA treatment or, in case no virus could be isolated, at the earliest available time point after PMPA treatment was withdrawn. All virus isolates tested were found to have wild-type susceptibility to PMPA in vitro (Table 3). Because reduced susceptibility of SIV to PMPA is associated with the development of an arginine-to-lysine amino acid substitution (K65R) in RT (47, 48), the region of RT encompassing amino acid 65 was sequenced: all these virus isolates obtained following short-term PMPA treatment had the wild-type amino acid arginine (K) at position 65 of RT (Table 3).
TABLE 3.
Phenotypic and genotypic PMPA susceptibility of virus isolates obtained from SIV-infected infant macaques following short-term PMPA treatment
| Animal no. | PMPA treatment perioda | Time point (day) of virus isolateb | Phenotype IC95 (μM)c | RT codon 65d |
|---|---|---|---|---|
| 30020 | Days 5–19 | 26 | 40 | K |
| 30022 | 26 | 40 | K | |
| 30023 | 26 | 40 | K | |
| 30024 | 19 | 40 | K | |
| 30053 | Days 5–65 | 79 | 45 | K |
| 30054 | 79 | 40 | K | |
| 30055 | 79 | 40 | K | |
| 30061 | 65 | 40 | K | |
| 30074 | Days 5–65 | 65 | 45 | K |
| 30077 | NA | NA | NA |
Animals were started on short-term PMPA treatment (30 mg/kg subcutaneously once daily) 5 days after oral wild-type SIVmac251 inoculation for a total duration of 14 or 60 days.
Virus was isolated from PBMC at the end of PMPA treatment or at the first available time point after PMPA withdrawal. NA, not available (no virus could be isolated from animal 30077 during PMPA treatment or at time of euthanasia at day 65).
In vitro susceptibility to PMPA is indicated by the 95% inhibitory concentration. SIVmac mutants with K65R mutation in RT and fivefold-reduced susceptibility to PMPA, which emerged in SIVmac251-infected infant macaques following prolonged PMPA treatment, are described elsewhere (47). The IC95 of PMPA for wild-type SIVmac251 was 30 to 50 μM (with a K at RT codon 65) and the IC95 of PMPA for mutant SIVmac was 150 to 250 μM (with an R at RT codon 65).
Amino acid at codon 65 of RT. K, arginine; R, lysine.
The short course of PMPA treatment resulted in enhanced antiviral immune responses for the SIV-infected infant macaques, as these animals made detectable and strong (titer, ≥1:25,600) anti-SIV IgG responses (Fig. 1). The animals which received only 14 days of PMPA treatment experienced a very rapid increase in anti-SIV IgG following PMPA withdrawal (titer, ≥1:6,400 by 7 weeks); in three animals (30020, 30022, and 30024), anti-SIV IgG levels started to decline as viral RNA levels increased at about 24 weeks of age (Fig. 1). Of the animals which received 60 days of PMPA treatment, the two animals (30054 and 30055) which had virus levels at day 5 lower than the other two animals in their group had a relatively slower rise in anti-SIV IgG titer while on PMPA treatment, presumably due to a lower expression of viral antigens. But following PMPA withdrawal, anti-SIV IgG levels in all these animals equaled or exceeded those of the animals which had received only 14 days of treatment and remained high (≥102,400) throughout the 24 weeks of observation (Fig. 1). All PMPA-treated animals made strong IgG responses following cholera toxin B subunit immunization (Fig. 1).
Importantly, all the SIV-infected infant macaques that received short-term PMPA treatment were healthy at 24 weeks of age; there were no significant clinical signs of AIDS, and CD4+ and CD8+ T-cell counts were normal (>500/μl; data not shown). The short course of PMPA treatment did not result in detectable toxicity. All PMPA-treated animals were euthanized at 24 weeks of age in order to determine infectious virus levels in lymphoid tissues and to conduct histopathology (Tables 1 and 2). Three of the animals which had received only 2 weeks of PMPA treatment and which had developed relatively high virus levels at 24 weeks of age (Fig. 1; Table 2) demonstrated histopathological evidence of opportunistic infections (Table 1, group B); the fourth animal (no. 30023), which had the lowest virus levels (Fig. 1; Table 2), manifested only lymphoid hyperplasia. The only significant finding for the four animals that had received 60 days of PMPA treatment was lymphoid hyperplasia (Table 1, group C-I). As expected, there was a strong, positive association between virus levels in peripheral blood and spleen and lymph nodes (Table 2). However, virus levels in the thymus in all the PMPA-treated infant macaques were low, which was unlike those of the untreated control animals (Table 3). PCR analysis of proviral DNA gave similar results; while proviral DNA could be detected consistently in PBMC, spleens, and lymph nodes of all these PMPA-treated animals, no proviral DNA could be detected in thymus lysates of animals 30023, 30054, and 30055 (Fig. 2). While the CD4+/CD8+ T-cell ratio in the thymuses of all SIV-infected control animals was less than 1, this ratio was higher (>1) in most PMPA-treated animals at time of euthanasia; only one animal (30022), which had high viremia and signs of opportunistic infections at time of death, had a CD4+/CD8+ T-cell ratio <1 in the thymus at time of euthanasia (Table 2). The CD4+/CD8+ T-cell ratios in other lymphoid tissues and in peripheral blood were more variable and did not differ significantly from those of PMPA-treated and untreated animals at time of euthanasia; absolute CD4+ T-cell counts in peripheral blood were less reliable parameters, as the untreated SIV-infected animals often had high CD4+ T-cell counts due to lymphocytosis (data not shown).
FIG. 2.
Detection of proviral DNA in SIV-infected infant macaques. Newborn macaques were inoculated orally with uncloned SIVmac251. Animals of group A were untreated control animals and were euthanized at the time of the development of simian AIDS. Groups B and C were started on short-term PMPA treatment 5 days after SIV inoculation, for a duration of 14 days (B) or 60 days (C). Animals of groups B and C were euthanized either at the end of PMPA treatment (day 65; C-II) or at 24 weeks of age (B, C-I), prior to the development of clinical simian AIDS. PCR results are presented as number of positive reactions out of total number of replicates. Solid borders indicate at least one positive reaction. Abbreviations: d, day; wks, weeks; Mes., Inguin., and Axill.: mesenteric, inguineal, and axillary lymph node, respectively.
Two animals (30074 and 30077) were given 60 days of PMPA treatment starting at day 5 and were euthanized at the end of PMPA treatment to measure virus levels in lymphoid tissues (Table 1, group C-II); neither of these animals had clinical signs of AIDS at the time of necropsy. One of these animals (30074) had detectable but low virus levels in most lymphoid tissues at the end of PMPA treatment (Table 2). For the other animal (30077), virus was isolated from PBMC only at 5 days of age (approximately one infected cell per 106 PBMC); no virus could be isolated from this animal during PMPA treatment or from lymphoid tissues collected at necropsy at the end of the 60 days of PMPA treatment (Table 2). Proviral DNA was not detected at time of euthanasia by PCR in PBMC, spleen, thymus, and inguineal lymph node of animal 30077, but a low level of proviral DNA was detected in the mesenteric and axillary lymph nodes (Fig. 2); because no infectious virus could be isolated from 107 mononuclear cells of these lymphoid tissues, this proviral DNA may have been defective. This animal had made a detectable anti-SIV IgM response (titer, 1:200 at 2 weeks of age; data not shown), but was the only PMPA-treated SIV-infected animal that did not mount a detectable anti-SIV IgG response. It is unclear whether virus levels in animal 30077 would have increased again if this animal had been monitored after PMPA withdrawal or whether animal 30077 had experienced PMPA-induced transient viremia (50).
DISCUSSION
The results of the present study provide strong evidence that very early events during viral infection determine the ultimate disease course in simian AIDS. Studies with older macaques have already suggested an association between early virus replication and the subsequent disease course (17, 18, 21, 29). The demonstration that the severity of acute HIV-1 illness in HIV-infected adults correlates with the long-term prognosis also suggests the importance of early events in HIV pathogenesis (38, 44). In the present study, we used short-term antiviral drug treatment as an intervention tool to temporarily disturb primary viremia. The finding that this short-term reduction of SIV primary viremia induces long-term therapeutic effects is the strongest evidence of a causal relationship between very early events in viral infection and the subsequent rate of disease progression. Our results strongly support the immediate use of potent antiviral drug treatment for human newborns of HIV-infected women and, by extension, for adults following exposure to HIV.
In the present study, groups of newborn macaques were inoculated orally with SIVmac251. In untreated control animals, SIV replicated rapidly to high titers. This high viremia during the initial weeks of infection probably leads to rapid and early dissemination of virus to all lymphoid tissues. The inability of most untreated newborn macaques to mount detectable anti-SIV IgG responses also indicates virus-induced immunosuppression during these initial weeks of infection (30). This inability to control virus replication resulted in rapidly progressive fatal immunodeficiency within approximately 3 months. The fulminant disease course observed in the four untreated newborn macaques in the present study is representative of a much larger number of historical control animals inoculated at birth with uncloned SIVmac251 (25, 47, 52, 53).
To determine the effects of short-term drug administration during the primary infection stage, groups of SIVmac251-infected newborn macaques were started on short-term PMPA treatment 5 days after virus inoculation; PMPA treatment was continued for either 14 or 60 days. The rationale for waiting 5 days before starting PMPA administration was that previous studies had demonstrated that short-term PMPA treatment of newborn or juvenile macaques starting near the time of virus inoculation was highly effective in preventing the establishment of persistent SIV infection (41, 42, 45, 50). Therefore, in the present study, we started PMPA treatment at a time when animals were SIV infected (as determined by detectable virus isolation from the peripheral blood) but prior to the development of peak viremia. The present study demonstrates that even when virus infection cannot be prevented, early short-term drug administration still has a dramatic impact on the long-term disease course of SIV-infected newborn macaques.
As expected, early PMPA administration starting 5 days after SIV inoculation greatly reduced virus levels in the peripheral blood throughout the drug treatment period. Even though virus levels increased in most animals once PMPA treatment was withdrawn, animals stayed healthy for at least 6 months. Several factors may contribute to this delayed disease progression. The early short-term suppression of virus replication by PMPA treatment may have reduced the establishment of virus reservoirs in lymphoid tissues. In this context, the untreated SIVmac251-infected infants had high virus levels in all lymphoid tissues which were tested, including the thymus. This systemic virus dissemination is consistent with findings from our previous studies with SIVmac251-infected infant macaques (49). In contrast, virus levels in the thymus of the short-term PMPA-treated animals at 6 months of age were low or undetectable, even for the animals in which virus levels in peripheral blood and other lymphoid tissues had increased following PMPA withdrawal; in addition, the PMPA-treated animals maintained higher CD4+/CD8+ T-cell ratios in the thymus. Also for HIV-infected infants, a strong association was found between thymic dysfunction and disease progression (28). Considering the role of the thymus in the ontogeny of immune responses, it is likely that early drug treatment can limit virally induced depletion of thymus cells and thereby have a long-term beneficial effect on the immune system. A beneficial effect of antiviral drug treatment on thymopoiesis has been demonstrated in HIV-infected human thymic implants in the SCID-hu mouse model (56).
Another important finding of the present study was that early short-term drug treatment enhanced the development of SIV-specific IgG responses. This observation extends our previous reports that prolonged antiviral drug treatment of SIV-infected infant macaques augmented antiviral immune responses (47, 52). Because the infant macaque model is predictive of the immunogenicity of antigens for human infants (54), similar results can be expected for HIV-infected human infants. The development of SIV-specific immune responses in the present study may have limited the rebound in viremia once PMPA treatment was withdrawn. In HIV-infected adults, an inverse relationship between the strength of HIV-specific CD4+ T-cell proliferative responses (indicators of T-helper-cell function) and virus levels exists. It has been demonstrated that early drug intervention enhanced these HIV-specific immune responses, while drug intervention during chronic infection did not restore these responses (36). Because T-helper-cell responses are required for the production of most antiviral antibodies, the results of our studies with newborn macaques provide strong in vivo evidence that virus-specific CD4+ T-helper-cell responses are severely suppressed in untreated SIV-infected newborn macaques, and that early drug intervention enhances these antiviral antibody responses, possibly by preventing CD4+ T-cell destruction and dysfunction. Virus-specific CD4+ T-helper-cell responses presumably contribute to the control of viremia and a delay in disease progression. Our data suggest that some level of viral antigen expression is needed during drug treatment to stimulate the strongest antiviral immune responses. It has also been shown in HIV-infected infants receiving early, aggressive combination therapy that the development of HIV-specific antibodies may be dampened in those infants for whom virus levels became undetectable (24). These findings suggest that it may be worthwhile to investigate whether boosting these antiviral immune responses by vaccination during the period of drug treatment would further limit the rebound in viremia following drug withdrawal.
Our results indicate that a longer duration of PMPA treatment early in infection results in longer-lasting therapeutic benefits following withdrawal of drug administration. The SIV-infected infant macaques that received 60 days of PMPA treatment had generally lower plasma RNA levels when PMPA treatment was stopped than most animals that received only 14 days of PMPA treatment. In addition, the animals which had received 60 days of PMPA treatment had only generalized lymphoid hyperplasia at 24 weeks of age, while three of the four animals that received only 14 days of PMPA treatment had histopathologic evidence of more advanced immune dysfunction as indicated by the presence of opportunistic infections.
It was previously demonstrated that the outcome of neonatal SIV infection is largely dependent upon the virulence of the virus inoculum (25, 30). The virus inoculum which we used in this study, uncloned SIVmac251, is the most virulent virus isolate we have available to inoculate newborn macaques. Demonstration of efficacy against a highly virulent virus isolate is the best indicator of the success of a therapeutic intervention. The disease course in the SIVmac251-infected newborn macaques which received short-term PMPA treatment became similar to that seen in juvenile or adult macaques following infection with virulent SIVmac251. It is of interest that in each group of PMPA-treated animals, there was one animal which, even after PMPA withdrawal, maintained low virus levels which are typically seen in SIV-infected adult macaques with very slow disease progression. The demonstration that short-term drug intervention drastically changed the disease course with the most virulent virus isolate in a highly vulnerable host (i.e., a newborn macaque) suggests that early drug treatment following infection with a less virulent virus isolate is likely to result in even more pronounced long-term benefits, and that similar strategies are likely to be even more effective in an adult population (1). Support for this hypothesis comes from two studies, which demonstrated that 16 weeks of stavudine (d4T) treatment of HIV-2 infected macaques, and 4 weeks of 9-(2-phosphonylmethoxyethyl)adenine (PMEA) treatment of SIVmac239-infected macaques induced sustained suppression of viral replication in most animals following drug withdrawal (18, 55).
Current Public Health Service guidelines recommend using antiviral drugs for health-care workers following occupational exposure to HIV (e.g., through needle-stick) (8). Due to a lack of efficacy data, however, there is no recommendation for or against antiviral drug administration following nonoccupational (such as sexual or intravenous-drug-use) exposure to HIV (7); in addition, the guidelines question the usefulness of initiating antiviral drug treatment when the time interval between exposure and initiation of drug administration is beyond 36 to 72 h (7). Our data obtained with SIV-infected macaques are strong evidence in support of prompt antiviral drug use following any exposure to HIV. Even in cases where it may no longer be possible to eradicate the initial infection, short-term drug administration starting in the first few days after exposure may produce profound long-lasting clinical benefits.
The present study allays the concerns that early drug intervention will rapidly select for the emergence of drug-resistant mutants, which would reduce the efficacy of drug treatment at a later stage of the disease. The 14 or 60 days of PMPA administration during early SIV infection did not result in the detectable emergence of viral mutants with reduced susceptibility to PMPA. The initiation of drug treatment at 5 days is more likely to result in a slower emergence of drug-resistant viral mutants than initiating drug treatment once widespread systemic virus dissemination has occurred and many more viral variants exist (9). In this context, we have previously demonstrated that prolonged PMPA treatment of SIV-infected newborn macaques starting 3 weeks after oral SIVmac251 inoculation resulted in the emergence of SIV K65R mutants with low-level (fivefold) reduced susceptibility to PMPA after 5 to 15 weeks of treatment. We have, however, also observed that PMPA treatment of infant macaques still had therapeutic efficacy against infection with these low-level PMPA-resistant SIV mutants (48). Recent data from phase I/II human trials have demonstrated very potent antiviral effects of PMPA for HIV-infected patients, and no emergence of PMPA-resistant HIV mutants was observed during up to 4 weeks of treatment (13, 14, 26). In a phase II trial with the related analog PMEA, very few patients developed HIV mutants with reduced drug susceptibility to PMEA following 6 to 12 months of treatment, but even these patients had sustained suppression of virus levels (27). Altogether, these data suggest that short-term PMPA administration during the initial stages of HIV infection will not abrogate the potential of using PMPA treatment again to strongly suppress virus replication at a later stage of the disease course.
In conclusion, our data demonstrate that early short-term PMPA treatment limited systemic spread of the virus, reduced virus-induced immunosuppression, enhanced antiviral immune responses, and significantly delayed disease progression for SIV-infected newborn macaques. Together, these observations further support the use of potent anti-HIV drug therapy for human newborns and adults as soon as possible following HIV exposure. Especially when financial resources are limited, even treatment for only a few weeks is superior to postponing treatment until systemic infection is established.
ACKNOWLEDGMENTS
We thank E. Agatep, L. Antipa, D. Bennett, C. Berardi, J. Bernales, L. Brignolo, R. Buchholz, B. Capuano, I. Cazares, K. Christe, Z. Dehqanzada, S. Dillard-Telm, D. Florence, C. Oxford, L. Reay, G. Rogers, C. Valverde, T. Vogt, T. Wang, C. Young, and Colony Services of the California Regional Primate Research Center for expert technical assistance and J. Booth (Chiron) for performing viral RNA measurements.
This research was supported by an E. Glaser Scientist Award and NIH grant AI39109 to M.L.M. and NIH grant RR00169 to the California Regional Primate Research Center.
REFERENCES
- 1.Ammann A J. Human immunodeficiency virus infection/AIDS in children: the next decade. Pediatrics. 1994;93:930–935. [PubMed] [Google Scholar]
- 2.Blanche S, Rouzioux C, Guihard Moscato M-L, Veber F, Mayaux M-J, Jacomet C, Tricoire J, Deville A, Vial M, Firtion G, de Crepy A, Douard D, Robin M, Courpotin C, Ciraru-Vigneron N, le Deist F, Griscelli C the HIV Infection in Newborns French Collaborative Study Group. A prospective study of infants born to women seropositive for human immunodeficiency virus type 1. N Engl J Med. 1989;320:1643–1648. doi: 10.1056/NEJM198906223202502. [DOI] [PubMed] [Google Scholar]
- 3.Borkowsky W, Krasinki K, Paul D, Holzman R, Moore T, Bebenroth D, Lawrence R, Chandwani S. Human immunodeficiency virus type 1 antigenemia in children. J Pediatr. 1989;114:940–945. doi: 10.1016/s0022-3476(89)80434-2. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Update: perinatally acquired HIV/AIDS—United States, 1997. Morbid Mortal Weekly Rep. 1997;46:1086–1092. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Update: trends in AIDS incidence—United States, 1996. Morbid Mortal Weekly Rep. 1997;46:862–867. [Google Scholar]
- 6.Centers for Disease Control and Prevention. Guidelines for the use of antiretroviral agents in pediatric HIV infection. Morbid Mortal Weekly Rep. 1998;47(RR-4):1–43. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Management of possible sexual, injecting-drug-use, or other nonoccupational exposure to HIV, including considerations related to antiretroviral therapy. Public Health Service statement. Morbid Mortal Weekly Rep. 1998;47(RR-17):1–14. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Public Health Service guidelines for the management of health-care worker exposures to HIV and recommendations for postexposure prophylaxis. Morbid Mortal Weekly Rep. 1998;47(RR-7):1–34. [PubMed] [Google Scholar]
- 9.Coffin J M. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995;267:483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 10.Connor E M, Sperling R S, Gelber R, Kiselev P, Scott G, O’Sullivan M J, VanDyke R, Bey M, Shearer W, Jacobson R L, Jiminez E, O’Neill E, Bazin B, Delfraissy J-F, Culnane M, Coombs R, Elkins M, Moye J, Stratton P, Balsley J for the Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N Engl J Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 11.Dailey P J, Zamroud M, Kelso R, Kolberg J, Urdea M. Proceedings of the 13th Annual Symposium on Nonhuman Primate Models of AIDS, Monterey, Calif., 5 to 8 November 1995. 1995. Quantitation of simian immunodeficiency virus (SIV) RNA in plasma of acute and chronically infected rhesus macaques using a branched DNA (bDNA) signal amplification assay, abstr. 99. [Google Scholar]
- 12.Dawson-Saunders B, Trapp R G. Basic and clinical biostatistics. Norwalk, Conn: Appleton & Lange; 1990. [Google Scholar]
- 13.Deeks S G, Barditch-Crovo P, Lietman P S, Collier A, Safrin S, Coleman R, Cundy K C, Kahn J O. Proceedings of the 5th Conference on Retroviruses and Opportunistic Infections, Chicago, Ill., 1 to 5 February 1998. 1998. The safety and efficacy of PMPA prodrug monotherapy: preliminary results of a phase I/II dose-escalation study, abstr. 772. [Google Scholar]
- 14.Deeks S G, Barditch-Crovo P, Lietman P S, Hwang F, Cundy K C, Rooney J F, Hellmann N S, Safrin S, Kahn J O. Safety, pharmacokinetics, and antiretroviral activity of intravenous 9-[2-(R)-(phosphonomethoxy)propyl]adenine, a novel anti-human immunodeficiency virus (HIV) therapy, in HIV-infected adults. Antimicrob Agents Chemother. 1998;42:2380–2384. doi: 10.1128/aac.42.9.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein L G, Boucher C A, Morrison S H, Connor E M, Oleske J M, Lange J M A, van der Noordaa J, Bakker M, Dekker J, Scherpbier H, van den Berg H, Boer K, Goudsmit J. Persistent human immunodeficiency virus type 1 antigenemia in children correlates with disease progression. Pediatrics. 1988;82:919–924. [PubMed] [Google Scholar]
- 16.European Collaborative Study. Children born to women with HIV-1 infection: natural history and risk of transmission. Lancet. 1991;337:253–260. [PubMed] [Google Scholar]
- 17.Hirsch V M, Fuerst T R, Sutter G, Carroll M W, Yang L C, Goldstein S, Piatak M J, Elkins W R, Alvord W G, Montefiori D C, Moss B, Lifson J D. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J Virol. 1996;70:3741–3752. doi: 10.1128/jvi.70.6.3741-3752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joag S V, Li Z, Foresman L, Pinson D M, Stephens E B, Raghavan R, Navé J-F, Casara P, Narayan O. Early treatment with 9-(2-phosphonylmethoxyethyl)adenine reduces virus burdens for a prolonged period in SIV-infected rhesus macaques. AIDS Res Hum Retroviruses. 1997;13:241–246. doi: 10.1089/aid.1997.13.241. [DOI] [PubMed] [Google Scholar]
- 19.Kinloch-de Loës S, Hirschel B J, Hoen B, Cooper D A, Tindall B, Carr A, Saurat J-H, Clumeck N, Lazzarin A, Mathiesen L, Raffi F, Antunes F, Von Overbeck J, Lüthy R, Glauser M, Hawkins D, Baumberger C, Yerly S, Perneger T V, Perrin L. A controlled trial of zidovudine in primary human immunodeficiency virus infection. N Engl J Med. 1995;333:408–413. doi: 10.1056/NEJM199508173330702. [DOI] [PubMed] [Google Scholar]
- 20.Lafeuillade A, Poggi C, Tamalet C, Profizi N, Tourres C, Costes O. Effects of a combination of zidovudine, didanosine, and lamivudine on primary human immunodeficiency virus type 1 infection. J Infect Dis. 1997;175:1051–1055. doi: 10.1086/516442. [DOI] [PubMed] [Google Scholar]
- 21.Lifson J D, Nowak M A, Goldstein S, Rossio J L, Kinter A, Vasquez G, Wiltrout T, Brown C, Schneider D, Wahl L, Lloyd A L, Williams J, Elkins W R, Fauci A S, Hirsch V M. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J Virol. 1997;71:9508–9514. doi: 10.1128/jvi.71.12.9508-9514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lohman B L, Higgins J, Marthas M L, Marx P A, Pedersen N C. Development of simian immunodeficiency virus isolation, titration, and neutralization assays which use whole blood from rhesus monkeys and an antigen capture enzyme-linked immunosorbent assay. J Clin Microbiol. 1991;29:2187–2192. doi: 10.1128/jcm.29.10.2187-2192.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lü X, Kiyono H, Lu D, Kawabata S, Torten J, Srinivasan S, Dailey P J, McGhee J R, Lehner T, Miller C J. Targeted lymph-node immunization with whole inactivated simian immunodeficiency virus (SIV) or envelope and core subunit antigen vaccines does not reliably protect rhesus macaques from vaginal challenge with SIVmac251. AIDS. 1998;12:1–10. doi: 10.1097/00002030-199801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luzuriaga K, Bryson Y, Krogstad P, Robinson J, Stechenberg B, Lamson M, Cort S, Sullivan J. Combination treatment with zidovudine, didanosine, and nevirapine in infants with human immunodeficiency virus type 1 infection. N Engl J Med. 1997;336:1343–1349. doi: 10.1056/NEJM199705083361902. [DOI] [PubMed] [Google Scholar]
- 25.Marthas M L, Van Rompay K K A, Otsyula M, Miller C J, Canfield D, Pedersen N C, McChesney M B. Viral factors determine progression to AIDS in simian immunodeficiency virus-infected newborn rhesus macaques. J Virol. 1995;69:4198–4205. doi: 10.1128/jvi.69.7.4198-4205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller M, Cherrington J M, Lamy P D, Mulato A S, Anton K E. Proceedings of the 12th World AIDS Conference, Geneva, Switzerland, 28 June to 3 July 1998. 1998. Genotypic changes in HIV RT which develop during Preveon (adefovir dipivoxil) therapy do not decrease susceptibility to PMPA, abstr. 41218. [Google Scholar]
- 27.Mulato A S, Lamy P D, Miller M D, Li W-X, Anton K E, Hellmann N S, Cherrington J M. Genotypic and phenotypic characterization of human immunodeficiency virus type 1 variants isolated from AIDS patients after prolonged adefovir dipivoxil therapy. Antimicrob Agents Chemother. 1998;42:1620–1628. doi: 10.1128/aac.42.7.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nahmias A J, Clark W S, Kourtis A P, Lee F K, Cotsonis G, Ibegbu C, Thea D, Palumbo P, Vink P, Simonds R J, Nesheim S R. Thymic dysfunction and time of infection predict mortality in human immunodeficiency virus-infected infants. J Infect Dis. 1998;178:680–685. doi: 10.1086/515368. [DOI] [PubMed] [Google Scholar]
- 28a.National Research Council Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Resources. Guide for the care and use of laboratory animals. Washington, D.C: National Academy Press; 1996. [Google Scholar]
- 29.Nowak M A, Lloyd A L, Vasquez G M, Wiltrout T A, Wahl L M, Bischofberger N, Williams J, Kinter A, Fauci A S, Hirsch V M, Lifson J D. Viral dynamics of primary viremia and antiretroviral therapy in simian immunodeficiency virus infection. J Virol. 1997;71:7518–7525. doi: 10.1128/jvi.71.10.7518-7525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otsyula M G, Miller C J, Marthas M L, Van Rompay K K A, Collins J R, Pedersen N C, McChesney M B. Virus-induced immunosuppression is linked to rapidly fatal disease in infant rhesus macaques infected with simian immunodeficiency virus. Pediatr Res. 1996;39:630–635. doi: 10.1203/00006450-199604000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Pachl C, Todd J A, Kern D G, Sheridan P J, Fong S-J, Stempein M, Hoo B, Besemer D, Yeghiazarian T, Irvine B, Kolberg J, Kokka R, Neuwald P, Urdea M S. Rapid and precise quantification of HIV-1 RNA in plasma using a branched DNA signal amplification assay. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;8:446–454. doi: 10.1097/00042560-199504120-00003. [DOI] [PubMed] [Google Scholar]
- 32.Palumbo P E, Kwok S, Waters S, Wesley Y, Lewis D, McKinney N, Bardeguez A, Connor E, Oleske J M. Viral measurement by polymerase chain reaction-based assays in human immunodeficiency virus-infected infants. J Pediatr. 1995;126:592–595. doi: 10.1016/s0022-3476(95)70357-8. [DOI] [PubMed] [Google Scholar]
- 33.Palumbo P E, Raskino C, Fiscus S, Pahwa S, Fowler M G, Spector S A, Englund J A, Baker C J. Predictive value of quantitative plasma HIV RNA and CD4+ lymphocyte count in HIV-infected infants and children. JAMA. 1998;279:756–761. doi: 10.1001/jama.279.10.756. [DOI] [PubMed] [Google Scholar]
- 34.Perrin L, Rakik A, Yerly S, Baumberger C, Kinloch-de Loes S, Pechere M, Hirschel B. Combined therapy with zidovudine and L-697, 661 in primary HIV infection. AIDS. 1996;10:1233–1237. doi: 10.1097/00002030-199609000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Pollack H, Zhan M X, Ilmet-Moore T, Ajuang-Simbiri K, Krasinski K, Borkowsky W. Ontogeny of anti-human immunodeficiency virus (HIV) antibody production in HIV-1-infected infants. Proc Natl Acad Sci USA. 1993;90:2340–2344. doi: 10.1073/pnas.90.6.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 37.Saag M S, Crain M J, Decker W D, Campbell-Hill S, Robinson S, Brown W E, Leuther M, Whitley R J, Hahn B H, Shaw G M. High-level viremia in adults and children infected with human immunodeficiency virus: relation to disease stage and CD4+ lymphocyte levels. J Infect Dis. 1991;164:72–80. doi: 10.1093/infdis/164.1.72. [DOI] [PubMed] [Google Scholar]
- 38.Schacker T W, Hughes J P, Shea T, Coombs R W, Corey L. Biological and virologic characteristics of primary HIV infection. Ann Intern Med. 1998;128:613–620. doi: 10.7326/0003-4819-128-8-199804150-00001. [DOI] [PubMed] [Google Scholar]
- 39.Scott G B, Hutto C, Makuch R W, Mastrucci M T, O’Connor T, Mitchell C D, Trapido E J, Parks W P. Survival in children with perinatally acquired human immunodeficiency virus type 1 infection. N Engl J Med. 1989;321:1791–1796. doi: 10.1056/NEJM198912283212604. [DOI] [PubMed] [Google Scholar]
- 40.Tindall B, Carr A, Goldstein D, Penny R, Cooper D A. Administration of zidovudine during primary HIV-1 infection may be associated with a less vigorous immune response. AIDS. 1993;7:127–128. doi: 10.1097/00002030-199301000-00020. [DOI] [PubMed] [Google Scholar]
- 41.Tsai C-C, Emau P, Follis K E, Beck T W, Benveniste R E, Bischofberger N, Lifson J D, Morton W R. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl)adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72:4265–4273. doi: 10.1128/jvi.72.5.4265-4273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai C-C, Follis K E, Beck T W, Sabo A, Grant R F, Bischofberger N, Benveniste R E. Prevention of simian immunodeficiency virus infection in macaques by 9-(2-phosphonylmethoxypropyl)adenine (PMPA) Science. 1995;270:1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 43.UNAIDS/WHO. Report on the global HIV/AIDS epidemic. Geneva, Switzerland: UNAIDS; 1997. . December. [Google Scholar]
- 44.Vanhems P, Lambert J, Cooper D A, Perrin L, Carr A, Hirschel B, Vizzard J, Kinloch-de Loës S, Allard R. Severity and prognosis of acute human immunodeficiency virus type 1 illness: a dose-response relationship. Clin Infect Dis. 1998;26:323–329. doi: 10.1086/516289. [DOI] [PubMed] [Google Scholar]
- 45.Van Rompay K K A, Berardi C J, Aguirre N L, Bischofberger N, Lietman P S, Pedersen N C, Marthas M L. Two doses of PMPA protect newborn macaques against oral simian immunodeficiency virus infection. AIDS. 1998;12:F79–F83. doi: 10.1097/00002030-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Van Rompay K K A, Berardi C J, Dillard-Telm S, Tarara R P, Canfield D R, Valverde C R, Montefiori D C, Stefano Cole K, Montelaro R C, Miller C J, Marthas M L. Passive immunization of newborn rhesus macaques prevents oral simian immunodeficiency virus infection. J Infect Dis. 1998;177:1247–1259. doi: 10.1086/515270. [DOI] [PubMed] [Google Scholar]
- 47.Van Rompay K K A, Cherrington J M, Marthas M L, Berardi C J, Mulato A S, Spinner A, Tarara R P, Canfield D R, Telm S, Bischofberger N, Pedersen N C. 9-[2-(Phosphonomethoxy)propyl]adenine therapy of established simian immunodeficiency virus infection in infant rhesus macaques. Antimicrob Agents Chemother. 1996;40:2586–2591. doi: 10.1128/aac.40.11.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Rompay, K. K. A., J. M. Cherrington, M. L. Marthas, P. D. Lamy, P. J. Dailey, D. R. Canfield, R. P. Tarara, N. Bischofberger, and N. C. Pedersen. 9-[2-(Phosphonomethoxy)propyl]adenine (PMPA) therapy prolongs survival of infant macaques inoculated with simian immunodeficiency virus with reduced susceptibility to PMPA. Antimicrob. Agents Chemother., in press. [DOI] [PMC free article] [PubMed]
- 49.Van Rompay, K. K. A., and M. L. Marthas. Unpublished data.
- 50.Van Rompay K K A, Marthas M L, Lifson J D, Berardi C J, Vasquez G M, Agatep E, Dehqanzada Z A, Cundy K C, Bischofberger N, Pedersen N C. Administration of 9-[2-(phosphonomethoxy)propyl]adenine (PMPA) for prevention of perinatal simian immunodeficiency virus infection in rhesus macaques. AIDS Res Hum Retroviruses. 1998;14:761–773. doi: 10.1089/aid.1998.14.761. [DOI] [PubMed] [Google Scholar]
- 51.Van Rompay K K A, Marthas M L, Ramos R A, Mandell C P, McGowan E K, Joye S M, Pedersen N C. Simian immunodeficiency virus (SIV) infection of infant rhesus macaques as a model to test antiretroviral drug prophylaxis and therapy: oral 3′-azido-3′-deoxythymidine prevents SIV infection. Antimicrob Agents Chemother. 1992;36:2381–2386. doi: 10.1128/aac.36.11.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Rompay K K A, Otsyula M G, Marthas M L, Miller C J, McChesney M B, Pedersen N C. Immediate zidovudine treatment protects simian immunodeficiency virus-infected newborn macaques against rapid onset of AIDS. Antimicrob Agents Chemother. 1995;39:125–131. doi: 10.1128/aac.39.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Rompay K K A, Otsyula M G, Tarara R P, Canfield D R, Berardi C J, McChesney M B, Marthas M L. Vaccination of pregnant macaques protects newborns against mucosal simian immunodeficiency virus infection. J Infect Dis. 1996;173:1327–1335. doi: 10.1093/infdis/173.6.1327. [DOI] [PubMed] [Google Scholar]
- 54.Vella P P, Ellis R W. Immunogenicity of Haemophilus influenzae type b conjugate vaccines in infant rhesus monkeys. Pediatr Res. 1991;29:10–13. doi: 10.1203/00006450-199101000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Watson A, McClure J, Ranchalis J, Scheibel M, Schmidt A, Kennedy B, Morton W R, Haigwood N L, Hu S-L. Early postinfection antiviral treatment reduces viral load and prevents CD4+ cell decline in HIV type 2-infected macaques. AIDS Res Hum Retroviruses. 1997;13:1375–1381. doi: 10.1089/aid.1997.13.1375. [DOI] [PubMed] [Google Scholar]
- 56.Withers-Ward E, Amado R F, Koka P S, Jamieson B D, Kaplan A H, Chen I S Y, Zack J A. Transient renewal of thymopoiesis in HIV-infected human thymic implants following antiviral therapy. Nat Med. 1997;3:1102–1109. doi: 10.1038/nm1097-1102. [DOI] [PubMed] [Google Scholar]