Abstract
The prevalence of non-alcoholic fatty liver disease (NAFLD) is continually increasing due to the global obesity epidemic. NAFLD comprises a systemic metabolic disease accompanied frequently by insulin resistance and hepatic and systemic inflammation. Whereas simple hepatic steatosis is the most common disease manifestation, a more progressive disease course characterized by liver fibrosis and inflammation (i.e. non-alcoholic steatohepatitis) is present in 10–20% of affected individuals. NAFLD furthermore progresses in a substantial number of patients towards liver cirrhosis and hepatocellular carcinoma. Whereas this disease now affects almost 25% of the world’s population and is mainly observed in obesity and type 2 diabetes, NAFLD also affects lean individuals. Pathophysiology involves lipotoxicity, hepatic immune disturbances accompanied by hepatic insulin resistance, a gut dysbiosis, and commonly hepatic and systemic insulin resistance defining this disorder a prototypic systemic metabolic disorder. Not surprisingly many affected patients have other disease manifestations, and indeed cardiovascular disease, chronic kidney disease, and extrahepatic malignancies are all contributing substantially to patient outcome. Weight loss and lifestyle change reflect the cornerstone of treatment, and several medical treatment options are currently under investigation. The most promising treatment strategies include glucagon-like peptide 1 receptor antagonists, sodium–glucose transporter 2 inhibitors, Fibroblast Growth Factor analogues, Farnesoid X receptor agonists, and peroxisome proliferator–activated receptor agonists. Here, we review epidemiology, pathophysiology, and therapeutic options for NAFLD.
Keywords: Non-alcoholic liver disease, NAFLD, Cardio vascular disease, Treatment, Pathophysiology, Diagnosis
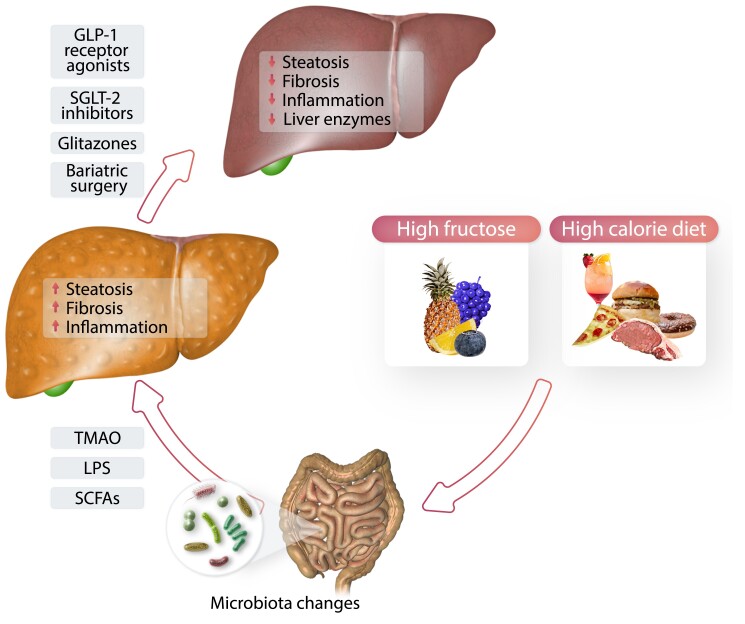
Graphical Abstract
Graphical Abstract.
1. Introduction
The prevalence of non-alcoholic fatty liver disease (NAFLD) is increasing globally and is expected to become the leading cause of liver transplantation by 2030, with expanding costs for the healthcare systems.1 NAFLD comprises a large spectrum of disease entities from simple hepatic steatosis to non-alcoholic steatohepatitis (NASH), liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC). NAFLD has an estimated prevalence of 25% in the general population2 with even higher prevalence in populations with metabolic diseases. Patients with type 2 diabetes (T2D) exhibit a NAFLD prevalence up to 75%,3 and severely obese patients show prevalence rates of even 90%.4,5 A recently published cross-sectional population-based study estimated the prevalence of advanced fibrosis in Germany at 1%.6 NAFLD has evolved as a prototypic systemic disease in the past decade, and importantly extrahepatic diseases such as cardiovascular disease (CVD) or extrahepatic malignancies are the major contributors to mortality in this population.7–9 The stringent associations of NAFLD with its mortality-driving co-morbidities are not well understood but may include various aspects including continuous low-grade inflammation observed in NAFLD.10,11
NAFLD is defined by an excessive hepatic fat accumulation, associated with insulin resistance (IR) and evidence of steatosis based on imaging techniques or histology. Furthermore, secondary causes of hepatic steatosis like alcohol consumption (>30 g for men and >20 g for women) need to be ruled out.12 In 2020, Eslam et al. proposed alternative diagnostic criteria for NAFLD and also suggested an alternative term: metabolic associated fatty liver disease (MAFLD). Instead of the exclusion of alcohol use, ‘positive criteria’ were defined. MAFLD is present in patients with observed hepatic steatosis [as detected by ultrasound, computed tomography, magnetic resonance spectroscopy, or controlled attenuation parameter (CAP, FibroScan)] and overweight [body mass index (BMI) ≥25 kg/m2 in Caucasians or BMI ≥23 kg/m2 in Asians] or T2D. In lean/normal weight patients, two of the following factors need to be present in addition to hepatic steatosis: increased waist circumference, arterial hypertension, elevated triglycerides, decreased plasma high-density lipoprotein cholesterol (HDL-c), pre-diabetes, elevated homoeostasis model assessment of insulin resistance (HOMAR) score, or increased plasma high-sensitivity C-reactive protein. A controversial discussion has evolved around this new, inclusive, diagnostic definition.13 Some studies indicate that using the new definition rather than the old one results in a higher detection of patients suffering from liver disease,14 which would result in better/optimized patient care. On the other side, the new definition criteria fail to include NASH, the aggressive form of NAFLD including inflammation and liver injury. However, there are still some unmet needs for this new definition and therefore we will use the term NAFLD throughout this review. Historically, NAFLD was and still is associated mainly with obese individuals; however, in the past years, the recognition of lean NAFLD as an entity of NAFLD has emerged. Recently, a new clinical practice guideline has been published,15 which supports the importance of diagnosing lean NAFLD patients [e.g. NAFLD and BMI <25 (non-Asian) and <23 kg/m2 (Asian)] and also identifies co-morbidities such as T2D, dyslipidaemia, hypertension, and fibrotic changes of the liver. However, a screening of otherwise healthy people for lean NAFLD is currently not recommended but should be considered in T2D patients older than 40.15
1.1. Pathophysiology of NAFLD
The pathophysiology of NAFLD is complex and heterogenous, already illustrated by the fact that NAFLD comprises a clinical spectrum from simple steatosis to cirrhosis as end stage of liver disease. Many different factors are involved in inducing metabolic associated changes in the liver. An overconsumption of nutrients can lead to dysbiosis in the gastrointestinal tract; further a translocation of microbial-associated molecular patterns to the liver via the portal vein and into the systemic circulation via an increased permeability of the intestinal barrier can induce pro-inflammatory reactions in the liver. On the other side, certain dietary components can also directly trigger relevant disease mechanisms in liver tissue.16–18
1.1.1. Lipotoxicity
One of the characterizing features of NAFLD on histopathological level is the accumulation of lipid droplets in hepatocytes.19 Therefore, a possible disease-driving role of lipids and lipid-derived compounds has been assumed since a long time. Harbouring a SNP in PNPLA3 (rs738409, I148M) increases the genetic susceptibility towards the development of NAFLD.20 This protein is in close proximity to lipid droplets within the hepatocyte.21,22 The I148m alteration in PNPLA3 leads to an altered remodelling of fatty acids in the hepatocytes; further this variant leads to an accumulation of PNPLA3 on lipid droplets, as the degradation of the protein via the ubiquitination pathway is reduced compared with the wild-type protein.21–23 The knockdown of the protein resolves steatosis in an experimental murine steatosis model, indicating that a knockout/inhibition of the enzyme would be a possible treatment target.22 Recently, the germinal centre kinase III (GCKIII) kinases Mammalian sterile 20-like (MST)-3 and MST4 were described to correlate positively with increased histopathological disease severity in NAFLD patients.24–27 These kinases associate with lipid droplets within the hepatocyte26–28 and control lipid-induced metabolic stress in hepatocytes. siRNA silencing experiments in human hepatocytes showed that reduced levels of MST3, MST4, and serine/threonine-protein kinase 24 (STK24) led to a decrease in triacylglycerol (TAG) synthesis and thereby to a reduction in lipid droplet formation. Further, it seems that these three proteins inhibit β-oxidation and thereby drive oxidative stress, which is a key pathomechanism of lipotoxicity in NAFLD.24–29
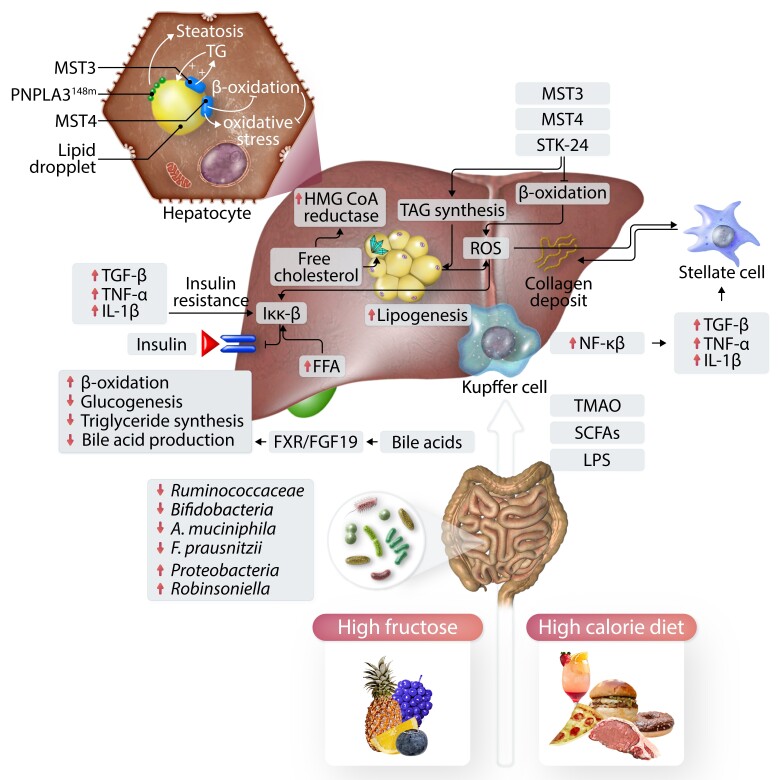
Another compound with possible lipotoxic functions is free cholesterol.30 The expression of 3-hydroxy-3-methylglutaryl (HMG) CoA reductase, the rate-limiting enzyme in cholesterol synthesis, is up-regulated in liver tissue of NAFLD patients compared with lean and obese controls.31 This up-regulation was paralleled by a dephosphorylation, thus more activation of HMG CoA reductase and an increase in free cholesterol synthesis.31 An accumulation of excess free cholesterol can lead to the development of cholesterol crystals in lipid droplets, which was associated with fibrosing NASH in a small human cohort.32 Further, free cholesterol could drive sterile inflammation by interacting with YAP-TAZ, which was also markedly increased in liver tissue of human NAFLD patients and murine livers of NAFLD models.33–36 Free cholesterol but not free fatty acids (FFAs) or triglycerides sensitized the liver towards the development of steatohepatitis induced by tumour necrosis factor (TNF) and fatty acid synthetase (FAS) in rodent models. This is due to depletion of mitochondrial glutathione,37 indicating an inflammation driving role of free cholesterol. Decreased glutathione levels can lead to augmented reactive oxygen stress (ROS) production and thereby to pro-inflammatory processes within the cell.37 Cholesterol is metabolized into bile acids (BAs), which are then secreted into the gut. In the gut, BAs act in the small intestine and play an important role in the uptake of cholesterol, fat, and vitamins (fat soluble). Primary BAs are metabolized by the intestinal microbiome to secondary BAs and also influence the constituency of the microbiome.38,39 In the terminal ileum, almost all BAs are actively reabsorbed.38 BA can act as signalling molecule through different receptors, such as Farnesoid X receptor (FXR) or the G protein–coupled bile acid receptor 1 (GPBAR1 also known as TGR5).40 Reduced hepatic fat accumulation as a result of reduced lipogenesis is observed after activation of FXR.41 Ileal FXR influences hepatic metabolism also through the production of fibroblast growth factor 15 (FGF15; FGF19 in humans) in the small intestine and subsequent increased oxidation of fatty acids and decreased hepatic lipogenesis.40 In mice, nor-ursodeoxycholic acid, by targeting mTORC1 in CD8+ T cells, ameliorated experimental cholestatic liver injury, indicating therapeutic mechanisms beyond hepatocyte metabolism42 (Figure 1).
Figure 1.
The pathogenesis of non-alcoholic fatty liver disease (NAFLD) is multifactorial. Diet and dietary components effect intestinal microbiota and influence hepatic inflammation and steatosis. FFAs, reactive oxygen species (ROS), and low-grade inflammation mediate insulin resistance by altering IKK-β. Increased lipogenesis and free cholesterol further add cellular stress (lipotoxicity). LPS activates hepatic Kupffer cells to produce pro-inflammatory cytokines. SCFAs and TMAO are metabolites derived from diet components through the intestinal microbiota. Together, different mechanisms induce inflammation (e.g. production of pro-inflammatory cytokines), which activates stellate cells to produce collagen and induce fibrogenesis. FFA, free fatty acids; FXR, Farnesoid X receptor; IKK-β, inhibitor of nuclear factor kappa-B kinase subunit beta; IL-1b, interleukin 1 beta; LPS, lipopolysaccharide; MST-3 and MST-4, GCKIII kinases Mammalian sterile 20-like 3 and 4; NF-κB, nuclear factor of activated B cells; ROS, reactive oxygen species; SCFA, Short chain fatty acids; TAG, triacylglycerol; TGFβ, transforming growth factor beta; TMAO, trimethylamine-N-oxide; TNF-α, tumour necrosis factor alpha.
1.1.2. Dietary components affecting NAFLD
Besides overconsumption of calories and consecutive weight gain, fructose is a key player in the development and progression of NAFLD. Fructose is derived from the diet via sweetened beverages and processed food. Fructose increases lipogenesis by enhancing the available substrates for fatty acid synthesis via aldolase B and ketohexokinase action and also by activating transcription factors such as sterol regulatory element-binding protein 1c (SREBP1c) and others.43 A recent small study with paediatric and adolescents NAFLD patients described that the intakes of total calories, fat, and carbohydrates were similar between NAFLD and NASH patients; however, NASH patients had higher total intake of fructose, sugar, sucrose, and glucose.44 A meta-analysis including over 2000 individuals described that an excess of energy delivered by sugar-sweetened beverages (mainly by fructose) leads to an increase of liver fat.45
1.1.3. Microbiome, the intestine, and NAFLD
A plethora of studies has underlined the importance of dysbiosis in the development of different stages of liver disease.17,46–54 These microbial changes were described on phylum, family, genus, and species level. For instance, Proteobacteria seem to be increased in NAFLD,47–49,52 whereas Ruminococcaceae or Bifidobacteriaceae were described to be decreased in NAFLD patients compared with healthy controls.53,55Faecalibacterium prausnitzii, a rather anti-inflammatory bacterial strain, is decreased in NAFLD patients,55,56 while Robinsoniella is an example for a genus to be increased in NAFLD.52
Two murine landmark studies from the early 2000s could show that the microbiome plays an essential role for the development of experimental NAFLD and body fat storage. One of these studies described that germfree mice that were colonized with cecal microbiome from conventionally raised mice showed an increase in body weight.57 Li et al.58 demonstrated that the probiotic VSL#3 protected against high-fat diet-induced liver damage in ob/ob mice. In the meantime, various mainly pre-clinical studies could prove that interference with the intestinal microbiome offers a possibility to influence the course of NAFLD and related diseases. Treatment with Akkermansia muciniphila did ameliorate liver disease, dyslipidaemia, and IR in different mouse models.59,60 In a double-blind randomized proof of concept study enrolling 40 overweight humans, pasteurized A. muciniphila improved insulin sensitivity and reduced plasma cholesterol.61 An important role of the microbiota in the development of liver disease was observed by the group of Friedman. They observed that the transfer of human microbiome, obtained from infants born to obese mothers 2 weeks after birth, into germfree mice induced hepatic inflammation and an increased susceptibility to inflammation and obesity induced by a western-style diet.62 In a recent study, Sookoian et al.63 showed a distinct microbial profile in liver tissue of NAFLD patients linked to obesity. Several strains could be associated with histologic inflammation. The liver microbiome is potentially populated from the gut and might shape the hepatic immune system64 (Figure 1).
Bacteria-derived metabolites can influence inflammatory and metabolic processes in the liver and other organs. The faecal metabolomic signature of NAFLD patients is altered when compared with healthy individuals. Some metabolic active substances are produced by bacterial enzymes out of the dietary components such as butyrate, propionate, and acetate, so-called short chain fatty acids (SCFAs). These metabolites are increased in the faeces of NAFLD patients65 and are bioactive agents, mainly by the binding to G protein–coupled receptors (GPCRs).66 However, it is not quite understood if SCFAs are drivers of disease progression in NAFLD or could also be beneficial as another recent study described an inverse relation between systemic SCFA levels and severity of liver disease in 74 cirrhosis patients.67 Moreover, SCFAs are mainly produced out of dietary fibres, and fibre intake was associated with a lower risk of mortality in chronic liver disease in a recently published cohort study using the NIH-AARP Diet and Health Study.68 Furthermore, it is believed that SCFAs have beneficial effects on obesity and obesity-related diseases.69 Another dietary-derived metabolite shown to play a role in NAFLD and related disease is trimethylamine-N-oxide (TMAO), which is produced in the liver out of trimethylamine (TMA). The intestinal microbiota are able to metabolize choline, carnitine, and phosphatidylcholine into TMA. A prospective study with 4007 participants could show a positive correlation between the baseline TMAO level and the risk for a major cardiovascular event (death, stroke, or myocardial infarction), which could be explained by an increased platelet activation through TMAO.70,71 Recently, it was demonstrated that TMAO was also measurable in faeces of mice fed a native starch diet72 as an earlier study could show that the genome of certain bacteria includes a TMA monooxygenase, which could indicate a non-hepatic source of systemic TMAO.73 Increased circulating TMAO levels were associated with the severity of NAFLD in a recently published study based on a case-control study with 60 NAFLD cases and 35 controls and a cross-sectional study with 1628 Chinese adults.74 A possible mechanistical explanation for the role of TMAO in metabolic diseases could be the activation of protein kinase R (PKR)–like endoplasmic reticulum kinase (PERK) through binding of TMAO.75 This is interesting as ER stress, which is partly coordinated by PERK, plays an important role in the development of NAFLD and related diseases such as T2D.76,77 A recent study with 307 healthy men from the Men’s Lifestyle Validation Study could identify microbial taxa such as Alistipes shahii being associated with TMAO concentrations.78
Another possible role for the gut in the development of NAFLD is via an increased intestinal permeability and thereby the translocation of bacteria and bacterial products via the portal vein into the liver and the systemic circulation. In a cross-sectional study using patients from the FLORINASH cohort, an increase in 16S rDNA concentration in patients with fibrosis was described in the discovery cohort comprising of 50 patients and the validation cohort with 71 patients.79 Schierwagen et al.80 described a systemic microbiome that seemed to be circulating in patients who received a transjugular intrahepatic portosystemic shunt (TIPS) procedure. Studies over 30 years ago had demonstrated endotoxaemia in patients with chronic liver disease as a surrogate marker of increased intestinal permeability.81,82 In a recent meta-analysis summarizing 14 studies with adult and paediatric patients, an increased intestinal permeability was shown in NAFLD patients compared with healthy controls.83 Lipopolysaccharide (LPS) induces nuclear factor of activated B-cell (NF-κB) activation through binding to toll-like receptor 4 (TLR4). In a study with 25 NASH and 25 simple steatosis patients, it was found that serum LPS levels were higher in the NASH cohort; additionally, there was a higher number of TLR4 expressing macrophages in liver biopsies of NAFLD patients compared with normal livers.84 An important role of TLR4 signalling in the development of liver disease has also mechanistically been described in mice studies, as TLR4-deficient mice are protected from experimental NAFLD and also alcohol-induced liver disease.85–87 The induction of TLR-4 in hepatic Kupffer cells (hKCs) leads to the production of pro-inflammatory cytokines enhancing hepatocyte dysfunction, necrosis, and apoptosis of hepatocytes and neutrophil recruitment into the liver. Moreover, hepatic stellate cells (HSCs) are activated by cytokines resulting in generation of extracellular matrix proteins leading to fibrosis/cirrhosis.88 hKC and HSC do also ‘communicate’ with each other, and this cross talk might be driving pro-inflammatory and fibrotic processes in the liver.89 This is partly also regulated by TLR4. LPS-dependent production of chemokines in HSC leads to the recruitment of hKC, which in turn produce transforming growth factor beta (TGFβ) and thereby activate HSC.90 Recently, MER proto-oncogene, tyrosine kinase (MerTK) was identified to play an important role in this cross talk, as its activation modulated the secreted proteins in macrophages and thereby promoted a pro-fibrogenic phenotype in human HSC in vitro.91
1.1.4. Insulin resistance
NAFLD patients often also present with other features of the metabolic syndrome, and the liver is a central organ for metabolism, so a close relationship between NAFLD and IR is rather expected. IR is one of the main players in the pathophysiology of NAFLD as initially described by Marchesini et al.92 A rise of FFAs can induce hepatic IR in humans.93 Hepatic IR was associated with intrahepatic diacylglycerol (DAG) content in liver biopsies from obese, non-diabetic individuals.94 DAG content in the liver was correlated with protein kinase c epsilon-type (PKC-ε) activation.94 This axis was also described in a rodent model, where hepatic steatosis, induced by a short-term fat feeding, leads to activation of PKC-ε and c-Jun N-terminal kinases 1 (JNK1) and a possible interference with insulin receptor substrates 1 and 2 (IRS-1 and IRS-2) to the development of IR.95 An important driver of IR in the liver is inflammation, as mice expressing a constitutively active inhibitor of nuclear factor kappa-B kinase subunit beta (IKK-β) only in hepatocytes develop hepatic IR,96 while mice lacking IKK-β in hepatocytes are protected against hepatic IR after a high-fat diet, while they develop IR in muscle and fat.97 IKK-β is activated by oxidative stress,98 which is elevated in NAFLD patients.99 Further, IKK-β can also be activated by pro-inflammatory cytokines such as TNF, which are also elevated NAFLD patients.100 As NAFLD is extremely frequent in patients with T2D,101,102 IR seems also to be an attractive target for therapeutic modulation of NAFLD (see below) (Figure 1).
1.2. Clinical diagnosis of NAFLD
Today, besides liver biopsy, different non-invasive tests (NITs) can be used to diagnose NAFLD-like serum biomarkers, transient elastography (TE), and magnetic resonance elastography (MRE).
1.2.1. Serum tests
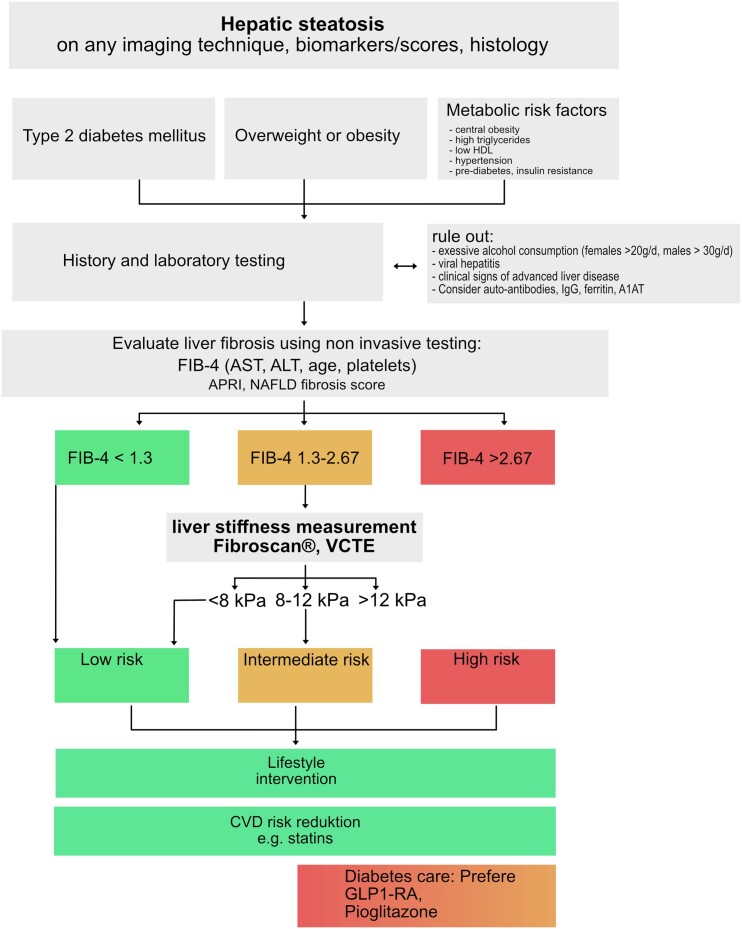
Serum tests comprise simple and inexpensive (non-patented) tests such as aspartat aminotransferase (AST)/alanin aminotransferase (ALT) ratio, AST to platelet ratio index (APRI), Fibrosis-4 (FIB-4), and NAFLD fibrosis score (NFS) compared with patented tests such as the FibroTest®, Fibrometer®, and Hepascore®. In a recent meta-analysis, Xiao et al.103 compared the performances of FIB-4, NFS, and APRI for the diagnoses of advanced fibrosis in NAFLD patients with summary AUROCS of 0.84, 0.84, and 0.77, respectively.103 The FIB-4 (age, AST, ALT, and platelet) can be used as a guidance to define patients who need further hepatic evaluation. Higher FIB-4 scores suggest advanced liver disease and a possible need for referral to a haepatologist. In patients with suspected NAFLD, a FIB-4 score < 1.3 rules out severe liver disease and no referral to a haepatologist or further diagnostic workup is needed. FIB-4 should be repeated in 1–3 years. In patients with suspected NAFLD and FIB-4 ≥ 1.3, TE for further workup is needed104,105 (Figure 2).
Figure 2.
Diagnostic algorithm adapted from Kanwal et al.106 and the EASL CPG.12 A1AT, alpha 1 antitrypsin; ALT, alanin aminotransferase; AST, aspartat aminotransferase; CVD, cardio vascular disease; HDL-c, high-density lipoprotein cholesterol; IgG, immunoglobulin G; VCTE, vibration controlled transient elastography.
1.2.2. Transient elastography
TE is a non-invasive tool for evaluating liver stiffness. It has a high applicability of >95% (in patients who are not morbidly obese), is easy to perform, and provides results in real time.12,104 Increased liver stiffness values are associated with liver fibrosis but can also occur in other conditions. However, liver stiffness is a physical property of the tissue, which not depends only on the amount of liver fibrosis but is also affected by inflammation, obstructive cholestasis, food ingestion, exercise, or venous congestion.104 TE enables evaluation of liver fibrosis in a broader population and is thereby feasible in view of NAFLD epidemic.107 Of note, TE should be repeated regularly also in patients already diagnosed with cirrhosis, as an increase in portal hypertension is a leading cause of cirrhosis-related complications.108,109
1.2.3. Magnetic resonance elastography
MRE can usually be done on a regular MRI machine. Compared with liver biopsy and TE, MRE examines the whole liver, which makes results more robust. Another advantage is the higher applicability in difficult examination conditions like presence of ascites and obesity than TE. On the other side, MRE is costly and time-consuming.
1.2.4. Biopsy
Liver biopsy is an invasive procedure with a mortality risk of ∼0.2%. Major bleedings occur in ∼0.6%.110 Today, liver biopsy is not universally needed to diagnose NAFLD because of the increasing benefit of NITs. Liver biopsy is indicated if NITs are discordant, to rule out other confounding liver diseases, defining stages of liver fibrosis or study purposes.104,111
1.3. NAFLD and CVD
Beside well-known liver-related mortality, CVD is a common cause for death in NAFLD patients. CVD-associated mortality in NAFLD patients increased by 14% from 2008 to 2018.112 Simon et al.113 showed recently increased overall mortality in all histological stages of NAFLD. Studies have proved the role of NAFLD in different cardiac disease manifestations like left ventricular dysfunction (LVD), atherosclerotic CV disease, and ischaemic heart disease. This suggests that NAFLD could be an independent predictor of CVD.114,115 In a recent meta-analysis including 34 043 patients with NAFLD, it was shown that NAFLD patients displayed an increased risk of both fatal and non-fatal CV events compared with non-NAFLD patients. Interestingly, this study furthered showed an increased risk of CV events in individuals with a greater severity of liver disease.9,116 Further, studies could show that especially hepatic fibrosis was associated with CVD and also liver-related outcome.117,118 Although the clinical association seems to be solid, a clear pathophysiological link between NAFLD and CVD is not established. It is rather thought to be a mixture between metabolic dysfunction, low-grade inflammation, dysregulated microbiome, and altered metabolism of (microbiome) derived products (e.g. TMAO; see above).9
1.4. Management of NAFLD
Although the prevalence of NAFLD and NASH is substantially increasing5 and is already a global burden, effective drug therapies are still missing. Cardiovascular disease and malignancies are the leading causes for death in patients with NAFLD.119 Therefore, the main treatment goal is to reduce CVD risk and malignancy risk as well as hepatic steatosis and inflammation. Here, we summarize some possible therapeutic strategies in NAFLD/NASH but will not discuss the importance of other CVD risk decreasing therapies such as statins, etc. Today, an increasing number of therapeutic options are available for the treatment of NAFLD. Important to note, most of them are not yet approved for the treatment of liver disease (Table 1).
Table 1.
Available therapeutic options in NAFLD
| Liver enzymes | Steatosis | Inflammation | Fibrosis | Adverse events | Beneficial clinical aspects | |
|---|---|---|---|---|---|---|
| GLP1 receptor agonists | + | + | + | + | Gastrointestinal | Weight loss, reduction of CV events |
| SGLT2 inhibitors | + | + | ? | ? | Genitourinary infections, dehydration | Reduction of CV events, nephron-protection, weight loss |
| Glitazones | + | + | + | + | Weight gain (mild), oedema, heart failure, bone fractures | reduction of CV events |
| Bariatric surgery | + | + | + | + | Invasive procedure, malnutrition | Weight loss |
CV, cardio vascular; GLP1, glucagon-like peptide 1; SGLT2, sodium–glucose transporter 2.
1.4.1. Probiotics
Multiple pre-clinical studies showed that the intestinal microbiota influences the course of NAFLD. Nevertheless, probiotics are not generally recommended for treating patients with NAFLD. Only few clinical studies tested probiotics in patients with NAFLD. VSL#3,120 different strains of Lactobacilli,121 and Lactobacillus bulgaricus and Streptococcus thermophilus122 were shown to be effective in reducing liver enzymes but not liver steatosis or fibrosis. More clinical trials are needed to define beneficial bacterial strains effective in NAFLD and NASH.
1.4.2. Lifestyle change
An unhealthy lifestyle was associated early with NAFLD.123 Therefore, lifestyle change is mandatory in every patient with NAFLD. In a prospective study including 293 patients with biopsy-proven NASH, Vilar-Gomez et al.124 showed a reduction of hepatic steatosis and inflammation after a recommended lifestyle change within 12 months. Interestingly, the degree of weight loss was independently associated with the degree of NASH. All patients who achieved a weight loss ≥ 10% had a reduction of NAFLD Activity Score (NAS, histologic score), 90% had resolution of NASH, and 45% had regression of fibrosis.124 Sustained weight loss is challenging because it requires a transformation of behavioural patterns but depicts a cornerstone in NAFLD treatment. Generally, NAFLD patients are recommended to lose 7–10% of their body weight.12 Further, patients are advised to avoid alcohol consumption12,125 and high fructose intake.126 Physical activity was shown not only to reduce liver fat127,128 but also to reduce risk of CVD, obesity, and T2D.129 The European Association for the Study of the Liver (EASL) recommends over 150 min/week of moderate intensity physical activity (three to five sessions per week) combining aerobic and resistance training.12,130 Analysing data from 304 patients, Huber et al.6 could report a correlation between biopsy-proven lobular inflammation, diabetes, age, and sex with a lower health-related quality of life in NAFLD patients. The prospect for improvement of the quality of life could help to motivate patients to introduce and maintain the sometimes tedious changes in lifestyle.
1.4.3. SGLT2 inhibitors
Sodium–glucose transporter 2 (SGLT2) inhibitors inhibit the SGLT2 transporter and promote urinary glucose excretion. Hereby, blood levels of glucose are decreased and IR can be improved in patients with T2DM.131 Kuchay et al. investigated the effect of empagliflozin on liver fat content in patients with T2D and NAFLD. Empagliflozin significantly reduced liver fat compared with controls (standard of care).132 In a meta-analysis including seven RTCs, the effect of SGLT2 inhibitors on NAFLD was investigated. Compared with placebo or reference therapy, empagliflozin, canagliflozin, or ipragliflozin showed a small improvement in liver fat content, assessed by ultrasound, FibroScan, and MRE. Furthermore, SGLT2 inhibitor treatment went along with a reduction of body weight (2–3 kg) and HbA1c reduction (0.8–1.0%). In all RCTs, SGLT2 inhibitors were associated with a reduction of transaminases,133 suggesting a possible amelioration of hepatic injury.
1.4.4. Glucagon-like peptide 1 receptor agonists
Glucagon-like peptide 1 receptor agonists (GLP1-RAs) seem to exert the most promising beneficial effects on NAFLD or NASH. GLP1-RAs mimic the effects of physiological GLP1, e.g. stimulation of insulin secretion, inhibition of glucagon, gastrointestinal secretions, and motility. Furthermore, it reduces food intake by enhancing satiety.134 In a multi-centre placebo-controlled Phase 2 trial including obese patients with biopsy-proven NASH, liraglutide 1.8 mg/day for 48 weeks was effective to induce histological resolution of NASH and significantly improved histologic scores of NASH compared with those receiving placebo.135 Semaglutide 0.1, 0.2, or 0.4 mg was tested in patients with biopsy-confirmed NASH and liver fibrosis of Stages F1–F3. NASH resolution without worsening of fibrosis was achieved in 40% of the 0.1-mg group, 36% in the 0.2-mg group, 59% in the 0.4-mg group, and 17% in the placebo group. In the 0.4-mg group, the mean per cent weight loss was 13%.136 Important to note, liraglutide and other long-acting GLP1-RAs have been proved to reduce risk of adverse CVD and renal outcomes in patients with T2D.137
1.4.5. BAs, BA metabolites, and FXR agonists
As also described above, BA signalling plays an important role in NAFLD development. FXR is a major regulator of BA metabolism and is involved in lipid and glucose metabolism.138 Different non-BA FXR agonists like tropifexor,139 cilofexor,140 and nidufexor141,142 have been tested in NAFLD and were proved to reduce liver fat content. Obeticholic acid (OCA), a modified BA and FXR agonist, was able to reduce fibrosis and histological features of liver disease in NASH patients.143–145 However, in one of these trials, an increase of very low-density lipoprotein (VLDL) and low-density lipoprotein cholesterol (LDL-c) particles and a decrease of HDL-c was observed during treatment but was reverted after discontinuation of the study drug, indicating a shift of lipoproteins from the liver to the systemic circulation.146 A Phase 2 dose finding study with 198 patients with NAFLD could show a reduction of ALT after 12 weeks of treatment with nor-ursodeoxycholic acid compared with placebo.147 In a 12-week, randomized, placebo-controlled study MET409, a non-BA agonist was shown to significantly reduce liver fat content compared with control group.148
1.4.6. PPAR agonists
Peroxisome proliferator–activated receptors (PPARs) are transcription factors of nuclear hormone receptors with three subtypes PPAR-α, PPAR-γ, and PPAR-β/δ, which regulate lipid metabolism, energy homoeostasis, insulin sensitization, and glucose metabolism. Pioglitazone (PPAR-γ agonist) is a potent insulin sensitizer and is used in treatment of T2D. In a recent meta-analysis, pioglitazone was proved to be effective in reducing liver fibrosis and NASH.149–151 Interestingly, similar effects could be shown also in patients without T2D.152 Furthermore, pioglitazone displays protective effects on the vasculature, decreasing the risk of ischaemic stroke in patients with T2D or prediabetes.153 Pioglitazone lowers levels of triglyceride and LDL-c and increases HDL-c. Common side effects are weight gain, lower limb oedema as well as bone fractures, predominantly in post-menopausal women.153 The pan-PPAR agonist Lanifibranor is under clinical investigation in a phase III study. In a phase IIb study, Lanifibranor was effective in reducing NASH fibrosis, liver enzyme levels inflammatory, and fibrosis biomarkers.154
1.4.7. Bariatric surgery
Weight loss is the cornerstone of NAFLD therapy,155,156 and the most potent therapy to induce weight loss is bariatric surgery.157 Although bariatric surgery is not a first-line therapy for NAFLD, it can be discussed for selected patients, especially if they qualify for surgery out of other reasons (co-morbidities or excessive obesity). An improvement of liver disease after bariatric surgery was described in many studies157; in a small clinical study from our clinic, we observed an improvement of liver histology after bariatric surgery, which was paralleled by an decrease of pro-inflammatory cytokines.158,159 A recent study from Germany described a histopathological resolution of NASH in 84% of observed patients 5 years after bariatric surgery, even in 45.5% of patients who had bridging fibrosis at baseline (i.e. a sign of advanced liver disease), fibrosis disappeared after 5 years in the follow-up biopsy.160 Together, these data indicate that bariatric surgery could be an appropriate therapeutic option for selected patients with NAFLD.
1.4.8. Therapeutic options in the future
Thyroid hormones are involved in the regulation of hepatic triglyceride and cholesterol metabolism. Resmetirom, a thyroid receptor beta (TR-beta) agonist, was shown to improve liver steatosis161 and also lowered LDL-c and triglyceride concentration.162 FGF21 was proved to be effective in different animal models of obesity and NAFLD. The FGF21 variant LY2405319 was tested in patients with T2D mellitus. Here, LY2405319 improved lipid profiles and tended to decrease body weight, fasting insulin, and fasting glucose.163 Although more clinical data are needed, FGF21 could be an interesting therapeutic target in the future.
2. Conclusion
NAFLD is one of the major and relevant human diseases associated with an altered lifestyle with a rapidly growing incidence and prevalence in most countries of the world. NAFLD can cause a dramatic burden of disease throughout the different stages of chronic liver disease, including cirrhosis and associated complications such as HCC and decompensation (e.g. oesophageal variceal bleeding, ascites, and hepatic encephalopathy). Besides hepatic complications, the metabolic syndrome, T2D, and CVD are commonly observed in NAFLD patients. Although our understanding of the underlying mechanisms in the context of NAFLD is increasing, it is still not fully understood how NAFLD influences T2D and CVD on a pathophysiological level. One of the leading hypotheses is a sub-clinical pro-inflammatory environment arising from lipotoxicity, IR, and the intestinal microbiota. The main cornerstone in the treatment is lifestyle modification including weight loss, dietary intervention, and regular physical exercise. Although this is a difficult goal to obtain for many patients, it should be recommended and supported by the treating physicians. A range of therapeutic options, from conservative management and medical intervention towards operative procedures (e.g. bariatric surgery), has been developed with varying outcomes especially for the conservative treatment options. The pipeline of new therapeutic approaches is broad with some promising candidates. Taken together, NAFLD is a major healthcare issue that will rise in the future; thus, an increased awareness from physicians of different specialties is needed to tackle this disease.
Acknowledgements
This work was supported by the excellence initiative VASCage (Centre for Promoting Vascular Health in the Ageing Community), an R&D K-Centre (COMET program—Competence Centers for Excellent Technologies) funded by the Austrian Ministry for Transport, Innovation and Technology, the Austrian Ministry for Digital and Economic Affairs and the federal states Tyrol, Salzburg, and Vienna.
Contributor Information
Christoph Grander, Department of Internal Medicine I, Gastroenterology, Hepatology, Endocrinology & Metabolism, Medical University Innsbruck, Anichstrasse 35, Innsbruck 6020, Austria.
Felix Grabherr, Department of Internal Medicine I, Gastroenterology, Hepatology, Endocrinology & Metabolism, Medical University Innsbruck, Anichstrasse 35, Innsbruck 6020, Austria.
Herbert Tilg, Department of Internal Medicine I, Gastroenterology, Hepatology, Endocrinology & Metabolism, Medical University Innsbruck, Anichstrasse 35, Innsbruck 6020, Austria.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011;141:1249–1253. [DOI] [PubMed] [Google Scholar]
- 2. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 3. Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol 2017;14:32–42. [DOI] [PubMed] [Google Scholar]
- 4. Portillo-Sanchez P, Bril F, Maximos M, Lomonaco R, Biernacki D, Orsak B, Subbarayan S, Webb A, Hecht J, Cusi K. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J Clin Endocrinol Metab 2015;100:2231–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- 6. Huber Y, Schulz A, Schmidtmann I, Beutel M, Pfeiffer N, Münzel T, Galle PR, Wild PS, Lackner KJ, Schattenberg JM. Prevalence and risk factors of advanced liver fibrosis in a population-based study in Germany. Hepatol Commun 2022;6:1457–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mantovani A, Dauriz M, Sandri D, Bonapace S, Zoppini G, Tilg H, Byrne CD, Targher G. Association between non-alcoholic fatty liver disease and risk of atrial fibrillation in adult individuals: an updated meta-analysis. Liver Int 2019;39:758–769. [DOI] [PubMed] [Google Scholar]
- 8. Mantovani A, Petracca G, Csermely A, Beatrice G, Bonapace S, Rossi A, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of new-onset heart failure: an updated meta-analysis of about 11 million individuals. Gut 2022:gutjnl-2022-327672. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 9. Targher G, Byrne CD, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut 2020;69:1691–1705. [DOI] [PubMed] [Google Scholar]
- 10. Wójcik-Cichy K, Koślińska-Berkan E, Piekarska A. The influence of NAFLD on the risk of atherosclerosis and cardiovascular diseases. Clin Exp Hepatol 2018;4:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tarantino G, Savastano S, Colao A. Hepatic steatosis, low-grade chronic inflammation and hormone/growth factor/adipokine imbalance. World J Gastroenterol 2010;16:4773–4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) . EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–1402. [DOI] [PubMed] [Google Scholar]
- 13. Younossi ZM, Rinella ME, Sanyal AJ, Harrison SA, Brunt EM, Goodman Z, Cohen DE, Loomba R. From NAFLD to MAFLD: implications of a premature change in terminology. Hepatology 2021;73:1194–1198. [DOI] [PubMed] [Google Scholar]
- 14. Yamamura S, Eslam M, Kawaguchi T, Tsutsumi T, Nakano D, Yoshinaga S, Takahashi H, Anzai K, George J, Torimura T. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int 2020;40:3018–3030. [DOI] [PubMed] [Google Scholar]
- 15. Long MT, Noureddin M, Lim JK. AGA clinical practice update: diagnosis and management of nonalcoholic fatty liver disease in lean individuals: expert review. Gastroenterology 2022;163:764–774.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Méndez-Sánchez N, Bugianesi E, Gish RG, Lammert F, Tilg H, Nguyen MH, Sarin SK, Fabrellas N, Zelber-Sagi S, Fan JG, Shiha G, Targher G, Zheng MH, Chan WK, Vinker S, Kawaguchi T, Castera L, Yilmaz Y, Korenjak M, Spearman CW, Ungan M, Palmer M, El-Shabrawi M, Gruss HJ, Dufour JF, Dhawan A, Wedemeyer H, George J, Valenti L, Fouad Y, Romero-Gomez M, Eslam M. Global multi-stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol Hepatol 2022;7:388–390. [DOI] [PubMed] [Google Scholar]
- 17. Tilg H, Adolph TE, Dudek M, Knolle P. Non-alcoholic fatty liver disease: the interplay between metabolism, microbes and immunity. Nat Metab 2021;3:1596–1607. [DOI] [PubMed] [Google Scholar]
- 18. Tilg H, Effenberger M. From NAFLD to MAFLD: when pathophysiology succeeds. Nat Rev Gastroenterol Hepatol 2020;17:387–388. [DOI] [PubMed] [Google Scholar]
- 19. Brunt EM, Tiniakos DG. Histopathology of nonalcoholic fatty liver disease. World J Gastroenterol 2010;16:5286–5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. BasuRay S, Smagris E, Cohen JC, Hobbs HH. The PNPLA3 variant associated with fatty liver disease (I148M) accumulates on lipid droplets by evading ubiquitylation. Hepatology 2017;66:1111–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. BasuRay S, Wang Y, Smagris E, Cohen JC, Hobbs HH. Accumulation of PNPLA3 on lipid droplets is the basis of associated hepatic steatosis. Proc Natl Acad Sci U S A 2019;116:9521–9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruhanen H, Perttilä J, Hölttä-Vuori M, Zhou Y, Yki-Järvinen H, Ikonen E, Käkelä R, Olkkonen VM. PNPLA3 mediates hepatocyte triacylglycerol remodeling. J Lipid Res 2014;55:739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cansby E, Kulkarni NM, Magnusson E, Kurhe Y, Amrutkar M, Nerstedt A, Ståhlman M, Sihlbom C, Marschall HU, Borén J, Blüher M, Mahlapuu M. Protein kinase MST3 modulates lipid homeostasis in hepatocytes and correlates with nonalcoholic steatohepatitis in humans. FASEB J 2019;33:9974–9989. [DOI] [PubMed] [Google Scholar]
- 25. Caputo M, Cansby E, Kumari S, Kurhe Y, Nair S, Ståhlman M, Kulkarni NM, Borén J, Marschall HU, Blüher M, Mahlapuu M. STE20-type protein kinase MST4 controls NAFLD progression by regulating lipid droplet dynamics and metabolic stress in hepatocytes. Hepatol Commun 2021;5:1183–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Amrutkar M, Chursa U, Kern M, Nuñez-Durán E, Ståhlman M, Sütt S, Borén J, Johansson BR, Marschall HU, Blüher M, Mahlapuu M. STK25 is a critical determinant in nonalcoholic steatohepatitis. FASEB J 2016;30:3628–3643. [DOI] [PubMed] [Google Scholar]
- 27. Amrutkar M, Kern M, Nuñez-Durán E, Ståhlman M, Cansby E, Chursa U, Stenfeldt E, Borén J, Blüher M, Mahlapuu M. Protein kinase STK25 controls lipid partitioning in hepatocytes and correlates with liver fat content in humans. Diabetologia 2016;59:341–353. [DOI] [PubMed] [Google Scholar]
- 28. Amrutkar M, Cansby E, Nuñez-Durán E, Pirazzi C, Ståhlman M, Stenfeldt E, Smith U, Borén J, Mahlapuu M. Protein kinase STK25 regulates hepatic lipid partitioning and progression of liver steatosis and NASH. FASEB J 2015;29:1564–1576. [DOI] [PubMed] [Google Scholar]
- 29. Mahlapuu M, Caputo M, Xia Y, Cansby E. GCKIII kinases in lipotoxicity: roles in NAFLD and beyond. Hepatol Commun 2022;6:2613–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Horn CL, Morales AL, Savard C, Farrell GC, Ioannou GN. Role of cholesterol-associated steatohepatitis in the development of NASH. Hepatol Commun 2022;6:12–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Min HK, Kapoor A, Fuchs M, Mirshahi F, Zhou H, Maher J, Kellum J, Warnick R, Contos MJ, Sanyal AJ. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab 2012;15:665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ioannou GN, Landis CS, Jin GY, Haigh WG, Farrell GC, Kuver R, Lee SP, Savard C. Cholesterol crystals in hepatocyte lipid droplets are strongly associated with human nonalcoholic steatohepatitis. Hepatol Commun 2019;3:776–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ioannou GN. The role of cholesterol in the pathogenesis of NASH. Trends Endocrinol Metab 2016;27:84–95. [DOI] [PubMed] [Google Scholar]
- 34. Alsamman S, Christenson SA, Yu A, Ayad NME, Mooring MS, Segal JM, Hu JK, Schaub JR, Ho SS, Rao V, Marlow MM, Turner SM, Sedki M, Pantano L, Ghoshal S, Ferreira DDS, Ma HY, Duwaerts CC, Espanol-Suner R, Wei L, Newcomb B, Mileva I, Canals D, Hannun YA, Chung RT, Mattis AN, Fuchs BC, Tager AM, Yimlamai D, Weaver VM, Mullen AC, Sheppard D, Chen JY. Targeting acid ceramidase inhibits YAP/TAZ signaling to reduce fibrosis in mice. Sci Transl Med 2020;12:eaay8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mooring M, Fowl BH, Lum SZC, Liu Y, Yao K, Softic S, Kirchner R, Bernstein A, Singhi AD, Jay DG, Kahn CR, Camargo FD, Yimlamai D. Hepatocyte stress increases expression of yes-associated protein and transcriptional coactivator with PDZ-binding motif in hepatocytes to promote parenchymal inflammation and fibrosis. Hepatology 2020;71:1813–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang X, Zheng Z, Caviglia JM, Corey KE, Herfel TM, Cai B, Masia R, Chung RT, Lefkowitch JH, Schwabe RF, Tabas I. Hepatocyte TAZ/WWTR1 promotes inflammation and fibrosis in nonalcoholic steatohepatitis. Cell Metab 2016;24:848–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marí M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A, Enrich C, Fernandez-Checa JC, García-Ruiz C. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab 2006;4:185–198. [DOI] [PubMed] [Google Scholar]
- 38. Arab JP, Karpen SJ, Dawson PA, Arrese M, Trauner M. Bile acids and nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatology 2017;65:350–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tian Y, Gui W, Koo I, Smith PB, Allman EL, Nichols RG, Rimal B, Cai J, Liu Q, Patterson AD. The microbiome modulating activity of bile acids. Gut Microbes 2020;11:979–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simbrunner B, Trauner M, Reiberger T. Review article: therapeutic aspects of bile acid signalling in the gut-liver axis. Aliment Pharmacol Ther 2021;54:1243–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li T, Chiang JYL. Bile acid-based therapies for non-alcoholic steatohepatitis and alcoholic liver disease. Hepatobiliary Surg Nutr 2020;9:152–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhu C, Boucheron N, Müller AC, Májek P, Claudel T, Halilbasic E, Baazim H, Lercher A, Viczenczova C, Hainberger D, Preglej T, Sandner L, Alteneder M, Gülich AF, Khan M, Hamminger P, Remetic J, Ohradanova-Repic A, Schatzlmaier P, Donner C, Fuchs CD, Stojakovic T, Scharnagl H, Sakaguchi S, Weichhart T, Bergthaler A, Stockinger H, Ellmeier W, Trauner M. 24-Norursodeoxycholic acid reshapes immunometabolism in CD8(+) T cells and alleviates hepatic inflammation. J Hepatol 2021;75:1164–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Herman MA, Samuel VT. The sweet path to metabolic demise: fructose and lipid synthesis. Trends Endocrinol Metab 2016;27:719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Porto A, Pan Z, Zhou W, Sokol RJ, Klaczkiewicz K, Sundaram SS. Macronutrient and micronutrient intake in adolescents with non-alcoholic fatty liver disease: the association with disease severity. J Pediatr Gastroenterol Nutr 2022;75:666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee D, Chiavaroli L, Ayoub-Charette S, Khan TA, Zurbau A, Au-Yeung F, Cheung A, Liu Q, Qi X, Ahmed A, Choo VL, Blanco Mejia S, Malik VS, El-Sohemy A, de Souza RJ, Wolever TMS, Leiter LA, Kendall CWC, Jenkins DJA, Sievenpiper JL. Important food sources of fructose-containing sugars and non-alcoholic fatty liver disease: a systematic review and meta-analysis of controlled trials. Nutrients 2022;14:2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aron-Wisnewsky J, Vigliotti C, Witjes J, Le P, Holleboom AG, Verheij J, Nieuwdorp M, Clément K. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol 2020;17:279–297. [DOI] [PubMed] [Google Scholar]
- 47. Hoyles L, Fernández-Real JM, Federici M, Serino M, Abbott J, Charpentier J, Heymes C, Luque JL, Anthony E, Barton RH, Chilloux J, Myridakis A, Martinez-Gili L, Moreno-Navarrete JM, Benhamed F, Azalbert V, Blasco-Baque V, Puig J, Xifra G, Ricart W, Tomlinson C, Woodbridge M, Cardellini M, Davato F, Cardolini I, Porzio O, Gentileschi P, Lopez F, Foufelle F, Butcher SA, Holmes E, Nicholson JK, Postic C, Burcelin R, Dumas ME. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat Med 2018;24:1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shen F, Zheng RD, Sun XQ, Ding WJ, Wang XY, Fan JG. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int 2017;16:375–381. [DOI] [PubMed] [Google Scholar]
- 49. Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, Dulai PS, Caussy C, Bettencourt R, Highlander SK, Jones MB, Sirlin CB, Schnabl B, Brinkac L, Schork N, Chen CH, Brenner DA, Biggs W, Yooseph S, Venter JC, Nelson KE. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab 2017;25:1054–1062.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Del Chierico F, Nobili V, Vernocchi P, Russo A, De Stefanis C, Gnani D, Furlanello C, Zandonà A, Paci P, Capuani G, Dallapiccola B, Miccheli A, Alisi A, Putignani L. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology 2017;65:451–464. [DOI] [PubMed] [Google Scholar]
- 51. Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L, Zhou J, Ni S, Liu L, Pons N, Batto JM, Kennedy SP, Leonard P, Yuan C, Ding W, Chen Y, Hu X, Zheng B, Qian G, Xu W, Ehrlich SD, Zheng S, Li L. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014;513:59–64. [DOI] [PubMed] [Google Scholar]
- 52. Raman M, Ahmed I, Gillevet PM, Probert CS, Ratcliffe NM, Smith S, Greenwood R, Sikaroodi M, Lam V, Crotty P, Bailey J, Myers RP, Rioux KP. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2013;11:868–875.e1–3. [DOI] [PubMed] [Google Scholar]
- 53. Alferink LJM, Radjabzadeh D, Erler NS, Vojinovic D, Medina-Gomez C, Uitterlinden AG, de Knegt RJ, Amin N, Ikram MA, Janssen HLA, Kiefte-de Jong JC, Metselaar HJ, van Duijn CM, Kraaij R, Murad S D, Microbiomics M, Metagenomics P. And hepatic steatosis in a population-based study of 1,355 adults. Hepatology 2021;73:968–982. [DOI] [PubMed] [Google Scholar]
- 54. Lee G, You HJ, Bajaj JS, Joo SK, Yu J, Park S, Kang H, Park JH, Kim JH, Lee DH, Lee S, Kim W, Ko G. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat Commun 2020;11:4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology 2013;57:601–609. [DOI] [PubMed] [Google Scholar]
- 56. Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 2011;54:562–572. [DOI] [PubMed] [Google Scholar]
- 57. Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A 2004;101:15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, Desimone C, Song XY, Diehl AM. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology 2003;37:343–350. [DOI] [PubMed] [Google Scholar]
- 59. Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, Myridakis A, Delzenne NM, Klievink J, Bhattacharjee A, van der Ark KC, Aalvink S, Martinez LO, Dumas ME, Maiter D, Loumaye A, Hermans MP, Thissen JP, Belzer C, de Vos WM, Cani PD. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med 2017;23:107–113. [DOI] [PubMed] [Google Scholar]
- 60. Kim S, Lee Y, Kim Y, Seo Y, Lee H, Ha J, Lee J, Choi Y, Oh H, Yoon Y. Akkermansia muciniphila prevents fatty liver disease, decreases serum triglycerides, and maintains gut homeostasis. Appl Environ Microbiol 2020;86:e03004-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, de Barsy M, Loumaye A, Hermans MP, Thissen JP, de Vos WM, Cani PD. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med 2019;25:1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Soderborg TK, Clark SE, Mulligan CE, Janssen RC, Babcock L, Ir D, Young B, Krebs N, Lemas DJ, Johnson LK, Weir T, Lenz LL, Frank DN, Hernandez TL, Kuhn KA, D'Alessandro A, Barbour LA, El Kasmi KC, Friedman JE. The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nat Commun 2018;9:4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sookoian S, Salatino A, Castaño GO, Landa MS, Fijalkowky C, Garaycoechea M, Pirola CJ. Intrahepatic bacterial metataxonomic signature in non-alcoholic fatty liver disease. Gut 2020;69:1483–1491. [DOI] [PubMed] [Google Scholar]
- 64. Leinwand JC, Paul B, Chen R, Xu F, Sierra MA, Paluru MM, Nanduri S, Alcantara CG, Shadaloey SA, Yang F, Adam SA, Li Q, Bandel M, Gakhal I, Appiah L, Guo Y, Vardhan M, Flaminio Z, Grodman ER, Mermelstein A, Wang W, Diskin B, Aykut B, Khan M, Werba G, Pushalkar S, McKinstry M, Kluger Z, Park JJ, Hsieh B, Dancel-Manning K, Liang FX, Park JS, Saxena A, Li X, Theise ND, Saxena D, Miller G. Intrahepatic microbes govern liver immunity by programming NKT cells. J Clin Invest 2022;132:e151725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rau M, Rehman A, Dittrich M, Groen AK, Hermanns HM, Seyfried F, Beyersdorf N, Dandekar T, Rosenstiel P, Geier A. Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease. United European Gastroenterol J 2018;6:1496–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The orphan G protein–coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 2003;278:11312–11319. [DOI] [PubMed] [Google Scholar]
- 67. Juanola O, Ferrusquía-Acosta J, García-Villalba R, Zapater P, Magaz M, Marín A, Olivas P, Baiges A, Bellot P, Turon F, Hernández-Gea V, González-Navajas JM, Tomás-Barberán FA, García-Pagán JC, Francés R. Circulating levels of butyrate are inversely related to portal hypertension, endotoxemia, and systemic inflammation in patients with cirrhosis. FASEB J 2019;33:11595–11605. [DOI] [PubMed] [Google Scholar]
- 68. Liu X, Yang W, Petrick JL, Liao LM, Wang W, He N, Campbell PT, Zhang ZF, Giovannucci E, McGlynn KA, Zhang X. Higher intake of whole grains and dietary fiber are associated with lower risk of liver cancer and chronic liver disease mortality. Nat Commun 2021;12:6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 2015;11:577–591. [DOI] [PubMed] [Google Scholar]
- 70. Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WHW, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 2016;165:111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Koay YC, Chen YC, Wali JA, Luk AWS, Li M, Doma H, Reimark R, Zaldivia MTK, Habtom HT, Franks AE, Fusco-Allison G, Yang J, Holmes A, Simpson SJ, Peter K, O'Sullivan JF. Plasma levels of trimethylamine-N-oxide can be increased with ‘healthy’ and ‘unhealthy’ diets and do not correlate with the extent of atherosclerosis but with plaque instability. Cardiovasc Res 2021;117:435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chen Y, Patel NA, Crombie A, Scrivens JH, Murrell JC. Bacterial flavin-containing monooxygenase is trimethylamine monooxygenase. Proc Natl Acad Sci U S A 2011;108:17791–17796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chen YM, Liu Y, Zhou RF, Chen XL, Wang C, Tan XY, Wang LJ, Zheng RD, Zhang HW, Ling WH, Zhu HL. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci Rep 2016;6:19076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen S, Henderson A, Petriello MC, Romano KA, Gearing M, Miao J, Schell M, Sandoval-Espinola WJ, Tao J, Sha B, Graham M, Crooke R, Kleinridders A, Balskus EP, Rey FE, Morris AJ, Biddinger SB. Trimethylamine N-oxide binds and activates PERK to promote metabolic dysfunction. Cell Metab 2019;30:1141–1151.e5. [DOI] [PubMed] [Google Scholar]
- 76. Harding HP, Ron D. Endoplasmic reticulum stress and the development of diabetes: a review. Diabetes 2002;51:S455–S461. [DOI] [PubMed] [Google Scholar]
- 77. Lebeaupin C, Vallée D, Hazari Y, Hetz C, Chevet E, Bailly-Maitre B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J Hepatol 2018;69:927–947. [DOI] [PubMed] [Google Scholar]
- 78. Li J, Li Y, Ivey KL, Wang DD, Wilkinson JE, Franke A, Lee KH, Chan A, Huttenhower C, Hu FB, Rimm EB, Sun Q. Interplay between diet and gut microbiome, and circulating concentrations of trimethylamine N-oxide: findings from a longitudinal cohort of US men. Gut 2022;71:724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lelouvier B, Servant F, Païssé S, Brunet AC, Benyahya S, Serino M, Valle C, Ortiz MR, Puig J, Courtney M, Federici M, Fernández-Real JM, Burcelin R, Amar J. Changes in blood microbiota profiles associated with liver fibrosis in obese patients: a pilot analysis. Hepatology 2016;64:2015–2027. [DOI] [PubMed] [Google Scholar]
- 80. Schierwagen R, Alvarez-Silva C, Madsen MSA, Kolbe CC, Meyer C, Thomas D, Uschner FE, Magdaleno F, Jansen C, Pohlmann A, Praktiknjo M, Hischebeth GT, Molitor E, Latz E, Lelouvier B, Trebicka J, Arumugam M. Circulating microbiome in blood of different circulatory compartments. Gut 2019;68:578–580. [DOI] [PubMed] [Google Scholar]
- 81. Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol 1991;12:162–169. [DOI] [PubMed] [Google Scholar]
- 82. Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol 1987;4:8–14. [DOI] [PubMed] [Google Scholar]
- 83. De Munck TJI, Xu P, Verwijs HJA, Masclee AAM, Jonkers D, Verbeek J, Koek GH. Intestinal permeability in human nonalcoholic fatty liver disease: a systematic review and meta-analysis. Liver Int 2020;40:2906–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Carpino G, Del Ben M, Pastori D, Carnevale R, Baratta F, Overi D, Francis H, Cardinale V, Onori P, Safarikia S, Cammisotto V, Alvaro D, Svegliati-Baroni G, Angelico F, Gaudio E, Violi F. Increased liver localization of lipopolysaccharides in human and experimental NAFLD. Hepatology 2020;72:470–485. [DOI] [PubMed] [Google Scholar]
- 85. An L, Wirth U, Koch D, Schirren M, Drefs M, Koliogiannis D, Nieß H, Andrassy J, Guba M, Bazhin AV, Werner J, Kühn F. The role of gut-derived lipopolysaccharides and the intestinal barrier in fatty liver diseases. J Gastrointest Surg 2022;26:671–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Liu J, Zhuang ZJ, Bian DX, Ma XJ, Xun YH, Yang WJ, Luo Y, Liu YL, Jia L, Wang Y, Zhu ML, Ye DW, Zhou G, Lou GQ, Shi JP. Toll-like receptor-4 signalling in the progression of non-alcoholic fatty liver disease induced by high-fat and high-fructose diet in mice. Clin Exp Pharmacol Physiol 2014;41:482–488. [DOI] [PubMed] [Google Scholar]
- 87. Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology 2001;34:101–108. [DOI] [PubMed] [Google Scholar]
- 88. Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med 2000;343:1467–1476. [DOI] [PubMed] [Google Scholar]
- 89. Matsuda M, Seki E. Hepatic stellate cell-macrophage crosstalk in liver fibrosis and carcinogenesis. Semin Liver Dis 2020;40:307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Seki E, Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol 2012;590:447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pastore M, Caligiuri A, Raggi C, Navari N, Piombanti B, Di Maira G, Rovida E, Piccinni MP, Lombardelli L, Logiodice F, Rombouts K, Petta S, Marra F. Macrophage MerTK promotes profibrogenic cross-talk with hepatic stellate cells via soluble mediators. JHEP Rep 2022;4:100444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med 1999;107:450–455. [DOI] [PubMed] [Google Scholar]
- 93. Roden M, Stingl H, Chandramouli V, Schumann WC, Hofer A, Landau BR, Nowotny P, Waldhäusl W, Shulman GI. Effects of free fatty acid elevation on postabsorptive endogenous glucose production and gluconeogenesis in humans. Diabetes 2000;49:701–707. [DOI] [PubMed] [Google Scholar]
- 94. Kumashiro N, Erion DM, Zhang D, Kahn M, Beddow SA, Chu X, Still CD, Gerhard GS, Han X, Dziura J, Petersen KF, Samuel VT, Shulman GI. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A 2011;108:16381–16385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem 2004;279:32345–32353. [DOI] [PubMed] [Google Scholar]
- 96. Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 2005;11:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 2005;11:191–198. [DOI] [PubMed] [Google Scholar]
- 98. Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res 2011;21:103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Albano E, Mottaran E, Vidali M, Reale E, Saksena S, Occhino G, Burt AD, Day CP. Immune response towards lipid peroxidation products as a predictor of progression of non-alcoholic fatty liver disease to advanced fibrosis. Gut 2005;54:987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Duan Y, Pan X, Luo J, Xiao X, Li J, Bestman PL, Luo M. Association of inflammatory cytokines with non-alcoholic fatty liver disease. Front Immunol 2022;13:880298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Loomba R, Abraham M, Unalp A, Wilson L, Lavine J, Doo E, Bass NM. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology 2012;56:943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124–131. [DOI] [PubMed] [Google Scholar]
- 103. Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology 2017;66:1486–1501. [DOI] [PubMed] [Google Scholar]
- 104.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; Clinical Practice Guideline Panel; Chair; EASL Governing Board representative; Panel members. EASL Clinical practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J Hepatol 2021;75:659–689. [DOI] [PubMed] [Google Scholar]
- 105. Mózes FE, Lee JA, Selvaraj EA, Jayaswal ANA, Trauner M, Boursier J, Fournier C, Staufer K, Stauber RE, Bugianesi E, Younes R, Gaia S, Lupșor-Platon M, Petta S, Shima T, Okanoue T, Mahadeva S, Chan WK, Eddowes PJ, Hirschfield GM, Newsome PN, Wong VW, de Ledinghen V, Fan J, Shen F, Cobbold JF, Sumida Y, Okajima A, Schattenberg JM, Labenz C, Kim W, Lee MS, Wiegand J, Karlas T, Yılmaz Y, Aithal GP, Palaniyappan N, Cassinotto C, Aggarwal S, Garg H, Ooi GJ, Nakajima A, Yoneda M, Ziol M, Barget N, Geier A, Tuthill T, Brosnan MJ, Anstee QM, Neubauer S, Harrison SA, Bossuyt PM, Pavlides M. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut 2022;71:1006–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kanwal F, Shubrook JH, Adams LA, Pfotenhauer K, Wai-Sun Wong V, Wright E, Abdelmalek MF, Harrison SA, Loomba R, Mantzoros CS, Bugianesi E, Eckel RH, Kaplan LM, El-Serag HB, Cusi K. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology 2021;161:1657–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ginès P, Castera L, Lammert F, Graupera I, Serra-Burriel M, Allen AM, Wong VW, Hartmann P, Thiele M, Caballeria L, de Knegt RJ, Grgurevic I, Augustin S, Tsochatzis EA, Schattenberg JM, Guha IN, Martini A, Morillas RM, Garcia-Retortillo M, de Koning HJ, Fabrellas N, Pich J, Ma AT, Diaz MA, Roulot D, Newsome PN, Manns M, Kamath PS, Krag A. Population screening for liver fibrosis: toward early diagnosis and intervention for chronic liver diseases. Hepatology 2022;75:219–228. [DOI] [PubMed] [Google Scholar]
- 108.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406–460. [DOI] [PubMed] [Google Scholar]
- 109. de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C. Baveno VII—renewing consensus in portal hypertension. J Hepatol 2022;76:959–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. West J, Card TR. Reduced mortality rates following elective percutaneous liver biopsies. Gastroenterology 2010;139:1230–1237. [DOI] [PubMed] [Google Scholar]
- 111. Castera L. Non-invasive tests for liver fibrosis in NAFLD: creating pathways between primary healthcare and liver clinics. Liver Int 2020;40:77–81. [DOI] [PubMed] [Google Scholar]
- 112. Golabi P, Paik JM, Eberly K, de Avila L, Alqahtani SA, Younossi ZM. Causes of death in patients with non-alcoholic fatty liver disease (NAFLD), alcoholic liver disease and chronic viral hepatitis B and C. Ann Hepatol 2022;27:100556. [DOI] [PubMed] [Google Scholar]
- 113. Simon TG, Roelstraete B, Khalili H, Hagström H, Ludvigsson JF. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut 2021;70:1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ma J, Hwang SJ, Pedley A, Massaro JM, Hoffmann U, Chung RT, Benjamin EJ, Levy D, Fox CS, Long MT. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J Hepatol 2017;66:390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Tana C, Ballestri S, Ricci F, Di Vincenzo A, Ticinesi A, Gallina S, Giamberardino MA, Cipollone F, Sutton R, Vettor R, Fedorowski A, Meschi T. Cardiovascular risk in non-alcoholic fatty liver disease: mechanisms and therapeutic implications. Int J Environ Res Public Health 2019;16:3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol 2016;65:589–600. [DOI] [PubMed] [Google Scholar]
- 117. Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, Ishigami M, Toyoda H, Wai-Sun Wong V, Peleg N, Shlomai A, Sebastiani G, Seko Y, Bhala N, Younossi ZM, Anstee QM, McPherson S, Newsome PN. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology 2020;158:1611–1625.e2. [DOI] [PubMed] [Google Scholar]
- 118. Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, Kechagias S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol 2017;67:1265–1273. [DOI] [PubMed] [Google Scholar]
- 119. Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61:1547–1554. [DOI] [PubMed] [Google Scholar]
- 120. Loguercio C, Federico A, Tuccillo C, Terracciano F, D'Auria MV, De Simone C, Del Vecchio Blanco C. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol 2005;39:540–543. [DOI] [PubMed] [Google Scholar]
- 121. Loguercio C, De Simone T, Federico A, Terracciano F, Tuccillo C, Di Chicco M, Cartenì M. Gut-liver axis: a new point of attack to treat chronic liver damage? Am J Gastroenterol 2002;97:2144–2146. [DOI] [PubMed] [Google Scholar]
- 122. Aller R, De Luis DA, Izaola O, Conde R, Gonzalez Sagrado M, Primo D, De La Fuente B, Gonzalez J. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci 2011;15:1090–1095. [PubMed] [Google Scholar]
- 123. Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol 2005;42:132–138. [DOI] [PubMed] [Google Scholar]
- 124. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015;149:367–378.e365; quiz e314–e365. [DOI] [PubMed] [Google Scholar]
- 125. Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010;51:1972–1978. [DOI] [PubMed] [Google Scholar]
- 126. Howard BV, Wylie-Rosett J. Sugar and cardiovascular disease: a statement for healthcare professionals from the committee on nutrition of the council on nutrition, physical activity, and metabolism of the American Heart Association. Circulation 2002;106:523–527. [DOI] [PubMed] [Google Scholar]
- 127. Perseghin G, Lattuada G, De Cobelli F, Ragogna F, Ntali G, Esposito A, Belloni E, Canu T, Terruzzi I, Scifo P, Del Maschio A, Luzi L. Habitual physical activity is associated with intrahepatic fat content in humans. Diabetes Care 2007;30:683–688. [DOI] [PubMed] [Google Scholar]
- 128. Gerber L, Otgonsuren M, Mishra A, Escheik C, Birerdinc A, Stepanova M, Younossi ZM. Non-alcoholic fatty liver disease (NAFLD) is associated with low level of physical activity: a population-based study. Aliment Pharmacol Ther 2012;36:772–781. [DOI] [PubMed] [Google Scholar]
- 129. Wahid A, Manek N, Nichols M, Kelly P, Foster C, Webster P, Kaur A, Friedemann Smith C, Wilkins E, Rayner M, Roberts N, Scarborough P. Quantifying the association between physical activity and cardiovascular disease and diabetes: a systematic review and meta-analysis. J Am Heart Assoc 2016;5:e002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kistler KD, Brunt EM, Clark JM, Diehl AM, Sallis JF, Schwimmer JB. Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am J Gastroenterol 2011;106:460–468; quiz 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, Takasu T, Imamura M, Li Q, Tomiyama H, Kobayashi Y, Noda A, Sasamata M, Shibasaki M. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol 2013;715:246–255. [DOI] [PubMed] [Google Scholar]
- 132. Kuchay MS, Krishan S, Mishra SK, Farooqui KJ, Singh MK, Wasir JS, Bansal B, Kaur P, Jevalikar G, Gill HK, Choudhary NS, Mithal A. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT trial). Diabetes care 2018;41:1801–1808. [DOI] [PubMed] [Google Scholar]
- 133. Mantovani A, Byrne CD, Scorletti E, Mantzoros CS, Targher G. Efficacy and safety of anti-hyperglycaemic drugs in patients with non-alcoholic fatty liver disease with or without diabetes: an updated systematic review of randomized controlled trials. Diabetes Metab 2020;46:427–441. [DOI] [PubMed] [Google Scholar]
- 134. Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet 1987;2:1300–1304. [DOI] [PubMed] [Google Scholar]
- 135. Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016;387:679–690. [DOI] [PubMed] [Google Scholar]
- 136. Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, Sanyal AJ, Sejling AS, Harrison SA. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med 2021;384:1113–1124. [DOI] [PubMed] [Google Scholar]
- 137. Zhu J, Yu X, Zheng Y, Li J, Wang Y, Lin Y, He Z, Zhao W, Chen C, Qiu K, Wu J. Association of glucose-lowering medications with cardiovascular outcomes: an umbrella review and evidence map. Lancet Diabetes Endocrinol 2020;8:192–205. [DOI] [PubMed] [Google Scholar]
- 138. Chávez-Talavera O, Tailleux A, Lefebvre P, Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology 2017;152:1679–1694.e3. [DOI] [PubMed] [Google Scholar]
- 139. Xiao Y, Wang Y, Liu Y, Wang W, Tian X, Chen S, Lu Y, Du J, Cai W. A nonbile acid Farnesoid X receptor agonist tropifexor potently inhibits cholestatic liver injury and fibrosis by modulating the gut-liver axis. Liver Int 2021;41:2117–2131. [DOI] [PubMed] [Google Scholar]
- 140. Patel K, Harrison SA, Elkhashab M, Trotter JF, Herring R, Rojter SE, Kayali Z, Wong VW, Greenbloom S, Jayakumar S, Shiffman ML, Freilich B, Lawitz EJ, Gane EJ, Harting E, Xu J, Billin AN, Chung C, Djedjos CS, Subramanian GM, Myers RP, Middleton MS, Rinella M, Noureddin M. Cilofexor, a nonsteroidal FXR agonist, in patients with noncirrhotic NASH: a phase 2 randomized controlled trial. Hepatology 2020;72:58–71. [DOI] [PubMed] [Google Scholar]
- 141. Fiorucci S, Biagioli M, Sepe V, Zampella A, Distrutti E. Bile acid modulators for the treatment of nonalcoholic steatohepatitis (NASH). Expert Opin Investig Drugs 2020;29:623–632. [DOI] [PubMed] [Google Scholar]
- 142. Chianelli D, Rucker PV, Roland J, Tully DC, Nelson J, Liu X, Bursulaya B, Hernandez ED, Wu J, Prashad M, Schlama T, Liu Y, Chu A, Schmeits J, Huang DJ, Hill R, Bao D, Zoll J, Kim Y, Groessl T, McNamara P, Liu B, Richmond W, Sancho-Martinez I, Phimister A, Seidel HM, Badman MK, Joseph SB, Laffitte B, Molteni V. Nidufexor (LMB763), a novel FXR modulator for the treatment of nonalcoholic steatohepatitis. J Med Chem 2020;63:3868–3880. [DOI] [PubMed] [Google Scholar]
- 143. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 2015;385:956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Rinella ME, Dufour JF, Anstee QM, Goodman Z, Younossi Z, Harrison SA, Loomba R, Sanyal AJ, Bonacci M, Trylesinski A, Natha M, Shringarpure R, Granston T, Venugopal A, Ratziu V. Non-invasive evaluation of response to obeticholic acid in patients with NASH: results from the REGENERATE study. J Hepatol 2022;76:536–548. [DOI] [PubMed] [Google Scholar]
- 145. Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, Bedossa P, Geier A, Beckebaum S, Newsome PN, Sheridan D, Sheikh MY, Trotter J, Knapple W, Lawitz E, Abdelmalek MF, Kowdley KV, Montano-Loza AJ, Boursier J, Mathurin P, Bugianesi E, Mazzella G, Olveira A, Cortez-Pinto H, Graupera I, Orr D, Gluud LL, Dufour JF, Shapiro D, Campagna J, Zaru L, MacConell L, Shringarpure R, Harrison S, Sanyal AJ. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019;394:2184–2196. [DOI] [PubMed] [Google Scholar]
- 146. Siddiqui MS, Van Natta ML, Connelly MA, Vuppalanchi R, Neuschwander-Tetri BA, Tonascia J, Guy C, Loomba R, Dasarathy S, Wattacheril J, Chalasani N, Sanyal AJ. Impact of obeticholic acid on the lipoprotein profile in patients with non-alcoholic steatohepatitis. J Hepatol 2020;72:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Traussnigg S, Schattenberg JM, Demir M, Wiegand J, Geier A, Teuber G, Hofmann WP, Kremer AE, Spreda F, Kluwe J, Petersen J, Boettler T, Rainer F, Halilbasic E, Greinwald R, Pröls M, Manns MP, Fickert P, Trauner M. Norursodeoxycholic acid versus placebo in the treatment of non-alcoholic fatty liver disease: a double-blind, randomised, placebo-controlled, phase 2 dose-finding trial. Lancet Gastroenterol Hepatol 2019;4:781–793. [DOI] [PubMed] [Google Scholar]
- 148. Harrison SA, Bashir MR, Lee KJ, Shim-Lopez J, Lee J, Wagner B, Smith ND, Chen HC, Lawitz EJ. A structurally optimized FXR agonist, MET409, reduced liver fat content over 12 weeks in patients with non-alcoholic steatohepatitis. J Hepatol 2021;75:25–33. [DOI] [PubMed] [Google Scholar]
- 149. Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, Webb A, Portillo-Sanchez P. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes Mellitus: a randomized trial. Ann Int Med 2016;165:305–315. [DOI] [PubMed] [Google Scholar]
- 150. Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, Austin AS, Freeman JG, Morgan L, Webber J. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 2008;135:1176–1184. [DOI] [PubMed] [Google Scholar]
- 151. Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Musso G, Cassader M, Paschetta E, Gambino R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta-analysis. JAMA Intern Med 2017;177:633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. DeFronzo RA, Inzucchi S, Abdul-Ghani M, Nissen SE. Pioglitazone: the forgotten, cost-effective cardioprotective drug for type 2 diabetes. Diab Vasc Dis Res 2019;16:133–143. [DOI] [PubMed] [Google Scholar]
- 154. Francque SM, Bedossa P, Ratziu V, Anstee QM, Bugianesi E, Sanyal AJ, Loomba R, Harrison SA, Balabanska R, Mateva L, Lanthier N, Alkhouri N, Moreno C, Schattenberg JM, Stefanova-Petrova D, Vonghia L, Rouzier R, Guillaume M, Hodge A, Romero-Gómez M, Huot-Marchand P, Baudin M, Richard MP, Abitbol JL, Broqua P, Junien JL, Randomized AMA. Controlled trial of the pan-PPAR agonist lanifibranor in NASH. N Engl J Med 2021;385:1547–1558. [DOI] [PubMed] [Google Scholar]
- 155. Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet 2021;397:2212–2224. [DOI] [PubMed] [Google Scholar]
- 156. Tilg H. How to approach a patient with nonalcoholic fatty liver disease. Gastroenterology 2017;153:345–349. [DOI] [PubMed] [Google Scholar]
- 157. Chauhan M, Singh K, Thuluvath PJ. Bariatric surgery in NAFLD. Dig Dis Sci 2022;67:408–422. [DOI] [PubMed] [Google Scholar]
- 158. Moschen AR, Molnar C, Wolf AM, Weiss H, Graziadei I, Kaser S, Ebenbichler CF, Stadlmann S, Moser PL, Tilg H. Effects of weight loss induced by bariatric surgery on hepatic adipocytokine expression. J Hepatol 2009;51:765–777. [DOI] [PubMed] [Google Scholar]
- 159. Moschen AR, Molnar C, Geiger S, Graziadei I, Ebenbichler CF, Weiss H, Kaser S, Kaser A, Tilg H. Anti-inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression. Gut 2010;59:1259–1264. [DOI] [PubMed] [Google Scholar]
- 160. Lassailly G, Caiazzo R, Ntandja-Wandji LC, Gnemmi V, Baud G, Verkindt H, Ningarhari M, Louvet A, Leteurtre E, Raverdy V, Dharancy S, Pattou F, Mathurin P. Bariatric surgery provides long-term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology 2020;159:1290–1301. e5. [DOI] [PubMed] [Google Scholar]
- 161. Harrison SA, Bashir MR, Guy CD, Zhou R, Moylan CA, Frias JP, Alkhouri N, Bansal MB, Baum S, Neuschwander-Tetri BA, Taub R, Moussa SE. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2019;394:2012–2024. [DOI] [PubMed] [Google Scholar]
- 162. Harrison SA, Bashir M, Moussa SE, McCarty K, Pablo Frias J, Taub R, Alkhouri N. Effects of resmetirom on noninvasive endpoints in a 36-week phase 2 active treatment extension study in patients with NASH. Hepatol Commun 2021;5:573–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, Kharitonenkov A, Bumol T, Schilske HK, Moller DE. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab 2013;18:333–340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.