Abstract
Gestational diabetes mellitus (GDM) is a major pregnancy complication affecting approximately 14.0% of pregnancies around the world. Air pollution exposure, particularly exposure to , has become a major environmental issue affecting health, especially for vulnerable pregnant women. Associations between exposure and adverse birth outcomes are generally assumed to be the same throughout a large geographical area. However, the effects of air pollution on health can very spatially in subpopulations. Such spatially varying effects are likely due to a wide range of contextual neighborhood and individual factors that are spatially correlated, including SES, demographics, exposure to housing characteristics and due to different composition of particulate matter from different emission sources. This combination of elevated environmental hazards in conjunction with socioeconomic-based disparities forms what has been described as a “double jeopardy” for marginalized sub-populations. In this manuscript our analysis combines both an examination of spatially varying effects of a) unit-changes in exposure and examines effects of b) changes from current exposure levels down to a fixed compliance level, where compliance levels correspond to the Air Quality Standards (AQS) set by the U.S. Environmental Protection Agency (EPA) and World Health Organization (WHO) air quality guideline values. Results suggest that exposure reduction policies should target certain “hotspot” areas where size and effects of potential reductions will reap the greatest rewards in terms of health benefits, such as areas of southeast Los Angeles County which experiences high levels of exposures and consist of individuals who may be particularly vulnerable to the effects of air pollution on the risk of GDM.
Keywords: Gestational diabetes mellitus; Spatially varying effects; Air pollution exposures; Bayesian modeling, multi-level models
1. Introduction
Gestational diabetes mellitus (GDM) is defined as diabetes diagnosed in the second or third trimester of pregnancy that was not clearly overt diabetes prior to gestation (American Diabetes Association Professional Practice Committee, 2021). GDM is a major pregnancy complication affecting approximately 14.0% of pregnancies around the world (Wang et al., 2022) and 7.6% of pregnancies in the U.S. (Casagrande et al., 2018) and is associated with higher risk of short- and long-term adverse health outcomes in both mothers and their offspring (Daly et al., 2018; Farahvar et al., 2019; Mirghani Dirar and Doupis, 2017; Tobias et al., 2017). Mothers who have GDM are more likely to develop preeclampsia during pregnancy, as well as type 2 diabetes, metabolic syndrome, and cardiovascular diseases after delivery (Daly et al., 2018; Farahvar et al., 2019; Lai et al., 2016; Mirghani Dirar and Doupis, 2017; Tobias et al., 2017). For offspring, a series of increased risk of adverse health outcomes in relation to GDM are reported, including preterm birth, macrosomia, neonatal hypoglycemia, hyperbilirubinemia and hypocalcemia (Farrar et al., 2016; Martino et al., 2016; Yang et al., 2019), childhood autism, obesity, as well as diabetes and cardiometabolic disorders later in life (Clausen et al., 2008; Farahvar et al., 2019; Jo et al., 2019; Metzger, 2007; Nijs and Benhalima, 2020; Tam et al., 2017; Xiang et al., 2015; Xu et al., 2014).
Air pollution has become a major environmental issue affecting health, especially for vulnerable pregnant women. Exposure to has been shown to be associated with higher risk of adverse pregnancy and birth outcomes, such as GDM (Sun et al., 2021), hypertensive disorders of pregnancy (Bai et al., 2020), term low-birth weight ((Wilhelm et al., 2012)), and preterm birth (Lamichhane et al., 2015,(Ritz et al., 2007)). Generally, associations between exposure and adverse birth outcomes are assumed to be the same throughout a large geographical area. However, the effects of air pollution on health can very spatially in subpopulations. For example, (Coker et al., 2015) found that the association of exposure with term low-birth weight were greater within the urban core of Central and Southern Los Angeles County census tracts where large percentages of people of lower socioeconomic status (SES) resided. Such spatially varying effects are likely due to a wide range of contextual neighborhood and individual factors that are spatially correlated, including SES, demographics, exposure to violence ((Messer et al., 2006)), access to healthy food ((Walker et al., 2010)) or green space ((Hystad et al., 2014)), housing characteristics and due to different composition of particulate matter from different emission sources. To the best of our knowledge, no study has explored the spatial variation in effects (not just concentration levels) on GDM in a large obstetric population. Simply estimating global pollutant effects may obscure disparities suggested by spatial patterns. In addition, identifying effect “hotspots”, i.e., areas where policy interventions are likely to reap the biggest rewards in terms of reducing incidence of GDM, could help guide spatially targeted public health interventions and protect susceptible subpopulations.
While effects of unit-level reductions in exposure may vary spatially in a large geographical region, one must also consider the reality that base-line levels of air pollution exposures vary spatially as well. Such spatially varying levels of exposure mean that exposure reduction policies may result in larger reductions in some regions over others. One goal of exposure reduction policies is to reduce exposures from current levels down to compliance levels where compliance levels corresponds to National Ambient Air Quality Standards (NAAQS) set by the U.S. Environmental Protection Agency (EPA) (US EPA, 2020) and World Health Organization (WHO) air quality guideline values ((WHO)). These compliance levels are designed to guide policy makers in limiting the amount of in the air, and thus protect public health by reducing exposure to harmful air pollutants. Here, ambient air quality standards are important in that they define the maximum amount of pollutant that can be present in outdoor air without harming human health. (CARB, 2023; Brook et al., 2010; Krewski, 2009; Laden et al., 2006).
Air pollution studies consistently show that communities of color and those containing large proportions of low-income residents are exposed to higher levels of air pollution as they are often located near major sources of exposures such as highways and industrial facilities (Hajat et al., 2015). In addition, these disadvantaged communities are more susceptible to the harmful effects of elevated exposures to social stressors such as poor diet, poor quality of housing stock, being lower on the social hierarchy, and other lifestyle factors such as smoking and the weathering effects of racism (Morello-Frosch et al., 2011; Geronimus et al., 2006). This combination of elevated environmental hazards in conjunction with socioeconomic-based disparities forms what has been described as a “double jeopardy” for marginalized sub-populations, suggesting that individuals living in disadvantaged communities would suffer worse health effects compared to more advantaged people, even at the same levels of exposures (Li et al., 2022; Su et al., 2016; Molitor et al., 2011; Morello-Frosch and Bill, 2006). As such it is important to look at how air pollution reduction policies can affect individuals both in terms of addressing elevated exposure levels facing vulnerable subpopulations and deal with how such reductions differentially affect individuals because of their socio-economic status. Here, double jeopardy refers to inequalities in both exposure and susceptibility. In this manuscript our analysis combines both an examination of spatially varying effects of a) unit-changes in exposure and examines effects of b) changes from current exposure levels down to a fixed compliance levels, Here, different individuals may experience different levels of exposure reductions based on individual exposure reduction needs. By looking at impacts of exposure reduction in regard to both amount and effect of reduction we appeal to certain aspects of the concept of equity in exposure effect modeling, which refers to “inequities in health systematically put groups of people who are already socially disadvantaged (for example, by virtue of being poor, female, and/or members of a disenfranchised racial, ethnic, or religious group) at further disadvantage with respect to their health.” (Ramirez et al., 2008). This approach considers the possibility that ethnic minorities and people living in low and middle-income areas may be more vulnerable to air pollution due, in part, to the higher levels of air pollution to which they are exposed (Samet, 2004). As such, we examine spatially varying differential effects of exposure reduction on health in a manner that considers both subpopulation vulnerability and elevated exposure levels. Our reasons for examining such effects are two-fold: (1) We expect that different regions (census tracts in our case) will have different base-line levels of and thus have different potentials for reduction, with regions containing very high levels of exposure having greater potential for large exposure reductions as a result of policy enforcement; (2) Unit-level effects of policy on exposure reductions and health improvements will likely be greater in areas of low SES compared to areas of high SES where individuals are somewhat insulated from effects of high exposure and will likely benefit less from measures used to reduce exposures. In this way, we focus on real-world issues related to effects of exposure reduction policy, with an eye towards the fact that policy implementation may result in greater health benefits in some regions than others. Note here that our results will be beneficial to policy makers as we establish links between exposure and GDM and provide information regarding areas where policies related to air pollution reduction intervention will likely reap the biggest rewards in terms of health benefits related to GDM.
Our examination focuses on spatially varying reductions down to compliance, but in a manner which takes into account the spatially varying effects that each unit of reduction has on GDM. Thus, in this paper, we aim to examine the spatially varying effects of on GDM in southern California with the broad hypothesis that such effects and policy-based reduction will vary spatially both in size and effect, and such variations will be affected by contextual-based factors such as living in a lower SES community and issues such as diminished access to care or occupational status.
2. Methods
2.1. Study population and GDM outcome
This retrospective cohort study used electronic health records (EHRs) from all Kaiser Permanente Southern California (KPSC) facilities, including women who gave birth to singleton children between January 1, 2008, and December 31, 2017. We excluded women who were not KPSC members or with gestational age ≤ 20 or ≥ 47 weeks (), with multiple birth (), with stillbirth (), without address data (), with incomplete covariates data (), or lived in rural areas (). We also excluded pregnancies with preexisting diabetes () or missing GDM lab test results (). In total, 341,909 women were included in this analysis. GDM diagnosis was based on KPSC laboratory tests using two criteria for GDM testing: the Carpenter-Coustan criteria (Carpenter and Coustan, 1982) or the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria (Metzger, 2010). More details of this population and GDM diagnosis have been described in our previous work (Sun et al., 2022). This study was approved by the Institutional Review Board of KPSC and the University of California, Irvine.
2.2. exposure
Historical ambient monthly measurements from 2007 to 2018 were obtained from the fine-resolution geoscience-derived models (Meng et al., 2019; van Donkelaar et al., 2019), which provide validated and publicly available outputs at a 1-km resolution over North America based on satellite remote sensing, GEOS-Chem chemical transport modeling and ground-based monitors with a geographically weighted regression. Simulated relative composition using relative-humidity-dependent and composition-dependent fixed size distribution was applied to the hybrid mass to produce constituents. All the 1-km grids that are within or intersecting a census tract were averaged to assess the census tract-level air pollution exposures. Air pollution estimates were spatiotemporally linked to each woman based on the geocoded maternal residential addresses. We calculated entire-pregnancy and trimester-specific and entire-pregnancy exposures by averaging the air pollution measurements in each specific time period: entire-pregnancy (from the date of conception to the date of delivery); the first trimester (1st - 3rd gestational months) and second trimester (4th - 6th gestational months). Our previous work showed that the patterns of the association between GDM and air pollution were similar for different exposure windows during pregnancy (Sun et al., 2021). In addition, most pregnant women were routinely screened for GDM between 24 and 28 weeks of gestation. Therefore, our main exposure for the GDM risk is total mass in the first and second trimester. As a sensitivity, we also analyzed other constituents: sulfate, nitrate, ammonium, organic matter, and black carbon. Correlations between various constituents are displayed in the plot in Appendix F.
2.3. Covariates
Pregnancy-related covariates and potential confounders were selected a priori based on the existing literature (Eze et al., 2015; Thiering and Heinrich, 2015; Zhang et al., 2020) and abstracted from the KPSC EHRs: maternal age, race/ethnicity (African American, Asian, Hispanic, non-Hispanic white, and others including Pacific Islanders, Native American/Alaskan and mothers with multiple race/ethnicities specified) and educational level (≤8th grade, 9th grade to high school, college <4 years, and college ≥4 years); median household income in the block group of residence at birth (CDC, 2023); pre-pregnancy body mass index [BMI, kg/m2: underweight (<18.5), normal (18.5–24.9), overweight (25.0–29.9) and obese (≥30.0)]; maternal smoking status during pregnancy (never smoker, ever smoker, smoking during pregnancy, and passive smoker); insurance type; season of conception (warm: May–October; cool: November–April) and year of infant birth.
2.4. Statistical analysis
Here we employ hierarchical methods in the spirit of Spatially Varying Coefficient (SVC) models (Coker et al., 2015; Franco-Villoria et al., 2019a) in a Bayesian setting to estimate the varying effects of exposure in a unified multivariate manner. We model “intercepts”, or baseline levels of log-odds of GDM, and “slopes”, or spatially varying effects of increases in exposure, jointly via a level-2 multivariate normal distribution. Note that these spatially varying slopes reflects effect modification by spatially varying environmental conditions. (For a book-level discussion of hierarchical models and joint modeling of intercepts and slopes, see Gelman and Jennifer, 2006). Our analysis suggests that both intercepts and slopes are highly correlated, necessitating the multivariate approach. (For example, in analyzing exposures the correlation coefficient between intercepts and slopes was .) Since the intercepts and slopes are modeled jointly, our model incorporates a kind of smoothing or “borrowing of strength” where parameter estimates corresponding to a region in question can inform estimates of other regions, and where estimates corresponding to regions with higher sample sizes exert more influence in the smoothing process compared to regions with smaller sample sizes.
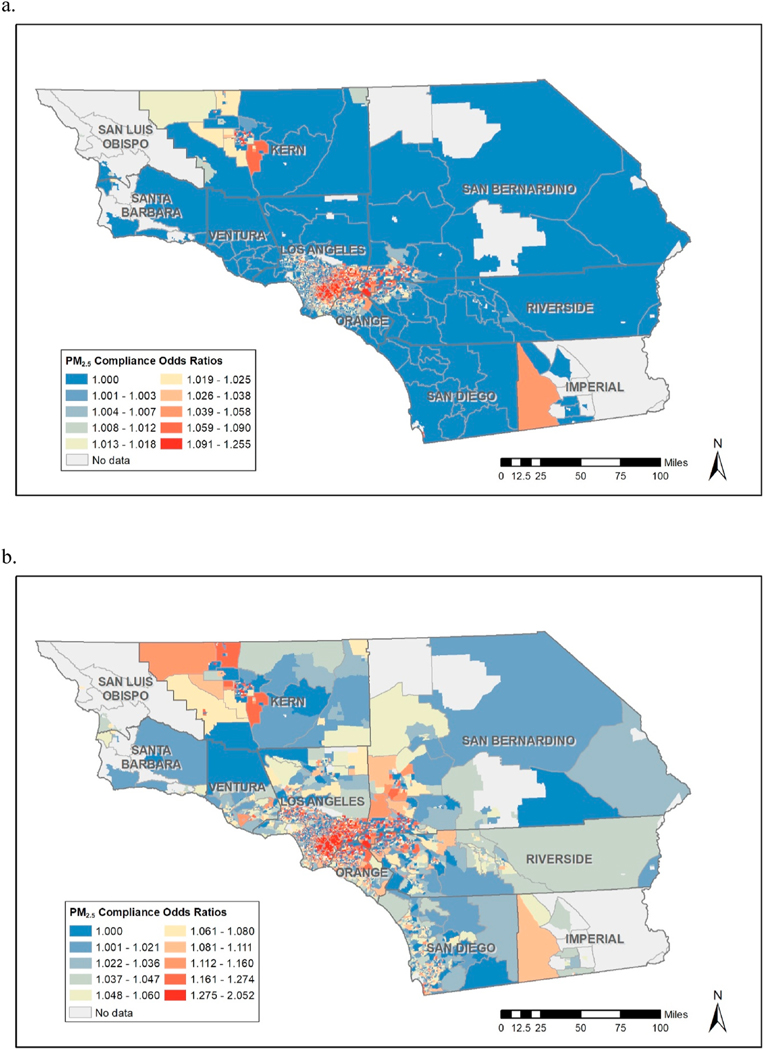
Here, one could improve inference by incorporating spatial smoothing techniques, which utilize information regarding regions (in our case census tracts) in a manner that nearby regions influence region-specific parameter estimates more than those farther away, via models such as the Conditional Auto Regression (CAR) model (Besag et al., 1991) or the related Besag-York-Mollie (BYM) model (J. Besag, York, and Molli\’e 1991). It would seem to make sense to use these approaches to model both sets of parameters (intercepts and slopes) in a spatial manner as was done in (Franco-Villoria et al., 2019a). However, modeling both intercepts and slopes in this manner is potentially problematic due to issues of “spatial confounding” (Hodges et al., 2010), where, for example, spatially varying intercepts terms can compete with slopes (effects of exposure) and thus explain away or and “wash out” these important, real effects. This is less of an issue when utilizing the common disease mapping models employed by (Franco-Villoria et al., 2019a) where the focus in on predicting outcomes (relative risks) related to disease counts. Note that Franco-Villoria et al. did address this issue to some extent via the use of careful chosen informative prior specifcation via Penalized-Complexity (PC) priors. (Simpson et al., 2017). An alternative strategy to dealing with such confounding issues would be to adopt the approach of Coker et al. (2015) and only model exposure effects (slopes) spatially, thus sidestepping this spatial confounding issue to some extent. However, here we wish to model relatively highly correlated spatially varying intercept terms along with effect (slope) parameters which would suggest that if spatial smoothing were desired one should model these intercepts and slopes jointly with multivariate spatial model such as the Multivariate-CAR model (Palmí-Perales et al., 2021). However, these models are difficult to fit, complicated in a Bayesian setting regarding prior specification, and may still suffer from spatial confounding issues. Here, for the most part, we forgo these complexities related to spatial smoothing and fit standard hierarchical models employed by Gelman and Jennifer (2006), which are sufficiently complex to capture the spatially varying effects for our analyses. We do, however, as a sensitivity analysis include an analysis utilizing spatial smoothing on slopes only as done by Coker et al. (2015) (See Fig. 4).
Fig. 4.
Spatial Smoothing: Compliance Odds Ratios for total mass corresponding to the standard of (a) the U.S. EPA: 12 μg/m3 and (b) the WHO: 5 μg/m3.
One aspect of standard hierarchal models (including SVC models) and of regression-based models in general is that effects are generally measured in terms of effects on health, for individual , for one unit change in exposure . For example, if denotes exposure then the “beta” coefficient (slope) in a regression model will indicate predicted change in health outcome (or perhaps log odds of equal to one) for a one-unit change (reduction) in exposure, say the change of to . This is true weather or not the effect parameters vary by region. However, in this paper we examine spatially varying effects of single-unit reductions of exposure but in addition we also examine effects of bringing exposures down from current levels to “compliance” levels, namely exposure levels consistent with Air Quality Standards (AQS) set by the U.S. Environmental Protection Agency (EPA) (US EPA, 2020), and World Health Organization (WHO) air quality guideline values (WHO).
In the case of total , individual-level compliance, denoted by , is set to a secondary annual standard of , as defined by the U.S. EPA as an area that meets the standard “if the three-year average of its annual average concentration is less than or equal to the level of the standard.” (US EPA, 2020). We examine effects of changes from current levels of exposure down to compliance levels (with compliance defined above), or, more generally, the effect of going from exposure levels to , where (current exposure level) and . As a sensitivity analysis, we also define compliance as , which is the guideline values for fine particulate matter as specified by the WHO (WHO).
We outline our model as follows. For individual , we denote for presence of GDM and otherwise, denotes probability of GDM for individual , denotes the neighborhood-level exposure. Our model is:
| (1) |
where and represent baseline (intercepts) exposure levels and exposure effects (slopes) respectively for the region to which individual belongs (with census tract as region in this case), and corresponds to baseline covariate adjustments corresponding to mother’s age, race/ethnicity, insurance type, education, BMI, smoking status, household income at birth, season of conception, and year of infant birth. We model intercepts and slope terms via standard random effects constructs with error terms which incorporates a multivariate structure. Our slopes and intercepts are as:
| (2) |
| (3) |
We jointly model intercepts and slopes as multivariate normal where is modeled as multivariate normal as,
| (4) |
Our analysis is conducted via the Bayesian software package R-INLA (Rue et al., 2009), a well-established software package which provides a fast Bayesian estimation of parameters for models utilized in complex spatially-orient problems. We were able to jointly model the error terms via use of the recently developed “copy” command in INLA (See Gómez-Rubio, 2020) Default flat priors were utilized, though all continuous covariates were standardized (subtracting mean and dividing by standard deviation), including exposures, which to some extent alleviated issues related to invertedly providing highly informative prior specifications. (Compliance levels were subjected to the same standardization.)
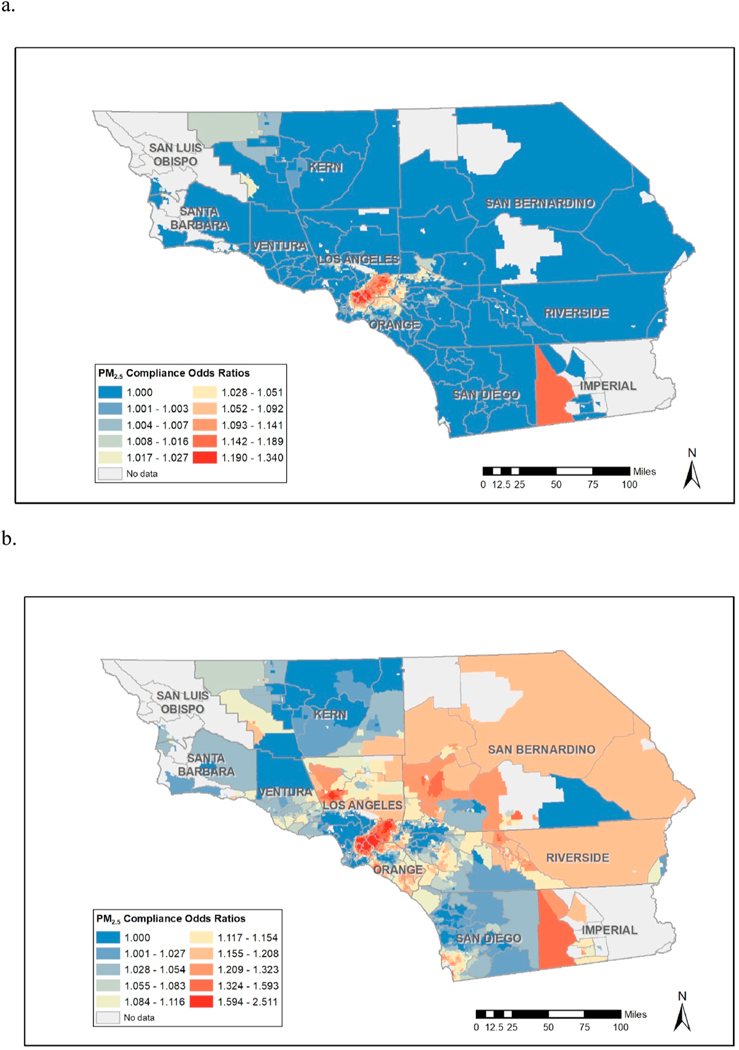
Using the R-INLA package, we are able to compute relevant odds ratios (OR’s) corresponding to unit reductions in exposure (Fig. 1a) and exceedance probabilities corresponding to the likelihood that the corresponding odds ratio (OR) is greater than one, namely for each census tract, , in a manner consistent with (Coker et al., 2015) (See Fig. 1b.). In order to examine compliance effects, or effects on GDM for exposure reduction from current levels down to compliance level , we simply compute odds ratios corresponding to the average exposure for region as compared to compliance, namely the odds ratio appropriate for an individual with exposure for region compared to , corresponding to reductions , where or (before standardization). We dub these ratios as Compliance Odds Ratios (COR’s) as they incorporate different reductions for different individuals ( for individual ) throughout space and incorporate different unit-level effects corresponding to the region to which individual belongs. We note that for some census tracts, the average exposure levels are already below compliance. Since we are not interested in examining interventions which may correspond to exposure increases, we set in these areas, thus denoting that no reduction in exposure is required to achieve compliance levels. For a small number of regions, the point estimates for are negative, meaning that for these few areas the model is suggesting a protective effect of air pollution (though usually these protective effects are associated with high exceedance probabilities, i.e., high uncertainty.) Such effects may be due to exposure misclassification, residual confounding, or perhaps model misspecification. Note that we are modeling linear effects per region (but not a linear effect across the whole geographical space is as often done) and this linearly could be distorting the exposure/response relationship at low levels of (See for example, (Weichenthal et al., 2022), for a discussion on supralineasr effects of very low levels of .). In these circumstances, where the is negative, we fix as we are trying to focus on areas where exposure reductions have the largest effect on reducing likelihood of GDM. With these restrictions in place we can then compute COR’s for all census tracts which thus enable us to produce maps which display “hotspot” areas where reductions of individual-level exposures levels down to compliance present the greatest benefit in terms of reduction in probability of GDM.
Fig. 1.
total mass (a) odds ratios corresponding to unit changes on exposure and (b) exceedance probabilities corresponding unit reductions in exposure .
3. Results
We first reiterate that all continuous variables used in the model, including exposures, have been standardized by subtracting off the relevant means and dividing by the standard deviations. Also, the correlation between intercepts and slopes is positive 0.426 (0.308, 0.538) (equation (4)), suggesting that higher effects of exposure reduction are aligned with higher baseline levels of GDM.
The distribution of selected demographic and pregnancy characteristics is presented in Table 1. Among 341,909 women included in our study population, 37,711 (11.0%) cases of GDM with clinical diagnosis were identified. The mean of maternal age in our study was 30.2 years. Compared to the entire cohort, GDM cases were found more frequently among older mothers, Asian or Hispanic mothers, mothers who lived in low-income neighborhoods, and overweight or obese mothers. Individual residential exposure levels of air pollution metrics during entire pregnancy were shown in Table 2. Overall, air pollution exposures were higher among mothers with GDM, younger mothers, African American or Hispanic mothers, mothers with low education, mothers who lived in low-income neighborhoods, and obese mothers for and chemical constituents. In addition, total mass and constituents, including nitrate, organic matter and black carbon, were higher among mothers who conceived in warm season, while sulfate levels were higher for mothers who conceived in cool season.
Table 1.
Descriptive statistics for selected population characteristics by Gestational diabetes (GDM) groups, 2008–2017.
| Characteristics | Total births |
GDM |
Non-GDM |
|||
|---|---|---|---|---|---|---|
| N | mean/percent | N | mean/percent | N | mean/percent | |
| Maternal age, years | 341,909 | 30.21 | 37,711 | 32.56 | 304,198 | 29.91 |
| Median household income, $ | 341,909 | 60,137.68 | 37,711 | 58,474.69 | 304,198 | 60,343.84 |
| Maternal race/ethnicity | ||||||
| African American | 25,263 | 0.07 | 1962 | 0.05 | 23,301 | 0.08 |
| Non-Hispanic Asian | 44,210 | 0.13 | 7680 | 0.20 | 36,530 | 0.12 |
| Hispanic | 172,342 | 0.50 | 20,712 | 0.55 | 151,630 | 0.50 |
| Non-Hispanic white | 91,432 | 0.27 | 6568 | 0.17 | 84,864 | 0.28 |
| Multiple/other | 8662 | 0.03 | 789 | 0.02 | 7873 | 0.03 |
| Maternal education | ||||||
| ≤8th grade | 3521 | 0.01 | 738 | 0.02 | 2783 | 0.01 |
| 9th grade – high school | 103,794 | 0.30 | 11,070 | 0.29 | 92,724 | 0.30 |
| College (<4 years) | 77,773 | 0.23 | 8581 | 0.23 | 69,192 | 0.23 |
| College (4 years) | 110,240 | 0.32 | 12,477 | 0.33 | 97,763 | 0.32 |
| College | 46581 | 0.14 | 4845 | 0.13 | 41736 | 0.14 |
| Smoking | ||||||
| Never Smoker | 284,956 | 0.83 | 31,509 | 0.84 | 253,447 | 0.83 |
| Ever Smoker | 39,273 | 0.11 | 4410 | 0.12 | 34,863 | 0.11 |
| Smoking during pregnancy | 17,680 | 0.05 | 1792 | 0.05 | 15,888 | 0.05 |
| Pre-pregnancy BMI in categories | ||||||
| Underweight (<18.5) | 8525 | 0.02 | 473 | 0.01 | 8052 | 0.03 |
| Normal (18.5–24.9) | 150,602 | 0.44 | 10,421 | 0.28 | 140,181 | 0.46 |
| Overweight (25.0–29.9) | 96,246 | 0.28 | 11,233 | 0.30 | 85,013 | 0.28 |
| Obese (≥30.0) | 86,536 | 0.25 | 15,584 | 0.41 | 70,952 | 0.23 |
| Season of conception | ||||||
| Cool season | 173,652 | 0.51 | 19,707 | 0.52 | 153,945 | 0.51 |
| Warm season | 168,257 | 0.49 | 18,004 | 0.48 | 150,253 | 0.49 |
| Year of infant birth | ||||||
| 2008 | 31,368 | 0.09 | 3483 | 0.09 | 27,885 | 0.09 |
| 2009 | 30,680 | 0.09 | 3265 | 0.09 | 27,415 | 0.09 |
| 2010 | 30,687 | 0.09 | 3397 | 0.09 | 27,290 | 0.09 |
| 2011 | 32,313 | 0.09 | 4557 | 0.12 | 27,756 | 0.09 |
| 2012 | 33,833 | 0.10 | 4435 | 0.12 | 29,398 | 0.10 |
| 2013 | 33,580 | 0.10 | 3446 | 0.09 | 30,134 | 0.10 |
| 2014 | 35,166 | 0.10 | 3668 | 0.10 | 31,498 | 0.10 |
| 2015 | 36,542 | 0.11 | 3828 | 0.10 | 32,714 | 0.11 |
| 2016 | 38,455 | 0.11 | 3658 | 0.10 | 34,797 | 0.11 |
| 2017 | 39,285 | 0.11 | 3974 | 0.11 | 35,311 | 0.12 |
Table 2.
Individual air pollution levels during pregnancy by selected population characteristics.
| Characteristics | total mass | sulfate | nitrate | ammonium | organic matter | black carbon |
|---|---|---|---|---|---|---|
| Total births | 12.93 (2.53) | 1.28 (0.26) | 2.42 (0.62) | 0.95 (0.31) | 5.43 (1.27) | 1.50 (0.61) |
| Gestational diabetes | ||||||
| Yes | 13.20 (2.44) | 1.29 (0.26) | 2.49 (0.60) | 0.98 (0.30) | 5.51 (1.22) | 1.59 (0.62) |
| No | 12.91 (2.54) | 1.28 (0.26) | 2.42 (0.63) | 0.95 (0.31) | 5.41 (1.28) | 1.49 (0.61) |
| Maternal age | ||||||
| <25 | 13.22 (2.55) | 1.30 (0.27) | 2.47 (0.64) | 0.98 (0.31) | 5.57 (1.29) | 1.54 (0.60) |
| 25–35 | 12.89 (2.54) | 1.28 (0.26) | 2.42 (0.62) | 0.95 (0.31) | 5.40 (1.27) | 1.49 (0.61) |
| >35 | 12.82 (2.48) | 1.27 (0.26) | 2.40 (0.59) | 0.93 (0.30) | 5.36 (1.24) | 1.50 (0.62) |
| Maternal race/ethnicity | ||||||
| African American | 13.20 (2.23) | 1.28 (0.25) | 2.45 (0.53) | 0.97 (0.27) | 5.55 (1.17) | 1.59 (0.57) |
| Asian | 12.95 (2.37) | 1.28 (0.26) | 2.43 (0.57) | 0.95 (0.29) | 5.40 (1.20) | 1.54 (0.60) |
| Hispanic | 13.34 (2.48) | 1.30 (0.26) | 2.51 (0.61) | 0.98 (0.30) | 5.60 (1.26) | 1.57 (0.62) |
| Non-Hispanic white | 12.14 (2.59) | 1.24 (0.26) | 2.26 (0.65) | 0.89 (0.32) | 5.09 (1.29) | 1.33 (0.57) |
| Multiple/other | 12.38 (2.51) | 1.26 (0.26) | 2.30 (0.61) | 0.89 (0.31) | 5.20 (1.25) | 1.36 (0.58) |
| Maternal education | ||||||
| ≤8th grade | 13.74 (2.39) | 1.34 (0.28) | 2.57 (0.58) | 1.06 (0.29) | 5.92 (1.29) | 1.63 (0.52) |
| 9th grade – high school | 13.07 (2.54) | 1.30 (0.26) | 2.45 (0.64) | 0.97 (0.31) | 5.50 (1.29) | 1.49 (0.59) |
| College (<4 years) | 13.18 (2.53) | 1.29 (0.26) | 2.47 (0.62) | 0.97 (0.30) | 5.53 (1.28) | 1.56 (0.62) |
| College (4 years) | 12.74 (2.51) | 1.27 (0.26) | 2.38 (0.62) | 0.93 (0.31) | 5.34 (1.25) | 1.47 (0.61) |
| > College | 12.68 (2.48) | 1.25 (0.25) | 2.37 (0.59) | 0.92 (0.30) | 5.28 (1.23) | 1.48 (0.62) |
| Block group household income | ||||||
| ≤ $43,839 | 13.39 (2.37) | 1.29 (0.25) | 2.50 (0.60) | 0.97 (0.29) | 5.66 (1.21) | 1.60 (0.61) |
| $43,839–$56,170 | 13.22 (2.46) | 1.29 (0.26) | 2.48 (0.59) | 0.97 (0.29) | 5.57 (1.25) | 1.55 (0.61) |
| $56,170–$71,582 | 12.81 (2.54) | 1.27 (0.26) | 2.40 (0.62) | 0.94 (0.30) | 5.38 (1.27) | 1.48 (0.61) |
| ≥ $71,582 | 12.33 (2.60) | 1.26 (0.27) | 2.33 (0.65) | 0.93 (0.33) | 5.09 (1.27) | 1.38 (0.57) |
| Smoking | ||||||
| Never Smoker | 12.97 (2.53) | 1.28 (0.26) | 2.43 (0.62) | 0.95 (0.31) | 5.43 (1.27) | 1.51 (0.61) |
| Ever Smoker | 12.75 (2.53) | 1.27 (0.25) | 2.37 (0.62) | 0.93 (0.31) | 5.35 (1.26) | 1.47 (0.60) |
| Smoking during pregnancy | 12.90 (2.54) | 1.29 (0.26) | 2.40 (0.64) | 0.96 (0.31) | 5.45 (1.28) | 1.49 (0.58) |
| Pre-pregnancy BMI | ||||||
| Underweight (<18.5) | 12.85 (2.53) | 1.27 (0.27) | 2.40 (0.61) | 0.96 (0.30) | 5.40 (1.28) | 1.50 (0.60) |
| Normal (18.5–24.9) | 12.80 (2.55) | 1.27 (0.26) | 2.39 (0.62) | 0.94 (0.31) | 5.37 (1.28) | 1.49 (0.61) |
| Overweight (25.0–29.9) | 13.00 (2.51) | 1.28 (0.26) | 2.44 (0.62) | 0.96 (0.31) | 5.45 (1.27) | 1.51 (0.61) |
| Obese (≥30.0) | 13.09 (2.49) | 1.29 (0.26) | 2.46 (0.62) | 0.96 (0.30) | 5.49 (1.25) | 1.51 (0.61) |
| Season of conception | ||||||
| Cool season | 12.89 (2.40) | 1.41 (0.23) | 2.38 (0.57) | 0.95 (0.30) | 5.29 (1.21) | 1.45 (0.61) |
| Warm season | 12.99 (2.66) | 1.15 (0.23) | 2.47 (0.66) | 0.95 (0.31) | 5.57 (1.32) | 1.55 (0.61) |
Mean (standard deviation). The units are μg/m3 for total mass and constituents.
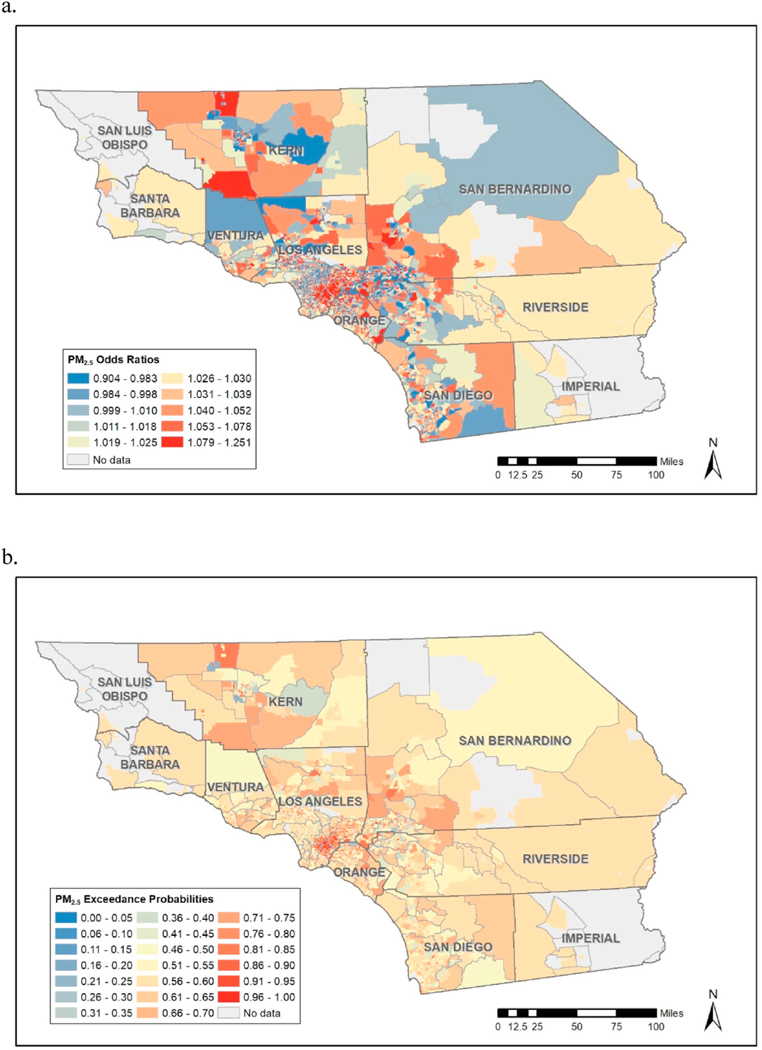
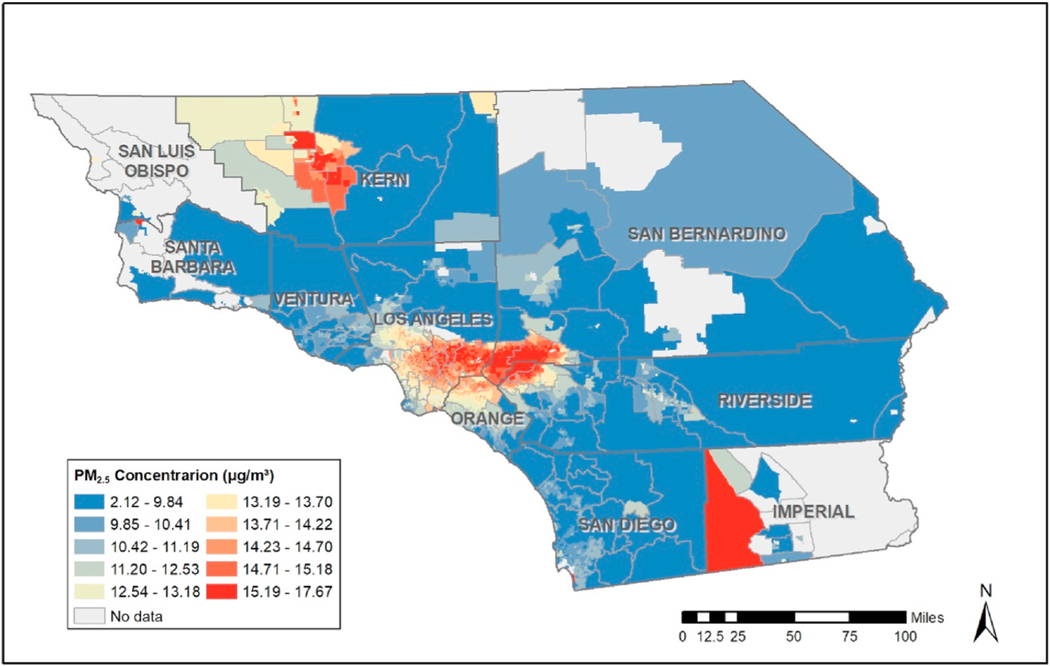
Table 3 provides results from the regression analysis corresponding to “fixed” effects, though our focus here is on examination of spatially varying slopes which indicate spatially varying effects of exposure, which consist of linear combinations of the fixed and random effects in equation (3), which can be computed via the “lincomb” function in R-INLA. These slopes form the basis for computation of the kind of spatially varying Odds Ratios (OR’s) which address our interest in spatially varying effects of exposure reductions. Fig. 1a below provides estimates these OR’s corresponding to unit reductions in exposure, which could be used to inform decisions as to where policy interventions would be most beneficial. However, Fig. 1b reveals that many of the areas in Fig. 1 with high OR’s have relatively low exceedance probabilities, , suggesting high levels of uncertainty regarding some of these OR estimates. Fig. 1a and b thus suggest that policy-interventions be focused in areas such as southeast Los Angeles County and parts of Kern, San Bernadino, Riverside and San Diego Counties. However, as Fig. 2 reveals, some of these areas, such as parts of Riverside and San Diego Counties, have relatively low levels of exposure, making these areas less relevant from a “hotspot” exposure reduction point of view. Further, certain southwest parts of Kern County with the highest exposures (representing potentially prime areas for exposure reductions) are associated with some of the lowest exceedance probabilities. If we focus on the COR maps corresponding to (Fig. 3a) we see that areas in southeast Los Angeles County have high OR, high exceedance probabilities, and display high levels of exposures. This is a somewhat disadvantaged area of Los Angeles County that has a high population of Hispanics. The COR map thus provides perhaps the most useful display of areas where individual-level exposure reductions are likely to reap the greatest rewards in terms reduction in prevalence of GDM. We further note that the COR map related to (Fig. 3b) represents much more extreme exposure reductions, and this map suggest interventions in additional areas of potential intervention if such large exposure reductions are to be proposed in the hotspot areas of intervention. When we examine constituents (see relevant Appendices), we see high exceedance probabilities effects of ammonium, black carbon, and nitrate in the same area of southeast Los Angeles in a manner similar to what was observed for total mass, though for sulfate and organic matter the exceedance probabilities are low throughout the whole study region, suggesting that these components are less influential regarding the overall effect of exposure on likelihood of GDM.
Table 3.
Mean (95% Credible Interval). Continuous variables, including air pollution exposures in the first and second trimester, have been standardized.
| Characteristics | total mass | sulfate | nitrate | ammonium | organic matter | black carbon |
|---|---|---|---|---|---|---|
| Intercept | −3.23 (−3.38, −3,09) | −3.26 (−3.40, −3.11) | −3.24 (−3.39, −3.09) | −3.24 (−3.36, −3.08) | −3.24 (−3.39, −3.09) | −3.23 (−3.38, −3.08) |
| Exposure | 0.03 (0.01, 0.04) | −0.03 (−0.04, −0.02) | 0.04 (0.02, 0.05) | 0.03 (0.01, 0.04) | 0.04 (0.02, 0.05) | 0.07 (0.05, 0.08) |
| Maternal age | 0.49 (0.48, 0.51) | 0.49 (0.48, 0.51) | 0.49 (0.48, 0.51) | 0.49 (0.48, 0.51) | 0.49 (0.48, 0.51) | 0.49 (0.48, 0.51) |
| Block group household income | −0.08 (−0.10, −0.06) | −0.08 (−0.10, −0.07) | −0.08 (−0.10, −0.06) | −0.08 (−0.10, −0.07) | −0.08 (−0.10, −0.06) | −0.08 (−0.09, −0.06) |
| Year of infant birth | −0.09 (−0.10, −0.08) | −0.11 (−0.12, −0.10) | −0.10 (−0.11, −0.09) | −0.09 (−0.10, −0.08) | −0.10 (−0.11, −0.09) | −0.09 (−0.10, −0.07) |
| Maternal race/ethnicity | ||||||
| African American | – | – | – | – | – | – |
| Asian | 1.29 (1.23, 1.35) | 1.29 (1.23, 1.35) | 1.29 (1.23, 1.35) | 1.29 (1.23, 1.35) | 1.29 (1.23, 1.35) | 1.29 (1.23, 1.35) |
| Hispanic | 0.52 (0.47, 0.57) | 0.52 (0.47, 0.58) | 0.52 (0.47, 0.57) | 0.52 (0.47, 0.57) | 0.52 (0.47, 0.57) | 0.52 (0.47, 0.57) |
| Non-Hispanic white | 0.19 (0.13, 0.24) | 0.18 (0.12, 0.24) | 0.19 (0.13, 0.24) | 0.19 (0.13, 0.24) | 0.19 (0.13, 0.24) | 0.20 (0.14, 0.26) |
| Multiple/other | 0.39 (0.29, 0.48) | 0.38 (0.29, 0.48) | 0.39 (0.29, 0.48) | 0.39 (0.29, 0.48) | 0.39 (0.29, 0.48) | 0.40 (0.30, 0.49) |
| Maternal education | ||||||
| ≤8th grade | – | – | – | – | – | – |
| 9th grade – high school | −0.21 (−0.30, −0.11) | −0.21 (−0.30, −0.11) | −0.21 (−0.30, −0.11) | −0.21 (−0.30, −0.12) | −0.21 (−0.30, −0.11) | −0.21 (−0.30, −0.12) |
| College (<4 years) | −0.23 (−0.32, −0.14) | −0.23 (−0.32, −0.13) | −0.23 (−0.32, −0.13) | −0.23 (−0.33, −0.14) | −0.23 (−0.32, −0.13) | −0.24 (−0.33, −0.15) |
| College (4 years) | −0.33 (−0.42, −0.24) | −0.33 (−0.42, −0.23) | −0.33 (−0.42, −0.24) | −0.33 (−0.42, −0.24) | −0.33 (−0.42, −0.23) | −0.34 (−0.43, −0.25) |
| > College | −0.48 (−0.57, −0.38) | −0.48 (−0.57, −0.38) | −0.48 (−0.57, −0.38) | −0.48 (−0.57, −0.38) | −0.48 (−0.57, −0.38) | −0.49 (−0.59, −0.39) |
| Smoking | ||||||
| Smoking during pregnancy | – | – | – | – | – | – |
| Never Smoker | −0.01 (−0.04, 0.03) | −0.01 (−0.04, 0.03) | −0.01 (−0.04, 0.03) | −0.01 (−0.04, 0.03) | −0.01 (−0.04, 0.03) | −0.01 (−0.04, 0.03) |
| Ever Smoker | 0.05 (−0.00, 0.11) | 0.05 (−0.00, 0.10) | 0.05 (−0.00, 0.11) | 0.05 (−0.00, 0.11) | 0.05 (−0.00, 0.10) | 0.05 (−0.01, 0.10) |
| Pre-pregnancy BMI in categories | ||||||
| Underweight (<18.5) | – | – | – | – | – | – |
| Normal (18.5–24.9) | 0.22 (0.11, 0.32) | 0.22 (0.11, 0.32) | 0.22 (0.11, 0.32) | 0.22 (0.11, 0.32) | 0.22 (0.11, 0.32) | 0.22 (0.11, 0.32) |
| Overweight (25.0–29.9) | 0.83 (0.72, 0.93) | 0.83 (0.72, 0.93) | 0.83 (0.72, 0.93) | 0.83 (0.72, 0.93) | 0.83 (0.72, 0.93) | 0.83 (0.72, 0.93) |
| Obese (≥30.0) | 1.40 (1.30, 1.50) | 1.40 (1.30, 1.51) | 1.40 (1.39, 1.50) | 1.40 (1.30, 1.51) | 1.40 (1.30, 1.50) | 1.40 (1.30, 1.51) |
| Season of conception | ||||||
| Cool season | – | – | – | – | – | – |
| Warm season | −0.06 (−0.08, −0,04) | −0.03 (−0.06, −0.01) | −0.05 (−0.08, −0.03) | −0.06 (−0.09, −0.04) | −0.06 (−0.08, −0.03) | −0.07 (−0.09, −0.04) |
Fig. 2.
Average census tract exposure levels for total mass.
Fig. 3.
Compliance Odds Ratios for total mass corresponding to the standard of (a) the U.S. EPA: 12 μg/m3 and (b) the WHO: 5 μg/m3.
Though issues of the aforementioned spatial confounding are a continual concern in these kind of analyzes, we did, as a sensitivity analysis, analyze the exposures using a model with random intercepts and slopes (“effects”) as before, but this time without the joint multivariate normal specification and modeled the slopes using a spatially smoothed intrinsic Conditional Autoregressive (iCAR) model (Besag et al., 1991). We used “vague” uniform priors on the variance component for the random intercepts and on the variance component of the spatial model used for the slopes. The results were nearly identical with alternative priors such as the half normal. (Both priors are specified and coded in (Gómez-Rubio, 2020) Results were quite similar to those obtained without the spatial smoothing, though there is an obvious effect of smoothing on results. For a comparison, see smoothed graphs of compliance effect for Compliance OR’s for values of 12 ug/m3 and 5 ug/m3 (Fig. 4) and to jointly modeled (with no spatial smoothing) plots of similar OR’s in Fig. 3. We did experiment with addition of spatial smoothing terms for the intercepts; however, results were somewhat sensitive to prior specification and presence/absence of smoothing constructs. In models with spatially smoothed effects (or “slopes”) the spatially distributed error terms are weighted by the exposure and are thus not expected to be orthogonal to it. As such, spatial confounding is less of an issue in this situation. Nevertheless, while we recognize the value of incorporation of spatially smoothed terms into the modeling process, one must be extra cautious regarding issues related to model and prior specification when these spatially smoothed error terms are included in these kinds of models.
4. Discussion
Our analysis provides insights as to where policy interventions, in terms of reducing prevalence of GDM, will be most effective among a cohort of women living throughout Southern California. A Bayesian statistical model was fit providing spatially varying effects of unit level reduction in exposure, in conjunction with OR’s which take into account effects of exposure reductions down to compliance levels. This combination of size and effect of air pollution reductions helps focus on areas which are likely to benefit most from exposure policy interventions.
To the best of our knowledge, no study has explored the spatial variation in effects on GDM in a large obstetric population. Spatial modeling approaches have been used in previous studies to investigate spatial structure in relationship between air pollution and health outcomes. The major advantage gained in using this approach is to not only examine spatial varying effects of air pollution on health but also develop public health interventions to optimize the potential benefits and promote environmental health equity. First, our findings imply that uniform air quality regulation and intervention may not be sufficiently protective to susceptible population subgroups, and that such policies may need to be spatially tailored for these subregions. Second, our approach could identify ‘hotspots’ to help guide spatially targeted interventions to protect susceptible subpopulations from outdoor air pollution (e.g., use of air filter and purifier to reduce indoor air pollution at home and workplace). Further, potential public health interventions (e.g., earlier GDM screening, and healthier lifestyles) could be also conducted to reduce the risk of air pollution on GDM among subpopulations who live in the hotspot regions. In summary, interventions targeting the hotspot areas may translate into a more pronounced reduction of GDM in southern California and maximize the benefits of reducing air pollution exposure during pregnancy.
Our results also underscore the need to prioritize such hotspot regions for addressing environmental health disparities. For example, these hotspots of southeast Los Angeles County have a high population of Hispanics, who may be particularly vulnerable to air pollution on the risk of GDM (Sun et al., 2021). Moreover, some hotspot regions in Kern County, and southern Los Angeles County and downwind areas are disadvantaged communities with higher level, but which also lack an air quality monitoring network (Sun et al., 2022). Therefore, such hotspot regions are in the greatest need of public health interventions and air pollution regulations to close the gap of environmental health inequity.
One could examine interactions between SES-based susceptibility factors and exposure effects throughout space, which could improve interpretability of results. However, such an analysis is quite complex, may require additional data, and is thus beyond the scope of the paper. We note that in our previous work we have utilized Bayesian Profile Regression (BRP) (Molitor et al., 2010) to construct SES-based clusters and to examine effects of exposure for each cluster in question. Such work can be quite involved as effects of exposure can vary according to SES cluster and other, unobserved factors as well (Coker et al., 2016) and careful selection of SES variables must be considered.
Our study has its limitations as the data are observational, and as such one cannot be sure that all necessary confounders have been accounted for or that the confounders used where adjusted using the proper mathematical form. From a modeling perspective, we assumed logit-linear models at the CT-level, though modeling such linearity at the small-region level does not mean that we assume such a linear relationship between exposures and log-outcome throughout the entire study region. Also, potential non-linear time trends in exposures and log-odds of GDM where not explicitly modeled and spatially smoothing techniques where forgone to sidestep issues of spatial confounding. As such, there could be some loss of information compared to what would be obtained using richer, though perhaps harder to interpret models. Further, many areas contain high amounts of parameter estimation error (and thus low exceedance probabilities), corresponding relevant quantities such as OR’s COR’s, etc. This may be due to low effects sizes in these areas, or it may simple be a data issue related to low regional sample sizes or a lack of exposure-level variability, making effect estimation difficult for these areas in question. Of further note is the fact that we only considered and its constituents, and no other gaseous pollutants such as O3 and NO2. However, if limited resources are available for intervention purposes, our results can still point policy makers towards areas where exposure reduction is likely to have a high impact on health improvement, as opposed to areas where the success of such interventions is highly uncertain.
While our study contains weaknesses, it contains many strengths as well. In particular, our study contains a relatively large number of individuals, and represents a large and diverse set of regions throughout southern California. In addition, the high-quality and comprehensive information on diagnosis, demographic characteristics, and individual lifestyle from the KPSC EHRs can minimize the screening biases and enable deeper understanding of air pollution and GDM by adjusting for a wide range of confounders. Moreover, well-validated air pollution models can provide a wide range of chemical compositions of and enhance the accuracy for the air pollution exposure assessments. Further, both WHO and national compliance levels of were considered in this analysis.
There is a long tradition in environmental epidemiology research where effects of exposures are summarized based on the concept of “statistical significance” and often accompanied with a p-value and an associated confidence interval. However, from an intervention perspective, interest often lies in the size of the relevant effect and how that effect varies spatially regarding SES and other geographically varying modifiers. Such spatially varying effects and associated modeling frameworks are well established. However, due to potential complexities in implementation, such methods are often underutilized. In this paper we provide an example of how to model spatially varying effects using a relatively simple model, but also combine such analysis with consideration for spatially varying amounts of exposure reductions based on the idea that more highly exposed areas provide greater opportunity for exposure reduction compared to regions which already experience low levels of exposure and are thus unlikely to be greatly affected by exposure-reduction oriented policies. This approach, examining both size and effect of exposure reductions, provided a meaningful and useful set of maps and results that can be utilized to provide targeted intervention policies designed to optimize effects of exposure reductions on health.
Supplementary Material
Acknowledgement
This study was supported by the National Institute of Environmental Health Sciences (NIEHS; R01ES030353). Any opinions, findings, and conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the NIEHS.
Footnotes
Credit author
John Molitor and Yi Sun provided conceptualization, modeling, formal analysis, and writeup. Virgilio Gómez Rubio, Tarik Benmarhnia, Jiu-Chiuan Chen, Chantal Avila, David A. Sacks, Vicki Chiu, Jeff Slezak, Darios Getahun, and Jun Wu aided in conceptualization, modeling, and writeup. Darios Getahun and Jun Wu provided funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2023.116091.
Data availability
The data that has been used is confidential.
References
- American Diabetes Association Professional Practice Committee. 2021. “2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022.” Diabetes Care 45 (Supplement_1): S17–38. 10.2337/dc22-S002. [DOI] [PubMed] [Google Scholar]
- Bai W, Li Y, Niu Y, Ding Y, Yu X, Zhu B, Sun Z, 2020. Association between ambient air pollution and pregnancy complications: a systematic review and meta-analysis of cohort studies. Environ. Res 185, 109471 10.1016/j.envres.2020.109471. [DOI] [PubMed] [Google Scholar]
- Brook Robert D., Rajagopalan Sanjay, Pope C. Arden, Brook Jeffrey R., Bhatnagar Aruni, Diez-Roux Ana V., Holguin Fernando, et al. 2010. “Particulate Matter Air Pollution and Cardiovascular Disease: An Update to the Scientific Statement from the American Heart Association.” Circulation 121 (21): 2331–78. 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Besag J, York JC, and Molli\’e A. 1991. “Bayesian Image Restoration, with Two Applications in Spatial Statitics (with Discussion).” Ann. I. Stat. Math 43 (1): 1–59. [Google Scholar]
- Carpenter MW, Coustan DR, 1982. Criteria for screening tests for gestational diabetes. Am. J. Obstet. Gynecol 144 (7), 768–773. 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- Casagrande SS, Linder B, Cowie CC, 2018. Prevalence of gestational diabetes and subsequent Type 2 diabetes among U.S. women. Diabetes Res. Clin. Pract 141, 200–208. 10.1016/j.diabres.2018.05.010. [DOI] [PubMed] [Google Scholar]
- “CDC WONDER.” Accessed May 22, 2023. https://wonder.cdc.gov/.
- Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, Damm P, 2008. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care 31 (2), 340–346. 10.2337/dc07-1596. [DOI] [PubMed] [Google Scholar]
- Coker Eric, Ghosh Jokay, Jerrett Michael, Virgilio Gomez-Rubio Bernardo Beckerman, Cockburn Myles, Liverani Silvia, et al. 2015. “Modeling Spatial Effects of on Term Low Birth Weight in Los Angeles County.” Environmental Research 142 (October): 354–64. 10.1016/j.envres.2015.06.044. [DOI] [PubMed] [Google Scholar]
- Coker Eric, Liverani Silvia, Ghosh Jo Kay, Jerrett Michael Beckerman Bernardo, Li Arthur, Ritz Beate, and Molitor John. 2016. “Multi-Pollutant Exposure Profiles Associated with Term Low Birth Weight in Los Angeles County.” Environment International 91 (May): 1–13. 10.1016/j.envint.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Daly B, Toulis KA, Thomas N, Gokhale K, Martin J, Webber J, Nirantharakumar K, 2018. Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: a population-based cohort study. PLoS Med. 15 (1), e1002488 10.1371/journal.pmed.1002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eze IC, Hemkens LG, Bucher HC, Hoffmann B, Schindler C, Künzli N, Probst-Hensch NM, 2015. Association between ambient air pollution and diabetes mellitus in Europe and North America: systematic review and meta-analysis. Environ. Health Perspect 123 (5), 381–389. 10.1289/ehp.1307823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahvar S, Walfisch A, Sheiner E, 2019. Gestational diabetes risk factors and long-term consequences for both mother and offspring: a literature review. Expet Rev. Endocrinol. Metabol 14 (1), 63–74. 10.1080/17446651.2018.1476135. [DOI] [PubMed] [Google Scholar]
- Farrar D, Simmonds M, Bryant M, Sheldon TA, Tuffnell D, Golder S, Lawlor DA, 2016. Hyperglycaemia and risk of adverse perinatal outcomes: systematic review and meta-analysis. BMJ 354, i4694. 10.1136/bmj.i4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Villoria Maria, Ventrucci Massimo, and Rue Håvard. 2019a. “A Unified View on Bayesian Varying Coefficient Models.” Electronic Journal of Statistics 13 (2): 5334–59. 10.1214/19-EJS1653. [DOI] [Google Scholar]
- Gelman Andrew, Jennifer Hill, 2006. Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge University Press. [Google Scholar]
- Geronimus Arline T., Hicken Margaret, Keene Danya, and Bound John. 2006. “‘Weathering’ and Age Patterns of Allostatic Load Scores Among Blacks and Whites in the United States.” American Journal of Public Health 96 (5): 826–33. 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Rubio Virgilio., 2020. Bayesian Inference with INLA. CRC Press. [Google Scholar]
- “Inhalable Particulate Matter and Health ( and ) | California Air Resources Board.” Accessed March 1, 2023. https://ww2.arb.ca.gov/resources/inhalable-particulate-matter-and-health.
- Hajat Anjum, Hsia Charlene, and O’Neill Marie S. 2015. “Socioeconomic Disparities and Air Pollution Exposure: A Global Review.” Current Environmental Health Reports 2 (4): 440–50. 10.1007/s40572-015-0069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges James S., and Reich Brian J. 2010. “Adding Spatially-Correlated Errors Can Mess Up the Fixed Effect You Love.” The American Statistician 64 (4): 325–34. 10.1198/tast.2010.10052. [DOI] [Google Scholar]
- Hystad Perry, Davies Hugh W., Frank Lawrence, Josh Van Loon Ulrike Gehring, Tamburic Lillian, and Brauer Michael. 2014. “Residential Greenness and Birth Outcomes: Evaluating the Influence of Spatially Correlated Built-Environment Factors.” Environmental Health Perspectives 122 (10): 1095–1102. 10.1289/ehp.1308049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krewski D. 2009. “Evaluating the Effects of Ambient Air Pollution on Life Expectancy.” N Engl J Med 360 (4): 413–15. 10.1056/NEJMe0809178. [DOI] [PubMed] [Google Scholar]
- Jo H, Eckel SP, Chen JC, Cockburn M, Martinez MP, Chow T, McConnell R, 2019. Gestational diabetes mellitus, prenatal air pollution exposure, and autism spectrum disorder. Environ. Int 133 (Pt A), 105110 10.1016/j.envint.2019.105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laden Francine, Schwartz Joel, Speizer Frank E., and Dockery Douglas W. 2006. “Reduction in Fine Particulate Air Pollution and Mortality: Extended Follow-up of the Harvard Six Cities Study.” American Journal of Respiratory and Critical Care Medicine 173 (6): 667–72. 10.1164/rccm.200503-443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai FY, Johnson JA, Dover D, Kaul P, 2016. Outcomes of singleton and twin pregnancies complicated by pre-existing diabetes and gestational diabetes: a population-based study in Alberta, Canada, 2005–11. J. Diabetes 8 (1), 45–55. 10.1111/1753-0407.12255. [DOI] [PubMed] [Google Scholar]
- Lamichhane DK, Leem JH, Lee JY, Kim HC, 2015. A meta-analysis of exposure to particulate matter and adverse birth outcomes. Environ Health Toxicol 30, e2015011. 10.5620/eht.e2015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Xiaoying, Baumgartner Jill, Christopher Barrington-Leigh Sam Harper, Robinson Brian, Shen Guofeng, Sternbach Talia, et al. 2022. “Socioeconomic and Demographic Associations with Wintertime Air Pollution Exposures at Household, Community, and District Scales in Rural Beijing, China.” Environmental Science & Technology 56 (12): 8308–18. 10.1021/acs.est.1c07402. [DOI] [PubMed] [Google Scholar]
- Martino J, Sebert S, Segura MT, García-Valdés L, Florido J, Padilla MC, Campoy C, 2016. Maternal body weight and gestational diabetes differentially influence placental and pregnancy outcomes. J. Clin. Endocrinol. Metab 101 (1), 59–68. 10.1210/jc.2015-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Li C, Martin RV, van Donkelaar A, Hystad P, Brauer M, 2019. Estimated long-term (1981–2016) concentrations of ambient fine particulate matter across North America from chemical transport modeling, satellite remote sensing, and ground-based measurements. Environ. Sci. Technol 53 (9), 5071–5079. 10.1021/acs.est.8b06875. [DOI] [PubMed] [Google Scholar]
- Messer Lynne C., Laraia Barbara A., Kaufman Jay S., Eyster Janet, Holzman Claudia, Culhane Jennifer, Elo Irma, Burke Jessica G., and O’Campo Patricia. 2006. “The Development of a Standardized Neighborhood Deprivation Index.” Journal of Urban Health: Bulletin of the New York Academy of Medicine 83 (6): 1041–62. 10.1007/s11524-006-9094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger BE, 2007. Long-term outcomes in mothers diagnosed with gestational diabetes mellitus and their offspring. Clin. Obstet. Gynecol 50 (4). [DOI] [PubMed] [Google Scholar]
- Metzger BE, 2010. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33 (3), 676. 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirghani Dirar A, Doupis J, 2017. Gestational diabetes from A to Z. World J. Diabetes 8 (12), 489–511. 10.4239/wjd.v8.i12.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitor John, Papathomas Michail, Jerrett Michael, Sylvia Richardson, 2010. Bayesian Profile Regression with an Application to the National Survey of Children’s Health, 3. Biostatistics (Oxford, England, pp. 98–484. 10.1093/biostatistics/kxq013. [DOI] [PubMed]
- Molitor John, Jason G Su Nuoo-Ting Molitor, Virgilio Gómez Rubio Sylvia Richardson, Hastie David, Morello-Frosch Rachel, and Jerrett Michael. 2011. “Identifying Vulnerable Populations through an Examination of the Association between Multipollutant Profiles and Poverty.” Environmental Science & Technology 45 (18): 7754–60. 10.1021/es104017x. [DOI] [PubMed] [Google Scholar]
- Morello-Frosch Rachel, and Jesdale Bill M. 2006. “Separate and Unequal: Residential Segregation and Estimated Cancer Risks Associated with Ambient Air Toxics in U.S. Metropolitan Areas.” Environmental Health Perspectives 114 (3): 386–93. 10.1289/ehp.8500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello-Frosch Rachel, Zuk Miriam, Jerrett Michael, Shamasunder Bhavna, and Kyle Amy D. 2011. “Understanding the Cumulative Impacts of Inequalities in Environmental Health: Implications for Policy.” Health Affairs (Project Hope) 30 (5): 879–87. 10.1377/hlthaff.2011.0153. [DOI] [PubMed] [Google Scholar]
- Nijs H, Benhalima K, 2020. Gestational diabetes mellitus and the long-term risk for glucose intolerance and overweight in the offspring: a narrative review. J. Clin. Med 9 (2), 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmí-Perales Francisco, Gómez-Rubio Virgilio, and Martinez-Beneito Miguel A. 2021. “Bayesian Multivariate Spatial Models for Lattice Data with INLA.” Journal of Statistical Software 98 (June): 1–29. 10.18637/jss.v098.i02. [DOI] [Google Scholar]
- Ramirez Laura K. Brennan, Baker Elizabeth A., and Metzler Marilyn. 2008. “Promoting Health Equity: A Resource to Help Communities Address Social Determinants of Health: (540452013–001).” American Psychological Association. 10.1037/e540452013-001. [DOI]
- Ritz B, Wilhelm M, Hoggatt KJ, and Ghosh JK 2007. “Ambient Air Pollution and Preterm Birth in the Environment and Pregnancy Outcomes Study at the University of California, Los Angeles.” Am J Epidemiol 166 (9): 1045–52. [DOI] [PubMed] [Google Scholar]
- Rue H, Martino S, 2009. Approximate Bayesian Inference for Latent Gaussian Models by Using Integrated Nested Laplace Approximations. J R Stat Soc Ser B Stat Methodol 71. 10.1111/j.1467-9868.2008.00700.x. [DOI] [Google Scholar]
- Samet JM, and White RH 2004. “Urban Air Pollution, Health, and Equity.” Journal of Epidemiology & Community Health 58 (1): 3–5. 10.1136/jech.58.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson Daniel, Rue Håvard, Riebler Andrea, Martins Thiago G., and Sørbye Sigrunn H. 2017. “Penalising Model Component Complexity: A Principled, Practical Approach to Constructing Priors.” Statistical Science 32 (1): 1–28. 10.1214/16-STS576. [DOI] [Google Scholar]
- Su Jason, Meng G, Ying-Ying, Pickett Melissa Seto Edmund, Ritz Beate, Michael Jerrett., 2016. Identification of Effects of Regulatory Actions on Air Quality in Goods Movement Corridors in California. Environmental Science & Technology (50 (16):), 96–8687. 10.1021/acs.est.6b00926. [DOI] [PubMed] [Google Scholar]
- Sun Y, Li X, Benmarhnia T, Chen JC, Avila C, Sacks DA, Wu J, 2021. Exposure to air pollutant mixture and gestational diabetes mellitus in Southern California: results from electronic health record data of a large pregnancy cohort. Environ. Int 158, 1873–6750 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Mousavi A, Masri S, Wu J, 2022. Socioeconomic disparities of low-cost air quality sensors in California, 2017–2020. Am. J. Publ. Health 112 (3), 434–442. 10.2105/AJPH.2021.306603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam WA-OX, Ma RCW, Ozaki R, Li AM, Chan MHM, Yuen LY, Chan JCN, 2017. Utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in offspring. Diabetes Care 40 (5), 679–686. 10.2337/dc16-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiering E, Heinrich J, 2015. Epidemiology of Air Pollution and Diabetes, pp. 1879–3061 (Electronic)). [DOI] [PubMed]
- Tobias DK, Stuart JJ, Li S, Chavarro J, Rimm EB, Rich-Edwards J, Zhang C, 2017. Association of history of gestational diabetes with long-term cardiovascular disease risk in a large prospective cohort of US women. JAMA Intern. Med 177 (12), 1735–1742. 10.1001/jamainternmed.2017.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA, OAR. 2020. “National Ambient Air Quality Standards (NAAQS) for PM.” Other Policies and Guidance. April 13, 2020. https://www.epa.gov/pm-pollution/national-ambient-air-quality-standards-naaqs-pm.
- van Donkelaar A, Martin RV, Li C, Burnett RT, 2019. Regional estimates of chemical composition of fine particulate matter using a combined geoscience-statistical method with information from satellites, models, and monitors. Environ. Sci. Technol 53 (5), 2595–2611. 10.1021/acs.est.8b06392. [DOI] [PubMed] [Google Scholar]
- Walker Renee E., Keane Christopher R., and Burke Jessica G. 2010. “Disparities and Access to Healthy Food in the United States: A Review of Food Deserts Literature.” Health & Place 16 (5): 876–84. 10.1016/j.healthplace.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Wang H, Li N, Chivese T, Werfalli M, Sun H, Yuen L, 2022. IDF diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by international association of diabetes in pregnancy study group’s criteria. Diabetes Res. Clin. Pract 183, 109050 10.1016/j.diabres.2021.109050. [DOI] [PubMed] [Google Scholar]
- Weichenthal Scott, Pinault Lauren, Christidis Tanya, Burnett Richard T., Brook Jeffrey R., Chu Yen, Crouse Dan L., et al. 2022. “How Low Can You Go? Air Pollution Affects Mortality at Very Low Levels.” Science Advances 8 (39): eabo3381. 10.1126/sciadv.abo3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2020. WHO | WHO Global Urban Ambient Air Pollution Database (Update 2016), 21. WHO. World Health Organization. http://www.who.int/airpollution/data/cities-2016/en/. [Google Scholar]
- Wilhelm Michelle, Ghosh Kay, Jo Jason Su, Cockburn Myles, Jerrett Michael, Beate Ritz, 2012. Traffic-Related Air Toxics and Term Low Birth Weight in Los Angeles County, California. Environmental Health Perspectives 120 (1), 38–132. 10.1289/ehp.1103408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang AH, Wang X, Martinez MP, Walthall JC, Curry ES, Page K, Getahun D, 2015. Association of maternal diabetes with autism in offspring. JAMA 313 (14), 1425–1434. 10.1001/jama.2015.2707. [DOI] [PubMed] [Google Scholar]
- Xu G, Jing J, Bowers K, Liu B, Bao W, 2014. Maternal diabetes and the risk of autism spectrum disorders in the offspring: a systematic review and meta-analysis. J. Autism Dev. Disord 44 (4), 766–775. 10.1007/s10803-013-1928-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G-R, Dye TD, Li D, 2019. Effects of pre-gestational diabetes mellitus and gestational diabetes mellitus on macrosomia and birth defects in Upstate New York. Diabetes Res. Clin. Pract 155, 107811 10.1016/j.diabres.2019.107811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wang Q, He S, Wu K, Ren M, Dong H, Huang C, 2020. Ambient air pollution and gestational diabetes mellitus: a review of evidence from biological mechanisms to population epidemiology. Sci. Total Environ 719, 137349 10.1016/j.scitotenv.2020.137349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.