Abstract
Rationale
Disparities in patient selection for advanced therapeutics in health care have been identified in multiple studies, but it is unclear if disparities exist in patient selection for extracorporeal membrane oxygenation (ECMO), a rapidly expanding critical care resource.
Objectives
To determine if disparities exist in patient selection for ECMO based on sex, primary insurance, and median income of the patient’s neighborhood.
Methods
In a retrospective cohort study using the Nationwide Readmissions Database 2016–2019, we identified patients treated with mechanical ventilation (MV) and/or ECMO with billing codes. Patient sex, insurance, and income level for patients receiving ECMO were compared with the patients treated with MV only, and hierarchical logistic regression with the hospital as a random intercept was used to determine odds of receiving ECMO based on patient demographics.
Results
We identified 2,170,752 MV hospitalizations with 18,725 cases of ECMO. Among patients treated with ECMO, 36.1% were female compared with 44.5% of patients treated with> MV only (adjusted odds ratio [aOR] for ECMO, 0.73; 95% confidence interval [CI], 0.70–0.75). Of patients treated with ECMO, 38.1% had private insurance compared with 17.4% of patients treated with MV only. Patients with Medicaid were less likely to receive ECMO than patients with private insurance (aOR, 0.55; 95% CI, 0.52–0.57). Patients treated with ECMO were more likely to live in the highest-income neighborhoods compared with patients treated with MV only (25.1% vs. 17.3%). Patients living in the lowest-income neighborhoods were less likely to receive ECMO than those living in the highest-income neighborhoods (aOR, 0.63; 95% CI, 0.60–0.67).
Conclusions
Significant disparities exist in patient selection for ECMO. Female patients, patients with Medicaid, and patients living in the lowest-income neighborhoods are less likely to be treated with ECMO. Despite possible unmeasured confounding, these findings were robust to multiple sensitivity analyses. On the basis of previous work describing disparities in other areas of health care, we speculate that limited access in some neighborhoods, restrictive/biased interhospital transfer practices, differences in patient preferences, and implicit provider bias may contribute to the observed differences. Future studies with more granular data are needed to identify and modify drivers of observed disparities.
Keywords: artificial respiration, mechanical ventilators, implicit bias
Since its inception in the 1970 s, extracorporeal membrane oxygenation (ECMO) has been thought of as a revolutionary technology with tremendous promise for severe respiratory and/or cardiovascular failure. Despite the optimism, the ideal patient for ECMO remains unclear, especially because large clinical trials have not shown a clear mortality benefit associated with ECMO (1, 2). Nonetheless, ECMO use has grown rapidly in critical care units with a great deal of focus on ECMO availability during the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) coronavirus disease (COVID-19) pandemic (3–10).
ECMO is one of the most advanced, complex, resource-intensive, and expensive interventions available to critically ill patients with respiratory and/or cardiovascular failure (11). As such, not all hospitals are ECMO capable. Given the limited availability of ECMO, optimal patient selection is critical. Sometimes, mortality prediction tools such as the Respiratory ECMO Survival Prediction (RESP) score are used for ECMO patient selection, despite the RESP score having been validated only in a population already receiving ECMO and not in all patients receiving MV (12, 13). Demographic factors, such as sex, insurance, and income, are not supposed to affect patient selection for ECMO. However, multiple studies have highlighted disparities in treatments for critical illness. Female patients are less likely to be admitted to the intensive care unit (ICU) and to receive lung-protective ventilation for acute respiratory distress syndrome (ARDS) (14–18). In addition, female patients, underinsured patients, and patients in lower-income strata are also less likely to be treated with advanced heart failure therapies such as left ventricular assist devices and heart transplant (19–26). Given the recent rapid expansion of ECMO programs, ensuring equitable access to ECMO is critical to reducing health disparities among critically ill patients (3, 4, 6, 27).
To better understand real-world patient selection for ECMO, we conducted a retrospective population study investigating the impact of sex, insurance status, and neighborhood income on patient selection for ECMO in the United States from 2016 to 2019. We hypothesized that in the absence of disparities, sex, insurance status, and income for patients receiving ECMO will be similar to those treated with only mechanical ventilation (MV) and will not be associated with the odds of receiving ECMO.
Methods
Full details are available in the Methods section of the data supplement.
Patients
Using the Nationwide Readmissions Database (NRD) from 2016 to 2019, we conducted a retrospective population-level cohort study of adult patients (⩾18 yr) who received ECMO. The NRD is an all-payer administrative discharge database compiled by the Healthcare Cost and Utilization Project (HCUP) containing 100% of all nonfederal discharges from contributing states (60.4% of all U.S. hospitalizations in 2019) (28). Data sources that rely on a percentage sample of hospitalized patients may poorly estimate extremely rare procedures (e.g., one case of ECMO in a 20% sample of patients from a hospital does not necessarily translate to that hospital performing five ECMO cases in total). Discharge billing codes were used to identify patients who received MV and ECMO (see Table E1 in the data supplement).
Outcomes
The primary outcome was the receipt of ECMO. The primary exposures were patient sex, patient primary insurance, and median income quartile for the zip code in which the patient lived (29). We also compared hospital mortality by demographic category for patients treated with ECMO and those treated with MV only to determine whether differential survival patterns may contribute to ECMO patient selection.
Statistical Analysis
We report categorical and continuous variables with percentages, means, medians, standard deviations, and interquartile ranges as appropriate. The percentage of patients receiving ECMO in a specific demographic category was compared with that of patients who received MV only. To determine the odds of ECMO based on demographic category, hierarchical logistic regression with the hospital as a random intercept was performed with risk adjustment that included patient age, individual Elixhauser comorbidities, and HCUP-specific measures of mortality risk and severity of illness (29–31). Refer to the Methods section of the data supplement for details on variable selection. Separate models were used to determine the association of sex, payer status, and neighborhood income with ECMO use. To address potential unmeasured confounding, we also report E-values for each of the primary analyses (32–34).
Sensitivity Analyses
Although the NRD provides the largest population to evaluate ECMO implementation, it has multiple limitations, including limited risk adjustment for severity of illness. Therefore, we conducted multiple sensitivity analyses across several categories, including 1) analyses to investigate the intersection of demographic categories; 2) analyses to evaluate the association of ECMO patient selection with patient demographics in different subgroups of patients in the NRD; 3) multiple sensitivity analyses to evaluate model overfit and residual confounding, including inverse probability of treatment weight (IPTW) analysis; and 4) multiple sensitivity analyses using seven state inpatient databases (SIDs) from 2018 and 2019 (Arizona, California, Florida, Iowa, Maryland, Mississippi, and New York) to perform analyses not possible with the larger NRD (35–37). Analyses using the SIDs allow more granular risk adjustment and exploration of potential racial and ethnic disparities. The full details of all of the sensitivity analyses and their justifications can be found in the Methods section of the data supplement. Briefly, some of the sensitivity analyses included evaluation of the association between ECMO patient selection and the intersection of sex and neighborhood income, based on previous studies suggesting that intersectional approaches may identify even greater disparities, restriction of the analysis to only hospitals that performed at least one ECMO case (i.e., ECMO-capable hospitals), restricting the analysis to only nonelective admissions, difference in ECMO patient selection by type of ECMO (venovenous [VV] vs. venoarterial [VA]) in 2019 when different billing codes for each were first introduced (Table E1), restricting the analysis to only patients with pneumonia or sepsis present on admission, the least absolute shrinkage and selection operator variable selection method to reduce the number of covariates, the use of the SID data subset for more granular severity of illness risk adjustment with acute organ failures present on admission than is capable with the NRD (Table E2), and many other sensitivity analyses (38–40). The fundamental purpose of the extensive number of sensitivity analyses is to recognize the limitations of the NRD and provide multiple different vantage points of evaluating potential disparities and determining if all analyses provide similar conclusions.
SAS version 9.4 (SAS Institute) was used for all statistical analyses. All tests were two tailed with α = 0.05. The Colorado Multiple Institutional Review Board waived approval and consent (Regional and National Utilization and Trends in Critical Care, COMIRB 20-2949, February 26, 2021).
Results
Patient-Level Use
Between 2016 and 2019, 2,170,752 cases of MV with 18,725 cases of ECMO were identified in the NRD. Patients treated with ECMO tended to be younger than patients treated with MV only (Tables 1 and E4). Patients treated with ECMO were less likely to have cancer and chronic lung disease but more likely to have chronic heart failure (Table E4). Among patients treated with ECMO, 36.1% were female compared with 44.5% of patients treated with MV only. Female patients were less likely to be treated with ECMO compared with male patients (adjusted odds ratio [aOR], 0.73; 95% confidence interval [CI], 0.70–0.75) (Tables 2 and E5).
Table 1.
Patient characteristics*
| Characteristic | ECMO (n = 18,725) | Mechanical Ventilation Only (n = 2,170,752) |
|---|---|---|
| Mean age, yr (SD) | 54.1 (15.7) | 62.6 (16.7) |
| Female, % | 36.1 | 44.5 |
| Primary insurance, % | ||
| Medicare | 36.5 | 57.7 |
| Medicaid | 18.2 | 17.4 |
| Private insurance | 38.1 | 17.4 |
| Other | 7.2 | 7.5 |
| Median income of patient zip code, %† | ||
| Quartile 1 | 24.5 | 32.7 |
| Quartile 2 | 24.6 | 26.8 |
| Quartile 3 | 25.8 | 23.2 |
| Quartile 4 | 25.1 | 17.3 |
| Intersectional identity, % | ||
| Female, income quartile 1 | 9.6 | 14.8 |
| Male, income quartile 1 | 14.9 | 17.9 |
| Female, income quartile 2 | 8.8 | 12.0 |
| Male, income quartile 2 | 15.8 | 14.8 |
| Female, income quartile 3 | 9.2 | 10.3 |
| Male, income quartile 3 | 16.7 | 12.9 |
| Female, income quartile 4 | 8.7 | 7.5 |
| Male, income quartile 4 | 16.4 | 9.7 |
| Median Elixhauser score (IQR)‡ | 11.0 (6.0–17.0) | 10.0 (5.0–16.0) |
| Metastatic cancer, % | 1.0 | 3.8 |
| Chronic heart failure, % | 37.9 | 26.6 |
| Chronic lung disease, % | 19.4 | 32.3 |
| Diabetes, % | 28.0 | 33.7 |
| Hypertension, % | 46.8 | 59.0 |
| Obesity, % | 23.0 | 19.1 |
| Mean HCUP measure of risk of mortality (SD)§ | 3.8 (0.5) | 3.7 (0.7) |
| Mean HCUP measure of severity of illness (SD)§ | 3.9 (0.4) | 3.8 (0.6) |
Definition of abbreviations: ECMO = extracorporeal membrane oxygenation; HCUP = Healthcare Cost and Utilization Project; IQR = interquartile range; SD = standard deviation.
Refer to Table E4 for full patient characteristics by exposure group, including individual Elixhauser comorbidities.
Cutoffs for median income quartiles vary by year. Quartile 1 indicates the lowest income level, and quartile 4 is the highest income level. Full documentation of income levels can be found at https://www.hcup-us.ahrq.gov/db/vars/zipinc_qrtl/nrdnote.jsp.
Calculated without the arrhythmia comorbidity.
The risk of mortality measure and severity of illness measures were developed by HCUP and calculated on the basis of diagnosis-related groups and other billing codes. The score ranges from 0 to 4. More information about these measures can be found at https://www.hcup-us.ahrq.gov/db/nation/nrd/nrddde.jsp.
Table 2.
Adjusted odds of extracorporeal membrane oxygenation use, based on patient demographics
| Demographic Category | Multivariable Hierarchical Logistic Regression aOR (95% CI) |
E-Value Estimates* | IPTW Analysis aOR (95% CI) |
|---|---|---|---|
| Sex | |||
| Female | 0.73 (0.70–0.75) | 2.08 | 0.77 (0.75–0.79)† |
| Male | Reference‡ | — | Reference |
| Primary insurance | |||
| Medicare | 0.50 (0.48–0.52) | 3.41 | — |
| Medicaid | 0.55 (0.52–0.57) | 3.04 | 0.57 (0.55–0.58)§ |
| Other | 0.64 (0.60–0.68) | 2.50 | — |
| Private insurance | Reference‖ | — | Reference |
| Median income of patient zip code¶ | |||
| Quartile 1 | 0.63 (0.60–0.67) | 2.55 | 0.64 (0.61–0.66)** |
| Quartile 2 | 0.78 (0.74–0.81) | 1.88 | — |
| Quartile 3 | 0.87 (0.83–0.91) | 1.56 | — |
| Quartile 4 | Reference†† | — | Reference |
| Intersectionality Identity¶ | |||
| Female, income quartile 1 | 0.50 (0.46–0.53) | 3.41 | —‡‡ |
| Male, income quartile 1 | 0.64 (0.61–0.68) | 2.50 | — |
| Female, income quartile 2 | 0.56 (0.53–0.60) | 2.97 | — |
| Male, income quartile 2 | 0.80 (0.76–0.85) | 1.81 | — |
| Female, income quartile 3 | 0.64 (0.60–0.69) | 2.50 | — |
| Male, income quartile 3 | 0.89 (0.84–0.94) | 1.50 | — |
| Female, income quartile 4 | 0.74 (0.69–0.79) | 2.04 | — |
| Male, income quartile 4 | Reference§§ | — | — |
Definition of abbreviations: aOR = adjusted odds ratio; CI = confidence interval; ECMO = extracorporeal membrane oxygenation; IPTW = inverse probability of treatment weighting.
The E-value estimates the strength that an unmeasured confounder would need to possess in order to shift the observed association to the null. For example, an unmeasured confounder would need to have an adjusted odds ratio of 2.08 in order to shift the observed adjusted association of ECMO use and patient sex from 0.73 to the null (1.00). E-values are calculated from the estimates obtained from multivariable hierarchical logistic regression models (the primary analysis) (32–34).
See Table E8 for standardized mean differences achieved by weighting observations on the inverse probability of the exposure (female vs. male).
See Table E5 for full model parameter estimates including fit statistics.
See Table E9 for standardized mean differences achieved by weighting observations on the inverse probability of the exposure (Medicaid vs. private insurance). Inverse probability of treatment weighting has better performance characteristics for binary exposures, so the analysis focused on patients with Medicaid compared with patients with private insurance.
See Table E6 for full model parameter estimates including fit statistics.
Cutoffs for median income quartiles vary by year. Quartile 1 indicates the lowest income level, and quartile 4 is the highest income level. Full documentation of income levels can be found at https://www.hcup-us.ahrq.gov/db/vars/zipinc_qrtl/nrdnote.jsp.
See Table E10 for standardized mean differences achieved by weighting observations on the inverse probability of the exposure (lowest income quartile 1 vs. highest income quartile 4). Inverse probability of treatment weighting has better performance characteristic for binary exposures, so the analysis focused on patients with lowest income quartile 1 compared with patients with highest income quartile 4.
See Table E7 for full model parameter estimates including fit statistics.
Given smaller cohort sizes for each intersectional category, IPTW was not performed for the intersectional analysis.
See Table E11 for full model parameter estimates including fit statistics.
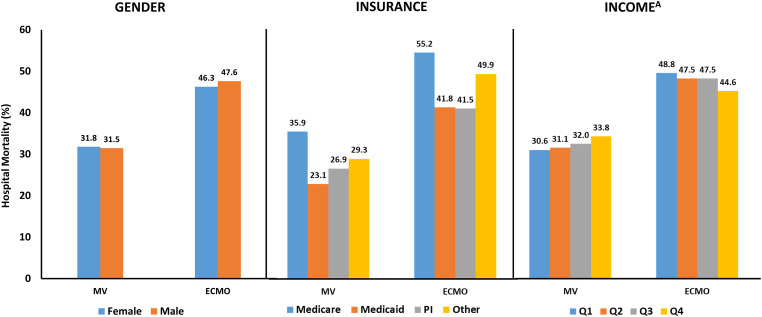
Patients treated with ECMO were less likely to have Medicare as their primary insurance compared with patients treated with MV only (36.5% vs. 57.7%) (Table 1). However, patients treated with ECMO had higher rates of private insurance than patients treated with MV (38.1% vs. 17.4%). Patients with Medicaid (aOR, 0.55; 95% CI, 0.52–0.57) and Medicare (aOR, 0.50; 95% CI, 0.48–0.52) had lower odds of being treated with ECMO than patients with private insurance (Tables 2 and E6). Patients treated with ECMO were also less likely to live in the lowest-income neighborhoods than patients treated with MV only (24.5% vs. 32.7%) and more likely to live in the highest-income neighborhoods (25.1% vs. 17.3%) (Table 1). Patients living in the lowest-income neighborhood had lower odds of receiving ECMO than patients living in the highest-income neighborhoods (aOR, 0.63; 95% CI, 0.60–0.67) (Tables 2 and E7). Despite differences in ECMO use by demographic category, there were minimal differences in hospital mortality based on sex, primary insurance, or income for either patients treated with ECMO or MV only (Figure 1).
Figure 1.
Hospital mortality by demographic and exposure category. Minimal differences were observed in hospital mortality for patients treated with extracorporeal membrane oxygenation (ECMO) or mechanical ventilation (MV) only based on sex. Patients with Medicare had higher mortality in exposure groups likely related to patient age. No differences were observed in hospital mortality for patients treated with ECMO between those with Medicaid and those with private insurance (PI), despite different use rates. Only relatively small differences in mortality were observed on the basis of median income quartiles (Q). AIncome refers to the median income quartile for that patient’s home zip code. Cutoffs for median income quartiles vary by year. Quartile 1 indicates the lowest income level, and quartile 4 is the highest income level. Full documentation of income levels can be found at https://www.hcup-us.ahrq.gov/db/vars/zipinc_qrtl/nrdnote.jsp.
Sensitivity Analyses
We conducted extensive sensitivity analysis to address some of the shortcomings of the NRD and explore other aspects of demographic disparities. When using IPTW to reduce differences between exposure groups, we observed similar differences in patient selection as the primary analysis (Tables 2 and E8–E10). The application of an intersectional framework to disparities revealed greater variation between intersectional identities along the axes of sex and income. When compared with male patients living in the highest-income neighborhoods, female patients living in lower-income quartile neighborhoods had lower odds of receiving ECMO (aOR, 0.50; 95% CI, 0.46–0.53) (Tables 2 and E11). Female patients compared with male patients had lower odds of ECMO at all income levels. When demographics for patients treated with VA ECMO, VV ECMO, and MV only were compared for 2019, similar differences based on demographic category were observed, although they appeared to be larger for VA ECMO and slightly smaller for VV ECMO (Table 3).
Table 3.
Differences in patient selection by extracorporeal membrane oxygenation type in 2019
| VA ECMO (n = 3,469) | VV ECMO (n = 1,816) | Mechanical Ventilation Only (n = 552,248) | |
|---|---|---|---|
| Female, % | 34.0 | 38.4 | 44.2 |
| Primary insurance, % | |||
| Medicare | 39.6 | 26.3 | 57.2 |
| Medicaid | 15.9 | 26.1 | 18.9 |
| Private insurance | 37.5 | 39.5 | 17.3 |
| Other | 7.0 | 8.1 | 7.7 |
| Median income of patient zip code, %* | |||
| Quartile 1 | 22.5 | 29.2 | 32.7 |
| Quartile 2 | 24.9 | 24.6 | 26.1 |
| Quartile 3 | 25.6 | 24.2 | 23.6 |
| Quartile 4 | 27.0 | 22.0 | 17.6 |
| Intersectional identity, %* | |||
| Female, income quartile 1 | 8.5 | 12.3 | 14.7 |
| Male, income quartile 1 | 14.0 | 16.8 | 18.0 |
| Female, income quartile 2 | 8.6 | 9.0 | 11.6 |
| Male, income quartile 2 | 16.3 | 15.7 | 15.6 |
| Female, income quartile 3 | 8.3 | 9.0 | 10.4 |
| Male, income quartile 3 | 17.3 | 15.2 | 13.2 |
| Female, income quartile 4 | 8.8 | 8.2 | 7.6 |
| Male, income quartile 4 | 18.1 | 13.8 | 10.0 |
Definition of abbreviations: ECMO = extracorporeal membrane oxygenation; VA = venoarterial; VV = venovenous.
Cutoffs for median income quartiles vary by year. Quartile 1 indicates the lowest income level, and quartile 4 is the highest income level. Full documentation of income levels can be found at https://www.hcup-us.ahrq.gov/db/vars/zipinc_qrtl/nrdnote.jsp.
With the SIDs database, we found patients identified as Black were less likely to receive ECMO than patients identified as White (aOR, 0.72; 95% CI, 0.65–0.79), but no differences were seen on the basis of ethnicity (Tables 4, E13, and E14). When the SIDs were used to replicate the primary analysis with more granular risk adjustment, similar sex, primary insurance, and income differences were observed with ECMO patient selection (Tables 4 and E15–E17).
Table 4.
State inpatient database analyses*
| ECMO (n = 5,254) | Mechanical Ventilation Only (n = 588,609) | aOR for ECMO (95% CI) | |
|---|---|---|---|
| Race, %† | |||
| White | 64.0 | 66.3 | Reference |
| Black | 14.7 | 17.9 | 0.72 (0.65–0.79) |
| Asian | 5.4 | 5.0 | 0.91 (0.78–1.07) |
| Native American/PI | 0.6 | 0.8 | 0.74 (0.48–1.14) |
| Other | 15.4 | 10.0 | 0.98 (0.88–1.09) |
| Ethnicity, %‡ | |||
| Hispanic | 17.4 | 17.5 | 0.92 (0.84–1.01) |
| Not Hispanic | 82.6 | 82.5 | Reference |
| Sex, %§ | |||
| Female | 34.0 | 43.1 | 0.77 (0.72–0.83) |
| Male | 66.0 | 56.9 | Reference |
| Primary insurance, %‖ | |||
| Medicare | 33.6 | 56.5 | 0.51 (0.46–0.56) |
| Medicaid | 21.3 | 20.5 | 0.57 (0.52–0.63) |
| Other | 38.6 | 16.3 | 0.46 (0.37–0.56 |
| Private insurance | 6.6 | 6.7 | Reference |
| Median income of patient zip code¶** | |||
| Quartile 1 | 25.6 | 36.0 | 0.54 (0.47–0.62) |
| Quartile 2 | 27.2 | 29.0 | 0.70 (0.61–0.80) |
| Quartile 3 | 23.9 | 20.9 | 0.83 (0.73–0.94) |
| Quartile 4 | 23.4 | 14.1 | Reference |
Definition of abbreviations: aOR = adjusted odds ratio; CI = confidence interval; ECMO = extracorporeal membrane oxygenation; PI = Pacific Islander.
The Nationwide Readmissions Database is a compilation of individual State Inpatient Databases (SIDs) also published by the Healthcare Cost and Utilization Project. This analysis focused on seven SIDs (Arizona, California, Florida, Iowa, Maryland, Mississippi, and New York) that contain race and ethnicity data as well as present on admission indicators for International Classification of Diseases, Tenth Revision, Clinical Modification, diagnosis codes, allowing more robust risk adjustment. See the data supplement for details of the subgroup analysis.
See Table E13 for full model parameter estimates including fit statistics.
See Table E14 for full model parameter estimates including fit statistics.
See Table E15 for full model parameter estimates including fit statistics.
See Table E16 for full model parameter estimates including fit statistics.
See Table E17 for full model parameter estimates including fit statistics.
Cutoffs for median income quartiles vary by year. Quartile 1 indicates the lowest income level, and quartile 4 is the highest income level. Full documentation of income levels can be found at https://www.hcup-us.ahrq.gov/db/vars/zipinc_qrtl/nrdnote.jsp.
The disparities observed in the primary analysis persisted with additional sensitivity analyses (Table 5) when the patient cohort was restricted to ECMO-capable hospitals (Tables E18–E20), restricted to nonelective admissions (Tables E21–E23), restricted to pneumonia or sepsis present on admission with the SIDs (Tables E24–E26), stratification and adjustment by hospital type (for profit, teaching, and urban/rural designation) (Tables E27–E32), etc. Results of additional sensitivity analyses can be found in the data supplement.
Table 5.
Key subgroup sensitivity analyses
| Exposure | ECMO | Mechanical Ventilation Only | aOR (95% CI) |
|---|---|---|---|
| ECMO-capable hospitals (n = 1,089,225)* | |||
| Female (%) | 36.1 | 43.5 | 0.72 (0.70–0.75) |
| Primary insurance, % | |||
| Medicare | 36.5 | 56.2 | 0.50 (0.48–0.52) |
| Medicaid | 18.2 | 17.3 | 0.54 (0.52–0.57) |
| Other | 7.2 | 7.8 | 0.64 (0.60–0.68) |
| Private insurance | 38.1 | 18.7 | Reference |
| Median income of patient zip code, %† | |||
| Quartile 1 | 24.5 | 32.0 | 0.63 (0.60–0.67) |
| Quartile 2 | 24.6 | 26.4 | 0.77 (0.73–0.81) |
| Quartile 3 | 25.8 | 23.3 | 0.87 (0.83–0.91) |
| Quartile 4 | 25.1 | 18.3 | Reference |
| Nonelective admissions (n = 2,032,224)‡ | |||
| Female, % | 35.5 | 44.5 | 0.71 (0.68–0.73) |
| Primary insurance, % | |||
| Medicare | 33.5 | 57.5 | 0.47 (0.44–0.49) |
| Medicaid | 19.8 | 17.8 | 0.55 (0.52–0.57) |
| Other | 7.8 | 7.7 | 0.64 (0.60–0.68) |
| Private insurance | 38.9 | 17.1 | Reference |
| Median income of patient zip code, %† | |||
| Quartile 1 | 24.8 | 32.7 | 0.62 (0.59–0.66) |
| Quartile 2 | 24.3 | 26.7 | 0.76 (0.72–0.80) |
| Quartile 3 | 25.6 | 23.2 | 0.85 (0.81–0.90) |
| Quartile 4 | 25.3 | 17.3 | Reference |
| Pneumonia or sepsis present on admission§ (n = 294,401) | |||
| Female, % | 35.6 | 44.0 | 0.75 (0.67–0.84) |
| Primary insurance, % | |||
| Medicare | 22.5 | 60.9 | 0.39 (0.33–0.45) |
| Medicaid | 29.0 | 19.9 | 0.57 (0.50–0.65) |
| Other | 7.6 | 5.4 | 0.54 (0.44–0.67) |
| Private insurance | 41.0 | 13.9 | Reference |
| Median income of patient zip code, %† | |||
| Quartile 1 | 25.6 | 36.7 | 0.53 (0.43–0.66) |
| Quartile 2 | 30.4 | 28.8 | 0.81 (0.66–1.00) |
| Quartile 3 | 22.5 | 20.5 | 0.92 (0.74–1.14) |
| Quartile 4 | 21.6 | 14.1 | Reference |
Definition of abbreviations: aOR = adjusted odds ratio; CI = confidence interval; ECMO = extracorporeal membrane oxygenation.
See Tables E18–E20 for full model details.
Cutoffs for median income quartiles vary by year. Quartile 1 indicates the lowest income level, and quartile 4 is the highest income level. Full documentation of income levels can be found at https://www.hcup-us.ahrq.gov/db/vars/zipinc_qrtl/nrdnote.jsp.
See Tables E21–E23 for full model details.
See Tables E24–E26 for full model details. Pneumonia/sepsis analysis was performed using the state inpatient databases.
Discussion
Using a nationally representative database with multiple subgroup and sensitivity analyses, we observed significant disparities in patient selection for ECMO. Female patients, patients with Medicaid, and patients living in the lowest-income neighborhoods were far less likely to receive ECMO than male patients, patients with private insurance, and patients living in the highest-income neighborhoods. The differences in ECMO use were even larger based on an intersectional identity of sex and neighborhood income. Differences in ECMO use persisted when ECMO was broken down into VA and VV, although they were slightly smaller for patients treated with VV ECMO. Although a population-level analysis with the NRD can only approximate real-world experiences, the multitude of sensitivity findings across different cohorts and statistical approaches is strongly suggestive that female patients, patients with Medicaid, and patients living in lower-income neighborhoods are less likely to receive ECMO. These findings raise concern about the manner in which ECMO, a rapidly growing critical care resource, is being implemented in the United States (3, 10).
Several studies have already highlighted disparities in several areas of critical care. Multiple investigations have shown that female patients with ARDS are less likely to receive low tidal volume ventilation (15–17, 41). Moreover, female patients and patients with Medicaid or who are uninsured are less likely to receive advanced heart failure therapies (19–26). In these studies, investigators have suggested that differences in recognition or treatment of advanced disease may stem from availability of resources as well as provider bias. We speculate that several factors contribute to the differential use in ECMO by sex, insurance, and neighborhood income that was observed in this study: reduced access, restrictive transfer practices, patient preferences, and implicit provider bias.
In this study, patients with Medicaid compared with private insurance and patients living in lower-income versus higher-income neighborhoods were significantly less likely to be treated with ECMO. We speculate that these groups likely have reduced access to hospitals that perform ECMO and therefore are less likely to receive ECMO. Only about half of patients receiving MV are admitted to ECMO-capable hospitals. ECMO is one of the most resource-intensive interventions in health care, most hospitals are not ECMO capable, ECMO-capable hospitals tend to be clustered in major cities and far from rural areas (fewer than 15 cases of ECMO in rural hospitals in this study), and few safety net hospitals have ECMO capabilities (4, 42, 43). Patients with Medicaid, those living in lower-income neighborhoods, and patients identified as Black are more likely to use safety net hospitals. Therefore, some of the observed disparity may be less related to specific patient selection patterns and more related to ECMO availability in certain types of hospitals and in certain geographic regions. Patients living in rural areas likely experienced even greater disparities in access to ECMO, although we were unable to quantify this because there were fewer than 15 patients in rural hospitals who received ECMO throughout the entire study period. However, disparities persisted across other hospital types and when adjusting for hospital characteristics.
We speculate that a second major driver of the observed differences in ECMO use relates to restrictive transfer practices and is intricately linked to issues related to ECMO access faced by lower-income patients with Medicaid. When patients with advanced respiratory or cardiovascular failure present to a hospital without ECMO capabilities, the goal is often to transfer such patients to ECMO-capable hospitals, and patients who are not transferred can have higher mortality (6). However, ICU beds in ECMO-capable hospitals can often be in short supply, and some patients may be accepted for transfer over others (9, 27). The Emergency Medical Treatment and Labor Act is designed to prevent U.S. hospitals from considering insurance status or ability to pay in hospital transfer decisions when the receiving hospital can provide a higher level of care needed by the patient (e.g., ECMO) (44). Despite the Emergency Medical Treatment and Labor Act, several studies have shown that racial and ethnic minorities, female patients, and patients with Medicaid or those who are uninsured are less likely to be accepted for transfer even for conditions with a potential mortality benefit associated with transfer such as ECMO (45–47). We speculate that female patients and patients with Medicaid or who are uninsured may be less likely to receive ECMO because they are less likely to be referred for transfer by hospitals without ECMO capabilities or accepted for transfer by ECMO-capable hospitals. Although inappropriate restrictive transfer practices may account for some of the disparities, the fact that disparities persisted even within ECMO-capable hospitals suggests that ECMO availability and restrictive transfer practices only partially account for disparities observed in this study.
It is also likely that patient and family preferences partially contribute to the observed differences in ECMO use. Female patients have been shown to be more likely to have advanced directives that limit aggressive care (24, 48). Moreover, previous studies have shown that female patients are less likely to receive aggressive therapies that require substantial support during or after treatment than male patients because of a perceived lack of social support from spouses, partners, and families, and this observation may contribute to sex differences (22, 23, 25).
Last, we speculate that implicit provider bias may also contribute to some of the observed differences in ECMO use. Several studies have demonstrated that female patients, patients who are underinsured, and patients in lower socioeconomic strata are less likely to be identified as having ARDS, less likely to be treated with lung-protective ventilation settings, and less likely to receive more common critical care interventions (41, 49–51). These studies suggest that the differences may stem from implicit provider bias and misconceptions about the incidence of diseases such as ARDS in specific populations. We speculate that the same provider-based factors potentially limit female patients, patients with Medicaid, and patients from lower-income neighborhoods from being selected for ECMO or referred to ECMO-capable hospitals.
A major limitation of this study is the potential for residual confounding. Although the NRD is the largest dataset with a 100% representation of ECMO cases in the states included, importantly including low case volume hospitals that may not be represented in national registries, it is limited in its ability to assess for various confounders such as severity of acute illness. Some studies suggest that patients from lower socioeconomic strata may present with higher severity of illness, whereas other studies have found no difference (52, 53). In the primary analysis, crude HCUP-derived markers of severity of illness were used to adjust for severity of illness, but these measures have extremely poor discrimination among patients receiving MV. Use of the acute organ failures present on admission in the SIDs database better approximates physiologic severity-of-illness markers such as Sequential Organ Failure Assessment in predicting mortality among ICU patients and resulted in the same disparities seen in the primary analyses (38). However, almost all measures of severity of illness (e.g., acute organ failures, Sequential Organ Failure Assessment, and even the RESP score) were designed to predict mortality and not the outcome in this study, namely receipt of ECMO.
Because no clear “score” exists to adjust analyses with receipt of ECMO as the outcome, we employed multiple sensitivity analyses to reduce the difference between exposure groups and to create a more homogeneous population. This included IPTW analyses that dramatically reduced differences in measured covariates between exposure groups and confirmed results from the primary analysis. The sensitivity analyses also included analyzing disparities among nonelective admissions only (likely reducing post–cardiac surgery and bridge to transplant admissions) and among patients with pneumonia or sepsis on admission. Both approaches likely created more homogeneous populations. Last, we quantified the potential for unmeasured confounding by reporting the E-value estimate, a measure of the strength that an unmeasured confounder would need to have to shift an observed difference to the null, for each of the primary analyses. This approach showed that only extremely large unmeasured confounders would nullify the associations observed in this study. Although the potential for residual confounding will always exist in retrospective observational studies, the multitude of methods employed in this study provides far greater confidence in the primary findings.
Limitations
In addition to unmeasured confounding, this study has several additional limitations. The NRD is an administrative database relying on billing codes that could result in misclassification bias. The SIDs analysis did find racial disparities in ECMO patient selection, but we were unable to differentiate between hospital-assigned race and self-identified race. Although the difference could lead to some bias in the estimates, because most patients were initiated on MV close to admission, we suspect that a substantial percentage of patients may not have been able to self-identify their race. We chose to use the NRD because it is the largest fully representative dataset that captures ECMO at all facilities as well as the larger pool from which ECMO patients are selected (i.e., patients receiving MV). We also did not have access to advance directives that might limit a patient’s eligibility for ECMO. However, there is no evidence of such large differences in rates of advance directives between demographic groups that could account for the differences in ECMO use observed in this study. Although we were able to differentiate VA ECMO from VV ECMO in 2019, we could not determine the “at-risk” population for each, nor were we able to further subdivide the ECMO population based on indication. Although not technically a limitation, the question of model overfit is also an issue, given the large number of patients in the cohort and covariates in the primary analysis. To address the specific issue of model fit, we conducted several additional sensitivity analyses with different iterations of the least absolute shrinkage and selection operator method, which confirmed findings from the primary analysis. We also conducted extensive sensitivity analyses that increase the possibility of a type I statistical error for at least one of the statistical tests. However, because all of the analyses arrived at the same conclusion, there can be greater confidence in the findings.
Conclusions
ECMO is a rapidly growing critical care resource, and some data would suggest that access to ECMO significantly improved mortality during the COVID-19 pandemic (3, 6). Despite growth in ECMO programs, little effort has been made to ensure equitable access, despite multiple examples of disparities in the provision of other critical care resources and treatments. This study highlights multiple demographic disparities in adult patient selection for ECMO that might be driven by lack of access, restrictive transfer policies, patient preference, and implicit provider bias. Multiple major medical societies have issued statements highlighting the critical need to understand and address healthcare inequities across the spectrum of care, many even highlighting the fact that any inequity for some patients is a threat to all patients (54–58). Despite several limitations, this study highlights the potential for large-scale disparities in adult ECMO patient selection that warrant further investigations to ensure equitable access.
Footnotes
Supported by National Institutes of Health grant K23HL141704 (primary funding source [A.B.M.]) and by National Institutes of Health grant R01NR016459 (I.S.D.). The views expressed in this article represent those of the authors and do not communicate an official position or opinion of the National Institutes of Health. The sponsors had no role in study design, data analysis, or manuscript preparation.
Author Contributions: A.B.M., J.K.T., T.C.L., and I.S.D. conceived the study. A.B.M. was responsible for data collection and analysis. A.B.M., J.K.T., G.D., and I.S.D. were responsible for data interpretation. J.K.T. and A.B.M. drafted the article. A.B.M., J.K.T., G.D., and I.S.D. provided critical revisions and meaningful input for the final draft. A.B.M. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. A.B.M. conducted all aspects of data analysis. All authors approved the final draft of the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. CESAR trial collaboration Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet . 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 2. Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. EOLIA Trial Group, REVA, and ECMONet Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med . 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 3. Stentz MJ, Kelley ME, Jabaley CS, O’Reilly-Shah V, Groff RF, Moll V, et al. Trends in extracorporeal membrane oxygenation growth in the United States, 2011-2014. ASAIO J . 2019;65:712–717. doi: 10.1097/MAT.0000000000000872. [DOI] [PubMed] [Google Scholar]
- 4. Thiagarajan RR, Barbaro RP, Rycus PT, Mcmullan DM, Conrad SA, Fortenberry JD, et al. ELSO member centers Extracorporeal Life Support Organization registry international report 2016. ASAIO J . 2017;63:60–67. doi: 10.1097/MAT.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 5. Bertini P, Guarracino F, Falcone M, Nardelli P, Landoni G, Nocci M, et al. ECMO in COVID-19 patients: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth . 2022;36:2700–2706. doi: 10.1053/j.jvca.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gannon WD, Stokes JW, Francois SA, Patel YJ, Pugh ME, Benson C, et al. Association between availability of ECMO and mortality in COVID-19 patients eligible for ECMO: a natural experiment. Am J Respir Crit Care Med . 2022;205:1354–1357. doi: 10.1164/rccm.202110-2399LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCarthy FH, McDermott KM, Kini V, Gutsche JT, Wald JW, Xie D, et al. Trends in U.S. extracorporeal membrane oxygenation use and outcomes: 2002-2012. Semin Thorac Cardiovasc Surg . 2015;27:81–88. doi: 10.1053/j.semtcvs.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rush B, Wiskar K, Berger L, Griesdale D. Trends in extracorporeal membrane oxygenation for the treatment of acute respiratory distress syndrome in the United States. J Intensive Care Med . 2017;32:535–539. doi: 10.1177/0885066616631956. [DOI] [PubMed] [Google Scholar]
- 9.Stunson M.2021. https://www.star-telegram.com/news/coronavirus/article254459828.html
- 10.Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project. 2022. https://www.hcup-us.ahrq.gov/db/nation/nis/HCUP-NIS2016-2020-DXandPRfreqs.xlsx
- 11.Learish J.2020. https://www.cbsnews.com/pictures/most-expensive-medical-procedures-without-insurance
- 12. Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med . 2014;189:1374–1382. doi: 10.1164/rccm.201311-2023OC. [DOI] [PubMed] [Google Scholar]
- 13. Brunet J, Valette X, Buklas D, Lehoux P, Verrier P, Sauneuf B, et al. Predicting survival after extracorporeal membrane oxygenation for ARDS: an external validation of RESP and PRESERVE scores. Respir Care . 2017;62:912–919. doi: 10.4187/respcare.05098. [DOI] [PubMed] [Google Scholar]
- 14. Lat TI, McGraw MK, White HD. Gender differences in critical illness and critical care research. Clin Chest Med . 2021;42:543–555. doi: 10.1016/j.ccm.2021.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McNicholas BA, Madotto F, Pham T, Rezoagli E, Masterson CH, Horie S, et al. LUNG SAFE Investigators and the ESICM Trials Group Demographics, management and outcome of females and males with acute respiratory distress syndrome in the LUNG SAFE prospective cohort study. Eur Respir J . 2019;54:1900609. doi: 10.1183/13993003.00609-2019. [DOI] [PubMed] [Google Scholar]
- 16. Walkey AJ, Wiener RS. Risk factors for underuse of lung-protective ventilation in acute lung injury. J Crit Care . 2012;27:323.e1–323.e9. doi: 10.1016/j.jcrc.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Valentin A, Jordan B, Lang T, Hiesmayr M, Metnitz PG. Gender-related differences in intensive care: a multiple-center cohort study of therapeutic interventions and outcome in critically ill patients. Crit Care Med . 2003;31:1901–1907. doi: 10.1097/01.CCM.0000069347.78151.50. [DOI] [PubMed] [Google Scholar]
- 18. Reinikainen M, Niskanen M, Uusaro A, Ruokonen E. Impact of gender on treatment and outcome of ICU patients. Acta Anaesthesiol Scand . 2005;49:984–990. doi: 10.1111/j.1399-6576.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 19. Breathett K, Yee E, Pool N, Hebdon M, Crist JD, Yee RH, et al. Association of gender and race with allocation of advanced heart failure therapies. JAMA Netw Open . 2020;3:e2011044. doi: 10.1001/jamanetworkopen.2020.11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Colvin M, Smith JM, Hadley N, Skeans MA, Uccellini K, Goff R, et al. OPTN/SRTR 2018 annual data report: heart. Am J Transplant . 2020;20:340–426. doi: 10.1111/ajt.15676. [DOI] [PubMed] [Google Scholar]
- 21. Givens RC, Dardas T, Clerkin KJ, Restaino S, Schulze PC, Mancini DM. Outcomes of multiple listing for adult heart transplantation in the United States: analysis of OPTN data from 2000 to 2013. JACC Heart Fail . 2015;3:933–941. doi: 10.1016/j.jchf.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Knoepke CE, Siry-Bove B, Mayton C, Latimer A, Hart J, Allen LA, et al. Variation in left ventricular assist device postdischarge caregiver requirements: results from a mixed-methods study with equity implications. Circ Heart Fail . 2022;15:e009583. doi: 10.1161/CIRCHEARTFAILURE.122.009583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ladin K, Emerson J, Berry K, Butt Z, Gordon EJ, Daniels N, et al. Excluding patients from transplant due to social support: results from a national survey of transplant providers. Am J Transplant . 2019;19:193–203. doi: 10.1111/ajt.14962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vallabhajosyula S, Dunlay SM, Barsness GW, Miller PE, Cheungpasitporn W, Stulak JM, et al. Sex disparities in the use and outcomes of temporary mechanical circulatory support for acute myocardial infarction-cardiogenic shock. CJC Open . 2020;2:462–472. doi: 10.1016/j.cjco.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Steinberg RS, Nayak A, Burke MA, Aldridge M, Raja Laskar S, Bhatt K, et al. Association of race and gender with primary caregiver relationships and eligibility for advanced heart failure therapies. Clin Transplant . 2022;36:e14502. doi: 10.1111/ctr.14502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Flint K, Chaussee EL, Henderson K, Breathett K, Khazanie P, Thompson JS, et al. Social determinants of health and rates of implantation for patients considering destination therapy left ventricular assist device. J Card Fail . 2021;27:497–500. doi: 10.1016/j.cardfail.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fink S.2021.
- 28.Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project. https://www.hcup-us.ahrq.gov/nrdoverview.jsp
- 29.Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project. https://www.hcup-us.ahrq.gov/db/nation/nrd/nrddde.jsp
- 30. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care . 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidityicd10/comorbidity_icd10.jsp
- 32. Mathur MB, Ding P, VanderWeele TJ. Website and R package for computing E-values. Epidemiology . 2018;29:e45–e47. doi: 10.1097/EDE.0000000000000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med . 2017;167:268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 34. Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA . 2019;321:602–603. doi: 10.1001/jama.2018.21554. [DOI] [PubMed] [Google Scholar]
- 35.Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project. http://www.hcup-us.ahrq.gov/sidoverview.jsp
- 36.Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project. https://www.hcup-us.ahrq.gov/db/state/siddist/Introduction_to_SID.pdf
- 37.California Department of Health Care Access and Information. https://hcai.ca.gov/data-and-reports/
- 38. Bosch NA, Law AC, Rucci JM, Peterson D, Walkey AJ. Predictive validity of the Sequential Organ Failure Assessment score versus claims-based scores among critically ill patients. Ann Am Thorac Soc . 2022;19:1072–1076. doi: 10.1513/AnnalsATS.202111-1251RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tibshirani R. Regression shrinkage and selection via the Lasso. J R Stat Soc B . 1996;58:267–288. [Google Scholar]
- 40.López N, Gadsden VL.2016. https://nam.edu/health-inequities-social-determinants-and-intersectionality/
- 41. Modra LJ, Higgins AM, Pilcher DV, Bailey MJ, Bellomo R. Sex differences in mortality of ICU patients according to diagnosis-related sex balance. Am J Respir Crit Care Med . 2022;206:1353–1360. doi: 10.1164/rccm.202203-0539OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farmer B.2021. https://www.npr.org/sections/health-shots/2021/09/06/1033832562/covid-icu-ecmo-life-support-shortage-hospitals
- 43. Gandjian M, Williamson C, Xia Y, Maturana C, Chervu N, Verma A, et al. Association of hospital safety net status with outcomes and resource use for extracorporeal membrane oxygenation in the United States. J Intensive Care Med . 2022;37:535–542. doi: 10.1177/08850666211007062. [DOI] [PubMed] [Google Scholar]
- 44.Centers for Medicare and Medicaid Services. 2022. https://www.cms.gov/Regulations-and-Guidance/Legislation/EMTALA
- 45. Hanmer J, Lu X, Rosenthal GE, Cram P. Insurance status and the transfer of hospitalized patients: an observational study. Ann Intern Med . 2014;160:81–90. doi: 10.7326/M12-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shannon EM, Schnipper JL, Mueller SK. Identifying racial/ethnic disparities in interhospital transfer: an observational study. J Gen Intern Med . 2020;35:2939–2946. doi: 10.1007/s11606-020-06046-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shannon EM, Zheng J, Orav EJ, Schnipper JL, Mueller SK. Racial/ethnic disparities in interhospital transfer for conditions with a mortality benefit to transfer among patients with Medicare. JAMA Netw Open . 2021;4:e213474. doi: 10.1001/jamanetworkopen.2021.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mehta AB, Cooke CR, Douglas IS, Lindenauer PK, Wiener RS, Walkey AJ. Association of early do-not-resuscitate orders with unplanned readmissions among patients hospitalized for pneumonia. Ann Am Thorac Soc . 2017;14:103–109. doi: 10.1513/AnnalsATS.201608-617OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lyon SM, Benson NM, Cooke CR, Iwashyna TJ, Ratcliffe SJ, Kahn JM. The effect of insurance status on mortality and procedural use in critically ill patients. Am J Respir Crit Care Med . 2011;184:809–815. doi: 10.1164/rccm.201101-0089OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Malnoske ML, Quill CM, Barwise AK, Pietropaoli AP. Disparities in lung-protective ventilation in the United States. Cureus . 2022;14:e29834. doi: 10.7759/cureus.29834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Swart P, Deliberato RO, Johnson AEW, Pollard TJ, Bulgarelli L, Pelosi P, et al. Impact of sex on use of low tidal volume ventilation in invasively ventilated ICU patients—a mediation analysis using two observational cohorts. PLoS One . 2021;16:e0253933. doi: 10.1371/journal.pone.0253933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bein T, Hackner K, Zou T, Schultes S, Bösch T, Schlitt HJ, et al. Socioeconomic status, severity of disease and level of family members’ care in adult surgical intensive care patients: the prospective ECSSTASI study. Intensive Care Med . 2012;38:612–619. doi: 10.1007/s00134-012-2463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Quenot JP, Helms J, Labro G, Dargent A, Meunier-Beillard N, Ksiazek E, et al. IVOIRE Trial Investigators and the CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) Influence of deprivation on initial severity and prognosis of patients admitted to the ICU: the prospective, multicentre, observational IVOIRE cohort study. Ann Intensive Care . 2020;10:20. doi: 10.1186/s13613-020-0637-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.CHEST. 2020. https://www.chestnet.org/Newsroom/CHEST-News/2020/06/CHESTs-Pledge-for-Equity
- 55. Celedón JC, Burchard EG, Schraufnagel D, Castillo-Salgado C, Schenker M, Balmes J, et al. American Thoracic Society and the National Heart, Lung, and Blood Institute An American Thoracic Society/National Heart, Lung, and Blood Institute workshop report: addressing respiratory health equality in the United States. Ann Am Thorac Soc . 2017;14:814–826. doi: 10.1513/AnnalsATS.201702-167WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Celedón JC, Roman J, Schraufnagel DE, Thomas A, Samet J. Respiratory health equality in the United States. The American Thoracic Society perspective. Ann Am Thorac Soc . 2014;11:473–479. doi: 10.1513/AnnalsATS.201402-059PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.American Medical Association. https://www.ama-assn.org/system/files/2021-05/ama-equity-strategic-plan.pdf
- 58.American Heart Association. https://www.heart.org/en/about-us/2024-health-equity-impact-goal