Abstract
Background
Parvovirus is a common childhood infection that could be very dangerous to the fetus, if pregnant women become infected. The spectrum of effects range from pure red blood cell aplasia with hydrops fetalis to meningoencephalitis, with many symptoms in between. Severe anemia in the setting of pure red blood cell aplasia is one of the more common effects that neonatal experience (if infected intrapartum), with the current gold standard treatment being intrauterine or postnatal packed red blood cell (PRBC) transfusions, yet intravenous immunoglobulin (IVIG) may be a superior treatment option.
Case presentation
A preterm infant was born at 26th week of gestational age via emergency Cesarean section due to hydrops fetalis, with parvovirus B19 exposure one month prior. The infant tested positive for IgM antibodies against parvovirus B19. Among many other serious complications of both hydrops fetalis and premature delivery, the infant had severe unremitting anemia, and received many PRBC transfusion over the course of his 71-day-long neonatal intensive care unit stay. During a follow up appointments as outpatient, his blood tests showed persistent high copies of parvovirus B19. He was then supported with PRBC transfusions and treated with IVIG. After three doses of IVIG, the infant’s parvovirus B19 viral copy numbers have dramatically reduced and the infant did not require any more PRBC transfusions.
Conclusions
IVIG infusion effectively treated the parvovirus B19 infection and restored erythropoiesis making the child transfusion independent. Furthermore, since IVIG is safe and readily crosses the placenta, further studies are needed to determine if IVIG should be considered as an alternative prenatal treatment for congenital parvovirus B19 infection.
Keywords: IVIG, Perinatal infection, Parvovirus B19, Congenital parvovirus infection, Congenital anemia, Fetal anemia, Hydrops fetalis, Neonatal anemia
Background
Parvovirus B19 is a common cause of childhood infections mostly presenting in immunocompetent children as an intense red facial rash known as erythema infectiosum [1]. However, in the event that a pregnant woman becomes infected with parvovirus B19, the effects on the fetus have the possibility of causing serious disease [2–5].
Although parvovirus has minimal to no adverse effects on a healthy pregnant mother, it is transmitted via the placenta to the fetus. The transmission rate to fetus is about 33%, and the risk for fetal death can be as high as 10% in most studies except a Danish population study where they found no association between maternal parvovirus infection and disease in the infants [2, 4, 6–8]. The virus suppresses erythroid precursors in the early stages of hematopoiesis, which may lead to pure red blood cell aplasia and severe anemia [9]. If the infant of an affected mother survives, he/she may develop thrombocytopenia, myocarditis, hepatitis, vasculitis, meningoencephalitis, or sepsis-like syndrome as well [2].
Here we report a case of severe congenital anemia secondary to gestational parvovirus B19 infection, review of current knowledge on the management of parvovirus induced congenital anemia and use of intravenous immunoglobulins (IVIG) to treat the infection and resultant anemia.
Case presentation
The child was born at an outside institution at 26th week of gestational age via emergency cesarean section secondary to hydrops fetalis to a 24-year-old mother. This was her second child and mother reported her older child having symptoms consistent with parvovirus infection 1 month prior to delivery. Mother’s evaluation at time of delivery showed presence of parvovirus B19 IgG antibodies but IgM antibodies were not detected.
Hydrops fetalis was thought to be related to congenital parvovirus infection. Percutaneous umbilical cord blood sampling showed hemoglobin of 3.1 g/dL and platelet count of 12,000/µL. An attempt to give in-utero-transfusion failed and emergency delivery was carried out. Baby was born with Apgar scores of 2, 6, and 6 at 1, 5, and 10 min of life, respectively. He was then transferred to neonatal intensive care unit (NICU) started on transfusion support with packed red blood cells (PRBC).
In the NICU, child was found to have positive IgM antibodies for parvovirus B19 but IgG was negative. A polymerase chain reaction (PCR) assay done on blood for parvovirus B19 showed 7.7 109 IU/mL copy number. His neonatal course was complicated by grade 2 intraventricular hemorrhage and abdominal wall defect. He required extensive transfusion support and antibiotics for suspected sepsis in addition to respiratory and nutritional support. He was transferred to the neonatal intensive care unit of our institution at the chronologic age of 56 days. He continued to require PRBC transfusion support while in our NICU and was finally discharged home at 71 days of age.
At 121 days of life, the child presented to the emergency room with severe anemia and cardiac failure. He received PRBC transfusion in addition to cardiac and respiratory support and fully recovered from the event. He was sent home 5 days after admission with close follow-up by our Pediatric Hematology/Oncology team.
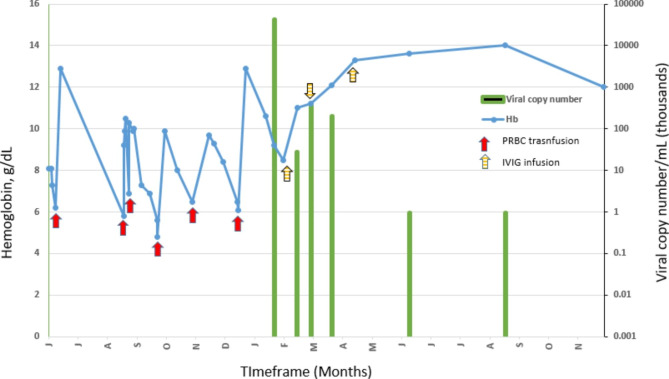
During his outpatient follow-up the child remained anemic with severe reticulocytopenia and required repeated PRBC transfusions. Further investigations did not show any evidence of an inherited bone marrow failure syndrome. His blood PCR for parvovirus B19 was elevated at 4.3 × 106 IU/mL. We opted to treat the child with IVIG and monitor response by parvovirus B19 PCR, blood hemoglobin, and reticulocyte measurements. After three doses of IVIG parvovirus B19 was undetectable in the blood and child became transfusion independent with full recovery of erythropoiesis (See Fig. 1).
Fig. 1.
Representation of laboratory test results and interventions
Discussion and conclusions
The most common and serious clinical manifestation of fetal anemia secondary to parvovirus B19 is nonimmune hydrops fetalis. Severe anemia due to immune or non-immune etiologies causing hypoxemia and cardiogenic heart failure may lead to hydrops fetalis and ultimately death in utero [10]. Hydrops fetalis is often diagnosed via prenatal ultrasound as scalp and skin edema, ascites, pleural effusion, placentomegaly, and polyhydramnios [11]. About 90% of hydrops fetalis cases are due to non-immune mediated causes such as parvovirus as preventative measures such as anti-D immune globulin treatment are now in place to decrease immune mediated cases [12].
Parvovirus B19 is highly tropic to human bone marrow and replicates only in erythroid progenitor cells [13, 14]. Resultant pure red cell aplasia is secondary to failure of erythropoiesis. It is a normocytic, normochromic anemia in which the bone marrow is unable to make erythroblasts. In the peripheral blood, there is an absence of reticulocytes. Platelet and leukocyte precursors however, are less likely affected. It has been hypothesized that due to viral induction of apoptosis, parvovirus B19 replication stops the erythrocyte development at the giant pronormoblast stage. Non-erythroid lineages may be affected as well, although these are less efficient in supporting B19 viral replication [13, 15]. Postnatal infections with parvovirus B19 can also cause pure red cell aplasia. Transient erythroblastopenia of childhood is a self-limiting and benign disease which occurs when there is a temporary suppression of erythropoiesis, resulting in reticulocytopenia in the blood [16, 17].
Current management of anemia in children with severe anemia due to parvovirus B19 infection is PRBC transfusion support as needed. Society of Obstetricians and Gynaecologists of Canada guidelines in fact recommend early delivery and intrauterine transfusions as the recommended intervention in hydrops fetalis or fetal anemia following parvovirus infection [18]. Intrauterine transfusions remain the standard of care for non-immune hydrops fetalis associated with severe fetal anemia with significant risk for mortality and morbidity [19–21].
Intravenous immunoglobulins is a group of pooled antibodies from healthy donors with various exposures. Ideally, IVIG contains antibodies against a broad variety of infectious agents, including but not limited to parvovirus. Children, who do not have prior parvovirus exposure and or antibodies against the virus, have no defense mechanism to stop the virus from attacking erythrocytes and causing anemia. IVIG contains neutralizing antibody against parvovirus B19 and has been reported to be effective for chronic B19 infection-related anemia in immunocompromised hosts. It has been used in infants and children with chronic and refractory anemia due to parvovirus infection [5, 22, 23]. In an interesting study, parvovirus antibody enriched immunoglobulins were successfully used in the form of fetal intraperitoneal infusion to treat hydrops fetalis associated with parvovirus B19 infection [24].
IVIG readily crosses the placenta and is available to the fetus. It has also been used successfully for treatment of fetus in the case of hemolytic disease of the newborn due to Rh-alloimmunization. IVIG in this setting is thought to help by diluting maternal antibodies and by inducing competition at the placenta and therefore reducing transplacental transfer of maternal antibodies resulting in lower maternal alloantibody levels and by blocking fetal macrophage function [25, 26]. It would be intriguing to see if IVIG infused to the pregnant woman intravenously would also treat fetal anemia caused by congenital parvovirus infection as IVIG has been successfully used in pregnant woman with minimal adverse effects for a variety of indications [25].
In conclusion, use of IVIG in the setting of congenital parvovirus B19 induced anemia appears to be effective in reducing and potentially eliminating the need for PRBC transfusions. Prenatal IVIG infusion to the mothers of fetuses affected by parvovirus B19 infection to prevent fetal anemia and its potential complications is a subject for future research.
Acknowledgements
Not applicable.
Abbreviations
- PRBC
packed red blood cell
- IVIG
intravenous immunoglobulin
- NICU
Neonatal intensive care unit
- PCR
Polymerase chain reaction
Authors’ contribution
SA reviewed case & drafted the manuscript.
MC reviewed the case, collected, analyzed, interpreted the data and finalized the manuscript.
LG reviewed the data analyzed and its interpretation, contributed to the manuscript.
RA reviewed the data analyzed and its interpretation, contributed to the manuscript.
Funding
Not applicable.
Data Availability
Data used is not publicly available to protect the personal health information. Fully de-identified clinical test results may be provided upon request.
Declarations
Ethics approval and consent to participate
Waived as the manuscript does not contain identifiable personal health information.
Competing interests
SA has no competing interests.
MC has no competing interests.
LG has no competing interests.
RA has no competing interests.
Consent for publication
As per institutional guidelines, consent not applicable the manuscript does not contain identifiable personal health information.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Servey JT, Reamy B, Hodge J. Clinical presentations of parvovirus B19 infection. Am Fam Physician. American Academy of Family Physicians; 2007;75. [PubMed]
- 2.Ornoy A, Ergaz Z. Parvovirus B19 infection during pregnancy and risks to the fetus. Birth Defects Res John Wiley and Sons Inc. 2017;109:311–23. doi: 10.1002/bdra.23588. [DOI] [PubMed] [Google Scholar]
- 3.Hudson AC, Montegudo AE, Steele RW. Congenital human parvovirus B19 infection with persistent viremia. Clin Pediatr (Phila). SAGE Publications Inc.; 2015;54:409–13. [DOI] [PubMed]
- 4.Attwood LO, Holmes NE, Hui L. Identification and management of congenital parvovirus B19 infection. Prenat Diagn. Volume 40. John Wiley and Sons Ltd; 2020. pp. 1722–31. [DOI] [PubMed]
- 5.Nadimpalli SS, Miller RS, Kamath VM, Farkouh CR, Nhan-Chang CL, Rathe JA et al. Congenital Parvovirus B19 Infection: Persistent Viremia and Red Blood Cell Aplasia. Open Forum Infect Dis [Internet]. Open Forum Infect Dis; 2015 [cited 2022 Sep 11];2. Available from: https://pubmed.ncbi.nlm.nih.gov/26288800/. [DOI] [PMC free article] [PubMed]
- 6.Staroselsky A, Klieger-Grossmann C, Garcia-Bournissen F, Koren G. Exposure to fifth disease in pregnancy. Can Fam Physician. 2009;55:1195–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Lassen J, Bager P, Wohlfahrt J, Böttiger B, Melbye M. Parvovirus B19 infection in pregnancy and subsequent morbidity and mortality in offspring. Int J Epidemiol. 2013;42:1070–6. doi: 10.1093/ije/dyt117. [DOI] [PubMed] [Google Scholar]
- 8.Lassen J, Jensen AKV, Bager P, Pedersen CB, Panum I, Norgaard-Pedersen B, et al. Parvovirus B19 infection in the first trimester of pregnancy and risk of fetal loss: a population-based case-control study. Am J Epidemiol. 2012;176:803–7. doi: 10.1093/aje/kws177. [DOI] [PubMed] [Google Scholar]
- 9.Bascietto F, Liberati M, Murgano D, Buca D, Iacovelli A, Flacco ME et al. Outcome of fetuses with congenital parvovirus B19 infection: systematic review and meta-analysis. Ultrasound in Obstetrics and Gynecology. John Wiley and Sons Ltd; 2018;52:569–76. [DOI] [PubMed]
- 10.Swearingen C, Colvin ZA, Leuthner SR. Nonimmune Hydrops Fetalis. Clin Perinatol [Internet]. Clin Perinatol; 2020 [cited 2022 Sep 11];47:105–21. Available from: https://pubmed.ncbi.nlm.nih.gov/32000919/. [DOI] [PubMed]
- 11.Xu J, Raff TC, Muallem NS, Neubert AG. Hydrops fetalis secondary to parvovirus B19 infections. J Am Board Family Pract Am Board Family Med. 2003;16:63–8. doi: 10.3122/jabfm.16.1.63. [DOI] [PubMed] [Google Scholar]
- 12.Erythroblastosis Fetalis - PubMed [Internet]. [cited 2022 Sep 11]. Available from: https://pubmed.ncbi.nlm.nih.gov/30020664/.
- 13.Yaegashi N. Pathogenesis of nonimmune hydrops fetalis caused by intrauterine B19 infection. Tohoku Journal of Experimental Medicine. Volume 190. Tohoku University Medical Press; 2000. pp. 65–82. [DOI] [PubMed]
- 14.Brown KE, Young NS. Parvoviruses and bone marrow failure. Stem Cells Wiley-Blackwell. 1996;14:151–63. doi: 10.1002/stem.140151. [DOI] [PubMed] [Google Scholar]
- 15.Chisaka H, Morita E, Yaegashi N, Sugamura K. Parvovirus B19 and the pathogenesis of anaemia. Rev Med Virol. 2003;13:347–59. doi: 10.1002/rmv.395. [DOI] [PubMed] [Google Scholar]
- 16.Wranne L. Transient Erythroblastopenia in Infancy and Childhood. Scand J Haematol. 1970;7:76–81. doi: 10.1111/j.1600-0609.1970.tb01872.x. [DOI] [PubMed] [Google Scholar]
- 17.Prassouli A, Papadakis V, Tsakris A, Stefanaki K, Garoufi A, Haidas S, et al. Classic transient erythroblastopenia of childhood with human parvovirus B19 genome detection in the blood and bone marrow. J Pediatr Hematol Oncol. 2005;27:333–6. doi: 10.1097/01.mph.0000169249.72858.8c. [DOI] [PubMed] [Google Scholar]
- 18.Crane J, Mundle W, Boucoiran I, Gagnon R, Bujold E, Basso M, et al. Parvovirus B19 infection in pregnancy. J Obstet Gynecol Can Elsevier Inc. 2014;36:1107–16. doi: 10.1016/S1701-2163(15)30390-X. [DOI] [PubMed] [Google Scholar]
- 19.Hellmund A, Geipel A, Berg C, Bald R, Gembruch U. Early Intrauterine Transfusion in Fetuses with Severe Anemia Caused by Parvovirus B19 Infection. Fetal Diagn Ther [Internet]. Fetal Diagn Ther; 2018 [cited 2022 Sep 11];43:129–37. Available from: https://pubmed.ncbi.nlm.nih.gov/28633140/. [DOI] [PubMed]
- 20.Prefumo F, Fichera A, Fratelli N, Sartori E. Fetal anemia: diagnosis and management. Best pract res clin Obstet Gynaecol. Bailliere Tindall Ltd. 2019;58:2–14. doi: 10.1016/j.bpobgyn.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Lindenburg ITM, van Kamp IL, van Zwet EW, Middeldorp JM, Klumper FJCM, Oepkes D. Increased perinatal loss after intrauterine transfusion for alloimmune anaemia before 20 weeks of gestation. BJOG [Internet]. BJOG; 2013 [cited 2022 Sep 11];120:847–52. Available from: https://pubmed.ncbi.nlm.nih.gov/23551577/. [DOI] [PubMed]
- 22.Manchanda A, Datta V, Jhunjhunwala K, Saili A, Kumar A, Agarwal N. Parvovirus B19 nonimmune hydrops in a neonate. Indian J Pediatr [Internet]. Indian J Pediatr; 2007 [cited 2022 Sep 11];74:585–6. Available from: https://pubmed.ncbi.nlm.nih.gov/17595505/. [DOI] [PubMed]
- 23.Janssen O, Lin J. Postnatal IVIG treatment for persistent anaemia in neonate due to congenital parvovirus infection. BMJ Case Rep NLM (Medline); 2021;14. [DOI] [PMC free article] [PubMed]
- 24.Matsuda H, Sakaguchi K, Shibasaki T, Takahashi H, Kawakami Y, Furuya K. Intrauterine therapy for parvovirus B19 infected symptomatic fetus using B19 IgG-rich high titer gammaglobulin. J Perinat Med. 2005;33:561–3. doi: 10.1515/JPM.2005.100. [DOI] [PubMed] [Google Scholar]
- 25.D’Mello RJ, Hsu CD, Chaiworapongsa P, Chaiworapongsa T. Update on the use of intravenous immunoglobulin in pregnancy. Neoreviews Am Acad Pediatr. 2021;22:e7–24. doi: 10.1542/neo.22-1-e7. [DOI] [PubMed] [Google Scholar]
- 26.van der Zwiers C, van Kamp IL, van Geloven N, Lopriore E, Smoleniec J et al. Postponing Early intrauterine Transfusion with Intravenous immunoglobulin Treatment; the PETIT study on severe hemolytic disease of the fetus and newborn. Am J Obstet Gynecol. Mosby Inc.; 2018;219:291.e1-291.e9. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used is not publicly available to protect the personal health information. Fully de-identified clinical test results may be provided upon request.