Abstract
Rationale
In numerous cohorts, lung function decline is associated with all-cause and cardiovascular-cause mortality, but the association between the decrease in forced expiratory volume in 1 second (FEV1) and cancer-cause mortality, particularly after occupational/environmental exposure(s), is unclear. Exposure to dust/smoke from the World Trade Center (WTC) disaster caused inflammation and lung injury in Fire Department of the City of New York rescue/recovery workers. In addition, prior research found that >10% of the cohort experienced greater than twice the age-related decrease in FEV1 (⩾64 ml/yr).
Objectives
To evaluate the association of longitudinal lung function with all-cause and cancer-cause mortality after exposure to the WTC disaster.
Methods
We conducted a prospective cohort study using longitudinal prebronchodilator FEV1 data for 12,264 WTC-exposed firefighters and emergency medical service providers. All-cause and cancer-cause mortality were ascertained using National Death Index data from September 12, 2001, through December 31, 2021. Joint longitudinal survival models evaluated the association of baseline FEV1 and change in FEV1 from baseline with all-cause and cancer-cause mortality adjusted for age, race/ethnicity, height, smoking, work assignment (firefighters vs. emergency medical service providers), and WTC exposure.
Results
By December 31, 2021, 607 of the 12,264 individuals in the cohort (4.9%) had died (crude rate = 259.5 per 100,000 person-years), and 190 of 12,264 (1.5%) had died from cancer (crude rate = 81.2 per 100,000 person-years). Baseline FEV1 was ⩾80% predicted in 10,970 of the 12,264 (89.4%); final FEV1 was ⩾80% in 9,996 (81.5%). Lower FEV1 at baseline was associated with greater risk for all-cause mortality (hazard ratio [HR] per liter = 2.32; 95% confidence interval [95% CI] = 1.98–2.72) and cancer-cause mortality (HR per liter = 1.99; 95% CI = 1.49–2.66). Longitudinally, each 100-ml/yr decrease in FEV1 was associated with an 11% increase in all-cause mortality (HR = 1.11; 95% CI = 1.06–1.15) and a 7% increase in cancer-cause mortality (HR = 1.07; 95% CI = 1.00–1.15). Compared with FEV1 decrease <64 ml/yr, those with FEV1 decrease ⩾64 ml/yr had higher all-cause (HR = 2.91; 95% CI = 2.37–3.56) and cancer-cause mortality (HR = 2.68; 95% CI = 1.90–3.79).
Conclusions
Baseline FEV1 and longitudinal FEV1 decrease are associated with increased risk of all-cause and cancer-cause mortality in a previously healthy occupational cohort, the majority of whom had normal lung function, after intense exposure to dust/smoke. Further investigation is needed to define pathways by which lung function impacts mortality after an irritant exposure.
Keywords: Lung function decline, cancer mortality, all-cause mortality, WTC exposure
World Trade Center (WTC) exposure was followed by lung injury with an immediate decrease in forced expiratory volume in 1 second (FEV1) among rescue/recovery workers (1). Lung function stabilized for most, but some exposed workers continued to experience FEV1 decrease ⩾64 ml/yr (i.e., accelerated FEV1 decrease), double the expected age-related decrease of 32 ml/yr (2). Postexposure accelerated FEV1 decrease is associated with chronic inflammation, and, in other studies, increased the hazard of incident chronic obstructive pulmonary disease (COPD) and asthma (3). Cancer incidence is increased in WTC-exposed cohorts, but cancer-cause mortality is reduced compared with the general population (4, 5), possibly related to aggressive case ascertainment, close case management during treatment, and the “healthy worker” effect. The association of baseline lung function and change in lung function over time with all-cause and cause-specific mortality in WTC cohorts has not been defined.
Increased all-cause mortality among those with impaired lung function and accelerated lung function decline has been found in the general population and other occupational cohorts (6–12). Sircar and colleagues found a dose–response trend when evaluating the association between FEV1 decrease and mortality among coal miners using selected cutoff points of <30 ml/yr, 30–90 ml/yr, and >90 ml/yr (13). Marott and colleagues observed that individuals in whom COPD developed later in adulthood had a greater risk of mortality than those in whom COPD never developed or those in whom COPD developed in early adulthood (8). In other studies, FEV1 decrease was associated with lung cancer mortality, but the association between lung function decline and mortality from other cancers is unclear (7, 14).
The Fire Department of the City of New York (FDNY) WTC Health Program has collected extensive longitudinal data during the two decades following the terrorist attack and building collapse on September 11, 2001, that have been an invaluable resource in the study of latent health effects of exposure to WTC dust and smoke such as pulmonary and cardiovascular diseases and cancer (15, 16). Few studies, if any, have explored the association between lung function and mortality in a cohort with normal baseline lung function, and none have examined this association in a WTC-exposed cohort. The primary objective of this study was to evaluate the association between lung function at baseline and changes over time using longitudinal measurements, with all-cause and cancer-cause mortality as outcomes in an occupational cohort with normal baseline lung function and WTC exposure: an exposure with a well-established association to lung injury (17). Importantly, the extensive longitudinal data available on this cohort were needed to investigate whether baseline FEV1, changes in FEV1 over time, or both are independently associated with all-cause and cause-specific mortality.
Methods
Study Population
The source population included firefighters and emergency medical service (EMS) providers who were actively employed by the FDNY as of September 11, 2001, and responded to the WTC site between September 11, 2001, and July 24, 2002. Ethnoracial groups other than White, Black, and Hispanic were excluded from analyses because of small numbers (n = 77 participants). Participants without pulmonary function testing (PFT) were excluded (n = 38). All participants provided informed written consent. The final study population included 12,264 participants (Figure E1 in the data supplement). The Montefiore Medical Center/Albert Einstein College of Medicine Institutional Review Board approved this study, which followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guideline.
PFT: Spirometry
Participants had longitudinal medical monitoring including prebronchodilator spirometry to assess lung function. The FDNY protocol is described elsewhere (1, 18); briefly, calibration techniques were consistent throughout follow-up (19), and only high-quality grades of “A” and “B” were included in the present evaluation. FEV1 was used for all analyses because of its reproducibility and because it is most commonly used in longitudinal analyses (20). We included only the latter of two examinations separated by <9 months to reduce the potentially exaggerated influence on FEV1 decrease of examinations performed in rapid succession. PFTs that met the above criteria and were conducted between September 11, 2001, and December 31, 2021 (end of the study), were included in the study. Percent predicted normative values for FEV1 were calculated based on race-, sex-, and age-based prediction equations from the Third National Health and Nutrition Examination Survey (21).
Mortality Ascertainment
Dates and causes of death were ascertained via linkages to the National Death Index through December 31, 2021. Causes of death from the International Classification of Diseases, 10th Revision were classified in conformity with the National Institute of Occupational Safety and Health (NIOSH) major death categories (22, 23). Outcomes for primary analyses were were all-cause mortality and mortality from cancer (“cancer-cause mortality,” NIOSH categories 2–10). Additionally, deaths from diseases of the heart (NIOSH category 16) and from respiratory-system diseases (NIOSH category 18) were analyzed.
Other Cohort Characteristics
Demographic data including age as of September 11, 2001; sex; height; and race/ethnicity were acquired from the FDNY employee database. Smoking behaviors were obtained via self-administered surveys conducted at periodic medical evaluations. Those who consistently reported never smoking were classified accordingly. Because few participants continued to smoke after WTC exposure, current and former smokers were classified together as “ever-smokers” in all analyses. Initial time of arrival at the WTC disaster site is a measure of exposure intensity (24).
Statistical Methods
Cohort characteristics were evaluated as counts/proportions and means/standard deviations as appropriate. For all analyses, baseline lung function was defined as the initial PFT measurement after September 11, 2001.
Joint longitudinal survival modeling was employed to assess the association between baseline FEV1 and the rate of change of FEV1 with mortality (25–30). The longitudinal and time-to-event submodels were linked using a random-effects shared-parameter models framework assuming that the risk of an event at a given time depends on the longitudinal response predicted at that time. For longitudinal FEV1 measurements and survival time, we used a linear mixed-effects model with a random intercept and a random slope (over time) and a piecewise exponential proportional hazards model with the longitudinal process of FEV1, respectively, sharing the same random effects. Time zero was at a participant’s baseline PFT. In addition, we decomposed the longitudinal FEV1 response as baseline FEV1 and change in FEV1 over time and assessed their associations with survival outcomes. Model equations are provided in the data supplement. Joint modeling allows us to evaluate the time-varying covariates on the risk of death while accounting for individual heterogeneity in longitudinal and survival submodels and provides efficient estimates with reduced bias (25–31).
In the primary analysis, the survival outcome was all-cause mortality. Piecewise exponential proportional hazards models were fit using seven distinct intervals such that the numbers of deaths were partitioned equally for each period. Follow-up began at the time of participants’ baseline PFT and ended at death or the end of the follow-up period for the study (i.e., December 31, 2021), whichever occurred first. Except for the last examination, in the longitudinal submodel, person-time accrual for each repeated measure was computed as the difference (in years) between successive PFTs. For the final examination, person-time was calculated as the difference between the final PFT in the study period and the end of follow-up (i.e., death or December 31, 2021). The primary analysis controlled for age; race/ethnicity; smoking; height; work assignment (EMS/firefighter) as of September 11, 2001; and WTC arrival time. Adjusted cumulative mortality plots were created using results from the joint longitudinal survival model. Seven curves were plotted for participants with FEV1 rates of decline in the 5th, 10th, 25th, 50th, 75th, 90th, and 95th percentiles.
In secondary analyses, the associations between baseline FEV1 and FEV1 trajectories with cancer-related, heart disease–related, and respiratory causes of death were estimated. Two sensitivity analyses were conducted: 1) removing PFT measurements within 3 years of the end of the follow-up for an individual to eliminate the potential bias incurred from reverse causality (i.e., removing measurements that were possibly after the onset of impaired lung function caused by incident cancer or other serious disease) and 2) requiring at least three PFT measurements to assess the extent to which selection bias affected results in the full models.
In an additional analysis, rates of change in FEV1 were estimated for each participant using linear regression. Whereas all PFT measurements were included for the primary analysis, for this additional analysis, only participants with at least three FEV1 measurements were included to improve the precision of estimates of rates of change and to be consistent with previous WTC research evaluating pulmonary function decline (2, 3). Rates of decline were then stratified by participants who had accelerated lung function decline, defined as ⩾64 ml/yr, double the age-related decline observed in our prior WTC-related research (2, 3). Piecewise exponential survival models controlling for age on September 11, 2001; race/ethnicity; sex; smoking; and WTC arrival time were used to estimate mortality hazard ratios (HRs) of participants with a ⩾64-ml/yr decrease compared with the rest of the cohort.
Results
Cohort Characteristics
The study population included 12,264 WTC-exposed firefighters and EMS providers who were actively employed on September 11, 2001. Cohort characteristics are presented in Table 1. There were more firefighters (n = 10,301) than EMS providers (n = 1,963). The mean age of the cohort was 39.7 years (standard deviation [SD], 7.8) on September 11, 2001. Most participants were never-smokers (63.8%).
Table 1.
Selected cohort characteristics
| Variable | Overall (N = 12,264) | Firefighters (n = 10,301) | EMS (n = 1,963) |
|---|---|---|---|
| Age on 9/11/2001, yr | 39.7 ± 7.8 | 40.4 ± 7.4 | 35.9 ± 8.6 |
| PFT examinations | 11.2 ± 4.1 | 11.1 ± 3.9 | 12.0 ± 5.2 |
| Post-9/11 follow-up, yr | 19.1 ± 2.6 | 19.1 ± 2.6 | 18.9 ± 2.5 |
| Race/ethnicity | |||
| White | 10,756 (87.7) | 9,701 (94.2) | 1,055 (53.7) |
| Black | 696 (5.7) | 268 (2.6) | 428 (21.8) |
| Hispanic | 812 (6.6) | 332 (3.2) | 480 (24.5) |
| Sex | |||
| Male | 11,862 (96.7) | 10,277 (99.8) | 1,585 (80.7) |
| Female | 402 (3.3) | 24 (0.2) | 378 (19.3) |
| Smoking status | |||
| Never | 7,819 (63.8) | 6,804 (66.1) | 1,015 (51.7) |
| Current/former | 4,445 (36.2) | 3,497 (33.9) | 948 (48.3) |
| WTC exposure | |||
| 9/11 a.m. | 2,076 (16.9) | 1,672 (16.2) | 404 (20.6) |
| 9/11 p.m. | 6,028 (49.2) | 5,444 (52.8) | 584 (29.8) |
| 9/12 | 2,050 (16.7) | 1,794 (17.4) | 256 (13.0) |
| 9/13–9/24 | 1,793 (14.6) | 1,264 (12.3) | 529 (26.9) |
| 9/25 to site close | 275 (2.2) | 87 (0.8) | 188 (9.6) |
| Unknown | 42 (0.3) | 40 (0.4) | 2 (0.1) |
Definition of abbreviations: EMS = emergency medical service providers; PFT = pulmonary function test; WTC = World Trade Center.
Values presented as mean ± SD where applicable. Values in parentheses are percentages.
By December 31, 2021, 607 of the 12,264 individuals in the cohort (4.9%) had died, for a crude death rate of 259.5 per 100,000 person-years; 190 of 12,264 individuals (1.5%) died of cancer, for a crude death rate of 81.2 per 100,000 person-years. Crude mortality rate during the follow-up period was lower in firefighters than in EMS providers (4.5% vs. 7.4%). Of 607 total deaths included in the analyses, 190 (31.3%) were cancer-related. There were 131 (21.6%) heart disease–related and 30 (4.9%) respiratory causes of death.
Most of the cohort had normal lung function on their initial PFT (Table 2), with FEV1% predicted ⩾80% in 10,970 of 12,264 (89.4%) and FEV1/FVC ratio ⩾70% in 11,751 (97.6%). The median FEV1 rate of change was a 34.7-ml/yr loss (interquartile range, 22.1–49.5-ml/yr loss). At the time of the participants’ last PFT, the proportion of the cohort with FEV1% predicted ⩾80% and FEV1/FVC ratio ⩾70% had substantially decreased (Table 2). On average, 19.1 (SD, 2.6) years elapsed from participants’ first PFT to the end of follow-up. The median (interquartile range) numbers of years elapsed from the last PFT to the end of follow-up were 1.3 (0.6–3.0) and 1.9 (0.8–4.1) for living and deceased participants, respectively.
Table 2.
Longitudinal lung function
| Stratum of FEV1% Predicted | First PFT* |
Last PFT* |
||
|---|---|---|---|---|
| n (%) | FEV1 (ml) | n (%) | FEV1 (ml) | |
| 0–59 | 79 (0.6) | 1,892 ± 436.2 | 309 (2.5) | 1,718 ± 403.5 |
| 60–69 | 204 (1.7) | 2,585 ± 367.2 | 502 (4.1) | 2,344 ± 361.7 |
| 70–79 | 1,011 (8.2) | 3,067 ± 387.8 | 1,457 (11.9) | 2,719 ± 387.1 |
| 80–89 | 2,606 (21.3) | 3,497 ± 432.1 | 2,823 (23.0) | 3,106 ± 438.0 |
| 90–99 | 3,652 (29.8) | 3,884 ± 451.7 | 3,391 (27.7) | 3,431 ± 466.7 |
| 100–109 | 2,822 (23.0) | 4,231 ± 503.8 | 2,422 (19.8) | 3,731 ± 489.0 |
| 110–119 | 1,307 (10.7) | 4,609 ± 566.6 | 1,023 (8.3) | 3,973 ± 561.0 |
| 120–140 | 583 (4.8) | 5,030 ± 667.0 | 337 (2.8) | 4,187 ± 641.4 |
| FEV1/FVC <70%† | 288 (2.4) | – | 1,142 (9.3) | – |
| Vital status | FEV1% Predicted | FEV1% Predicted | ||
| Deceased | 607 | 90.5 ± 16.1 | 607 | 84.9 ± 17.9 |
| Alive | 11,657 | 96.2 ± 13.6 | 11,657 | 92.3 ± 14.8 |
Definition of abbreviations: FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; PFT = pulmonary function test (spirometry).
Values presented as mean ± SD where applicable.
A total of 262 participants had one PFT and thus this PFT contributed to the first and last measurement calculations.
Totals of 12,039 and 12,249 participants had valid PFT results for their first and final visits, respectively.
Joint Longitudinal Survival Models
Lung function and all-cause mortality
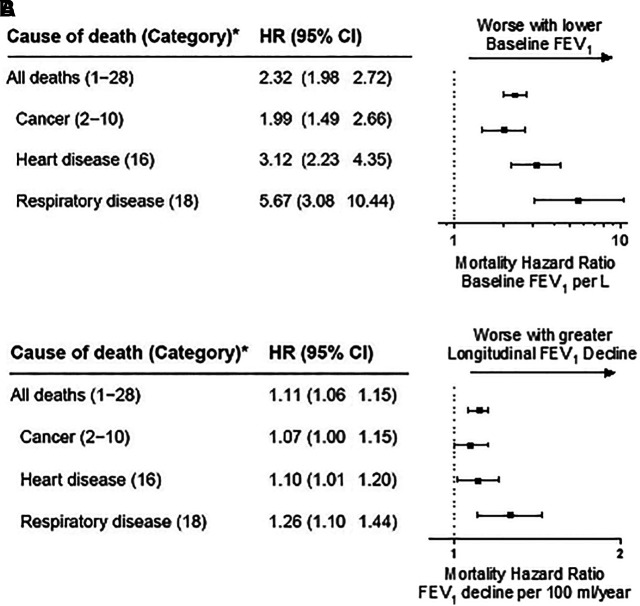
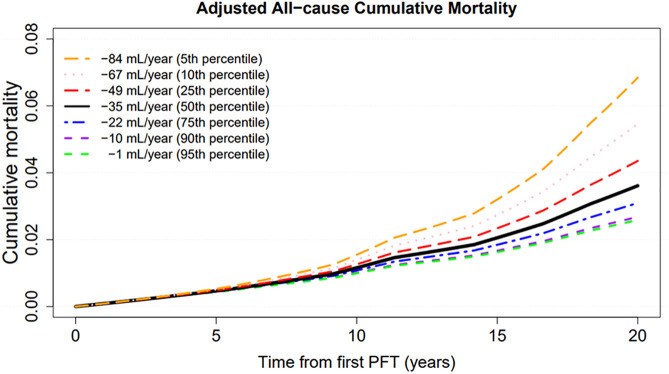
Participants contributed 233,931 person-years of follow-up. Baseline lung function was associated with a 2.3-fold increase in all-cause mortality per 1-L lower FEV1 (Figure 1A). A 100-ml/yr longitudinal decline in lung function from baseline was independently associated with an 11% increase in mortality (Figure 1B). In the longitudinal submodel, the 50th percentile of FEV1 slope was a 35-ml/yr decline, the fifth percentile of FEV1 slope was a 1-ml/yr decline, and the 95th percentile of FEV1 slope was an 84-ml/yr decline (Figure 2). Cumulative all-cause mortality increased with greater rates of decrease in longitudinal FEV1 across the range.
Figure 1.
Joint longitudinal survival models evaluating the association between baseline forced expiratory volume in 1 second (A) and longitudinal change from baseline (B) and mortality. Models control for age on September 11, 2001; race/ethnicity; height; smoking (ever vs. never); work assignment on September 11, 2001 (firefighters vs. emergency medical service providers); and World Trade Center initial arrival time. *Category corresponds with National Institute of Occupational Safety and Health life table analysis system major categories (22). FEV1 = forced expiratory volume in 1 second; HR = hazard ratio.
Figure 2.
Results from joint longitudinal survival models for World Trade Center (WTC)–exposed Fire Department of New York responders evaluating lung function decline and all-cause mortality. Seven curves correspond with results from cumulative mortality plots for individuals whose forced expiratory volume in 1 second decreased at the 5th (orange), 10th (pink), 25th (red), 50th (black), 75th (blue), 90th (purple), and 95th (green) percentiles. The model controls for age on September 11, 2001 (centered at 40 yr), race (centered at White), smoking (centered at never), work assignment on September 11, 2001 (centered at firefighters), height (centered at 180 cm), and WTC arrival time (centered at initial arrival between September 13, 2001, and July 25, 2002). PFT = pulmonary function test (spirometry).
Lung function and cause-specific mortality
As with the findings for all-cause mortality, baseline FEV1 was associated with a 1.99-fold increased hazard for cancer-cause mortality per 1-L lower FEV1 (Figure 1A). Similarly, each 100-ml/yr decrease in longitudinal FEV1 was associated with a 7% increase in cancer-cause mortality (Figure 1B). Each 1-L lower baseline FEV1 was associated with a 3.1- and 5.7-fold increase in heart disease–related and respiratory-disease mortality, respectively. Every 100-ml/yr longitudinal decline in lung function was associated with a 10% increased mortality for heart disease and 26% increased mortality for respiratory disease (Figure 1B).
Sensitivity analyses with the removal of PFT measurements within 3 years of the end of the follow-up period and requiring at least three PFT measurements yielded similar results to the main models (Table E1 in the data supplement).
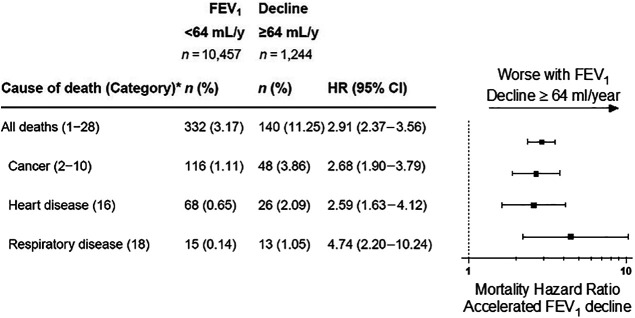
Mortality by accelerated lung function decline
We observed 1,244 participants (10.1%) whose lung function declined by ⩾64 ml/yr (i.e., accelerated FEV1 decrease). The crude all-cause cumulative mortality rate for those with an FEV1 decrease <64 ml/yr was 3.2%, compared with 11.3% for those with accelerated FEV1 decrease. Multivariable analyses adjusted for age on September 11, 2001; race/ethnicity; sex; smoking (ever vs. never); and initial WTC arrival time demonstrated that, compared with expected FEV1 decrease, accelerated FEV1 decrease was associated with a 2.9-fold increase in all-cause mortality (Figure 3). Accelerated FEV1 decrease was also associated with a 2.7-fold increase in cancer-cause mortality, a 2.6-fold increase in heart disease–cause mortality, and a 4.7-fold increase in respiratory-cause mortality. Small numbers of deaths during the first 20 years after exposure limited the power to observe the statistical significance of the association for many specific cancer types.
Figure 3.
Accelerated lung function decline and mortality by cause of death. Accelerated lung function decline is defined by losses in forced expiratory volume in 1 second (FEV1) of ⩾64-ml per year in an individual; n = 563 participants contributed two or fewer pulmonary function tests and thus were not included in the analysis to improve the precision of slope (i.e., rate of change) estimates. Crude death proportions are presented. Piecewise exponential survival models control for age on September 11, 2001; race/ethnicity; sex; World Trade Center site arrival time; and smoking. The top three cancer causes of death were cancers of the digestive organs (category 3; n = 61), hematopoietic/lymphatic system (category 10; n = 25), and respiratory system (category 4; n = 23). *Category corresponds with National Institute of Occupational Safety and Health life table analysis system major categories.
Discussion
Longitudinal data over a 20-year period provide a unique resource for assessing the association of lung function with all-cause and cause-specific mortality. Similar to longitudinal studies without WTC exposure and using different modeling techniques (6, 8), this prospective cohort study of 12,264 WTC-exposed FDNY responders found that greater FEV1 decrease is associated with increased all-cause, heart disease–cause, and respiratory disease–cause mortality. This investigation also revealed a significant association of FEV1 decrease and cancer-cause mortality, findings that are even more important given this cohort’s normal baseline lung function and low mortality rates (32). It is plausible that prior studies analyzing pulmonary function and mortality longitudinally were unable to observe associations with long-latency outcomes like cancer-cause mortality as a result of relatively few years of follow-up. The joint longitudinal survival model used in the present study enabled us to evaluate the independent contributions to mortality of the first post–WTC exposure baseline FEV1 and FEV1 decrease. Interestingly, baseline FEV1 was strongly associated with mortality even though only 10.5% of the cohort had relatively abnormal lung function (i.e., baseline post-WTC FEV1 <80% predicted). Further analyses are needed to assess if pre–WTC exposure FEV1 is also associated with mortality.
Longitudinal lung function is well characterized in this cohort, with an average of 11 FEV1 measurements. We observed a twofold greater risk of death from all causes per 1-L lower FEV1 at baseline and an 11% increased hazard per 100-ml/yr decrease in FEV1 after controlling for age, race/ethnicity, height, smoking, arrival time at the WTC site, and work assignment. We have previously shown that accelerated FEV1 decrease (i.e., FEV1 loss of ⩾64 ml/yr) is associated with incident respiratory diseases such as COPD, asthma, and asthma/COPD overlap syndrome (2, 3). The present study demonstrates that accelerated FEV1 decrease is associated with a greater than 2.5-fold increased risk for cancer-, heart disease-, and respiratory-cause mortality, a magnitude of risk similar to that seen in the primary analysis. These findings demonstrate that baseline FEV1 and FEV1 decrease are important independent risk factors for all-cause and cause-specific mortality.
There are several biologically plausible explanations for the association between lung function and mortality from diseases in multiple organ systems. WTC-related lung injury is associated with inflammatory biomarkers in serum obtained even years after September 11, 2001 (3, 33). There is extensive evidence that individuals with chronic inflammation (34–36) have a worse disease prognosis. Systemic chronic inflammation produced by physical inactivity, poor diet, environmental and industrial toxicants, and psychological stress can lead to lung disease, heart disease, and cancer that result in most of the world’s disability and mortality (37). Among patients with COPD, Eickhoff and colleagues found significant impairment of vasodilation and a possible increased risk of cardiovascular disease due to airflow obstruction and systemic inflammation (38). Further, a recent study demonstrated that exposure to WTC dust increases clonal hematopoiesis (39). This is defined by the outgrowth of hematopoietic stem cells with somatic mutations of growth-inhibiting genes, including DMNT3A and TET2. Myeloid stem cells with these somatic mutations expand and then develop into inflammatory monocytes. Risk factors for clonal hematopoiesis include old age and cigarette smoking. DNMT3A and TET2 mutations were also the most common mutations in large population studies of patients with COPD, heart disease, and cancer (34, 35, 40, 41). The investigation of this cohort may have implications for other environmental/occupational cohorts such as studies of the respiratory health effects of airborne hazards after burn pit exposures in the Southwest Asia theater of military operations (42).
Although we cannot fully determine the mechanistic association between lung function and mortality, these findings are notable for many reasons and could provide a rationale for pharmacologic and lifestyle interventions among participants with accelerated FEV1 decrease. Previously, we showed that treatment with inhaled corticosteroids and long-acting β-agonists did not slow lung function decline in most patients in this cohort and did not improve respiratory symptoms if started after 2010 (43, 44). The accelerated FEV1 decrease phenotype and its association with increased mortality, even in patients with normal lung function, is concerning and could meet the definition of pre-COPD, arguing for monitoring and the development of improved therapies that could then allow for early intervention (8).
This study has distinctive strengths over prior work evaluating the association between lung function and mortality. First, our study featured a large sample size, excellent cohort retention, and repeated measurements for 20 years of follow-up, something lacking in prior studies examining the association between lung function and mortality. Second, the FDNY has excellent mortality capture. Complete demographic data including social security numbers were used for National Death Index linkages. Death data were also validated by independent FDNY records. Third, our study featured rigorous quality assurance, standardization, and consistency for spirometry testing procedures. Because spirometry is effort-dependent, a standardized process is paramount when studying outcomes longitudinally. Fourth, the joint longitudinal survival methodology accounted for dependencies between the longitudinal exposure process (lung function) and the time-to-event outcome process (death). By modeling the two concurrently, bias is reduced. Specifically, a given set of covariates at each time interval is used to predict mortality such that the exposure precedes the outcome. Although all longitudinal FEV1 measurements are used in the model, future FEV1 values are not used to predict an underlying biological process that leads to death. Further, joint modeling also preserves temporality by allowing each repeated FEV1 measurement in the longitudinal model to predict mortality at each respective time interval, thereby reducing biases produced by informative censoring (45). This method enables estimation of the association between FEV1 trajectories and survival and allows modeling of baseline FEV1 and rate of change in FEV1 over time (i.e., FEV1 slope) in the prediction of survival. Finally, the consistent results we obtained regarding the known associations with heart and respiratory disease mortality (6, 8), shown in other studies to be associated with lung function, lend strength to the validity of our methods.
There are some limitations to this work. First, we were underpowered to detect an association between lung function decline and mortality for specific cancer types or other less common causes of death. Continued follow-up will be important for future work. Second, unmeasured confounding, particularly related to continued workplace exposures among firefighters and EMS providers who remained active in the years following September 11, 2001, could not be discounted. We believe, however, that this bias was likely to be minimal across differing lung function trajectories. Third, we cannot fully rule out informative missingness in the pulmonary function data. However, the short amount of average time elapsed from the last PFT to the end of follow-up (<2 yr) demonstrates that it would have not introduced substantial bias. Further, the potential for missingness may bias toward the null because the deceased individuals had, on average, slightly longer times between the last PFT and the end of follow-up (i.e., death date), suggesting that any additional measurements would have shown worse lung function. Finally, a sensitivity analysis after removing PFT measurements within 3 years of the end of the follow-up period found a similar effect, reducing the potential bias incurred from reverse causality.
In summary, this study provides evidence that lung function decline is associated with all-cause mortality and cancer-cause mortality after controlling for important confounders. We found that baseline FEV1 and change in FEV1 over time are associated with all-cause mortality, cancer-cause mortality, and mortality from heart and lung disease. Systemic inflammation affecting the respiratory and cardiovascular systems, as well as poor cancer control produced by WTC-associated clonal hematopoiesis, is a possible mechanism that contributes to all-cause and cause-specific mortality. Although aspects of the WTC exposure are unique, our study design could benefit the monitoring of other cohorts with occupational/environmental exposures (13, 42, 46). Further research can test the hypothesis that chronic inflammation is a common cause of loss of lung function and increased mortality rates.
Footnotes
Supported by National Institute for Occupational Safety and Health grants U01 OH011682, U01 OH011480, and U01 OH011302 and contracts 200-2011-39383, 200-2011-39378, 200-2017-93326, 200-2017-93426, and 200-2017-93432; and National Center for Advancing Translational Sciences grant UL1 TR002556.
Author Contributions: R.Z.-O. and D.J.P. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: D.G.G., C.B.H., J.C., R.Z.-O., D.J.P., and M.D.W. Acquisition, analysis, or interpretation of data: D.G.G., C.B.H., R.Z.-O., H.C., D.J.P., and M.D.W. Drafting of the manuscript: D.G.G., C.B.H., R.Z.-O., D.J.P., and M.D.W. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: D.G.G., C.B.H., J.C., R.Z.-O., M.C., and M.D.W. Obtained funding: D.J.P. Administrative, technical, or material support: D.J.P. and M.D.W. Study supervision: R.Z.-O., D.J.P., and M.D.W.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Aldrich TK, Gustave J, Hall CB, Cohen HW, Webber MP, Zeig-Owens R, et al. Lung function in rescue workers at the World Trade Center after 7 years. N Engl J Med . 2010;362:1263–1272. doi: 10.1056/NEJMoa0910087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zeig-Owens R, Singh A, Aldrich TK, Hall CB, Schwartz T, Webber MP, et al. Blood leukocyte concentrations, FEV1 decline, and airflow limitation. A 15-year longitudinal study of World Trade Center-exposed firefighters. Ann Am Thorac Soc . 2018;15:173–183. doi: 10.1513/AnnalsATS.201703-276OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weiden MD, Singh A, Goldfarb DG, Putman B, Zeig-Owens R, Schwartz T, et al. Serum Th-2 cytokines and FEV1 decline in WTC-exposed firefighters: a 19-year longitudinal study. Am J Ind Med . 2021;64:845–852. doi: 10.1002/ajim.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li J, Yung J, Qiao B, Takemoto E, Goldfarb DG, Zeig-Owens R, et al. Cancer incidence in World Trade Center rescue and recovery workers: 14 years of follow-up. J Natl Cancer Inst . 2022;114:210–219. doi: 10.1093/jnci/djab165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldfarb DG, Zeig-Owens R, Kristjansson D, Li J, Brackbill RM, Farfel MR, et al. Cancer survival among World Trade Center rescue and recovery workers: a collaborative cohort study. Am J Ind Med . 2021;64:815–826. doi: 10.1002/ajim.23278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Costanzo S, Magnacca S, Bonaccio M, Di Castelnuovo A, Piraino A, Cerletti C, et al. Moli-sani Study Investigators Reduced pulmonary function, low-grade inflammation and increased risk of total and cardiovascular mortality in a general adult population: prospective results from the Moli-sani study. Respir Med . 2021;184:106441. doi: 10.1016/j.rmed.2021.106441. [DOI] [PubMed] [Google Scholar]
- 7. Whittaker HR, Bloom C, Morgan A, Jarvis D, Kiddle SJ, Quint JK. Accelerated FEV1 decline and risk of cardiovascular disease and mortality in a primary care population of COPD patients. Eur Respir J . 2021;57:2000918. doi: 10.1183/13993003.00918-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marott JL, Ingebrigtsen TS, Çolak Y, Vestbo J, Lange P. Lung function trajectories leading to chronic obstructive pulmonary disease as predictors of exacerbations and mortality. Am J Respir Crit Care Med . 2020;202:210–218. doi: 10.1164/rccm.201911-2115OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vasquez MM, Zhou M, Hu C, Martinez FD, Guerra S. Low lung function in young adult life is associated with early mortality. Am J Respir Crit Care Med . 2017;195:1399–1401. doi: 10.1164/rccm.201608-1561LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mannino DM, Reichert MM, Davis KJ. Lung function decline and outcomes in an adult population. Am J Respir Crit Care Med . 2006;173:985–990. doi: 10.1164/rccm.200508-1344OC. [DOI] [PubMed] [Google Scholar]
- 11. Schünemann HJ, Dorn J, Grant BJ, Winkelstein W, Jr, Trevisan M. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest . 2000;118:656–664. doi: 10.1378/chest.118.3.656. [DOI] [PubMed] [Google Scholar]
- 12. Rodriguez BL, Masaki K, Burchfiel C, Curb JD, Fong KO, Chyou PH, et al. Pulmonary function decline and 17-year total mortality: the Honolulu Heart Program. Am J Epidemiol . 1994;140:398–408. doi: 10.1093/oxfordjournals.aje.a117262. [DOI] [PubMed] [Google Scholar]
- 13. Sircar K, Hnizdo E, Petsonk E, Attfield M. Decline in lung function and mortality: implications for medical monitoring. Occup Environ Med . 2007;64:461–466. doi: 10.1136/oem.2006.031419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Purdue MP, Gold L, Järvholm B, Alavanja MC, Ward MH, Vermeulen R. Impaired lung function and lung cancer incidence in a cohort of Swedish construction workers. Thorax . 2007;62:51–56. doi: 10.1136/thx.2006.064196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen HW, Zeig-Owens R, Joe C, Hall CB, Webber MP, Weiden MD, et al. Long-term cardiovascular disease risk among firefighters after the World Trade Center disaster. JAMA Netw Open . 2019;2:e199775. doi: 10.1001/jamanetworkopen.2019.9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zeig-Owens R, Webber MP, Hall CB, Schwartz T, Jaber N, Weakley J, et al. Early assessment of cancer outcomes in New York City firefighters after the 9/11 attacks: an observational cohort study. Lancet . 2011;378:898–905. doi: 10.1016/S0140-6736(11)60989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lioy PJ, Weisel CP, Millette JR, Eisenreich S, Vallero D, Offenberg J, et al. Characterization of the dust/smoke aerosol that settled east of the World Trade Center (WTC) in lower Manhattan after the collapse of the WTC 11 September 2001. Environ Health Perspect . 2002;110:703–714. doi: 10.1289/ehp.02110703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aldrich TK, Vossbrinck M, Zeig-Owens R, Hall CB, Schwartz TM, Moir W, et al. Lung function trajectories in World Trade Center-exposed New York City firefighters over 13 years: the roles of smoking and smoking cessation. Chest . 2016;149:1419–1427. doi: 10.1016/j.chest.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Banauch GI, Hall C, Weiden M, Cohen HW, Aldrich TK, Christodoulou V, et al. Pulmonary function after exposure to the World Trade Center collapse in the New York City Fire Department. Am J Respir Crit Care Med . 2006;174:312–319. doi: 10.1164/rccm.200511-1736OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vollmer WM, McCamant LE, Johnson LR, Buist AS. Long-term reproducibility of tests of small airways function. Comparisons with spirometry. Chest . 1990;98:303–307. doi: 10.1378/chest.98.2.303. [DOI] [PubMed] [Google Scholar]
- 21. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med . 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 22. Robinson CF, Schnorr TM, Cassinelli RT, II, Calvert GM, Steenland NK, Gersic CM, et al. Tenth revision U.S. mortality rates for use with the NIOSH life table analysis system. J Occup Environ Med . 2006;48:662–667. doi: 10.1097/01.jom.0000229968.74906.8f. [DOI] [PubMed] [Google Scholar]
- 23.National Center for Health Statistics. National Death Index user’s guide. Hyattsville, MD: National Center for Health Statistics; 2013. https://www.cdc.gov/nchs/data/ndi/NDI_Users_Guide.pdf [Google Scholar]
- 24. Prezant DJ, Weiden M, Banauch GI, McGuinness G, Rom WN, Aldrich TK, et al. Cough and bronchial responsiveness in firefighters at the World Trade Center site. N Engl J Med . 2002;347:806–815. doi: 10.1056/NEJMoa021300. [DOI] [PubMed] [Google Scholar]
- 25. Garcia-Hernandez A, Rizopoulos D. %JM: a SAS macro to fit jointly generalized mixed models for longitudinal data and time-to-event responses J Stat Softw 2018. 84 1 29 30450020 [Google Scholar]
- 26. Ibrahim JG, Chu H, Chen LM. Basic concepts and methods for joint models of longitudinal and survival data. J Clin Oncol . 2010;28:2796–2801. doi: 10.1200/JCO.2009.25.0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Song X, Davidian M, Tsiatis AA. A semiparametric likelihood approach to joint modeling of longitudinal and time-to-event data. Biometrics . 2002;58:742–753. doi: 10.1111/j.0006-341x.2002.00742.x. [DOI] [PubMed] [Google Scholar]
- 28. Wulfsohn MS, Tsiatis AA. A joint model for survival and longitudinal data measured with error. Biometrics . 1997;53:330–339. [PubMed] [Google Scholar]
- 29. Henderson R, Diggle P, Dobson A. Joint modelling of longitudinal measurements and event time data. Biostatistics . 2000;1:465–480. doi: 10.1093/biostatistics/1.4.465. [DOI] [PubMed] [Google Scholar]
- 30. Xu J, Zeger SL. Joint analysis of longitudinal data comprising repeated measures and times to events. J R Stat Soc Ser C Appl Stat . 2001;50:375–387. [Google Scholar]
- 31. Tsiatis AA, Davidian M. Joint modeling of longitudinal and time-to-event data: an overview. Stat Sin . 2004;14:809–834. [Google Scholar]
- 32. Colbeth HL, Zeig-Owens R, Hall CB, Webber MP, Schwartz TM, Prezant DJ. Mortality among fire department of the city of New York rescue and recovery workers exposed to the World Trade Center Disaster, 2001-2017. Int J Environ Res Public Health . 2020;17:6266. doi: 10.3390/ijerph17176266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nolan A, Naveed B, Comfort AL, Ferrier N, Hall CB, Kwon S, et al. Inflammatory biomarkers predict airflow obstruction after exposure to World Trade Center dust. Chest . 2012;142:412–418. doi: 10.1378/chest.11-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuhnert S, Mansouri S, Rieger MA, Savai R, Avci E, Díaz-Piña G, et al. Association of clonal hematopoiesis of indeterminate potential with inflammatory gene expression in patients with COPD. Cells . 2022;11:2121. doi: 10.3390/cells11132121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miller PG, Qiao D, Rojas-Quintero J, Honigberg MC, Sperling AS, Gibson CJ, et al. COPDGene Study Investigators, National Heart, Lung, and Blood Institute Trans-Omics for Precision Medicine Consortium Association of clonal hematopoiesis with chronic obstructive pulmonary disease. Blood . 2022;139:357–368. doi: 10.1182/blood.2021013531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leng S, Diergaarde B, Picchi MA, Wilson DO, Gilliland FD, Yuan JM, et al. Gene promoter hypermethylation detected in sputum predicts FEV1 decline and all-cause mortality in smokers. Am J Respir Crit Care Med . 2018;198:187–196. doi: 10.1164/rccm.201708-1659OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med . 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eickhoff P, Valipour A, Kiss D, Schreder M, Cekici L, Geyer K, et al. Determinants of systemic vascular function in patients with stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2008;178:1211–1218. doi: 10.1164/rccm.200709-1412OC. [DOI] [PubMed] [Google Scholar]
- 39. Jasra S, Giricz O, Zeig-Owens R, Pradhan K, Goldfarb DG, Barreto-Galvez A, et al. High burden of clonal hematopoiesis in first responders exposed to the World Trade Center disaster. Nat Med . 2022;28:468–471. doi: 10.1038/s41591-022-01708-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kar SP, Quiros PM, Gu M, Jiang T, Mitchell J, Langdon R, et al. Genome-wide analyses of 200,453 individuals yield new insights into the causes and consequences of clonal hematopoiesis. Nat Genet . 2022;54:1155–1166. doi: 10.1038/s41588-022-01121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med . 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Academies of Sciences, Engineering, and Medicine. Consensus study report: respiratory health effects of airborne hazards exposures in the Southwest Asia theater of military operations. Washington, DC: National Academies Press; 2020. [PubMed] [Google Scholar]
- 43. Goldfarb DG, Putman B, Lahousse L, Zeig-Owens R, Vaeth BM, Schwartz T, et al. Lung function decline before and after treatment of World Trade Center associated obstructive airways disease with inhaled corticosteroids and long-acting beta agonists. Am J Ind Med . 2021;64:853–860. doi: 10.1002/ajim.23272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Putman B, Lahousse L, Singh A, Zeig-Owens R, Hall CB, Fazzari MJ, et al. Dyspnea and inhaled corticosteroid and long-acting β-agonist therapy in an occupational cohort: a longitudinal study. Ann Am Thorac Soc . 2020;17:770–773. doi: 10.1513/AnnalsATS.201910-794RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang P, Shen W, Boye ME. Joint modeling of longitudinal outcomes and survival using latent growth modeling approach in a mesothelioma trial. Health Serv Outcomes Res Methodol . 2012;12:182–199. doi: 10.1007/s10742-012-0092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gam KB, Kwok RK, Engel LS, Curry MD, Stewart PA, Stenzel MR, et al. Exposure to oil spill chemicals and lung function in deepwater horizon disaster response workers. J Occup Environ Med . 2018;60:e312–e318. doi: 10.1097/JOM.0000000000001292. [DOI] [PMC free article] [PubMed] [Google Scholar]