Abstract
Rationale
Poor adherence limits the effectiveness of continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea (OSA). A better understanding of CPAP adherence is needed to develop novel strategies to improve it.
Objectives
To determine if the chronotype (morning, evening, or intermediate) of patients with OSA is associated with differences in CPAP adherence. If such an association exists, determine the mechanisms underlying this association.
Methods
We performed a secondary analysis of the APPLES (Apnea Positive Pressure Long-term Efficacy Study) clinical trial. We assessed chronotype using the Morningness-Eveningness Questionnaire (MEQ) among participants randomized to the CPAP arm with daily adherence data (n = 469). Evening (MEQ ⩽ 41), intermediate (41 < MEQ < 59), and morning type (MEQ ⩾ 59) categories were the exposures. We modeled daily CPAP use (hours per night) over a 6-month period, using a linear mixed model, adjusted for covariates (e.g., age, sex, marital status). To assess mechanisms of the association, we performed mediation analyses using sleep duration, weekend catch-up sleep, depression, and other factors.
Results
Most participants were obese men with severe OSA (body mass index of 32.3 ± 7.3 kg/m2, 65% male, and apnea–hypopnea index 39.8 ± 24.6/h). Participants were 44% morning, 47% intermediate, and 8% evening chronotype. Participants with the morning chronotype reported the shortest sleep duration on weekends (7.3 vs. 7.6 and 7.9 h/night) compared with the intermediate and evening types. Participants with the morning chronotype exhibited a 40-min/night higher CPAP use (P = 0.001) than persons with the intermediate chronotype. This relationship was mildly attenuated (32.8 min/night; P = 0.011) after adjustment for covariates. None of the selected factors (e.g., sleep duration, weekend catch-up sleep) exhibited a significant mediation effect.
Conclusions
Morning chronotype is associated with a clinically meaningful increase in CPAP adherence compared with other chronotypes. Mechanisms of this association require further study. Chronotype may be a novel predictor of CPAP adherence.
Clinical trial registered with www.clinicaltrials.gov (NCT 00051363).
Keywords: OSA, chronotype, CPAP, adherence
Obstructive sleep apnea (OSA) affects nearly 1 billion adults worldwide (1). Untreated OSA impairs daytime function and quality of life and is a risk factor for neurocognitive decline, diabetes, stroke, heart failure, and cancer (2–6). Continuous positive airway pressure (CPAP) is the first-line therapy for moderate to severe OSA, prescribed to approximately 85% of patients with newly diagnosed disease (7). Self-reported sleepiness, objective sleepiness, and functional status improve with each hour of CPAP use per night (8). However, even in idealized environments, such as clinical trials of CPAP therapy, only 39–42% of patients use CPAP for >4 hours per night, markedly limiting effectiveness (9–11). Understanding the factors that influence CPAP adherence is important if we are to improve CPAP effectiveness and mitigate the burden of OSA.
In recent years, the biopsychosocial model has been used to better understand adherence to medical therapy, including CPAP (12). Biomedical factors such as age, sex, and OSA severity are notably inconsistent predictors of CPAP adherence (12). On the other hand, psychosocial factors, such as beliefs about treatment, self-efficacy, and social support are more consistent predictors of adherence (12). Although interventions to address the psychosocial factors have been developed (13, 14), a recent systematic review reveals no meaningful improvement in adherence rates since objective CPAP monitoring was introduced (15). Notably, current models of CPAP adherence explain only a fraction (11–58%) of the variance in CPAP use (12, 16), suggesting that other, yet-to-be-identified, factors may be important.
Recent data suggest that novel biological metrics may help predict adherence. For example, a low arousal threshold, an endotype of OSA, is associated with poor CPAP use (17). Another novel biological factor may be an individual’s chronotype or preference of sleep–wake timing (18). Chronotype is categorized into morning, evening, or intermediate type. Morning and intermediate chronotype are more common, with 29.6–62.1% of adults being classified as morning chronotype and 35.7–64.5% as intermediate chronotype versus 2.2–5.9% as evening chronotype in various study populations (19–21). Compared with individuals with an evening chronotype, those with a morning chronotype exhibit earlier sleep onset times and earlier times for optimal physical and cognitive function independent of environmental factors (22). Chronotype can also influence health-promoting behaviors, such as eating a healthy diet, exercising, avoidance of drug use, and safer sexual practices (23–26). For example, persons with the morning chronotype (vs. evening) consume significantly less alcohol (27) and exhibit higher self-regulation (28). It is plausible that an individual’s chronotype may influence other behaviors, such as adherence to CPAP therapy. Notably, the morning chronotype is also associated with longer sleep duration, more regular sleep, and lower rates of mood disorders (e.g., depression, bipolar disorder). In turn, these factors are associated with increased CPAP adherence (29–33), suggesting potential mechanisms by which chronotype may influence adherence. Understanding the role of chronotype in CPAP adherence may help to better identify and treat those at risk of nonadherence.
Our primary aim was to determine whether chronotype is associated with CPAP adherence. We hypothesized that the morning chronotype will be associated with increased CPAP use independently of other biopsychosocial adherence determinants. Our secondary aim was to examine the potential mechanisms of this association. We hypothesized that increased CPAP adherence in persons with a morning chronotype is mediated through metrics of sleep (e.g., longer sleep duration, less insomnia) or lower rates of comorbidities such as depression and obesity, which are differentially associated with chronotypes and thus are plausible as underlying mechanisms for observed differences in CPAP adherence (29–34).
Some of the results of this study have been previously reported in the form of an abstract (35).
Methods
Study Design, Participants, and Analytic Sample
We performed a secondary analysis of the APPLES (Apnea Positive Pressure Long-term Efficacy Study) trial. The detailed methodology for APPLES has been previously published (9). Briefly, the study randomized 1,105 participants to active versus sham CPAP stratified by sex, race (White vs. non-White), and OSA severity (moderate, 15.1–30.0; severe, >30.0; using American Academy of Sleep Medicine Task Force [1999] OSA diagnostic criteria), with follow-up of 6 months. Individuals working night or rotating shifts were excluded from enrollment in APPLES. The primary outcome of APPLES was neurocognitive function, and detailed assessments of CPAP adherence and potential covariates were measured. Participants randomized to the active CPAP arm (n = 558) were eligible for our study. Figure 1 displays the reasons for exclusion that yielded a final analytic sample of 469 participants (see Tables E1 and E2).
Figure 1.
Analytic sample selection. Patients were disqualified (n = 12) from parent APPLES (Apnea Positive Pressure Long-term Efficacy Study) cohort for the following reasons: no continuous positive airway pressure (CPAP) download data, new medical condition (e.g., intracranial hemorrhage, psychosis), safety reasons, and drowsy driving risk. Patients were dropped (n = 65) from parent APPLES cohort due to: CPAP intolerance during titration study without CPAP attempt at home, dropped or lost to follow-up (<30 d of follow-up), insufficient time with follow-up (<30 d), new medical condition precluding use, seeking non–positive airway pressure treatment, withdrawal before study start, moving, or death. MEQ = Morningness-Eveningness Questionnaire.
Exposure
Our primary exposure was chronotype assessed by the validated Morningness-Eveningness Questionnaire (MEQ) (22). We analyzed chronotype as a categorical variable with subdivisions into evening type (MEQ ⩽ 41), intermediate type (41 < MEQ < 59), and morning type (MEQ ⩾ 59) categories (22). Because of the distribution of the chronotypes (small sample of evening types; see Table 1), we selected the intermediate chronotype as the reference.
Table 1.
Clinical characteristics stratified by Morning-Eveningness Questionnaire chronotype
| Characteristic | Variable Type | Chronotype |
P Value for Comparisons between Chronotypes*†‡ | ||
|---|---|---|---|---|---|
| Morning (n = 207) | Intermediate (n = 223) | Evening (n = 39) | |||
| Demographics and medical history | |||||
| Age, yr | COV | 56.3 ± 11.3 56 (49–64.5) |
50.3 ± 11.5 50 (42–58.5) |
47.9 ± 13.4 47 (41–58) |
<0.001* 0.426† <0.001‡ |
| Sex | COV | 0.815* 0.071† 0.111‡ |
|||
| Male | 137 (66.2) | 151 (67.7) | 20 (51.3) | ||
| Female | 70 (33.8) | 72 (32.3) | 19 (48.7) | ||
| Race/ethnicity | COV | 0.127* 0.236† 0.003‡ |
|||
| White | 169 (81.6) | 165 (74) | 28 (71.8) | ||
| Asian | 6 (2.90) | 17 (7.62) | 7 (17.9) | ||
| Black | 18 (8.70) | 24 (10.8) | 2 (5.13) | ||
| Hispanic | 9 (4.35) | 14 (6.28) | 2 (5.13) | ||
| Other | 5 (2.42) | 3 (1.35) | 0 (0) | ||
| Marital status | COV | <0.001* 0.005† <0.001‡ |
|||
| Married | 146 (70.5) | 121 (54.3) | 11 (28.2) | ||
| Other | 61 (29.5) | 102 (45.7) | 28 (71.8) | ||
| Level of education, yr | COV | 15.6 ± 2.67 16 (14–18) |
15.6 ± 2.38 16 (14–17.2) |
16.1 ± 2.57 16 (14–18) |
0.987* 0.337† 0.335‡ |
| BMI, kg/m2 | MED | 31.2 ± 6.48 30.1 (26.8–34.8) |
32.7 ± 7.19 31.4 (27.5–36.8) |
34.9 ± 10.4 33 (27.4–38) |
0.095* 0.449† 0.105‡ |
| Anxiety | MED | 10 (4.83) | 25 (11.2) | 2 (5.13) | 0.026* 0.401† 1‡ |
| Depression | MED | 28 (13.5) | 33 (14.8) | 5 (12.8) | 0.828* 0.930† 1‡ |
| Sleep characteristics (self-reported) | |||||
| Total sleep duration, h | MED | 7.03 ± 1.14 7 (6.28–8) |
7.04 ± 1.24 7.28 (6.28–8) |
6.95 ± 1.38 7.21 (6.14–8) |
0.477* 0.923† 0.766‡ |
| Weekend sleep duration, h | MED | 7.31 ± 1.27 7 (6.5–8) |
7.63 ± 1.49 8 (7–8.5) |
7.90 ± 1.97 8 (7–9) |
0.013* 0.272† 0.015 |
| Weekday sleep duration, h | MED | 6.93 ± 1.15 7 (6–8) |
6.81 ± 1.28 7 (6–8) |
6.58 ± 1.31 7 (6–8) |
0.637* 0.302† 0.197‡ |
| Sleep efficiency, % | MED | 77.3 ± 12.0 79.6 (70.2–85.8) |
79.5 ± 13.8 83.1 (73.8–88.6) |
72.0 ± 16.2 76.7 (65.8–82.8) |
0.010* 0.002† 0.074‡ |
| Weekend catch-up sleep, h | MED | 0.381 ± 0.696 0 (0–1) |
0.826 ± 1.12 1 (0–1.5) |
1.32 ± 1.44 1 (0–2) |
<0.001* 0.061† <0.001‡ |
| ESS score | MED | 10.5 ± 4.16 10 (8–13) |
10.3 ± 4.64 10 (7–14) |
9.31 ± 4.46 9 (6.5–13) |
0.986* 0.601† 0.601‡ |
| ESS score > 10 | MED | 99 (47.8) | 111 (49.8) | 18 (46.2) | 0.758* 0.807† 0.986‡ |
| MWT, min | MED | 17.3 ± 3.66 20 (15.3–20) |
17 ± 3.96 20 (14.6–20) |
16.8 ± 3.85 18.9 (13.7–20) |
0.703* 0.539† 0.414‡ |
| Symptoms (sleep questionnaire) | |||||
| Insomnia | MED | 81 (39.1) | 85 (38.1) | 19 (48.7) | 0.907* 0.229† 0.283‡ |
| Feeling unrested during the day | MED | 36 (17.4) | 63 (28.3) | 16 (41.0) | 0.011* 0.157† 0.002 |
| Insufficient sleep | MED | 20 (9.66) | 41 (18.4) | 6 (15.4) | 0.014* 0.822† 0.434‡ |
| Fatigue | MED | 10 (4.83) | 17 (7.62) | 7 (17.9) | 0.315* 0.08† 0.001‡ |
| Sleep apnea characteristics (PSG) | |||||
| PSG TST, min | MED | 373 ± 62.6 378 (338–414) |
380 ± 67.6 394 (349–424) |
341 ± 78.6 358 (297–399) |
0.067* 0.003† 0.067‡ |
| PSG sleep efficiency, % | MED | 77.3 ± 12.0 83.1 (65.1–83.2) |
79.5 ± 13.8 83.1 (73.7–88.8) |
72.0 ± 16.2 79.6 (70.0–85.8) |
0.085* 0.001† 0.022‡ |
| AHI, events/h | COV | 37.8 ± 21.9 34.6 (20–51.2) |
42.2 ± 27.3 35.4 (22.2–54.2) |
39.1 ± 24.4 33.4 (22.2–54.2) |
0.812* 1† 1‡ |
| Time <85% O2 saturation, % | COV | 2.19 ± 7.09 0.06 (0–0.943) |
2.24 ± 5.84 0.108 (0–1.27) |
2.58 ± 5.11 0.09 (0–3.02) |
0.671* 0.735† 0.671‡ |
| Minimum O2 saturation, % | COV | 81.5 ± 7.17 83 (78–87) |
80.8 ± 7.34 82 (77–86) |
78.8 ± 10.8 82 (74.5–87) |
0.73* 0.73† 0.73‡ |
| Arousal threshold, % eupneic ventilation | COV | 145 ± 28.5 137 (126–158) |
146 ± 31 140 (124–160) |
136 ± 23.8 126 (122–145) |
0.64* 0.066† 0.086‡ |
Definition of abbreviations: AHI = apnea–hypopnea index; BMI = body mass index; COV = covariate; ESS = Epworth Sleepiness Scale; MED = mediator; MWT = maintenance of wakefulness test; PSG = polysomnogram; TST = total sleep time; Weekend catch-up sleep = weekend sleep duration minus weekday sleep duration.
Categorical data are presented as n (%); continuous data are presented as mean ± standard deviation (normal distribution) or median (interquartile range).
P value < 0.05 for morning chronotype compared to intermediate chronotype.
P value < 0.05 for evening chronotype compared to intermediate chronotype.
P value < 0.05 for evening chronotype compared to morning chronotype.
Outcome
CPAP adherence was defined as the daily CPAP use (hours per night) over a 6-month follow-up period. CPAP use was tracked using Encore Pro SmartCards (Phillips Respironics, Inc.) that were returned by the participant twice monthly.
Statistical Approach and Analysis
Descriptive analyses for normally and nonnormally distributed continuous variables are presented as mean ± standard deviation and median (interquartile range). Categorical variables are presented with frequency and percentage. Chi-square and analysis of variance tests were used for univariate comparisons across the three MEQ chronotype categories as appropriate. Significance was taken at P values <0.05.
Our primary aim was to evaluate the association between chronotype and CPAP adherence. This was performed using a linear mixed model, accounting for correlation of repeated measures within the individual over time (36). The fixed effects include MEQ chronotype and time (days), and random effects include a random intercept and a random slope for time (days). Intermediate chronotype was selected as the reference group.
Covariate and Mediator Variables
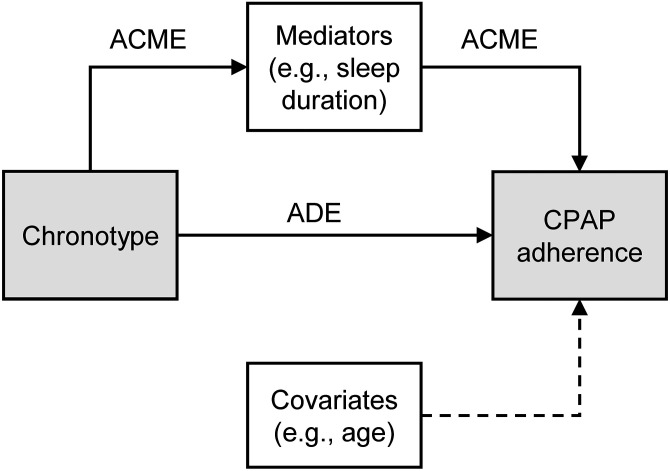
A conceptual framework for the relationship between the chronotype, CPAP adherence, mediators, and covariates is presented in Figure 2. We hypothesized that the chronotype may affect CPAP adherence by either direct or mediated pathways. Accordingly, factors that can influence CPAP adherence were categorized into two domains based on plausible relationships: covariates (e.g., age, sex, ethnicity [37, 38], marital status [39], level of education [40], OSA severity [apnea–hypopnea index, time spent with arterial oxygen saturation <85%] [41], and arousal threshold [17]), and mediators (e.g., sleep duration, weekend catch-up sleep, depression). Self-reported weekday and weekend sleep duration (calculated from sleep onset and sleep offset times), catch-up sleep (hours per night), and sleep efficiency (percentage) were ascertained from patient questionnaires with questions such as “At what time do you usually fall asleep on weekdays or your workdays?” Total sleep time and sleep efficiency were also obtained from standard polysomnography (PSG) at study baseline. The assessment method for each variable is described in the data supplement (see Tables E3 and E4). To distinguish between sleep measures obtained from surveys versus PSG, the remainder of the manuscript refers to self-reported and PSG sleep metrics, respectively.
Figure 2.
Direct acyclic graph of a structural model of mediation for the association between chronotype and CPAP adherence. Mediators include sleep duration, weekend catch-up sleep (difference between weekend and weekday sleep duration), sleep efficiency, insomnia, body mass index, depression, anxiety, and patient-reported symptoms: fatigued, awakening unrested, feeling of insufficient sleep. Covariates and confounders include age, sex, race, ethnicity, marital status, level of education, apnea–hypopnea index, time spent with arterial oxygen saturation <85%, arousal threshold (percentage eupneic ventilation). ACME = average causal mediation effect; ADE = average direct effect; CPAP = continuous positive airway pressure.
Mediation Analyses
To assess the potential mechanisms by which the chronotype may influence CPAP adherence, we constructed a causal mediation model (Figure 2), with CPAP adherence as the outcome. Using linear mixed modeling, adjusted for the above covariates, we quantified the average direct effect, the average causal mediating effect, and the proportion of mediation adjusted for the previously listed covariates for potential mediators. This was performed using the R package mediation (42). In this approach, the average causal mediating effect is the expected difference in CPAP adherence when each potential mediator (e.g., subjective sleep duration) took the value that would occur when a chronotype changed, while the chronotype itself is held constant. This allows for the separation of the mediation effect from the direct effect because any change in the outcome is attributed to the mediation effect given no change in chronotype. The significance of the causal mediation effect is calculated using the Monte Carlo method (43). Potential mediators included factors that may be influenced by chronotype and may impact CPAP adherence as reported in the literature, including subjective sleep duration (29), weekend catch-up sleep (difference between weekend and weekday sleep duration) (44), objective sleep duration and efficiency based on PSG (29), insomnia (45), body mass index (BMI) (46), depression (29), anxiety (47), and patient-reported symptoms including fatigue, awakening unrested, and feeling of insufficient sleep (48).
Sensitivity Analyses
To investigate the possibility our selected mediation factors were acting as confounders, we performed a sensitivity analysis using a fully adjusted model using all characteristics noted in Table 1. In addition, to assess whether the definition of the outcome influenced the relationship between the chronotype and CPAP adherence, we also performed a sensitivity analysis using the Centers for Medicare and Medicaid Services (CMS) adherence definition (⩾4 h/night for >70% of the nights). For the CMS adherence definition analysis, we applied a mixed effects logistic regression model for both the original model and the fully adjusted model described above. Finally, because we found the prevalence of the evening chronotype to be low (<10%), we performed post hoc analyses (primary and mediation) comparing participants with the morning chronotype to the reference group: a combination of individuals with intermediate and evening chronotypes.
Results
Participant Characteristics by Chronotype
Our analytic sample included 469 participants. Most were men with obesity and severe OSA (BMI, 32.3 ± 7.3; 65% male; apnea–hypopnea index, 39.8 ± 24.6/h). Most of the participants had a morning or intermediate chronotype (207 [44%] and 223 [47%], respectively), with only 39 participants (8%) having an evening chronotype (Table 1). The participants with an evening chronotype were on average 8 years younger than the participants with a morning chronotype. Participants with an evening chronotype also had the lowest proportion of reporting a status of married (28.2% vs. 70.5% and 54.3% for participants with morning and intermediate types, respectively). No differences were observed in sex, BMI, or level of education. Participants with the morning chronotype reported the shortest self-reported sleep duration on weekends (7.3 vs. 7.6 and 7.9 h/night) and were less likely to feel unrested during the day (17.4% vs. 28.3% and 41.0%) compared with the those with the intermediate and evening chronotypes. No differences in self-reported weekday sleep duration were noted. Notably, no significant differences were noted in insomnia or sleepiness (Table 1). Anxiety was highest among participants with an intermediate chronotype. No significant differences in sleep apnea characteristics were noted, except for lower total PSG sleep time for participants with an evening compared with an intermediate chronotype.
Chronotype and CPAP Adherence
Compared with the intermediate chronotype, the morning chronotype was associated with higher adherence to CPAP over 6 months (Table 2 and Figure 3). Specifically, the participants with the morning chronotype exhibited a 40.5 min/night higher CPAP use (Table 2; P = 0.001) than participants with the intermediate chronotype. This relationship was mildly attenuated (32.8 min/night; P = 0.011) after adjustment for predetermined covariates. There was no difference in adherence between participants with the intermediate and evening chronotypes.
Table 2.
Association between chronotype and continuous positive airway pressure adherence over 6 months
| Chronotype | Unadjusted |
Adjusted* |
Fully Adjusted† |
|||
|---|---|---|---|---|---|---|
| β‡ (95% CI) | P Value | β (95% CI) | P Value | β (95% CI) | P Value | |
| Intermediate (n = 223) | Reference | n/a | Reference | n/a | Reference | n/a |
| Morning (n = 207) | 40.5 (16.3 to 64.8) | 0.001 | 32.8 (7.8 to 57.9) | 0.011 | 31.9 (6.3 to 57.5) | 0.017 |
| Evening (n = 39) | 8.3 (−35.2 to 51.9) | 0.708 | 22.6 (−21.8 to 67.1) | 0.325 | 23.2 (−23.0 to 69.4) | 0.366 |
Definition of abbreviations: CI = confidence interval; n/a = not applicable.
Adjusted for age, sex, ethnicity, apnea–hypopnea index, time spent with arterial oxygen saturation <85%, arousal threshold, marital status, and level of education.
Adjusted for age, sex, ethnicity, apnea–hypopnea index, time spent with arterial oxygen saturation <85%, arousal threshold, marital status, level of education plus mediators (self-reported sleep duration, sleep efficiency, insomnia, weekend catch-up sleep, depression, anxiety, body mass index, fatigue, sleepiness [Epworth Sleepiness Scale score]).
Continuous positive airway pressure adherence measured in minutes per night over 6 months; β units are minutes per night compared to reference.
Figure 3.
Adherence to continuous positive airway pressure (CPAP) over 6 months according to chronotype (morning, intermediate, evening). Each line is a fitted regression curve, with shaded areas representing the 95% confidence interval for the curve. Morning (n = 207, green line), intermediate (n = 223, blue line), and evening (n = 39, red line) chronotypes as categorized by the Morningness-Eveningness Questionnaire. Data points reflect weekly averages of CPAP adherence for each chronotype.
Mediators of the Association between Chronotype and CPAP Adherence
Because no significant differences were observed between the intermediate and evening chronotypes, the mediation analyses were only performed on a sample including the participants with the morning and evening chronotypes. None of our a priori selected factors (e.g., self-reported or PSG sleep duration, self-reported weekend catch-up sleep) exhibited a significant mediation effect of the association between the morning chronotype and higher CPAP adherence (Table 3).
Table 3.
Adjusted average direct and causal mediation effects for associations between chronotype and continuous positive airway pressure adherence
| Mediator/Association | Morning Chronotype (n = 207) β (95% CI) |
P Value |
|---|---|---|
| Self-reported sleep duration, h | ||
| ADE | 32.7 (10.5 to 58.2) | <0.001 |
| ACME | 0.2 (−4.7 to 4.8) | 0.940 |
| Proportion mediated, % | 0.003 (−0.178 to 0.16) | 0.940 |
| Self-reported sleep efficiency, % | ||
| ADE | 33.9 (11.5 to 59.4) | <0.001 |
| ACME | −0.8 (−4.2 to 1.8) | 0.540 |
| Proportion mediated, % | −0.016 (−0.158 to 0.04) | 0.540 |
| Insomnia, yes/no | ||
| ADE | 33.6 (11.5 to 59.4) | <0.001 |
| ACME | −0.5 (−5.0 to 3.0) | 0.920 |
| Proportion mediated, % | −0.007 (−0.204 to 0.10) | 0.920 |
| Self-reported weekend catch-up sleep, h | ||
| ADE | 36.6 (13.5 to 63.0) | <0.001 |
| ACME | −4.2 (−10.8 to 0.6) | 0.120 |
| Proportion mediated, % | −0.121 (−0.567 to 0.02) | 0.120 |
| Depression, yes/no | ||
| ADE | 32.6 (10.2 to 58.2) | <0.001 |
| ACME | 0.045 (−1.5 to 1.2) | 0.860 |
| Proportion mediated, % | 0.002 (−0.055 to 0.06) | 0.860 |
| Anxiety, yes/no | ||
| ADE | 30.3 (7.8 to 56.4) | 0.020 |
| ACME | 1.0 (−1.7 to 5.4) | 0.560 |
| Proportion mediated, % | 0.021 (−0.077 to 0.27) | 0.560 |
| BMI, kg/m2 | ||
| ADE | 32.9 (10.3 to 58.2) | <0.001 |
| ACME | 0.2 (−1.3 to 2.4) | 0.920 |
| Proportion mediated, % | 0.001 (−0.046 to 0.13) | 0.920 |
| Fatigue, yes/no | ||
| ADE | 33.2 (10.9 to 59.4) | <0.001 |
| ACME | −0.2 (−2.3 to 1.8) | 0.760 |
| Proportion mediated, % | −0.004 (−0.092 to 0.09) | 0.760 |
| Sleepiness, ESS score | ||
| ADE | 32.3 (10.2 to 58.2) | <0.001 |
| ACME | 1.2 (−1.4 to 4.2) | 0.400 |
| Proportion mediated, % | 0.032 (−0.040 to 0.15) | 0.400 |
Definition of abbreviations: ACME = average causal mediation effect; ADE = average direct effect or direct effect of morning chronotype on adherence after accounting for impact of the potential mediator; ESS = Epworth Sleepiness Scale; Weekend catch-up sleep = weekend sleep duration minus weekday sleep duration.
Adjusted for age, sex, ethnicity, apnea–hypopnea index, time spent with arterial oxygen saturation <85%, arousal threshold, marital status, and level of education.
Sensitivity Analyses
Sensitivity analysis using CPAP adherence defined by CMS criteria confirmed that participants with the morning chronotype showed increased CPAP adherence compared with participants with the intermediate chronotype (odds ratio, 3.01; P = 0.001), with minimal attenuation after adjustment for other covariables (odds ratio, 2.31; P = 0.026) (see Table E5). To assess the possibility that our selected mediation factors were acting as confounders, a fully adjusted model using all characteristics showed 31.9 min/night increased adherence for the morning versus the intermediate chronotype (Table 2). Finally, combining individuals with the evening and intermediate chronotypes as a reference (see Table E6) did not meaningfully alter the results, with a morning chronotype exhibiting a 39.3 min/night higher CPAP use (see Table E7 and Figure E1; P = 0.001) than the reference group. As with our primary analysis, none of the potential mediation factors exhibited significant mediation effects (see Table E8).
Discussion
Overview
The key finding of our study is that the morning chronotype is associated with greater CPAP adherence than an intermediate chronotype. The observed 40-minute increase in CPAP use for participants with the morning chronotype is clinically meaningful. The association between CPAP use and chronotype appears robust. The magnitude of the relationship attenuated only mildly, to 32 min/night, after adjustment for factors that may act as covariates (e.g., age, sex, marital status) mediators (e.g., depression, self-reported sleep duration, weekend catch-up sleep, PSG total sleep time). Moreover, the relationship between the morning chronotype and CPAP adherence remains significant in a sensitivity analysis using an alternate, insurance-driven definition for adherence. Finally, our mediation analysis shows that the proposed mediators, including self-reported sleep duration, weekend catch-up sleep, or depression, do not change the association between chronotype and CPAP adherence.
Novel Association between Chronotype and CPAP Adherence
We observed that the morning chronotype is associated with improved CPAP adherence compared with the intermediate type. The lack of statistical differences in adherence for the evening versus the intermediate chronotype could be due to low sample size of the evening type in our sample (n = 39, 8%). These findings align with prior investigations demonstrating that persons with the morning chronotype exhibit better adherence to health-promoting behaviors than persons with the evening chronotype (23–26). For example, one cross-sectional study of more than 172 middle-aged adults showed that the morning chronotype was associated with better adherence to regular physical activity, eating a healthy diet, and less smoking compared with evening chronotypes (46). Similarly, the morning chronotype is associated with increased resilience and optimism scores compared with the intermediate and evening types (49). These associations suggest that persons with a morning chronotype may exhibit higher capacity to face adversity and adapt positively, which may be relevant for CPAP adherence. Our results indicate that chronotype may be a novel candidate for understanding and ultimately improving CPAP adherence. Chronotype is easily assessed by a short survey (MEQ) and can therefore identify patients at elevated risk of CPAP nonadherence.
Mediators of the Association between Chronotype and CPAP Adherence
There are several plausible mechanisms by which chronotype influences CPAP adherence, including associations between chronotype and sleep disruption, health choices, addiction, and mental-emotional health (50–52). For example, persons with a morning chronotype exhibit behaviors and conditions that may result in higher CPAP adherence, such as longer sleep duration, higher sleep quality, less insomnia, and less social jetlag (50, 51, 53). Of particular note is social jetlag, which is seen more commonly in persons with an evening chronotype versus persons with an intermediate or morning chronotype and is associated with worse sleep and other health-related outcomes that may further impact CPAP adherence (51). Beyond sleep disruption, disorders such as depression and anxiety are associated with lower CPAP adherence and are overrepresented among persons with an evening chronotype (54).
We evaluated several potential mediators of the association between chronotype and CPAP adherence, including measures of sleep duration (self-reported and PSG-based), sleep disruption, mood disorders, and impaired daytime symptoms (e.g., sleepiness and fatigue). None of the tested measures mediated the observed association between chronotype and CPAP adherence. One plausible explanation is that differences between chronotypes in the general population may not be reflected in those with OSA. For example, we had predicted that the shorter workday sleep that has been demonstrated in persons with an evening chronotype (33) would result in fewer hours of use and therefore lower calculated adherence. However, in this analysis, we did not observe that sleep duration was a significant contributor to adherence using self-reported or PSG methods (Table 3). Self-reported sleep duration did not differ among persons with any chronotype (Table 1). It is also plausible that differences in habitual sleep duration are not captured by self-report and require objective measures, such as longitudinal actigraphy (see Strengths and Limitations below). Persons with the evening chronotype exhibited a nonsignificantly shorter total sleep time on PSG (341 vs. 373 min; P = 0.067) versus morning chronotype and lower total sleep time (341 vs. 380 min; P = 0.003) compared with the intermediate chronotype. Such differences may be due to the timing of PSG administration typically designed for the majority of the population (morning or intermediate chronotypes) rather than due to habitual shortening of sleep duration. In addition to sleep duration, we evaluated self-reported weekend catch-up sleep as a surrogate measure of social jetlag. Consistent with prior work, weekend catch-up sleep differed among the chronotypes (55) but was not a mediator of the relationship between chronotype and CPAP adherence.
Another explanation for the lack of identifying the mediation may be simply a lack of potential mediators in our dataset. For example, measures of health choices and addiction were not available for testing. Prospective testing of mediation by sleep disruption domains, disorders of mood and mental health, health choice behaviors, and addiction is a key next step in understanding the association between chronotype and CPAP adherence.
Strengths and Limitations
Our study has several strengths, which include accounting for the effects of sleep duration (self-reported and single-night objective PSG total sleep time) on CPAP adherence (a determinant of CPAP use) as well as important covariates that may confound the relationship, including age, sex, and marital status. The association between CPAP use and chronotype appears robust, as supported by adjustment for potential covariates and sensitivity analyses. An additional strength of the study is the daily CPAP adherence data permitting an objective quantification of CPAP use and accounting for correlation of repeated measures within the individual over time.
Because of the nature of our study, secondary data analysis of the APPLES trial, we could not perform longitudinal objective sleep duration measurements in participants’ sleeping environment, including longitudinal assessment of sleep while on CPAP therapy. Lack of these data may have limited our ability to determine whether objective sleep duration is a mechanism for chronotypes’ association with adherence. Similarly, because the APPLES trial was not designed to investigate the association of chronotype with CPAP adherence, the potential mediators used in our analysis are limited to those collected in APPLES, and some mechanisms (e.g., physical activity) could not be addressed (56, 57). Our dataset also included low numbers of persons with an evening chronotype, as determined via standard MEQ cutoffs (22). This may have limited our ability to assess differences in evening versus other chronotypes. Notably, in the general population, the morning and intermediate chronotypes are more common, with 29.6–62.1% of participants being classified as having a morning chronotype and 35.7–64.5% as having an intermediate chronotype versus 2.2–5.9% as having an evening chronotype in various study populations (19–21).
To minimize bias and reduce data loss, we reviewed CPAP use and reasons participants were “dropped” from the per-protocol analysis in the original APPLES. Of the 119 dropped individuals, we were able to include data for 54 participants with CPAP usage (see data supplement for review process and criteria). Nonetheless, 65 participants were excluded from analyses because of no CPAP use or other reasons (e.g., death or moving away). Although the chronotype distribution between included and excluded persons did not differ (χ2 P value = 0.2), exclusion of these participants may limit generalizability of our findings. Finally, participants in the APPLES trial include >80% White persons, and thus our findings may not apply across other racial groups.
We point out that our study is the first to describe chronotype distribution among patients with OSA. In addition, MEQ is validated, correlates with dim-light melatonin onset (58), and is associated with clinically important outcomes, such as response to therapy for depression and adherence to dietary intervention, as described above (46).
Future research should prospectively validate the novel findings we observed in this study, adequately sample the evening chronotype, and collect objective measures of the circadian clock (e.g., dim-light melatonin onset or actigraphy-based metrics). Importantly, this work should explore potential mechanistic pathways between circadian rhythmicity and CPAP adherence, which might include mediators such as health attitudes and cognitive emotional skills like resilience.
Clinical Implications
This knowledge may help identify those likely to succeed with CPAP and those at risk of poor CPAP adherence and contribute to the development of novel interventions to improve CPAP adherence. As noted by Crawford and colleagues (12), the use of a biopsychosocial profile with tailored interventions can translate into a more individualized and multidisciplinary approach to CPAP adherence. The 40-min/night increase in CPAP adherence observed in our study is large compared with other previously assessed variables, especially biomedical factors such as the use of auto-adjusting PAP (11–13 min/night) (59, 60). Of the almost 30 million U.S. adults with OSA, approximately 85% of those diagnosed are prescribed CPAP; however, only 40–60% will remain adherent with treatment over the long term. The human and economic impact of untreated OSA in the United States is enormous, ranging from motor vehicle and workplace accidents to lost productivity ($86.9 billion in 2015) (7).
Conclusions
The impact of individual chronotype on CPAP adherence is a novel finding. The mechanism behind this association needs to be further delineated, and prospective longitudinal studies are needed. Ultimately, chronotype can be integrated into biopsychosocial profiles that can be individualized to improve CPAP adherence.
Acknowledgments
Acknowledgment
The authors thank Kara S. Griffin for her invaluable assistance in extracting and cleaning the data from the APPLES trial used in our analyses.
Footnotes
Supported by National Heart, Lung, and Blood Institute grants K23 HL138229 (M.P.K.), K24HL132093 (H.K.Y.), and K23HL159259 (A.Z.); the Doris Duke Charitable Foundation and Yale Center for Clinical Investigation Fund to Retain Clinical Scientists award 2015216 (M.P.K. and A.Z.); National Institute on Drug Abuse (NIDA) grant K23DA045957 (S.B.); and the Parker B. Francis Foundation (A.Z.).
Author Contributions: Conception and design of the work: A.Z., H.K.Y., M.P.K., and O.A. data collection, data management, trait analysis, and statistical analysis: A.Z., C.K., J.C., A.D., and Z.X. Data interpretation: all authors. Drafting manuscript: M.P.K., A.Z., O.A., H.K.Y., and S.B. Critical revision of manuscript: all authors.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med . 2019;7:687–698. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA . 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davies SK, Ang JE, Revell VL, Holmes B, Mann A, Robertson FP, et al. Effect of sleep deprivation on the human metabolome. Proc Natl Acad Sci USA . 2014;111:10761–10766. doi: 10.1073/pnas.1402663111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med . 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 5. Arzt M, Woehrle H, Oldenburg O, Graml A, Suling A, Erdmann E, et al. SchlaHF Investigators prevalence and predictors of sleep-disordered breathing in patients with stable chronic heart failure: the SchlaHF registry. JACC Heart Fail . 2016;4:116–125. doi: 10.1016/j.jchf.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 6. Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farré R. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med . 2012;186:190–194. doi: 10.1164/rccm.201201-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Academy of Sleep Medicine. 2016. http://www.aasmnet.org/sleep-apnea-economic-impact.aspx
- 8. Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep . 2007;30:711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kushida CA, Nichols DA, Holmes TH, Quan SF, Walsh JK, Gottlieb DJ, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep (Basel) . 2012;35:1593–1602. doi: 10.5665/sleep.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. SAVE Investigators and Coordinators CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med . 2016;375:919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 11. Bakker JP, Weaver TE, Parthasarathy S, Aloia MS. Adherence to CPAP: what should we be aiming for, and how can we get there? Chest . 2019;155:1272–1287. doi: 10.1016/j.chest.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 12. Crawford MR, Espie CA, Bartlett DJ, Grunstein RR. Integrating psychology and medicine in CPAP adherence—new concepts? Sleep Med Rev . 2014;18:123–139. doi: 10.1016/j.smrv.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 13. Wallace DM, Shafazand S, Aloia MS, Wohlgemuth WK. The association of age, insomnia, and self-efficacy with continuous positive airway pressure adherence in black, white, and Hispanic U.S. Veterans. J Clin Sleep Med . 2013;9:885–895. doi: 10.5664/jcsm.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bakker JP, Wang R, Weng J, Aloia MS, Toth C, Morrical MG, et al. Motivational enhancement for increasing adherence to CPAP: a randomized controlled trial. Chest . 2016;150:337–345. doi: 10.1016/j.chest.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg . 2016;45:43. doi: 10.1186/s40463-016-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olsen S, Smith S, Oei T, Douglas J. Health belief model predicts adherence to CPAP before experience with CPAP. Eur Respir J . 2008;32:710–717. doi: 10.1183/09031936.00127507. [DOI] [PubMed] [Google Scholar]
- 17. Zinchuk AV, Chu JH, Liang J, Celik Y, Op de Beeck S, Redeker NS, et al. Physiological traits and adherence to sleep apnea therapy in individuals with coronary artery disease. Am J Respir Crit Care Med . 2021;204:703–712. doi: 10.1164/rccm.202101-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms . 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 19. Paine SJ, Gander PH, Travier N. The epidemiology of morningness/eveningness: influence of age, gender, ethnicity, and socioeconomic factors in adults (30–49 years) J Biol Rhythms . 2006;21:68–76. doi: 10.1177/0748730405283154. [DOI] [PubMed] [Google Scholar]
- 20. Taillard J, Philip P, Chastang JF, Bioulac B. Validation of Horne and Ostberg morningness-eveningness questionnaire in a middle-aged population of French workers. J Biol Rhythms . 2004;19:76–86. doi: 10.1177/0748730403259849. [DOI] [PubMed] [Google Scholar]
- 21. Yu JH, Yun CH, Ahn JH, Suh S, Cho HJ, Lee SK, et al. Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. J Clin Endocrinol Metab . 2015;100:1494–1502. doi: 10.1210/jc.2014-3754. [DOI] [PubMed] [Google Scholar]
- 22. Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol . 1976;4:97–110. [PubMed] [Google Scholar]
- 23. Rodríguez-Muñoz PM, Carmona-Torres JM, Rivera-Picón C, Fabbian F, Manfredini R, Rodríguez-Borrego MA, et al. Associations between chronotype, adherence to the Mediterranean diet and sexual opinion among university students. Nutrients . 2020;12:1900. doi: 10.3390/nu12061900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Broms U, Pennanen M, Patja K, Ollila H, Korhonen T, Kankaanpää A, et al. Diurnal evening type is associated with current smoking, nicotine dependence and nicotine intake in the population based national FINRISK 2007 study. J Addict Res Ther . 2012;S2:002. doi: 10.4172/2155-6105.s2-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nguyen-Louie TT, Brumback T, Worley MJ, Colrain IM, Matt GE, Squeglia LM, et al. Effects of sleep on substance use in adolescents: a longitudinal perspective. Addict Biol . 2018;23:750–760. doi: 10.1111/adb.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taylor BJ, Bowman MA, Brindle A, Hasler BP, Roecklein KA, Krafty RT, et al. Evening chronotype, alcohol use disorder severity, and emotion regulation in college students. Chronobiol Int . 2020;37:1725–1735. doi: 10.1080/07420528.2020.1800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adan A. Chronotype and personality factors in the daily consumption of alcohol and psychostimulants. Addiction . 1994;89:455–462. doi: 10.1111/j.1360-0443.1994.tb00926.x. [DOI] [PubMed] [Google Scholar]
- 28. Owens JA, Dearth-Wesley T, Lewin D, Gioia G, Whitaker RC. Self-regulation and sleep duration, sleepiness, and chronotype in adolescents. Pediatrics . 2016;138:e20161406. doi: 10.1542/peds.2016-1406. [DOI] [PubMed] [Google Scholar]
- 29. Weiss C, Woods K, Filipowicz A, Ingram KK. Sleep quality, sleep structure, and PER3 genotype mediate chronotype effects on depressive symptoms in young adults. Front Psychol . 2020;11:2028. doi: 10.3389/fpsyg.2020.02028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boudebesse C, Lajnef M, Geoffroy PA, Bellivier F, Nieto I, Gard S, et al. French Academic Centres of Expertise for Bipolar Disorders (FACE-BD) Collaborators Chronotypes of bipolar patients in remission: validation of the French version of the circadian type inventory in the FACE-BD sample. Chronobiol Int . 2013;30:1042–1049. doi: 10.3109/07420528.2013.798330. [DOI] [PubMed] [Google Scholar]
- 31. Baek JH, Kim JS, Kim MJ, Ryu S, Lee K, Ha K, et al. Lifetime characteristics of evening-preference and irregular bed-rise time are associated with lifetime seasonal variation of mood and behavior: comparison between individuals with bipolar disorder and healthy controls. Behav Sleep Med . 2016;14:155–168. doi: 10.1080/15402002.2014.974179. [DOI] [PubMed] [Google Scholar]
- 32. Law M, Naughton M, Ho S, Roebuck T, Dabscheck E. Depression may reduce adherence during CPAP titration trial. J Clin Sleep Med . 2014;10:163–169. doi: 10.5664/jcsm.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, et al. Epidemiology of the human circadian clock. Sleep Med Rev . 2007;11:429–438. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 34. Zhang R, Cai X, Lin C, Yang W, Lv F, Wu J, et al. The association between metabolic parameters and evening chronotype and social jetlag in non-shift workers: a meta-analysis. Front Endocrinol (Lausanne) . 2022;13:1008820. doi: 10.3389/fendo.2022.1008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Adekolu O, Xu Z, Chu J, Kushida C, Yaggi H, Knauert M, et al. Influence of chronotype on CPAP adherence. Sleep . 2021;44:A174–A175. [Google Scholar]
- 36. Babbin SF, Velicer WF, Aloia MS, Kushida CA. Identifying longitudinal patterns for individuals and subgroups: an example with adherence to treatment for obstructive sleep apnea. Multivariate Behav Res . 2015;50:91–108. doi: 10.1080/00273171.2014.958211. [DOI] [PubMed] [Google Scholar]
- 37. Patel SR, Bakker JP, Stitt CJ, Aloia MS, Nouraie SM. Age and sex disparities in adherence to CPAP. Chest . 2021;159:382–389. doi: 10.1016/j.chest.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. May AM, Gharibeh T, Wang L, Hurley A, Walia H, Strohl KP, et al. CPAP adherence predictors in a randomized trial of moderate-to-severe OSA enriched with women and minorities. Chest . 2018;154:567–578. doi: 10.1016/j.chest.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gentina T, Bailly S, Jounieaux F, Verkindre C, Broussier PM, Guffroy D, et al. Marital quality, partner’s engagement and continuous positive airway pressure adherence in obstructive sleep apnea. Sleep Med . 2019;55:56–61. doi: 10.1016/j.sleep.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 40. Wozniak DR, Lasserson TJ, Smith I. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev . 2014;1:CD007736. doi: 10.1002/14651858.CD007736.pub2. [DOI] [PubMed] [Google Scholar]
- 41. Wild MR, Engleman HM, Douglas NJ, Espie CA. Can psychological factors help us to determine adherence to CPAP? A prospective study. Eur Respir J . 2004;24:461–465. doi: 10.1183/09031936.04.00114603. [DOI] [PubMed] [Google Scholar]
- 42. Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. mediation: R package for causal mediation analysis J Stat Softw 2014. 59 1 38 26917999 [Google Scholar]
- 43. Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychol Methods . 2010;15:309–334. doi: 10.1037/a0020761. [DOI] [PubMed] [Google Scholar]
- 44. Oh YH, Kim H, Kong M, Oh B, Moon JH. Association between weekend catch-up sleep and health-related quality of life of Korean adults. Medicine (Baltimore) . 2019;98:e14966. doi: 10.1097/MD.0000000000014966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boland EM, Bertulis K, Leong SH, Thase ME, Gehrman PR. Preliminary support for the role of reward relevant effort and chronotype in the depression/insomnia comorbidity. J Affect Disord . 2019;242:220–223. doi: 10.1016/j.jad.2018.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Muscogiuri G, Barrea L, Aprano S, Framondi L, Di Matteo R, Laudisio D, et al. On behalf of the Opera Prevention Project Chronotype and adherence to the Mediterranean diet in obesity: results from the Opera Prevention Project. Nutrients . 2020;12:1354. doi: 10.3390/nu12051354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Antypa N, Vogelzangs N, Meesters Y, Schoevers R, Penninx BW. Chronotype associations with depression and anxiety disorders in a large cohort study. Depress Anxiety . 2016;33:75–83. doi: 10.1002/da.22422. [DOI] [PubMed] [Google Scholar]
- 48. Fárková E, Šmotek M, Bendová Z, Manková D, Kopřivová J. Chronotype and social jet-lag in relation to body weight, appetite, sleep quality and fatigue. Biol Rhythm Res . 2021;52:1205–1216. [Google Scholar]
- 49. Antúnez JM, Navarro JF, Adan A. Circadian typology is related to resilience and optimism in healthy adults. Chronobiol Int . 2015;32:524–530. doi: 10.3109/07420528.2015.1008700. [DOI] [PubMed] [Google Scholar]
- 50. Partonen T. Chronotype and health outcomes. Curr Sleep Med Rep . 2015;1:205–211. [Google Scholar]
- 51. Caliandro R, Streng AA, van Kerkhof LWM, van der Horst GTJ, Chaves I. Social jetlag and related risks for human health: a timely review. Nutrients . 2021;13:4543. doi: 10.3390/nu13124543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gulick D, Gamsby JJ. Racing the clock: the role of circadian rhythmicity in addiction across the lifespan. Pharmacol Ther . 2018;188:124–139. doi: 10.1016/j.pharmthera.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 53. Alvaro PK, Roberts RM, Harris JK. The independent relationships between insomnia, depression, subtypes of anxiety, and chronotype during adolescence. Sleep Med . 2014;15:934–941. doi: 10.1016/j.sleep.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 54. Taillard J, Sagaspe P, Philip P, Bioulac S. Sleep timing, chronotype and social jetlag: impact on cognitive abilities and psychiatric disorders. Biochem Pharmacol . 2021;191:114438. doi: 10.1016/j.bcp.2021.114438. [DOI] [PubMed] [Google Scholar]
- 55. Koo DL, Yang KI, Kim JH, Kim D, Sunwoo JS, Hwangbo Y, et al. Association between morningness-eveningness, sleep duration, weekend catch-up sleep and depression among Korean high-school students. J Sleep Res . 2021;30:e13063. doi: 10.1111/jsr.13063. [DOI] [PubMed] [Google Scholar]
- 56. Henson J, Rowlands AV, Baldry E, Brady EM, Davies MJ, Edwardson CL, et al. CODEC Investigators Physical behaviors and chronotype in people with type 2 diabetes. BMJ Open Diabetes Res Care . 2020;8:e001375. doi: 10.1136/bmjdrc-2020-001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pépin JL, Jullian-Desayes I, Sapène M, Treptow E, Joyeux-Faure M, Benmerad M, et al. Multimodal remote monitoring of high cardiovascular risk patients with OSA initiating CPAP: a randomized trial. Chest . 2019;155:730–739. doi: 10.1016/j.chest.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 58. Reiter AM, Sargent C, Roach GD. Concordance of chronotype categorisations based on dim light melatonin onset, the Morningness-Eveningness Questionnaire, and the Munich Chronotype Questionnaire. Clocks Sleep . 2021;3:342–350. doi: 10.3390/clockssleep3020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Morandi A, Pandharipande PP, Jackson JC, Bellelli G, Trabucchi M, Ely EW. Understanding terminology of delirium and long-term cognitive impairment in critically ill patients. Best Pract Res Clin Anaesthesiol . 2012;26:267–276. doi: 10.1016/j.bpa.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 60. Smith HA, Boyd J, Fuchs DC, Melvin K, Berry P, Shintani A, et al. Diagnosing delirium in critically ill children: validity and reliability of the Pediatric Confusion Assessment Method for the Intensive Care Unit. Crit Care Med . 2011;39:150–157. doi: 10.1097/CCM.0b013e3181feb489. [DOI] [PMC free article] [PubMed] [Google Scholar]