Abstract
Rationale
Population-based data on the epidemiology of nontuberculosis mycobacterial (NTM) infections are limited, particularly with respect to variation in NTM infection among racial groups and socioeconomic strata. Wisconsin is one of a handful of states where mycobacterial disease is notifiable, allowing large, population-based analyses of the epidemiology of NTM infection in this state.
Objectives
To estimate the incidence of NTM infection in Wisconsin adults, describe the geographic distribution of NTM infection across the state, identify the frequency and type of infection caused by different NTM species, and investigate associations between NTM infection and demographics and socioeconomic status.
Methods
We conducted a retrospective cohort study using laboratory reports of all NTM isolates from Wisconsin residents submitted to the Wisconsin Electronic Disease Surveillance System from 2011 to 2018. For the analyses of NTM frequency, multiple reports from the same individual were enumerated as separate isolates when nonidentical, collected from different sites or collected more than one year apart.
Results
A total of 8,135 NTM isolates from 6,811 adults were analyzed. Mycobacterium avium complex accounted for 76.4% of respiratory isolates. The M. chelonae-abscessus group was the most common species isolated from skin and soft tissue. The annual incidence of NTM infection was stable over the study period (from 22.1 per 100,000 to 22.4 per 100,000). The cumulative incidence of NTM infection among Black (224 per 100,000) and Asian (244 per 100,000) individuals was significantly higher compared with that among their White counterparts (97 per 100,000). Total NTM infections were significantly more frequent (P < 0.001) in individuals from disadvantaged neighborhoods, and racial disparities in the incidence of NTM infection generally remained consistent when stratified by measures of neighborhood disadvantage.
Conclusions
More than 90% of NTM infections were from respiratory sites, with the vast majority caused by M. avium complex. Rapidly growing mycobacteria predominated as skin and soft tissue pathogens and were important minor respiratory pathogens. We found a stable annual incidence of NTM infection in Wisconsin between 2011 and 2018. NTM infection occurred more frequently in non-White racial groups and in individuals experiencing social disadvantage, suggesting that NTM disease may be more frequent in these groups as well.
Keywords: nontuberculous mycobacteria, epidemiology, pulmonary, Wisconsin
Nontuberculous mycobacteria (NTM) are a group of bacteria widely distributed in the environment, where they are found in soil, natural bodies of water, and municipal water supplies (1). This group includes all species of the genus Mycobacterium aside from members of the M. tuberculosis complex and the agents of Hansen’s disease (leprosy). In humans, a subset of NTM species (approximately 20%) can cause pulmonary, extrapulmonary, or disseminated disease (2). Over the past decade, the use of genetic sequencing has led to a rapid expansion in unique NTM species and subspecies, which now number more than 190 (3). Across different regions, some studies have reported increases in the incidence or prevalence of NTM disease (4–12), whereas others have not (13, 14). Increasing NTM frequency may be an artifact of improved detection of NTM species with newer molecular techniques, such as polymerase chain reaction and mass spectrometry, or increased screening. However, a true increase in NTM disease could also be due to changes in the physical environment, widespread chlorination of water supplies (15), climate perturbation, aging populations, or broader use of immune-suppressive drugs.
Pathogenic NTM species produce a variety of disease presentations, including skin and soft tissue infection, lymphadenitis, pneumonia, catheter-related bloodstream infection, osteomyelitis, and central nervous system and disseminated infection (16, 17). In adults, most NTM infections are pulmonary, while in children, lymphadenitis is the most common presentation (18). Well-documented risk factors for NTM disease in adults include structural lung abnormalities (e.g., chronic obstructive pulmonary disease, bronchiectasis), chest wall deformities, primary immunodeficiencies (e.g., molecular lesions in the interleukin-12 or interferon-γ signaling pathways), and acquired immunodeficiencies (e.g., advanced human immunodeficiency virus [HIV] infection or use of tumor necrosis factor blockers, steroids, or other immune-suppressive agents) (9, 19, 20). In contrast, cutaneous disease most frequently occurs in normal hosts accidentally inoculated during trauma, surgery, or cosmetic procedures (21, 22). On occasion, disseminated disease may present with cutaneous lesions in immune-suppressed hosts.
Unlike M. tuberculosis, which spreads directly from person to person and does not survive outside the host, with rare exceptions (23), NTM infection is acquired through environmental exposure and is not communicable. Proximity to surface water, higher daily evapotranspiration amounts, higher amounts of copper or sodium and lower amounts of manganese in the soil, and frequency of pathogenic NTM in showerhead biofilms have been associated with elevated incidence of NTM infection (15, 24). Although it is well recognized that social determinants are risk factors for certain communicable infectious diseases, such as hepatitis C, HIV infection, and tuberculosis (25–28), it is not known whether there are social determinants of risk of NTM disease in the United States.
Wisconsin is one of a handful of states in which NTM disease notification to health officials is mandated by statute. The aim of this study was to estimate the incidence of NTM infection in Wisconsin adults, describe the geographic distribution of NTM infection across the state, and identify the frequency and type of infection caused by different NTM species. We also investigated associations between NTM infection and demographics and socioeconomic status.
Methods
Study Design
We conducted a retrospective cohort study using laboratory reports of all NTM isolates from Wisconsin residents submitted to the Wisconsin Electronic Disease Surveillance System (WEDSS) from 2011 to 2018.
Data Collection
WEDSS is an electronic public health system designed to manage data submitted by health professionals and clinical laboratories reporting the occurrence of reportable diseases and conditions and is maintained by the Wisconsin Department of Health Services. Inputs to this data system include NTM laboratory reports received from the Wisconsin State Laboratory of Hygiene (approximately 40% of all NTM isolates), other laboratories in Wisconsin with the capability to isolate and identify mycobacteria (approximately 50% of all NTM isolates), and national reference laboratories (approximately 10% of isolates). Clinical data were generally not reported or not fully captured when a laboratory transmitted isolation data to WEDSS. Data extracted from WEDSS included case identification number, age, gender, race, street address, county of residence, date of specimen collection, specimen type (tissue site), mycobacterial species, and reporting laboratory. These data originated from requisition forms that were completed by laboratory personnel at the facilities submitting samples and used information from patients’ medical records. Reports from non–Wisconsin residents and individuals less than 18 years old or of unknown age were excluded. Isolates of M. gordonae were excluded. Duplicate reports of identical species isolated from the same site within one year in any given individual were also removed. Multiple reports from the same individual were included and enumerated as separate infections if 1) species were distinct (nonidentical), 2) the isolates were collected from different sites (e.g., lung and skin), or 3) isolates were collected more than one year apart when calculating annual incidence. Given the lack of clinical data, cases of NTM disease were not defined in this study. As specimens were generally collected from patients in whom NTM infection was possible or suspected, instances of NTM isolation are defined as cases of NTM infection. Therefore, the terms “isolation” and “infection” are used interchangeably and do not necessarily indicate disease. It is possible that some laboratory isolations of NTM were reported for individuals without corresponding clinical syndromes. However, it is very likely that isolation from skin, lymph nodes, or usually sterile sites represents true disease.
Throughout most of the study period, molecular assays were routinely used to identify acid-fast organisms. Those assays were unable to distinguish among M. avium, M. chimaera, and M. intracellulare. Therefore, those three species were grouped together as “M. avium complex (MAC)” in this analysis. In addition, laboratory methods in use before 2015 generally failed to discriminate among the three M. abscesses subspecies (M. abscessus abscessus, M. abscessus bolletii, and M. abscessus massiliense) and M. chelonae. Thus, these species are grouped together under the rubric “M. chelonae-abscessus group.”
Area Deprivation Index Score
A state-level area deprivation index (ADI) score of 1 (least disadvantaged) through 10 (most disadvantaged) was assigned to each individual in the database with a home street address reported, using the online tool Neighborhood Atlas (29, 30). The ADI score provides a comparative ranking of socioeconomic status at the census block group level, determined using an individual’s street address. After assignment of the ADI score, street address data were removed to deidentify individuals in the data set.
Data Analysis
Demographic features of study subjects and characteristics of NTM isolates were summarized by calculating proportions, medians, and interquartile ranges using the software program Stata version 16 (StataCorp). Cumulative incidence (per 100,000 adult residents) of NTM infection over the study period was calculated using data from the American Community Survey, which used survey data collected over five years from 2010 to 2014 to determine the population denominators, including for racial and age subgroups in Wisconsin (31). Characteristics of individuals at the time of their first reported isolates were used in the estimation of cumulative incidence and for demographic analysis. To avoid overcounting individuals when making comparisons by race and ADI, and thereby biasing our conclusions, individuals were enumerated only once during the study time period, to provide a cumulative estimate of individuals having at least one NTM infection during an eight-year period. For comparisons across race, race was imputed when missing by sequential imputation using a monotone-missing pattern and 30 imputations (32). The multinomial logistic regression models used had four categories of race and included age, sex, year of infection, and national ADI scores. Results of these models were combined using rules described by Rubin (33). The ADI score was used to investigate whether NTM infection was associated with socioeconomic disadvantage. The cumulative incidence of individuals with NTM infection was compared across racial groups, ADI scores, and racial groups stratified by ADI scores using the Fisher exact test. The proportional frequency of NTM infection within racial groups was compared with the expected distributions in ADI scores using the chi-square goodness-of-fit test.
Ethical Considerations
The study design, including the use of deidentified human data, was approved by the Institutional Review Board of the University of Wisconsin School of Medicine and Public Health and by the Wisconsin Department of Health Services.
Results
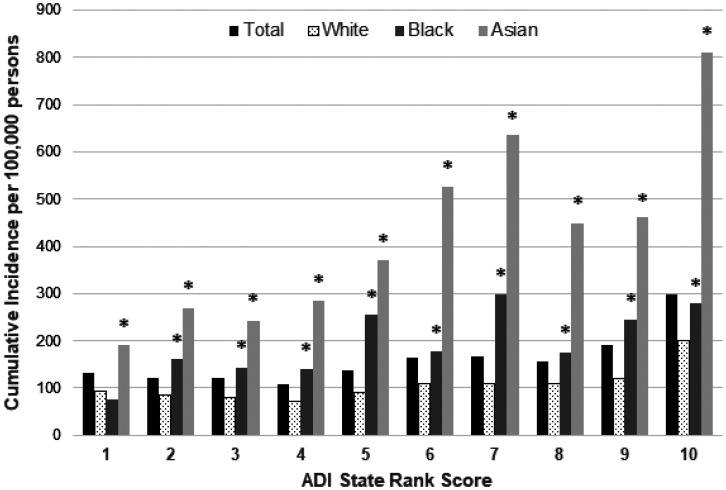
A total of 8,135 NTM isolates from 6,811 individuals were analyzed. As shown in Table 1, there was a slight female predominance in the overall cohort (52.1%) and in the subgroups with respiratory or skin and soft tissue NTM, which was not statistically significant. The median age of study participants was 66 years. Race was ascertained in 69.8% of individuals overall and 55.4% of those with skin and soft tissue infection. There was a more than twofold higher cumulative incidence of NTM infection in Black (224 per 100,000) and Asian (244 per 100,000) individuals compared with their White counterparts (97 per 100,000) (Table 1). Results were similar in analyses using imputed race (see Table E1 in the data supplement). Total NTM infections were significantly more frequent (P < 0.001) in individuals with the highest (most disadvantaged) ADI scores compared with the expected incidence on the basis of the population distribution of ADI scores (Figure 1). Across all ADI scores, Asian individuals had the highest cumulative incidence of NTM infection. Black individuals had a significantly higher incidence than White individuals in nine ADI scores (Figure 1).
Table 1.
Demographic characteristics of Wisconsin adults with nontuberculosis mycobacterial infection, 2011–2018
| Total |
Respiratory |
SST |
||||
|---|---|---|---|---|---|---|
| Number (%) of Persons | Cumulative Incidence per 100,000 (95% CI) | Number (%) of Persons | Cumulative Incidence per 100,000 (95% CI) | Number (%) of Persons | Cumulative Incidence per 100,000 (95% CI) | |
| Total | 6,811 (100) | 154 (150–157) | 6,088 (100) | 137 (134–141) | 294 (100) | 6.6 (5.9–7.4) |
| Gender | ||||||
| Female | 3,545 (52.1) | 157 (152–163) | 3,183 (52.3) | 141 (136–146) | 151 (51.4) | 6.7 (5.6–7.8) |
| Male | 3,238 (47.5) | 148 (143–154) | 2,879 (47.3) | 132 (127–137) | 143 (48.6) | 6.6 (5.5–7.6) |
| Not reported | 28 (0.4) | — | 26 (0.4) | — | 0 (0.0) | — |
| Age, yr, median (IQR) | 66 (54–76) | — | 67 (56–76) | — | 58 (45–71) | — |
| Race | ||||||
| Unascertained | 2,058 (30.2) | — | 1,774 (29.1) | — | 131 (44.6) | — |
| Ascertained | 4,753 (69.8) | — | 4,314 (70.9) | — | 163 (55.4) | — |
| White | 3,833 (80.6) | 96.5 (93.4–99.5) | 3,466 (80.3) | 87.2 (84.3–90.1) | 140 (85.9) | 3.5 (2.9–4.1) |
| Black | 563 (11.8) | 224 (205–242)* | 507 (11.8) | 202 (184–219)* | 16 (9.8) | 6.4 (3.2–9.5) |
| Asian | 247 (5.2) | 244 (214–275)* | 241 (5.6) | 238 (208–268)* | 1 (0.6) | — |
| Other | 67 (1.4) | — | 60 (1.4) | — | 3 (1.8) | — |
| Native American | 25 (0.5) | 71.7 (43.6–99.8) | 25 (0.6) | 71.7 (43.6–99.8) | 0 (0.0) | — |
| Multiple | 11 (0.2) | 23.7 (9.7–37.7) | 9 (0.2) | 19.4 (6.7–32.0) | 2 (1.2) | — |
| Pacific Islander | 7 (0.1) | 656 (170–1,142) | 6 (0.1) | 562 (112–1,012) | 1 (0.6) | — |
Definition of abbreviations: CI = confidence interval; IQR = interquartile range; SST = skin and soft tissue.
Respiratory samples included sputum, bronchoalveolar lavage, and tracheal aspirate specimens.
Statistically significant difference (P < 0.001) compared with White.
Figure 1.
Cumulative incidence of Wisconsin adults with nontuberculosis mycobacterial infection by race and ADI, 2011–2018 (1 = least disadvantaged score, 10 = most disadvantaged score). Categorization was based on the individual’s address at the time of the initially recovered sample if multiple samples were obtained from an individual. *P < 0.05 (Fisher exact test) comparing incidence by race, with White as the reference. ADI = area deprivation index.
Sixty NTM species were identified, of which 41 are depicted in Table 2. Most isolates, 7,337 (90.2%), were from respiratory sites, and 798 (9.8%) were from nonrespiratory (NR) sites. MAC was the most frequent species overall and the most common respiratory NTM, constituting 72.2% of total and 76.4% of respiratory isolates. The M. chelonae-abscessus group was second in frequency, constituting 9.6% of total and 7.3% of respiratory isolates. Rapidly growing mycobacteria (RGM), comprising the M. chelonae-abscessus and M. fortuitum groups and other species such as M. mucogenicum, accounted for 12.5% of total respiratory isolates but 52.9% of non-MAC respiratory isolates. Two important pathogenic NTM, M. xenopi and M. kansasii, accounted for 163 (9.4%) and 64 (3.7%) of the non-MAC respiratory isolates, respectively.
Table 2.
Frequency of total, respiratory, and nonrespiratory isolates by nontuberculosis mycobacteria species in Wisconsin adults, 2011–2018
| NTM Species | Number (Column %) of Isolates |
||
|---|---|---|---|
| Total (N = 8,135) | Respiratory (n = 7,337) | Nonrespiratory (n = 798) | |
| M. avium complex | 5,872 (72.2) | 5,608 (76.4) | 264 (33.1) |
| M. chelonae-abscessus grp | 779 (9.6) | 534 (7.3) | 245 (30.7) |
| M. fortuitum grp | 483 (5.9) | 365 (5.0) | 118 (14.8) |
| M. mucogenicum | 172 (2.1) | 132 (1.8) | 40 (5.0) |
| M. xenopi | 165 (2.0) | 163 (2.2) | 2 (0.3) |
| M. peregrinum | 116 (1.4) | 111 (1.5) | 5 (0.6) |
| M. kansasii | 70 (0.9) | 64 (0.9) | 6 (0.8) |
| M. terrae complex | 39 (0.5) | 35 (0.5) | 4 (0.5) |
| M. marinum | 34 (0.4) | 0 | 34 (4.3) |
| M. porcinum | 33 (0.4) | 27 (0.4) | 6 (0.8) |
| M. neoaurum | 24 (0.3) | 16 (0.2) | 8 (1.0) |
| M. nebraskense | 19 (0.2) | 16 (0.2) | 3 (0.4) |
| M. arupense | 18 (0.2) | 16 (0.2) | 2 (0.3) |
| M. simiae complex | 15 (0.2) | 15 (0.2) | 0 |
| M. peregrinum-septicum | 14 (0.2) | 10 (0.1) | 4 (0.5) |
| M. szulgai | 12 (0.2) | 10 (0.1) | 2 (0.3) |
| M. goodii | 9 (0.1) | 5 (0.1) | 4 (0.5) |
| M. lentiflavum | 9 (0.1) | 8 (0.1) | 1 (0.1) |
| M. paraffinicum | 9 (0.1) | 9 (0.1) | 0 |
| M. smegmatis | 6 (0.1) | 2 (0.1) | 4 (0.5) |
| M. celatum | 5 (0.1) | 5 (0.1) | 0 |
| M. franklinii | 4 (0.1) | 3 (0.1) | 1 (0.1) |
| M. malmoense | 4 (0.1) | 3 (0.1) | 1 (0.1) |
| M. immunogenum | 4 (0.1) | 4 (0.1) | 0 |
| M. genavense | 3 (0.1) | 0 | 3 (0.4) |
| M. interjectum | 3 (0.1) | 3 (0.1) | 0 |
| M. nonchromogenicum | 3 (0.1) | 3 (0.1) | 0 |
| M. asiaticum | 2 (0.1) | 2 (0.1) | 0 |
| M. cookii | 2 (0.1) | 2 (0.1) | 0 |
| M. elephantis | 2 (0.1) | 2 (0.1) | 0 |
| M. europaeum | 2 (0.1) | 2 (0.1) | 0 |
| M. flavescens | 2 (0.1) | 0 | 2 (0.3) |
| M. gastri | 2 (0.1) | 2 (0.1) | 0 |
| M. kubicae | 2 (0.1) | 2 (0.1) | 0 |
| M. kumamotonense | 2 (0.1) | 2 (0.1) | 0 |
| M. llatzerense | 2 (0.1) | 2 (0.1) | 0 |
| M. obuense | 2 (0.1) | 2 (0.1) | 0 |
| M. parascrofulaceum | 2 (0.1) | 2 (0.1) | 0 |
| M. phlei | 2 (0.1) | 2 (0.1) | 0 |
| M. ulcerans | 2 (0.1) | 1 (0.1) | 1 (0.1) |
| Unspecified | 167 (2.1) | 138 (1.7) | 34 (4.0) |
Definition of abbreviations: grp = group; M. = Mycobacterium; NTM = nontuberculosis mycobacteria.
Respiratory samples included sputum, bronchoalveolar lavage, and tracheal aspirate specimens. All the following species had one isolate: M. agri, M. austroafricanum, M. branderi, M. canariasense, M. chlorophenolicum, M. conceptionense-houstonense, M. farcinogenes-senegalense, M. gilvum, M. haemophilum, M. iranicum, M. mageritense, M. monacense, M. palustre, M. phocaicum, M. saskatchewanense, M. senegalense, M. shimoidei, and M. thermoresistibile.
Although most species were isolated predominantly from respiratory sites, exceptions to this pattern were seen for M. marinum (100% NR), M. smegmatis (67% NR), M. genavense (100% NR), M. flavescens (100% NR), and M. ulcerans (50% NR) (Table 2). The M. chelonae-abscessus group and M. neoaurum also had relatively high rates of NR isolation (31.5% and 33.3%, respectively). As shown in Table 3, the M. chelonae-abscessus group was the most common species isolated from skin and soft tissue; head, ears, nose, and throat; musculoskeletal; and eye specimens. MAC was the most common isolate from the remaining tissue sites.
Table 3.
Most common nontuberculosis mycobacteria species isolated from nonrespiratory sites in Wisconsin adults, 2011–2018
| Site | Total (N = 798) [n (%)] |
Most Frequent Species |
|
|---|---|---|---|
| Name | n (%) | ||
| SST | 306 (38.3) | M. chelonae-abscessus grp | 132 (43.1) |
| M. fortuitum grp | 51 (16.7) | ||
| M. avium complex | 45 (14.7) | ||
| M. marinum | 33 (10.8) | ||
| Other | 193 (24.2) | M. avium complex | 104 (53.9) |
| M. chelonae-abscessus grp | 38 (19.7) | ||
| M. fortuitum grp | 31 (16.1) | ||
| Blood | 99 (12.4) | M. avium complex | 28 (28.3) |
| M. mucogenicum | 27 (27.3) | ||
| M. fortuitum grp | 16 (16.2) | ||
| M. chelonae-abscessus grp | 11 (11.1) | ||
| Gastrointestinal | 65 (8.1) | M. avium complex | 31 (47.7) |
| M. chelonae-abscessus grp | 14 (21.5) | ||
| M. fortuitum grp | 10 (15.4) | ||
| HENT | 37 (4.6) | M. chelonae-abscessus grp | 23 (62.2) |
| M. avium complex | 8 (21.6) | ||
| Pleural | 29 (3.6) | M. avium complex | 20 (69.0) |
| M. chelonae-abscessus grp | 3 (10.3) | ||
| Musculoskeletal | 22 (2.8) | M. chelonae-abscessus grp | 11 (50.0) |
| M. avium complex | 7 (31.8) | ||
| Genitourinary | 15 (1.9) | M. avium complex | 11 (73.3) |
| Eye | 12 (1.5) | M. chelonae-abscessus grp | 10 (83.3) |
| Lymph node | 12 (1.5) | M. avium complex | 7 (58.3) |
| CNS | 5 (0.6) | M. avium complex | 1 (20.0) |
| M. chelonae-abscessus grp | 1 (20.0) | ||
| M. fortuitum grp | 1 (20.0) | ||
| M. mucogenicum | 1 (20.0) | ||
| Unidentified NTM species | 1 (20.0) | ||
| Bone marrow | 1 (0.1) | M. avium complex | — |
| Heart | 1 (0.1) | M. avium complex | — |
Definition of abbreviations: CNS = central nervous system; grp = group; HENT = head, ears, nose, or throat; M. = Mycobacterium; NTM = nontuberculosis mycobacteria; SST = skin and soft tissue.
The annual incidence of NTM infections ranged from 22.1 per 100,000 adults in 2016 to 24.4 per 100,000 adults in 2017, showing a stable trend over the study period (see Figure E1). Across Wisconsin, there was significant geographic variation in MAC infection, with a higher annual incidence of MAC isolation in the southeastern and eastern counties of the state (Figure 2A). In contrast, the annual incidence of RGM isolation was more widely distributed across the state, with highest incidence in northwestern and southeastern counties in Wisconsin (Figure 2B).
Figure 2.
Annual incidence of infection with Mycobacterium avium complex (A) or rapidly growing mycobacteria (B), by Wisconsin county, 2011–2018.
Discussion
NTM are ubiquitous environmental bacteria and in humans may cause colonization or clinical disease, most commonly involving the lung. Both the risk of NTM disease and its clinical presentation may be driven by host factors (9, 19, 20), variable pathogenicity across NTM species (1, 9, 34), and environmental factors (1). In this study, 90% of all NTM were isolated from respiratory sites, consistent with previous reports showing that in adults, NTM infection of the lung is much more frequent than infection at other sites (18). The finding of higher rates of NTM infection in older individuals is also consistent with studies showing that pulmonary and nonpulmonary NTM diseases both increase with age (4–6, 10, 13, 35, 36). Several reports have indicated increasing incidence and prevalence of NTM infections across the United States (5, 6, 35), Canada (7, 8), Europe (4, 10, 11), and Asia (37, 38). It is unclear if these trends represent a true or artifactual increase in NTM disease or both. In this analysis of more than 8,000 NTM isolates collected from 6,811 Wisconsin adults from 2011 through 2018, the annual incidence of both total and respiratory NTM infection was stable over the relatively short study period.
We found increased incidence of NTM infection in Black and Asian individuals compared with White individuals. Increased rates of NTM disease in Asian and Pacific Islander subjects were also reported in a large study of pulmonary NTM prevalence among U.S. Medicare beneficiaries (39). However, in contrast to our findings, Black individuals overall in this Medicare population had a significantly lower prevalence of pulmonary NTM disease relative to White individuals. Interestingly, this finding was driven by a particularly low rate of NTM prevalence among Black women, which might reflect less access to care (39). The reason for the discrepancy between these earlier data and our finding that Black subjects had a significantly higher incidence of NTM infection than White subjects in most of the ADI scores is not clear and deserves further investigation. One consideration is that our study was not limited to Medicare beneficiaries, and reliance on a statewide laboratory database with broad population coverage may have allowed a more accurate comparison of racial differences in NTM infection. A more recent analysis of data from a large healthcare organization in Hawaii also demonstrated a higher incidence of NTM infection in Asian individuals compared with White individuals (40). The present study thus adds to a growing body of evidence that Asian individuals living in the United States are at higher risk for NTM infection.
Socioeconomic status, a measure that includes environmental risk factors for disease, has received much attention as a risk modifier of both communicable and noncommunicable diseases, such as tuberculosis (27, 28), diabetes (41), and asthma (42, 43). We found higher NTM incidence in those with the greatest social disadvantage according to ADI scoring. Elements of the physical environment in disadvantaged neighborhoods may increase the risk of NTM exposure or infection in those communities. Alternatively, this finding could be due to confounding factors and could have arisen from an unmeasured association between low socioeconomic status and biological or behavioral risk factors for NTM isolation, such as advanced HIV infection or chronic obstructive pulmonary disease (9, 19), which we were unable to analyze in this study. The potential association between socioeconomic disadvantage and NTM disease merits further investigation.
Previous epidemiologic studies in the United States have suggested that Wisconsin has a comparatively large burden of NTM disease (5, 24, 44). In one study of five states where NTM infections are reportable, Wisconsin had the highest prevalence of NTM isolation (5). In another study using a national managed care claims database, Wisconsin was among nine states with the highest prevalence of pulmonary NTM disease (45). Within Wisconsin, we detected a higher incidence of both MAC and RGM in Milwaukee and Winnebago counties, both of which contain large municipalities. An analysis of Medicare population data identified Milwaukee County as one of seven spatial clusters of NTM disease in the United States (24). Both Milwaukee and Winnebago counties lie immediately west of large bodies of water (Lake Michigan and Lake Winnebago, respectively). Surface water area has also been associated with higher NTM disease incidence (24).
Milwaukee and Winnebago counties also have large municipal water supplies. A recent study described frequent NTM colonization of household showerheads, especially those showerheads supplied by municipal as opposed to well water (15). The same study also described an association between pathogenic NTM recovered from showerheads and NTM disease prevalence across geographic regions. Of note, Dane County also has a large area of surface water and a large municipal water supply in Madison, the second most populous city in Wisconsin. However, Dane County had a notably lower incidence of MAC and rapid growers reported compared with Milwaukee and Winnebago counties, suggesting that additional risk factors modulate NTM infection.
MAC is often reported as the most prevalent isolate and the most frequent cause of NTM disease (1, 9, 34, 46). In our study, MAC represented 72.2% of total isolates and 76.4% of respiratory isolates. Together, the M. chelonae-abscessus and M. fortuitum groups were the most frequent species recovered from NR and skin and soft tissue sites, accounting for 45.5% and 59.8% of those categories, respectively. In contrast, M. marinum, a pathogen that generally causes infections restricted to the skin and soft tissue and adjacent structures, represented only 10.2% of the skin and soft tissue NTM. These findings are consistent with a small study of cutaneous NTM infections occurring between 1980 and 2009 in Olmstead County, Minnesota, which borders western Wisconsin. The majority (65% [15 of 24]) of cutaneous NTM infections in the third decade of that study (2000–2009) were caused by RGM. In contrast, M. marinum, a slow-growing species, was the most common NTM (64%) during the first two decades of study (1980–1999) (12). We found that RGM accounted for 46.5% of NR isolates but only 12.5% of respiratory isolates. Other investigators have also reported a predominance of RGM in nonpulmonary NTM disease (13, 38).
M. kansasii and M. xenopi are pathogenic NTM species with specific geographic distributions. Worldwide, M. kansasii has been reported to be most prevalent in Poland, the United Kingdom, and Brazil (46). Older studies showed M. xenopi to be common in southern Europe and Ontario, Canada (8, 46). Within the United States, M. kansasii and M. xenopi are relatively uncommon in most surveillance studies. In recent surveys of pulmonary NTM disease in Oregon and North Carolina, M. kansasii and M. xenopi constituted only 1.2–1.9% and 0.3%, respectively, of reported species (6, 13). A study of 4,200 NTM isolates reported to state health departments in Mississippi, Missouri, Ohio, and Wisconsin in 2014 found that only 2.4% were M. kansasii and 1.3% were M. xenopi (47). In contrast, a 10-year, retrospective study of patients with NTM lung disease revealed that M. kansasii accounted for 7.7% of cases (48). In the present study, we found a low proportion of M. kansasii (0.9%). M. xenopi represented 2.0% of all isolates. M. xenopi has been associated with high mortality, especially in HIV-positive patients, and aggressive treatment is recommended (16). We raise concern for M. xenopi as a possible emerging pathogen in Wisconsin.
Strengths and Limitations
A notable strength of our study is its reliance on a comprehensive statewide electronic database, WEDSS. As Wisconsin statutes mandate reporting of mycobacterial disease, usually accomplished through laboratory reporting into this database, our data represent a reasonable estimate of the total burden of NTM infection within the state, although reporting accuracy and adherence have not been studied. In addition, because some demographic data are also captured in this database, we were able to analyze NTM frequency among different racial groups and by socioeconomic status.
Our study has several important limitations. First, particularly for pulmonary NTM disease, clinical information (symptoms, chest imaging) is required to define a case of NTM disease. The absence of such data in this laboratory-based study may reduce the clinical relevance of the findings presented. On the other hand, we did not assume that recovery of the same respiratory isolate more than once counted as “disease,” an approach used by others with its own limitations (49). Furthermore, consideration of the type of respiratory specimen is an important factor that we were not able to assess in this study. Guidelines give more weight to samples acquired using invasive procedures, such as from bronchoalveolar lavage, in the diagnostic criteria for pulmonary NTM disease compared with expectorated sputum (16). Unequal access to healthcare and procedural testing for NTM disease across racial and socioeconomic groups deserves further study.
Second, race was not reported for 30.2% of included individuals, though the analysis in which missing race data were imputed produced similar results. Third, the population estimates for the incidence calculations do not take into account annual population changes during the study period but instead are the best estimates for the midpoint (2014) of this study. This does not greatly affect the conclusions drawn from our incidence estimates, as changes to the population during this period were negligible.
Fourth, a relatively high percentage of the Asian and Black population lives in Milwaukee County, an area with elevated NTM incidence. However, we were unable to evaluate the geographic risk of NTM infection independently of race. It is important that future investigations use methods and statistical approaches that allow the assessment of NTM infection risk across geographic locations independent of race and socioeconomic status and vice versa.
Finally, techniques for laboratory identification of NTM to the species and subspecies levels changed over the study period. Most notably, distinctions between 1) M. chimaera and M. intracellulare and 2) M. abscessus and M. chelonae were technically infeasible during most of the study period. For this reason, these species were combined into MAC and M. chelonae-abscessus groups, respectively. Given recent advances in the molecular identification of NTM, we anticipate that future epidemiologic studies will be able to analyze individual species of particular clinical relevance (50). For example, research on M. abscessus has revealed important differences in susceptibility to macrolide antibiotics across its three constituent subspecies, M. abscessus abscessus, M. abscessus bolletii, and M. abscessus massiliense (51, 52).
Conclusions
This comprehensive analysis of NTM infections in Wisconsin adults adds to our understanding of NTM epidemiology in several important ways. First, in contrast to reports of increasing rates of NTM disease, we found a stable annual incidence of NTM isolation over a relatively short 8-year interval. Second, our study provides evidence that RGM, including M. abscessus subspecies, M. fortuitum, and M. chelonae, now predominate as skin and soft tissue pathogens, as reported elsewhere (12). Third, RGM appear to be important minor respiratory pathogens. Fourth, we highlight M. xenopi as a potential emerging pathogen in this region. Fifth, we report wide variation in NTM isolation across the state, which suggests geographic heterogeneity in exposure. Finally, our data indicate higher rates of NTM infection among Black and Asian individuals and among individuals living in disadvantaged neighborhoods, with racial disparities persisting across measures of neighborhood disadvantage. This finding suggests that race and socioeconomic disparities independently influence the risk of NTM infection and thus may do so for NTM disease as well. Future work should investigate the interactions among environmental, racial, and social determinants of NTM infection and disease.
Acknowledgments
Acknowledgment
The authors thank Laura Louison, tuberculosis laboratory program coordinator at the Wisconsin State Laboratory of Hygiene, for clarifying various terms and laboratory techniques for identification of mycobacteria. The authors thank Andrew Swartz and Benjamin Anderson from the Wisconsin Department of Health Services, Geographic Information System Team, who assisted with all aspects of geocoding and ADI rank assignment.
Footnotes
Supported by National Institute of Allergy and Infectious Diseases award T32AI055397 (B.J.V.) and the University of Wisconsin School of Medicine and Public Health Shapiro Summer Research Program (K.P.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: D.G., S.N.G.-B., and E.A.M. conceived of the study. B.J.V., D.G., S.N.G.-B., and E.A.M. designed the study. K.P.H., J.T.-K., and S.N.G.-B. acquired the data. B.J.V., D.G., K.P.H., A.L.W., B.C.A., and S.N.G.-B. analyzed the data. B.J.V., D.G., and E.A.M. interpreted the data and drafted the manuscript. All authors provided intellectual input in the manuscript revisions.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Honda JR, Virdi R, Chan ED. Global environmental nontuberculous mycobacteria and their contemporaneous man-made and natural niches. Front Microbiol . 2018;9:2029. doi: 10.3389/fmicb.2018.02029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Daley CL, Griffith DE. Pulmonary non-tuberculous mycobacterial infections. Int J Tuberc Lung Dis . 2010;14:665–671. [PubMed] [Google Scholar]
- 3.German Collection of Microorganisms and Cell Cultures. 2020. https://lpsn.dsmz.de/genus/mycobacterium
- 4. Dakić I, Arandjelović I, Savić B, Jovanović S, Tošić M, Kurucin T, et al. Pulmonary isolation and clinical relevance of nontuberculous mycobacteria during nationwide survey in Serbia, 2010–2015. PLoS One . 2018;13:e0207751. doi: 10.1371/journal.pone.0207751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Donohue MJ, Wymer L. Increasing prevalence rate of nontuberculous mycobacteria infections in five states, 2008–2013. Ann Am Thorac Soc . 2016;13:2143–2150. doi: 10.1513/AnnalsATS.201605-353OC. [DOI] [PubMed] [Google Scholar]
- 6. Henkle E, Hedberg K, Schafer S, Novosad S, Winthrop KL. Population-based incidence of pulmonary nontuberculous mycobacterial disease in Oregon 2007 to 2012. Ann Am Thorac Soc . 2015;12:642–647. doi: 10.1513/AnnalsATS.201412-559OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marras TK, Chedore P, Ying AM, Jamieson F. Isolation prevalence of pulmonary non-tuberculous mycobacteria in Ontario, 1997 2003. Thorax . 2007;62:661–666. doi: 10.1136/thx.2006.070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marras TK, Mendelson D, Marchand-Austin A, May K, Jamieson FB. Pulmonary nontuberculous mycobacterial disease, Ontario, Canada, 1998–2010. Emerg Infect Dis . 2013;19:1889–1891. doi: 10.3201/eid1911.130737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med . 2015;36:13–34. doi: 10.1016/j.ccm.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ringshausen FC, Wagner D, de Roux A, Diel R, Hohmann D, Hickstein L, et al. Prevalence of nontuberculous mycobacterial pulmonary disease, Germany, 2009–2014. Emerg Infect Dis . 2016;22:1102–1105. doi: 10.3201/eid2206.151642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Ingen J, Hoefsloot W, Dekhuijzen PNR, Boeree MJ, van Soolingen D. The changing pattern of clinical Mycobacterium avium isolation in the Netherlands. Int J Tuberc lung Dis . 2010;14:1176–1180. [PubMed] [Google Scholar]
- 12. Wentworth AB, Drage LA, Wengenack NL, Wilson JW, Lohse CM. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clin Proc . 2013;88:38–45. doi: 10.1016/j.mayocp.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith GS, Ghio AJ, Stout JE, Messier KP, Hudgens EE, Murphy MS, et al. Epidemiology of nontuberculous mycobacteria isolations among central North Carolina residents, 2006–2010. J Infect . 2016;72:678–686. doi: 10.1016/j.jinf.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 14. Hermansen TS, Ravn P, Svensson E, Lillebaek T. Nontuberculous mycobacteria in Denmark, incidence and clinical importance during the last quarter-century. Sci Rep . 2017;7:6696. doi: 10.1038/s41598-017-06931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gebert MJ, Delgado-Baquerizo M, Oliverio AM, Webster TM, Nichols LM, Honda JR, et al. Ecological analyses of mycobacteria in showerhead biofilms and their relevance to human health. mBio . 2018;9:1–15. doi: 10.1128/mBio.01614-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ, Jr, Andrejak C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis . 2020;71:e1–e36. doi: 10.1093/cid/ciaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kasperbauer S, Huitt G. Management of extrapulmonary nontuberculous mycobacterial infections. Semin Respir Crit Care Med . 2013;34:143–150. doi: 10.1055/s-0033-1333576. [DOI] [PubMed] [Google Scholar]
- 18. López-Varela E, García-Basteiro AL, Santiago B, Wagner D, van Ingen J, Kampmann B. Non-tuberculous mycobacteria in children: muddying the waters of tuberculosis diagnosis. Lancet Respir Med . 2015;3:244–256. doi: 10.1016/S2213-2600(15)00062-4. [DOI] [PubMed] [Google Scholar]
- 19. Wu UI, Holland SM. Host susceptibility to non-tuberculous mycobacterial infections. Lancet Infect Dis . 2015;15:968–980. doi: 10.1016/S1473-3099(15)00089-4. [DOI] [PubMed] [Google Scholar]
- 20. Simner PJ, Woods GL, Wengenack NL. Mycobacteria. Microbiol Spectr . 2016;4 doi: 10.1128/microbiolspec.DMIH2-0016-2015. [DOI] [PubMed] [Google Scholar]
- 21. Jabbour SF, Malek AE, Kechichian EG, Tomb RR, Nasr MW. Nontuberculous mycobacterial infections after cosmetic procedures: a systematic review and management algorithm. Dermatol Surg . 2020;46:116–121. doi: 10.1097/DSS.0000000000001929. [DOI] [PubMed] [Google Scholar]
- 22. Everall I, Nogueira CL, Bryant JM, Sánchez-Busó L, Chimara E, Duarte RDS, et al. Genomic epidemiology of a national outbreak of post-surgical Mycobacterium abscessus wound infections in Brazil. Microb Genom . 2017;3:e000111. doi: 10.1099/mgen.0.000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bryant JM, Grogono DM, Greaves D, Foweraker J, Roddick I, Inns T, et al. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet . 2013;381:1551–1560. doi: 10.1016/S0140-6736(13)60632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adjemian J, Olivier KN, Seitz AE, Falkinham JO, III, Holland SM, Prevots DR. Spatial clusters of nontuberculous mycobacterial lung disease in the United States. Am J Respir Crit Care Med . 2012;186:553–558. doi: 10.1164/rccm.201205-0913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. 2016. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-23-6.pdf
- 26. Kakchapati S, Maharjan M, Rawal BB, Dixit SM. Social determinants and risk behaviors associated with prevalent Hepatitis C and HIV/HCV co-infection among male injection drug users in Nepal. Arch Public Health . 2017;75:39. doi: 10.1186/s13690-017-0206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Duarte R, Lönnroth K, Carvalho C, Lima F, Carvalho ACC, Muñoz-Torrico M, et al. Tuberculosis, social determinants and co-morbidities (including HIV) Pulmonology . 2018;24:115–119. doi: 10.1016/j.rppnen.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 28. Satyanarayana S, Thekkur P, Kumar AMV, Lin Y, Dlodlo RA, Khogali M, et al. An opportunity to end TB: using the sustainable development goals for action on socio-economic determinants of TB in high burden countries in WHO South-East Asia and the Western Pacific regions. Trop Med Infect Dis . 2020;5:1–19. doi: 10.3390/tropicalmed5020101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible—the Neighborhood Atlas. N Engl J Med . 2018;378:2456–2458. doi: 10.1056/NEJMp1802313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.University of Wisconsin School of Medicine and Public Health. 2020. https://www.neighborhoodatlas.medicine.wisc.edu/
- 31.U.S. Census Bureau. 2021. https://www.census.gov/programs-surveys/decennial-census/about/rdo.html
- 32. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med . 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 33.Rubin DB. 1987. [Google Scholar]
- 34. Kendall BA, Winthrop KL. Update on the epidemiology of pulmonary nontuberculous mycobacterial infections. Semin Respir Crit Care Med . 2013;34:87–94. doi: 10.1055/s-0033-1333567. [DOI] [PubMed] [Google Scholar]
- 35. Adjemian J, Frankland TB, Daida YG, Honda JR, Olivier KN, Zelazny A, et al. Epidemiology of nontuberculous mycobacterial lung disease and tuberculosis, Hawaii, USA. Emerg Infect Dis . 2017;23:439–447. doi: 10.3201/eid2303.161827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morimoto K, Hasegawa N, Izumi K, Namkoong H, Uchimura K, Yoshiyama T, et al. A laboratory-based analysis of nontuberculous mycobacterial lung disease in Japan from 2012 to 2013. Ann Am Thorac Soc . 2017;14:49–56. doi: 10.1513/AnnalsATS.201607-573OC. [DOI] [PubMed] [Google Scholar]
- 37. Ko RE, Moon SM, Ahn S, Jhun BW, Jeon K, Kwon OJ, et al. Changing epidemiology of nontuberculous mycobacterial lung diseases in a tertiary referral hospital in Korea between 2001 and 2015. J Korean Med Sci . 2018;33:e65. doi: 10.3346/jkms.2018.33.e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lai CC, Tan CK, Lin SH, Liu WL, Liao CH, Huang YT, et al. Clinical significance of nontuberculous mycobacteria isolates in elderly Taiwanese patients. Eur J Clin Microbiol Infect Dis . 2011;30:779–783. doi: 10.1007/s10096-011-1155-8. [DOI] [PubMed] [Google Scholar]
- 39. Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med . 2012;185:881–886. doi: 10.1164/rccm.201111-2016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blakney RA, Ricotta EE, Frankland TB, Honda S, Zelazny A, Mayer-Barber KD, et al. Incidence of nontuberculous mycobacterial pulmonary infection, by ethnic group, Hawaii, USA, 2005–2019. Emerg Infect Dis . 2022;28:1543–1550. doi: 10.3201/eid2808.212375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mendenhall E, Kohrt BA, Norris SA, Ndetei D, Prabhakaran D. Non-communicable disease syndemics: poverty, depression, and diabetes among low-income populations. Lancet . 2017;389:951–963. doi: 10.1016/S0140-6736(17)30402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brigham E, Allbright K, Harris D. Health disparities in environmental and occupational lung disease. Clin Chest Med . 2020;41:623–639. doi: 10.1016/j.ccm.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 43. Stempel H, Federico MJ, Szefler SJ. Applying a biopsychosocial model to inner city asthma: recent approaches to address pediatric asthma health disparities. Paediatr Respir Rev . 2019;32:10–15. doi: 10.1016/j.prrv.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 44. Adjemian J, Olivier KN, Prevots DR. Nontuberculous mycobacteria among patients with cystic fibrosis in the United States: screening practices and environmental risk. Am J Respir Crit Care Med . 2014;190:581–586. doi: 10.1164/rccm.201405-0884OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Winthrop KL, Marras TK, Adjemian J, Zhang H, Wang P, Zhang Q. Incidence and prevalence of nontuberculous mycobacterial lung disease in a large US managed care health plan, 2008–2015. Ann Am Thorac Soc . 2020;17:178–185. doi: 10.1513/AnnalsATS.201804-236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hoefsloot W, van Ingen J, Andrejak C, Angeby K, Bauriaud R, Bemer P, et al. Nontuberculous Mycobacteria Network European Trials Group The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J . 2013;42:1604–1613. doi: 10.1183/09031936.00149212. [DOI] [PubMed] [Google Scholar]
- 47. Donohue MJ. Increasing nontuberculous mycobacteria reporting rates and species diversity identified in clinical laboratory reports. BMC Infect Dis . 2018;18:163. doi: 10.1186/s12879-018-3043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Abate G, Stapleton JT, Rouphael N, Creech B, Stout JE, El Sahly HM, et al. Variability in the management of adults with pulmonary nontuberculous mycobacterial disease. Clin Infect Dis . 2021;72:1127–1137. doi: 10.1093/cid/ciaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis . 2009;49:e124–e129. doi: 10.1086/648443. [DOI] [PubMed] [Google Scholar]
- 50. Minias A, Żukowska L, Lach J, Jagielski T, Strapagiel D, Kim SY, et al. Subspecies-specific sequence detection for differentiation of Mycobacterium abscessus complex. Sci Rep . 2020;10:16415. doi: 10.1038/s41598-020-73607-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mougari F, Bouziane F, Crockett F, Nessar R, Chau F, Veziris N, et al. Selection of resistance to clarithromycin in Mycobacterium abscessus subspecies. Antimicrob Agents Chemother . 2016;61:e00943-16. doi: 10.1128/AAC.00943-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mougari F, Amarsy R, Veziris N, Bastian S, Brossier F, Berçot B, et al. Standardized interpretation of antibiotic susceptibility testing and resistance genotyping for Mycobacterium abscessus with regard to subspecies and erm41 sequevar. J Antimicrob Chemother . 2016;71:2208–2212. doi: 10.1093/jac/dkw130. [DOI] [PubMed] [Google Scholar]