Abstract
Metabotropic glutamate (mGlu) receptors are promising targets for the treatment of affective disorders and alcohol use disorder (AUD). Nonspecific ligands for Group II (mGlu2 and mGlu3) mGlu receptors have demonstrated consistent therapeutic potential for affective disorders in preclinical models. Disentangling the specific roles of mGlu2 versus mGlu3 receptors in these effects has persisted as a major challenge, in part due to pharmacological limitations. However, the recent development of highly specific allosteric modulators for both mGlu2 and mGlu3 receptors have enabled straightforward and rigorous investigations into the specific function of each receptor. Here, we review recent experiments using these compounds that have demonstrated both similar and distinct receptor functions in behavioral, molecular, and electrophysiological measures associated with basal function and preclinical models of affective disorders. Studies using these selective drugs have demonstrated that mGlu2 is the predominant receptor subclass involved in presynaptic neurotransmitter release in prefrontal cortex. By contrast, the activation of postsynaptic mGlu3 receptors induces a cascade of cellular changes that results in AMPA receptor internalization, producing long-term depression and diminishing excitatory drive. Acute stress decreases the mGlu3 receptor function and dynamically alters transcript expression for both mGlu2 (Grm2) and mGlu3 (Grm3) receptors throughout stress- and reward-related brain areas. Accordingly, both mGlu2 and mGlu3 negative allosteric modulators show acute antidepressant-like effects and potential prophylactic effects against acute and traumatic stressors. The wide array of effects displayed by these new allosteric modulators of mGlu2 and mGlu3 receptors suggest that these drugs may act through improving endophenotypes of symptoms observed across several neuropsychiatric disorders. Therefore, recently developed allosteric modulators selective for mGlu2 or mGlu3 receptors show promise as potential therapeutics for affective disorders and AUD.
Keywords: synaptic plasticity, G protein-coupled receptor, drug discovery, prelimbic cortex, gene expression, electrophysiology
Introduction
Mood disorders, including major depressive disorder (MDD) and anxiety disorders are a broad range of neuropsychiatric disorders characterized by symptoms relating to an individual’s emotional state. The 12-month prevalence for mood disorders and anxiety disorders are approximately 9.5% and 18.1%, respectively [1]. These disorders also display high comorbidity with other psychiatric diseases, notably including substance and alcohol use disorder (AUD) [2]. AUD is characterized by excessive and problematic drinking, and many individuals with AUD display protracted affective symptoms during abstinence. Mood disorders and AUD alike suffer from unsatisfactory treatment options and high rates of recurrence or relapse. Small molecules directed at metabotropic glutamate (mGlu) receptors have garnered significant interest as targets for developing new treatments for affective disorders and AUD [3–6]. The family of mGlu receptors consist of eight receptor subtypes that are classified into three groups (Group I – mGlu1 and mGlu5; Group II – mGlu2 and mGlu3; Group III – mGlu4, mGlu6, mGlu7 and mGlu8) [7]. In the early 1990s, rotationally constrained glutamate analogs were rapidly developed, optimized, and evaluated in rodent models relevant to several psychiatric diseases [4, 8, 9]. While these studies yielded considerable and consistent findings for the therapeutic potential of several classes of mGlu receptor ligands (reviewed in [4, 5]), early compounds suffered from poor receptor subtype selectivity because they targeted the highly conserved glutamate-binding orthosteric site. The first-in-class mGlu2/mGlu3 receptor ligands are non-selective, showing comparable activity at both receptor subtypes [9]. Over the last two decades, however, many drug discovery programs have pivoted their primary screens from radioligand displacement to functional assessments of receptor activity (e.g. calcium mobilization, cAMP production, potassium channel activation) [10, 11]. This technical innovation enabled the detection of molecules that interact with mGlu receptors not only near the glutamate-binding domain but also at other sites (i.e. “allosteric) that are less conserved between related receptor subtypes [12]. Through this approach, subtype-specific positive and negative allosteric modulators (PAMs and NAMs) have been identified for mGlu2 and mGlu3 receptors, including highly potent molecules suitable for systemic administration (Table 1). These groundbreaking compounds have enabled advances in the specific functional properties of mGlu receptor subtypes, while also providing significant evidence for the therapeutic potential of subtype-specific mGlu receptor modulators.
Table 1.
Effects of mGlu2 and mGlu3 receptors across brain circuits involved in mood disorders and alcohol use disorder
| Brain Region | Synaptic Plasticity |
|---|---|
| Prelimbic Prefrontal Cortex | ▪ mGlu2/3 agonism attenuates glutamate release and promotes LTD (31-43) ▪ mGlu2 regulates glutamate release (33-35,43) ▪ mGlu3 mediates postsynaptic LTD via AMPAR internalization (38-43) |
| Hippocampus | ▪ mGlu2/2 homodimers regulate glutamate release at mossy fiber (20) ▪ mGlu2/4 heterodimers regulate glutamate release in perforant path (46) ▪ mGlu3 activation promotes NMDAR-LTP (50, 51) ▪ Glial mGlu3 regulates adrenergic plasticity (54, 55) |
| Striatum | ▪ mGlu2 regulates glutamate and dopamine release (44, 58) ▪ mGlu2/3 agonism induces LTD in the nucleus accumbens (61) |
| Amygdala | ▪ mGlu2/3 agonism attenuates glutamate release and induces LTD in BLA (62), LA (63), CEA (64), and BNST (65) |
Abbreviations: BLA, basolateral amygdala; BNST, bed nucleus of the stria terminalis; CEA, central nucleus of the amygdala; LA, lateral amygdala; LTD, long-term depression; LTP, long-term potentiation
The mGlu2 and mGlu3 receptor subtypes are exclusively categorized as Group II mGlu receptors, evolutionarily related by high sequency homology and therefore shared orthosteric pharmacology. Canonical mGlu2 and mGlu3 receptor signaling proceeds through Gi protein-coupled effectors [7, 10]. Thus, receptor activation facilitates the inhibition of adenylate cyclase activity and cyclic adenosine monophosphate (cAMP) production, as well as the modulation of potassium and calcium channels. These and other signaling cascades affect a host of processes that regulate brain function and behavior, including neuronal intrinsic properties, synaptic plasticity mechanisms, and glial function [13]. mGlu2 and mGlu3 receptors are both expressed at presynaptic terminals, but mGlu3 receptors are also highly expressed at postsynaptic locations and on glia [14–16]. Group II mGlu receptors can form functional homodimers with themselves (ex. mGlu2-mGlu2 or mGlu3-mGlu3) or form heterodimers with the other subunit (mGlu2-mGlu3) [17–19]. The ability to pharmacologically target homodimers or heterodimers holds significant promise to modulate discrete neural circuits. While some allosteric modulators and nanobodies have been developed for mGlu2 homomers [20], we are not aware of the existence of similar tools for mGlu2-mGlu3 heterodimers or mGlu3 homomers. Similarly, the development of biased PAMs and/or NAMs that are biased towards specific effectors (as observed with mGlu5 PAMs [21]) could provide opportunities to minimize potential off target effects while retaining efficacy. More research needs to be carried out to characterize potential signaling bias of existing Group II compounds and to discover new chemical entities with these properties. For more details regarding mGlu2 and mGlu3 receptor signaling mechanisms, genetics and protein structure, or expression and localization, we direct the reader to comprehensive reviews [6, 7, 22, 23].
Recent studies have revealed that mGlu2 and mGlu3 receptors serve both similar and distinct roles across several neural circuits and behavioral outcomes involved in adaptations to stress and models of affective disorders. In this review, we first overview new pharmacological tools available to assess the function of mGlu2 and mGlu3 receptor sub-types. We then describe the current understanding of how each mGlu2 and mGlu3 receptor subtype regulates the physiology of the prefrontal cortex, hippocampus, striatum, and amygdala. We then summarize the acute antidepressant and anxiolytic effects of mGlu2 and mGlu3 drugs. Next, we summarize recent advances in understanding how both receptor subtypes are involved in adaptations to stress in preclinical models. Finally, we discuss recent findings of mGlu2 and mGlu3 receptors on alcohol-related behaviors. We conclude with a brief discussion of the clinical literature around mGlu2 and mGlu3 receptors and clinical trials that have been conducted using selective allosteric modulators of mGlu2 or mGlu3.
Pharmacology
In the mid-1990’s several agonists and antagonists targeting the Group II mGlu receptors were developed, paving the way for physiological and behavioral studies evaluating their basic function and therapeutic potential. The systemically active orthosteric agonists LY354740 and LY379268 (518 PubMed-indexed publications since 1997), along with the related antagonist LY341495 (454 publications since 1997), are among the most widely used mGlu receptor tool compounds in preclinical studies. Reviewed well by others [4, 5], these and related molecules have been used to build significant evidence that mGlu2/3 agonists can attenuate anxiety-like behavior and mGlu2/3 antagonists can deliver antidepressant-like effects. However, until recently, the relative contribution of mGlu2 vs mGlu3 receptors in these effects has not been well understood.
While the development of subtype-specific transgenic mouse lines has helped separate some functions of the distinct Group II mGlu receptors, compensatory adaptations have interfered with clear interpretations in some cases. With that in mind, the development of selective pharmacological tools to modulate only one receptor subtype has been invaluable in helping to understand the individual contributions of mGlu2 or mGlu3 receptors in affective behaviors and neural circuitry. The first series of Group II selective compounds were mGlu2 PAMs (originally termed ‘potentiators’). These mGlu2 PAMs (LY181837, LY487379, AZD8529 and Biphenyl-indanone A (BINA)) provided the unprecedented ability to ask questions about the specific role of a single Group II mGlu receptor subtype. More recently, highly selective and centrally penetrant NAMs have been developed for both mGlu2 and mGlu3 receptors [24]. These classes of compounds, notably including the mGlu2-selective NAM VU6001966 [25] and the mGlu3-selective NAMs VU0650786 [26] and VU6010572 [27], have enabled thorough and mechanistic preclinical studies that detail how mGlu2 and mGlu3 receptors separably influence neurophysiology (Table 1) and affective behaviors (Table 2).
Table 2.
Physiochemical and behavioral properties of novel mGlu2 and mGlu3 negative allosteric modulators
| Compound | Target | IC50 (μM) | Brain Kp | T1/2 (min) | Dose i.p. (mg/kg) | Behavioral Effects | Refence |
|---|---|---|---|---|---|---|---|
| VU6001966 | mGlu2 | 78 | 1.9 | 20 | 10 – 30 | ↔ immobility TST ↓ immobility FST |
Bollinger et al. J Med Chem Letters. 2017 |
| VU0650786 | mGlu3 | 392 | 1.7 | 49 | 10 – 56.5 | ↓ marble burying ↓ immobility TST and FST reverse anhedonia and amotivation after stressors |
Engers et al. J Med Chem. 2015 |
| VU6010572 | mGlu3 | 245 | 1.15 | ? | 1.8 – 3 (mouse/rat) | ↓ immobility TST ↑ open arm time EPM (rat) ↓ habituation to startle (rat) ↓ TMT-induced hyperactivity (rat) |
Engers et al. J Med Chem Letters. 2017 |
Note: Findings are from mouse studies unless otherwise specified
Abbreviations: EPM, elevated pus maze; FST, forced swim test; TMT, trimethylthiazoline predator odor; TST, tail suspension test
Function and Physiology
Prefrontal cortex
The prefrontal cortex (PFC) regulates the direction and vigor of cognitive and motivated behaviors by computing information related to internal state, environmental cues, and long-term goals [28]. Human studies have consistently detected population-level associations in PFC function with affective disorders, and both human and preclinical studies indicate the PFC is exquisitely sensitive to stressors of all magnitudes and modalities [29]. The PFC can be broadly split into orbitofrontal, ventral, dorsal, and cingulate regions, which roughly correspond to the orbitofrontal (OFC), infralimbic (ILC), prelimbic (PLC), and anterior cingulate (ACC) cortices. In general, the function of the anterior and ventral regions (OFC/ILC) is associated with autonomic and emotional responses, whereas the posterior and dorsal regions (PLC/ACC) are more involved in higher-order planning [30]. Preclinical physiology and plasticity studies have primarily focused on understanding how mGlu2 and mGlu3 receptors regulate PLC function and this section will be accordingly biased.
In rodent PFC, mGlu2/3 agonists rapidly attenuate glutamate release probability and induce sustained long-term depression (LTD) of excitatory transmission [31, 32]. Until recently, it was unclear whether these actions were related to each other and if either is mediated by one specific mGlu receptor subtype. Substantial evidence now indicates that presynaptic mGlu2 receptors regulate acute glutamate release probability. mGlu2 PAMs, like BINA, attenuate increases in glutamate release triggered by serotonergic activation in PLC slices [33, 34]. In addition, incubating PLC slices with the selective mGlu2 NAM VU6001966 increases glutamatergic tone and increases indices of presynaptic release probability onto pyramidal cells [35]. Similar findings have been obtained from synaptosomes prepared from whole cortex: both BINA and another mGlu2 PAM, LY566332, facilitated the inhibition of glutamate exocytosis from cortical synaptosomes [36]. In those studies, two mGlu3 NAMs also partially attenuated the effect of an mGlu2/3 agonist, suggesting mGlu2/3 heterodimers may assemble at presynaptic terminals in PFC, consistent with molecular studies [17–19]. Finally, acute presynaptic inhibition of glutamate release has also been observed in pyramidal cells within layers 2/3 of human cortex [37], demonstrating important conservation of mGlu2 receptor function in cortex across species.
In PLC slices, mGlu2/3 agonists also mediate LTD of excitatory transmission that persists for >30 minutes following agonist wash-out [38–40]. This LTD is almost entirely dependent on mGlu3 receptors (see next paragraph for exception). With respect to field potentials or excitatory postsynaptic currents evoked with electrical stimulation, selective mGlu3 NAMs completely block PFC LTD, while mGlu2 NAMs do not [38–40]. Similar findings have been observed in mGlu3 knockout mice but not mGlu2 knockout mice [38, 41]. Electron microscopy studies display high subcellular localization of mGlu3 receptors in dendrites and other postsynaptic compartments in monkey PFC [42], and several studies implicate the necessary participation of postsynaptic signaling cascades and the internalization of AMPA receptors as the final step in LTD expression. LTD was associated with decreased amplitude of spontaneous excitatory postsynaptic currents, did not affect NMDA receptor responses, and was blocked by a dynamin inhibitory peptide that disrupts endocytosis within the postsynaptic neuron [39]. LTD was not dependent on the mobilization of intracellular calcium stores but did require activation of the PI3K/Akt signaling pathway [40]. We also found that other molecules associated with postsynaptic signaling, including mGlu5 receptors, Glycogen Synthase Kinase 3, and Homer proteins, are critically involved in mGlu3-LTD [40]. Thus, cortical mGlu3 receptor activation initiates a cascade of intracellular signaling events that culminates in AMPA receptor internalization and reduced excitatory drive onto pyramidal cells.
The PFC receives glutamatergic input from a variety of cortical and subcortical afferents. Studies using optogenetics have identified that mGlu2 and mGlu3 receptors are differentially involved in regulating the strength of glutamate transmission at distinct inputs. We discovered that mGlu3 receptors induce LTD at isolated inputs from the basolateral amygdala (BLA), whereas transmission from the ventral hippocampus was not affected by an mGlu2/3 agonist [39]. Furthermore, by leveraging a combinatorial viral strategy to selectively express channelrhodopsin in the BLA and genetically reduce mGlu3 receptor expression in PLC, we demonstrated that mGlu3 receptors expressed in postsynaptic sites themselves are required for LTD. By contrast, PLC synapses arising from the mediodorsal thalamus (MDT) express LTD mediated by either mGlu2 or mGlu3 [43]. LTD inducted by an mGlu2/3 agonist was only partially blocked by either the mGlu3 NAM VU0650786 or two structurally distinct mGlu2 NAMs. Considering that mGlu2 NAMs increase measurements of glutamate release probability but mGlu3 NAMs do not, these data suggest that mGlu2 receptors mediate presynaptic LTD at MDT→PLC synapses. MDT-arising synapses are also regulated by mGlu2/mGlu4 receptor heterodimers, while BLA and other inputs to PLC are not [34]. Taken together, these findings indicate that synapses from the BLA and MDT both participate in postsynaptic mGlu3 LTD, and synapses from the MDT also engage in presynaptic mGlu2-dependent LTD, similar to phenomena reported at thalamic terminals in striatum [44].
Hippocampus
The hippocampus is a cortical brain area intimately linked with memory formation and retrieval. One of its key functions is converting short-term memories into long-term memories; thus, it is not surprising that hippocampus function has been associated with the development of PTSD and disorders associated with chronic stressful experiences [45]. The hippocampus has been one of the best-studied brain regions in preclinical electrophysiology and synaptic plasticity research, largely due to its defined, laminar structure and prominent location. The canonical path of information flow through the hippocampus proceeds through a glutamatergic tri-synaptic circuit: perforant path inputs from entorhinal cortex enter the hippocampus within the dentate gyrus; the mossy fiber carries dentate gyrus granule cell projections to CA3; the Schaffer collateral describes synapses from CA3 pyramidal cells to CA1; CA1 and subiculum pyramidal cells then project to a variety of cortical and subcortical structures [46].
mGlu2 and mGlu3 receptors are expressed throughout the hippocampus. mGlu2 receptors are expressed in the lateral perforant path, where they attenuate glutamate release probability onto granule cells in the dentate gyrus [47, 48]. Cells within the dentate gyrus display the highest levels of Grm2 transcript within the hippocampus [49], and, accordingly, mossy fiber synapses terminating in CA3 are also regulated by mGlu2 receptors [20]. Interestingly, recent studies using allosteric nanobodies and novel small molecules indicate that mGlu2 receptors assemble as distinct dimers across hippocampal synapses, with mGlu2/2 homodimers [20] regulating glutamate release at the mossy fiber synapses while mGlu2/4 heterodimers function within the performant path [47]. Historically, mGlu2 receptors were not thought to regulate glutamate release within the CA1 region, but recent studies have identified that transmission along the temporo-ammonic path (the direct input from entorhinal cortex to CA1) is sensitive to mGlu2 receptor modulation [50].
Grm3 transcript (encodes mGlu3) is broadly expressed throughout hippocampal subregions, but mGlu3 receptor functions have been described best within CA1. Despite being similarly expressed in post/peri-synaptic sites and in glia, hippocampal mGlu3 receptors regulate glutamate transmission in a distinct manner from their cortical counterparts. Recent research has identified that activation of mGlu3 receptors promotes the LTP induction at the Schaeffer collateral synapse [51, 52]. Subthreshold activation of mGlu3 receptors facilitates the induction of tetanus-induced LTP and saturating agonist application can increase glutamate transmission on its own. Like mGlu3-LTD in frontal cortex, mGlu3-LTP in CA1 is dependent on the coordinated activation of mGlu5 receptors [51], however mGlu3-LTP is also dependent on the activation of NMDA receptors and the mobilization of intracellular Ca2+ [52]. Studies using cell type-specific transgenic mice demonstrated that neuronal expression of mGlu3 and mGlu5 receptors is necessary for LY379268 to potentiate LTP and the restoration of deficits in trace fear learning [51]. Taken together, these studies indicate that neuronal mGlu3 receptors play an important role in regulating the strength of transmission through hippocampal circuits as well as trace fear learning.
mGlu3 receptors are the primary, if not only, mGlu receptor subtype expressed in astrocytes in adulthood [53]. Glial mGlu3 receptors were first implicated in regulating hippocampal plasticity by a series of early studies demonstrating Group II mGlu receptors can inhibit adrenergic facilitation of LTP [54, 55]. Recent studies using selective NAMs, knockout mice, and glial toxins, indicate these effects are exclusively mediated by mGlu3 receptors and require intact glia [56]. Coordinated activation of mGlu3 receptors and β receptors results in a large increase in cyclic AMP, release of adenosine, and activation of presynaptic A1 adenosine receptors to block the induction of LTP. Overall, the recent literature indicates that glial and neuronal mGlu3 receptors regulate hippocampal metaplasticity through divergent mechanisms and result in distinct circuit-level outcomes. As microglia have also been implicated in stress-related disorders, the ability of mGlu3 receptors to regulate microglia function [57] also merits further investigation.
Striatum and amygdala
While less is known regarding their signaling mechanisms or ability to participate in synaptic plasticity, recent studies using contemporary tools have investigated mGlu2 and mGlu3 receptors in other regions. The dorsal and ventral striatum are widely implicated in reward learning and mood disorders [58]. Within dorsal striatum, mGlu2 receptors, but not mGlu3 receptors attenuate glutamate and dopamine release probabilities [44, 59]. This plasticity appears to be related to the inhibition of presynaptic Ca2+ entry through P/Q-type channels [60]. In related findings, mGlu2, but not mGlu3, receptor knockout mice displayed enhanced novelty-induced locomotor activity and reduced hyperactivity following methamphetamine administration [61], suggesting mGlu2 receptor function has clear relevance for behaviors dependent on striatal function. Nonselective mGlu2/3 agonists have long been known to induce LTD in the nucleus accumbens [62], but the relative contribution of each receptor subtype has yet to be established.
mGlu2/3 agonists also attenuate glutamate transmission and induce LTD within several nuclei of the extended amygdala, including the BLA [63], the lateral amygdala [64], the central nucleus [65], and the bed nucleus of the stria terminalis [66]. These extended amygdala regions play an important role in mood disorders and AUD [67]. In most of these nuclei, the individual contribution of mGlu2 or mGlu3 receptors to LTD is unknown. In the lateral amygdala, studies using knockout rats, knockout mice, and the mixed compound LY541850 demonstrated that both mGlu2 and mGlu3 receptors can induce LTD [64]. In the BLA, studies have shown that an mGlu2 positive allosteric modulator can attenuate glutamate release [68] but it is not clear whether mGlu3 receptors may regulate plasticity as well.
Effects of Stress Exposure
Receptor expression
Stress is a major factor in the etiology of many affective disorders. Relatively few studies have directly assessed how chronic stress affects the function of mGlu2 and mGlu3 receptors. Studies examining chronic social isolation in male mice have related decreased prefrontal cortex Grm2 (encodes mGlu2) expression with depressive-like outcomes [69]. We have also demonstrated decreased Grm2 expression in ILC four weeks after an acute exposure to a predator odor stressor in male, Long-Evans rats [70]. In a related study, decreased Grm2 and mGlu2 protein expression was observed in the BLA in panic-prone rats [68]. Similarly, in the dentate gyrus, protein and transcript levels for mGlu2 receptors were decreased in mice following three weeks chronic restraint stress [71]. The same authors also found decreased mGlu2 receptor protein expression in hippocampus and PFC in mice that were susceptible to four weeks chronic unpredictable stress, whereas resilient mice displayed decreased PFC mGlu2 receptor expression, but intact hippocampal expression relative to controls [72]. These studies collectively suggest that mGlu2 receptor expression levels, across multiple brain regions, may be inversely related to stress exposure and depressive-like behaviors. Consistent with that hypothesis, epigenetic increases in Grm2 expression have been linked with antidepressant drug treatment. Studies from the Nicoletti lab have shown that the well-tolerated drug L-acetylcarnitine epigenetically increases hippocampal and PFC Grm2 expression in Flinders Sensitive Line rats and in mice exposed to chronic unpredictable stress [73], suggesting a bidirectional relationship between cortical mGlu2 receptor function and affective behaviors. Based on this literature, potentiating mGlu2 receptor function, via PAMs or alternative mechanisms, may be able to reverse stress-induced adaptations and related affective disturbances.
In addition to the effects of stress on mGlu2 receptors, stress also impacts mGlu3 expression (gene: Grm3). Our lab has found acute exposure to a predator odor stressor in rats downregulates Grm3 levels in the PLC and dorsal hippocampus two days following the stressor. By contrast, we detected upregulated Grm3 in the nucleus accumbens at the same timepoint, indicating that changes in regulation of mGlu3 receptor expression following traumatic stress are dependent on brain region. In addition, the time elapsed following predator odor is a crucial variable that regulates mGlu3 mRNA levels, as we found that Grm3 was upregulated in the insular cortex two weeks following stress exposure. [70]. Overall, traumatic stress dynamically alters mGlu3 receptor expression in cortex and striatum at early timepoints, and the durable effects in the insular cortex may have important ramifications for developing new treatments for PTSD. Nonetheless, more research will be needed to further identify the cell types involved in these phenomena (e.g. neurons vs. glia) and to better understand the effects of mild and/or chronic stress on mGlu3 receptor expression.
Physiology and synaptic plasticity
Studies have begun to address how stressful experiences alter the separable physiological functions of mGlu2 and mGlu3 receptor signaling. In mice, we have found that a single exposure to 20 minutes of immobilization stress, 30 minutes before sacrifice, impairs mGlu3-LTD within PLC [39, 40]. Stress also impaired mGlu3-LTD when animals were sacrificed one day later, and the plasticity recovered to comparable levels as controls following three days. Systemic administration with the mGlu3 NAM VU0650786 (30 mg/kg) 15 minutes prior to, or immediately following, 20 minutes restraint stress exposure restored the ability to induce mGlu3-LTD ex vivo [39], indicating that receptor activation during stress is necessary for the physiological changes in plasticity to occur. In addition, the stress-induced impairment in mGlu3-LTD was rescued via ex vivo application of the mGlu5 PAM VU0409551 [40]. Notably, several alternative mGlu5-dependent functions were intact following acute stress, indicating a selective alteration in signaling related to mGlu3-LTD. These findings raise the possibility that mGlu3 NAMs may be useful in preventing the consolidation of stress-induced adaptations to PFC function. Another exciting hypothesis for future studies is that mGlu3 PAMs or potentiators may be efficacious in ameliorating stress disturbances that have been consolidated or established. Unfortunately, the limited availability of selective mGlu3 PAMs has hindered efforts in clearly testing this hypothesis. In addition, whether acute and chronic stress affects mGlu2 and mGlu3 receptor-dependent plasticity in other cortical and subcortical areas remains an open area of inquiry.
Behavior
Newly developed mGlu2 and mGlu3 NAMs have shown promising results in preclinical animal experiments for their role in alleviating stress-induced adaptations in depressive-like behaviors. For example, an mGlu3 NAM (VU0650786, 10-30 mg/kg) dose-dependently blocked restraint stress-induced changes in motivation to work for palatable food on a progressive ratio schedule of reinforcement in male mice when administered 15-min before restraint stress [39]. In another study modeling chronic stress effects on reward behavior, both chronic corticosterone (CORT) exposure in the drinking water and chronic variable stress decreased sucrose preference. Treatment with either the mGlu2 NAM VU6001966 (10 mg/kg) or the mGlu3 NAM VU0650786 (30 mg/kg) one day before the sucrose preference test reversed the stress-induced anhedonia-like behavior [43]. Importantly, future studies should also assess how to best prevent or mitigate potential side effects related to mGlu2 or mGlu3 receptor inhibition, considering observed effects on cognitive functions including working memory [42] and extinction learning [38].
Similar to studies examining non-conditioned behaviors, studies assessing stress-induced behaviors in mGlu2 and mGlu3 knockout mice have yielded mixed results. In studies using mice on a CD1 background, mGlu2 knockouts showed resilience to the development of escape deficits induced by inescapable shock stress, while mGlu3 knockouts did not [74]. Furthermore, mGlu2 knockouts did not exhibit decreased escape behavior following CORT administration or anhedonia-like behavior induced by chronic social defeat stress [74]. By contrast, other knockout studies in mice on a C57BL/6J background have reached the opposite conclusion, that Grm2 genetic deletion enhances susceptibility to stress. Nasca et al. found that after four weeks of chronic unpredictable stress, mGlu2 knockout mice displayed increased immobility on the forced swim, decreased body weight, and greater fur coat deterioration relative to matched C57BL/6J controls [72]. Together, these data show that while mGlu2 receptor NAMs demonstrate therapeutic potential for treating anhedonia in MDD, additional mechanistic preclinical neuroscience research is needed to reconcile inconsistencies related to rodent genetic background.
Behavioral studies in traumatic stress models have revealed prophylactic anti-stress effects of mGlu3 NAM administration. Pretreatment with the mGlu3 NAM VU6010572 (3 mg/kg) prior to exposure to an acute predator odor stressor did not affect the engagement in stress-reactivity behavior during the stressor, but blocked freezing behavior when rats were re-exposed to the stressor context two weeks later [75]. As exposure to the scent of a predator has been used to model aspects of a traumatic stress experience [70, 76, 77], an intriguing hypothesis for future studies is that signaling triggered by mGlu3 receptors could be recruited during traumatic stressors and contribute to long-term adaptations related to PTSD. Consistent with that notion, mGlu3 receptor activation was found to block the reconsolidation of fear learning [56]. Molecularly, VU6010572 blocked increased Grin3B upregulation by predator odor exposure in the insular cortex and bed nucleus of the stria terminalis [75]. Grin3B is the gene that encodes for the NMDA receptor subunit GluN3B and has recently been identified as a predictive blood biomarker of PTSD symptomology following a traumatic experience [78]. Based on this, Grin3B expression represents an exciting potential biomarker to be deployed in future studies assessing the therapeutic potential for mGlu3 NAMs in treating PTSD and/or other affective disorders.
Antidepressant- and Anxiolytic-like Behavioral Effects
Passive coping behaviors
Their abilities to modulate adaptations to stress, and neural circuits related to anxiety, affect, and reward in general, suggest that mGlu2 and mGlu3 receptors are poised to regulate motivated behaviors [79]. Indeed, as discussed, systemic administration of mGlu2 and mGlu3 NAMs confers rapid antidepressant-like effects in rodent models. Several studies have shown that mGlu3 NAMs decrease passive coping behavior in acute rodent models. The mGlu3 NAM VU0650786 decreased time spent immobile in male mice in a forced swim test (56.6 mg/kg, 30-min pretreatment; all compounds administered i.p. unless otherwise noted) [26]. The structurally distinct mGlu3 NAM VU6010572 (3 mg/kg, 15-min pretreatment) also demonstrated antidepressant-like effects in a tail-suspension test in male mice [27], consistent with on-target mGlu3 receptor inhibition driving effects on passive coping behavior. In two studies that performed a head-to-head comparison, the mGlu3 NAMs VU0650786 (30 mg/kg) and VU6010572 (1.8-3 mg/kg), but not the mGlu2 NAM VU6001966 (10-30 mg/kg), increased the latency to immobility and decreased the total time spent immobile in the tail suspension test [27, 43]. Interestingly, both the mGlu2 NAM VU6001966 (10 mg/kg) and the mGlu3 NAM VU0650786 (30 mg/kg) administered 45 minutes before a forced swim test in male C57BL/6J mice decreased latency to float immobile and decreased total time immobile [43], suggesting that mGlu2 NAM effects on passive coping behavior may not generalize to all behavioral tasks. Chemogenetic inhibition of MDT→PFC circuitry blocked the behavioral effect of both the mGlu2 and mGlu3 NAMs in the forced swim test [43]. This finding suggests that, despite the differences in synaptic mechanisms, MDT→PFC circuitry is similarly involved in the antidepressant-like effects of both mGlu2 and mGlu3 NAMs.
Similar studies using genetic manipulations have yielded more mixed results than those using selective pharmacology. In a series of studies using male mice on a C57BL/6J background, mGlu3 knockouts displayed decreased immobility in the forced swim test [80]. In studies from another group, mGlu2 knockouts on a C57BL/6J background had no baseline differences in behavior in the forced swim test relative to controls [72]. By contrast, a recent set of studies using CD1 background knockout mice found that mGlu2 but not mGlu3 knockout mice exhibited reduced immobility in the forced swim test and fewer escape failures in a learned helplessness assay [74]. Thus, some evidence from knockout mouse suggests that both mGlu2 and mGlu3 NAMs have antidepressant-like potential, however these findings may critically depend based on genetic background. Finally, we observed that selective genetic knockdown of mGlu3 receptors within the PFC led to decreased immobility in both the forced swim and tail suspension tests in both male and female mice on a hybrid C57BL/6N x 6J background [41]. These studies provide evidence that mGlu3 receptor inhibition exerts antidepressant-like effects in both male and female rodents and implicate PFC circuits in mediating behavioral responses to mGlu3 receptor modulators.
Anxiety-like behaviors
A few studies assessing anxiety-like behaviors have produced evidence that selective inhibition of mGlu3 receptors may attenuate anxiety; however, to our knowledge selective mGlu2 NAMs have not been examined in similar studies. The mGlu3 NAM VU0650786 decreased marble burying behavior (10-56.5 mg/kg; 15-min pretreatment) in male mice [26], consistent with a potential decrease in anxiety-like behavior. In separate studies in rats, a single administration of mGlu3 NAM VU6010572 (3 mg/kg) increased time spent in the open arms of an elevated zero maze test when tested 2 weeks after treatment, demonstrating that this drug may have prophylactic effects in addition to its acute effects [75]. Studies using knockout mice corroborate the recent pharmacological studies to some extent. A modest anxiolytic-like effect was observed in male mGlu3 knockout mice on some but not all endpoints in the light-dark transition [80]. In a more comprehensive study with respect to anxiety-like behaviors, male mGlu3 receptor knockout mice were found to exhibit a trend increase in open arm time on the elevated plus maze, a shorter latency to enter the open arms, and a shorter latency to eat novel food in a hypophagia task [81]. By contrast, mGlu2 knockout mice in the same study were not different than controls on any measure of anxiety-like behavior. Finally, consistent with the modest anxiolytic-like effect observed in global knockouts, mGlu3 receptor knockdown in the mouse PFC also increased time spent in the open arms of the elevated zero maze but had no effect on the light-dark box test [41]. These convergent data from multiple laboratories suggest that mGlu3 NAMs have the potential to be developed as novel anxiolytic medications, but enthusiasm should be tempered due to the limited breadth across preclinical models related to anxiety. Some evidence from preclinical models also suggests that mGlu2 PAMs may have potential anxiolytic activity [82, 83].
Alcohol behaviors
Nonselective mGlu2/3 agonists have repeatedly been shown to reduce alcohol intake behaviors, including operant self-administration and cue-induced reinstatement, but the relative contribution of mGlu2 vs mGlu3 receptors in these effects remains considerably unclear [3, 6, 84, 85]. Interestingly, the mGlu2/3 agonist LY379268 (3 mg/kg, subcutaneous) was found to be more effective in reducing ethanol self-administration and reinstatement in dependent Wistar rats relative to non-dependent controls [85], suggesting that chronic ethanol exposure may upregulate mGlu2/3 receptor function. Consistent with this hypothesis, we recently discovered that prior exposure to intermittent drinking enhanced the ability of LY379268 to attenuate presynaptic glutamate release probability on intratelencephalic neurons within mouse PLC [86]. Considering this function is mediated by mGlu2 and not mGlu3 receptors [43], these findings provide a potential neurobiological substrate through which mGlu2-directed compounds could alter ethanol-related behaviors. Furthermore, intermittent alcohol vapor exposure decreases mGlu2 receptor expression in ILC pyramidal neurons and resulted in insensitivity to mGlu2/3 agonist-induced decreases in extracellular glutamate in the nucleus accumbens shell [87]. Viral-mediated restoration of mGlu2 receptor expression in the ILC reversed escalated alcohol-seeking.
Studies in rats have also demonstrated the potential for mGlu2 receptor potentiation to attenuated maladaptive drinking. The mGlu2-selective PAM, AZD8529 (20-40 mg/kg, subcutaneous), modestly decreased operant alcohol (20%) self-administration in male, Wistar rats [88]. More robustly, AZD8529, blocked cue-induced alcohol seeking in the same rats. This effect was not observed in alcohol preferring (P rats) rats, which lack expression of mGlu2 receptors [89], demonstrating the functional role of mGlu2 in the effect on cue-induced alcohol seeking [88]. Another mGlu2 PAM BINA (20 mg/kg), failed to show these effects on self-administration and cue-induced reinstatement, demonstrating that this effect may be dependent on the mGlu2 PAM used or other technical parameters between investigators [90]. Once selective mGlu3 PAMs are widely available, assessing whether these compounds can recapitulate the broad ability of mGlu2/3 agonists to reduce motivation to drink in preclinical models will be an important series of studies. Alternatively, it is possible that potentiation of receptor activity is insufficient to reduce drinking and we may find that agonist activity at mGlu2 or mGlu3 receptors is essential.
Several lines of convergent evidence indicate that mGlu3 receptors may be a promising target for AUD treatment development. Genetic knock out of mGlu3 receptors in mice blocked the conditioned place preference of alcohol [91], suggesting that mGlu3 receptors may be involved in the rewarding and/or interoceptive effects of alcohol. Both mGlu2/3 agonists and antagonists attenuate the interoceptive stimulus effects (i.e., the subjective effects) of alcohol in rats [92, 93]. In recent studies using the newly developed selective NAMs, both the mGlu2 NAM VU6001966 (6 mg/kg) and the mGlu3 NAM VU6010572 (12 mg/kg) attenuate the interoceptive stimulus effects of alcohol in rats (2 g/kg, intragastric) [94]. These data raise the possibility that these receptors are both involved in the interoceptive effects of alcohol, and/or their effects on cellular physiology can blunt the interoceptive effects of alcohol. Taken together, the ability of both mGlu2 and mGlu3 NAMs to attenuate the interoceptive effects of alcohol, produce acute and lasting antidepressant-like effects, and reverse stress-induced adaptations, may make these drugs ideal candidates for potentially treating affective disorders and AUD.
Clinical studies and looking ahead
While limited in number and interpretation, some findings from post-mortem studies have implicated mGlu2 and mGlu3 receptor function in affective disorders. One post-mortem study found decreased binding of the mGlu2/3 antagonist [3H]LY341495 in the anterior cingulate cortex of subjects that had MDD [101]. Another post-mortem study also found decreased mGlu2/3 receptor immunoreactivity in the PFC from individuals with MDD compared to controls [102]. Together, these studies indicate that depression is associated with decreased expression of Group II mGlu receptors in the brain, which are consistent with the effects of stress and depressive-like behavior in rodents. Given their divergence in regulating synaptic plasticity, glial function, and other neurobiological actions, future post-mortem studies should be designed to disentangle mGlu2 versus mGlu3 receptor expression, should assess cellular and subcellular location of the receptors, and should consider using more selective radioligands and/or assessing receptor expression at the transcript level. Future studies should also assess potential changes in mGlu2 and mGlu3 receptor expression in anxiety disorders and AUD.
Agonists of mGlu2/3 receptors have undergone clinical trials for several neuropsychiatric disorders. One clinical trial investigated the efficacy of LY354740 (an mGlu2/3 agonist) and LY544344 (an LY354740 prodrug) for generalized anxiety disorder. Patients treated with LY544344 showed significant improvements compared to baseline in Hamilton Anxiety and Clinical Global Impression—Improvement scores. Unfortunately, this trial was discontinued due to findings of convulsions in animal studies, despite the lack of any similar adverse events in humans [103]. One the other hand, while results have not yet been made public, two phase 1 trials recently assessed the Group II mGlu receptor antagonists BCI-838 and BCI-632/MGS0039 (NCT01546051 and NCT01548703). Similarly, results from a phase 2 trial for adjunct treatment in MDD with the Group II NAM decoglurant/RO4995819 (NCT01457677) were recently disclosed indicating no separation from a relatively large placebo response [95]. While clinical trials employing nonselective Group II compounds have yielded disappointing results, additional studies examining the antagonist TS-161 [96] are underway (NCT04821271), and findings from trials investigating selective Group II modulators give reason for more optimism. Treatment with the mGlu2 PAM JNJ-40411813/ADX71149 improved panic disorder symptoms in a small group of patients [68]. A larger trial assessing the mGlu2 PAM as an adjunct treatment in high-anxiety MDD was discontinued due to lack of separation from placebo [97], but the compound is currently enrolled in a large Phase 2 trial for adjunct treatment in epilepsy (NCT04836559) that will provide additional safety and tolerability data. Moving forward, trials employing new selective and potent NAMs, ideally as standalone treatments, would be ideal to test whether selective mGlu2 or mGlu3 inhibition can confer antidepressant and other therapeutic effects in the clinic.
The mGlu2 PAM AZD8529 has been evaluated in clinical trials of schizophrenia [98, 99] and smoking cessation (NCT02401022), but not for AUD. To our knowledge, the primary outcomes of the Phase 2 smoking cessation study have not yet been fully disclosed. In the schizophrenia trials, AZD8529 did not affect primary outcome measurements, including negative symptoms [99], but the compound did increase activation of the anterior cingulate cortex and striatum during a working memory task [98]. This finding demonstrates important proof-of-principle that mGlu2 PAMs can modulate reward circuitry in humans and may pave the way for the development of biomarkers for further trials. While efforts in developing suitable radiotracers are underway, until those tools can be widely deployed, functional biomarkers will be essential for assessing target engagement and potential utility in broad clinical populations. Considering the breadth and depth of preclinical literature supporting the potential utility of selective mGlu2 and mGlu3 allosteric modulators for treating mood disorders and AUD, we hope to see more clinical trials on the horizon.
Figure 1. Synaptic locus dissociation of mGlu2 and mGlu3 receptor functions in mouse prefrontal cortex.
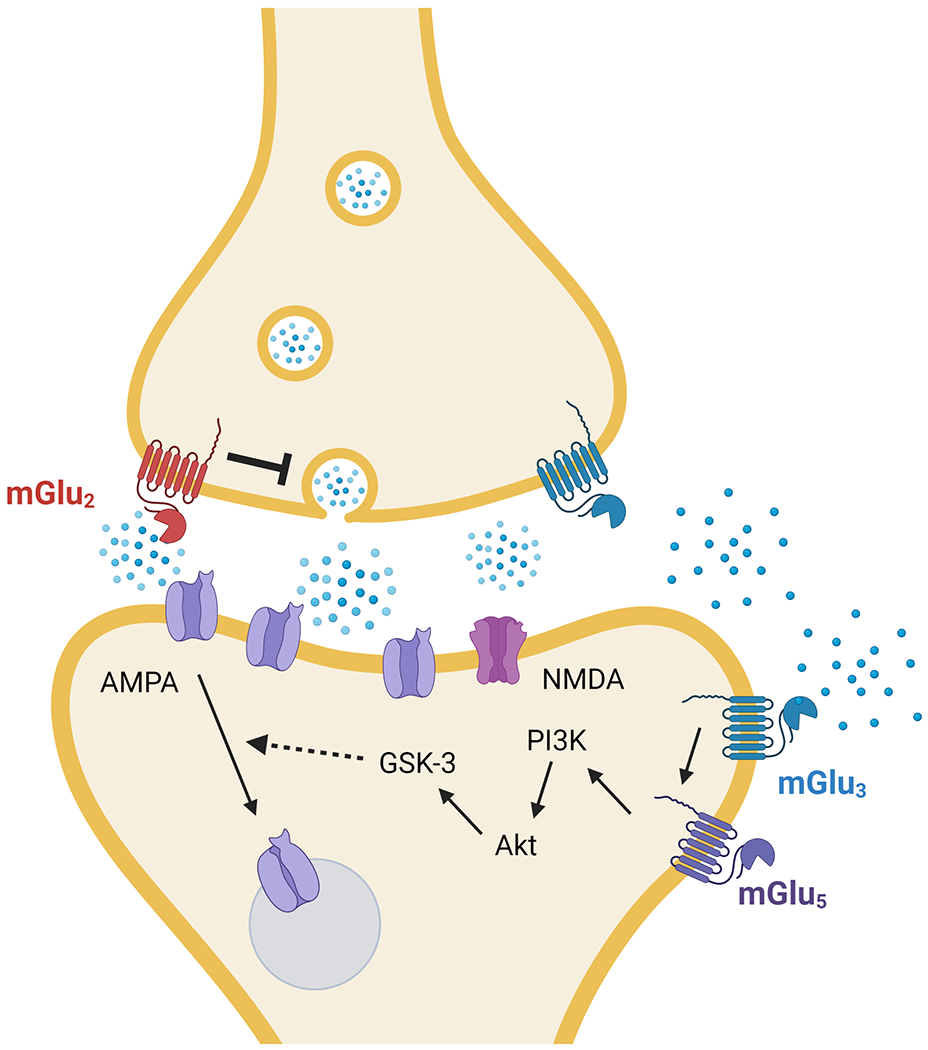
Presynaptic mGlu2 receptors gate glutamate release probability in PFC. At synapses arising from the MDT, presynaptic mGlu2 receptors can induce a long-term depression (LTD) of excitatory transmission. By contrast, neuronal mGlu3 receptors mediate a postsynaptic form of LTD. Postsynaptic mGlu3 LTD proceeds through the coordinated signaling of mGlu5 receptors, phosphoinositide 3-kinase (PI3K), Akt, glycogen synthase kinase-3 (GSK-3), and the internalization of AMPA receptors. mGlu3 LTD is also differentially expressed across long-range inputs to PFC, having been observed at synapses arising from the MDT and BLA, but not the ventral hippocampus.
Acknowledgements
This work was supported in part by the National Institute of Health AA027806 (MEJ) and AA026537 (JB) and by the Bowles Center for Alcohol Studies. RET was supported by AA029946. Figure created with BioRender.com.
Footnotes
Conflict of Interest
The authors declare no potential conflicts of interest.
References
- 1.Kessler RC, et al. , Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry, 2005. 62(6): p. 617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasin DS, et al. , Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry, 2007. 64(7): p. 830–42. [DOI] [PubMed] [Google Scholar]
- 3.Johnson KA and Lovinger DM, Allosteric modulation of metabotropic glutamate receptors in alcohol use disorder: Insights from preclinical investigations. Adv Pharmacol, 2020. 88: p. 193–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaki S, mGlu2/3 Receptor Antagonists as Novel Antidepressants. Trends in Pharmacological Sciences, 2017. 38(6): p. 569–580. [DOI] [PubMed] [Google Scholar]
- 5.Witkin JM, mGlu2/3 receptor antagonism: A mechanism to induce rapid antidepressant effects without ketamine-associated side-effects. Pharmacol Biochem Behav, 2020. 190: p. 172854. [DOI] [PubMed] [Google Scholar]
- 6.Joffe ME, et al. , Metabotropic Glutamate Receptors in Alcohol Use Disorder: Physiology, Plasticity, and Promising Pharmacotherapies. ACS Chem Neurosci, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niswender CM and Conn PJ, Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol, 2010. 50: p. 295–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witkin JM, et al. , In vitro pharmacological and rat pharmacokinetic characterization of LY3020371, a potent and selective mGlu2/3 receptor antagonist. Neuropharmacology, 2017. 115: p. 100–114. [DOI] [PubMed] [Google Scholar]
- 9.Schoepp DD, Jane DE, and Monn JA, Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology, 1999. 38(10): p. 1431–76. [DOI] [PubMed] [Google Scholar]
- 10.Nicoletti F, et al. , Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology, 2011. 60(7–8): p. 1017–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conn PJ and Roth BL, Opportunities and challenges of psychiatric drug discovery: roles for scientists in academic, industry, and government settings. Neuropsychopharmacology, 2008. 33(9): p. 2048–60. [DOI] [PubMed] [Google Scholar]
- 12.Conn PJ, et al. , Opportunities and challenges in the discovery of allosteric modulators of GPCRs for treating CNS disorders. Nature reviews. Drug discovery, 2014. 13(9): p. 692–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison PJ, et al. , The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3): expression, function and involvement in schizophrenia. J Psychopharmacol, 2008. 22(3): p. 308–22. [DOI] [PubMed] [Google Scholar]
- 14.Bruno V, et al. , The neuroprotective activity of group-II metabotropic glutamate receptors requires new protein synthesis and involves a glial-neuronal signaling. J Neurosci, 1997. 17(6): p. 1891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohishi H, et al. , Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: An in situ hybridization study. Journal of Comparative Neurology, 1993. 335(2): p. 252–266. [DOI] [PubMed] [Google Scholar]
- 16.Tamaru Y, et al. , Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience, 2001. 106(3): p. 481–503. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, et al. , Defining the Homo- and Heterodimerization Propensities of Metabotropic Glutamate Receptors. Cell Rep, 2020. 31(5): p. 107605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levitz J, et al. , Mechanism of Assembly and Cooperativity of Homomeric and Heteromeric Metabotropic Glutamate Receptors. Neuron, 2016. 92(1): p. 143–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doumazane E, et al. , A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB J, 2011. 25(1): p. 66–77. [DOI] [PubMed] [Google Scholar]
- 20.Scholler P, et al. , Allosteric nanobodies uncover a role of hippocampal mGlu2 receptor homodimers in contextual fear consolidation. Nat Commun, 2017. 8(1): p. 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sengmany K, et al. , Biased allosteric agonism and modulation of metabotropic glutamate receptor 5: Implications for optimizing preclinical neuroscience drug discovery. Neuropharmacology, 2017. 115: p. 60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferraguti F and Shigemoto R, Metabotropic glutamate receptors. Cell Tissue Res, 2006. 326(2): p. 483–504. [DOI] [PubMed] [Google Scholar]
- 23.Pin JP and Bettler B, Organization and functions of mGlu and GABAB receptor complexes. Nature, 2016. 540(7631): p. 60–68. [DOI] [PubMed] [Google Scholar]
- 24.Qunies AM and Emmitte KA, Negative allosteric modulators of group II metabotropic glutamate receptors: A patent review (2015 - present). Expert Opin Ther Pat, 2021. 31(8): p. 687–708. [DOI] [PubMed] [Google Scholar]
- 25.Bollinger KA, et al. , Design and Synthesis of mGlu2 NAMs with Improved Potency and CNS Penetration Based on a Truncated Picolinamide Core. ACS Med Chem Lett, 2017. 8(9): p. 919–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engers JL, et al. , Discovery of a Selective and CNS Penetrant Negative Allosteric Modulator of Metabotropic Glutamate Receptor Subtype 3 with Antidepressant and Anxiolytic Activity in Rodents. J Med Chem, 2015. 58(18): p. 7485–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engers JL, et al. , Design and Synthesis of N-Aryl Phenoxyethoxy Pyridinones as Highly Selective and CNS Penetrant mGlu3 NAMs. ACS Med Chem Lett, 2017. 8(9): p. 925–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe M and Sakagami M, Integration of cognitive and motivational context information in the primate prefrontal cortex. Cereb Cortex, 2007. 17 Suppl 1: p. i101–9. [DOI] [PubMed] [Google Scholar]
- 29.Popoli M, et al. , The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nature reviews. Neuroscience, 2011. 13(1): p. 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siddiqui SV, et al. , Neuropsychology of prefrontal cortex. Indian journal of psychiatry, 2008. 50(3): p. 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marek GJ, et al. , Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther, 2000. 292(1): p. 76–87. [PubMed] [Google Scholar]
- 32.Moghaddam B and Adams BW, Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science, 1998. 281(5381): p. 1349–52. [DOI] [PubMed] [Google Scholar]
- 33.Benneyworth MA, et al. , A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol Pharmacol, 2007. 72(2): p. 477–84. [DOI] [PubMed] [Google Scholar]
- 34.Xiang Z, et al. , Input-specific regulation of glutamatergic synaptic transmission in the medial prefrontal cortex by mGlu2/mGlu4 receptor heterodimers. Sci Signal, 2021. 14(677). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joffe ME, Winder DG, and Conn PJ, Contrasting sex-dependent adaptations to synaptic physiology and membrane properties of prefrontal cortex interneuron subtypes in a mouse model of binge drinking. Neuropharmacology, 2020: p. 108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olivero G, et al. , Immuno-pharmacological characterization of group II metabotropic glutamate receptors controlling glutamate exocytosis in mouse cortex and spinal cord. Br J Pharmacol, 2017. 174(24): p. 4785–4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bocchio M, et al. , Group II Metabotropic Glutamate Receptors Mediate Presynaptic Inhibition of Excitatory Transmission in Pyramidal Neurons of the Human Cerebral Cortex. Front Cell Neurosci, 2018. 12: p. 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker AG, et al. , Metabotropic glutamate receptor 3 activation is required for long-term depression in medial prefrontal cortex and fear extinction. Proc Natl Acad Sci U S A, 2015. 112(4): p. 1196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joffe ME, et al. , Metabotropic glutamate receptor subtype 3 gates acute stress-induced dysregulation of amygdalo-cortical function. Mol Psychiatry, 2019. 24(6): p. 916–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joffe ME, et al. , Mechanisms underlying prelimbic prefrontal cortex mGlu3/mGlu5-dependent plasticity and reversal learning deficits following acute stress. Neuropharmacology, 2019. 144: p. 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joffe ME, et al. , Frontal cortex genetic ablation of metabotropic glutamate receptor subtype 3 (mGlu3) impairs postsynaptic plasticity and modulates affective behaviors. Neuropsychopharmacology, 2021. 46(12): p. 2148–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin LE, et al. , mGluR2 versus mGluR3 Metabotropic Glutamate Receptors in Primate Dorsolateral Prefrontal Cortex: Postsynaptic mGluR3 Strengthen Working Memory Networks. Cereb Cortex, 2018. 28(3): p. 974–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joffe ME, et al. , mGlu2 and mGlu3 Negative Allosteric Modulators Divergently Enhance Thalamocortical Transmission and Exert Rapid Antidepressant-like Effects. Neuron, 2020. 105(1): p. 46–59 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson KA, Mateo Y, and Lovinger DM, Metabotropic glutamate receptor 2 inhibits thalamically-driven glutamate and dopamine release in the dorsal striatum. Neuropharmacology, 2017. 117: p. 114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joshi SA, et al. , A review of hippocampal activation in post-traumatic stress disorder. Psychophysiology, 2020. 57(1): p. e13357. [DOI] [PubMed] [Google Scholar]
- 46.Arszovszki A, Borhegyi Z, and Klausberger T, Three axonal projection routes of individual pyramidal cells in the ventral CA1 hippocampus. Frontiers in Neuroanatomy, 2014. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moreno Delgado D, et al. , Pharmacological evidence for a metabotropic glutamate receptor heterodimer in neuronal cells. Elife, 2017. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kew JN, et al. , Differential regulation of synaptic transmission by mGlu2 and mGlu3 at the perforant path inputs to the dentate gyrus and CA1 revealed in mGlu2 −/− mice. Neuropharmacology, 2002. 43(2): p. 215–21. [DOI] [PubMed] [Google Scholar]
- 49.McOmish CE, Demireva EY, and Gingrich JA, Developmental expression of mGlu2 and mGlu3 in the mouse brain. Gene Expr Patterns, 2016. 22(2): p. 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanna L, et al. , Differentiating the roles of mGlu2 and mGlu3 receptors using LY541850, an mGlu2 agonist/mGlu3 antagonist. Neuropharmacology, 2013. 66: p. 114–21. [DOI] [PubMed] [Google Scholar]
- 51.Dogra S, et al. , Activating mGlu3 Metabotropic Glutamate Receptors Rescues Schizophrenia-like Cognitive Deficits Through Metaplastic Adaptations Within the Hippocampus. Biol Psychiatry, 2021. 90(6): p. 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenberg N, Gerber U, and Ster J, Activation of Group II Metabotropic Glutamate Receptors Promotes LTP Induction at Schaffer Collateral-CA1 Pyramidal Cell Synapses by Priming NMDA Receptors. J Neurosci, 2016. 36(45): p. 11521–11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun W, et al. , Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science, 2013. 339(6116): p. 197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gereau R.W.t. and Conn PJ, A cyclic AMP-dependent form of associative synaptic plasticity induced by coactivation of beta-adrenergic receptors and metabotropic glutamate receptors in rat hippocampus. J Neurosci, 1994. 14(5 Pt 2): p. 3310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winder DG, et al. , Novel glial-neuronal signalling by coactivation of metabotropic glutamate and beta-adrenergic receptors in rat hippocampus. J Physiol, 1996. 494 (Pt 3): p. 743–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker AG, et al. , Co-Activation of Metabotropic Glutamate Receptor 3 and Beta-Adrenergic Receptors Modulates Cyclic-AMP and Long-Term Potentiation, and Disrupts Memory Reconsolidation. Neuropsychopharmacology, 2017. 42(13): p. 2553–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zinni M, et al. , mGlu3 receptor regulates microglial cell reactivity in neonatal rats. J Neuroinflammation, 2021. 18(1): p. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marchand WR and Yurgelun-Todd D, Striatal structure and function in mood disorders: a comprehensive review. Bipolar Disord, 2010. 12(8): p. 764–85. [DOI] [PubMed] [Google Scholar]
- 59.Johnson KA, et al. , Operant self-stimulation of thalamic terminals in the dorsomedial striatum is constrained by metabotropic glutamate receptor 2. Neuropsychopharmacology, 2020. 45(9): p. 1454–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kupferschmidt DA and Lovinger DM, Inhibition of presynaptic calcium transients in cortical inputs to the dorsolateral striatum by metabotropic GABA(B) and mGlu2/3 receptors. J Physiol, 2015. 593(10): p. 2295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Busceti CL, et al. , Behavioural and biochemical responses to methamphetamine are differentially regulated by mGlu2 and mGlu3 metabotropic glutamate receptors in male mice. Neuropharmacology, 2021. 196: p. 108692. [DOI] [PubMed] [Google Scholar]
- 62.Robbe D, et al. , Role of p/q-Ca2+ channels in metabotropic glutamate receptor 2/3-dependent presynaptic long-term depression at nucleus accumbens synapses. J Neurosci, 2002. 22(11): p. 4346–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li C and Rainnie DG, Bidirectional regulation of synaptic plasticity in the basolateral amygdala induced by the D1-like family of dopamine receptors and group II metabotropic glutamate receptors. J Physiol, 2014. 592(19): p. 4329–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lucas SJ, et al. , Selective activation of either mGlu2 or mGlu3 receptors can induce LTD in the amygdala. Neuropharmacology, 2013. 66: p. 196–201. [DOI] [PubMed] [Google Scholar]
- 65.Han JS, et al. , Enhanced group II mGluR-mediated inhibition of pain-related synaptic plasticity in the amygdala. Mol Pain, 2006. 2: p. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grueter BA and Winder DG, Group II and III metabotropic glutamate receptors suppress excitatory synaptic transmission in the dorsolateral bed nucleus of the stria terminalis. Neuropsychopharmacology, 2005. 30(7): p. 1302–11. [DOI] [PubMed] [Google Scholar]
- 67.Price JL and Drevets WC, Neurocircuitry of mood disorders. Neuropsychopharmacology, 2010. 35(1): p. 192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Molosh AI, et al. , Panic results in unique molecular and network changes in the amygdala that facilitate fear responses. Mol Psychiatry, 2020. 25(2): p. 442–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ieraci A, Mallei A, and Popoli M, Social Isolation Stress Induces Anxious-Depressive-Like Behavior and Alterations of Neuroplasticity-Related Genes in Adult Male Mice. Neural Plast, 2016. 2016: p. 6212983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tyler RE, et al. , Exposure to the predator odor TMT induces early and late differential gene expression related to stress and excitatory synaptic function throughout the brain in male rats. Genes Brain Behav, 2020. 19(8): p. e12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nasca C, et al. , Stress dynamically regulates behavior and glutamatergic gene expression in hippocampus by opening a window of epigenetic plasticity. Proc Natl Acad Sci U S A, 2015. 112(48): p. 14960–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nasca C, et al. , Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Mol Psychiatry, 2015. 20(6): p. 755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nasca C, et al. , L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proc Natl Acad Sci U S A, 2013. 110(12): p. 4804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Highland JN, et al. , Group II metabotropic glutamate receptor blockade promotes stress resilience in mice. Neuropsychopharmacology, 2019. 44(10): p. 1788–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tyler RE, et al. , The effects of predator odor (TMT) exposure and mGlu3 NAM pretreatment on behavioral and NMDA receptor adaptations in the brain. Neuropharmacology, 2022. 207: p. 108943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Endres T, Apfelbach R, and Fendt M, Behavioral Changes Induced in Rats by Exposure to Trimethylthiazoline, a Component of Fox Odor. Behavioral Neuroscience, 2005. 119(4): p. 1004–1010. [DOI] [PubMed] [Google Scholar]
- 77.Whitaker AM, Gilpin NW, and Edwards S, Animal models of post-traumatic stress disorder and recent neurobiological insights. Behavioural pharmacology, 2014. 25(5–6): p. 398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lori A, et al. , Transcriptome-wide association study of post-trauma symptom trajectories identified GRIN3B as a potential biomarker for PTSD development. Neuropsychopharmacology, 2021. 46(10): p. 1811–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chaki S, mGlu2/3 Receptor Antagonists as Novel Antidepressants. Trends Pharmacol Sci, 2017. 38(6): p. 569–580. [DOI] [PubMed] [Google Scholar]
- 80.Fujioka R, et al. , Comprehensive behavioral study of mGluR3 knockout mice: implication in schizophrenia related endophenotypes. Mol Brain, 2014. 7: p. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Filippis B, et al. , The role of group II metabotropic glutamate receptors in cognition and anxiety: comparative studies in GRM2(−/−), GRM3(−/−) and GRM2/3(−/−) knockout mice. Neuropharmacology, 2015. 89: p. 19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Galici R, et al. , Biphenyl-indanone A, a positive allosteric modulator of the metabotropic glutamate receptor subtype 2, has antipsychotic- and anxiolytic-like effects in mice. J Pharmacol Exp Ther, 2006. 318(1): p. 173–85. [DOI] [PubMed] [Google Scholar]
- 83.Johnson MP, et al. , Metabotropic glutamate 2 receptor potentiators: receptor modulation, frequency-dependent synaptic activity, and efficacy in preclinical anxiety and psychosis model(s). Psychopharmacology (Berl), 2005. 179(1): p. 271–83. [DOI] [PubMed] [Google Scholar]
- 84.Bäckström P and Hyytiä P, Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. European Journal of Pharmacology, 2005. 528(1): p. 110–118. [DOI] [PubMed] [Google Scholar]
- 85.Sidhpura N, Weiss F, and Martin-Fardon R, Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history of ethanol dependence. Biol Psychiatry, 2010. 67(9): p. 804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Joffe ME, Winder DG, and Conn PJ, Increased Synaptic Strength and mGlu2/3 Receptor Plasticity on Mouse Prefrontal Cortex Intratelencephalic Pyramidal Cells Following Intermittent Access to Ethanol. Alcohol Clin Exp Res, 2021. 45(3): p. 518–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meinhardt MW, et al. , Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. J Neurosci, 2013. 33(7): p. 2794–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Augier E, et al. , The mGluR2 Positive Allosteric Modulator, AZD8529, and Cue-Induced Relapse to Alcohol Seeking in Rats. Neuropsychopharmacology, 2016. 41(12): p. 2932–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou Z, et al. , Loss of metabotropic glutamate receptor 2 escalates alcohol consumption. Proc Natl Acad Sci U S A, 2013. 110(42): p. 16963–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Windisch KA and Czachowski CL, Effects of group II metabotropic glutamate receptor modulation on ethanol- and sucrose-seeking and consumption in the rat. Alcohol, 2018. 66: p. 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lainiola M, et al. , The lack of conditioned place preference, but unaltered stimulatory and ataxic effects of alcohol in mGluR3-KO mice. J Psychopharmacol, 2019. 33(7): p. 855–864. [DOI] [PubMed] [Google Scholar]
- 92.Jaramillo AA, et al. , Activation of mGluR2/3 following stress hormone exposure restores sensitivity to alcohol in rats. Alcohol, 2015. 49(6): p. 525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cannady R, et al. , Activation of group II metabotropic glutamate receptors inhibits the discriminative stimulus effects of alcohol via selective activity within the amygdala. Neuropsychopharmacology, 2011. 36(11): p. 2328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tyler RE, et al. , Predator odor (TMT) exposure potentiates interoceptive sensitivity to alcohol and increases GABAergic gene expression in the anterior insular cortex and nucleus accumbens in male rats. bioRxiv, 2022: p. 2022.02.16.480725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Umbricht D, et al. , Randomized, Double-Blind, Placebo-Controlled Trial of the mGlu2/3 Negative Allosteric Modulator Decoglurant in Partially Refractory Major Depressive Disorder. J Clin Psychiatry, 2020. 81(4). [DOI] [PubMed] [Google Scholar]
- 96.Watanabe M, et al. , Evaluation of the Safety, Tolerability, and Pharmacokinetic Profiles of TP0473292 (TS-161), A Prodrug of a Novel Orthosteric mGlu2/3 Receptor Antagonist TP0178894, in Healthy Subjects and Its Antidepressant-Like Effects in Rodents. Int J Neuropsychopharmacol, 2022. 25(2): p. 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kent JM, et al. , Efficacy and safety of an adjunctive mGlu2 receptor positive allosteric modulator to a SSRI/SNRI in anxious depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 2016. 67: p. 66–73. [DOI] [PubMed] [Google Scholar]
- 98.Wolf DH, et al. , Effect of mGluR2 positive allosteric modulation on frontostriatal working memory activation in schizophrenia. Mol Psychiatry, 2022. 27(2): p. 1226–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Litman RE, et al. , AZD8529, a positive allosteric modulator at the mGluR2 receptor, does not improve symptoms in schizophrenia: A proof of principle study. Schizophr Res, 2016. 172(1–3): p. 152–7. [DOI] [PubMed] [Google Scholar]


