Abstract
Rationale
Central sleep apnea (CSA) is pervasive during sleep at high altitude, disproportionately impacting men and associated with increased peripheral chemosensitivity.
Objectives
We aimed to assess whether biological sex affects loop gain (LGn) and CSA severity during sleep over 9–10 days of acclimatization to 3,800 m. We hypothesized that CSA severity would worsen with acclimatization in men but not in women because of greater increases in LGn in men.
Methods
Sleep studies were collected from 20 (12 male) healthy participants at low altitude (1,130 m, baseline) and after ascent to (nights 2/3, acute) and residence at high altitude (nights 9/10, prolonged). CSA severity was quantified as the respiratory event index (REI) as a surrogate of the apnea–hypopnea index. LGn, a measure of ventilatory control instability, was quantified using a ventilatory control model fit to nasal flow. Linear mixed models evaluated effects of time at altitude and sex on respiratory event index and LGn. Data are presented as contrast means with 95% confidence intervals.
Results
REI was comparable between men and women at acute altitude (4.1 [−9.3, 17.5] events/h; P = 0.54) but significantly greater in men at prolonged altitude (23.7 [10.3, 37.1] events/h; P = 0.0008). Men had greater LGn than did women for acute (0.08 [0.001, 0.15]; P = 0.047) and prolonged (0.17 [0.10, 0.25]; P < 0.0001) altitude. The change in REI per change in LGn was significantly greater in men than in women (107 ± 46 events/h/LGn; P = 0.02).
Conclusions
The LGn response to high altitude differed between sexes and contributed to worsening of CSA over time in men but not in women. This sex difference in acclimatization appears to protect females from high altitude–related CSA. These data provide fundamental sex-specific physiological insight into high-altitude acclimatization in healthy individuals and may help to inform sex differences in sleep-disordered breathing pathogenesis in patients with cardiorespiratory disease.
Keywords: loop gain, central sleep apnea, high altitude, periodic breathing
Each year more than 100 million people travel to high altitude for work or recreation (1), many of whom have unknown (i.e., preclinical) or preexisting medical conditions (2, 3). As the prevalence of cardiovascular (2), respiratory (3, 4), and metabolic (5) disease increases worldwide, so does the likelihood of adverse events occurring at high altitude, where human physiology is challenged by the environment and physical exertion. Medical emergencies in often remote high-altitude regions are met with barriers to prompt and optimal medical care. To best inform high-altitude travelers on risk management, it is necessary to fully understand human responses after ascent and acclimatization to high altitude.
Central sleep apnea (CSA) is ubiquitous during sleep at high altitude, occurring in >90% of otherwise healthy high-altitude sojourners (6). High-altitude CSA is considered to arise consequent to hypoxia-induced augmentation of chemoreflex sensitivity and is exacerbated with the increased chemosensitivity that accompanies ventilatory acclimatization (7). The resultant ventilatory instability produces alternating periods of apnea and hyperventilation during sleep. Ventilatory instability can be quantified using the engineering concept of loop gain (LGn) (8). The LGn, or instability of the ventilatory control system, is based on three components: 1) controller gain, which is the change in ventilation per change in arterial carbon dioxide tension or arterial oxygen tension as elicited by central and peripheral chemoreflexes; 2) plant gain, which is the change in arterial carbon dioxide tension or arterial oxygen tension per change in ventilation as achieved by alveolar ventilation; and 3) the circulatory delay between the lungs and central and peripheral chemoreceptors (9, 10). Instability-induced CSA can be due to changes in one or more of these control system components (e.g., heightened controller gain arising from ventilatory acclimatization to hypoxia) (9, 11). There are multiple validated techniques used to quantify LGn, with larger LGn values denoting increasingly unstable ventilatory control (12–14).
LGn in obstructive sleep apnea (OSA) and Hunter-Cheyne-Stokes respiration are well characterized (9); yet, the LGn and high-altitude CSA literature is limited and conflicting (10, 15, 16). The contrasting relationship between LGn and apnea–hypopnea index (AHI) at altitude could be influenced by the altitude or time spent at altitude and is further complicated by sex differences in respiratory control. For example, LGn is higher in men with OSA, congestive heart failure, treatment-emergent CSA, and non–rapid eye movement dominant apnea (13, 17, 18). Furthermore, the hypoxic ventilatory response (HVR; i.e., daytime controller gain) and AHI are greater in men at acute high altitude (19–21). These data suggest that larger HVRs lead to the greater CSA found in men. However, extrapolating these findings to prolonged stays at altitude is challenging where acclimatization alters peripheral chemosensitivity (11), and it ignores other LGn components (e.g., circulatory delay) that contribute to CSA pathogenesis.
The aim of this study was to determine how sex influences LGn and CSA during sleep after acute exposure to high altitude and after 9 days of acclimatization to 3,800 m. We hypothesized that at high altitude, the severity of CSA would worsen over time in men but not in women because of greater increases in LGn in men during sleep.
Methods
Ethical Clearance
This study abided by the Canadian Government Tri-Council policy on research ethics with human participants and the Declaration of Helsinki, except for registration in a database. Ethical clearance was received from the University of Calgary Conjoint Human Research Ethics Board (Protocol REB18-0374), the Mount Royal University Human Research Ethics Board (Protocol 101979), and the University of British Columbia Clinical Ethics Board (H19-01734). All participants provided written informed consent.
Participants and Study Design
This study was part of a larger research expedition to 3,800 m (White Mountains in California) in August 2019 in which participant demographics and cardiorespiratory and sleep measurements have been reported previously (22, 23). Eight women and 13 men were recruited for the present study. They had no medical history of cardiopulmonary, neurological, or metabolic disease. Participants were native lowlanders who had not ascended to high altitude in the past year and were not receiving carbonic anhydrase inhibitors (e.g., acetazolamide) or corticosteroids for prevention or treatment of acute mountain sickness (AMS). Inclusion in this study required volunteers to be healthy men or women older than 18 years of age who planned to stay at the research station for 10 consecutive days. Participants were excluded if they had a body mass index >35 kg/m2 or a history of smoking or were receiving prescription medication other than birth control. For logistical reasons, it was not possible to align measurements with ovarian phases. However, seven participating females had hormonal intrauterine devices, and one was not using any form of birth control.
Participants traveled from their respective lowland locations to Calgary, AB, Canada (1,130 m), where they underwent baseline measurements (i.e., baseline) before flying to Las Vegas, NV (610 m), where they stayed for one night. The following morning, participants ascended (∼5–6 h) to the Barcroft Field Station (White Mountains, California; 3,800 m) by vehicle. Participants resided at high altitude for 10 days, where metrics of sleep-disordered breathing were obtained on nights 2/3 (i.e., acute) and 9/10 (i.e., prolonged). Daytime ancillary data for AMS scores (24) and partial pressure of end-tidal carbon dioxide were also reported as previously detailed (23).
Data Analysis
Portable monitors (ApneaLink Air, Generation 3, ResMed) consisting of a nasal cannula for airflow, thoracic strain gauge for respiratory effort, and oxygen saturation as measured by pulse oximetry were used to collect cardiopulmonary data during sleep. The first 30 minutes and last 5 minutes of each recording were excluded from analysis, and only recordings ⩾90 minutes in duration were included. Signals were analyzed for events (e.g., apneas/hypopneas), airflow shapes, and LGn using a custom MATLAB (MathWorks) program as previously described (13, 25). CSA severity was determined from the respiratory event index (REI) and the oxygen desaturation index (ODI). REI was calculated as the average number of apneas and hypopneas per hour taken from the total recording time. REI was used as a surrogate measure of AHI and likely underestimates AHI because of the inability to differentiate sleep versus wakefulness states from the ApneaLink device. Apneas (⩾90% reduction in peak nasal pressure) and hypopneas (⩾30% reduction in peak nasal pressure) were required to be ⩾6 seconds in duration to account for increases in minute ventilation and the shorter-duration apneas typical of high altitude (5–15 s) (11, 26–28). Apneas/hypopneas were scored as central events because mild OSA (∼5 events/h) is abolished and replaced by CSA at 3,840 m (29). Event duration, cycle period, oxygen saturation as measured by pulse oximetry measures, hypoxic burden, and average nadir delay were also collected and are defined in the Methods section of the data supplement.
To determine the extent of airflow obstruction throughout apneic events, flow shape analysis was used to determine flow:drive, a measure estimating the percentage of airway patency (i.e., 100% represents no airflow obstruction, and 0% is complete obstruction) (25). Flow:drive before each apneic event and during the most obstructive part of each apnea was also calculated as BaselineFlowDrive and NadirFlowDrive, respectively. These indices were then used to determine how much worse obstruction became during each apneic event, which is reported as %ΔFlow:drive.
LGn was quantified during sleep using an established method (13, 14, 30) adapted for the ApneaLink device. Briefly, analysis of nasal pressure airflow signal provided an uncalibrated breath-by-breath ventilation signal (volume × respiratory rate); ventilation data were then used as the input to a ventilatory control model whose parameters (gain, response time constant, delay) were adjusted (least squares) such that the output ventilatory drive signal best matched future values of the ventilation signal. In essence, LGn was varied such that a ventilatory drive signal output from the model best fit each ventilatory overshoot that followed a prior hypopnea (input). The following key modifications to the analysis were made to suit the present study: 1) Data were analyzed in 3-minute rather than 7-minute windows to improve model fit quality (per preliminary visual inspection); 2) all windows during sleep were analyzed (regardless of the presence of scored events) to minimize potential selection bias; and 3) the ventilatory response to arousal was not included in model fitting.
From each window, we calculated LGn at the natural frequency with median values reported for each nighttime study. LGn captures the overall ventilatory control instability and reflects the combined impact of controller and plant gains and the influence of circulatory delay (13). We also calculated LGn magnitude variables, namely LGn at 1 cycle/min (LG1), 2 cycles/min (LG2), and 3 cycles/min (LG3) that describe the ventilatory control sensitivity in the absence of circulatory delays (13). LG1 closely resembles the cycling dynamics of OSA (31), whereas LG2 (19, 32) and LG3 (8, 11) more closely relate to the cycling dynamics of high-altitude CSA. In addition, we quantified circulatory delay, which represents the time delays due to blood transport from the lungs to peripheral chemoreceptors plus any chemoreflex response latency.
Statistical Analysis
Statistical analyses were performed using the R statistical language (R Foundation for Statistical Computing). Data are presented as the mean ± standard deviation, and contrasts are presented as mean with 95% confidence interval. Statistical significance was determined at P < 0.05. Participant characteristics and AMS scores by sex were compared using unpaired t tests and Wilcoxon signed-rank tests, respectively. Linear mixed effects models were used to determine changes in outcome parameters with time at altitude (baseline, acute, and prolonged exposure) and sex as fixed factors and participant identifier as a random factor. Where significant F-ratios were detected, Tukey’s honestly significant difference post hoc analysis was used to identify pairwise differences. Additional mixed effects models were conducted to determine the influence of sex on the relationship between CSA severity and LGn. Sex was a fixed factor, CSA metric was a continuous predictor, and participant identifier was a random factor.
Results
One male participant was excluded because of severe OSA, leaving 8 women and 12 men in the complete analysis. Demographic data are presented in Table 1. AMS scores were similar between men and women at high altitude (P > 0.53). Daytime partial pressure of end-tidal carbon dioxide was significantly higher in men than in women at baseline (3.1 [0.3, 5.9] mm Hg; P = 0.03) and acute altitude (3.8 [1.2, 6.4] mm Hg; P = 0.006) but not after prolonged altitude (−0.1 [−2.7, 2.5] mm Hg; P = 0.95).
Table 1.
Participant characteristics
| Characteristics | Females | Males | P Value |
|---|---|---|---|
| n | 8 | 12 | |
| Age, yr | 28 ± 8 | 31 ± 11 | 0.59 |
| Height, m | 1.66 ± 0.09 | 1.79 ± 0.08 | 0.003 |
| Weight, kg | 63 ± 8 | 83 ± 12 | 0.001 |
| BMI, kg/m2 | 22.8 ± 4.1 | 25.6 ± 2.9 | 0.10 |
| AMS score | |||
| Low altitude | 0 [0] | 0 [0] | NS |
| High altitude | — | — | — |
| Acute | 1 [2.25] | 2 [2.25] | 0.53 |
| Prolonged | 0 [0] | 0 [0] | 0.78 |
| Daytime PetCO2 (mm Hg) | |||
| Low altitude | 34 ± 3 | 38 ± 3 | 0.02 |
| High altitude | — | — | — |
| Acute | 29 ± 3* | 32 ± 3 | 0.01 |
| Prolonged | 27 ± 3* | 27 ± 2 | 0.95 |
Definition of abbreviations: AMS = acute mountain sickness scale; BMI = body mass index; P = probability of difference between male and female; PetCO2 = partial pressure of end-tidal carbon dioxide.
Data are presented as mean ± standard deviation. AMS scores are presented as median [interquartile range]. P < 0.05 compared with females.
n = 7.
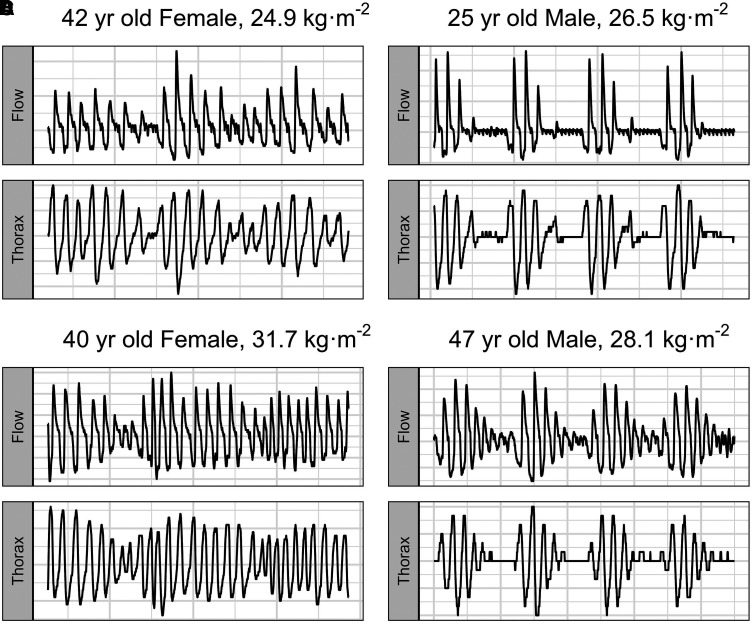
Ninety-second epoch nasal airflow and thoracic excursion traces for two male and two female subjects at prolonged altitude (Night 9) at 3,800 m are presented in Figure 1. Men experienced more obstructed airflow than women did (−8.5% [−0.6%, −16.3%]; P = 0.03), but obstruction did not increase at high altitude. Furthermore, the change in obstruction during apnea was not different for men compared with women (7.8% [−4.5%, 20.1%]; P = 0.18). BaselineFlowDrive and NadirFlowDrive are reported in Table E1 in the data supplement.
Figure 1.
Ninety-second epoch representative tracings for nasal airflow and thoracic excursions in two female (A and C) and two male (B and D) participants on night 9 at 3,800 m.
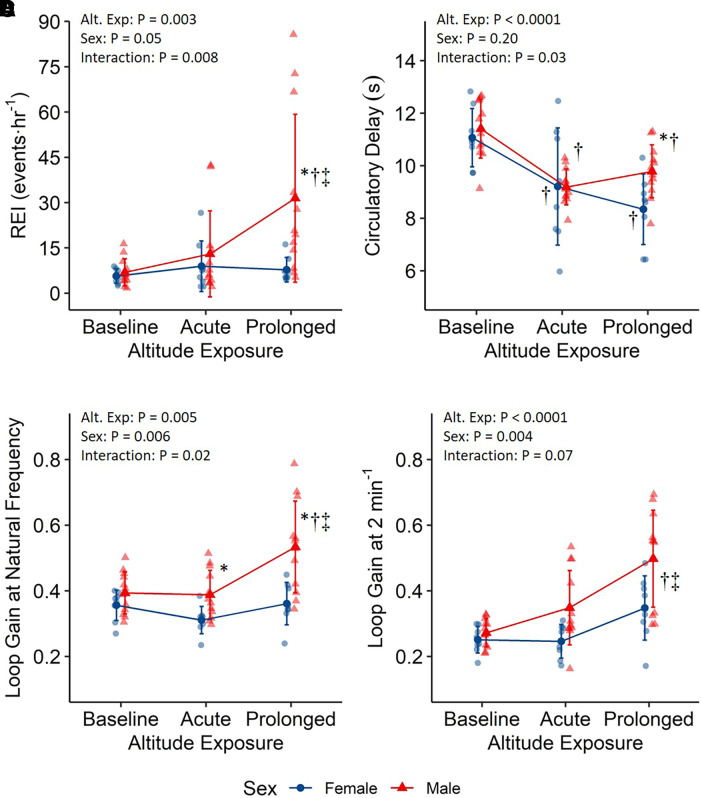
Metrics of sleep-disordered breathing are reported in Table 2, with altitude-by-sex interactions for REI (Figure 2A; P = 0.008) and ODI (P = 0.005). REI was comparable between men and women at baseline (1.2 [−12.2, 14.6] events/h; P = 0.86) and acute altitude (4.1 [−9.3, 17.5] events/h; P = 0.54) but became significantly greater in men at prolonged altitude (23.7 [10.3, 37.1] events/h; P = 0.0008). Women had similar REI between acute and prolonged altitude (−1.2 [−15.9, 13.6] events/h; P = 0.98), whereas REI increased in men (18.4 [6.4, 30.5] events/h; P = 0.002). The REI was predominantly hypopneas, with hypopneas making up >90% of REI events for all time points in both sexes. Results for hypopneas and ODI are reported in Table 1.
Table 2.
Sleep study, central sleep apnea, and loop gain measurements made at low altitude and at acute and prolonged high altitude in females and males
| Sex |
P Value |
|||||
|---|---|---|---|---|---|---|
| Variable | Altitude | Female | Male | Sex | Altitude | X |
| Total recording time, min | Baseline, 1,130 m | 389 ± 59 | 399 ± 70 | 0.32 | 0.0002 | 0.29 |
| Acute, 3,800 m | 401 ± 45 | 404 ± 39 | ||||
| Prolonged, 3,800 m*† | 364 ± 63 | 319 ± 68 | ||||
| REI, events/h | Baseline, 1,130 m | 5.7 ± 2.3 | 6.9 ± 4.5 | 0.05 | 0.003 | 0.008 |
| Acute, 3,800 m | 8.9 ± 8.4 | 13 ± 14.2 | ||||
| Prolonged, 3,800 m | 7.7 ± 4.0 | 31.4 ± 27.8*†‡ | ||||
| Hypopneas, events/h | Baseline, 1,130 m | 5.2 ± 2.4 | 6.4 ± 4.4 | 0.05 | 0.002 | 0.007 |
| Acute, 3,800 m | 8.4 ± 8.0 | 11.9 ± 12.4 | ||||
| Prolonged, 3,800 m | 7.5 ± 4.1 | 28.5 ± 23.9*†‡ | ||||
| ODI, events/h | Baseline, 1,130 m | 5 ± 3 | 7 ± 5 | 0.04 | <0.0001 | 0.005 |
| Acute, 3,800 m | 20 ± 12 | 28 ± 19* | ||||
| Prolonged, 3,800 m | 22 ± 9* | 52 ± 32*†‡ | ||||
| Flow:drive, % | Baseline, 1,130 m | 89 ± 5 | 78 ± 11 | 0.03 | 0.41 | 0.27 |
| Acute, 3,800 m | 88 ± 8 | 81 ± 10 | ||||
| Prolonged, 3,800 m | 86 ± 13 | 79 ± 6 | ||||
| %ΔFlow:drive, % | Baseline, 1,130 m | 24 ± 17§ | 28 ± 8 | 0.18 | 0.07 | 0.54 |
| Acute, 3,800 m | 23 ± 21 | 33 ± 17 | ||||
| Prolonged, 3,800 m | 29 ± 21 | 40 ± 10 | ||||
| Cycle period, s | Baseline, 1,130 m | 42 ± 4 | 43 ± 5 | 0.68 | <0.0001 | 0.002 |
| Acute, 3,800 m | 34 ± 8* | 29 ± 5†‡ | ||||
| Prolonged, 3,800 m | 29 ± 4*† | 31 ± 3* | ||||
| Loop gain | Baseline, 1,130 m | 0.36 ± 0.05 | 0.39 ± 0.06 | 0.0006 | 0.0005 | 0.02 |
| Ventilatory instability (LGn) | Acute, 3,800 m | 0.31 ± 0.04 | 0.39 ± 0.07‡ | |||
| Prolonged, 3,800 m | 0.36 ± 0.07 | 0.53 ± 0.14*†‡ | ||||
| Ventilatory sensitivity (LG2) | Baseline, 1,130 m | 0.25 ± 0.04 | 0.27 ± 0.04 | 0.004 | <0.0001 | 0.07 |
| Acute, 3,800 m | 0.25 ± 0.05 | 0.35 ± 0.11 | ||||
| Prolonged, 3,800 m*† | 0.35 ± 0.10 | 0.50 ± 0.15 | ||||
| Circulatory delay, s | Baseline, 1,130 m | 11.1 ± 1.1 | 11.4 ± 1.1 | 0.20 | <0.0001 | 0.03 |
| Acute, 3,800 m | 9.2 ± 2.2* | 9.2 ± 0.7* | ||||
| Prolonged, 3,800 m | 8.3 ± 1.3* | 9.8 ± 1.0†‡ | ||||
Definition of abbreviations: %ΔFlow:drive = noninvasive quantification of how the severity of airflow obstruction changes during an apnea using airflow shape analyses; Flow:drive = noninvasive quantification of the severity of airflow obstruction using airflow shape analyses; LG2 = loop gain at 2 cycles/min; ODI = oxygen desaturation index, >3% desaturation events; P = probability of difference between male and female; REI = respiratory event index, >6 seconds and >3% desaturation events; X = interaction effect of sex and altitude.
Data are presented as mean ± standard deviation.
P < 0.05 compared with baseline.
P < 0.05 compared with acute.
P < 0.05 compared with female.
n = 7.
Figure 2.
Central sleep apnea (A) and loop gain (B–D) measurements during low-altitude baseline (1,130 m) and acute (nights 2/3) and prolonged (nights 9/10) exposure to 3,800 m. *P < 0.05 compared with females. †P < 0.05 compared with baseline. ‡P < 0.05 compared with acute. Presented as mean ± standard deviation. REI = respiratory event index, >6 seconds and >3% desaturation events.
LGn metrics are reported in Table 2, with altitude-by-sex interactions for LGn (Figure 2C; P = 0.02) and circulatory delay (Figure 2B; P = 0.03) and significant main effects of altitude and sex for LG2 (Figure 2D; altitude, P < 0.0001; sex, P = 0.004). Baseline LGn (0.04 [−0.04, 0.11]; P = 0.33) was similar between men and women but was elevated in men with acute (0.08 [0.001, 0.15]; P = 0.047) and prolonged (0.17 [0.10, 0.25]; P < 0.0001) altitude. LGn was similar in women between acute and prolonged altitude (0.05 [−0.04, 0.14]; P = 0.40) but increased in men (0.15 [0.07, 0.22]; P < 0.0001). LG2 was elevated in men (0.09 [0.03, 0.15]; P = 0.005) compared with women and was greatest at prolonged altitude compared with baseline (0.16 [0.09, 0.23]; P < 0.0001) and acute altitude (0.13 [0.06, 0.19]; P = 0.0002). Circulatory delay was similar between sexes at baseline (0.3 [−0.8, 1.5] s; P = 0.56) and acute altitude (0 [−1.2, 1.1] s; P = 0.97) but was longer in men at prolonged altitude (1.4 [0.3, 2.6] s; P = 0.02). Circulatory delays decreased with high altitude (P < 0.0006), but there were no differences from acute to prolonged altitude in either men (0.6 [ 0.3, 1.5] s; P = 0.26) or women (−0.9 [−2.0, 0.2] s; P = 0.15). LG1 and LG3 differences were generally similar to LG2 results and are reported in Table E1.
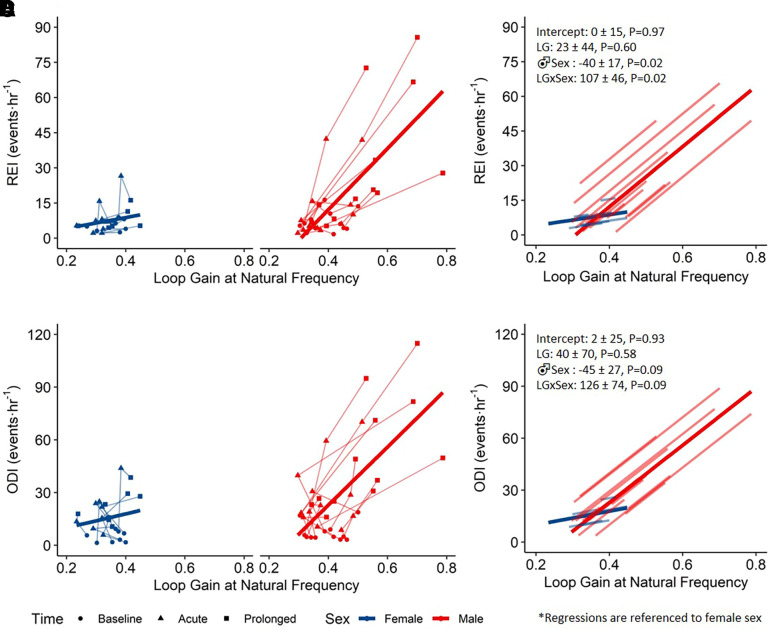
Figure 3 depicts individual data points and the linear mixed effects models that describe the association between REI/ODI and LGn across sex. The relationship between REI/ODI and LGn was significantly different across sex. In men but not women, heightened LGn was associated with greater REI. There was no association between REI/ODI and circulatory delay (P > 0.19; data not shown).
Figure 3.
Linear mixed models for the relationship between central sleep apnea severity and LGn. Within-subject individual data with group linear mixed effects models by sex for respiratory event index, >6 seconds and >3% desaturation events (REI) (A) and oxygen desaturation index, >3% desaturation events (ODI) (C). Individual data points correspond to measurements taken at baseline, acute altitude, or prolonged altitude. Lines fit to individual subject data and group data for REI (B) and ODI (D). LGn = loop gain at natural frequency.
Discussion
The principal findings were 1) LGn and CSA severity substantially increased in men, but not in women, over 9 days of acclimatization to high altitude; 2) heightened LGn in men was associated with CSA severity and CSA-related hypoxemia; and 3) circulatory delay was less in women than in men at prolonged altitude, which may offset the heightened controller gain in men and protect against ventilatory instability at high altitude. Our findings highlight distinct influences of sex on LGn at high altitude with acclimatization and may provide fundamental sex-specific physiological insight into sleep-disordered breathing pathogenesis for patients with cardiorespiratory disease.
During acclimatization to high altitude, men increased LGn and CSA severity, whereas women did not (Figure 2). Although sex differences in high-altitude CSA are not new (19–21, 28, 33), our research combines measures of LGn with CSA severity during acclimatization at 3,800 m. After acute exposure to 3,800 m, increased CSA severity was modest and did not differ between sexes. In contrast, previous studies have found greater AHI in men acutely at 4,559 m (20) and greater AHI and ODI in men both acutely and after prolonged exposure to 5,400 m (19). Comparatively, our altitude was lower, likely accounting for the relatively small increase in CSA severity that did not differ between sexes acutely. Notably, the dichotomy of CSA severity observed between sexes at prolonged altitude emulated previous work (19). It is also worth noting that the absence of sleep electroencephalograms likely contributed to the reduced quantification of CSA because sleep is often more fragmented at altitude (34, 35); thus, using REI instead of AHI underrepresents CSA severity. For LGn, sex differences have not been explored during high-altitude acclimatization. We present the novel finding that LGn did not change in women with altitude but progressively increased in men over time. Our findings are in alignment with prior studies that have assessed LGn in hypobaric/normobaric hypoxia. One study found that LGn increased from days 2–4 to days 12–14 at 5,050 m (10), whereas two other studies only assessed LGn on the first night at 2,020 m (16) and simulated 3,400 m (15). However, the relationship between LGn and CSA severity is conflicting. LGn was poorly correlated with increased AHI at 5,050 m (8 males/4 females; r2 = 0.11; P < 0.05) (10) but correlated with central apneas at 2,020 m (7 males; r2 = 0.84; P = 0.004) (16) and with AHI at simulated 3,400 m (14 males; r2 = 0.86; P < 0.001) (15). Furthermore, HVR has correlated with AHI acutely but not with prolonged altitude (10, 11, 36). Thus, our data in men agree with previous prolonged altitude work in which peripheral chemosensitivity was the major driver of increases in LGn (10). In addition, these results support CSA being a function of LGn, with the added novelty of women having inherently attenuated ventilatory instability compared with men at high altitude. This implies that male-related risk of ventilatory instability is present in healthy individuals and helps to explain why prior studies in OSA and congestive heart failure found higher LGn than in women resulting from augmented controller gain and longer circulatory delays (26).
Men are anatomically more susceptible to upper airway obstruction leading to sleep-disordered breathing (26). Our results demonstrate that men experienced more obstructed airflow than did women but that obstruction was not exacerbated at high altitude (26). This aligns with previous work in which sleep-disordered breathing at high altitude was predominantly central in nature as chemical stimuli dominate over airflow obstruction (29, 37). For our central events, however, there did seem to be morphological differences in central hypopneas (Figure 1) where female subjects had a gradual waxing-and-waning pattern of nasal flow and thoracic effort, whereas men followed a more polarized oscillation between hypopnea and hyperventilation. These morphological differences in which female subjects are better protected from sleep-disordered breathing may help inform our understanding of sex differences in hypopneas for individuals with cardiorespiratory disease.
How women maintained better breathing stability with acute and prolonged high-altitude exposure compared with men is unknown. Our results suggest that smaller increases in LGn and maintained reduction in circulatory delay may be contributing factors. Heightened controller gain is known to primarily drive LGn for hypoxia-induced CSA (9, 11), and previous work has linked greater HVRs in men to larger AHIs (20). This led to the concept that these differences in CSA are hormonally mediated because testosterone upregulates the HVR (20, 38). Previous investigations report similar HVRs between sexes at 2,100 m and after 6–7 days at 4,350 m (39); however, progesterone acts as a respiratory stimulant and thus could potentially offset observed sex differences (38). Reconciling these discrepant findings is difficult because ovarian phase, methodological differences in HVR assessment, and/or length of stay at altitude could influence results.
The elevated controller gain previously observed in women may be partially offset by shorter circulatory delays at prolonged altitude (9, 33), leading to relatively small and nonsignificant increases in LGn. Lombardi and colleagues proposed hormonal mediation of CSA severity because estrogen and testosterone increase and decrease cerebral blood flow (CBF), respectively (19). With LGn (Figure 3) and CBF (36, 40) relating to AHI/REI, hormonal influences could alter circulatory delay and stabilize ventilatory control in women, although this is unlikely, given similar delays between men and women at acute altitude. Hematological differences, however, may play a role (41). Cardiac output is augmented with acute hypoxia (28, 33, 42), thus lowering circulatory delays (9), but reduced blood volume and hypoxic pulmonary vasoconstriction with prolonged hypoxic exposures lower stroke volume, thereby decreasing cardiac output and CBF over time at altitude (36, 42). This would account for the circulatory delay response in men with altitude (Figure 2B), but why women decreased their circulatory delay at prolonged altitude remains unclear. Although speculative, hemoconcentration, blood volume, and cardiac output may underpin this divergence in circulatory delays at prolonged altitude (33); however, potential sex-related cardiovascular differences, particularly in women, remain understudied (42).
Methodological Considerations
Sex hormones were not controlled for, despite known respiratory influences (38). Feasibility precluded studying women in a chosen ovarian phase because the research expedition involved synchronized group ascent and residence. Notably, ovarian cycle phase does not correlate with CSA (19) or significantly interfere with chemoreflex activity (20). Notwithstanding these considerations, sex differences in LGn were highly significant and are unlikely underpinned by variations in cycling female sex hormones. Next, electroencephalograms were not collected, and sleep staging was not performed. Monitoring was kept minimal because our primary outcome of LGn can be quantified from minimal instrumentation (14). Furthermore, we wished to maximize the number of participants studied on a single night to reduce variability during acclimatization, and full polysomnography would hinder this goal. Polysomnography would have allowed measurement of sleep time instead of monitoring time for quantification of AHI instead of REI, with arousals likely causing these values to diverge with initial altitude exposure (34) but to converge at prolonged altitude (21, 35). To what extent arousals increase (34) or are similar to low-altitude levels after a night at altitude (21, 28, 35, 43) is equivocal. As a result, there may be inaccuracies in the measurement of REI and ODI, which are calculated on the basis of recording time rather than sleep time. Last, we cannot exclude the possibility that the lack of between-sex differences in some metrics may be due to data variability and the relatively small sample size of our study. Our sample size was limited by the number of recording monitors available and the number of participants who could be accommodated at the Barcroft Field Station.
Conclusions
Male and female subjects have differing LGn responses to high altitude whereby healthy men, but not healthy women, acclimatized to altitude-related hypoxia with a marked increase in LGn that manifested as an increase in CSA severity and CSA-related hypoxemia over time. Such sex differences were not detectable at sea level and were subtle upon acute ascent to 3,800 m. Our data indicate that women are protected from developing CSA by avoiding acclimatization-related increases in ventilatory sensitivity and by reducing their circulatory delays. Together these findings highlight the physiologically distinct pathogenesis of sleep-related breathing disorders between men and women and provide evidence of fundamental sex differences in high-altitude acclimatization.
Footnotes
Supported by the Alberta Government Student Temporary Employment Program and Mitacs through the Mitacs Accelerate program (J.D.B.). S.A.S. was funded by the National Institutes of Health, National Heart, Lung, and Blood Institute (R01HL146697), and the American Academy of Sleep Medicine Foundation (228-SR-20). C.L.H.S. was funded by an undergraduate research fellowship presented by the Stober Foundation. N.G.J. was funded by the Natural Sciences and Engineering Research Council of Canada BRAIN CREATE Program and a Parker B. Francis Foundation Post-Doctoral Fellowship from the Francis Family Foundation. G.E.F. was a Michael Smith Foundation for Health Research scholar. In addition, funding was provided by Natural Sciences and Engineering Research Council of Canada Discovery grants (T.A.D., RGPIN-2016–04915; R.J.A.W., RGPIN-03941; G.E.F., RGPIN-2014-05643 and RGPIN-2020-04010) and a University of Calgary University Research Grants Committee grant.
Author Contributions: J.D.B., N.G.J., R.J.A.W., T.A.D., and G.E.F. conceived and designed the research; J.D.B. performed data collection; J.D.B., S.A.S., R.M.A., C.L.H.S., and G.E.F. analyzed data; J.D.B., S.A.S., and G.E.F. interpreted the results of experiments; J.D.B. and G.E.F. prepared figures and tables; J.D.B. drafted the manuscript; J.D.B., S.A.S., R.M.A., C.L.H.S., B.M.S., N.G.J., R.J.A.W., T.A.D., and G.E.F. approved the final version of the manuscript.
Data Availability: The deidentified data that support the findings of this study are available from the corresponding author upon reasonable request from a qualified researcher.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Luks AM, Ainslie PN, Lawley JS, Roach RC, Simonson TS. Ward, Milledge and West’s high altitude medicine and physiology. Boca Raton, FL: CRC Press; 2021. [Google Scholar]
- 2. Cornwell WK, III, Baggish AL, Bhatta YKD, Brosnan MJ, Dehnert C, Guseh JS, et al. American Heart Association Exercise, Cardiac Rehabilitation, and Secondary Prevention Committee of the Council on Clinical Cardiology; and Council on Arteriosclerosis, Thrombosis and Vascular Biology Clinical implications for exercise at altitude among individuals with cardiovascular disease: a scientific statement from the American Heart Association. J Am Heart Assoc . 2021;10:e023225. doi: 10.1161/JAHA.121.023225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Luks AM, Hackett PH. Medical conditions and high-altitude travel. N Engl J Med . 2022;386:364–373. doi: 10.1056/NEJMra2104829. [DOI] [PubMed] [Google Scholar]
- 4. Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med . 2019;7:687–698. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou B, Lu Y, Hajifathalian K, et al. NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet . 2016;387:1513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Venkat D, Dhillon K, Rowley JA. Effects of high altitude on sleep and respiratory system. Curr Pulmonol Rep . 2021;10:103–109. [Google Scholar]
- 7. Sands SA, Edwards BA, Kee K, Stuart-Andrews C, Skuza EM, Roebuck T, et al. Control theory prediction of resolved Cheyne-Stokes respiration in heart failure. Eur Respir J . 2016;48:1351–1359. doi: 10.1183/13993003.00615-2016. [DOI] [PubMed] [Google Scholar]
- 8. Khoo MCK, Kronauer RE, Strohl KP, Slutsky AS. Factors inducing periodic breathing in humans: a general model. J Appl Physiol . 1982;53:644–659. doi: 10.1152/jappl.1982.53.3.644. [DOI] [PubMed] [Google Scholar]
- 9. Orr JE, Malhotra A, Sands SA. Pathogenesis of central and complex sleep apnoea. Respirology . 2017;22:43–52. doi: 10.1111/resp.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andrews G, Ainslie PN, Shepherd K, Dawson A, Swart M, Lucas S, et al. The effect of partial acclimatization to high altitude on loop gain and central sleep apnoea severity. Respirology . 2012;17:835–840. doi: 10.1111/j.1440-1843.2012.02170.x. [DOI] [PubMed] [Google Scholar]
- 11. Ainslie PN, Lucas SJE, Burgess KR. Breathing and sleep at high altitude. Respir Physiol Neurobiol . 2013;188:233–256. doi: 10.1016/j.resp.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 12. Sands SA, Edwards BA, Kee K, Turton A, Skuza EM, Roebuck T, et al. Loop gain as a means to predict a positive airway pressure suppression of Cheyne-Stokes respiration in patients with heart failure. Am J Respir Crit Care Med . 2011;184:1067–1075. doi: 10.1164/rccm.201103-0577OC. [DOI] [PubMed] [Google Scholar]
- 13. Terrill PI, Edwards BA, Nemati S, Butler JP, Owens RL, Eckert DJ, et al. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur Respir J . 2015;45:408–418. doi: 10.1183/09031936.00062914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wellman A, Eckert DJ, Jordan AS, Edwards BA, Passaglia CL, Jackson AC, et al. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol (1985) . 2011;110:1627–1637. doi: 10.1152/japplphysiol.00972.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown CV, Boulet LM, Vermeulen TD, Sands SA, Wilson RJA, Ayas NT, et al. Angiotensin II-type I receptor antagonism does not influence the chemoreceptor reflex or hypoxia-induced central sleep apnea in men. Front Neurosci . 2020;14:382. doi: 10.3389/fnins.2020.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rey de Castro J, Liendo A, Ortiz O, Rosales-Mayor E, Liendo C. Ventilatory cycle measurements and loop gain in central apnea in mining drivers exposed to intermittent altitude. J Clin Sleep Med . 2017;13:27–32. doi: 10.5664/jcsm.6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Won CHJ, Reid M, Sofer T, Azarbarzin A, Purcell S, White D, et al. Sex differences in obstructive sleep apnea phenotypes, the multi-ethnic study of atherosclerosis. Sleep (Basel) . 2020;43:zsz274. doi: 10.1093/sleep/zsz274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moro M, Gannon K, Lovell K, Merlino M, Mojica J, Bianchi MT. Clinical predictors of central sleep apnea evoked by positive airway pressure titration. Nat Sci Sleep . 2016;8:259–266. doi: 10.2147/NSS.S110032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lombardi C, Meriggi P, Agostoni P, Faini A, Bilo G, Revera M, et al. HIGHCARE Investigators High-altitude hypoxia and periodic breathing during sleep: gender-related differences. J Sleep Res . 2013;22:322–330. doi: 10.1111/jsr.12012. [DOI] [PubMed] [Google Scholar]
- 20. Caravita S, Faini A, Lombardi C, Valentini M, Gregorini F, Rossi J, et al. Sex and acetazolamide effects on chemoreflex and periodic breathing during sleep at altitude. Chest . 2015;147:120–131. doi: 10.1378/chest.14-0317. [DOI] [PubMed] [Google Scholar]
- 21. Li T, Tan L, Furian M, Zhang Y, Luo L, Lei F, et al. Sex-specific difference in the effect of altitude on sleep and nocturnal breathing in young healthy volunteers. J Clin Med . 2022;11:2869. doi: 10.3390/jcm11102869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bird JD, Kalker A, Rimke AN, Chan JS, Chan G, Saran G, et al. Severity of central sleep apnea does not affect sleeping oxygen saturation during ascent to high altitude. J Appl Physiol (1985) . 2021;131:1432–1443. doi: 10.1152/japplphysiol.00363.2021. [DOI] [PubMed] [Google Scholar]
- 23. Bird JD, Leacy JK, Foster GE, Rickards CA, Wilson RJA, O’Halloran KD, et al. Time course and magnitude of ventilatory and renal acid-base acclimatization following rapid ascent to and residence at 3,800 m over nine days. J Appl Physiol (1985) . 2021;130:1705–1715. doi: 10.1152/japplphysiol.00973.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roach RC, Hackett PH, Oelz O, Bärtsch P, Luks AM, MacInnis MJ, et al. Lake Louise AMS Score Consensus Committee The 2018 Lake Louise Acute Mountain Sickness score. High Alt Med Biol . 2018;19:4–6. doi: 10.1089/ham.2017.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mann DL, Terrill PI, Azarbarzin A, Mariani S, Franciosini A, Camassa A, et al. Quantifying the magnitude of pharyngeal obstruction during sleep using airflow shape. Eur Respir J . 2019;54:1802262. doi: 10.1183/13993003.02262-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev . 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dempsey JA, Smith CA, Przybylowski T, Chenuel B, Xie A, Nakayama H, et al. The ventilatory responsiveness to CO2 below eupnoea as a determinant of ventilatory stability in sleep. J Physiol . 2004;560:1–11. doi: 10.1113/jphysiol.2004.072371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Graf LC, Furian M, Bitos K, Mademilov M, Abdraeva A, Buenzli J, et al. Effect of altitude and acetazolamide on sleep and nocturnal breathing in healthy lowlanders 40 y of age or older. Data from a randomized trial. Sleep (Basel) . 2022 doi: 10.1093/sleep/zsac269. [DOI] [PubMed] [Google Scholar]
- 29. Burgess KR, Johnson PL, Edwards N. Central and obstructive sleep apnoea during ascent to high altitude. Respirology . 2004;9:222–229. doi: 10.1111/j.1440-1843.2004.00576.x. [DOI] [PubMed] [Google Scholar]
- 30. Orr JE, Sands SA, Edwards BA, Deyoung PN, Deacon N, Jen R, et al. Measuring loop gain via home sleep testing in patients with obstructive sleep apnea. Am J Respir Crit Care Med . 2018;197:1353–1355. doi: 10.1164/rccm.201707-1357LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Edwards BA, Sands SA, Eckert DJ, White DP, Butler JP, Owens RL, et al. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol . 2012;590:1199–1211. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kohler M, Kriemler S, Wilhelm EM, Brunner-LaRocca H, Zehnder M, Bloch KE. Children at high altitude have less nocturnal periodic breathing than adults. Eur Respir J . 2008;32:189–197. doi: 10.1183/09031936.00119807. [DOI] [PubMed] [Google Scholar]
- 33. Torlasco C, Bilo G, Giuliano A, Soranna D, Ravaro S, Oliverio G, et al. Effects of acute exposure to moderate altitude on blood pressure and sleep breathing patterns. Int J Cardiol . 2020;301:173–179. doi: 10.1016/j.ijcard.2019.09.034. [DOI] [PubMed] [Google Scholar]
- 34. Anholm JD, Powles AC, Downey R, III, Houston CS, Sutton JR, Bonnet MH, et al. Operation Everest II: arterial oxygen saturation and sleep at extreme simulated altitude. Am Rev Respir Dis . 1992;145:817–826. doi: 10.1164/ajrccm/145.4_Pt_1.817. [DOI] [PubMed] [Google Scholar]
- 35. Nussbaumer-Ochsner Y, Ursprung J, Siebenmann C, Maggiorini M, Bloch KE. Effect of short-term acclimatization to high altitude on sleep and nocturnal breathing. Sleep (Basel) . 2012;35:419–423. doi: 10.5665/sleep.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Burgess KR, Lucas SJE, Shepherd K, Dawson A, Swart M, Thomas KN, et al. Worsening of central sleep apnea at high altitude—a role for cerebrovascular function. J Appl Physiol (1985) . 2013;114:1021–1028. doi: 10.1152/japplphysiol.01462.2012. [DOI] [PubMed] [Google Scholar]
- 37. Burgess KR, Cooper J, Rice A, Wong K, Kinsman T, Hahn A. Effect of simulated altitude during sleep on moderate-severity OSA. Respirology . 2006;11:62–69. doi: 10.1111/j.1440-1843.2006.00785.x. [DOI] [PubMed] [Google Scholar]
- 38. Behan M, Wenninger JM. Sex steroidal hormones and respiratory control. Respir Physiol Neurobiol . 2008;164:213–221. doi: 10.1016/j.resp.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bhaumik G, Sharma RP, Dass D, Lama H, Chauhan SK, Verma SS, et al. Hypoxic ventilatory response changes of men and women 6 to 7 days after climbing from 2100 m to 4350 m altitude and after descent. High Alt Med Biol . 2003;4:341–348. doi: 10.1089/152702903769192296. [DOI] [PubMed] [Google Scholar]
- 40. Burgess KR, Lucas SJE, Burgess KME, Sprecher KE, Donnelly J, Basnet AS, et al. Increasing cerebral blood flow reduces the severity of central sleep apnea at high altitude. J Appl Physiol (1985) . 2018;124:1341–1348. doi: 10.1152/japplphysiol.00799.2017. [DOI] [PubMed] [Google Scholar]
- 41. Janssens JV, Bell JR, Weeks KL, Mellor KM, Delbridge LMD. The big picture: cardiac sex-age interactions and proteogenomic insights. Am J Physiol Heart Circ Physiol . 2022;323:H640–H642. doi: 10.1152/ajpheart.00418.2022. [DOI] [PubMed] [Google Scholar]
- 42. Williams AM, Levine BD, Stembridge M. A change of heart: mechanisms of cardiac adaptation to acute and chronic hypoxia. J Physiol . 2022;600:4089–4104. doi: 10.1113/JP281724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Latshang TD, Lo Cascio CM, Stöwhas A-C, Grimm M, Stadelmann K, Tesler N, et al. Are nocturnal breathing, sleep, and cognitive performance impaired at moderate altitude (1,630-2,590 m)? Sleep (Basel) . 2013;36:1969–1976. doi: 10.5665/sleep.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]