Abstract
Rationale
Positive airway pressure (PAP) is the first-choice treatment for obstructive sleep apnea (OSA). However, its real-world effectiveness is often questioned because of usage issues. The relationship between patient sleepiness and PAP usage has been assessed in relatively small and selected populations within the research context.
Objectives
To assess the impact of patient-reported sleep outcomes, particularly self-reported sleepiness and its change during therapy, on PAP usage in the real-world setting.
Methods
Deidentified data for U.S.-based patients receiving PAP therapy were examined. Eligible patients were registered in the myAir app and provided self-reported sleepiness at baseline and after 7, 14, 21, and 28 days of PAP between November 2019 and April 2020.
Results
A total of 95,397 registered patients met all eligibility criteria and were included in the analysis (mean age, 49.6 ± 13.0 yr; 61.6% male). Daytime sleepiness was the most common reason for PAP therapy initiation (57.1% of patients), and 42.2% of all patients had self-reported moderate to severe OSA. Self-reported sleepiness improved with PAP therapy in most patients over the assessment period, with 62.1% of patients reporting “no” or “slight” sleepiness at Day 28. There was a dose-dependent association between improvement in self-reported sleepiness at Day 28 and PAP usage, and this finding was maintained at Day 360. Self-reported sleepiness at Day 28 was associated with achieving U.S. Centers for Medicare & Medicaid Services compliance at 90 days (approximately 90% for those with no or slight sleepiness vs. <70% for those with residual very or extreme sleepiness); average daily PAP usage over 360 days was ⩾5.0 and ⩽3.7 hours, respectively, for those with no or slight versus very or extreme sleepiness.
Conclusions
This study demonstrates the feasibility of capturing patient-reported outcomes via a digital platform. Patient-reported outcomes appear to be associated with PAP usage, especially self-reported sleepiness and its response to therapy. Capturing patient-reported outcomes using digital solutions during the course of treatment has the potential to enhance patient outcomes by providing actionable insights.
Keywords: obstructive sleep apnea, patient-reported outcomes, adherence, positive airway pressure therapy, sleepiness
Obstructive sleep apnea (OSA) is a common condition that is estimated to affect up to 1 billion people worldwide (1). OSA has important neurocognitive and cardiovascular sequelae that are at least partially ameliorated by therapeutic interventions (2–4). The gold standard treatment is positive airway pressure (PAP) therapy. However, achieving adequate adherence with PAP has been an ongoing challenge in the field. A systematic review published in 2016 demonstrated that PAP adherence in clinical trials did not increase over the preceding 20 years, despite continuous improvements in technical innovation and support (5).
We and others have retrospectively analyzed large-scale real-world data to investigate patterns of PAP usage in various sleep-related breathing disorders and geographic areas (6–11). In contrast to clinical trial data, we observed excellent adherence in patients initiating PAP therapy, with 75% of patients achieving the U.S. Centers for Medicare & Medicaid Services (CMS) compliance threshold in the first 90 days (7). A subsequent study across the United States, Mexico, and Brazil found that >80% of patients meeting CMS criteria at 3 months were still using PAP therapy at 1 year, with more than three-quarters of all users being adherent at the 1-year follow-up (8). PAP usage trajectories are heterogeneous, and early identification of subgroups requiring support is important (10).
There is an extensive body of literature describing factors that may influence PAP adherence, including OSA disease characteristics, patient characteristics, treatment protocols, and technological factors (11–14). These data have resulted in a suite of strategies for enhancing PAP adherence, including educational and supportive interventions, cognitive behavior therapy interventions, technological interventions, and telemedicine (15–20). A meta-analysis has shown that continuous PAP (CPAP) reduced the Epworth Sleepiness Scale (ESS) score by an average of 2.9 points more than did placebo (P < 0.001) in patients with OSA and that those with moderate to severe OSA had a greater reduction than those with mild OSA (21). Furthermore, the dose–response relationship between PAP therapy and daytime sleepiness has been examined in a clinical trial context, which found that although patients with fewer hours of PAP use still may benefit, the percentage of nights with device usage of ⩾4 hours per night provided the best discrimination for predicting ESS normalization (22). The failure of CPAP treatment to normalize ESS in all patients has also been noted (23). However, relatively little attention has been directed to the specific relationship of changes in symptoms during PAP therapy on adherence and device usage. Use of PAP has been associated with a patient’s perception of OSA symptoms, the risks of sleep-disordered breathing, and the perceived benefits of therapy (17). PAP usage may also relate to treatment outcome expectations, self-efficacy, and coping mechanisms (17).
Inclusion of patient-reported outcomes in clinical research and practice ensures that aspects of health status that are relevant to patients are evaluated (24). Daytime sleepiness is an important symptom in OSA that can negatively impact several aspects of patient well-being, including mood, daily functioning, and cognition (24). Sleepiness is therefore an important patient-reported outcome in this setting. The current study investigated the relationship of the patient-reported outcome of sleepiness and its change during therapy on compliance and adherence to treatment with PAP.
Methods
Database and Patients
Deidentified therapy data were acquired from the AirView database (ResMed Inc.) for wirelessly connected AirSense 10 platforms (CPAP and automatically titrating PAP modes). To be eligible for this analysis, patients needed to register in the myAir app and provide responses to a question relating to the amount of daytime sleepiness at baseline and at least one other time of the weekly question for 4 weeks via the myAir app between November 2019 and April 2020. All eligible patients were followed for 360 days, and nonuse of PAP or missing PAP data over this period was assumed to be zero usage. All data communication and storage were encrypted to meet required international privacy and security standards. The study was reviewed by an institutional review board and deemed exempt from institutional review board oversight.
Self-reported Sleepiness and Motivations for Treatment
At baseline and on Days 7, 14, 21, and 28 of therapy, patients were asked to rate their degree of daytime sleepiness on the following 5-point scale: 0 = not at all sleepy; 1 = slightly sleepy; 2 = moderately sleepy; 3 = very sleepy; and 4 = extremely sleepy. Also at baseline, patients were asked to provide their motivation(s) for treatment, which included “daytime sleepiness,” “ongoing health issue or risk,” “disturbing my partner’s sleep,” and “restless sleep.”
Outcomes
The primary outcome was the association between change in self-reported sleepiness over time and compliance at Day 90 and adherence at Day 360 to PAP therapy. An improvement in self-reported sleepiness was defined as a change to a less severe category of sleepiness. Compliance was defined using the CMS definition (⩾4 h PAP use on 70% of nights in a consecutive 30-day period in the first 90 days of therapy). Adherence was defined as continued use of PAP at Day 360 based on nontermination of therapy, where therapy termination was defined as 30 consecutive days of zero PAP usage.
Data Extraction and Analysis
Self-reported age, sex, reason(s) for PAP therapy, body mass index (BMI), and OSA severity were captured at baseline, and responses to the sleepiness question were also obtained from within the app. Device usage data for calculation of adherence rates were extracted from the AirView database. In addition, the proportion of patients who met CMS compliance criteria at 90 days was determined (i.e., the compliance rate).
Baseline data are reported using descriptive statistics (mean ± standard deviation and median [interquartile range (IQR)]), or as number of patients (percentage). Statistical tests were conducted to determine whether relationships between different variables were statistically significant. Analyses of variance tests were used to compare mean values for a numerical variable (e.g., age, BMI, etc.) among multiple groups. Wilcoxon signed-rank tests were used to compare the paired difference of numerical variables (e.g., sleep score at baseline vs. at Day 28) between the matched samples. Chi-square tests were used to study the independence between two categorical variables (e.g., sex vs. baseline sleepiness). Log-rank tests were specifically used in survival analysis to compare the survival distribution (e.g., long-term adherence distribution) between multiple groups. Statistical significance was defined as P < 0.05. Eta-squared, a measure of effect size, was used to compare the amount of variation explained by sleepiness at baseline and sleepiness at Day 28 on PAP usage at 90 and 360 days. Higher eta-squared values signify greater effect sizes, with the reported benchmarks of small (0.01), medium (0.06), and large (⩾0.14) (25).
Univariable and multivariable logistic regression models were constructed to estimate the crude and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) to evaluate associations between sleepiness improvement and usage of PAP therapy at 90 days and 360 days. Wald chi-square was used to test the relationship between combined sleepiness change and the status of compliance at Day 90 and adherence at Day 360 to PAP therapy. Confounding variables were determined a priori based on previous literature, including age, sex, residual apnea–hypopnea index (AHI) in the first 28 days, 95th percentile pressure, median mask leak, and motivation for treatment. We did not include BMI and daytime sleepiness as motivation for treatment as confounders in the models because they had a high value in the variance inflation factor test (indicating multicollinearity).
Because some patients reported their baseline sleepiness after the commencement of PAP, we undertook a sensitivity analysis in a subset of patients who reported baseline sleepiness ⩽1 day after commencement of PAP (n = 14,693).
Statistical analyses were performed using R (v3.4.1) and Rstudio (v1.0.153) located on a ResMed server in San Diego.
Results
Population
Of 174,614 adult patients registered in myAir with setup from November 1, 2019, to April 20, 2020, and using AirSense 10 devices, 95,397 met all eligibility criteria and were included in the current analysis (Figure 1). The study population had a mean age of 49.6 ± 13.0 years, nearly two-thirds were male, and most were overweight or obese based on the median BMI (33.0 kg/m2 [IQR, 28.8–38.6]) (Table 1). Self-reported median AHI was 21.0/h (IQR, 11.7–40.0), and the most common indication for PAP therapy was daytime sleepiness, followed by restless sleep (Table 1). There were no important clinical differences between the baseline demographics and clinical characteristics of the myAir patients who were excluded (n = 50,429) compared with those who were included (n = 95,397) (data not shown).
Figure 1.
Flow chart showing patient inclusion in the analysis. PAP = positive airway pressure.
Table 1.
Patient demographic and clinical characteristics at baseline
| Characteristic | Patients (N = 95,397) |
|---|---|
| Age, yr | |
| Mean ± SD | 49.6 ± 13.0 |
| Median (IQR) | 49.0 (40.0–59.0) |
| Male sex, n (%) | 58,805 (61.6) |
| Self-reported body mass index, kg/m2 | |
| Mean ± SD | 34.5 ± 9.2 |
| Median (IQR) | 33.0 (28.8–38.6) |
| Self-reported AHI, /h | |
| Mean ± SD | 30.1 ± 26.5 |
| Median (IQR) | 21.0 (11.7, 40.0) |
| OSA severity, n (%) | |
| AHI < 5/h | 21,035 (22) |
| Mild (AHI 5 to <15/h) | 22,827 (23.9) |
| Moderate (AHI 15 to <30/h) | 18,962 (19.9) |
| Severe (AHI ⩾ 30/h) | 23,236 (24.4) |
| Missing AHI values | 9,337 (9.8) |
| Reason for PAP therapy,* n (%) | |
| Daytime sleepiness | 54,452 (57.1) |
| Restless sleep | 38,307 (40.2) |
| Disturbing partner’s sleep | 30,788 (32.3) |
| Ongoing health issue | 33,993 (35.6) |
Definition of abbreviations: AHI = apnea–hypopnea index; IQR = interquartile range; OSA = obstructive sleep apnea; PAP = positive airway pressure; SD = standard deviation.
Patients could choose more than one reason for using PAP therapy.
Patients who reported higher amounts of sleepiness at baseline and at Day 28 were younger and more likely to be female, and those who reported extreme sleepiness at baseline had a higher self-reported AHI (see Table E1 in the data supplement). At baseline, less sleepy patients were more likely to report their motivation for using PAP as disturbing their partner or an ongoing health issue, whereas the most common reason for using PAP therapy for very or extremely sleepy patients was restless sleep (Table E1).
Therapy Characteristics
During PAP (CPAP or automatically titrating PAP) usage, the average median pressure over the first 90 days was 8.3 cm H2O (IQR, 6.9–9.9 cm H2O), and OSA was effectively treated (median average residual AHI, 1.7/h; IQR, 0.9–3.1/h) (Table 2). Just over 80% of the study population achieved CMS compliance at 90 days (Table 2). Values for all therapy parameters over the first 360 days were similar to those over the first 90 days, and median daily usage of the first 360 days was 5.7 hours (IQR, 4.0–7.0 h) (Table 2).
Table 2.
Positive airway pressure therapy characteristics
| Characteristic | Patients (N = 95,397) |
|---|---|
| Over the first 90 d | |
| Average median pressure, cm H2O | |
| Mean ± SD | 8.6 ± 2.2 |
| Median (IQR) | 8.3 (6.9–9.9) |
| Average 95th percentile pressure, cm H2O | |
| Mean ± SD | 11.2 ± 2.5 |
| Median (IQR) | 10.9 (9.4–12.7) |
| Average residual AHI, events/h | |
| Mean ± SD | 2.6 ± 3.1 |
| Median (IQR) | 1.7 (0.9–3.1) |
| Average median leak, L/min | |
| Mean ± SD | 2.7 ± 4.6 |
| Median (IQR) | 1.0 (0.2–3.3) |
| Average 95% percentile leak, L/min | |
| Mean ± SD | 13.0 ± 11.0 |
| Median (IQR) | 10.4 (5.2–17.7) |
| Average daily usage, h | |
| Mean ± SD | 5.2 ± 2.3 |
| Median (IQR) | 5.7 (3.8–7.0) |
| Achieved CMS compliance, n (%) | 11,780 (80.2) |
| Over the first 360 d | |
| Average median pressure, cm H2O | |
| Mean ± SD | 8.5 ± 2.2 |
| Median (IQR) | 8.2 (6.8–9.8) |
| Average 95th percentile pressure, cm H2O | |
| Mean ± SD | 10.9 ± 2.4 |
| Median (IQR) | 10.8 (9.2–12.4) |
| Average residual AHI, events/h | |
| Mean ± SD | 2.2 ± 2.7 |
| Median (IQR) | 1.4 (0.8–2.6) |
| Average median leak, L/min | |
| Mean ± SD | 3.0 ± 5.0 |
| Median (IQR) | 1.2 (0.2–3.6) |
| Average 95% percentile leak, L/min | |
| Mean ± SD | 13.9 ± 11.6 |
| Median (IQR) | 11.2 (5.7–19.1) |
| Average daily usage, h | |
| Mean ± SD | 5.3 ± 2.2 |
| Median (IQR) | 5.7 (4.0–7.0) |
Definition of abbreviations: AHI = apnea–hypopnea index; CMS = Centers for Medicare & Medicaid Services; IQR = interquartile range; OSA = obstructive sleep apnea; PAP = positive airway pressure; SD = standard deviation.
Average daily usage is based on all days of the specified timeframe.
Sleepiness
Median (IQR) sleepiness score at baseline was 2.0 (1.0–3.0), and this decreased to 1.0 (1.0–2.0) at 28 days (P < 0.001). At Day 28, the sleepiness score had improved by a median of 1.0 points (IQR, 0.0–1.0) in those with moderate sleepiness at baseline, and by 2.0 (1.0–2.0) and 2.0 (1.0–3.0) points, respectively, in those who were very or extremely sleepy at baseline.
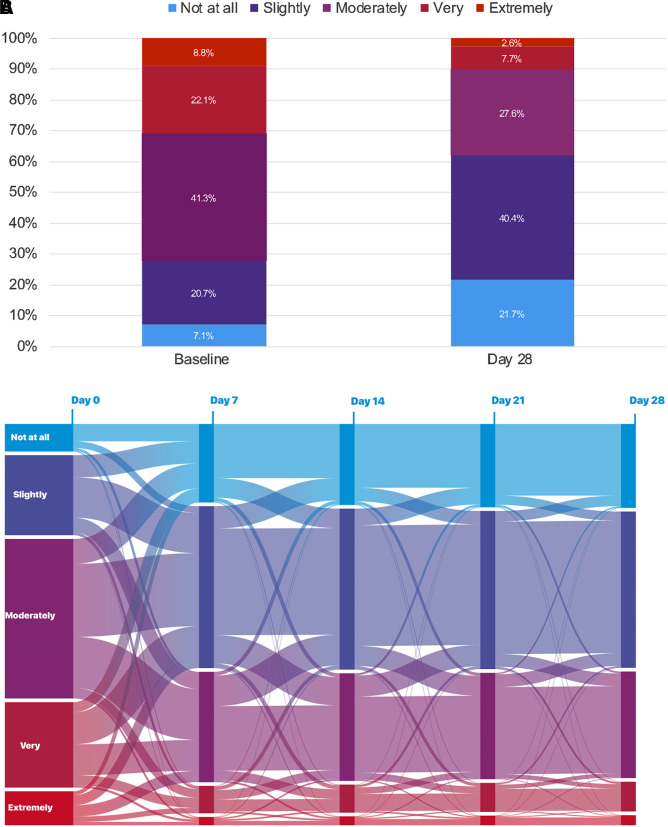
The proportion of patients with no self-reported sleepiness increased from 7.1% at baseline to 21.7% at Day 28, and the proportion of patients who said they felt only slightly sleepy also increased (Figure 2A). There were corresponding decreases from baseline to Day 28 in the proportions of patients who were moderately, very, or extremely sleepy (Figure 2A). Individual trajectories in self-reported sleepiness are visualized in a Sankey chart showing there are high amounts of both intra- and interindividual variability, as well as clusters based on the size of the bands across the follow-ups over the 28 days (Figure 2B). For example, among patients with moderate sleepiness at baseline, considerable variability is noted by Day 7, although the largest clusters were in the direction of improved sleepiness (slightly sleepy or no change). Less variability is evident after the first 7 days across all sleepiness categories.
Figure 2.
(A) Proportion of patients with different amounts of self-reported sleepiness at baseline and 28 days, and (B) Sankey chart showing individual patient data for change in self-reported sleepiness over time.
There was no overall change in sleepiness score in patients who were not sleepy or only slightly sleepy at baseline, and these patients generally remained not at all or slightly sleepy at Day 28 (Table E2). For those who were extremely sleepy at baseline, three-quarters (75.0%) improved to a moderate amount of sleepiness or better by Day 28 (Table E2). For each baseline sleepiness amount, PAP usage was associated with a dose-dependent increase in the proportion of patients with improvement in sleepiness at Day 28 (Figure 3).
Figure 3.
Association between positive airway pressure usage and self-reported sleepiness improvement in patients reporting sleepiness at baseline.
Multivariate analysis identified that older age (⩾43 vs. <43 yr), male sex, higher 95th percentile pressure (⩾8 vs. <8 cm H2O), disturbing partner’s sleep, and being moderately to extremely sleepy at baseline may be significant predictors of improvement in self-reported sleepiness during PAP therapy (Figure E1).
PAP Usage
Self-reported sleepiness at Day 28 was more closely associated with compliance with PAP at Day 90 and adherence to PAP at Day 360 than baseline sleepiness (Table 3). Looking at daily PAP usage over 90 days, eta-squared values (effect size) for self-reported sleepiness at baseline and Day 28 were 0.0027 (95% CI, 0.0021–0.0034) and 0.0573 (95% CI, 0.0545–0.0601), respectively. When PAP usage was evaluated over 360 days, eta-squared values for the effect size relating to self-reported sleepiness at baseline and Day 28 were 0.004 (95% CI, 0.0037–0.0053) and 0.0533 (95% CI, 0.0506–0.0563), respectively. These results suggest that sleepiness at Day 28 explains a greater amount of variation in daily PAP use than self-reported sleepiness at baseline (Figure E2).
Table 3.
Relationship of self-reported sleepiness and change at Day 28 on Centers for Medicare & Medicaid Services 90-day compliance and adherence to PAP at 360 days
| Patients, n (total %) | CMS Compliance at 90 d, n (line %) | Average Daily Usage over 90 d (h) | Adherence Rate at Day 360, (line %) | Average Daily Usage over 360 d, h | |
|---|---|---|---|---|---|
| Self-reported sleepiness at baseline | |||||
| Not at all | 6,785 (7.1) | 5,932 (87.4) | 5.6 ± 2.1 | 72.5 | 5.1 ± 2.5 |
| Slightly | 19,762 (20.7) | 16,889 (85.5) | 5.5 ± 2.1 | 70.9 | 4.9 ± 2.5 |
| Moderately | 39,400 (41.3) | 32,594 (82.7) | 5.3 ± 2.2 | 66.8 | 4.7 ± 2.6 |
| Very | 21,088 (22.1) | 17,072 (81.0) | 5.3 ± 2.2 | 64.3 | 4.6 ± 2.6 |
| Extremely | 8,362 (8.8) | 6,531 (78.1) | 5.1 ± 2.3 | 61.1 | 4.5 ± 2.7 |
| P value | <0.001* | <0.001* | <0.001† | <0.001‡ | <0.001† |
| Self-reported sleepiness at Day 28 | |||||
| Not at all | 20,710 (21.7) | 18,757 (90.6) | 5.9 ± 1.9 | 76.8 | 5.4 ± 2.3 |
| Slightly | 38,586 (40.4) | 33,553 (87.0) | 5.6 ± 2.0 | 71.6 | 5.0 ± 2.4 |
| Moderately | 26,301 (27.6) | 20,233 (76.9) | 4.9 ± 2.2 | 59.7 | 4.2 ± 2.6 |
| Very | 7,342 (7.7) | 5,047 (68.7) | 4.5 ± 2.4 | 50.3 | 3.7 ± 2.7 |
| Extremely | 2,458 (2.6) | 1,428 (58.1) | 3.8 ± 2.5 | 40.3 | 3.1 ± 2.7 |
| P value | <0.001* | <0.001* | <0.001† | <0.001‡ | <0.001† |
| Change in sleepiness at Day 28 | |||||
| Worse | 11,551 (12.1) | 8,674 (75.1) | 4.8 ± 2.3 | 58.80 | 4.2 ± 2.7 |
| No change§ | 24,678 (25.9) | 19,301 (78.2) | 5.0 ± 2.2 | 61.70 | 4.4 ± 2.6 |
| 1 level of Improvement | 30,965 (32.5) | 26,236 (84.7) | 5.5 ± 2.1 | 68.90 | 4.9 ± 2.5 |
| 2 levels of Improvement | 17,706 (18.6) | 15,449 (87.3) | 5.7 ± 2.0 | 71.90 | 5.1 ± 2.5 |
| 3 levels of Improvement | 5,389 (5.6) | 4,794 (89.0) | 5.9 ± 2.0 | 74.50 | 5.3 ± 2.4 |
| 4 levels of Improvement | 1,160 (1.2) | 1,066 (91.9) | 6.1 ± 1.8 | 79.60 | 5.7 ± 2.3 |
| P value | <0.001* | <0.001† | <0.001‡ | <0.001† |
Definition of abbreviation: CMS = Centers for Medicare & Medicaid Services.
Average daily usage is based on all days of the specified timeframe.
Chi-square test.
Analysis of variance test.
Log-rank test.
Excludes patients whose sleepiness change score is 0 with baseline of “not at all sleepy” (n = 3,948).
Regarding qualitative changes in sleepiness, the adjusted models (Table 4) demonstrated that those who were less sleepy over the 28 days had higher odds of being CMS compliant with PAP at 90 days (OR, 1.76; 95% CI, 1.73–1.80) as well as adherent to PAP at 360 days (OR, 1.51; 95% CI, 1.49–1.54), compared with patients who had no change in their sleepiness. Conversely, those who were more sleepy over 28 days had lower odds of being CMS compliant with PAP at 90 days (OR, 0.81; 95% CI, 0.78–0.83) as well as adherent to PAP at 360 days (OR, 0.87; 95% CI, 0.85–0.89) than those whose sleepiness did not change over time.
Table 4.
Change in sleepiness over 28 days versus compliance with positive airway pressure at 90 days and adherence to positive airway pressure at 360 days
| Sleepiness at Day 28 | No Change | Less Sleepy | More Sleepy | P for Trend |
|---|---|---|---|---|
| Patients, n* | 24,084 | 54,157 | 11,235 | |
| Outcome 1: PAP compliance at 90 d | ||||
| No. events | 18,925 | 46,757 | 8,488 | |
| Crude OR (95% CI) | 1 | 1.72 (1.69–1.76) | 0.84 (0.82–0.87) | <0.001 |
| Adjusted OR (95% CI) | 1 | 1.76 (1.73–1.8) | 0.81 (0.78–0.83) | <0.001 |
| Outcome 2: PAP adherence at 360 d | ||||
| No. events | 14,988 | 38,473 | 6,669 | |
| Crude OR (95% CI) | 1 | 1.49 (1.46–1.51) | 0.89 (0.87–0.91) | <0.001 |
| Adjusted OR (95% CI) | 1 | 1.51 (1.49–1.54) | 0.87 (0.85–0.89) | <0.001 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio; PAP = positive airway pressure.
Excludes patients whose sleepiness change score is 0 with baseline of “not at all sleepy” and patients who had missing data in any of the modeling variables (dependent and independent variables).
In addition, older age, male sex, higher 95th percentile pressure, and disturbing partner’s sleep were positively associated with higher odds of CMS compliance with PAP at 90 days and adherence to PAP at 360 days (Figures E3A and E3B, respectively). Those with a higher number of residual AHI events, greater mask leak, having restless sleep, or ongoing health risk as a motivation for using PAP were less likely to achieve CMS compliance at 90 days and more likely to be nonadherent to PAP at 360 days (Figures E3A and E3B, respectively).
Regarding quantitative changes in sleepiness, as shown in Table 5, in adjusted models with patients with no change as the reference group, as the amount of sleepiness score change increased, there were higher odds of being CMS compliant with PAP at 90 days and adherent to PAP at 360 days (P for trend <0.001 for both outcomes).
Table 5.
Change in sleepiness improvement score over 28 days versus compliance to positive airway pressure at 90 days and adherence to positive airway pressure at 360 days
| Sleepiness Score Change |
P for Trend | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| −4 | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | ||
| Patients, n* | 107 | 428 | 2,131 | 8,569 | 24,084 | 30,356 | 17,385 | 5,279 | 1,137 | |
| Outcome 1: PAP compliance at 90 d | ||||||||||
| No. events | 85 | 340 | 1,559 | 6,504 | 18,925 | 25,802 | 15,207 | 4,703 | 1,045 | |
| Crude OR (95% CI) | 1.05 (0.83–1.34) | 1.05 (0.93–1.19) | 0.74 (0.71–0.78) | 0.86 (0.83–0.88) | 1 | 1.54 (1.51–1.58) | 1.9 (1.85–1.96) | 2.23 (2.12–2.33) | 3.1 (2.77–3.46) | <0.001 |
| Adjusted OR (95% CI) | 1.2 (0.93–1.54) | 0.99 (0.88–1.12) | 0.71 (0.68–0.75) | 0.82 (0.79–0.85) | 1 | 1.55 (1.52–1.59) | 1.99 (1.94–2.05) | 2.41 (2.29–2.53) | 3.18 (2.85–3.56) | <0.001 |
| Outcome 2: PAP adherence at 360 d | ||||||||||
| No. events | 69 | 257 | 1,243 | 5,100 | 14,988 | 21,048 | 12,566 | 3,951 | 908 | |
| Crude OR (95% CI) | 1.1 (0.9–1.35) | 0.91 (0.83–1.01) | 0.85 (0.81–0.89) | 0.89 (0.87–0.92) | 1 | 1.37 (1.35–1.4) | 1.58 (1.55–1.62) | 1.81 (1.74–1.87) | 2.41 (2.23–2.59) | <0.001 |
| Adjusted OR (95% CI) | 1.21 (0.98–1.49) | 0.9 (0.81–1) | 0.85 (0.81–0.89) | 0.87 (0.85–0.9) | 1 | 1.37 (1.35–1.4) | 1.64 (1.6–1.67) | 1.93 (1.86–2) | 2.43 (2.25–2.62) | <0.001 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio; PAP = positive airway pressure.
Excludes patients whose sleepiness change score is 0 with baseline of “not at all sleepy” and patients who had missing data in any of the modeling variables (dependent and independent variables).
Analysis of the subset of patients (n = 14,693) who reported their baseline data ⩽1 day after commencement of PAP showed there were not significant differences in the overall findings (Table E3).
Discussion
This large-scale, real-world study showed that the change in self-reported sleepiness was associated with short- and long-term usage of PAP therapy. Specifically, perceived sleepiness and its change over the first 28 days of therapy appeared to have an important relationship with PAP usage over the short (28 d) and intermediate (90 d) terms, and this finding continued out to 1 year of follow-up (360 d). The results demonstrated that self-reported improvement in sleepiness may be a better predictor of ongoing PAP adherence than the baseline sleepiness. Our findings suggest that monitoring symptom change (or a lack thereof) may be clinically useful for identifying patients who require augmented management to further optimize treatment.
Subjective daytime sleepiness has been reported to be a significant predictor of long-term adherence to CPAP therapy in a retrospective study of patients with OSA (26). In contrast, an older study from the United Kingdom found that daytime sleepiness (determined using the ESS) was not significantly associated with continued use of CPAP, whereas the severity of OSA (based on the oxygen desaturation index) was significantly associated with long-term CPAP adherence within a multivariate analysis (27). The uniqueness of our findings was to suggest that the perceived change in sleepiness in the first 28 days after starting PAP might provide better insights about which patients will continue to use PAP therapy than a single baseline measure of sleepiness. For each amount of baseline sleepiness, the less sleepy patients felt at Day 28, the more compliant and adherent they were at Day 90 and Day 360, respectively, although in this type of study, it is not possible to disentangle the causality of relationships. Also, the greater the amount of improvement in sleepiness by Day 28, the more compliant and adherent they were at Day 90 and 360, respectively. Perhaps these results reflect the importance of sleepiness as a patient-reported outcome, such that improvements in sleepiness during PAP therapy provide positive reinforcement to adhere to treatment on an ongoing basis. The association between improvement in sleepiness during PAP therapy and ongoing adherence seen in the current study could be one explanation for the low adherence reported in clinical trials of PAP modalities in randomized clinical trials of PAP in nonsleepy patients with sleep-disordered breathing (28–30), who would have no subjective improvement in sleepiness to motivate them to continue adhering to PAP therapy.
Our study replicated the previously reported dose–response relationship between the number of hours of PAP usage and sleepiness improvement (22, 31). Our study extends this observation by noting that even the group that reported being only slightly sleepy at baseline showed an increase in the proportion of patients with sleepiness improvement, despite overall being low compared with the other groups, when PAP usage was ⩾4 h/night (Figure 3). These findings are consistent with data showing that self-reported sleepiness in patients with OSA is normalized by CPAP usage of at least 4–5 h/night (22, 23, 31). It is noteworthy that in the groups with more severe self-reported sleepiness at baseline, even lower PAP usage (⩾2 h/night) was associated with sleepiness improvement. Thus, any use of PAP is likely to be better than no use, but improvements in symptoms and clinical outcomes are likely to be greater with longer nightly durations of PAP therapy usage (32).
The end of the recruitment period for this study coincided with the start of the coronavirus disease (COVID-19)-related lockdowns. To check whether this issue had any meaningful impact on the study findings, a post hoc analysis was conducted to compare demographic characteristics and PAP usage between patients who started PAP and registered in myAir in April 2020 and those who did so before April 2020. This analysis did not show any statistically significant differences between the cohorts in terms of patient characteristics, PAP usage, self-reported sleepiness at baseline and Day 28, and improvement in sleepiness during PAP therapy. Therefore, the early part of lockdowns and restrictions did not appear to influence the study results.
Strengths and Limitations
Our study had a number of strengths, including the analysis of data from a large number of patients treated in a real-world setting and the objective measures of PAP adherence based on device metrics. However, several limitations also need to be taken into account when interpreting the current findings. First, the source of data means that the number of parameters with available information is small and was limited to machine-recorded data and a defined set of patient self-reported information (e.g., diagnostic AHI, BMI, motivation for PAP usage) captured via a digital platform. Another important limitation is the possible influence of known and unknown biases. The sample analyzed represented 55% of the total number of patients initiating therapy in the myAir system over the recruitment period. Those who chose to enroll with myAir and appropriately followed instructions to complete self-reported sleepiness questions may have been a group of more highly motivated, digitally able, and higher socioeconomic status individuals. Thus, there is the possibility of underrepresentation of low-income and minoritized populations. The possibility that these characteristics might influence adherence cannot be excluded and may limit the generalizability to these groups. A subset of patients registered for myAir after starting PAP therapy, and this could have influenced their baseline reporting of sleepiness. However, a sensitivity analysis in the subgroup of patients who registered in myAir within a day of commencing therapy (n = 14,693) showed no difference in outcomes (Table E3). Sleepiness was not objectively assessed in the current study and was instead reported by patients using a nonvalidated measure, so the minimal clinically important difference is not known. Nevertheless, the results of overall residual sleepiness in this study align reasonably well with other publications in which sleepiness was determined using a validated instrument, such as the ESS. In our study, the proportions of patients reporting that they were very or extremely sleepy at baseline and Day 28 were 30.9% and 10.3%, respectively. In a study by Gasa and colleagues, sleepiness was objectively rated using the ESS; 58% of patients were sleepy at baseline and 13% were sleepy at follow-up (33). This finding suggests that the self-reported sleepiness measure used in the current study adequately captured the patient experience. Furthermore, it is important to apply simple and convenient measures in the real world to engage patients with the app. Another limitation is that we cannot assess the specificity of the observed associations regarding sleepiness, as opposed to other patient-perceived benefits of treatment, as we did not have additional patient-reported outcomes to assess. Future studies should include additional patient-reported outcomes. Finally, it is important to note that the effects of improvement in sleepiness observed in this study are additional to those that might have occurred because of the use of the myAir app, which has been shown to improve adherence in previous studies (34, 35).
Conclusions
The results of this study highlight the importance of patient-reported outcomes as contributors to ongoing adherence to PAP therapy. Although baseline measurements alone are helpful, it is the tracking of changes in parameters such as sleepiness on PAP treatment that may provide greater predictive information about long-term adherence and can be actioned by care providers to optimize treatment. Digital technology is an efficient and cost-effective method to gather such information, and the insights derived from these data are likely to lead to further advances in PAP management of OSA.
Acknowledgments
Acknowledgment
Medical writing assistance was provided by Nicola Ryan, independent medical writer, funded by ResMed.
Footnotes
Supported by ResMed and by MIAI Grenoble Alpes grant ANR-19-P3IA-0003 (J.-L.P.). Representatives of ResMed were involved in the design of the study, study procedures, and analyses.
Author Contributions: Conception and design: P.A.C. and A.V.B. Analysis: Y.Y., V.V., and M.A.B. Interpretation: P.A.C., J.P.A., A.M., K.L.S., C.M.N., J.-L.P., and A.V.B. Drafting the first version of the manuscript: P.A.C. Review, editing, and approval of the manuscript: all authors. All authors made the decision to submit the manuscript for publication and assume responsibility for the accuracy and completeness of the analyses and for the fidelity of this report.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med . 2019;7:687–698. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet . 1999;353:2100–2105. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- 3. Labarca G, Saavedra D, Dreyse J, Jorquera J, Barbe F. Efficacy of CPAP for improvements in sleepiness, cognition, mood, and quality of life in elderly patients with OSA: systematic review and meta-analysis of randomized controlled trials. Chest . 2020;158:751–764. doi: 10.1016/j.chest.2020.03.049. [DOI] [PubMed] [Google Scholar]
- 4. Labarca G, Schmidt A, Dreyse J, Jorquera J, Enos D, Torres G, et al. Efficacy of continuous positive airway pressure (CPAP) in patients with obstructive sleep apnea (OSA) and resistant hypertension (RH): systematic review and meta-analysis. Sleep Med Rev . 2021;58:101446. doi: 10.1016/j.smrv.2021.101446. [DOI] [PubMed] [Google Scholar]
- 5. Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg . 2016;45:43. doi: 10.1186/s40463-016-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benjafield A, Liu D, Shi H, Woehrle H, Cistulli P, Armitstead J, et al. A big data analysis of ASV therapy pressures [abstract] Eur Respir J . 2018;52:PA2260. [Google Scholar]
- 7. Cistulli PA, Armitstead J, Pepin JL, Woehrle H, Nunez CM, Benjafield A, et al. Short-term CPAP adherence in obstructive sleep apnea: a big data analysis using real world data. Sleep Med . 2019;59:114–116. doi: 10.1016/j.sleep.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drager LF, Malhotra A, Yan Y, Pépin JL, Armitstead JP, Woehrle H, et al. medXcloud group Adherence with positive airway pressure therapy for obstructive sleep apnea in developing vs. developed countries: a big data study. J Clin Sleep Med . 2021;17:703–709. doi: 10.5664/jcsm.9008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pépin J-L, Woehlre H, Liu D, Armitstea J, Cistulli P, Benjafield A, et al. Compliance after switching from CPAP to ASV: big data analysis. ERJ Open Res . 2017;3:45. [Google Scholar]
- 10. Pépin JL, Bailly S, Rinder P, Adler D, Szeftel D, Malhotra A, et al. medXcloud Group CPAP therapy termination rates by OSA phenotype: a French nationwide database analysis. J Clin Med . 2021;10:936. doi: 10.3390/jcm10050936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Woehrle H, Arzt M, Graml A, Fietze I, Young P, Teschler H, et al. Predictors of positive airway pressure therapy termination in the first year: analysis of big data from a German homecare provider. BMC Pulm Med . 2018;18:186. doi: 10.1186/s12890-018-0748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Catcheside PG. Predictors of continuous positive airway pressure adherence. F1000 Med Rep . 2010;2:70. doi: 10.3410/M2-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mehrtash M, Bakker JP, Ayas N. Predictors of continuous positive airway pressure adherence in patients with obstructive sleep apnea. Lung . 2019;197:115–121. doi: 10.1007/s00408-018-00193-1. [DOI] [PubMed] [Google Scholar]
- 14. Shapiro GK, Shapiro CM. Factors that influence CPAP adherence: an overview. Sleep Breath . 2010;14:323–335. doi: 10.1007/s11325-010-0391-y. [DOI] [PubMed] [Google Scholar]
- 15. Hwang D, Chang JW, Benjafield AV, Crocker ME, Kelly C, Becker KA, et al. Effect of telemedicine education and telemonitoring on continuous positive airway pressure adherence: the Tele-OSA randomized trial. Am J Respir Crit Care Med . 2018;197:117–126. doi: 10.1164/rccm.201703-0582OC. [DOI] [PubMed] [Google Scholar]
- 16. Pestak R, Tyler C. What intervention is best for improving adherence to positive airway pressure devices for treatment of obstructive sleep apnea? Evidence-based Practice . 2018;21:64–65. [Google Scholar]
- 17. Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev . 2011;15:343–356. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sunwoo BY, Light M, Malhotra A. Strategies to augment adherence in the management of sleep-disordered breathing. Respirology . 2020;25:363–371. doi: 10.1111/resp.13589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weaver TE. Novel aspects of CPAP treatment and interventions to improve CPAP adherence. J Clin Med . 2019;8:2220. doi: 10.3390/jcm8122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Woehrle H, Ficker JH, Graml A, Fietze I, Young P, Teschler H, et al. Telemedicine-based proactive patient management during positive airway pressure therapy: impact on therapy termination rate. Somnologie (Berl) . 2017;21:121–127. doi: 10.1007/s11818-016-0098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med . 2003;163:565–571. doi: 10.1001/archinte.163.5.565. [DOI] [PubMed] [Google Scholar]
- 22. Pascoe M, Bena J, Andrews ND, Auckley D, Benca R, Billings ME, et al. Dose-response relationship between positive airway pressure therapy and excessive daytime sleepiness: the HomePAP study. J Clin Sleep Med . 2022;18:1027–1034. doi: 10.5664/jcsm.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Antic NA, Catcheside P, Buchan C, Hensley M, Naughton MT, Rowland S, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep (Basel) . 2011;34:111–119. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abma IL, van der Wees PJ, Veer V, Westert GP, Rovers M. Measurement properties of patient-reported outcome measures (PROMs) in adults with obstructive sleep apnea (OSA): a systematic review. Sleep Med Rev . 2016;28:18–31. doi: 10.1016/j.smrv.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 25. Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol . 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacobsen AR, Eriksen F, Hansen RW, Erlandsen M, Thorup L, Damgård MB, et al. Determinants for adherence to continuous positive airway pressure therapy in obstructive sleep apnea. PLoS One . 2017;12:e0189614. doi: 10.1371/journal.pone.0189614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kohler M, Smith D, Tippett V, Stradling JR. Predictors of long-term compliance with continuous positive airway pressure. Thorax . 2010;65:829–832. doi: 10.1136/thx.2010.135848. [DOI] [PubMed] [Google Scholar]
- 28. Cowie MR, Woehrle H, Wegscheider K, Angermann C, d’Ortho MP, Erdmann E, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med . 2015;373:1095–1105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. SAVE Investigators and Coordinators CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med . 2016;375:919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 30. Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunström E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea: the RICCADSA randomized controlled trial. Am J Respir Crit Care Med . 2016;194:613–620. doi: 10.1164/rccm.201601-0088OC. [DOI] [PubMed] [Google Scholar]
- 31. Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep . 2007;30:711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc . 2008;5:173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gasa M, Tamisier R, Launois SH, Sapene M, Martin F, Stach B, et al. Scientific Council of the Sleep Registry of the French Federation of Pneumology-FFP Residual sleepiness in sleep apnea patients treated by continuous positive airway pressure. J Sleep Res . 2013;22:389–397. doi: 10.1111/jsr.12039. [DOI] [PubMed] [Google Scholar]
- 34. Malhotra A, Crocker ME, Willes L, Kelly C, Lynch S, Benjafield AV. Patient engagement using new technology to improve adherence to positive airway pressure therapy: a retrospective analysis. Chest . 2018;153:843–850. doi: 10.1016/j.chest.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Woehrle H, Arzt M, Graml A, Fietze I, Young P, Teschler H, et al. Effect of a patient engagement tool on positive airway pressure adherence: analysis of a German healthcare provider database. Sleep Med . 2018;41:20–26. doi: 10.1016/j.sleep.2017.07.026. [DOI] [PubMed] [Google Scholar]